- 1Department of Chemistry and Bioscience, Aalborg University, Aalborg, Denmark
- 2Department of Forestry, University of Helsinki, Helsinki, Finland
On a global scale, species biodiversity is declining rapidly, including that of terrestrial invertebrates. Environmental heterogeneity is viewed as a key factor promoting biodiversity, and previous studies have shown how beavers can have a profound effect on both habitat heterogeneity and abundance and diversity of a plethora of water-related and terrestrial organisms. However, less is known about the effects of beavers and successional stages on the terrestrial invertebrate community. Here, we review existing knowledge and outline research trajectories to improve our understanding of how beavers affect the terrestrial invertebrate community with special focus on the importance of each successional stage that beavers provide on terrestrial invertebrates. Although beavers can have a large impact on the terrestrial invertebrate community, more studies are needed that take into consideration successional stages and with standardized sampling designs. A better understanding of how beaver activity affects the terrestrial invertebrate community can help in conservation of endangered species and restoration of biodiversity in terrestrial habitats.
1 Introduction
On a global scale, species biodiversity is strongly declining (Régnier et al., 2015; Cowie et al., 2022). Trends for terrestrial insects and invertebrates differ across regions and habitats, with some studies reporting declines of 75-98%, whereas in other regions patterns are less clear and dependent on species group (Lister and Garcia, 2018; Goulson, 2019; Høye et al., 2021). The reported declines are of major concern and much effort is currently going into species conservation and restoring biodiversity (Bakker and Svenning, 2018).
Environmental heterogeneity is viewed as a key factor promoting biodiversity in a landscape (Stein et al., 2014; Turner and Gardner, 2015; Hammill et al., 2018). This heterogeneity is typically created by variation in abiotic and biotic conditions within the patches of which the landscape is formed (Bartel et al., 2010; McCarthy et al., 2010). Environmental heterogeneity is also created by patch disturbance, which again can be caused by either abiotic or biotic actors (Turner et al., 1997; Kuuluvainen and Nummi, 2023). Beavers, Castor spp., exemplify biotic actors that cause patch disturbance (Remillard et al., 1987; Nummi and Kuuluvainen, 2013; Johnston, 2017; Kivinen et al., 2020) which compared to abiotic disturbances such as fire and storms are more predictable in the landscape (Nummi and Kuuluvainen, 2013). Beaver ecosystem engineering has a profound influence on the environment since it includes turning a terrestrial ecosystem to an aquatic one (Johnston, 2017; Brazier et al., 2021; Larsen et al., 2021; Wohl, 2021). Beavers build dams, and by doing so, they alter the geomorphology, hydrology and biogeochemistry of the ecosystem they inhabit (Puttock et al., 2017; Nummi et al., 2018; Brazier et al., 2021). Moreover, the flooding and subsequent drying of trees affect forest structure (Hyvönen and Nummi, 2008).
The activities of beavers have a facilitative effect on the abundance and diversity of a plethora of water related as well as terrestrial organisms, including mammals, waterbirds, aquatic invertebrates, and plants (Nummi and Holopainen, 2014; Stringer and Gaywood, 2016; Law et al., 2019; Nummi et al., 2019a; Nummi et al., 2019b). However, the effect of the beaver is not limited to organisms directly connected to the aquatic habitat. Mammals and birds, both terrestrial and semi-aquatic, have higher abundance and richness near beaver flowages (Nelner and Hood, 2011; Nummi et al., 2019a; Fedyń et al., 2022; Fedyn et al., 2023; Wikar et al., 2024), and can further enhance the richness and activity of carnivores (Fedyń et al., 2022). Moreover, the legacy of beaver engineering persists for years or even decades after the flood in the form of beaver meadows (Johnston, 2017; Kivinen et al., 2020; Wohl, 2021; Albertson et al., 2024).
Even though the importance of beavers on the environmental heterogeneity and on abundance and diversity of certain groups of organisms is well established, less is known about the terrestrial invertebrate community. This is surprising as beaver wetlands and non-beaver wetlands are different with respect to multiple parameters likely to impact terrestrial invertebrates, e.g. inundation patterns, deadwood availability, and habitat heterogeneity (Thompson et al., 2016; Bush et al., 2019; Kivinen et al., 2020; Åhlén et al., 2023). Beaver wetlands are dynamic habitats with high heterogeneity and many sub-habitats (Bush & Wissinger, 2016) which should provide niches for many species that would otherwise perish (Donkor & Fryxell, 1999; Johnston, 2017; Åhlén et al., 2023; Achury et al., 2023).
In this mini-review, we summarize the current knowledge on how beavers affect the terrestrial invertebrate community with special focus on the importance of each successional stage that beavers provide for terrestrial invertebrates following a beaver flood. We identified four distinct successional stages following beaver flooding inspired by Knudsen (1962), Kivinen et al. (2020), and Bush et al. (2019) (expanded in section 3. Successional stages in beaver wetlands). To identify relevant studies, we searched the literature for studies concerning beavers and terrestrial invertebrates. Lastly, we concluded on research gaps and discuss future directions and importance of including terrestrial invertebrates when evaluating the effects that beavers can have on biodiversity.
2 Methods
This mini-review was conducted using the search engine web of science. We use keywords on beaver wetlands (beaver dam, beaver pond, beaver wetland, OR beaver lake) and invertebrates (invertebrate, insect, arthropod, OR benthic fauna) on google scholar and web of science. The search was conducted in January 2024. Papers were included if they included data on terrestrial invertebrates. To determine this, we searched the method section of the paper to learn whether terrestrial or aquatic invertebrates were samples. We focused on studies that included a control area with no beaver activity, but made note of all studies that sampled terrestrial invertebrates. Studies only including aquatic invertebrates were excluded. Additionally, we scanned the reference lists of included papers to ensure we found all relevant publications. In total, 11 studies across the globe were found. Studies were published between 1978-2023, more than half of them since 2020, indicating a growing field of study.
We noted the organism group investigated in each study as well as the parameter studied in relation to the group (for example abundance, richness, activity). If a control was presented, we noted whether a difference was found between beaver and non-beaver wetlands with regards to each parameter.
3 Successional stages in beaver wetlands
The fact that a beaver patch goes through many stages of succession - both aquatic and terrestrial - means that in a landscape inhabited by beavers there are plenty of beaver patches in different successional stages. The environmental heterogeneity caused by beaver activities lays a foundation on which considerable species diversity can build (Bush et al., 2019; Nummi et al., 2019a; Kivinen et al., 2020). Within the aquatic realm, different successional stages have a documented effect on the plant community (Nummi, 1989; Ray et al., 2001). In water, few studies have investigated the effects of successional stages (Bush et al., 2019; Nummi et al., 2021b), and results show that water beetles show the highest abundance and richness in the early successional stage (Nummi et al., 2021b).
Terrestrial succession and an abundance of niches in beaver wetlands should create grounds for high terrestrial invertebrate diversity and abundance on both a temporal and spatial scale (Figure 1). The terrestrial succession after a beaver flood result in a great variability in microhabitats and create structural heterogeneity in the form of dead wood, meadows, saplings, and young trees (Chandler et al., 2009). Apart from succession-created temporal variability in the beaver patch, there is considerable spatial within-patch heterogeneity in beaver ponds (Willby et al., 2018). The high heterogeneity is due to the many niches and habitats found in and around beaver ponds, including the relatively deep water just behind the dam and in the original creek channel or pond, beaver-created foraging channels, flooded shrubs-swamps, shallow marshes of emergent vegetation, and wet meadows (Nummi, 1989; Hood and Larson, 2015; Bush and Wissinger, 2016; Johnston, 2017; Nummi et al., 2021b).
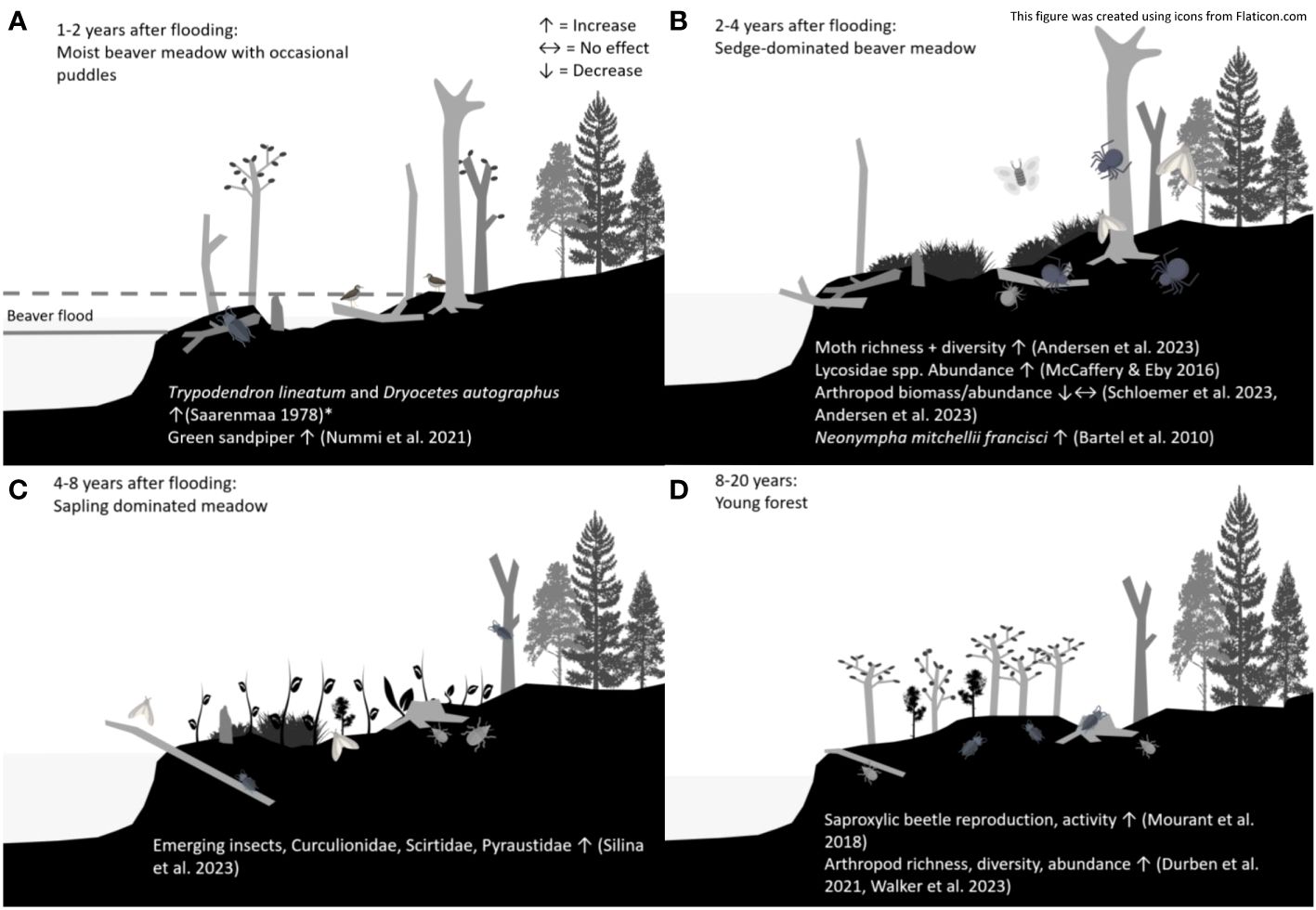
Figure 1 Successional stages of a beaver wetland following beaver abandonment. The first few years after beaver abandonment is characterized by a wet meadow stage with puddles (A). The sedge-dominated beaver meadow (B) follows, and favours moths, spiders and butterflies. After 4-8 years, saplings will sprout on the beaver meadow (C), and finally, if not re-occupied by beavers, a young forest will reappear (D). *Study took place during the beaver flood stage. This image was created using icons from Flaticon.com.
Initially, the beaver dam causes flooding, resulting in shallow water impounding areas adjacent to the beaver dam that may remain present for as long as the beaver pond is active (Knudsen, 1962; Johnston, 2017). This stage is characterized by shallow shores with lentic water interspersed with vegetation (Beard, 1953; Nummi & Hahtola, 2008), and at the edge of the flooded area a complex and extensive marsh zone can appear (Knudsen, 1962; Bush and Wissinger, 2016). In its early phase, this stage is important to ducks, amphibians, aquatic insects, and terrestrial invertebrates, and its late phase especially to fishes (Rosell et al., 2005; Stringer and Gaywood, 2016).
Flooding, however, might also have deleterious consequences for terrestrial organisms as is evident from several ecosystems. Flooding stress negatively impact plant growth and survival (Zhou et al., 2020; Aslam et al., 2023). Flooding changes the invertebrate community composition, but the effects are taxa dependent (Ellis et al., 2001). In grasslands, flooding negatively impact diversity, biomass, and abundance of the terrestrial soil microfauna as many species lack the physiological adaptations to withstand flooding and as a result evade flooded areas (Plum, 2005). In a mosaic floodplain landscape, areas with longer inundation periods had a lower ant species richness compared to drier sites (Ballinger et al., 2007).
After the beaver flooding phase, the first successional stage after dam breaching is the moist beaver meadow that appear during the first few years of water drawdown after beaver abandonment (Neff, 1957) (Figure 1A). Here, the water recedes, exposing soil which will soon be covered in sedges, grasses, and forbs, whereas shrubs and trees are not yet able to sprout (Knudsen, 1962; Johnston, 2017). This stage is important habitat for e.g. the invertivorous green sandpiper, Tringa ochropus which continues to thrive during the first years of beaver abandonment, utilizing the moist beaver meadows which still may contain shallow puddles (Nummi et al., 2021a). Further, the stage is potentially important for insects found in shallow water and moist soil, for example Tipulidae and Stratiomyiidae (Hodkinson, 1975).
When the soil dries out, we enter the drier beaver meadow stage (Figure 1B) (Bartel et al., 2010; Johnston, 2017), and after that the sapling and shrub stage (Hyvönen and Nummi, 2008) (Figure 1C). Beavers are herbivores that forage selectively, thus changing sapling recruitment in the areas surrounding the beaver flowage (Donkor and Fryxell, 1999). Beaver foraging initially increases the dominance of coniferous species, but the gaps created by the beaver disturbance facilitate regeneration of both deciduous and coniferous species (Donkor and Fryxell, 1999). Whereas succession following foraging of deciduous trees favors conifers, deciduous trees dominate the succession following a beaver flood (Hyvönen and Nummi, 2008). These beaver-created gaps and increased habitat heterogeneity could benefit saproxylic invertebrates; a study in temperate forests found canopy openness and habitat heterogeneity being the main factors affecting saproxylic beetle diversity (Seibold et al., 2016), and for red-listed saproxylic invertebrates in Sweden, it was noted that a high proportion of the species thrive in sun-exposed deadwood surfaces often found in snags (Jonsell et al., 1998). A special feature of beaver patches is the occurrence of snags in moist conditions; and while they have a documented positive effect on dead-wood related species such as pin lichens (Vehkaoja et al., 2017), little is known of invertebrates in these microhabitats. As the saplings mature, the next successional stage commences (Figure 1D), unless beavers re-enter an area and the area reverts to the first successional stages of a beaver wetland (Kivinen et al., 2020).
Deadwood formation is not limited to one successional stage but is a continuous process. Prolonged flooding leads to tree death (Glenz et al., 2006), and since beavers often occupy the same site over multiple years (Hood, 2020; Kivinen et al., 2020), deadwood formation is expected to accelerate during beaver occupancy. The legacy of the beaver continues after it leaves the area as the snags gradually get more degraded, and on a landscape level deadwood will continuously form as beavers occupy new patches (Thompson et al., 2016). It is also worth mentioning that deadwood of different stages and sizes are present in patches that have faced multiple beaver floods (Thompson et al., 2016; Kivinen et al., 2020).
4 Effects on terrestrial invertebrates
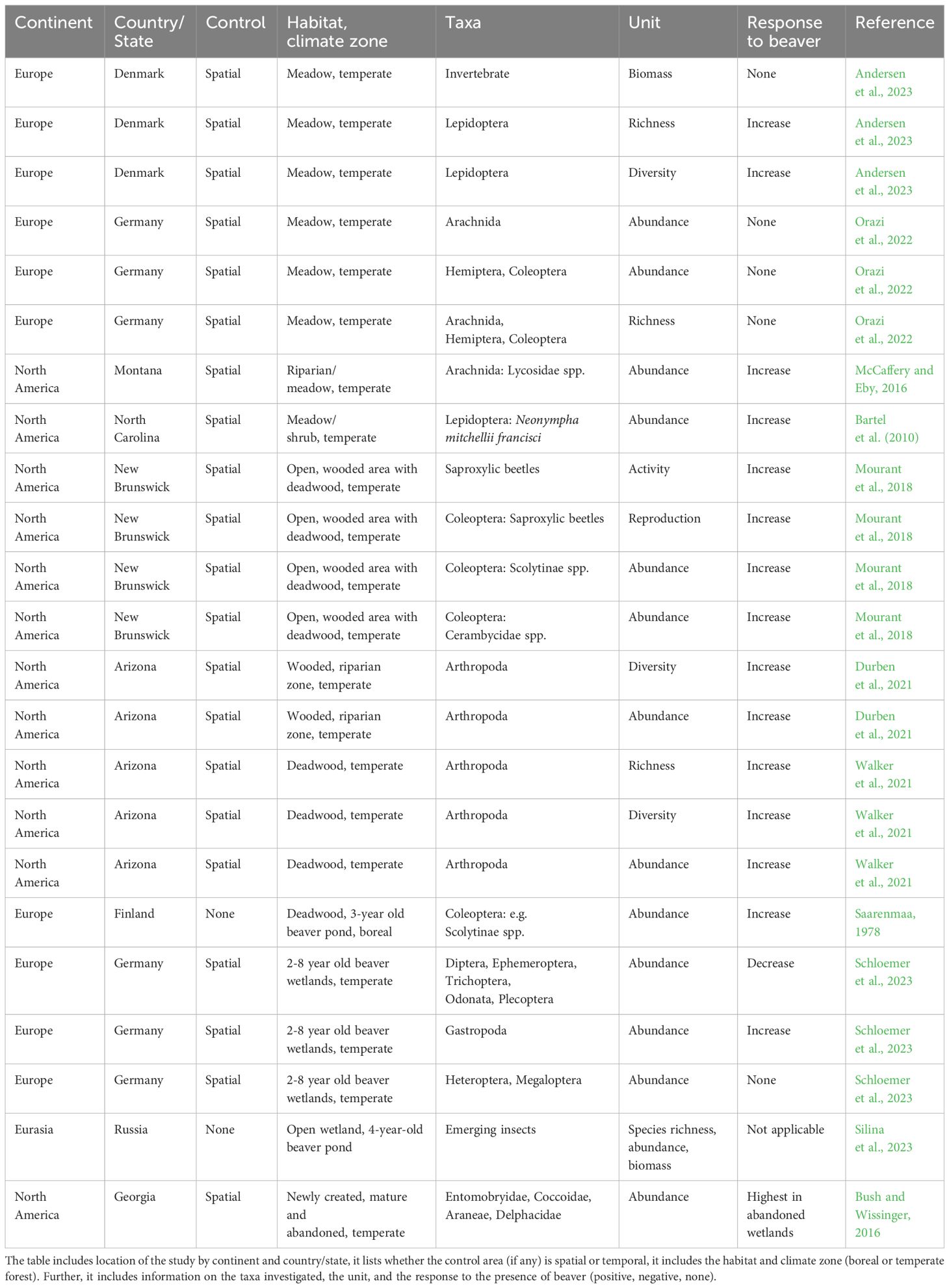
We found 11 studies on terrestrial invertebrates in beaver wetlands, most comparing a beaver wetland to a control wetland spatially separate from each other (Table 1). Of these studies, 5 were conducted in Europe, and 6 were conducted in the United States. The majority were conducted in the temporal zone rather than the boreal zone, and most studied abundance and richness at a taxonomic level above species. While none of the studies include all the successional stages created by the beaver in their study design, as a unity they enable us to elucidate some trends with regards to beavers and terrestrial invertebrates, and the importance of the different successional stages.
Bartel et al. (2010) included two successional stages in their study, comparing the effect of the early successional meadow stage and the late successional scrub stage on a butterfly, Neonympha mitchellii francisci (Figure 1B). The butterfly population size was correlated to the cover of certain Carex species, which in turn were more abundant in the early successional stage (Bartel et al., 2010). Several studies have compared areas with and without the presence of beavers. McCaffery and Eby (2016) showed an increased abundance of spiders at the family level, where Lycosidae spp. were found at higher abundance in areas with no beaver. The increase in Lycosidae abundance was explained by an increased food availability due to an increase in aquatic subsidies, which in turn increased the macroinvertebrate emergence rate (McCaffery and Eby, 2016). Andersen et al. (2023) found an increased richness and diversity of Lepidoptera in areas with beavers potentially explained by a higher habitat heterogeneity in beaver wetlands (as indicated by a higher variance in NDVI). Orazi et al. (2022) saw no difference with regards to the richness of Coleoptera, Hemiptera or Arachnida, but recorded a distinct Coleoptera community in beaver wetlands. Overall, these studies suggest that the effect of the presence of beavers might not be detected at broad taxonomic levels nor in all arthropod groups in the early successional stages of beaver meadows. For example, overall invertebrate biomass (Andersen et al., 2023) or order level abundance (Orazi et al., 2022) did not differ between beaver meadows and compatible control areas without beaver activities, whereas data at the family level detected significant differences [Coleoptera (Orazi et al., 2022), Lycosidae (McCaffery and Eby, 2016)].
Beaver induced flooding produces large volumes of deadwood in areas that are otherwise rarely disturbed (Thompson et al., 2016). Deadwood is an important habitat for numerous species, including saproxylic insects, where 800 species of Coleoptera, 500-1000 species of Diptera, and 500-1000 species of Hymenoptera are expected to be found in Finland alone (Siitonen, 2001), and many of these species are specialized in specific deadwood stages (Stokland et al., 2012). We therefore expect beaver activities to have an indirect impact on both the richness and abundance of saproxylic insects. Three studies examined the importance of successional stage in the sapling/young forest, where deadwood is abundant (Figures 1B, C), on terrestrial invertebrates (Mourant et al., 2018; Durben et al., 2021; Walker et al., 2021). Mourant et al. (2018) investigated activity and fecundity of saproxylic beetles in Canadian beaver wetlands dominated by mixed forest vegetation and deadwood stands. They found a higher abundance of both small emergence holes attributed to small beetle species, such as Scolytinae, and large emergence holes attributed to large beetles (Cerambycidae) in snags in beaver-modified habitats compared to control sites. The increased activity and reproduction was ascribed to the high-quality wooden debris present in beaver modified wetlands (Mourant et al., 2018). Saarenmaa (1978) examined trees from an area still flooded and found the moisture-liking insect species, Trypodendron lineatum and Dryocetes autographus, especially abundant in beaver-killed trees.
Ecosystems containing beaver-felled trees also have a positive impact on arthropod richness, diversity and abundance (Durben et al., 2021). According to Durben et al. (2021), beaver herbivory alters the chemical traits of saplings and stems compared to saplings not subjected to herbivory, with the added effect of attracting more arthropods. In a different study, Walker et al. (2021) found a higher arthropod richness, abundance and diversity within beaver-felled trees compared to unfelled trees. Beaver herbivory increases tree productivity and stress, which create genetically more heterozygous trees that in turn are attractive to the arthropod community (Walker et al., 2021). In conclusion, the richness and abundance of multiple invertebrate orders increase in deadwood dominated beaver wetlands compared to control wetlands and the mechanisms behind these increases are complex.
Two other studies are worth mentioning that have investigated terrestrial invertebrates in beaver wetlands but have not presented results from a non-beaver control. In their study on macroinvertebrates in beaver dams, Schloemer et al. (2023) focused on aquatic invertebrates but their sampling design, in which they sampled by the actual beaver dam, resulted in semi-terrestrial and terrestrial invertebrates also being sampled. For the sampled invertebrate orders, more terrestrial species were recorded in abandoned than active beaver wetlands. Terrestrial species included a beetle (Dianous coerulescens) and a snail (Vertigo antivertigo) (Schloemer et al., 2023). Silina et al. (2023) looked at the abundance, biomass, and diversity of emerging flying insects at different zones of a beaver pond in Russia. They recorded 162 species across 8 orders. Also worth mentioning is the study on aquatic invertebrates by Bush et al. (2019). They identified terrestrial Coleoptera: Anthicidae as an indicator-taxa in mature beaver wetlands, while Hemiptera: Pseudococcidae and Coleoptera: Curculionidae were identified as indicator species of abandoned beaver wetlands. Further, the study by Bush and Wissinger (2016) compared newly-formed, mature, and abandoned beaver wetlands and found terrestrial taxa present in both newly formed (Araneae) and abandoned (Entomobryidae, Coccoidae, Araneae, Delphacidae) beaver wetlands.
Another important aspect to remember is that terrestrial and aquatic habitats are connected, and that many species have aquatic larvae resulting in an influx of insects to the terrestrial realm. Overall, the abundance of emerging invertebrates may thus be higher in beaver wetlands compared to controls (Nummi, 1992; Nummi et al., 2011; McCaffery and Eby, 2016; Xiang et al., 2017). However, one study found no difference in overall aerial invertebrate biomass between temperate wetlands with and without beaver (Andersen et al., 2023).
5 Conclusions and future directions
This literature review indicates that beavers can have an impact on the terrestrial invertebrate community, and that the effect might be present across multiple successional stages. The number of studies conducted on terrestrial invertebrates is low compared to aquatic invertebrates in beaver wetlands. Further studies are needed to address the potential impact of beavers on the terrestrial community, especially studies taking into consideration successional stages. For example, currently we lack information on different species groups, such as above- and below-ground species, flying and non-flying species, and the importance of taxonomic resolution. It is also important to include successional stages, space, time, sampling effort in the sampling design. Lastly, we need more information on the different abiotic and biotic factors co-occurring with successional stages, such as temperature, humidity, nutrient level, and structural complexity. Further, the close link between the terrestrial and aquatic realms, with many invertebrate species having both an aquatic and terrestrial life form, and interactions across species should be taken into account. Even in the aquatic realm, where numerous studies have been conducted (see for example the review by Washko et al. (2022)), few studies take the different successional stages of the beaver wetland into account. Nummi et al. (2021b) included both newly formed, as well as old and abandoned beaver ponds in their study on water beetles and found the largest richness and diversity in newly formed ponds. In a study on beta-diversity, Bush et al. (2019) showed that various successional stages of beaver wetland had unique aquatic invertebrate communities and that beta-diversity was nearly twice as high for the entire community compared to each successional stage. Further, Hood and Larson (2014) studies active and abandoned beaver sites, and found beaver wetlands to have deeper ponds and higher species richness of aquatic invertebrates.
A better understanding of how successional stages caused by beaver activity affect the terrestrial invertebrate community can also help in conservation of endangered species and restoration of biodiversity. In recent years, rewilding has emerged aiming to restore natural processes and functions in ecosystems. Reintroduction of key ecosystem engineering species, such as beavers may help facilitate restoring biodiversity for terrestrial species but also across the aquatic and terrestrial realm.
Author contributions
LHA: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. PN: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. SB: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Dr. SB was supported by a grant from the Novo Nordic Foundation (Grant no. NNF21OC0070910).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achury R., Staab M., Blüthgen N., Weisser W. W. (2023). Forest gaps increase true bug diversity by recruiting open land species. Oecologia 202, 299–312. doi: 10.1007/s00442-023-05392-z
Åhlén I., Jarsjö J., Hambäck P. A. (2023). Connecting wetland flooding patterns to insect abundance using high-resolution inundation frequency data. Wetlands 43. doi: 10.1007/s13157-023-01716-0
Albertson L. K., Sklar L. S., Tumolo B. B., Cross W. F., Collins S. F., Woods H. A. (2024). The ghosts of ecosystem engineers: Legacy effects of biogenic modifications. Funct. Ecol. 38, 52–72. doi: 10.1111/1365-2435.14222
Andersen L. H., Ransborg C., Pertoldi C., Pagh S., Bahrndorff S. (2023). Can reintroduction of beavers improve insect biodiversity? J. Environ. Manage. 337. doi: 10.1016/j.jenvman.2023.117719
Aslam A., Mahmood A., Ur-Rehman H., Li C., Liang X., Shao J., et al. (2023). Plant adaptation to flooding stress under changing climate conditions: Ongoing breakthroughs and future challenges. Plants 12. doi: 10.3390/plants12223824
Bakker E. S., Svenning J. (2018). Trophic rewilding: Impact on ecosystems under global change. Philosophical Transactions of the Royal Society of London. Ser. B. Biol. Sci. 373. doi: 10.1098/rstb.2017.0432
Ballinger A., Lake P. S., Nally R. M. (2007). Do terrestrial invertebrates experience floodplains as landscape mosaics? Immediate and longer-term effects of flooding on ant assemblages in a floodplain forest. Oecologia 152, 227–238. doi: 10.1007/s00442-006-0648-0
Bartel R. A., Haddad N. M., Wright J. P. (2010). Ecosystem engineers maintain a rare species of butterfly and increase plant diversity. Oikos 119, 883–890. doi: 10.1111/j.1600-0706.2009.18080.x
Beard E. B. (1953). The importance of beaver in waterfowl management at the Seney National Wildlife Refuge. J. Wildlife Manage. 17, 398–436. doi: 10.2307/3797047
Brazier R. E., Puttock A., Graham H. A., Auster R. E., Davies K. H., Brown C. M. L. (2021). Beaver: Nature’s ecosystem engineers. Wiley Interdisciplinary Reviews. Water 8, e1494. doi: 10.1002/wat2.1494
Bush B. M., Stenert C., Maltchik L., Batzer D. P. (2019). Beaver-created successional gradients increase β-diversity of invertebrates by turnover in stream-wetland complexes. Freshw. Biol. 64, 1265–1274. doi: 10.1111/fwb.13302
Bush B. M., Wissinger S. A. (2016). “Invertebrates in beaver-created wetlands and ponds,” in Invertebrates in freshwater wetlands. Eds. Batzer D., Boix D. (Springer, Cham). doi: 10.1007/978-3-319-24978-0_12
Chandler R. B., King D. I., DeStefano S. (2009). Scrub-shrub bird habitat associations at multiple spatial scales in beaver meadows in Massachusetts. Auk 126, 186–197. doi: 10.1525/auk.2009.08083
Cowie R. H., Bouchet P., Fontaine B. (2022). The sixth mass extinction: Fact, fiction or speculation? Biol. Rev. 97, 640–663. doi: 10.1111/brv.12816
Donkor N. T., Fryxell J. M. (1999). Impact of beaver foraging on structure of lowland boreal forests of Algonquin Provincial Park, Ontario. For. Ecol. Manage. 118, 83–92. doi: 10.1016/S0378-1127(98)00487-3
Durben R. M., Walker F. M., Holeski L., Keith A. R., Kovacs Z., Hurteau S. R., et al. (2021). Beavers, bugs and chemistry: A mammalian herbivore changes chemistry composition and arthropod communities in foundation tree species. Forests 12. doi: 10.3390/f12070877
Ellis L. M., Crawford C. S., Molles M. C. Jr. (2001). Influence of annual flooding on terrestrial arthropod assemblages of a Rio Grande Riparian Forest. Regulated Rivers 17, 1–20. doi: 10.1002/(ISSN)1099-1646
Fedyń I., Przepióra F., Sobociński W., Wyka J., Ciach M. (2022). Eurasian beaver–A semi-aquatic ecosystem engineer rearranges the assemblage of terrestrial mammals in winter. Sci. Total Environ. 831. doi: 10.1016/j.scitotenv.2022.154919
Fedyn I., Przepiora F., Sobocinski W., Wyka J., Ciach M. (2023). Beyond beaver wetlands: The engineering activities of a semi-aquatic mammal mediate the species richness and abundance of terrestrial birds wintering in a temperate forest. For. Ecol. Manage. 529. doi: 10.1016/j.foreco.2022.120698
Glenz C., Schlaepfer R., Iorgulescu I., Kienast F. (2006). Flooding tolerance of central European tree and shrub species. For. Ecol. Manage. 235, 1–13. doi: 10.1016/j.foreco.2006.05.065
Goulson D. (2019). The insect apocalypse, and why it matters. Curr. Biol. 29, R967–R971. doi: 10.1016/j.cub.2019.06.069
Hammill E., Hawkins C. P., Greig H. S., Kratina P., Shurin J. B., Atwood T. B. (2018). Landscape heterogeneity strengthens the relationship between β-diversity and ecosystem function. Ecol. (Durham) 99, 2467–2475. doi: 10.1002/ecy.2492
Hodkinson I. D. (1975). A community analysis of the benthic insect fauna of an abandoned beaver pond. J. Anim. Ecol. 44, 533–551. doi: 10.2307/3610
Hood G. A. (2020). Not all ponds are created equal: Long-term beaver (Castor canadensis) lodge occupancy in a heterogeneous landscape. Can. J. Zoology 98, 210–218. doi: 10.1139/cjz-2019-0066
Hood G. A., Larson D. G. (2014). Beaver-created habitat heterogeneity influences aquatic invertebrate assemblages in boreal Canada. Wetlands (Wilmington N.C.) 34, 19–29. doi: 10.1007/s13157-013-0476-z
Hood G. A., Larson D. G. (2015). Ecological engineering and aquatic connectivity: A new perspective from beaver-modified wetlands. Freshw. Biol. 60, 198–208. doi: 10.1111/fwb.12487
Høye T. T., Loboda S., Koltz A. M., Gillespie M. A. K., Bowden J. J., Schmidt N. M. (2021). Nonlinear trends in abundance and diversity and complex responses to climate change in arctic arthropods. Proc. Natl. Acad. Sci. - PNAS 118. doi: 10.1073/pnas.2002557117
Hyvönen T., Nummi P. (2008). Habitat dynamics of beaver Castor canadensis at two spatial scales. Wildlife Biol. 14, 302–308. doi: 10.2981/0909-6396(2008)14[302:HDOBCC]2.0.CO;2
Johnston C. A. (2017). Beavers: Boreal ecosystem engineers. 1st ed (Cham: Springer International Publishing). doi: 10.1007/978-3-319-61533-2
Jonsell M., Weslien J., Ehnstrom B. (1998). Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodiversity Conserv. 7, 749–764. doi: 10.1023/A:1008888319031
Kivinen S., Nummi P., Kumpula T. (2020). Beaver-induced spatiotemporal patch dynamics affect landscape-level environmental heterogeneity. Environ. Res. Lett. 15, 94065. doi: 10.1088/1748-9326/ab9924
Knudsen G. J. (1962). Relationship of beaver to forests, trout and wildlife in Wisconsin (Wisconsin: Wisconsin State Conservation Dept.). Available at: https://hdl.handle.net/2027/wu.89058818287.
Kuuluvainen T., Nummi P. (2023). Strategies for the ecological restoration of the boreal forest facing climate change - chapter 17 (Cham, Switzerland: Springer International Publishing). doi: 10.1007/978-3-031-15988-6_17
Larsen A., Larsen J. R., Lane S. N. (2021). Dam builders and their works: Beaver influences on the structure and function of river corridor hydrology, geomorphology, biogeochemistry and ecosystems. Earth-Science Rev. 218. doi: 10.1016/j.earscirev.2021.103623
Law A., Levanoni O., Foster G., Ecke F., Willby N. J. (2019). Are beavers a solution to the freshwater biodiversity crisis? Diversity Distributions 25, 1763–1772. doi: 10.1111/ddi.12978
Lister B. C., Garcia A. (2018). Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. - PNAS 115, E10397–E10406. doi: 10.1073/pnas.1722477115
McCaffery M., Eby L. (2016). Beaver activity increases aquatic subsidies to terrestrial consumers. Freshw. Biol. 61, 518–532. doi: 10.1111/fwb.12725
McCarthy T. S., Tooth S., Kotze D. C., Collins N. B., Wandrag G., Pike T. (2010). The role of geomorphology in evaluating remediation options for floodplain wetlands: The case of ramsar-listed Seekoeivlei, Eastern South Africa. Wetlands Ecol. Manage. 18, 119–134. doi: 10.1007/s11273-009-9153-7
Mourant A., Lecomte N., Moreau G. (2018). Indirect effects of an ecosystem engineer: How the Canadian beaver can drive the reproduction of saproxylic beetles. J. Zoology 304, 90–97. doi: 10.1111/jzo.12506
Neff D. J. (1957). Ecological effects of beaver habitat abandonment in the Colorado Rockies. J. Wildlife Manage. 21, 80–84. doi: 10.2307/3797684
Nelner T. B., Hood G. A. (2011). Effect of agriculture and presence of American beaver Castor canadensis on winter biodiversity of mammals. Wildlife Biol. 17, 326–336. doi: 10.2981/09-097
Nummi P. (1989). Simulated effects of the beaver on vegetation, invertebrates and ducks. Annales Zoologici Fennici 26, 43–52.
Nummi P. (1992). The importance of beaver ponds to waterfowl broods: An experiment and natural tests. Annales Zoologici Fennici 29, 47–55.
Nummi P., Arzel C., Sauramo V. (2021a). Populations in stable and variable habitats: Green and common sandpiper in a beaver-influenced landscape. Global Ecol. Conserv. 28, e01678. doi: 10.1016/j.gecco.2021.e01678
Nummi P., Hahtola A. (2008). The beaver as an ecosystem engineer facilitates teal breeding. Ecography 31, 519–524. doi: 10.1111/j.0906-7590.2008.05477.x
Nummi P., Holopainen S. (2014). Whole-community facilitation by beaver: Ecosystem engineer increases waterbird diversity. Aquat. Conservation: Mar. Freshw. Ecosyst. 24, 623–633. doi: 10.1002/aqc.2437
Nummi P., Kattainen S., Ulander P., Hahtola A. (2011). Bats benefit from beavers: A facilitative link between aquatic and terrestrial food webs. Biodiversity Conserv. 20, 851–859. doi: 10.1007/s10531-010-9986-7
Nummi P., Kuuluvainen T. (2013). Forest disturbance by an ecosystem engineer: Beaver in boreal forest landscapes. Boreal Environ. Res. 18, 13–24.
Nummi P., Liao W., Huet O., Scarpulla E., Sundell J. (2019a). The beaver facilitates species richness and abundance of terrestrial and semi-aquatic mammals. Global Ecol. Conserv. 20, e00701. doi: 10.1016/j.gecco.2019.e00701
Nummi P., Liao W. F., van der Schoor J., Loehr J. (2021b). Beaver creates early successional hotspots for water beetles. Biodiversity Conserv. 30, 2655–2670. doi: 10.1007/s10531-021-02213-8
Nummi P., Suontakanen E. M., Holopainen S., Väänänen V. M. (2019b). The effect of beaver facilitation on common teal: Pairs and broods respond differently at the patch and landscape scales. Ibis 161, 301–309. doi: 10.1111/ibi.12626
Nummi P., Vehkaoja M., Pumpanen J., Ojala A. (2018). Beavers affect carbon biogeochemistry: Both short-term and long-term processes are involved. Mammal Rev. 48, 298–311. doi: 10.1111/mam.12134
Orazi V., Hagge J., Gossner M. M., Mueller J., Heurich M. (2022). A biodiversity boost from the Eurasian beaver (Castor fiber) in Germany’s oldest national park. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.873307
Plum N. (2005). Terrestrial invertebrates in flooded grassland: A literature review. Wetlands 25, 721–737. doi: 10.1672/0277-5212(2005)025[0721:TIIFGA]2.0.CO;2
Puttock A., Graham H. A., Cunliffe A. M., Elliott M., Brazier R. E. (2017). Eurasian beaver activity increases water storage, attenuates flow and mitigates diffuse pollution from intensively-managed grasslands. Sci. Total Environ. 576, 430–443. doi: 10.1016/j.scitotenv.2016.10.122
Ray A. M., Rebertus A. J., Ray H. L. (2001). Macrophyte succession in Minnesota beaver ponds. Can. J. Bot. 79, 487–499. doi: 10.1139/b01-018
Régnier C., Achaz G., Lambert A., Cowie R. H., Bouchet P., Fontaine B. (2015). Mass extinction in poorly known taxa. Proc. Natl. Acad. Sci. - PNAS 112, 7761–7766. doi: 10.1073/pnas.1502350112
Remillard M. M., Gruendling G. K., Bogucki D. J. (1987). “Disturbance by beaver (Castor canadensis) and increased landscape heterogeneity,” in Landscape heterogeneity and disturbance. Ed. Turner M. G. (Springer, New York), 103–122.
Rosell F., Bozser O., Collen P., Parker H. (2005). Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems. Mammal Rev. 35, 248–276. doi: 10.1111/j.1365-2907.2005.00067.x
Saarenmaa H. (1978). The occurrence of bark beetles (col., scolytidae) in a dead spruce stand flooded by beavers (Castor canadensis Kuhl). Silva Fennica 12. doi: 10.14214/sf.a14857
Schloemer S., Hörren T., Lorenz A. W., Hering D. (2023). The macroinvertebrate fauna of maintained and abandoned beaver dams. Hydrobiologia 850, 1763–1778. doi: 10.1007/s10750-023-05176-9
Seibold S., Bässler C., Brandl R., Büche B., Szallies A., Thorn S., et al. (2016). Microclimate and habitat heterogeneity as the major drivers of beetle diversity in dead wood. J. Appl. Ecol. 53, 934–943. doi: 10.1111/1365-2664.12607
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bulletins 49, 11–41. https://www.jstor.org/stable/20113262.
Silina A. E., Sushchik N. N., Gladyshev M. I., Kurina E. M., Kolmakova A. A., Seleznev D. G. (2023). Emergence of amphibious insects from an old beaver pond in the upper Khoper Valley under conditions of the forest steppe. Contemp. Problems Ecol. 16, 790–806. doi: 10.1134/S1995425523060197
Stein A., Gerstner K., Kreft H., Arita H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880. doi: 10.1111/ele.12277
Stokland J. N., Siitonen J., Jonsson B. G. (2012). Biodiversity in dead wood (1. publ. ed.) (Cambridge: Cambridge University Press). doi: 10.1017/CBO9781139025843
Stringer A. P., Gaywood M. J. (2016). The impacts of beavers Castor spp. on biodiversity and the ecological basis for their reintroduction to Scotland, UK. Mammal Rev. 46, 270–283. doi: 10.1111/mam.12068
Thompson S., Vehkaoja M., Nummi P. (2016). Beaver-created deadwood dynamics in the boreal forest. For. Ecol. Manage. 360, 1–8. doi: 10.1016/j.foreco.2015.10.019
Turner M. G., Gardner R. H. (2015). Landscape ecology in theory and practice. 2 (New York: Springer). doi: 10.1007/978-1-4939-2794-4
Turner M. G., Romme W. H., Gardner R. H., Hargrove W. W. (1997). Effects of fire size and pattern on early succession in Yellowstone National Park. Ecol. Monogr. 67, 411–433. doi: 10.1890/0012-9615(1997)067[0411:EOFSAP]2.0.CO;2
Vehkaoja M., Nummi P., Rikkinen J. (2017). Beavers promote Calicioid diversity in boreal forest landscapes. Biodiversity Conserv. 26, 579–591. doi: 10.1007/s10531-016-1259-7
Walker F. M., Durben R., Shuster S. M., Lindroth R. L., Whitham T. G. (2021). Heterozygous trees rebound the fastest after felling by beavers to positively affect arthropod community diversity. Forests 12, 694. doi: 10.3390/f12060694
Washko S., Willby N., Law A. (2022). How beavers affect riverine aquatic macroinvertebrates: A review. PeerJ 10, e13180. doi: 10.7717/peerj.13180
Wikar Z., Ciechanowski M., Zwolicki A. (2024). The positive response of small terrestrial and semi-aquatic mammals to beaver damming. Sci. Total Environ. 906, 167568. doi: 10.1016/j.scitotenv.2023.167568
Willby N. J., Law A., Levanoni O., Foster G., Ecke F. (2018). Rewilding wetlands: Beaver as agents of within-habitat heterogeneity and the responses of contrasting biota. Philos. Trans. R. Soc. B-Biological Sci. 373. doi: 10.1098/rstb.2017.0444
Wohl E. (2021). Legacy effects of loss of beavers in the continental United States. Environ. Res. Lett. 16, 25010. doi: 10.1088/1748-9326/abd34e
Xiang H., Zhang Y., Richardson J. S. (2017). Importance of riparian zone: Effects of resource availability at land-water interface. Riparian Ecol. Conserv. 3, 1–17. doi: 10.1515/remc-2016-0001
Keywords: wetlands, biodiversity, abundance, succession, Castor, insects
Citation: Andersen LH, Nummi P and Bahrndorff S (2024) Can beavers help improve terrestrial invertebrate diversity? Front. Ecol. Evol. 12:1396207. doi: 10.3389/fevo.2024.1396207
Received: 05 March 2024; Accepted: 03 May 2024;
Published: 21 May 2024.
Edited by:
Attila D. Sándor, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Michał Ciach, University of Agriculture in Krakow, PolandCopyright © 2024 Andersen, Nummi and Bahrndorff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Line Holm Andersen, bGlob2FuQGJpby5hYXUuZGs=
†These authors share last authorship
 Line Holm Andersen
Line Holm Andersen Petri Nummi
Petri Nummi Simon Bahrndorff
Simon Bahrndorff