
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol., 12 March 2024
Sec. Behavioral and Evolutionary Ecology
Volume 12 - 2024 | https://doi.org/10.3389/fevo.2024.1321115
This article is part of the Research TopicHuman Upright Walking From Past to PresentView all 11 articles
Investigations into the role of selection in the origin of human bipedalism using ape models have relied heavily on behavioral frequency data. However, analysis of video of wild apes has the advantage of capturing the details of the entirety of each rare, brief bipedal bout witnessed, not just the moment detected in observational studies. We used video to explore the behavioral context and effects of several variables on bipedalism across all ages in wild forest-dwelling chimpanzees from Ngogo, Uganda. We found, as in earlier studies, that adult chimpanzees used bipedalism in the context of foraging; however, unlike earlier studies, we found that while foraging was the predominant behavioral context during arboreal bipedalism, terrestrial bipedalism was more varied in contextual composition. We also found that these different behavioral contexts of bipedalism were associated with different variables. Specifically, foraging was associated with arboreality, hand assistance, and adulthood; antagonism was associated with adulthood, locomotion, and males; play was associated with terrestriality and subadulthood; and travel was associated with locomotion and females. Given that several variables influence bipedalism across multiple behavioral contexts in chimpanzees, it is likely that the early evolution of human bipedalism occurred under the influence of numerous factors. This exploratory study thus suggests that more comprehensive models should be used when reconstructing the transition to bipedalism from the Last Common Ancestor of humans and chimpanzees.
Bipedality is the hallmark of the human lineage and the origins of this behavior have often been investigated through behavioral ecological modeling of our closest living relatives, chimpanzees. Like other apes, chimpanzees navigate their habitat using a range of versatile positional behaviors that are critically linked to an upright (orthograde) trunk that enables highly mobile limbs to be used differentially, and bear weight in a variety of orientations (MacLatchy et al., 2023). One such orthograde behavior is bipedalism, which chimpanzees perform facultatively on arboreal and terrestrial substrates. As terrestrial travelers and frequent arboreal feeders who regularly move between these substrates, chimpanzees represent an ideal taxon to investigate the behavioral context of bipedality on both substrates.
Although the degree to which early hominins used arboreal substrates even after becoming adapted to terrestrial bipedality is debated (e.g., Susman et al., 1984; Latimer et al., 1987; Ward, 2002; Kappelman et al., 2016; Carlson et al., 2021), it is well accepted that the origin of terrestrial bipedalism must have involved the use of both arboreal and terrestrial substrates. The feasibility of engagement in both arboreal and terrestrial positional behaviors is supported by reconstructions of the environments of early bipeds, which include forest, woodland and savannah elements (e.g., Behrensmeyer and Reed, 2013; Domínguez-Rodrigo, 2014). It is reasonable to conjecture that locomotor versatility also characterized the human-chimpanzee Last Common Ancestor (LCA; MacLatchy et al., 2023), but postcranial fossil evidence from the relevant time period ~7-8 million years ago (Langergraber et al., 2012; Steiper and Seiffert, 2012) is sparse (Daver et al., 2022). Nonetheless, exploration of the behavioral contexts and conditions associated with chimpanzee bipedality could give insight into the factors that prompted overall locomotor versatility to be abandoned in favor of almost exclusive use of bipedalism.
Hypotheses related to the contextual origins of habitual bipedality are numerous. A (non-exhaustive) list includes travel (Rodman and McHenry, 1980), increased visibility (Dart, 1959), foraging and feeding (Du Brul, 1962; Jolly, 1970; Rose, 1974, 1976; Hunt, 1994, 1996; Wrangham, 1980), freeing the hands for carrying or tool use (Darwin, 1871; Etkin, 1954; Hewes, 1961; Washburn, 1967; Zihlman and Tanner, 1978; Tanner, 1981; Videan and McGrew, 2002; Carvalho et al., 2012) and aggression (including fight and display; Livingstone, 1962; Jablonski and Chaplin, 1993; Carrier, 2011). These five behavioral contexts are also associated with chimpanzee bipedalism, and so can be investigated in the wild. Other hypotheses, such as those involving thermoregulatory adaptation (Wheeler, 1984; Wackerly, 2019, 1991) were not amenable to this study’s project design, and so are not considered.
Kevin Hunt pioneered the use of behavioral frequency data from wild chimpanzees to generate hypotheses about the origins of bipedality. Hunt (1994, 1996, 1998) observed 97 instances (93 posture, 4 locomotion) of bipedality in 700 hours of observation on wild, forest-dwelling adult chimpanzees. Hunt (1996, 1998) found that overall, postural (stationary) bipedalism was in the context of foraging (80%), particularly while feeding on small items; occurred both arboreally (61%) and terrestrially (39%); and was hand assisted (60%). Hunt (1996, 1998) extended these findings to hominins, and concluded that postural bipedalism evolved to feed on small objects both arboreally and terrestrially. This foraging hypothesis has been further supported by behavioral frequency data from chimpanzees living in a drier habitat. In a study by Drummond-Clarke et al. (2022), 109 bipedal observations were made, and as in Hunt’s work, were predominantly postural (79% postural vs. 21% locomotor). Also as in Hunt’s work, bipedality occurred in a foraging context, and was overwhelmingly arboreal (86% of observations), despite trees being less available than in Hunt’s studies (Drummond-Clarke et al., 2022). These findings were thus viewed as strengthening hypotheses associating bipedal origins with forested habitats (Drummond-Clarke et al., 2022).
Chimpanzees are not the only ape for whom bipedalism has been examined using observational data. Thorpe and colleagues (2006, 2007) examined positional behavior in wild orangutans with a focus on locomotor bipedalism (N = 10 individuals with ~196 bouts) and found that bipedalism was associated with hand assistance on arboreal substrates using multiple, small diameter supports. These findings were used to develop the hypothesis that bipedal locomotion in humans may have evolved under these specific arboreal circumstances (Crompton et al., 2010). While this study added important comparative breadth, terrestrial contexts could not be included because of the rarity with which orangutans descend to the ground.
The present study includes the first substantial dataset to analyze multiple variables associated with the behavioral contexts of bipedal engagement in chimpanzees for both postural and locomotor bipedalism. We take the view that behavioral frequencies from extant primates, while fundamental in reconstructing extinct primate behavior (e.g., Rein et al., 2011; Hunt, 2016; Wuthrich et al., 2019), should not be the sole criterion used in assessing adaptive commitment in studies of locomotor evolution. Video analysis allows for the capture of all bipedal bouts witnessed by the investigator. This approach thus includes the bouts which can be feasibly documented during real time observations using the behavioral frequency methods discussed above. However, video records have the added advantage of including the short, hard to capture bouts that require repeated scrutiny of video frame-by-frame and that may be missed in real-time observations. Further, by ‘keeping the camera rolling’, brief, rare behaviors are more likely to be included in the data that is collected from this method compared to behavioral frequency methods.
In addition, while sex differences in bipedal frequencies have been investigated, this study is the first to examine bipedality in both adult and subadult apes. We thus include play as a behavioral context, along with travel, visibility, feeding/foraging and aggression. This study is also the first to examine the interplay between the variables of sex, substrate, age, hand assistance, and mode with regard to different bipedal contexts.
Ngogo, Kibale National Park, Uganda is a mid-altitude old growth rainforest with patches of regenerating secondary forest and grassland (Struhsaker, 1997; Lwanga, 2003). Chimpanzees primarily use the old growth rainforest. Video footage was captured using Canon XF400 camcorders set at 4K 60 fps from January to February 2020 by LS and LM resulting in the 31.8 hours of positional video used in this study. Video footage was obtained ad-libitum when there was a clear view of any individual(s) with minimal obstruction from vegetation with the exception of long resting bouts. An individual was then followed until they went out of view and there was no expectation the individuals would be visible again. The next closest individual was then recorded.
Video was examined frame by frame to capture all bouts of bipedal behavior where the weight distribution was clearly visible for all limbs, the individuals could be identified, and the context of the behavior could be clearly discerned either from narration of the video by the videographer or the video itself. Application of these criteria to our raw dataset resulted in 425 usable bouts, and sampled 106 individuals. At the time of the filming, the Ngogo study group consisted of ~200 individuals in two adjacent communities.
Bipedalism is defined as a positional behavior in which the body weight is borne primarily by the hindlimbs with no significant contribution from the forelimbs (i.e., not bearing more than their own weight) with the torso orientated from approximately 45 - 90 degrees (i.e., not pronograde) and the hip above the knees (i.e., so as not to include other orthograde modes such as squat; modified from Hunt et al., 1996). Bipedality in this study refers to both postural (stationary) and locomotor (movement with a change in location) modes. After we identified bipedality in the video footage, we collected the following variables: subject ID, sex, age, behavioral context, substrate height, mode (whether it was postural or locomotor), whether hands were used for assistance, and whether an object was being carried (Figure 1; Table 1).
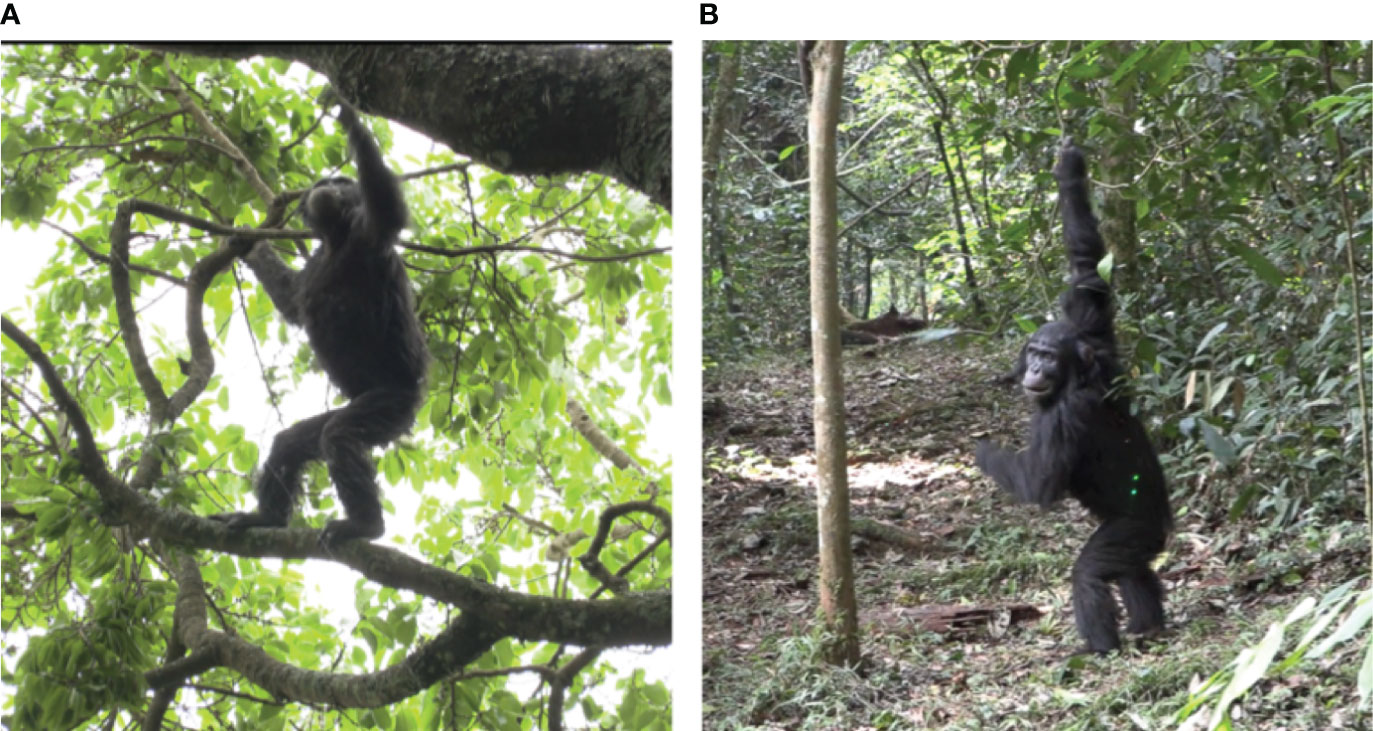
Figure 1 Bipedal chimpanzees at Ngogo, Kibale National Park, Uganda. (A) Bipedal chimpanzee on arboreal substrate in context of travel; (B) Bipedal chimpanzee on terrestrial substrate in context of feed/forage.
In order to investigate which variables (sex, substrate, age, hand assistance, mode) are associated with each behavioral context in which bipedalism was observed, we generated separate models for each context (foraging, antagonism, travel, play and visibility). We constructed binary logistic models in a generalized estimating equation context for the binary outcomes of each categorical outcome. The generalized estimating models were clustered by subject with an exchangeable working correlation matrix. Predictor variables were the categorical variables of sex, substrate, hand assistance, mode, and the covariate variable age. All models used SPSS version 28 and 29. As this is an exploratory study (Tukey, 1977), a correction for multiple comparisons is not warranted as it can lead to Type II errors (Jaeger and Halliday, 1998). Variables were considered to be significantly associated with a behavioral context when p < 0.050.
When all bipedal bouts were examined, feeding/foraging was the most prevalent behavioral context, occurring in 32.7% of the 425 bipedal bouts (Table 1). However, as illustrated in Figure 2, when data are assessed by age and by substrate (arboreal vs. terrestrial), behavioral context varied (Figure 2). Feeding/foraging was still the most prevalent behavioral context for both adults and subadults when arboreal. However, the effect of behavioral context on terrestrial bipedality was more varied, with visibility, play and antagonistic interactions all having increased occurrence. In the case of subadults, play was the predominant context of terrestrial bipedality (Figure 2).
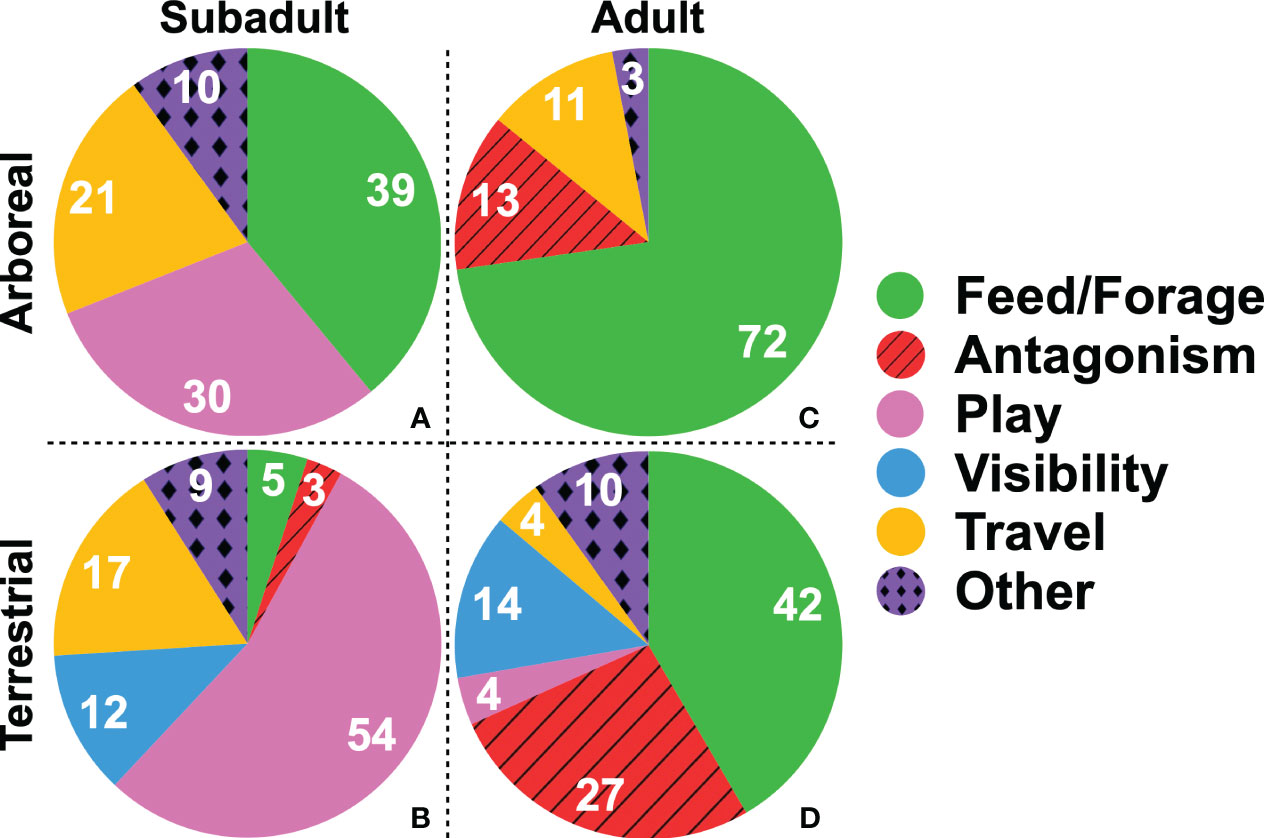
Figure 2 Contextual breakdown of bipedalism. The numbers in each piece of the pies indicated the total number of observations. (A) arboreal subadults; (B) terrestrial subadults; (C) arboreal adults; (D) terrestrial adults. Adults in this figure refer to individuals over 10, which includes adolescents as adolescent and adult positional behavior patterns do not differ statistically (Sarringhaus et al., 2014).
Bipedality in a foraging/feeding context was positively associated with hand assistance, arboreality, and increased age (hand assistance odds ratio = 1.990 (95% C.I. 1.101 – 3.597), p = 0.023; substrate odds ratio = 5.002 (95% C.I. 2.459 – 10.255), p < 0.001; age odds ratio = 1.065 (95% C.I 1.034 – 1.1097), p < 0.001; sex and mode p > 0.050, Figures 3; Supplementary Table S1).
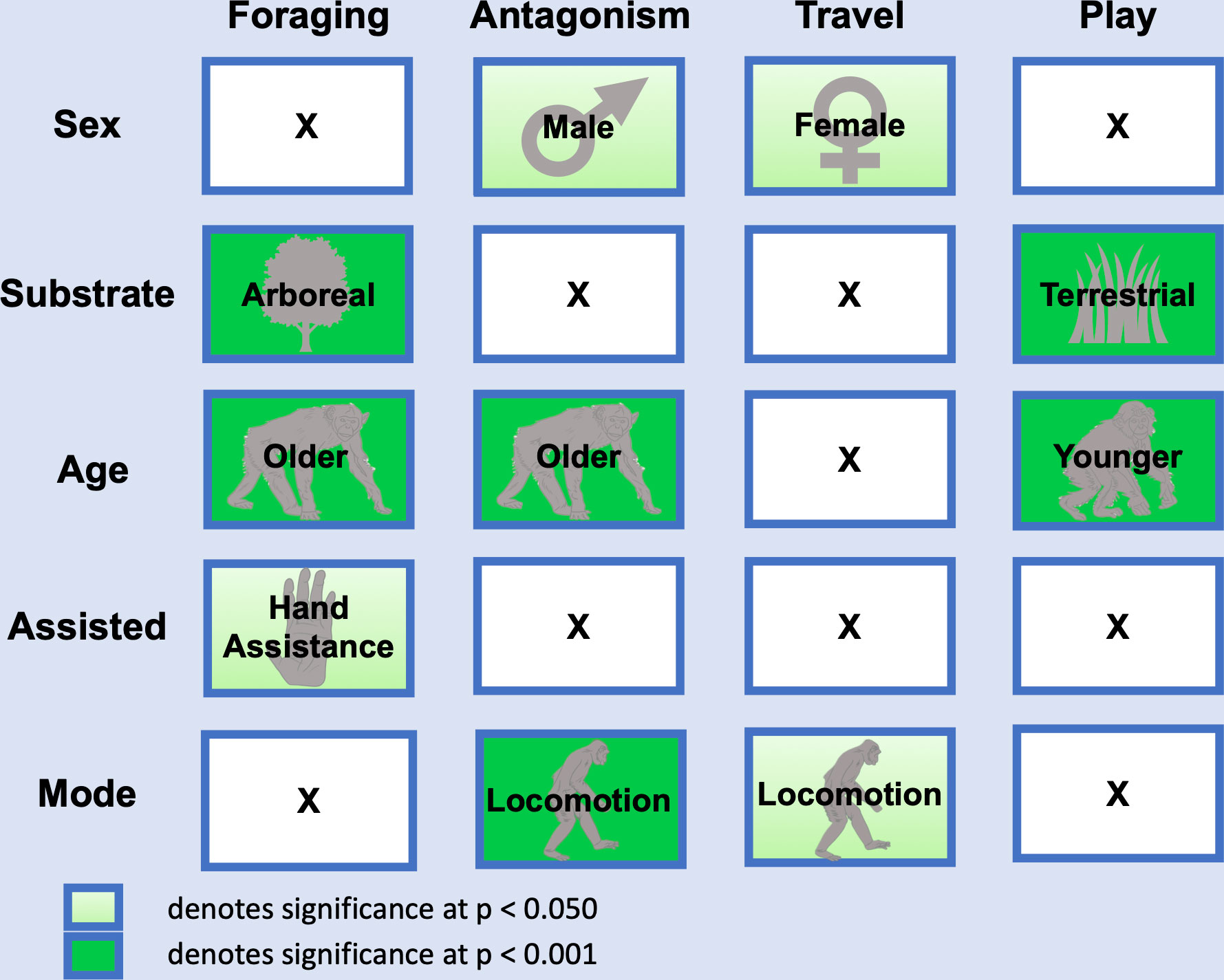
Figure 3 Generalized estimating equations model results for four contexts of bipedality. Context categories are on the top x axis (foraging, antagonism, travel, play) and variables are on the y axis (sex, substrate, age, assisted, mode). Variable category displayed is positively associated. S. Tucker created the category icons for older, younger, hand assistance, and locomotion.
Bipedality in an antagonistic context was positively associated with males, locomotion, and increased age (sex odds ratio 0.316 (95% C.I. 0.108 – 0.927, p = 0.036; mode odds ratio 9.600 (95% C.I. 3.560 – 25.890), p < 0.001; age odds ratio 1.070 (95% C.I. 1.041 – 1.099), p < 0.001; hand assistance and substrate p >0.050; Figures 3; Supplementary Table S1).
Bipedality in a travel context was positively associated with females and locomotion (sex odds ratio 1.932 (95% C.I. 1.053 – 3.545), p < 0.034; mode odds ratio 2.206 (95% C.I. 1.241 – 3.922), p = 0.007; hand assistance, substrate, and age p >0.050; Figures 3; Supplementary Table S1).
Bipedality in a play context was positively associated with terrestriality and negatively associated with age (substrate odds ratio 0.320 (95% C.I. 0.176 – 0.583), p < 0.001; age odds ratio 0.782 (95% C.I. 0.728 – 0.840), p < 0.001; sex, mode, and hand assistance p > 0.050; Figures 3; Supplementary Table S1).
Due to low occurrence, we were unable to analyze visibility or carrying in our behavioral context models (Table 1).
The video-based approach used in this study facilitated the collection of the largest sample of bipedal instances yet documented for any ape. It is apparent from this data set that bipedality in chimpanzees occurs under different behavioral contexts and is associated with multiple variables. Arboreal vs. terrestrial substrate use emerged as a particularly relevant variable (Figures 2, 3), and it was found that terrestrial bipedalism occurred under more contextually diverse circumstances than did arboreal bipedalism (Figure 2).
As in studies of great ape locomotion using behavioral frequency data, we found that arboreal bipedalism occurred chiefly in a feeding/foraging context (Hunt, 1996, 1998; Thorpe et al., 2007; Drummond-Clarke et al, 2022). Bipedality during feeding/foraging was principally by adult individuals and was also associated with hand usage on arboreal substrates, as in studies of both chimpanzees (Hunt, 1996, 1998) and orangutans (Thorpe et al., 2007).
Our examination of the behavioral contexts associated with terrestrial bipedalism revealed greater variability. In the case of adults, although a feeding/foraging context was the most common, it comprised a much smaller proportion of the total number of bipedal bouts when compared to the proportion found when using arboreal substrates (42% of observations when terrestrial vs. 72% when arboreal). Bipedality during antagonism was the second most common behavioral context for terrestrial bipedality in adults (27%) (Figure 2), providing support for the continued relevance of antagonistic behaviors in evolutionary reconstructions of the origin of bipedality (Jablonski and Chaplin, 1993; Carrier, 2011). Bipedalism in an antagonistic context was associated with adult males, as would be expected; chimpanzee society is hierarchical with males dominant over and therefore displaying more frequently than females (Goodall, 1986). Bipedalism during antagonism also occurs predominantly as a locomotor mode, presumably because displays incorporate steps, not just standing. Our results suggest that the role bipedalism may play during antagonism, particularly as a terrestrial locomotor mode, is worthy of further investigation.
While bipedal travel during antagonism is associated with males, our model also shows that females engage more commonly in bipedal locomotion overall (i.e., when travel is the primary context), which was not associated with substrate in our dataset. As far as we are aware, this is the first time this sex difference has been demonstrated in chimpanzees. Our qualitative notes indicate females travel bipedally while engaging in secondary contexts such as vacating from or moving toward a social situation; or when grabbing an infant from an arboreal substrate, and simultaneously looking up. Combining multiple contexts such as ‘visibility’ with ‘travel’ was not a feature of our study design, as each bipedal bout was coded as having a single predominant behavioral context. These qualitative notes suggest females may often employ bipedalism during travel in tandem with other, likely informative contexts; thus, subsequent modelling could benefit by assessing whether multiple behavioral contexts occur simultaneously.
As noted above, previous behavioral frequency studies in wild chimpanzees (Hunt, 1996, 1998; Drummond-Clarke et al, 2022) found that foraging was the primary behavioral context eliciting bipedalism. However, most of the bipedalism documented in these studies was postural rather than locomotor (ibid.). In the current video capture study, postural and locomotor modes of bipedalism had approximately equal representation in the sample, which we believe offers a new perspective as no single behavioral context plays an over-arching role in eliciting bipedality among chimpanzees (Figure 2). In addition, foraging is a major but not the only context which prompts significant, increased engagement in bipedalism. As discussed above, we found that more behavioral contexts were associated with terrestrial bipedalism compared to arboreal bipedalism. In addition, our data indicate that feeding/foraging was not preferentially associated with posture over locomotion per se; rather, feeding/foraging was associated with arboreal bipedalism, in older individuals. Thus, the high representation of postural over locomotor bouts in behavioral frequency data may have led to a focus on feeding/foraging at the expense of understanding other factors influencing bipedal locomotion. This finding underscores our recommendation above that future studies may benefit by examining the interplay of multiple variables when examining bipedality in primates.
Age was also found to be a highly relevant variable influencing bipedalism (Figures 2, 3). The major age trend we document is the role of play in eliciting bipedality in subadults. While the feeding/foraging context makes up a slightly larger proportion of all arboreal bipedal bouts than does play, subadults engaged in terrestrial bipedalism predominantly in the context of play. The strong association between play and terrestrial bipedalism in infants and juveniles merits further study; as Bezanson (2012, 2017) has noted, the role of play is under researched in studies of primate locomotor ontogeny and evolution. Rose (1976) incorporated play as a behavioral context in his foundational study on baboon bipedalism, however, that study included crouching, torso pronograde postures, and other positional behavioral modes omitted from the current study. We are unaware of any hypotheses related to the evolution of bipedalism that involve play. We propose play is ontogenetically critical for establishing the versatile orthograde behaviors that constitute ape positional behavior, and so may have facilitated the development of bipedalism in hominins.
The video capture method we employed allowed us to sample the rare chimpanzee behavior of bipedalism more than was previously feasible. It also allowed for a more comprehensive analysis of the variables influencing the behavioral contexts of bipedalism. We quantitatively demonstrate that terrestrial bipedalism is far more diverse with regards to behavioral context than arboreal bipedalism. Furthermore, our data suggests that different influences may be acting on subadults vs. adults and adult females vs. adult males.
The implications for hominin origins are as follows. First, bipedalism in the LCA, as in modern chimpanzees, may have been performed in multiple behavioral contexts, involving multiple variables, with males, females and subadults possibly being influenced in different ways. Likewise, as has been previously speculated (Napier, 1964), the transition to obligate bipedalism may have involved several behavioral contexts. Thus caution should be used when focusing on a single or very few variables when considering the origins of bipedality in the hominin lineage(s). Foraging almost certainly played a major role in the evolution of hominin bipedality; however, our data on chimpanzees show that other factors may have contributed as well.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by In accordance with IACUC from University of Michigan and James Madison University. The study was conducted in accordance with the local legislation and institutional requirements.
LS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. RS: Formal analysis, Visualization, Writing – review & editing. LM: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this project was supported by the Leakey Foundation, the University of Michigan, and the National Science Foundation 1850328. Funding for publication was provided by James Madison University.
This research was sponsored by the Ugandan National Council for Science and Technology, Uganda Wildlife Authority, and the Makerere University Biological Field Station. We particularly thank Sharifah Namaganda for her guidance in the field and for many productive discussions about chimpanzee positional behavior. We also thank John Mitani, Sam Angedakin, Kevin Lee, Emily Orlikoff and John Kingston for their intellectual contributions and field support. This work would not be possible without support from the Ngogo Chimpanzee Project and Ngogo Snare Team members. Corey Powell provided excellent statistical support, and we thank Sydney Tucker for providing the older, younger, hand assistance and locomotion icons used in Figure 3.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1321115/full#supplementary-material
Behrensmeyer A. K., Reed K. E. (2013). Reconstructing the habitats of Australopithecus: Paleoenvironments, site taphonomy, and faunas. Paleobiol. Australopithecus, 41–60. doi: 10.1007/978-94-007-5919-0_4
Bezanson M. (2012). The ontogeny of prehensile-tail use in Cebus capucinus and Alouatta palliata. Am. J. Primatol. 74, 770–782. doi: 10.1002/ajp.22028
Bezanson M. (2017). Primate positional behavior development and evolution. Annu. Rev. Anthropol. 46, 279–298. doi: 10.1146/annurev-anthro-102116-041503
Carlson K. J., Green D. J., Jashashvili T., Pickering T. R., Heaton J. L., Beaudet A., et al. (2021). The pectoral girdle of StW 573 (‘Little Foot’) and its implications for shoulder evolution in the Hominina. J. Hum. Evol. 158, 102983. doi: 10.1016/j.jhevol.2021.102983
Carrier D. R. (2011). The advantage of standing up to fight and the evolution of habitual bipedalism in hominins. PloS One 6, e19630. doi: 10.1371/journal.pone.0019630
Carvalho S., Biro D., Cunha E., Hockings K., McGrew W. C., Richmond B. G., et al. (2012). Chimpanzee carrying behaviour and the origins of human bipedality. Curr. Biol. 22, R180–R181. doi: 10.1016/j.cub.2012.01.052
Crompton R. H., Sellers W. I., Thorpe S. K. (2010). Arboreality, terrestriality and bipedalism. Philos. Trans. R. Soc. B: Biol. Sci. 365, 3301–3314. doi: 10.1098/rstb.2010.0035
Daver G., Guy F., Mackaye H. T., Likius A., Boisserie J. R., Moussa A., et al. (2022). Postcranial evidence of late Miocene hominin bipedalism in Chad. Nature 609, 94–100. doi: 10.1038/s41586-022-04901-z
Domínguez-Rodrigo M. (2014). Is the “Savanna Hypothesis” a dead concept for explaining the emergence of the earliest hominins? Curr. Anthropol. 55, 1. doi: 10.1086/674530
Drummond-Clarke R. C., Kivell T. L., Sarringhaus L., Stewart F. A., Humle T., Piel A. K. (2022). Wild chimpanzee behavior suggests that a savanna-mosaic habitat did not support the emergence of hominin terrestrial bipedalism. Sci. Adv. 8, eadd9752. doi: 10.1126/sciadv.add9752
Du Brul E. L. (1962). The general phenomenon of bipedalism. Am. Zoologist, 2, 205–208. doi: 10.1093/icb/2.2.205
Etkin W. (1954). Social behavior and the evolution of man’s mental faculties. Am. Nat. 88, 129–142. doi: 10.1086/281823
Goodall J. (1986). The chimpanzees of Gombe: patterns of behavior (Cambridge, Mass: Belknap Press of Harvard Univ. Press).
Hewes G. W. (1961). Food transport and the origin of hominid bipedalism. Am. Anthropologist. 63, 687–710. doi: 10.1525/aa.1961.63.4.02a00020
Hunt K. D. (1994). The evolution of human bipedality: ecology and functional morphology. J. Hum. Evol. 26, 183–202. doi: 10.1006/jhev.1994.1011
Hunt K. D. (1996). The postural feeding hypothesis: an ecological model for the evolution of bipedalism. South Afr. J. Sci. 92, 77–90.
Hunt K. D. (1998). “Ecological morphology of Australopithecus afarensis: traveling terrestrially, eating arboreally,” in Primate locomotion: Recent advances (Springer US, Boston, MA), 397–418.
Hunt K. D. (2016). Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. J. Anat. 228, 630–685. doi: 10.1111/joa.12454
Hunt K. D., Cant J. G., Gebo D. L., Rose M. D., Walker S. E., Youlatos D. (1996). Standardized descriptions of primate locomotor and postural modes. Primates 37, 363–387. doi: 10.1007/BF02381373
Jablonski N. G., Chaplin G. (1993). Origin of habitual terrestrial bipedalism in the ancestor of the Hominidae. J. Hum. Evol. 24, 259–280. doi: 10.1006/jhev.1993.1021
Jaeger R. G., Halliday T. R. (1998). On confirmatory versus exploratory research. Herpetologica, S64–S66.
Jolly C. J. (1970). The seed-eaters: a new model of hominid differentiation based on a baboon analogy. Man 5, 5–26. doi: 10.2307/2798801
Kappelman J., Ketcham R. A., Pearce S., Todd L., Akins W., Colbert M. W., et al. (2016). Perimortem fractures in Lucy suggest mortality from fall out of tall tree. Nature 537, 503–507. doi: 10.1038/nature19332
Langergraber K. E., Prüfer K., Rowney C., Boesch C., Crockford C., Fawcett K., et al. (2012). Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proc. Natl. Acad. Sci. 109, 15716–15721. doi: 10.1073/pnas.1211740109
Latimer B., Ohman J. C., Lovejoy C. O. (1987). Talocrural joint in African hominoids: implications for Australopithecus afarensis. Am. J. Phys. Anthropol. 74, 155–175. doi: 10.1002/ajpa.1330740204
Livingstone F. B. (1962). Reconstructing man’s Pliocene pongid ancestor. Am. Anthropol. 64, 301–305. doi: 10.1525/aa.1962.64.2.02a00050
Lwanga J. S. (2003). Forest succession in Kibale National Park, Uganda: implications for forest restoration and management. Afr. J. Ecol. 41, 9–22. doi: 10.1046/j.1365-2028.2003.00400.x
MacLatchy L., Cote S., Deino A., Kityo R., Mugume A., Rossie J., et al. (2023). The evolution of hominoid locomotor versatility: Evidence from Moroto, a 21 Ma site in Uganda. Science 380, eabq2835. doi: 10.1126/science.abq28
Rein T. R., Harrison T., Zollikofer C. P. (2011). Skeletal correlates of quadrupedalism and climbing in the anthropoid forelimb: implications for inferring locomotion in Miocene catarrhines. J. Hum. Evol. 61, 564–574. doi: 10.1016/j.jhevol.2011.07.005
Rodman P. S., McHenry H. M. (1980). Bioenergetics and the origin of hominid bipedalism. Am. J. Phys. Anthropol. 52, 103–106. doi: 10.1002/ajpa.1330520113
Rose M. D. (1974). “Postural adaptations in New and Old World monkeys,” in Primate Locomotion. Ed. Jenkins F. A. Jr. (Academic Press, New York), 201–222.
Rose M. D. (1976). Bipedal behavior of olive baboons (Papio anubis) and its relevance to an understanding of the evolution of human bipedalism. Am. J. Phys. Anthropol. 44, 247–261. doi: 10.1002/ajpa.1330440207
Sarringhaus L.A., MacLatchy L. M., Mitani J. C. (2014). Locomotor and postural development of wild chimpanzees. J. Hum. Evol. 66, 29–38.
Steiper M. E., Seiffert E. R. (2012). Evidence for a convergent slowdown in primate molecular rates and its implications for the timing of early primate evolution. Proc. Natl. Acad. Sci. 109, 6006–6011. doi: 10.1073/pnas.1119506109
Struhsaker T. T. (1997). Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation (Gainesville: University Press of Florida).
Susman R. L., Stern J. T., Jungers W. L. (1984). Arboreality and bipedality in the Hadar hominids. Folia Primatologica 43, 113–156. doi: 10.1159/000156176
Thorpe S. K., Crompton R. H. (2006). Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am. J. Phys. Anthropol. 131, 384–401. doi: 10.1002/ajpa.20422
Thorpe S. K., Holder R. L., Crompton R. H. (2007). Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science 316, 1328–1331. doi: 10.1126/science.1140799
Tukey J. W. (1977). Exploratory data analysis, Vol. 2. 131–160. Reading Mass: Addison-Wesley Publishing Co.
Videan E. N., McGrew W. C. (2002). Bipedality in chimpanzee (Pan troglodytes) and bonobo (Pan paniscus): testing hypotheses on the evolution of bipedalism. Am. J. Phys. Anthropol. 118, 184–190. doi: 10.1002/ajpa.10058
Wackerly N. (2019). Thermo-imaging bipedalism on the savanna: Chimpanzee locomotion at Fongoli, Senegal and implications for the evolution of hominin bipedalism. Iowa State University. Doctoral dissertation.
Ward C. V. (2002). Interpreting the posture and locomotion of Australopithecus afarensis: where do we stand? Am. J. Phys. Anthropol. 119 (S35), 185–215. doi: 10.1002/ajpa.10185
Washburn S. L. (1967). Behaviour and the origin of man. Proc. R. Anthropological Institute Great Britain Ireland 1967, 21–27. doi: 10.2307/3031724
Wheeler P. E. (1984). The evolution of bipedality and loss of functional body hair in hominids. J. Hum. Evol. 13, 91–98. doi: 10.1016/S0047-2484(84)80079-2
Wheeler P. E. (1991). The influence of bipedalism on the energy and water budgets of early hominids. J. Hum. Evol. 21, 117–136. doi: 10.1016/0047-2484(91)90003-E
Wrangham R. W. (1980). Bipedal locomotion as a feeding adaptation in gelada baboons, and its implications for hominid evolution. J. Hum. Evol. 9, 329–331. doi: 10.1016/0047-2484(80)90059-7
Wuthrich C., MacLatchy L. M., Nengo I. O. (2019). Wrist morphology reveals substantial locomotor diversity among early catarrhines: an analysis of capitates from the early Miocene of Tinderet (Kenya). Sci. Rep. 9, 3728. doi: 10.1038/s41598-019-39800-3
Keywords: bipedal, ape model, play, ontogeny, forage, chimpanzee, orthograde, human bipedality
Citation: Sarringhaus L, Srivastava R and MacLatchy L (2024) The influence of multiple variables on bipedal context in wild chimpanzees: implications for the evolution of bipedality in hominins. Front. Ecol. Evol. 12:1321115. doi: 10.3389/fevo.2024.1321115
Received: 13 October 2023; Accepted: 22 February 2024;
Published: 12 March 2024.
Edited by:
Nicholas Baird Holowka, University at Buffalo, United StatesReviewed by:
Caroline VanSickle, Des Moines University, United StatesCopyright © 2024 Sarringhaus, Srivastava and MacLatchy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren Sarringhaus, c2FycmlubGFAam11LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.