- 1School of Biotechnology, Kalinga Institute of Industrial Technology (KIIT), Bhubaneswar, India
- 2Huck Institute of Life Sciences, Pennsylvania State University, University Park, PA, United States
- 3School of Biological Sciences, University of Edinburgh, Edinburgh, United Kingdom
Introduction: How selection influences phenotypic plasticity is an important question in evolutionary biology. We report an experimental evolution study that examined how prolonged selection at cold vs. warm temperature impacts the thermal plasticity of traits like reproductive output, body size, and body water content in Drosophila melanogaster.
Methods: We conducted the study on two sets of large, outbred fly populations: one maintained at the standard fly rearing temperature, i.e., 25°C, and another selected at cold temperature, i.e., 17°C, for 3.5 years. Both selection lines were derived from the same ancestral population.
Results and discussion: We found that while 25°C selected females lay significantly fewer eggs in cold compared to warm or optimal rearing temperature of 25°C, the 17°C selected females have consistent or canalized fecundity levels across warm and cold conditions. Sustained fecundity levels across cold and warm conditions are potential marks of adaptation to a broader thermal range. While phenotypic plasticity may aid in adaptation to new environments, for traits such as fecundity, consistent levels across environments, that is, low plasticity, may be more adaptive. We also found that male flies from cold vs. warm selection regimes differ in their thermal plasticity. Plasticity of dry weight and body water content was reduced in the cold-selected males, indicating the evolution of canalized levels for these traits too. While canalized fecundity levels across temperatures can potentially help in thermal adaptation, the significance of reduced plasticity of male body size and water content needs to be investigated in the future.
Introduction
Temperature induces both plastic and evolutionary changes in organisms. Short-term or within-generation effect of temperature on a trait, that is, thermal plasticity, is observed in a wide variety of biological traits. Thermal plasticity is often viewed as an inevitable effect of temperature on different biological parameters like growth, metabolism, and physiology (Van Der Have and De Jong, 1996; Angilletta and Dunham, 2003; Zuo et al., 2012; Ghosh et al., 2013). Nonetheless, there is ample empirical evidence that suggests thermal plasticity could be adaptive, and different species harbor genetic variation controlling how a trait is influenced by temperature. Therefore, thermal plasticity can be shaped by selection (Nettle and Bateson, 2015; van Heerwaarden and Sgrò, 2017; McDonald et al., 2018; Lafuente et al., 2018; MacLean et al., 2019).
Whether selection can alter the level of plasticity for a trait is an interesting question in evolutionary biology. The impact of selection on plasticity is context-dependent (Leonard and Lancaster, 2020; Barley et al., 2021; Carbonell et al., 2021; Schaum et al., 2022). How selection changes the level of plasticity of a trait in a given population would depend on various factors, such as a) if the population is inhabiting a stable or unpredictable environment, b) if plasticity for the trait incurs any energetic cost in its bearers, c) if there is genetic variation for the extent of phenotypic plasticity in the trait and whether or not there are genetic constraints influencing plasticity of the trait, and d) whether high or low plasticity is favored for that given trait.
Direct selection for high or low plasticity may alter the plasticity of a trait. Moreover, selection for a trait per se—and not its plasticity—can also lead to evolved changes in the plasticity of the trait as a by-product of selection. For empirical investigation of the effect of selection on plasticity, temperature can be a suitable factor as it engenders both proximate and ultimate changes, reflecting thermal plasticity and thermal evolution. Ectothermic organisms experience a greater impact of temperature because they do not have internal thermoregulation the way endotherms do. The ectotherm Drosophila is particularly well-suited for studies focusing on temperature-induced plasticity and evolution, because of its a) amenability to studies manipulating temperature in the laboratory and (b) wide geographic range across latitudes and continents covering thermally diverse regions (James et al., 1997; Trotta et al., 2006; Mayekar et al., 2023). In Drosophila, cold developmental temperature leads to slow growth and metamorphosis, which translates into the emergence of bigger flies, demonstrating the temperature size rule (Atkinson, 1994; Kingsolver and Huey, 2008; Ghosh et al., 2013). Cold temperature during the adult stage usually leads to prolonged lifespan but reduced reproductive output or fecundity (Partridge et al., 1995; Mołoń et al., 2020). The plastic effects of cold temperature on growth, body size, and fecundity are mostly consistent across studies. However, the evolutionary impact of temperature may vary from population to population, depending upon the genetic composition and evolutionary history of the concerned population and also upon the specific thermal range considered for the study. Studies exploring the effect of temperature in Drosophila have often explored thermal selection response, clinal adaptation, or thermal plasticity, but it is rare to find research focusing on how thermal selection shapes the thermal plasticity of life history traits.
Studies performed on fly populations collected from different latitudes have added important information about thermal adaptation, but clinal adaptation may be influenced by factors other than temperature, like humidity, photoperiod, and altitude. Therefore, some of the evolved changes may have been shaped by factors other than temperature. In contrast, the experimental evolution approach enables one to study evolution in a carefully controlled laboratory setup, under the influence of clearly defined selection pressures (Garland and Rose, 2009; Kawecki et al., 2012; Lenski, 2017; Prasad and Joshi, 2003; Schlötterer, 2023). While experimental evolution studies may not mimic multidimensional and complex selection pressures experienced by populations in a natural environment, they nonetheless hold considerable merit in identifying evolutionary responses to specific selection pressures. Consequently, tracking the evolution of populations subjected to specific thermal regimes under regulated laboratory conditions for many generations can potentially help us understand the role temperature plays in shaping adaptive evolution.
While experimental evolution has been used by a number of research groups to study thermal evolution in Drosophila (James and Partridge, 1995; Partridge et al., 1995; Santos et al., 2006; Schlötterer, 2023), there is more than one reason that warrants renewed efforts to study thermal selection using experimental evolution. For example, some laboratory thermal selection studies were conducted for a very short duration (Fragata et al., 2014; Tobler et al., 2014), while others employed a small population size, making it difficult to draw meaningful conclusions at the evolutionary level (Cavicchi et al., 1995). One long-term thermal selection study conducted by Linda Partridge’s group performed extensive characterization of life history, growth, and size traits (James and Partridge, 1995; Partridge et al., 1995; Gilchrist et al., 1997). However, this study did not control for rearing densities that can potentially interfere with thermal selection, as warm temperature leads to crowding that, in turn, leads to evolutionary changes caused by high densities and not warm temperature per se [see Santos et al. (2004) for a discussion]. We used a well-replicated selection design for studying the laboratory thermal evolution of Drosophila, focusing majorly on how the plasticity of various traits evolves with thermal selection.
The thermal range of D. melanogaster ranges from ~11°C to ~32°C (Trotta et al., 2006; Klepsatel et al., 2019). While 25°C is optimal for the growth and survival of this species and can be considered warm, 17°C is cold, which extends the developmental time twofold compared to that at 25°C, produces bigger flies (Ghosh et al., 2013; McDonald et al., 2018), and suppresses fecundity (Mołoń et al., 2020; Partridge et al., 1995). In this study, we investigated how some life history traits of the fly and the plasticity thereof change as a result of evolving at 17°C vs. 25°C for many generations. Reproductive output is a direct measure of the Darwinian fitness of an organism, and in Drosophila, it can be measured as the number of eggs laid and/or as the offspring produced by the flies. We used fly fecundity, i.e., the number of eggs laid per female, as a measure of reproductive output or fitness of flies. Except for a few studies (Nunney and Cheung, 1997; Novoseltsev et al., 2005), fecundity is often measured as reproductive output during the early life of flies (Fragata et al., 2014; Klepsatel et al., 2019; Santos et al., 2020). However, we chose to measure eggs laid by individual females every day, up to 22 days of adult life, covering a large part of their lifespan, in order to get a clearer picture about the distribution of egg output across age. Another important life history trait in Drosophila is body size. Body size is positively correlated with a) female fecundity and b) male mating success in Drosophila (Pitnick, 1991; reviewed in Prasad and Joshi, 2003; Flatt, 2020) and shows plastic and evolutionary changes to rearing temperatures. Therefore, we investigated the evolved and plastic changes in the body size of flies caused by temperature. Different researchers have examined the variability in wing area, thorax length, and body weight to measure body size variation in flies. In this study, we measured body weight variation, similar to various earlier studies focusing on the life history traits in Drosophila (James et al., 1997; reviewed in Cavicchi et al., 1995; Watanabe and Riddle, 2021). However, there are some concerns that the body weight of a fly can vary considerably depending upon its age, activity, and in the case of females its egg, laying status (before and after peak fecundity). Taking these factors into consideration, we measured the weights of only freshly eclosed unmated flies, within 4 h of emergence from pupae, keeping age, activity, and reproductive status consistent.
Materials and methods
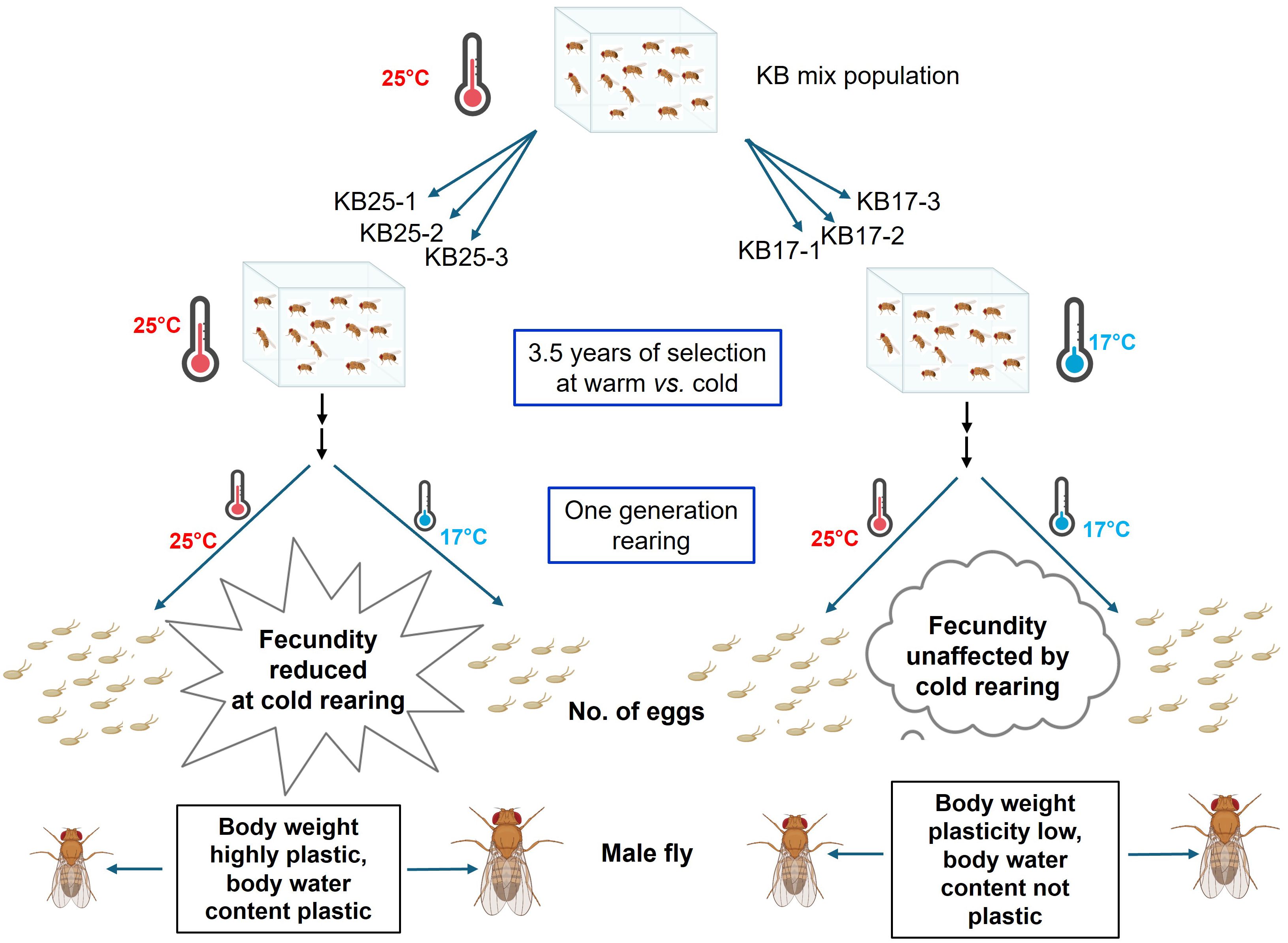
We employed a 2 × 2 full factorial design, in which flies from the two thermal selection regimes were reared and assayed at two treatment temperatures (17°C and 25°C). This design allowed us to investigate both a) the evolutionary effect of cold vs. warm selection and b) the plastic effect of cold and warm temperatures on traits like fecundity and body size of flies. While we examined the egg production of flies for fecundity, the wet and dry body masses of flies were assayed for body size. This also allowed us to quantify the relative content of water with respect to the body mass of the flies. The main effect of selection temperature revealed the evolutionary change in fecundity and body size traits, and a comparison of the trait values across treatment temperatures for a given selection regime allowed us to evaluate the extent of plasticity of the traits. The study design thus allowed us to find out whether selection in warm vs. cold has changed the extent of plasticity of the traits under study.
Study populations and selection regimes
We used six large, outbred (see Garland and Rose, 2009) laboratory populations of D. melanogaster: three populations allowed to evolve at 17°C (KIIT Base populations 17 or KB17 1–3) and three populations maintained at 25°C (KIIT Base populations 25 or KB25 1–3). Both KB17 and KB25 populations are descendants of MB (melanogaster Base) populations, whose ancestry has been described in detail in Sheeba et al. (1998) and Sarangi et al. (2016). MBs were maintained on cornmeal medium at 25°C on a 21-day discrete generation cycle, under constant light and high humidity in the laboratory of Amitabh Joshi for over 200 generations (Sheeba et al., 1998). Five replicate populations of MB were mixed and maintained for ~2 years, and two thermal selection lineages were initiated from the mixed population, namely, KB17 (KIIT Baseline 17) 1–3 and KB25 (KIIT Baseline 25) 1–3. KB17 populations were allowed to evolve at 17°C, on a discrete generation cycle of 24 days. KB25 populations were maintained at 25°C, on a 14-day discrete generation cycle. Both sets of populations were maintained on cornmeal medium, 24 h light, and high humidity. At the time of this study, both lineages had evolved for a little over 3.5 years at their respective temperatures. Drosophila melanogaster takes 17–20 days to develop from egg to adult at 17°C, whereas it takes 8–10 days to develop at 25°C. Thus, the generation time is much longer at 17°C. Except for temperature and generation time, the same protocol was followed for maintaining both sets of populations. For each replicate population, flies were reared in 25 vials containing food, and the larval density was controlled at ~70 per vial. Upon eclosion, flies from all 25 vials were transferred to plexiglass cages containing food smeared with supplementary live yeast-acetic acid paste. On the 14th day after the previous generation egg collection in KB25 populations and the 24th day after the previous egg collection in KB17 populations, eggs were collected for the next generation. Thus, egg collection was done on the 4th–6th day of adult age for KB25 and the 4th–7th day of adult age for KB17 populations. A day prior to the egg collection, each population cage was provided with a Petri dish filled with fly food. Flies were then allowed to oviposit on it. After 12 to 16 h, the food plate was taken out and placed under a microscope, and the food was cut into small pieces, each containing approximately 60–80 eggs. Each piece was placed inside a vial containing 6 mL of food. Twenty-five such vials were used for each replicate population. These vials were then incubated at a specific temperature (25°C or 17°C), and upon eclosion, all flies were transferred to a population cage for the next generation and the cycle was repeated.
Generation of flies for the experiments
Body weight and fecundity of both 25°C and 17°C selection lines were assayed at two treatment temperatures, namely, a) 25°C and b) 17°C. For comparing the selection lines at a) 25°C, flies from both 17°C and 25°C selection lines were reared at a common temperature of 25°C for one generation, and these flies were referred to as standardized flies. The progeny of standardized flies was reared and assayed at 25°C. This way both selection lines were standardized, and any non-genetic parental effect of divergent temperature on the progeny was eliminated, and only selection response or evolved changes between the two thermal selection lines could be identified (Rose, 1984). Similarly, to compare the selection response at 17°C, both 17°C and 25°C selected populations were standardized at 17°C for one generation, and their progeny was subsequently reared and assayed at 17°C. The larval density was controlled at ~70 per vial for the generation of flies for all our experiments.
All assays reported here were conducted between 56 and 63 generations of selection for KB17 populations. The KB25s, the predecessors of the MB flies and their ancestors, all had been maintained at the optimal rearing temperature of 25°C for decades. Hence, the number of generations is somewhat irrelevant for the KB25s, and they should rather be considered as control baseline or populations that had been adapted to 25°C for very long.
Fecundity assay
This assay was conducted after 56 generations of selection of the KB17 populations. Twenty vials of eggs were collected from all three replicates of KB17, over an oviposition window of 13–14 h at 17°C, and the vials were transferred to 25°C. From this step onward, the entire assay was conducted at 25°C. Upon eclosion, these flies were transferred to cages and were referred to as the standardized flies (for 25°C assay temperature). These flies were then provided with food and excess yeast-acetic acid paste for 3 days, and their eggs were collected in vials over a small oviposition window of 3 h. The flies growing from these eggs are referred to as assay flies. After 8 days of egg collection, flies started eclosing, and those eclosed in the first 4 h were discarded. After this point, every 4 h, freshly eclosed flies were transferred from the rearing vials to empty vials and subjected to chill coma for ~30 min by placing the vials in ice. After being immobilized by chill coma, the male and female flies were separated and then transferred to vials containing food. By this time, the flies recovered from the chill coma. This process was repeated every 4 h, till the next day, and males and females were thus collected in separate storage vials. Several such storage vials were maintained, each containing 30–40 flies. Once flies were collected in sufficient numbers covering the complete middle part of the eclosion time distribution, virgin collection was stopped. Similar to very early flies, very late eclosed flies too were excluded from the assay. Given the KB25 populations were already maintained at 25°C, no standardization was needed for them. Eggs were collected from them over an oviposition window of 3 h and incubated at 25°C. Once eclosed, these were our assay flies. A similar protocol was followed for virgin separation in these flies. Once the virgin collection was completed for both selection lines, one male and one female randomly drawn from the storage vials were introduced into one fecundity assay vial containing 2 mL of food. Fifteen such vials were set up for the fecundity assay for each selection regime and replicate population. Upon completion of oviposition for 24 h, each fly pair was transferred to a fresh food vial to allow for the next day’s egg laying, and the previous day’s vial was taken under a microscope and the eggs laid in it were counted. This cycle was repeated for 22 days. The assay was conducted at 25°C. If a male from a pair died, it was replaced with an unmated male of the same age. If a female died, it was not replaced, and data from the same vial were included in the analysis up to the death of the female. Similarly, for the treatment temperature of 17°C, eggs were collected directly from the generation 56 flies of the 17°C selection lines, and assay flies were generated at 17°C. Simultaneously, 25°C selected populations were standardized for a generation at 17°C, and the fecundity assay was performed using their offspring, also maintained at 17°C. KB17 flies were reared similarly at 17°C, and after virgin separation, a similar assay setup was done for them. Total fecundity was analyzed cumulatively over a period of 22 days using a 2 selection × 2 treatment temperature full factorial design. The total number of eggs laid by the female was averaged across 15 vials and used for the analysis. The fecundity data were also analyzed separately to investigate the age-specific pattern of egg production. For this, the weekly total fecundity was calculated for weeks 1, 2, and 3, and an ANOVA was performed that included age (week) as a variable. The data for day 22 were not included in the analysis.
Weight assays
The dry and wet weights of the flies from all replicates of KB25 and KB17 populations were assayed after 63 generations of cold selection. The assays were conducted at both 25°C and 17°C, and the weight of the progenies of standardized flies was assayed. The assay flies were collected within 4 h of eclosion, stored immediately in microcentrifuge tubes (MCTs), and freeze-killed by keeping the flies at −20°C for 40–45 min. Wet weight was assayed for males and females separately. For each combination of selection regime, treatment temperature, replicate population, and sex, eight MCTs were set up with five flies in each. After freeze-killing, these flies were taken out of the freezer and weighed immediately. Five flies were weighed at a time using a Sartorius Quintix 35 (d = 0.01 mg) balance, and the average weight per fly was calculated for each MCT. After measuring the wet weight of the flies, the dry weight of the same flies was also assayed. For this, after collection of the wet weight data, the flies were returned to their respective MCTs and placed in a hot air oven set at 70°C. They were kept at 70°C for 36 h, thus being dehydrated completely, and then taken out and weighed again.
Relative water content of the flies
The water content of the flies was calculated by measuring the water lost during dehydration in the hot air oven. The total dry weight of five flies stored in each MCT was subtracted from the wet weight of the same flies to obtain the water content (in mg) of flies in each MCT. The relative water content was calculated by dividing the water content by the wet weight of the flies in the respective MCT (in percent).
Statistical analysis
Mixed model analyses of variance (ANOVA) were performed for all the assays. Replicate population means were used for testing the significance of fixed factors and their interactions in all the analyses. Post-hoc comparisons were done using Tukey’s honest significant difference (HSD) test. Data analyses were carried out on JMP Pro 17. To analyze the fecundity data, 22 days’ total fecundity per female was averaged across vials to obtain replicate population means. ANOVA was performed on replicate population means. Selection and treatment temperature were treated as fixed factors, and replicate population was treated as a random factor nested within selection and treatment temperature. We also analyzed the week-wise fecundity data (weeks 1, 2, and 3) to investigate the effects of selection and treatment temperature on early fecundity, mid-stage fecundity, and late fecundity till 21 days of adult life. A three-way ANOVA was conducted with selection, treatment, and age (week post-eclosion) as fixed factors, with the random factor population replicate nested within all three of these.
For both wet weight and dry weight measurements, the mean weight per fly from each MCT was averaged across eight MCTs to obtain the replicate population mean. ANOVA was performed on replicate population means of individual fly weight, with selection, treatment temperature, and sex being treated as fixed factors. The replicate population was treated as a random factor nested within all three fixed factors. Similar analyses were performed separately for wet weight and dry weight data. The relative water content data were obtained in percentage and therefore were subjected to arcsine-square root transformation to meet the normality assumption of ANOVA. Population means were used for testing the significance of fixed factors such as selection, treatment temperature, sex, and their interactions.
Results
Total fecundity
After evolving at 17°C for 56 generations, KB17 populations did not show any significant difference in total fecundity from KB25 populations (p = 0.1672) (Supplementary Tables 1; 4A, B), but treatment temperature had a significant main effect on fecundity (p = 0.0001) as fecundity was significantly less at 17°C treatment compared to 25°C. However, the interaction of selection temperature and treatment temperature was significant (p = 0.0356), and post-hoc comparisons showed that KB25 populations exhibit a significant drop in fecundity from 25°C to 17°C treatment temperature (p < 0.05), but KB17 populations did not show any significant change between 25°C and 17°C treatment (Figure 1).
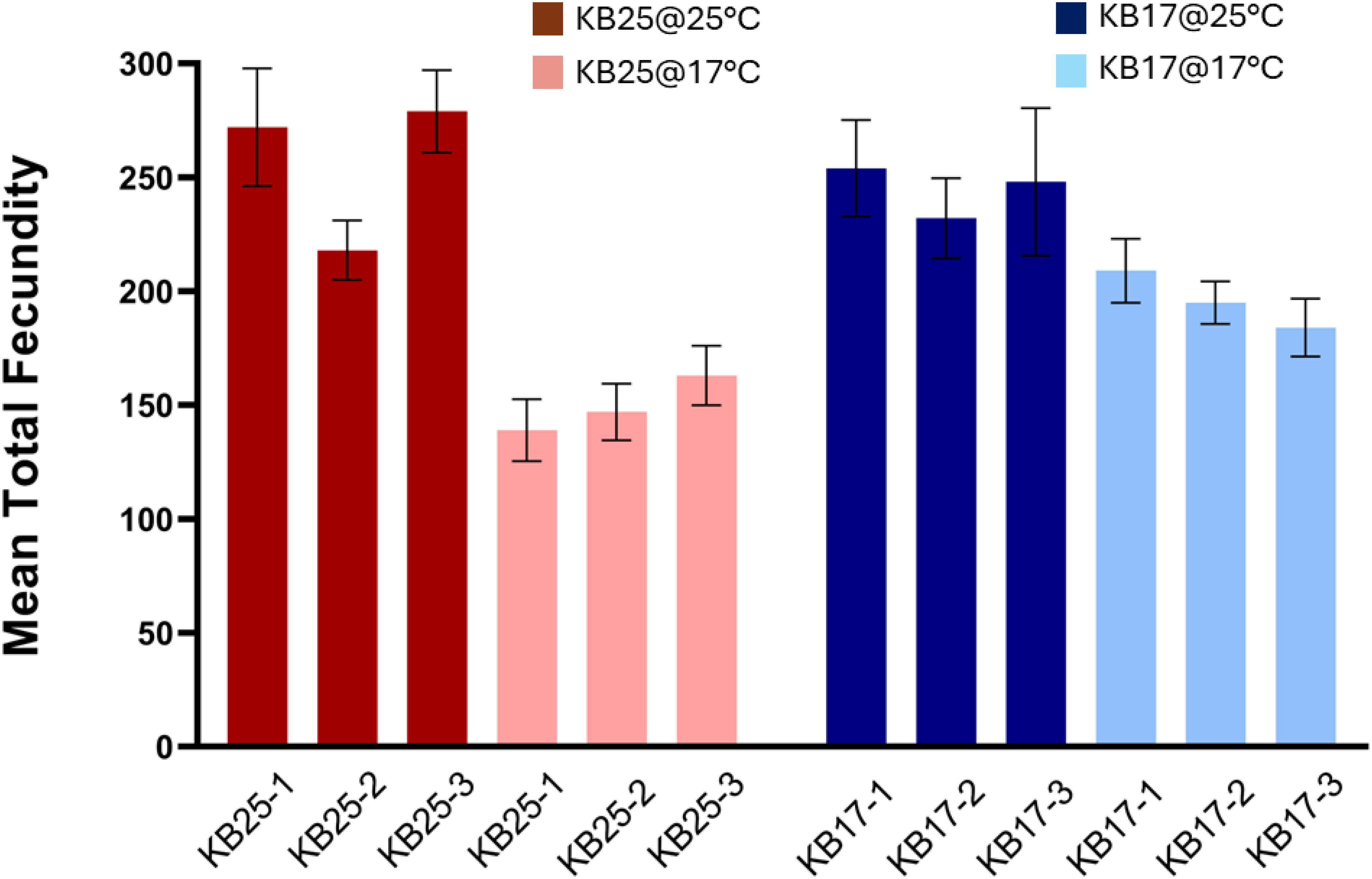
Figure 1. Mean total egg production per female in KB25 and KB17 replicate populations, assayed at 25°C and 17°C. The error bars represent standard error across replicate vials.
Age-specific fecundity
Separate analysis of the week-wise fecundity data showed significant effects of treatment temperature, age (week), and interaction of treatment temperature and age (p < 0.0001, for all three effects) (Supplementary Tables 2; 4A, B). The 17°C treatment reduced the overall fecundity compared to 25°C (Figure 2). Pooled over treatments and selection regimes, post-hoc comparisons showed that fecundity was significantly higher during the first week compared to weeks 2 and 3 (p < 0.05). However, the interaction of treatment temperature and age showed that fecundity declined significantly across weeks only in the 25°C treatment. In contrast, fecundity did not differ significantly across weeks in the 17°C treatment. The main effect of selection and its interactions was not significant for the week-wise fecundity data (Figure 2).
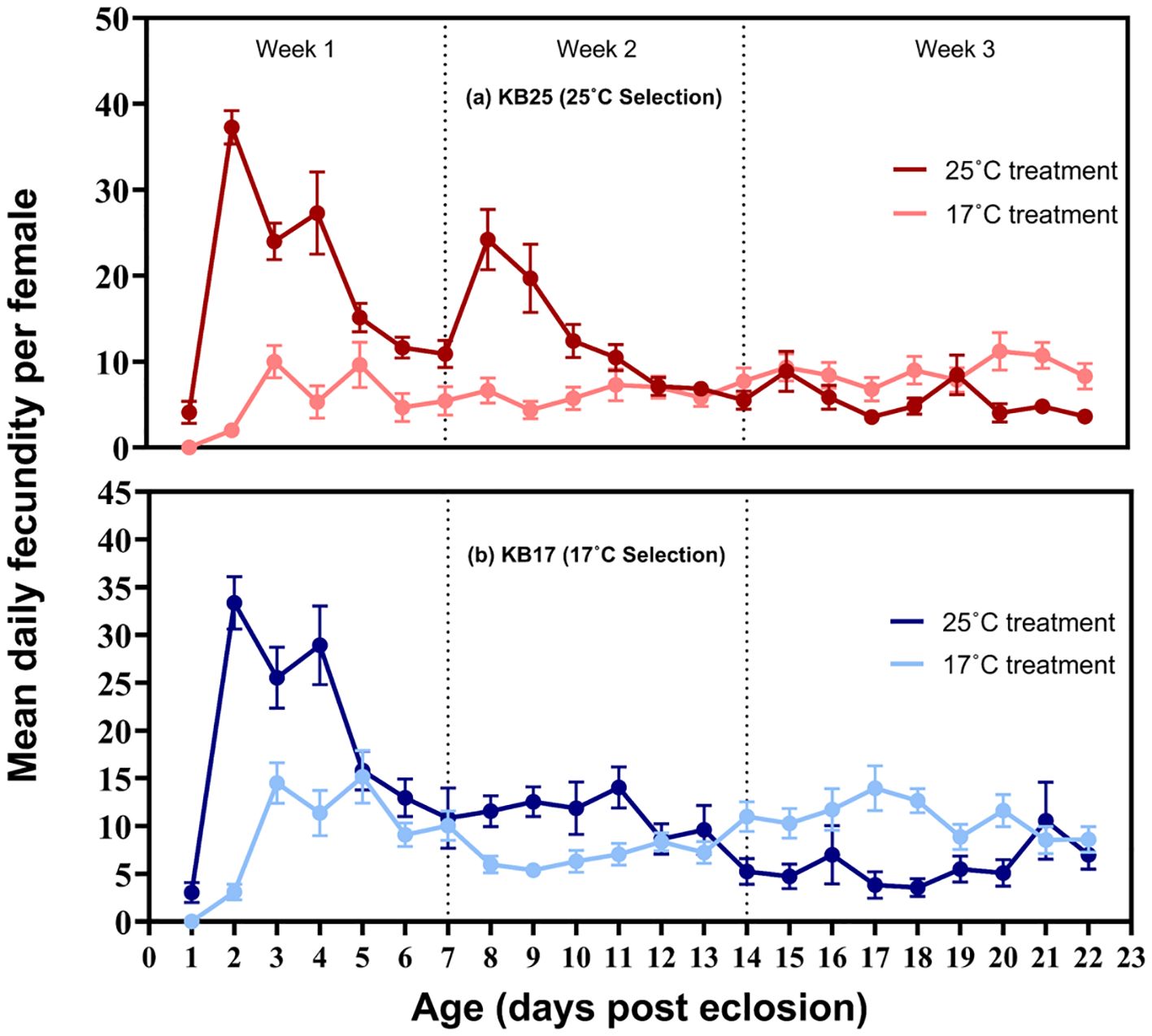
Figure 2. Mean daily egg production per female of (A) KB25 and (B) KB17 populations, assayed at 17°C and 25°C. The error bars represent standard error across replicate populations.
Wet weight
After 63 generations of selection at 17°C, the wet weight of the flies was strongly affected by selection temperature, treatment temperature, and sex (Figures 3A, B). Overall, the flies of KB17 populations were significantly heavier than their counterparts from KB25 (p = 0.0093), and the flies reared at 17°C had significantly greater wet weight than those reared at 25°C (p < 0.0001) (Supplementary Table 5). Females had significantly greater wet weight than males (p < 0.0001) (Figures 3A, B). Fixed factor interactions were not significant (Supplementary Table 3).
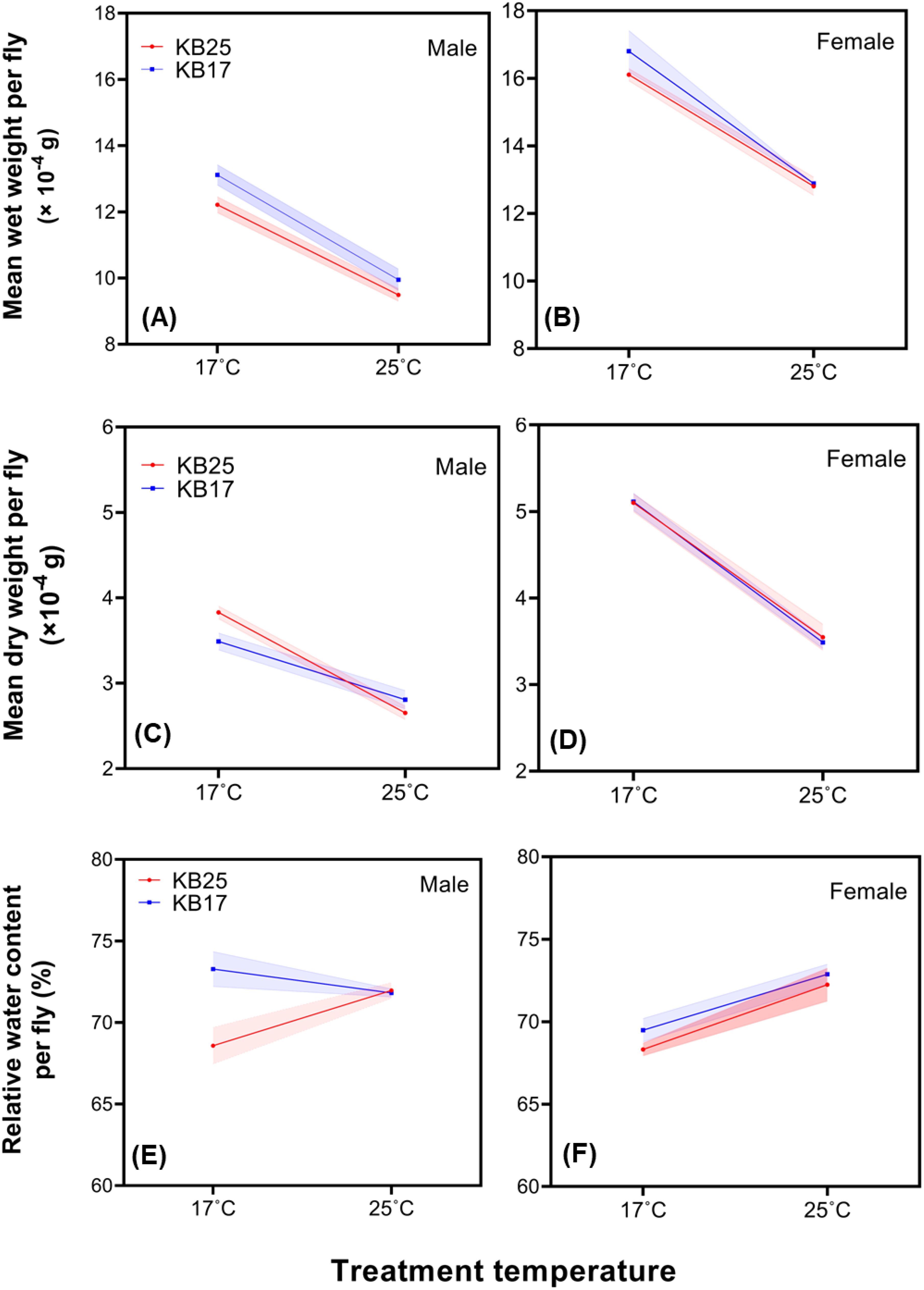
Figure 3. Mean wet weight of individual (A) male and (B) female fly at eclosion, mean dry of individual (C) male and (D) female fly at eclosion, and mean relative water content per (E) male and (F) female fly at eclosion, in KB25 and KB17 populations, reared at 25°C and 17°C. The error bars represent standard error of the three replicate population means.
Dry weight
The dry weight of the flies was strongly affected by treatment temperature and sex, but not selection temperature (Supplementary Tables 3, 5). Flies reared at 17°C had significantly greater dry weight than those reared at 25°C (p < 0.0001) (Figures 3C, D). Females were significantly heavier than males across conditions (p < 0.0001). Two-way interaction between treatment and sex (p < 0.0001) and three-way interaction among selection, treatment, and sex were significant (p = 0.025) (Supplementary Table 3). Both males and females of the KB25 population and females of the KB17 population had 44% to 47% greater dry weight at 17°C than at 25°C. However, KB17 population males had only 24% increase in dry weight in the same thermal range (Figures 3C, D).
Relative water content of the flies
For water content per unit wet weight of flies, the main effects of selection temperature and treatment temperature were significant (p = 0.0031 and p = 0.0001, respectively) (Supplementary Tables 3, 5; Figures 3E, F). The two-way interactions between selection temperature and treatment temperature and between sex and treatment temperature were significant (p = 0.0083, for both interactions) and so was the three-way interaction among selection, treatment temperature, and sex (p = 0.0303). KB25 males and females had significantly less water content (68%–69%) when reared at 17°C compared to 25°C (71%–72%) (p < 0.05). Similarly, KB17 females had significantly less water content (69%) when reared at 17°C compared to 25°C (73%) (p < 0.05), but KB17 males did not show a significant change in relative water content when reared at 17°C vs. 25°C (72%–73%) (Figures 3E, F). Consequently, the relative water content of KB17 and KB25 males was not significantly different at 25°C, but it was significantly higher in KB17 males than KB25 males at 17°C (p < 0.05).
Discussion
Evolution of fecundity
Cold temperature reduced the pooled fecundity of the two selection regimes, but the interaction of selection and treatment temperature revealed that the impact of cold treatment on the fecundity of warm- and cold-evolved flies was different. The control groups, i.e., the warm selection lines, suffered a significant decline in fecundity when assayed at cold temperature, in comparison to their fecundity in warmer conditions. In contrast, the cold selection lines did not suffer any significant reduction in fecundity at cold treatment temperature compared to the warm treatment. For D. melanogaster rearing, 25°C is the laboratory optimum, and the findings from our control populations show that 17°C represses their fecundity, as observed in numerous previous studies (Nunney and Cheung, 1997; Klepsatel et al., 2019; Mołoń et al., 2020). However, the cold-selected populations seem to have evolved the ability to withstand cold, such that cold temperature does not cause a significant reduction in their egg production. This clearly indicates that the cold-evolved flies have adapted to cold as a result of selection. The sustained fecundity of the cold-adapted flies transitioning from warm to cold environments, an attribute not observed in the warm-evolved controls, hints at an evolved ability to neutralize the cold’s suppressive effect. There could be various possibilities, such as a) cold perception that potentially modulates egg production might have diverged in the two selection lines or cold tolerance might have improved in cold selected flies, and/or b) cold selection might have led to bigger flies such that an increased size buffers against the repressive effect of cold on fecundity, as body size and fecundity are positively correlated in flies (Pitnick, 1991; Lefranc and Bundgaard, 2000; Flatt, 2020). There was a main effect of selection temperature on the wet weight of flies in our study, indicating that KB17 flies had evolved a higher wet weight compared to KB25. This could be one of the factors contributing to an increased fecundity of cold-evolved flies, while there could be possible alternative or additional mechanisms accounting for their thermally canalized fecundity. We also evaluated the progeny survivorship in a later generation (gen 78) and found no significant effect of selection, treatment temperature, or their interaction on the trait (Chattopadhyay et al., unpubl. data). This indicates that the different effects of cold temperature on the reproductive output of warm- and cold-selected populations were primarily exerted through an effect of cold on egg production and not through progeny survivorship.
Comparison of different thermal selection studies
Interestingly, one study conducted by the research group of Mauro Santos involved the selection of Drosophila subobscura populations at 13°C, 18°C, and 22°C for over 4 years. In this study, warm-adapted populations showed greater net fitness at all three test temperatures, while cold-adapted populations had low fitness in the warm environment (Santos, 2007). On the other hand, in a thermal selection study under laboratory conditions conducted by Linda Partridge’s research group, cold-adapted populations of D. melanogaster lived longer and laid more eggs compared to warm-adapted flies, at their maintenance temperature, i.e., 18°C (Partridge et al., 1995). Similarly, the warm-adapted flies (25°C) lived longer and laid more eggs than warm-adapted flies when tested at 25°C. Thus, while our study suggests “colder is better,” Santos’ work suggests “warmer is better,” and Partridge’s findings indicate the existence of a trade-off between adaptation to cold vs. warm environments. The contrasting findings from the different studies suggest that thermal adaptation may manifest diverse evolutionary patterns as it can vary across populations and species.
Age-specific fecundity pattern
Apart from total fecundity, our study revealed a noticeable impact of treatment temperature on the age-specific pattern of fecundity. The distribution of fecundity along the age axis in wild-caught Drosophila and those maintained in the laboratory tends to be positively skewed. A triangular shape of the lifetime fecundity distribution characterized by an early peak is a typical feature of iteroparous insects (Dixon and Agarwala, 2002). This peak has been shown to be triggered by the onset of mating in flies (Modak, 2009). In our study, the typical early-life spike in fecundity was observed at 25°C treatment, which also coincides with the age of egg collection in our fly populations. Surprisingly, we did not observe such a spike at the 17°C treatment for either of the selection lines, which suggests that the early-life fecundity spike may not be canalized across conditions and can be absent in colder environments. When flies were reared and assayed at 25°C, fecundity was the highest during the first week, after which it showed a gradual decline during weeks 2 and 3. At 17°C, in contrast, the pooled fecundity did not differ across 3 weeks, and the data rather show a somewhat moderate steady fecundity level at 17°C throughout the duration of the assay, which is different from the pattern observed at 25°C. These differences between the 25°C and 17°C treatments can be suitably explained by the lifespan of the flies at the said temperatures. Cold temperature increases the lifespan in flies, and a trade-off between lifespan and reproductive output is well-documented in evolutionary biology (reviewed in Prasad and Joshi, 2003). In conjunction with the existence of this trade-off, flies appear to maintain a relatively steady yet reduced egg production throughout their entire lifespan at cold temperature. In contrast, at warm temperature, flies lay a maximum number of eggs early in life which subsequently dwindles to a lesser egg output for the remaining part of their lifespan. The strategy at cold temperature seems to be conserving resources, facilitating a prolonged lifespan and an extended period of egg production. The energy needs to support a longer lifespan perhaps do not allow flies to exhaust too much resource early in life. The age-specific fecundity pattern, therefore, may correspond to the energy need of the flies to live and reproduce during their respective lifespans at warm vs. cold conditions. It was observed that this temperature-dependent age-wise fecundity pattern remained unaffected by selection. However, it is important to note that in our cold selection regime, neither a long lifespan nor a consistent egg output throughout the lifespan was relevant to fitness, because similar to KB25, eggs for the next generation were collected from KB17 populations on the 4th–7th day of adult life. It is possible that a somewhat flat pattern of fecundity as a plastic response to cold environmental temperature is hardwired in flies. Whether continued cold selection in which a) only early-life fecundity is favored and b) a longer lifespan is not relevant to fitness can alter this pattern can be potentially investigated in these populations in the future. It is important to note that some flies from the KB25-1 and KB25-3 populations approximately days 8–9 laid a large number of eggs that led to a smaller second peak in the daily fecundity pattern (Figure 2). In the MB populations and their ancestor JBs, eggs were collected after 10–12 days of eclosion, which led to the evolution of a subsidiary peak at that age (Prasad and Joshi, 2003). We speculate that the small second peak observed in the KB25 populations could be a result of their evolutionary history. However, it was not found in KB17 populations.
Evolution and plasticity of body size and relative water content
Cold temperature of 17°C led to greater wet weight of flies both at plastic and evolutionary levels. Dry weight on the other hand was influenced only by the treatment temperature implying the plastic influence of cold temperature, but it remained unaffected by cold selection. Females being heavier than males under all conditions is expected and hence does not require much discussion. As revealed by the interactions of selection temperature, treatment temperature, and sex, KB17 males showed significantly less thermal plasticity of dry weight compared to KB17 females and KB25 flies. While cold vs. warm selection did not alter wet weight plasticity, this indicates that cold selection however lowered the dry weight plasticity, albeit only in male flies. At present, it is not very clear why cold selection led to reduced plasticity of the dry weight, albeit only in the male flies. In future generations, investigating how body weight plasticity evolves further may reveal some more details about this trend. Male Drosophila take a longer time to develop, yet they are smaller and lighter than females. Flies become sexually mature and start mating within a few hours of eclosion, and it is suggested that the reproductive maturation of males takes more time than that of females, accounting for the longer development time of the former (reviewed in Prasad and Joshi, 2003). How the development time difference of the two sexes fares across the two selection regimes and treatments would be worth investigating, in order to gain more insight about the reduced size plasticity observed only in cold-evolved males.
Apart from the weights, the trend observed for relative water content was interesting. KB25 flies as well as KB17 females had a plastic reduction in relative body water content when developing in cold temperature. Some earlier studies suggest that a reduced body water content may be a sign of cold tolerance in insects in freezing temperatures as it may help to reduce the damage caused by water crystal formation (Worland, 1996). However, it is not clear why flies would have a lower water content at a tolerable cold temperature like 17°C than at a warmer temperature of 25°C. Given that both KB25 flies and KB17 females showed a small but significant reduction in relative water content when developing at 17°C, this could be common in Drosophila, but that KB17 males instead evolved the same body water percentage across 25°C and 17°C is surprising. Whether or not these flies have evolved a different metabolism, retaining more water at cold temperatures compared to the females of their population and the ancestral flies from which they have evolved, would be worth investigating in the future. Our work thus indicates that the cold-evolved males have diverged from the warm-evolved populations both in terms of thermal plasticity of dry weight and relative body water content.
To sum up, we show that while cold temperature suppresses the reproductive output of D. melanogaster, prolonged cold selection can lead to improved egg production in cold temperature, showing clear evidence of adaptation to the cold environment. The fecundity of the cold-evolved flies also remained unaffected under warm conditions. Therefore, compared to warm-adapted populations that suffer a significant decrease in egg production from warm to cold conditions, cold-adapted populations evolved fecundity levels that were less thermally plastic or more canalized. We speculate that the evolution of canalized or more consistent fecundity levels across environmental conditions can potentially aid in range expansion of a species. While for some traits phenotypic plasticity may aid in adaptation, for others, canalization might be more adaptive. In addition to fecundity, we found that male flies of cold-selected populations evolved reduced plasticity of dry body weight and relative body water content. Future studies exploring the connections between weight, body composition, temperature, and metabolism can potentially help us understand the significance of the evolution of low plasticity in these traits.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
RR: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. AC: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. SS: Investigation, Methodology, Writing – review & editing. AM: Investigation, Methodology, Writing – review & editing. PB: Investigation, Methodology, Writing – review & editing. SG: Investigation, Methodology, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a fellowship to SG under the Department of Science & Technology Government of India, DST Women Scientist A scheme, SR/WOS-A/LS-1179/2015(G), and a grant from the Science and Engineering Research Board (DST-SERB), Government of India, under start-up research grant, SRG/2020/001573.
Acknowledgments
We thank Pragya Giri, Proteek Sen, and Subrath Ranjan for standardizing fly population maintenance and media preparation and Ranjit Pradhan and Manas Ranjan Mallik for their general help in the laboratory. We thank two anonymous reviewers for their suggestions to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1452948/full#supplementary-material
References
Angilletta M. J., Dunham A. E. (2003). The temperature-size rule in ectotherms: simple evolutionary explanations may not be general. Am. Nat. 162, 332–342. doi: 10.1086/377187
Atkinson D. (1994). Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res. 25, 1–58. doi: 10.1016/S0065-2504(08)60212-3
Barley J. M., Cheng B. S., Sasaki M., Gignoux-Wolfsohn S., Hays C. G., Putnam A. B., et al. (2021). Limited plasticity in thermally tolerant ectotherm populations: evidence for a trade-off. Proc. R. Soc. B 288. doi: 10.1098/RSPB.2021.0765
Carbonell J. A., Wang Y. J., Stoks R. (2021). Evolution of cold tolerance and thermal plasticity in life history, behaviour and physiology during a poleward range expansion. J. Anim. Ecol. 90, 1666–1677. doi: 10.1111/1365-2656.13482
Cavicchi S., Guerra D., La Torre V., Huey R. B. (1995). Chromosomal analysis of heat-shock tolerance in drosophila melanogaster evolving at different temperatures in the laboratory. Evolution 49, 676–684. doi: 10.1111/J.1558-5646.1995.TB02304.X
Dixon A. F. G., Agarwala B. K. (2002). Triangular fecundity function and ageing in ladybird beetles. Ecol. Entomology 27, 433–440. doi: 10.1046/J.1365-2311.2002.00429.X
Flatt T. (2020). Life-history evolution and the genetics of fitness components in drosophila melanogaster. Genetics 214, 3–48. doi: 10.1534/GENETICS.119.300160
Fragata I., Simões P., Lopes-Cunha M., Lima M., Kellen B., Bárbaro M., et al. (2014). Laboratory selection quickly erases historical differentiation. PloS One 9, 96227. doi: 10.1371/JOURNAL.PONE.0096227
Garland T., Rose M. R. (2009). Experimental EvolutionConcepts, Methods, and Applications of Selection Experiments. Univ. of California Press, Berkeley, California. doi: 10.1525/CALIFORNIA/9780520247666.001.0001
Ghosh S. M., Testa N. D., Shingleton A. W. (2013). Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proc. R. Soc. B: Biol. Sci. 280. doi: 10.1098/RSPB.2013.0174
Gilchrist G. W., Huey R. B., Partridge L. (1997). Thermal Sensitivity of Drosophila melanogaster: Evolutionary Responses of Adults and Eggs to Laboratory Natural Selection at Different Temperatures. Physiol. Zool. 70, 403–414. doi: 10.1086/515853
James A. C., Azevedo R. B., Partridge L. (1997). Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics. 146 (3), 881–890. doi: 10.1093/genetics/146.3.881
James A. C., Partridge L. (1995). Thermal evolution of rate of larval development in Drosophila melanogaster in laboratory and field populations. J. Evolutionary Biol. 8, 315–330. doi: 10.1046/J.1420-9101.1995.8030315.X
Kawecki T. J., Lenski R. E., Ebert D., Hollis B., Olivieri I., Whitlock M. C. (2012). Experimental evolution. Trends Ecol. Evol. 27, 547–560. doi: 10.1016/J.TREE.2012.06.001
Kingsolver J. G., Huey R. B. (2008). Size, temperature, and fitness: three rules (Accessed 2 November 2023).
Klepsatel P., Girish T. N., Dircksen H., Gáliková M. (2019). Reproductive fitness of Drosophila is maximised by optimal developmental temperature. J. Exp. Biol. 222. doi: 10.1242/JEB.202184
Lafuente E., Duneau D., Beldade P. (2018). Genetic basis of thermal plasticity variation in Drosophila melanogaster body size. PloS Genet. 14, e1007686. doi: 10.1371/JOURNAL.PGEN.1007686
Lefranc A., Bundgaard J. (2000). The influence of male and female body size on copulation duration and fecundity in Drosophila melanogaster. Hereditas 132, 243–247. doi: 10.1111/J.1601-5223.2000.00243.X
Lenski R. E. (2017). Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. ISME J. 11, 2181–2194. doi: 10.1038/ismej.2017.69
Leonard A. M., Lancaster L. T. (2020). Maladaptive plasticity facilitates evolution of thermal tolerance during an experimental range shift. BMC Evol. Biol. 20, 1–11. doi: 10.1186/S12862-020-1589-7
MacLean H. J., Sørensen J. G., Kristensen T. N., Loeschcke V., Beedholm K., Kellermann V., et al. (2019). Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Philos. Trans. R. Soc. B 374. doi: 10.1098/RSTB.2018.0548
Mayekar H. V., Solanki P. S., Arya H., Aradhya R., Suravajhala P., Loeschcke V., et al. (2023). Tropical High-Altitude Insects Show Limited Capacity to Handle High Temperatures. bioRxiv 2022.09.10.507406. doi: 10.2139/SSRN.4604588
McDonald J. M. C., Ghosh S. M., Gascoigne S. J. L., Shingleton A. W. (2018). Plasticity through canalization: The contrasting effect of temperature on trait size and growth in Drosophila. Front. Cell Dev. Biol. 6. doi: 10.3389/FCELL.2018.00156/PDF
Modak S. G. (2009). Evolution of life-history traits, canalization and reproductive isolation in laboratory populations of drosophila melanogaster selected for faster pre-adult development and early reproduction. Available online at: https://libjncir.jncasr.ac.in/jspui/handle/10572/755 (Accessed 18 September 2023).
Mołoń M., Dampc J., Kula-Maximenko M., Zebrowski J., Mołoń A., Dobler R., et al. (2020). Effects of temperature on lifespan of drosophila melanogaster from different genetic backgrounds: links between metabolic rate and longevity. Insects 11, 1–18. doi: 10.3390/INSECTS11080470
Nettle D., Bateson M. (2015). Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proc. R. Soc. B: Biol. Sci. 282. doi: 10.1098/RSPB.2015.1005
Novoseltsev V. N., Arking R., Carey J. R., Novoseltseva J. A., Yashin A. I. (2005). Individual fecundity and senescence in drosophila and medfly. journals gerontology. Ser. A Biol. Sci. Med. Sci. 60, 953. doi: 10.1093/GERONA/60.8.953
Nunney L., Cheung W. (1997). The effect of temperature on body size and fecundity in female drosophila melanogaster: evidence for adaptive plasticity. Evolution 51, 1529–1535. doi: 10.1111/J.1558-5646.1997.TB01476.X
Partridge L., Barrie B., Barton N. H., Fowler K., French V. (1995). Rapid laboratory evolution of adult life-history traits in drosophila melanogaster in response to temperature. Evolution 49, 538–544. doi: 10.1111/J.1558-5646.1995.TB02285.X
Pitnick S. (1991). Male size influences mate fecundity and remating interval in Drosophila melanogaster. Anim. Behav. 41, 735–745. doi: 10.1016/S0003-3472(05)80340-9
Prasad N. G., Joshi A. (2003). What have two decades of laboratory life-history evolution studies onDrosophila melanogaster taught us? J. Genet. 82, 45–76. doi: 10.1007/BF02715881
Rose M. R. (1984). Laboratory evolution of postponed senescence in drosophila melanogaster. Evol. (N Y) 38, 1004–1010. doi: 10.1111/J.1558-5646.1984.TB00370.X
Santos M. (2007). Evolution of total net fitness in thermal lines: Drosophila subobscura likes it “warm. J. evolutionary Biol. 20, 2361–2370. doi: 10.1111/J.1420-9101.2007.01408.X
Santos M. A., Carromeu-Santos A., Quina A. S., Santos M., Matos M., Simões P. (2020). High developmental temperature leads to low reproduction despite adult temperature. J. Therm. Biol. 95, 102794. doi: 10.1016/j.jtherbio.2020.102794
Santos M., Brites D., Laayouni H. (2006). Thermal evolution of pre-adult life history traits, geometric size and shape, and developmental stability in Drosophila subobscura. J. Evolutionary Biol. 19, 2006–2021. doi: 10.1111/J.1420-9101.2006.01139.X
Santos M., Iriarte P. F., Céspedes W., Balanyà J., Fontdevila A., Serra L. (2004). Swift laboratory thermal evolution of wing shape (but not size) in Drosophila subobscura and its relationship with chromosomal inversion polymorphism. J. Evolutionary Biol. 17, 841–855. doi: 10.1111/J.1420-9101.2004.00721.X
Sarangi M., Nagarajan A., Dey S., Bose J., Joshi A. (2016). Evolution of increased larval competitive ability in Drosophila melanogaster without increased larval feeding rate. J. Genet. 95, 491–503. doi: 10.1007/S12041-016-0656-8/METRICS
Schaum C. E., Buckling A., Smirnoff N., Yvon-Durocher G. (2022). Evolution of thermal tolerance and phenotypic plasticity under rapid and slow temperature fluctuations. Proc. R. Soc. B 289. doi: 10.1098/RSPB.2022.0834
Schlötterer C. (2023). How predictable is adaptation from standing genetic variation? Experimental evolution in Drosophila highlights the central role of redundancy and linkage disequilibrium. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 378. doi: 10.1098/RSTB.2022.0046
Sheeba V., Aravinda Madhyastha N. A., Joshi A. (1998). Oviposition preference for novel versus normal food resources in laboratory populations of Drosophila melanogaster. J. Biosci. 23, 93–100. doi: 10.1007/BF02703000/METRICS
Tobler R., Franssen S. U., Kofler R., Orozco-Terwengel P., Nolte V., Hermisson J., et al. (2014). Massive Habitat-Specific Genomic Response in D. melanogaster Populations during Experimental Evolution in Hot and Cold Environments. Mol. Biol. Evol. 31, 364–375. doi: 10.1093/MOLBEV/MST205
Trotta V., Calboli F. C. F., Ziosi M., Guerra D., Pezzoli M. C., David J. R., et al. (2006). Thermal plasticity in Drosophila melanogaster: A comparison of geographic populations. BMC Evolutionary Biol. 6. doi: 10.1186/1471-2148-6-67
Van Der Have T. M., De Jong G. (1996). Adult size in ectotherms: temperature effects on growth and differentiation. J. Theor. Biol. 183, 329–340. doi: 10.1006/JTBI.1996.0224
van Heerwaarden B., Sgrò C. M. (2017). The quantitative genetic basis of clinal divergence in phenotypic plasticity. Evolution 71, 2618–2633. doi: 10.1111/EVO.13342
Watanabe L. P., Riddle N. C. (2021). GWAS reveal a role for the central nervous system in regulating weight and weight change in response to exercise. Sci. Rep. 11, 1–13. doi: 10.1038/s41598-021-84534-w
Worland M. R. (1996). The relationship between water content and cold tolerance in the arctic collembolan Onychiurus arcticus (Collembola: Onychiuridae). Available online at: http://www.eje.cz/artkey/eje-199603-0006_The_relationship_between_water_content_and_cold_tolerance_in_the_arctic_collembolan_Onychiurus_arcticus_Collem.php (Accessed August 4, 2024).
Keywords: thermal plasticity, thermal adaptation, Drosophila, experimental evolution, fecundity, body size, canalization, body water
Citation: Roy R, Chattopadhyay A, Deb Sharma S, Mondal A, Biswas P and Ghosh SM (2025) Reduction in thermal plasticity of life history traits in response to cold selection: an experimental evolution study using Drosophila melanogaster. Front. Ecol. Evol. 12:1452948. doi: 10.3389/fevo.2024.1452948
Received: 21 June 2024; Accepted: 26 December 2024;
Published: 24 January 2025.
Edited by:
Miguel Brun-Usan, Autonomous University of Madrid, SpainReviewed by:
David M. Rand, Brown University, United StatesRoland Zimm, Institute of Functional Genomics Lyon, France
Copyright © 2025 Roy, Chattopadhyay, Deb Sharma, Mondal, Biswas and Ghosh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shampa M. Ghosh, c2hhbXBhLmdob3NoQGtpaXRiaW90ZWNoLmFjLmlu
†These authors share first authorship
 Rishav Roy
Rishav Roy Aradhya Chattopadhyay
Aradhya Chattopadhyay Sreebes Deb Sharma
Sreebes Deb Sharma Aharna Mondal
Aharna Mondal Payel Biswas1,3
Payel Biswas1,3 Shampa M. Ghosh
Shampa M. Ghosh