- 1Peking University–Hong Kong University of Science and Technology (PKU-HKUST) Shenzhen-Hong Kong Institution, Shenzhen, China
- 2Guangdong Provincial Key Laboratory of Conservation and Precision Utilization of Characteristic Agricultural Resources in Mountainous Areas, School of Life Science, Jiaying University, Meizhou, China
- 3Guangdong Forestry Survey and Planning Institute, Guangzhou, China
- 4Green Infrastructure Institute, Peking University Shenzhen Institute, Shenzhen, China
- 5Urban Planning and Design Institute of Shenzhen (UPDIS), Shenzhen, China
- 6Guangzhou Yuanheng Natural Resources Technology Co., Ltd, Guangzhou, China
Introduction: Under the impacts of high intensity human activities, mangrove natural protected areas are pivotal strategies for biodiversity conservation and play a significant role in preserving bird diversity. Mangrove natural protected areas in Guangdong Province, China, lie along the migratory path of the East Asian-Australasian Flyway, serving as breeding, feeding, and resting grounds for birds. Variations in bird responses to environmental factors are significant.
Methods: To comprehensively understand these variances, redundancy analysis was employed, focusing on bird diversity surveys conducted from March 2022 to February 2023 in natural protected areas of Guangdong to examine how driving factors such as mangrove habitat landscape, community structure, water quality, and soil sedimentation affected the diversity of avian species.
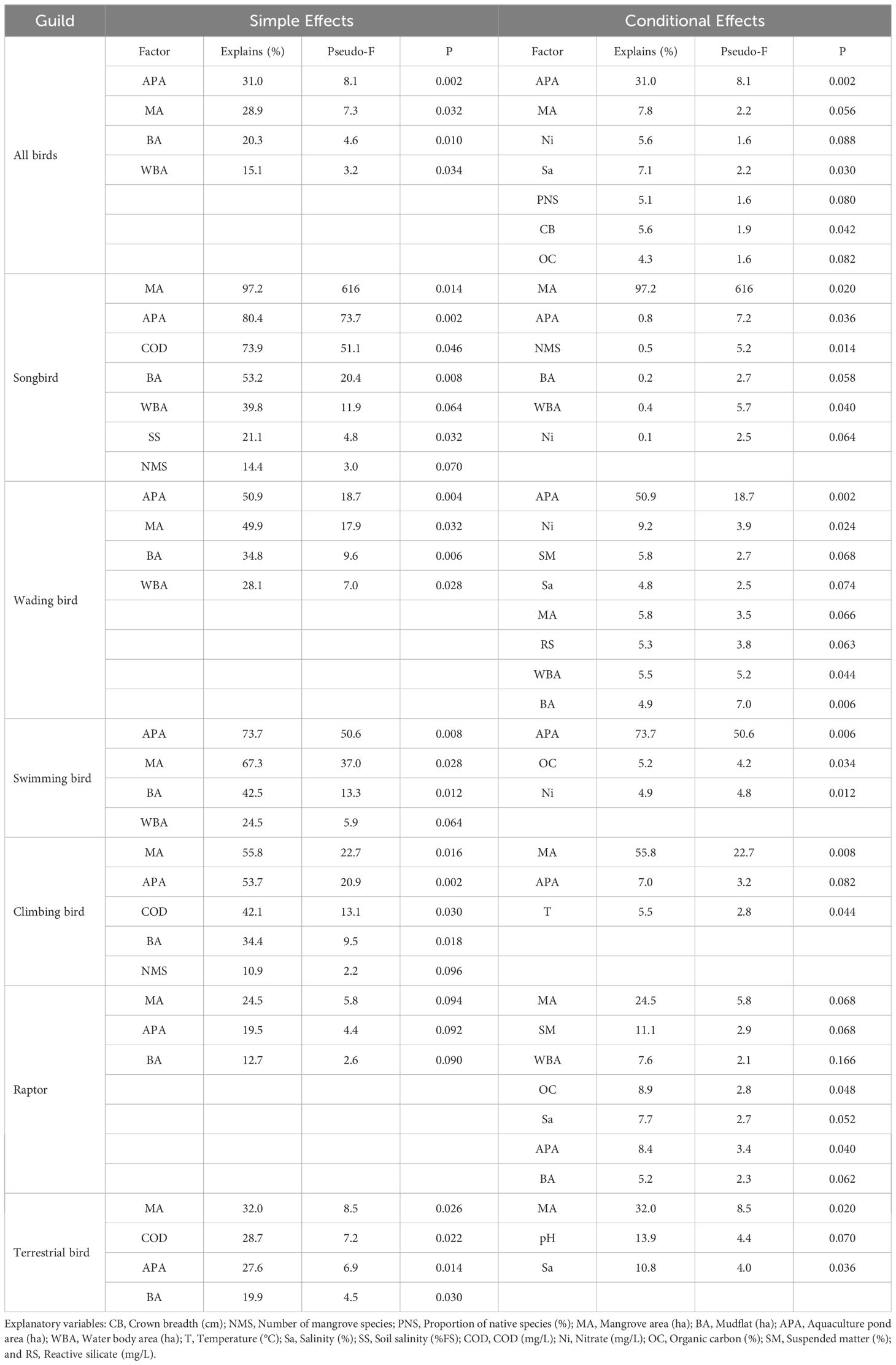
Results: The findings were as follows: (1) A total of 193 bird species spanning 17 orders and 53 families were documented, including 74 songbirds, 60 wading birds, 27 swimming birds, 17 climbing birds, 10 raptors, and 5 terrestrial birds. (2) Regarding the impact of simple effects on all bird species, aquaculture pond area, mangrove extent, and mudflat area emerged as significant factors driving bird diversity, with explanatory rates of 31.0%, 28.9%, and 20.3%, respectively. Notably, the aquaculture pond area was the main driver of bird diversity, with an explanatory rate of 31.0%. (3) Mangrove extent has emerged as a pivotal factor shaping the songbird diversity, climbing birds, raptors, and terrestrial birds, whereas the aquaculture pond area was pivotal for wading birds, swimming birds, and others.
Discussion: To enhance mangrove bird diversity protection, management agencies overseeing natural mangrove protected areas should adopt science-based approaches when managing mangrove, mudflats, and aquaculture pond areas in mangrove forest protection and restoration plans. This would prevent extensive mangrove planting, which encroaches on non-mangrove habitats. Additionally, the scientific management of aquaculture ponds should accommodate diverse bird habitats through measures, such as water level adjustments.
1 Introduction
In recent decades, global bird diversity has declined, primarily because of rapid socioeconomic development (Yasué, 2006; Ma et al., 2014; Hamza et al., 2015; Wei et al., 2017; Jackson et al., 2021). This decline is particularly pronounced in the coastal regions of China. Despite its location in the migratory route of the East Asian-Australasian Flyway and its significant role in global migratory bird protection, China has experienced a noteworthy decline in the diversity of nearly 19% of waterbirds due to the degradation of coastal habitats resulting from human disturbance (Ma et al., 2014). Notably, the decline in bird diversity in the mangrove ecosystems of South China is particularly severe.
As integral components of coastal wetlands, mangroves serve multiple functions, including resistance to natural disaster risk (Menéndez et al., 2018), acting as a carbon sink (Gu et al., 2022), maintaining high biodiversity (Mancini et al., 2018; Jackson et al., 2021; Yang et al., 2021), and even becoming a refuge for some flagship species (Thompson and Stefanie, 2019). Owing to the fragility of mangrove ecosystems, restoration of their original state is challenging (Ren, 2009). Consequently, the Chinese government has designated over 90% of mangroves into the system of natural protected areas through constructing natural protected areas (Li et al., 2013). Simultaneously, expanding mangrove planting within these areas facilitates rapid ecosystem restoration and structural reshaping (Leung, 2015), thereby laying the groundwork for improving bird habitat quality and restoring biodiversity. Guangdong Province, which has the largest mangrove distribution in China with an area of 12092.95 ha, accounting for 41.9% of China’s mangroves (excluding Hong Kong, Macao, and Taiwan), harbors over 50% of its mangroves within protected areas. This region serves as a crucial stopover and provisioning site for migratory birds and is important for the conservation of both Chinese and global avian migrants.
Previous studies have demonstrated that mangrove bird diversity is influenced by a range of complex factors, including landscape features (Yang et al., 2021, 2022; Ahmed et al., 2023; Wanjiru et al., 2023), mangrove community structure (Etezadifar and Ahmad, 2013; Leung and Nora, 2013; Mancini et al., 2018), water environment (Caussy, 2009), and soil sedimentary environment (Weinstein and Daniel, 2002; Cannicci et al., 2008). Optimizing the ratio of mangroves to mudflat areas through scientific management can enhance bird diversity (Yang et al., 2021). Mangroves with singular habitats may provide limited microhabitats and specialized ecological niches, resulting in low bird species richness and simplified structures (Jayasilan et al., 2015). Additionally, the relatively small average height of mangrove canopies may affect egret canopy utilization (Mancini et al., 2018). Furthermore, water (Caussy, 2009) and soil conditions (Weinstein and Daniel, 2002; Cannicci et al., 2008) can affect bird diversity by affecting benthic organism diversity. These studies primarily examined the effects of individual factors on bird diversity; however, complex interactions among these factors exist within a nonlinear system. Different bird species exhibit distinct responses to shared driving factors (Hamza et al., 2015), highlighting the need for researchers to comprehensively understand these variations and identify key drivers of bird diversity to inform future mangrove bird conservation and habitat restoration efforts.
Therefore, based on the study of bird diversity in protected mangrove areas in Guangdong Province, we utilized the redundancy analysis (RA) analysis method to explore how various factors, such as mangrove habitat landscape, mangrove community structure, water environment, and soil sedimentary environment, affect different types of bird diversity. By analyzing various bird responses to these factors, we identified the key drivers of bird diversity and enhanced future conservation efforts.
2 Regional overview and methods
2.1 Regional overview
China has experienced mangrove destruction, protection, and restoration. Despite efforts to restore mangrove ecosystem functions by planting native species in natural protected areas, over 80% of restored mangroves remain as degraded secondary forests (Sui and Zhang, 2001). Additionally, species such as Sonneratia apetala were selected as the main species for recovery. As a result, some natural protection areas still retain large areas of Sonneratia apetala (Chen et al., 2017).
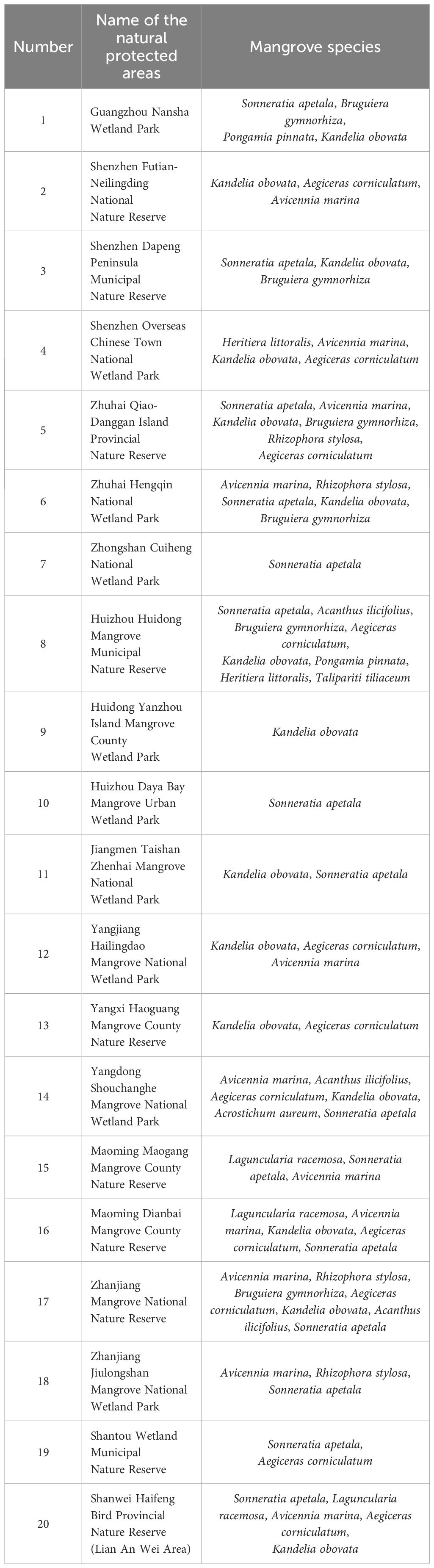
Guangdong Province, situated in the southernmost region of mainland China, has a mainland coastline spanning 4114.3 km and an island coastline spanning 1649.5 km. Mangrove species prevalent in Guangdong include Kandelia obovata, Aegiceras corniculatum, Avicennia marina, Bruguiera gymnorhiza, Rhizophora stylosa, Acanthus ilicifolius, Acrostichum aureum, and Sonneratia apetala. These mangrove resources are distributed across 46 counties (cities and districts) in 13 prefecture-level cities along the coast, totaling 12092.95 ha, representing 41.9% of China’s mangrove area (excluding Hong Kong, Macao, and Taiwan). Notably, 50.20% of these mangroves are situated within 20 natural protected areas, including the Zhanjiang Mangrove National Nature Reserve, Zhuhai Qi’ao-Dangan Island Provincial Nature Reserve, and Jiangmen Taishan Zhenhai Mangrove National Wetland Park (Table 1 and Figure 1). All 20 naturally protected areas were included in this study. In some of these protected areas, abandoned aquaculture ponds are not used for economic farming, Instead, managers utilize tides to facilitate the exchange of aquatic resources inside and outside abandoned aquaculture ponds, providing food for birds inhabiting mangrove forests. These aquaculture ponds are a crucial part of the mangrove ecosystems. Additionally, some of these protected areas are connected to land rich in grasslands and shrublands. For example, Zhanjiang Jiulongshan Mangrove the National Wetland Park is connected to such land, and Galliformes inhabit areas between mangroves and grassland, with mangroves serving as a supplemental food source.
2.2 Methods
2.2.1 Determine of survey line transects, survey quadrats, and sampling points
2.2.1.1 Bird survey line transects
In ArcGIS 10.2, the coastal area of Guangdong Province was partitioned into 10 × 10 km grids using the kilometer grid method (Yang et al., 2021). These grids were overlaid with the boundary ranges of 20 natural protected areas. If an entire protected area fell within a grid, a 6 km line transect traversing the mangrove ecosystem was established within that grid. A total of 41 transect lines were included in the survey. Specifically, 21 line transects were established in the Zhanjiang Mangrove National Nature Reserve, 2 in the Jiangmen Taishan Zhenhaiwan Mangrove National Wetland Park, and 1 in each of the remaining protected areas.
2.2.1.2 Mangrove community survey quadrats
Mangrove coverage within the specified grids was assessed using ArcGIS 10.2, with measurements performed for the length of coverage in each grid. Within each grid, 5 quadrats, measuring 20 m × 20 m, were selected at regular intervals. In total 205 survey samples were collected.
2.2.1.3 Sampling points for water and soil environmental factors sampling points
5 collection points for water and soil environmental factors were selected from the aforementioned quadrats. In total, 205 survey samples were collected.
2.2.2 Survey methods
2.2.2.1 Bird diversity
Bird surveys were conducted during both the breeding period (March to May 2022) and wintering period (October to December 2022), with two surveys completed during each period. All surveys were performed by the same staff members to ensure the accuracy of observations. Each survey sample line was 6 km long, and the survey was conducted on foot. Surveys were conducted either in the morning (7:00–11:30) or afternoon (3:00–6:30), each lasting up to 3 h, with low tide identified as the optimal time for assessing bird diversity (Jimenez et al., 2015; Fonseca et al., 2017; Horn et al., 2020). Tide tables from the official website of the China Marine Service Network (Ocean.cnss.com.cn) were checked to determine the low-tide timings for survey scheduling. Field survey equipment included TSN841 20-60x monocular telescopes and 1000 m telephoto lenses for recording assistance. In this study, the species names and individual numbers of birds were collected. Due to the presence of 20 protected natural areas along the migratory route of the East Asian-Australasian Flyway, these areas play a significant role in global migratory bird protection. This is particularly important for special birds, such as migrating geese, ducks, and swifts, which can use mangroves for rest or as a food source. Field personnel comprised experienced researchers with expertise in bird observation. Bird identification references included A Field Guide to the Birds of China (MacKinnon and Phillipps, 2000). Resident type references include A Checklist on the Classification and Distribution of the Birds in China(Fourth Edition) (Zheng, 2023).
The surveyed birds were categorized into six ecological types based on their habits and morphological characteristics, namely songbirds, terrestrial birds, climbing birds, swimming birds, wading birds, and raptors (Table 2). Songbirds, primarily passerine birds, are adept at singing via the syrinx control. Terrestrial birds have relatively short beaks, typically living in flocks, and can be either terrestrial or arboreal, they primarily move on the ground in search of food, and include species, such as Galliformes. Climbing birds, such as Caprimulgiformes, Apodiformes, Piciformes and Coraciiformes, have two toes pointing forward and two toes pointing backward, which facilitates tree climbing. Swimming birds, including Anseriformes, Anseriformes, Podicipediformes, and Lariformes, have webbed toes and wide or sharp beaks, that enable them to excel at swimming, diving, and foraging in water, although most are not adept at walking on land. Wading birds, adapted to live in shallow waters or along shores, have long beaks, necks, and legs, making them suitable for wading but not for swimming, this group includes Ciconiiformes, Gruiformes, and Charadriiformes. Raptors, including Accipitriformes and Falconiformes, have powerful hooked beaks, large wings, strong feet, and sharp talons, they prey on other birds, mice, rabbits, and snakes, or feed on carrion.
2.2.2.2 Mangrove plant species and community
Mangroves are crucial ecosystems for bird reproduction, roosting, and feeding, and bird habitat utilization is closely linked to vegetation structure. Investigating the impact of mangrove communities on bird diversity involves assessing crown width, crown height, tree height, diameter at breast height (ground diameter), and mangrove plant species composition (Etezadifar and Ahmad, 2013; Leung and Nora, 2013; Mancini et al., 2018). Therefore, measurements of all mangrove plants were conducted within the survey quadrats during March-May and October-December 2022, and their average values were calculated.
2.2.2.3 Water and soil environmental factors
The physical and chemical conditions of mangrove water and soil environments not only indicate the ability of mangroves to accumulate and purify harmful substances, but also affect the biodiversity of the biological community it supports (Weinstein and Daniel, 2002; Cannicci et al., 2008). Previous studies have typically investigated parameters such as pH, water temperature, salinity, dissolved oxygen, soil salinity, chemical oxygen demand, nitrite, nitrate, ammonia, and suspended solids in aquatic environments (Caussy, 2009), as well as organic matter, sulfide, inorganic phosphorus, and reactive silicate in sedimentary environments (Weinstein and Daniel, 2002; Cannicci et al., 2008) to assess their effects on biodiversity. Hence, water quality and soil sediment environmental data were collected during the wet season (June–August) and dry season (November–December) of 2022, and the average values of these factors within each protected area were computed.
Water environmental factor detection method: At high water levels, water samples (1 L) were collected in brown glass bottles at each sampling point. A portable water quality detector (COD Ammonia Nitrogen Total Phosphorus Total Nitrogen analyzer) was used on-site to test pH, water temperature, salinity, dissolved oxygen, soil salinity, chemical oxygen demand, nitrite, nitrate, ammonia, suspended matter, and other factors. Data were recorded accordingly.
Sedimentary environmental factor detection method: Samples were collected at low tide, and surface sediment samples (10 cm) were taken from each sampling point. These samples were then brought back to the laboratory for the analysis of sedimentary environmental factors. The following methods were employed in the analysis of various components: thermal conductivity for organic matter, spectrophotometry for active silicate, the iodine method for sulfide, and digestion-molybdenum-antimony resistance spectrophotometry for inorganic phosphorus.
2.2.2.4 Mangrove habitat area
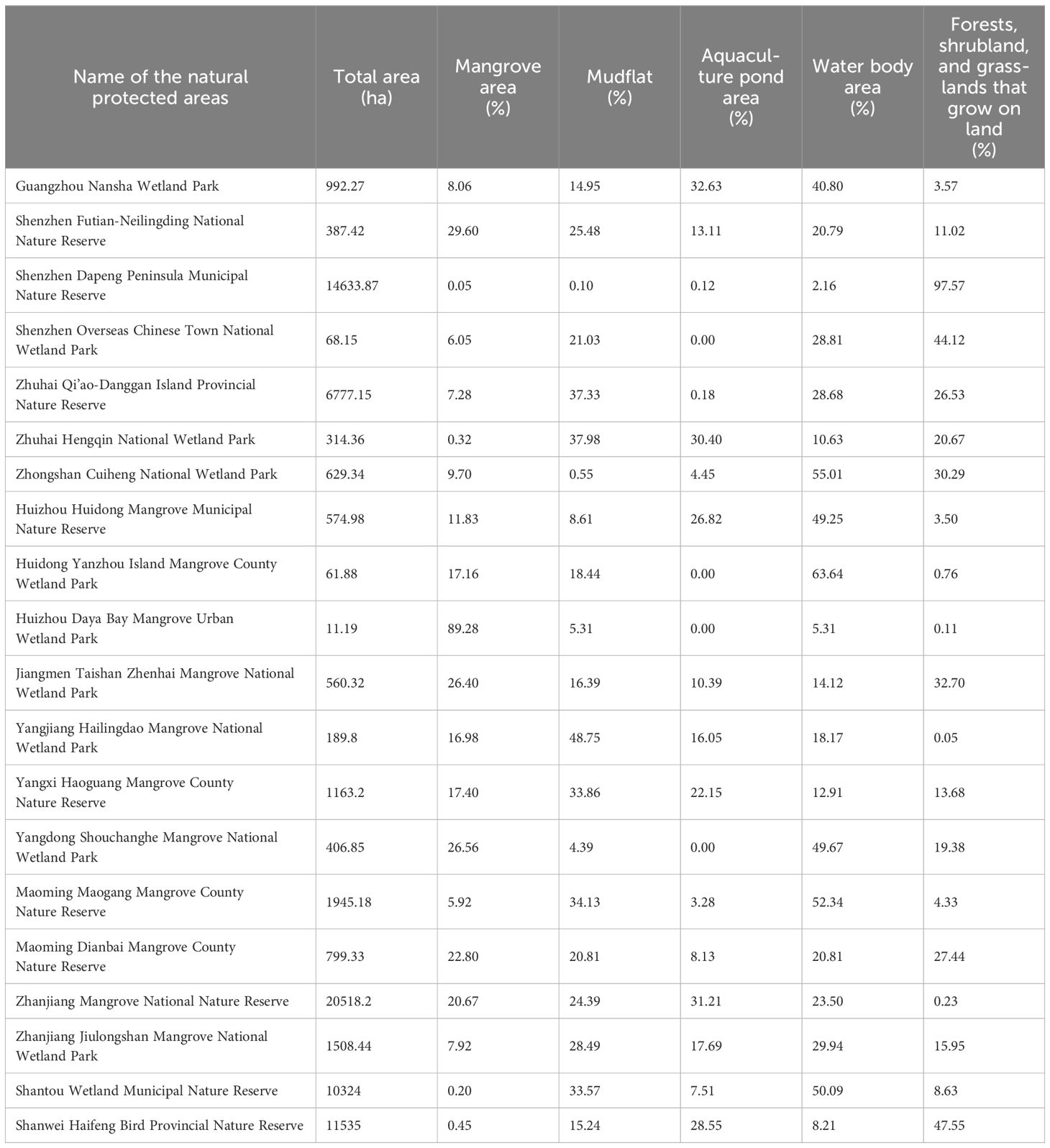
Within mangrove ecosystems, factors such as mangrove area, mudflat extent, aquaculture pond coverage, and open water areas (excluding aquaculture) play significant roles in shaping bird diversity. Remote sensing imagery for 2022 was acquired from the Data Center for Resources and Environmental Sciences, at the Chinese Academy of Sciences (RESDC, http://www.resdc.cn). Geometric and radiometric corrections were performed using the ENVI 5.3 software. The processed data underwent on-site verification, with mangroves, mudflats, aquaculture ponds, and open water areas quantified within each natural protection area.
2.2.3 Data processing
2.2.3.1 Correlation analysis of environmental factors
The Kolmogorov-Smirnov normal distribution test was performed for all factors, with a significance level of 0.05. The results showed that mangrove area, aquaculture pond area, open water area, sulfide, inorganic phosphorus, and other factors did not follow a normal distribution, whereas the remaining factors did. Pearson’s correlation analysis was used to examine correlations between environmental factors with a normal distribution, and rank correlation analysis was employed for those with a non-normal distribution. All analyses were conducted using SPSS 16.0 software. The analysis revealed that 5 environmental factors, including crown value, diameter at breast height (ground diameter), tree height, nitrate, and soil salinity, were correlated with other factors and were therefore excluded from further analysis.
2.2.3.2 Data standardization
Given the diverse types and dimensions of environmental factors, standardizing initial data is imperative (Cheng et al., 2021). The range method was employed to standardize the quantitative indices. A higher positive index value corresponds to a greater function, whereas a lower negative index value corresponds to a smaller function. Equations 1 and 2 can be used to standardize positive and negative indicators, respectively.
where is the standardized value of the environmental factor, and its value ranges from 0 to 1; is the j value of the i indicator; is the minimum value of the i indicator; and is the maximum value of the i indicator.
2.2.3.3 Redundancy analysis
To identify the primary drivers of bird diversity, RA was employed to assess the correlation between birds and their drivers, a widely used method in environmental studies (Zhang et al., 2016; Wang et al., 2021). First, detrended correspondence analysis (DCA) determined the linearity or unimodal response model of the analysis. The standardized sum-transformed species population square matrix served as the response variable. For the explanatory variables, we adopted a log10 transformation []. Given that the first four DCA axes exhibited maximum gradient lengths of less than 2, RA was selected for further analysis of the driving factors and bird diversity. To incorporate more factors into the analysis, a forward selection procedure identified variables with significant effects (P< 0.1), excluding those deemed insignificant (P > 0.1), because in very significant cases(P<0.05), only a few factors were included in the analysis. The significance of each variable was evaluated using the RA and a Monte Carlo permutation test with 999 permutations. DCA and RA analyses were predominantly conducted using CANOCO version 5.0.
3 Results
3.1 Land-use type
As shown in Table 2, the land-use types of each nature reserve were diverse, and the mangrove ecosystem was coupled with the terrestrial ecosystem. This coupling provides complex habitat requirements for various bird species.
3.2 Bird diversity
A comprehensive survey recorded 193 bird species, belonging to 17 orders and 53 families. Among these, there were 74 songbirds, 60 wading birds, 27 swimming birds, 17 climbing birds, 10 raptors, and 5 terrestrial birds (Table 3).
Among the recorded species, 28 were listed in “China’s National Key Protected Species List”, namely Eastern white pelican (Pelecanus onocrotalus), Saunders’s gull (Larus saundersi), Spotted greenshank (Tringa guttife), and Eurasian spoonbill (Platalea leucorodia). Additionally, 78 species were included in the “Agreement Between the Government of the People’s Republic of China and the Government of Japan for the Protection of Migratory Birds and Habitat Environment” (1981), including Bean goose (Anser fabalis), Fork-tailed swift (Apus pacificus), Common moorhen (Gallinula chloropus), Black-headed gull (Larus ridibundus), and Besra (Accipiter virgatus). Furthermore, 34 recorded species were listed in the “Agreement Between the Government of the People’s Republic of China and the Government of Australia for the Protection of Migratory Birds and Their Habitat Environment” (1986). This indicated the significant role played by the coastal areas of Guangdong Province in safeguarding the biodiversity of birds in China and globally.
3.3 Resident types of birds
The 193 species of birds consisted of 93 species of winter visitors (49.18%), 15 species of summer visitors (7.77%), 70 species of residents (36.27%), 13 species of passage migrants (6.74%), and 2 species of vagrant visitors (1.04%). Mangrove ecosystems within protected areas of Guangdong Province are pivotal for preserving the diversity of migratory bird species.
3.4 Characteristics of driving factors
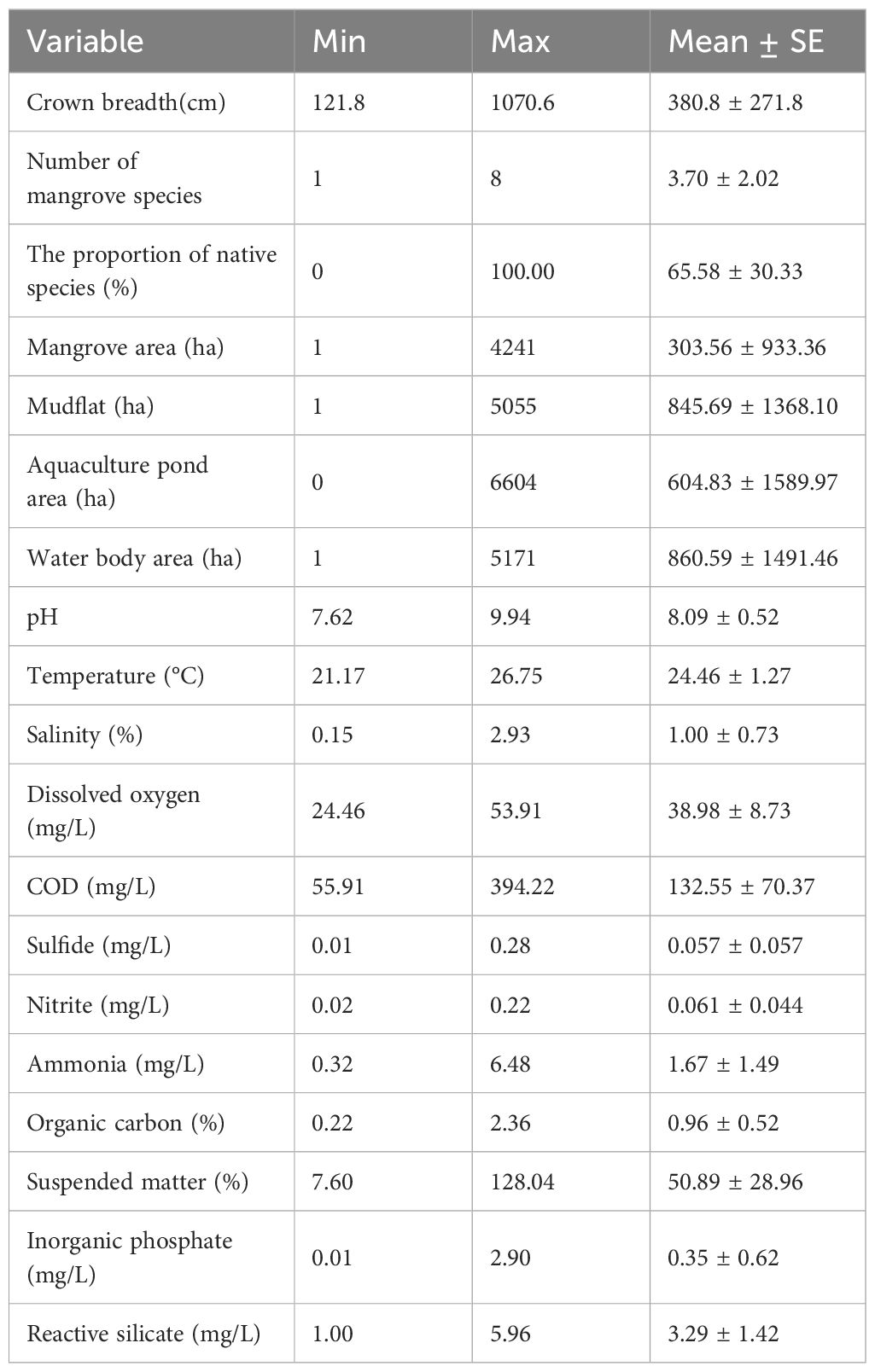
The basic conditions for each driving factor are listed in Table 4, where each factor shows its minimum, maximum, and average values. Data were collected from all transects.
3.5 Bird diversity and RA analysis of environmental factors
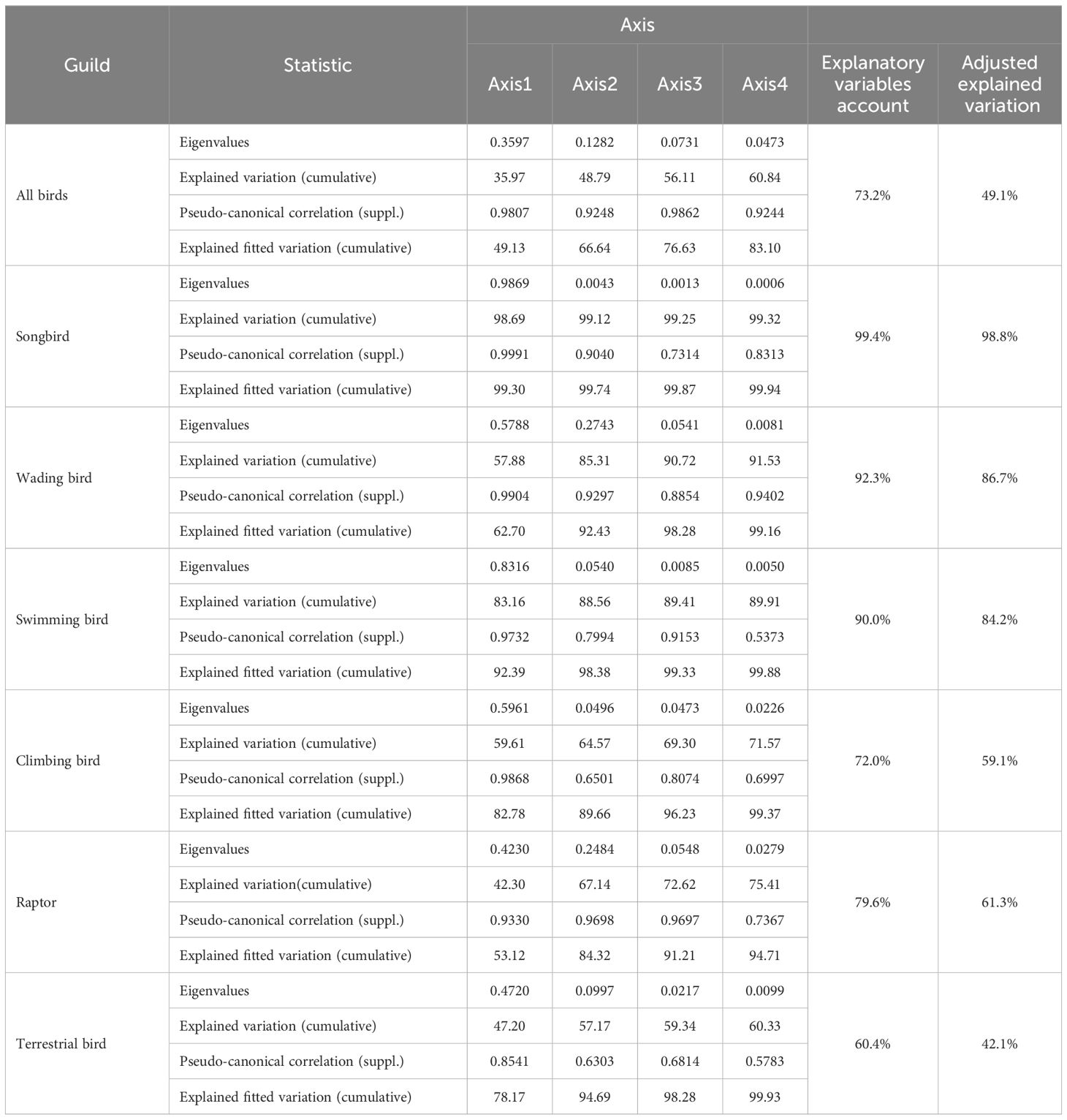
This study examined the effects of environmental factors and human disturbances on various bird categories, including birds, songbirds, wading birds, swimming birds, climbing birds, raptors, and terrestrial birds, using the RA method. The sorting results of RA are presented in Table 5.
The cumulative explanation of the variance in the species-environment relationship for all typical axes for all birds was approximately 83.10%. Significance of the Monte Carlo permutation tests for all positive axes was observed (p< 0.05). The eigenvalues of the first two canonical axes significantly exceeded those of the remaining axes, signifying their principal explanatory roles. These two axes accounted for approximately 49.13% and 17.51% of the variation, respectively, demonstrating their efficacy in elucidating relationships between species and environmental variables.
Similarly, for songbirds, wading birds, swimming birds, climbing birds, raptors, and terrestrial birds, the cumulative explanation of the variance in the species-environment relationship across all typical axes exceeded 90%. Monte Carlo permutation tests indicated significance (p< 0.05), and the first two typical axes effectively elucidated the relationship between species and environmental variables.
3.6 Relationships between birds and habitat characteristics
In Figure 2, the red arrow represents the environmental factor, and the blue line indicates the species factor. The length of the arrow indicates the strength of the correlation between the bird diversity and environmental factors. The angle formed between the arrow and ranking axis reflects the magnitude of the correlation between the environmental factor and ranking axis. A small angle indicates a strong correlation. Furthermore, the quadrant in which the arrow is located indicates whether the correlation between the azimuth environmental factor and ranking axis is positive or negative.
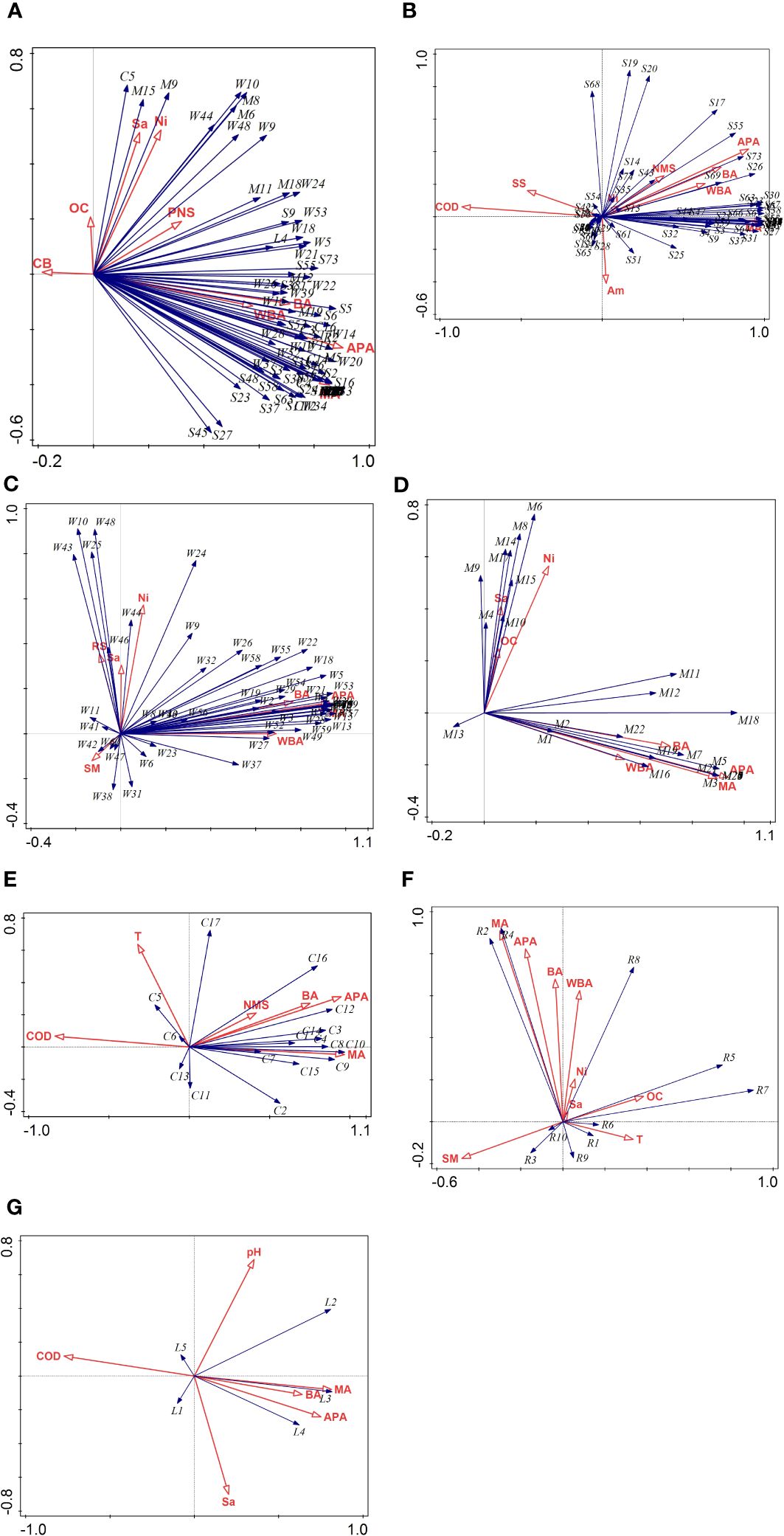
Figure 2. Relationships between birds and habitat characteristics. (A) All birds, (B) Songbird, (C) Wading bird, (D) Swimming bird, (E) Climbing bird, (F) Raptor, (G) Terrestrial bird. Explanatory variables: CB, Crown breadth (cm); NMS, Number of mangrove species; PNS, Proportion of native species (%); MA, Mangrove area (ha); BA, Mudflat (ha); APA, Aquaculture pond area(ha); WBA, Water body area(ha); T, Temperature (°C); Sa, Salinity (%); SS, Soil salinity (%FS); COD, (mg/L); Ni, Nitrate (mg/L); OC, Organic carbon (%); SM, Suspended matter (%); RS, Reactive silicate (mg/L). Sonbirds: S1-Pericrocotus divaricatus, S2-Dicrurus macrocercus, S3-Dicrurus hottentottus, S4-Lanius cristatus, S5-Lanius Schach, S6-Lanius tephronotus, S7-Garrulus glandarius, S8-Cyanopica cyanus, S9-Urocissa erythrorhyncha, S10-Dendrocitta formosae, S11-Pica pica, S12-Corvus pectoralis, S13-Corvus macrorhynchos, S14-Parus major, S15-Parus minor, S16-Alauda gulgula, S17-Cisticola juncidis, S18-Prinia superciliaris, S19-Prinia flaviventris, S20-Prinia inornate, S21-Orthotomus sutorius, S22-Pnoepyga pusilla, S23-Hirundo rustica, S24-Cecropis daurica, S25-Pycnonotus jocosus, S26-Pycnonotus sinensis, S27-Pycnonotus aurigaster, S28-Hemixos castanonotus, S29-Hypsipetes leucocephalus, S30-Phylloscopus fuscatus, S31-Phylloscopus proregulus, S32-Phylloscopus inornatus, S33-Phylloscopus humei, S34-Hemitesia pallidipes, S35-Horornis fortipes, S36-Aegithalos concinnus, S37-Zosterops erythropleurus, S38-Zosterops japonicus, S39-Pomatorhinus ruficollis, S40-Stachyris ruficeps, S41-Alcippe morrisonia, S42-Garrulax canorus, S43-Garrulax perspicillatus, S44-Acridotheres cristatellus, S45-Sturnus sericeus, S46-Sturnus cineraceus, S47-Sturnus nigricollis, S48-Sturnia sinensis, S49-Zoothera aurea, S50-Turdus hortulorum, S51-Turdus merula, S52-Calliope calliope, S53-Copsychus saularis, S54-Kittacincla malabarica, S55-Phoenicurus auroreus, S56-Rhyacornis fuliginosus, S57-Saxicola torquatus, S58-Saxicola stejnegeri, S59-Monticola rufiventris, S60-Muscicapa dauurica, S61-Aethopyga latouchii, S62-Lonchura striata, S63-Lonchura punctulate, S64-Passer montanus, S65-Motacilla tschutschensis, S66-Motacilla cinerea, S67-Motacilla alba, S68-Anthus richardi, S69-Anthus hodgsoni, S70-Anthus cervinus, S71-Emberiza cioides, S72-Emberiza fucata, S73-Emberiza pusilla, S74-Emberiza spodocephala. Wading birds: W1-Rallus indicus, W2-Porzana pusilla, W3-Amaurornis phoenicurus, W4-Amaurornis akool, W5-Gallinula chloropus, W6-Fulica atra, W7-Haematopus ostralegus, W8-Ibidorhyncha struthersii, W9-Himantopus Himantopus, W10-Recurvirostra avosetta, W11-Vanellus cinereus, W12-Pluvialis fulva, W13-Pluvialis squatarola, W14-Charadrius dubius, W15-Charadrius alexandrines, W16-Charadrius mongolus, W17-Charadrius leschenaultia, W18-Actitis hypoleucos, W19-Gallinago megala, W20-Gallinago gallinago, W21-Limosa limosa, W22-Limosa lapponica, W23-Numenius phaeopus, W24-Numenius Arquata, W25-Numenius madagascariensis, W26-Tringa erythropus, W27-Tringa tetanus, W28-Tringa stagnatilis, W29-Tringa nebularia, W30-Tringa guttifer, W31-Tringa ochropus, W32-Tringa glareola, W33-Calidris tenuirostris, W34-Calidris alba, W35-Calidris ruficollis, W36-Eurynorhynchus pygmeus, W37-Calidris temminckii, W38-Calidris ferruginea, W39-Calidris alpina, W40-Calidris canutus, W41-Phalaropus lobatus, W42-Glareola maldivarum, W43-Ciconia boyciana, W44-Phalacrocorax carbo, W45-Phalacrocorax Pelagicus, W46-Pelecanus onocrotalus, W47-Platalea leucorodia, W48-Platalea minor, W49-Ixobrychus sinensis, W50-Ixobrychus cinnamomeus, W51-Nycticorax nycticorax, W52-Butorides striata, W53-Ardeola bacchus, W54-Bubulcus ibis, W55-Ardea cinerea, W56-Ardea purpurea, W57-Ardea alba, W58-Ardea intermedia, W59-Egretta garzetta, W60-Egretta eulophotes. Swimming birds: M1-Anser fabalis, M2-Cygnus columbianus, M3-Tadorna tadorna, M4-Tadorna ferruginea, M5-Anas strepera, M6-Anas Penelope, M7-Anas platyrhynchos, M8-Anas zonorhyncha, M9-Anas acuta, M10-Anas crecca, M11-Anas clypeata, M12-Spatula querquedula, M13-Sibirionetta Formosa, M14-Aythya marila, M15-Aythya fuligula, M16-Tachybaptus ruficollis, M17-Podiceps cristatus, M18-Larus ridibundus, M19-Larus saundersi, M20-Larus crassirostris, M21-Sterna albifrons, M22-Hydroprogne caspia, M23-Sterna hirundo, M24-Chlidonias hybrida, M25-Chlidonias leucopterus, M26-Chlidonias niger, M27-Chlidonias hybrida. Climbing birds: C1-Apus pacificus, C2-Apus nipalensis, C3-Centropus sinensis, C4-Centropus bengalensis, C5-Eudynamys scolopaceus, C6-Surniculus lugubris, C7-Cuculus Micropterus, C8-Phaenicophaeus tristis, C9-Upupa epops, C10-Merops philippinus, C11-Halcyon coromanda, C12-Halcyon smyrnensis, C13-Halcyon pileate, C14-Alcedo atthis, C15-Ceryle rudis, C16-Jynx torquilla, C17-Remiz consobrinus. Raptors: R1-Pandion haliaetus, R2-Elanus caeruleus, R3-Accipiter trivirgatus, R4-Accipiter soloensis, R5-Accipiter virgatus, R6-Circus spilonotus, R7-Milvus migrans, R8-Spilornis cheela, R9-Falco tinnunculus, R10-Falco Subbuteo. Terrestrial birds: L1-Phasianus colchicus, L2-Streptopelia orientalis, L3-Streptopelia tranquebarica, L4-Spilopelia chinensis, L5-Macropygia unchall.
As shown in Figure 2A and Table 6, for all birds, the aquaculture pond, mangrove, and mudflat areas were identified as significant drivers of bird diversity, with interpretation rates of 31.0%, 28.9%, and 20.3%, respectively, in terms of simple effects. Regarding conditional effects, aquaculture pond area emerged as the main factor, explaining 31.0% of the bird diversity. Hence, the area of aquaculture ponds significantly affected the bird diversity.
Various bird species exhibit distinct sensitivities to identical driving factors.
(1) Songbirds. The importance of factors driving songbird diversity, such as mangrove area, aquaculture pond area, COD, mudflat area, water body area, and soil salinity, is evident from Figure 2B and Table 6, particularly in terms of the degree of impact of simple effects. The interpretation rates of these factors were 97.2%, 80.4%, 73.9%, 53.2%, 39.8%, 21.1%, and 14.4%, respectively. Among these, mangroves stood out as the primary driver, with an interpretation rate of 97.2%. Therefore, mangrove areas played a crucial role in the conservation of songbird diversity.
(2) Wading birds. In terms of the impact of simple effects, Figure 2C and Table 6 illustrate that the aquaculture pond area, mangrove area, mudflat area, and water body area significantly influenced the diversity of wading birds. Specifically, aquaculture pond areas, mangrove areas, mudflat areas, and water body areas had interpretation rates of 50.9%, 49.9%, 34.8%, and 28.1%, respectively. In terms of their impact on conditional effects, the aquaculture pond area stood out as the primary driver, with an interpretation rate of 50.9%. Hence, the aquaculture pond area was pivotal in driving bird diversity and plays a significant role in this regard.
(3) Swimming birds. As shown in Figure 2D and Table 6, the aquaculture pond area, mangrove area, mudflat area, and water body area significantly contributed to the diversity of swimming birds in terms of their degree of impact on simple effects. The interpretation rates of these factors were 73.7%, 67.3%, 42.5%, and 24.5%, respectively. Regarding conditional effects, the aquaculture pond area emerged as the primary driver, with an interpretation rate of 73.7%. Thus, the aquaculture pond area was a key factor affecting the diversity of swimming birds.
(4) Climbing birds. As shown in Figure 2E and Table 6, mangrove area, aquaculture pond area, COD, mudflat area, and number of mangrove species significantly contributed to the diversity of climbing birds in terms of their degree of impact on simple effects. The interpretation rates of these factors were 55.8%, 53.7%, 42.1%, 34.4%, and 10.9%, respectively. Regarding conditional effects, the mangrove area emerged as the main driver, with an interpretation rate of 55.8%. Therefore, the mangrove area was a key factor influencing the diversity of climbing birds and plays a crucial role in their conservation efforts.
(5) Raptors. As shown in Figure 2F and Table 6, the mangrove area and aquaculture pond area were the main drivers of raptor diversity in terms of the impact degree of simple effects. The interpretation rates of these factors were 24.5%, 19.5%, and 12.7%, respectively. Regarding conditional effects, the mangrove area stood out as the main driver, with an interpretation rate of 24.5%. Although the mangrove area was the primary factor influencing raptor diversity, its contribution was comparatively lower than that of other types of bird diversity.
(6) Terrestrial birds. As shown in Figure 2G and Table 6, the mangrove area, COD, aquaculture pond area, and mudflat area were the primary drivers of terrestrial bird diversity in terms of their degree of impact on simple effects. The interpretation rates of these factors were 32.0%, 28.7%, 27.6%, and 19.9%, respectively. Regarding conditional effects, the mangrove area emerged as the main driver, with an interpretation rate of 32.0%. Thus, the mangrove area was the primary factor influencing terrestrial bird diversity, although its contribution was relatively lower than that of other types of bird diversity.
In summary, the influence of specific driving factors on the diversity of different types of birds varies. Overall, mangrove areas emerged as the primary driving factor for land-dwelling birds, such as songbirds, climbing birds, raptors, and terrestrial birds, whereas the aquaculture pond area significantly affected water birds such as wading birds and swimming birds. Additionally, the water area, mudflat area, and other factors also play crucial roles in shaping bird diversity.
4 Discussion
4.1 Areas of aquaculture ponds, mangroves, and mudflats are important factors driving the diversity of birds
This study examined the impact of driving factors, such as mangrove habitat landscape factors, mangrove community structure, water environment, and soil sedimentary environment, on bird diversity. RA revealed that the areas of aquaculture ponds, mangroves, and mudflats significantly affected bird diversity, with interpretation rates of 31.0%, 28.9%, and 20.3%, respectively. Therefore, these factors were crucial for maintaining bird diversity.
Aquaculture pond areas can significantly affect bird diversity. Although some studies have indicated that large-scale coastal aquaculture pond construction can occupy bird habitats and reduce diversity levels (Zhang and Ouyang, 2019), others have suggested that these ponds can serve as important bird habitats (Yasué and Dearden, 2009; Lehnen and Krementz, 2013; Navedo et al., 2013; Fonseca and Navedo, 2020) and supplementary foraging sites (Walton et al., 2015; Wei et al., 2017; Fonseca and Navedo, 2020; Wang et al., 2020). This may be because birds can access high-energy food resources in aquaculture ponds (Mander et al., 2007; Ehmke et al., 2016). Different from profit-driven aquaculture pond construction within mangrove forests (Van et al., 2015), aquaculture ponds within naturally protected areas prioritize ecological benefits over economic gains. In China, the government has repurposed aquaculture ponds within protected areas, utilizing them as supplemental habitats, exchanging fish resources through tidal activity (Yang et al., 2022), or providing annual fish and shrimp supplements to sustain bird diversity. For example, the Guangdong Haifeng Avian Natural Reserve conserves nearly 90% of its tidal exchange ponds, and attracts over 300 bird species and 40,000 birds annually. Similarly, the Zhuhai Qi’ao-Dangan Island Provincial Nature Reserve has converted portions of its Sonneratia apetala forest into aquaculture ponds to attract geese, ducks, and herons. Therefore, in managing mangrove ecosystems, it is essential to preserve mangrove vegetation, beaches, water, and aquaculture ponds. These ponds should be retained through leasing or redemption and utilized to facilitate the exchange of aquatic biological resources with the tide. This approach enhances the foraging grounds available to the birds.
Mangrove areas can significantly influence bird diversity, serving as vital ecosystems for breeding, inhabiting, and feeding on numerous bird species. However, the extent of habitat utilization depends on mangrove area and vegetation structure (Mancini et al., 2018). Our findings revealed a close correlation between mangrove bird diversity and mangrove area, with mangrove community structure, such as species count, crown width and proportion of native species, making a relatively modest contribution to bird diversity. China has experienced mangrove destruction, protection, and restoration. Despite efforts to restore mangrove ecosystem functions by planting native species in natural protected areas, over 80% of restored mangroves remain as degraded secondary forests (Sui and Zhang, 2001). Consequently, the mangrove community structure remains relatively simple, with limited contribution to bird diversity. Mangroves play pivotal roles in driving bird diversity. As mangrove cover areas expand, bird diversity increases (Zou et al., 2008), fostering three-dimensional complex habitats owing to larger patch areas, that offer diverse microhabitats (Jo et al., 2012; Jayasilan et al., 2015) and ecological niches, thus enhancing niche heterogeneity and accommodating various bird breeding and habitat needs (Chen et al., 2017; Mancini et al., 2018). To further bolster biodiversity, the Chinese government plans to expand mangrove planting in naturally protected areas through initiatives such as A Specific Project for the Protection and Restoration of Mangroves (2020–2025). For example, the Zhuhai Qi’ao-Dangan Island Provincial Nature Reserve intends to plant 50 ha of mangroves in 2023 to maintain high biodiversity levels. Notably, in the Zhanjiang Mangrove National Nature Reserve, extensive mangrove planting efforts have corresponded to an upward trend in bird diversity (Li et al., 2013).
Mudflat areas have emerged as the main factor affecting bird diversity. Existing research confirms that mudflats offer abundant food resources for birds (Burger, 2018), with larger areas harboring more benthic organisms and better resistance to declines in benthic animal density owing to bird foraging (Fonseca et al., 2017). Particularly during low tides, mudflats provide optimal foraging conditions, as more invertebrates become accessible (Jimenez et al., 2015). Despite serving as a vital foraging ground for birds in protected areas, managing mudflats in these areas poses new challenges. For instance, in some protected areas, non-forest lands, including aquaculture ponds, mudflats, and shallow water areas, are being converted into large-scale mangrove planting areas, encroaching on bird foraging grounds and subsequently diminishing bird diversity levels. Consequently, the scientific management of mangrove areas remains an area of ongoing investigation and discussion.
The water area, environmental factors, and soil sedimentary environments contribute to bird diversity to some extent. Water areas significantly affect waterfowls, such as geese, ducks, gulls, and herons, providing essential elements such as varied water levels, hiding conditions, and food sources (Yang et al., 2022). However, its influence on terrestrial birds was comparatively limited (Table 6 and Figure 2). Despite the relatively weak response of birds to water and soil environments, this does not mean that their effect on bird diversity is insignificant. Previous studies have indicated that water quality (Caussy, 2009) and soil sediment conditions (Weinstein and Daniel, 2002; Cannicci et al., 2008) affect bird diversity by influencing benthic organism diversity. Certain waterfowl species, such as loons and large wading birds, thrive at moderate salinity levels (20–40 ppt), with abundance declining sharply beyond 100 ppt, as high salinity can lead to rapid dehydration and compromise feather waterproofing. Soil organic matter content correlates with the abundance of species such as crabs (Cannicci et al., 2008), which are vital food sources for certain birds. Further research is required to fully understand the effects of these factors on bird diversity.
4.2 Driving factors of diversity in different types of birds
The impact of specific driving factors on different bird types varied. However, mangrove areas have emerged as the primary driver for land-dwelling birds such as songbirds, climbers, raptors, and terrestrial birds, whereas aquaculture pond areas predominate for water birds such as wading birds and swimming birds. Various bird types exhibit distinct responses to these driving factors and are influenced by specific ecological considerations.
(1) Mangrove areas can significantly affect the diversity of songbirds, climbing birds, raptors, terrestrial birds, and other land-dwelling bird species.
The extent of mangrove areas typically determines the internal or marginal effects within mangrove ecosystems (Li et al., 2013), profoundly influencing the diversity of birds inhabiting mangroves, including songbirds, climbing birds, raptors, and terrestrial birds. Larger patches of mangroves play a more crucial role in reducing fragmentation and isolation between patches, than smaller patches do. Small mangrove areas may hinder communication among mangrove organisms, particularly by affecting ground-nesting birds, such as terrestrial birds (Uezu et al., 2005). Conversely, larger mangrove areas encompass a greater variety of mangrove species and support diverse mangrove communities, catering to the breeding, roosting, foraging, and hiding requirements of terrestrial birds (Jo et al., 2012; Jayasilan et al., 2015). Moreover, the complex structure of mangrove communities promotes the growth of benthic organisms and insects (Cannicci et al., 2008), which are essential components of the food chain in mangrove ecosystems. The increased biomass of these organisms enhances bird diversity (Hamza et al., 2015). For example, in the Zhanjiang Mangrove National Nature Reserve, a decline in the mangrove area led to a sharp decrease in falcon and eagle populations, highlighting the role of mangrove areas in raptor decline. After the restoration of extensive mangrove areas, populations of songbirds, such as Sternus nigricollis increased by 10%–20%, and populations of Streptopelia chinensis increased by approximately 30% (Li et al., 2013). This evidence demonstrates the importance of expanding mangrove plantations within protected areas to support terrestrial bird populations.
(2) Aquaculture ponds play a pivotal role in driving waterbirds, particularly wading and swimming birds.
Aquaculture ponds serve as important habitats for maintaining the diversity of water birds, including wading and swimming species (Mander et al., 2007; Ehmke et al., 2016), and as supplements or alternatives to natural foraging grounds. Currently, aquaculture ponds within natural mangrove protected areas in Guangdong Province have transitioned from economic to ecological significance. Owing to factors such as body size, beak length, and leg length, wading and swimming birds exhibit varied preferences for using these ponds. Studies have indicated that small shorebirds prefer water depths below 5 cm, large shorebirds between 5 cm–10 cm, geese and ducks between 10 cm–15 cm, diving ducks over 20 cm, and herons between 0 cm–30 cm (Therkildsen and Bregnballe, 2006). Consequently, the Guangzhou Nansha Wetland Park, Zhongshan Cuiheng National Wetland Park, and other natural protection areas adjust their water levels to create diverse depths, catering to the foraging requirements of different bird species. Additionally, management agencies employ extensive approaches to minimize human interference, such as prohibiting pesticide and fertilizer application, and promoting the growth of reeds and aquatic grasses, which offer shelter for birds. Similar initiatives in places, such as the Mai Po Nature Reserve in Hong Kong, have successfully enhanced waterbird abundance through artificial aquaculture pond management (Wei et al., 2017).
5 Conclusion
Natural protected areas play a crucial role in upholding bird diversity amid high-intensity human activities, forming an integral part of biodiversity conservation strategies. Our findings revealed notable variations in how different bird types respond to common environmental drivers. Specifically, the extent of aquaculture pond area, mangrove coverage, and mudflat area emerged as pivotal factors influencing bird diversity, with interpretation rates of 31.0%, 28.9%, and 20.3%, respectively. Mangrove coverage significantly influenced the diversity of songbirds, climbing birds, raptors, terrestrial birds, and others, whereas the area of aquaculture ponds notably affected wading and swimming birds. Although birds exhibited relatively subdued responses to water and soil environments, the significance of these factors in shaping their diversity cannot be ignored. Further investigations is warranted to elucidate the precise impact of these drivers on bird diversity. Although other environmental factors, such as COD, nitrate concentration, and salinity, were less significant drivers of bird diversity compared to aquaculture pond area, mangrove coverage, and mudflat area, they still had an impact. Investigating how these factors affect bird diversity is a worthwhile topic for future studies.
The Chinese government aims to expand mangrove planting within protected natural areas under the Specific Project for the Protection and Restoration of Mangroves (2020-2025). To enhance the protection of mangrove bird diversity, management authorities should judiciously oversee mangroves, mudflat, and aquaculture pond areas while formulating mangrove forest protection and restoration plans. This approach ensures that large-scale mangrove planting does not encroach upon the habitats of non-mangrove species. Furthermore, scientific management of aquaculture ponds, including water level regulation, is essential to meet diverse bird habitat requirements.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
XY: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. RW: Methodology, Software, Writing – review & editing, Conceptualization. CZ: Data curation, Writing – original draft. MQ: Formal analysis, Writing – original draft. JL: Data curation, Writing – original draft. RY: Data curation, Writing – review & editing. YZ: Methodology, Writing – original draft. GH: Resources, Writing – original draft. KT: Methodology, Writing – review & editing. LY: Methodology, Writing – original draft. SY: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work has received financial supports from “ Research and demonstration of mangrove ecological restoration and function enhancement technology in Guangdong, China(2020B020214001)”, “National Natural Science Foundation of China (52078004)”, and the “Science, Technology and Innovation Commission of Shenzhen Municipality (KCXFZ20211020164205009)”.
Conflict of interest
Author SY was employed by the company Guangzhou Yuanheng Natural Resources Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed M. U., Alam M. I., Debnath S., Debrot A. O., Rahman M. M., Ahsan M. N., et al. (2023). The impact of mangroves in small-holder shrimp ponds in south-west Bangladesh on productivity and economic and environmental resilience. Aquaculture 180, 739464. doi: 10.1016/j.aquaculture.2023.739464
Burger J. (2018). Use of intertidal habitat by four species of shorebirds in an experimental array of oyster racks, reefs and controls on Delaware Bay, New Jersey: Avoidance of oyster racks. Sci. Total Environment 624, 1234–1243. doi: 10.1016/j.scitotenv.2017.12.188
Cannicci S., Burrows D., Fratini S., Smith T. J., Offenberg J., Cannicci S., et al. (2008). Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat Bot. 89, 186–200. doi: 10.1016/j.aquabot.2008.01.009
Caussy L. (2009). Ecological assessment of the mangrove wetland of Pointe d’Esny and its ability to absorb pollutants. (Mauritius: University of Mauritus)
Chen L., Yan T., Xiong Y., Zhang Y., Lin G. (2017). Food sources of dominant macrozoobenthos between native and non-native mangrove forests: A comparative study. Estuar. Coast. Shelf Sci. 187, 160–167. doi: 10.1016/j.ecss.2016.12.012
Cheng W., Gang W., Dai L., Liu H., Li Y., Qiu C., et al. (2021). Study on the effect of habitat function change on waterbird diversity and guilds in Yancheng coastal we tlands based on structure–function coupling-Science Direct. Ecol. Indicators 122, 107223. doi: 10.1016/j.ecolind.2020.107223
Ehmke G., Grainne S. M., Tomas B., Daniel I., Michael A. W. (2016). An obligate beach bird selects sub- inter- and supra-tidal habitat elements. Estuarine Coast. Shelf Sci. 181, 266–276. doi: 10.1016/j.ecss.2016.12.012
Etezadifar F., Ahmad B. (2013). Nest-site selection of Western Reef Heron (Egretta gularis) in relation to mangrove (Avicennia marina) structure in the Persian Gulf: Implication for management. For. Ecol. Management 310, 74–79. doi: 10.1016/j.foreco.2013.07.060
Fonseca J., Enzo B., David S., Juan G. N. (2017). Effects of tidal cycles on shorebird distribution and foraging behaviour in a coastal tropical wetland: Insights for carrying capacity assessment. Estuar. Coast. Shelf Ence 198, 279–287. doi: 10.1016/j.ecss.2017.09.016
Fonseca J., Navedo J. G. (2020). Shorebird predation on benthic invertebrates after shrimp-pond harvesting: Implications for semi-intensive aquaculture management. J. Environ. Management 262, 110290.1–90.7. doi: 10.1016/j.jenvman.2020.110290
Gu X., Zhao H., Peng C., Guo X., Lin Q., Yang Q., et al. (2022). The mangrove blue carbon sink potential: Evidence from three net primary production assessment methods. For. Ecol. Management 545, 119848. doi: 10.1016/j.foreco.2021.119848
Hamza F., Hammouda A., Selmi S. (2015). Species richness patterns of waterbirds wintering in the gulf of Gabes in relation to habitat and anthropogenic features. Estuar. Coast. Shelf Sci. 165, 254–260. doi: 10.1016/j.ecss.2015.05.025
Horn S., Philipp S., Moritz M., Leonie E., Ragnhild A., Stefan G., et al. (2020). Species composition of foraging birds in association with benthic fauna in four intertidal habitats of the Wadden Sea. Estuarine Coast. Shelf Sci. 233, 1–15. doi: 10.1016/j.ecss.2019.106537
Jackson M. V., Fuller R. A., Gan X., Li J., Mao D., David S. M., et al. (2021). Dual threat of tidal flat loss and invasive Spartina alterniflora endanger important shorebird habitat in coastal mainland China. J. Environ. Management 278, 111549. doi: 10.1016/j.jenvman.2020.111549
Jayasilan M. A., Noske R., Lawes M. (2015). The role of habitat heterogeneity in structuring mangrove bird assemblages. Diversity 7, 118–136. doi: 10.3390/d7020118
Jimenez A., Robert W. E., Corinna F., Karen R., Ronald C. Y. (2015). Intertidal biofilm distribution underpins differential tide-following behavior of two sandpiper species (Calidris mauri and Calidris alpina) during northward migration. Estuar. Coast. Shelf Ence 155, 8–16. doi: 10.1016/j.ecss.2014.12.038
Jo S. T., Louise S. V., Loren M. S., Scott T., David A. H. (2012). Influence of local and landscape characteristics on avian richness and density in wet playas of the Southern Great Plains, USA. Wetlands 32, 605–618. doi: 10.1007/s13157-012-0280-1
Lehnen S. E., Krementz D. G. (2013). Use of aquaculture ponds and other habitats by autumn migrating shorebirds along the lower Mississippi river. Environ. Management 52, 417–426. doi: 10.1007/s00267-013-0087-8
Leung J. Y. S. (2015). Habitat heterogeneity affects ecological functions of macrobenthic communities in a mangrove: Implication for the impact of restoration and afforestation. Global Ecol. Conserv. 4, 423–433. doi: 10.1016/j.gecco.2015.08.005
Leung J. Y. S., Nora F. Y. T. (2013). Influence of plantation of an exotic mangrove species, Sonneratia caseolaris (L.) Engl., on macrobenthic infaunal community in Futian Mangrove National Nature Reserve, China. J. Exp. Mar. Biol. ecol. 448, 1–9. doi: 10.1016/j.jembe.2013.06.006
Li M. S., Mao L. J., Shen W. J., Liu S. Q., Wei A. S. (2013). Change and fragmentation trends of Zhanjiang mangrove forests in southern China using multi-temporal Landsat imagery, (1977–2010). Estuar. Coast. Shelf Sci. 130, 111–120. doi: 10.1016/j.ecss.2013.03.023
Ma Z., Melville D. S., Liu J., Chen Y., Yang H., Ren W., et al. (2014). Ecosystems Managerment Rethinking China’s new great wall. Science 346, 912–914. doi: 10.1126/science.1257258
MacKinnon J., Phillipps K. (2000). A field guide to the birds of China (Oxford: Oxford University Press).
Mancini P. L., Resisneto A. S., Fischer L. G., Luís F. S., Yara S. N. (2018). Differences in diversity and habitat use of avifauna in distinct mangrove areas in Sao Sebastiao, Sao Paulo, Brazil. Ocean Coast. Management 164, 79–91. doi: 10.1016/j.ocecoaman.2018.02.002
Mander L., Nicholas D. C., James A., Krysia M. (2007). Assessing the development of newly created habitat for wintering estuarine birds. Estuarine Coast. Shelf Sci. 75, 163–174. doi: 10.1016/j.ecss.2007.04.028
Menéndez P., Iñigo J. L., Michael W. B., Saul T. O., Antonio E., Siddharth N., et al. (2018). Valuing the protection services of mangroves at national scale: The Philippines. Ecosystem Services 34, 24–36. doi: 10.1016/j.ecoser.2018.09.005
Navedo J. G., Arranz D., Herrera A. G., Salmón P., Juanes J. A., Masero J. A. (2013). Agroecosystems and conservation of migratory waterbirds: importance of coastal pastures and factors influencing their use by wintering shorebirds. Biodiversity Conserv. 22, 1895–1907. doi: 10.1007/s10531-013-0516-2
Ren H. (2009). Sonneratia apetala Buch.Ham in the mangrove ecosystems of China: An invasive species or restoration species? Biol. Plantarum 8, 1243–1248. doi: 10.1016/j.ecoleng.2009.05.008
Sui S., Zhang Q. (2001). The mangrove wetland resources and their conservation in China. J. Natural Resour. 16, 28–36. doi: 10.11849/zrzyxb.2001.01.005
Therkildsen O. R., Bregnballe T. (2006). The importance of salt-marsh wetness for seed exploitation by dabbling ducks Anas sp. J. Ornithol. 147, 591–598. doi: 10.1007/s10336-006-0083-3
Thompson B. S., Stefanie M. R. (2019). Beyond ecosystem services: Using charismatic megafauna as flagship species for mangrove forest conservation. Environ. Sci. Policy 102, 9–17. doi: 10.1016/j.envsci.2019.09.009
Uezu A., Metzger J. P., Vielliard J. M. E. (2005). Effects of structural and functional connectivity and patch size on the abundance of seven Atlantic Forest bird species. Biol. Conserv. 123, 507–519. doi: 10.1016/j.biocon.2005.01.001
Van O. P. E., Siahainenia A. J., Sualia I., Tonneijck F. H., Van D. P. S., Groot R. S., et al. (2015). Effects of different management regimes on mangrove ecosystem services in Java, Indonesia. Ocean Coast. Manage. 116, 353–367. doi: 10.1016/j.ocecoaman.2015.08.003
Walton M. E. M., Vilas C., Cañavate P., Gonzalez O. E., Prieto A., Van B. A. J., et al. (2015). A model for the future: Ecosystem services provided by the aquaculture activities of Veta la Palma, Southern Spain. Aquaculture 448, 382–390. doi: 10.1016/j.aquaculture.2015.06.017
Wang C., Gang W., Dai L., Liu H., Li Y., Qiu C., et al. (2021). Study on the effect of habitat function change on waterbird diversity and guilds in Yancheng coastal wetlands based on structure–function coupling. Ecol. Indicators 122, 107223. doi: 10.1016/j.ecolind.2020.107223
Wang C., Wang G., Dai L., Liu H., Zhao Y. (2020). Diverse usage of waterbird habitats and spatial management in Yancheng coastal wetlands. Ecol. Indicators 117, 106583. doi: 10.1016/j.ecolind.2020.106583
Wanjiru C., Ivan N., Sonja R., William H., Mark H. (2023). Where to fish in the forest? Tree characteristics and contiguous seagrass features predict mangrove forest quality for fishes and crustaceans. J. Appl. Ecol. 60, 1340–1351. doi: 10.1111/1365-2664.14421
Wei P., Zan Q., Nora F. Y. T., Paul K. S. S., Li M. (2017). Impact of habitat management on waterbirds in a degraded coastal wetland. Mar. pollut. bulletin 124, 645–652. doi: 10.1016/j.marpolbul.2017.02.068
Weinstein M. P., Daniel A. K. (2002). “Influences of Vegetation and Abiotic Environmental Factors on Salt Marsh Invertebrates,” in Concepts and Controversies in Tidal Marsh Ecology. (Boston: Kluwer Academic Publishers). doi: 10.1007/0-306-47534-0:661-707
Yang X., Duan Z., Hu Y., Liu J., Xu Y., Hu H., et al. (2021). Mangrove planting strategies should consider the optimal ratio between the area of tidal flats and the area of mangroves. Ocean Coast. Manage. 213, 105875. doi: 10.1016/j.ocecoaman.2021.105875
Yang X., Duan Z., Li S., Zhang C., Qu M., Hua G., et al. (2022). Factors driving the abundance of wintering waterbirds in coastal Areas of Guangdong province, China. Front. Ecol. Evolution 09. doi: 10.3389/fevo.2021.808105
Yasué M. (2006). Environmental factors and spatial scale influence shorebirds’ responses to human disturbance. Biol. Conserv. 128, 47–54. doi: 10.1016/j.biocon.2005.09.015
Yasué M., Dearden P. (2009). The importance of supratidal habitats for wintering shorebirds and the potential impacts of shrimp aquaculture. Environ. Management 43, 1108–1121. doi: 10.1007/s00267-008-9255-7
Zhang C., Yuan Y., Zeng G., Liang J., Guo S., Lu H., et al. (2016). Influence of hydrological regime and climatic factor on waterbird abundance in Dongting Lake Wetland, China: Implications for biological conservation. Ecol. Engineering 90, 473–481. doi: 10.1016/j.ecoleng.2016.01.076
Zhang L., Ouyang Z. (2019). Focusing on rapid urbanization areas can control the rapid loss of migratory water bird habitats in China. Global Ecol. Conserv. 20, e00801. doi: 10.1016/j.gecco.2019.e00801
Zheng G. (2023). A checklist on the classification and distribution of the birds of China. 4th ed. (Beijing: Science Press).
Keywords: mangrove, natural protected areas, bird, habitat, diversity, factor
Citation: Yang X, Wen R, Zhang C, Qu M, Luo J, Yu R, Zhao Y, Hua G, Tan K, Yu L and Ye S (2024) Exploring the driving factors of bird diversity in mangrove natural protected areas in Guangdong Province, China. Front. Ecol. Evol. 12:1421189. doi: 10.3389/fevo.2024.1421189
Received: 23 April 2024; Accepted: 22 July 2024;
Published: 10 December 2024.
Edited by:
Daniel Murdiyarso, IPB University, IndonesiaReviewed by:
Scott Rush, Mississippi State University, United StatesAni Mardiastuti, IPB University, Indonesia
Copyright © 2024 Yang, Wen, Zhang, Qu, Luo, Yu, Zhao, Hua, Tan, Yu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rushu Wen, MzMyNTM2MDIxQHFxLmNvbQ==
 Xitao Yang
Xitao Yang Rushu Wen2*
Rushu Wen2*