- 1College of Agriculture, Hawassa University, Hawassa, Ethiopia
- 2College of Forestry and Natural Resources, Wondo Genet, Hawassa University, Hawassa, Ethiopia
- 3Department of Sustainable Agriculture and Biodiversity Conservation, School of Life Sciences and Bioengineering, The Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
- 4Ecology of Tropical Agricultural Systems, Hans Ruthenberg Institute, University of Hohenheim, Stuttgart, Germany
- 5Department of Physical Geography, Stockholm University, Stockholm, Sweden
Globally, bush encroachment poses a great threat to the conservation of biodiversity and rangeland productivity. However, control methods of encroaching woody species have rarely been experimentally quantified. We assessed the impact of tree thinning intensities on tree mortality, and the herbaceous community in Borana rangelands, an Ethiopian savannah ecosystem. At two 1.4 ha areas of mono-specific Vachellia drepanolobium stands, we set up 20 m x 10 m experimental plots with four tree-thinning treatments (0%, 33%, 67%, and 100% tree removal), with three replications in a randomized complete block design (RCBD) across two sites. The 0% plot was left uncleared and used as control. Over two growing periods, we monitored resulting tree mortality, coppicing, seedling mortality, and recruitment as well as herbaceous layer attributes (diversity, biomass) and the rangeland conditions. Tree thinning intensity significantly increased abundance of the dominant desirable grass species. Total herbaceous and grass species richness, diversity and biomass were significantly improved under high (100%) and moderate (67%) tree removal intensity. We conclude that tree thinning at moderate intensity (67%) was most effective in enhancing mortality of encroached trees, and improving grass diversity, and herbaceous biomass. We stress that effective tree thinning requires post-thinning management and repeated bush control measures. Our findings contribute to development of recommendations on controlling bush encroachment, species restoration, and rangeland productivity in Ethiopian rangelands.
1 Introduction
Woody plant encroachment (WPE) is one of the most extensive forms of rangeland degradation in dry and semi-arid regions (Kellner et al., 2022). Woody plant encroachment is an increase in the density, cover, and biomass of native or alien woody plant species at the expense of herbaceous species, particularly grasses (Briske et al., 2020; Wieczorkowski and Lehmann, 2022). Globally, bush encroachment poses a threat to rangelands (O’Connor et al., 2020; Wieczorkowski and Lehmann, 2022), particularly in the arid and semi-arid region of East African savannas (Hare et al., 2020; Utaile et al., 2023). Over the past three decades, woody plants have encroached across some 7.5 million km2 of African rangeland (Venter et al., 2018). The underlying causes of woody encroachment are numerous and complex, including intense herbivore pressure (O’Connor et al., 2014), suppression of fire (Scholtz et al., 2018), drought (Case et al., 2020), rising atmospheric carbon dioxide concentrations (Devine et al., 2017), and specific plant species adaptation (Archer et al., 2017).
The ecological consequences of WPE are highly variable and can be either positive, negative or neutral, depending on the type of encroaching species, tree density, and environmental conditions and grazing pressure (Maestre et al., 2016). While trees at low densities can have positive effects on under-storey herbaceous plants, soils and ecological functions (Treydte et al., 2007, Treydte et al., 2008) at high densities these effects often become negative (Riginos et al., 2009) and can reduce biodiversity and productivity of plants (Yusuf et al., 2015; Utaile et al., 2021). This consequently affects species diversity conservation, livestock production (Selemani, 2018), and the livelihoods of many communities (O’Connor et al., 2014).
The mechanism of WPE is driven by various factors, with fire suppression and overgrazing documented as the most significant (Luvuno et al., 2018). Overgrazing reduces grass biomass, fire fuel and grass competition, thereby favouring the establishment of tree seedlings (Higgins et al., 2000; O’Connor et al., 2014). Additionally, herbivory by browsers can limit woody plant growth, whereas a reduction in browsing pressure can lead to increased woody plant recruitment (Staver and Bond, 2014).
As woody cover advances into savannas, trees begin to strongly influence microhabitat under their canopies, altering resource redistribution and adding to habitat heterogeneity on smaller and larger scales (Treydte et al., 2007). The microhabitat changes primarily benefit grasses but later tend to support woody plant growth (Blaser et al., 2013). Under lower tree density, particularly in water-limited savannas, trees can play a facilitative role; for instance, trees can bring water from deep sources closer to the surface by hydraulic lift, providing grasses with better water access (Barron-Gafford et al., 2017). Additionally, leguminous trees in savannas are capable of fixing atmospheric nitrogen, making it available for plant uptake and potentially improving grass production, species composition, soil quality (e.g. soil carbon and nitrogen) and forage quality (Treydte et al., 2007, Treydte et al., 2008; Quero et al., 2013). Trees can also help to protect the topsoil from erosion by facilitating herbaceous ground vegetation cover (Marquart et al., 2019). Trees can facilitate their own growth through shading, hydraulic lift, and nutrient enrichment (Vadigi and Ward, 2013).
As tree cover increases, the ecosystem functions and structure such as herbaceous biomass can decline, while carbon sequestration, biodiversity and soil fertility often increase (e.g.,Ding and Eldridge, 2019). However, often an increased woody cover is considered as undesirable processes in rangelands by communities whose livelihoods depend on mainly herbaceous layers for their livestock (Hare et al., 2021a).
Rainfall and soil moisture influence the balance between trees and grasses. In mesic savannas, where there is high productivity due to high rainfall and nutrient availability (Biancari et al., 2024), both fire or herbivory act to limit woody plant recruitment (Luvuno et al., 2018; Staver et al., 2021). Under such mesic environment, fire alone can play a key role as a landscape level disturbance and limit woody plant recruitment (Lehmann et al., 2011). In arid and semi-arid savannas, limited moisture restricts both grass and tree establishment, making herbivory the main constraint on woody growth due to low-intensity fires (O’Connor et al., 2014).
Soil properties also play a role: seasonal waterlogging and shallow soils favour grasses, while sandy soils, which allow deep water infiltration, support woody establishment by reducing grass competition (Holdo and Nippert, 2023). In addition, low nutrient levels in sandy soils lower grass productivity and fire intensity (Sankaran et al., 2008). On a global scale, increased CO2 levels have further favoured woody species over grasses by enhancing photosynthetic efficiency in trees over grass (Bond and Midgley, 2012). This process can have significant ecological consequences on rangeland ecosystem structure and structure.
Mitigation strategies for restoring and improving rangeland productivity through the control of encroaching woody plants have been promoted (Eldridge and Ding, 2021; Hare et al., 2020). Globally, several woody plant control methods such as prescribed fire, browsing animals, mechanical tree removal, herbicides, or various combinations of these, have been used (Archer and Predick, 2014). Tree thinning, which involves a reduction in the density of a tree to a predetermined level, as opposed to clearing woody plants, has been promoted to control bush encroachment in savanna rangelands (Smit, 2014; Ward, 2005). Thinning act as disturbance that reduce resources competition (e.g., water, nutrients and light) (Mndela et al., 2022a). This release of competition promotes increased herbaceous biomass and improves rangeland condition. However, the effectiveness of tree thinning often varies across ecosystems, with thinning more effective in high rainfall mesic areas than arid-environment (Ding and Eldridge, 2019). tree thinning intensities, the identity of the encroaching plant (Daryanto et al., 2019; Ding and Eldridge, 2019), and environmental conditions (e.g., soil properties, rainfall, and grazing pressure) (Eldridge and Ding, 2021) can strongly influence the outcome of an encroachment management option. The effectiveness of woody plant removal is further relatively short-lived (< 5 years), depending on which variables are measured (Daryanto et al., 2019; Ding and Eldridge, 2023). As the total removal of all woody plants in arid regions could have a negative long-term impact on the rangelands’ productivity and biodiversity (Ding et al., 2020) maintaining a certain density of the woody layer might be desirable to promote forage production and various ecosystems functions. Yet, only a few studies have investigated how the vegetation of rangelands responds to varying levels of tree thinning intensities in East African savannahs (Ding and Eldridge, 2019; Hare et al., 2021b). Further, little is known about how tree removal affects herbaceous communities (e.g. diversity, biomass, and rangeland condition.
The Borana savanna rangelands of southern Ethiopia support a large population of diversified livestock species (Liao et al., 2018). Livestock, as main source of livelihoods for many communities (Liao et al., 2018), mainly depend on rangeland resources, which are becoming scarcer due to recent woody plant species encroachment (Yusuf et al., 2011). Several native encroaching woody plant species have been identified (Hare et al., 2020) and one of those encroaching Acacia species, V. drepanolobium is the most prominent invading species in Ethiopia and other East African countries (Riginos et al., 2009; Kimaro et al., 2019; Hare et al., 2021b). An earlier study by Liao et al. (2018) on the expansion of woody encroachment within the Borana rangeland estimates that about 80% of the landscape was encroached upon in 2013. A shrub cover of 40% and/or 2400 woody plants/hectare has been considered as a borderline between non-encroached and encroached condition (Roques et al., 2001) with 2500 tree equivalents/hectare indicating highly encroached condition (Richter et al., 2001). The increase in woody plants is most likely due to a combination of factors, including changes in grazing pressure, fire suppression, drought, and climatic factors (Angassa, 2012) as well as an adaptation to distinct soils (Dalle et al., 2005). In southern Ethiopia, pastoralists, agro-pastoralists, and numerous institutions have used total tree clearing and grazing exclusion to control invading plants and restore the productivity of the rangelands. Yet, successful results have not been achieved, particularly in the long term (e.g., Angassa and Oba, 2009).
Our objectives were to: (i) evaluate the impact of different intensities of tree thinning on the overall response of V. drepanolobium woody tree mortality in the Borana rangelands, southern Ethiopia, and to (ii) investigate how different intensities of thinning treatments affect the herbaceous vegetation (plant species diversity, biomass). We asked the following questions: (i) Does the intensity of bush thinning of V. drepanolobium influence the regeneration potential of this native woody plant encroacher species? Many woody plants can regenerate using coppicing and seedling establishment following thinning (Mokgosi, 2018). These regeneration strategies make it difficult to control bush encroachment in the long run. Thus, understanding how woody plants regenerate through seedling recruitment or coppicing can be useful (Mokgosi, 2018). (ii) How are grass production, and diversity affected by different thinning intensities? We hypothesized that higher tree thinning intensities can control bush encroachment and improve the diversity, biomass, and rangeland conditions of herbaceous species in savannah rangelands.
We set up experimental field sites of different thinning treatments in the Borana rangelands, Ethiopia, and monitored responses of both woody and grassy vegetation over one year. Our results will support the decision-making process in designing effective bush control strategies (Nghikembua et al., 2023; Ward et al., 2022).
2 Materials and methods
2.1 Study area
The study was conducted in the Borana rangelands of southern Ethiopia (Figure 1), a rangeland covering a total area of 95,000 km2, bordering Somalia and Kenya (Megersa et al., 2014; Tuffa and Treydte, 2017). The Borana rangelands are situated within the altitudinal range of 750–2000 m above sea level (Dalle et al., 2006b) and experience an arid and semi-arid climate with pockets of sub-humid zones (Megersa et al., 2014). The inter-annual rainfall varies, with an average annual rainfall of 527 mm (Dalle et al., 2006a) and a high coefficient of variability (18% to 69%) (Angassa and Oba, 2008). The rainfall in Borana is bimodal with a long rainy season between March and May, and a short rainy season between September and November. The mean annual rainfall of the Yabello district within the Borana rangelands, where the study was conducted, was 645 mm (Tuffa and Treydte, 2017).The average annual temperature of the area varies from 19 to 26°C. Drought is common in the area, with isolated dry years (<400 mm) happening once every 5 years but becoming more frequent (Tuffa and Treydte, 2017). The region is characterised by limited availability of surface water; the major sources of water for humans and livestock are deep wells and ponds (Homann, 2004). The soils of the study area are derived from ancient alluvial and volcanic materials (FAO, 1986) with shallow red sandy loam in the uplands and vertisols in the bottomlands (Dalle et al., 2006b).
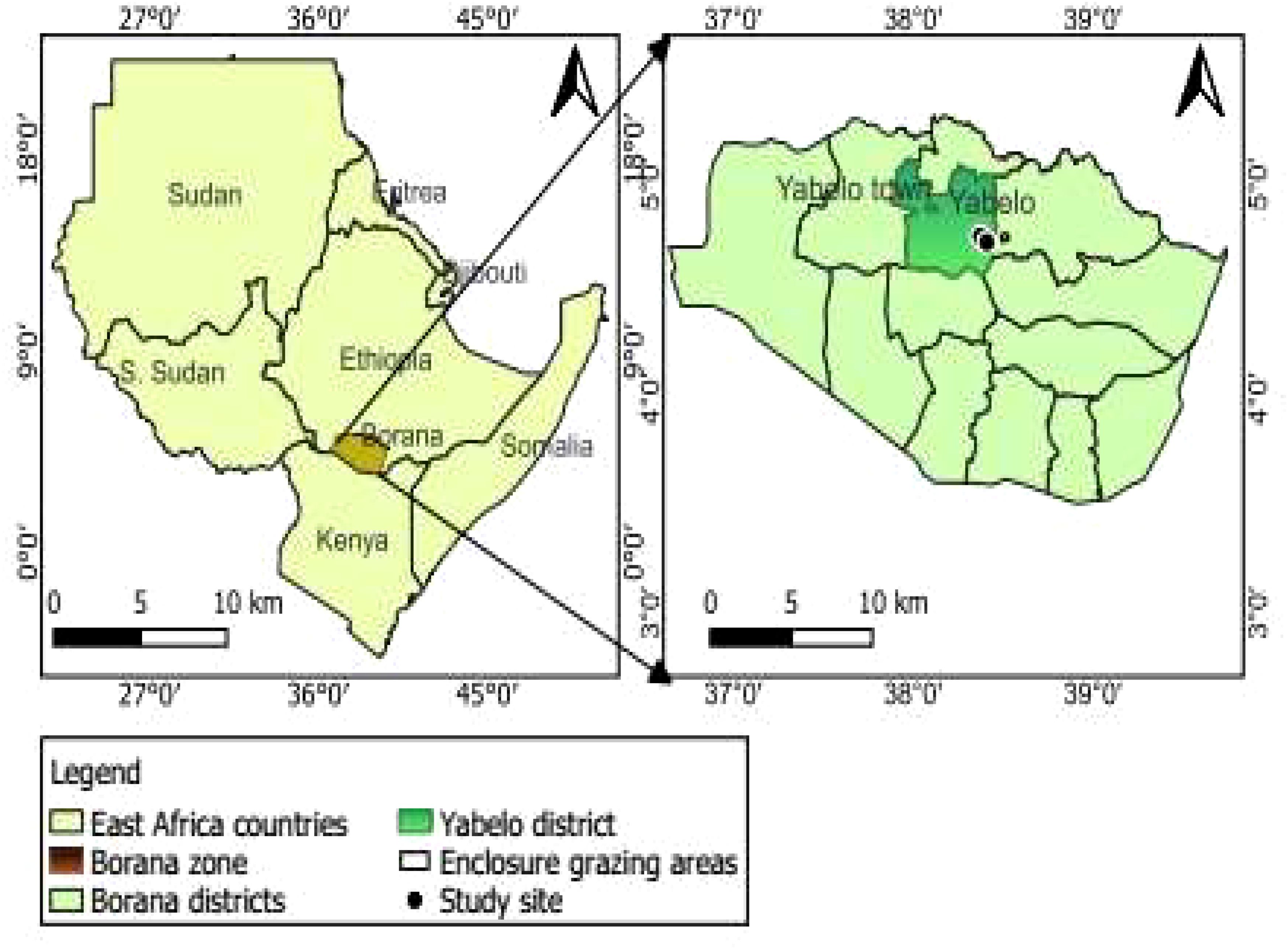
Figure 1. Map of the study area, the Borana rangelands of southern Ethiopia. In this area, we set up experimental plots of different tree thinning treatments to understand responses of both woody and grassy vegetation in the year 2020.
The livelihood of the Borana pastoralists mainly depends on livestock production in this tropical savanna with varying proportions of open grassland and perennial herbaceous and woody vegetation, composed of Vachellia-Commiphora small-leaved deciduous woodlands (Coppock, 1994). Encroachment of woody plants is a major challenge, causing a decline in herbaceous species, rangeland productivity, range condition, and livestock production (Tefera et al., 2007; Yusuf et al., 2011) and fire is not an effective bush layer management tool any more (Angassa and Oba, 2008). The suppression of fire caused the proliferation of woody plants including Vachellia brevispica, V. bussei, V. drepanolobium, V. etbaica, V. mellifera, V. nilotica, V. seyal, and Commiphora africana (Yusuf et al., 2015; Feyisa et al., 2017). Vachella drepanolobium is a perennial, leguminous, thorny plant that reaches up to 7 m height, mostly found on black cotton soils containing heavy clay soils, where it grows in mono-dominant stands and makes up 98% of the individual tree species (Hare et al., 2020). Vachellia drepanolobium is aggressively encroaching into rangelands, suppressing grass production and affecting the productivity of the rangelands (Beyene and Mlambo, 2010). This species has a low forage value because it is heavily protected against herbivores by spines and ants (Okello, 2007) but provides the main sources of feed for black rhinos (Diceros bicornis) and giraffes (Giraffa camelopardalis) in the Laikipia plateau of Kenya (Birkett, 2002).
2.2 Site selection and experimental layout
The study was conducted in the year 2020 over two growing seasons in the Borana rangelands of the Yabello district, Dida Hara Kebele Administration (KA), which has a total area of 985 km2 and is located between 1,200 and 1,600 m.a.s.l (Angassa and Oba, 2010). The first growing period was between March and May (long rainy season), while the second growing period was between September and November (short rainy season).The experimental sites were selected within the community enclosure named Kelo Olla Doyo Dube, which extended over 4.3 km2 and was established more than 25 years ago. This enclosure has two distinct soil types, black cotton vertisol, known for its water retention, and red soil with a diverse grass, shrubs, bush and tree (Oba, 2012). The tree density within the enclosure varied, creating a mosaic of habitats such as savanna, shrub and bushland, and woodland. We selected two representative grazing areas through participatory mapping together with local elders, who knew the history and nature of the rangeland. The enclosure was fenced, and was only grazed by few animals during the dry season (Angassa and Oba, 2010). We selected two experimental sites that had similar soil types (vertisol), topography, rainfall, vegetation type, grazing potential and no history of earlier disturbances such as bush clearing and burning of the grazing areas. The vegetation of both sites was primarily savanna, dominated by Vachellia drepanolobium trees and various herbaceous species.
The human population settlements in the Dida-Hara area are semi-permanent settlements with an average livestock holding per household of 13 cattle, 11 small ruminants (goats and sheep), and 2 camels (Tefera et al., 2007). The majority of the open communal rangelands in the areas have deteriorated as a result of the high stocking rate, which was estimated to be 0.235 Tropical Livestock Units ha-1 (1 TLU = 250 kg) (Homann et al., 2008). Despite the unregulated stocking rate on the enclosure grazing land, overgrazing was not a threat within the enclosure (Angassa and Oba, 2010) and, thus we assumed that grazing effects after thinning could be excluded as a factor in our analyses.
At each of the two sites, we selected an 1.42 ha experimental area that had mono-specific stands of V. drepanolobium. We used a randomized complete block design (RCBD) with four levels of thinning treatment replicated three times in two areas. In each site, we established three10 m x 20 m (200 m2) plot per treatment, with 3 m-wide firebreaks between the plots and a 5 m-wide distance between replication. Each plot received one of four randomly assigned tree thinning treatments, representing different intensities: 1) non-thinned or control (0% tree removal), 2) low thinning (33% tree removal), 3) moderate thinning (67% tree removal), and 4) high thinning (100% total tree removal) (Wieczorkowski and Lehmann, 2022). The thinning levels were determined from the measured tree density of the control plot, and trees were removed to the equivalents of 33%, 67%, and 100% (Smit, 2005; Hare et al., 2021b; Monegi et al., 2022; Supplementary Table S1). Before the tree-thinning operation, the density of trees, number of seedlings, and height of individual trees within each plot were counted and measured. During thinning, we marked and tagged each cut tree with aluminium tags at the base of the trunk, below the cutting point. Using a local axe, trees were cut at the base (0.5 m above ground), and the stumps were debarked, at the end of the dry season in February 2020. While cutting, we did not favour any particular tree sizes and made sure the remaining trees were scattered to reduce competition (Hare et al., 2020). The experimental plots were fenced off and protected to prevent disturbance from human and livestock grazing and browsing. During the post-thinning periods, the number of V. drepanolobium trees, saplings, seedlings, and plant heights were counted and measured at the end of each growing period (i.e. both at end of main (May) and short rainy season (November). We monitored V. drepanolobium tree and shrub mortality (TreeMort), coppicing (TreeCopp) (referring to regrowth in response to treatment effects), seedling mortality (SeedMort) and new seedling recruitment (SeedRec), whereas seedling refers to the pre-reproductive stage of plants less than 0.5 m in height (Angassa and Oba, 2010). Cumulative regeneration (TreeCum) refers to the sum of coppicing and new seedling recruitment.
2.3 Grass species composition
The grass species composition was assessed in three 1 x 1 m quadrats randomly located within each treatment plot. Individual number of distinct tufts of grass species were counted and identified during both the main (May) and short rainy season (November), i.e., growing period 1 and 2, respectively. The identified grass species were classified according to life forms (annual and perennial) and ecological status into (i) decreasers (desirable species likely to decrease with heavy grazing pressure), (ii) increasers (intermediately desired species likely to increase under heavy grazing intensity), and (iii) invaders (undesirable species likely to increase under heavy grazing intensity) based on the histories of particular grass species (Jenkins et al., 1974; Tainton, 1981) and the opinions of livestock herders in local communities.
2.4 Herbaceous dry matter yield
Dry matter (DM) biomass of the herbaceous vegetation (grasses and forbs) was determined in each treatment plot by harvesting biomass from three 1 m2 quadrats at ground level. The harvested fresh material was weighed and hand-separated into grasses and forbs. The grasses were further separated into different species, and the proportional contribution of each species was determined. The samples of collected grasses and forbs were oven-dried at Hawassa University, College of Agriculture, in the Animal Nutrition Laboratory at 65°C for 24 hours and weighed. Identification and nomenclature of plant species was followed by Hedberg and Edwards (1995) and Guide to the Grasses of Ethiopia (Froman and Persson, 1974) and Grasses Common to Arero Area (Jenkins et al., 1974).
2.5 Data analysis
Response of each individual tree that was cut in our treatments was evaluated in terms of plant mortality, i.e., Percentage of tree mortality (number of individual trees killed after being cut divided by the number of all trees cut in the treatment plot multiplied by 100). Percentage of trees coppicing (number of individual trees that coppiced after being cut divided by the number of all trees cut in the plot) (Angassa and Oba, 2009; Hare et al., 2020). A total of 24 sample units (4 treatments x 6 replicates, i.e.,(six plots per thinning treatment that aggregated from two sites) were considered for the data analysis on the response of woody trees to tree thinning. For the herbaceous layer response variables we included sites, growing period and treatment a total of 48 sample units (4 thinning intensities x 3 replications x 2 sites x 2 growing periods). For data analysis, biomass data were averaged from three 1m² plots within each large quadrat. Following tree thinning, we would expect that there would be a higher herbaceous yield and species diversity in the first growing period as this received more rainfall than in the second period. Data were checked for normality, homogeneity of variance, and assumptions of linearity. For data that did not fulfil the normality assumption, we used square root transformation for tree mortality, and log transformation for DM biomass. The woody plant parameters, herbaceous species composition (abundance of grass and forbs), diversity, and DM biomass parameters were analysed using the general linear mixed model procedure of the Statistical Analysis System (SAS, 9.4)
To assess the impact of thinning intensity, as the dependent variables were woody species attributes (e.g., TreeMort, TreeCopp, TreeCum, SeedMort, and SeedRec).We also assessed, herbaceous layer characteristics, i.e., grass and forb diversity, and DM biomass, categorized by desirability (highly desirable, intermediate, and less desirable grasses according to the opinions of livestock herders in local communities. Thinning intensities were treated as the independent variables.
We used a General Linear Mixed Model (GLMM) with binomial distributions and logit link function for TreeMort, TreeCopp, SeedMort, with thinning intensity considered as fixed factor while replication and site (blocking) as a random factor. In our study, we focused on the overall impact of thinning treatments rather than comparing specific sites or growing periods, as both sites were selected from the same enclosure. The model was Yijkl=μ+αi+γj+δk+Yijkl. Where, Yijkl is response variable for the lth observation in the kth replication within the jth site, under the ith thinning intensity. In the model, μ represents overall mean.αi indicates the mixed effects of the ith thinning intensity. γj represents random effect of the jth site. δk denotes random effect of the kth replication within the site.eijkl indices residual error term. TreeCum and SeedRec were analysed using GLMM with normal distribution. Summary of independent and dependent variables with description and units is presented in Table 1.
Table 1. Summary of independent and dependent variables of woody plant species and herbaceous layer with description and units.
Similarly, we used GLMM for herbaceous vegetation characteristics (biomass, diversity), with thinning intensity and growth period, and their interaction considered as fixed factors while site and replication as a random factor. The model was different from woody vegetation and described as Yijklm=μ+αi+βj+αβij+γk+δl+eijklm. Where, Yijklm represents the response variable for the mth observation in the kth site and lth replication, under the ith thinning intensity and jth growth period. μ denoted the overall mean, αi and βj represents the fixed effect of the ith thinning intensity and the jth growth period. αβij is a fixed effect indicates the interaction between the thinning intensity and growth period. γk and δl indicates the random effect of the kth site and the lth replication (blocking effect), respectively. Finally, eijklm is the residual error term.
Herbaceous species that were recorded in each of the treatment plots at the end of the second growing period were ordinated using Correspondence Analysis (CA) (Greenacre, 2007) using PAST 4.13 software (Hammer et al., 2001). Total herbaceous species, grass, and forb species alpha diversity, richness, and evenness were determined using the Shannon-Wiener index (Kent, 1992) using PAST 3 software and analysed using GLMM. We assessed the effects of thinning intensity on herbaceous vegetation species abundance using repeated measures permutational analysis of variance (PERMANOVA) with a Bray-Curtis dissimilarity measure using log-transformed species abundance data. To complement the PERMANOVA results, the species abundance across the different thinning intensity using GLMM. Tukey-HSD post hoc test was used to test for differences in woody responses, herbaceous (grass and forb) and biomass parameters and the mean differences were considered at P < 0.05.
3 Results
3.1 Tree thinning intensity on woody species parameters
Tree thinning intensities significantly (F = 35.008; P < 0.001) affected tree mortalities (F = 50.3; P < 0.001), with almost 60% of trees that were cut dying in the highly thinned treatment and about 50% dying in the moderately thinned treatment while only 2% died a natural death in the control plots (Figure 2, Table 2). Tree coppicing was by about one fourth higher in the low thinned treatment compared to highly thinned treatments (F = 35.249; P < 0.001; Figure 2). Cumulative regeneration (sum of trees coppicing and seedling recruitments) was about twice as high (45%) in the highly thinned plots than in the low thinned plots (22%) and (0%) in the control treatment (F = 43.441; P < 0.001). Seedling recruitment showed weaker trends, with recruitment being about twice as high in highly thinned areas compared to low thinned areas (F = 5.958; P = 0.004; Figure 3). Seedling mortality showed a significant difference among treatments (F = 222.420; P = 0.001; Figure 3) and we recorded an average seedling mortality of 23% ha-1 across all treatments. Highly thinned plot significantly reduced seedling mortality, resulting in a mortality rate more than four times lower than the control.
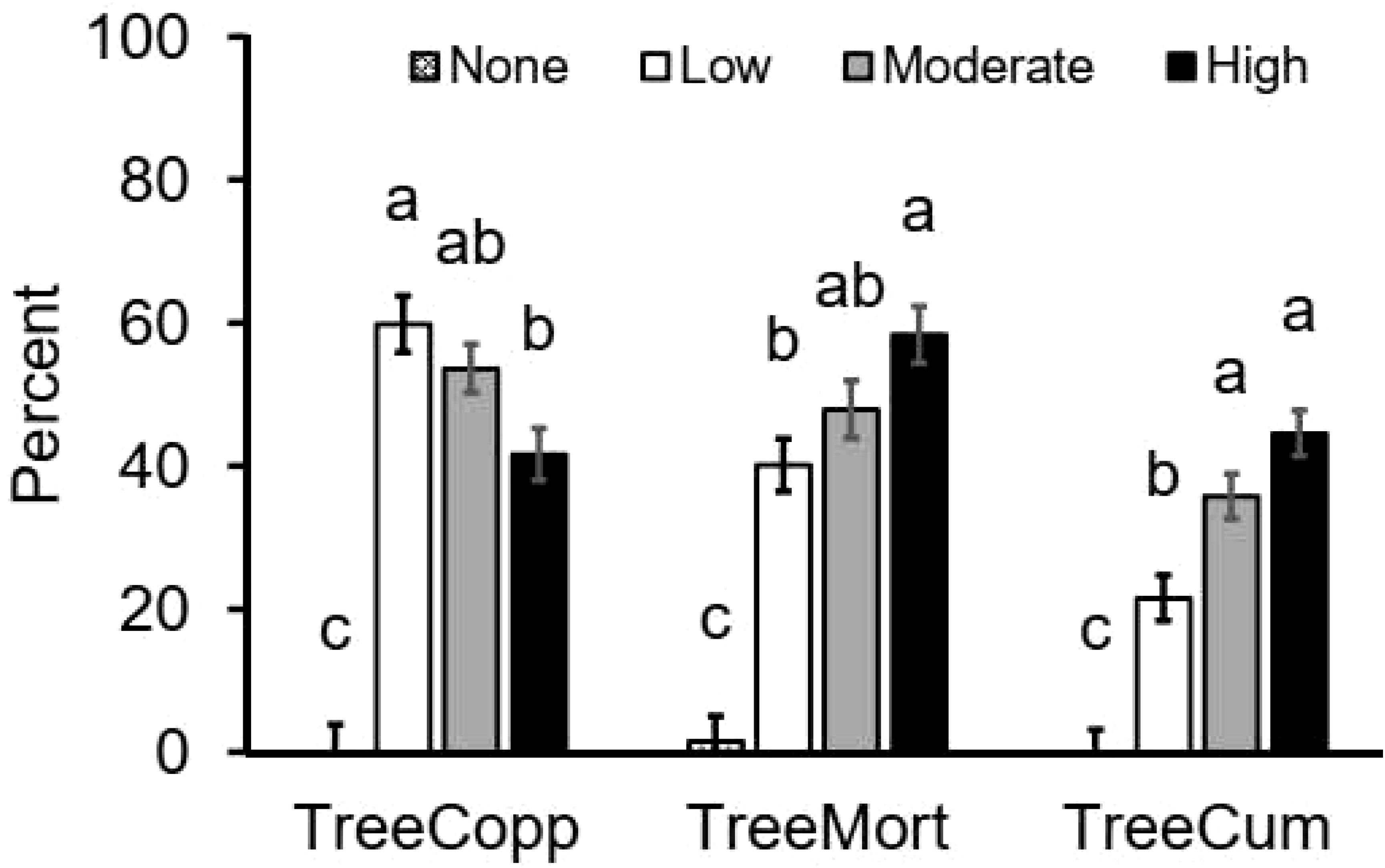
Figure 2. Average (± SE) percent (% of all cut trees ha-1) of woody vegetation response: tree and shrub mortality (TreeMort), tree coppicing (TreeCopp), cumulative regeneration (TreeCum) to tree thinning intensities (None: 0% tree removal or control, Low: 33% tree removal, Moderate: 67% tree removal and High: 100% tree removal). Different letters represent significant differences among thinning treatments at P < 0.05 based on Tukey-HSD post- hoc test. n = 24.
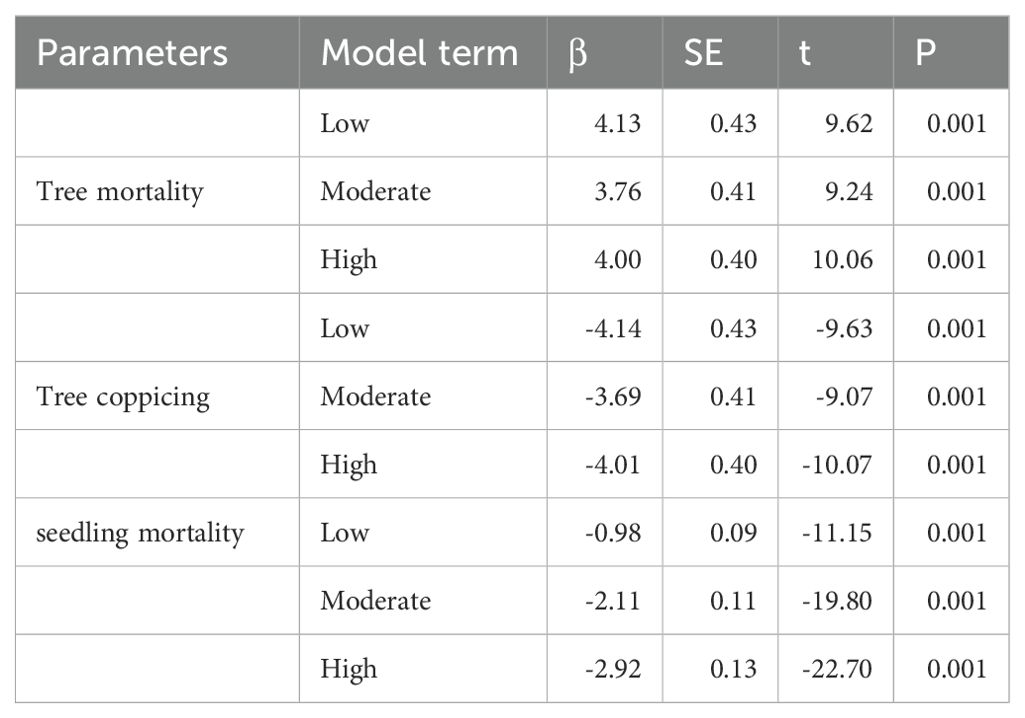
Table 2. Parameter estimates (β) and test statistics (t) for predictors in the generalized linear mixed models assessing the effects of thinning intensity on tree mortality, coppicing, and seedling mortality (N = 24).
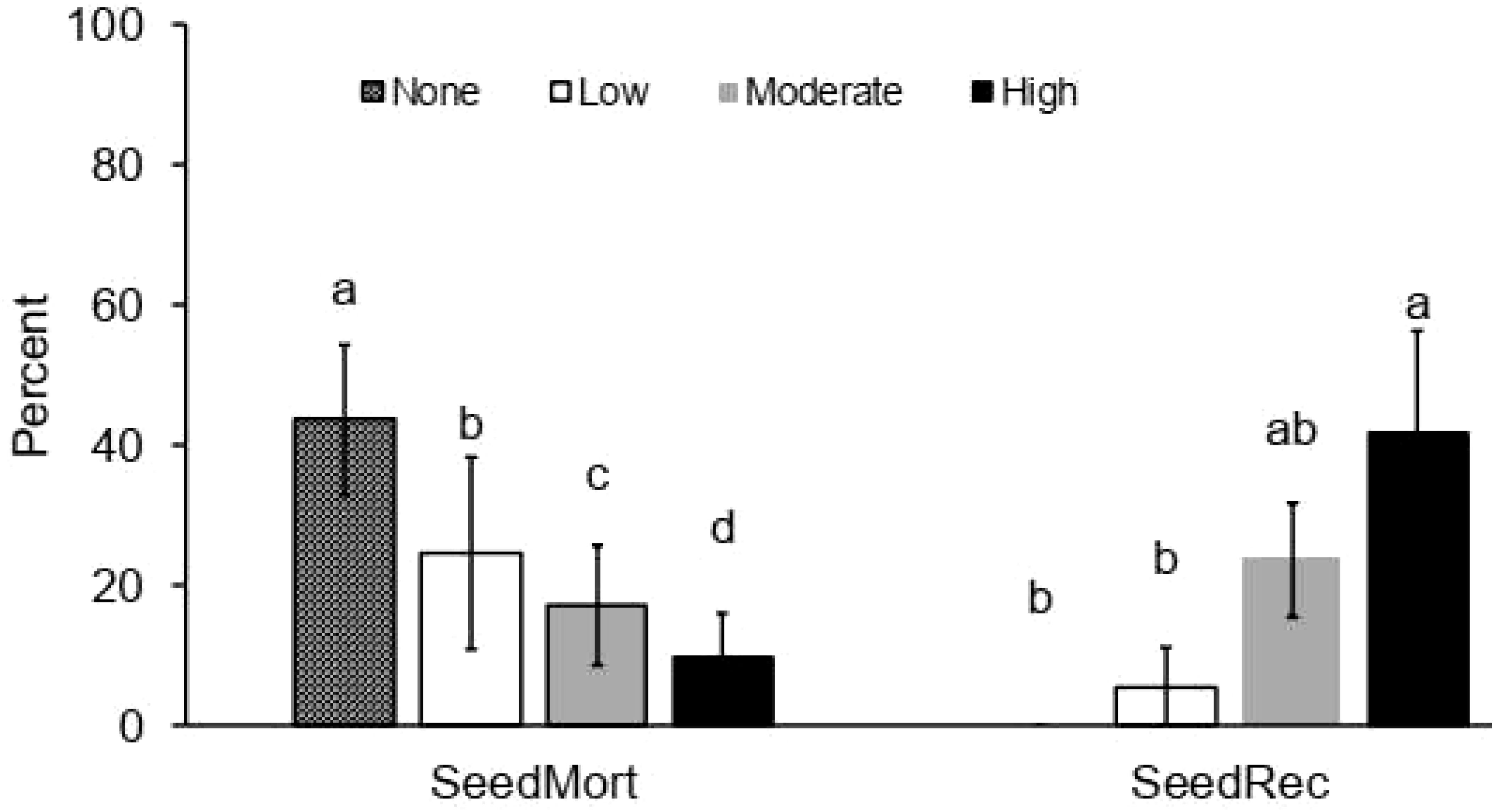
Figure 3. Average percent (± SE, of the initial seedlings ha-1) response of seedlings parameters [(seedling mortality (SeedMort), and new seedling recruitment (SeedRec)], to tree thinning intensities (None: 0% tree removal or control, Low: 33% tree removal, Moderate: 67% tree removal and High: 100% tree removal). Different letters represent significant differences among thinning treatments at P < 0.05 based on Tukey-HSD post- hoc test. n = 24.
3.2 Tree-thinning intensity on herbaceous species composition and diversity
Across all treatments, we identified a total of 36 herbaceous species, 21 grasses and 15 forb species (Table 3; Supplementary Table S2). The grass species comprised 75% perennials and 25% annuals, and they were composed of 30% highly desirable, 35% intermediately desirable, and 35% less desirable grass species. The dominant grass species in our study were Cenchrus ciliaris, Chrysopogon aucheri, Pennisetum mezianum, Pennisetum stramineum, Digitaria milanjiana, Cynodon dactylon, Eragrostis papposa, and Sporobolus pyramidalis, contributing in total to 81% of the total abundance of all grasses. The PERMANOVA analysis demonstrated significant differences in herbaceous species abundances among different thinning intensities, with the thinning treatment explaining 64% of the total variation in species abundances (F = 8.23, P = 0.0001, R² = 0.6414). This indicates that a strong effect of thinning intensity on herbaceous vegetation abundances. Post hoc pairwise PERMANOVA tests further showed that high thinning intensity significantly differed from both the control (F = 17.69, P = 0.0006) and low thinning intensity (F = 6.639, P = 0.0006). Similarly, moderate thinning intensity differed significantly from the control (F = 11.1, P = 0.0006) and low thinning intensity (F = 3.274, P = 0.0174). However, the comparison between moderate and high thinning intensities was not statistically significant (F = 2.69, P = 0.068). The correspondence analysis (CA) results did not separate the herbaceous species by the thinning intensities as only 79.90% of the total variance in species abundance was explained by the model (Supplementary Figure S1). Bush thinning intensity significantly altered herbaceous species abundance of some species such as C. ciliaris, C. aucheri, E. papposa, P. mezianum, and P. stramineum, which were the most frequently occurring herbaceous species in the highly thinned plot, whereas D. milanjiana (F = 11.3; P < 0.001) was the most frequent species in the control (Supplementary Table S2). We found that abundance of C. ciliaris and Chrysopogon aucheri was more than five times as high in highly thinned areas than in none (Table 2). Moreover, P. mezianum and P. stramineum were more than seven times as abundant in highly thinned areas compared to control (Table 1). However, compared to the highly thinned plot, D.milanjiana was more than twice as high in the none-thinned treatment (Table 1; Supplementary Table S2).
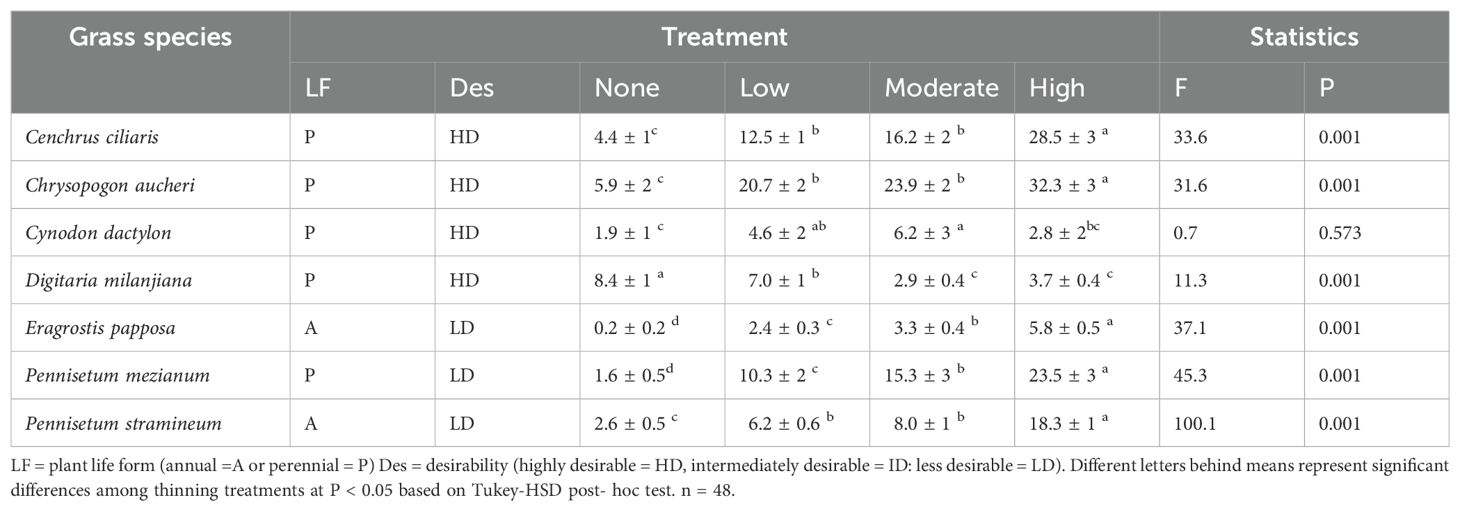
Table 3. The average (± SE) abundance of the most frequent grass species recorded per plot (number of individuals per species per m2) in response to the different thinning intensities (None: 0% tree removal or control, Low: 33% tree removal, Moderate: 67% tree removal and High: 100% tree removal).
We further found that bush thinning significantly influenced overall herbaceous (F = 23.734; P < 0.001), grass (F = 24.768; P < 0.001), and forb (F = 9.036; P < 0.001) species richness, with grass species richness being almost twice as high in highly thinned treatments compared to control (Figure 4). The diversity of grass and forb species (F = 13.453; p < 0.001, F = 4.733;P < 0.006, respectively) was also higher in the thinned plots (low, moderate and high tree removal) compared to the control but with no significant differences among the thinned treatments. Grass diversity was 20% higher in the high tree removal treatment (2.10 ± 0.06), 13% higher in the moderate (1.95 ± 0.06), and 14% higher in the low (1.98 ± 0.06), compared to the control (1.68 ± 0.06). Similarly, forb diversity increased by 21% in the high treatment (1.65 ± 0.10) and 28% in the moderate (1.76 ± 0.10), relative to the control (1.30 ± 0.10).
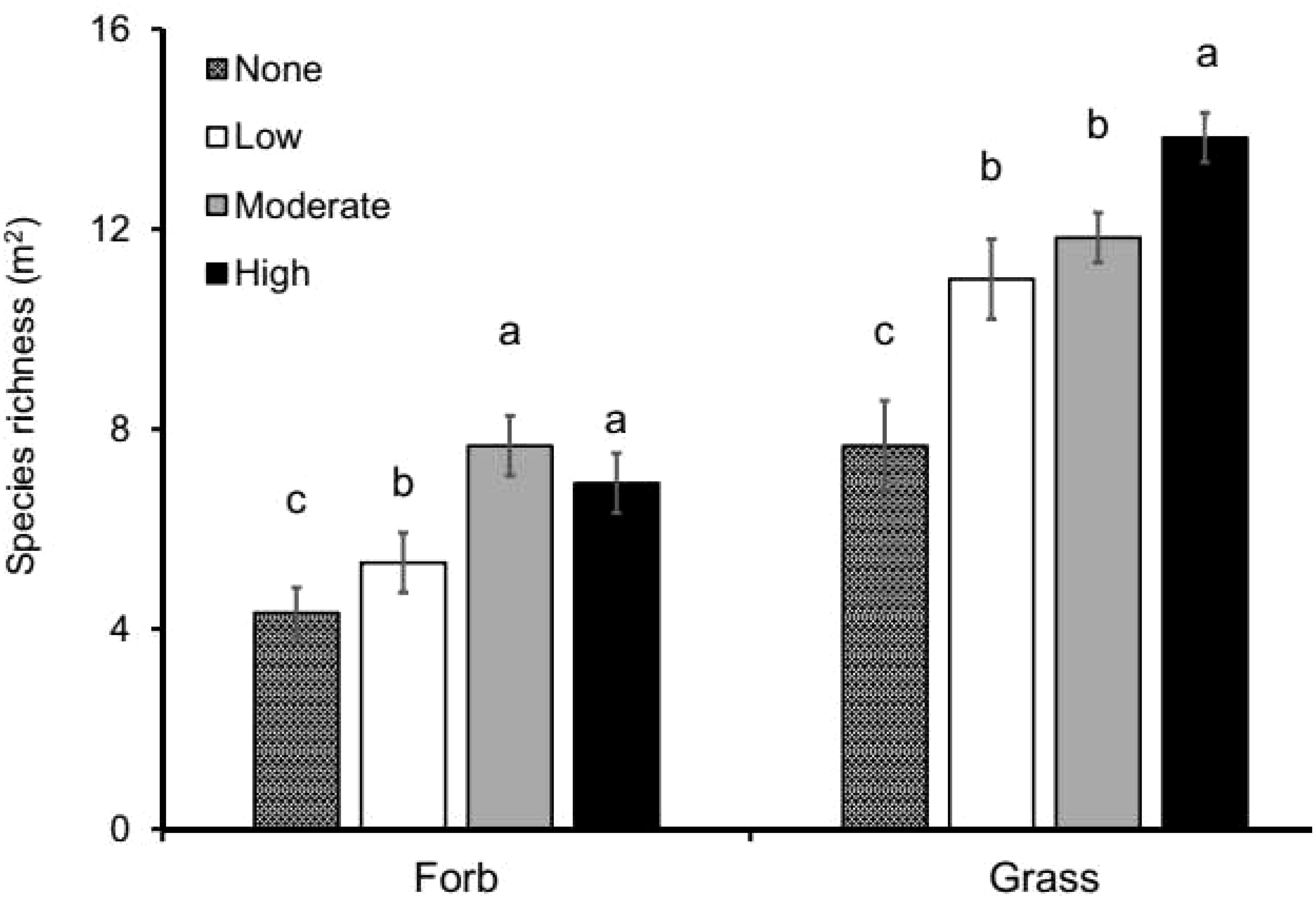
Figure 4. Average (± SE) grass and forb species richness per plot (m2) as response to tree thinning intensities (None: 0% tree removal or control, Low: 33% tree removal, Moderate: 67% tree removal and High: 100% tree removal). Different letters represent significant differences among thinning treatments at P < 0.05 based on Tukey-HSD post- hoc test. n = 48.
3.3 Tree-thinning intensity on herbaceous biomass
Thinning intensity significantly influenced grass biomass. Grass DM biomass was more than twice as high in the highly thinned treatments compared to all other treatments (F =267.417; P < 0.0001; Figure 5) while forb DM biomass (F = 31.062; P < 0.0001; Figure 5) was about twice as high in both moderately and highly thinned treatments compared to the other treatments. The DM biomass of highly desirable (F = 327.044; P < 0.0001), intermediate (F = 43.965;P < 0.0001) and less desirable (F = 106.108; P < 0.0001) grasses was all more than twice as large in the highly thinned treatment compared to the other treatments (Figure 5).
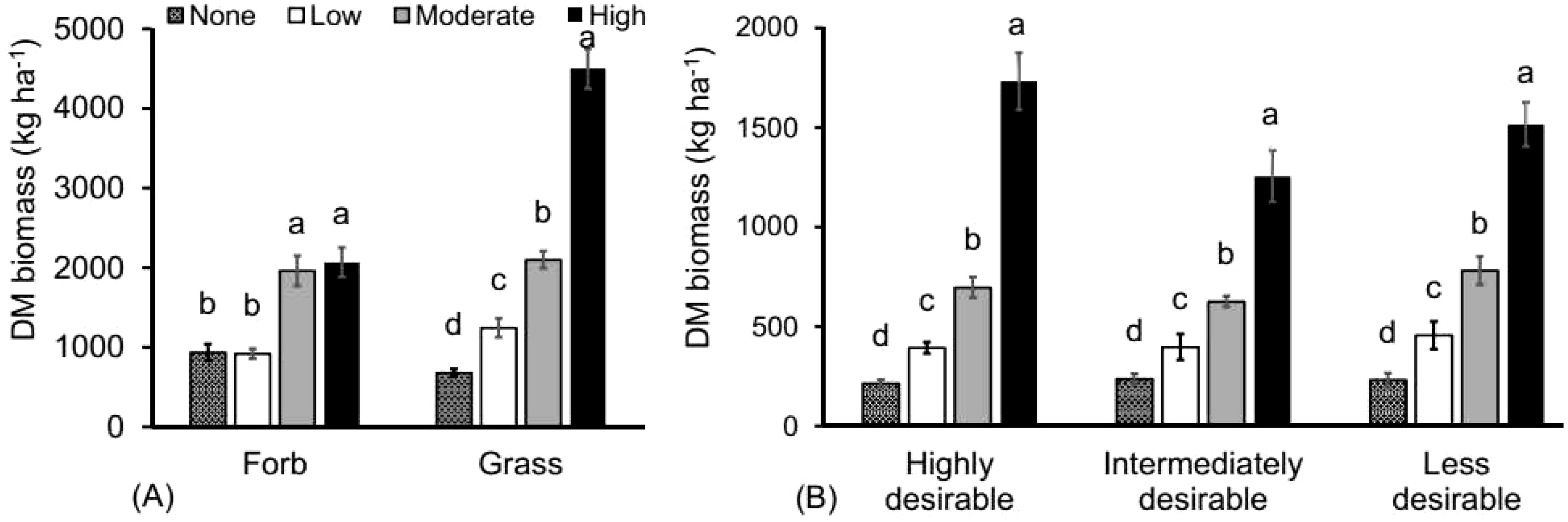
Figure 5. The effect of tree thinning intensities on (A) average (± SE) forb and grass DM biomass yield (kg ha-1) and (B) DM biomass based on desirability according to pastoralists. Different letters represent significant differences among thinning treatments (None: 0% tree removal or control, Low: 33% tree removal, Moderate: 67% tree removal and High: 100% tree removal) at P < 0.05 based on Tukey-HSD post- hoc test. n = 48.
3.4 Growing period and thinning intensity on herbaceous species
Growing period significantly affected overall grass biomass (F = 21.837; p = 0.001), biomass of highly desirable (F = 69.301; P = 0.0001), intermediately desirable (F = 3.589; P = 0.011) and less desirable grass species (F = 30.242; P = 0.0001) (Figure 6). The growing period a substantial impact on grass species diversity and richness (F = 8.459; P = 0.006 and F = 15.759; P = 0.001, respectively). It also greatly affected forb richness and diversity (F = 15.213; P = 0.0001 and F = 3.575; P = 0.066, respectively) (Figure 7), with all those trends being more pronounced in the second growing season than in the first (Figure 7). There was an interactive effect between thinning intensity and season (Wilks’ λ = 0.001; F = 4.068; P = 0.001) with highly desirable grass biomass (F = 21.166; P < 0.001) and grass diversity (F = 3.642; P = 0.021) being higher in the second growing season but the other variables did not show any significant interactive effect (P > 0.05) (data not presented).
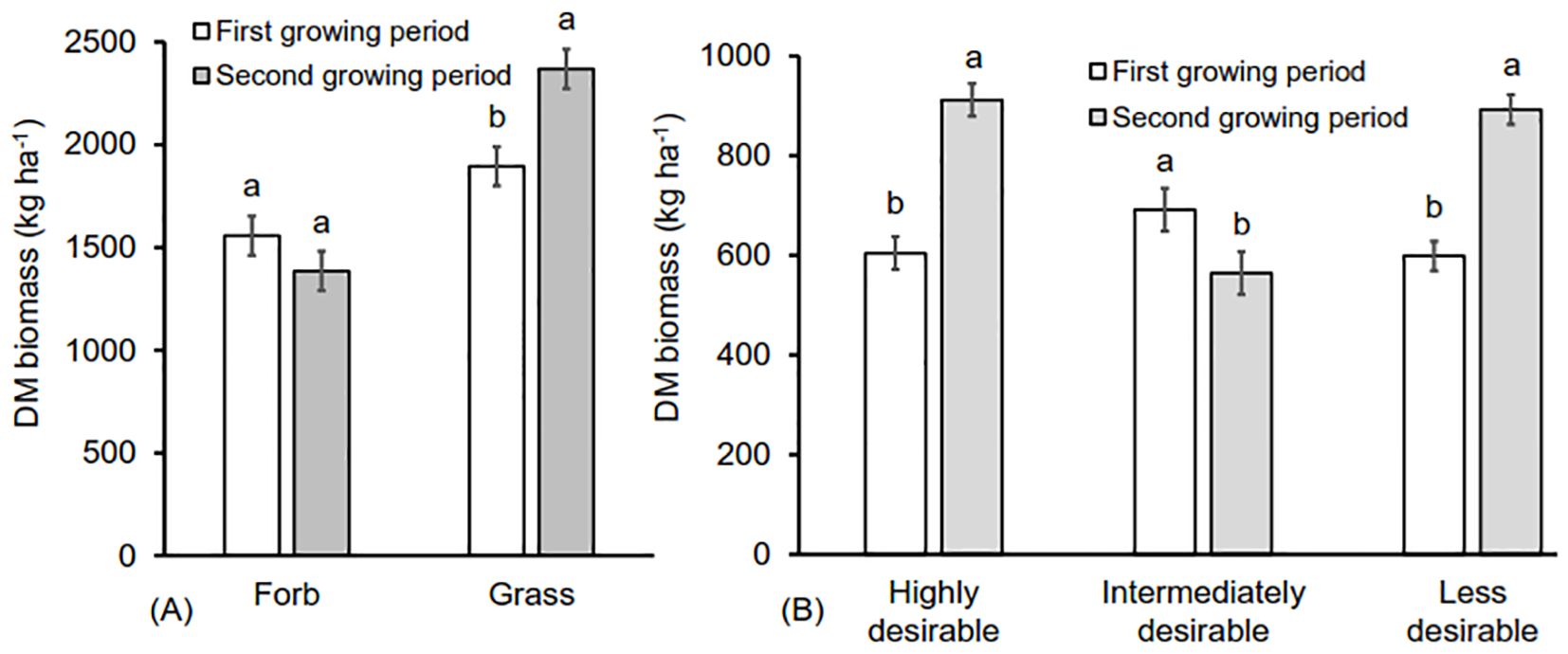
Figure 6. The effect of growing period (First growing period = March and May (white bars), Second growing period = September and November (gray bars)) on (A) an average (± SE) forbs, and grass biomass (kg ha-1) and (B) on grass biomass in terms of desirability. Desirable (highly desirable= High, Intermediate desirable = Intermediate: Less desirable =Less). Different letters represent significant differences in means between growing periods at P < 0.05 based on Tukey-HSD post- hoc test. n = 48.
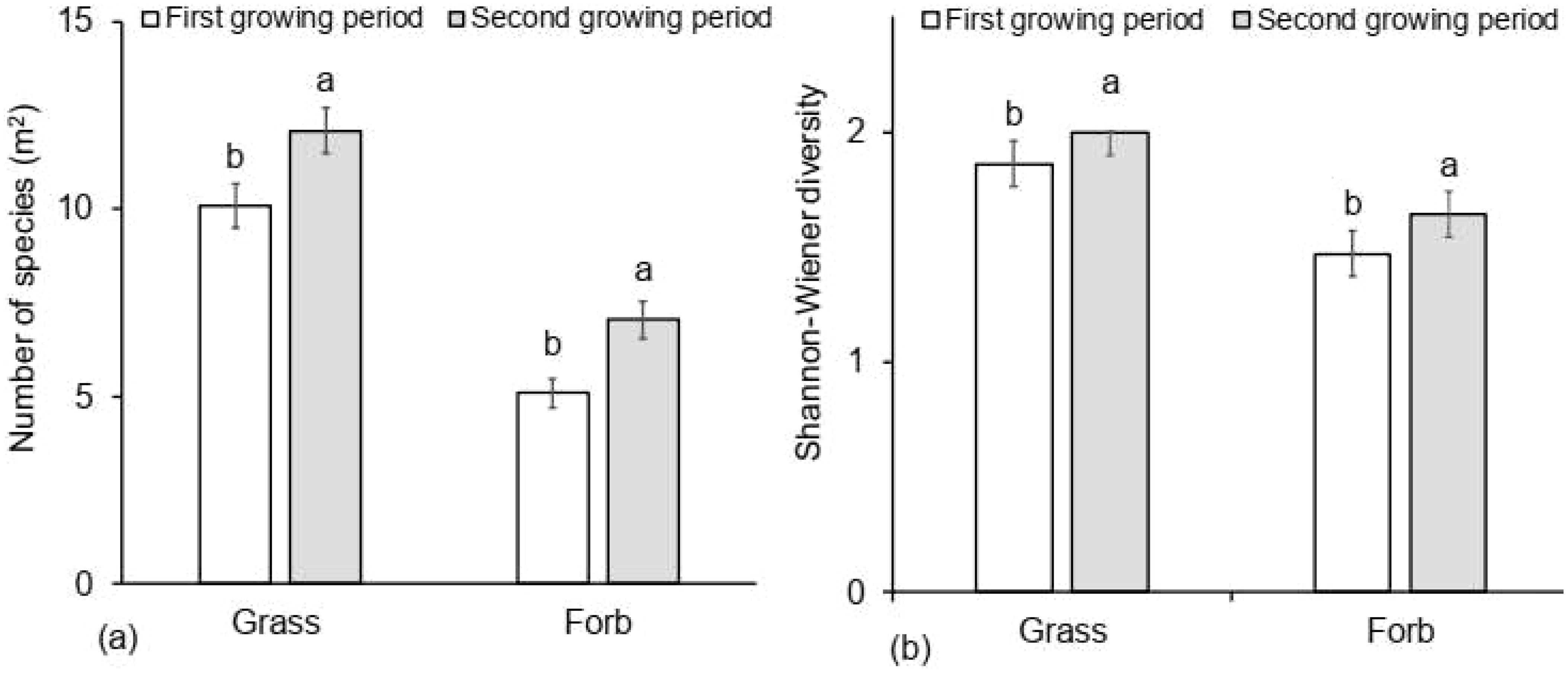
Figure 7. Average (± SE) number of grass and forb species richness (A, B) diversity per plot (m2) response to growing period (First growing period = March and May (white bars), Second growing period = September and November gray bars)). Different letters represent significant differences among thinning treatments at P < 0.05 based on Tukey-HSD post- hoc test.). n = 48.
4 Discussion
4.1 Response of woody species to tree thinning intensity
Our results demonstrated that thinning at high and moderate densities significantly increased the encroacher species’ tree mortality, resulting in lower tree cover and density. Thus, these management options likely reduced the competition with native tree species in the future (Archer and Predick, 2014). Tree thinning also strongly increased tree mortality in the studies of southern Ethiopia and Burkina Faso (Hare et al., 2020; Savadogo et al., 2008, respectively). The slight V. drepanolobium tree mortality we found in the non-thinned treatment (2%) may be attributed to self-thinning of this species, stem-boring insect attacks (Kenfack et al., 2021), drought, and wildlife damage (Wahungu et al., 2011). Minimal woody re-infestation was also reported by Nghikembua et al. (2021) and Hare et al (2021a) after thinning activities in Namibia and Ethiopia.
Our study further revealed that tree coppicing was high in the low and moderately thinned treatments, which is a survival strategy used by many woody plant species after severe disturbances like cutting (Hare et al., 2021b; Mokgosi, 2018). Trees with large stump sizes have strong resprouting capability (Boys, 2022; Monegi et al., 2024) which we confirmed in our study (see also Kahumba, 2010). Hence, we recommend a follow-up treatment after thinning to suppress this coppicing effect. We found a higher seedling recruitment in the highly and moderately thinned treatments than in the low and the none-thinned treatments. Tree thinning can reduce competition, which allows seedlings to establish in areas where mature trees and shrubs had previously been present (e.g. Dwyer and Mason, 2018; Nghikembua et al., 2020). Our results also indicated that V. drepanolobium had higher cumulative regeneration capability (sum of coppicing and seedling recruitment) in the highly and moderately thinned treatment than in the other treatments. This regeneration potential is of great concern as it can lead to re-establishment of woody plants, if not controlled regularly, and will result in re-encroaching of the bush, to a state worse than before the thinning operation.
We found seedling mortality differ among treatments, in support to studies who reported that dense tree stands limit tree seedling establishment by outcompeting seedlings for resources (Brudvig and Asbjornsen, 2009). Tree thinning can promote tree seedling establishment and growth (Kambatuku et al., 2011; Smit, 2014) as well as promote grass production. We expected that thinning would increase seedling survival due to competitive release from existing trees (shading, competition for moisture and nutrients). The higher herbaceous layer biomass after tree thinning maybe reduced tree mortality and facilitated tree seedling survival in our study (see also Tomlinson et al., 2019). The increased growth of grasses and forbs might cover little tree seedlings from being discovered. As grasses might cover little tree seedlings from being discovered and eaten by herbivores (Riginos and Young, 2007; Cramer and Bond, 2013), thinning could lead to reduced seedling mortality. Hence, we recommend a close follow-up after treatment to better understand these facilitation or competition processes.
4.2 Response of herbaceous species to thinning intensity
Our results demonstrated that tree thinning can enhance the abundance of specific grass species, similar to research in South Africa (Ndhlovu et al., 2016; Mndela et al., 2022a; Monegi et al., 2023b). The suppressive effects of V. drepanolobium on herbaceous species were visible after thinning in increased recruitment, cover and growth of herbaceous plants, diversity and forage productivity (Smit, 2005). The observed changes in the abundance and composition of particular grass and forb species in our study likely resulted from the reduction in the suppressive effect of woody plants on the herbaceous layer, increased nutrient supply (Smit, 2014), light and water availability (Stephens et al., 2016) and the presence of seeds in the soil seed banks (Bassett et al., 2020; Mndela et al., 2023). Removing woody plants showed variable outcomes on the herbaceous layer in general (Eldridge et al., 2011; Soliveres and Eldridge, 2014), as the outcomes depend on many factors including the species that are encroaching (Eldridge et al., 2011), the removal methods and the thinning intensity used (Ding and Eldridge, 2019), soil type, and rainfall (Eldridge et al., 2011; Soliveres and Eldridge, 2014). Our results showed that thinning intensities increased some desirable grass species abundances such as Cenchrus ciliaris, C. aucheri (highly desirable), P. mezianum, P. stramineu (intermediately desirable). The abundance of C. aucheri and C. ciliaris agrees with the report of Beyene and Mlambo (2010) in southern Ethiopia. Additionally, the abundance of P. stramineum, and P. mezianum, was similar to the findings of Riginos et al. (2009) in a Vachellia drepanolobium-dominant savanna in Laikipia, Kenya. Of the dominant grass species, C. aucheri, C. ciliaris, and C. dactylon are known as the most palatable and valuable forage species in the Borana rangelands (Angassa, 2012). These findings concur with (Monegi et al., 2023b; Stephen and Seleteng, 2023) who reported that bush encroachment (e.g. control plot) suppresses palatable grasses in particular. Hence, we recommend tree thinning to promote the presence of those desirable grass species in the Borana rangelands.
The observed increase in the abundance of P. mezianum in our study at moderate tree thinning is similar to results from Kenya, which reported that P. mezianum was mainly found in low V. drepanolobium tree densities (Riginos et al., 2009). Pennisetum stramineum and P. mezianum are perennial, bunch-type growth forms, shade-tolerant, and less palatable grasses (Odadi et al., 2007), which prefer low tree densities (Riginos et al., 2009). As V. drepanolobium is a nitrogen-fixing tree (Riginos et al., 2009), and P. mezianum and P. stramineum perform best in nitrogen-rich soils (Riginos et al., 2009; Smith et al., 2013), the nitrogen-rich soils after thinning might have further promoted the growth of these species in our study. However, we claim that these species will likely decline in abundance over time in a highly tree-thinned plot as a result of the post-tree clearing impact on soil fertility (Ndhlovu et al., 2016).
We found that the abundance of P. maximum substantially increased in moderate and high tree-thinned plots, similar to findings by Smit (2005). However, our results contradict with Treydte et al. (2007; Treydte et al, 2008) and Hare et al. (2021b) who reported that P. maximum prefers to grow under tree canopies and it also prefers tall trees rather than growing under shrubs or bushes, and should become less abundant, particularly in the highly thinned treatment, once trees are cut. An increase in abundance of P. maximum in thinned areas may be attributed to its ability to colonize fertile land created by N-fixing tree species (Smit, 2005).
Our results also revealed that D. milanjiana frequently occurred in the non-thinned and lightly thinned treatment, suggesting that these species are shade-loving and their growth is enhanced by high tree density (Erfanzadeh et al., 2021). Species such as B. insculpta, H. contortus, and T. triandra showed inconsistent trends in our study, thus implying that species-specific responses play a large role after thinning, and a longer duration of our experimental period might have shown different results for these species. Cynodon dactylon was abundant at moderate and low thinning treatments, suggesting that moderate shade/tree cover might be important for this species, which is high in nutrients and preferred by many livestock and wildlife species (Treydte et al., 2006).
We also demonstrated that tree thinning enhanced the frequency occurrence of several forb species, e.g., Abutilon hirtum, Commelina africana and Cyathula orthacantha while Indigofera volkensii decreased in highly cleared treatments. The increased abundance of many forb species in highly thinned treatment could be related to release from tree competition, light availability and nutrient supply, which creates a favourable condition for recruitment by altering habitat conditions (Stephens et al., 2016; Mndela et al., 2023). The increases in forb abundance can be affected by simultaneous grass growth (Smit, 2005; Dreber et al., 2011), and grazing intensities. Thus, long-term monitoring might show an increase of grasses in comparison to forbs.
Our results on higher herbaceous layer diversity in thinned areas correspond with those of Utaile et al. (2021), who reported increases in herbaceous richness in low-encroached savanna rangelands in Nech-Sar National Park in East Africa. Our results are further consistent with earlier studies (e.g. Angassa, 2012; Kelil Gobelle, 2021), which found that bush clearing increased species diversity in the Borana rangeland of southern Ethiopia. This suggests that thinning at moderate and high treatments can increase grass and forb species diversity and richness.
4.3 Response of herbaceous biomass to thinning intensity
As expected, we found that biomass of the herbaceous layer increased following tree thinning, similar to results by (Richter et al., 2001). The highly and moderately thinned treatments had the highest biomass production. This is in agreement with (Angassa, 2012) and (Mndela et al., 2022a), who reported that total bush-clearing enhanced biomass condition in Ethiopia. A decline in bare ground cover as reported by Smit (2005); Ndhlovu et al. (2016); Marquart et al. (2023) in our study suggests that tree thinning has potentials to recover herbaceous vegetation on bare soil and increase herbaceous biomass production (Nghikembua et al., 2020; Hare et al., 2021b; Pillay and Ward, 2021; Mndela et al., 2022b; Monegi et al., 2022). Although a complete clearing treatment produced more herbaceous biomass than the other thinning levels in our study, the positive effects of woody species in dry savannas proposes that some trees and shrubs might be beneficial for overall rangeland quality (Treydte et al., 2007). Trees benefit other plants by creating microsites favourable for the establishment of herbaceous plants that livestock graze on (Ding and Eldridge, 2019). We highlight that a legacy effect might promote high beneath-canopy nutrients and biomass of grasses after tree-cutting, but would disappear after a few years (Ndhlovu et al., 2016). Total removal of all woody plants may have a negative long-term impact on the productivity of rangelands (Smit, 2014) and biodiversity Kutt and Martin (2010). This is particularly true in cases where, such as in our study, the V. drepanolobium trees are legumes and are capable of fixing nitrogen, improving soil quality for neighbouring grasses, provide shading and browsing and other ecosystem services, and particularly tall trees should remain in the system (Treydte et al., 2008).
We found that herbaceous vegetation responses were stronger after thinning in the second growing period (Smit, 2005; Davies et al., 2012), which corresponds with the findings of earlier studies (Hare et al., 2021a), who suggested that variation in climatic conditions (rainfall and seasonality) and soil moisture contents following tree thinning greatly influenced grass species composition and biomass production. Similarly, Angassa and Oba (2010), suggest that variation in rainfall substantially affected herbaceous biomass, grass basal cover, and richness, as well as the diversity and evenness of herbaceous species.
5 Conclusion
Our study showed that tree thinning resulted in reducing woody plant density and increased the abundance of several desirable grass species. Further, thinning was related to higher grass species diversity and richness as well as herbaceous biomass, with higher values the more trees were removed. Our overall rangeland condition was best in areas where 100% of V. drepanolobium trees had been thinned. However, although a complete tree removal treatment produced more biomass and better than the other thinning intensities in our example, we recommend to maintain a certain density of particularly tall trees as our short study duration might not have taken the positive legacy effect of trees on the grass layer into account. Our study suggests that a tree thinning intensity of 67% may help control encroaching trees by increasing mortality and suppressing seedling growth, thereby enhancing biomass production of herbaceous species and maintaining diversity. Our study suggests that a tree thinning intensity of 67% may help control encroaching trees by increasing mortality and suppressing seedling growth, thereby enhancing biomass production of herbaceous species and maintaining diversity. We finally conclude that the thinning intensities in one-off interventions showed positive results but were not effective in controlling bush encroachment completely, indicating the need for post-thinning management (e.g., use of fire and browsing) and repeated actions. Our results help with recommendations on controlling bush encroachment, biodiversity restoration, and rangeland productivity in Ethiopian rangelands and other rangelands in similar conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
TAba: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. TAbe: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. AT: Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was financed by the German-Ethiopian SDG Graduate School “Climate Change Effects on Food Security (CLIFOOD) project, which was coordinated by the Food Security Center (FSC) of the University of Hohenheim (Germany) and the Hawassa University supported by the DAAD with funds from the Federal Ministry for Economic Cooperation and Development (BMZ) of Germany.
Acknowledgments
The authors gratefully acknowledge the local pastoral communities for permitting the experimental fieldwork and the coordinators of the CLIFOOD project for facilitating logistics
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1461573/full#supplementary-material
References
Angassa A. (2012). Effects of grazing intensity and bush encroachment on herbaceous species and rangeland condition in southern Ethiopia. Land Degrad. Dev. 25, 438–451. doi: 10.1002/ldr.2160
Angassa A., Oba G. (2008). Herder perceptions on impacts of range enclosures, crop farming, fire ban and bush encroachment on the rangelands of Borana, Southern Ethiopia. Hum. Ecol. 36, 201–215. doi: 10.1007/S10745-007-9156-Z
Angassa A., Oba G. (2009). Bush encroachment control demonstrations in southern Ethiopia: 1. Woody species survival strategies with implications for herder land management. Afr. J.Ecol. 47, 63–76. doi: 10.1111/j.1365-2028.2007.00919.x
Angassa A., Oba G. (2010). Effects of grazing pressure, age of enclosures and seasonality on bush cover dynamics and vegetation composition in southern Ethiopia. J. Arid Environ. 74, 111–120. doi: 10.1016/j.jaridenv.2009.07.015
Archer S. R., Andersen E. M., Predick K. I., Schwinning S., Steidl R. J., Woods S. R. (2017). Woody Plant Encroachment : Causes and Consequences. Rangeland systems: Processes, management and challenges. (Cham, Switzerland: Springer International Publishing) 25–84. doi: 10.1007/978-3-319-46709-2
Archer S. R., Predick K. I. (2014). An ecosystem services perspective on brush management:Research priorities for competing land-use objectives. J. Ecol. 102, 1394–1407. doi: 10.1111/1365-2745.12314
Barron-Gafford G. A., Sanchez-Cañete E. P., Minor R. L., Hendryx S. M., Lee E., Sutter L. F., et al. (2017). Impacts of hydraulic redistribution on grass–tree competition vs facilitation in a semi-arid savanna. New Phytol. 215, 1451–1461. doi: 10.1111/nph.14693
Bassett T. J., Landis D. A., Brudvig L. A. (2020). Effects of experimental prescribed fire and tree thinning on oak savanna understory plant communities and ecosystem structure. For. Ecol. Manage. 464 (September 2019), 118047. doi: 10.1016/j.foreco.2020.118047
Beyene S., Mlambo V. (2010). Encroachment of Acacia brevispica and Acacia drepanolobium in Semi-Arid Rangelands of Ethiopia and their Influence on Sub-Canopy Grasses. Research. J. Bot. 5, 1–13. doi: 10.3923/rjb.2010.1.13
Biancari L., Aguiar M. R., Eldridge D. J., Oñatibia G. R., Le Bagousse-Pinguet Y., Saiz H., et al. (2024). Drivers of woody dominance across global drylands. Sci. Adv. 10, eadn6007. doi: 10.1126/sciadv.adn6007
Birkett A. (2002). The impact of giraffe, rhino and elephant on the habitat of a black rhino sanctuary in Kenya. Afr. J. Ecol. 40, 276–282. doi: 10.1046/j.1365-2028.2002.00373.x
Blaser W. J., Sitters J., Hart S. P., Edwards P. J., Olde Venterink H. (2013). Facilitative or competitive effects of woody plants on understorey vegetation depend on N-fixation, canopy shape and rainfall. J. Ecol. 101, 1598–1603. doi: 10.1111/1365-2745.12142
Bond W. J., Midgley G. F. (2012). Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philos. Trans. R. Soc B Biol. Sci. 367 (1588), 601–612. doi: 10.1098/rstb.2011.0182
Boys J. M. (2022). Sustainable wood harvesting principles with the aim to restore rangeland in the Thornbush Savanna of Namibia (Bloemfontein, South Africa: University of the Free State).
Briske D. D., Coppock D. L., Illius A. W., Fuhlendorf S. D. (2020). Strategies for global rangeland stewardship: Assessment through the lens of the equilibrium–non-equilibrium debate. J. Appl. Ecol. 57, 1056–1067. doi: 10.1111/1365-2664.13610
Brudvig L. A., Asbjornsen H. (2009). The removal of woody encroachment restores biophysical gradients in Midwestern oak savannas. J. Appl. Ecol. 46, 231–240. doi: 10.1111/j.1365-2664.2008.01590.x
Case M. F., Wigley B. J., Wigley-Coetsee C., Carla and Staver A. (2020). Could drought constrain woody encroachers in savannas? Afr. J. Range Forage Sci. 37, 19–29. doi: 10.2989/10220119.2019.1697363
Coppock D. L. (1994). “The borana plateau of southern Ethiopia: synthesis of pastoral research,” in Development and change 1980-91. Systems study no. 5 (International Livestock Centre for Africa, Addis Ababa), 374.
Cramer M. D., Bond W. J. (2013). N-fertilization does not alleviate grass competition induced reduction of growth of African savanna species. Plant Soil. 366, 563–574. doi: 10.1007/s11104-012-1456-4
Dalle G., Maass B. L., Isselstein J. (2005). Plant communities and their species diversity in the semi-arid rangelands of Borana lowlands, southern Oromia, Ethiopia. Com. Ecol. 6, 167–176. doi: 10.1556/ComEc.6.2005.2.5
Dalle G., Maass B. L., Isselstein J. (2006a). Encroachment of woody plants and its impact on pastoral livestock production in the Borana lowlands, southern Oromia, Ethiopia. Afr. J. Ecol. 44, 237–246. doi: 10.1111/j.1365-2028.2006.00638.x
Dalle G., Maass B. L., Isselstein J. (2006b). Rangeland condition and trend in the semi-arid Borana lowlands, southern Oromia, Ethiopia. Afr. J. Range Forage Sci. 23, 49–58. doi: 10.2989/10220110609485886
Daryanto S., Fu B., Zhao W., Wang L. (2019). One-hundred years after shrub encroachment: Policy directions towards sustainable rangeland-use. Land Use Policy 84, 71–78. doi: 10.1016/j.landusepol.2019.03.008
Davies K. W., Bates J. D., Nafus A. M. (2012). Mowing wyoming big sagebrush communities with degraded herbaceous understories: Has a threshold been crossed? Rangeland Ecol. Manage. 65, 498–505. doi: 10.2111/REM-D-12-00026.1
Devine A. P., McDonald R. A., Quaife T., Maclean I. M. D. (2017). Determinants of woody encroachment and cover in African savannas. Oecologia 183, 939–951. doi: 10.1007/s00442-017-3807-6
Ding J., Elridge D. (2023). The success of woody plant removal depends on encroachment stage and plant traits. Nat. Plants. 9, 58–67. doi: 10.1038/s41477-022-01307-7
Ding J., Eldridge D. J. (2019). Contrasting global effects of woody plant removal on ecosystem structure, function and composition. Perspect. Plant Ecol. Evol. Sys. 39, 125460. doi: 10.1016/j.ppees.2019.125460
Ding J., Travers S. K., Delgado-Baquerizo M., Eldridge D. J. (2020). Multiple trade-offs regulate the effects of woody plant removal on biodiversity and ecosystem functions in global rangelands. Global Change Biol. 26, 709–720. doi: 10.1111/gcb.14839
Dreber N., Oldeland J., Van Rooyen G. M. W. (2011). Species, func-tional groups and community structure in seed banks of the aridNama Karoo: Grazing impacts and implications for rangeland resto-ration. Agric Ecosyst. Environ. 141, 399–409. doi: 10.1016/j.agee.2011.04.004
Dwyer J. M., Mason R. (2018). Plant community responses to thinning in densely regenerating Acacia harpophylla forest. Restor. Ecol. 26, 97–105. doi: 10.1111/rec.12536
Eldridge D. J., Bowker M. A., Maestre F. T., Roger E., Reynolds J. F., Whitford W. G. (2011). Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 14, 709–722. doi: 10.1111/j.1461-0248.2011.01630.x
Eldridge D. J., Ding J. (2021). Remove or retain: ecosystem effects of woody encroachment and removal are linked to plant structural and functional traits. New Phytol. 229, 2637–2646. doi: 10.1111/nph.17045
Erfanzadeh R., Yazdani M., Arani A. M. (2021). Effect of different shrub species on theirsub-canopy soil and vegetation properties in semiarid regions. Land Degrad. Dev. 32, 3236–3247. doi: 10.1002/ldr.3977
Feyisa K., Beyene S., Angassa A., Said M. Y., de Leeuw J., Abebe A., et al. (2017). Effects of enclosure management on carbon sequestration, soil properties and vegetation attributes in East African rangelands. Catena 159, 9–19. doi: 10.1016/j.catena.2017.08.002
Food and Agricultural Organization (FAO). (1986). Soil map of the world. Revised Legend. Technical Paper 20 (Wageningen, The Netherlands: ISRIC).
Froman B., Persson S. (1974). “An illustrated guide to the grasses of Ethiopia,” in An illustrated guide to the grasses of Ethiopia (Assela, Ethiopia: Chilalo Agricultural Development Unit (CADU)).
Hammer Ø, Harper D. A. T., Ryan P.D. (2001). “Past: paleontological statistics software package for education and data analysis,” in Palaeontologia electronica, vol. 4. , 9. Available at: http://palaeo-lectronica.org/2001_1/past/issue1_01.htm (Accessed March 5, 2023).
Hare M. L., Wang Y. D., Xu X. W., Yuan Y., Na Z., Gedda A. E. (2021b). Do bush control techniques have an effect on the density, cover and recruitment of woody plants in a semi-arid savanna? The case of a semi-arid savanna, southern Ethiopia. Front. Environ. Sci. 9, 1-14. doi: 10.3389/fenvs.2021.777146
Hare M. L., Xu X., Wang Y., Gedda A. I. (2020). The effects of bush control methods on encroaching woody plants in terms of die-off and survival in Borana rangelands, southern Ethiopia. Pastoralism 10, 1–14. doi: 10.1186/s13570-020-00171-4
Hare M. L., Xu X. W., Wang Y. D., Yuan Y., Gedda A. E. (2021a). Do woody tree thinning and season have effect on grass species’ Composition and biomass in a semi-arid savanna? The case of a semi-arid savanna, southern Ethiopia. Front. Environ. Sci. 9. doi: 10.3389/fenvs.2021.692239
Hedberg I., Edwards S. (1995). Flora of Ethiopia and Eritrea, vol. 7. Poaceae (Graminaceae) (Addis Ababa, Ethiopia: National Herbarium, Addis Ababa University). 420.
Higgins S. I., Bond W. J., Trollope W. S. (2000). Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J. Ecol. 88 (2), 213–229. doi: 10.1046/j.1365-2745.2000.00435.x
Holdo R. M., Nippert J. B. (2023). Linking resource-and disturbance-based models to explain tree–grass coexistence in savannas. New Phytol. 237, 1966–1979. doi: 10.1111/nph.v237.6
Homann S. (2004). Indigenous knowledge of Borana pastoralists in natural resource management : a case study from southern Ethiopia (Germany: Doctoral dissertation, Justus Liebig University Giessen).
Homann S., Rischkowsky B., Steinbach J., Kirk M., Mathias E. (2008). Towards endogenous livestock development : borana pastoralists ‘ Responses to environmental and institutional changes. Hum. Ecol. 36, 503–520. doi: 10.1007/s10745-008-9180-7
Jenkins P. M., Bisrat G., Bekele D. (1974). Grasses common to arero area southern Ethiopia (Addis Ababa, Ethiopia: Ministry of Agriculture).
Kahumba A. (2010). Comparison of the rehabilitative effects of mechanical and chemical methods of bush control on degraded highland savanna rangelands in Namibia (MSc thesis)). University of Namibia Windhoek, Namibia. Available at: http://hdl.handle.net/11070/507.
Kambatuku J. R., Cramer M. D., Ward D. (2011). Savanna tree-grass competition is modified by substrate type and herbivory. J. Veg. Sci. 22, 225–237. doi: 10.1111/j.1654-1103.2010.01239.x
Kelil Gobelle S. (2021). Impacts of bush management on herbaceous plant diversity and biomass and, soil organic carbon and nitrogen in borana rangelands, southern Ethiopia. J. Plant Sci. 9, 38. doi: 10.11648/j.jps.20210902.12
Kellner K., Fouché J., Tongway D., Boneschans R., van Coller H., van Staden N. (2022). Landscape function analysis: responses to bush encroachment in a semi-arid savanna in the molopo region, South Africa. Sustainability. 14, 1-24. doi: 10.3390/su14148616
Kenfack D., Arellano G., Kibet S., Kimuyu D., Musili P. (2021). Understanding the monodominance of Acacia drepanolobium in East African savannas: insights from demographic data. Trees - Struct. Funct. 35, 1439–1450. doi: 10.1007/s00468-021-02127-6
Kent M. P. C. (1992). Vegetation description and analysis: a practical approach (Belhaven: Press. London).
Kimaro H. S., Asenga A. M., Munishi L., Treydte A. C. (2019). Woody encroachment extent and its associated impacts on plant and herbivore species occurrence in maswa game reserve Vol. 9 (Tanzania: Canadian Center of Science and Education (CCSE)), 63–76. doi: 10.5539/enrr.v9n3p63
Kutt A. S., Martin T. G. (2010). Bird foraging height predicts bird species response to woody vegetation change. Biodivers Conserv. 19, 2247–2262. doi: 10.1007/s10531-010-9840-y
Lehmann C. E., Archibald S. A., Hoffmann W. A., Bond W. J. (2011). Deciphering the distribution of the savanna biome. New Phytol. 191 (1), 197–209. doi: 10.1111/j.1469-8137.2011.03689.x
Liao C., Clark P. E., DeGloria S. D. (2018). Bush encroachment dynamics and rangeland management implications in southern Ethiopia. Ecol.Evol. 8, 11694–11703. doi: 10.1002/ece3.4621
Luvuno L., Biggs R., Stevens N., Esler K. (2018). Woody Encroachment as a Social-Ecological Regime Shift. Sustainability 10 (7), 2221.1-16. doi: 10.3390/su10072221 10.3390/su10072221
Maestre F. T., Eldridge D. J., Soliveres S. (2016). commentary a multifaceted view on the impacts of shrub encroachment. Applied Vegetation Science 19, 369–370. doi: 10.1111/avsc.12254
Marquart A., Eldridge D. J., Travers S. K., Val J., Blaum N. (2019). Large shrubs partly compensate negative effects of grazing on hydrological function in a semi-arid savanna. Basic Appl. Ecol. 38, 58–68. doi: 10.1016/j.baae.2019.06.003
Marquart A., Van Coller H., Van Staden N., Kellner K. (2023). Impacts of selective bush control on herbaceous diversity in wildlife and cattle land use areas in a semi-arid Kalahari savanna. J. Arid Environ. 208, 104881. doi: 10.1016/j.jaridenv.2022.104881
Megersa B., Markemann A., Angassa A., Ogutu J. O., Piepho H. P., Valle Zaráte A. (2014). Impacts of climate change and variability on cattle production in southern Ethiopia: Perceptions and empirical evidence. Agric. Syst. 130, 23–34. doi: 10.1016/J.AGSY.2014.06.002
Mndela M., Madakadze I. C., Nherera-Chokuda F. V., Dube S., Ramoelo A., Mangwane M., et al. (2022a). Short-term responses of herbaceous vegetation to bush clearing in semi-arid rangelands of South Africa. Pastoralism. 12. doi: 10.1186/s13570-022-00235-7
Mndela M., Madakadze I. C., Tjelele J. T., Mangwane M., Nherera-Chokuda F., Dube S., et al. (2022b). Responses of grass productivity traits to bush clearing in semi-arid rangelands in North-West Province of South Africa. Rangeland J. 44, 33–45. doi: 10.1071/RJ21053
Mndela M., Mangwane M., Ngcobo N., Rasekgokga N. J., Monegi P. (2023). Soil seed banks along a woody plant removal gradient in a semi-arid savanna of South Africa: Implications for restoration. Acta. Oecologica. 118, 103891. doi: 10.1016/j.actao.2023.103891
Mokgosi R. O. (2018). Effects of bush encroachment control in a communal managed area in the Taung region (North West Province, South Africa: Doctoral dissertation, North-West University).
Monegi P., Mkhize N. R., Tjelele T. J., Ward D., Tsvuura Z. (2022). The impact of tree removal on standing grass biomass, seedling establishment and growth of woody species. Rangeland J. 44, 25–32. doi: 10.1071/RJ21003
Monegi P., Mkhize N. R., Tjelele J. T., Ward D., Tsvuura Z. (2023b). Resprouting response among savanna tree species in relation to stem size, woody removal intensity and herbicide application. Plants 12, 1-10. doi: 10.3390/plants12193451
Monegi P., Mkhize N. R., Tjelele J. T., Ward D., Monegi P. (2024). Grass dynamics along a woody-plant density reduction gradient in a South African savanna. Afr. J. Range Forage Sci. 41, 117–124. doi: 10.2989/10220119.2023.2262534
Ndhlovu T., Milton S. J., Esler K. J. (2016). Effect of Prosopis (mesquite) invasion and clearing on vegetation cover in semi-arid Nama Karoo rangeland, South Africa. Afr. J. Range forage Sci. 33, 11–19. doi: 10.2989/10220119.2015.1036460
Nghikembua M. T., Marker L. L., Brewer B., Leinonen A., Mehtätalo L., Appiah M., et al. (2021). Restoration thinning reduces bush encroachment on freehold farmlands in north-central Namibia. Forestry. Int. J. For. Res. 94, 551–564. doi: 10.1093/forestry/cpab009
Nghikembua M. T., Marker L. L., Brewer B., Leinonen A., Mehtätalo L., Appiah M., et al. (2023). Response of woody vegetation to bush thinning on freehold farmlands in north − central Namibia. Sci. Rep. 13, 297. doi: 10.1038/s41598-022-26639-4
Nghikembua M. T., Marker L. L., Brewer B., Mehtätalo L., Appiah M., Pappinen A. (2020). Response of wildlife to bush thinning on the north central freehold farmlands of Namibia. For. Ecol. Manage. 473, 118330. doi: 10.1016/j.foreco.2020.118330
Oba G. (2012). Harnessing pastoralists' indigenous knowledge for rangeland management: three African case studies. Pastor. Res. Policy Pract. 2, 1–25. doi: 10.1186/2041-7136-2-1
O’Connor T. G., Puttick J. R., Hoffman M. T. (2014). Bush encroachment in southern Africa: Changes and causes. Afr. J. Range Forage Sci. 31, 67–88. doi: 10.2989/10220119.2014.939996
O’Connor R. C., Taylor J. H., Nippert J. B. (2020). Browsing and fire decreases dominance of. Ecology. 101, 1–11. doi: 10.1002/ecy.2935
Odadi W. O., Young T. P., Okeyo-Owuor J. B. (2007). Effects of wildlife on cattle diets in Laikipia rangeland, Kenya. Rangeland Ecol. Manage. 60, 179–185. doi: 10.2111/05-044R3.1
Okello B. D. (2007). Effects of herbivores, fire and harvesting on the population dynamics of Acacia drepanolobium sjoestedt in Laikipia, Kenya (Doctoral dissertation) (Pietermaritzburg, KwaZulu-Natal, South Africa: University of KwaZulu-Natal ).
Pillay T., Ward D. (2021). Grass competition is more important than fire for suppressing encroachment of Acacia sieberiana seedlings. Plant Ecol. 222, 149–158. doi: 10.1007/S11258-020-01094-1
Quero J. L., Maestre F. T., Ochoa V., García-Gómez M., Delgado-Baquerizo M. (2013). On the importance of shrub encroachment by sprouters, climate, species richness and anthropic factors for ecosystem multifunctionality in semi-arid Mediterranean ecosystems. Ecosyst. 16, 1248–1261. doi: 10.1007/s10021-013-9683-y
Richter C. G. F., Snyman H. A., Smit G. N. (2001). The influence of tree density on the grass layer of three semi-arid savanna types of southern africa. Afr. J. Range Forage Sci. 18, 103–109. doi: 10.2989/10220110109485762
Riginos C., Grace J. B., Augustine D. J., Young T. (2009). Local versus landscape-scale effects of savanna trees on grasses. J. Ecol. 97, 1337–1345. doi: 10.1111/j.1365-2745.2009.01563.x
Riginos C., Young Æ.T.P (2007). Positive and negative effects of grass, cattle, and wild herbivores on Acacia saplings in an East African savanna. Oecologia 985–995, 985–995. doi: 10.1007/s00442-007-0799-7
Roques K. G., O'connor T. G., Watkinson A. R. (2001). Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 38 (2), 268–280. doi: 10.1046/j.1365-2664.2001.00567.x
Sankaran M., Ratnam J., Hanan N. (2008). Woody cover in African savannas: the role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 17 (2), 236–245. doi: 10.1111/j.1466-8238.2007.00360.x
Savadogo P., Tiveau D., Sawadogo L., Tigabu M. (2008). Herbaceous species responses to long-term effects of prescribed fire, grazing and selective tree cutting in the savanna-woodlands of West Africa. Perspect. Plant Ecol. Evol. Syst. 10, 179–195. doi: 10.1016/j.ppees.2008.03.002
Scholtz R., Polo J. A., Tanner E. P., Fuhlendorf S. D. (2018). Grassland fragmentation and its influence on woody plant cover in the southern Great Plains, USA. Landscape Ecol. 33, 1785–1797. doi: 10.1007/s10980-018-0702-4
Selemani I. S. (2018). Ecological implications of bush encroachment on foraging behavior of dairy cows and goats at SUA farm, Morogoro, Tanzania. Grassl.Forrajes Trop. 6, 169–176. doi: 10.17138/tgft(6)169-176
Smit G. N. (2005). Tree thinning as an option to increase herbaceous yield of an encroached semi-arid savanna in South Africa. BMC Ecol. 5, 1–15. doi: 10.1186/1472-6785-5-4
Smit N. (2014). Response of Colophospermum mopane to different intensities of tree thinning in the Mopane Bushveld of southern Africa. Afr. J. Range Forage Sci. 31, 173–177. doi: 10.2989/10220119.2014.899513
Smith M. D., van Wilgen B. W., Burns C. E., Govender N., Potgieter A. L., Andelman S., et al. (2013). Long-term effects of fire frequency and season on herbaceous vegetation in savannas of the Kruger National Park, South Africa. J. Plant Ecol. 6, 71–83. doi: 10.1093/jpe/rts014
Soliveres S., Eldridge D. J. (2014). Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understorey plant communities and soil function? Funct. Ecol. 28, 530–537. doi: 10.1111/1365-2435.12196
Staver A. C., Bond W. J. (2014). Is there a ‘browse trap’? Dynamics of herbivore impacts on trees and grasses in an African savanna. J. Ecol. 102 (3), 595–602. doi: 10.1111/1365-2745.12230
Staver A. C., Abraham J. O., Hempson G. P., Karp A. T., Faith J. T. (2021). The past, present, and future of herbivore impacts on savanna vegetation. J. Ecol. 109, 2804–2822. doi: 10.1111/1365-2745.13685
Stephen R. J., Seleteng P. C. L. (2023). Herbaceous vegetation changes along a bush encroachment intensity gradient in a montane area. June. Afr.J. Ecol. 61, 1–12. doi: 10.1111/aje.13192
Stephens G. J., Johnston D. B., Jonas J. L., Paschke M. W. (2016). Understory responses to mechanical treatment of pinyon-juniper in northwestern Colorado. Rangeland Ecol. Manage. 69, 351–359. doi: 10.1016/j.rama.2016.06.003
Tainton N. M. (1981). “The assessment of veld condition,” in Veld and pasture management in South Africa. Ed. Tainton M. N. (Shuter and Shooter, Ltd: Pietermaritzburg, South Africa), 46–55.
Tefera B., Snyman H.A.Ã., Smit G. N. (2007). Cattle-rangeland management practices and perceptions of pastoralists towards rangeland degradation in the Borana zone of southern Ethiopia. J. Environ. Manage. 82, 481–494. doi: 10.1016/j.jenvman.2006.01.008
Tomlinson K. W., Sterck F. J., Barbosa E. R. M., de Bie S., Prins H. H. T., van Langevelde F. (2019). Seedling growth of savanna tree species from three continents under grass competition and nutrient limitation in a greenhouse experiment. J. Ecol. 107, 1051–1066. doi: 10.1111/1365-2745.13085
Treydte A. C., Bernasconi S. M., Kreuzer M., Edwards P. J. (2006). Diet of the common warthog (Phacochoerus africanus) on former cattle grounds in a Tanzanian savanna. J. Mammal. 87, 889–898. doi: 10.1644/05-MAMM-A-336R2.1
Treydte A. C., Heitko I. M. A., Prins H. H. T., Ludwig F. (2007). Trees improve grass quality for herbivores in African savannas. Perspect. Plant Ecol. Evol. Syst. 8, 197–205. doi: 10.1016/j.ppees.2007.03.001
Treydte A. C., Van Beeck F. A. L., Ludwig F., Heitkönig I. M. A. (2008). Improved quality of beneath-canopy grass in South African savannas: Local and seasonal variation. J. Veg. Sci. 19, 663–670. doi: 10.3170/2008-8-18435
Tuffa S., Treydte A. C. (2017). Modeling Boran cattle populations under climate change and varying carrying capacity. Ecol. Model. 352, 113–127. doi: 10.1016/j.ecolmodel.2017.03.009
Utaile Y. U., Honnay O., Muys B., Cheche S. S., Helsen K. (2021). Effect of Dichrostachys cinerea encroachment on plant species diversity, functional traits and litter decomposition in an East-African savannah ecosystem. J. Veg. Sci. 32, e12949. doi: 10.1111/jvs.12949
Utaile Y. U., Honnay O., Shibru S., Kenny C. (2023). Assessing removal methods for controlling Dichrostachys cinerea encroachment and their impacts on plant communities in an East- African savannah ecosystem. Appl. Veg. Sci. 26, e12720. doi: 10.1111/avsc.12720
Vadigi S., Ward D. (2013). Shade, nutrients, and grass competition are important for tree sapling establishment in a humid savanna. Ecosphere. 4 (11), 1–27. doi: 10.1890/ES13-00239.1
Venter Z. S., Cramer M. D., Hawkins H. J. (2018). Drivers of woody plant encroachment over Africa. Nat.Commun. 9, 1–7. doi: 10.1038/s41467-018-04616-8
Wahungu G. M., Mureu L. K., Kimuyu D. M., Birkett A., Macharia P. G., Burton J. (2011). Survival, recruitment and dynamics of Acacia drepanolobium Sjøstedt seedlings at Olpejeta Conservancy, Kenya, between 1999 and 2009. Afr. J. Ecol. 49, 227–233. doi: 10.1111/j.1365-2028.2010.01254.x
Ward D. (2005). Do we understand the causes of bush encroachment in African savannas? African. J. Range Forage Sci. 22, 101–105. doi: 10.2989/10220110509485867
Ward D., Pillay T., Mbongwa S., Kirkman K., Hansen E., Van M. (2022). Rangeland ecology management reinvasion of native invasive trees after a tree-thinning experiment in an african savanna. Rangeland Ecol. Manage. 81, 69–77. doi: 10.1016/j.rama.2022.01.004
Wieczorkowski J. D., Lehmann C. E. R. (2022). Encroachment diminishes herbaceous plant diversity in grassy ecosystems worldwide. Global Change Biol. 28, 5532–5546. doi: 10.1111/gcb.16300
Yusuf H., Treydte A. C., Demissew S., Woldu Z. (2011). Assessment of woody species encroachment in the grasslands of Nechisar National Park, Ethiopia. Afr. J. Ecol. 49, 397–409. doi: 10.1111/j.1365-2028.2011.01271.x
Keywords: bush encroachment, restoration, tree removal, Vachellia drepanolobium, Ethiopia
Citation: Abate T, Abebe T and Treydte A (2025) How much to cut? Finding an optimal thinning intensity of encroaching woody species for the herbaceous community in an East African savanna. Front. Ecol. Evol. 12:1461573. doi: 10.3389/fevo.2024.1461573
Received: 08 July 2024; Accepted: 26 November 2024;
Published: 06 January 2025.
Edited by:
Darren Norris, Universidade Federal do Amapá, BrazilReviewed by:
Craig Morris, Agricultural Research Council of South Africa (ARC-SA), South AfricaCorli Coetsee, South African National Parks, South Africa
Copyright © 2025 Abate, Abebe and Treydte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teshome Abate, dGVzaG9tZWFiYXRlQGdtYWlsLmNvbQ==
 Teshome Abate
Teshome Abate Tesfaye Abebe1
Tesfaye Abebe1