- 1Faculty of Environmental Sciences, Czech University of Life Sciences Prague, Praha, Czechia
- 2Institute of Biological Sciences, University of Zielona Góra, Zielona Góra, Poland
Urban areas are known to have high levels of noise pollution, which can impact an animal’s antipredator behavior. Noise can either distract the animal or mask the sounds of a predator, increasing the animal’s vulnerability to predation. However, the prey may increase vigilance in noisier environments, thus reducing energy and time spent on other activities. Alert Distance (AD) refers to the distance at which an animal becomes alert to a potential predator approaching. Flight Initiation Distance (FID) is the distance from the potential predator at which the animal flees. We studied the impact of ambient noise pollution on the AD and a corrected FID (FID/AD) of Eurasian Magpies (Pica pica) using a field investigator as a potential predator walking towards birds at a constant speed. We found that the noise level did not affect the AD. Still, noise had a negative effect on the Eurasian Magpies’ FID/ADs, suggesting that noise may slow their reaction to a potential threat but not their ability to detect it. Thus, our research highlights that urban noise pollution can increase an individual's vulnerability to predation, even when predators are still detectable. Ambient noise may distract the bird by diverting some of its limited attention and causing a delayed response to the predators. Alternatively, noise could be masking auditory cues that would have otherwise been added together with visual cues to cause an enhanced response. More research is necessary to understand the effects of noise pollution on the antipredator behavior of birds in urban areas, taking into account the specific strategies and adaptations of each species.
1 Introduction
Urban areas are filled with anthropogenic sounds from traffic, industrial, and commercial activities (Warren et al., 2006). Sounds are not inherently problematic, as most animals use them for communication and survival (Sun and Narins, 2005; Sordello et al., 2020). However, after a certain threshold, human-made sounds begin to cause disruptions to wildlife and, thus, turn into “noise pollution” (Sordello et al., 2020). The extent of disturbance is relative and varies based on species tolerance levels (Sordello et al., 2020). Noise pollution may interfere with wildlife by masking their natural sounds, affecting communication and essential auditory signals crucial for survival (Sun and Narins, 2005; Slabbekoorn and Ripmeester, 2008; Barber et al., 2010). Additionally, noise has been observed to elevate stress levels in numerous animal species, leading to complex and diverse implications for their physiological systems (Kight and Swaddle, 2011).
Birds are an example of fauna adversely affected by noise, as they rely on acoustic communication and signals to interact with their environment (Francis et al., 2009; Hu and Cardoso, 2009; Petrelli et al., 2017). Birds use their songs to communicate with mates, brood, and conspecifics, attract partners, and establish dominance (Catchpole and Slater, 2003; Slabbekoorn and Ripmeester, 2008). Auditory cues also serve birds to detect approaching predators and send and receive signals to and from their conspecifics about predation threats (Hollén and Radford, 2009). Birds are considered model organisms in urban ecology and good indicators of habitat quality as they are easy to spot and study and are responsive to anthropogenic habitat alterations (Marzluff, 2008; Croci et al., 2008).
Intense ambient noise may impact an animal’s antipredator behavior in various ways (Shannon et al., 2016). On the one hand, noise may hinder the individual’s ability to detect threats (either by masking auditory cues of the predator or by distracting the prey; Barber et al., 2010; Chan et al., 2010a; Zhou et al., 2019), increasing the predation threat for the animal (Chan et al., 2010a; Chan et al., 2010b). On the other hand, noise may lead the animal to augment its vigilance (as an attempt to compensate for its compromised hearing or by perceiving the noise as a direct threat in itself; Meillère et al., 2015; Kern and Radford, 2016; Shannon et al., 2016; Evans et al., 2018). However, increased vigilance would entail additional energy costs and keep the animal from optimal foraging and other activities (Kern and Radford, 2016). Thus, urban noise may disturb an animal’s antipredator behavior in two opposing, unfavorable manners.
Several studies investigated the impacts of noise pollution on the antipredator behavior of birds (Gravolin et al., 2014; Meillère et al., 2015; Petrelli et al., 2017; Evans et al., 2018; Zhou et al., 2019; Merrall and Evans, 2020). Various methods were used, such as observing vigilant behavior (Evans et al., 2018), willingness to visit feeders (Merrall and Evans, 2020), response to alarm calls (Zhou et al., 2019), and others. Few have used the Flight Initiation Distance (hereafter FID; Gravolin et al., 2014; Meillère et al., 2015; Petrelli et al., 2017).
FID is among the most widely investigated antipredator behaviors used extensively in behavioral ecology studies to assess fear, risk-taking, evolution, or adaptation (Møller, 2021). FID is the distance from an advancing danger (usually a researcher approaching the animal under standard conditions) that leads an animal to flee (Blumstein, 2003). Alert Distance (hereafter AD) is the distance before FID, at which the animal becomes aware of the predator and actively observes it before fleeing (Fernández-Juricic et al., 2001). The FID must compromise between flight costs and benefits to stay put and resume current activity (Møller, 2008). For example, in urban areas, where humans are commonly around, energetically costly constant fleeing would put birds at a disadvantage at the expense of optimal foraging (Cooper and Frederick, 2007). Thus, urban birds have lower fear responses than their rural counterparts and prioritize investing their energy in other activities, such as foraging and reproducing (Cooper and Frederick, 2007; Tryjanowski et al., 2016; Morelli et al., 2019; Díaz et al., 2021). They save energy by delaying flight and actively monitoring the predator until the costs of remaining put are higher than those of fleeing current activity (Price, 2008).
Although a few studies investigated the impacts of noise pollution on the FID of birds (Meillère et al., 2015; Petrelli et al., 2017), no study has also studied its impact on AD (but see Shannon et al., 2016 in a study on Prairie Dogs; Cynomys ludovicianus). Most studies do not account for AD and use FID as a proxy for the animals’ capacity to detect danger since the two are highly correlated (Blumstein et al., 2005). However, we hypothesize that high levels of noise pollution may have different impacts on the alertness and antipredator response of animals and that FID alone may not be sufficient to reflect the effects of noise on the attention of individuals. For example, noise may reduce the birds’ capacity to perceive the sounds of approaching predators (Barber et al., 2010; Chan et al., 2010b; Zhou et al., 2019), leading to a lower AD. However, birds exposed to more noise may be more stressed, leading them to flee sooner after they detect the danger, as shown in previous studies (Meillère et al., 2015).
Therefore, we think it is valuable to study the impacts of urban noise on AD and FID in a bird study regarding noise pollution, which has not yet been done to our knowledge. This approach may highlight two opposing ways urban noise may impact an animal’s antipredator behavior (either by distracting the animal or causing it to increase its vigilance) that are not mutually exclusive.
Many corvid species, including the Eurasian Magpie (Pica pica), have spread into urban areas (Benmazouz et al., 2021; Abou Zeid et al., 2023). Their behavioral flexibility has allowed them to adapt to urban areas by modifying their behavior (Benmazouz et al., 2021). For example, Eurasian Magpies tend to raise the heights of their nests in trees as urbanization intensity increases to avoid disturbances from humans and predation from Hooded Crows (Corvus cornix; Šálek et al., 2020; Xu et al., 2020; Ciebiera et al., 2021). Thus, Eurasian Magpies are ideal subjects for studying urban noise’s ecological implications on animal behavior.
This study aims to investigate the effect of noise pollution on Eurasian Magpies’ antipredator behavior in urban areas during the breeding season. The specific objectives are 1) to study the variation of AD and FID/AD of Eurasian Magpies across a gradient of urban noise and 2) to investigate the consistency of the impact of noise on threat detection and speed of the escape response. We hypothesize two potential reactions of Eurasian Magpies against increased noise: a) reduction in the birds’ AD due to masking or distraction or b) increase in AD due to heightened vigilance. Additionally, we expect that FID/AD may be increased so the Eurasian Magpies can compensate for reduced hearing by reacting more quickly to perceived threats. We think noise’s impact on threat detection may not be congruent with the escape response, even though the two may be highly correlated. To investigate these hypotheses, we modeled the effect of ambient noise on the AD and FID/AD of Eurasian Magpies in Prague, taking into account other confounding variables, such as the Starting Distance (hereafter SD, the distance that separates the researcher from the bird at the start of the sampling), the age of the individual, and the site surveyed.
2 Methods
2.1 Study area and field data collection
The fieldwork was conducted in Prague, Czechia, during the breeding season of 2022 (with more than 95% of data collected from mid-May until mid-July and before noon). Public parks and green areas with large Eurasian Magpie populations were surveyed on days that were not rainy or strongly windy (Beauford number ≤ 3). Standardized AD and FID collection methods were used (Blumstein, 2003). Only Eurasian Magpie individuals who were on the ground were sampled. When an individual was detected, the field researcher (FAZ, wearing similar inconspicuous dark clothes) began the collection by dropping a small marker (made of cotton and cloth not to attract the bird’s attention) behind their back to mark the Starting Distance (SD). The researcher began approaching the bird slowly and at a steady speed. Another marker was dropped when the bird started to exhibit alert behavior to the advancing person (looking at the field investigator, displaying cautious behavior, turning its head, etc.; Fernández-Juricic et al., 2001). A third marker was dropped when the bird escaped (by jumping or flying). Then, the researcher would stand in the bird’s last occupied spot and collect information regarding the noise level. The ambient noise level (dBA) was measured using a Multifunctional environment meter 13/464/0 from Brannan by collecting the minimum and maximum noise levels detected by the tool in 1 minute right after each individual was approached. The mean noise level was calculated as the average of the maximum and minimum collected within the minute. This was done to ensure that measured noise reflected the noise levels when the individual was approached. We also took note of the individual’s age (juvenile vs. adult). Juveniles were identified by smaller body sizes and shorter tails. Additionally, we counted all Eurasian Magpies in the flock near the sampled individual and the density of people present within a radius of 50 meters around the sampling point. After taking note of all these variables, the investigator measured the SD, AD, and FID from the birds’ last perch to the respective marker using a surveyor’s tape. We did not approach individuals who were alert before the start of the collection (Morelli et al., 2022). To reduce pseudo-replication bias, we avoided resampling the same individuals. Sites (parks or other green areas) with only one observation were also dropped.
2.2 Statistical analyses
We calculated the ratio of FID to AD (FID/AD). Since the maximum distance the individual can take flight at is constrained by the AD, it is necessary to use a corrected flight distance (Shannon et al., 2016). Several authors have used the distance separating AD and FID (AD – FID) as a corrected flight distance (also termed Buffer Distance or Assessment Interval; Fernández-Juricic et al., 2002; Shannon et al., 2016; Tätte et al., 2019). However, calculating the corrected flight distance in this way gives an absolute number and does not relay the relationship between FID and AD. Therefore, a better way to adjust FID for AD is to look at the proportions (FID/AD). We used the FID/AD ratio as a corrected flight response measure. High FID/AD indicates a small difference between FID and AD, suggesting a fast escape after threat detection. In contrast, smaller FID/AD shows a larger difference between the two measures and, thus, a slower escape after threat detection.
SD and AD were root square transformed to approach a normal distribution. All continuous variables were scaled and centered. Pearson’s correlation coefficients were calculated to check the correlation among the SD and AD and SD and FID/AD measures.
Generalized Linear Mixed Models (hereafter GLMMs) were performed to assess the impact of ambient urban noise on the Eurasian Magpies’ AD and FID/AD. AD and FID/AD were the response variables, while the mean noise level was the predictor. The age of the individual (juvenile or adult) and the SD were also predictors, as they were previously demonstrated to impact the antipredator responses of birds (Blumstein, 2003; Kalb et al., 2019). Since all data was collected in Prague, we can assume a similar predation risk across the collected data. Additionally, the site (or park) was used as a random factor to reduce confounding variables (such as the variability among human activity in different sites and the type of vegetation cover), which may impact the antipredator behavior (Radvan et al., 2023).
Since most Eurasian Magpies sampled were alone or in very small flocks (93% of sampled individuals had two or fewer conspecifics nearby), we have not included the flock size in the models. Similarly, we have not included the human density around the sampled individuals since human density was similar across observations, with 75% of observations having five or fewer people within a 50 m radius around the sampled individual.
The models were fit using the R package “lme4” (Bates et al., 2014). The R package “lmerTest” was used to derive p-values using Satterthwarte’s degrees of freedom method (Kuznetsova et al., 2017). The Variation Inflation Factor (VIF) was calculated from the “car” package to assess multicollinearity among the predictors, but none was detected (all VIF < 5; Fox et al., 2007). Plots of residuals against fitted values were evaluated visually for further model validation. Cook’s distance values were used to detect influential observations, but none were found. The conditional R2 (the proportion of variance explained by fixed and random effects and marginal R2 (the ratio of variance presented by the fixed effects only) were calculated to explore the models’ performance using the r2 function from the ‘performance’ package of R (Lüdecke et al., 2021)
All analyses were performed using R software version 4.3.0 (R Core Team, 2022).
3 Results
Initially, we sampled 169 Eurasian Magpie individuals at 13 different sites. After removing sites with single observations, 167 individuals remained (138 adults and 29 juveniles) at 11 sites. On average, around 6 Eurasian Magpies were sampled in one session. Mean ambient sound levels ranged from 43.0 to 63.2 dBA, averaging 50.4 ± 5.1 (SD) dBA. Min noise ranged from 38.0 to 56.7 and averaged 44.9 ± 4.1 dBA. Max noise ranged from 45.6 to 77.0, averaging 55.9 ± 7.2 dBA. The average SD, AD, and FID/AD values for adults, juveniles, and all data are presented in Table 1.
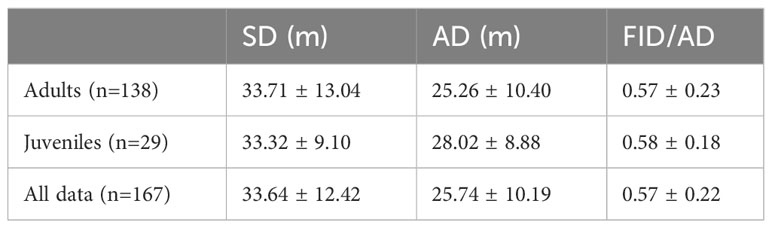
Table 1 The mean Starting Distance (SD), Alert Distance (AD), and Flight Initiation Distance (FID)/AD ± Standard Deviation for adults, juveniles, and all Eurasian Magpie individuals.
SD and AD were positively correlated (r(165) = 0.84, p-value < 0.001; Figure 1). SD was negatively correlated with the FID/AD ratio (r(165) = -0.37, p-value < 0.001; Figure 1).
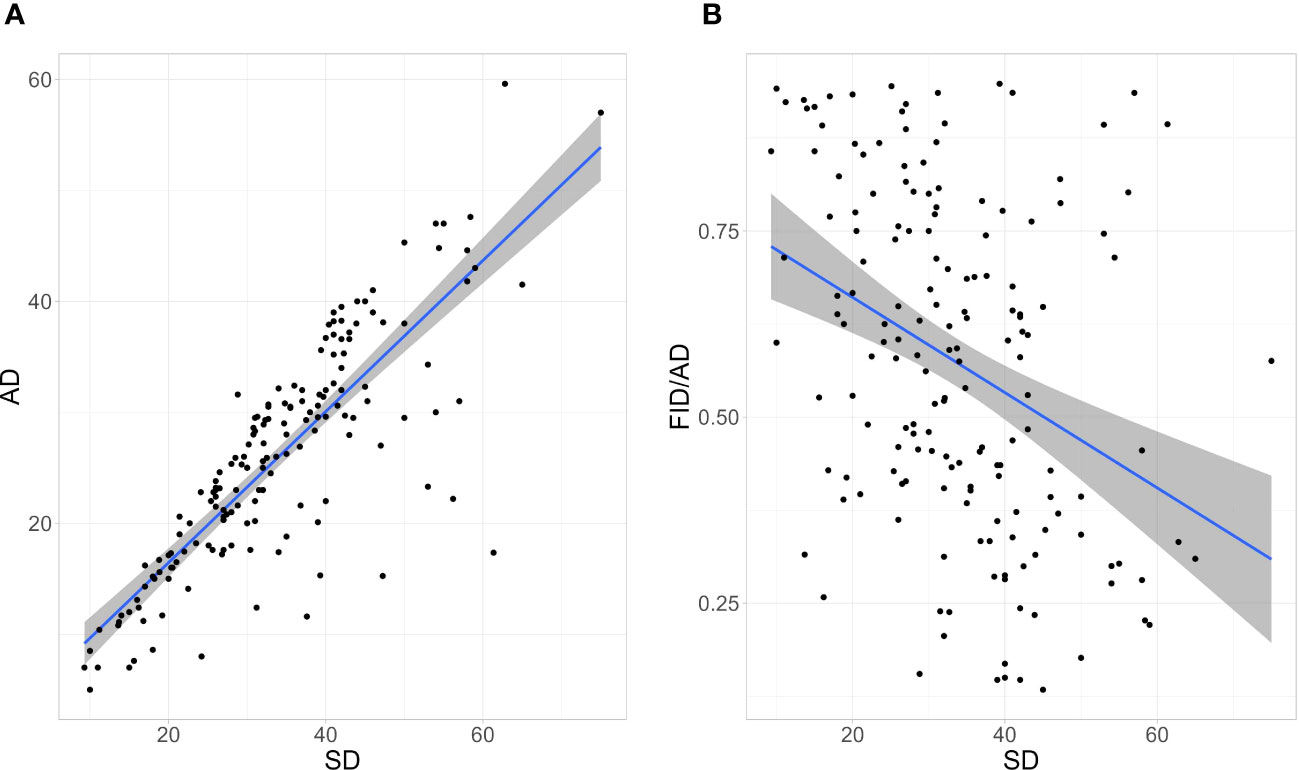
Figure 1 The correlation between (A) Alert Distance (AD) and Starting Distance (SD) and (B) SD and the Flight Initiation Distance/Alert Distance (FID/AD) ratio of Eurasian Magpies surveyed. Envelopes around linear regression lines represent the 95% Confidence Intervals. n = 167.
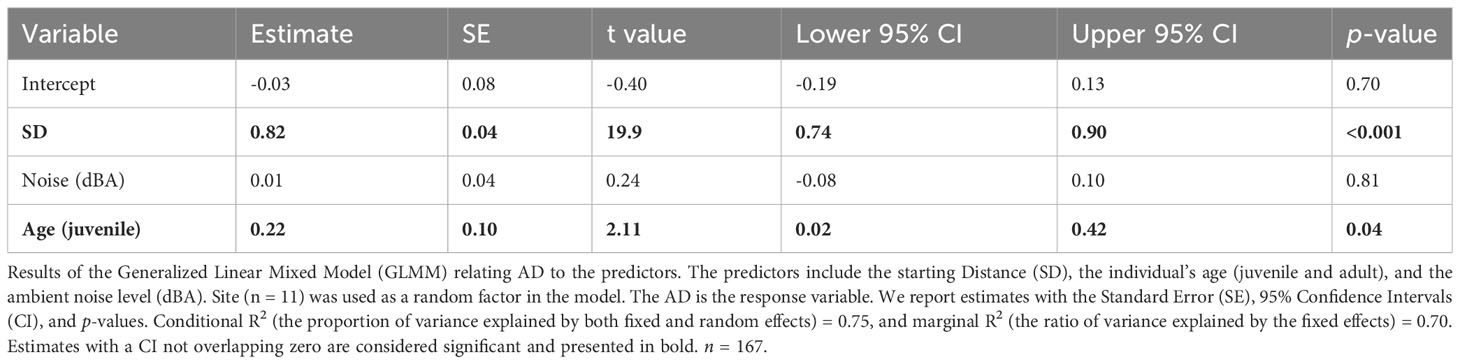
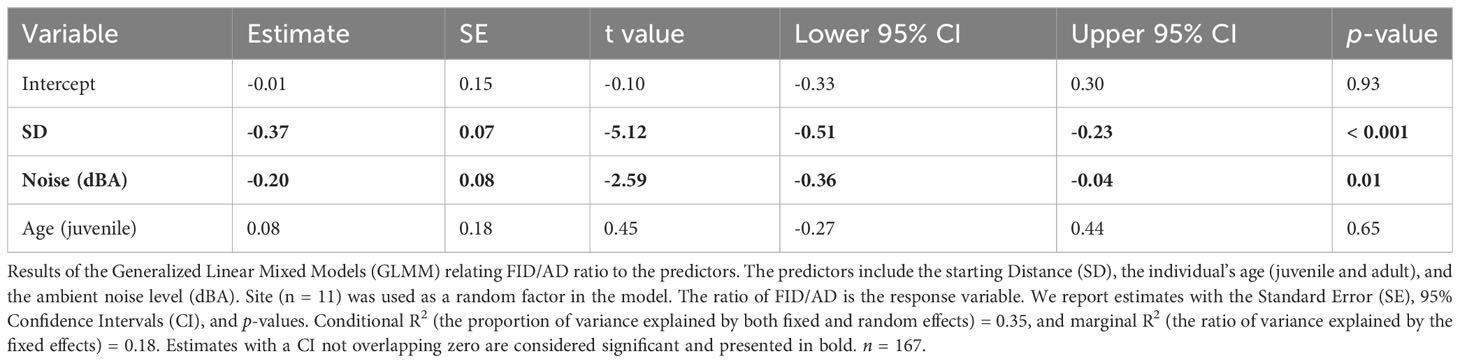
As for the results of the GLMMs the SD had a significant positive impact on the AD (Table 2); juveniles had significantly longer ADs than adults (Table 2). The noise level did not show a significant effect on the AD of the Eurasian Magpies (Table 2). The SD and noise levels had a significant negative effect on the FID/AD ratio, while the age did not seem to have any (Table 3; Figure 2).
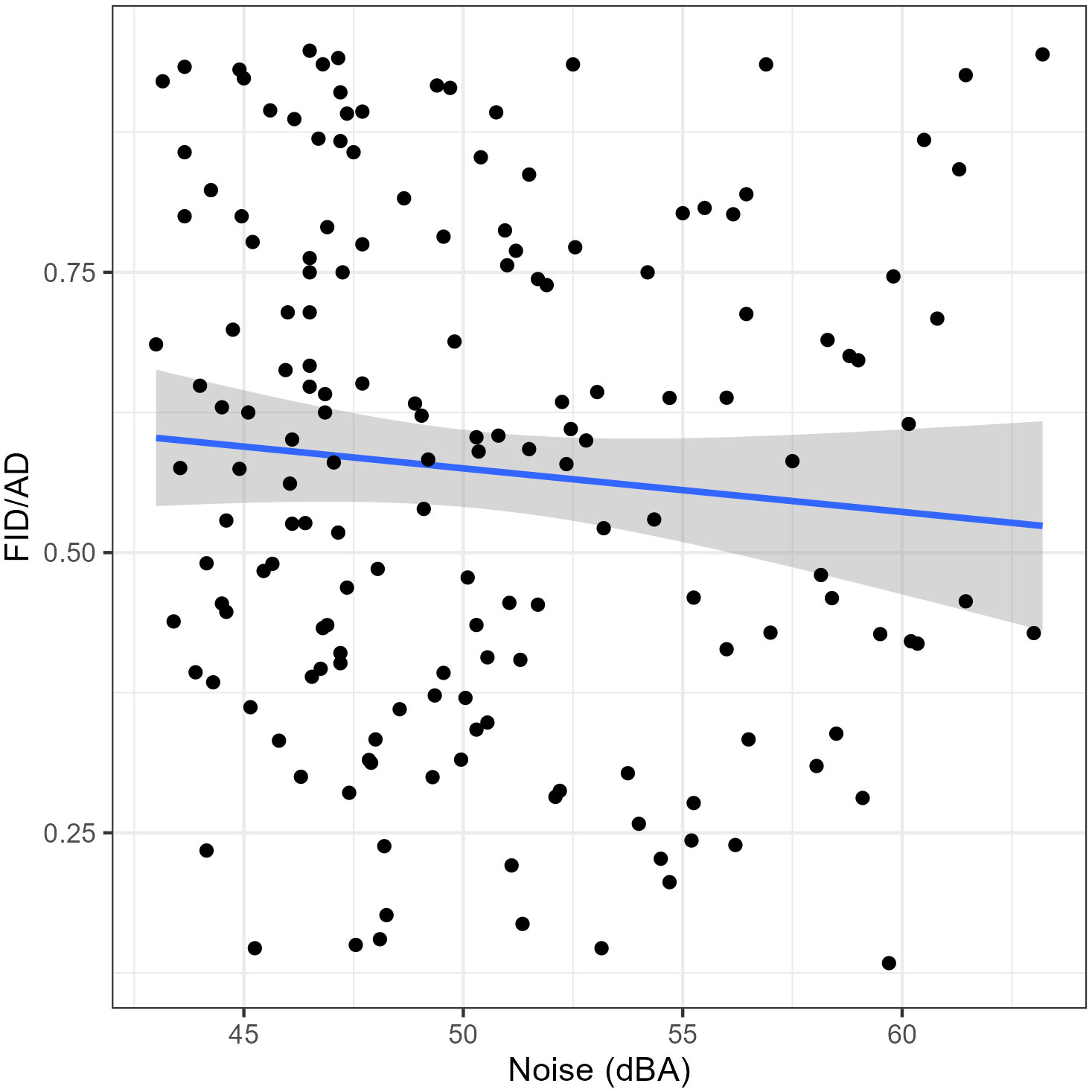
Figure 2 The association between the Flight Initiation Distance/Alert Distance (FID/AD) ratios and the noise level (dBA). The envelope around the linear regression line represents the 95% Confidence Interval. n = 167.
4 Discussion
Surviving predation attempts requires efficiently detecting predators and assessing their danger level (Lukas et al., 2021). Here, we studied the impacts of ambient urban noise on Eurasian Magpies’ alertness to predators and antipredator response through AD and FID/AD under a gradient of urban noise. We found no significant effect of noise on AD, but increased noise negatively impacted the Eurasian Magpies’ FID/AD ratios.
4.1 Noise and alertness
We found no impact of the ambient noise levels on the AD of the Eurasian Magpies surveyed. Several studies have shown that many birds (especially ground foraging species) spend more time vigilant and visually scanning for predators in noisier environments and less time feeding to compensate for their reduced hearing abilities (Quinn et al., 2006; Ware et al., 2015; Klett-Mingo et al., 2016; Partan, 2017; Evans et al., 2018). We had predicted that such an increase in vigilance might lead to a rise in the ADs of the Eurasian Magpies. Tätte et al. (2019) found that although birds in urban areas were more vigilant (assessed by the proxy of the head-raising behavior of birds), there was no correlation between the increase in vigilance and the detection of predators (AD). Some birds may be able to detect threats while foraging with their heads down due to their wide visual field, making them less reliant on sound cues, and thus, they may not need to compensate for reduced hearing by increasing vigilance (Lima and Bednekoff, 1999; Blumstein et al., 2004; Fernández-Juricic et al., 2004). However, this also means that those birds may detect a predator before displaying vigilant behavior or looking directly at it, which is the criteria for measuring AD (Blumstein et al., 2004). Therefore, we can not entirely deny the possible impact of noise pollution on the Eurasian Magpies’ capacity to detect danger. Still, there seems to be no variation in their displayed vigilant behavior. The noise may still impact the predator detection capacity of the Eurasian Magpies, but it could need larger samples to confirm or a different technique to assess (Blumstein et al., 2004).
4.2 Noise and escape
Although the Eurasian Magpies did not seem to have modified alertness, they responded more slowly to threats and had significantly smaller FID/AD ratios under noisier conditions. Meillère et al. (2015) found an opposite trend in breeding female House Sparrows (Passer domesticus). However, the birds were rural birds that were exposed to chronic noise for only two months, while in our study, the Eurasian Magpies surveyed are urban individuals that have been exposed to urban noise longer and have adapted to the presence of humans in urban parks and may view them as less of a threat than the rural House Sparrows. In addition, different species may react differently to noise. Another study found a negative correlation between the level of noise and the FID of ground foraging bird species, indicating a delayed escape, similar to our results, but found a different trend in flycatching and canopy-gleaning species (Petrelli et al., 2017). Therefore, the impact of noise pollution on the antipredator behavior of birds may be species-specific. A similar pattern to our study was discovered in a non-avian species, the Caribbean Hermit Crab (Coenobita clypeatus), which was slower to respond to simulated images of a silent predator when exposed to noise and the authors suggested the distracted prey hypothesis (Chan et al., 2010a; Chan et al., 2010b). Since individuals have limited attention, they must divide it among relevant stimuli and processes (i.e., foraging and vigilance; Dukas, 2004; Washburn and Taglialatela, 2006). Additional stimuli, such as anthropogenic noise, may distract an animal by causing it to involuntarily shift some of its limited attention to it and away from the relevant tasks at hand, which would increase its vulnerability to predation (Dukas, 2004; Chan et al., 2010b). Increased noise may also be related to increased traffic or human presence, which could create additional simultaneous visual distractions to which the bird may be diverting some of its attention. Here, we also measured the AD to confirm whether the impact of noise on the escape behavior can also reflect the alertness levels of the individuals. We found no noise effect on the Eurasian Magpies’ AD. Therefore, our results do not directly support the distracted prey hypothesis in terms of their capacity to detect the predator. The Eurasian Magpies studied may have taken longer to assess the level of threat the approaching person poses due to their attention being divided among several tasks and their brain processes being overwhelmed at increased noise levels. In this case, it could be that the noise is not interfering with their capacity to receive visual stimuli from the predator but rather distracting and slowing down their decision-making process or execution of their response (Dukas, 2002). Tätte et al. (2019) found that birds in urban areas were more vigilant but delayed their escape after detecting the threat and suggested that the increased distractions in urban areas, including noise, may explain their results (Chan et al., 2010b). Our study supports their suggestion as Eurasian Magpies took longer to assess threat as background noise increased.
Another non-mutually exclusive explanation could be that the background noise may be masking relevant auditory cues, such as the footfalls of the approaching predator (Barber et al., 2010; Zhou et al., 2019). Although the Eurasian Magpies still seem to detect the predator normally, their perception of auditory cues may be impaired under higher noise. Animals resort to multisensory integration to lessen environmental uncertainty (Munoz and Blumstein, 2012; Partan, 2017). Multisensory integration relies on different stimuli from several sensory modalities during decision-making, such as during antipredator behavior (Munoz and Blumstein, 2012). Stimuli are considered “redundant” if they lead to a similar response in the same direction (i.e., escaping the threat; Partan et al., 2009). When presented together, redundant stimuli interact, leading to three possible behaviors of the recipient: equivalence (response is not different from when stimuli are presented alone), enhancement (response is more intense), and antagonism (response is reduced; Partan and Marler, 2005; Munoz and Blumstein, 2012). At lower noise, the Eurasian Magpies would receive auditory and visual cues from the predator, which may lead to an enhanced response and cause them to flee faster from farther distances than Eurasian Magpies approached at noisier conditions. In other avian and non-avian studies, visual and auditory cues were shown to lead to an enhanced antipredator response. For example, Free-living Hoatzins (Opisthocomus hoazin) were more alert and escaped more quickly when approached by loud tourists than silent ones (Karp and Root, 2009). In addition, when combined, auditory and visual cues of predators instigated stronger and faster antipredator responses from a fish species when presented separately (Lukas et al., 2021), and wild squirrels’ response to conspecific’s alarm call was enhanced in the presence of both auditory and visual elements (Partan et al., 2009). In our study, higher level urban noise may have been masking auditory predator cues and, thus, decreasing the information received by the Eurasian Magpies even in the presence of normally perceived visual cues and preventing an enhanced response, which would explain the decreased FID/AD ratio at higher levels of noise while AD is unaffected. However, to confirm that combined auditory and visual predator cues cause an enhanced antipredator behavior in urban Eurasian Magpies, future experiments must follow the “multiple stimuli framework” proposed by Munoz and Blumstein (2012).
Finally, it is crucial to stress that while we made diligent efforts to control variables, the nature of our fieldwork study introduces the possibility of uncontrolled confounding variables. Consequently, the observed impact of noise on FID/AD may be influenced by other unaccounted-for variables that may have emerged during the fieldwork. We suggest future controlled settings where the researchers manipulate the noise levels to confirm better direct effects of noise on the antipredator behavior of Eurasian Magpies.
Here, we did not include human density in the models as we found a similar human activity across observations. We believe that human density was similar as we have visited the field under comparable weather conditions and times of the day. Additionally, we believe that any slight difference in human density across different parks would be accounted for within the random factor of the park.
4.3 Antipredator behavior and age
We found that juvenile Eurasian Magpies had significantly longer ADs than adults. Their FID/AD ratios were not significantly different, meaning that younger birds detect threats earlier but take a similar time to respond. Other studies found conflicting results between juvenile and adult antipredator behavior where some found that juveniles were more vigilant (similar to our results, i.e., de Jong et al., 2021; Mohring et al., 2022), others found the opposite to be true (i.e., Koch and Paton, 2014; Kalb et al., 2019), while some found no significant differences between the two age groups (i.e., Biondi et al., 2020). In some species, behavioral plasticity allows individuals to change their behavior across their lifetime based on different selective pressures (Petelle et al., 2013). In urban areas, juveniles may still have not habituated well to the increased presence of humans and may still be wearier of people than their adult counterparts. Eurasian Magpies may increase their tolerance to people throughout their lives. In urban areas where humans are increasingly present and generally harmless, birds would benefit from reducing their fear of humans to decrease energy loss and missed opportunities due to constant fleeing (Cooper and Frederick, 2007; Díaz et al., 2021). Alternatively, adults and juveniles may have different priorities while balancing the trade-off between vigilance and other activities, especially when adults are foraging for offsprings during the breeding season, such as when the experiment was conducted.
5 Conclusions
During the breeding season, we assessed the effects of urban noise pollution on the antipredator behavior of Eurasian Magpies in Prague. We found that noise may not interfere with Eurasian Magpies’ capacity to detect danger but increases their time to respond to it. We propose that the impact of noise on the escape behavior may not always reflect the same pattern in its capacity to detect the predator and suggest that future studies investigating the impact of noise on the escape behavior consider both aspects of the antipredator behavior. We also recommend future studies to compare different aspects of attention and to find the best proxies of predator detection (such as using telemetric eye trackers) since birds may detect the approaching person before displaying alertness to it, and AD may not always be a very precise measure of predator detection (Yorzinski and Platt, 2014; Tätte et al., 2019).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because no Eurasian Magpies were caught or trapped. All individuals studied were present in public parks or green areas. The AD and FID of foraging Eurasian Magpies were determined by slowly approaching them until they flushed (by jumping or flying away). The experiments only cause brief and minimal disturbance to the birds and are no different than the regular background disturbance urban birds face in public parks by other visitors. Thus, the field experiments comply with the current laws of the Czechia and require no special permits.
Author contributions
FA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YB: Conceptualization, Methodology, Supervision, Writing – review & editing. AS: Investigation, Writing – review & editing. FM: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The project was supported by IGA Faculty of Environmental Sciences CZU Prague “Investigating the impacts of urban noise pollution on the antipredator behavior of foraging corvids -No. 2022B0001”.
Acknowledgments
We would like to thank the two reviewers for their suggestions, which have greatly improved the quality of our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1345971/full#supplementary-material
References
Abou Zeid F., Morelli F., Ibáñez-Álamo J. D., Díaz M., Reif J., Jokimäki J., et al. (2023). Spatial overlap and habitat selection of corvid species in european cities. Animals 13, 1192. doi: 10.3390/ani13071192
Barber J. R., Crooks K. R., Fristrup K. M. (2010). The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189. doi: 10.1016/j.tree.2009.08.002
Bates D., Maechler M., Bolker B., Walker S. (2014). lme4: Linear mixed-effects models using Eigen and S4. R Packageversion 1. 1–4. Available at: https://cran.r-project.org/web/packages/lme4/index.html.
Benmazouz I., Jokimäki J., Lengyel S., Juhász L., Kaisanlahti-Jokimäki M.-L., Kardos G., et al. (2021). Corvids in urban environments: A systematic global. Animals 11 (11), 1–24. doi: 10.3390/ani11113226
Biondi L. M., Fuentes G. M., Córdoba R. S., Bó M. S., Cavalli M., Paterlini C. A., et al. (2020). Variation in boldness and novelty response between rural and urban predatory birds: The Chimango Caracara, Milvago chimango as study case. Behav. Processes 173, 104064. doi: 10.1016/j.beproc.2020.104064
Blumstein D. T. (2003). Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl Manage 67, 852–857. doi: 10.2307/3802692
Blumstein D. T., Fernandez-Juricic E., Ledeeà O., Larsen E., Rodriguez-Prieto I., Zugmeyer C. (2004). Avian risk assessment: effects of perching height and detectability. Ethology 110, 273–285. doi: 10.1111/j.1439-0310.2004.00970.x
Blumstein D. T., Fernández-Juricic E., Zollner P. A., Garity S. C. (2005). Inter-specific variation in avian responses to human disturbance. J. Appl. Ecol. 42, 943–953. doi: 10.1111/j.1365-2664.2005.01071.x
Catchpole C. K., Slater P. J. B. (2003). Bird song: biological themes and variations (Cambridge, United Kingdom: Cambridge university press).
Chan A. A. Y. H., David Stahlman W., Garlick D., Fast C. D., Blumstein D. T., Blaisdell A. P. (2010a). Increased amplitude and duration of acoustic stimuli enhance distraction. Anim. Behav. 80, 1075–1079. doi: 10.1016/j.anbehav.2010.09.025
Chan A. A. Y. H., Giraldo-Perez P., Smith S., Blumstein D. T. (2010b). Anthropogenic noise affects risk assessment and attention: The distracted prey hypothesis. Biol. Lett. 6, 458–461. doi: 10.1098/rsbl.2009.1081
Ciebiera O., Czechowski P., Morelli F., Piekarski R., Bocheński M. (2021). Selection of urbanized areas by magpie pica pica in a medium size city in Poland. Animals 11, 1738. doi: 10.3390/ani11061738
Cooper W. E., Frederick W. G. (2007). Optimal flight initiation distance. J. Theor. Biol. 244, 59–67. doi: 10.1016/j.jtbi.2006.07.011
Croci S., Butet A., Clergeau P. (2008). Does urbanization filter birds on the basis of their biological traits? Condor 110, 223–240. doi: 10.1525/cond.2008.8409
de Jong M. E., Nicolaus M., Fokkema R. W., Loonen M. J. J. E. (2021). State dependence explains individual variation in nest defence behaviour in a long-lived bird. J. Anim. Ecol. 90, 809–819. doi: 10.1111/1365-2656.13411
Díaz M., Grim T., Markó G., Morelli F., Alamo J. D. I., Jokimäki J. (2021). Effects of climate variation on bird escape distances modulate community responses to global change. Sci. Rep. 1–10, 12826. doi: 10.1038/s41598-021-92273-1
Dukas R. (2002). Behavioural and ecological consequences of limited attention. Philos. Trans. R. Soc. B: Biol. Sci. 357, 1539–1547. doi: 10.1098/rstb.2002.1063
Dukas R. (2004). Causes and consequences of limited attention. Brain Behav. Evol. 63, 197–210. doi: 10.1159/000076781
Evans J. C., Dall S. R. X., Kight C. R. (2018). Effects of ambient noise on zebra finch vigilance and foraging efficiency. PloS One 13, e0209471. doi: 10.1371/journal.pone.0209471
Fernández-Juricic E., Erichsen J. T., Kacelnik A. (2004). Visual perception and social foraging in birds. Trends Ecol. Evol. 19, 25–31. doi: 10.1016/j.tree.2003.10.003
Fernández-Juricic E., Jimenez M. D., Lucas E. (2001). Alert distance as an alternative measure of bird tolerance to human disturbance: Implications for park design. Environ. Conserv. 28, 263–269. doi: 10.1017/S0376892901000273
Fernández-Juricic E., Jimenez M. D., Lucas E. (2002). Factors affecting intra- and inter-specific variations in the difference between alert distances and flight distances for birds in forested habitats. Can. J. Zool 80, 1212–1220. doi: 10.1139/z02-104
Fox J., Friendly G. G., Graves S., Heiberger R., Monette G., Nilsson H., et al. (2007). The car package Vol. 1109 (Vienna, Austria: R Foundation for Statistical Computing), 1431.
Francis C. D., Ortega C. P., Cruz A. (2009). Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419. doi: 10.1016/j.cub.2009.06.052
analysisGravolin I., Key M., Lill A. (2014). Boldness of urban Australian magpies and local traffic volume. Avian Biol. Res. 7, 244–250. doi: 10.3184/175815514X14151981691872
Hollén L. I., Radford A. N. (2009). The development of alarm call behaviour in mammals and birds. Anim. Behav. 78, 791–800. doi: 10.1016/j.anbehav.2009.07.021
Hu Y., Cardoso G. C. (2009). Are bird species that vocalize at higher frequencies preadapted to inhabit noisy urban areas? Behav. Ecol. 20, 1268–1273. doi: 10.1093/beheco/arp131
Kalb N., Anger F., Randler C. (2019). Flight initiation distance and escape behavior in the black redstart (Phoenicurus ochruros). Ethology 125, 430–438. doi: 10.1111/eth.12867
Karp D. S., Root T. L. (2009). Sound the stressor: How Hoatzins (Opisthocomus hoazin) react to ecotourist conversation. Biodivers Conserv. 18, 3733–3742. doi: 10.1007/s10531-009-9675-6
Kern J. M., Radford A. N. (2016). Anthropogenic noise disrupts use of vocal information about predation risk. Environ. pollut. 218, 988–995. doi: 10.1016/j.envpol.2016.08.049
Kight C. R., Swaddle J. P. (2011). How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14, 1052–1061. doi: 10.1111/ele.2011.14.issue-10
Klett-Mingo J. I., Pavón I., Gil D. (2016). Great tits, Parus major, increase vigilance time and reduce feeding effort during peaks of aircraft noise. Anim. Behav. 115, 29–34. doi: 10.1016/j.anbehav.2016.02.021
Koch S. L., Paton P. W. C. (2014). Assessing anthropogenic disturbances to develop buffer zones for shorebirds using a stopover site. J. Wildlife Manage. 78, 58–67. doi: 10.1002/jwmg.631
Kuznetsova A., Brockhoff P. B., Christensen R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw 82 (13), 1–26. doi: 10.18637/jss.v082.i13
Lima S. L., Bednekoff P. A. (1999). Back to the basics of antipredatory vigilance: can nonvigilant animals detect attack? Anim. Behav. 58, 537–543. doi: 10.1006/anbe.1999.1182
Lüdecke D., Ben-Shachar M. S., Patil I., Waggoner P., Makowski D. (2021). performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw 6 (60), 3139. doi: 10.21105/joss.03139
Lukas J., Romanczuk P., Klenz H., Klamser P., Arias Rodriguez L., Krause J., et al. (2021). Acoustic and visual stimuli combined promote stronger responses to aerial predation in fish. Behav. Ecol. 32, 1094–1102. doi: 10.1093/beheco/arab043
Marzluff J. M. (2008). Island biogeography for an urbanizing world how extinction and colonization may determine biological diversity in human-dominated landscapes. In Marzluff J. M., et al. Urban Ecosyst. Boston, MA: Springer. doi: 10.1007/978-0-387-73412-5_23
Meillère A., Brischoux F., Angelier F. (2015). Impact of chronic noise exposure on antipredator behavior: An experiment in breeding house sparrows. Behav. Ecol. 26, 569–577. doi: 10.1093/beheco/aru232
Merrall E. S., Evans K. L. (2020). Anthropogenic noise reduces avian feeding efficiency and increases vigilance along an urban–rural gradient regardless of species' tolerances to urbanisation. J. Avian Biol. 51, 1–8. doi: 10.1111/jav.02341
Mohring B., Angelier F., Jaatinen K., Steele B., Lönnberg E., Öst M. (2022). Drivers of within-and among-individual variation in risk-taking behaviour during reproduction in a long-lived bird. Proc. R. Soc. B 289, 20221338. doi: 10.1098/rspb.2022.1338
Møller A. P. (2008). Flight distance of urban birds, predation, and selection for urban life. Behav. Ecol. Sociobiol 63, 63–75. doi: 10.1007/s00265-008-0636-y
Møller A. P. (2021). Risk-taking behaviour as a central concept in evolutionary biology. World at Our Fingertips, 301–314. doi: 10.1093/oso/9780198851738.003.0017
Morelli F., Benedetti Y., Díaz M., Grim T., Ibáñez-Álamo J. D., Jokimäki J., et al. (2019). Contagious fear: Escape behavior increases with flock size in European gregarious birds. Ecol. Evol. 9, 6096–6104. doi: 10.1002/ece3.5193
Morelli F., Mikula P., Blumstein D. T., Díaz M., Markó G., Jokimäki J., et al. (2022). Flight initiation distance and refuge in urban birds. Sci. Total Environ. 842, 156939. doi: 10.1016/j.scitotenv.2022.156939
Munoz N. E., Blumstein D. T. (2012). Multisensory perception in uncertain environments. Behav. Ecol. 23, 457–462. doi: 10.1093/beheco/arr220
Partan S. R. (2017). Multimodal shifts in noise: switching channels to communicate through rapid environmental change. Anim. Behav. 124, 325–337. doi: 10.1016/j.anbehav.2016.08.003
Partan S. R., Larco C. P., Owens M. J. (2009). Wild tree squirrels respond with multisensory enhancement to conspecific robot alarm behaviour. Anim. Behav. 77, 1127–1135. doi: 10.1016/j.anbehav.2008.12.029
Partan S. R., Marler P. (2005). Issues in the classification of multimodal communication signals. Am. Nat. 166, 231–245. doi: 10.1086/431246
Petelle M. B., McCoy D. E., Alejandro V., Martin J. G. A., Blumstein D. T. (2013). Development of boldness and docility in yellow-bellied marmots. Anim. Behav. 86, 1147–1154. doi: 10.1016/j.anbehav.2013.09.016
Petrelli A. R., Levenhagen M. J., Wardle R., Barber J. R., Francis C. D. (2017). First to flush: The effects of ambient noise on songbird flight initiation distances and implications for human experiences with nature. Front. Ecol. Evol. 5. doi: 10.3389/fevo.2017.00067
Price M. (2008). The impact of human disturbance on birds: A selective review. Aust. Zoologist 34, 163–196. doi: 10.7882/fs.2008.023
Quinn J. L., Whittingham M. J., Butler S. J., Cresswell W. (2006). Noise, predation risk compensation and vigilance in the chaffinch fringilla coelebs. J. Avian Biol. 37, 601–608. doi: 10.1111/j.2006.0908-8857.03781.x
Radvan M., Rendall A. R., Weston M. A. (2023). The habitat connectivity hypothesis of escape in urban woodland birds. Behav. Ecology 34 (2), 297–305. doi: 10.1093/beheco/arac127
R Core Team. (2022). R: A language and environment for statistical computing. Available online at: https://www.R-project.org/.
Šálek M., Grill S., Riegert J. (2020). Nest-site selection of an avian urban exploiter , the Eurasian magpie Pica pica , across the urban-rural gradient. J. Vertebr Biol. 70 (1), 20086.11. doi: 10.25225/jvb.20086
Shannon G., Crooks K. R., Wittemyer G., Fristrup K. M., Angeloni L. M. (2016). Road noise causes earlier predator detection and flight response in a free-ranging mammal. Behav. Ecol. 27, 1370–1375. doi: 10.1093/beheco/arw058
Slabbekoorn H., Ripmeester E. A. P. (2008). Birdsong and anthropogenic noise: Implications and applications for conservation. Mol. Ecol. 17, 72–83. doi: 10.1111/j.1365-294X.2007.03487.x
Sordello R., Ratel O., de Lachapelle F. F., Leger C., Dambry A., Vanpeene S. (2020). Evidence of the impact of noise pollution on biodiversity: A systematic map. Environ. Evid 9, 1–27. doi: 10.1186/s13750-020-00202-y
Sun J. W. C., Narins P. M. (2005). Anthropogenic sounds differentially affect amphibian call rate. Biol. Conserv. 121, 419–427. doi: 10.1016/j.biocon.2004.05.017
Tätte K., Ibáñez-Álamo J. D., Markó G., Mänd R., Møller A. P. (2019). Antipredator function of vigilance re-examined: vigilant birds delay escape. Anim. Behav. 156, 97–110. doi: 10.1016/j.anbehav.2019.08.010
Tryjanowski P., Møller A. P., Morelli F., Biaduń W., Brauze T., Ciach M., et al. (2016). Urbanization affects neophilia and risk-taking at bird-feeders. Sci. Rep. 6, 28575. doi: 10.1038/srep28575
Ware H. E., McClure C. J. W., Carlisle J. D., Barber J. R., Daily G. C. (2015). A phantom road experiment reveals traffic noise is an invisible source of habitat degradation. Proc. Natl. Acad. Sci. U.S.A. 112, 12105–12109. doi: 10.1073/pnas.1504710112
Warren P. S., Katti M., Ermann M., Brazel A. (2006). Urban bioacoustics: It's not just noise. Anim. Behav. 71, 491–502. doi: 10.1016/j.anbehav.2005.07.014
Washburn D. A., Taglialatela L. A. (2006). “Attention as it is manifest across species,” in Comparative cognition: Experimental explorations of animal intelligence (Oxford, United Kingdom: Oxford University Press), 127–142. doi: 10.1093/acprof:oso/9780195377804.003.0008
Xu Y., Cao Z., Wang B. (2020). Effect of urbanization intensity on nest-site selection by Eurasian Magpies (Pica pica). Urban Ecosyst. 23, 1099–1105. doi: 10.1007/s11252-020-00996-2
Yorzinski J. L., Platt M. L. (2014). Selective attention in peacocks during predator detection. Anim. Cognit. 17, 767–777. doi: 10.1007/s10071-013-0708-x
Keywords: flight initiation distance, alert distance, Pica pica, alertness, escape behavior, threat detection, vigilance
Citation: Abou-Zeid F, Benedetti Y, Siretckaia A and Morelli F (2024) Urban noise slows down the antipredator reaction of Eurasian Magpies. Front. Ecol. Evol. 12:1345971. doi: 10.3389/fevo.2024.1345971
Received: 28 November 2023; Accepted: 06 March 2024;
Published: 20 March 2024.
Edited by:
Zoltan Elek, University of Szeged, HungaryReviewed by:
Oded Berger-Tal, Ben-Gurion University of the Negev, IsraelFelipe N. Moreno-Gómez, Universidad Católica del Maule, Chile
Copyright © 2024 Abou-Zeid, Benedetti, Siretckaia and Morelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farah Abou-Zeid, ZmFyYWguYWJvdXplaWQyMUBnbWFpbC5jb20=
 Farah Abou-Zeid
Farah Abou-Zeid Yanina Benedetti
Yanina Benedetti Anastasiia Siretckaia
Anastasiia Siretckaia Federico Morelli
Federico Morelli