- 1Centre for Research on Environmental Ecology and Fish Nutrition (CREEFN) of the Ministry of Agriculture and Rural Affairs, Shanghai Ocean University, Shanghai, China
- 2Key Laboratory of Freshwater Aquatic Genetic Resources, Ministry of Agriculture and Rural Affairs, Shanghai Ocean University, Shanghai, China
- 3Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-culture of Aquaculture Animals, Shanghai Ocean University, Shanghai, China
The characteristics of macrobenthic community structure can accurately indicate the ecological health of aquatic environments. To elucidate the spatiotemporal responses of macrobenthic communities and environmental factors in reservoirs, this study investigated macrobenthos and environmental parameters in Yinghu Lake during spring (May), summer (August), and autumn (November). The results showed that the trophic level index (TLI), total nitrogen (TN) and total phosphorus (TP) were significantly higher and pH was significantly lower (P < 0.05) at the developed sites (DS) than at the undeveloped sites (UDS). The survey identified 32 macrobenthos species representing 3 phylums and 5 orders. PERMANOVA analyses showed that the macrobenthic community structure of Yinghu Lake differed significantly between regions, Limnodrilus hoffmeisteri from the gathering collectors (20.47%) and Corbicula fluminea from the filtering collectors (7.82%) were the primary species driving the differences. The results of the two-way ANOVA indicated that species richness, the Margalef index (D), and the functional feeding group Margalef index (FFG-D) were significantly lower in summer than in autumn (P < 0.05). FFG-D was also significantly lower at the DS compared to the UDS (P < 0.05), while the interaction effects of season and region on these indicators were not significant (P > 0.05). Redundancy analysis (RDA) and generalized additive modelling (GAM) indicated that the permanganate index (CODMn) and total dissolved solids altered the macrobenthic community structure towards oligochaete and gathering collectors-dominated assemblages. Moreover, macrobenthic diversity was constrained by high total dissolved solids concentrations, sediment organic carbon (OC_s), soluble reactive phosphorus (SRP), low sediment total phosphorus (TP_s), high water temperature, and deep water. In summary, the spatiotemporal variations in water quality and macrobenthos communities in the reservoir were influenced by natural conditions and anthropogenic disturbances. This study provides valuable insights into the spatiotemporal dynamics of macrobenthic communities and contributes to a more comprehensive understanding of the role of biodiversity in maintaining the stability of large reservoir ecosystems.
1 Introduction
Reservoirs are artificial bodies of water created by constructing barriers at narrow points, primarily for storing excess water during floods and regulating water flow (Zhang et al., 2021). Since the 1950s, China has been actively promoting the construction of reservoirs. To date, China has built more than 100,000 reservoirs of various types, ranking first in the world (Song et al., 2022). While huge reservoirs which are larger than 100,000,000 m3 in volume, comprise only 5% of the total number, they account for 92% of the total capacity, significantly impacting flood management, water supply, irrigation, power generation, fish farming, river sediment regulation, and ecological enhancement (Kim et al., 2023).
The subtropical monsoon climate is distinguished by irregular weather and substantial seasonal fluctuations in rainfall, which result from the alternating influence of tropical oceanic and polar continental air masses (Qian et al., 2024). During hot and rainy summer conditions, the accumulation of precipitation in the river basin or the thawing of snow on mountain glaciers can lead to an increase in river levels (Wang et al., 2023). The rivers carry large amounts of sediment into the reservoirs during this process, and the water, chemical and even biological composition of the reservoirs is altered to a large extent by strong dynamic disturbances and high nutrient influxes (Ding et al., 2022; Drummond et al., 2022). The study conducted by (Tang et al., 2023) on the Yangtze-Three Gorges Reservoir demonstrated that fluctuations in flood control can increase the nitrogen-phosphorus ratio in the water, potentially leading to eutrophication in the upper part of the reservoir.
Moreover, human economic activities have the potential to cause significant alterations in land use patterns within reservoir watersheds. As the human population increases within the reservoir watershed so increases anthropogenic development (Chu and Karr, 2017; Verburg et al., 2015). Urbanized land use can increase the proportion of impermeable surfaces, significantly affecting nutrient and sediment levels entering watercourses, leading to increased nutrient loading to water bodies (Daramola et al., 2022). These activities have the potential to impact all facets of ecological services in reservoir systems, encompassing nutrient cycling, energy transfer, and biodiversity (Raymond et al., 2016; Wang et al., 2023). Nevertheless, reservoir ecosystem is highly complex. Therefore, it is frequently advantageous to identify dependable indicators that can furnish researchers with a comprehensive insight into reservoir systems affected by land-use changes and seasons.
Macrobenthos is a valuable and excellent tool for monitoring projects in freshwater ecosystems (Li et al., 2016). Macrobenthic fauna is highly sensitive to aquatic ecosystem stressors such as pollutant levels, fragmentation and eutrophication, and are influenced by physico-chemical parameters based on species diversity, allowing them to show marked differences in habitat preferences (Mely et al., 2023). However, when just taxonomic structural indicators, such as the composition and quantity of aquatic species, were utilized as response variables, macrobenthic fauna did not exhibit a sensitive reaction to environmental pressures. In contrast, functional structural indicators were able to detect and respond to these stresses with sensitivity, which provides a complementary relationship between environmental stress and aquatic organisms (Lamouroux and Souchon, 2002). The concept of functional feeding group (FFG) of macrobenthos was first proposed by Cummins and Klug (1979) in the 1970s, and their taxonomic composition is closely related to local environmental factors such as water quality, hydrology, substrate quality and nutrient inputs. Over the past few years, there has been a growing interest in examining the influence of ecosystem stressors influence macrobenthos FFG. The study by Bendary et al. (2023) noted that the distribution of FFG can be affected by the pollution level in a water body. However, there are fewer studies on the effects of seasonal and anthropogenic disturbances on the spatial and temporal distribution of macrobenthic organisms and their FFG in deep-water reservoirs.
Yinghu Lake is a significant reservoir located in China, rated as an AAAA picturesque area. It is classified as a Large-Scale (Type 1) Reservoir due to its total storage capacity exceeding 1 billion m³, its ability to irrigate over 100,000 hectares of farmland, provide flood control for over 133,000 hectares of area, and its installed hydropower capacity of no less than 1.2 million kilowatts, alongside its critical role in supporting urban and industrial water needs. It is located 16 kilometers southwest of the city of Ankang, Shanxi Province, China (Liu et al., 2019). The aquatic environment of Yinghu Lake, a crucial water source protection region for the South-to-North Water Diversion Project, is significantly influenced by seasonal precipitation fluctuations and anthropogenic activities, but the spatial and temporal variations within its ecosystem remain unidentified. Therefore, this work analyses the spatial and temporal fluctuations of the reservoir water environment, macrobenthic communities, and the responses of macrobenthic communities to environmental factors, using Yinghu Lake, China, as the case study. The primary aims of the study were (i) to analyze the spatial and temporal variations of aquatic environmental factors in the reservoir region; (ii) to investigate the effects of spatiotemporal variations in deep-water reservoirs on the distribution of macrobenthic communities, including FFG; and (iii) to elucidate the impact of diverse environmental drivers on macrobenthic communities. Our findings will establish a scientific basis for the strategic utilization and management of benthic biological resources in large reservoirs, as well as for monitoring ecological quality.
2 Materials and methods
2.1 Study area
Yinghu Lake, located in the upper reaches of the Han River, the largest tributary of the Yangtze River, is the largest freshwater lake in northwest China. It has a controlled catchment area of 35,700 km², a total storage capacity of 3.2 billion m³, a maximum flood storage capacity of 980 million m³, a normal water level of 330 m, an average depth of 48.5 m, and a maximum depth of 80 m. The reservoir supplies 75% of the water for the central route of the South-to-North Water Diversion Project (Liu et al., 2019). As a large multipurpose reservoir integrating power generation, flood control, water supply, navigation, and tourism, Yinghu Lake was included in China’s first batch of 15 key lake ecological and environmental protection projects in 2014. The reservoir area encompasses 34 administrative villages, where untreated domestic sewage from local communities is discharged directly into the lake. Additionally, water quality is further affected by tourism-related waste, including increased domestic sewage from tourist facilities, trash left by visitors, pollutants from recreational activities, as well as feed and waste from aquaculture and livestock farming, and agricultural runoff, all of which exacerbate nutrient enrichment. Furthermore, Yinghu Lake is located within the subtropical monsoon climate zone, characterized by significant seasonal hydrological variations (Liu et al., 2019). During summer (July-September), rainfall accounts for approximately 50% of the annual precipitation in the watershed. The reservoir manages inflowing flood peaks to adapt to variations in rainfall. These anthropogenic activities and natural changes collectively influence water quality, nutrient levels, and macrobenthic communities.
We established twelve sampling sites along the riverine sections of Yinghu Lake, evenly distributed from upstream to downstream, and covering the main tributaries. And we sampled macrobenthos and their ecological variables at these sites (Figure 1). We obtained land use data from the Zenodo website (https://zenodo.org/), which contains the 2022 Yinghu Lake watershed surface cover types at a 30 m resolution. We used ArcGIS (ver. 10.8) to calculate land use within a 1 km radius around each sampling point (Nelson Mwaijengo et al., 2020) and determined the percentage coverage of land use types in the buffer zone. We categorized the sampling sites into developed sites (DS) including S1, S4, S5, S6, S7, S8, S10, and S11, and undeveloped sites (UDS) including S2, S3, S9, and S12 based on the presence or absence of impervious surfaces. The actual land use conditions of each sampling site are presented in Supplementary Table A.1.
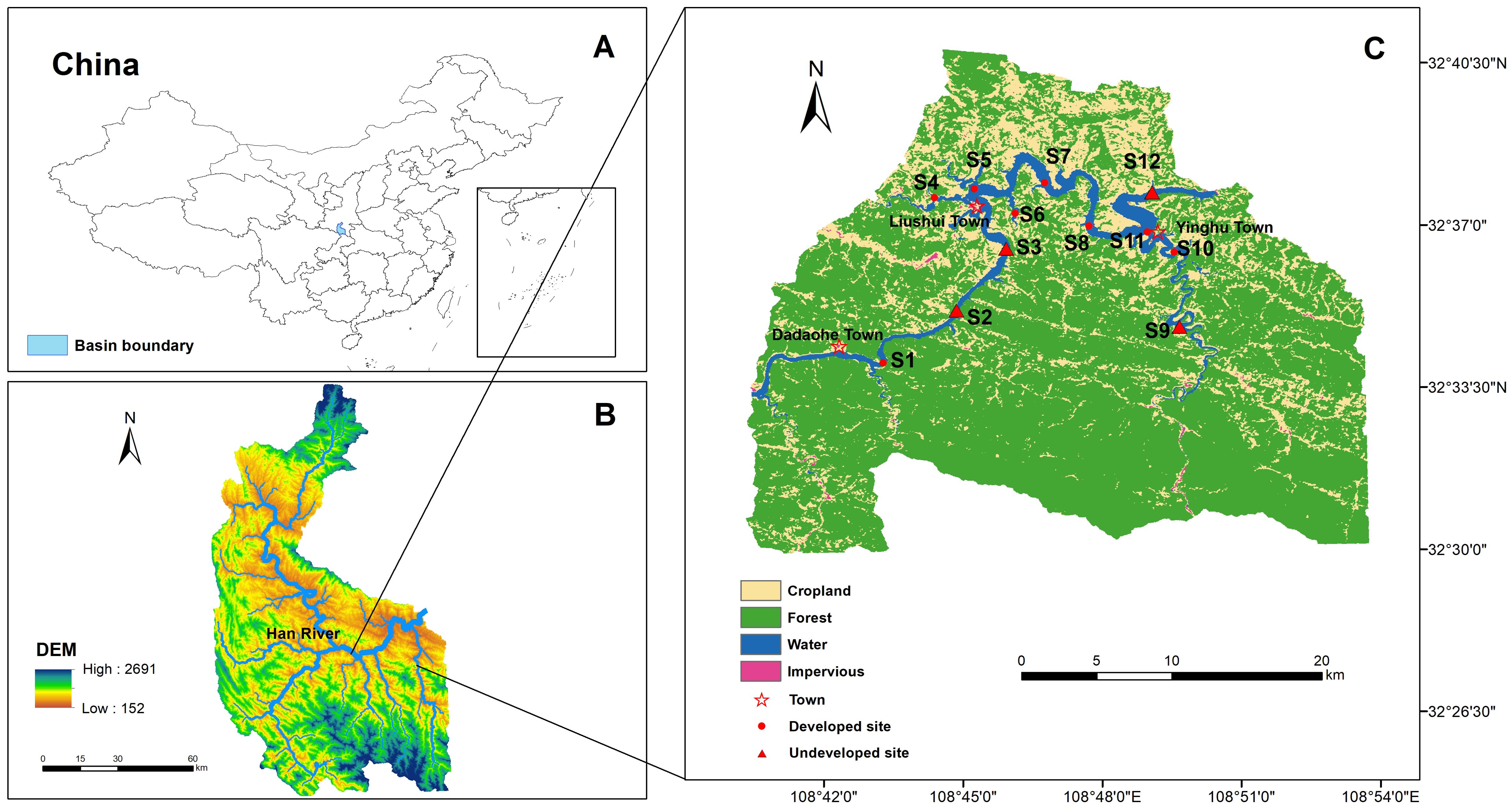
Figure 1. Distribution of land use types and sampling sites in the Yinghu Lake watershed. (A) Location of Yinghu Lake Basin in China, (B) Map of the Yinghu Lake Basin, (C) Sampling sites in the Yinghu Lake and the surrounding land use types.
2.2 Water sample collection and measurement of physicochemical parameters
We conducted sampling in 2023 during February (winter), May (spring), August (summer), and November (autumn). The winter data were excluded from the study because the plastic storage bags for sediment samples at multiple sites ruptured, leading to the mixing of sediment samples from different sites and compromising subsequent data analysis. To prevent such issues, samples from each site were stored separately during the other seasons, ensuring data integrity for later measurements and correlation analyses with macrobenthic organisms. Water samples were collected in layers (0 m, 4 m, 8 m, 12 m, 16 m and 20 m) using a 5L acrylic water sampler (GWS-117, CN).
Total phosphorus (TP), soluble reactive phosphorus (SRP), total nitrogen (TN), ammoniacal nitrogen (NH4+-N), nitrate nitrogen (NO3–N), nitrite nitrogen (NO2–N) and the permanganate index (CODMn) were measured according to Standard Methods (Rice et al., 2012). We measured water temperature (hereafter Temperature), dissolved oxygen (DO), conductivity (EC), total dissolved solids (TDS), pH, and turbidity (hereafter Turb) using a multi-parameter water quality analyzer (YSI ProDSS, USA), and calculated the mean values for each water layer for subsequent analyses. We determined chlorophyll a (Chl-a) using the BBE Algae Analyzer (FluoroProbe, GER), Secchi depth (SD) using a Secchi disk, and water depth (Depth) using a sonar depth gauge (SM-5A, USA).
2.3 Macrobenthos sample collection, identification, classification based on FFG
We collected macrobenthos in the study area using a 1/16 m² grab sampler (PBS-411, CN), taking two collections per sample site and mixing them into one sample. At each sample point, we cleaned the samples by filtering them through a nylon mesh with a pore size of 450 μm. We placed the residue into 250 ml plastic bottles and immediately fixed it in 10% formalin. Subsequently, we rinsed off the formalin, picked out the benthos, and collected them in petri dishes. We counted all organisms at each site and identified them to the lowest possible taxonomic unit (Thorp and Covich, 2010). We classified FFG based on their taxonomic characteristics and feeding types (Barbour et al., 1996), with major FFG including predators, scrapers, filtering collectors, and gathering collectors (Pratiwi et al., 2024).
2.4 Sediment collection and determination
During the collection of macrobenthos samples, we simultaneously collected small sediment samples and transported them to the laboratory under refrigerated storage at 4 °C. In the laboratory, we naturally air-dried the sediment samples, ground them, and sieved them through a 100 - mesh sieve. We used the sieved powder samples to measure the mechanical composition using the densitometer method and extracted the proportion of 0.002 - 0.02 mm fines. We measured the total carbon of sediment (TC_s), organic carbon of sediment (OC_s), and total nitrogen of sediment (TN_s) using an elemental analyzer (Vario Macro-CHNS, GER). We measured total phosphorus of sediment (TP_s) using the molybdenum-antimony colorimetric method (Rice et al., 2012).
2.5 Ecological diversity index
We determined the dominant species based on the dominance degree Y ≥ 0.02. The formula for calculating Y statistics was as follows:
where ni is the number of individuals of a species in the sample; fi is the frequency of occurrence of the species; and N is the total number of individuals of all species in the sample.
We used the Richness of macrobenthic species, Abundance, Margalef index of macrobenthic fauna (D), and Margalef index of FFG (FFG-D) to describe the diversity of macrobenthos at different sampling sites (Yi et al., 2018).The formula general for Margalef index (D) was as follows:
where S is species number. When calculating the Margalef index of FFG (FFG-D), the formula remains unchanged, but S is replaced by the number of FFG, and N representing the total number of individuals across all FFG in the sample.
We used the ‘DescTools’ and ‘rstatix’ packages in R (ver. 4.3.3) to test the significance of spatial and temporal differences in environmental factors and macrobenthic bioindicators using a two-way ANOVA (Analysis of Variance). We applied a T-test to analyze the significance of differences in macrobenthic communities across different land use classes, considering P < 0.05 as significant (Tomanova et al., 2006). We organized the macrobenthos abundance data from the three seasons using the ‘vegan’ package in R for Non-metric Multidimensional Scaling (NMDS) analysis, aiming to examine differences in macrobenthos ecosystems across the three seasons. We performed a Hellinger transformation of abundance before the analyses. We used PERMANOVA to assess the significance of differences in macrobenthic communities across different spatial and temporal scales. We identified species with the most significant contribution to differences across spatial and temporal scales using similarity percentage analysis (SIMPER). We conducted Detrended Correspondence Analysis (DCA) and Redundancy Analysis (RDA) using CANOCO (version 5) to explore the relationship between environmental factors and macrobenthic community structure (including FFG). Prior to analyses, we transformed data on environmental variables of percentage type (e.g., substrate composition) by an inverse chord square root transformation, and we applied a log (x+1) transformation to species abundance and other physico-chemical environmental variables (except pH).
We used a Generalized Additive Model (GAM) to fit the association between the response variables (diversity indices and abundance of FFG) and environmental conditions. We included the explanatory variables using the “mgcv” package in R. The formula general for GAM was as follows (Politou et al., 2008):
where g(Y) is the connection function, Y = lg (y+1), where y is the response variable; ɑ is the constant functional intercept; si(xi) is the spline smoothing function for each environmental factor; and θ is the random error.
We first examined the relationship between each environmental factor and the Margalef index to test whether the effect of individual environmental factors on the community Margalef index was significant, with a significance level of 0.1. We then applied the forward selection method for adding variables. To avoid the effect of covariance, we excluded environmental variables with absolute values of correlation coefficients (R) in Pearson exceeding 0.4 from the same model. We comprehensively evaluated the impact of the resulting models based on the corrected coefficient of determination (Adj-R2), Deviance Explained (DE), and Akaike Information Criterion (AIC), and then fitted the best model (Maravelias et al., 2007; Martínez-Rincón et al., 2012).
3 Results
3.1 Environmental factors
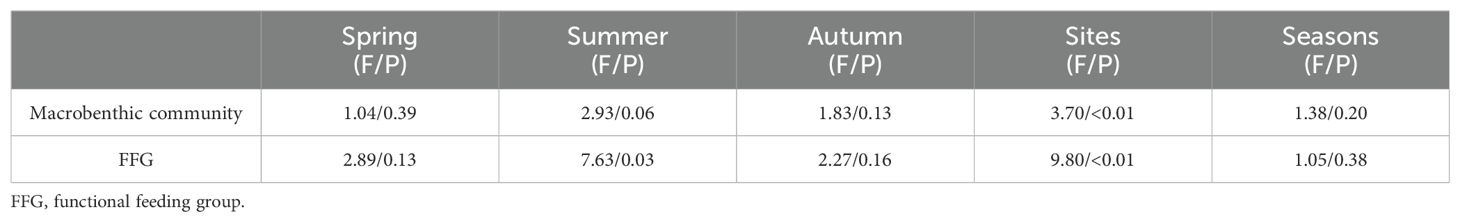
Our results indicated that NO3–N, NO2–N, Temperature, DO, pH, Chl-a, Secchi depth and TLI had varied among seasons (P < 0.05). DO, pH, Secchi depth, and NO3–N were significantly lower during the summer compared to the spring and autumn seasons, Temperature, TLI, and Chl-a were highest during the summer, and NO2–N was highest during the spring season. The rest of the environmental factors did not vary among seasons (P > 0.05). We observed differences between land use development classes in TN, TP, pH, and TLI (P < 0.05). TN, TP, and TLI significantly higher in DS than in UDS (P < 0.05), and pH significantly higher in UDS than in DS (P < 0.05), while the rest of the environmental factors were similar among land use classes. Secchi depth was influenced by a season-by-land use class interaction, and the rest of the environmental factors were insignificant (P > 0.05, Table 1). Environmental factors data for each sampling site during the three seasons is presented in (Supplementary Tables A.2–A.4).

Table 1. Environmental factors with significant spatial and temporal differences in Yinghu Lake (Mean ± SE).
3.2 Community composition of macrobenthos
3.2.1 Species composition and dominant populations
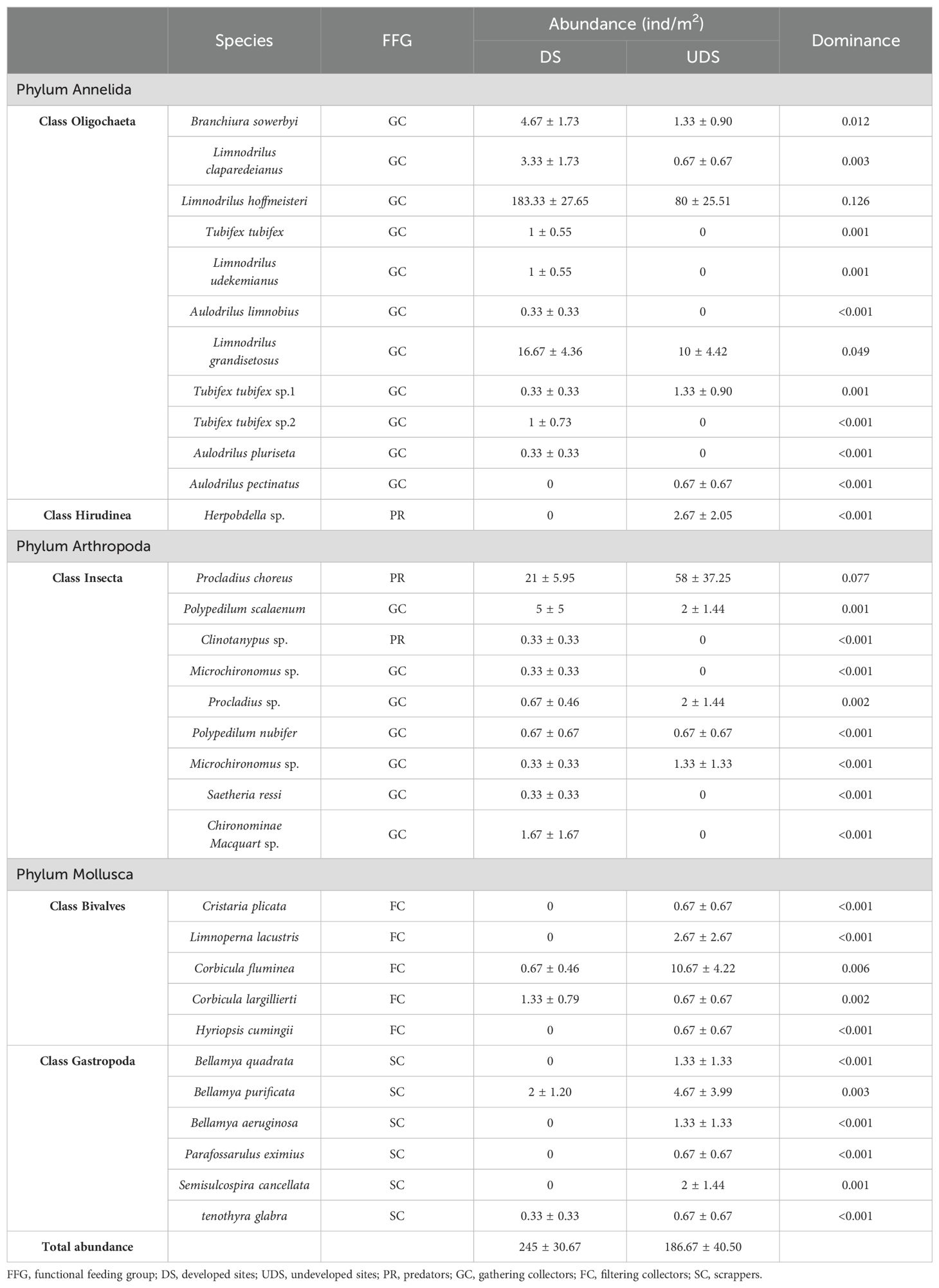
We collected a total of 32 species of macrobenthos from 3 phyla and 5 orders during the survey. Oligochaeta was dominant in Yinghu Lake, followed by Insecta, while Bivalvia, Gastropoda, and Hirudinea were less abundant. Limnodrilus hoffmeisteri, Procladius choreus and Limnodrilus grandisetosus were the dominant species in Yinghu Lake. In terms of spatial distribution, abundance was higher at DS compared to UDS, with Oligochaeta, which tolerate pollution better, were more abundant at DS, and Hirudinea, Insecta, Gastropoda, and Bivalvia more abundant at UDS. Tubifex tubifex, Limnodrilus udekemianus, Aulodrilus limnobius, Tubifex sp.2, Aulodrilus pluriseta, Clinotanypus sp., Microchironomus sp., Saetheria ressi, and Chironominae Macquart sp. were present at the DS only, and Aulodrilus pectinatus, Herpobdella sp., Cristaria plicata, Limnoperna lacustris, Hyriopsis cumingii, Bellamya quadrata, Bellamya aeruginosa and Parafossarulus eximius were present at the UDS only (Table 2).
NMDS analysis of the macrobenthic community data resulted in a coefficient of stress of 0.123, indicating a certain degree of interpretability of the results, and PERMANOVA analysis indicated that there were no significant differences in macrobenthic community structure between the three seasons and between sites within each season, and overall spatially, the macrobenthic communities in the two land use classes were significantly different (P < 0.05, Table 3), and were able to see that some of the UDS were on the left side of the plot (Figure 2). SIMPER analysis showed that L. hoffmeisteri (20.47%) and Corbicula fluminea (7.82%) were the main species driving the differences. T-test of macrobenthos in both land use classes showed that L. hoffmeisteri were significantly more abundant at DS (183.33 ind/m2) than at UDS (80 ind/m2), and C. fluminea were significantly less abundant at DS (0.67 ind/m2) than at UDS (10.67 ind/m2).
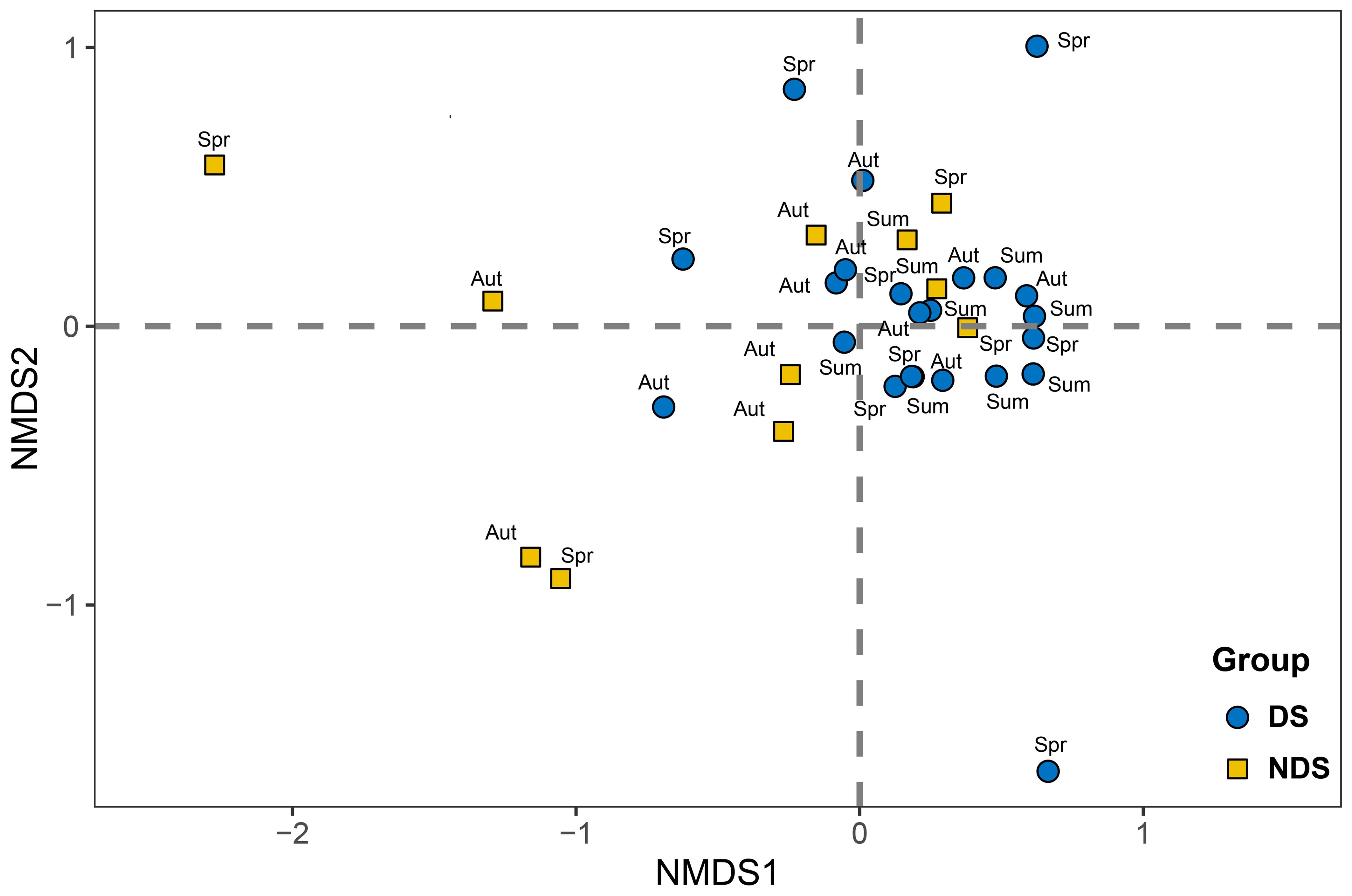
Figure 2. Non-metric multidimensional scale ranking of macrobenthic communities in Yinghu Reservoir of different flood seasons. DS, Developed sites; UDS, Undeveloped sites; Spr, Spring; Sum, Summer; Aut, Autumn.
3.2.2 FFG of macrobenthos
As shown in Figure 3, gathering collectors showed the highest abundance across all three seasons and was consistently higher at DS than at UDS. Predators, scrapers, and filtering collectors followed a similar trend across seasons, with higher abundances at UDS than at DS. Predators was 5.73 times more abundant at UDS than at DS during the summer. In addition, scrapers was only present at UDS, and filtering collectors was 16 times more abundant at UDS than at DS. In the autumn, scrapers was 5.2 times more abundant at UDS, and filtering collectors was 3.67 times more abundant at UDS. PERMANOVA analysis indicated that FFG composition was spatially significant (P < 0.05), but did not differ significantly across seasons. When analyzing specific season, results showed no significant differences in FFG composition between DS and UDS during the spring and autumn (P > 0.05). The FFG composition significantly differed between DS and UDS in the summer (P < 0.05, Table 3). According to SIMPER analysis, gathering collectors (12.10%) and filtering collectors (9.17%) were the main FFG driving the variability. In addition, T-test results revealed that gathering collectors abundance was significantly higher at DS, while filtering collectors abundance was significantly higher at UDS (P < 0.05).
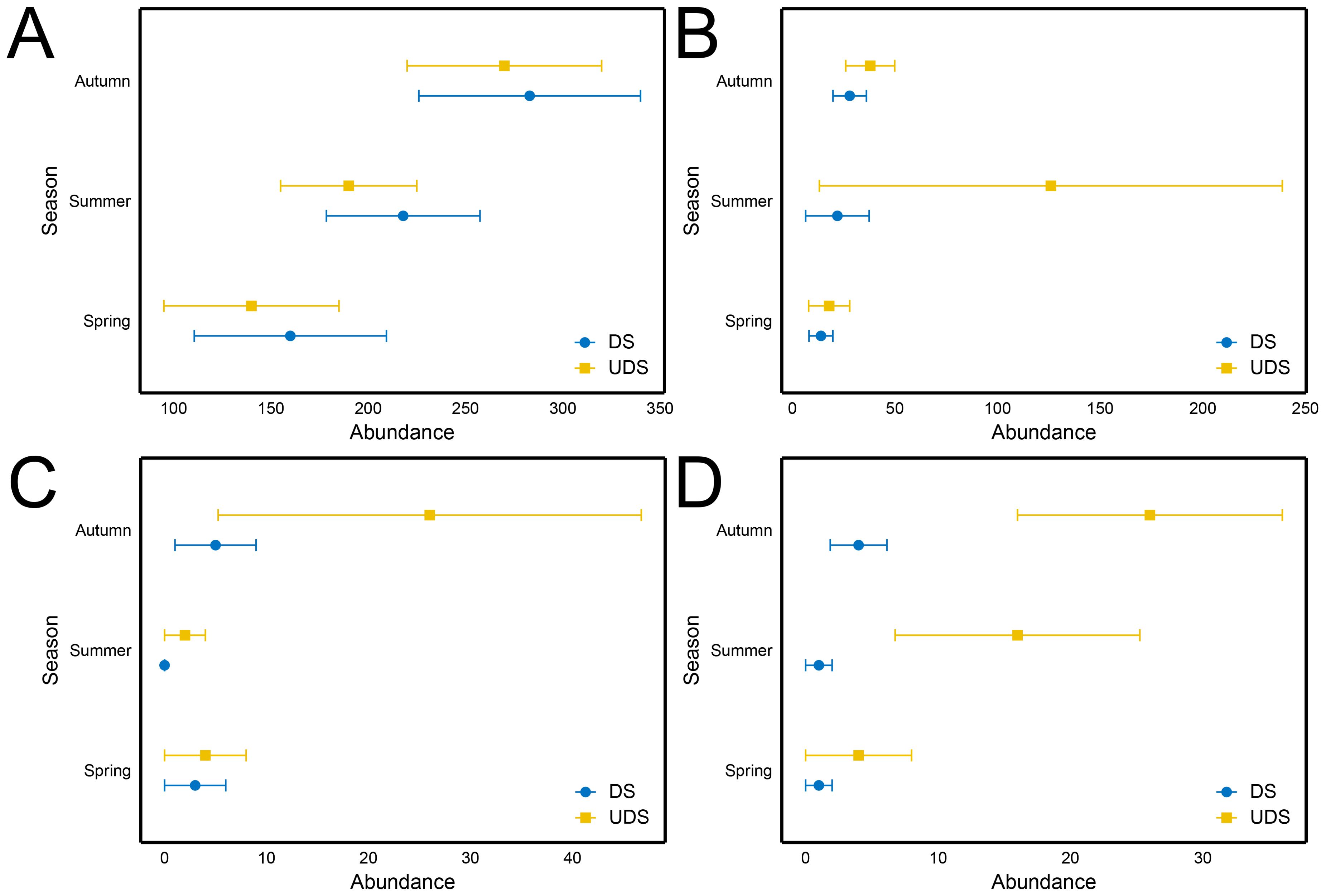
Figure 3. Seasonal variations in the abundance of macrobenthic FFG in Yinghu Lake. (A) Gathering collectors, (B) Predators, (C) Scrapers, (D) Filtering collectors, DS, Developed sites; UDS, Undeveloped sites.
3.2.3 Bioindicator
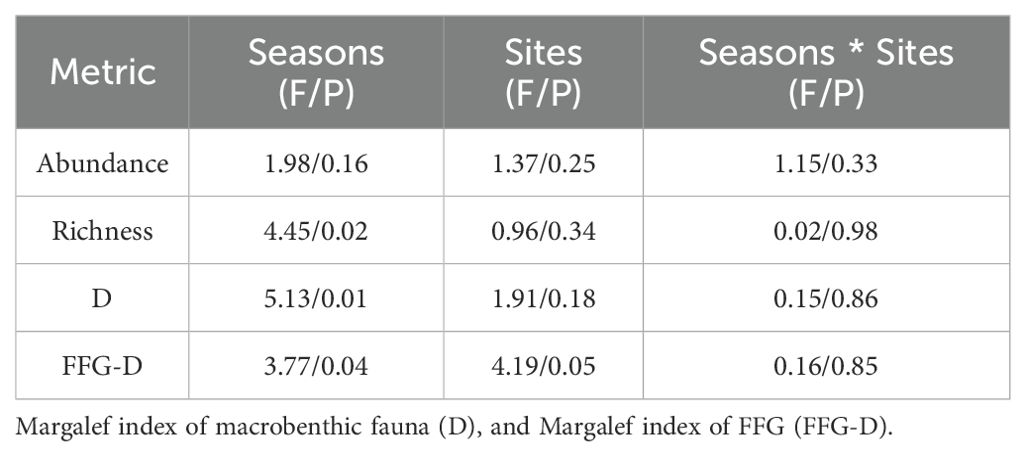
Richness, D, and FFG-D had lower values at DS than at UDS across seasons. The value of the abundance of DS was higher than that of UDS in the spring and autumn, and the abundance of DS was lower than that of UDS in the summer. And the values of all four metrics were higher in the autumn than in the spring (Figure 4). Two-way ANOVA on the four bioindicators showed that the species of macrobenthic abundance, richness, D, and FFG-D varied significantly from one season to another (P < 0.05). The values of all the above indices in the summer were significantly lower than those in the autumn (P < 0.05). In contrast, there was no significance in the spring compared to the other seasons (P > 0.05). Spatially, FFG-D was significantly lower at DS than at UDS (P < 0.05), and the rest of the indicators did not differ significantly in spatial variation, interactions between seasons and regions were not significant for any metric (P > 0.05, Table 4). In summary, differences in the two land use classes can affect macrobenthic diversity distinctly, with more pronounced variances arising from seasonal variations.
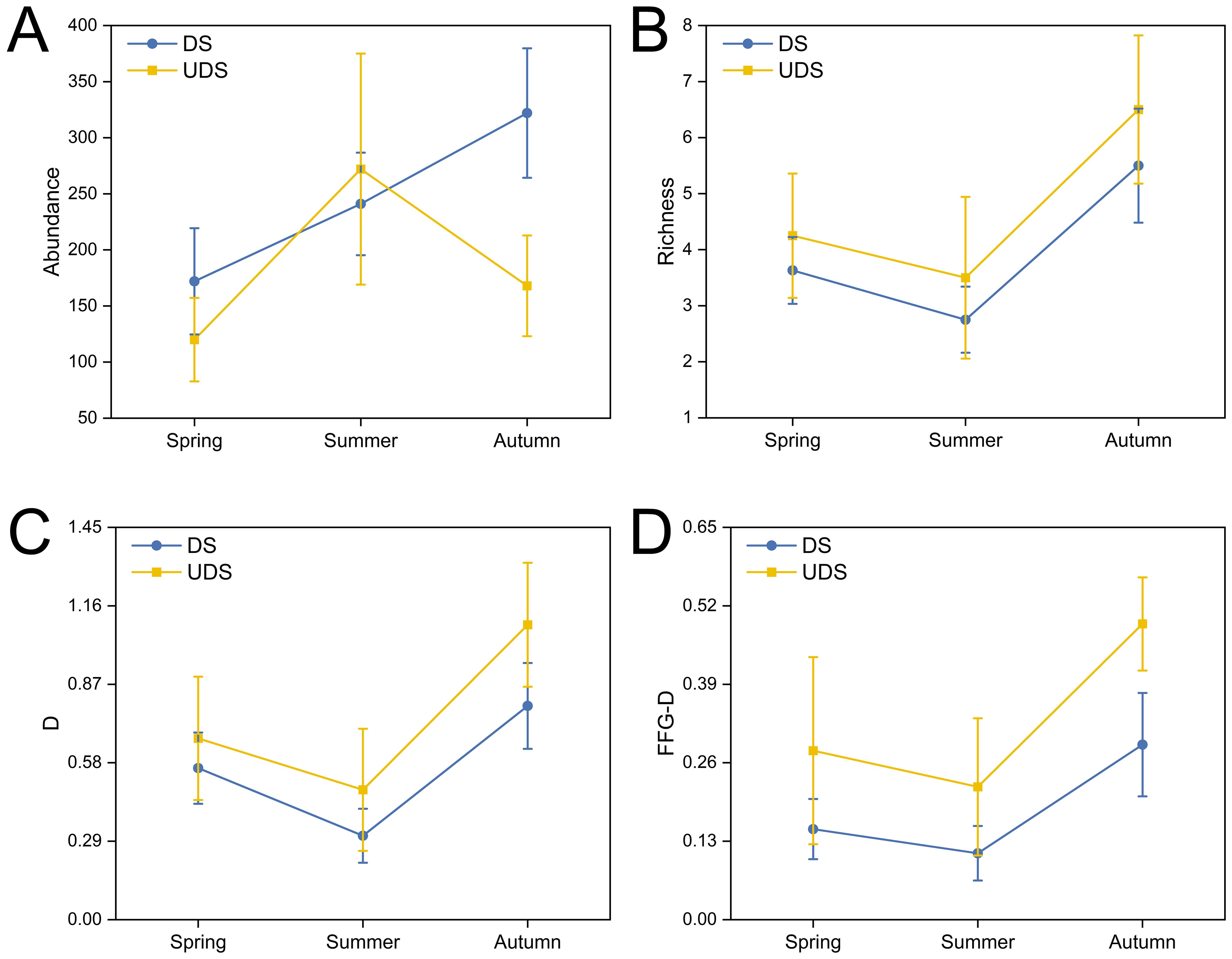
Figure 4. Spatial and temporal variations of biological indicators (mean + SE) in Yinghu Lake. (A) Abundance, (B) Richness, (C) Margalef index of macrobenthic fauna (D), (D) Margalef index of FFG (FFG-D).
3.3 Relationships between macrobenthic communities and environmental factors
We performed a detrended correspondence analysis (DCA) on the macrobenthic community data, yielding principal axis lengths of 2.1 and 1.8, respectively. We then conducted a redundancy analysis (RDA) for the macrobenthic communities and FFG. The redundancy analysis results between macrobenthic communities and environmental factors showed that the first two axes explained a total of 24.76% of the variation (Figure 5A). Among the environmental variables, Chl-a (F=4.3, P < 0.01) was the most closely related to macrobenthos with a contribution of 9.9%, followed by TP_s (F=3.3, P < 0.01) with a contribution of 8.8% and CODMn (F=3.3, P < 0.01) with a contribution of 8.3%. From the results, it can be seen that L. hoffmeisteri, L. grandisetosus and B. sowerbyi were positively correlated with CODMn. C. fluminea were positively correlated with TP_s. Macrobenthic communities were negatively correlated with Chl-a. Redundancy analysis of FFG with environmental factors showed that the first two axes explained a total of 24.35% of the variation (Figure 5B). Among the environmental variables, TP_s (F=4.1, P < 0.01), total dissolved solids (F=3.1, P < 0.05) and Chl-a (F=2.6, P < 0.05) were the environmental factors most closely related to FFG, and their contributions were 16.3%, 11.4% and 9.5%, respectively. From the results, it can be seen that gathering collectors was positively correlated with total dissolved solids, while predators, scrapers and filtering collectors were positively correlated with TP_s, and FFG was negatively correlated with Chl-a. Compared with UDS, DS were mainly located in the lower left part of the ordination diagram and had a stronger relationship with total dissolved solids.
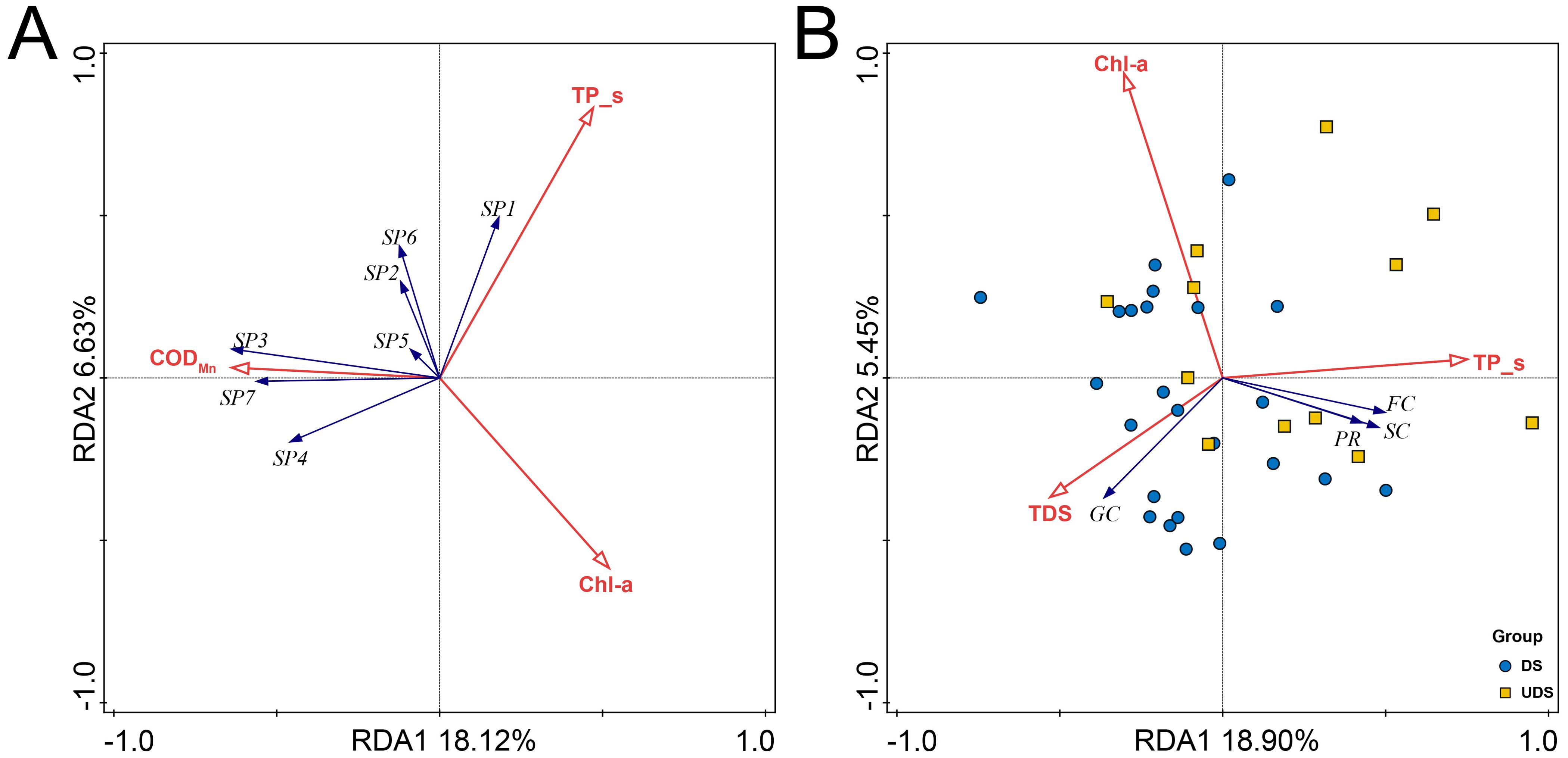
Figure 5. Redundancy analysis of macrobenthic communities (A) and FFG (B) in response to environmental variables. SP1, C. fluminea; SP2, P. choreus; SP3, L. hoffmeisteri; SP4, L. grandisetosus; SP5, L. claparedeianus; SP6, B. purificata; SP7, B. sowerbyi; PR, predators; GC, gathering collectors; FC, filtering collectors; SC, scrappers.
3.4 Relationship between diversity indices and environmental factors
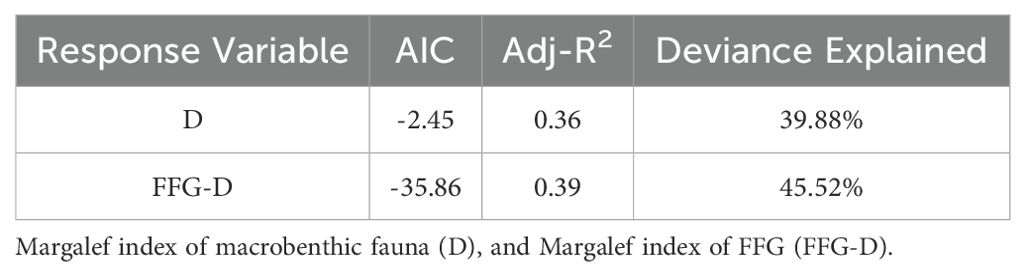
As the Margalef diversity index performs well in distinguishing differences between communities (Yi et al., 2018), the Margalef index was selected to reflect changes in macrobenthic diversity. We then examined the influence of environmental factors on both the D and FFG-D indices. The results indicated that SRP, Temperature, total dissolved solids, pH, Secchi depth, OC_s, and TP_s significantly influenced D (P < 0.1); SRP, Temperature, DO, EC, total dissolved solids, Depth, and TP_s were the environmental factors that significantly influenced FFG-D (P < 0.1). Since the correlation coefficients of DO with Temperature and EC, as well as EC and total dissolved solids were > 0.4, these two variables could not be added to the model simultaneously. We selected Temperature and total dissolved solids for the following analysis and performed forward stepwise regression to screen the optimal model for each. The Temperature with the lowest AIC was first added to the model of D, and the addition of total dissolved solids and OC_s on top of this significantly improved the model performance (P < 0.05). SRP, pH, and TP_s were removed because adding these environmental factors did not improve model performance, and the effect of this environmental factor on D was not significant after addition (P > 0.1). The final model was optimal with the addition of Temperature, total dissolved solids, and OC_s, and the AIC was minimal, with a cumulative model deviation of 39.88%. D was linearly negatively correlated with Temperature, total dissolved solids, and OC_s (Figure 6A). We first added SRP with the minimum AIC to the FFG-D model, and the model’s performance significantly improved after adding Depth, Temperature, and TP_s. We then removed total dissolved solids, as it did not improve the model’s performance. The final optimal model, which included SRP, Depth, Temperature, and TP_s with the minimum AIC, explained 45.52% of the cumulative deviation of the model (Table 5). FFG-D was linearly negatively correlated with SRP and Temperature, and linearly positively correlated with TP_s. FFG-D exhibited the highest index values at a depth of approximately 20 m, followed by a gradual decline, and then a slight increase after reaching about 60 meters (Figure 6B).
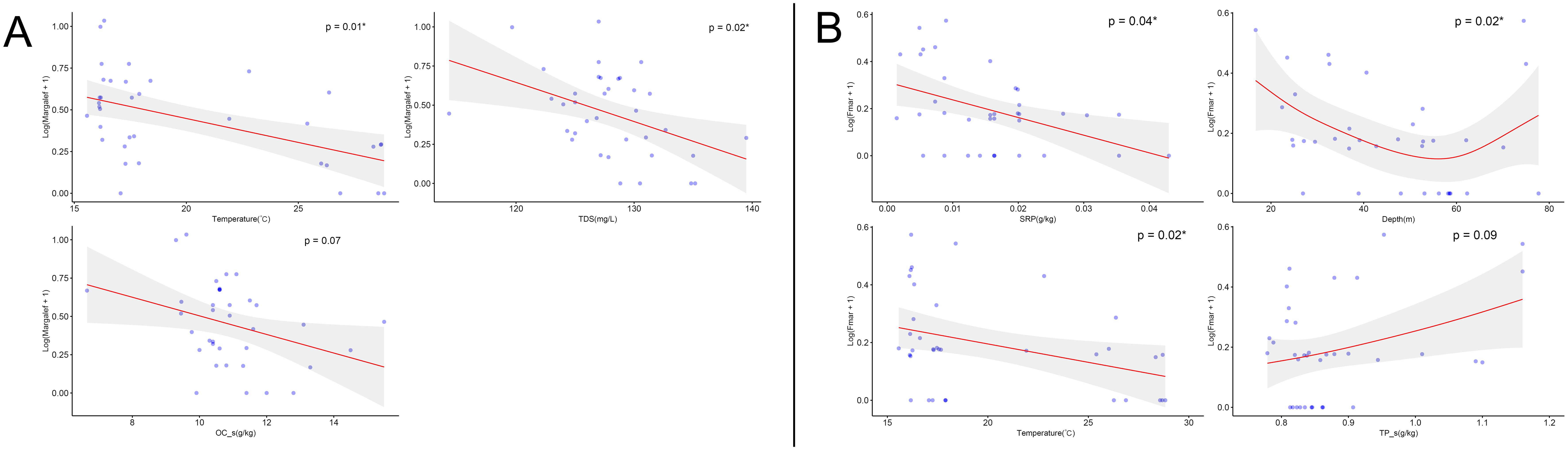
Figure 6. Response of biodiversity indices to environmental factors based on the GAM model. (A) Margalef index of macrobenthic fauna (D), (B) Margalef index of FFG (FFG-D). Shaded areas indicate 95% confidence limits.
4 Discussion
4.1 Characteristics of environmental factors
Urban areas characterized by non-permeable pavements facilitate the rapid transport of pollutants into water bodies via surface runoff. In aerobic conditions, microorganisms break down organic matter, resulting in the production of CO2. Simultaneously, certain nitrogenous compounds undergo oxidation to nitrate through nitrification in the water column, which generates hydrogen ions, leading to a decreased pH at the DS compared to the UDS (Gałuszka and Migaszewski, 2020). Furthermore, the direct or indirect discharge of domestic sewage and aquaculture waste into the reservoir area at the DS frequently causes the enrichment of organic constituents, resulting in water quality degradation and hydrological disruption in the reservoir (Dudgeon et al., 2006). Furthermore, due to the impervious surfaces of developed areas, the increased rainfall and elevated temperatures during summer facilitate the transport of pollutants via surface runoff, significantly increasing the likelihood of nutrient pollution at the DS (Yang et al., 2020). The mean TLI value at the DS throughout the summer was the highest for the whole year (44.68), with the most pronounced difference in pollutant concentrations (TN and TP) between the two regions. However, during the hydrologically stable spring and autumn seasons, these differences were relatively smaller. Additionally, the TLI values at the UDS in spring were higher than those at the DS, likely due to sampling conducted after rainfall, resulting in poorer water quality. Sampling points S2 and S3, located upstream, were directly influenced by non-point source pollution, whereas the S9 and S12 sites, situated in the lower reaches of the mainstem and tributaries, experienced slower flow rates, potentially facilitating nutrient retention (Wang et al., 2020, 2021).
4.2 Macrobenthic community structure
The main groups of macrobenthos in Yinghu Lake are Oligochaeta and Insecta, of which, L. hoffmeisteri, L. grandisetosus and P. choreus are the representative species of these two orders, and they are the dominant species in Yinghu Lake. The distinct structural and functional characteristics of chironomid larvae and some oligochaetes enable these r-selected organisms to recover more rapidly from environmental disturbances and to thrive in deep-water habitats (Hayford et al., 2015). Furthermore, chironomid larvae eventually metamorphose into adults, which possess strong dispersal capabilities (Cummins and Klug, 1979), which may contribute to their apparent prevalence in deep-water reservoirs.
Impervious surfaces significantly impact the ecosystem, resulting in distinct habitat environments between DS and UDS. Additionally, Yinghu Reservoir is located in a monsoon climate zone, where temperature, rainfall, and wind exhibit significant seasonal variations (Chi et al., 2017; Sun et al., 2024). Substrate diversity, nutrient concentrations, and hydromorphological characteristics largely determine the community structure of macrobenthic fauna (Mathooko and Mavuti, 1992). As a result, the changes in macrobenthic community structure across different land use vary significantly under different seasons. At the DS, domestic sewage discharge and industrial production directly degrade water quality, leading to a decline in pollution-sensitive macrobenthic fauna due to reduced food availability and potential toxicity or harm (Pratiwi et al., 2024). Differences between the two regions were primarily reflected in the proportion of pollution-tolerant individuals. L. hoffmeisteri, a widely known pollution-tolerant species, exhibited significantly higher abundance at the DS with higher trophic state indices compared to the UDS. In contrast, less pollution-tolerant mollusks were predominantly found at the UDS, where the abundance of C. fluminea was significantly higher than at the DS and served as a dominant species at the UDS, which indicate that the spatial distribution of macrobenthic communities in Yinghu Lake exhibited significant differences. When habitats are degraded, the abundance of semi-tolerant taxa decreases, and habitat-sensitive species like C. fluminea may serve as effective indicators of habitat degradation. Additionally, under the influence of climate variability, differences in community structure between the DS and UDS were most pronounced during the summer, compared to the other two seasons. Given that the DS dominate the study area, the community composition was primarily characterized by Oligochaeta. Furthermore, the sediment properties did not show significant seasonal variation, and the habitat uniformity influenced the distribution patterns of macrobenthic fauna (Hayford et al., 2015), leading to a non-significant difference in the macrobenthic community structure of Yinghu Lake across seasons.
4.3 FFG of macrobenthos
Typically, changes in trophic structure are a community’s response to specific changes in its food sources or disturbance patterns (Cummins and Klug, 1979; Mendoza and Araújo, 2019), and FFG more accurately represents the vulnerability of macrobenthos to environmental stressors. In Yinghu Lake, the FFG exhibited significant spatial variation. During the summer, with increased rainfall and rising temperatures, the FFG composition between the two regions showed a significant difference. There were no significant differences between the two regions for predators and scrapers, although higher abundance was observed at the UDS than at the DS. Collectors are typically organisms that gather organic particles or microorganisms (Pratiwi et al., 2024). The increased organic matter at the DS offers abundant nourishment for gathering collectors, which thrive in such environments due to their high contamination tolerance, diminutive size, brief lifecycle, and incessant reproduction, facilitating swift community recovery and sustained survival. Nevertheless, filtering collectors, being collectors as well, exhibit greater environmental demands and face disadvantages in interspecific competition with gathering collectors, which are primarily composed of smaller, pollution-tolerant organisms (Wang et al., 2021a), leading to an inverse distribution pattern between filtering collectors and gathering collectors. Temporally, gathering collectors and filtering collectors abundance peaked in the autumn when hydrology was stable. Pratiwi et al. (2024) observed that the abundance of predators correlates with the species present in the habitat. The water pollution has led to a decline in biodiversity at the DS (Wang et al., 2012), thereby diminishing the availability of food sources for predators and subsequently reducing their abundance. Scrapers are species that consumes sedimentary organic matter and algae, is preferentially supported during steady hydrological conditions (Rahman et al., 2022), paralleling the fluctuations in the relative abundance of scrapers in Yinghu Lake. The stable hydrological conditions during autumn facilitated the deposition of organic matter. Following the autumnal deposition, phytoplankton and supplementary organic matter, including decomposed leaves, accumulate at the bottom of reservoirs, resulting in a peak abundance of scrapers during the autumn. Spatially, scrapers exhibited reduced abundance at DS compared to UDS due to its lower tolerance to pollution.
4.4 Diversity of macrobenthic communities
Richness, D and FFG-D were all significantly lower during the summer compared to the autumn. This result is similar to conclusions drawn from studies on changes in macrobenthic communities due to variations in rainfall or changes in reservoir water level regulation (Milner et al., 2013; Quadroni et al., 2016; Sun et al., 2024), indicating that variations in seasons may be the predominant factor affecting the diversity of macrobenthic communities. Variations in macrobenthic diversity indices are contingent upon environmental stability (Duan et al., 2009). Compared to the summer, the spring and autumn seasons exhibited more stable hydromorphological characteristics, resulting in higher diversity indices during these seasons. Additionally, spring sampling conducted after rainfall revealed that nutrient concentrations in the water were influenced, leading to lower water quality in the spring compared to the autumn. This may explain why structural indices in the spring were lower than in the autumn. The r-selected macrobenthos, which dominate the abundance in Yinghu Lake, are less affected by environmental disturbances, resulting in no significant differences in abundance across the three seasons. Spatially, there were significant differences in the FFG-D between the two regions, indicating that changes in land use patterns and human activities have altered habitat conditions, thereby constraining the survival of various organism groups, which in turn has led to spatial differences in species diversity (Wang et al., 2021b). Due to environmental change, richness, D, and FFG-D at DS were lower than those at UDS across all three seasons.
4.5 Relationship between macrobenthos and environmental factors
Environmental factors directly influence the composition, life cycle and distribution of macrobenthic communities (Cooper et al., 2007). Evaluations of the correlation between environmental variables and macrobenthos can accurately determine the ecological and biological condition of sampling locations (Poikane et al., 2016; Yang et al., 2020). Previous studies have shown that nutrients have a strong influence on macrobenthic community structure (Poikane et al., 2016). CODMn serves as a crucial metric for assessing the organic matter concentration in water, mostly utilized in evaluating river pollution and analyzing the characteristics of industrial effluent (Kawabe and Kawabe, 1997). Pollution-tolerant species among the principal macrobenthic communities (L. hoffmeisteri, B. sowerbyi, and P. choreus of oligochaetes) had a positive correlation with CODMn. Moreover, the discharge of municipal and industrial wastewater elevates the total dissolved solids in water (Chen et al., 2022), and overly high total dissolved solids adversely affects aquatic species and water quality (Pratiwi et al., 2024). The DS was primarily composed of gathering collectors, and total dissolved solids was positively correlated with DS, aligning with prior research findings. Generally, CODMn and total dissolved solids concentrations in DS are relatively high, providing abundant food sources for the more pollution-tolerant gathering collectors and oligochaetes, while inhibiting the growth of the less pollution-tolerant filtering collectors. UDS were mainly dominated by filtering collectors, scrapers and predators and were somewhat negatively correlated with gathering collectors abundance.
Prior research has demonstrated that elevated TP_s and TN concentrations led to diminished DO levels in the benthic environment and increased toxicity in both sediment and water column, potentially resulting in the decline of macrobenthic fauna (Jinlin et al., 2003). This study found a positive correlation between scrapers, represented by the B. purificata, and filtering collectors, dominated by the C. fluminea, with TP_s. Phosphorus, as a vital ingredient for living organisms, enhances the stability and complexity of benthic food web structures, ensuring a sufficient food supply for predators while supporting increased mollusk biomass. The better quality of the aquatic environment at the UDS encouraged the growth of filtering collectors that have specific environmental requirements, leading to competition for food resources between filtering collectors and gathering collectors. This competition subsequently inhibited the growth and reproduction of gathering collectors, which occupy lower ecological niches. Theoretically, Chl-a promotes photosynthesis in algal organisms, increasing primary productivity and providing a food source for filter-feeding benthos. However, excessive Chl-a concentrations are commonly associated with eutrophication of aquatic systems, resulting in a decline in macrobenthic abundance due to environmental stresses (Carvalho et al., 2011).
The key environmental factors selected based on GAM are similar to those in previous studies of macrobenthic community diversity as affected by anthropogenic disturbance as well as changes in the natural environment (Alvarez-Cabria et al., 2011; Fu et al., 2015, 2022; Yi et al., 2018). Both the Margalef index of macrobenthos and the FFG Margalef index showed a negative correlation with Temperature, suggesting that seasonal changes significantly affected macrobenthic diversity. During the summer with the highest temperature, alterations in the aquatic environment due to precipitation and anthropogenic gate operations subsequently affected biodiversity (Milner et al., 2013). During the autumn with the lowest temperature, a period characterized by stable hydrological conditions and abundant food resources, which favored the growth of macrobenthic organisms. Environmental factors such as OC_s, SRP, and total dissolved solids, influenced by anthropogenic activities (Cooper et al., 2007; Yuan et al., 2019), are marked by a decline in water quality resulting from heightened stressor intensity, which affects the diversity of macrobenthos (Firmiano et al., 2021). However, TP_s, also subject to anthropogenic disturbance, showed a positive correlation with FFG diversity. TP_s successfully sustained a significant diversity of FFG throughout the monitoring range, likely due to the concentration of TP_s in Yinghu Lake not reaching a detrimental threshold for aquatic life. Consequently, the scope of environmental component sampling may be broadened to improve the reliability of the model. In addition, depth was identified as a significant environmental factor limiting the diversity of FFG in Yinghu Lake, aligning with the conclusions of Petridis and Sinis (1993). Water depth influences the physical characteristics of macrobenthic habitats, DO, and food supply, significantly altering the structure of benthic communities (Cui et al., 2008; Ohtaka et al., 2006). It is noteworthy that the diversity index was slightly greater when the water depth exceeded a certain threshold. They may be due to deeper waters having less variation in environmental parameters (Nalepa, 1989), and certain oligochaetes have a competitive advantage under these conditions, resulting in a slight increase in their diversity.
Variations in natural conditions and the intensity of human disturbances lead to changes in water environmental factors and food resources for macrobenthic fauna in deep-water reservoirs. These are the primary factors influencing changes in the community structure and trophic composition of macrobenthic fauna. Notably, environmental factors with significant differences between DS and UDS (e.g., TN and TP) may be important drivers of macrobenthic community structure, but did not show significant correlations in this study. This may be due to the complex ecological roles of these factors or potential lag effects in the response of macrobenthic fauna. Additionally, undetected pollutants in the current dataset (e.g., heavy metals and organic pollutants) may exhibit significant differences between the two regions and directly or indirectly influence macrobenthic community composition (Bendary et al., 2023). Therefore, it is essential to expand the range of detected indicators and implement continuous monitoring measures to maintain macrobenthic biodiversity and prevent further ecological degradation.
5 Conclusion
Macrobenthos serve as significant bioindicators of ecological health and can evaluate the stability and resilience of reservoir ecosystems. The results of this study demonstrate that seasonal changes and human disturbances influenced the water environment of the reservoir area, as well as the living conditions and availability of food resources for macrobenthic organisms. Moreover, all bioindicators of macrobenthos, excluding abundance, were affected by human disturbances across all three seasons. The RDA results indicate that CODMn, total dissolved solids, Chl-a, and TP_s significantly influence the macrobenthic community, with relatively low levels of TP_s potentially serving as a limiting factor for the macrobenthic community. Nutrient indices, specifically CODMn and total dissolved solids, indicate that anthropogenic disturbances alter macrobenthic community structure, favoring assemblages that are more tolerant to pollution. The modeling of macrobenthic community diversity and FFG diversity in relation to environmental factors using Generalized Additive Model revealed a significant influence of seasonal variations on macrobenthic diversity. Moreover, elevated concentrations of total dissolved solids and OC_s were found to restrict macrobenthic diversity, while FFG diversity was constrained by high levels of SRP and low levels of TP_s. In summary, the poorer water quality at DS led to an increase in the relative abundance of Oligochaeta within the macrobenthic community structure, accompanied by a decline in macrobenthic diversity. During the summer, the differences in community structure became even more pronounced. Alterations in macrobenthic communities and FFG serve as a crucial approach for examining shifts in water quality within deepwater reservoirs, as well as the effects of human activities on aquatic ecosystem functions from a community ecology perspective. Systematic monitoring of relevant indicators in large reservoirs and the formulation of rational management plans are essential for enhancing the ecological environment of the reservoir area and facilitating the sustainable exploitation of macrobenthic fauna resources. Furthermore, it is necessary to conduct further studies on subtropical reservoirs encompassing different types and gradients of human impacts, while expanding the range of detection indicators to enhance the generalizability of the findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
RC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. FH: Data curation, Software, Visualization, Writing – original draft, Writing – review & editing. XW: Investigation, Writing – review & editing. HL: Investigation, Writing – review & editing. ZY: Investigation, Writing – review & editing. ZH: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. QL: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We gratefully acknowledge the funding for this study provided by the National Key Research and Development Program of China (No. 2023YFD2400900).
Acknowledgments
We thank everyone who helped with and took part in this research endeavor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1509130/full#supplementary-material
References
Alvarez-Cabria M., Barquín J., Juanes J. A. (2011). Microdistribution patterns of macroinvertebrate communities upstream and downstream of organic effluents. Water Res. 45, 1501–1511. doi: 10.1016/j.watres.2010.11.028
Barbour M. T., Gerritsen J., Griffith G. E., Frydenborg R., Mccarron E., White J. S., et al. (1996). A framework for biological criteria for Florida streams using benthic macroinvertebrates. J. North Am. Benthol. Soc 15, 185–211. doi: 10.2307/1467948
Bendary R. E., Ibrahim S. M., Goher M. E., Elsaied H. E., El Shabrawy G. M., El Mordy M. A., et al. (2023). Taxonomic and functional structure of macrobenthic invertebrate communities and their response to environmental variables along the subbranches of the Nile River (rayahs), Egypt. Environ. Sci. pollut. Res. Int. 30, 28803–28817. doi: 10.1007/s11356-022-24140-z
Carvalho S., Pereira P., Pereira F., De Pablo H., Vale C., Gaspar M. B. (2011). Factors structuring temporal and spatial dynamics of macrobenthic communities in a eutrophic coastal lagoon (Óbidos lagoon, Portugal). Mar. Environ. Res. 71, 97–110. doi: 10.1016/j.marenvres.2010.11.005
Chen S. S., Kimirei I. A., Yu C., Shen Q., Gao Q. (2022). Assessment of urban river water pollution with urbanization in East Africa. Environ. Sci. pollut. Res. Int. 29, 40812–40825. doi: 10.1007/s11356-021-18082-1
Chi S., Li S., Chen S., Chen M., Zheng J., Hu J. (2017). Temporal variations in macroinvertebrate communities from the tributaries in the Three Gorges Reservoir Catchment, China. Rev. Chil. Hist. Nat. 90. doi: 10.1186/s40693-017-0069-y
Chu E. W., Karr J. R. (2017). “Environmental impact: Concept, consequences, measurement ☆,” in Reference Module in Life Sciences (Amsterdam, Netherlands: Elsevier). doi: 10.1016/b978-0-12-809633-8.02380-3
Cooper M. J., Uzarski D. G., Burton T. M. (2007). Macroinvertebrate community composition in relation to anthropogenic disturbance, vegetation, and organic sediment depth in four Lake Michigan drowned river-mouth wetlands. Wetlands (Wilmington) 27, 894–903. doi: 10.1672/0277-5212(2007)27[894:mccirt]2.0.co;2
Cui Y.-D., Liu X.-Q., Wang H.-Z. (2008). Macrozoobenthic community of Fuxian Lake, the deepest lake of southwest China. Limnologica 38, 116–125. doi: 10.1016/j.limno.2007.10.003
Cummins K. W., Klug M. J. (1979). Feeding ecology of stream invertebrates. Annu. Rev. Ecol. Syst. 10, 147–172. doi: 10.1146/annurev.es.10.110179.001051
Daramola J., Adepehin E. J., Ekhwan T. M., Choy L. K., Mokhtar J., Tabiti T. S. (2022). Impacts of land-use change, associated land-use area and runoff on watershed sediment yield: Implications from the Kaduna Watershed. Water (Basel) 14, 325. doi: 10.3390/w14030325
Ding Y., Wang H., Zhang Q., Chai B., Lei X., Ye M., et al. (2022). Effects of dissolved oxygen on phosphorus transformation in reservoir sediments: novel insights on bacterial community and functional genes. J. Soils Sediments 22, 2094–2104. doi: 10.1007/s11368-022-03233-9
Drummond J. D., Aquino T., Davies-Colley R. J., Stott R., Krause S. (2022). Modeling contaminant microbes in rivers during both baseflow and stormflow. Geophys. Res. Lett. 49, e2021GL096514. doi: 10.1029/2021GL096514
Duan X., Wang Z., Xu M., Zhang K. (2009). Effect of streambed sediment on benthic ecology. Int. J. Sediment Res. 24, 325–338. doi: 10.1016/s1001-6279(10)60007-8
Dudgeon D., Arthington A. H., Gessner M. O., Kawabata Z.-I., Knowler D. J., Lévêque C., et al. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc 81, 163–182. doi: 10.1017/S1464793105006950
Firmiano K. R., Castro D. M. P., Linares M. S., Callisto M. (2021). Functional responses of aquatic invertebrates to anthropogenic stressors in riparian zones of Neotropical savanna streams. Sci. Total Environ. 753, 141865. doi: 10.1016/j.scitotenv.2020.141865
Fu L., Jiang Y., Ding J., Liu Q., Peng Q. Z., Kang M. Y., et al. (2015). Spatial variation of macroinvertebrate community structure and associated environmental conditions in a subtropical river system of southeastern China. Knowl. Manage. Aquat. Ecosyst. 2015, 17. doi: 10.1051/kmae/2015013
Fu X., Yang W., Zheng L., Liu D., Li X. (2022). Spatial patterns of macrobenthos taxonomic and functional diversity throughout the ecotones from river to lake: A case study in Northern China. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.922539
Gałuszka A., Migaszewski Z. M. (2020). “Rivers and lakes: acidification,” in Managing Water Resources and Hydrological Systems (Boca Raton, FL, USA: CRC Press, Taylor & Francis Group), 87–103. doi: 10.1201/9781003045045-9
Hayford B. L., Caires A. M., Chandra S., Girdner S. F. (2015). Patterns in benthic biodiversity link lake trophic status to structure and potential function of three large, deep lakes. PloS One 10, e0117024. doi: 10.1371/journal.pone.0117024
Jinlin X., Xinguo M. E. I., Chuanlin H. U. (2003). Comparative study on the community structure and biodiversity of zoobenthos in lakes of different pollution states. J. Lake Sci. 15, 160–168. doi: 10.18307/2003.0210
Kawabe M., Kawabe M. (1997). Temporal and spatial characteristics of chemical oxygen demand in Tokyo Bay. J. Oceanogr. 53, 19–26. doi: 10.1007/bf02700745
Kim S., Kim S., Hwang S., Lee H., Kwak J., Song J.-H., et al. (2023). Impact assessment of water-level management on water quality in an estuary reservoir using a watershed-reservoir linkage model. Agric. Water Manage. 280, 108234. doi: 10.1016/j.agwat.2023.108234
Lamouroux N., Souchon Y. (2002). Simple predictions of instream habitat model outputs for fish habitat guilds in large streams. Freshw. Biol. 47, 1531–1542. doi: 10.1046/j.1365-2427.2002.00880.x
Li Y., Li Y., Xu Z., Li L. (2016). Assessment of the Huntai River in China using a multimetric index based on fish and macroinvertebrate assemblages. J. Freshw. Ecol. 31, 169–190. doi: 10.1080/02705060.2015.1070109
Liu Z., Lyu J., Jia Z., Wang L., Xu B. (2019). Risks analysis and response of forecast-Based Operation for Ankang Reservoir flood control. Water (Basel) 11, 1134. doi: 10.3390/w11061134
Maravelias C. D., Tsitsika E. V., Papaconstantinou C. (2007). Environmental influences on the spatial distribution of European hake (Merluccius merluccius) and red mullet (Mullus barbatus) in the Mediterranean. Ecol. Res. 22, 678–685. doi: 10.1007/s11284-006-0309-0
Martínez-Rincón R. O., Ortega-García S., Vaca-Rodríguez J. G. (2012). Comparative performance of generalized additive models and boosted regression trees for statistical modeling of incidental catch of wahoo (Acanthocybium solandri) in the Mexican tuna purse-seine fishery. Ecol. Modell. 233, 20–25. doi: 10.1016/j.ecolmodel.2012.03.006
Mathooko J. M., Mavuti K. M. (1992). Composition and seasonality of benthic invertebrates, and drift in the Naro Moru River, Kenya. Hydrobiologia 232, 47–56. doi: 10.1007/bf00014611
Mely S. S., Hossain M. B., Rahman M., Albeshr M. F., Arai T. (2023). Changes of macrobenthic diversity and functional groups in saltmarsh habitat under different seasons and climatic variables from a subtropical coast. Sustainability 15. doi: 10.3390/su15097075
Mendoza M., Araújo M. B. (2019). Climate shapes mammal community trophic structures and humans simplify them. Nat. Commun. 10, 5197. doi: 10.1038/s41467-019-12995-9
Milner A. M., Robertson A. L., Mcdermott M. J., Klaar M. J., Brown L. E. (2013). Major flood disturbance alters river ecosystem evolution. Nat. Clim. Change 3, 137–141. doi: 10.1038/nclimate1665
Nalepa T. F. (1989). Estimates of macroinvertebrate biomass in Lake Michigan. J. Great Lakes Res. 15, 437–443. doi: 10.1016/S0380-1330(89)71499-4
Nelson Mwaijengo G., Msigwa A., Njau K. N., Brendonck L., Vanschoenwinkel B. (2020). Where does land use matter most? Contrasting land use effects on river quality at different spatial scales. Sci. Total Environ. 715, 134825. doi: 10.1016/j.scitotenv.2019.134825
Ohtaka A., Nishino M., Kobayashi T. (2006). Disappearance of deep profundal zoobenthos in Lake Ikeda, southern Kyushu, Japan, with relation to recent environmental changes in the lake. Limnology (Tokyo) 7, 237–242. doi: 10.1007/s10201-006-0180-2
Petridis D., Sinis A. (1993). Benthic macrofauna of Tavropos reservoir (central Greece). Hydrobiologia 262, 1–12. doi: 10.1007/bf00010985
Poikane S., Johnson R. K., Sandin L., Schartau A. K., Solimini A. G., Urbanič G., et al. (2016). Benthic macroinvertebrates in lake ecological assessment: A review of methods, intercalibration and practical recommendations. Sci. Total Environ. 543, 123–134. doi: 10.1016/j.scitotenv.2015.11.021
Politou C.-Y., Tserpes G., Dokos J. (2008). Identification of deep-water pink shrimp abundance distribution patterns and nursery grounds in the eastern Mediterranean by means of generalized additive modelling. Hydrobiologia 612, 99–107. doi: 10.1007/s10750-008-9488-8
Pratiwi D., Sumiarsa D., Oktavia D., Fatharani R. H., Sunardi (2024). Effect of land use type on macrobenthos assemblages, distribution, and functional guild in Upstream Citarum River. Ecol. Indic. 160, 111849. doi: 10.1016/j.ecolind.2024.111849
Qian R., Cai F., Wen Y., Bejarano M. D., Wu S., Yang Q., et al. (2024). The functional diversity of plants dispersed via three upland rivers in humid subtropical monsoon climate. Hydrobiologia. 851, 4639–4651. doi: 10.1007/s10750-024-05615-1
Quadroni S., Brignoli M. L., Crosa G., Gentili G., Salmaso F., Zaccara S., et al. (2016). Effects of sediment flushing from a small Alpine reservoir on downstream aquatic fauna: Effects of Sediment Flushing on Aquatic Fauna. Ecohydrology 9, 1276–1288. doi: 10.1002/eco.1725
Rahman M. K., Hossain M. B., Majumdar P. R., Mustafa M. G., Noman M. A., Albeshr M. F., et al. (2022). Macrobenthic assemblages, distribution and functional guilds from a freshwater-dominated tropical estuary. Diversity (Basel) 14, 473. doi: 10.3390/d14060473
Raymond P. A., Saiers J. E., Sobczak W. V. (2016). Hydrological and biogeochemical controls on watershed dissolved organic matter transport: pulse-shunt concept. Ecology 97, 5–16. doi: 10.1890/14-1684.1
Rice E. W., Bridgewater L., Association, A. P. H (2012). Standard methods for the examination of water and wastewater Vol. 10 (Washington, DC: American public health association).
Song C., Fan C., Zhu J., Wang J., Sheng Y., Liu K., et al. (2022). A comprehensive geospatial database of nearly 100 000 reservoirs in China. Earth Syst. Sci. Data 14, 4017–4034. doi: 10.5194/esSecchidepth-14-4017-2022
Sun C., Xia L., Zhang M., He Q., Yu N., Xiang H., et al. (2024). The impacts of different seasons on macroinvertebrate community structure and functional diversity in the Jingui River, China. Glob. Ecol. Conserv. 51, e02876. doi: 10.1016/j.gecco.2024.e02876
Tang X., Li R., Wang D., Jing Z., Zhang W. (2023). Reservoir flood regulation affects nutrient transport through altering water and sediment conditions. Water Res. 233, 119728. doi: 10.1016/j.watres.2023.119728
Thorp J. H., Covich A. P. (2010). “Preface,” in Ecology and Classification of North American Freshwater Invertebrates (Amsterdam, Netherlands: Elsevier), xiii–xxiv. doi: 10.1016/b978-0-12-374855-3.00026-1
Tomanova S., Goitia E., Helešic J. (2006). Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia 556, 251–264. doi: 10.1007/s10750-005-1255-5
Verburg P. H., Crossman N., Ellis E. C., Heinimann A., Hostert P., Mertz O., et al. (2015). Land system science and sustainable development of the earth system: A global land project perspective. Anthropocene 12, 29–41. doi: 10.1016/j.ancene.2015.09.004
Wang A., Yang D., Tang L. (2020). Spatiotemporal variation in nitrogen loads and their impacts on river water quality in the upper Yangtze River basin. J. Hydrol. (Amst.) 590, 125487. doi: 10.1016/j.jhydrol.2020.125487
Wang B., Liu D., Liu S., Zhang Y., Lu D., Wang L. (2012). Impacts of urbanization on stream habitats and macroinvertebrate communities in the tributaries of Qiangtang River, China. Hydrobiologia 680, 39–51. doi: 10.1007/s10750-011-0899-6
Wang K., Pang Y., Yi Y., Yang S., Wang Y., He C., et al. (2023). Response of dissolved organic matter chemistry to flood control of a large river reservoir during an extreme storm event. Water Res. 230, 119565. doi: 10.1016/j.watres.2023.119565
Wang Y., Liu J.-J., Li B.-L., Liu W., Zuo Y.-F., Kong D.-X., et al. (2021a). Relationships between characteristics of macrobenthic assemblages and environmental variables in the Heihe River Basin, China. Aqua 70, 710–730. doi: 10.2166/aqua.2021.022
Wang Y., Liu J.-J., Liu W., Feng Q., Li B.-L., Lu H., et al. (2021b). Spatial variation in macrobenthic assemblages and their relationship with environmental factors in the upstream and midstream regions of the Heihe River Basin, China. Environ. Monit. Assess. 193, 53. doi: 10.1007/s10661-020-08822-0
Yang Y., Yi Y., Zhou Y., Wang X., Zhang S., Yang Z. (2020). Spatio-temporal variations of benthic macroinvertebrates and the driving environmental variables in a shallow lake. Ecol. Indic. 110, 105948. doi: 10.1016/j.ecolind.2019.105948
Yi Y., Sun J., Yang Y., Zhou Y., Tang C., Wang X., et al. (2018). Habitat suitability evaluation of a benthic macroinvertebrate community in a shallow lake. Ecol. Indic. 90, 451–459. doi: 10.1016/j.ecolind.2018.03.039
Yuan T., Vadde K. K., Tonkin J. D., Wang J., Lu J., Zhang Z., et al. (2019). Urbanization impacts the physicochemical characteristics and abundance of fecal markers and bacterial pathogens in surface water. Int. J. Environ. Res. Public Health 16, 1739. doi: 10.3390/ijerph16101739
Keywords: Yinghu Lake, macrobenthos, functional feeding group (FFG), GAM modelling, human activities
Citation: Chen R, Hu F, Wang X, Lin H, Ye Z, Hu Z and Liu Q (2025) Spatial and temporal distributions of macrobenthic communities and their environmental driving factors in deepwater reservoirs: a case study of Yinghu Lake, China. Front. Ecol. Evol. 13:1509130. doi: 10.3389/fevo.2025.1509130
Received: 10 October 2024; Accepted: 24 January 2025;
Published: 11 February 2025.
Edited by:
Michael J. Anteau, United States Department of the Interior, United StatesReviewed by:
Baoquan Li, Chinese Academy of Sciences (CAS), ChinaXuhui Dong, Guangzhou University, China
Copyright © 2025 Chen, Hu, Wang, Lin, Ye, Hu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongjun Hu, empodUBzaG91LmVkdS5jbg==; Qigen Liu, cWdsaXVAc2hvdS5lZHUuY24=
†These authors have contributed equally to this work
 Ruoyu Chen
Ruoyu Chen Fangzheng Hu
Fangzheng Hu Xinyu Wang1,2
Xinyu Wang1,2 Qigen Liu
Qigen Liu