- 1Department of Agricultural, Food and Environmental Sciences, University of Perugia, Perugia, Italy
- 2CREA- Plant Protection and Certification, Milano, Italy
Introduction: Crop Wild Relatives (CWR) have great socioeconomic importance for humans harbouring a broad spectrum of diversity and being important elements of different habitats. Beta vulgaris subsp. maritima ((L.) Arcangeli), also known as sea beet, is an important CWR of cultivated beets (GP-1). The high adaptability of this taxon to different environmental conditions, as well as its tolerance/resistance to different biotic and abiotic stresses, makes it a vital source for sugar beet improvement. Aim of this work was to analyse the in situ and ex situ status of sea beet population conservation in Italy, so as to guide protection activities and new collecting missions.
Methods: Geographical distribution data of populations were that occur in the wild and conserved in genebanks were assembled from different databases and submitted to data quality control. Distribution, habitat characterization, land cover and use of the involved sites were then evaluated to provide insight into the current condition of areas hosting this CWR diversity. The presence of populations within vs. outside Italian protected areas was also evaluated. A density analysis of the records was performed and the adequateness of sea beet ex situ conservation, in terms of number of conserved accessions, was finally estimated.
Results: A collection of 138 B. vulgaris subsp. maritima high quality georeferenced records were obtained, mainly distributed into Mediterranean Biogeographic Region. About 22% of the considered populations occurs in protected sites of the Natura 2000 Network, while about 15% in areas recorded in the Official list of protected natural areas (EUAP). Occurrences within protected areas are founded primarily in natural environments, whereas those outside are mainly located in urban and cultivated areas. The comparison of distribution and density analysis results revealed the presence of several gaps between sites hosting in situ populations and sites where ex situ conserved accessions were originally collected.
Discussion: Here presented data indicates that the protection status for sea beet in Italy can be considered only partially adequate; more proactive protection measures should be foreseen to increase the role of protected areas in safeguarding in situ conservation. “Out of reserve management” for populations outside protected areas should also be developed, as well as new collecting missions carried out.
Introduction
The domestication process of plant species determined a considerable reduction in the genetic variability of most crops compared to their wild counterparts (Olsen and Gross, 2008; Dempewolf et al., 2014). In the context of plant breeding, this phenomenon, commonly referred to as the “domestication bottleneck,” has raised concerns because breeders need access to the maximum genetic diversity possible to tackle future food production challenges. However, a wide spectrum of diversity for many different traits can still be found in the genomes of wild plant species that are closely related to crops also known as “crop wild relatives” (CWR). Indeed, the inclusion of CWR into breeding programs can significantly widen the source of genetic variation and selection towards the achievement of the different breeding targets. Disease and pest resistance improvement in wheat, rice, potato, tomato, sunflower and lettuce, as well the improvement of yield in wheat and rice and of tolerance to abiotic stress in rice, tomato, barley and chickpea are among the many available examples of successful use of CWR in plant breeding (Hajjar and Hodgkin, 2007 and references therein; Tirnaz et al., 2022 and references therein). Starting in the ‘40s-’50s of the last century, a notable increase in breeding achievements related to CWR use was gained from the ‘60s and ‘70s (Harlan, 1976, 1984; Prescott-Allen and Prescott-Allen, 1981; Hoyt, 1988). Nowadays, genome sequencing, pangenome construction and de novo domestication facilitate traits/gene selection from both closely and remotely wild relatives related to crops (Tirnaz et al., 2022). Finally, CWRs plays a vital role in traditional agro-ecosystems thanks to natural genetic exchange with landraces that also contributed to increasing their diversity after domestication.
With the assumption that any species belonging to the same genus of a given crop is a CWR (Maxted et al., 2006), the number of CWR species could account for about the 20% of the world’s flora corresponding to about 50,000-60,000 species (FAO, 2017); about 11,000 of these species are believed to be valuable for food security at the global level while about 700 are of a high value in terms of their potential use in plant breeding programs (Maxted and Kell, 2009; Engels and Thormann, 2020). An alarming 75% of CWR taxa are under threat in the wild, and climate change is expected to intensify this issue (Jarvis et al., 2008). The significance of preserving CWR is now increasingly recognized as a top priority (IPBES, 2022); noteworthy, conserving these species in their natural habitats is critical to ensure their ongoing evolution (Maxted and Kell, 2009).
B. vulgaris subsp. maritima ((L.) Arcangeli) (“sea beet”, 2n=2x=18) is a highly variable, allogamous, wind pollinated taxon; populations are composed of several genotypes (Andrello et al., 2016) and can have a mixture of annual, biennial, and perennial plants which helps the species to survive in extreme conditions (Letschert and Frese, 1993). The taxon is widely diffused along the coasts of the Mediterranean Sea, the European Atlantic Ocean and the western portion of the Baltic Sea, on sand and pebble beaches, saltmarshes drift-line, sea rocks and cliffs (Becker-Dillingen, 1928; Ulbrich, 1934; Biancardi et al., 2012a; Monteiro et al., 2018).
Besides a subspecies of B. vulgaris (Euro+Med DataBase. - the information resource for Euro-Mediterranean plant diversity, available at https://europlusmed.org/cdm_dataportal/taxon/2584ce98-01b2-4344-bc9e-d13f359293c2 [Accessed: 02/05/2023]), this taxon has been considered a synonym of Beta vulgaris L. (e.g. World Flora Online, available at http://www.worldfloraonline.org [Accessed on 02/05/2023]), or a species by other authors (e.g. Biancardi et al., 2012), available at http://dryades.units.it/floritaly [Accessed: 02/05/2023]).
This valuable CWR of cultivated beets belongs to the genus Beta L. (Amaranthaceae, Betoideae) that has been suggested as priority at global level for the economic importance of the related crops and the conservation status (Castañeda-Álvarez et al., 2016). Its remarkable resistance to drought and soil salinity makes it extremely competitive in coastal habitats, when compared to other species (Biancardi et al., 2012a), but, as showed by some studies conducted in central Italy by Pirone (1995), Biondi et al. (2002) and Landucci et al. (2012), it can also colonize the hinterland, with populations occurring in clayey gully systems with marine clay deposits. Indeed, even if its diffusion progressively decreases going inland, where most forms derive from hybridization with cultivated ones, this taxon shows a large environmental adaptability.
This wild taxon is fully sexually compatible and able to give rise to fully fertile progenies when crossed with the many B. vulgaris cultivated forms; as such it is placed in the GP-1 according to Harlan and de Wet (1971). Wild beet has been already successfully used as a source of useful trats in cultivated beet breeding (Biancardi et al., 2012; Capistrano-Gossmann et al., 2017; Dempewolf et al., 2017 and references therein, respectively) as well as for broadening the genetic diversity of the crop (Campbell, 2010). Sugar beet, the most important crop within the genus Beta, was developed in a very short period towards a high productive crop (Biancardi et al., 2012b). To maintain the adaptability of this crop, the continuous and systematic incorporation of genetic variability from close relatives into its gene pool has been proposed (Fénart et al., 2008). It is above all urgent to adapt the cultivation of beet to climate change, especially regarding drought resistance.
The high genetic variability of sea beet, associated with adaptability to different stressing climatic and environmental conditions (e.g. hydric, saline and nutritional stresses) (Hanson and Wyse, 1982; Stevanato et al., 2014) is a vital source of useful traits for beet improvement. Resistance to different biotic stresses, including cercospora (Cercospora beticola Sacc.), downy mildew (Peronospora farinosa (Fr.) Fr.), powdery mildew (Erysiphe betae (Vañha) Weltzien), root-rot (Rhizoctonia solani Kühn) and rhizomania (Beet Necrotic Yellow Vein Virus (BNYVV)) as well salinity tolerance are some of the traits with breeding value present in the wild beets (Biancardi et al., 2012a).
The phenotypic and genotypic variability of B. vulgaris subs. maritima was studied for Madeira’s archipelago populations (Ascarini et al., 2021), while the usefulness of its cytogenetic diversity for breeding and conservation programs was investigated by Monteiro et al. (2018 and reference therein). Furthermore, it is a heritage of evolution and an important component of coastal ecosystems, where it provides food and habitat for native wildlife and contributes to consolidate coastal line, having per se an intrinsic value and as such deserving to be maintained.
In Italy B. vulgaris subs. maritima is quite diffused (Bartolucci et al., 2018) although it is not protected since protection priorities in the country are based on the IUCN status of ‘endangered’ or ‘vulnerable’ (Landucci et al., 2014; Ciancaleoni et al., 2021). However, it is noteworthy that this taxon is at high risk of genetic erosion in those areas where the production of sugar beet seed is carried out and, among others, also due to touristic and agricultural activities. Thus, appropriate long-term conservation strategies should be adopted to protect and maintain B. vulgaris subsp. maritima populations in Italy.
In situ conservation is the most appropriate and effective protection strategy for CWR as it preserves both the population and the evolutionary processes by managing organisms in their natural surroundings (CBD, 1992). Understanding the geographical distribution of CWR populations (i.e. the punctual occurrence of populations in the wild) allows the development of targeted strategies that are crucial for effective in situ conservation. Examining where CWR populations are present in protected areas allows to identify areas where conservation efforts might already be in place or areas that require additional protection measures; moreover it helps to identify the site-based population vulnerability, considering factors such as habitat degradation, human activity, and climate change impacts which undermines taxa in situ survival (Bilz et al., 2011; Dempewolf et al., 2014; Aguirre-Gutiérrez et al., 2017). Indeed, Protected Areas play a crucial role in in situ conservation as they often provide a legal framework and management structures that can help in ensuring the conservation of CWR populations. Without additional data, it is reasonable to presume that populations residing within protected areas are safer than those outside.
Among existing protected areas, it is noteworthy to mention those belonging to the Natura 2000, a network of protected areas covering Europe’s most valuable and threatened species and habitats. Natura 2000 is the largest coordinated network of protected areas in the world, extending across all 27 EU Member States, both on land and at sea. The sites within Natura 2000 are designated under the “Birds Directive” 79/409/EEC (European Commission, 1979) and the “Habitats Directive” 92/43/EEC (European Commission, 1992).
In addition, all the Italian officially recognised National, Regional and Interregional, marine and terrestrial, protected natural areas are listed in the ‘VI Official The official list of Nationally protected natural areas of Italy (EUAP) which is drawn up, and periodically updated, by the Ministry of Ecological Transition (Ministero della Transizione Ecologica, MiTE) — Directorate for the Protection of Nature, which collects National, Regional and Interregional Italian protected natural areas, marine and terrestrial, officially recognised (Repubblica italiana, 1991).
However, the status of a population cannot be exactly defined even if the population falls within a protected area. The information about presence should be completed with other data about the state of the site, to avoid too binary assumptions about the state of protection of the taxon (Langhammer et al., 2007). Therefore, combining the knowledge of population occurring sites, protected areas distribution and level of CWR taxa diversity within protected areas can guide the selection of best areas for implementing CWR in situ protection (Maxted, 2003). Indeed, although the level of protection within protected areas may in general be passive, they still offer essential safeguards against habitat destruction, overexploitation, and other anthropogenic pressures (Maxted et al., 2008).
Mapping taxa distribution in relation to Land Use and Land Cover (LULC) is another way to gain a perspective on the state of the sites hosting CWR diversity, so to understand which are the most “sensitive” areas where populations may be most at risk due to anthropic or environmental pressures connected to LULC. In this regard, it has been already shown that many populations of CWR of different species of interest are currently occurring outside protected areas (Heywood and Dulloo, 2005; Raggi et al., 2022b, 2024) including road and field edges as well as cultivated fields. Different actions can be put in place to establish a certain level of protection even in these areas by means of the ‘Out of Reserve Management’ approach (Hale and Lamb, 1997) so to reduce the level of risk and the related threats.
As part of comprehensive CWR conservation planning, also the ex situ conservation plays an important role; this conservation strategy is complementary to in situ, by securing the preservation of target populations as well as facilitating access to their diversity for both research and breeding activities (Maxted et al., 1997; Brush, 2004; Phillips et al., 2016; Schwartz et al., 2017).
Aims of this work were to explore B. vulgaris subsp. maritima distribution across Italy by means of population distribution data available in different databases. Regarding the potential role of protected areas in advancing CWR in situ conservation, the presence of populations in protected sites was evaluated and discussed, taking into account the land cover and use of the involved sites. A comparison between the distribution of the sites hosting populations in situ and where ex situ conserved accessions were originally collected was also performed to highlight possible gap in the ex situ conservation of this species.
Materials and methods
Data acquisition
We acknowledge for this taxon the rank of subspecies [B. vulgaris L. subsp. maritima (L.) Arcang.], in agreement with the Portal of the Flora of Italy (available at https://dryades.units.it/floritaly/index.php [Accessed: 02/05/2023]). In the first part of this study, we explored the taxon distribution over the whole Italian territory (mainland and islands), employing a methodology similar to the one described by Raggi et al. (2022b). In brief, in situ occurrences were estimated using data from different online databases: the Global Biodiversity Information Facility (GBIF), ‘VegItaly’, ‘Genesys’ and the ‘National Biodiversity Network’ database.
GBIF (available at http://www.gbif.org/) is an international network and data infrastructure which provides open access to chorological data of all types of life on Earth presents in genebanks, botanic gardens, museums, and universities. Part of the Global Index of Vegetation-Plot Databases, VegItaly is coordinated by the Italian Society of Vegetation Science (https://www.scienzadellavegetazione.it/vegitaly/); it organizes all data derived from the different approaches used in the field of vegetation science into an organic structure. Currently, VegItaly is hosted in the anArchive project (http://www.anarchive.it), a database system dedicated to the archiving of botanical data (taxonomic data, floristic reports, vegetation surveys). Genesys is a database holding information on ex situ accessions conserved worldwide (available at https://www.genesys-pgr.org/). The ‘National Biodiversity Network’ is a database, collated by the Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA) accessible on the Italian National Geoportal (available at http://geoviewer.nnb.isprambiente.it/mapreacter) that is promoted by the Ministry of Ecological Transition (Ministero della Transizione Ecologica, MiTE), the Italian Ministry in charge of protecting nature.
All retrieved data were organised and standardized in a unique database. For each record of occurrence (i.e. database entry) the following fields were annotated: i) database origin (DATABASE-ORIGIN), code of the donor (INSTCODE) and accession number (ACCENUMB); ii) taxonomy and nomenclature including genus (GENUS), species (SPECIES) and species author (SPAUTHOR), subtaxa (SUBTAXA) and subtaxa author (SUBTAUTHOR); iii) geographic coordinates: latitude (DECLATITUDE) and longitude (DECLONGITUDE), iv) other information on population collection site including nation (ORIGCTY), administrative region (ADMREGION) and collection site (COLLSITE) and v) acquisition date (ACQDATE). It is here anticipated that only records with sufficiently accurate geographical coordinates (i.e. georeferenced records) were targeted and analysed.
To create high-quality datasets, different filters were applied to the collated raw data in the Quality Control (QC) process as suggested by Rubio Teso and colleagues (2020); in particular, occurrences belonging to the following classes were removed: (i) corresponded to cultivated, (i.e. not wild) materials; (ii) located outside Italian national borders; (iii) characterised by low-quality geographical coordinates (i.e. ≤ 2 decimal digits or stated error > 500 meters) and (iv) dated before 1970. Records from GBIF (v) with major known issues (i.e. invalid basis of record, institution match fuzzy) and (vi) coming from unsupervised sources (i.e. iNaturalist) or from the System-wide Information Network for Genetic Resources (SINGER) were also eliminated. Duplicates were simplified keeping records more recent and with more information available.
Records that survived QC were used to create two databases:
1) “In situ database”, merging records of populations that occur in the wild from GBIF, National Biodiversity Network and Genesys; the inclusion of entries from Genesys among in situ populations is motivated by the fact that ex situ conserved accessions (listed in Genesys) come from collections of populations occurring in the wild (i.e. in situ) (Rubio Teso et al., 2020; Raggi et al., 2024);
2) “Ex situ database”, including records representing the actual ex situ conserved populations (only coming from Genesys). Geographical data here refer to the original geographical coordinates of collection sites (i.e. of sites where ex situ conserved accessions were originally collected).
Populations distribution
Occurrence records stored in the two above mentioned databases were imported into QGIS software 3.16.15-Hannover (QGIS Development Team, 2021) specifying the geographic reference system WGS84 (EPSG: 4326) not projected, compliant with the LAT/LONG DD format.
A spatial consistency verification followed, where B. vulgaris susp. maritima occurrence records with the following characteristics were removed: (i) placed outside Italian national borders, as defined in the polygonal shape file (scale of 1:1 000 000) of the administrative borders of Italy; (ii) in the country centroid or (iii) in the sea. The few records near close the coasts (<1 km) were manually repositioned along the coast (Raggi et al., 2022b).
Occurrence of the considered populations in the different Italian Biogeographic Regions was tested in QGIS according to the “Biogeographical regions, Europe 2016, version 1” geospatial vector retrieved from the European Environment Agency (https://www.eea.europa.eu/en/datahub/datahubitem-view/11db8d14-f167-4cd5-9205-95638dfd9618, v. January 2016).
Protection status of population hosting sites
Two different cartography layers were used to infer the protection status of the sites hosting sea beet occurrences taking advantage of the Vector overlay function in QGIS and using the geographic coordinates of each record in the in situ database.
The status ‘inside’ or ‘outside’ protected area was tested using the geospatial vector of:
i) Natura 2000 Network retrieved from the European Environment Agency web site (available at https://www.eea.europa.eu/data-and-maps/data/natura-14/natura-2000-spatial-data, v. end 2021). The file accounts for delineations of the “Habitats Directive” and of the EMERALD Network, set up under the ‘Convention on the Conservation of European Wildlife and Natural Habitats’ (i.e., Bern Convention) (Council of Europe, 1979);
ii) ’VI Official The official list of Nationally protected natural areas of Italy (EUAP). It is worth noting that EUAP areas and Natura 2000 sites are not necessarily overlapped; indeed, in Italy, about half of Natura 2000 sites fall outside the EUAP network.
Habitat characterization and land use and land cover of population hosting sites
The characterization of the habitats occurring in the sites hosting B. vulgaris subsp. maritima populations was estimated using the Vector overlay function in QGIS according to ‘Carta della Natura’ (1:250.000) (Amadei et al., 2003), provided by ‘Istituto Superiore per la Protezione e la Ricerca Ambientale’, which describes the habitats and ecosystems insisting on the Italian territory.
Land Cover (LC) of the same sites was also estimated using the same QGIS function and according the ESA CCI Land Cover, level 1, v2.0.7 (2015) (ESA, 2017) (spatial resolution 300m). This source gives information on the land cover according to 22 primary and 14 secondary use classifications (maps.elie.ucl.ac.be/CCI/viewer/download/ESACCI-LC-Ph2-PUGv2_2.0.pdf).
Land Use and Land Cover (LULC) were also estimated according to the Corine Land Cover (CLC) 2018 (status layer, spatial resolution 100m) that utilize instead 44 different classes at the European scale to give information on territory cover and use (https://land.copernicus.eu/en/products/corine-land-cover/clc2018).
All categorizations were nested within the primary category of ‘inside’ or ‘outside’ protected areas. Results for sites ‘inside’ or ‘outside’ protected areas were graphically summarised using histograms.
In situ vs ex situ conservation
A density analysis of the records from the in situ and ex situ databases was performed in QGIS as described in Raggi et al. (2022a, b). Briefly, a grid of 10×10 km was obtained from the standard ones available at the European Environment Agency website (European Environment Agency, 2017) and the number of CWR records per cell was calculated using the Count Points in Polygon tool. The position of cells containing ≥1 records were shown over the Italian territory and coloured according to a purposely developed colour scale. Finally, the adequateness of sea beet ex situ conservation in terms of number of conserved accessions was estimated by comparing the number of populations occurring in the wild and conserved ex situ across the different Italian regions.
Results
Data acquisition and populations distribution
After the application of QC filters, the query of GBIF, Genesys and National Biodiversity Network databases generated a collection of 138 B. vulgaris subsp. maritima in situ records (Table S1, Supplementary Materials); most of the records came from Genesys (46%) followed by GBIF (33%) while 21% were obtained from the shared data management system of the Italian ‘National Biodiversity Network’ database. As for the ex situ, a total of 63 records from Genesys were kept after QC (Table S2, Supplementary Materials).
With 122 of the total 138 sites (88%), the Mediterranean Biogeographic Region hosts the highest number of B. vulgaris subsp. maritima populations in nature, followed by the Continental one (12%). Geographical distribution of sea beet in situ and ex situ occurrence records across Italy is available in Figure 1.
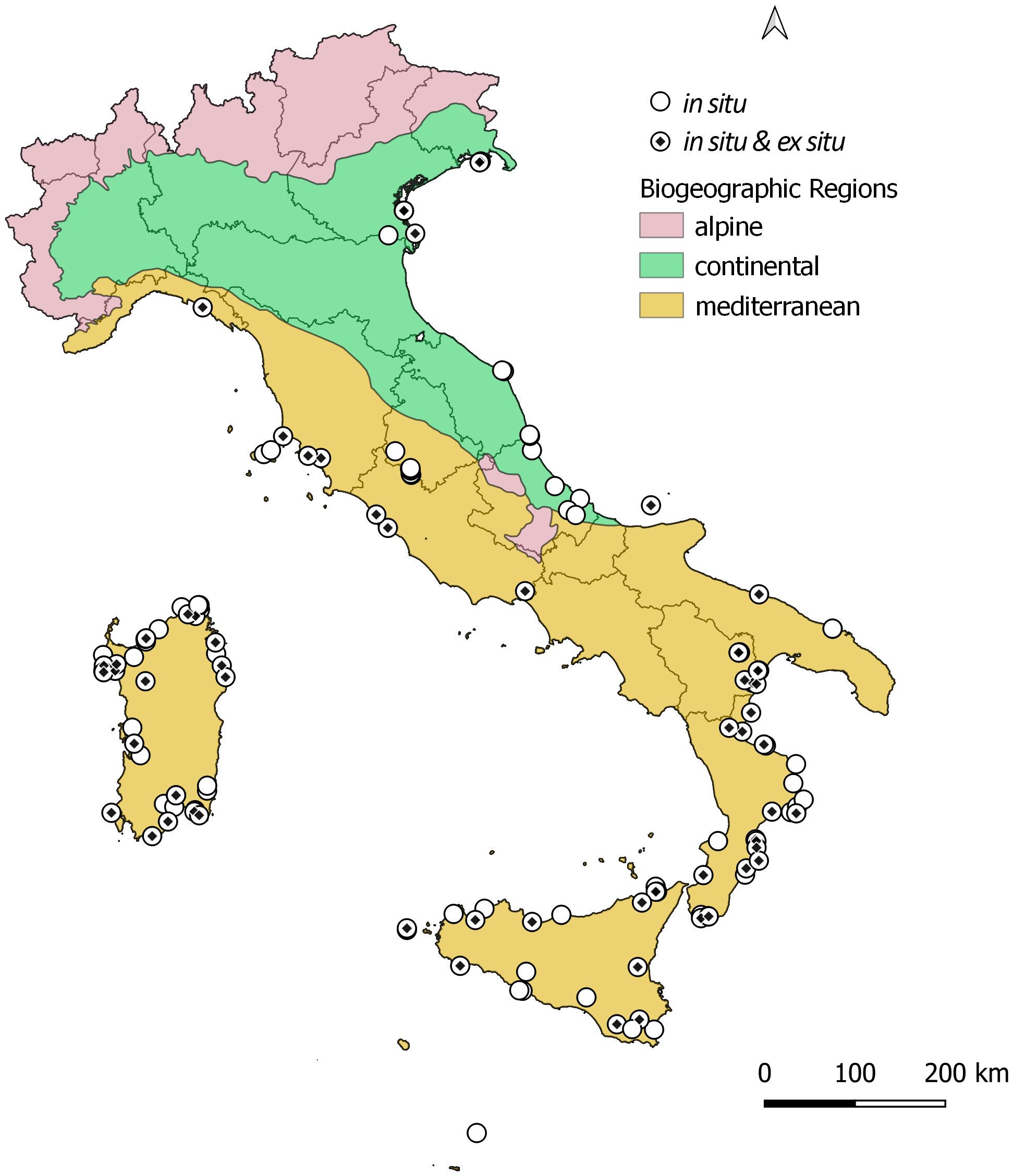
Figure 1. Geographical distribution of the 138 in situ and 63 ex situ B. vulgaris subsp. maritima occurrence records, multiple sites with similar geographic coordinates appear as a single locality. Symbols and colours are according to the figure legend. Italian Region perimeters are also shown.
Protection status of population hosting sites
As for the protection status, 30 of the 138 sites hosting Beta vulgaris subsp. maritima populations (22%) are in protected areas of the Natura 2000 Network (Figure 2A pay chart) and 21 populations (15%) in areas listed in the EUAP (Figure 2B pay chart). When Natura 2000 Network and EUAP are considered together, 32 sites (23% of the total) are the object of protection due to extensive overlapping of protected areas.
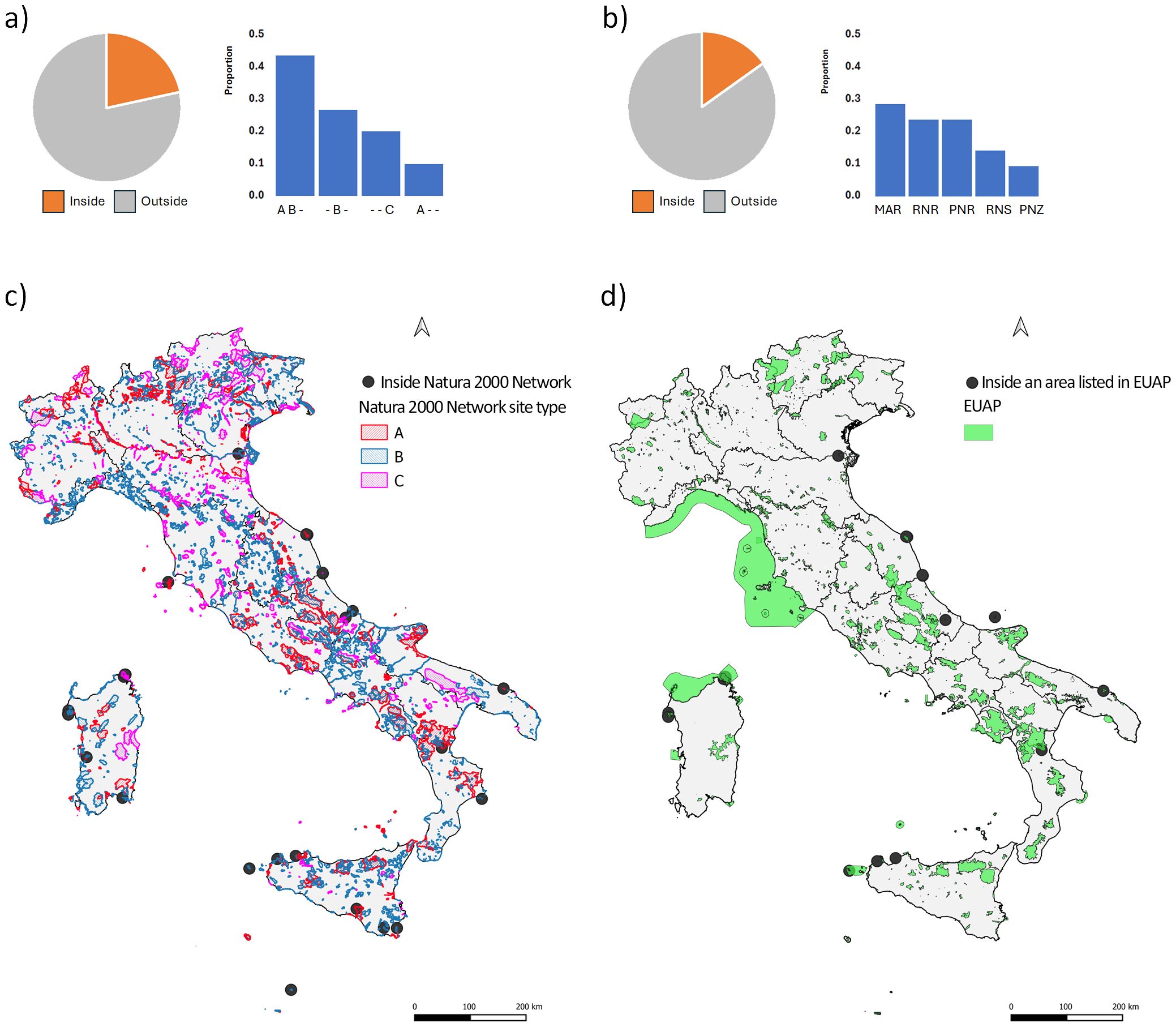
Figure 2. Pie graphs showing the proportion of B. vulgaris subsp. maritima in situ records inside (orange) and outside (light grey) protected areas of Natura 2000 Network (A) and of EUAP (B) together with histogram representing records distribution in the different types of protected areas. Letters associated to the Natura 2000 sites histograms are as follows: “A” = designed “Special Protection Area” (SPA); “B” = “Sites of Community Importance” (SCI) or “Special Area of Conservation” (SAC); “C” = SCI/SAC is the same as designated SPA. Acronyms associated to the EUAP areas histograms are as follows: “MAR” = Marine Reserve; “RNR” = Regional Natural Reserves; “PNR” = Regional and Interregional Natural Parks; “SNR” = State Nature Reserves; “PNZ” = National Parks. Geographical distribution of sites hosting Beta vulgaris subsp. maritima populations located inside Natura 2000 Network (C) and inside areas listed in EUAP (D).
The highest proportion (0.43) of B. vulgaris subsp. maritima in situ occurrences included in the Natura 2000 Network is in sites listed, at the same time, as Special Protection Areas (SPAs, A type) of the “Birds Directive” (European Commission, 1979) and Sites of Community Importance (SCIs, B type) or Special Areas of Conservation (SACs, B type) of the “Habitat directive” (European Commission, 1992) (Figure 2A, histograms). Because of the coastal distribution of this taxon, Marine Reserve (MAR) is the EUAP category hosting the highest proportion of sea beet populations followed by Regional Natural Reserves (RNR) and Regional and Interregional Natural Parks (PNR) (Figure 2B histograms). The distribution of here analysed sites hosting Beta vulgaris subsp. maritima populations, and located inside considered protected areas, well resembles the distribution of this taxon across the Italian territory along the coasts of the Mediterranean Sea and in the major Italian islands Sicily and Sardinia (Figures 2C, D, respectively).
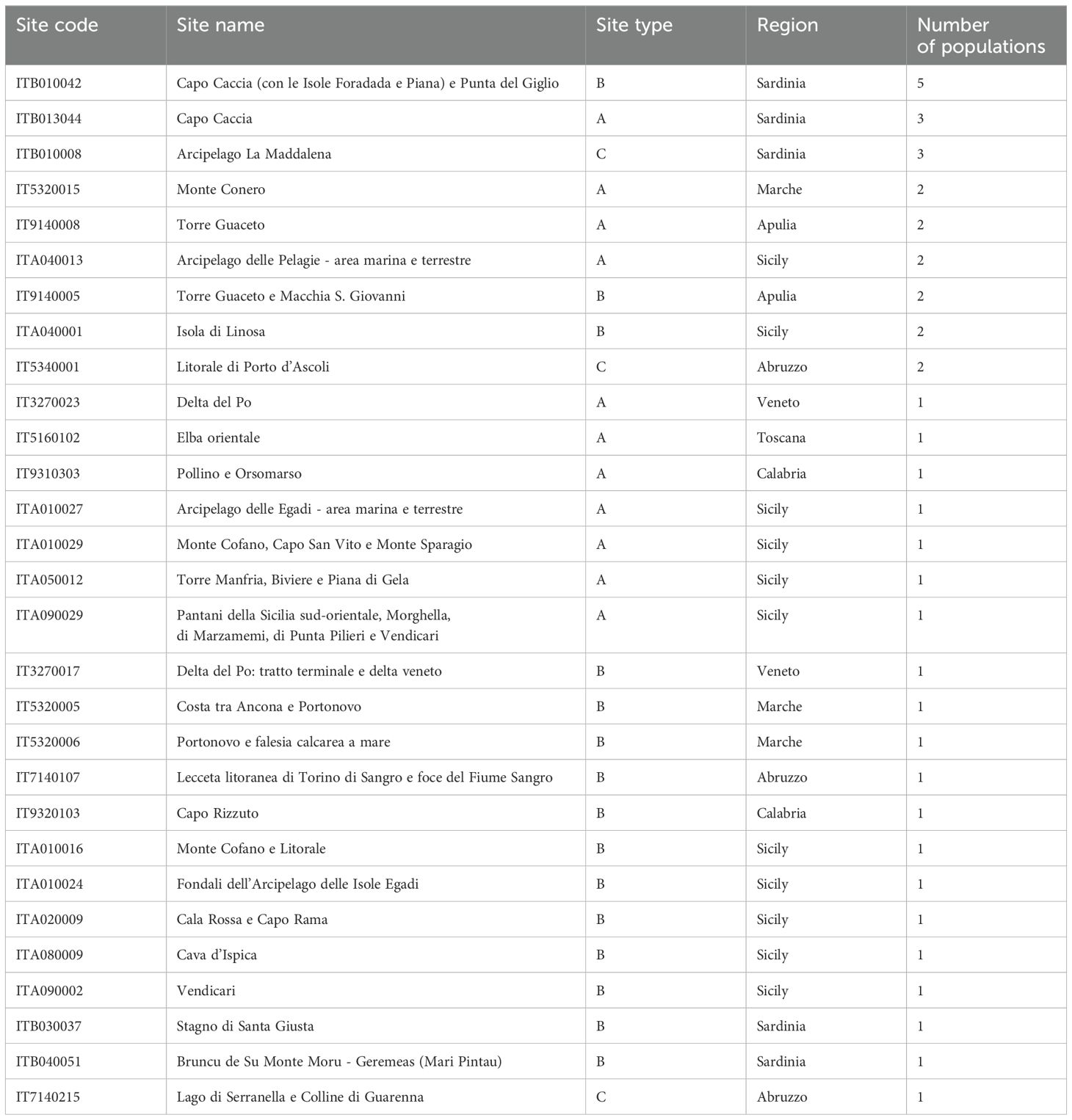
The complete list of Natura 2000 Network sites hosting B. vulgaris subsp. maritima populations is reported in Table 1. The Natura 2000 SAC-B site ‘Capo Caccia (con le Isole Foradada e Piana) e Punta del Giglio’ (ITB010042), hosts the highest number of occurrences (5) distributed across two cells. With an extension of about 7,004 ha, this site is also included in the ‘Parco Naturale Regionale di Porto Conte’ in the municipality Alghero (north Sardinia). Another site of interest is the SCI-SPA-C ‘Arcipelago La Maddalena’(ITB010008), hosting 3 occurrences; it almost completely corresponds to the borders of the homonymous National Park, which in turn coincides with the entire municipality of La Maddalena at the northern end of Sardinia.
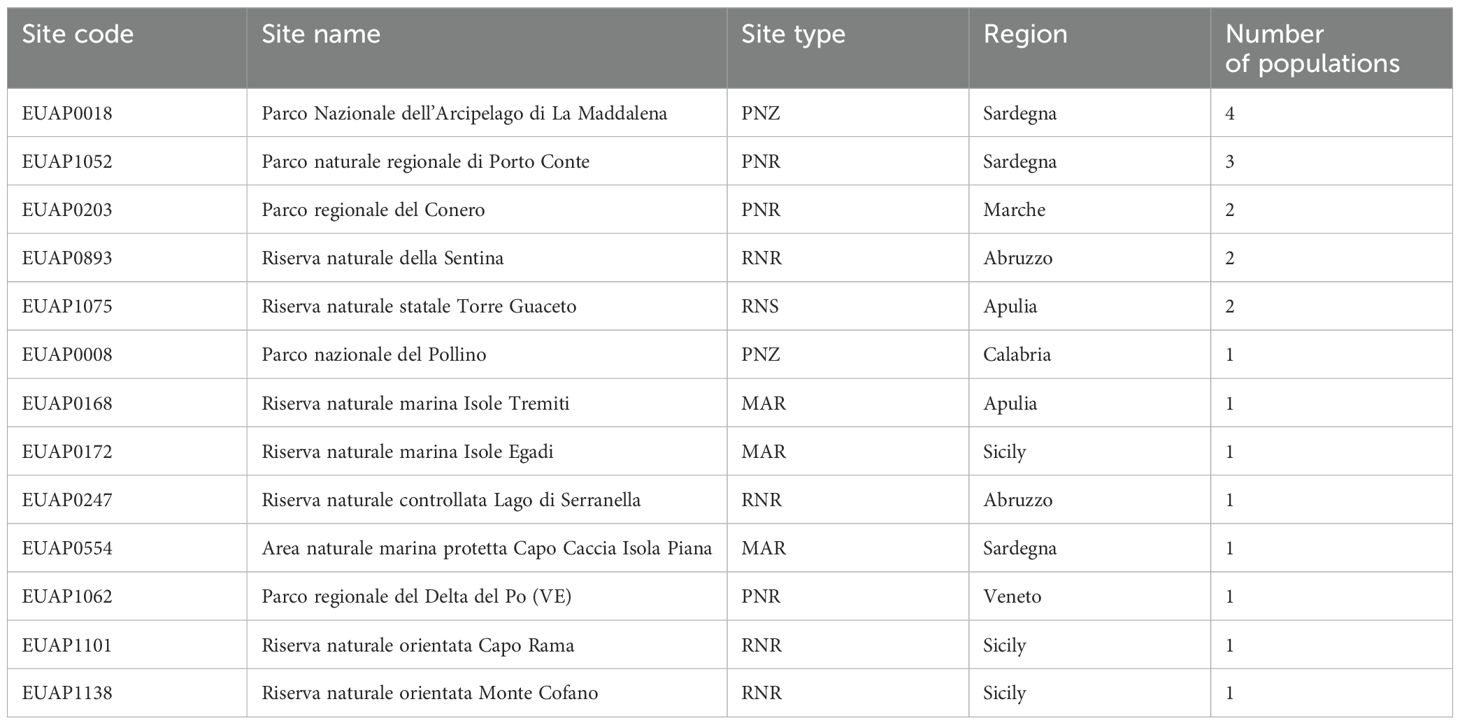
With 4 and 3 occurrences, respectively, ‘Parco Nazionale dell’Arcipelago di La Maddalena’ and ‘Parco naturale regionale di Porto Conte’ are the two most significant areas listed in the EUAP for the protection of this taxon (Table 2) followed by ‘Parco regionale del Conero’, ‘Riserva naturale della Sentina’ and ‘Riserva naturale statale Torre Guaceto’ each one characterised by the presence of two populations.
Habitat characterization and land use and land cover of population hosting sites
When categories of “Carta della Natura” are considered, the landscape of sites hosting B. vulgaris subsp. maritima populations included in sites of the Natura 2000 Network mainly corresponds to coastal plains (0.25), small islands (0.22) and isolated coastal relief (0.16); if coastal plains are also the most common when sites outside protected areas are considered (0.26), frequencies of the other categories are quite different with small islands and isolated coastal reliefs almost only occurring within protected areas (Figure 3A).
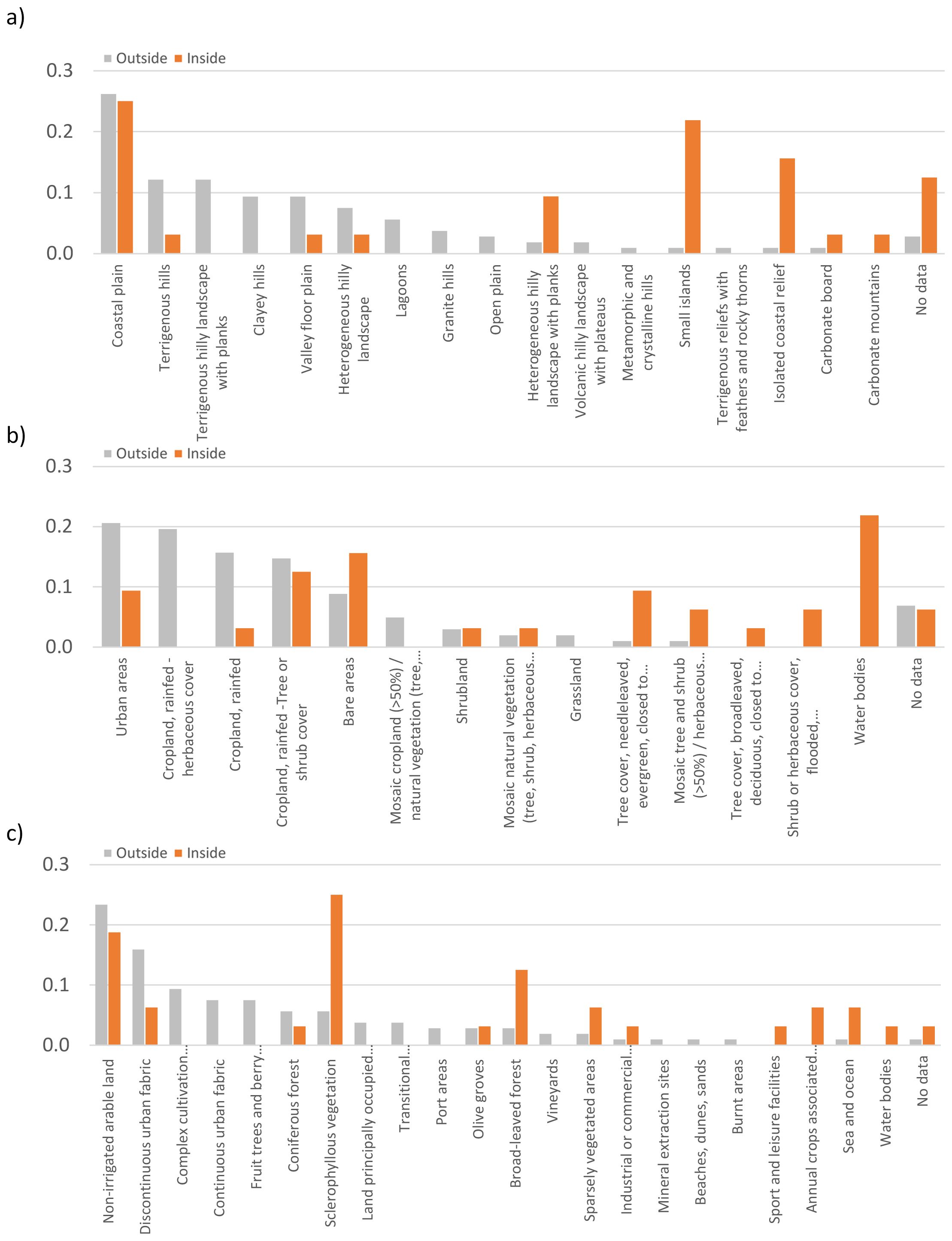
Figure 3. Histograms of the 138 B. vulgaris subsp. maritima in situ records in the different categories of ‘Carta della Natura’ (A), ESA CCI Land Cover (B) and CLC (C); distribution of sites with respect to the Natura 2000 Network are as follows: inside (orange) and outside (light grey).
Results of the LULC analysis based on ESA CCI Land Cover showed that protected sites hosting this taxon mainly belong to the categories water bodies (0.22), bare areas (0.16) and cropland, rainfed -tree or shrub cover (0.13). Outside protected areas, most of the sites correspond to urban areas (0.21) and different categories of rainfed cropland (0.50) accounting together for almost three-quarters of the total (Figure 3B).
Finally, according to the Corine Land Cover categories, sites hosting this taxon, and located within Natura 2000 Network, are mainly characterized by sclerophyllous vegetation (0.25), non-irrigated arable land (0.19) and broad-leaved forest (0.13) while sites outside Natura 2000 mainly correspond to cultivated and urban areas also according to this categorisation (Figure 3C).
In situ vs ex situ conservation
After superimposing the selected grid to the Italian territory, in situ records intercepted a total of 104 cells with number of records per cell ranging from 1 - common to most of the cells (0.79) - to a maximum of 4 only observed in two cells located in Umbria and Basilicata regions (Figure 4A). The application of the same procedure showed that ex situ records intercept 57 cells mainly including 1 record (0.89) or 2 records at maximum (0.11) (Figure 4B). The visual comparison of the density analysis results for the two databases revealed the presence of several gaps between in situ occurrence and sites where ex situ conserved accessions were originally collected (Figures 4A, B).
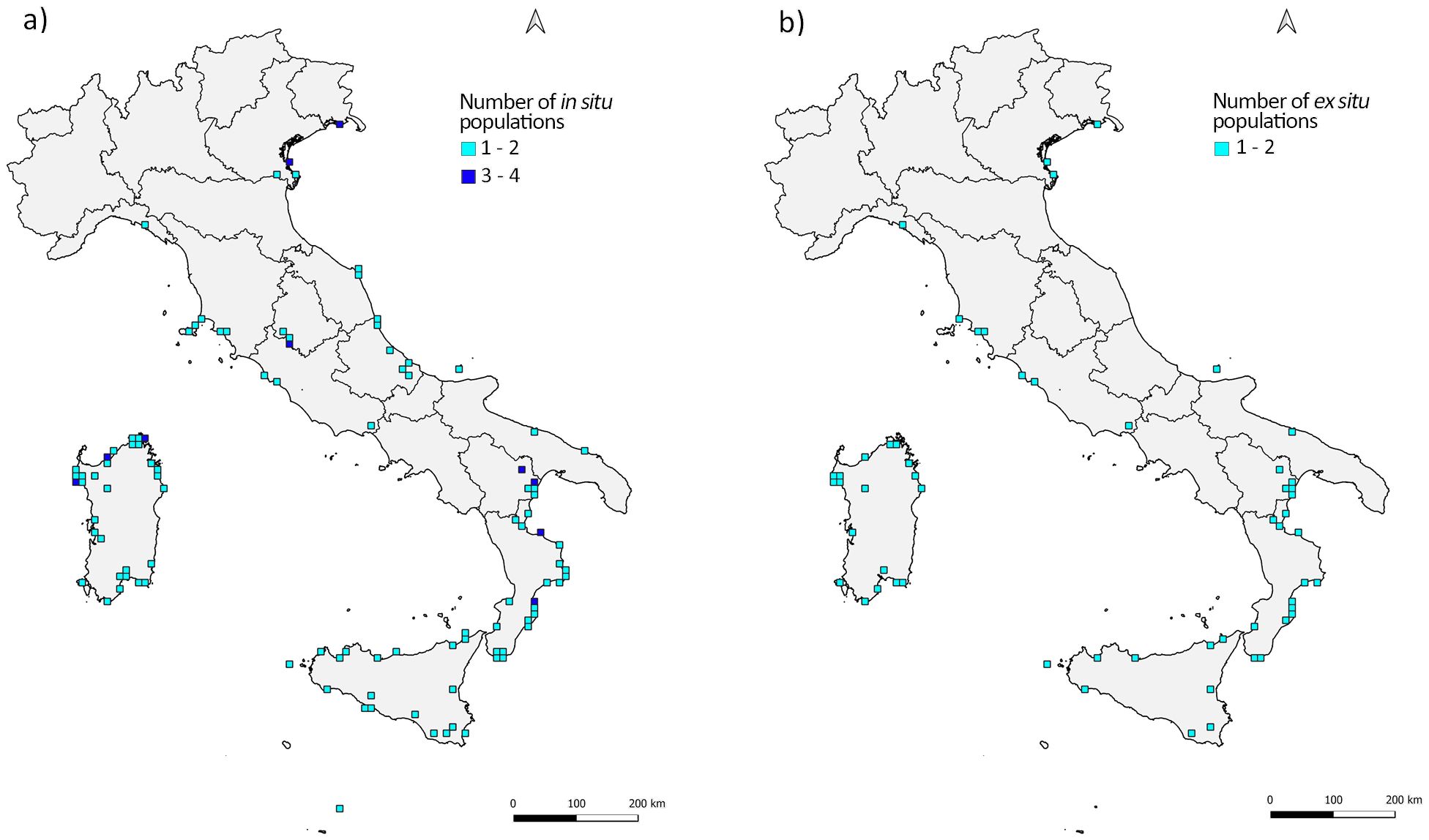
Figure 4. Number of populations in each 10 × 10 km square of the grid (density) in situ (A) and of sites where collections of ex situ conserved samples occurred (B). Symbols and colours are according to the figure legend.
In particular, according to the collated georeferenced records, in situ occurrences of this taxon are scattered over 13 Italian regions and are mainly located in Sardinia (42 records, mainly in the provinces of Sassari Cagliari), Calabria (27 records, mainly in the province of Reggio Calabria, Cosenza and Crotone) and Sicily (23 records, mainly in the provinces of Trapani, Agrigento and Messina) while ex situ conserved samples were collected in only 10 different Italian regions, especially in Sardinia, Calabria and Sicily; none of the collections regarded Abruzzo, Umbria, or Marche regions where in situ populations do exist (Figure 5). Main gaps, in terms of difference in number of populations recorded in situ and conserved ex situ, exist in Sicily, Sardinia and Calabria (Figure 5). As for ex situ conserved materials, most of the collections were performed during the middle-end of the ‘80s, few during the ‘90s while the others between the end of the ‘90s and the first years of the 2000s (Figure S1, Supplementary Materials).
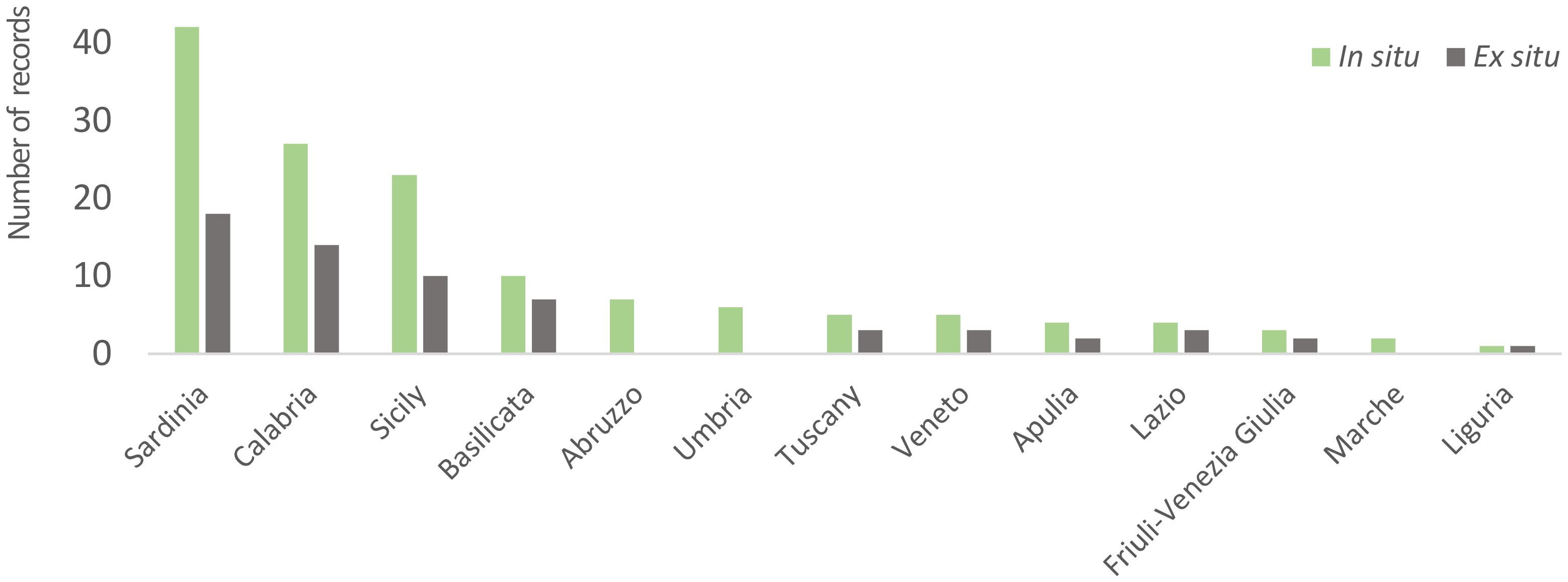
Figure 5. Number of in situ and ex situ records of Beta vulgaris subsp. maritima in the different Italian regions.
Discussion
We carried out a population distribution analysis for Beta vulgaris subsp. maritima to thoroughly assess its present distribution and conservation status and offer a data-driven perspective for planning future conservation actions. The applied filtering process was fundamental to provide a trustable dataset; several filters were indeed applied to remove cultivated materials, accessions with low-quality geographical coordinates or invalid basis of records. Thanks to the strict quality control applied, effective databases of sea beet population occurrences in nature (in situ database) and accessions conserved in genebanks (ex situ database) were developed. Results of collated data show that, based on the current knowledge, as many as 138 populations still exist in Italy distributed across a wide area in the country from 36.90 to 45.68 decimal degrees N and from 8.15 to 17.77 decimal degrees E. According to Rubio Teso and colleagues (2021) more than 1000 populations still exist in situ at the European level distribute in 17 different countries with an average value of populations per country lower than what is reported for Italy, herein.
The ecological richness of Italian populations, located across the Italian territory and in two distinct Biogeographic Regions, should, however, be better preserved in situ than it currently is. In fact, only 23% of the populations are currently experiencing a form of in situ protection, occurring in a site within a protected area. Considering the entire European territory, the level of protection of this taxon is slightly lower, with 19% of populations occurring within the Natura 2000 Network, according to a recent work of Rubio Teso et al. (2020).
Higher percentages of protection have been recently reported by Raggi and colleagues (2022b) for some CWR species of Brassica genus (e.g. 86% for B. montana, 75% for B. insularis and 57% for B. rupestris). Wild beet has not been considered by any type of red-list assessment: it results as not evaluated both by the Red list of threatened vascular plants in Italy (Orsenigo et al., 2021) and by the Threatened and extinct policy plants at the European and EU 27 level (Bilz et al., 2011). This exclusion is possibly the consequence of the wider distribution of wild beet and does not underpin the possibility to create new protected areas to safeguard populations of this taxon in our country. Additionally, it is worth highlighting that the protection status resulting from the presence of a population within a protected area leans predominantly towards a passive conservation (Maxted et al., 2008). Notably, discernible population management practices are absent within areas designated under Natura 2000 or EUAP. This underlines the need for a more proactive and comprehensive approach to conservation efforts, including the implementation of effective population management strategies within these designated areas to boost the safeguarding of sea beet in a manner that aligns with sustainable and ecologically sound practices (Maxted et al., 2008; Iriondo et al., 2021). It should be important to carry out some forms of active protection, with population census monitoring at least. This would be particularly important for populations included in National and Regional parks. Indeed, by declaring an active role in safeguarding genetic resources of primary importance, these parks would see their role in protecting nature enforced, and their utility for the community made more relevant and appreciated. In addition, a more thorough assessment of the actual existence and distribution of populations should be carried out in each Region where the species was recorded in the past. Protection of genetic resources, as from Italian laws, is a responsibility of each Region Government which should be made better elicited to carry out this task. Presently most of them have regional laws to protect genetic resources, but funds to implement them are lacking everywhere (Bertacchini et al., 2011).
In general, the vulnerability of a taxon within a particular site strictly depends on the state of the site, and it is a key factor for guiding effective conservation planning and management. Some of the core elements to assess the site-based vulnerability of a species include the degree of human intrusion and disturbance, natural system modifications, presence of invasive species, degree of pollution, geological events, and the effects of climate change and severe weather occurring within the target site (Langhammer et al., 2007). Conducting a site-based vulnerability assessment can assist in prioritizing conservation actions and effectively allocating limited resources to areas where they are most urgently needed.
Interesting enough, our data show that B. vulgaris subsp. maritima occurrences within protected areas are primarily in natural environments, whereas those outside protected areas are mainly in anthropic and cultivated areas. Given that most sea beet populations outside protected areas are found in urban and cultivated areas, and that these populations contribute to in situ conservation through their diversity, it is urgently necessary to establish a certain level of protection even in these areas, considering their high level of anthropization. Since the institution of further protected areas might not be feasible or affordable, due to economic and management constraints, and also due to the absence of this species from red lists, positive results in protecting the taxon might be achieved by means of the ‘Out of Reserve Management’ approach (Hale and Lamb, 1997) through the application of management plans to reduce the level of risk and the related threats. It is therefore necessary to develop an approach that integrates agroecosystems and marginal zones, encompassing urban and barren areas. For example, as already suggested by Perrino and Wagensommer (2021) for Beta macrocarpa Guss., an “eco-agricultural-based conservation strategy” could involve the cooperation with farmers to develop the cultivation of this taxon, which is also a valuable food and natural source of bioactive molecules (Bouchmaa et al., 2022). On the other hand, cooperation with farmers needs policy-based measures to become truly viable.
As already suggested for the sustainable conservation of other relevant Italian CWR taxa (Raggi et al., 2022b), agro-ecosystems, as well as other anthropogenic habitats, need to be incorporated into conservation strategies to achieve effective long-term biodiversity conservation and associated ecosystem services (Scherr and McNeely, 2008; Mellink et al., 2017). Solutions can be also proposed involving local botanical gardens and amateur botanists for assessing the populations’ existence, or citizens for taking care of populations located outside protected areas. This, of course, requires an enhancement of awareness and respect for the environment and its biological resources. An eco-agricultural strategy applied to the conservation of Beta vulgaris subsp. maritima could include cooperation with farmers whose lands are characterized by the presence of the taxon, through the realization of protected spots of natural habitat within the farm’s territory.
The ex situ protection level of this taxon seems less concerning, given that 46% of recorded populations have already been collected and stored within gene banks. Indeed, this percentage is relatively high when compared with the ex situ conservation status of other relevant CWR taxa (Castañeda-Álvarez et al., 2016; Zair et al., 2021) and when compared to percentages reported by Raggi et al. (2022b) for other CWR taxa occurring in Italy and characterized by a number of populations similar to those here reported: Brassica villosa Biv (18.91%), Brassica rupestris Raf. (14.75%), Brassica montana Pourr. (7.14%) and Brassica insularis Moris (1.08%). As for B. vulagris subsp. maritima ex situ conservation, however, it should also be noted that many collections have been carried out over two decades ago and meanwhile an astonishing change in the climatic conditions is occurring worldwide with measurable effects on plants (Li et al., 2001; Nevo et al., 2012; Ciancaleoni et al., 2018). It is important to question how past collections adequately represent the diversity needed for present breeding purposes, particularly concerning the urgent need to adapt crops to climate change and unpredictability, along with the associated new biotic and abiotic stresses. Accordingly, populations still existing in situ (both CWR and landraces) would be most probably better suitable to answer the needs of present breeding work being evolved over time in response to climatic and related biotic pressures (Catullo et al., 2019; Lovell et al., 2023 and references therein). In this context, population genetic studies could contribute to determining the presence of valuable unique genetic variation in conserved materials as well as to test how well ex situ conserved accessions cover the diversity existing in situ so also to guide future collection missions. However, CWR genetic analyses are complicated due to the high costs of a comprehensive taxon’s range sampling, the high required number of individual plants per sample (Maxted et al., 2008) and the limited number of specifically developed genotyping facilities (Cortés and Barnaby, 2023). On the other hand, ecogeographic diversity (Korona, 1996) of the sites hosting B. vulgaris subsp. maritima could provide and indirect estimation of population genetic diversity (Parra-Quijano et al., 2012).
Although there is a relatively high number of ex situ conserved accessions for this taxon, particularly the availability of wild beet ex situ resources should be increased. This consideration is warranted due to the overall value of related crops and the fact that less than half of the populations analyzed here have been the target of collecting missions in the past. New collections should be carried out starting from areas more degraded or not target of previous collecting missions, such as the sites located in Umbria, Marche and Abruzzo. In addition to these sites, the results of analyses conducted in the present work also reveal the presence of numerous gaps in ex situ conserved materials for Sicily, Sardinia and Calabria, the three Regions hosting the highest number of populations. In this regard, it is advisable that ex situ accessions adequately reflect this remarkable diversity and abundance of B. vulgaris subsp. maritima still existing in situ.
Conclusions
In this study, we present the findings of a comprehensive analysis concerning the distribution of Beta vulgaris subsp. maritima across Italy. Given that Beta genus is of great relevance at the global levels and that Italy has a central position within the Mediterranean biodiversity hotspot our findings raise some concern. Indeed, here presented data indicates that the protection status for sea beet in Italy can be considered only partially adequate. Indeed, quite a limited proportion of the target populations occurs in sites of the Natura 2000 Network or areas listed in the EUAP where populations only experience passive protection while more proactive protection measures should be foreseen to increase the role of protected areas in safeguard CWR in situ. As such, a more proactive approach is needed for the thorough safeguarding of sea beet in Italy. This should include surveying populations and their site status, actively managing populations in protected areas, promoting “out of reserve management” for populations outside protected areas, and collecting new populations not previously stored ex situ. In fact, even if the ex situ protection level of this taxon seems less concerning, almost half of the recorded collection missions have been carried out over three decades ago and, meanwhile, an astonishing change in the climatic conditions is occurring with measurable effects on plants. Genetic diversity analyses on in situ and ex situ conserved materials would help in shedding light on this relevant point related to the conservation and use of B. vulgaris subsp. maritima.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CZ: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. LR: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AG: Writing – original draft, Writing – review & editing. GS: Writing – original draft, Writing – review & editing. DG: Funding acquisition, Writing – original draft, Writing – review & editing. VN: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by: University of Study of Perugia through the ‘Fondo d’Ateneo per la Ricerca di Base 2020’, Progetto ‘FLora Autoctona: dall’analisi del Germoplasma alla Conservazione ex-situ’ (FLAG); European Union’s Horizon 2020 research and innovation programme under the Grant Agreement No: 774271 ‘Networking, partnerships and tools to enhance in situ conservation of European plant genetic resources’ (Farmer’sPride).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1399341/full#supplementary-material
References
Aguirre-Gutiérrez J., van Treuren R., Hoekstra R., van Hintum T. J. L. (2017). Crop wild relatives range shifts and conservation in Europe under climate change. Divers. Distrib. 23, 739–750. doi: 10.1111/ddi.12573
Amadei M., Bagnaia R., Laureti L., Lugeri F., Lugeri N., Feoli E., et al. (2003). Il Progetto Carta della Natura alla scala 1:250.000 Metodologia di realizzazione. Available online at: https://www.isprambiente.gov.it/resolveuid/c5ec7a1bce1b41e89dcf25d8640cc1dd.
Andrello M., Henry K., Devaux P., Desprez B., Manel S. (2016). Taxonomic, spatial and adaptive genetic variation of Beta section Beta. Theor. Appl. Genet. 129, 257–271. doi: 10.1007/s00122-015-2625-7
Ascarini F., Nóbrega H. G. M., Leite I. S., Freitas G., Ragonezi C., Amely Zavattieri M., et al. (2021). Assessing the diversity of sea beet (Beta vulgaris L. ssp. maritima) populations. J. Agric. Sci. Technol. 23, 685–698.
Bartolucci F., Peruzzi L., Galasso G., Albano A., Alessandrini A., Ardenghi N. M. G., et al. (2018). An updated checklist of the vascular flora native to Italy. Plant Biosyst. 152, 179–303. doi: 10.1080/11263504.2017.1419996
Becker-Dillingen (1928). Handbuch des Hackfruchtbaues und Handelapfl anzbaues (Berlin, Germany: Verlag Paul Parey).
Bertacchini E., Saccone D., Santagata W. (2011). Embracing diversity, correcting inequalities: towards a new global governance for the UNESCO World Heritage. Int. J. Cult. Policy 17, 278–288. doi: 10.1080/10286632.2010.528833
Biancardi E., Panella L., Lewellen R. (2012a). Beta maritima. The Origin of Beets (New York, NY, USA: Springer New York Dordrecht Heidelberg). doi: 10.1007/978-1-4614-0842-0
Biancardi E., Panella L., Lewellen R. (2012b). “History and current importance,” in Beta maritima. The Origin of Beets (Springer New York Dordrecht Heidelberg, New York, NY, USA), 1–74. doi: 10.1007/978-1-4614-0842-0_1
Bilz M., Kell S. P., Maxted N., Lansdown R. V. (2011). European Red List of Vascular Plants (Luxembourg: Publications Office of the European Union). doi: 10.2779/8515
Biondi E., Calandra R., Gigante D., Pignattelli S., Rampiconi E., Venanzoni R. (2002). Il paesaggio vegetale della Provincia di Terni (Terni, Italy: Arti Grafiche Sandro Iezzi).
Bouchmaa N., Mrid R., Kabach I., Zouaoui Z., Karrouchi K., Chtibi H., et al. (2022). Beta vulgaris subsp. maritima: A Valuable Food with High Added Health Benefits. Appl. Sci. 12, 1866. doi: 10.3390/app12041866
Brush S. (2004). Farmers’ Bounty: Locating Crop Diversity in the Contemporary World (New Haven, CT: Yale University Press). Available at: https://www.jstor.org/stable/j.ctt1np9rd. doi: 10.12987/yale/9780300100495.001.0001
Campbell L. G. (2010). Registration of seven sugarbeet germplasms selected from crosses between cultivated sugarbeet and wild beta species. J. Plant Regist. 4, 149–154. doi: 10.3198/jpr2009.11.0673crg
Capistrano-Gossmann G. G., Ries D., Holtgräwe D., Minoche A., Kraft T., Frerichmann S. L. M., et al. (2017). Crop wild relative populations of Beta vulgaris allow direct mapping of agronomically important genes. Nat. Commun. 8, 15708. doi: 10.1038/ncomms15708
Castañeda-Álvarez N., Khoury C., Achicanoy H., Bernau V., Dempewolf H., Eastwood R., et al. (2016). Global conservation priorities for crop wild relatives. Nat. Plants 2, 1–6. doi: 10.1038/nplants.2016.22
Catullo R. A., Llewelyn J., Phillips B. L., Moritz C. C. (2019). The potential for rapid evolution under anthropogenic climate change. Curr. Biol. 29, R996–R1007. doi: 10.1016/j.cub.2019.08.028
CBD (1992). Convention on Biological Diversity: Text and Annexes. Secretariat of the Convention on Biological Diversity (Rio de Janeiro, Brazil: United Nations environment program). Available at: http://www.cbd.int/convention/.
Ciancaleoni S., Raggi L., Barone G., Donnini D., Gigante D., Domina G., et al. (2021). A new list and prioritization of wild plants of socioeconomic interest in Italy: toward a conservation strategy. Agroecol. Sustain. Food Syst. 45, 1300–1326. doi: 10.1080/21683565.2021.1917469
Ciancaleoni S., Raggi L., Negri V. (2018). Assessment of spatial–temporal variation in natural populations of Brassica incana in south Italy: implications for conservation. Plant Syst. Evol. 304, 1–15. doi: 10.1007/s00606-018-1505-4
Cortés A. J., Barnaby J. Y. (2023). Editorial: Harnessing genebanks: High-throughput phenotyping and genotyping of crop wild relatives and landraces. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1149469
Council of Europe (1979). Bern Convention – The Convention on the Conservation of European Wildlife and Natural Habitats (Strasbourg: CoE).
Dempewolf H., Baute G., Anderson J., Kilian B., Smith C., Guarino L. (2017). Past and future use of wild relatives in crop breeding. Crop Sci. 57, 1070–1082. doi: 10.2135/cropsci2016.10.0885
Dempewolf H., Eastwood R. J., Guarino L., Khoury C. K., Müller J. V., Toll J. (2014). Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 38, 369–377. doi: 10.1080/21683565.2013.870629
Engels J. M. M., Thormann I. (2020). Main challenges and actions needed to improve conservation and sustainable use of our crop wild relatives. Plants 9, 1–26. doi: 10.3390/plants9080968
ESA (2017). Land Cover CCI Product User Guide Version 2. Available online at: https://maps.elie.ucl.ac.be/CCI/viewer/download/ESACCI-LC-Ph2-PUGv2_2.0.pdf.
European Commission (1979). Council Directive 79/409/EEC of 2 April 1979 on the conservation of wild birds. Off. J. Eur. Communities 103, 1–18.
European Commission (1992). Council Directive 92 /43 /EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities 35, 7–51.
European Environment Agency (2017). EEA Reference Grid. Available online at: https://www.eea.europa.eu/data-and-maps/data/eea-reference-grids-2.
FAO (2017). Voluntary Guidelines for the Conservation and Sustainable Use ofCrop Wild Relatives and Wild Food Plants (Rome, Italy: Food and Agriculture Organization of the United Nations).
Fénart S., Arnaud J. F., De Cauwer I., Cuguen J. (2008). Nuclear and cytoplasmic genetic diversity in weed beet and sugar beet accessions compared to wild relatives: New insights into the genetic relationships within the Beta vulgaris complex species. Theor. Appl. Genet. 116, 1063–1077. doi: 10.1007/s00122-008-0735-1
Hajjar R., Hodgkin T. (2007). The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156, 1–13. doi: 10.1007/s10681-007-9363-0
Hale P., Lamb D. (1997). Conservation Outside Nature Reserves. Eds. Hale P., Lamb D. (Brisbane, AU: Centre for Conservation Biology, University of Queensland).
Hanson A., Wyse R. (1982). Biosynthesis, translocation, and accumulation of betaine in sugar beet and its progenitors in relation to salinity. Plant Physiol. 70, 1191–1198. doi: 10.1104/pp.70.4.1191
Harlan J. R. (1976). Genetic resources in wild relatives of crops 1. Crop Sci. 16, 329–333. doi: 10.2135/cropsci1976.0011183X001600030004x
Harlan J. R. (1984). “Evaluation of wild relatives of crop plants,” in Conservation and Evaluation. Eds. Holden J., Williams J. (George Allen and Unwin, London), 212–222.
Harlan J. R., de Wet J. M. J. (1971). TOWARD A RATIONAL CLASSIFICATION OF CULTIVATED PLANTS. Taxon 20, 509–517. doi: 10.2307/1218252
Heywood V., Dulloo E. (2005). In situ conservation of wild plant species a critical global review of good practices IPGRI Technical Bulletin No. 11 (Rome, IT: FAO and IPGRI).
Hoyt E. (1988). Conserving the wild relatives of crops. Eds. Williams J., McCusker A., Stapleton P., Heywood V., Synge H. (Rome, Italy: IBPGR/ IUCN/WWF).
IPBES (2022). Summary for Policymakers of the Thematic Assessment Report on the Sustainable Use of Wild Species of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Eds. Fromentin J.M., Emery M.R., Donaldson J., Danner M.C., Hallosserie A., Kieling D., et al (Bonn, Germany: IPBES secretariat). doi: 10.5281/zenodo.6425599
Iriondo J. M., Magos Brehm J., Dulloo M. E., Maxted N. (2021). “Crop wild relative population management guidelines,” in Farmer’s Pride Networking, partnerships tools to Enhanc. situ Conserv. Eur. plant Genet. Resour, 132. Available at: http://www.farmerspride.eu/.
Jarvis A., Lane A., Hijmans R. (2008). The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ. 126, 13–23. doi: 10.1016/j.agee.2008.01.013
Korona R. (1996). Adaptation to structurally different environments. Proc. R. Soc London. Ser. B Biol. Sci. 263, 1665–1669. doi: 10.1098/rspb.1996.0243
Landucci F., Panella L., Gigante D., Donnini D., Venanzoni R., Torricelli R., et al. (2012). Floristic and vegetation databases as tools for CWR surveys: a case study from Central Italy. Crop Wild Relat. Newsl. 8, 22–23. University of Birmingham. ISSN: 1742-3694.
Landucci F., Panella L., Lucarini D., Gigante D., Donnini D., Kell S., et al. (2014). A prioritized inventory of crop wild relatives and wild harvested plants of Italy. Crop Sci. 54, 1628–1644. doi: 10.2135/cropsci2013.05.0355
Langhammer P., Bakarr M., Bennun L., Brooks T., Clay R., Darwall W., et al. (2007). Identification and Gap Analysis of Key Biodiversity Areas Targets for Comprehensive Protected Area Systems. No. 15. Ed. Valentine P. (Gland, Switzerland: IUCN).
Letschert J., Frese L. (1993). Analysis of morphological variation in wild beet (Beta vulgaris L.) from Sicily. Genet. Resour. Crop Evol. 40, 15–24. doi: 10.1007/BF00053460
Li Y. C., Krugman T., Fahima T., Beiles A., Korol A. B., Nevo E. (2001). Spatiotemporal allozyme divergence caused by aridity stress in a natural population of wild wheat, Triticum dicoccoides, at the ammiad microsite, Israel. Theor. Appl. Genet. 102, 853–864. doi: 10.1007/s001220000474
Lovell R. S. L., Collins S., Martin S. H., Pigot A. L., Phillimore A. B. (2023). Space-for-time substitutions in climate change ecology and evolution. Biol. Rev. 98, 2243–2270. doi: 10.1111/brv.13004
Maxted N. (2003). Conserving the genetic resources of crop wild relatives in European protected areas. Biol. Conserv. 113, 411–417. doi: 10.1016/S0006-3207(03)00123-X
Maxted N., Ford-Lloyd B. V., Hawkes J. G. (1997). “Complementary conservation strategies,” in Plant genetic conservation: the in situ approach. Eds. Maxted N., Ford-Lloyd B. V., Hawkes J. G. (Chapman & Hall, London, UK), 20–55.
Maxted N., Ford-Lloyd B. V., Jury S., Kell S., Scholten M. (2006). Towards a definition of a crop wild relative. Biodivers. Conserv. 15, 2673–2685. doi: 10.1007/s10531-005-5409-6
Maxted N., Iriondo J. M., De Hond L., Dulloo M. E., Lefebvre V., Asdal A. (2008). “Genetic reserve management,” in Conserving Plant Genetic Diversity in Protected Areas: Population Management of Crop Wild Relatives. Eds. Iriondo J. M., Maxted N., Dulloo M. E. (CABI, Wallingford), 65–87. doi: 10.5860/choice.46-0870
Maxted N., Kell S. P. (2009). Establishment of a global network for the in situ conservation of crop wild relatives: status and needs. Available online at: http://www.fao.org/docrep/013/i1500e/i1500e18a.pdf.
Mellink E., Riojas-López M. E., Cárdenas-García M. (2017). Biodiversity conservation in an anthropized landscape: Trees, not patch size drive, bird community composition in a low-input agroecosystem. PloS One 12, 1–17. doi: 10.1371/journal.pone.0179438
Monteiro F., Frese L., Castro S., Duarte M. C., Paulo O. S., Loureiro J., et al. (2018). Genetic and genomic tools to asssist sugar beet improvement: The value of the crop wild relatives. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00074
Nevo E., Fu Y.-B., Pavlicek T., Khalifa S., Tavasi M., Beiles A. (2012). Evolution of wild cereals during 28 years of global warming in Israel. Proc. Natl. Acad. Sci. U. S. A. 109, 3412–3415. doi: 10.1073/pnas.1121411109
Olsen K. M., Gross B. L. (2008). Detecting multiple origins of domesticated crops. Proc. Natl. Acad. Sci. U. S. A. 105, 13701–13702. doi: 10.1073/PNAS.0807439105/ASSET/C09ED446-05D4-4EB0-8701-A6A4B1C89758/ASSETS/GRAPHIC/ZPQ9990849670002.JPEG
Orsenigo S., Fenu G., Gargano D., Montagnani C., Abeli T., Alessandrini A., et al. (2021). Red list of threatened vascular plants in Italy. Plant Biosyst. 155, 310–335. doi: 10.1080/11263504.2020.1739165
Parra-Quijano M., Iriondo J. M., Torres E. (2012). Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet. Resour. Crop Evol. 59, 205–217. doi: 10.1007/s10722-011-9676-7
Perrino E. V., Wagensommer R. P. (2021). Crop wild relatives (Cwr) priority in Italy: Distribution, ecology, in situ and ex situ conservation and expected actions. Sustain 13, 1–21. doi: 10.3390/su13041682
Phillips J., Asdal Å., Magos Brehm J., Rasmussen M., Maxted N. (2016). In situ and ex situ diversity analysis of priority crop wild relatives in Norway. Divers. Distrib. 22, 1112–1126. doi: 10.1111/ddi.12470
Pirone G. (1995). Vegetazione dei calanchi di Atessa (Abruzzo) e problematiche sintassonomiche della vegetazione calanchiva appenninica in fitoclimi temperato-mediterranei di transizione. Fitosociologia 30, 221–232.
Prescott-Allen R., Prescott-Allen C. (1981). In Situ Conservation of Crop Genetic Resources, A Report to the International Board for Plant Genetic Resources (IBPGR) (Gland, Switzerland: IUCN).
QGIS Development Team (2021). QGIS Geographic Information System. Available online at: http://qgis.osgeo.org.
Raggi L., Pacicco L. C., Caproni L., Alvarez-mu C., Barata A. M., Batir-rusu D., et al. (2022a). Analysis of landrace cultivation in Europe : A means to support in situ conservation of crop diversity. Biol. Conserv. 267, 109460. doi: 10.1016/j.biocon.2022.109460
Raggi L., Zucchini C., Gigante D., Negri V. (2022b). In situ occurrence and protection of crop wild relatives in Italian sites of natura 2000 network : Insights from a data- driven approach. Front. Plant Sci. 1–17. doi: 10.3389/fpls.2022.1080615
Raggi L., Zucchini C., Sayde E., Gigante D., Negri V. (2024). Priority areas for the establishment of genetic reserves to actively protect key crop wild relative species in Italy. Glob. Ecol. Conserv. 50, e02836. doi: 10.1016/j.gecco.2024.e02836
Rubio Teso M., Álvarez Muñiz C., Gaisberger H., Kell S., Lara-Romero C., Magos-Brehm J., et al. (2020). “Crop wild relatives in Natura 2000 network,” in Farmer’s Pride Networking, partnerships tools to Enhanc. situ Conserv. Eur. plant Genet. Resour. Available at: https://more.bham.ac.uk/farmerspride/wp-content/uploads/sites/19/2020/10/MS19_Crop_Wild_Relatives_in_the_Natura_2000_Network.pdf.
Rubio Teso M., Álvarez Muñiz C., Gaisberger H., Kell S. P., Lara-Romero C., Magos Brehm J., et al. (2021). “European crop wild relative diversity: towards the development of a complementary conservation strategy,” in Farmer’s Pride Networking, partnerships tools to Enhanc. situ Conserv. Eur. plant Genet. Resour. Available at: http://www.farmerspride.eu.
Scherr S. J., McNeely J. A. (2008). Biodiversity conservation and agricultural sustainability: Towards a new paradigm of “ecoagriculture” landscapes. Philos. Trans. R. Soc B Biol. Sci. 363, 477–494. doi: 10.1098/rstb.2007.2165
Schwartz K. R., Christien E., Rockwood L., Wood T. C. (2017). Integrating in-situ and ex-situ data management processes for biodiversity conservation. Front. Ecol. Evol. 5. doi: 10.3389/fevo.2017.00120
Stevanato P., Broccanello C., Biscarini F., Del Corvo M., Sablok G., Panella L., et al. (2014). High-throughput RAD-SNP genotyping for characterization of sugar beet genotypes. Plant Mol. Biol. Rep. 32, 691–696. doi: 10.1007/s11105-013-0685-x
Tirnaz S., Zandberg J., Thomas W. J. W., Marsh J., Edwards D., Batley J. (2022). Application of crop wild relatives in modern breeding: An overview of resources, experimental and computational methodologies. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1008904
Ulbrich E. (1934). “Chenopodiaceae,” in Die Natürlichen Pfl anzenfamilien. Eds. Engler A., Harms H. (Wilhelm Engelmann, Leipzig, Germany), 375–584.
Keywords: sea beat, CWR, in situ conservation, ex situ conservation, GIS analysis
Citation: Zucchini C, Raggi L, Grassi A, Spataro G, Gigante D and Negri V (2024) In situ occurrence and conservation of Beta vulgaris subsp. maritima ((L.) Arcangeli) in Italy. Front. Ecol. Evol. 12:1399341. doi: 10.3389/fevo.2024.1399341
Received: 11 March 2024; Accepted: 25 July 2024;
Published: 19 August 2024.
Edited by:
Purabi Saikia, Banaras Hindu University, IndiaReviewed by:
Petr Smýkal, Palacký University in Olomouc, CzechiaSamantha S. Hauser, Embark Veterinary, Inc., United States
Copyright © 2024 Zucchini, Raggi, Grassi, Spataro, Gigante and Negri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Raggi, bG9yZW56by5yYWdnaUB1bmlwZy5pdA==
†These authors have contributed equally to this work
 Cecilia Zucchini1†
Cecilia Zucchini1† Lorenzo Raggi
Lorenzo Raggi Daniela Gigante
Daniela Gigante Valeria Negri
Valeria Negri