
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 January 2025
Sec. Behavioral and Evolutionary Ecology
Volume 12 - 2024 | https://doi.org/10.3389/fevo.2024.1488373
The lifetime fitness of an individual is determined by the integrated results of survival and reproduction. Improving our understanding of variation in survival senescence within and between species will therefore provide greater insight into the evolution of different life history strategies. Survival is influenced by multiple factors, consequently, variation in patterns of senescence is expected between individuals and sexes and across mating systems and the continuum of life history strategies. To date there is little consensus regarding the mechanisms driving the evolution of sex differences in actuarial senescence, necessitating the need for studies of sex-specific senescence for species across a wide range of life histories. The Weddell seal is a species of long-lived mammal that displays moderate polygyny and little sexual size dimorphism, which makes it an unusual species compared to other long-lived mammals that share the polygynous mating system. Here we used 37 years of data for 1,879 female and 1,474 male Weddell seals from Erebus Bay, Antarctica, to estimate and compare sex-specific patterns of survival rates using basis splines which allow flexible modeling of age-specific patterns. We found that males had lower rates of survival throughout life and higher rates of actuarial senescence after early adulthood compared to females. These results add to our understanding of sex-specific survival rates in the species and contribute information for a long-lived, polygynous species that should aid in achieving a broader understanding of aging between sexes and across the tree of life.
Patterns of senescence, the progressive deterioration of an organism that leads to a decline of fitness components with increasing age, may be driven by the optimization of energy allocation, reproductive schedules, background mortality rates, or a combination of these mechanisms (Lemaître et al., 2015; Monaghan et al., 2008). A large body of research has accumulated that suggests actuarial senescence, an increase in mortality with age (Gaillard and Lemaître, 2017; Ricklefs, 2010; Ronget and Gaillard, 2020) is common among many species (Jones et al., 2014; Nussey et al., 2013; Promislow, 1991). Patterns of senescence have been shown to vary interspecifically across the slow-fast continuum of life history strategies in birds and mammals (Jones et al., 2008). Intraspecific variation in patterns of senescence may occur across populations (Cayuela et al., 2020; Lemaître et al., 2013), different environments (Garratt et al., 2015) and sexes (Clutton-Brock and Isvaran, 2007). Of particular interest is the origin and evolution of sex differences in senescence. Although, there is evidence for a sex-bias in lifespan, there is less consensus regarding sex-differences in the onset and rate of actuarial senescence among mammals and more broadly vertebrates (Lemaître et al., 2020). Several hypotheses have been presented to explain the evolution of sex-bias in senescence stemming from evolutionary theories of senescence, but a dearth of studies and consistent evidence of sex-bias in senescence make this an open topic of research (Lemaître et al., 2015).
Three dominant theories have been put forth to explain the evolution of senescence. The mutation accumulation theory suggests that deleterious mutations accumulate at older ages due to increased baseline mortality as individuals age, which leads to weak selection against mutations arising at older ages (Medawar, 1952). In an extension of the mutation accumulation theory, antagonistic pleiotropic genes that are deleterious in old age but contribute positively to survival and reproduction at young ages were posited to explain senescence (Williams, 1957). The disposable soma theory hypothesizes that irreparable damage accumulates to the soma due to the trade-off between allocating limited resources to self-maintenance, reproduction, and other activities and that optimal allocation of resources to these activities is based on the expected lifespan of an individual (Kirkwood, 1977; Kirkwood and Austad, 2000). More recently the disposable soma theory has been broadened beyond the cellular level and reinterpreted within the context of life history evolution suggesting that trade-offs in energy allocation throughout life may explain patterns of senescence (Baudisch and Vaupel, 2012; Gaillard and Lemaître, 2020; Lemaître et al., 2015). There is now an increasing understanding that the process of senescence can be explained by the latter three theories presented above (Gaillard and Lemaître, 2020; Lemaître and Gaillard, 2017).
Common among the evolutionary theories to explain senescence is the idea that reproductive effort early in life leads to greater declines in fitness traits at older ages. Based on this theory it is predicted that the rate and onset of senescence among individuals and species will be driven by patterns of reproduction, with the onset of actuarial senescence predicted to occur at the age of first reproduction (Hamilton, 1966; Williams, 1957). Reproduction schedules are shaped by resource demands of reproduction (gamete production and parental care), and sexual selection that drives mating strategies is therefore likely to account for variation in senescence patterns among species and drive observed differences between males and females within a species (Bonduriansky et al., 2008; Brooks and Garratt, 2017). In species where males exhibit strong competition for mating opportunities or territoriality it is expected that males will experience an earlier onset or greater rate of actuarial senescence compared to females due to greater energetic allocation to reproduction (Bonduriansky et al., 2008; Brooks and Garratt, 2017). Alternatively, it could be argued that in species where one sex provides a greater amount of energy towards parental care, often females in mammals (Gittleman and Thompson, 1988), that sex will show stronger senescence due to the greater resource demands of reproduction. There is some evidence for this hypothesis in monogamous bird species for which males provide more parental care (Pardo et al., 2013). Sexual size dimorphism is often associated with mating strategies and has also been offered as an explanation for sex-bias in senescence (Loison et al., 1999; Promislow, 1992). The larger sex must achieve a greater size through either more rapid development or a longer growth period, which likely comes at a physiological cost either in terms of an energetic trade-off or damage to the soma and may lead to an earlier onset and/or higher rate of senescence (Lemaître et al., 2020; Marais et al., 2018). The sex hormones that mediate sex-specific differences in reproduction may also contribute to sex differences in actuarial senescence (Brooks and Garratt, 2017). Testosterone has been assumed to lower survival of males through physiological costs such as reduced immunocompetence (Foo et al., 2017) or the behavioral changes it induces such as increased aggression and risk taking (Bonduriansky et al., 2008). There are many explanations for how sex differences in senescence may arise, with predictions for a given species being strongly tied to the life history of the species and the relative energetic expenditures of each sex towards reproduction and somatic maintenance.
There is now an abundance of evidence for a sex-difference in lifespan in wild animals with many males exhibiting shorter lives compared to females in polygynous species (Lemaître and Gaillard, 2013), while males have similar or longer lives compared to females in monogamous species (Clutton-Brock and Isvaran, 2007). Evidence for a relationship between senescence and mating system is accumulating with results showing a stronger rate of actuarial senescence (Clutton-Brock and Isvaran, 2007) or earlier onset of senescence (Tidière et al., 2015) for males of polygynous compared to monogamous species although there is conflicting evidence for these predicted patterns (Lemaître et al., 2020) as well as sexual selection as the origin of these patterns (Lemaître and Gaillard, 2013). For example, in polygynous, large herbivores, male wild boars (Sus scrofa) exhibit an earlier onset, but similar rate of actuarial senescence to females (Gamelon et al., 2014), whereas male red deer (Cervus elaphus) exhibit an increased rate but similar onset of actuarial senescence compared to females (Catchpole et al., 2004). There is also contrasting evidence for the relationship between mating system and sex-specific senescence among monogamous species. Male wandering albatross (Diomedea exulans) (Pardo et al., 2013) exhibit a higher rate of senescence compared to females. In contrast, in meerkats (Suricata suricatta) (Thorley et al., 2020) and Atlantic puffins (Fratercula arctica) (Landsem et al., 2023) there is no sex difference in senescence patterns following predictions laid out earlier. Based on the conflicting results regarding the origins of sex-differences in actuarial senescence, it seems likely that these differences might not be so easily explained by a single measure such as mating system but may reflect the complex array of mechanisms that govern life histories and reproductive schedules.
Early work estimating and comparing sex-specific senescence patterns has relied on cross sectional data (Clutton-Brock and Isvaran, 2007), but cross-sectional data can lead to misleading estimates of age-specific patterns in vital rates and longitudinal data is now considered ideal for estimating senescence patterns (Nussey et al., 2013, Nussey et al., 2008). An accurate estimation of age-specific vital rates should not only be based upon longitudinal data but should also account for other sources of variation that might bias estimates. Individual heterogeneity is a potential source of variation that may bias the age-specific patterns in vital rates at the population level if not accounted for in models (Cam et al., 2002). An example of this bias has been shown to occur when the quality of individuals within a cohort varies and below average individuals disappear from the population earlier in life, resulting in an increase of the average quality of the population as the cohort ages (Vaupel and Yashin, 1985). This progressive disappearance can mask age-specific patterns in vital rates at the individual level (Forslund and Pärt, 1995; Péron et al., 2010). Empirical studies that use longitudinal data and can account for bias created by individual heterogeneity are needed to progress our understanding of the evolution of senescence.
Here we use long-term data from a population of Weddell seals (Leptonychotes weddelli) in Erebus Bay, Antarctica to estimate and compare sex-specific actuarial senescence. Weddell seals are a long-lived marine mammal that aggregate in breeding colonies during the austral spring to give birth and breed. Weddell seals are easily approached by humans and have high site fidelity, which allows individuals to be monitored throughout life. We estimate and compare the age-specific survival patterns of males and females from early life to death using multi-decadal data on large samples of animals individually marked at birth and monitored annually to better understand sex differences in patterns of actuarial senescence in this long-lived species that exhibits modest polygyny. We employ a modeling approach that provides greater flexibility in the shape of the relationship between age and survival than previous research from this population (Brusa et al., 2020; Paterson et al., 2018). Using longitudinal data on individuals we account for individual heterogeneity in vital rates using mixture classes, which results in estimation of within-individual patterns of aging. This modeling approach allows for accurate estimation of actuarial senescence that can be compared between sexes.
Weddell seals provide an interesting case study for investigating sex-specific actuarial senescence as they don’t strictly follow the general patterns of highly polygynous species. Weddell seals show modest polygyny with males defending underwater breeding territories (Harcourt et al., 2007a). Males use aggressive competition to retain positions under the ice, which is associated with large energetic demands and physical costs. Males lose up to 24% of their initial body mass over the breeding season (Harcourt et al., 2007b) and males are often found hauled out on the ice with lacerations to their body (Harcourt et al., 2007a). The reproductive strategies of male Weddell seals lead to moderate variance in successful siring of pups in any given year (Gelatt, 2001; Harcourt et al., 2007a). Given the polygynous mating system of Weddell seals and the male-male combat for mating opportunities one prediction is that male Weddell seals exhibit a higher rate of senescence compared to females. This higher rate could be due to greater energy allocation to reproduction through combat and territory guarding, continued damage to the soma from competition in accordance with the disposable soma theory, the physiological costs of testosterone in accordance with antagonistic pleiotropy or a combination of all three. In male Weddell seals the onset of senescence has been reported to occur at three years old (Brusa et al., 2020), which is the age of sexual maturity in males (Gelatt, 2001), and we predict that we will observe a similar onset in this study.
Weddell seals are a particularly interesting species in that attributes of the mating system and sexual size dimorphism do not clearly predict whether males or females will senesce more strongly. Contrary to the commonly observed male-biased, sexual-size dimorphism of polygynous species, Weddell seals do not exhibit strong sexual-size dimorphism (Harcourt et al., 2007b; Hill, 1987) with some early research suggesting that females may be slightly larger (Stirling, 1975). As capital breeders, reproduction is energetically intensive for females with mothers nursing pups for ~30-45 days while primarily fasting, resulting in ~34% of body mass loss over the course of lactation (Macdonald et al., 2020). Therefore, females are not only subject to the extreme energetic demands of gestation and lactation but also the energetic requirements of a similar body size to males. Given the large energetic requirements of females, an alternative prediction under the energy allocation theory of senescence is that females are subject to greater energetic demands and will exhibit a higher rate of actuarial senescence compared to males. Previous research estimating age-specific survival of females started modeling from the average age of first reproduction at age seven and found immediate declines in survival probability (Paterson et al., 2018), although sexual maturity can occur at three years old (Gelatt, 2001). Therefore, the onset of actuarial senescence in females will likely occur between ages three and seven and we predict that it will occur later in this age range given very few females in this population give birth to a pup prior to age six.
This study focuses on a population of Weddell seals located in Erebus Bay, Antarctica (-77.62° to -77.87°S, 166.3° to 167.0°E) that has been monitored annually since 1969 (Siniff et al., 1977). Seals form pupping and breeding colonies each austral spring, from October-December, on the land-fast ice using perennial cracks that form from tidal movement to access the ice surface (Cameron and Siniff, 2004). Females give birth to pups starting in mid-October through November (Rotella et al., 2016) and remain with their pups for approximately five to six weeks to nurse (Stirling, 1969a). Males join the pupping colonies shortly after the pupping season begins to establish underwater territories and breed with females (Stirling, 1969a). Estimates of the operational sex ratio (male to female) vary between 2.8:1 and 8.9:1 (Hill, 1987). Male and female Weddell seals in this population can become sexually mature at 3 years of age (Gelatt, 2001). Males have been recorded siring offspring as early as four years old (Harcourt et al., 2007a). The mean age of first reproduction for females is 7-8 years-old (Hadley et al., 2006), giving birth at five years of age is rare, and just a few females have given birth at four years of age. There is evidence for negative density dependence in this population, but it is not known which vital rates are most influenced (Rotella et al., 2009). Theory suggests that in long-lived mammals, adult survival rates are the last to respond to increased density (Eberhardt, 2002), and recent research does show that annual variation in adult female survival has been consistently low across a 30-year period (Rotella et al., 2012), suggesting probabilities of reproduction and temporary emigration may respond more strongly to population density (Paterson et al., 2018).
Since 1968 Weddell seals have been individually marked in the hind flipper and resighted (Siniff et al., 1977). Since 1982 each pup born in the study area has been marked and most pups are marked within 1-2 days of birth (Garrott et al., 2012). The number of pups marked each year has fluctuated between 169 and 820 pups over the past 30 years with increases in the long-term average between the first decade of marking and the most recent decade (Levinson et al. In review). Estimates from 1990-2000 suggest there is a core adult population of 600 females and 200 males (Cameron and Siniff, 2004), but, given the increasing number of pups born each year, the core population size has likely increased in the study area during the past two decades. Individuals with broken, worn, or missing tags have been retagged when encountered to preserve the annual live-encounter histories for known individuals. Six to eight study area wide resighting surveys have been conducted each year approximately 3-5 days apart starting in early November since 1973 (Cameron and Siniff, 2004). All animal handling activities were approved by NOAA National Marine Fisheries Service (permit number: 21158 and previous permits) and the Institutional Animal Care and Use committee of Montana State University (protocol number:2017-11 and previous permits). The extensive mark-resight efforts allow ages to be known for virtually every individual born in the study area. Weddell seals exhibit strong adult philopatry to Erebus Bay if they were born to this population (Cameron and Siniff, 2004; Hadley et al., 2007). Pre-breeding females, mothers with pups, females on sabbatical from reproduction, as well as males are all detected at high rates in the study area over the course of multiple surveys (Brusa et al., 2020; Cameron and Siniff, 2004; Rotella et al., 2009). The nearest colony outside the study area is located at Marble Point, which is approximately 40 miles from the nearest edge of the study area. Historically dispersal between the Erebus Bay population and outside colonies is suggested to be minimal (Stirling, 1969a). Little is known regarding temporary emigration in males although in females, temporary emigration varies with age, reproductive status, and environmental variation (Chambert et al., 2015; Paterson et al., 2018). For example, young and old females are estimated to emigrate temporarily at a higher rate (Chambert et al., 2015).
We constructed extensions of Cormack-Jolly-Seber (CJS) models using a Bayesian framework to estimate age- and year-specific apparent survival and detection rates independently for males and females. CJS modeling cannot distinguish between death and permanent emigration so estimates of survival from populations where permanent emigration occurs will be biased low and the resulting survival estimates are considered apparent survival (Lebreton et al., 1992). Although the population is open to temporary emigration and possibly to low levels of permanent emigration (Cameron and Siniff, 2004; Chambert et al., 2015), Weddell seals display high philopatry to the Erebus Bay study area such that apparent survival estimates are expected to be quite close to estimates of true survival. We evaluated two competing model structures (see below) and estimates from the best-supported models were used for comparison between male and female Weddell seals. We compared patterns of estimated annual survival rates and rates of actuarial senescence, measured as the slope of change in survival rate with age (Ronget and Gaillard, 2020).
Due to low survival and detection rates of seals during the first few years of life the inclusion of an individual in the data set was conditioned on recapture of the individual at age 3 or older. With regards to age-specific variation in survival, we modeled survival with a spline function starting at age 3 years. To model age-specific patterns of survival and detection we used basis splines, which provide a flexible modeling approach that allows for a covariate to have a nonlinear relationship with the response variable and does not require any prior assumption about the shape of the relationship (Hastie and Tibshirani, 1990). The shape of the spline is determined by the data and is made up of segments that are connected by knots, or pivot points (Hastie and Tibshirani, 1990). The spline for age-specific survival included eight internal knots, placed at evenly spaced sample quantiles. The number of knots was chosen to allow a smooth flexible function without overfitting the relationship. Temporal variation in survival was accounted for by including a random effect for year (see below for specific model details).
A female Weddell seal’s probability of survival varies with reproductive state such that females that reproduced in the previous year exhibit slightly lower probabilities of survival compared to females that skipped reproduction (Paterson et al., 2018). Reproductive histories are unknown for males in this population, so to ensure survival estimates were comparable between males and females, reproductive state variables were not included in models. We accounted for heterogeneity in survival by adding a finite mixture of two survival classes to the basic survival model, allowing individuals in the same heterogeneity class to share an intercept. Finite mixture models allow individuals with similar vital rates to be clustered together in a finite number of heterogeneity classes (Pledger et al., 2003). Random effects have previously been used to model individual heterogeneity in survival for this population (Brusa et al., 2020; Paterson et al., 2018), but this method assumes that individual intercepts are normally distributed, which can be violated if there is any clustering for the trait being assessed (Hamel et al., 2016). To understand if models using finite mixtures perform similarly to those using random effects to account for individual heterogeneity in this population, we chose to use a finite mixture model to account for individual heterogeneity. Two survival classes were chosen to account for individual heterogeneity as we hypothesize the population is made up of individuals that exhibit high or low survival, similar to other survival analyses that have used mixture models to account for individual heterogeneity (Gimenez et al., 2018).
We evaluated a base model for age-related variation in detection rates that included a basis spline identical to that used to model survival. We chose a spline to allow greater flexibility in age -related patterns of detection compared to the logarithmic functional form of age or age bin models previously used to model detection for males (Brusa et al., 2020) and females (Hadley et al., 2007; Rotella et al., 2012) in this population. We also evaluated a model that included heterogeneity in detection by adding a finite mixture of three detection classes to the base detection model. Three detection classes were considered as there is evidence for greater individual variation in detection and we hypothesize that the population may be made up of individuals with low, average, and high detection. In both models a random effect for year was included to account for temporal variation in detection.
Model convergence was assessed using visual inspection of trace plots, the potential scale-reduction factor (Gelman and Rubin, 1992), and the Geweke diagnostic with the R package ggmcmc (Fernández-i-Marín, 2016). Model selection was carried out using the Watanabe-Akaike Information Criterion (WAIC) a fully Bayesian information criterion, based on the posterior predictive distribution (Hooten and Hobbs, 2015; Watanabe, 2010). Additionally, WAIC is appropriate for model selection when evaluating hierarchical and mixture models (Hooten and Hobbs, 2015; Watanabe, 2013). We fit all models in the R computing environment (R Core Team, 2023) using the package NIMBLE (de Valpine et al., 2023; de Valpine et al., 2017). All regression coefficients on age for survival and detection, along with the survival intercept were assigned weakly informative priors. The prior for proportion of individuals in each heterogeneity class was assumed to come from a uniform Dirichlet distribution. Random effects of year were assumed to be normally distributed around a mean of zero with unique standard deviations. Standard deviations for random effects of year were assigned weakly informative priors , truncated above zero to be positive values. Three chains were run for 240,000 iterations each with every fifth sample saved for posterior predictions resulting in 48000 samples from each chain. The first 12,000 iterations of each chain were removed as burn-in. Due to the large number of dependent nodes in the model that are used to calculate a WAIC score, every 20th sample was saved to the posterior distribution and subsequently used to calculate a WAIC score resulting in a posterior of 9,000 samples per chain after a burn-in of 3,000 for a total of 27,000 samples.
Goodness of fit was evaluated for the most supported model using posterior predictive checks (Conn et al., 2018; Gelman et al., 2013). For each of the 6,000 iterations from the posterior distribution used to calculate WAIC, we calculated survival and detection probabilities for each individual in each year using the estimated parameters, including estimated survival and detection class. Binomial trials were conducted for each individual in each year using the predicted probabilities to simulate whether an individual survived from one season to the next and was detected if alive. These 6,000 simulated data sets were then summarized by age class, and the number of individuals seen at each age was then compared to the corresponding number in the actual field data. The four age classes used for posterior checks corresponded to four biologically relevant periods of life. Early life (3-6 years), recruitment ages (7-12 years), prime reproductive ages (13-20 years), and late life (21 years and older). We calculated the Bayesian P-value as the proportion of simulated data sets for which the number of detected individuals in an age class was equal or greater than that observed in the data providing a measure of how well the most supported model predicted observed encounter histories. Bayesian P-values were above 0.90 for females during the early life period, but within an acceptable range for all other periods of life for males and females (0.33-0.80), which included the ages of interest for making inferences regarding senescent patterns. The overestimate of females seen during early life could be a result of overestimating detection or survival probabilities, but it occurs during a time when survival is quite high and senescence is quite weak. Given survival and detection classes were included in the calculation of survival and detection probabilities used for the posterior predictive checks it suggests the mixture classes adequately estimated individual heterogeneity in survival and detection.
We used capture-mark-recapture encounter histories from 1,474 male and 1,879 female Weddell seals tagged as pups between 1982 and 2018 to model vital rates. Seals were monitored through 2019 at which point our data included 6,159 observations for males and 12,191 observations for females after birth. Encounter histories started at the age of 3 years and individuals still alive at the end of the monitoring period were accounted for by right censoring these data. The maximum age recorded was 28 years for males and 31 years for females. Encounter histories for individual males ranged from one observation (n=386) to 17 observations (n=4), and females ranged from one observation (n=336) to 27 observations (n=2).
Parameter estimates are represented by the mean of the posterior distribution and are presented on the probability scale unless otherwise noted. Results are presented with 95% highest density intervals (Meredith and Kruschke, 2020). Survival rates can be biased low if marks are lost throughout the study period (Arnason and Mills, 1981). Estimated tag-retention rates () for the study population are quite high with the probability of retaining at least one tag being 0.989 for adult females and 0.982 for adult males (Cameron, 2001; Cameron and Siniff, 2004). Survival rate estimates presented below are corrected for tag loss using the adjustment equation (Arnason and Mills, 1981).
The model that received the most support for both males and females included splines to model age- specific survival and detection as well as a mixture of two survival classes and three detection classes (Table 1; Supplementary Figures S1–S3). A large proportion of individuals were estimated to be in the high survival class, 0.75 (0.52, 0.94) of females and 0.88 (0.63, 1.0) of males (Supplementary Figures S4, S5). Given the large proportion of individuals in the high survival class, we only report estimates for that class here, but the patterns reported here are the same as those estimated for the low survival class.
Patterns of age-specific survival were similar between male and female Weddell seals early in life before diverging and provided clear evidence of senescence. Annual apparent survival for females was estimated to be highest at three years old with survival estimated to be 0.98 (95% HDI: 0.96, 1.0) for individuals in the high survival class and 0.72 (0.45, 0.88) for the low survival class. Estimates of male survival are lower than females for all ages although the highest density intervals for each sex moderately overlap from ages 3-14 years (Figure 1). Apparent survival of males is estimated to be highest at 6 years old, 0.96 (0.93, 0.98) for the high survival class and 0.55 (0.12, 0.90) for the low survival class. This indicates onset of senescence for females is at 3 years old and males at 6 years old, although the highest density intervals widely overlap point estimates at younger ages so it’s possible the age of onset of senescence may be earlier or later. Earlier in life senescence was stronger in females, but after 12 years of age the rate of annual senescence, calculated as the change in annual probability of survival between successive ages, changes direction (Supplementary Table S1). Senescence in males continues to increase after 12 years old whereas the rate of decline in annual survival probabilities for females was slight from age 12 to 19 (apparent survival at 12 years ()= 0.94 [0.91,0.98]; = 0.88 [0.86, 0.91]). Female senescence in survival rates accelerated with rates declining steadily from age 20 years through the oldest age of 31 years old. In contrast, survival rates for males continued to decline in steady fashion from ages 12 to 19 ( = 0.92 [0.90, 0.96]; = 0.82 [0.77, 0.86]). Males experience an acceleration in senescence after age 19 years old as well with apparent survival declining more rapidly to the oldest observed age of 28 years.
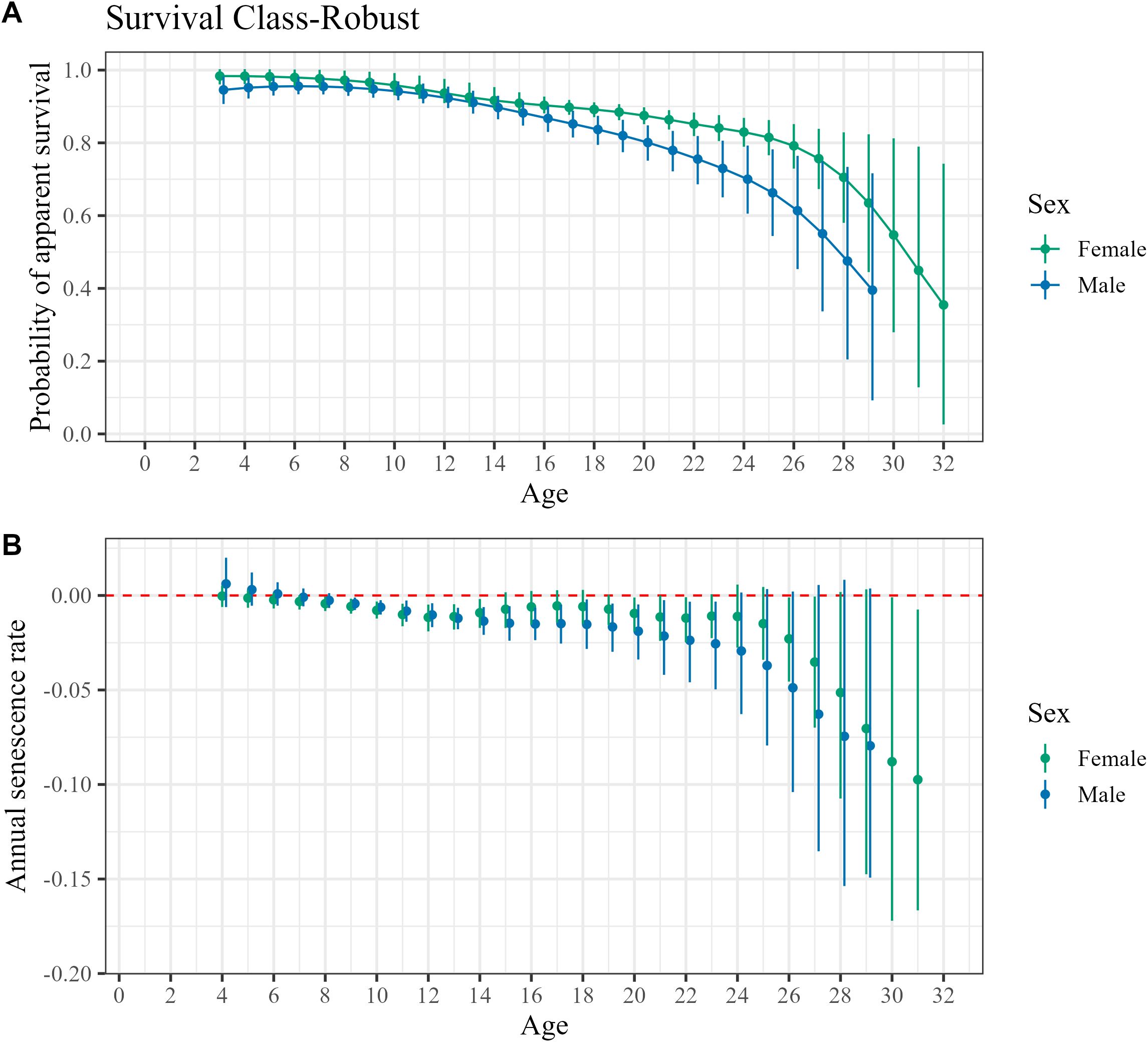
Figure 1. Comparisons of male and female (A) probability of apparent survival for ages observed and (B) calculated rate of senescence between each age, with the dashed red line representing no change in probability of survival. Error bars represent 95% highest density intervals.
Detection of males and females increases steadily from age three years to 12 years before stabilizing and declining at the oldest ages (Supplementary Figure S6). Male detection was estimated to be lower than females with larger uncertainty surrounding estimates of detection probability at young and old ages. There was support for individual heterogeneity in probability of detection with most females being assigned to the high detection class and most males being assigned to the average detection class (Supplementary Figure S6). There was much greater annual variation in probability of detection compared to survival. Annual variation in probability of survival was low and ranged from 0.94 (0.91, 0.98) to 0.91 (0.87, 0.96) for a 12-year-old female and between 0.94 (0.90, 0.98) and 0.79 (0.65, 0.92) for a 12-year-old male (Figure 2). Annual variation in detection was greater than survival with probability of detection in the high detection class ranging from 0.99 (0.99, 1.0) to 0.67 (0.60, 0.73) for a 12-year-old female and 0.94 (0.88, 0.98) to 0.43 (0.34, 0.53) for a 12-year-old male.
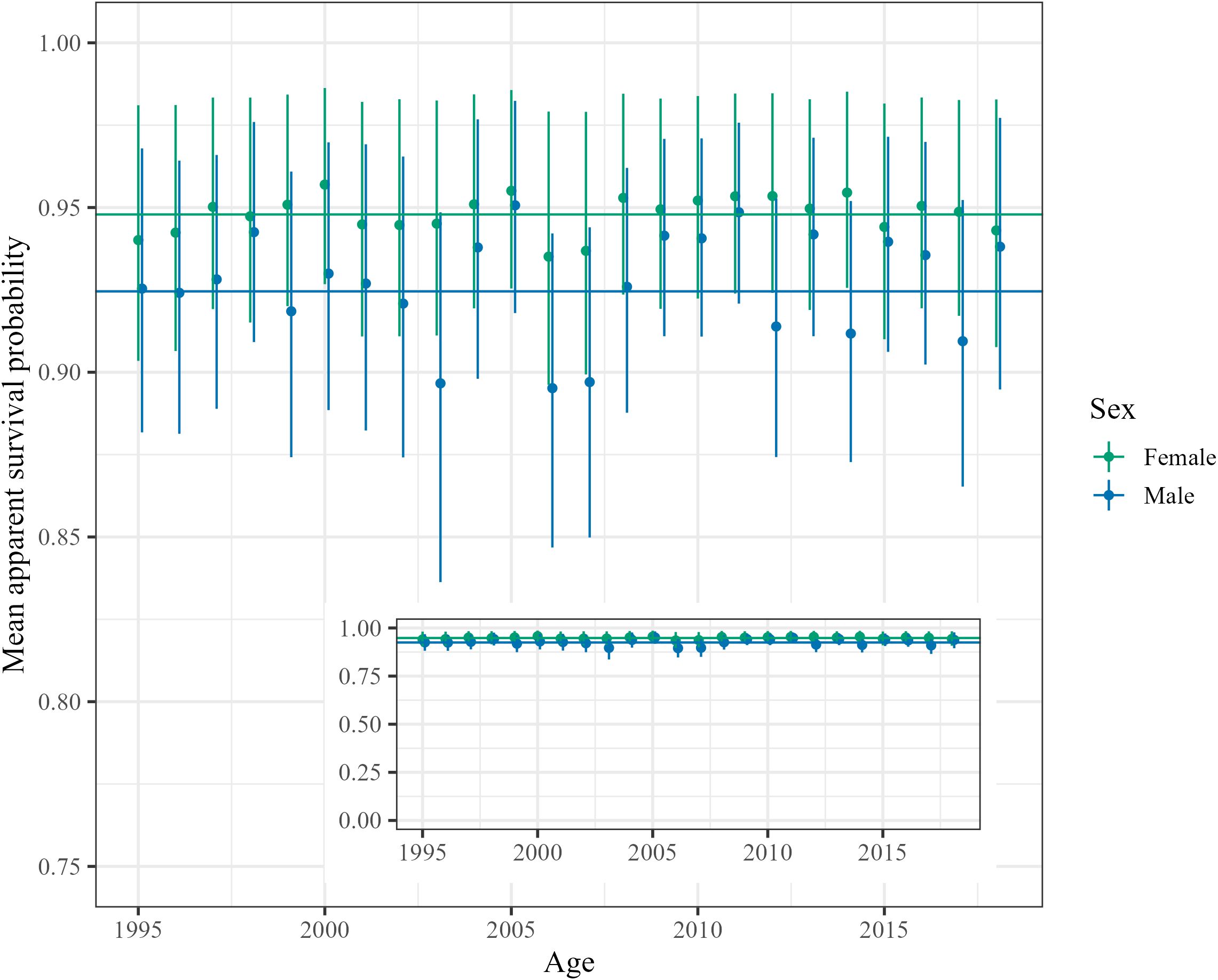
Figure 2. Annual variation in probability of apparent survival for a 12-year old male (blue) and female (green). Horizontal lines represent survival probability with a random effect of year equal to zero. Only years for which the survival probability of a 12-year old individual could be estimated are included. Error bars represent 95% highest density intervals.The inset graph shows estimates on an axis from 0-1 to help visualize the annual variation in probability of apparent survival.
Using data from a long-term mark recapture study of Weddell seals, we confirmed predictions of sex-specific patterns of actuarial senescence for a moderately polygynous species. Our research built upon previous work from this population that estimated actuarial senescence in males (Brusa et al., 2020) and compared senescence patterns to those estimated for females after age of first reproduction (Paterson et al., 2018). We calculated age-specific survival probabilities of individuals recaptured at age three years or older for males and females separately and used basis splines, a more flexible modeling approach that does not force age specific survival to take a certain shape. These methods allowed us to make a full comparison of male and female senescence patterns and place the results within the context of evolutionary theories for senescence.
Our findings broadly align with previous work from this population. We found that male and female Weddell seal survival probabilities peak around the age of sexual maturity and that there are some differences in the rate and pattern of senescence between the sexes, similar to previous work from this population (Brusa et al., 2020). Our estimate of peak male apparent survival is 0.96 (0.94, 0.97) at six years old for an individual in the high survival class which is a slightly higher probability of survival and later age of onset of senescence compared to the previously reported estimate of 0.94 (95% CI: 0.92, 0.96) at two years old (Brusa et al., 2020). We found that females exhibited a decline in probability of survival from ages 3-6 years old compared to the estimated increase in probability of survival for males adding to our understanding of survival patterns for females prior to the average age of first reproduction. Although it is possible permanent emigration of males at the youngest ages could bias estimates resulting in an underestimation of survival the highest density intervals are wider at these ages and the observed increase may simply be the result of less precise estimates at the youngest ages. We found a near plateau in probability of survival for females between the ages of 12 to 19 years old, which is slightly different from results of previous work that found more rapid declines in survival during these ages (Paterson et al., 2018). It is important to note that previous estimates of age specific survival for females only included females that reproduced at least once in their lives and also accounted for breeding state and temporary emigration (Paterson et al., 2018). To make estimates for females and males as comparable as possible, we did not account for breeding state or temporary emigration in the female model and it’s possible that not accounting for these state transitions may have resulted in a plateau. If a greater proportion of females skipped reproduction between the ages of 12 to 19 years old and therefore avoided survival costs of reproduction this might result in the plateau of survival probabilities, we observed. However, the explanation that the absence of state transitions accounts for the plateau in survival probabilities seems unlikely given that females exhibit a peak in probability of reproduction during these ages (Paterson et al., 2018). Additionally, the probability of temporary emigration is estimated to be 0.04 (95% credible interval: 0.03-0.07) on average, with the lowest levels of temporary emigration estimated for females at ~14 years old (Chambert et al., 2015).
The onset of senescence is estimated to be slightly earlier for females occurring between ages of three and four years old, compared to ages between six and seven years old for males. The onset of senescence for females occurs at the age of first ovulation recorded in the literature (Gelatt, 2001) although our records indicate it is very rare for individuals in our study population to begin reproduction at this age. For females the age of onset occurs several years earlier than the mean age of first reproduction which is estimated to be approximately seven years old for females (Hadley et al., 2006; Paterson et al., 2018). The estimated onset of senescence for males does coincide with the approximate age when males have been recorded competing for territories (Gelatt, 2001). Interestingly, males as young as four years are known to have sired pups (Harcourt et al., 2007a), so the average age that onset of senescence occurs is after the age of sexual maturity, perhaps because a small proportion of young males actually attempt to sire pups at such ages. It is notable that while males and females can become sexually mature at the same age, there is a difference in the onset of senescence. There is now evidence that predictions for the onset of senescence at reproductive maturation do not hold in all species (Gaillard and Lemaître, 2017), and others have found the onset of senescence to be plastic (Hammers et al., 2013). Given that Weddell seal females exhibit a large range in the age of first reproduction (Hadley et al., 2006), our findings suggest that the onset of senescence may be related to age at first breeding and not to the age at which individuals become reproductively mature. Changes in the probability of survival from ages three to ten years are quite small for both males and females so although there is a small difference in onset of senescence between sexes the pattern of senescence appears to be quite similar during this early life period. Our findings suggest that onset of senescence may be related to the age of first reproduction but that the costs to survival of sexual maturity and first reproduction are likely low given the small declines in survival rates during the early reproductive years.
We found that the senescence rate of males and females diverge around 12 years old (Figure 1). Male survival probabilities continue to decline at an increasing rate, but females show a diminished rate of senescence with a near plateau in survival probabilities from ages 12-19 years. This pattern of a decline in mortality rate during prime ages after age of first reproduction has been exhibited in other large mammals, and it’s hypothesized that this is due to risk avoidance by females whereby they modify reproductive effort in response to environmental conditions (Gaillard et al., 2003). Our findings are in agreement with this hypothesis as Weddell seal females will often skip reproduction every few years or during environmentally challenging conditions, which may allow them to avoid energetic costs of reproduction and provide the chance to focus energy on somatic maintenance (Chambert et al., 2015, Chambert et al., 2013). The population of individual females greater than 11 years old will be made up of nearly all experienced breeders (Hadley et al., 2006) therefore, it may be that females are able to slow the rate of senescence during prime age due to reproductive experience and improved energetic efficiency during lactation (Macdonald et al., 2020), freeing up resources for somatic maintenance. Additionally, there is evidence that non-reproductive adult female Weddell seals carry higher blood oxygen stores compared to males, which translates to a slight increase in the calculated aerobic dive limit (Hindle et al., 2011). The aerobic dive limit can constrain the time and efficiency of foraging for animals that forage underwater (Hindle et al., 2011), and it is possible this difference in oxygen stores may contribute to the observed differences in survival rates of males and females at these prime ages.
Unlike females, males exhibit intense competition for access to mating opportunities, with competition often resulting in deep lacerations to the body (Harcourt et al., 2007a). During the mating season males can lose almost a quarter of their body mass (Harcourt et al., 2007b) likely because they are allocating more energy to competition and foregoing opportunities to forage in order to spend more time defending mating territories. Therefore, an explanation for increased rate of senescence of prime age males may be in line with the energetic allocation hypothesis (Bonduriansky et al., 2008). It seems that while energetic allocation towards mating and not somatic maintenance may play a part in the rate of senescence for males during this period the mass loss of males is much lower to that of females and so energetic allocation alone as measured by body mass loss does not fully explain the increased rate of senescence compared to females in the prime and old ages. Another mechanism that might explain the increased senescence of males compared to females during prime ages may be that of reduced immune response of males due to increased testosterone production of sexually mature males (Metcalf et al., 2020; Tidière et al., 2020). As males accumulate mating seasons, they accumulate damage from successive years of competition with other males. Therefore the increased rate of senescence for males, especially at older ages, may be due to the accumulating deterioration of the soma from the damage of competition, and reduced energetic allocation towards somatic repair combined with reduced immune response (Bonduriansky et al., 2008), which would be in line with the disposable soma theory of senescence.
We found evidence that senescence rates of males and females increase after 19 years of age. Males show strong increases in senescence rates after 19 years old whereas for females the increase in senescence rate is more moderate out to 25 years old at which point the rate of senescence occurs more rapidly. The increase in rate of senescence for both males and females is not surprising as damage to the soma and accumulation of mutations at older ages is expected based on evolutionary theory (Baudisch and Vaupel, 2012; Kirkwood, 1977; Kirkwood and Austad, 2000; Williams, 1957). Results from a physiology study suggests a potential mechanism behind the faster rate of senescence exhibited by older males. While older female and male Weddell seals (ages 19+ years) both show muscular senescence, males also exhibit lower densities of myofiber compared to females, which was hypothesized to be the result of elevated muscle stress due to competition for mating territories (Hindle et al., 2009). Evidence for muscular senescence (Hindle et al., 2009) and advanced tooth wear (Stirling, 1969b) at older ages suggest physical wear and tear may reduce foraging efficiency and the ability to maintain breathing holes at old ages, which could potentially reduce survival rates of both sexes.
Our finding that male survival was lower than females throughout life and that the maximum observed male lifespan was three years shorter than for females aligns with results from the literature for a species that displays a polygynous mating system (Gaillard and Lemaître, 2020; Lemaître and Gaillard, 2013; Tidière et al., 2015). This result also aligns with life history theory that predicts in species where the burden of sexual reproduction is greater or variance in reproductive success is lower for females, males are expected to sacrifice longer lifespans for greater mating success. In species where females experience greater costs from sexual reproduction, females are predicted to adopt more low-risk strategies that allow greater reproductive success over a longer time (Bonduriansky et al., 2008). Sexual selection is not only predicted to be a driver of observed lifespan but also contribute to sex differences in senescence patterns.
We generally found support for the hypothesis that sex differences in senescence rates of Weddell seals could be explained by sexual selection. As part of sexual selection, sexual size dimorphism is considered a potential driver behind sex differences in senescence rates, but we found no evidence to support this hypothesis given there is little difference in size between the sexes (Harcourt et al., 2007b; Hill, 1987; Stirling, 1975), and still found that males exhibited stronger actuarial senescence after early adulthood. Instead, our results match predictions that for a species with high sexual competition the competing sex will experience increased senescence and mortality due to the energetic trade off of somatic maintenance and maintenance of sexual traits (Bonduriansky et al., 2008 Although female Weddell seals lose a larger proportion of their mass over the course of lactation mobilizing a greater energetic investment into reproduction (Macdonald et al., 2020) they also display a flexible, risk averse reproductive tactic, skipping reproduction to regain body reserves (Chambert et al., 2015, Chambert et al., 2013). Males appear to lose a lower proportion of mass over the course of the breeding season compared to females, but males may also show less flexibility in competition for mating opportunities, putting forth energy towards competition and reproduction on an annual basis accumulating damage to the soma. It is not currently known if males show a more flexible pattern of reproduction, skipping mating and the necessary competition in some years, but a better understanding of the relative amount and timing of reproductive effort expressed by each sex should shed some light on this question.
Although our results generally follow predictions for a moderately polygynous species factors beyond mating system may influence the patterns of senescence observed. Authors suggest that sex differences in aging rates may be due to a complex interaction between environment and sex-specific reproductive costs and not mating system (Lemaître et al., 2020). This interaction between environment and sex-specific reproductive costs may be at play in Weddell seals as males adopt the risky behavior of fighting for mating territories accumulating wounds and within the potentially pathogen rich environment of a seal colony this may lead to a greater rate of senescence. Sex specific differences in senescence have been hypothesized to be driven by sex differences in response to pathogens (Tidière et al., 2020). Blood samples from this population during the breeding season show evidence of increased indicators of stress and lower levels of nourishment in males (Mellish et al., 2011). This evidence in conjunction with some evidence for reduced immune response of males linked to testosterone levels (Metcalf et al., 2020; Tidière et al., 2020) could also explain the increased rates of senescence we found for male Weddell seals.
Although our study cannot distinguish the mechanisms leading to sex differences in both longevity and strength of senescence of Weddell seals our results generally align with predictions that life history strategies, specifically sexual selection leads to greater senescence in the sex that competes for opportunities to mate and has less investment in offspring (Bonduriansky et al., 2008; Brooks and Garratt, 2017; Bronikowski et al., 2022) and with more recent suggestions that differences may arise from an interaction between sex-specific physiological pathways and environmental conditions (Lemaître et al., 2020). To further understand the mechanisms behind sex differences in senescence patterns of Weddell seals it would be useful to improve our knowledge regarding patterns of reproductive effort and understanding of the relationship between reproductive effort and survival for male Weddell seals. Currently, we cannot measure either reproductive effort or reproductive success of males because mating occurs in the water. Should this information become more easily measured in this species, perhaps through genomic methods, it would be useful to investigate survival costs of reproduction for males. Additionally, longitudinal physiology studies provide an opportunity to better understand physiologic pathways of senescence in the Weddell seal.
Our work adds to a growing number of studies that explore sex differences in survival senescence within a species (Fay et al., 2020; Gamelon et al., 2014; Pardo et al., 2013; Reichard et al., 2022; Thorley et al., 2020; Tompkins and Anderson, 2019) and can be used in the future to add to studies across species (Clutton-Brock and Isvaran, 2007; Gaillard et al., 2003; Lemaître et al., 2020; Lemaître and Gaillard, 2013; Tidière et al., 2015). These results suggest that for a long-lived, moderately polygynous mammal, differences in actuarial senescence between the sexes may be driven primarily by sexual selection, but that environmental conditions may also be involved. Given that explanations for patterns of sex-specific senescence across species lack consistent evidence there is a need for additional studies that include species across a wide range of different life-history strategies. It seems that within mating systems the sex differences in amount and timing of energetic allocation towards reproduction may be quite different across species and may explain why sex-specific senescence rates cannot be fully explained by the mating system itself. Clearly more work is needed to elucidate the mechanisms driving patterns in sex-specific senescence and our results provide a useful data point towards that end.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Dryad Digital Repository, doi: 10.5061/dryad.4b8gthtp1.
The animal study was approved by Institutional Animal Care and Use Committee of Montana State University and NOAA National Marine Fisheries. The study was conducted in accordance with the local legislation and institutional requirements.
KM: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. JR: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing. WL: Formal analysis, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work presented here and the field work that provided the data used in our analyses were supported by a series of grants from the National Science Foundation, Office of Polar Programs (Grant Nos. 1640481 and 2147553) and prior NSF Grants to R. A. Garrott, JR, D. B. Siniff, and J. Ward Testa.
We thank the many graduate students and field technicians who have collected data on this project. We would like to thank Terrill Paterson who provided feedback on data analysis and assisted in coding. Logistical support for fieldwork in Antarctica was provided by Lockheed Martin, Raytheon Polar Services Company, Antarctic Support Associates, the United States Navy and Air Force and Petroleum Helicopters Incorporated. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1488373/full#supplementary-material
Arnason A. N., Mills K. H. (1981). Bias and loss of precision due to tag loss in jolly–seber estimates for mark–recapture experiments. Can. J. Fish. Aquat. Sci. 38, 1077–1095. doi: 10.1139/f81-148
Baudisch A., Vaupel J. W. (2012). Getting to the root of aging. Science 338, 618–619. doi: 10.1126/science.1226467
Bonduriansky R., Maklakov A., Zajitschek F., Brooks R. (2008). Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 22, 443–453. doi: 10.1111/j.1365-2435.2008.01417.x
Brooks R. C., Garratt M. G. (2017). Life history evolution, reproduction, and the origins of sex-dependent aging and longevity. Ann. New York Acad. Sci. 1389, 92–107. doi: 10.1111/nyas.13302
Bronikowski A. M., Meisel R. P., Biga P. R., Walters J. R., Mank J. E., Larschan E., et al (2022). Sex-specific aging in animals: Perspective and future directions. Aging Cell 21, e13542. doi: 10.1111/acel.13542
Brusa J. L., Rotella J. J., Garrott R. A., Paterson J. T., Link W. A. (2020). Variation of annual apparent survival and detection rates with age, year and individual identity in male Weddell seals (Leptonychotes weddellii) from long-term mark-recapture data. Population Ecol. 62, 134–150. doi: 10.1002/1438-390X.12036
Cam E., Link W. A., Cooch E. G., Monnat J., Danchin E., Travis E. J. (2002). Individual covariation in life-history traits: seeing the trees despite the forest. Am. Nat. 159, 96–105. doi: 10.1086/324126
Cameron M. F. (2001). Dynamics of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica (Ph.D.) (United States – Minnesota: University of Minnesota).
Cameron M. F., Siniff D. B. (2004). Age-specific survival, abundance, and immigration rates of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica. Can. J. Zoology 82, 601–615. doi: 10.1139/z04-025
Catchpole E. A., Fan Y., Morgan B. J. T., Clutton-Brock T. H., Coulson T. (2004). Sexual dimorphism, survival and dispersal in red deer. JABES 9, 1–26. doi: 10.1198/1085711043172
Cayuela H., Lemaître J.-F., Bonnaire E., Pichenot J., Schmidt B. R. (2020). Population position along the fast–slow life-history continuum predicts intraspecific variation in actuarial senescence. J. Anim. Ecol. 89, 1069–1079. doi: 10.1111/1365-2656.13172
Chambert T., Rotella J. J., Garrott R. A. (2015). Female Weddell seals show flexible strategies of colony attendance related to varying environmental conditions. Ecology 96, 479–488. doi: 10.1890/14-0911.1
Chambert T., Rotella J. J., Higgs M. D., Garrott R. A. (2013). Individual heterogeneity in reproductive rates and cost of reproduction in a long-lived vertebrate. Ecol. Evol. 3, 2047–2060. doi: 10.1002/ece3.615
Clutton-Brock T., Isvaran K. (2007). Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B: Biol. Sci. 274, 3097–3104. doi: 10.1098/rspb.2007.1138
Conn P. B., Johnson D. S., Williams P. J., Melin S. R., Hooten M. B. (2018). A guide to Bayesian model checking for ecologists. Ecol. Monogr. 88, 526–542. doi: 10.1002/ecm.1314
de Valpine P., Paciorek C. J., Turek D., Michaud N., Anderson-Bergman C., Obermeyer F., et al. (2023). NIMBLE: MCMC, particle filtering, and programmable hierarchical modeling. Zenodo. doi: 10.5281/ZENODO.1211190
de Valpine P., Turek D., Paciorek C. J., Anderson-Bergman C., Lang D. T., Bodik R. (2017). Programming with models: writing statistical algorithms for general model structures with NIMBLE. J. Comput. Graphical Stat 26, 403–413. doi: 10.1080/10618600.2016.1172487
Eberhardt L. L. (2002). A paradigm for population analysis of long-lived vertebrates. Ecology 83, 2841–2854. doi: 10.1890/0012-9658(2002)083[2841:APFPAO]2.0.CO;2
Fay R., Schaub M., Border J. A., Henderson I. G., Fahl G., Feulner J., et al. (2020). Evidence for senescence in survival but not in reproduction in a short-lived passerine. Ecol. Evol. 10, 5383–5390. doi: 10.1002/ece3.6281
Fernández-i-Marín X. (2016). ggmcmc: analysis of MCMC samples and bayesian inference. J. Stat. Software 70, 1–20. doi: 10.18637/jss.v070.i09}
Foo Y. Z., Nakagawa S., Rhodes G., Simmons L. W. (2017). The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. 92, 551–571. doi: 10.1111/brv.12243
Forslund P., Pärt T. (1995). Age and reproduction in birds — hypotheses and tests. Trends Ecol. Evol. 10, 374–378. doi: 10.1016/S0169-5347(00)89141-7
Gaillard J.-M., Lemaître J.-F. (2017). The Williams’ legacy: A critical reappraisal of his nine predictions about the evolution of senescence. Evolution 71, 2768–2785. doi: 10.1111/evo.13379
Gaillard J.-M., Lemaître J.-F. (2020). An integrative view of senescence in nature. Funct. Ecol. 34, 4–16. doi: 10.1111/1365-2435.13506
Gaillard J.-M., Loison A., Festa-Bianchet M., Yoccoz N. G., Solberg E. (2003). Ecological correlates of life span in populations of large herbivorous mammals. Population Dev. Rev. 29, 39–56.
Gamelon M., Focardi S., Gaillard J.-M., Gimenez O., Bonenfant C., Franzetti B., et al. (2014). Do age-specific survival patterns of wild boar fit current evolutionary theories of senescence? Evolution 68, 3636–3643. doi: 10.1111/evo.12519
Garratt M., Lemaître J.-F., Douhard M., Bonenfant C., Capron G., Warnant C., et al. (2015). High juvenile mortality is associated with sex-specific adult survival and lifespan in wild roe deer. Curr. Biol. 25, 759–763. doi: 10.1016/j.cub.2014.11.071
Garrott R. A., Rotella J. J., Siniff D. B., Parkinson C. L., Stauffer G. E. (2012). Environmental variation and cohort effects in an Antarctic predator. Oikos 121, 1027–1040. doi: 10.1111/j.1600-0706.2011.19673.x
Gelatt T. S. (2001). Male reproductive success, relatedness, and the mating system of Weddell seals in McMurdo Sound, Antarctica (Ph.D.) (United States – Minnesota: University of Minnesota).
Gelman A., Carlin J. B., Stern H. S., Dunson D. B., Vehtari A., Rubin D. B. (2013). Bayesian data analysis. 3rd ed. (Philadelphia, PA, United States: Chapman and Hall/CRC).
Gelman A., Rubin D. B. (1992). Inference from iterative simulation using multiple sequences. Statist. Sci. 7, 457–472. doi: 10.1214/ss/1177011136
Gimenez O., Cam E., Gaillard J.-M. (2018). Individual heterogeneity and capture–recapture models: what, why and how? Oikos 127, 664–686. doi: 10.1111/oik.04532
Gittleman J. L., Thompson S. D. (1988). Energy allocation in mammalian reproduction. Am. Zoologist 28, 863–875. doi: 10.1093/icb/28.3.863
Hadley G. L., Rotella J. J., Garrott R. A. (2007). Evaluation of reproductive costs for Weddell seals in Erebus Bay, Antarctica. J. Anim. Ecol. 76, 448–458. doi: 10.1111/j.1365-2656.2007.01219.x
Hadley G. L., Rotella J. J., Garrott R. A., Nichols J. D. (2006). Variation in probability of first reproduction of Weddell seals. J. Anim. Ecol. 75, 1058–1070. doi: 10.1111/j.1365-2656.2006.01118.x
Hamel S., Yoccoz N. G., Gaillard J.-M. (2016). Assessing variation in life-history tactics within a population using mixture regression models: a practical guide for evolutionary ecologists. Biol. Rev. 92, 754–775. doi: 10.1111/brv.12254
Hamilton W. D. (1966). The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45. doi: 10.1016/0022-5193(66)90184-6
Hammers M., Richardson D. S., Burke T., Komdeur J. (2013). The impact of reproductive investment and early-life environmental conditions on senescence: support for the disposable soma hypothesis. J. Evolutionary Biol. 26, 1999–2007. doi: 10.1111/jeb.12204
Harcourt R. G., Kingston J. J., Cameron M. F., Waas J. R., Hindell M. A. (2007a). Paternity analysis shows experience, not age, enhances mating success in an aquatically mating pinniped, the weddell seal (Leptonychotes weddellii). Behav. Ecol. Sociobiology 61, 643–652. doi: 10.1007/s00265-006-0294-x
Harcourt R. G., Kingston J. J., Waas J. R., Hindell M. A. (2007b). Foraging while breeding: alternative mating strategies by male Weddell seals? Aquat. Conserv: Mar. Freshw. Ecosyst. 17, S68–S78. doi: 10.1002/aqc.915
Hastie T. J., Tibshirani R. J. (1990). Generalized additive models (New York: Chapman & Hall/CRC Press).
Hill S. E. B. (1987). Reproductive ecology of weddell seals (leptonychotes weddelli) in mcmurdo sound, Antarctica (Ph.D.) (United States – Minnesota: University of Minnesota).
Hindle A. G., Horning M., Mellish J. A. E., Lawler J. M. (2009). Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii). J. Exp. Biol. 212, 790–796. doi: 10.1242/jeb.025387
Hindle A. G., Mellish J.-A. E., Horning M. (2011). Aerobic dive limit does not decline in an aging pinniped. J. Exp. Zool. 315A, 544–552. doi: 10.1002/jez.703
Hooten M. B., Hobbs N. T. (2015). A guide to Bayesian model selection for ecologists. Ecol. Monogr. 85, 3–28. doi: 10.1890/14-0661.1
Jones O. R., Gaillard J.-M., Tuljapurkar S., Alho J. S., Armitage K. B., Becker P. H., et al. (2008). Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol. Lett. 11, 664–673. doi: 10.1111/j.1461-0248.2008.01187.x
Jones O. R., Scheuerlein A., Salguero-Gómez R., Camarda C. G., Schaible R., Casper B. B., et al. (2014). Diversity of ageing across the tree of life. Nature 505, 169–173. doi: 10.1038/nature12789
Landsem T. L., Yoccoz N. G., Layton-Matthews K., Hilde C. H., Harris M. P., Wanless S., et al. (2023). Raising offspring increases ageing: Differences in senescence among three populations of a long-lived seabird, the Atlantic puffin. J. Anim. Ecol. 92, 774–785. doi: 10.1111/1365-2656.13884
Lebreton J.-D., Burnham K. P., Clobert J., Anderson D. R. (1992). Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 62, 67–118. doi: 10.2307/2937171
Lemaître J.-F., Berger V., Bonenfant C., Douhard M., Gamelon M., Plard F., et al. (2015). Early-late life trade-offs and the evolution of ageing in the wild. Proc. R. Soc. B: Biol. Sci. 282, 20150209. doi: 10.1098/rspb.2015.0209
Lemaître J.-F., Gaillard J.-M. (2013). Male survival patterns do not depend on male allocation to sexual competition in large herbivores. Behav. Ecol. 24, 421–428. doi: 10.1093/beheco/ars179
Lemaître J.-F., Gaillard J.-M. (2017). Reproductive senescence: new perspectives in the wild. Biol. Rev. 92, 2182–2199. doi: 10.1111/brv.12328
Lemaître J.-F., Gaillard J.-M., Lackey L. B., Clauss M., Müller D. W. H. (2013). Comparing free-ranging and captive populations reveals intra-specific variation in aging rates in large herbivores. Exp. Gerontology 48, 162–167. doi: 10.1016/j.exger.2012.12.004
Lemaître J.-F., Ronget V., Tidière M., Allainé D., Berger V., Cohas A., et al. (2020). Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl. Acad. Sci. U.S.A. 117, 8546–8553. doi: 10.1073/pnas.1911999117
Loison A., Festa-Bianchet M., Gaillard J.-M., Jorgenson J. T., Jullien J.-M. (1999). Age-specific survival in five populations of ungulates: evidence of senescence. Ecology 80, 2539–2554. doi: 10.1890/0012-9658(1999)080[2539:ASSIFP]2.0.CO;2
Macdonald K. R., Rotella J. J., Garrott R. A., Link W. A. (2020). Sources of variation in maternal allocation in a long-lived mammal. J. Anim. Ecol. 89, 1927–1940. doi: 10.1111/1365-2656.13243
Marais G. A. B., Gaillard J.-M., Vieira C., Plotton I., Sanlaville D., Gueyffier F., et al. (2018). Sex gap in aging and longevity: can sex chromosomes play a role? Biol. Sex Differ 9, 33. doi: 10.1186/s13293-018-0181-y
Mellish J.-A. E., Hindle A. G., Horning M. (2011). Health and condition in the adult Weddell seal of McMurdo Sound, Antarctica. Zoology 114, 177–183. doi: 10.1016/j.zool.2010.11.007
Metcalf C. J. E., Roth O., Graham A. L. (2020). Why leveraging sex differences in immune trade-offs may illuminate the evolution of senescence. Funct. Ecol. 34, 129–140. doi: 10.1111/1365-2435.13458
Monaghan P., Charmantier A., Nussey D. H., Ricklefs R. E. (2008). The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378. doi: 10.1111/j.1365-2435.2008.01418.x
Nussey D. H., Coulson T., Festa-Bianchet M., Gaillard J.-M. (2008). Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol. 22, 393–406. doi: 10.1111/j.1365-2435.2008.01408.x
Nussey D. H., Froy H., Lemaitre J.-F., Gaillard J.-M., Austad S. N. (2013). Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Res. Reviews Special Issue: Invertebrate Models Aging 12, 214–225. doi: 10.1016/j.arr.2012.07.004
Pardo D., Barbraud C., Weimerskirch H. (2013). Females better face senescence in the wandering albatross. Oecologia 173, 1283–1294. doi: 10.1007/s00442-013-2704-x
Paterson J. T., Rotella J. J., Link W. A., Garrott R. (2018). Variation in the vital rates of an Antarctic marine predator: the role of individual heterogeneity. Ecology 99, 2385–2396. doi: 10.1002/ecy.2481
Péron G., Crochet P.-A., Choquet R., Pradel R., Lebreton J.-D., Gimenez O. (2010). Capture–recapture models with heterogeneity to study survival senescence in the wild. Oikos 119, 524–532. doi: 10.1111/j.1600-1706.2009.17882.x
Pledger S., Pollock K. H., Norris J. L. (2003). Open capture-recapture models with heterogeneity: I. Cormack-Jolly-Seber Model. Biometrics 59, 786–794. doi: 10.1111/j.0006-341X.2003.00092.x
Promislow D. E. L. (1991). Senescence in natural populations of mammals: A comparative study. Evolution 45, 1869–1887. doi: 10.1111/j.1558-5646.1991.tb02693.x
Promislow D. E. L. (1992). Costs of sexual selection in natural populations of mammals. Proc. R. Soc. London. Ser. B: Biol. Sci. 247, 203–210. doi: 10.1098/rspb.1992.0030
Reichard M., Blažek R., Žák J., Cellerino A., Polačik M. (2022). The sources of sex differences in aging in annual fishes. J. Anim. Ecol. 91, 540–550. doi: 10.1111/1365-2656.13656
Ricklefs R. E. (2010). Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl. Acad. Sci. 107, 10314–10319. doi: 10.1073/pnas.1005862107
Ronget V., Gaillard J.-M. (2020). Assessing ageing patterns for comparative analyses of mortality curves: Going beyond the use of maximum longevity. Funct. Ecol. 34, 65–75. doi: 10.1111/1365-2435.13474
Rotella J. J., Link W. A., Chambert T., Stauffer G. E., Garrott R. A. (2012). Evaluating the demographic buffering hypothesis with vital rates estimated for Weddell seals from 30 years of mark–recapture data. J. Anim. Ecol. 81, 162–173. doi: 10.1111/j.1365-2656.2011.01902.x
Rotella J. J., Link W. A., Nichols J. D., Hadley G. L., Garrott R. A., Proffitt K. M. (2009). An evaluation of density-dependent and density-independent influences on population growth rates in weddell seals. Ecology 90, 975–984. doi: 10.1890/08-0971.1
Rotella J. J., Paterson J. T., Garrott R. A. (2016). Birth dates vary with fixed and dynamic maternal features, offspring sex, and extreme climatic events in a high-latitude marine mammal. Ecol. Evol. 6, 1930–1941. doi: 10.1002/ece3.1985
Siniff D. B., DeMaster D. P., Hofman R. J., Eberhardt L. L. (1977). An analysis of the dynamics of a weddell seal population. Ecol. Monogr. 47, 319–335. doi: 10.2307/1942520
Stirling I. (1969a). Ecology of the weddell seal in mcMurdo sound, Antarctica. Ecology 50, 573–586. doi: 10.2307/1936247
Stirling I. (1969b). Tooth wear as a mortality factor in the weddell seal, leptonychotes weddelli. J. Mammalogy 50, 559–565. doi: 10.2307/1378783
Stirling I. (1975). Factors affecting the evolution of social behaviour in the Pinnipedia. Rapp. P.V. Reun. Cons. Int. Explor. Mer 169, 205–212.
Thorley J., Duncan C., Sharp S. P., Gaynor D., Manser M. B., Clutton-Brock T. (2020). Sex-independent senescence in a cooperatively breeding mammal. J. Anim. Ecol. 89, 1080–1093. doi: 10.1111/1365-2656.13173
Tidière M., Badruna A., Fouchet D., Gaillard J.-M., Lemaître J.-F., Pontier D. (2020). Pathogens shape sex differences in mammalian aging. Trends Parasitol. 36, 668–676. doi: 10.1016/j.pt.2020.05.004
Tidière M., Gaillard J.-M., Müller D. W. H., Lackey L. B., Gimenez O., Clauss M., et al. (2015). Does sexual selection shape sex differences in longevity and senescence patterns across vertebrates? A Rev. New Insights captive ruminants. Evol. 69, 3123–3140. doi: 10.1111/evo.12801
Tompkins E. M., Anderson D. J. (2019). Sex-specific patterns of senescence in Nazca boobies linked to mating system. J. Anim. Ecol. 88, 986–1000. doi: 10.1111/1365-2656.12944
Vaupel J. W., Yashin A. I. (1985). Heterogeneity’s ruses: some surprising effects of selection on population dynamics. Am. Statistician 39, 176–185. doi: 10.1080/00031305.1985.10479424
Watanabe S. (2010). Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory. J. Mach. Learn. Res. 11, 3571–3594.
Watanabe S. (2013). A widely applicable bayesian information criterion. J. Mach. Learn. Res. 14, 867–897.
Keywords: actuarial senescence, sex-specific survival, life history, Weddell seal, mark-recapture
Citation: Macdonald KR, Rotella JJ and Link WA (2025) A comparison of sex-specific senescence patterns in a long-lived marine mammal. Front. Ecol. Evol. 12:1488373. doi: 10.3389/fevo.2024.1488373
Received: 29 August 2024; Accepted: 11 December 2024;
Published: 16 January 2025.
Edited by:
David Andrew Gray, California State University, Northridge, United StatesReviewed by:
Pierre Bize, Swiss Ornithological Institute, SwitzerlandCopyright © 2025 Macdonald, Rotella and Link. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaitlin R. Macdonald, a2FpdGxpbi5yLm1hY2RvbmFsZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.