Abstract
Hummingbirds have crucial ecological importance in natural and human-altered habitats in the Neotropics. Their unique biological characteristics imply a delicate energetic balance that drove the evolution of species-specific dominance and territorial behavior tactics that, in turn, shape the diversity and composition of nectarivorous communities. Understanding these factors could help improve conservation strategies, particularly important for eroding communities in cities. Our objective is to evaluate whether a species of territorial hummingbird, the Sparkling Violetear (Colibri coruscans), is able to modulate its aggressive behavior in relation to the identity of the species that invades its feeding territory, comparing between dry (relative depletion of nectar resources) and wet seasons, and analyzing the possible ecological factors that determine this response. Considering the maximization of energy efficiency, we hypothesize that the territorial aggressive responses of Sparkling Violetears will vary in relation to the territoriality and behavioral dominance of each intruder species, and that aggressive responses in the dry season will be greater compared to the wet season. We elicited aggressive behavioral responses with territorial songs playbacks from the four species that compose the urban nectarivorous bird community, including songs from their own species, characterizing eight behaviors that varied in aggressive intensity. We quantified the aggressive response in two ways: the number of observation events in which territorial Sparkling Violetears performed each behavior and by constructing an “aggressiveness score” for each territorial individual in each observation event. Territorial aggressive response varied significantly in relation to the identity of the intruding species, but the seasonal effect was only observed, as a more aggressive response in the dry season, towards heterospecific playbacks. We analyzed several hypotheses that could explain the species-specific aggressive response, concluding that the “risk to take-over” hypothesis, through wing morphology and maneuverability, best explains the modulation of the aggressive response in relation to the territoriality and behavioral dominance of each intruder species. These results are useful for urban planning if we elucidate the ecological conditions that could promote the coexistence of subordinate species with dominant ones.
1 Introduction
In the Neotropics, hummingbirds represent an important component of nectarivorous communities and their ecology has been widely studied, particularly in relation to their energy physiology (Schuchmann, 1999; Altshuler and Dudley, 2002; Suarez and Gass, 2002; Videler, 2005; Shankar et al., 2022). The highly demanding flight, small size and dependence on nectar as the main food source imply important constraints on the balance between energy intake and expenditure, leading to the optimization of the time and energy budgets dedicated to different activities (Wolf and Hainsworth, 1971; Powers and McKee, 1994; Shankar et al., 2020). The organization of budgets is not fixed, but flexible enough to respond short- and long-term environmental changes (Ewald and Orians, 1983; Ewald, 1985; Shankar et al., 2019).
Such flexibility also implies the behavioral tactics that a particular species engages in aggressive territorial behaviors against different species in its community (e.g. Lyon et al., 1977; Ewald and Carpenter, 1978; Ewald and Bransfield, 1987; Powers and McKee, 1994; Dearborn, 1998; Camfield, 2006; Mendiola-Islas et al., 2016). High competition for the nectar resource drove the evolution of different territorial and dominance tactics in hummingbirds (Feinsinger and Colwell, 1978; Bribiesca et al., 2019; Sargent et al., 2021), and each species, in relation to its specific tactics, would be expected to behave differentially compared to the tactics of the other species.
Dominance hierarchies and territorial to non-territorial strategies shape the diversity and composition of the hummingbird communities (e.g. Lyon, 1976; Abrahamczyk and Kessler, 2015; Martin et al., 2017; López-Segoviano et al., 2018, 2023; Fernandez-Duque et al., 2024). Several morphological and behavioral traits were hypothesized to predict each species’ particular position on the territorial and dominance spectrum in hummingbird communities (Feinsinger and Chaplin, 1975; Dearborn, 1998; Martin and Ghalambor, 2014; Bribiesca et al., 2019; Márquez-Luna et al., 2019; Sargent et al., 2021). Understanding the ecological drivers that explain the composition of nectarivorous communities through hierarchical dominance could help understand their vulnerability to environmental changes such as urbanization. Previous studies on this topic are scarce, and focusing mainly on feeding ecology, they point to a simplification of hummingbird communities favoring the most generalist and behaviorally dominant species (Maruyama et al., 2019; Puga-Caballero et al., 2020). However, no study to date has directly evaluated behavioral responses related to heterospecific hierarchical dominance. Nectarivorous are particularly important because the persistence of ecosystems functions and services depends to a high degree on pollination (Potts et al., 2016; Wenzel et al., 2020). A better understanding of the ecological factors that shape natural nectarivorous communities could improve conservation and management strategies for these communities in human-altered environments (Vitorino et al., 2021; Leimberger et al., 2022; Adedoja and Mallinger, 2024).
In the urban ecosystem of La Paz (Bolivia), a high-altitude city in the tropical Andes, the nectarivorous bird community is mainly composed of three hummingbirds and a nectar-robber passerine: the Giant Hummingbird (Patagona gigas), the Sparkling Violetear (Colibri coruscans), the Red-tailed Comet (Sappho sparganurus), and the Grey-bellied Flowerpiercer (Diglossa carbonaria). This simplified nectarivorous community allows us to evaluate whether a territorial hummingbird species modulate aggressive interactions in relation the recognition of the intruder species, and from here understand the ecological drives that determine the assembly of this urban nectarivorous community. The Sparkling Violetear is the most successful urban colonizing species since it is the most abundant, frequent and ubiquitous within the city (Villegas and Garitano-Zavala, 2010), and is recognized as a territorial and dominant species (Hainsworth, 1977; Fjeldså and Krabbe, 1990; Schuchmann, 1999; Bribiesca et al., 2019; Züchner et al., 2020; Sargent et al., 2021).
Our approach is to elicit aggressive behaviors in territorial individuals of Sparkling Violetear using the playback of territorial songs from conspecific and heterospecific birds. It is important to include, in addition to hummingbird species, the territorial song playback of the Grey-bellied Flowerpiercer, because aggressive interactions between Diglossa flowerpiercers and hummingbirds are common in Neotropical nectarivorous communities (Colwell, 1973; Fjeldså and Krabbe, 1990; Céspedes et al., 2019), which makes sense considering the significant energetic cost of nectar resource depletion due to nectar-robbing (Hazlehurst and Karubian, 2018). The acoustic playback technique has been widely used for various purposes in bird studies (Falls, 1992; De Rosa et al., 2022), including interspecific recognition and provocation of conspecific and heterospecific aggressive behaviors in songbirds (e.g. Emlen, 1972; Martin et al., 1996; Freeman, 2016; Louder et al., 2020). In the case of hummingbirds, playback techniques have mainly used to understand conspecific acoustic communication (Duque and Carruth, 2022), but very few studies have used this technique to determine heterospecific recognition (e.g. Cruz-Yepez et al., 2020). To our knowledge no previous studies used playback techniques to elicit heterospecific aggressive behaviors in hummingbirds.
The objective of our study is to evaluate whether territorial Sparkling Violetears are able to recognize the territorial songs of the four species of birds in their nectarivorous community, -emulating with playback an intrusion into feeding territories-, and if they react behaviorally accordingly. Furthermore, compare the response between the dry season (relative depletion of nectar resources) and the wet season as a natural variation in the value of the territories. Upon the assumption that natural selection could favor the ability to recognize the songs of each species and react differentially to maximize energy efficiency, we hypothesize that the aggressive responses of territorial Sparkling Violetears will vary in relation to territoriality and behavioral dominance of each species, also called “risk of take-over” hypothesis (Dearborn, 1998). Briefly, this hypothesis states that territory owners will have a more intense aggressive response against intruders of territorial species that represent the greatest threat of taking over the territory. Furthermore, if the energy balance between energy consumption and expenditure is involved, aggressive responses in the dry season will be greater compared to the wet season.
2 Materials and methods
2.1 Study site and species
The city of La Paz is located in western of Bolivia in an inter-Andean valley between 3,200 and 4,100 m (Figure 1). Although it is the administrative capital of the country, it does not have more than one million inhabitants (United Nations, 2020). La Paz has a marked seasonality governed by rain, with a peak rainfall between November and February and the dry season between May and August. As is particularly the case in the high mountain environments of the South American Andes, the phenology of flowering species is strongly marked by this seasonality, with flowering occurring for most species during the wet season (Morellato et al., 2013). The birds of La Paz breed mainly during the wet season.
Figure 1
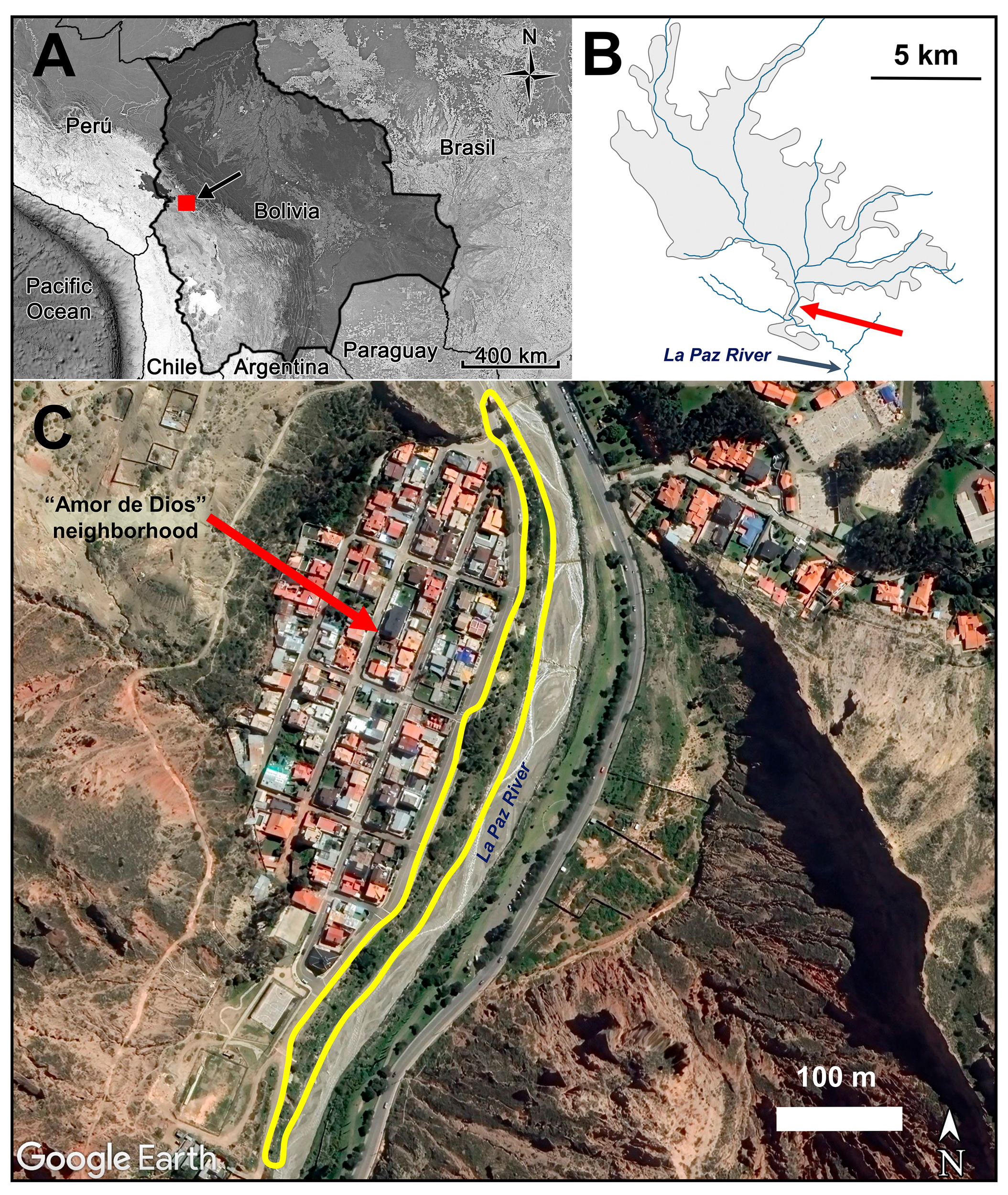
Position of the city of La Paz in Bolivia indicated by a black arrow (A); the red square represents the map in (B) in which the gray shadow polygon represents the area occupied by the city of La Paz in 2016, including the main rivers (in blue) that converge to the La Paz River in a north to south direction. The red arrow shows the position of our study site which appears in detail in (C) as a satellite image (Google Earth Pro 7.3.2, 2019, image date December 2016), in which the yellow polygon shows the secondary thicket of shrubs with the presence of territorial Sparkling Violetears.
Our study site was a secondary thicket of shrubs (-16.554509S -68.092369W to -16.554509S -68.093817W) next to a suburban residential neighborhood called “Amor de Dios”, which develops linearly 650 m in a north to south direction along the western bank of the La Paz River at 3,230 m altitude (Figure 1). This thicket of shrubs was dominated by the Tree tobacco (Nicotiana glauca), a small native evergreen tree that grows up to five meters. This species has yellow ornithophilic flowers that bloom throughout the year, being one of the few plant species that offers flowers during the dry season, representing an important and defensible nectar resource for the nectarivorous species, even in regions where the species is exotic (Sánchez Sánchez and Lara, 2024).
The three hummingbird species of our local community had been reported previously to feed on Tree tobacco (Fjeldså and Krabbe, 1990; Schuchmann, 1999), and in La Paz, the four nectarivorous species feed on it (pers. obs.). The four species vary widely in mass, wing and beak morphology, as well in abundance and frequency (Table 1). The Sparkling Violetear is a medium-sized hummingbird, widespread, abundant and common, distributed in the Andes from Venezuela to Argentina between 1,700 to 4,500 m, and it is found from wild to highly altered habitats by humans (Fjeldså and Krabbe, 1990; BirdLife International, 2016; Züchner et al., 2020). In the city of La Paz and its surroundings inhabits the entire urban gradient with the exception of areas deprived of vegetation, being the most abundant and common species compared to the other nectarivorous species (Villegas and Garitano-Zavala, 2010).
Table 1
| Giant Hummingbird | Sparkling Violetear | Red-tailed Comet | Grey-bellied Flowerpiercer | |
|---|---|---|---|---|
| Mass (g) (a) | 20.2 (10) | 6.7 (16) | 5.3 (10) | 11.0 (22) |
| Wing length (cm) (b) | 13.57 (6) | 7.54 (28) | 6.32 (5) | 6.83 (15) |
| Beak length (cm) (b) | 4.52 (6) | 2.91 (28) | 2.01 (5) | 1.44 (15) |
| Hand-wing index (b) | 64.5 | 62.5 | 63.1 | 16.9 |
| LWD (g/cm2) (c) | 0.021467 | 0.023313 | 0.027490 | |
| Local abundance (d) | 16 | 89 | 20 | 29 |
| Local frequency (d) | 14.4 | 53.8 | 18.3 | 18.3 |
| Behavioral dominance | BD (e) | BD (e) | NBD (e) | BD (f) |
| Year-round residence | M (g) | R (h) | R (h) | R (h) |
| Territoriality(h) | ** | *** | * | *** |
Morphological, behavioral and ecological information of the four bird nectarivorous species studied in the city of La Paz, three hummingbirds and one passerine species specialized as nectar-robber, the Grey-bellied Flowerpiercer.
Morphometric data include means of mass, wing length, beak length and hand-wing index for the four nectarivorous species, and wing disc loading (LWD) for the three hummingbirds; in parenthesis the number of individuals considered without distinction of gender. Information for the birds’ populations in the city of La Paz includes relative abundance and relative frequency. Behavioral data include classifications as behaviorally dominant (BD) to non-behaviorally dominant (NBH), year-round resident (R) to seasonally migrant (M), and territoriality/kleptoparasitism classified on an arbitrary scale between mainly territorial (***), occasionally territorial (**), and frequently kleptoparasitic (*) because species could move plastically between both strategies.
(a)Data obtained from Dunning (2008). (b)Data obtained from Tobias et al. (2022). (c)Calculated only for hummingbirds using the formula: (Feinsinger and Chaplin, 1975). with data from Tobias et al. (2022). (d)Data available in Villegas and Garitano-Zavala (2010) for the city of La Paz, this information was obtained from 104 observation stations randomly distributed from the hardest core to the peri-urban habitats of the city of La Paz, where local abundance is the sum of abundances measured across all the observation stations and frequency is the percentage of observation stations in which each species was observed. (e)Classification proposed by Bribiesca et al. (2019). (f)Classification inferred from information in Hilty (2011) and our own field observations. (g)Classification following the proposal of Williamson et al. (2024) for the Giant Hummingbird populations that correspond to La Paz; (h)Classification inferred from the behavioral descriptions of Fjeldså and Krabbe (1990), Schuchmann (1999) and Hilty (2011), and our own field observations.
All four species can behave as territorial or kleptoparasitic depending on certain environmental factors, but Sparkling Violetear and Grey-bellied Flowerpiercer are more frequently territorial (Fjeldså and Krabbe, 1990; Schuchmann, 1999; Hilty, 2011). The level of behavioral dominance, understood as the relative heterospecific hierarchical position of each species among all other species in nectarivorous bird communities (Bribiesca et al., 2019) is also variable between the species, as well is the level of residence. We include such behavioral information obtained from the literature or personal observations in Table 1. The Sparkling Violetear is a territorial and behaviorally dominant species (Bribiesca et al., 2019) that defends three types of territories: nesting, display and feeding (Schmidt-Marloh and Schuchmann, 1980; Zerda-Ordoñez, 1994); the first is defended only by females, the second only by males and the third is held and defended individually by anyone. We worked only with feeding territories without differentiation of males and females because sexes are very alike and hard to distinguish (Hainsworth, 1977; Schuchmann, 1999). Feeding territories are relatively small ranging between 130 to 640 m2 (Zerda-Ordoñez, 1994).
2.2 Study design
Hummingbirds have learning components in their acoustic communication (Duque and Carruth, 2022), which allows for geographic and dialect variations as has been shown to occur in Sparkling Violetear, who has the ability to recognize neighbors through their songs (Gaunt et al., 1994). This situation could potentially alter aggressive responses towards the known neighbors (Monte et al., 2023). For this reason, we do not use recordings of songs from La Paz. Instead, we obtained the territorial songs from the xeno-canto website (http://www.xeno-canto.org/), selecting recordings with the clearest territorial song without overlapped songs from other birds. All the selected songs came from Andean places in South America, and were: for the Giant Hummingbird song XC17922, for the Sparkling Violetear song XC257806, for the Red-tailed Comet song XC19534, and for the Grey-bellied Flowerpiercer song XC2093. With the clearest songs we edited a continuous three-minute track for the playbacks of each species. In hummingbirds, both sexes emit songs, particularly in feeding territories (Schuchmann, 1999), but there are no studies on sexual differences in hummingbird songs with the sole exception of Anna’s Hummingbird (Glassman et al., 2024). In this way, the songs deposited in xeno-canto.org for our studied species do not specify the sex.
We tested the effectiveness of each playback of our “foreign” songs by conducting a preliminary behavioral test on six Sparkling Violetears defending feeding territories at a different location (the University Campus of the Universidad Mayor de San Andrés in La Paz). These preliminary observations informed us that the songs of the four species could be recognized by local Sparkling Violetears despite their geographical origin. They also allowed us to prepare a complete ethogram with all the aggressive behaviors elicited by the playbacks, and informed us that the three-minute tracks were sufficient for a complete behavioral recording. In Table 2 we describe the eight aggressive behaviors identified, and to each we assign an integer value as a behavior score assuming the level of associated costs deduced from previously published information (Schmidt-Marloh and Schuchmann, 1980; Fjeldså and Krabbe, 1990; Zerda-Ordoñez, 1994; Schuchmann, 1999; Züchner et al., 2020). Behavior score ranged from those in which the individual performed only a few movements without leaving the perch (score of 1) to those in which the individual left the perch and flew toward the sound source (score of 5).
Table 2
| Behavior name (code) | Description of the behavior | BS/IV |
|---|---|---|
| Tail movement (TM) | Perched on a branch, spreads and flaps the tail | 1/No |
| Wings movement (WM) | Perched on a branch, flaps the wings | 1/No |
| Vocalizing on perch static song “1” (V1) | Perched on a branch, emits monotonous songs with repetitive metallic chips described as “tzirp” by Fjeldså and Krabbe (1990), or as “tlik” by Züchner et al. (2020). | 2/Yes |
| Vocalizing on perch static song “2” (V2) | Perched on a branch, emits songs with repetitive notes described as “rrt” by Fjeldså and Krabbe (1990), or as “dijit” by Züchner et al. (2020). | 2/Yes |
| Vocalizing while flying to feed (VF) | While flying to drink nectar from flowers, emits a vocalization described as “drrr … drrr…” by Züchner et al. (2020). | 3/Yes |
| Agonistic display “1” (vertical flight) (A1) | Departing up from perch vertically performs while flying an eight-shaped display emitting a complex song described as “tzeezee-zirr” by Fjeldså and Krabbe (1990); after that returns to the same perch. | 4/No |
| Agonistic display “2” (horizontal flight) (A2) | As the agonistic display “1”, including the same song, but performed horizontally; after that returns to the same perch or towards another perch. | 4/No |
| Exploring the sound source (ES) | Flies towards the sound source and explores it. | 5/No |
Description of the eight aggressive behaviors of territorial individuals of Sparkling Violetear elicited by the playback of the songs of the four species belonging to the local community of nectarivorous birds of La Paz.
The behavior score (BS) and the intensity value (IV) are specified, the latter only when vocalizations are emitted with the behavior. We assign BS to each behavior in relation to the assumed cost, and the IV as the number of notes of vocalizations produced during the performance of the behavior.
We worked only on weekends in the dry (lower supply of flowers) and wet seasons from May 2015 to February 2017. We visited the study site only one day in the weekends, without rain or strong wind, and from now on we refer to each visit as “observation events”. At our study site, at least four territorial Sparkling Violetears defending feeding territories were present at each of our observation events. Feeding territories generally involved one to three adjacent Tree tobacco individuals. The control and defense of a feeding territory is recognizable because the individual perches visibly on top of the nectar source, constantly emits the “Static song 1” (Table 2) and performs many feeding rounds (Zerda-Ordoñez, 1994). We did not capture or mark individuals because not all individuals observed in each observation event were able to remain at the study site for the entire study period, and continued capture in such a small patch would potentially disrupt the normal behavior of territorial individuals. We neither control each territory nor each individual in relation to the characteristics and quality of the territories occupied at a particular event, because the territory-owner relationship is likely to vary widely over time.
Our response units or “subjects” is each observation event. Such observation events began half an hour after sunrise when an observer wearing camouflage clothing, who was always the same person, randomly located the first solitary territorial Sparkling Violetear defending its feeding territory, regardless of its sex and territory occupied. It is important to note that our evaluations involve a certain moment in the life of the selected individuals, a moment in which they are clearly demonstrating possession advertisement behaviors and a predisposition to defend their feeding territory. Our evaluations involve the simulation of invasion at that small moment in its life by an individual of a potentially competing nectarivorous species. The observer held a WS-887 speaker (WSTER, Shenzhen Haozhijie Electronic Technology Co., Ltd., Shenzhen, China, 7cm length by 6.6 cm diameter, black color, output 5W, frequency response: 280 to 16 kHz, transmission range 50 m) connected to cell phone through Bluetooth on a branch two meters below the perched individual. The observer hid five meters away so as not to be seen by the bird, but at the same time to be able to observe all its behavioral responses to playback. After at least five minutes and when the territorial Sparkling Violetear was quiet and still on its perch, the observer activated the speaker remotely and played the track of a single nectarivorous species without pauses for the entire three minutes. During this period, we recorded all territorial behaviors performed by the observed individual using the previously obtained ethogram. If we detected any other external stimulus in addition to our playback during the three minutes, such as the physical presence or vocalizations of nectarivorous species, or others disturbances related to people, pets or any other animal species we eliminated such data. We recorded the behaviors as presence/absence, and in the case of the behaviors accompanied by vocalizations (V1, V2 and VF), we also recorded the total number of individual notes of the vocalizations emitted in each behavior type during the observation event as a measure of behavioral intensity (Table 2). The songs of Sparkling Violetear in V1 and V2 behaviors, as well as the vocalizations emitted while flying in VF, are composed of simple, repetitive notes in a non-fixed number, allowing them to be easily counted.
Once we completed the work with the first territorial Sparkling Violetear, we looked for another territorial individual that was at least 100 m away, and we repeated the same procedure changing the track to the song of another nectarivorous species. Since the speaker has a range of 50 m, we assume that the first playback has no effect on the second individual. We repeated this procedure until four different territorial Sparkling Violetears were completed for each observation event such that each of them was exposed to the song playback of only one of the four nectarivorous bird species. Because individuals were not marked, we separated the observation events by at least one week to avoid habituation and responses that might depend on previous playbacks, increasing the chances that each of the behavioral responses in consecutive observation events was independent. Due to the possibility that the same individuals occupied the same territory or adjacent territories in the same season of each year, we also avoided in each observation event to follow the same order of song playbacks according to the north-south direction of the thicket of shrubs. We are aware that despite all these precautions to control the effect of confounding factors, it is still possible that in the same season of the same year, an individual has been subjected to the song of the same species more than once, causing a pseudoreplication, however, we consider this possibility small. For each season of a year, we carried out four observation events, obtaining four independent behavioral responses for each of the territorial song of the four species of nectarivorous birds, and as we repeated the procedure in two consecutive years, we finally obtained eight independent behavioral responses per dry and wet season and playback.
2.3 Data analysis
We adopted two approaches to behavioral data analysis. First, we considered the “number of observation events” (out of a total of eight per season) in which each of the eight aggressive behaviors was elicited in response to playbacks (positive responses) versus the absence of the behavior (negative responses). Our second approach was the construction of an “aggressiveness score” calculated for each territorial Sparkling Violetear in each observation event, taking as reference the method applied by Emlen (1972) for the construction of his “hybrid index” to quantify behavioral responses of the Indigo Bunting (Passerina cyanea) towards playbacks. But unlike Emlen (1972), our aggressiveness score value per observation event is the sum of the scores (BS values in Table 2) of all types of aggressive behaviors performed during the three-minutes playback (rather than using only the value of the behavior with the maximum score), adding for behaviors V1, V2 and VF, when present, the log10 of the number of individual notes produced in the vocalizations as measure of intensity (IV values in Table 2).
We applied Generalized Lineal Models (GLM) to analyze both types of data. For the “number of observation events” we used a Binomial probability distribution with a Logit link function, introducing into the model the main effects of the three fixed factors as predictors: nectarivorous species to which the playback belongs (four levels), season of the year (two levels), and provoked aggressive behavior (eight levels), plus the interaction between species*season with the Bonferroni test as post-hoc (Model 1 in Table 3). We will not consider the interactions with the behavior factor due to a quasi-complete separation in the data making the validity of the model fit uncertain. We subsequently ran this same model with only three nectarivorous species excluding the Sparkling Violetear data (Model 2 in Table 3). For the “aggressiveness score” we used a Normal probability distribution with Identity link function because the deviance residual distribution had a normal distribution (Shapiro-Wilk’s test = 0.970; p=0.122). We used a full factorial model with the factors nectarivorous species and season as predictors, plus the interaction between species*season with the Bonferroni test as post-hoc (Model 3 in Table 3). We subsequently ran this same model with only three nectarivorous species excluding the Sparkling Violetear data (Model 4 in Table 3). We performed all statistical analysis and graphs with IBM SPSS Statistics v 29 (IBM Corporation, 2023), considering a significance threshold value of 0.05.
Table 3
| Likelihood Ratio Chi-Square | d.f. | p-value | Akaike’s Information Criterion (AIC) | Model’s deviance (D) | Nule model’s deviance (Do) | Variation explained by the model (%) | |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| Model fit statistics | 120.95 | 14 | <0.001 | 195.80 | 74.40 | 195.36 | 61.9 |
| Species | 29.12 | 3 | <0.001 | ||||
| Behaviors | 93.18 | 7 | <0.001 | ||||
| Season | 3.15 | 1 | 0.076 | ||||
| Species*Season | 2.75 | 3 | 0.432 | ||||
| Model 2 | |||||||
| Model fit statistics | 96.50 | 12 | <0.001 | 139.22 | 55.28 | 151.78 | 63.6 |
| Species | 16.71 | 2 | <0.001 | ||||
| Behaviors | 78.90 | 7 | <0.001 | ||||
| Season | 4.44 | 1 | 0.035 | ||||
| Species*Season | 0.92 | 2 | 0.632 | ||||
| Model 3 | |||||||
| Model fit statistics | 25.42 | 7 | <0.001 | 358.42 | 765.10 | 1138.15 | 32.8 |
| Species | 22.89 | 3 | <0.001 | ||||
| Season | 1.12 | 1 | 0.289 | ||||
| Species*Season | 2.50 | 3 | 0.475 | ||||
| Model 4 | |||||||
| Model fit statistics | 18.58 | 5 | 0.002 | 259.84 | 471.08 | 693.81 | 32.1 |
| Species | 15.89 | 2 | <0.001 | ||||
| Season | 3.18 | 1 | 0.075 | ||||
| Species*Season | 0.57 | 2 | 0.751 | ||||
Statistical results for the Generalized Lineal Models (GLM) of four different models.
Model 1 is “number of observation events” variable ~ species + behaviors + season + species*season using a binomial probability distribution with logit link function considering data from all four nectarivorous species; model 2 is the same as model 1 but without considering Sparkling Violetear data. Model 3 is “aggressiveness score” variable ~ species + season + species*season using a normal probability distribution with identity link function considering data from all four nectarivorous species; model 4 is the same as model 3 but without considering Sparkling Violetear data. The percentage of variation explained by each model was calculated as: .
3 Results
The model for the number of events in which each behavior was elicited in territorial individuals of Sparkling Violetear (Model 1) was significant (Table 3) and explained 62% of the variation. The model for the individual reaction of each territorial Sparkling Violetear in each observation event quantified as “aggressiveness score” (Model 3) explained only 33% of the variation because the responses of individuals varied widely among them, as shown by the dispersion measures (Table 4); but despite that the model was significant (Table 3). Both models consistently show the same pattern, the aggressive response varied significantly in relation to the song identity of the nectarivorous bird species (Table 3), being significantly lower towards the songs of the Red-tailed Comet, and significantly higher towards the songs of the Grey-bellied Flowerpiercer and its own species; the response towards the Giant Hummingbird was between these two extremes (Figure 2).
Table 4
| Species | Sparkling Violetear | Grey-bellied Flowerpiercer | Giant Hummingbird | Red-tailed Comet | |||||
|---|---|---|---|---|---|---|---|---|---|
| Season | D | W | D | W | D | W | D | W | |
| Aggresiveness score | Mean | 7.50 | 8.74 | 8.17 | 5.62 | 5.39 | 4.50 | 2.85 | 1.38 |
| SD | 2.34 | 6.04 | 1.53 | 3.87 | 2.14 | 5.22 | 2.43 | 3.51 | |
| Min | 3.30 | 0.00 | 6.08 | 1.00 | 2.70 | 0.00 | 0.00 | 0.00 | |
| Max | 10.4 | 16.2 | 10.5 | 12.2 | 8.18 | 12.9 | 7.48 | 10.0 | |
| Proportion of observation events | Behava | ||||||||
| TM | 0.13 | 0.13 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| WM | 0.25 | 0.25 | 0.13 | 0.25 | 0.00 | 0.25 | 0.00 | 0.13 | |
| V1 | 0.63 | 0.50 | 0.75 | 0.50 | 0.75 | 0.50 | 0.50 | 0.13 | |
| V2 | 0.88 | 0.50 | 1.00 | 0.13 | 0.75 | 0.13 | 0.38 | 0.13 | |
| VF | 0.00 | 0.50 | 0.50 | 0.25 | 0.00 | 0.38 | 0.00 | 0.00 | |
| A1 | 0.25 | 0.13 | 0.00 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | |
| A2 | 0.25 | 0.13 | 0.13 | 0.13 | 0.00 | 0.00 | 0.00 | 0.13 | |
| ES | 0.13 | 0.50 | 0.00 | 0.13 | 0.13 | 0.13 | 0.13 | 0.00 | |
| Totalb | 0.31 | 0.33 | 0.33 | 0.20 | 0.20 | 0.17 | 0.13 | 0.06 | |
Descriptive statistics of the “aggressiveness score” and values of the proportion of the “number of observation events” in which aggressive behaviors were executed by territorial individuals of Sparkling Violetear towards the intrusion simulated by playback the songs of the four species of nectarivorous birds of their community, for the dry (D) and wet (W) seasons.
Aggressive behaviors are: Tail movement (TM), Wings movement (WM); Vocalizing on perch song “1” (V1), Vocalizing on perch song “2” (V2), Vocalizing while flying to feed (VF), Agonistic display “1” (A1), Agonistic display “2” (A2), and Exploring the sound source (ES). (a) is the proportional value of each behavior performed in the total of eight observation events per each intruder species per season, (b) is the proportional value for the total aggressive behaviors performed in each season towards each intruder species.
Figure 2
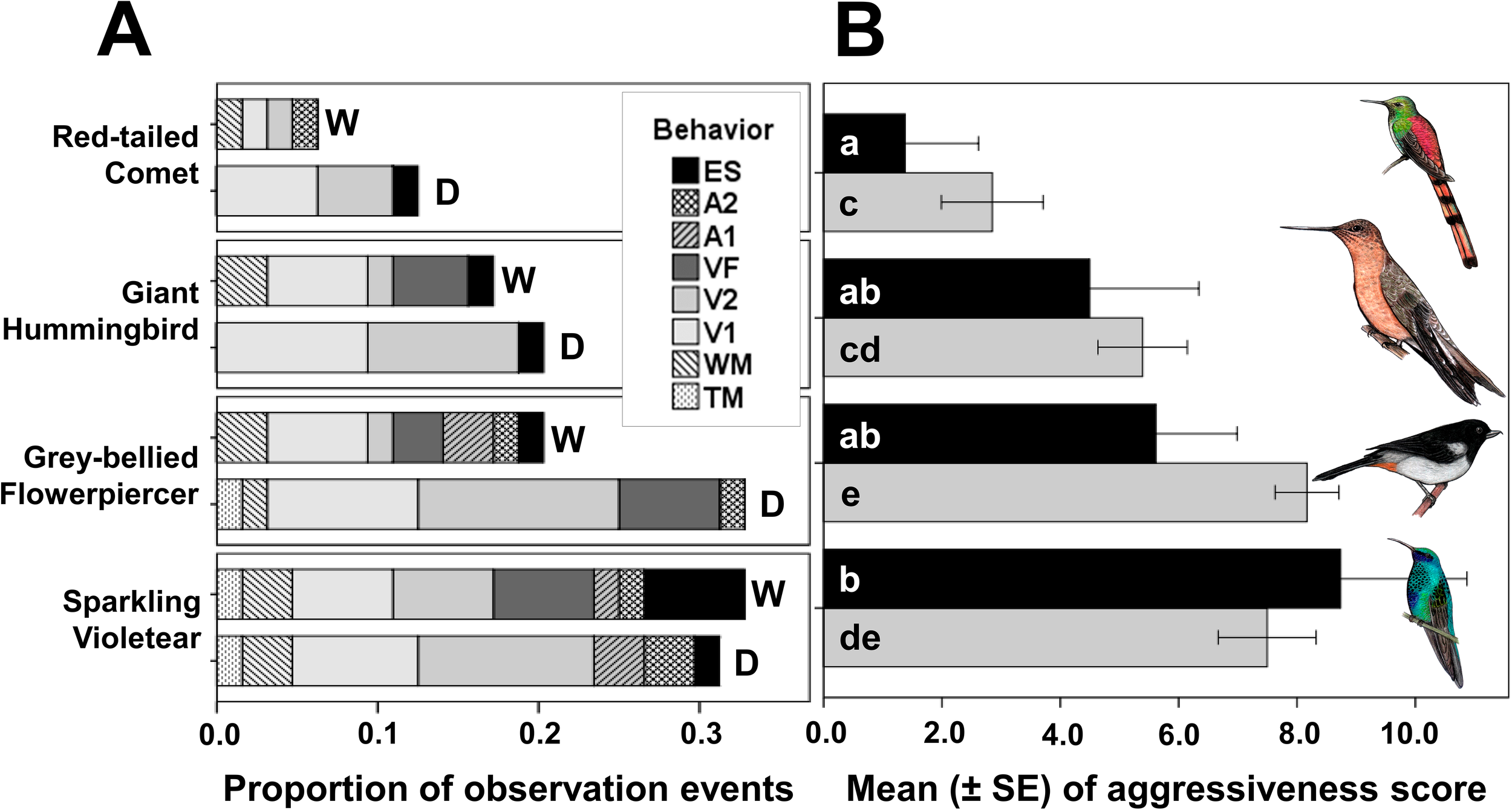
Proportion of the “number of observation events” in which each of the eight aggressive behaviors of Sparkling Violetear were elicited by the playbacks of territorial songs of each of the four species of the nectarivorous bird community (A), separated by wet (W) and dry (D) seasons. Bars of the mean (± SE) of the “aggressiveness score” of Sparkling Violetear territorial individuals towards the songs of each of the four species of nectarivorous birds and seasons (B), wet season in black and dry season in gray. a, b shows the subgroups for wet season and c - e the subgroups for the dry season. Behavior abbreviations: Exploring the sound source (ES), Agonistic display “2” (A2), Agonistic display “1” (A1), Vocalizing while flying to feed (VF), Vocalizing on perch song “2” (V2), Vocalizing on perch song “1” (V1), Wings movement (WM), and Tail movement (TM). The drawings of the birds used under the generous authorization of the artist Daniela Ticona.
Although overall, the aggressive response of territorial Sparkling Violetears against the playbacks appeared to be more intense in the dry season compared to the wet season (Figure 2), the difference was not statistically significant for either the number of observation events (Model 1) nor for the aggressiveness score (Model 3) (Table 3). But interestingly, Sparkling Violetear’s seasonal pattern of aggressive response to the songs of its own species was opposite to that of the other three species, increasing in the wet season. Even though both models do not detect a significant interaction between the factors species and season (Table 3) that could explain the absence of a significant effect of season, when we extracted the Sparkling Violetear data in the analyses, that is, comparing the behavioral responses only with the other three nectarivorous species except its own, we detected that aggressive responses were significantly higher in the dry season only in the number of observation events model (Model 2 in Table 3).
There were significant differences in the number of events in which each behavior was elicited (Models 1 and 2 in Table 3). The two aggressive behaviors significantly most elicited by playbacks of all four species in both seasons were the vocalizations on perch V1 and V2 (Table 4 and Figure 2A), which accounted for 58.6% of all elicited behaviors. The least elicited behavior was tail movement on perch (TM) directed only against its own species and the Grey-bellied Flowerpiercer (Table 4). The entire repertoire of aggressive behaviors occurred only towards the songs of its own species and the songs of the Grey-bellied Flowerpiercer, and only five of them towards the Red-tailed Comet and the Giant Hummingbird. The agonistic displays were elicited only towards its own species and the Grey-bellied Flowerpiercer, with the exception of a single occasion elicited toward the song of the Red-tailed Comet. When territorial Sparkling Violetear individuals were confronted with the songs of their own species, vocalizations while feeding occurred only in the wet season, and exploration of the sound source was four times greater compared to the dry season (Table 4 and Figure 2A).
4 Discussion
Playbacks of territorial songs of the four species of the La Paz nectarivorous community elicited behavioral responses in territorial Sparkling Violetears that ranged from simple body postural changes on perch to agonistic displays and exploration of sound source. Similar to the studies of Cruz-Yepez et al. (2020), who elicited aggressive responses to songs among subspecies of the Wedge-tailed Sabrewing (Campylopterus curvipennis) complex, and the study by Duque et al. (2020) on the responses of the Ecuadorian Hillstar (Oreotrochilus chimborazo) to high-frequency conspecific acoustic signals, we obtained behavioral responses that could be interpreted as attention, advertisement, and exploration towards the sound signal of a potential competitor. In our study the preferred behaviors used were advertisement carried out while perching, which implies energy savings by not reacting with costly behaviors if they are not necessary, and escalating to behaviors in flight only depending on the intruding species. Similar to the aforementioned studies (Cruz-Yepez et al., 2020; Duque et al., 2020) the most aggressive behavior elicited was the exploration of the sound source. More aggressive behaviors, such as chasing or fighting, were not elicited because they occur when visual recognition signals are also present (Grether, 2011). In future studies it would be interesting to combine acoustic and visual signals to obtain a more complete repertoire to better understand the modulation of the aggressive behaviors.
We obtained that the model based on the number of occasions in which each aggressive behavior was elicited, as well the model for the total aggressive response of each territorial individual summarized as the aggressiveness score showed similar results. The aggressiveness score that we propose is a way to quantify in a single number the total set of behaviors executed by each individual, but the score is not free of errors since it is based on quantifying behaviors with several assumptions. Probably the high standard deviations obtained, the lower percentage of variability explained by the model and the difficulty to obtain significant differences between seasons are related to this. Nevertheless, the score has the advantages offered by continuous quantitative measures, we believe that this is an interesting way to quantify behaviors and that the validity of these indices could be further investigated.
In the dry season, when other food resources are scarce (Morellato et al., 2013) and therefore established territories in Tobacco Tree patches were more valuable, apparently the territorial Sparkling Violetears were more aggressive against the songs of the three nectarivorous species other than their own. In our study, the depletion of nectar resources is only inferred from general floral phenology, but measurements of the availability of nectar in territories as an energetic source, as well its variation in time and space, could confirm such inference. In studies in which the energetic value of hummingbird territories was manipulated, territorial individuals also increased more energetically costly aggressive behaviors against competitors when the relative food value of the owner’s territory increased (Ewald and Carpenter, 1978; Ewald and Orians, 1983; Ewald and Bransfield, 1987; Powers and McKee, 1994; Dearborn, 1998; Camfield, 2006). The pattern was the opposite when songs of their own species were played, including a higher proportion of flying exploration of the sound source. This is probably because in the wet season territorial individuals not only defended feeding territories, males also attracted, controlled and defended their potential or actual reproductive partners, and females defended their nesting territories (Schmidt-Marloh and Schuchmann, 1980; Zerda-Ordoñez, 1994). Because in our study we did not differentiate males and females and because males behave aggressively towards females even in the breeding season (Schmidt-Marloh and Schuchmann, 1980), we cannot disentangle the proportion of aggressive behaviors related to different motivations.
The variations in the aggressive responses of territorial Sparkling Violetears were very consistent depending on the nectarivorous species to which the territorial song playbacks belonged. Clearly, the most intense aggressive response was elicited against playbacks of its own species and the Grey-bellied Flowerpiercer, while the least aggressive responses occurred against playbacks of the Red-tailed Comet. The aggressive responses against the songs of the Giant Hummingbird were intermediate between both extremes.
The modulation of the interspecific aggressive response towards the opponent depends on the balance between the benefits of obtaining nectar and the costs of time and energy invested in aggressive behaviors as well as the risks of possible injuries (Wolf, 1978). Dearborn (1998) synthesized the possible reasons that could explain the variations in the interspecific aggressive responses of territorial hummingbirds into five hypotheses and their respective predictions. The “risk of take-over” hypothesis predicts a more intense aggressive response against territorial species that pose the greatest threat of territory taking. The “wing disc loading (LWD)” hypothesis predicts more aggressive responses against species with lower LWD than the owner because less maneuverability is assumed in aggressive encounters. The “intruder detectability” hypothesis predicts that smaller species are more difficult for the owner to detect, so less aggressive behavior could be elicited against them. The “potential for resource removal” hypothesis predicts that species with a better capability to extract nectar relative to beak morphology could elicit more aggressive responses. Finally, the “cost of engagement” hypothesis predicts an inverse relationship between the intensity of aggression and the risk of injuries to the territory owner; this hypothesis is mainly related to the body mass and predicts a less aggressive response to opponents larger than the owner. In our study, we cannot test the “intruder detectability” hypothesis because we simulated territory invasion with acoustic cues only, and therefore the information about visual cues for intruder detectability and recognition did not vary.
Our results on the ordering of intruder species according to the level of aggressive responses of territorial Sparkling Violetear individuals do not support three of the other four hypotheses. For the “cost of engagement” hypothesis, we should have observed the most aggressive responses against the Red-tailed Comet and the least aggressive response against the Giant Hummingbird considering their respective masses (Table 1). For the “wing disc loading (LWD)” hypothesis, and considering only the hummingbirds of our community, we would expect greater aggressiveness towards the Giant Hummingbird and less towards the Red-tailed Comet (Table 1). For the “potential for resource removal” hypothesis, we would expect more aggressive responses against the Giant Hummingbird, the species with the longest beak (Table 1). However, short beaks are not a limitation because access through the floral opening is not the only way to access the nectar. In fact, as a nectar-robber, the Grey-bellied Flowerpiercer gains access by piercing the base of the corolla and periodically revisiting the hole for nectar (Hilty, 2011), and the Red-tailed Comet can also obtain nectar from the holes made from other nectar robbers (Tellería et al., 2024). It is not possible to conclude about resource removal potential based solely on beak morphology in our nectarivorous community.
The only hypothesis we propose fully explains our results is the “risk of take-over”. Although Dearborn (1998) only refers to the level of territoriality of each species, the aggressive social organization of nectarivorous bird communities also depends on the hierarchical dominance (Wolf, 1978). We propose that the level of aggressiveness of the behavioral responses elicited in territorial Sparkling Violetears increased from less territorial and submissive species to the more territorial and behaviorally dominant species, and that aggressive behaviors were executed from those of lowest to highest energetic cost, following the same progressive aggressiveness (Figure 3). From this proposal, it is possible to predict that the bird species in the nectarivorous community studied are ordered in territoriality and dominance according to: first, Sparkling Violetear equal to Grey-bellied Flowerpiercer, second, the Giant Hummingbird, and third, the Red-tailed Comet (Figure 3).
Figure 3

The proposed relationship between the two components of the risk of take-over, territoriality and behavioral dominance, on the decisions of territorial Sparkling Violetears to perform behaviors of variable level of aggressiveness in relation to the identity of the intruder species simulated by playback (acoustic cues). On the right are the behavior categories that were used most frequently in relation the level of aggressiveness and the playback of the territorial songs of each nectarivorous species: Sparkling Violetear (SV), Grey-bellied Flowerpiercer (G-b F), Giant Hummingbird (GH) and Red-tailed Comet (R-t C). Fighting was not observed in this study, but it is assumed that it would be elicited in combination with visual cues. The drawings of the birds used under the generous authorization of the artist Daniela Ticona.
The Sparkling Violetear is a territorial and behaviorally dominant species over other hummingbirds (Schuchmann, 1999; Bribiesca et al., 2019; Sargent et al., 2021). Bribiesca et al. (2019) propose that mass is a supertrait that explains the behavioral dominance in hummingbirds, and Sargent et al. (2021) predicts that the largest hummingbirds would be the most territorial. However, the Sparkling Violetear it is not the largest species in our nectarivorous community, but rather the Giant Hummingbird, which was also described as a behaviorally dominant (Bribiesca et al., 2019) and territorially aggressive species (Fjeldså and Krabbe, 1990; Schuchmann, 1999). Although the high aggressive response against playbacks of its own species could be explained by the expected maximum overlap in resource use with conspecifics (Peiman and Robinson, 2010), this does not explain the almost equal high level of aggressiveness against the Grey-bellied Flowerpiercer. What is the reason why the more aggressive response of the territorial Sparkling Violetears against the playbacks of the Giant Hummingbird was not elicited?
Sargent et al. (2021) propose that a territorial species will face selection for large body size and greater maneuverability with the trade-off of higher flight costs. Since body size alone does not explain the ordering of aggressiveness in our nectarivorous community relative to the “risk of take-over” hypothesis, it is important to consider also maneuverability and flight costs. Feinsinger and Chaplin (1975) propose a positive relationship between wing disc loading (LWD) and maneuverability, and hence territoriality. The Giant Hummingbird has the lowest LWD value in our hummingbird community (Table 1), suggesting disadvantages in its maneuverability and territoriality. As was observed that LWD was not an adequate predictor of maneuverability and competitive ability of hummingbird species (Altshuler et al., 2004), Sargent et al. (2021) propose, instead, that wing loading (the ratio of body weight to wing area) would be a better predictor due its direct and positive relation with aspect ratio. When data on wing area or wing span (variables necessary to calculate wing loading) are not available, the “hand wing index” (HWI) is a good indicator of the aspect ratio and, from here, a proxy of the bird characteristics such as aerial lifestyles and dispersal (Sheard et al., 2020; Weeks et al., 2022). HWI data are available for our species (Tobias et al., 2022), and from here, the Giant Hummingbird has the highest HWI value among all our hummingbird species (Table 1). This means that the Giant Hummingbird has a high aspect ratio associated with longer and narrower wings that predispose the species to fly long distances at the expense of maneuverability and territoriality. In fact, Fjeldså and Krabbe (1990) describe the flight of the Giant Hummingbird as slow, deep wing strokes combined with glides that resembles swallows.
The morphology of the wings could better explain why the Giant Hummingbird is not the most territorial species in our nectarivorous community if we consider the flight energetics. Several studies were conducted on the costs of hovering flight in hummingbirds using different methodologies (Videler, 2005). Considering hovering metabolic rates reported for several hummingbird species, Voigt and Winter (1999) propose a mean mass-specific hovering flight cost of 393 ± 98.8W kg-1. For Sparkling Violetear, Berger (1985) report a metabolic cost in hovering of 1.90 W, which represents a mass-specific hovering flight cost of 223.53 W kg-1 (datum obtained using the author’s reported mass). For the Giant Hummingbird, Fernández et al. (2011) reported a metabolic cost in hovering of 2.92 W, which represents 166.86 W kg-1 (datum obtained using the mass reported by the authors). Obviously, the absolute energy cost of the Giant Hummingbird is higher than the Sparkling Violetear, but it is more energy efficient considering the cost per gram.
Our interpretation from the aggressive responses of territorial Sparkling Violetears suggests that the Giant Hummingbird, although it is the largest species in the community studied, is not the most territorial and behaviorally dominant because the morphology of its wings and its flight energy indicate an optimal strategy to fly long distances. In La Paz it is common to observe individuals of Sparkling Violetear chasing and effectively expelling Giant Hummingbirds from their territories with fast and very well maneuvered flights, while the latter show little resistance to this (pers. obs.). Some evidence could support this proposal, the Sparkling Violetear is a resident species but the Giant Hummingbird is capable of traveling long distances seasonally (Fjeldså and Krabbe, 1990; Schuchmann, 1999; Williamson et al., 2024), being one of the only three species of South American hummingbirds that migrate long latitudinal distances (Rodriguez-Flores et al., 2019). Several other studies reported that the Giant Hummingbird was chased or behaved submissively in front Sparkling Violetear (Hainsworth, 1977; Wester and Claßen-Bockhoff, 2006). Territoriality is probably conditioned in the Giant Hummingbird because it is attracted to large flowers such as cacti and bromeliads, or other flowering plants that flower in short periods of local aggregations with abundant nectar supplies (Fjeldså and Krabbe, 1990; Schuchmann, 1999). It was recently proposed to split the Giant Hummingbird species into two, a Northern non-migratory and a Southern migratory (Williamson et al., 2024). The differences in territoriality and behavioral dominance previously reported for this species (Fjeldså and Krabbe, 1990; Schuchmann, 1999; Bribiesca et al., 2019) are probably related to these different populations. According to the proposal of Williamson et al. (2024) the populations in La Paz correspond to the migratory one, however, it is important to note that in La Paz, Giant Hummingbirds are present during the dry season (per.obs.). It would be interesting to evaluate in the future the differences in territoriality and dominance behavior of Giant Hummingbird populations and the ecological conditions related to it.
In other hummingbird communities it was also reported that the largest species was not the behaviorally dominant (Fernandez-Duque et al., 2024), proposing that wing morphology explains the dominance hierarchy better than mass. The evolutionary distance between clades of contending species is another factor that could explain when smaller species win over larger ones (Martin and Ghalambor, 2014; Márquez-Luna et al., 2019). Situations that do not exclude cases in which the largest hummingbird species are actually the behaviorally dominant ones in their communities (López-Segoviano et al., 2018). Thus, the composition of each nectarivorous community, the morphological and ecological adaptations of each species, linked to the ecological conditions and the spatial and temporal variations of nectar resources, configure the hierarchical dominance in each community.
The Grey-bellied Flowerpiercer is a resident and territorial species that aggressively defends small patches of flowers against any other small nectar consumers (Hilty, 2011). Defending floral patches is crucial for this specialized nectar-robber because the presence of the long hook at the tip of the bill –which allows it to extract nectar at the base of the corollas- is a compromise for the consumption of other food sources as fruits (Schondube and Martínez del Rio, 2003). Comparisons of wing morphology between hummingbirds and passerines are very difficult because hummingbirds have extreme morphology with a hand wing length of more than 80% of the total wing length, being around 70% in songbirds (Videler, 2005). In any case, the low HWI value of the Grey-bellied Flowerpiercer (Table 1) implies a high maneuverability capacity. In La Paz the most intense fights for feeding territories occur between individuals of Sparkling Violetear and Grey-bellied Flowerpiercer, and it is common to see changes in the owners of territories between individuals of both species (pers. obs.). We propose that the Grey-bellied Flowerpiercer represents a high risk to take-over the territory of a territorial Sparkling Violetear, and explains why the level of aggression against the songs of this species was approximately the same as against the songs of their own species.
The Red-tailed Comet is the species that might pose the least risk to take-over Sparkling Violetear’s territories. Although it was described as a species that aggressively defends territories, such aggressive behaviors focus on individuals of its own species or smaller hummingbird species (Contino, 1975), being submissive to larger species, particularly Violetears and Hillstars (Contino, 1975; Fjeldså and Krabbe, 1990). Bribiesca et al. (2019) classify this species as non-behaviorally dominant, and the HWI value indicates an intermediate maneuverability ability (Table 1), suggesting also a better capacity for long distance flights. Indeed, this species establish territories only when clumped defensible nectar resources temporarily appears, moving widely after that to search for other nectar sources (Contino, 1975; Fjeldså and Krabbe, 1990). In La Paz, we observed this pattern of behavior in the temporary flowering of Mutisia orbignyana, Agalinis lanceolata and Tecoma fulva (per. obs.). We propose that the aggressive responses of the territorial Sparkling Violetears predict that the behavioral resource-defense strategy of the Red-tailed Comet is primarily based on “traveling exploitation” rather than “stationary interference” (Sargent et al., 2021), proposal which should be evaluated in the future.
According to our interpretation of the results, Sparkling Violetear individuals are capable, first, of recognizing through acoustic signals the nectarivorous species that potentially invade their territories, and second, of modulating their aggressive response based on the potential risk of take-over that each intruder species represents (Figure 3). That means not investing energy unnecessarily in individuals of more “inoffensive” species in order to balance their energy budgets. Considering that each hummingbird community is different at each location within the range of a particular species, it is difficult to support the idea that the responses would be innate; instead, they must come from learning from previous experiences. Hummingbirds have sufficient learning and memory abilities to optimize their foraging in the field in relation to spatial and temporal distribution as well as energetic reward from nectar sources (Sandlin, 2000; Healy and Hurly, 2003). Learning skills are also related to reacting and adapting to changes in food rewards (Jelbert et al., 2014), and learning skills related to song production (Johnson and Clark, 2020). Such evidence makes plausible the possibility that the modulation of aggressive responses in territorial Sparkling Violetears comes from previous experiences with territorial behaviors, dominance, and territory invasion attempts by conspecifics or individuals of other species. As this prediction depends on the level of individual experience, it would be interesting to evaluate these responses in the future in relation to the age and experience of the individuals.
Although our results were obtained in a single shrub thicket, and their extrapolation to other bird nectarivorous communities elsewhere should be taken with caution, they still have an important connotation for urban planning focused on the objective of designing biodiversity-friendly cities. Urbanization tends to favor behaviorally dominant bird species that displace their closely related subordinates (Martin and Bonier, 2018), and linked to the presence of non-native plant species as an opportunistic nectar source (Anselmo et al., 2023), urbanization favors generalist hummingbird to the detriment of specialized ones (Puga-Caballero et al., 2020; Vitorino et al., 2021). Modifying and simplifying hummingbird assemblages has the potential to alter interactions with flowering plants (Leimberger et al., 2022) and ultimately affect pollination as an ecosystem service in cities (Wenzel et al., 2020). The Sparkling Violetear as a generalist and behaviorally dominant species is the most successful hummingbird in the city of La Paz (Villegas and Garitano-Zavala, 2010), and with our results it is possible to understand the behavioral substrate that causes it. Our findings could be contrasted in other settings in the future to evaluate what factors could modify such responses. To promote more complex and ecologically functional nectarivorous bird communities in the city of La Paz, we need to advance our understanding of the ecological conditions that behaviorally subordinate species need to coexist with dominant ones.
Finally, it is important to note that in our study we had several limitations regarding the control of the sex of the territorial individuals and the songs used, as well as the control of the spatial and temporal relationship of the territorial individuals with their territories. Behavioral responses could be modulated in addition to the identity of the intruder with the quality of the territories and the time for which territorial individuals are owners. Considering the possibility of sexual (Glassman et al., 2024) and geographic (Gaunt et al., 1994) differences in songs, our study opens the possibility of study with playback sex-related and dialectal effects on behavioral responses.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because we work with animals in the wild, we do not capture them, we do not manipulate them and we do not sacrifice them. Our study was entirely observational.
Author contributions
LT: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ÁG-Z: Conceptualization, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are very grateful to Rodrigo Calbimonte who helped us obtain the data in the field. Andrea Salazar helped us with the design of Figure 1. Daniela Ticona provides us drawings of the bird species for Figures 2 and 3. We are very grateful to two reviewers who helped to substantially improve the manuscript.
Conflict of interest
The handling editor RO-A declared a past co-authorship with the author AG-Z.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AbrahamczykS.KesslerM. (2015). Morphological and behavioural adaptations to feed on nectar: how feeding ecology determines the diversity and composition of hummingbird assemblages. J. Ornithol.156, 333–347. doi: 10.1007/s10336-014-1146-5
2
AdedojaO. A.MallingerR. E. (2024). Can trait matching inform the design of pollinator-friendly urban green spaces? A review and synthesis of the literature. Ecosphere15, e4734. doi: 10.1002/ecs2.4734
3
AltshulerD. L.DudleyR. (2002). The ecological and evolutionary interface of hummingbird flight physiology. J. Exp. Biol.205, 2325–2336. doi: 10.1242/jeb.205.16.2325
4
AltshulerD. L.StilesF. G.DudleyR. (2004). Of hummingbirds and helicopters: hovering costs, competitive ability, and foraging strategies. Am. Nat.163, 16–25. doi: 10.1086/380511
5
AnselmoP. A.CardosoJ. C. F.SiqueiraP. R.MaruyamaP. K. (2023). Non-native plants and illegitimate interactions are highly relevant for supporting hummingbird pollinators in the urban environment. Urban For. Urban Gree.86, 128025. doi: 10.1016/j.ufug.2023.128025
6
BergerM. (1985). “Sauerstoffverbrauchvon Kolibris (Colibri coruscans und Colibri thalassinus) beim Horizontalflug,” in Bird flight – vogelflug, BIONA-report. Ed. NachtigallW. (Gustav Fischer, Sttutgart and New York), 307–314.
7
BirdLife International (2016). Colibri coruscans, The IUCN Red List of Threatened Species 2016: e.T22687114A93140619 (Accessed May 03, 2024).
8
BribiescaR.Herrera-AlsinaL.Ruiz-SanchezE.Sánchez-GonzálezL. A.SchondubeJ. E. (2019). Body mass as a supertrait linked to abundance and behavioral dominance in hummingbirds: a phylogenetic approach. Ecol. Evol.9, 1623–1637. doi: 10.1002/ece3.4785
9
CamfieldA. F. (2006). Resource value affects territorial defense by Broad-tailed and Rufous hummingbirds. J. Field Ornithol.77, 120–125. doi: 10.1111/j.1557-9263.2006.00031.x
10
CéspedesL. N.PavanL. I.HazlehurstJ. A.JankowskiJ. E. (2019). The behavior and diet of the Shining Sunbeam (Aglaeactis cupripennis): A territorial high-elevation hummingbird. Wilson J. Ornithol.131, 24–34. doi: 10.1676/18-79
11
ColwellR. K. (1973). Competition and coexistence in a simple tropical community. Am. Nat.107, 737–760. doi: 10.1086/282872
12
ContinoF. (1975). Observaciones sobre la conducta de Sappho sparganura en el Cerro de Santa Barbara, Jujuy, Argentina. Hornero11, 265–270. doi: 10.56178/eh.v11i4.1255
13
Cruz-YepezN.GonzálezC.OrnelasJ. F. (2020). Vocal recognition suggests premating isolation between lineages of a lekking hummingbird. Behav. Ecol.31, 1046–1053. doi: 10.1093/beheco/araa050
14
DearbornD. C. (1998). Interspecific territoriality by a Rufous-Tailed Hummingbird (Amazilia tzacatl): Effects of Intruder Size and Resource Value. Biotropica30, 306–313. doi: 10.1111/j.1744-7429.1998.tb00064.x
15
De RosaA.CastroI.MarslandS. (2022). The acoustic playback technique in avian fieldwork contexts: a systematic review and recommendations for best practice. Ibis164, 371–387. doi: 10.1111/ibi.13033
16
DunningJ. B. (2008). CRC handbook of avian masses. 2nd. ed (Boca Raton, FL: CRC Press-Taylor & Francis Group).
17
DuqueF. G.CarruthL. L. (2022). Vocal communication in hummingbirds. Brain Behav. Evol.19, 241–252. doi: 10.1159/000522148
18
DuqueF. G.Rodriguez-SaltosC. A.UmaS.NasirI.MonterosM. F.WilczynskiW.CarruthL. L (2020). High-frequency hearing in a hummingbird. Sci. Adv.6, eabb9393. doi: 10.1126/sciadv.abb9393
19
EmlenS. T. (1972). An experimental analysis of the parameters of bird song eliciting species recognition. Behaviour41, 130–171. doi: 10.1163/156853972X00248
20
EwaldP. W. (1985). Influence of asymmetries in resource quality and age on aggression and dominance in Black-chinned Hummingbirds. Anim. Behav.33, 705–719. doi: 10.1016/S0003-3472(85)80001-4
21
EwaldP. W.BransfieldR. J. (1987). Territory quality and territorial behavior in two sympatric species of hummingbirds. Behav. Ecol. Sociobiol.20, 285–293. doi: 10.1007/BF00292181
22
EwaldP. W.CarpenterF. L. (1978). Territorial responses to energy manipulations in the Anna hummingbird. Oecologia31, 277–292. doi: 10.1007/BF00346248
23
EwaldP. W.OriansG. H. (1983). Effects of resource depression on use of inexpensive and escalated aggressive behavior: experimental tests using Anna Hummingbirds. Behav. Ecol. Sociobiol.12, 95–101. doi: 10.1007/BF00343199
24
FallsJ. B. (1992). “Playback: A historical perspective,” in Playback and studies of animal communication. Ed. McGregorP. K. (Springer, Boston, MA), 11–33. doi: 10.1007/978-1-4757-6203-7_2
25
FeinsingerP.ChaplinS. B. (1975). On the relationship between wing disc loading and foraging strategy in hummingbirds. Am. Nat.109, 217–224. doi: 10.1086/282988
26
FeinsingerP.ColwellR. K. (1978). Community organization among Neotropical nectar-feeding birds. Am. Zool.18, 779–795. doi: 10.1093/icb/18.4.779
27
FernándezM. J.DudleyR.BozinovicF. (2011). Comparative energetics of the giant hummingbird (Patagona gigas). Physiol. Biochem. Zool.84, 333–340. doi: 10.1086/660084
28
Fernandez-DuqueF.MillerE. T.Fernandez-DuqueM.FalkJ.VenableG.RabinowiczS.et al. (2024). Phenotype predicts interspecific dominance hierarchies in a cloud-forest hummingbird guild. Ethology130, e13410. doi: 10.1111/eth.13410
29
FjeldsåJ.KrabbeN. (1990). Birds of the high andes (Svendborg: University of Copenhagen Zoological Museum).
30
FreemanB. G. (2016). Strong asymmetric interspecific aggression between two sympatric New Guinean robins. Ibis158, 75–81. doi: 10.1111/ibi.12318
31
GauntS. L. L.BaptistaL. F.SánchezJ. E.HernandezD. (1994). Song learning as evidenced from song sharing in two hummingbird species (Colibri coruscans and C. thalassinus). Auk111, 87–103. doi: 10.2307/4088508
32
GlassmanS. R. Y.DomerA.DudleyR. (2024). Vocal dimorphism in Anna’s hummingbirds. J. Avian Biol.2024, e03268. doi: 10.1111/jav.03268
33
GretherG. F. (2011). The neuroecology of competitor recognition. Integr. Comp. Biol.51, 807–818. doi: 10.1093/icb/icr060
34
HainsworthF. R. (1977). Foraging efficiency and parental care in Colibri coruscans. Condor79, 69–75. doi: 10.2307/1367532
35
HazlehurstJ. A.KarubianJ. O. (2018). Impacts of nectar robbing on the foraging ecology of a territorial hummingbird. Behav. Process.149, 27–34. doi: 10.1016/j.beproc.2018.01.001
36
HealyS. D.HurlyT. A. (2003). “Cognitive ecology: foraging in hummingbirds as a model system,” in Advances in the study of behavior 3. Eds. SlaterP. J. B.RosenblattJ. S.SnowdonC. T.RoperT. J. (Academic Press, London), 325–359.
37
HiltyS. L. (2011). “Family thraupidae (Tanagers),” in Handbook of the Birds of the World. Tanagers to New world blackbirds. Eds. HoyoJ.d.ElliottA.SargatalJ. (Lnyx Edicions, Barcelona), 46–329.
38
IBM Corporation (2023). IMB SPSS statistics version 29.0 (Armonk, NY: IBM Corporation).
39
JelbertS. A.HurlyT. A.MarshallR. E. S.HealyS. D. (2014). Wild, free-living hummingbirds can learn what happened, where and in which context. Anim. Behav.89, 185–189. doi: 10.1016/j.anbehav.2013.12.028
40
JohnsonK. E.ClarkC. J. (2020). Ontogeny of vocal learning in a hummingbird. Anim. Behav.167, 139–150. doi: 10.1016/j.anbehav.2020.07.010
41
LeimbergerK. G.DalsgaardB.TobiasJ. A.WolfC.BettsM. G. (2022). The evolution, ecology, and conservation of hummingbirds and their interactions with flowering plants. Biol. Rev.97, 923–959. doi: 10.1111/brv.12828
42
López-SegovianoG.Arenas-NavarroM.Nuñez-RosasL. E.ArizmendiM.delC. (2023). Implications of dominance hierarchy on hummingbird-plant interactions in a temperate forest in Northwestern Mexico. PeerJ11, e16245. doi: 10.7717/peerj.16245
43
López-SegovianoG.BribiescaR.ArizmendiM.d. C. (2018). The role of size and dominance in the feeding behaviour of coexisting hummingbirds. Ibis160, 283–292. doi: 10.1111/ibi.12543
44
LouderM. I. M.LafayetteM.LouderA. A.UyF. M. K.BalakrishnanC. N.YasukawaK.et al. (2020). Shared transcriptional responses to con- and heterospecific behavioral antagonists in a wild songbird. Sci. Rep.10, 4092. doi: 10.1038/s41598-020-60231-y
45
LyonD. L. (1976). A montane hummingbird territorial system in Oaxaca, Mexico. Wilson Bull.88, 280–299. Available at: http://www.jstor.org/stable/4160743.
46
LyonD. L.CrandallJ.McKoneM. (1977). A test of the adaptiveness of interspecific territoriality in the Blue-throated Hummingbird. Auk94, 448–454. doi: 10.1093/auk/94.3.448
47
Márquez-LunaU.LaraC.CorcueraP.ValverdeP. L. (2019). Factors affecting the dominance hierarchy dynamics in a hummingbird assemblage. Curr. Zool.65, 261–268. doi: 10.1093/cz/zoy057
48
MartinP. R.BonierF. (2018). Species interactions limit the occurrence of urban-adapted birds in cities. P. Natl. Acad. Sci. U.S.A.115, E11495–E11504. doi: 10.1073/pnas.1809317115
49
MartinP. R.FotheringhamJ. R.RatcliffeL.RobertsonR. J. (1996). Response of American redstarts (suborder Passeri) and Least flycatchers (suborder Tyranni) to heterospecific playback: the role of song in aggressive interactions and interference competition. Behav. Ecol. Sociobiol.39, 227–235. doi: 10.1007/s002650050285
50
MartinP. R.FreshwaterC.GhalamborC. K. (2017). The outcomes of most aggressive interactions among closely related bird species are asymmetric. PeerJ5, e2847. doi: 10.7717/peerj.2847
51
MartinP. R.GhalamborC. K. (2014). When David beats Goliath: the advantage of large size in interspecific aggressive contests declines over evolutionary time. PloS One9, e108741. doi: 10.1371/journal.pone.0108741
52
MaruyamaP. K.BonizárioC.MarconA. P.D’AngeloG.da SilvaM. M.da Silva NetoE. N.et al. (2019). Plant-hummingbird interaction networks in urban areas: Generalization and the importance of trees with specialized flowers as a nectar resource for pollinator conservation. Biol. Conserv.230, 187–194. doi: 10.1016/j.biocon.2018.12.012
53
Mendiola-IslasV.LaraC.CorcueraP.ValverdeP. L. (2016). Residency in white-eared hummingbirds (Hylocharis leucotis) and its effect in territorial contest resolution. PeerJ4, e2588. doi: 10.7717/peerj.2588
54
MonteA.da SilvaM. L.GahrM. (2023). Absence of song suggests heterogeneity of vocal-production learning in hummingbirds. J. Ornithol.164, 721–727. doi: 10.1007/s10336-023-02057-9
55
MorellatoL. P. C.CamargoM. G. G.GresslerE. (2013). “A review of plant phenology in South and Central America,” in Phenology: an integrative environmental science. Ed. SchwartzM. D. (Springer, Dordrecht), 91–113. doi: 10.1007/978-94-007-6925-0_6
56
PeimanK. S.RobinsonB. W. (2010). Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol.85, 133–158. doi: 10.1086/652374
57
PottsS. G.Imperatriz-FonsecaV.NgoH. T.AizenM. A.BiesmeijerJ. C.BreezeT. D.et al. (2016). Safeguarding pollinators and their values to human well-being. Nature540, 220–229. doi: 10.1038/nature20588
58
PowersD. R.McKeeT. (1994). The effect of food availability on time and energy expenditures of territorial and non-territorial hummingbirds. Condor96, 1064–1075. doi: 10.2307/1369115
59
Puga-CaballeroA.ArizmendiM.delC.Sánchez-GonzálezL. A. (2020). Phylogenetic and phenotypic filtering in hummingbirds from urban environments in Central Mexico. Evol. Ecol.34, 525–541. doi: 10.1007/s10682-020-10055-z
60
Rodriguez-FloresC. I.OrnelasJ. F.WethingtonS.ArizmendiM.d. C. (2019). Are hummingbirds generalists or specialists? Using network analysis to explore the mechanisms influencing their interaction with nectar resources. PloS One14, e0211855. doi: 10.1371/journal.pone.0211855
61
Sánchez SánchezM.LaraC. (2024). Exotic and native plants play equally important roles in supporting and structuring plant-hummingbird networks within urban green spaces. PeerJ12, e16996. doi: 10.7717/peerj.16996
62
SandlinE. A. (2000). Cue use affects resource subdivision among three coexisting hummingbird species. Behav. Ecol.11, 550–559. doi: 10.1093/beheco/11.5.550
63
SargentA. J.GroomD. J. E.Rico-GuevaraA. (2021). Locomotion and energetics of divergent foraging strategies in hummingbirds: A review. Integr. Comp. Biol.61, 764–748. doi: 10.1093/icb/icab124
64
Schmidt-MarlohD.SchuchmannK. L. (1980). Zur Biologie des Blauen Veilchenohr-kolibris (Colibri coruscans). Bonn. Zool. Beitr.31, 61–77.
65
SchondubeJ. E.Martínez del RioC. (2003). The flowerpiercers’ hook: an experimental test of an evolutionary trade-off. P. R. Soc Lond. B Bio.270, 195–198. doi: 10.1098/rspb.2002.2231
66
SchuchmannK.-L. (1999). “Family trochilidae (Hummingbirds),” in Handbook of the birds of the world. Barn-owls to hummingbirds. Eds. HoyoJ.d.ElliottA.SargatalJ. (Lynx Edicions, Barcelona), 468–680.
67
ShankarA.CisnerosI. N. H.ThompsonS.GrahamC. H.PowersD. R. (2022). A heterothermic spectrum in hummingbirds. J. Exp. Biol.225, jeb243208. doi: 10.1242/jeb.243208
68
ShankarA.GrahamC. H.CanepaJ. R.WethingtonS. M.PowersD. R. (2019). Hummingbirds budget energy flexibly in response to changing resources. Funct. Ecol.33, 1904–1916. doi: 10.1111/1365-2435.13404
69
ShankarA.PowersD. R.DávalosL. M.GrahamC. H. (2020). The allometry of daily energy expenditure in hummingbirds: An energy budget approach. J. Anim. Ecol.89, 1254–1261. doi: 10.1111/1365-2656.13185
70
SheardC.Neate-CleggM. H. C.AlioravainenN.JonesS. E. I.VincentC.MacGregorH. E. A.et al. (2020). Ecological drivers of global gradients in avian dispersal inferred from wing morphology. Nat. Commun.11, 2463. doi: 10.1038/s41467-020-16313-6
71
SuarezR. K.GassC. L. (2002). Hummingbird foraging and the relation between bioenergetics and behaviour. Comp. Biochem. Phys. A133, 335–343. doi: 10.1016/S1095-6433(02)00165-4
72
TelleríaL.CalbimonteR.Montaño-CentellasF. (2024). Nectar robbing by the Red-tailed Comet Sappho sparganurus: the value of citizen science to document infrequent behavior in birds. Ornitol. Neotrop.35, 20–22. doi: 10.58843/ornneo.v35i1.1287
73
TobiasJ. A.SheardC.PigotA. L.DevenishA. J. M.YangJ.SayolF.et al. (2022). AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett.25, 581–597. doi: 10.1111/ele.13898
74
United Nations (2020). Demographic yearbook, 70th issu (New York, NY: Department of Economic and Social Affairs).
75
VidelerJ. J. (2005). Avian flight (New York: Oxford University Press).
76
VillegasM.Garitano-ZavalaÁ. (2010). Bird community responses to different urban conditions in La Paz, Bolivia. Urban Ecosyst.13, 375–391. doi: 10.1007/s11252-010-0126-7
77
VitorinoB. D.da FrotaA. V. B.MaruyamaP. K. (2021). Ecological determinants of interactions as key when planning pollinator-friendly urban greening: A plant-hummingbird network example. Urban For. Urban Gree.64, 127298. doi: 10.1016/j.ufug.2021.127298
78
VoigtC. C.WinterY. (1999). Energetic cost of hovering flight in nectar-feeding bats (Phyllostomidae: Glossophaginae) and its scaling in moths, birds and bats. J. Comp. Physiol. B169, 38–48. doi: 10.1007/s003600050191
79
WeeksB. C.O’BrienB. K.ChuJ. J.ClaramuntS.SheardC.TobiasJ. A. (2022). Morphological adaptations linked to flight efficiency and aerial lifestyle determine natal dispersal distance in birds. Funct. Ecol.36, 1681–1689. doi: 10.1111/1365-2435.14056
80
WenzelA.GrassI.BelavadiV. V.TscharntkeT. (2020). How urbanization is driving pollinator diversity and pollination–A systematic review. Biol. Conserv.241, 108321. doi: 10.1016/j.biocon.2019.108321
81
WesterP.Claßen-BockhoffR. (2006). Hummingbird pollination in Salvia haenkei (Lamiaceae) lacking the typical lever mechanism. Plant Syst. Evol.257, 133–146. doi: 10.1007/s00606-005-0366-9
82
WilliamsonJ. L.GyllenhaalE. F.BauernfeindS. M.BautistaE.BaumannM. J.GadekC. R.et al. (2024). Extreme elevational migration spurred cryptic speciation in giant hummingbirds. P. Natl. Acad. Sci. U.S.A.121, e2313599121. doi: 10.1073/pnas.2313599121
83
WolfL. L. (1978). Aggressive social organization in nectarivorous birds. Am. Zool.18, 765–778. doi: 10.1093/icb/18.4.765
84
WolfL. L.HainsworthF. R. (1971). Time and energy budgets of territorial hummingbirds. Ecology52, 980–988. doi: 10.2307/1933803
85
Zerda-OrdoñezE. (1994). Historia natural del Tominejo, Colibri coruscans coruscans (Gould) (Aves, Trochilidae). Universitas Scientiarum2, 65–85.
86
ZüchnerT.BoesmanP. F. D.KirwanG. M. (2020). “Sparkling violetear (Colibri coruscans),” in Birds of the world. Eds. HoyoJ.d.ElliottA.SargatalJ.ChristieD. A.JuanaE. (Cornell Lab of Ornithology, Ithaca, NY). doi: 10.2173/bow.spvear1.01
Summary
Keywords
agonistic behavior, behavioral dominance, Bolivia, Diglossa, hummingbird, nectar-robbing, territoriality, urban ecology
Citation
Tellería L and Garitano-Zavala Á (2024) Playback-elicited heterospecific aggressive responses in urbanized Sparkling Violetear are modulated in relation the risk of take-over hypothesis. Front. Ecol. Evol. 12:1434518. doi: 10.3389/fevo.2024.1434518
Received
18 May 2024
Accepted
15 August 2024
Published
04 September 2024
Volume
12 - 2024
Edited by
Rubén Ortega-Álvarez, National Autonomous University of Mexico, Mexico
Reviewed by
Wiebke Schuett, University of Sussex, United Kingdom
Carlos Lara, Autonomous University of Tlaxcala, Mexico
Updates
Copyright
© 2024 Tellería and Garitano-Zavala.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Álvaro Garitano-Zavala, agaritanozavala@umsa.bo
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.