
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 20 November 2024
Sec. Conservation and Restoration Ecology
Volume 12 - 2024 | https://doi.org/10.3389/fevo.2024.1460363
Introduction: Identifying habitat of migratory species to effectively support conservation and management requires careful consideration of (1) the data used to inform habitat models, (2) the biology of the organism, (3) land tenure, and (4) the needs of the target audience.
Methods: To provide this information for western U.S. monarch butterflies, a population undergoing decline, we modeled habitat during the spring and fall migrations. Our approach controlled for biases in citizen science locality data, the principal source of monarch observation data, and incorporated needs for milkweed host plants in the spring and nectar plants in the fall.
Results and Discussion: The results showed the distribution of habitat for spring and fall migration, where the Coast Range and Central Valley in California and riparian areas throughout the range were particularly important. Just 29% of predicted habitat for spring and fall migrations, combined, overlapped between the two seasons. Although the U.S. federal government manages 53% of the land in the western U.S., government land makes up just 11.7% of the spring migration range and 23.5% of the fall migration range. State and local governments and non-governmental organizations (NGOs) manage an additional 4.2% of the spring and 4.0% of the fall migration habitat. Thus, like eastern monarchs, western monarchs rely heavily on land under private ownership for their migration and to be successful, monarch conservation efforts must embrace a public-private approach. Among federal agencies, the Bureau of Land Management (BLM; 5.9% of spring and 9.7% of fall habitat) and Forest Service (3.3% of spring and 9.2% of fall habitat) manage the greatest shares. Less than half of the government and NGO owned habitat for both migrations is managed for biodiversity conservation rather than multiple uses (spring, 46.0%; fall, 36.5%). We created custom model outputs for the BLM to highlight areas of both regional and local importance for migrating monarchs in each BLM administrative unit, enabling managers across the agency to contribute to recovery. The outcomes provide input at a relevant spatial scale to support actions such as habitat restoration, riparian zone protection, and pesticide use reduction to enhance conditions for migrating monarchs on both government and private lands.
Migratory animals present many challenges to conservation practitioners charged with their protection. Populations of these species are subject to varying threats in geographic areas often separated by hundreds or thousands of kilometers as well as during their transit between these areas (Martin et al., 2007). Effective conservation for these species requires knowledge about their full life cycle, including where they occur at different times of the year, resources required by all life stages, and which life stage limits population increase the most (Crouse et al., 1987; Runge et al., 2014; Reynolds et al., 2017). These life cycle details can be difficult to obtain due to needs for coordination among authorities, the challenging nature of tracking animals during their migrations, and geographic variation in data availability.
The migratory monarch butterfly (Danaus plexippus plexippus) undergoes iconic migrations in North America, and population declines present an example of the complexities of managing a migratory species (Pelton et al., 2019; Crossley et al., 2022). The monarch population west of the Rocky Mountains overwinters at sites primarily along the central and southern California coast. In the spring, individuals migrate throughout the western U.S. across multiple generations and continue to breed through the summer before returning to the coast in the fall (Pelton et al., 2019). In addition, some migrate to the same central Mexican overwintering sites as populations from east of the Rockies, but it is not confirmed that monarchs from Mexico migrate back to the western U.S (Morris et al., 2015; Billings, 2019; Pelton et al., 2019).
Conservation of the western monarch population requires protection of the overwintering sites, the breeding sites, and the migratory pathways. The overwintering sites are well known (Pelton et al., 2019), and recent models focusing on the milkweed (Asclepias spp.) hostplant have identified important breeding areas (Dilts et al., 2019). However, the areas used during migration for this population are not well understood (Pelton et al., 2019). An existing continental-scale model provides a useful overview of the seasonal expansion and contraction of this population (Castañeda et al., 2019) but is too coarse to inform managers responsible for individual tracts of land. Ideally, model outputs would be at scales of tens to hundreds of meters and readily available as GIS layers that allow users to zoom to an area of interest. A site suitability model for western monarchs fitted with limited locality, climate, and land use data for a single annual cycle (2016-2017) is also available (Kesler and Bunch, 2023) but again is too coarse for tract-level management and may not reflect multi-year patterns of habitat use. Knowing the routes through which monarchs migrate is key to identifying potential sources of mortality as well as areas for habitat conservation and/or enhancement (Howard and Davis, 2008).
Approaches to filling any such conservation knowledge gaps should be cognizant of the actors who would undertake desired land management interventions (Hulme, 2014; Raymond et al., 2015; Cvitanovic et al., 2016). The western monarch is widespread across 7 U.S. states (Idaho, Utah, Arizona, Nevada, Washington, Oregon, California) as well as the western portions of 4 Rocky Mountain states (Montana, Wyoming, Colorado, New Mexico). The federal government manages much of this land (e.g., 53% of the 7 westernmost states; Congressional Research Service, 2020), and state, local, and non-governmental organizations (NGOs) also manage sizable tracts of land. Any attempt to identify where western monarch migration takes place should therefore include outputs aimed at the spatial scale and technological tools used by managers for these agencies to facilitate uptake and efficient siting of conservation interventions.
Several challenges face the development of a representative habitat model for the migratory stage of this population’s life cycle. First, both the spring and fall migration are dependent on different food sources: milkweed in the spring (for larval food and as a major nectar source) and nectar from diverse plants in the fall (Xerces Society, 2018; Lukens et al., 2024). Second, most recent observation data for monarchs, milkweeds, and fall flowers that provide nectar are from citizen scientists, which, while prolific, are typically spatially biased to human population centers and areas with easy access (Mair and Ruete, 2016; Tiago et al., 2017; Young et al., 2019). Third, nonmigratory subpopulations of monarchs are known from several areas within the range of western monarchs (Crone and Schultz, 2021; Steele et al., 2023); observations of these individuals could confound models aimed at understanding the behavior of migrants. Fourth, as with other wide-ranging species, depicting distributions in ways that are meaningful to local managers can be challenging due the mismatch in scale between outputs (maps covering hundreds of thousands of square kilometers) and on-the-ground conservation interventions (actions taking place over areas of less than a square kilometer to perhaps dozens of square kilometers).
Here we present new habitat models for the spring and fall migration stages of the western monarch life cycle. We used these results to assess the proportion of the migratory ranges that are under the management of federal, state, and governmental land management agencies and NGOs versus private landowners. In addition, we describe a method to adjust the scale of our output to administrative management units of a major federal land management agency, the Bureau of Land Management (BLM). This scaling method can be adapted for scaling results of other spatial analyses for targeting on-the-ground conservation interventions.
We used locality data from 10 sources (see Acknowledgements for list) primarily made up of citizen science observations from across the 11 state range of the western monarch. We used data from eggs, larvae, and adults in the spring because any life stage indicates the presence of migratory individuals during this period, although some records may refer to nonmigrants. For the fall, we used only adult records to restrict modeling to migrating adults returning to overwintering sites. We restricted records to the years 2010-2021 to focus on recent migration patterns and used records from March – June for spring and August – October for fall migration. We discarded duplicate records from the same site and date, those from within 5 km of known overwintering sites on the California coast (CDFW, 2023) to avoid including overwintering individuals, and records annotated with a spatial uncertainty of greater than 1 km as a balance between filtering out valuable records and avoiding spurious effects on outputs generated at 330-m resolution. We used the R Package CoordinateCleaner (Zizka et al., 2019, R Core Team, 2022) to eliminate records over bodies of water, from state centroids, museums (which may erroneously be recorded as localities from specimens housed there), and botanic gardens (because citizen scientists may post records of captive individuals). Recognizing that nonmigratory populations occur in the San Francisco Bay and Los Angeles, California, and Phoenix, Arizona (Morris et al., 2015; Crone and Schultz, 2021; James et al., 2021), areas we eliminated records from the urban footprint of these regions.
To help control for spatial bias in record location caused by human population centers and patterns of collection/observation such as roads and parks, we used records of all Nymphalids, the family that monarchs belong to, in a target group correction approach (Ponder et al., 2001; Anderson, 2003; Phillips et al., 2009). Patterns of spatial bias in monarch records were associated with large clusters of points around metropolitan areas such as the San Francisco Bay area, Los Angeles, Phoenix, Salt Lake City, as national parks and highways. Target group correction estimates sampling effort for species related to the target species to identify areas of high geographical bias. We assumed that the spatial bias in Nymphalid records mirrors the spatial bias in monarch records, and visual inspection revealed similar clustering around metropolitan areas, highways and outdoor recreation areas. We obtained Nymphalid records through the Global Biodiversity Information Facility (GBIF; https://doi.org/10.15468/dd.u6h9qd) from the same 2010-2021 time period as for monarchs and filtered them using CoordinateCleaner. To create a bias layer, we used the MASS R package kde2d function (Venables and Ripley, 2002) to estimate a bias layer based on kernel density estimation, using a 10km standard deviation. This bias layer was then used to down weight the monarch records by sampling records in proportion to the estimated bias.
For both spring and fall models, we used a library of 108 terrestrial and palustrine 330-m resolution environmental predictor layers that represented climate, topography, hydrography, soils, land cover (listed in Supplementary Table 1). For the spring models, we also included a summed milkweed habitat suitability layer generated by summing the individual milkweed species outputs from Dilts et al. (2019) and made available by the Xerces Society. This layer approximates milkweed species diversity, which we assume is a useful indicator of milkweed abundance.
For the fall models, we created a nectar abundance layer by modeling the distribution of 11 species and 6 genera of fall-flowering plants derived from the Xerces Society Monarch Nectar Plant Database (Table 1). These species are the most frequently recorded food sources for migrating monarchs. Again, we used records from GBIF from the same time period and cleaned in the same manner as for the monarch and Nymphalid records and incorporating bias correction using angiosperm records overlapping the August to October migration period (GBIF, https://doi.org/10.15468/dd.yfj6ba). We selected predictor variables based on their importance in preliminary models, with highly correlated variables excluded through a pairwise selection process.
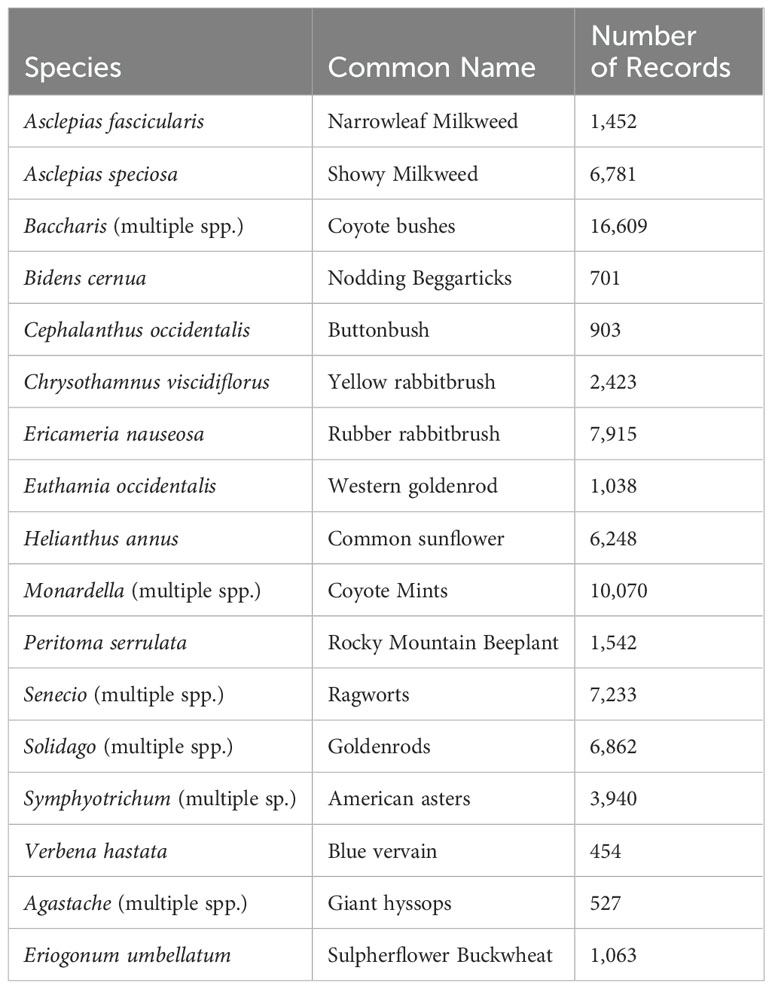
Table 1. Fall nectar plants for western monarchs used and the number of GBIF records used for each to create a nectar abundance layer.
Recognizing that monarchs expand outward from their coastal overwintering sites during the course of the spring, we generated 4 separate monthly models for this season. We created a single fall model because the migration takes place broadly across the western U.S. during this period (i.e., not initiating from a limited area). We used R scripts to implement methods described in Hamilton et al. (2022) to run models with the random forests (RF) algorithm with the randomForest R package (Breiman, 2001; Liaw and Wiener, 2002). R scripts providing details on model tuning and variable selection are available on GitHub (https://zenodo.org/doi/10.5281/zenodo.12659562). Modeling followed methods described in Hamilton et al., 2022). We built random forests models using classification trees and incorporated downsampling, fitting trees with a bootsrapped sample of presence points with an equal number of background points (Chen et al., 2004; Valavi et al., 2021). We selected background points from within the modeling extent area, avoiding samples from within HUC-10 hydrological units within 500 m of presences. The number of variables selected randomly at each split (mtry tuning) was selected to minimize out-of-bag (OOB) error using an initial setting of mtry=6 and increasing by 2 at each step. To account for spatial accuracy, we buffered points by their reported error radius in meters, with overlapping polygons merged and presences selected from within polygons to better sample environmental variation relative to locational accuracy, which also reduced pseudoreplication caused by multiple presence points in close vicinity. We built final models based on 2,000 trees following preliminary models based on 1,000 trees from which we removed variables by selecting the most important variable within sets of correlated variables, and removed variables based on mean decrease in accuracy, removing the lower 25th percentile of predictor variables. We assessed validation using leave-one-out jackknifing and calculated specificity, sensitivity, true skill statistic, kappa, and area under the curve. We generated mapped outputs of the final models from the values of environmental variables utilized in the models, and represent the relative probability of detecting monarchs at that site, or similarity to observed areas where monarchs have been documented based on the results of the multiple trees used to generate the model.
The extent of the milkweed layer, which covered the westernmost 7 states (Arizona, Utah, Idaho and the more western states), restricted the modeling extent for the spring models. The fall model included those 7 states plus the area west of the Continental Divide in Montana south through New Mexico. Output resolution was 330 m for all models.
Two monarch experts from the Xerces Society reviewed preliminary output and provided suggestions from improving the models, such as removing out-of-range records from west of the Cascade Mountains in Washington. We then reran the models to generate final continuous spatial predictions. To simplify the outputs for managers, we converted these continuous predictions to binary representations of habitat by applying a probability threshold that minimized the false positive rate (1- specificity) based on the receiver operating characteristic (ROC) curve, above which all values were classified as habitat (Hamilton et al., 2022). In addition, we created a single spring output where we assigned each pixel the maximum value from across the 4 monthly models, and then thresholded the combined output utilizing records from across the 4 spring months.
For validation statistics, we calculated area under the ROC curve (AUC), true skill statistic (TSS), overall accuracy, specificity, and sensitivity for each model using a jackknife procedure dropping records from geographically clustered groups of points (Hamilton et al., 2022). Also, we generated functional response plots for the 10 most important environmental predictor variables in each model using the partialPlot function of the randomForest R package.
We calculated overlap and unique spring and fall areas of habitat by overlaying the binary single spring and fall models, using only the area where predictions were made for both seasons in the westernmost 7 states (i.e., not including the portions of Montana, Wyoming, Colorado, and New Mexico that were modeled only for the fall).
We evaluated the stewardship of government and other agencies by overlaying the PAD-US 3.0 (USGS GAP, 2022) layer on the single spring and fall binary models. We considered GAP statuses 1 and 2 to represent areas managed for conservation and GAP status 3 to represent areas managed for multiple uses. We then calculated the percentage of the single spring and fall habitat that was managed by federal, state, and local governmental agencies and NGOs, and then separately across the major federal land management agencies for each of these two protection statuses. We did not consider tribal lands as these are not well delineated in the PAD-US data layer.
Recognizing that the BLM manages a substantial portion of the western U.S. and that land use decisions are made at the field office level, we performed an analysis to identify the most important areas for migrating monarchs within each field office unit. The BLM divides each of the 7 focal states into 9-22 nonoverlapping field office units, which collectively cover the entire land area of these states regardless of the amount of land in each managed by the agency. We first identified the top 10% of model suitability scores in each field office unit separately for the fall and spring seasons. We called any part of this area already identified as habitat in the binary model described above as being of “regional” significance. We called the remaining portion of the area with the 10% highest suitability as being of “local” significance.
From 30,067 initial records, the locality data sources yielded 8,473 unique fall monarch records and 1,716 unique spring records that fulfilled our criteria. Maps of the monarch records, Nymphalid bias map, and relative fall nectar plant densities are available in the Supplementary Figures 1-3. Monthly spring models show temporal expansion of monarchs across the western U.S. from March through June (Figures 1, 2). Monarch migration habitat is concentrated in the Coast Range and northern Central Valley of California in March and April before appearing across much of the central and northern portion of the range in the Willamette Valley, Oregon; the Columbia Plateau, Washington; Snake River, Idaho; west-central Nevada, and the Rocky Mountain foothills east of Great Salt Lake, Utah, in May. By June, habitat in California becomes less emphasized and migration habitat appears in Arizona.
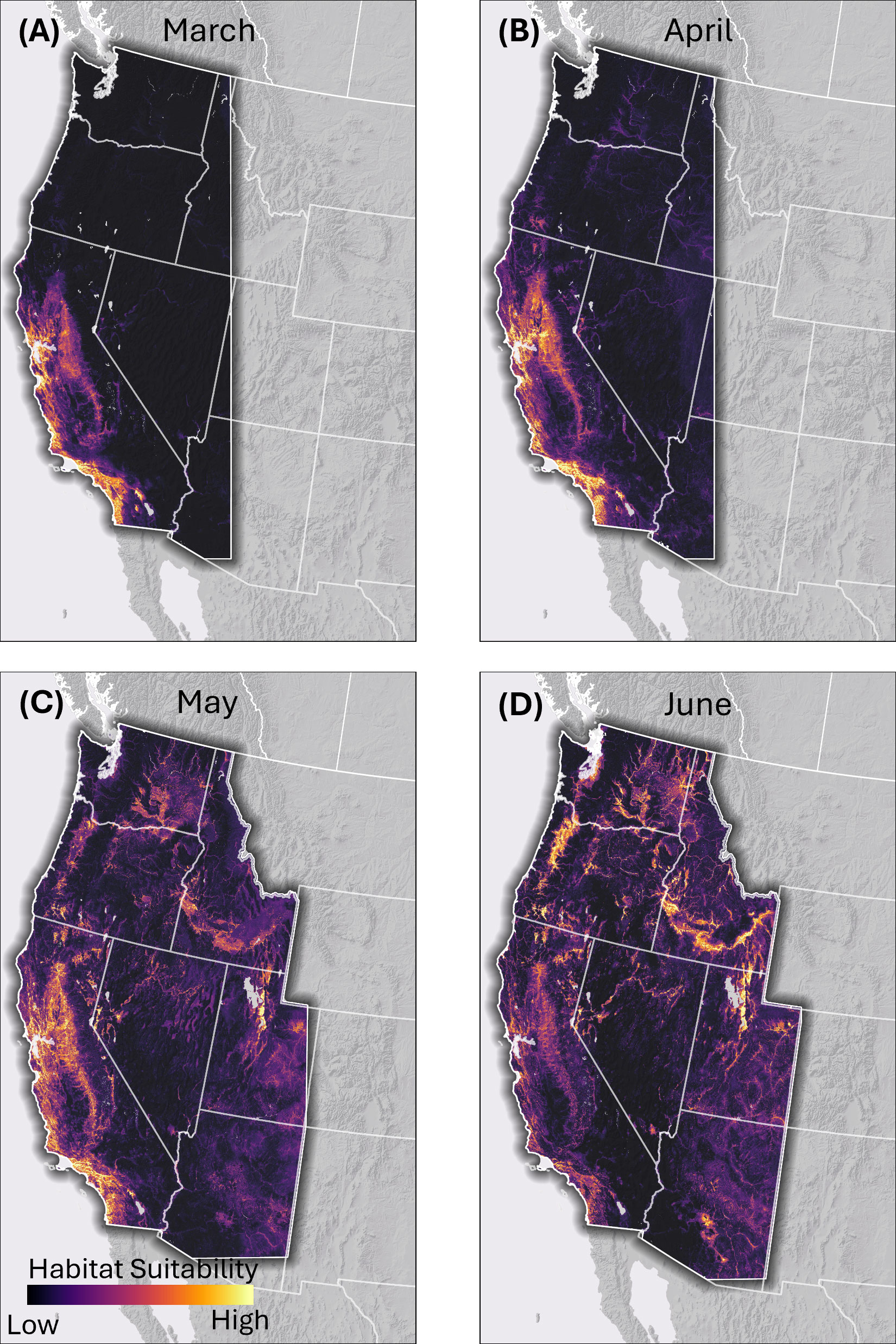
Figure 1. Continuous habitat suitability model output for migrating western monarch habitat in the spring based on the output of random forest modeling. (A) March, (B) April, (C) May, (D) June. Lighter colors represent areas of higher predicted suitability and darker areas lower suitability in relation to similarity to locations monarchs have been documented at during the spring period.
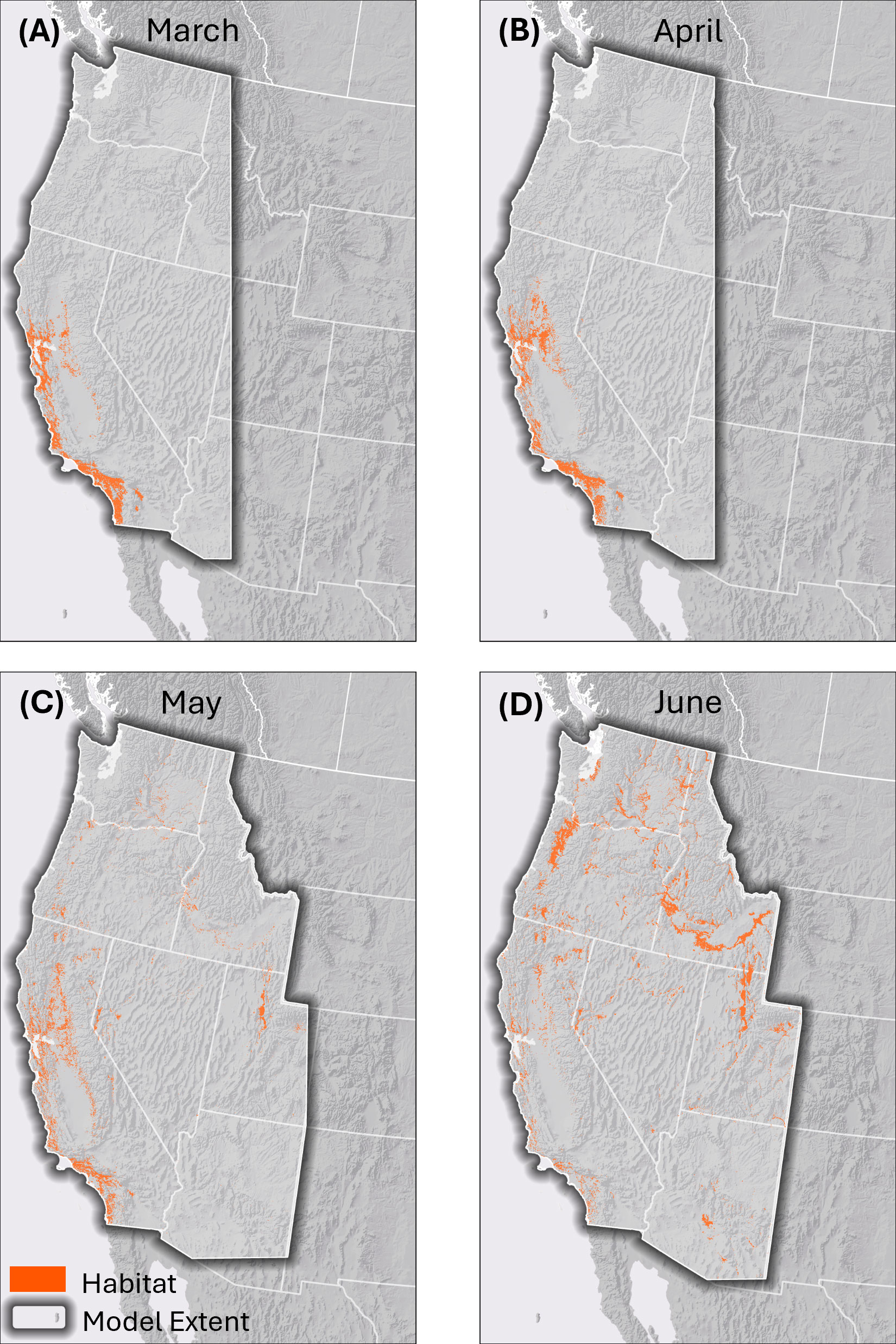
Figure 2. Thresholded binary habitat models for western monarchs across the spring migration season. (A) March, (B) April, (C) May, (D) June. These maps represent areas of high predicted suitability based on a threshold of the continuous model output (Figure 1) that minimizes the probability of false positives, in order to highlight the most likely areas of suitability across the broad extent of the modeling area.
The fall model shows concentrations of habitat in similar areas to the spring models with the addition of areas in western Colorado, the Wasatch and Uinta Mountains in Utah, and the Apache Highlands in Arizona (Figure 3). The combined, thresholded binary spring and fall model outputs are shown in Figure 4.
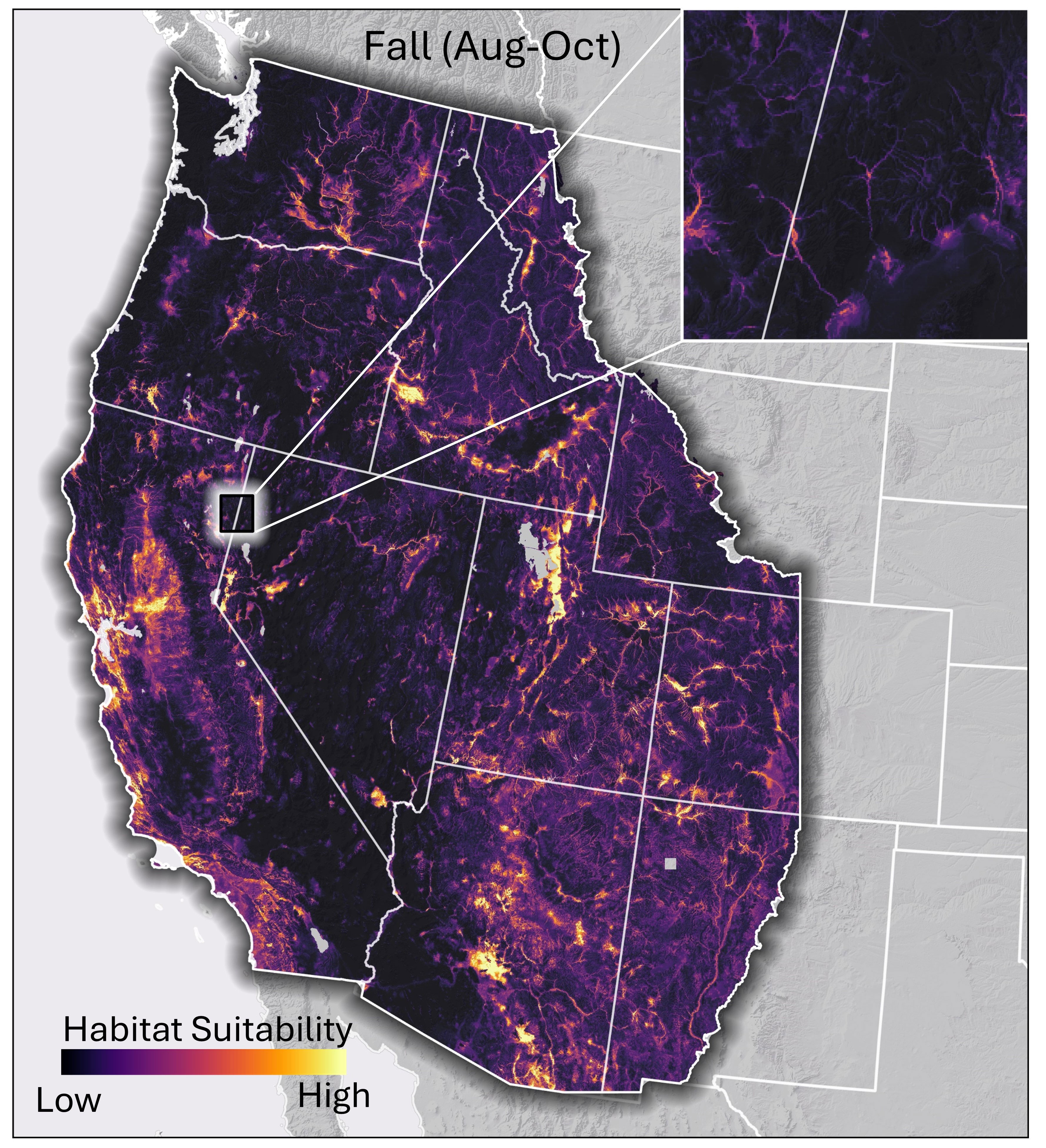
Figure 3. Continuous habitat suitability model output for migrating western monarch habitat in the fall based on the output of random forest modeling. Lighter colors represent areas of higher predicted suitability and darker areas lower suitability in relation to similarity to locations monarchs have been documented at during the fall period. Inset map illustrates habitat predictions in riparian areas.
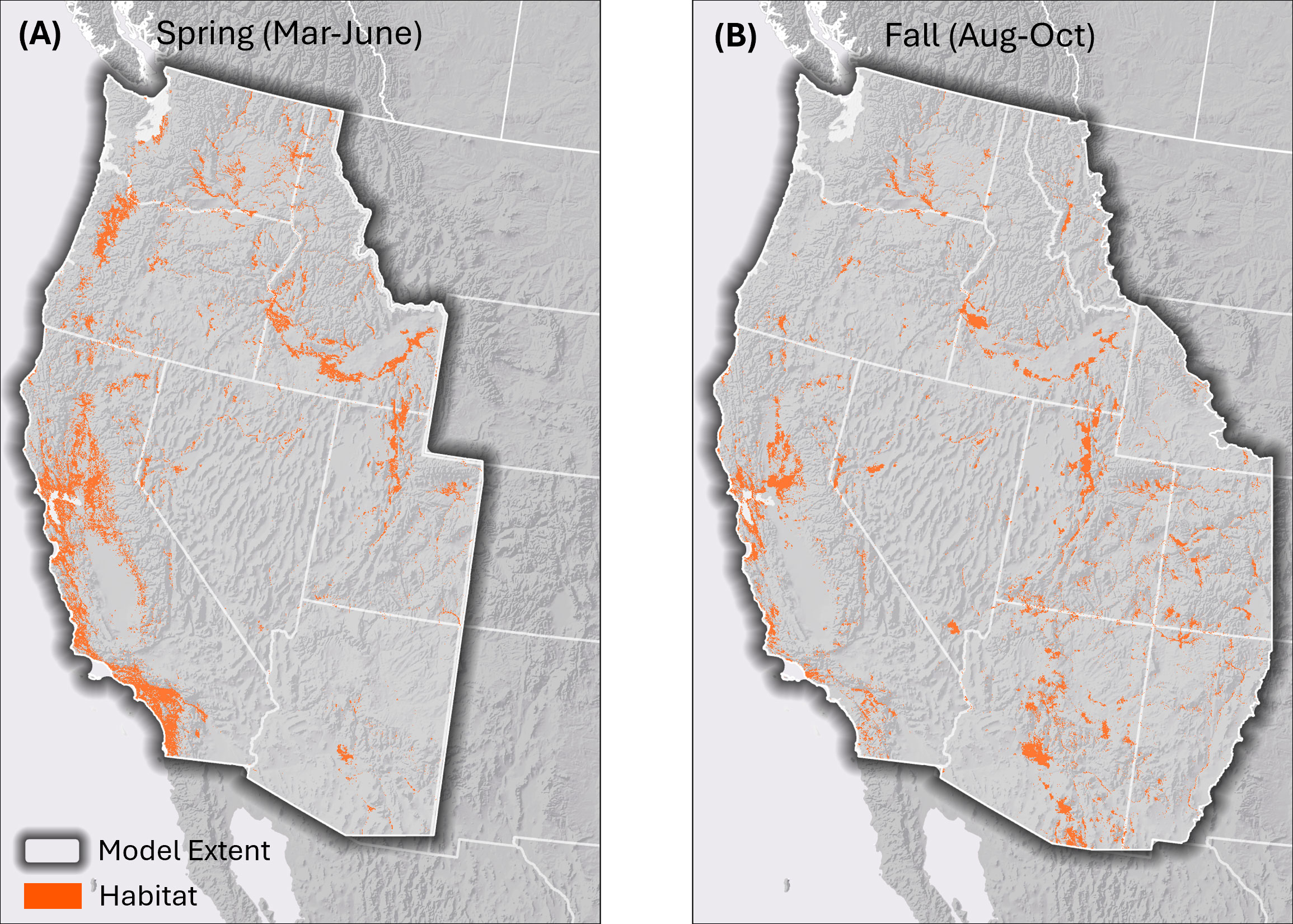
Figure 4. Single binary (A) spring and (B) fall habitat models for western monarchs during the migration seasons. These maps represent areas of high predicted suitability based on a threshold of the continuous model output (Figure 1) that minimizes the probability of false positives, in order to highlight the most likely areas of suitability across the broad extent of the modeling area.
The validation statistics showed generally good performance, with AUC values ranging from 0.87-0.96 (Table 2).
The environmental predictors with the greatest importance in the models varied seasonally (Supplementary Figures 4-8; functional response plots in Supplementary Figures 9-13). In March and April, climatic predictors including temperature, precipitation, and climatic water deficit were all among the most influential in the models. Distance to the ocean was also important in these early months of the migration. In April, summed milkweed habitat suitability first appeared as an important predictor, and this variable increased in importance for the May and June models. In May and June, fewer climatic predictors were important than earlier in the season, and ecological predictors such as coniferous forest and canopy cover (for which a lack of such cover was important in predicting habitat), distance to streams, and distance to woody wetlands became important.
For the fall model, nectar abundance was the most important predictor. Climatic and ecological variables, as well as distance to ocean, were also important (Supplementary Figure 8; functional response plot in Supplementary Figure 13). All three important climatic variables related to moisture: climatic water deficit and precipitation in wettest and driest quarters. Shrub cover measured at two different scales (10- and 100-cell means around a central focal raster cell); was the most important ecological variable for this model.
The area of predicted habitat for the binary output is 121,427 km2 in the spring and 89,287 km2 in the fall. Of the areas predicted for monarch migratory habitat in both seasons in the 7 westernmost states, 45% is habitat only for spring migrants, 26% is habitat only for fall migrants, and 29% is habitat for both migrations. Predicted habitat for the spring only is concentrated in the Willamette Valley, Oregon, along the northern and eastern margins of the Central Valley, along the Coast Range, and in southern California (Supplementary Figure 14). Habitat predicted uniquely for the fall was concentrated in river valleys, many with irrigated agricultural areas (and not in forested mountain ranges), in central and southern Utah south through central Arizona (Supplementary Figure 14).
Federal agencies own 11.7% of the habitat indicated by the spring model and 23.5% of the habitat indicated by the fall model (Figure 5, complete details in Supplementary Table 2). Among the federal agencies, the BLM owns the greatest amount, 5.9% of spring and 9.7% of fall habitat, followed by the U.S. Forest Service (3.3% and 9.2%, respectively) (Figure 5). State and local governmental agencies and NGOs own much less habitat, ranging from 0.6-2.7% combined of the habitat for each season (Figure 5). Collectively, governmental agencies and NGOs own 15.9% of the spring migration habitat and 27.5% of the fall migration habitat (Supplementary Table 2). All remaining habitat is under private ownership. Notably, the majority of government and NGO-owned habitat is managed for multiple uses rather than conservation (Figure 5). Across all stewardship classes (other than private), only 46.0% of spring and 36.5% of fall habitat is managed expressly for conservation.
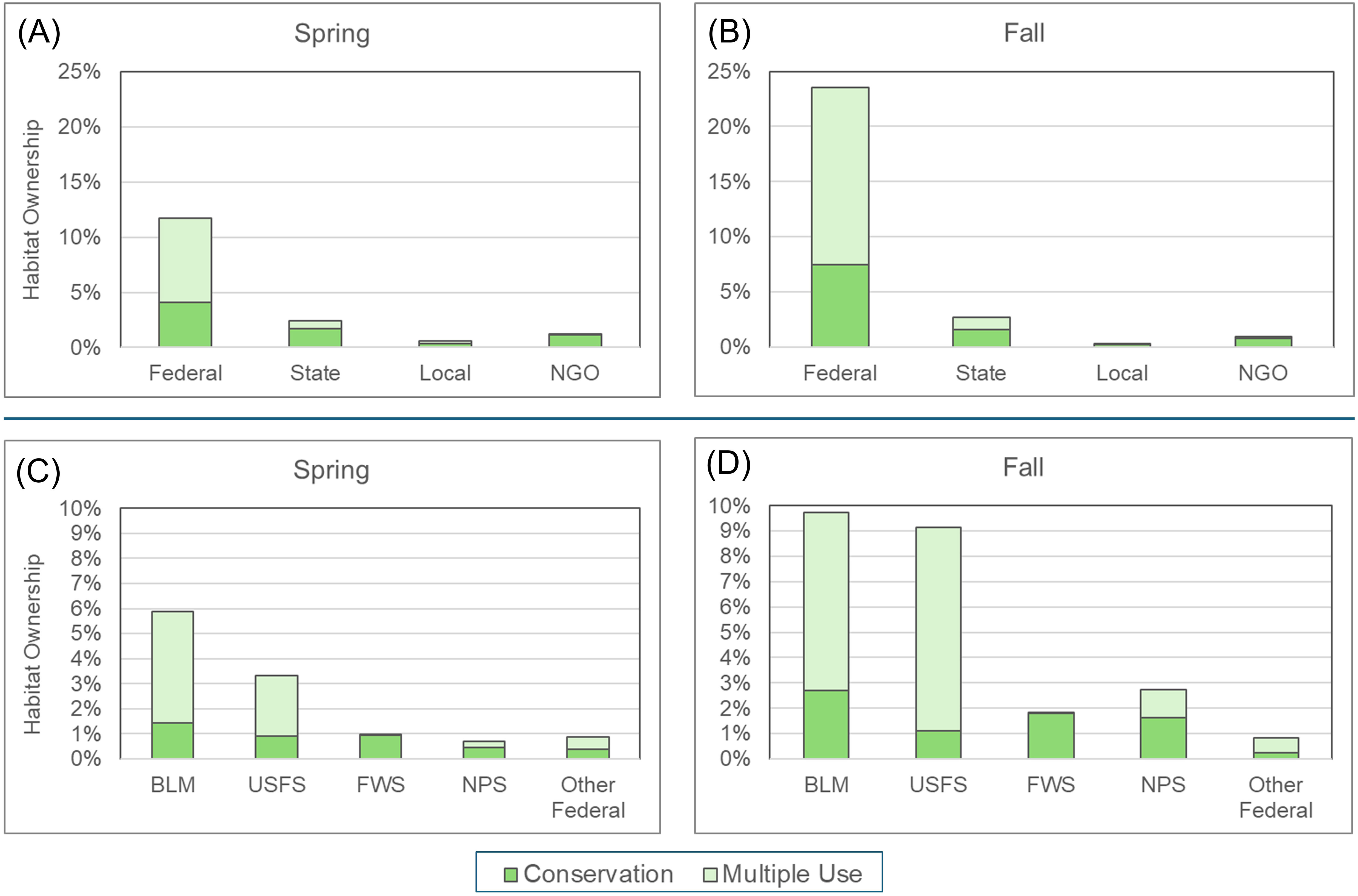
Figure 5. Stewardship of habitat for spring and fall migrating western monarchs. Top: major management classes for (A) spring and (B) fall migration. All habitat not covered by any of these classes is presumed to be under private ownership. Bottom: details on federal government agency management for (C) spring and (D) fall migration. Areas designated for conservation are from the Protected Areas Database (PAD) Gap categories 1 and 2; areas designated for multiple use are PAD Gap category 3. NGO, non-governmental organization; BLM, Bureau of Land Management; USFS, United States Forest Service; FWS, Fish and Wildlife Service; NPS, National Park Service. “Other Federal” includes, for example, the Department of Defense and Bureau of Reclamation.
Model output shows areas of locally important habitat for migrating western monarchs in each BLM administrative unit, filling gaps in regions lacking habitat of regional importance (Figure 6). Overall, the model output for combined regional and locally important habitat were similar for the spring and fall migration. In the spring, the parts of California near the overwintering sites (Coast Range, northern Central Valley, and southern California) are of regional importance whereas during the fall many of these same areas are highlighted as being of local importance. East of the Sierra Nevada and Cascade Mountains, most of the areas highlighted as being of either regional or local importance are associated with rivers or riparian areas (Figure 6).
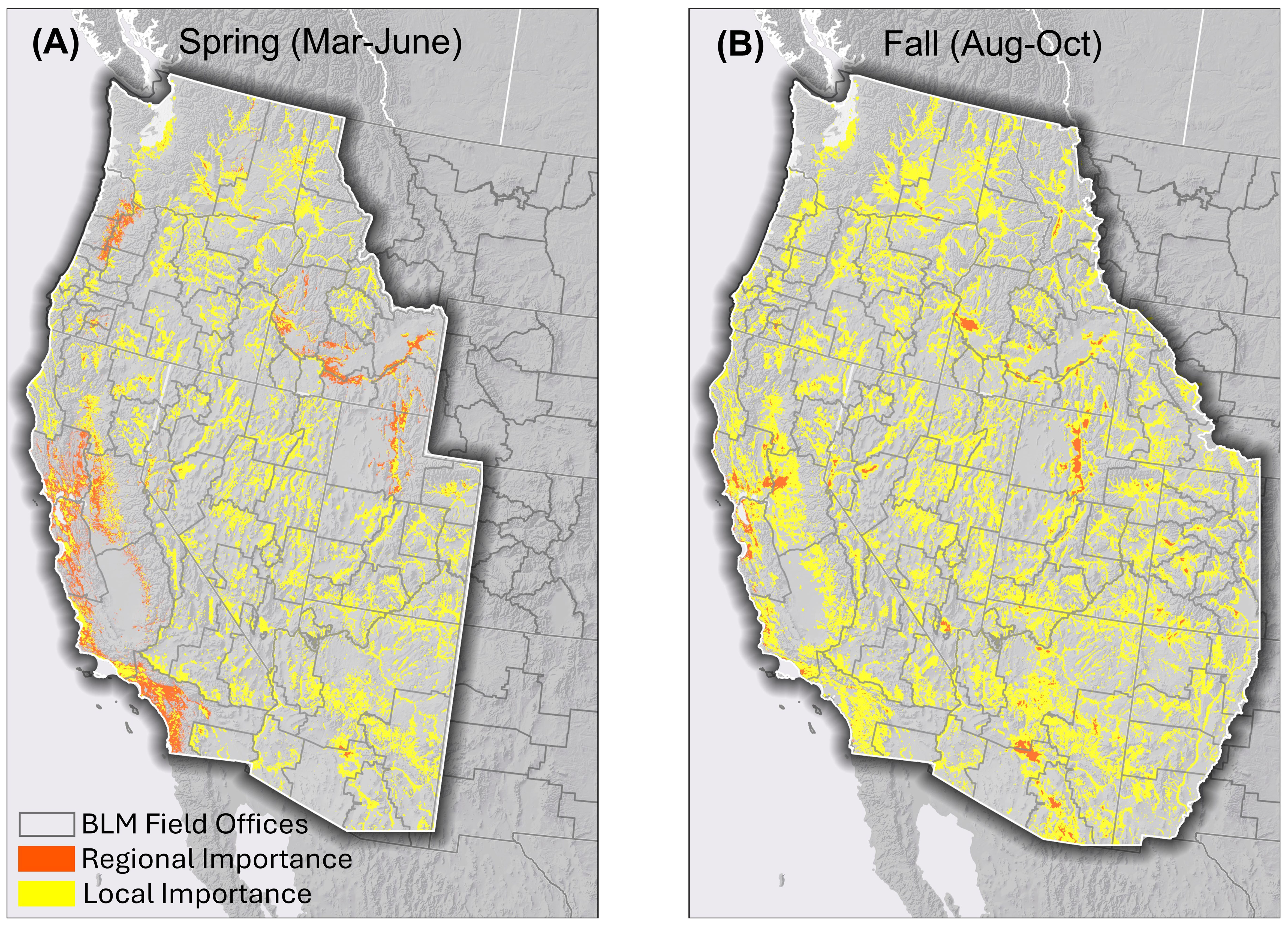
Figure 6. Areas of regional and local importance for (A) spring and (B) fall migrating western monarchs for Bureau of Land Management land managers. Areas of regional importance are the same as those shown in Figure 4. Areas of local importance are the portion of the most suitable 10% of habitat (upper 10% of predicted model scores) within each BLM field office unit that is not of regional importance.
This study produced, for the first time at such a high resolution (330 m), models of habitats that are important to migrating western monarchs, and at the same time, an analysis of land stewardship of migration habitat (Pelton et al., 2019). The models revealed that, according to the thresholding approach we used, western monarchs use somewhat different areas in the spring than in the fall. We found that most migratory habitat is on private land, in contrast to the overall land tenure pattern of the federal government managing more than half of the land. Among governmental and non-governmental agencies, the BLM and USFS have the greatest stewardship of this habitat. Land managers can use these results (see Data Availability statement for access to GIS versions) to inform parcel-level decisions for migrating monarchs (Xerces Society, 2018) such as reduce pesticide use; protect riparian zones; restore and enrich milkweed and floral resources, especially in drought years when nectar availability is reduced (Brower et al., 2015); and provide shrubs and trees for roosting and shade (Pelton et al., 2019).
Although we reduced bias toward human population centers and thus nonmigratory monarchs, fully controlling for the spatial bias in citizen science records would be challenging for this species. Thus, our models may still retain some bias towards areas with high densities of citizen science observations from urban areas in California, Arizona, and Utah. In addition to the large number of observers, human population centers in the desert ecoregions of the arid western U.S. have numerous irrigated parks and gardens that artificially attract monarchs due to increased milkweed and nectar plant availability compared to intervening drier natural areas. Resolving this issue was beyond the scope of this study, which is aimed at managers of public lands, few of which occur in population centers. Finer scale predictions of migration habitat near more populated areas are likely to vary depending on how resident and migrating monarchs are treated and the land use layers incorporated. In addition, results in sparsely populated areas such as the Great Basin may be sensitive to the approach to bias correction taken as well as the lower density of observations within these areas.
Our results broadly coincide with previous descriptions of western monarch migration, with some important differences. The monthly, North America-wide models of Castañeda et al. (2019) show a similar spring expansion from the California coast. However, that model showed virtually the entire Central Valley as providing habitat in March whereas the present model showed habitat being restricted to the northern and eastern portions of that area. Also, the Castañeda et al. (2019) model did not predict habitat outside of California in May. Monarchs clearly are more widespread in May since we compiled 182 May records of monarchs outside of California and consequently our model predicted areas of migratory habitat from across the range. The Dilts et al. (2019) model for the entire non-overwintering period highlighted most of the same areas as ours did, although the former model predicted medium suitability habitat in areas of the Sonora Desert in southeastern California and southeastern Washington where ours did not. The MaxEnt algorithm used by Dilts et al. (2019) can predict areas of habitat further from reference records than random forests (Fitzgibbon et al., 2022; Zhao et al., 2022). Notably, there appeared to be no reference records in these two areas (Dilts et al., 2019). Our finding that in Idaho, the Snake River Plain is the most important habitat for migrating monarchs was remarkably similar to a previous modeling effort focused on that state (Svancara et al., 2019). Also, distance to water predictors were important in both exercises.
A striking finding is the near absence of predicted areas of regional importance for migrating monarchs in the Great Basin of Nevada and western Utah. Few records of monarchs from the migratory seasons exist from this region (380 across both spring and fall in our compilation). Monarch records prior to 2005 appeared to be as common in the Great Basin as almost anywhere else in the range in an earlier compilation (Dingle et al., 2005), which suggests a possible decline in monarch passage through that region. In the future, it would be interesting to identify areas of potential historical importance based on older records which may be little occupied currently. Our identification of important areas for migratory monarchs occurring on the northern and eastern edges of the Great Basin and foothills of the Rocky Mountains poses the question of where these migrants are going to and from. The model results suggest that a direct migration between Idaho/Utah and the California coast may be taking place to a lesser extent than in the past (Crone et al., 2019).
Observations of tagged monarchs from Washington moving southeast in the fall (James and Kappen, 2021) raise the possibility that some individuals migrate from the northern portion of the range through eastern Utah and then either west through Arizona to the California coast, bypassing most of the Great Basin, or south to central Mexico. The spring model outputs are consistent with monarchs following one or both of these routes in reverse during that season. Tagging studies of fall monarchs in Idaho and Utah could further clarify details about migratory monarchs in this part of the range. Targeted surveys for migrating monarchs within the Great Basin would also be helpful to confirm that the low density of records and corresponding predicted habitat reported here represent accurate depictions of the migration and are not an artifact of sparse observer activity.
The stewardship analysis revealed that nearly 85% of spring and over 70% of fall migratory habitat is under private ownership, in contrast to the high degree of federal land ownership in the region. The Coast Range and Central Valley, both of which are primarily privately owned, are of major importance for the first spring generation (Crone et al., 2019) and provide the last habitats that most fall migrating western monarchs cross before arriving at their coastal overwintering areas. Further, monarch migration habitat coincides with major agricultural production areas in the western U.S.: the Central Valley (California), Willamette Valley (Oregon), Columbia Plateau (Washington), Snake River Plain (Idaho), Footslopes of the Wasatch Front (Utah), and the greater Phoenix area (Arizona). Future modeling efforts may wish to further explore this relationship by considering NDVI, which would identify irrigated land, as a predictor variable (Maselli et al., 2020). The situation for western monarchs is therefore similar to that of eastern monarchs, which also occur primarily on private agricultural land (Spaeth et al., 2022). Given the broad scope of private land ownership across the migratory range of the western monarch, outreach efforts such as land stewardship assistance to promote healthy habitat and reduce threats for monarchs on private lands will be key to the survival of the population (Xerces Society, 2018).
On federal land, the BLM and USFS are the agencies with the greatest responsibility for overseeing habitat for migrating western monarchs. The mandates of both the BLM and USFS include sustaining productivity and multiple use, and thus it is not surprising that the majority of western monarch migration habitat managed by these agencies is designated as multiple use. Managers of these agencies must leverage the tools at their disposal to ensure that uses such as livestock grazing, recreation, and logging incorporate practices that protect and restore monarch habitat. Encouragingly, the BLM announced on 18 April 2024 a new Public Lands Rule that, for the first time, places conservation on equal footing with other uses (BLM, 2024), giving managers of multiple use lands more authority to prioritize actions that benefit at-risk species such as the monarch.
Recent counts of monarchs at the California overwintering sites reveal that the population, while fluctuating in recent years, is far less numerous than in the 1980s (Pelton et al., 2019; Crone and Schultz, 2021; James, 2024). Indeed, the U.S. Fish and Wildlife Service determined that the species warrants listing as a threatened or endangered species and added it to their candidate list (USFWS, 2020). The decline imperils a highly visible, iconic migratory phenomenon that has few parallels among North American insects. The detailed maps of habitat for migrating monarchs described here contribute to the conservation toolbox available to practitioners on both public and private lands.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://natureserve.maps.arcgis.com/home/item.html?id=bdef109d5111490ba7cd01c9fec3d899.
The manuscript presents research on animals that do not require ethical approval for their study.
PM: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. HC: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. BY: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the National Fish and Wildlife Foundation grant 0126.21.073611.
We thank staff at the BLM, California Department of Fish and Wildlife, Monarch Joint Venture, U.S. Fish and Wildlife Service, and the Xerces Society for Invertebrate Conservation for fruitful conversations about the design and implementation of this study. We thank the following for providing locality data for monarchs, the milkweed layer, and list of fall nectar plants: Arizona Heritage Data Management System, Butterflies and Moths of North America, Global Biodiversity Information Facility, Idaho Natural Heritage Program, Journey North, Monarch Joint Venture, Monarch Larval Monitoring Project, SCAN-Bugs, Southwest Monarch Study, Western Monarch Milkweed Mapper. We thank T. Cornelisse, E. Pelton, M. Tarjan, and two reviewers for helpful comments on previous drafts of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government or the National Fish and Wildlife Foundation and its funding sources. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government, or the National Fish and Wildlife Foundation, or its funding sources.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1460363/full#supplementary-material
Anderson R. P. (2003). Real vs. artefactual absences in species distributions: tests for Oryzomys albigularis (Rodentia: Muridae) in Venezuela. J. Biogeogr. 30, 591–605. doi: 10.1046/j.1365-2699.2003.00867.x
Billings J. (2019). Opening a window on southwestern monarchs: fall migrant monarch butterflies, Danaus plexippus (L.), tagged synchronously in southeastern Arizona migrate to overwintering regions in either southern California or central Mexico. J. Lepid. Soc 73, 257–267. doi: 10.18473/lepi.73i4.a1
Brower L. P., Fink L. S., Kiphart R. J., Pocius V., Zubieta R. R., Ramírez M. I. (2015). “Effect of the 2010–2011 drought on the lipid content of monarchs migrating through Texas to overwintering sites in Mexico,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly. Eds. Oberhauser K. S., Nail K. R., Altizer S. (Cornell Univ. Press, Ithaca, NY), 117–129.
Bureau of Land Management (BLM) (2024). Public lands rule. Available online at: https://www.blm.gov/public-lands-rule (Accessed May 28, 2024).
California Department of Fish and Wildlife (CDFW) (2023). California Natural Diversity Database (CNDDB). Available online at: https://map.dfg.ca.gov/rarefind/view/RareFind.aspx (Accessed April 26, 2023).
Castañeda S., Botello F., Sánchez-Cordero V., Sarkar S. (2019). Spatio-temporal distribution of monarch butterflies along their migratory route. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00400
Chen C., Liaw A., Breiman L. (2004). Using random forest to learn imbalanced data. Technical report (Berkely, CA: Univ. California, Berkeley, Dept. of Statistics). Available at: https://statistics.berkeley.edu/sites/default/files/tech-reports/666.pdf.
Congressional Research Service (2020). Federal land ownership: overview and data (Washington, D.C: United States Congress). Available at: https://sgp.fas.org/crs/misc/R42346.pdf.
Crone E. E., Pelton E. M., Brown L. M., Thomas C. C., Schultz C. B. (2019). Why are monarch butterflies declining in the West? Understanding the importance of multiple correlated drivers. Ecol. Appl. 29, e01975. doi: 10.1002/eap.1975
Crone E. E., Schultz C. B. (2021). Resilience or catastrophe? A possible state change for monarch butterflies in western North America. Ecol. Lett. 24, 1533–1538. doi: 10.1111/ele.13816
Crossley M. S., Meehan T. D., Moran M. D., Glassberg J., Snyder W. E., Davis A. K. (2022). Opposing global change drivers counterbalance trends in breeding North American monarch butterflies. Glob. Change Biol. 28, 4726–4735. doi: 10.1111/gcb.16282
Crouse D. T., Crowder L. B., Caswell H. (1987). A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1223. doi: 10.2307/1939225
Cvitanovic C., McDonald J., Hobday A. J. (2016). From science to action: Principles for undertaking environmental research that enables knowledge exchange and evidence-based decision-making. J. Environ. Manage. 183, 864–874. doi: 10.1016/j.jenvman.2016.09.038
Dilts T. E., Steele M. O., Engler J. D., Pelton E. M., Jepsen S. J., McKnight S. J., et al. (2019). Host plants and climate structure habitat associations of the western monarch butterfly. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00188
Dingle H., Zalucki M. P., Rochester W. A., Armijo-Prewitt T. (2005). Distribution of the monarch butterfly, Danaus plexippus (L.) (Lepidoptera: Nymphalidae), in western North America. Biol. J. Linn Soc 85, 491–500. doi: 10.1111/j.1095-8312.2005.00512.x
Fitzgibbon A., Pisut D., Fleisher D. (2022). Evaluation of maximum entropy (Maxent) machine learning model to assess relationships between climate and corn suitability. Land 11, 1382. doi: 10.3390/land11091382
Hamilton H., Smyth R. L., Young B. E., Howard T. G., Tracey C., Breyer S., et al. (2022). Increasing taxonomic diversity and spatial resolution clarifies opportunities for protecting imperiled species in the US. Ecol. Appl. 32, e2534. doi: 10.1002/eap.2534
Howard E., Davis A. K. (2008). The fall migration flyways of monarch butterflies in eastern North America revealed by citizen scientists. J. Insect Conserv. 13, 279–286. doi: 10.1007/s10841-008-9169-y
Hulme P. E. (2014). Bridging the knowing-doing gap: know-who, know-what, know-why, know-how and know-when. J. Appl. Ecol. 51, 1131–1136. doi: 10.1111/1365-2664.12321
James D. G. (2024). Monarch butterflies in western North America: a holistic review of population trends, ecology, stressors, resilience and adaptation. Insects 14, 40. doi: 10.3390/insects15010040
James D. G., Kappen L. (2021). Further insights on the migration biology of monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) from the Pacific Northwest. Insects 12, 161. doi: 10.3390/insects12020161
James D. G., Schaefer M. C., Easton K. K., Carl A. (2021). First Population study on winter breeding monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the urban south bay of San Francisco, California. Insects 12, 946. doi: 10.3390/insects12100946
Kesler K. K., Bunch R. (2023). Mapping the migration: A western monarch butterfly site suitability study. Int J Appl Geospatial Res 14, 1–22. doi: 10.4018/ijagr.316769
Lukens L., Thieme J., Thogmartin W. E. (2024). Milkweed and floral resource availability for monarch butterflies (Danaus plexippus) in the United States. Front. in. Ecol. Evol. 12. doi: 10.3389/fevo.2024.1330583
Mair L., Ruete A. (2016). Explaining spatial variation in the recording effort of citizen science data across multiple taxa. PloS One 11, e0147796. doi: 10.1371/journal.pone.0147796
Martin T. G., Chadès I., Arcese P., Marra P. P., Possingham H. P., Norris D. R. (2007). Optimal conservation of migratory species. PloS One 2, e751. doi: 10.1371/journal.pone.0000751
Maselli F., Chiesi M., Angeli L., Fibbi L., Rapi B., Romani M., et al. (2020). An improved NDVI-based method to predict actual evapotranspiration of irrigated grasses and crops. Agr. Water Manage. 233, 106077. doi: 10.1016/j.agwat.2020.106077
Morris G. M., Kline C., Morris S. M. (2015). Status of Danaus plexippus population in Arizona. J. Lepid. Soc 69, 91–107. doi: 10.18473/lepi.69i2.a10
Pelton E. M., Schultz C. B., Jepsen S. J., Black S. H., Crone E. E. (2019). Western monarch population plummets: status, probable causes, and recommended conservation actions. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00258
Phillips S. J., Dudík M., Elith J., Graham C. H., Lehmann A., Leathwick J., et al. (2009). Sample selection bias and presence-only distribution models: implications for background and pseudoabsence data. Ecol. Appl. 19, 181–197. doi: 10.1890/07-2153.1
Ponder W. F., Carter G. A., Flemons P., Chapman R. R. (2001). Evaluation of museum collection data for use in biodiversity assessment. Conserv. Biol. 15, 648–657. doi: 10.1046/j.1523-1739.2001.015003648.x
Raymond C. M., Lechner A. M., Lockwood M., Carter O., Harris R. M. B., Gilfedder L. (2015). Private land manager capacity to conserve threatened communities under climate change. J. Environ. Manage. 159, 235–244. doi: 10.1016/j.jenvman.2015.04.048
R Core Team (2022). R 4.2: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Reynolds M. D., Sullivan B. L., Hallstein E., Matsumoto S., Kelling S., Merrifield M., et al. (2017). Dynamic conservation for migratory species. Sci. Adv. 3, e1700707. doi: 10.1126/sciadv.1700707
Runge C. A., Martin T. G., Possingham H. P., Willis S. G., Fuller R. A. (2014). Conserving mobile species. Front. Ecol. Environ. 12, 395–402. doi: 10.1890/130237
Spaeth K. E. Jr, Barbour P. J., Moranz R., Dinsmore S. J., Williams C. J. (2022). Asclepias dynamics on US rangelands: implications for conservation of monarch butterflies and other insects. Ecosphere 13, e03816. doi: 10.1002/ecs2.3816
Steele C., Ragonese I. G., Majewska. A. A. (2023). Extent and impacts of winter breeding in the North American monarch butterfly. Curr. Opin. Insect Sci. 59, 101077. doi: 10.1016/j.cois.2023.101077
Svancara L. K., Abatzoglou J. T., Waterbury B. (2019). Modeling current and future potential distributions of milkweeds and the monarch butterfly in Idaho. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00168
Tiago P., Ceia-Hasse A., Marques T. A., Capinha C., Pereira H. M. (2017). Spatial distribution of citizen science casuistic observations for different taxonomic groups. Sci. Rep. 7, 12832. doi: 10.1038/s41598-017-13130-8
U.S. Fish and Wildlife Service (USFWS) (2020). Endangered and threatened wildlife and plants; 12-month finding for the monarch butterfly. Federal Register 85, 81813–81822.
U.S. Geological Survey Gap Analysis Project (USGS GAP) (2022). Protected Areas Database of the United States (PAD-US) 3.0: U.S. Geological Survey data release. doi: 10.5066/P9Q9LQ4B
Valavi R., Elith J., Lahoz-Monfort J. J., Guillera-Arroita G. (2021). Modelling species presence-only data with random forests. Ecography 44, 1731–1742. doi: 10.1111/ecog.05615
Venables W. N., Ripley B. D. (2002). Modern Applied Statistics with S. 4th ed. (New York: Springer). Available at: https://www.stats.ox.ac.uk/pub/MASS4/.
Xerces Society (2018). Managing for monarchs in the West: best management practices for conserving the monarch butterfly and its habitat (Portland, OR: The Xerces Society for Invertebrate Conservation). Available at: https://www.xerces.org/sites/default/files/2018-06/18-009_01-Monarch_BMPs_Final_Web.pdf.
Young B. E., Dodge N., Hunt P. D., Ormes M., Schlesinger M. D., Shaw H. Y. (2019). Using citizen science data to support conservation in environmental regulatory contexts. Biol. Conserv. 237, 57–62. doi: 10.1016/j.biocon.2019.06.016
Zhao Z., Xiao N., Shen M., Li J. (2022). Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total Environ. 842, 156867. doi: 10.1016/j.scitotenv.2022.156867
Keywords: Bureau of Land Management, citizen science, milkweed, nectar plants, public lands, species habitat models, threatened species
Citation: McIntyre PJ, Ceasar H and Young BE (2024) Mapping migration habitat for western monarch butterflies reveals need for public-private approach to conservation. Front. Ecol. Evol. 12:1460363. doi: 10.3389/fevo.2024.1460363
Received: 05 July 2024; Accepted: 15 October 2024;
Published: 20 November 2024.
Edited by:
Junhong Bai, Beijing Normal University, ChinaReviewed by:
Darius Semmens, United States Department of the Interior, United StatesCopyright © 2024 McIntyre, Ceasar and Young. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruce E. Young, YnJ1Y2VfeW91bmdAbmF0dXJlc2VydmUub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.