- Department of Plant Protection Biology, Swedish University of Agricultural Sciences, Alnarp, Sweden
During decision-making, animals may use either innate or plastic behaviours. This has been suggested to be important for generalist phytophagous insects where females need to assess a large range of cues during host plant selection for oviposition. To facilitate the choice, generalists may thus use innate preference hierarchies among host plants combined with phenotypic plasticity based on earlier host experience, but if populations differ in whether they rely on innate or plastic factors during decision-making is not well-known. Females from an Egyptian population of the polyphagous moth Spodoptera littoralis has previously been found to shift preference between plants in their innate preference hierarchy depending on larval host plant experience. We studied the innate preference hierarchy for three host plants (cotton, cabbage and cowpea), and whether the hierarchy shifts based on larval host plant experience, in a Northern range margin population (Italy) and a core population (Kenya) of S. littoralis, to see if and how these traits vary across populations. In addition, we studied larval performance on the three host plants in all three populations. The Italian and Kenyan populations had different innate preference hierarchies, and both hierarchies differed from the hierarchy that has previously been found in the Egyptian population. Furthermore, the host plant selection of ovipositing females was affected by larval diet in the Italian and Kenyan population, but the larval host plant experience did not completely shift the preference hierarchy as in the Egyptian population. This indicates that not only host plant preference per se, but also phenotypic plasticity during host plant selection can vary between populations. We further found that the populations responded differently to larval diet for some performance traits. However, it was only the Italian population that showed indications of any link between preference and performance, as they had slower development on their least preferred host plant. Overall, preference divergence between populations seems not to be driven by local variation in larval performance.
1 Introduction
Animal decision-making, for example during the selection of a partner, habitat or food, is a complex procedure where individuals may rely on either innate (genetic) or plastic (non-genetic) behaviour to facilitate the process (Blumstein and Bouskila, 1996; Mendelson et al., 2016). One example of behavioural plasticity during decision-making is experience-based responses. Such behavioural responses are induced, and can be altered, by environmental experiences throughout an organism’s life-time and could be considered as learning (Dukas, 1998). The relative importance of, and dependence on, either innate or plastic behaviours in decision-making may depend on cost and benefits, environmental heterogeneity, availability of reliable environmental cues and genetic variation for plasticity (Berrigan and Scheiner, 2004). Thus, the use of innate or experience-based behavioural could potentially vary between populations due to e.g., resources species community or genetic population differentiation. For example, woodpecker finches, Actospiza pallida, from a population where food resources are variable have been found to be more flexible in a learning task than finches from a population where food resources are stable (Tebbich and Teschke, 2014). However, to our knowledge, little is yet known about the intra-specific variation in experience-based plasticity.
One species group that may be suitable for studies of intraspecific variation in decision-making is phytophagous insects. Female host plant selection during oviposition involves assessment of sensory cues, such as volatile, visual and gustatory stimuli which are translated to a decision on host-plant suitability. For example, females of the diamondback moth, Plutella xylostella, select an oviposition site depending on glucosinate content of the host plant and leaf direction (Badenes-Pérez and Heckel, 2023). Specialist insects need to assess cues from a limited range of plant species, while generalists need to assess cues from a wider range of species during host plant selection (Bernays, 2001). Interestingly, generalist insects can be less accurate in their assessment of plant quality (Janz and Nylin, 1997; Janz, 2003; Schäpers et al., 2016), which may depend on neural limitations in the insect brain to handle multiple plant species cues (Bernays, 1998). To facilitate host selection and to limit the range of plant cues to focus on, generalists often show innate preference hierarchies among plant species and prefer some species over others (Janz et al., 1994; Nylin et al., 2005; Wiklund et al., 2018). Another possibility for ovipositing females to facilitate the host plant selection is to rely on experience-based phenotypic plasticity (Bernays, 1998), including learning (West and Cunningham, 2002; Dukas, 2013). Such plastic preferences can be induced by, for example, adult female feeding experience (Gamberale-Stille et al., 2019), mating experience (Proffit et al., 2015) or larval feeding experience (Anderson and Anton, 2014).
While the final host plant preference of phytophagous species may differ between populations throughout the species range (Sylla et al., 2019), it is, to our knowledge, seldom studied if and how populations also can differ in dependence on innate and experience-based responses underlying the decision of host plant preference (but see examples of such studies by Badenes-Pérez et al., 2020; Braem and Van Dyck, 2023). The innate preference may for example be selected by the variation in plant species community, and how different host plants affect offspring survival and performance (Gripenberg et al., 2010; Friberg et al., 2015), while the costs and benefits of maintaining preference plasticity may be affected by factors such as the predictability of plant species (Cunningham and West, 2008). Understanding if, and how, innate and experience-based responses during decision-making differs between populations and environments, would contribute to explain not only geographic variation in plant-insect interactions but also the cognitive ecology behind decision-making in insects.
The Egyptian cotton leaf-worm, Spodoptera littoralis, is a generalist and a pest on numerous crops and wild plants. It is present throughout Africa and the Middle East, and has also established in southern Europe (CABI, 2019). Both larvae and adults of S. littoralis from Egypt have a distinct and consistent innate preference hierarchy (Anderson et al., 2013; Thöming et al., 2013). This innate preference hierarchy may shift depending on larval diet and adult mating experience (Thöming et al., 2013; Proffit et al., 2015; Lhomme et al., 2018) where the preference shift is mediated by plant emitted volatiles (Lhomme et al., 2018). This experience-based plasticity has repeatedly been shown for the Egyptian population of S. littoralis, both in laboratory and field experiments (Anderson et al., 2013; Thöming et al., 2013; Proffit et al., 2015), and both the innate preference hierarchy and the experience-based preference plasticity seems to be stable in this population.
The intriguing host plant plasticity in S. littoralis, makes it a suitable system to address novel questions on decision-making during generalist host plant selection. Here, we ask if the innate preference hierarchy and the relative importance of innate and experience-based components of host plant selection differ between populations that inhabit environments with different host plant communities and climate across the geographic range of S. littoralis. We studied host plant selection in a S. littoralis population in the species Northern range (Italy) as well as in a core population (Kenya) and compared our findings with previous results from a population which is geographically situated in between (Egypt). In addition, we studied larval development, survival and offspring longevity on the same plant species in all three populations, to address if preference and performance covaries among populations.
2 Materials and methods
2.1 Study species
We used S. littoralis individuals from a range margin population in Italy, a geographical core population in Kenya and a population in between, Egypt. The species is common in Egypt and in Kenya where it is a pest on various crops. The population in Italy was first recorded in 1968, which is in the northern range margin of the species (Inserra and Barbagallo, 1968) as it is not known to diapause (CABI, 2019) and temperature is considered to be a determining factor for range expansion (ElShahed et al., 2023). For this study, S. littoralis larvae were collected from alfalfa (Medicago sativa) and pepper (Capsicum annuum) fields in the Campania region, Italy, and from amaranth (Amaranthus dubius) in Machakos County, Kenya and brought to the laboratory at SLU, Sweden. The Egyptian population originated from the Alexandria region and persists in the lab at SLU, where laboratory reared Egyptian individuals show no difference in preference and performance in comparison to wild-caught individuals (Thöming et al., 2013). The regions that the three populations originate from has different climatic conditions, where the Italian population experiences Mediterranean hot summer climate, the Kenyan population experiences temperate highland tropical climate and the Egyptian population originates from a region with subtropical desert climate (weatherandclimate.com). All individuals were raised in climate chambers with controlled settings (25 C, 60% RH, 12:8 L:D) and fed either host plant material or artificial diet (Hinks and Byers, 1976) according to the experiment design (see below). Pupae were sexed and the sexes were kept in separate chambers until eclosion.
As host plants in the current study, we used cotton (Gossypium hirsutum), cabbage (Brassica oleracea) and cowpea (Vigna unguiculata). All three host plants are cultivated in Kenya and Egypt while only cotton and cowpea are cultivated in Italy. These plants were selected as previous studies on the Egyptian population have shown that both preference and performance differ on these host plant species, as well as whether they may induce an experience-based preference or not. In the Egyptian population, ovipositing females prefer cowpea over cotton while cabbage is the least preferred plant (Thöming et al., 2013). This innate preference hierarchy between cotton and cowpea could be shifted, since larvae fed on cotton prefers cotton over cowpea as adults (Anderson et al., 2013; Thöming et al., 2013) where the preference shift may be mediated by odour alone (Lhomme et al., 2018). In contrast, larvae fed on cabbage still do not prefer this plant as adults, thus cabbage does not induce any plastic preference (Thöming et al., 2013). Moreover, performance in the Egyptian population differs depending on host plant species, where host plant has been shown to affect larval development, mating propensity and fecundity (Karlsson Green et al., 2021), as well as immune response (Karlsson Green, 2021). All plants in the current study were cultivated from seeds in controlled greenhouse environment (25°C, 70% RH, 16:8 L:D) until 4-6 weeks old. Plants were used in the experiment in the non-flowering stage and when they had 6-8 true leaves. A schematic outline of the populations and plants used in the different experiments is found in the supplementary information (Supplementary Figure S1).
2.2 Host plant preference
To determine the innate host plant preference hierarchies in Italian and Kenyan S. littoralis, and examine if those hierarchies could be altered depending on larval diet, we conducted an oviposition choice experiment where larvae were fed either an artificial diet (Hinks and Byers, 1976) or detached cotton leaves until pupation. After eclosion, two-four days old adults were allowed to mate during 24 hours in a H*W*L 8*12*25 cm sized plastic box, one pair per box. The following day, females were introduced to oviposition cages (30*30*30 cm) where each female had the choice of two detached leaves each from two different plant species (i.e., in total four leaves per cage), positioned in the cage corners and kept in tubes with water to stay fresh. The two leaves from the same plant species were placed in diagonally opposing corners, and the direction was randomised between cages to avoid any position effect. The leaves of cotton, cabbage and cowpea differ in shape and texture, but the leaves selected for the oviposition experiment had similar area and previous studies have shown that oviposition experiments with detached leaves generate the same results as with intact plants in this species (Thöming et al., 2013). Honey water was provided for the female in the centre of the cage and replenished daily. Females were allowed to oviposit during three consecutive days and each day egg clutches were collected, by gently transferring a paper underneath the clutch, and weighed. The plant species on which a female had deposited the most egg weight on after three days was noted as the preferred plant. The experiments were conducted with the 1st-3rd generation of laboratory reared individuals.
To investigate the innate host plant preference hierarchy, individuals that had been fed artificial diet (Italian population total N = 58, Kenyan population total N= 19) were given a choice between cotton and cabbage (Italian N = 12, Kenyan N = 7), cotton and cowpea (Italian N = 14, Kenyan N = 4) or cabbage and cowpea (Italian N = 32, Kenyan N = 8). To study if females altered their preference based on larval diet experience, females that had been fed cotton (Italian population total N = 11, Kenyan N = 13) were given a choice between cotton and cowpea and their response was compared to females that had been fed artificial diet (N above). With animals from the Italian population, we performed a new experiment to address if females reared on cabbage leaves could alter their preference depending on larval experience. Ovipositing females were in this experiment only given the choice between cabbage and cowpea (N = 13), otherwise handling was as above. In total, 82 females from the Italian population and 32 females from the Kenyan population participated in the host plant preference experiment.
2.3 Host plant performance
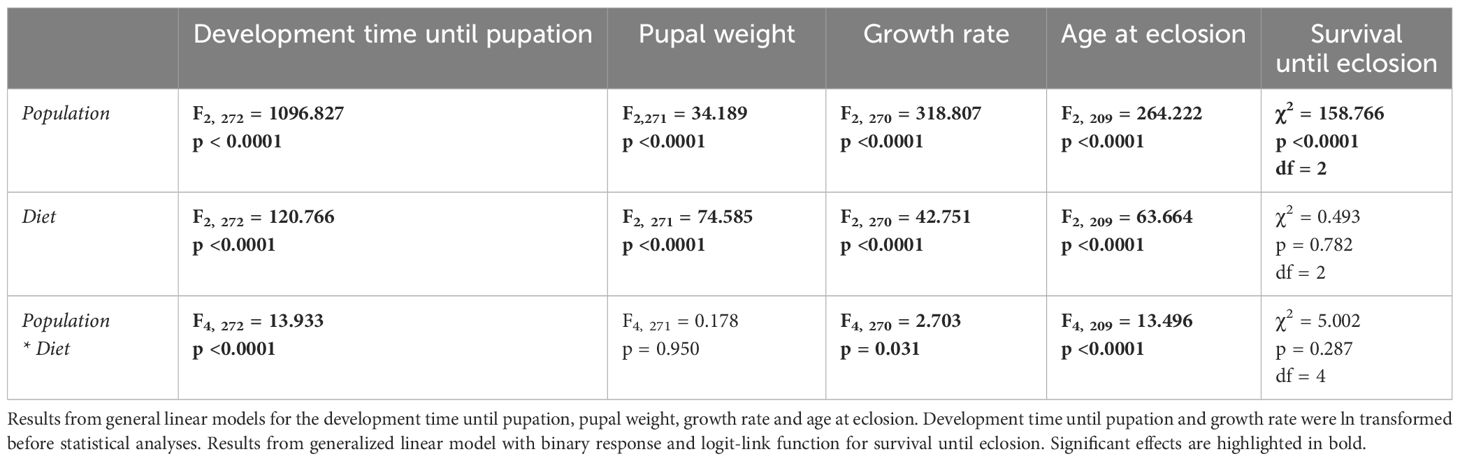
To study if the three populations differed in performance on different host plant species, we compared development and life-span in animals from the Italian, Kenyan and Egyptian population when fed detached leaves of either cotton, cabbage or cowpea. Larvae were reared in groups in H*W*L 8*12*25 cm sized plastic boxes until the second instar when they were separated and reared individually in 1.5 dl plastic cups until pupation. After eclosion, adults were kept in individual containers, only given access to water on a piece of cotton and monitored until they died. During the experiment, development time until pupation (number of days from hatching until pupation), pupal weight (mg), sex, age at eclosion (number of days from hatching until adulthood), survival until eclosion (whether individuals survive until adulthood or not) and adult life-span (number of days from eclosion until death) was noted. Growth rate was calculated as the ratio between pupal weight and development time until pupation (mg/days). In total, 120 individuals from each population were studied (40 individuals on each diet) and thus an overall total of 360 individuals were used in the experiment.
2.4 Statistical analyses
The innate host plant preferences indices in the Italian and the Kenyan populations, respectively, were calculated for individuals that were fed artificial diet as the sum of the preferences for each plant from all plant choice combinations ∑ (P2− 50%) (Thöming et al., 2013). This approach allowed us to combine the results of each choice combination and to make a comparison with previous studies on the Egyptian population. To study if individuals alter their oviposition preference based on larval diet, and if this differs between populations, we analysed their preference, i.e., which plant species they had oviposited most eggs on, as a binary response (preferred plant = 1, non-preferred plant = 0) with generalized linear models with logit as link function. We performed one model for the oviposition preference between cotton and cowpea for females fed cotton or artificial diet, in both the Kenyan and Italian populations, where explanatory variables were population, diet and their interaction. In addition, we performed another model for the preference between cabbage and cowpea for females that had been fed cabbage or artificial diet in the Italian population only, where diet was the only explanatory variable. Finally, we tested the hypotheses that the outcome of the host plant choice experiment differs from random for the females that had been fed cotton (one analysis of females from Italy and one analysis of females from Kenya) and in the females that had been fed cabbage with a likelihood ratio-test. The egg weights underlying the binary data for the GLM models are displayed in the Supplementary Information, Supplementary Figure S2.
To analyse if the Italian, Kenyan and Egyptian populations differed in performance on the three different host plants, we performed separate general linear models with development time until pupation, pupal weight, growth rate and age at eclosion as response variables and population, diet and their interaction as explanatory variables. Homoscedasticity was assessed with Levene’s test. As development time until pupation, growth rate, and adult life-span had unequal variances, development time until pupation and growth rate were ln transformed before the analyses. Normality of residuals was confirmed by visual inspection of Q-Q plots. One outlier was removed from the analyses of development time until pupation and growth rate. Tukey’s HSD test was used to analyse significant differences between means. The effects of population, diet and their interaction on adult life span were analysed with Kruskal-Wallis test after grouping the data in nine treatment groups (three populations * three diets). Multivariate comparisons between these nine groups were analysed with Dunn’s test for all pairs with joint ranks. We analysed survival until eclosion as a binary response with a generalized linear model and logit link function. Included in the model as explanatory variables were population, diet and their interaction. A posthoc z-test was conducted to analyse significant differences between means. One outlier was excluded from the analysis of development time. All analyses were performed in JMP version 15pro.
3 Results
3.1 Host plant preference
We found that the Italian and Kenyan populations had different preference hierarchies for the tested plants (Figure 1). The Italian population preferred cowpea the most and then preferred cabbage over cotton while the Kenyan population preferred cabbage the most, then cowpea and last cotton (Figure 1).
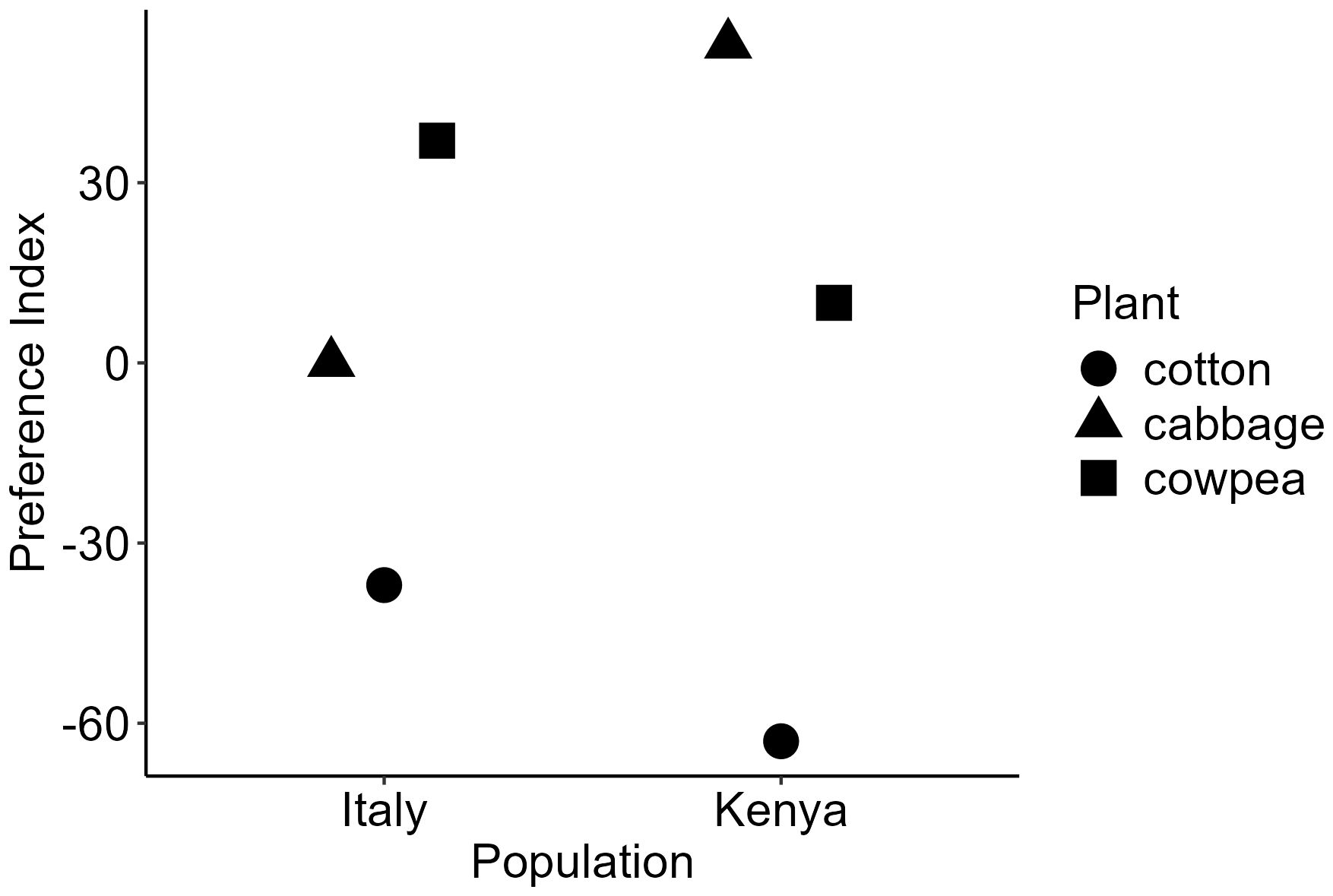
Figure 1. Innate preference hierarchy for cotton, cabbage and cowpea for the populations from Italy and Kenya. Preference hierarchies were calculated based on the outcome of oviposition choices between pairwise plant species as in Thöming et al., 2013).
When we addressed experience-based plasticity in the choice between cotton and cowpea for females that had been fed cotton or artificial diet as larvae, we found that Italian and Kenyan females used their innate preference for cowpea and not their larval host plant experience (cotton) during oviposition, as they in general did not prefer the plant they had been fed as larvae (Figure 2A). Diet did, however, still have a significant effect in the choice between cotton and cowpea (GLM χ2 = 5.625 df = 1 p = 0.018), as some females choose cotton more often when they had been fed this plant. The proportion of females that chose cotton was not larger than the proportion that chose cowpea (Figure 2A) and the choice was not significantly different from a random outcome neither for the Italian females (Likelihood Ratio χ2 = 0.829 df = 1 p = 0.363, Figure 2A) nor the Kenyan females (Likelihood Ratio χ2 = 0.077 df = 1 p = 0.781, Figure 2A). The overall preference hierarchy between cowpea and cotton was thus not shifted by larval feeding experience. Neither population (GLM χ2 = 2.625 df = 1 p = 0.105) nor the interaction between population and diet (GLM χ2 = 1.510 df = 1 p = 0.219) had significant effects on the oviposition choice.
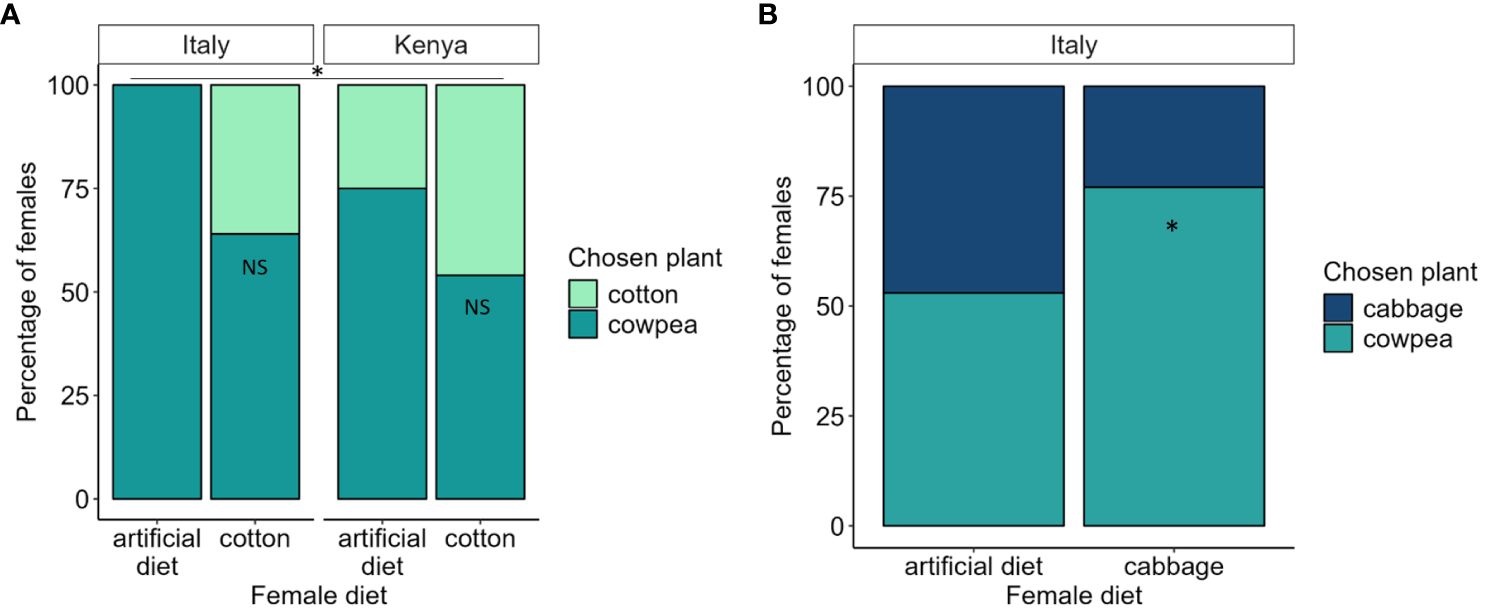
Figure 2. Chosen plant during ovipostion depending on larval diet. (A) The proportion of females in the Italian and Kenyan populations that has been feed artificial or cotton diet that choose to oviposit on cotton (light green) or cowpea (dark green) (B). The proportion of females in the italian population that has been fed artificial or cabbage diet that choose to oviposit on cabbage (dark blue) or cowpea (turqoise). Asterisk above bars indicate significant differences between diets at p < 0.05 in generalized linear models. Asterisk within bars indicate an outcome in the choice that is significantly different from a random choice in Likelihood Ratio test, while NS indicate an outcome that is not different from a random choice.
For the choice between cabbage and cowpea for Italian females that had been reared on either cabbage or artificial diet as larvae, diet did not have a significant effect on the oviposition choice (GLM χ2 = 2.264 df = 1 p = 0.132, Figure 2B). The choice between cabbage and cowpea differed, however, from a random outcome for the females that were fed cabbage as larvae (Likelihood Ratio χ2 = 3.977 df = 1 p = 0.046, Figure 2B), i.e., those females preferred cowpea over cabbage.
3.2 Host plant performance
We found that the populations differed in how they responded to the different plant diets for some of the performance variables, as the interaction between population and diet was significant in the analyses of larval development time until pupation, growth rate and age at eclosion (Table 1; Figures 3A–C). Posthoc analyses indicated that individuals from the Egyptian population developed significantly different on each host plant; slowest on cotton and fastest on cabbage (Figure 3A). Individuals from the Italian population also developed slowest on cotton but did not differ in development time on cabbage and cowpea (Figure 3A). Individuals from the Kenyan population developed equally fast on all three diets (Figure 3A). For growth rate, the Egyptian population was the only population where individuals that had a significantly different growth rate between all three diets, where they had the highest growth rate on cabbage and the slowest growth rate on cowpea (Figure 3B). Individuals from the Italian population had the slowest growth rate on cowpea but there was no difference between development on cabbage and cotton (Figure 3B). In the Kenyan population, individuals had the fastest growth rate on cotton and slowest on cowpea but the development time on cabbage did not significantly differ from development on the other two (Figure 3B). Individuals from the Kenyan population had the same age at eclosion irrespective of diet, while individuals from the Egyptian and Italian populations eclosed at the oldest age when feeding cotton (Figure 3C). Individuals from the Italian population had equally young age at eclosion when feeding cabbage and cowpea while individuals from the Egyptian population had a younger age when feeding cabbage than when feeding cowpea (Figure 3C). For adult life-span, the Kruskal-Wallis test showed significant differences among the nine treatment groups (χ2 = 60.158, df = 8, p < 0.0001) and the Dunn test showed that individuals from Egypt feeding cotton had a significantly longer adult life span (mean 9.36 days ± 1.78 SD) than individuals from Egypt feeding cowpea (mean 7.34 days ± 2.21 SD: z = -3.600, p = 0.011), from Italian individuals feeding cabbage (mean 7.28 days ± 1.72 SD, z = -3.703, p = 0.008) and cowpea (z = -5.360, p < 0.0001), as well as from Kenyan individuals feeding cowpea (z = -4.723, p <0.0001), cabbage (z =-4.235, p = 0.001) and cotton (z = 5.139, p < 0.0001). Individuals from Egypt feeding cabbage also had a significantly longer life span than individuals from Kenya feeding cotton (z = -3.530, p = 0.015). The populations did, however, not differ in pupal weight or survival until eclosion depending on host plant diet (Table 1).
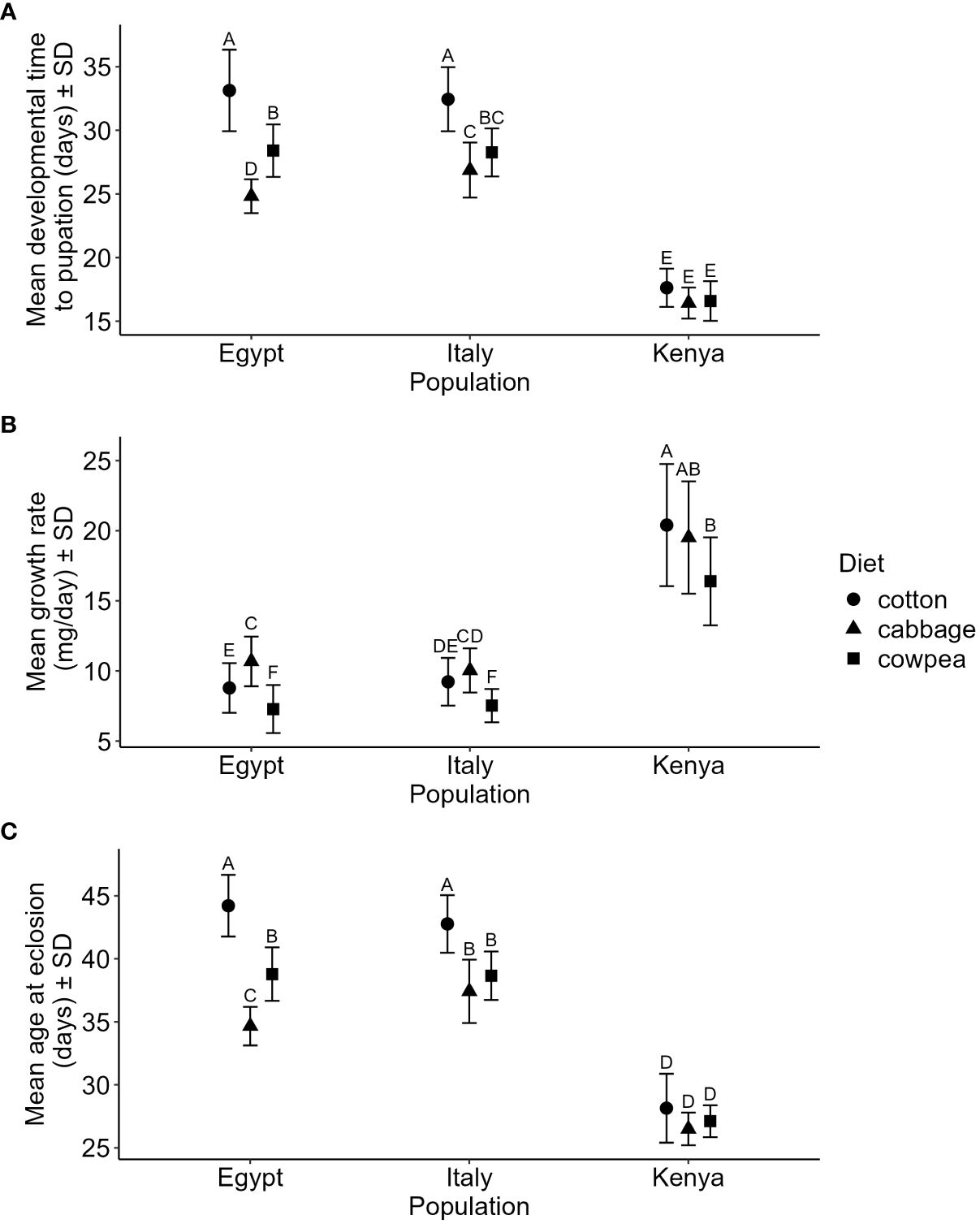
Figure 3. Differences in performance between the populations from Italy, Kenya and Egypt depending on the three plant diets. The figures depict the interaction between population and host plant diet for (A) mean development time until pupation, (B) mean growth rate and (C) mean age at eclosion. Growth rate was calculated as pupal weight divided by development time until pupation. Error bars denote two standard deviations from the mean. Different letters above bars indivate significant differences between treatments. Results from general linear models (Table 1). The figures represent the raw data but statistical analyses were made on ln transformed values for development time until pupation and growth rate.
Furthermore, there were general differences between the populations, irrespective of host plant diet (Table 1), where Tukey’s posthoc analyses revealed that individuals from Kenya had the significantly fastest development time (mean days ± SD for Kenya: 16.85 ± 0.28; Italy: 29.10 ± 0.19; Egypt: 28.81 ± 0.19) and highest pupal weight (mean mg ± SD for Kenya: 308.04 ± 0.28; Italy: 259.15 ± 4.25; Egypt: 253.29 ± 4.27), thus also the highest growth rate (mean mg/day ± SD for Kenya: 18.43 ± 0.31; Italy: 8.95 ± 0.21; Egypt: 8.95 ± 0.21) and youngest age at eclosion (mean days ± SD for Kenya: 27.35 ± 0.51; Italy: 39.46 ±0.22; Egypt: 39.13 ± 0.21). Individuals from Kenya also had the lowest probability of survival (mean % ± SD for Kenya: 16 ± 0.03; Italy: 82 ± 0.04; Egypt: 83 ± 0.03), while Italian and Egyptian individuals did not differ significantly from each other in these aspects. The host plant diet was, furthermore, found to significantly affect development time until pupation, pupal weight, growth rate and age at eclosion (Table 1). Posthoc analyses revealed that all three diets differed significantly from each other here in terms of development time (mean days ± SD for cabbage: 24.44 ± 0.24; cotton: 30.19 ± 0.23; cowpea: 25.48 ± 0.21), pupal weight (mean mg ± SD for cabbage: 274.20 ± 5.28; cotton: 303.63 ± 5.18; cowpea: 224.16 ± 4.70) and age at eclosion (mean days ± SD: cabbage: 35.55 ± 0.40; cotton: 41.99 ± 0.32; cowpea: 37.29 ± 0.30) where cotton-fed individuals took longest time to reach the pupal stage and to eclose but had the highest pupal weight. Individuals on cowpea diet did furthermore have a significantly lower growth rate than individuals on the other two host plant diets (mean ± SD for cabbage: 11.74 ± 0.25; cotton: 11.05 ± 0.25; cowpea: 9.52 ± 0.23). Diet did however not affect survival until eclosion (Table 1).
4 Discussion
This study found different innate host plant preference hierarchies in ovipositing females from two geographically separated populations of S. littoralis. With regards to experience-based plasticity, the Kenyan and Italian populations were, however, similar as a larval cotton diet affected preference between cotton and cowpea but not to the extent that they preferred cotton over cowpea. These two populations thus seem to mainly rely on innate preferences during host selection, but the ability for experience-based host selection is still present in the populations. This is different to previous studies on the Egyptian population, which shifts preference hierarchy based on larval host plant experience. Reliance on experience-based plasticity thus varies between populations in this species. The difference between populations in host plant preference does not seem to be driven by larval performance. However, there were large general differences between populations in performance and to some extent the populations differed in performance depending on host plant diet.
The ovipositing Italian females showed strongest preference for cowpea, and then preferred cabbage over cotton. In the Kenyan population, females instead preferred cabbage the most, and then cowpea over cotton. The hierarchies thus differed from each other and also differed to the previously studied preference hierarchy in the Egyptian population, since females of that population prefers cowpea over cotton and then cabbage (Thöming et al., 2013). Host plant use may differ between populations simply due to geographic variation in plant species availability (Wiklund et al., 2018) but research has shown that genetic differentiation between populations in host plant preference and performance may occur (Singer et al., 1989; Singer and McBride, 2010; Logarzo et al., 2011; Izzo et al., 2014). This may be due to differences between populations in abilities to process oviposition cues, such as plant odour or leaf morphology, or in ability to manage plant secondary metabolites. To understand what has driven population divergence in host plant preference, local availability of host plant species as well as phylogenetic relatedness between moth populations would need to be addressed. For example, while all three studied host plants are cultivated in Kenya and Egypt, cotton is currently not grown in Italy (Proto et al., 2000; Sannino, 2003) and individuals with a strong innate preference for cotton may thus have been selected against when this area was colonised. Moreover, cabbage is one of few species that is cultivated also during winter periods in Italy (Sannino, 2003) and a preference for cabbage may thus instead have been selected for in this region. The preference hierarchy in the Italian population, where cabbage is preferred over cotton, may thus reflect adaptations to the available crops in the novel environment. However, it does not explain the preference hierarchy in the Kenyan population where S. littoralis is a common pest on both cabbage and cotton (F. Obala pers. comm.) and where all host plants are grown, just as in Egypt. Since the Italian population is a newer population in the margin of S. littoralis’ geographic range, the traits in this population may also result from neutral processes such as founder effects (Ahern et al., 2009).
We furthermore found that also the ability to alter the preference hierarchy depending on larval experience was similar in the Italian and Kenyan populations. Although the proportion of ovipositing Italian and Kenyan females that preferred cotton over cowpea increased when fed cotton as larvae, and there was no preference of cowpea over cotton, they did not constitute a larger proportion than the females that still preferred cowpea. This is contrary to the Egyptian population, where the overall preference shifts towards cotton over cowpea if females have experienced cotton as larvae (Thöming et al., 2013). In the Egyptian population, preference induction does not occur with the least preferred plant species, which in that population is cabbage (Thöming et al., 2013). Considering that cotton was the least preferred plant species among Italian and Kenyan females, this could be the reason why preference induction for cotton was not more prominent in these populations. When we tested the possibility of preference induction for cabbage in the Italian population, we did not either see any significant differences in preference between females that had been fed artificial diet or cabbage. There was, however, a preference of cowpea over cabbage in females that had been fed cabbage, which means that the females that had previous experience of cabbage did not prefer for this plant. That we did not see any effect of diet in our analysis of preference differences depending on larval diet, indicates that even if there is a preference in the cabbage fed females, there is no statistical difference between this group and females fed artificial diet. With a larger sample size, this could perhaps be detected. Taken together, our results indicate a less plastic host selection in the Kenyan and Italian populations, even if the ability to use larval experience-based preferences indeed exists also in these populations. Our results imply that not only plant preferences per se varies between populations, but also that the basis for decision-making during host plant choice has differentiated, whether in space or time. This novel insight adds to our understanding of generalist host plant choice as well as of the cognitive ecology of insects; even if S. littoralis across its species range is able to detect and evaluate the same host plant species, the actual decision-making process may vary at a local scale.
We hypothesise that maintenance and variation of experience-based plasticity within the species level could depend on matches between plant predictability in the agricultural landscape and moth generation, but also founder effects and standing genetic variation during range expansion. Usage of earlier experience during oviposition is a form of learning (West and Cunningham, 2002) and is stipulated to be especially beneficial in pest insects where cultivated plants are predictably available in space and time (Cunningham and West, 2008). Although the theoretical work so far has been done on adult experience during host plant choice (Cunningham et al., 2001; West and Cunningham, 2002; Cunningham et al., 2004; Cunningham and West, 2008), we suggest that matching between moth generation time and crop predictability also affects whether it is beneficial to maintain a larval experience-based host plant choice. In a predictable environment, where the same crop species is available both during the larval and adult stages, it may be beneficial to rely on larval experience but if crops are different when individuals reach the adult stage, it may be better to rely on the innate host plant preference. In addition, trade-offs between search time and dispersal may affect which strategy is best to use (Braem and Van Dyck, 2023). Whether it is most beneficial to rely on the innate or the plastic preference may thus vary in time and space, depending on generation time of both crop species and moths. This may lead to variation in decision-making strategies being maintained both among and within populations, since a single strategy is not favoured at all times. To explore these questions further, pest insects with an experience-based host plant choice may provide suitable study systems. In addition, knowledge from such research questions may contribute to our understanding of the transition of species into pests (Petit et al., 2017).
The populations did not only differ in host plant preferences but also in how they performed on different host plant species where the Italian and Egyptian populations showed more plasticity in performance than the Kenyan, which often performed similarly on the three different host plants. There were only limited indications of that performance matched the preference hierarchies of the populations, as the Italian larvae developed slowest on the least preferred plant. Overall, our study is in line with previous work indicating that preference and performance are not always positively correlated for generalist species (Gripenberg et al., 2010; Friberg et al., 2015), as the populations overall preferred cowpea the most but in general performed best on cabbage then on cotton and worst on cowpea. Moreover, our results indicate that generalist populations maintain the ability to perform well on host plants that are not available in their geographic area, as the Italian population still perform well on cotton. In addition, the Egyptian population, which is kept in the laboratory and fed artificial diet, had comparable host plant performance to the Italian population. To fully understand the relation between host plant preference and performance in nature, indirect interactions with, for example, natural enemies need to be considered (Khallaf et al., 2023). In addition, it would be interesting for future studies to address the mechanisms behind population differences in performance; for example, if populations differ in abilities to handle secondary metabolites.
There were also general differences between the populations, where the Kenyan population developed faster and became heavier but had a lower survival. These findings may either be a local adaptation, or a plastic response to the novel laboratory conditions. Of notable importance is, however, that the sex ratio in this population was highly skewed with almost 100% female individuals, which strongly suggest a sex ratio distorter such as Wolbachia in the population. Wolbachia infection may vary between populations (e.g., (Woger et al., 2020) and has been suggested to be more common in areas closer to the equator (Ahmed et al., 2015). Wolbachia may also affect performance in insects, such as immune function in S. exempta (Graham et al., 2012) and life-history traits in Hymenoptera (Miura and Tagami, 2004) and could potentially be one reason why the Kenyan population differed extensively in performance.
In summary, the current results together with previous studies on S. littoralis indicate differences between populations in host plant preference and performance per se, but also in plasticity in these traits. Despite the small sample size, these findings could be discussed in the light of range expansion as our studied populations ranges from the Northern margin to the geographic core of S. littoralis’ geographic area. Plasticity has for example been suggested to act as a buffer against environmental fluctuations and be advantageous in novel environments (West-Eberhard, 2003; Bock et al., 2018; Corl et al., 2018), which may lead us to expect a higher degree of plasticity in the Italian population but plasticity could also be lost during colonisations of new areas (Wan et al., 2018). We did, however, not see that the range margin population in Italy was special in terms of plasticity instead, the Italian and Kenyan populations seem to have similar plasticity during host plant selection, while the Kenyan population seems to be less plastic than the others in performance. Intraspecific variation in experience-based plasticity during host plant selection is likely a complex interplay between population differentiation, trade-offs, memory modulation and environmental variation (e.g (Petit et al., 2017; Braem and Van Dyck, 2023). To fully understand population variation in host plant selection mechanisms in S. littoralis, an assessment of both local variation in host plant communities and moth generations, as well as founder effects and dispersal, needs to be undertaken. In addition, the possibility of host associated differentiation could be addressed, which is the case in the related S. frugiperda (Pashley, 1986). This would require experiments with a higher number of populations across the species geographic range as well as molecular methods to determine the relationship between populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because Swedish law does not require ethical approval on research on insects.
Author contributions
KKG: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. CP: Investigation, Visualization, Writing – review & editing. ML: Investigation, Writing – review & editing. PA: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financed by grant 2014-6418 to KKG from the Swedish Research Council and Marie Sklodowska Curie Actions, Cofund, Project INCA 600398 and by the Linnaeus grant “Insect Chemical Ecology, Ethology and Evolution”, funded by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas).
Acknowledgments
We thank Elin Isberg, Elisabeth Marling, Ida Rönnqvist and Mostafa Torbati for assistance with experiments and Magne Friberg and Mattias Larsson, as well as the two reviewers Emilie Dion and Francisco Rubén Badenes-Pérez, for helpful comments on an earlier draft of this text.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1426923/full#supplementary-material
Supplementary Figure 1 | Schematic outline of the populations and host plants used in the different experiments. Individuals from three populations that are geographically separated in the geographic range of S. littoralis were used in the study (Kenya, Italy and Egypt) and three host plant species (cotton, cabbage and cowpea). The study addressed variation in host plant preference during oviposition (experiment 1), where both innate preference hierarchies and experience-based plasticity was addressed and larval host plant performance (experiment 2). The figure indicate which plant species are cultivated in the populations and in which experiment the different plants were used. The Kenyan and Italian populations were used in all experiments and the Egyptian population was only used in the host plant performance study.
Supplementary Figure 2 | Mean egg weights deposited on either plant species in a host plant choice experiment with females that were reared on either artificial diet or host plant diet. (A) The mean egg weight deposited on cotton or cowpea by females from the Italian and Kenyan populations that were reared on either artificial or cotton diet. (B) The mean egg weight deposited on cabbage or cowpea by females from the Italian population that were reared on either artificial or cabbage diet.
References
Ahern R. G., Hawthorne D. J., Raupp M. J. (2009). Founder effects and phenotypic variation in Adelges cooleyi, an insect pest introduced to the eastern United States. Biol. Invasions. 11, 959–971. doi: 10.1007/s10530-008-9308-0
Ahmed M. Z., Araujo-Jnr E. V., Welch J. J., Kawahara A. Y. (2015). Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 12. doi: 10.1186/s12983-015-0107-z
Anderson P., Anton S. (2014). Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant Cell Environ. 37, 1826–1835. doi: 10.1111/pce.12342
Anderson P., Sadek M. M., Larsson M., Hansson B. S., Thöming G. (2013). Larval host plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 85, 1169–1175. doi: 10.1016/j.anbehav.2013.03.002
Badenes-Pérez F. R., Gershenzon J., Heckel D. G. (2020). Plant glucosinolate content increases susceptibility to diamondback moth (Lepidoptera: Plutellidae) regardless of its diet. J. Pest Sci. 93, 491–506. doi: 10.1007/s10340-019-01139-z
Badenes-Pérez F. R., Heckel D. G. (2023). Intraspecific and interstage similarities in host-plant preference in the diamondback moth (Lepidoptera: Plutellidae). Horticulturae 9, 39. doi: 10.3390/horticulturae9010039
Bernays E. A. (1998). The value of being a resource specialist: Behavioral support for a neural hypothesis. Am. Nat. 151, 451–464. doi: 10.1086/286132
Bernays E. A. (2001). Neural limitations in phytophagous insects: Implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 46, 703–727. doi: 10.1146/annurev.ento.46.1.703
Berrigan D., Scheiner S. M. (2004). “Modeling the evolution of phenotypic plasticity,” in Phenotypic plasticity: Functional and conceptual approaches. DeWitt T. J., Scheiner S. M. (Eds.) (Oxford University Press). doi: 10.1093/oso/9780195138962.001.0001
Blumstein D. T., Bouskila A. (1996). Assessment and decision making in animals: A mechanistic model underlying behavioral flexibility can prevent ambiguity. Oikos 77, 569–576. doi: 10.2307/3545948
Bock D. G., Kantar M. B., Caseys C., Matthey-Doret R., Rieseberg L. H. (2018). Evolution of invasiveness by genetic accommodation. Nat. Ecol. Evol. 2, 991–999. doi: 10.1038/s41559-018-0553-z
Braem S., Van Dyck H. (2023). Larval and adult experience and ecotype affect oviposition behavior in a niche-expanding butterfly. Behav. Ecol. 34, 547–561. doi: 10.1093/beheco/arad022
CABI (2019). Spodoptera littoralis (Wallingford, UK: CAB International). Available at: www.cabi.org/isc.
Corl A., Bi K., Luke C., Challa A. S., Stern A. J., Sinervo B., et al. (2018). The genetic basis of adaptation following plastic changes in coloration in a novel environment. Curr. Biol. 28, 2970–297+. doi: 10.1016/j.cub.2018.06.075
Cunningham J. P., Moore C. J., Zalucki M. P., West S. A. (2004). Learning, odour preference and flower foraging in moths. J. Exp. Biol. 207, 87–94. doi: 10.1242/jeb.00733
Cunningham J. P., West S. A. (2008). How host plant variability influences the advantages to learning: A theoretical model for oviposition behaviour in Lepidoptera. J. Theor. Biol. 251, 404–410. doi: 10.1016/j.jtbi.2007.11.009
Cunningham J. P., West S. A., Zalucki M. P. (2001). Host selection in phytophagous insects: A new explanation for learning in adults. Oikos 95, 537–543. doi: 10.1034/j.1600-0706.2001.950319.x
Dukas R. (1998). Cognitive ecology: The evolutionary ecology of information processing and decision making (Chicago: University of Chicago Press).
Dukas R. (2013). Effects of learning on evolution: Robustness, innovation and speciation. Anim. Behav. 85, 1023–1030. doi: 10.1016/j.anbehav.2012.12.030
ElShahed S. M., Mostafa Z. K., Radwan M. H., Hosni E. M. (2023). Modeling the potential global distribution of the Egyptian cotton leafworm, Spodoptera littoralis under climate change. Sci. Rep. 13, 17314. doi: 10.1038/s41598-023-44441-8
Friberg M., Posledovich D., Wiklund C. (2015). Decoupling of female host plant preference and offspring performance in relative specialist and generalist butterflies. Oecologia 178, 1181–1192. doi: 10.1007/s00442-015-3286-6
Gamberale-Stille G., Schäpers A., Janz N., Nylin S. (2019). Selective attention by priming in host search behavior of 2 generalist butterflies. Behav. Ecol. 146, 142–149. doi: 10.1093/beheco/ary146
Graham R. I., Grzywacz D., Mushobozi W. L., Wilson K. (2012). Wolbachia in a major african crop pest increases susceptibility to viral disease rather than protects. Ecol. Lett. 15, 993–1000. doi: 10.1111/j.1461-0248.2012.01820.x
Gripenberg S., Mayhew P. J., Parnell M., Roslin T. (2010). A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393. doi: 10.1111/j.1461-0248.2009.01433.x
Hinks C. F., Byers J. R. (1976). Biosystematics of the genus exoa (Lepidoptera: Noctuidae): V. Rearing procedures, and life cycles of 36 species. Can. Entomolgist. 108, 12. doi: 10.4039/ent1081345-12
Inserra R., Barbagallo S. (1968). Una massiccia infestazione di Spodoptera littoralis (Boisduval) (Lepidoptera Noctuidae) insidia le colture della sicilia sud-orientale. Tecnica. Agricola. 20, 478–493.
Izzo V. M., Mercer N., Armstrong J., Chen Y. H. (2014). Variation in host usage among geographic populations of Leptinotarsa decemlineata, the Colorado potato beetle. J. Pest Sci. 87, 597–608. doi: 10.1007/s10340-014-0578-2
Janz N. (2003). The cost of polyphagy: Oviposition decision time vs error rate in a butterfly. Oikos 100, 493–496. doi: 10.1034/j.1600-0706.2003.12290.x
Janz N., Nylin S. (1997). The role of female search behaviour in determining host plant range in plant feeding insects: A test of the information processing hypothesis. Proc. R. Soc. B-Biol. Sci. 264, 701–707. doi: 10.1098/rspb.1997.0100
Janz N., Nylin S., Wedell N. (1994). Host-plant utilization in the comma butterfly - sources of variation and evolutionary implications. Oecologia 99, 132–140. doi: 10.1007/BF00317093
Karlsson Green K. (2021). The effects of host plant species and larval density on immune function in the polyphagous moth Spodoptera littoralis. Ecol. Evol. 11, 10090–10097. doi: 10.1101/2021.06.03.446956
Karlsson Green K., Houot B., Anderson P. (2021). What should a poor mother do? Influence of host plant quality on oviposition strategy and behavior in a polyphagous moth. bioRxiv.: 2021.06.03.446956.
Khallaf M. A., Sadek M. M., Anderson P. (2023). Predator efficacy and attraction to herbivore-induced volatiles determine insect pest selection of inferior host plant. iScience 26, 106077. doi: 10.1016/j.isci.2023.106077
Lhomme P., Carrasco D., Larsson M., Hansson B., Anderson P. (2018). A context-dependent induction of natal habitat preference in a generalist herbivorous insect. Behav. Ecol. 29, 360–367. doi: 10.1093/beheco/arx173
Logarzo G. A., Casalinuovo M. A., Piccinali R. V., Braun K., Hasson E. (2011). Geographic host use variability and host range evolutionary dynamics in the phytophagous insect Apagomerella versicolor (Cerambycidae). Oecologia 165, 387–402. doi: 10.1007/s00442-010-1782-2
Mendelson T. C., Fitzpatrick C. L., Hauber M. E., Pence C. H., Rodríguez R. L., Safran R. J., et al. (2016). Cognitive phenotypes and the evolution of animal decisions. Trends Ecol. Evol. 31, 850–859. doi: 10.1016/j.tree.2016.08.008
Miura K., Tagami Y. (2004). Comparison of life history characters of Arrhenotokous and Wolbachia-associated Thelytokous Trichogramma kaykai Pinto and Stouthamer (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. America 97, 765–769. doi: 10.1603/0013-8746(2004)097[0765:COLHCO]2.0.CO;2
Nylin S., Nygren G. H., Windig J. J., Janz N., Bergström A. (2005). Genetics of host-plant preference in the comma butterfly Polygonia c-album (Nymphalidae), and evolutionary implications. Biol. J. Linn. Soc. 84, 755–765. doi: 10.1111/j.1095-8312.2004.00433.x
Pashley D. P. (1986). Host-associated genetic differentiation in Fall Armyworm (Lepidoptera: Noctuidae): A sibling species complex? Ann. Entomol. Soc. America 79, 898–904. doi: 10.1093/aesa/79.6.898
Petit C., Dupas S., Thiéry D., Capdevielle-Dulac C., Le Ru B., Harry M., et al. (2017). Do the mechanisms modulating host preference in holometabolous phytophagous insects depend on their host plant specialization? A quantitative literature analysis. J. Pest Sci. 90, 797–805. doi: 10.1007/s10340-017-0833-4
Proffit M., Khallaf M. A., Carrasco D., Larsson M. C., Anderson P. (2015). ‘Do you remember the first time?’ Host plant preference in a moth is modulated by experiences during larval feeding and adult mating. Ecol. Lett. 18, 365–374. doi: 10.1111/ele.12419
Proto M., Supino S., Malandrino O. (2000). Cotton: A flow cycle to exploit. Ind. Crops Products. 11, 173–178. doi: 10.1016/S0926-6690(99)00060-6
Sannino L. (2003). Spodoptera littoralis in Italia: Possibili ragioni della crescente diffusione e mezzi di lotta. Informatore. Fitopatol. 53, 28–31.
Schäpers A., Nylin S., Carlsson M. A., Janz N. (2016). Specialist and generalist oviposition strategies in butterflies: Maternal care or precocious young? Oecologia 180, 335–343. doi: 10.1007/s00442-015-3376-5
Singer M. C., McBride C. S. (2010). Multitrait, host-associated divergence among sets of butterfly populations: Implications for reproductive isolation and ecological speciation. Evolution 64, 921–933. doi: 10.1111/j.1558-5646.2009.00866.x
Singer M. C., Thomas C. D., Billington H. L., Parmesan C. (1989). Variation among conspecific insect populations in the mechanistic basis of diet breadth. Anim. Behav. 37, 751–759. doi: 10.1016/0003-3472(89)90061-4
Sylla S., Brevault T., Monticelli L. S., Diarra K., Desneux N. (2019). Geographic variation of host preference by the invasive tomato leaf miner Tuta absoluta: Implications for host range expansion. J. Pest Sci. 92, 1387–1396. doi: 10.1007/s10340-019-01094-9
Tebbich S., Teschke I. (2014). Coping with uncertainty: Woodpecker finches (Cactospiza pallida) from an unpredictable habitat are more flexible than birds from a stable habitat. PloS One 9, e91718. doi: 10.1371/journal.pone.0091718
Thöming G., Larsson M. C., Hansson B. S., Anderson P. (2013). Comparison of plant preference hierarchies of male and female moths and the impact of larval rearing hosts. Ecology 94, 1744–1752. doi: 10.1890/12-0907.1
Wan J. S. H., Fazlioglu F., Bonser S. P. (2018). Loss of plasticity in life-history strategy associated with secondary invasion into stressful environments in invasive narrowleaf plantain (Plantago lanceolata l.). Austral Ecol. 43, 752–762. doi: 10.1111/aec.12599
weatherandclimate.com. information retreived for Campania, Italy, Machakos, Kenya, and Alexandria, Egypt, the 5th of July 2024.
West S. A., Cunningham J. P. (2002). A general model for host plant selection in phytophagous insects. J. Theor. Biol. 214, 499–513. doi: 10.1006/jtbi.2001.2475
West-Eberhard M.-J. (2003). Developmental plasticity and evolution. Developmental plasticity and evolution (New York: Oxford University Press, Inc). doi: 10.1093/oso/9780195122343.001.0001
Wiklund C., Noren K., Ryman N., Friberg M. (2018). Local monophagy and between-site diversity in host use in the european swallowtail butterfly, Papilo machaon. Biol. J. Linn. Soc. 123, 179–190. doi: 10.1093/biolinnean/blx115
Keywords: animal decision-making, experience-based plasticity, host plant preference, host plant performance, Lepidoptera, phenotypic plasticity, range expansion, Spodoptera
Citation: Karlsson Green K, De Pasqual C, Litto M and Anderson P (2024) Population comparison of innate and plastic host plant preference and performance in a polyphagous insect. Front. Ecol. Evol. 12:1426923. doi: 10.3389/fevo.2024.1426923
Received: 02 May 2024; Accepted: 16 July 2024;
Published: 22 August 2024.
Edited by:
Roberto Romani, University of Perugia, ItalyReviewed by:
Emilie Dion, National University of Singapore, SingaporeFrancisco Rubén Badenes-Pérez, Spanish National Research Council (CSIC), Spain
Copyright © 2024 Karlsson Green, De Pasqual, Litto and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristina Karlsson Green, a3Jpc3RpbmEua2FybHNzb24uZ3JlZW5Ac2x1LnNl
†Present address: Maria Litto, Institute of Organismic and Molecular Evolution, Johannes Gutenberg University, Mainz, Germany
 Kristina Karlsson Green
Kristina Karlsson Green Chiara De Pasqual
Chiara De Pasqual Peter Anderson
Peter Anderson