
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 28 March 2025
Sec. Conservation and Restoration Ecology
Volume 13 - 2025 | https://doi.org/10.3389/fevo.2025.1456048
The yellow-spotted river turtle (Podocnemis unifilis) is widely distributed across the Amazon, Orinoco, and Essequibo River basins. Studies from the Amazon and Orinoco regions highlight the species’ importance to local communities for food, income, and cultural heritage, as well as the significant threats it faces. To expand knowledge in the Essequibo River basin and assist with population management, the goal of this study was to assess turtle and egg consumption, as well as nest and turtle numbers, in the South Rupununi River in Guyana, and finally to propose sustainable management strategies that balance conservation goals with community needs, by comparing egg consumption rates with potential flood-related losses. Based on interviews conducted with 125 out of 185 Wapichan households from Sand Creek community, our findings showed that 12.0% of households (n = 15) collect annually an average of 41.87 eggs per household, while 22.4% of households (n = 28) harvest an average of 3.32 turtles per household per year. Households with more children tend to consume higher amounts of turtle eggs and meat, and those engaging in turtle harvesting report higher levels of turtle meat consumption. The primary motivation for turtle capture is consumption, particularly during culturally significant occasions, though turtles are also used for local trade, as pets, and for their shells. At the community level, the estimated annual consumption of 929 eggs is lower than the estimated 1,210 eggs lost annually to flooding on monitored beaches. However, the estimated 138 turtles harvested village-wide exceeds the number of adult turtles observed per survey day in 2021 (n = 13) and 2022 (n = 19). Our analysis suggests that during years with early floods, local egg demand could be met by rescuing at-risk nests located near the river, without increasing natural egg mortality. To offset wild turtle harvests, we recommend hatching at least 182 rescued eggs ex-situ and managing them through extensive farming systems. This approach could reduce adult turtle harvests, particularly of females. To achieve sustainable management, we propose monitoring all beaches where eggs are harvested, implementing a nest rescue program during floods, and establishing extensive turtle farming systems. These measures could shift egg harvesting from wild populations to controlled ex-situ programs, helping to conserve the yellow-spotted river turtle while supporting community needs.
The yellow-spotted river turtle (Podocnemis unifilis) is a widely distributed species found in the Amazon, Orinoco, and Essequibo River basins (Pearse et al., 2006). The life cycle of this species is closely tied to the hydrological patterns of each region, with female turtles initiating nesting when waters begin to recede during the dry season (Ponce de Leão et al., 2019). Nesting occurs primarily on exposed sand beaches, but it can also nest along the edges of lakes, river channels, and vegetated river bank areas, with clutch size ranging from 20 to 30 eggs, with larger females typically laying larger clutches (Braga-Pereira et al., 2024; Erickson and Baccaro, 2016; Hernández et al., 2010).
During the nesting period, yellow-spotted river turtles and their eggs become highly vulnerable to human collection and exploitation. Residents in both rural and urban, rely on turtle meat and eggs as a source of food, which significantly diversifies the predominantly fish-based diets of riverine communities (Murrieta, 1998), in addition to being a source of medicine, and income (Alho, 1985; Pezzuti et al., 2010; Rebêlo and Pezzuti, 2000; Conway-Gómez, 2007; Fachín-Terán and Vogt, 2004). Additionally, the shells of the turtles are utilized in crafting traditional tools (e.g. spinning tools) (Fachín-Terán-Terán and Vogt, 2004). This chelonian species plays a central role in the cultural identity of South American communities, being a highly important item in social practices and celebrations (Freitas et al., 2020).
In the Amazon, during the 18th and 19th centuries, the demand for turtle meat, eggs, and oil led to a sharp decline in river turtle populations (Freitas et al., 2020; Casal et al., 2013; El Bizri et al., 2020a). Despite regulatory efforts in the 20th century, illegal harvesting persisted due to inadequate enforcement (El Bizri et al., 2020a; Kemenes and Pezzuti, 2007). The sale of turtle meat in Amazonian local markets and the collection of juveniles for the pet trade further exacerbated population declines (García-Martin et al., 2021). Presently, the yellow-spotted river turtle is classified as Vulnerable (A1acd), with a recommended revision to be classified as Endangered (Rhodin et al., 2018), as the population may continue to experience severe (≥ 50%) and rapid (< 50 years) future losses across 60% of the pan-Amazonian range (Norris et al., 2019a).
The decline in yellow-spotted river turtle populations is not solely attributed to unsustainable exploitation. Factors such as deforestation (Fagundes et al., 2018), water pollution, increased boat traffic, infrastructure development, and climate change with extreme water level rises, pose additional threats (Fachín Terán and von Mülhen 2003, Rachmansah et al., 2020; Páez et al., 2015; Eisemberg et al., 2016; Norris et al., 2018a). In particular, increasingly frequent alterations in the seasonal Amazon flood pulse are known to strongly affect freshwater turtle recruitment along seasonally flooded rivers (Bodie, 2001; Semlitsch and Bodie, 2003; Steen et al., 2012). Flooding of P. unifilis nests may cause from 10% to 100% mortality depending on the length of the flood (Pignati et al., 2013; Caputo et al., 2005; Páez and Bock, 1998). Thus, although the widespread distribution of these species demonstrates an adaption to flooding, rapid river level rise is a major factor affecting turtle nest mortality.
In flood-prone areas, relocating eggs to artificial hatcheries is crucial to safeguard them from flooding events by meticulously designing the hatcheries to closely replicate natural habitats, using local sand, and maintaining consistent humidity levels and other environmental conditions that affect temperature (Braga-Pereira et al, 2024). From a sustainable use perspective, some authors have suggested that nests that would otherwise be lost due to environmental factors such as flooding could be sufficient to satisfy the local human demand. As such, the rescue nests that appear at risk of being flooded could be harvested sustainably to satisfy consumption needs (Norris et al, 2020; Thorbjarnarson and Da Silveira 2000; Caputo et al., 2005) and potentially also be hatched for a later release to contribute to population re-stocking.
To assess this hypothesis, the goal of this study was to i) provide the first quantitative assessment of turtle and egg consumption in the Essequibo River basin, ii) monitor nesting beaches to quantify potential losses of eggs due to flooding events, iii) compare these losses with local consumption rates and ex- situ nesting success, and iv) propose recommendations for sustainable resource management in Sand Creek, a Wapichan community located in Essequibo, Guyana. Here, we contribute to filling an important gap concerning the ecology and use of yellow-spotted river turtles in the Essequibo River basin, knowing that most research has been carried out in the Amazon and Orinoco sections of the distribution range. This research was led by the village council of Sand Creek as part of their ongoing turtle conservation community project with support from the South Rupununi Conservation Society and the Sustainable Wildlife Management Programme.
This study was conducted in Sand Creek (latitude: 2.991994°S, longitude: 59.523020°W), a riverine Wapichan community located along the Rupununi River, in the South Rupununi within the Essequibo river basin in Guyana (Figure 1). The customary land of Sand Creek overlaps the Kanuku Mountains National Park. Community members in Sand Creek (185 households at the time of the study) traditionally live off activities like fishing, hunting, farming, and fruit tree cultivation (Henfrey, 2002). However, recent years have witnessed the region’s transition toward a cash-based economy, resulting in an increased engagement in wage labor, particularly in government employment, small-scale businesses, and construction. Nevertheless, subsistence lifestyles continue to dominate.
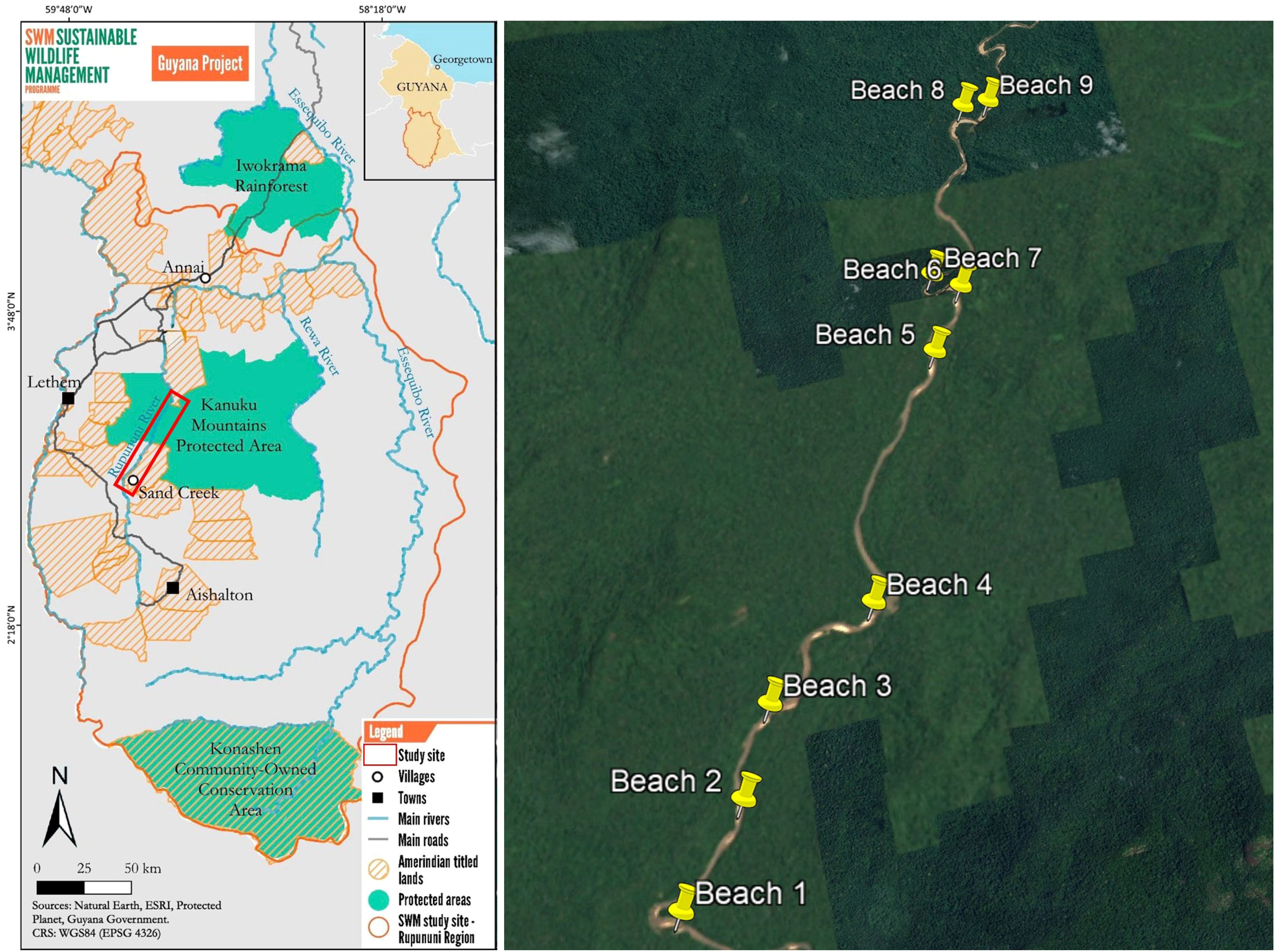
Figure 1. Map of the study area. On the left, the Rupununi Region, with its three National Protected Areas (Iwokrama Rainforest, Kanuku Mountains Protected Area, and Konashen Community-Owned), main rivers (Rupununi River a tributary of the Essequibo), and the location of the monitored nesting beaches highlighted in the red box and the center of the village where the interviews were conducted highlighted with a yellow star. On the right is, a satellite image (source: Google Earth) of the Sand Creek (South Rupununi), showing the nine monitored nesting beaches along the Rupununi River, as indicated in the map.
The Rupununi region exhibits two distinct seasons, characterized by a well-defined rainy season extending from May to August, accounting for the majority of the annual precipitation, ranging from 1500 to 2000mm. This period leads to recurrent river flooding events, inundating low-lying savannahs and forests. Conversely, dry seasons are marked by frequent wildfires, which traverse extensive portions of the landscape, generating a mosaic of burned and unburned areas. The region is home to six freshwater turtle species: Yellow-spotted River Turtle (Podocnemis unifilis), Giant Amazon River Turtle (Podocnemis expansa), Painted Wood Turtle (Rhinoclemmys punctularia), Mata Mata (Chelus fimbriata), Twist-necked Turtle (Platemys platycephala), and Toadhead Turtle (Mesoclemmys gibba) (Millar et al., 2024).
This study was conducted from December 2020 to April 2023 in the village of Sand Creek. Three main data collection activities were carried out: (1) interviews (December 2020–January 2021) to assess turtle and egg harvesting; (2) river-based surveys (2021–2022) along the Rupununi River to estimate turtle abundance through counts of basking individuals; and (3) nesting monitoring (January–April 2023) on nine beaches to evaluate nests, predation, and flooding impacts (Figure 1). Each of these activities is described in detail below.
Structured interviews were conducted between December 2020 and January 2021 and were completed with 67.56% (n = 125) of the 185 households residing in Sand Creek at that time. The study was led by the village council, after consultation and consent by all community members. The local monitors visited each household in the village and interviewed all available households at a time convened by the household head. Some households were not interviewed because they were absent from the village for a long period, and no subsequent arrangement for a visit could be made. All local monitors were residents of the village and interviewed in either English or Wapichan depending on the preference of the interviewee.
At the time of the study, the Kanuku Mountains National Park had a management plan, but it had no specific mention of turtle use. As such, the harvesting of eggs and turtles was not regulated by national park rules, suggesting a low likelihood of response bias stemming from fear of reprisal during this study. The questions included social characteristics of the household (number of children per household, the age and gender of the primary household provider), frequency of turtle harvesting and consumption, average number of turtles harvested per year, average number of eggs caught per year, turtle harvesting methods, reasons for harvesting turtles and eggs, and most common harvesting months.
To conduct the count of individuals the monitors started at the first beach and then drifted down to the last beach counting turtles identified on the way. To reduce any potential bias, the survey was always done in this direction so that the boat engine would not disturb turtles. In 2021, a total of 49 hours and 53min of monitoring was carried out over 26 days (average of 1.9 hours per day, SD=0.88). In 2022, a total of 56 hours and 49min of monitoring was carried out over 16 days (average of 3.0 hours per day, SD=1.39). Combined, the monitoring across both years totaled 106 hours and 42 minutes over 42 days, with an overall average of 2.5 hours per day.
The sex of each individual was identified by the spots on their head. Juveniles have the brightest spots and they are small in size, adult males keep some of these spots and adult females lose the spots completely and are significantly larger than males. The monitors used binoculars and eyesight to identify the spots. However, there is probably a degree of inaccuracy as the turtles often submerge once the rangers get close to them. The rangers were instructed to be cautious and select “don’t know” as an answer if they were not sure about the turtle’s sex and life stage.
Between January 29 and April 29, 2023, a team of 4 to 5 local monitors surveyed 9 beaches along a 10.82 km stretch of the Rupununi River. These beaches were located approximately 26 km in a straight line from Sand Creek Village, with Beach 1 marking the starting point of the surveyed area. Visits were carried out every day from ~5:30 am to ~9:00 am. The water level was monitored daily and recorded based on visual observations made by the rangers during their routine activities.
Water levels fluctuated throughout the monitoring period until complete flooding occurred. As the water level rose, the nests at risk of being flooded were collected and translocated to another area in situ out of the risk of being flooded, and when it was not possible, nests were translocated to a hatchery in Sand Creek. For each nest, we recorded the date of nesting, the date of collection, the nest location, the distance to the river, and the clutch size. We also recorded the nest GPS coordinates (latitude and longitude) using a handheld GPS device with ±5-meter accuracy. The distance to the river was calculated based on the coordinates of the nest and the nearest point on the riverbank, measured directly using the GPS. During the time of the study, the water level was so high, that as many nests as possible were rescued.
The number of eggs hatching was determined by counting the total number of successfully hatched eggs in each nest, whether translocated or those left in situ that were not flooded. Predated nests were identified through signs such as broken eggshells or remnants of partially eaten eggs outside the nest, disturbed nest sites with nearby wild animal tracks, and excavation marks or human footprints (Norris et al., 2019b). Clutch size was determined by counting the total number of hatched eggs and parts of the eggs in the case of those predated. Nests classified as flooded were those submerged by rising water levels during incubation or hatching.
To ensure the successful development of hatchlings, we implemented a protocol to collect eggs from nests at risk of flooding within the first 12 hours of nesting (Braga-Pereira et al., 2024). The eggs were carefully retrieved and transferred to a hatchery that aimed to mimic natural conditions. Although no active monitoring of nest parameters was conducted, basic measures were implemented. The nest parameters of depth and sand type were replicated in the hatchery by using beach sand and keeping the nesting under the shade of native trees to simulate environmental shading patterns. The eggs were placed at their original depth and orientation to support natural sex ratios, recognizing that temperature varies with depth and plays a crucial role in sex determination. However, no measures were previously taken to monitor or actively control temperature and humidity.
During the incubation period in artificial hatcheries, the egg mortality rate was 0.14, calculated by dividing the number of non-viable eggs by the total number of eggs incubated. After hatching, juveniles were transferred to artificial water tanks, where their mortality rate over the one-month rearing period was 0.05, calculated by dividing the number of juvenile deaths by the total number of hatchlings at the start of the rearing period. We highlight that this juvenile mortality rate is lower than that in the wild (Andrade, 2008; Iverson, 1991; Mogollones et al., 2010; Shine and Iverson, 1995), as turtles in the tanks are protected from predators, receive adequate feeding, and are shielded from diseases. Following this, the juveniles were released into their natural habitat.
To delve into the relationship between turtle meat consumption or egg harvesting, and the social characteristics of the interviewees, we employed a Generalized Linear Model (GLM) with a binomial distribution. This involved two models with distinct response variables (binary variable: yes/no): one considering turtle meat consumption and the other considering egg harvesting. The sampling unit for both models was each individual household, as interviews were conducted at the household level. In both models, the predictor variables included the number of children per household and the age and gender of the primary household provider. Also, egg collection was used as a predictor variable for the meat consumption model, and turtle harvesting and turtle meat consumption for the egg collection model. Engagement in eggs collection, turtle harvesting, turtle meat consumption, and gender were analysed as a binary variable (yes/no, female/male). The number of children per household and age were treated as continuous variables. We used GLMs to analyze relationships between variables without hierarchical data, allowing us to explore the direct effects of predictors like household size and the gender of the primary provider on binary outcomes (e.g., yes/no for consumption or harvesting).
We checked for collinearity among predictor variables by calculating correlation coefficients (r) and testing their significance. No strong correlations (r>0.7) were found, ensuring that multicollinearity would not interfere with the model’s performance. We used residual checks to assess the suitability of the models. We assessed model performance and selected the most parsimonious models using an information-theoretic approach. Starting with a global model including all predictors ((eggs ~ gender + age + children + hunting_turtles + eat_turtle) and (hunting_turtles ~ gender + age + children + eggs + eat_turtle)), we used the dredge function in the MuMIn package (Barton, 2020) to generate all possible model combinations. For each model, the Akaike Information Criterion (AIC), specifically the corrected version (AICc), was calculated, and ΔAICc values were determined as the difference between the AICc of each model and the AICc of the best-supported model (ΔAICc = AICc - AICc_min). Models with ΔAICc < 2 were considered to have substantial support. This approach enabled us to identify the most parsimonious models explaining variation in the response variable. All analyses were performed in R ver. 3.5.3 (R Development Core Team, 2019) using the MuMin e lme4 (Oksanen et al., 2013) packages.
We calculated the annual average number of eggs that would have flooded in the study area (Eflood) if no rescue had taken place using the formula:
Where, Nflood is the total number of nests in flooded areas and Cavg is the average clutch size.
To compare egg harvest levels (Eharvest) with the number of eggs lost due to flooding, we first calculated the average egg harvest per household per year (Eavg) using the formula:
Where Einter is the total annual egg harvest reported in interviews and Htotal is the number of households reporting egg harvesting in interviews.
Since not all households were interviewed, we calculated the total egg harvest (Eharvest) by first determining the percentage of interviewed households that harvest turtle eggs (Pharvest). This percentage was then multiplied by the total number of village households (Vhouseholds) and the average egg harvest per household (Eavg), using the formula Etotal = Eavg × Pharvest × Vhouseholds.
We also calculated the number of eggs that should be hatched in the artificial hatcheries annually to meet the community’s turtle consumption needs (Eartif). For the calculation, we considered the number of individuals consumed annually in the village (Ttotal= Tavg × Pharvest × Vhouseholds) and the cumulative mortality rate of the turtle (Tmort), through the equation Eartif > Tconsum/(1- Tmort). For the cumulative mortality rate, we considered the hatching failure rate (0.14), the juvenile mortality rate in tanks (0.05), and the adult mortality rate in the wild (0.07, in accordance with Andrade, 2008). Sequential calculations were applied to estimate cumulative mortality across life stages. i) For eggs, the mortality rate was 0.14.
ii) Juvenile mortality was calculated based on the hatching success rate (1 - 0.14 = 0.86) and the proportion of dying juveniles (0.05), resulting in:
iii) Adult mortality was determined from the juvenile survival rate (0.86 - 0.043 = 0.817) and the proportion of dying adults (0.07), giving:
Thus, the cumulative mortality (Tmort) across all stages was 0.14 + 0.043 + 0.057 = 0.24.
This research was reviewed and approved by the CIFOR Research Ethics Committee (https://www.cifor.org/fileadmin/downloads/CIFOR-Research-Ethics.pdf) and follows the Free Prior and Informed Consent and social safeguards approach from the Sustainable Wildlife Management (SWM) Programme (https://www.fao.org/3/cb7248en/cb7248en.pdf). Community meetings and coordination with communal authorities were carried out before conducting interviews to agree on procedures. The participants gave informed consent to participate in the study.
Among the 125 households interviewed, only 12.0% (n=15) reported harvesting eggs regularly each year. These households collectively harvested a total of 628 eggs, leading to a mean harvest of 41.87 eggs per household per year (mode=30; SD=45.86; ranging from 6 to 180). The number of eggs harvested was significantly related to the number of children in each household, with larger harvests observed in homes with more children (Figure 2A). Model selection identified multiple models with substantial support (ΔAICc < 2). The most parsimonious model (A1) included the number of children as the sole predictor, with a positive effect size of 0.011 (Table 1). This finding highlights the role of household composition, particularly the presence of children, in influencing egg harvesting practices. Egg harvesting occurs annually from December to February.
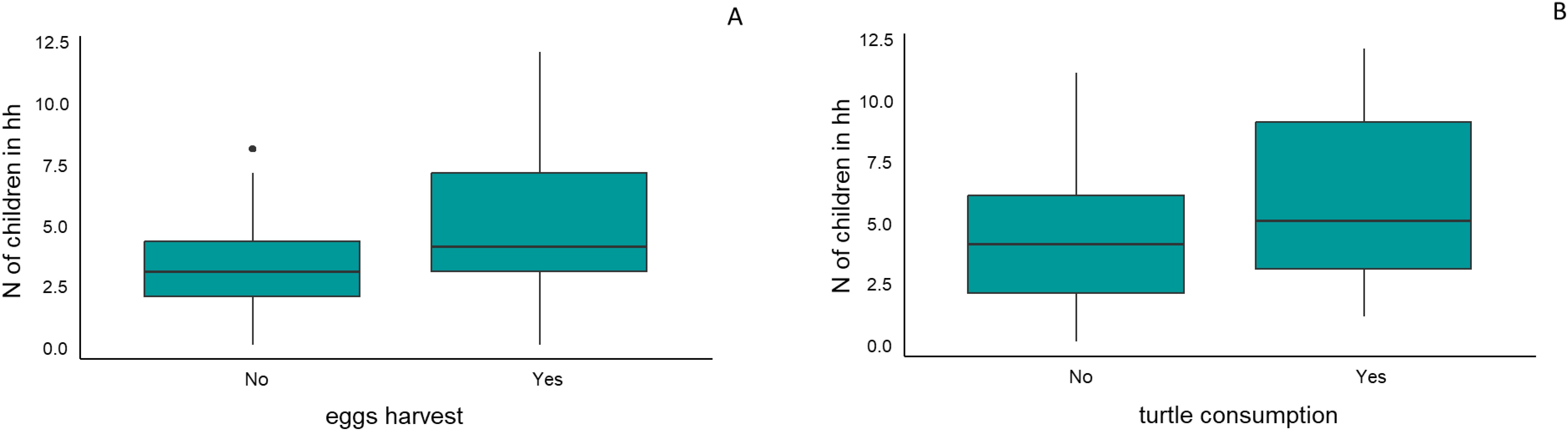
Figure 2. Relationship Between Household Size and Turtle Egg Harvesting or Turtle Consumption. The graph shows a significant positive correlation between the number of children in households (hh) and turtle egg harvesting (A). This correlation is also positive, but not significant, between the number of children in households and turtle consumption (B). Households with more children are more likely to engage in both activities. The horizontal line in each box represents the median, while the box edges indicate the first (Q1) and third (Q3) quartiles. Whiskers show the data range within 1.5 times the interquartile range, with points beyond them as outliers.
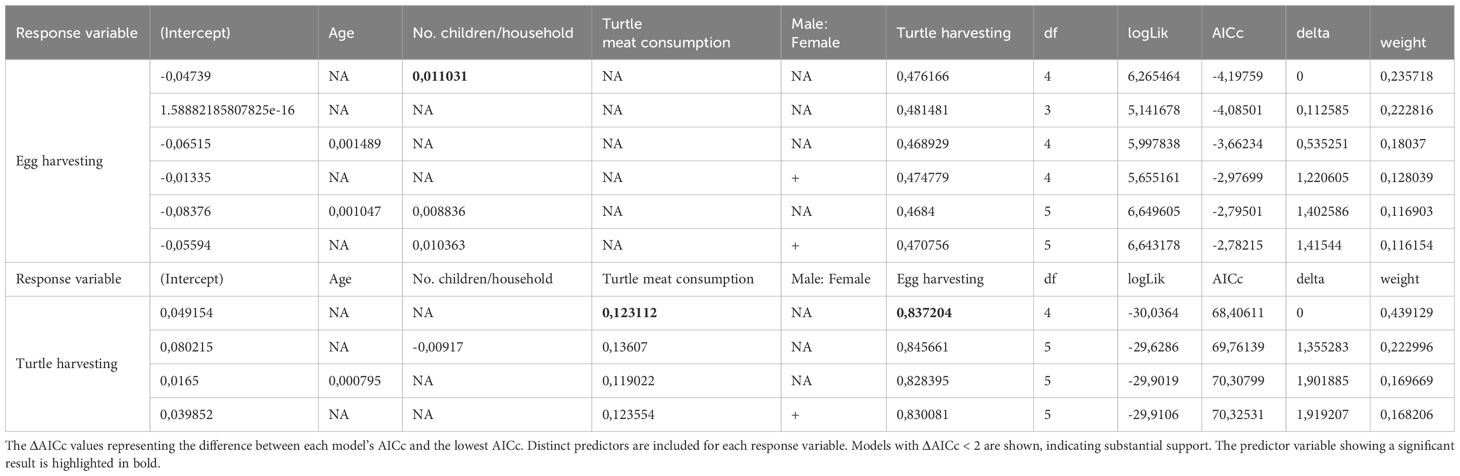
Table 1. Results from the best-supported models assessing the impact of interviewees’ characteristics on egg harvesting (response variable A) and turtle harvesting (response variable B).
Turtle harvesting was reported in 22.4% of the interviews (n = 28), resulting in a total of 93 individuals of P. unifilis being harvested during 2020 and early 2021 (average=3.32 turtle/household; mode=5; SD=1.53; ranging:1-8 turtles). Among the interviews, 66.4% (n=83) reported consuming turtle meat. The consumption was higher in houses of people who harvest turtles (19.2%, n=24) (Table 1). However, in 44.8% (n=56) houses people consume turtle meat, but do not capture, obtaining turtles or turtle meat either through purchase in the village or as gifts. Additionally, in only 2.4% (n=3) houses people capture, but do not consume the turtle and in 32.2% (n=41) of households, people do not harvest and consume turtles. Turtle meat consumption was also significantly higher in households with more children (Table 1; Figure 2B). Model selection identified a single best-supported model (AICc = 68.4, ΔAICc = 0.00, weight = 0.439), which included turtle harvesting and egg consumption as predictors. Turtle harvesting had a positive effect (β = 0.1231), as did egg consumption (β = 0.8372). The period of highest turtle capture was also from December to February, or during special events such as Christmas, New Year celebration, Easter, and birthday celebrations.
(Intercept) represents the expected value of the dependent variable when all predictors are zero or absent. age, children, eat_turtle, eggs, gender: These are the estimated coefficients for each predictor variable included in the model. Positive coefficients indicate a direct relationship with the dependent variable, while negative coefficients indicate an inverse relationship. Larger coefficients (in absolute value) suggest a stronger influence on the dependent variable, while smaller coefficients indicate weaker effects. NA means the predictor was not included in the model. “df” refers to degrees of freedom, “logLik” is the log-likelihood, “AICc” is the corrected Akaike Information Criterion, and “delta” is the difference from the best AICc model. “Weight” is the normalized probability of being the best model.
Among the 28 households that capture turtles, 89% (n = 25) reported exclusively capturing P. unifilis, while the remaining 11% (n = 3) captured both P. unifilis and P. expansa. Also, of these 28 interviewed households, the majority (64.3%, n = 18) indicated that their primary motivation for harvesting was subsistence meat, followed by local meat trade (21.4%, n=6), animals use as pets (10.7%, n=3), and shell use (3,6%, n=1). At Sand Creek, the harvesting techniques “Hook and Line”, “Arrow and Bow”, and “Seine Net” each received an equal number of citations (31.2%, n=10) making them the most used techniques while diving (4.2%) and cast net (2.1%) were less cited.
A total of 103 nests were identified in the nine beaches during the surveys in 2023 (average 11.44 nests/beach, SD= 5) and 55 of these nests were in flooded areas. We identified an average of 22 eggs/nest (SD= 5) from 98 nests in 2023 (Table 2). Considering the 55 nests in flooded zones in 2023 and the clutch size of 22 eggs/nest, the estimated total number of eggs lost to flooding, if no rescue had been made, would be approximately 1,210 eggs (55 nests * 22 eggs per nest). Additionally, we recorded that 110 eggs were predated by wild animals and humans on the monitored beaches; however, the total number of eggs harvested by people also included nests from unmonitored nesting beaches.
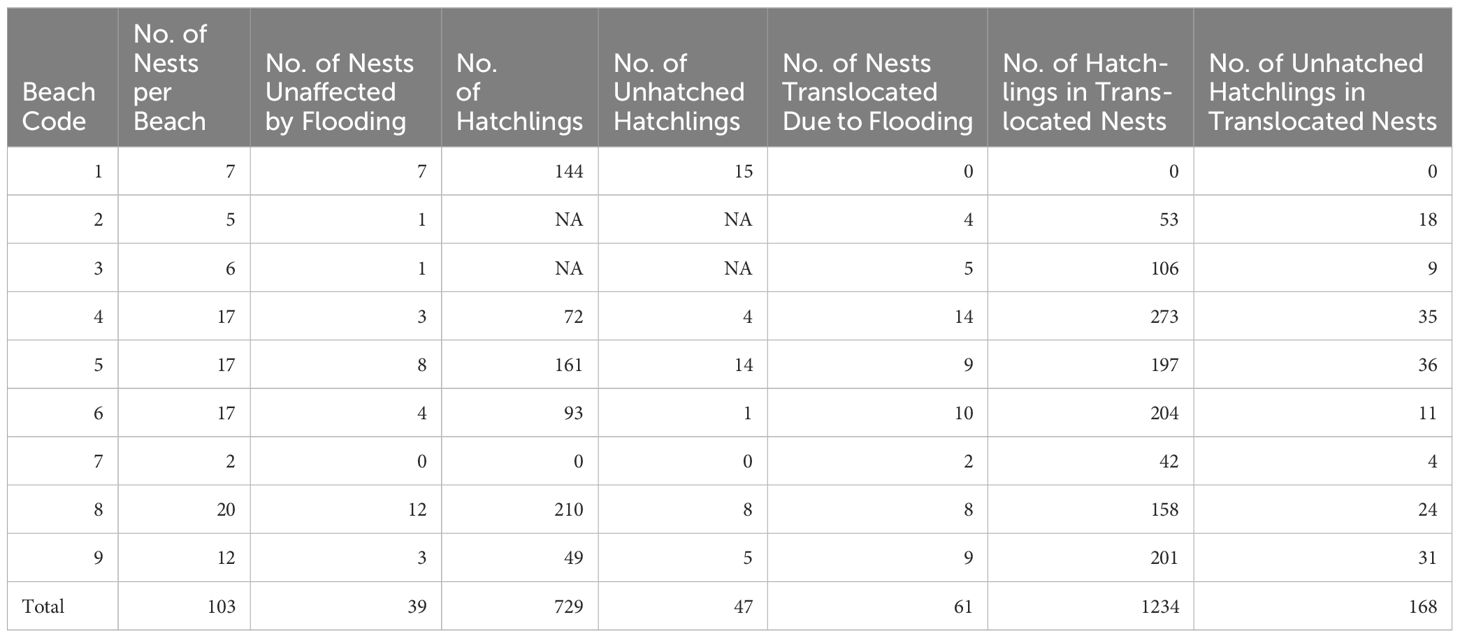
Table 2. Summary of nest status, hatchlings, and turtle mortality across monitored beaches, including the impact of flooding and nest translocation efforts.
On average, 13 turtles (SD = 9.6) were recorded per day over 26 monitoring days between January and March 2021, and 19 turtles (SD = 16.3) were recorded per day over 16 monitoring days between January and April 2022. In total, we recorded 634 sightings, of which the life stage and sex could be identified for 69.24% (n= 439 individuals) of the observed individuals. Out of this total (n = 439), adults represented the majority at 50.79% (n = 223), with females making up 42.37% (n = 186) and males 12.98% (n = 57). Juveniles accounted for 45.10% (n = 198). During the days of monitoring in March we recorded a higher number of turtles, suggesting potential seasonal variations in turtle activity. Juveniles were the most frequently observed group, with notable peaks also in March.
In the study area, considering the average number of eggs harvested/household/year (=41.87), the percentage of interviewed households capturing eggs (=12%), and the total of households in the study site (n=185), the total number of eggs estimated as consumed (Eharvest) in the study area was 929.51 (Eharvest =41.87*0.12*185). This value is lower than the estimated 1,210 eggs that could be lost annually on the monitored beaches due to flood if no rescue actions were undertaken. Since we monitored only 9 beaches, the local people may be harvesting nests on other beaches, so our assessment is a conservative measure.
Considering the average number of turtles harvested/household/year (=3.32), the percentage of interviewed households that harvest turtles (=22.4%), and the total of households in the study site (n=185) the total number of turtles estimated as captured (Ttotal) in the study area was 138 (Ttotal=3.32*0.22*185). As such, considering the turtle cumulative mortality (Tmort) of 0.24, we estimate that a minimum of 182 eggs (Eartif >Tconsum/(1- Tmort)= 138/1-0.24 = 138/0.76 = 181.57) should be hatched in ex-situ hatcheries and farmed in extensive systems to meet annual turtle consumption and reduce harvesting from the wild.
Our, study provides the first quantitative assessment of turtle meat and egg harvesting in a Wapichan community situated in the South Rupununi. The data is drawn from 125 households, shedding light on the significance of this practice. Notably, P. unifilis emerges as the most commonly caught chelonian species in the Rupununi, in accordance with the captures across broader Amazonian regions, as documented in several prior studies (El Bizri et al., 2020a; Chaves et al., 2019; El Bizri et al., 2020b; Peres, 2000; Pantoja-Lima et al., 2014).
Our study quantified egg consumption at 41.87 eggs per household per year, a figure consistent with the range found in the eastern Brazilian Amazon (n= 53.28; Norris and Michalski, 2013). Nevertheless, the observation eggs are collected mostly for own consumption, and consumed mainly among households with more children, suggests that this constitutes a seasonally important element for the food security of the most vulnerable households. Turtles’ meat and eggs constitute a seasonal food source due to the higher availability of suitable nesting and reproduction sites in the dry season, such as sandy and muddy beaches, or other dried and open habitats (Pezzuti et al., 2010). In Rupununi turtle females begin laying eggs typically from December to March, when water levels recede in the region. This period coincides with the highest capture of turtles and eggs, aligning with local traditions and festivities in the Rupununi. Similarly, in communities in Negro River Basin- Brazil, turtle consumption is 3 times higher in the dry season compared to the wet season (Pezzuti et al., 2010).
From a management perspective, the estimated annual consumption of turtle eggs by the community (929 eggs) is within the estimated losses to flooding on monitored beaches (1,210 eggs) in the absence of nest rescue actions. This equivalence suggests that the community’s harvest of eggs that would otherwise be lost to floods may not exert additional pressure on the turtle population. This aligns with other authors working in the Amazon basin (Norris et al, 2020; Thorbjarnarson and Da Silveira 2000; Caputo et al., 2005), suggesting that the rescue of turtle eggs during the seasonal floods could have covered the local demand for eggs and may provide an additional number of saved hatchlings.
We need to ensure that only eggs from flooded zones are being harvested, however considering that our study monitored only nine beaches, and harvesting likely occurs on additional beaches, the percentage of harvest eggs is lower compared to eggs potentially being lost due to flood. Expanding monitoring efforts to include other beaches used by local communities could provide a clearer picture of the overall impact and potential sustainability of this practice. Additionally, we are aware that floods do not happen equally each year, with the same length and intensity, and as such, it is impossible to predict the number of eggs that can be rescued each year. In drier years, there may be no need to rescue any eggs, while during early and intense flooding years, all nests would have to be rescued. This management approach cannot therefore totally replace harvest from the wild, particularly during drier years, but can certainly reduce pressure during flooded years.
Considering the drastic changes to river flow regimes it seems reasonable for management actions to include contingency plans for recovery and release of submerged P. unifilis eggs and hatchlings (Norris et al., 2020). The hatchlings could either be released into the river contributing to population recovery or farmed in extensive farming systems (ponds, lakes) for subsequent consumption by community members, therefore reducing harvest from the wild, particularly of females, as our results revealed a strong preference for capturing adult females due to their ease of capture during the dry season when they lay eggs and their larger size, resulting in a higher meat yield. The high capture of adult female of P. unifilis has been linked to population declines in other Amazonian regions, such as the Purus and Juruá rivers (Andrade et al., 2022).
In addition to managing eggs and hatchlings, the protection of subadults and adults is critical for long-term population stability. However, we recognize that introducing immediate harvesting bans or quotas could alienate communities that rely on turtles for cultural and subsistence purposes. To address this, our project employs a step-change approach, starting with community-focused initiatives such as nest monitoring, head-starting, and environmental education through turtle festivals. These efforts aim to foster a sense of affinity for the species and build community support for conservation. Future discussions on protecting adult turtles will emphasize community-driven regulations, including measures to safeguard reproductive females, which are vital for population recovery. Effective enforcement of these measures will require community buy-in to ensure long-term compliance. Integrating farming strategies into conservation efforts can play a pivotal role in addressing this issue by reducing direct pressure on wild populations while preserving cultural practices (Chaves et al., 2018). This holistic approach not only supports the recovery of turtle populations but also aligns conservation goals with the needs and traditions of local communities, ensuring sustainable outcomes over the long term.
To implement sustainable farming practices, we would adopt successful methods from neighboring countries, such as Brazil, where preservation projects are managed by local communities and aim to increase the populations of the yellow-spotted river turtle (P. unifilis), six-tubercled river turtle (P. sextuberculata), giant South American river turtle (P. expansa), and red-headed Amazon side-necked turtle (P. erythrocephala). These projects also facilitate the sustainable consumption of these animals to ensure food security and subsistence. For example, the captive breeding promoted by the Pé-de-Pincha project not only increases the chelonian population in the wild but also addresses the cultural demand in floodplain areas for turtle meat and eggs. Additionally, the initiative aims to meet emerging trends in national and international markets, which increasingly seek production alternatives that promote and value the management of wildlife by traditional populations (Andrade, 2008; Andrade et al., 2022).
This approach has shown promise in other regions with the establishment of turtle extensive farming units, which must still maintain optimal nutrition for the turtles, with close monitoring to ensure the animals’ well-being and monitor predation (Pamphilio Júnior, 2017; Brasiliense et al., 2023). With an estimated age at maturity between 3 to 9 years for P. unifilis, extensive turtle farms can help meet the demand for turtle meat, especially during cultural events when consumption reaches its peak. In Guyana, where the species is neither protected nor its harvest regulated by a quota system, it is important to develop community-driven sustainable use systems that can match traditional subsistence use and conservation. With the improvement of transportation means and increased access to markets in the Rupnuni expected for the next years (Braga-Pereira et al., 2024), it will become crucial to monitor turtle trade through the licensing system in place for wildlife trade, but also, ensuring that communities monitor potential illegal trade.
Community-based conservation projects allied to official protection programs have been restoring populations of chelonians of the genus Podocnemis throughout the Amazon since 1974 (Andrade et al., 2022). Successful wildlife conservation is closely linked to economic gains and the intrinsic values of local communities (Norris et al., 2018a; 2018b; 2019b; Campos-Silva et al., 2020). Thus, management options that take into consideration the cultural importance of turtle consumption and the needs for food and income, such as that presented in this study, are more likely to be sustained over the long run and adopted by community members generation after generation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by CIFOR Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by This research was reviewed and approved by CIFOR Research Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
NV: Data curation, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – original draft. NM: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. RR: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. FB-P: Data curation, Formal Analysis, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This research received financial support from the European Union as part of the Sustainable Wildlife Management Programme, an initiative of the Organization of African, Caribbean, and Pacific States (OACPS). Additional funding was provided through co-sponsorship from the French Facility for Global Environment and the French Development Agency, facilitated by the Food and Agriculture Organization of the United Nations (FAO), the French Agricultural Research Centre for International Development (CIRAD), the Centre for International Forestry Research (CIFOR), and the Wildlife Conservation Society (WCS).
This work owes its existence to the invaluable collaboration of the Indigenous Communities of South Rupununi, in partnership with South Rupununi Conservation Society (SRCS), and Caiman House (who support SRCS in the nest monitoring project).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alho C. J. R. (1985). Conservation and management strategies for commonly exploited Amazonian turtles. Biol. Conserv. 32, 291–298. doi: 10.1016/0006-3207(85)90019-9
Andrade P. C. M. (2008). Criação e manejo de quelônios no Amazonas: Projeto diagnóstico da criação de animais silvestres no Estado do Amazonas (Manaus: IBAMA).
Andrade P. C. M., de Oliveira P. H. G., de Lima A. C., da Mota Duarte J. A., da Silva Azevedo S. H., de Oliveira A. B. (2022). Community-based conservation and management of chelonians in the Amazon. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.769328
Bodie J. R. (2001). Stream and riparian management for freshwater turtles. J. Environ. Manage. 62 (4), 443–455.
Braga-Pereira F., Roberts R. A., Millar N., van Vliet N. (2024). Nesting trends and predation risks among yellow-spotted river turtles in essequibo river basin. Global Ecol. Conserv. 50, e02820.
Brasiliense A. R. P., Mendonça R. P., Almeida P. E. M., Damasceno L. F., Hoshino M. D. F. G., Yoshioka E. T. O. (2023). Different dietary protein levels for Podocnemis unifilis subadult farming: Hematological and biochemical assessment. J. Appl. Aquacult. 35, 674–686. doi: 10.1080/10454438.2021.2016544
Campos-Silva J. V., Hawes J. E., Freitas C. T., Andrade P. C., Peres C. A. (2020). Community-based management of amazonian biodiversity assets. Participatory Biodiversity Conservation: Concepts Experiences Perspect., 99–111.
Caputo F. P., Canestrelli D., Boitani L. (2005). Conserving the terecay (Podocnemis unifilis, Testudines: Pelomedusidae) through a community-based sustainable harvest of its eggs. Biol. Conserv. 126, 84–92. doi: 10.1016/j.biocon.2005.05.005
Casal A. C., Fornelino M. M., Restrepo M. F. G., Torres M. A. C., Velasco F. G. (2013). Uso histórico y actual de las tortugas charapa (Podocnemis expansa) y terecay (Podocnemis unifilis) en la Orinoquia y la Amazonia. Biota Colombiana 14, 45–64. doi: 10.21068/bc.v14i1.275
Chaves W. A., Monroe M. C., Sieving K. E. (2019). Wild meat trade and consumption in the central amazon, Brazil. Hum. Ecol. 47 (5), 733–746.
Chaves W. A., Valle D. R., Monroe M. C., Wilkie D. S., Sieving K. E., Sadowsky B. (2018). Changing wild meat consumption: An experiment in the Central Amazon, Brazil. Conserv. Lett. 11, e12391. doi: 10.1111/conl.12391
Conway-Gómez K. (2007). Effects of human settlements on abundance of Podocnemis unifilis and P. expansa turtles in northeastern Bolivia. Chelonian Conserv. Biol. 6, 199–205. doi: 10.2744/1071-8443(2007)6[199:EOHSOA]2.0.CO;2
Eisemberg C. C., MaChado Balestra R. A., Famelli S., Pereira F. F., Bernardes V. C. D., Vogt R. C. (2016). Vulnerability of giant South American turtle (Podocnemis expansa) nesting habitat to climate-change-induced alterations to fluvial cycles. Trop. Conserv. Sci. 9, 194008291666713. doi: 10.1177/194008291666713
El Bizri H. R., Morcatty T. Q., Ferreira J. C., Mayor P., Vasconcelos Neto C. F. A., Valsecchi J., et al. (2020a). Social and biological correlates of wild meat consumption and trade by rural communities in the Jutaí River Basin, Central Amazonia. J. Ethnobiol. 40, 183–196. doi: 10.2993/0278-0771-40.2.183
El Bizri H. R., Morcatty T. Q., Valsecchi J., Mayor P., Ribeiro J. E., Vasconcelos Neto C. F., et al. (2020b). Urban wild meat consumption and trade in Central Amazonia. Conserv. Biol. 34, 438–448. doi: 10.1111/cobi.13420
Erickson J., Baccaro F. (2016). Nest predation of the yellow-spotted Amazon River turtle (Podocnemis unifilis, Troschel 1848) by the fire ant (Solenopsis eminate, Fabricius 1804) in the Brazilian Amazon. Herpetol. J. 26, 183–186.
Fachín-Terán A., Vogt R. C. (2004). Estrutura populacional, tamanho e razão sexual de podocnemis unifilis (Testudines, podocnemididae) no rio guaporé (RO), norte do brasil. Phyllomedusa 3 (1), 29–42.
Fachín Terán A., von Mülhen E. M. (2003). Reproducción de la Taricaya Podocnemis unifilis Troschel 1848 (Testudines: Podocnemididae) en la várzea del medio Solimões, Amazonas, Brasil. Ecol. Aplicada 2, 125–132.
Fagundes C. K., Vogt R. C., de Souza R. A., De Marco P. Jr. (2018). Vulnerability of turtles to deforestation in the Brazilian Amazon: Indicating priority areas for conservation. Biol. Conserv. 226, 300–310. doi: 10.1016/j.biocon.2018.08.009
Freitas C. T., Lopes P. F. M., Campos-Silva J. V., Noble M. M., Dyball R., Peres C. A. (2020). Co-management of culturally important species: A tool to promote biodiversity conservation and human well-being. People Nat. 2, 61–81. doi: 10.1002/pan3.10064
García-Martín J. M., Sarmiento-Ramírez J. M., Diéguez-Uribeondo J. (2021). Beyond sea turtles: Fusarium keratoplasticum in eggshells of podocnemis unifilis, a threatened amazonian freshwater turtle. J. Fungi 7 (9), 742.
Henfrey T. B. (2002). Ethnoecology, resource use, conservation and development in a wapishana community in the south rupununi, guyana. (UK: University of Kent at Canterbury).
Hernández O., Espinosa-Blanco A. S., Lugo M., Jiménez-Oraa M., Seijas A. E. (2010). Artificial incubation of yellow-headed sideneck turtle Podocnemis unifilis eggs to reduce losses to flooding and predation, Cojedes and Manapire Rivers, southern Venezuela. Conserv. Evidence 7, 100–105.
Iverson J. B. (1991). Life history and demography of the yellow mud turtle (Kinosternon flavescens) in the Nebraska Sandhills. Herpetologica 47, 371–393.
Kemenes A., Pezzuti J. C. B. (2007). Estimate of trade traffic of Podocnemis (Testudines, Podocnemididae) from the Middle Purus River, Amazonas, Brazil. Chelonian Conserv. Biol. 6, 259–262. doi: 10.2744/1071-8443(2007)6[259:EOTTOP]2.0.CO;2
Millar N., Roberts A., Braga F., van Vliet N. (2024). 4.3. management of river turtles in the rupununi (Wildlife and People in the Rupununi), 197.
Mogollones S. C., Rodríguez D. J., Hernández O., Barreto G. R. (2010). A demographic study of the Arrau turtle (Podocnemis expansa) in the Middle Orinoco River, Venezuela. Chelonian Conserv. Biol. 9, 79–89. doi: 10.2744/ccb-0778.1
Murrieta R. S. S. (1998). O dilema do papa-chibé: Consumo alimentar, nutrição e práticas de intervenção na Ilha de Ituqui, baixo Amazonas, Pará. Rev. Antropol. 41, 1–30. doi: 10.1590/s0034-77011998000100004
Norris D., Michalski F. (2013). Socio-economic and spatial determinants of anthropogenic predation on Yellow-spotted River Turtle (Podocnemis unifilis) nests in the Brazilian Amazon: Implications for sustainable conservation and management. Zool. (Curitiba 30, 482–490. doi: 10.1590/S1984-46702013000500003
Norris D., Michalski F., Gibbs J. P. (2018a). Beyond harm’s reach? Submersion of river turtle nesting areas and implications for restoration actions after Amazon hydropower development. PeerJ 6, e4228. doi: 10.7717/peerj.4228
Norris D., Michalski F., Gibbs J. P. (2018b). Community involvement works where enforcement fails: Conservation success through community-based management of Amazon river turtle nests. PeerJ 6, e4856. doi: 10.7717/peerj.4856
Norris D., Michalski F., Gibbs J. P. (2019a). Community-based actions save Yellow-spotted River Turtle (Podocnemis unifilis) eggs and hatchlings flooded by rapid river level rises. PeerJ 8, e9921. doi: 10.7717/peerj.9921
Norris D., Michalski F., Gibbs J. P. (2020). Community based actions save yellow-spotted river turtle (Podocnemis unifilis) eggs and hatchlings flooded by rapid river level rises. PeerJ 8, e9921.
Norris D., Peres C. A., Michalski F., Gibbs J. P. (2019b). Prospects for freshwater turtle population recovery are catalyzed by pan-Amazonian community-based management. Biol. Conserv. 233, 51–60. doi: 10.1016/j.biocon.2019.02.022
Oksanen J., Guillaume Blanchet F., Kindt R., Legendre P., Friendly M., McGlinn D., et al. (2013). vegan: Community ecology package (Version 2.0-10). Available online at: https://cran.r-project.org/web/packages/vegan/index.html (Accessed March 17, 2005).
Páez V. P., Book B. C. (1998). Temperature effect on incubation period in the yellow-spotted river turtle, podocnemis unifilis, in the colombian amazon. Chelonian Conserv. Biol. 3, 31–36.
Páez V. P., Lipman A., Bock B. C., Heppell S. S. (2015). A plea to redirect and evaluate conservation programs for South America’s podocnemidid river turtles. Chelonian Conserv. Biol. 14, 205–216.
Pamphilio Júnior H. R. M. (2017). Crescimento compensatório e efeito fisiológico da restrição e privação alimentar em tracajá (Podocnemis unifilis) durante o cultivo. Universidade Federal do Amapá. Available online at: http://repositorio.unifap.br:80/jspui/handle/123456789/494 (Accessed March 15, 2025).
Pantoja-Lima J., Aride P. H., de Oliveira A. T., Félix-Silva D., Pezzuti J. C. B., Rebelo G. (2014). Chain of commercialization of Podocnemis spp. turtles (Testudines: Podocnemididae) in the Purus River, Amazon Basin, Brazil: Current status and perspectives. J. Ethnobiol. Ethnomed. 10, 1–12. doi: 10.1186/1746-4269-10-8
Pearse D. E., Arndt A.D, Valenzuela N., Miller B. A., Cantarelli V., Sites J. W. Jr. (2006). Estimating population structure under nonequilibrium conditions in a conservation context: Continent-wide population genetics of the giant Amazon river turtle, Podocnemis expansa (Chelonia; Podocnemididae). Mol. Ecol. 15, 985–1006. doi: 10.1111/j.1365-294X.2006.02869.x
Peres C. A. (2000). Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conserv. Biol. 14, 240–253. doi: 10.1046/j.1523-1739.2000.98485.x
Pezzuti J. C., Lima J. P., da Silva D. F, Begossi A. (2010). Uses and taboos of turtles and tortoises at Negro River, Amazonas, Brazil. J. Ethnobiol. 30, 153–168. doi: 10.2993/0278-0771-30.1.153
Pignati M. T., Fernandes L. F., Miorando P. S., Ferreira P. D., Pezzuti J. C. B. (2013). Nesting site and hatching success of podocnemis unifilis (Testudines: Podocnemididae) in a floodplain area in lower amazon river, pará, Brazil. South Am. J. Herpetology 8 (3), 175–185.
Ponce De Leão S., Famelli S., Vogt R. C. (2019). Home range of yellow-spotted Amazon River turtles (Podocnemis unifilis)(Testudines: Podocnemididae) in the Trombetas River biological reserve, Pará, Brazil. Chelonian Conserv. Biol. 18 (1), 10–18.
Rachmansah A., Norris D., Gibbs J. P. (2020). Population dynamics and biological feasibility of sustainable harvesting as a conservation strategy for tropical and temperate freshwater turtles. PloS One 15, e0229689. doi: 10.1371/journal.pone.0229689
R Development Core Team (2019). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Rebêlo G. H., Pezzuti J. C. B. (2000). Percepções sobre o consumo de quelônios na Amazônia: Considerações para o manejo atual. Ambiente e Sociedade 6, 85–104.
Rhodin A. G. J., Stanford C. B., Dijk Van P. P., Eisemberg C., Luiselli L., Mittermeier R. A., et al. (2018). Global conservation status of turtles and tortoises (Order Testudines). Chelonian Conserv. Biol. 17, 135–161. doi: 10.2744/CCB-1348.1
Semlitsch R. D., Bodie J. R. (2003). Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 17 (5), 1219–1228.
Shine R., Iverson I. B. (1995). Patterns of survival, growth and maturation in turtles. Oikos 72, 343–348. doi: 10.2307/3546119
Steen D. A., Gibbs J. P., Buhlmann K. A., Carr J. L., Compton B. W., Congdon J. D., et al. (2012). Terrestrial habitat requirements of nesting freshwater turtles. Biol. Conserv. 150 (1), 121–128.
Keywords: community-based conservation, Podocnemis unifilis, Guyana, sustainable use, nest relocation, consumption
Citation: van Vliet N, Millar N, Roberts RA and Braga-Pereira F (2025) Balancing conservation and traditional use of yellow-spotted river turtle (Podocnemis unifilis) in Southern Rupununi, Guyana. Front. Ecol. Evol. 13:1456048. doi: 10.3389/fevo.2025.1456048
Received: 27 June 2024; Accepted: 27 February 2025;
Published: 28 March 2025.
Edited by:
Darren Norris, Universidade Federal do Amapá, BrazilReviewed by:
Renato Richard Hilário, Universidade Federal do Amapá, BrazilCopyright © 2025 van Vliet, Millar, Roberts and Braga-Pereira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franciany Braga-Pereira, ZnJhbmJyYWdhODNAeWFob28uY29tLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.