- 1College of Ecology and Environment, Tibet University, Lhasa, China
- 2Institute of Aquatic Sciences, Tibet Autonomous Region Academy of Agricultural and Animal Husbandry Sciences, Lhasa, China
Introduction: The study of how soil fauna interact with soil ecosystems is an important research field. At present, there has been little research on the diversity and distribution patterns of soil macroarthropods and on the relationship between soil fauna and environmental factors in Tibet.
Methods: The data collection for soil macroarthropods and soil samples was conducted using the plum blossom five-point sampling method. Subsequently, the gathered data were meticulously organized and analyzed through a blend of ecological and statistical approaches.
Results: In total, 2880 soil macroarthropods were captured from 19 plots in the Nianchu River Basin, and the soil macroarthropod Hill numbers was at its lowest in spring. Sea buckthorn forest habitats had the highest Hill numbers. We found that Carabidae, Lycosidae, and Formicidae were always dominant species during seasonal changes in the Nianchu River Basin. Among the four different habitat types studied, Carabidae always appeared across the four studied habitat types and was one of the most significant taxa. The niche range of soil macroarthropods was wetland habitat > farmland habitat > sea buckthorn forest habitat > grassland habitat, and their community within sea buckthorn forest habitats was stable. The niche breadth of soil macroarthropods was the largest in summer, and the community was stable. Soil temperature and soil water content were the most important factors affecting the alpha diversity of soil macroarthropods, while altitude and soil temperature were the most important factors affecting their beta diversity.
Discussion: In summary, the results provide a comprehensive overview of the seasonal and habitat dynamics of soil macroarthropods in the Nianchu River Basin; it is strongly believed that the research carried out in this paper can contribute valuable information toward further research on the soil fauna diversity and ecological functions in this area and provide a strong scientific basis for the protection and sustainable development of the Nianchu River Basin ecosystem.
1 Introduction
Soil is one of the major components of a terrestrial ecosystem, and, more importantly, it provides the basic living conditions for the soil fauna. Soil macroarthropods are those that contact the soil surface or that live in the soil for a certain period of their life and have a certain impact on the soil (Yin, 2001; Coyle et al., 2017; Luo et al., 2018). Soil macroarthropods play an indispensable role in the material circulation and energy exchange of the soil ecosystem, contributing to soil looseness, maintaining the physical and chemical properties of the soil ecosystem, aiding in the reproduction of biological population, reflecting soil pollution, regulating the function of soil microorganisms, and indicating environmental and global ecological changes (Davis et al., 2006; Riutta et al., 2012; Slade and Riutta, 2012; Tiegs et al., 2013; Wang et al., 2020). Although soil macroarthropods are the most visible components of the soil environment, they are still under-represented in global surveys (Phillips et al., 2019), because there is no accepted standard of survey methods in the world that accounts for them (Anderson and Ingram, 1994; Rombke et al., 2006), and also because the role of soil macroarthropods in ecosystems has been previously underestimated, leading to a lack of quantitative assessment regarding them, and thereby hindering our understanding of the role of soil macroarthropods in global ecosystems.
In recent years, extensive investigations and research on soil fauna have been conducted by numerous scholars. These studies have primarily focused on their spatiotemporal distribution patterns (Pequeno et al., 2017; Yang et al., 2021) and diversity (Araújo et al., 2010; Yin et al., 2017), as well as the impact of different land use patterns on soil macroarthropod community characteristics (Jiang et al., 2015; Kelly et al., 2020; Li et al., 2020) and the relationships between soil fauna and other environmental factors (Yang et al., 2020; Tang et al., 2020). As ecological restoration efforts are being implemented in Northwest China, significant changes are expected to occur among the animal groups and populations in the local soil ecosystem. However, limited data are available regarding the impact on local soil fauna, leaving ample room for future research in Northwest China. Moreover, little research has been conducted on the characteristics of the soil macroarthropod community in the Qinghai–Tibet Plateau, and no studies have investigated the characteristics and spatial distributions of soil macroarthropods in the Nianchu River Basin in Tibet thus far.
The Nianchu River Basin, situated in the southern part of the Tibet Autonomous Region on the Tibetan Plateau, holds the distinction of being the largest river among the tributaries on the right bank of the Yarlung Zangbo River. It is bordered by the Karorah Snow Mountain and Yamdro–Moyunco Valley to the east, the Himalayas to the south, the State of Bhutan and the Lower Buqu Valley to the west, and the Yarlung Zangbo River main stream basin to the north. This region is of great agricultural significance in Tibet; its main crop is highland barley, and it is characterized by dense cultivated land and a dense population. The Nianchu River Basin spans an area of approximately 11,130 square kilometers and features an average basin slope of 6.1% (Yang et al., 2012). The Nianchu River stretches for 217 kilometers and exhibits a total drop of 1,322 meters. The study area falls within the plateau’s temperate semi-arid climate zone, known for its low temperatures, significant daily temperature variations, limited precipitation intensity, intense solar radiation, and high evaporation rates. The average annual temperature is about 7.2°C at the confluence and 2.4°C at the source of the river, and the temperature is gradually rising from the upper to the lower reaches. The average annual precipitation is approximately 365 mm, with uneven distribution across the region; however, the wet and dry seasons are more distinct, and the annual precipitation is mainly distributed in June to September (Liu and Chen, 1995; Wang and Liu, 2000; Li et al., 2018).
This study firstly investigated the season and habitat dynamics of the soil macroarthropod community in the Nianchu River Basin, Tibet. Traditional morphological methods were used to investigate the community composition, ecological division, and diversity of soil macroarthropods over different habitats and seasons in the Nianchu River Basin. The differences in the physical and chemical properties of the soil in different seasons and different habitat types were studied. The effects of environmental factors on the niche and diversity of soil macroarthropod communities were further determined. By deepening our understanding of soil fauna communities, this research will provide essential theoretical support for biodiversity conservation, deepen our understanding of the environmental adaptability of macroarthropods living in the soil habitats of the Tibetan Plateau, and could also be a vital addition to global biogeographic diversity information.
2 Materials and methods
2.1 Study area and sampling
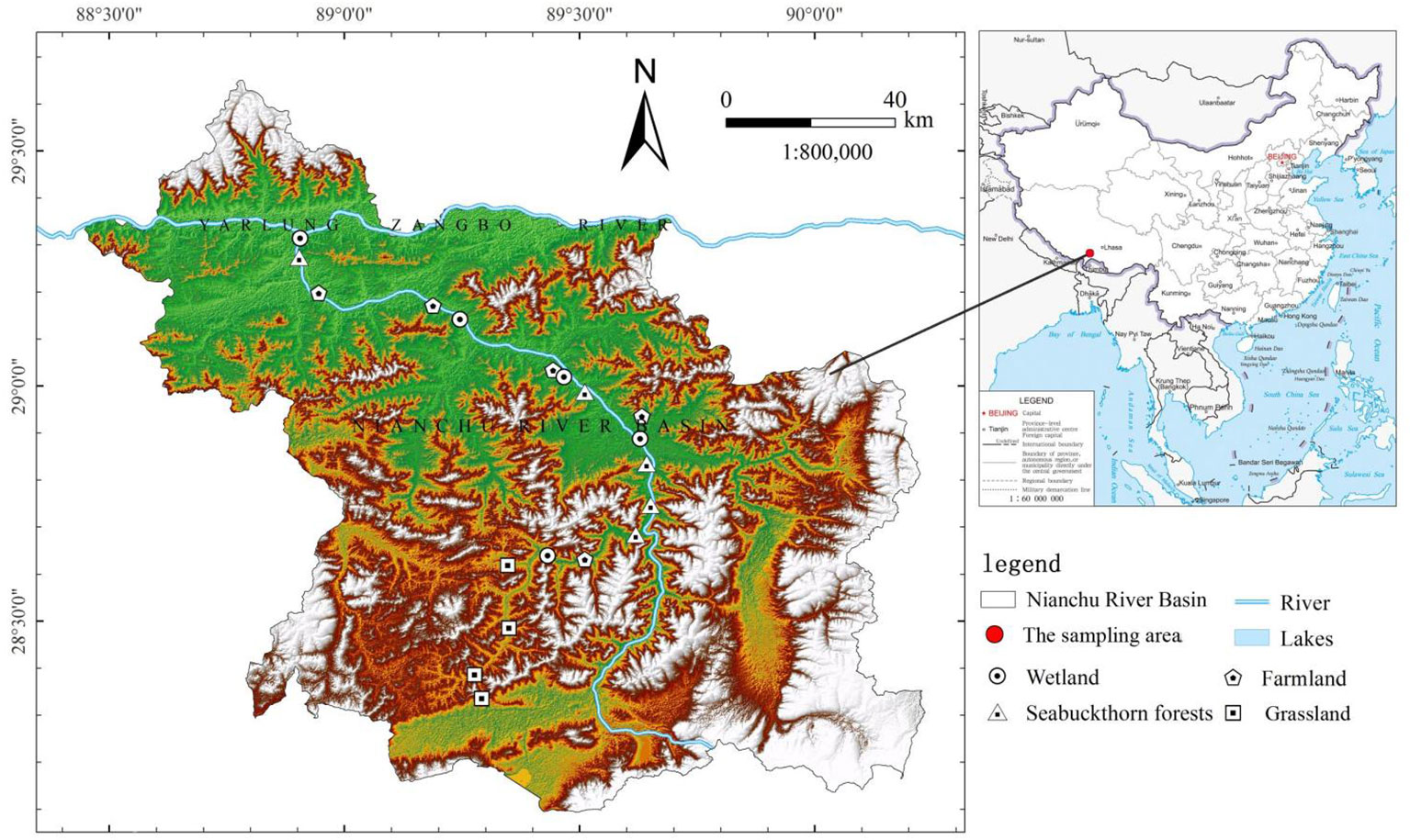
The study area is located in the Nianchu River Basin (28°10’~29°20’ N, 88°35’~90°15’ E) in Tibet, China. According to the geographical location and the habitat types in the Nianchu River Basin, 95 sampling sites in 19 of the first class quadrats were selected, including grassland (GL; 4 first-order quadrats with 20 sampling points), wetland (WL; 5 first-order quadrats with 25 sampling points), farmland (FL; 5 first-order quadrats with 25 sampling points), and sea buckthorn forest (SF; 5 first-order quadrats with 25 sampling points) areas. In compliance with the United States Department of Agriculture’s soil classification standards, this research categorizes the soil of the grassland sample areas as Gelisols. It classifies the soil from the wetland sampling sites and the farmland sample plots as Histosols, while designating the soil in the sea buckthorn forest sample areas as Spodosols. Three periods of sampling were carried out, in spring, summer, and autumn of 2020. The distribution of quadrats in each habitat type is shown in Figure 1. The soil samples were collected using a cylindrical ring knife (30 cm in height, 5 cm in diameter), and five small pieces were placed. The soil samples were mixed, stored in a sealed bag, brought back to the laboratory in the dark for air drying, and transported to the laboratory for further processing.
2.2 Laboratory processing of samples and measurement of environmental parameters
Soil macroarthropods were extracted from each soil core using modified Tullgren extractors (Tullgren Funnel Unit, BURKARD, UK). All extracted faunal samples were preserved in 75% ethanol and subsequently sorted under a dissecting microscope (Eclipse E200, Nikon, Japan). In each sample plot (20 m * 20 m), five quadrats (20 cm * 20 cm) were selected according to the five-point plum blossom pattern sampling method. Soil samples (0~10 cm) were collected from each quadrat. Soil macroarthropods on the surface and inside of each sample were picked up using tweezers and stored in collection tubes with 75% alcohol. These samples were brought back to the laboratory for morphological identification. After the soil macroarthropods were removed from the collection tubes, they were repeatedly cleaned with 95% alcohol, and their characteristics were observed under Leica stereo (Leica Camera Co., LTD., EZ4W, Berlin, Germany). For morphological identification, specialized bibliographies were consulted (Yin, 1992; Yin, 1998; Yin, 2000), the samples was sorted and identified at the order, family level, and the number of individuals and the species of each individual were recorded.
The soil surface temperature was measured using a soil temperature gun (Hong Kong Xima Instrument Co., LTD., AS700, Hongkong, China). The latitude, longitude, and altitude were recorded using a global positioning system (Beijing Jiaming Avionics Technology Co. LTD., GPSMAP631csx, Beijing, China). The soil moisture content was measured using a soil moisture tester (Liaoning Saias Instrument Co., LTD., SBS-SE, Liaoning, China). The organic matter and total nitrogen in the soil was measured using an acid burette. The effective phosphorus was extracted with sodium bicarbonate-molybdenum antimony spectrophotometry. The rapidly available potassium was measured with a quick-acting potassium atomic absorption spectrophotometer, and the pH was measured with a pHS-3C meter (Shanghai Leimi Electronic Technology Co., LTD., Shanghai, China). The determination of soil organic matter is conducted through acid titration, adhering to the NY/T 1121.6-2006 standard. Total nitrogen content is quantified using the Kjeldahl method as per HJ 717-2014, utilizing an acid titration approach. The measurement of available phosphorus is achieved through sodium bicarbonate extraction followed by antimony molybdenum colorimetric analysis, in accordance with HJ 704-2014. For available potassium, atomic absorption spectrophotometry is employed, guided by the NY/T 889-2004 standard. Finally, the soil pH value is accurately assessed using a pHS-3C pH meter, as specified in NY/T 1121.2-2006.
2.3 Statistical analysis
Statistical analyses were conducted in R-4.2.1 [https://cran.r-project.org/(accessed on 21 June 2022)]. Alpha diversity indices (including the richness index, Shannon–Wiener diversity index, Pielou evenness index, and Simpson dominance index) were calculated using “vegan” package in R. Two-way analysis of variance was used to determine the significance of the associations of the alpha diversity indices with seasons and habitat types. Alpha diversity measurements were conducted using the “iNEXT” function within the iNEXT package for R, ensuring thorough and complete sampling across all examined habitats. Principal coordinate analysis (PCoA) and analysis of similarities (ANOSIM) were performed based on the Bray–Curtis distance using the “vegan” package in R. The niche breadth stability was calculated for each community using the “spaa” and “microeco” packages in R. To delve deeper into the community dynamics of soil macroarthropod, we evaluated their structural variations by season and habitat type through the calculation of Hill numbers. We performed a detailed calculation of Hill numbers for macroarthropod communities in soil, encompassing: Species Richness (Hill number N0, q=0), Exponential Shannon-Wiener Index (Hill number N1, q=1), Inverse Simpson’s Concentration Index (Hill number N2, q=2), and Inverse Berger-Parker Index (Hill number N3, q=3) and the environmental parameters were calculated using regression analysis. Multiple samples were compared using the nonparametric Kruskal–Wallis test, followed by Dunn’s multiple comparison test. Mantel tests were used to determine correlations between environmental variables and selected characteristics of macroarthropod composition in the “linkET” package in R. Sampling sites were mapped in ArcMap 10.6.1.
3 Results
3.1 High variation in soil macroarthropod diversity in different seasons and habitats
In total, 2880 samples of soil macroarthropods, among these samples, 7 samples were identified at the genus level, 37 groups were identified at the family level, and 14 groups were identified at the order level, were captured in the Nianchu River Basin (Supplementary Table 4). The Hill numbers of these soil macroarthropods varied among different samples for the calculated indices (Supplementary Table 2): richness indices ranged from 5 to 17. The diversity indices revealed that the exponential Shannon index of soil macroarthropods spanned from 1.38 to 9.18. In contrast, the inverse Simpson and Berger-Parker indices of soil macroarthropods fluctuated between 1.22 to 7.21 and 1.17 to 1.64, respectively. Seasonal analysis revealed that both the exponential Shannon-Wiener index and the inverse Simpson index reached their zenith during summer, eclipsing the figures observed in both spring and autumn. In contrast, autumn witnessed the acme of the inverse Berger-Parker index, notably surpassing its counterparts in the spring and summer seasons (Figure 2A). Considering different habitats, the Seabuckthorn forest demonstrated superior values in the exponential Shannon index, the inverse Simpson index, and the inverse Berger-Parker index, distinctly outperforming the three other habitats under study (Figure 2B). In general, the diversity of soil macroarthropods exhibited variations in seasonal and habitat-dependent manners.
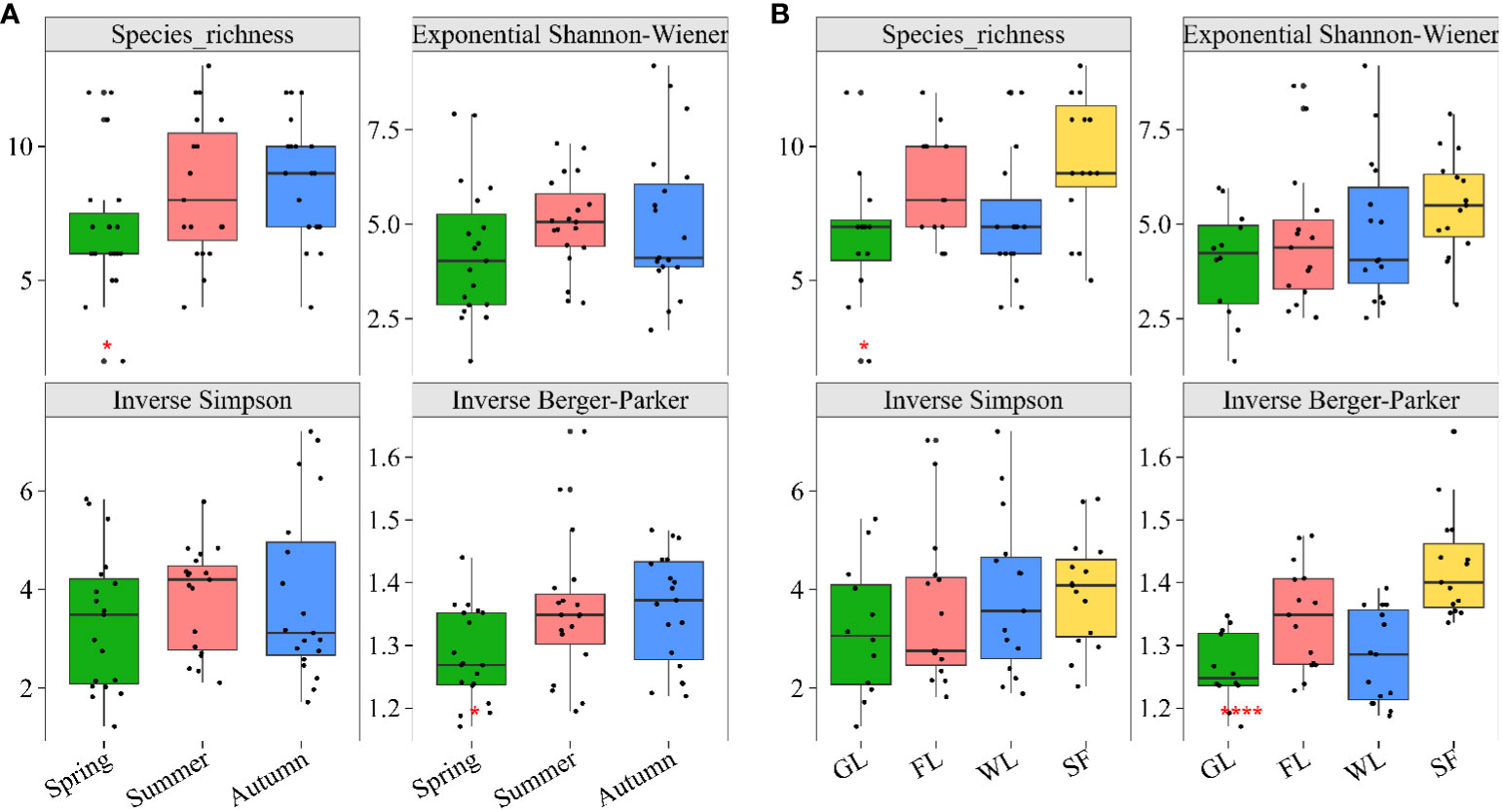
Figure 2 Hill numbers of soil macroarthropods. Species richness, Exponential Shannon-Wiener, Inverse of Simpson´s concentration index, and Inverse Berger-Parker index of soil macroarthropods in different (A) seasons and (B) habitats. The solid dots positioned outside the boundaries of the boxplot serve as indicators of outliers, highlighting data points that stand apart from the overall distribution pattern.
3.2 Habitat and seasonal variation in soil macroarthropod communities in Nianchu River Basin
ANOSIM showed that the composition of soil macroarthropod communities in the Nianchu River Basin exhibited significant variation (P <0.05), depending on season and habitat type. Habitat type (ANOSIM R2 = 0.14) had a greater effect than collection site (ANOSIM R2 = 0.084) on the composition of soil macroarthropod communities. PCoA, conducted based on Bray–Curtis distance to investigate differences in soil macroarthropod communities among the studied seasons and habitat types, showed that the PCoA1 and PCoA2 explained 16.9% and 14.2%, respectively, of the total variation in soil macroarthropod communities (Figures 3A, B). There was no significant difference in the soil macroarthropod community between spring and summer, but a significant difference was observed between spring and autumn and summer and autumn (Figure 3A). However, soil macroarthropod communities showed significant difference among habitat types (Figure 3B).
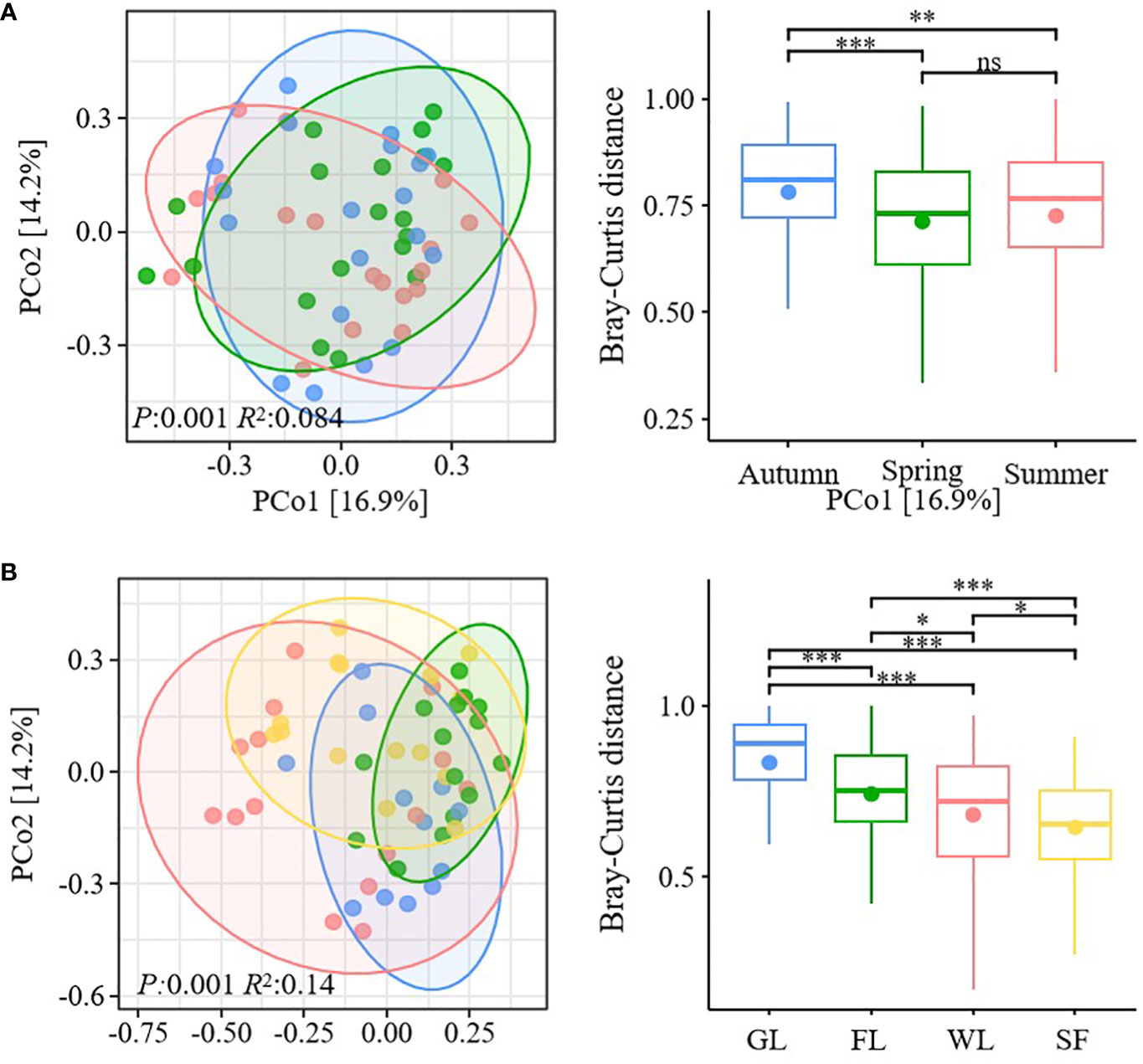
Figure 3 Beta diversity of soil macroarthropods. (A) Principal coordinate analysis (PCoA) and based on Bray–Curtis distance analysis of the differences in soil macroarthropod communities in the three studied seasons. (B) Principal coordinate analysis (PCoA) and based on Bray–Curtis distance analysis of the differences in soil macroarthropod communities in the different habitat types studied. In the boxplot, the presence of solid dots is indicative of the mean values. The symbols *, **, *** and **** are used to denote varying degrees of statistical significance. ns, not significant.
There were some observations regarding the high variation in the relative abundance of soil macroarthropods exhibited across different seasons and habitat types (Figure 4A). In spring, the predominant groups were Carabidae (24.75%), Lycosidae (22.58%), Formicidae (15.90%), Dipteralarva (9.62%), Lithobiomorpha_X (5.35%), and Orthoptera_X (3.29%). In summer, the predominant groups were Formicidae (17.53%), Carabidae (12.62%), Lithobiomorpha_X (11.31%), Lycosidae (10.95%), and Hemiptera_X (9.85%). Lastly, in autumn, the predominant groups were Lycosidae (16.82%), Formicidae (15.12%), Lygaeidae (13.16%), Carabidae (9.40%), and Lepidopteralarva (7.62%) (Figure 4B; Supplementary Tables 3, 4). The predominant groups comprising the proportion of relative abundance and community composition of soil macroarthropods demonstrated differences across three seasons. Analysis of the relative abundance of soil macroarthropods in the different habitat types studied showed that, in grassland habitat types, the predominant groups were Carabidae (15.28%), Meloidae (14.91%), Lygaeidae (11.46%), Formicidae (10.87%), and Hemiptera_X (10.76%). In farmland habitat types, the predominant groups were Lycosidae (25.95%), Carabidae (13.16%), Dipteralarva (12.62), Lithobiomorpha_X (9.69%), and Lepidopteralarva (9.51%). In wetland habitat types, the predominant groups were Formicidae (25.29%), Carabidae (24.51%), Lycosidae (11.63%), Orthoptera_X (6.67%), and Lithobiomorpha_X (6.54%). Lastly, in sea buckthorn forest habitat types, the predominant groups were Formicidae (24.95%), Lycosidae (20.96%), Lithobiomorpha_X (11.44%), Carabidae (9.36%), and Dipteralarva (9.02%) (Figure 4C; Supplementary Tables 3, 4). Located in the upper reaches of the Nianchu River Basin, the grassland habitat is situated at a comparatively higher elevation. This geographic positioning contributes to a more homogeneous and limited variety of species. Consequently, this leads to a reduced relative abundance of species in the grassland habitat when contrasted with the diversity found in the other three habitats.
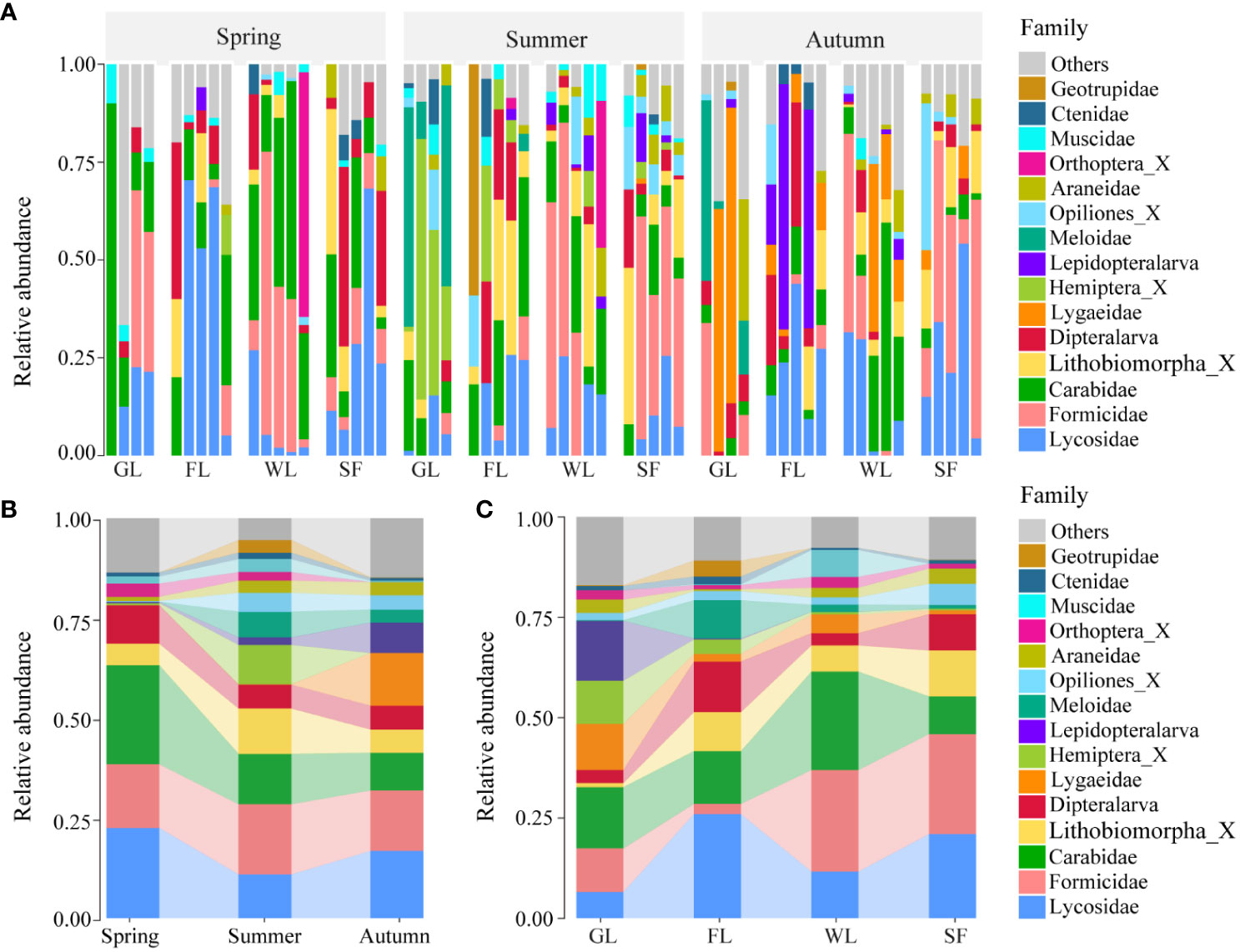
Figure 4 Habitat and seasonal variation in soil macroarthropod abundance (Orthoptera_X is a family of Orthoptera, Lithobiomorpha_X is a family of Lithobiomorpha, Hemiptera_X is a family of Hemiptera, Opiliones_X is a family of Opiliones). (A) Relative abundance of major groups of soil macroarthropods in the different seasons and habitat types studied. (B) Relative abundance of major groups of soil macroarthropods among the three studied seasons. (C) Relative abundance of major groups of soil macroarthropods among the four studied habitat types.
3.3 Niche breadth and community stability of soil macroarthropods
Niche breadth reflects the number of spatial resource slots occupied by different species and the extent and uniformity of their spatial distribution. The competition among species maintains the state of community balance and species diversity and can maintain the stability of species composition over a long period of time. The niche breadth of soil macroarthropods varied among the different seasons studied, but ranged from 1.00 to 10.26 for the different indices. The community stability index of soil macroarthropods ranged from 0.2 to 0.51 among the different studied seasons. The niche breadth of soil macroarthropods was the largest in summer, and the community was relatively stable (Figure 5; Supplementary Tables 6, 7). The niche breadth of soil macroarthropods varied among different habitat types, ranging from 1.00 to 9.83 for the different indices. The community stability index of soil macroarthropods ranged from 0.2 to 0.51 among the different studied habitat types. The niche breadth of soil macroarthropods was relatively large in wetland habitat types, and the community stability of soil macroarthropods in sea buckthorn forest habitats was relatively strong (Figure 5; Supplementary Tables 5, 8).
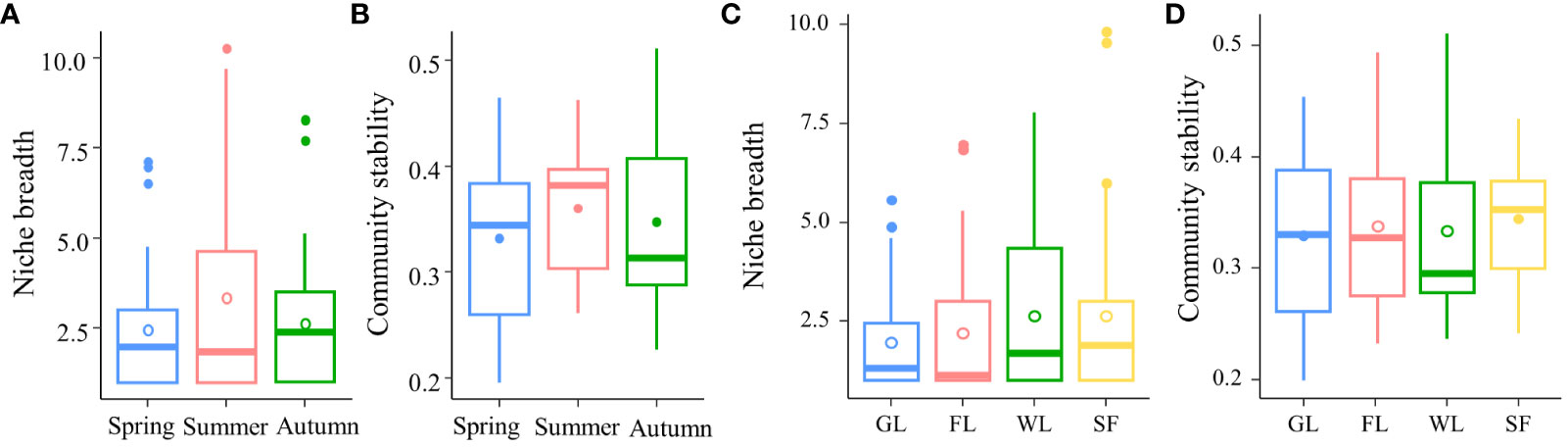
Figure 5 Niche breadth and community stability of soil macroarthropods. (A) Niche breadth of soil macroarthropods among the different studied seasons. (B) Community stability of soil macroarthropods in the different seasons studied. (C) Niche breadth of soil macroarthropods in the different habitat types studied. (D) Community stability of soil macroarthropods in the different habitat types studied. In the diagram, solid dots are used to denote outliers, illustrating values that deviate significantly from the norm, whereas hollow dots are employed to signify the mean values, representing the average tendency of the data.
3.4 Links between soil macroarthropod communities and environmental factors
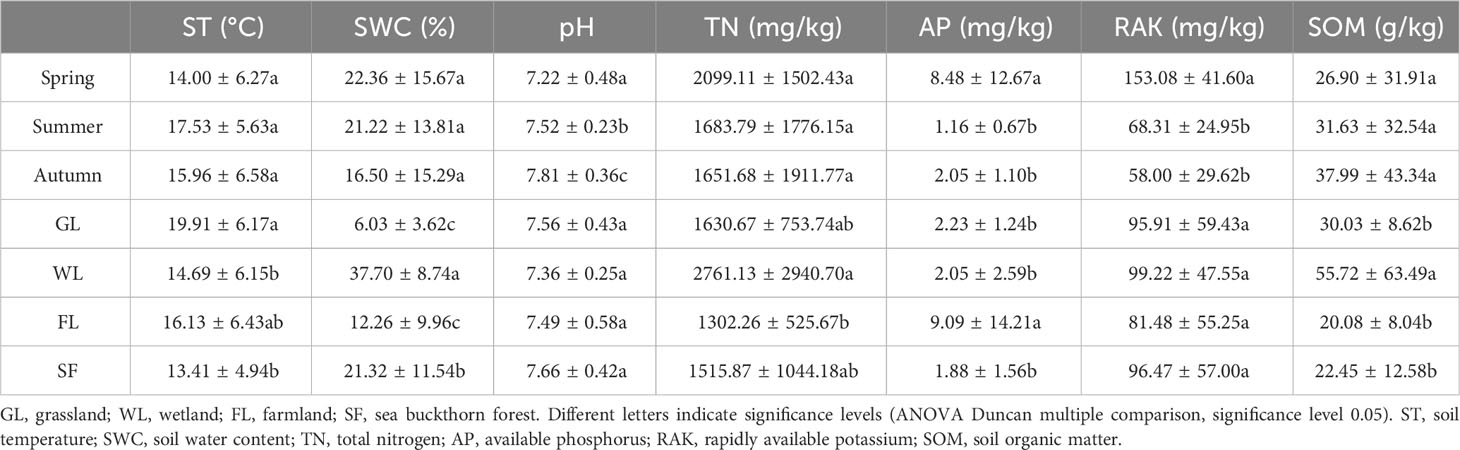
Comparisons of the average environmental factors, by season and habitat type, are presented in Table 1 (Supplementary Table 1). Environmental factors such as pH, available phosphorus, and rapidly available potassium showed significant seasonal changes (P < 0.05), whereas other factors, such as total nitrogen, soil water content, and soil temperature, did not. The pH values were highest in autumn and lowest in spring. The rapidly available potassium values were higher in spring than in summer and autumn. In contrast, soil temperature, total nitrogen, soil organic matter, and available phosphorus showed significant changes among the different habitat types investigated (P < 0.05). The soil temperature values in grassland habitat types were significantly higher than those in wetland and sea buckthorn forest habitat types, while the soil water content values in wetland habitats were significantly higher than those in sea buckthorn forest habitats. The total nitrogen values in wetland habitats were significantly higher than those in farmland habitats, and the available phosphorus values in farmland habitats were significantly higher than in grassland, wetland, and sea buckthorn forest habitats. Additionally, the soil organic matter values in wetland habitats were significantly higher than in grassland, farmland, and sea buckthorn forest habitats. There were no significant differences observed in pH and available potassium values among the different habitat types studied.
We analyzed the relationships between alpha and beta diversity for the macroarthropods and environmental factors. Regarding alpha diversity, the Simpson, Richness, Shannon, and Pielou indices for macroarthropods were most significantly affected by ST and SWC levels (Figures 6A, B). This result indicates a narrow alpha diversity for soil macroarthropods in soil with extremely high ST and SWC levels. The alpha diversity of macroarthropods was also negativity correlated with RAK, indicating a high alpha diversity for macroarthropods in areas with low RAK values. For beta diversity, the Jaccard and Bray–Curtis distances of soil macroarthropod communities positivity correlated with ALT and ST values, indicating that temperature is a main factor influencing the diversity of soil macroarthropods in the Nianchu River Basin (Figure 6C).
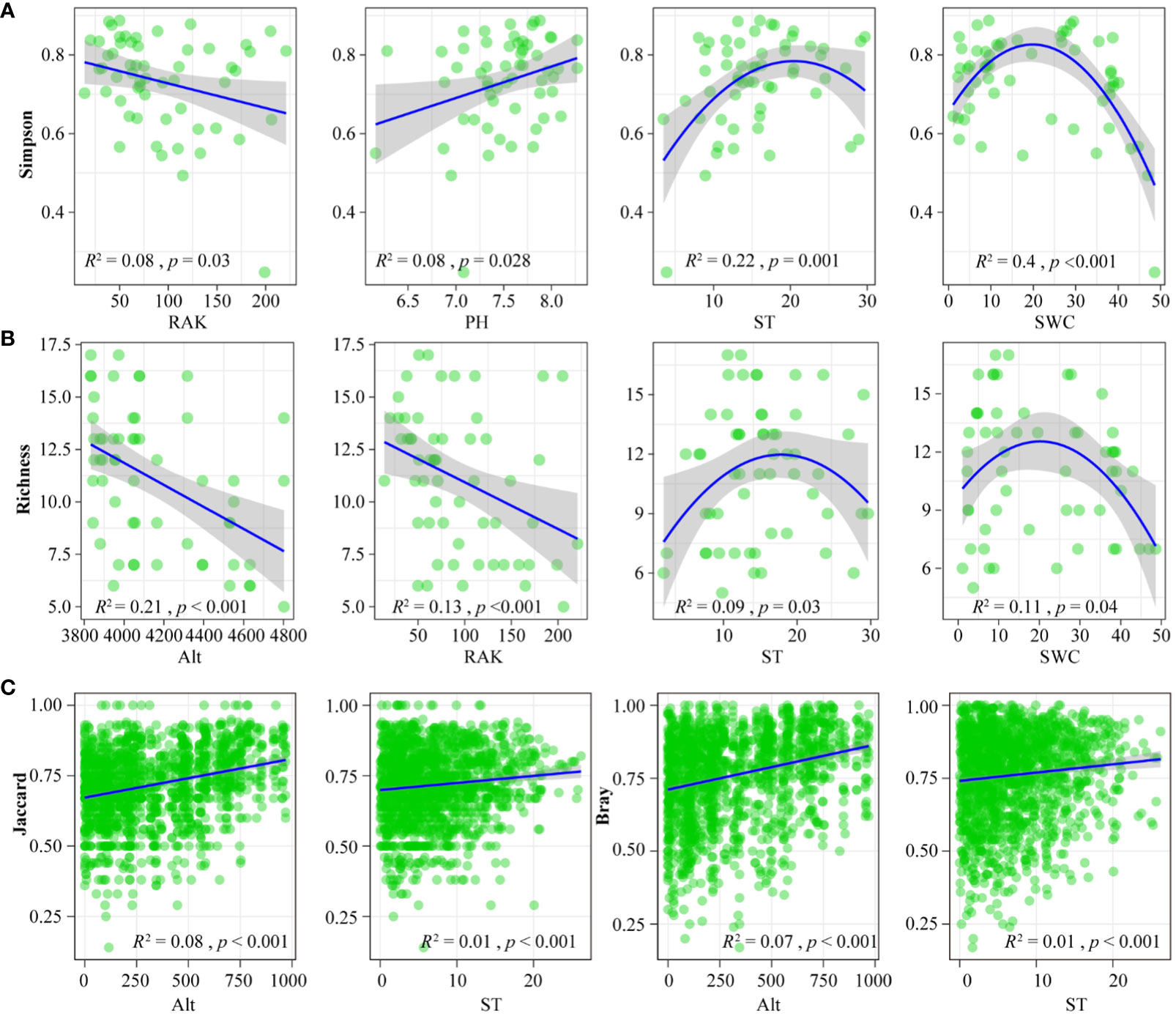
Figure 6 Relationship between soil macroarthropod diversity and environmental factors. (A) Relationship between soil macroarthropod Inverse Simpson and environmental factors. (B) Relationship between soil macroarthropod Richness diversity and environmental factors. (C) Relationship between soil microarthropod beta diversity (Based on Jaccard and Bray) and environmental factors.
Meanwhile, Mantel tests were used to explore the effects of environmental factors on the variation in soil macroarthropod communities among the different seasons and habitat types studied. With regards to seasons, soil macroarthropod community variation showed significant correlations with soil temperature and altitude in autumn (Figure 7A). With regards to habitat, soil macroarthropod community variation showed significant correlations with rapidly available potassium in grassland habitats, and with soil water content in farmland and wetland habitats (Figure 7B). These results suggest that temperature and soil water content are the most important environmental factors affecting the variation in soil macroarthropod communities in the Nianchu River Basin.
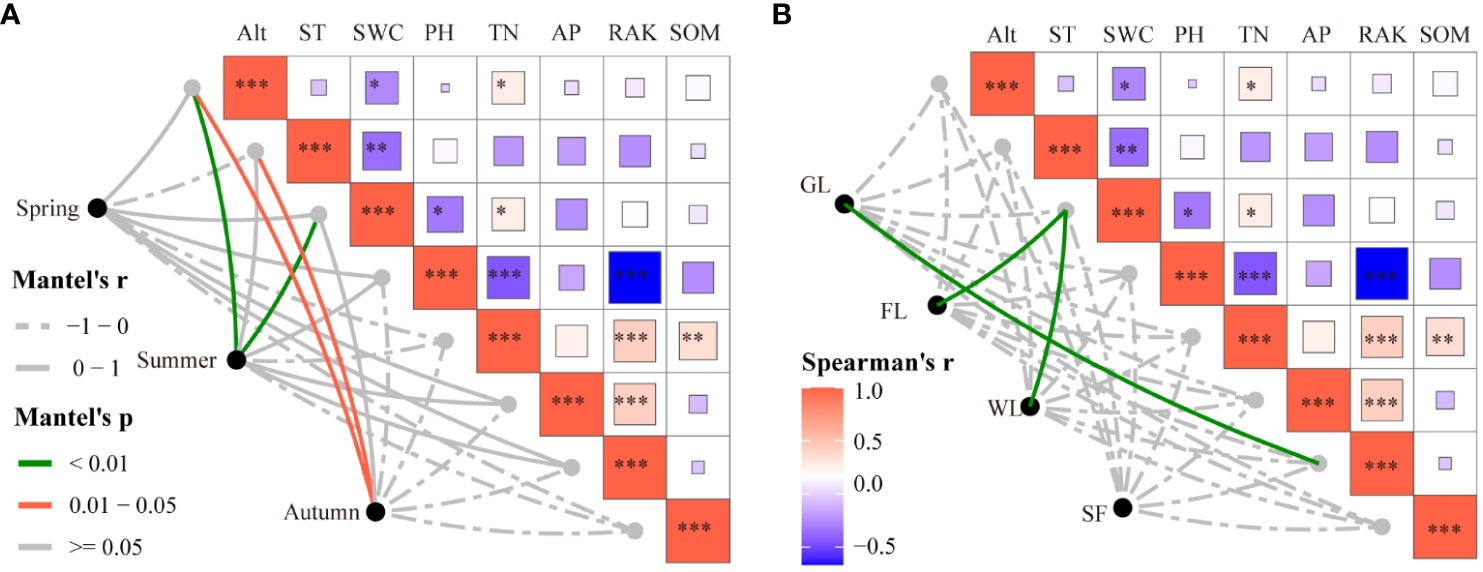
Figure 7 Environmental drivers of soil macroarthropods communities. (A) Evaluated using Mantel tests for the different seasons studied. (B) Evaluated using Mantel tests for the different habitat types studied. The symbols *, **, and *** are used to denote varying degrees of statistical significance.
4 Discussion
4.1 Comprehensive analysis on diversity of soil macroarthropods in Nianchu River Basin
The primary characteristics of soil macroarthropod communities include species composition, quantitative characteristics, and comprehensive characteristics. Soil macroarthropod community structures are closely related to diversity and the living environment; its community structure characteristics are additionally affected by geographical location, vegetation type, and environmental factors, leading to differences in the animal structure characteristics of the soil. The number of soil macroarthropods, the number of groups, and their diversity indices can objectively reflect the species composition among different habitats (Liu et al., 2008; Oxbrough et al., 2010; Culliney, 2013). As far as we know, this is the first study on the diversity, as well as the seasonal and biogeographical dynamics, of soil macroarthropods in the Nianchu River Basin. These results provide new research ideas regarding the dynamics of soil organisms and their adaptability to the environment in the river ecosystem of the Qinghai Tibet Plateau. Over the three seasons considered in this study, a total of 2880 soil macroarthropod specimens were captured in the Nianchu River Basin. Dominant species had obvious controlling effects on the formation of community structures and environments, and they are an important part of the basic characteristics of the community. In this study, the dominant groups, consisting of Carabidae, Formicidae, and Lycosidae, were abundantly distributed among the four habitats and three seasons investigated, and they seemed to be the most important soil animal groups in the Nianchu River Basin, playing an important role in the ecosystem.
4.2 Temporal and spatial patterns of soil macroarthropods in the Nianchu River Basin
Climate conditions are generally considered to be the key determinants of soil animal communities (Lavelle et al., 1997). Soil macroarthropods are affected by climate factors, such as temperature (Irmler, 2006), precipitation, solar radiation (Salmon et al., 2008) and soil moisture (Huhta et al., 1967). Our research clearly shows the spatial and temporal pattern of soil macroarthropods in the Nianchu River Basin. From spring to autumn, the composition of soil macroarthropod communities fluctuated significantly. Similarly, there are seasonal differences in soil animal communities in other rivers (Ye and Liu, 2021). The diversity index is an important tool with which to reflect the functional organization of soil animal communities. The larger the biodiversity index, the more abundant the species, the more complex their community structure, and the more uniform the distribution of the various taxa. The evenness of the distribution can reflect that of individual numbers of each species, and the dominance usually indicates the status and role of a species within the community. In this study, the Hill number serves as a comprehensive approach to assessing biodiversity, encapsulating both species richness and evenness. An upward trend in this metric during the summer months signifies a dual phenomenon: a rise in the variety of soil macroarthropod species, coupled with a more equitable distribution across these species. Many studies have shown that seasonal increases in temperature and precipitation can lead to a decrease in the number of individuals and groups of some soil animals, thereby affecting their community diversity (Bokhorst et al., 2008; Liao et al., 2013). The Nianchu River Basin has distinct seasons, and the rainy season is concentrated in summer. The appropriate temperature and soil moisture cause increases in the density and diversity index of soil macroarthropods in summer. Different ecosystems have different seasonal effects on soil macroarthropods (Noti et al., 1997). The seasonal variation in vegetation types directly affected the diversity of soil macroarthropods among the four studied habitat types in the Nianchu River Basin. The differences in the seasonal dynamics of plant communities may also lead to differences in the microclimate, resource availability, and soil properties among different habitats, thereby directly or indirectly affecting soil animal communities (Irmler, 2006). The study found that sea buckthorn forest habitats had the highest diversity and Hill number of soil macroarthropods. An elevated Hill number in sea buckthorn forests suggests not only a variety of species but also a balanced distribution among these species. This balance is crucial for maintaining ecosystem functions, as it ensures that no single species dominates to the detriment of others. This suggests a unique ecological niche or favorable conditions in these forests that support a diverse arthropod community.
4.3 Temperature and water content are important determinants of soil macroarthropod communities in the Nianchu River Basin
During the long-term adaptation of soil macroarthropods to their living environment, these creatures affected soil physical and chemical properties through their physiological activities, and, in turn, changes in soil nutrient content and other physical and chemical properties also made an impact on the spatial scale, difference distribution, and diversity index of the soil macroarthropods (Li et al., 2017; Lu et al., 2018; Tresch et al., 2019; Ye and Liu, 2021). Soil macroarthropods are significantly affected by several environmental factors, including altitude, soil temperature, and soil water content (Wang et al., 2013; Hao et al., 2020; Xu et al., 2021). Soil water content is the main environmental factor affecting soil macroarthropod communities (Setdila et al., 1995; Wall et al., 2010). The temperature difference between day and night in the Qinghai Tibet Plateau region is relatively large, and the average temperature is relatively low. A suitable temperature is more conducive to the survival of soil animals, and, as the temperature increases, the number of individual soil animals increases (Qian and Wang, 1995; Wang et al., 2021). Different types of soil animals have different requirements for soil moisture content, because different soil macroarthropods have different ecological niches and resource utilization capacities; thus, there are differences in their responses to environmental changes. Differences in soil moisture content can affect the number and species of soil animals, and high or low moisture can affect their number and distribution (Huang et al., 2009). In this study, the most important factors affecting the α diversity for soil macroarthropods were soil temperature and soil water content, and the higher the soil temperature and soil water content, the lower the diversity index. Altitude and soil temperature were the main factors affecting the β diversity for soil macroarthropods. Soil macroarthropod communities showed significant correlations with soil temperature and altitude in autumn, as well as with rapidly available potassium in grassland habitats, and soil water content in farmland and wetland habitats. Other environmental factors also have a certain impact on soil macroarthropod communities, because the effects of environmental factors on soil animals are the results of not one, but multiple factors. Nevertheless, the effect turned negative when the amount of nutrients exceeded that required for soil animals to survive (Villenave et al., 2010; Ayuke et al., 2011). The differences in soil physical and chemical factors among different habitats had different effects on the spatial distribution of soil macroarthropods.
5 Conclusions
From the above experiments and analyses, the following conclusions can be drawn. Firstly, the composition and distribution of soil macroarthropods vary among habitat types. The Hill number of Soil macroarthropod was lowest in summer. Compared with other habitats, the Hill number in sea buckthorn forest habitats was the highest, and the community was relatively stable. The niche breadth of soil macroarthropods was largest in summer, and the community was relatively stable. Soil temperature and soil water content are the most important factors affecting the α diversity of soil macroarthropods, while altitude and soil temperature are the most important factors affecting the β diversity of soil macroarthropods. In addition, we found spatial and temporal heterogeneity in the community composition of soil macroarthropods. Among the environmental factors, altitude, soil temperature, and soil water content are the most important environmental factors affecting the spatiotemporal differences in soil macroarthropod communities. The relationships between soil macroarthropod communities and environmental factors indicated that the adaptive mechanism between soil macroarthropod communities and their living environment has long been present in the Qinghai–Tibet Plateau.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
Z-ZW: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. PZ: Formal analysis, Methodology, Writing – review & editing. KH: Investigation, Supervision, Writing – review & editing. S-YZ: Conceptualization, Formal analysis, Writing – review & editing. BP: Conceptualization, Data curation, Funding acquisition, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a comprehensive scientific investigation project supported by the Chinese central government for the investigation and maintenance mechanism evaluation of biodiversity in the “One River and Four Rivers” watershed [Tibetan Caijiao refers to (2021) 1 and Tibetan Caijiao refers to (2019)01].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1297609/full#supplementary-material
References
Anderson J. M., Ingram J. S. I. (1994). Tropical soil biology and fertility: A handbook of methods. J. Ecology. 154 (4), 265. doi: 10.1097/00010694-199404000-00012
Araújo V., Bandeira A., Vasconcellos A. (2010). Abundance and stratification of soil macroarthropod in a Caatinga Forest in Northeast Brazil. Braz. J. Biol. 70, 737–746. doi: 10.1590/S1519-69842010000400006
Ayuke F. O., Brussaard L., Vanlauwe B., Six J., Lelei D. K., Kibunja C. N., et al. (2011). Soil fertility management: Impacts on soil macrofauna, soil aggregation and soil organic matter allocation. Appl. Soil Ecology. 48, 53–62. doi: 10.1016/j.apsoil.2011.02.001
Bokhorst S., Huiskes A., Convey P. (2008). Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem. 40, 1547–1556. doi: 10.1016/j.soilbio.2008.01.017
Coyle D. R., Nagendra U. J., Taylor M. K., Campbell J. H., Cunard C. E., Joslin A. H., et al. (2017). Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biol. Biochem. 110, 116–133. doi: 10.1016/j.soilbio.2017.03.008
Culliney T. W. (2013). Role of arthropods in maintaining soil fertility. Agriculture. 3, 629–659. doi: 10.3390/agriculture3040629
Davis C. A., Austin J. E., Buhl D. A. (2006). Factors influencing soil invertebrate communities in riparian grasslands of the central platte river floodplain. Wetlands. 26, 438–454. doi: 10.1672/0277-5212(2006)26[438:FISICI]2.0.CO;2
Hao B. B., Cao S. P., Li Y., Liu J., Qiang Q. H., Liu C. H. (2020). Spatial and temporal changes of large and medium-sized soil fauna in different years of returning farmland to forest land in wuqi county. J. arid land Resour. Environ. 34, 130–136. doi: 10.13448/j.cnki.jalre.2020.76
Huang Q. X., Bu Z. G., Hou X. J. (2009). Relationship between soil animal communities and soil physicochemical properties in different green areas of Baoding City. Hebei For. Fruit Res. 24 (02), 195–199.
Huhta V., Karppinen E., Nurminen M. (1967). Effect of silvicultural practices upon arthropos, annelid and nematode populations in coniferous forest soil. Annales Zoologici Fen-nici. 4, 87–145.
Irmler U. (2006). Climatic and litter fall effects on Collembolan and Oribatid mite species and communities in a beech wood based on a 7 years investigation. Eur. J. Soil Biol. 42, 51−62. doi: 10.1016/j.ejsobi.2005.09.016
Jiang M. B., Wang X. H., Liu S., Yun H., Sun X. Q., Zhao C. Y., et al. (2015). Diversity and abundance of soil animals as influenced by long-term fertilization in grey desert soil, China. Sustainability. 7, 10837–10853. doi: 10.3390/su70810837
Kelly C., Schipanski M., Kondratieff B., Sherrod L., Schneekloth J., Fonte S. J. (2020). The effects of dryland cropping system intensity on soil function and associated changes in macrofauna communities. Soil Sci. Soc. America J. 84 (6), 1854–1870. doi: 10.1002/saj2.20133
Lavelle P., Bignel D., Lepagge M. (1997). Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 33, 159−193.
Li W., Dou Z., Cu I. L., Zhao X., Zhang M., Zhang Y., et al. (2020). Soil fauna diversity at different stages of reed restoration in a lakeshore wetland at Lake Taihu China. Ecosystem Health Sustainability. 6, 16. doi: 10.1080/20964129.2020.1722034
Li H. G., Yin X. Q., Ma C., Guo Y. M. (2017). Ecological distribution characteristics of soil fauna community under different land use patterns in Changbai Mountain and hilly region. Acta pedologica sinica. 54, 1018–1028.
Li L., Zha X. Y. Z., Zhuo M., Shi J. Q., Bai M. T. Z., Zha X. O. Z. (2018). Changes of glaciers and glacial lakes in the Nianchu River Basin in Tibet and their response to climate change. J. plateau mountain meteorology. 38, 28–34.
Liao C. H., Li J. X., Yang Y. P. (2013). The community of soil animal in tropical rain forest in Jianfeng Mountain, Hainan Island, China : Relationship between seasonal change of community structure and climatic factors. Acta Ecologica Sinica. 23 (1), 139–147.
Liu T. Q., Chen A. L. (1995). Characteristics of annual precipitation in the Chuhe River Basin. J. Chengdu Univ. Sci. Technology. 4, 12–18.
Liu J. L., Yin X. Q., Qiu L. L. (2008). Relationship between large soil animals and soil factors in Zuojia Nature Reserve. J. Soil science. 45, 130–136.
Lu K. L., Teng Y., Li J. L. (2018). The impact of enclosure on the diversity of soil macrofauna in the degraded typical steppe of Inner Mongolia. Chin. J. Ecology. 37, 2680–2689.
Luo M. J., Li S. S., Qiang Q. H., Liu C. H. (2018). Nanniwan wetland soil animal community composition and relationship of soil physical and chemical properties. J. Ecol. environment. 27, 1432–1439.
Noti M. L., André, Henri M., Dufrêne, Marc (1997). Soil oribatid mite communities (Acari: Oribatida) from high Shaba (Zare) in relation to vegetation. Appl. Soil Ecology. 5 (1), 81–96. doi: 10.1016/S0929-1393(96)00122-9
Oxbrough A., Irwin S., Kelly T. C., Halloran J. O. (2010). Ground-dwelling invertebrates in reforested conifer plantations. For. Ecol. Management. 259, 2111–2121. doi: 10.1016/j.foreco.2010.02.023
Pequeno P., Franklin E., Norton R. A., Morais J. D., Guilherme D. R. (2017). Spatial abundance pattern of a common soil arthropod changes suddenly with season in a tropical rainforest. Pedobiologia. 63, 46–51. doi: 10.1016/j.pedobi.2017.07.002
Phillips H. R., Guerra C. A., Bartz M. L., Briones M. J., Brown G., Crowther T. W. (2019). Global distribution of earthworm diversity. Science. 366, 480–485. doi: 10.1126/science.aax4851
Qian F. S., Wang Z. Y. (1995). The relationship between soil animals and soil environment in Shuidong jujube garden. J. Appl. Ecology. 1, 44–50.
Riutta T., Slade E. M., Bebber D. P., Taylor M. E., Morecroft M. D. (2012). Experimental evidence for the interacting effects of forest edge, moisture and soil macrofauna on leaf litter decomposition. Soil Biol. Biochem. 49, 124–131. doi: 10.1016/j.soilbio.2012.02.028
Rombke J., Sousa J. P., Schouten T., Riepert F. (2006). Monitoring of soil organisms: a set of standardized field methods proposed by ISO. Eur. J. Soil Biol. 42, S61–S64. doi: 10.1016/j.ejsobi.2006.07.016
Salmon S., Artuso N., Frizzera L. (2008). Relationships between soil fauna communities and humus forms: Response to forest dynamics and solar radiation. Soil Biol. Biochem. 40, 1707–1715. doi: 10.1016/j.soilbio.2008.02.007
Setdila H., Marshall V. G., Trofymow J. A. (1995). Influence of micro- and macro-habitat factors on collembolan communities in Douglas-fir stumps during forest succession. Appl. Soil Ecology. 2, 227–242. doi: 10.1016/0929-1393(95)00053-9
Slade E. M., Riutta T. (2012). Interacting effects of leaf litter species and macrofauna on decomposition in different litter environments. Basic Appl. Ecology. 13, 423–431. doi: 10.1016/j.baae.2012.06.008
Tang T. W., Feng Y., Wu X. G., Pan K. W., Zhang L., Zhang X. H., et al. (2020). Community characteristics of soil macrofauna in urban forest of chengdu in winter. Chin. J. Appl. Environ. Biol. 26, 1207–1217. doi: 10.19675/j.cnki.1006-687x.2019.11015
Tiegs S. D., Entrekin S. A., Reeves G. H., Kuntzsch D., Merritt R. W. (2013). Litter decomposition, and associated invertebrate communities, in wetland ponds of the copper river delta, alaska (USA). Wetlands. 33, 1151–1163. doi: 10.1007/s13157-013-0470-5
Tresch S., Frey D., Bayon R., Zanetta A., Rasche F., Fliessbach A., et al. (2019). Litter decomposition driven by soil fauna, plant diversity and soil management in urban gardens. Sci. Total Environment. 658, 1614–1629. doi: 10.1016/j.scitotenv.2018.12.235
Villenave C., Saj S., Pablo A. L., Sall S., Djigal D., Chotte J. L., et al. (2010). Influence of long-term organic and mineral fertilization on soil nematofauna when growing Sorghum bicolor in Burkina Faso. Biol. Fertility Soils. 46, 659–670. doi: 10.1007/s00374-010-0471-y
Wall D. H., Bradford M. A., John M., Trofymow J. A., Zou X. (2010). Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Global Change Biol. 14, 2661–2677. doi: 10.1111/j.1365-2486.2008.01672.x
Wang Z. Z., He K., Zhu S. Y., Pu B. (2021). Characteristics of soil macrofauna community in wetland of Nianchu River Basin, Tibet. J. Arid Land Resour. Environment. 35, 184–190.
Wang X. L., Liu S. C. (2000). Investigation of water resources in Nianchu River Basin in Tibet. Northeast Water Resour. Hydropower. 9, 22–23.
Wang Y., Wang Q. G., Sun Y., Xing Y. J. (2020). Relationship between ecological function of soil fauna and various environmental factors of terrestrial ecosystem. Chin. Agric. Sci. bulletin. 36, 54–59.
Wang W. J., Yang W. Q., Tan B., Liu R. L., Wu F. Z. (2013). Relationship between litter decomposition and soil fauna community structure in subtropical evergreen broad-leaved forest of Sichuan Basin. Acta ecologica sinica. 33, 5737–5750. doi: 10.5846/stxb201305301244
Xu S. B., Li Y. H., Feng X. Y., Li L., Zhu L. Q. (2021). Study on functional groups and influencing factors of Small and medium soil Fauna in Baotianman. J. Nanyang Normal University. 20, 21–30.
Yang G. G., Dou P. P., Ma Y., Wang H. J., Lin D. M. (2020). Characteristics and influencing factors of soil fauna community in subtropical evergreen broad-leaved forest in Jinfo Mountain. Acta ecologica sinica. 40, 762–7610.
Yang X., Shao M. A., Li T. C., Gan M., Chen M. Y. (2021). Community characteristics and distribution patterns of soil fauna after vegetation restoration in the northern Loess Plateau. Ecol. Indicators. 122, 107236. doi: 10.1016/j.ecolind.2020.107236
Yang D. D., Zhou C. P., Hua O. Y., Chuanyou. C. (2012). Analysis of annual runoff variation characteristics in the Nianchu River Basin of Qinghai-Tibet Plateau. J. Resour. Ecology. 3, 80–86. doi: 10.5814/j.issn.1674-764x.2012.01.012
Ye Y., Liu W. H. (2021). The relationship between soil animal community characteristics and environmental factors in subtropical evergreen broad-leaved forest in Xijiang River Basin. J. Northwest Forestry University. 36, 29–35.
Keywords: soil macroarthropods, diversity, community composition, Nianchu River basin, Qinghai-Tibet Plateau
Citation: Wang Z-Z, Zhang P, He K, Zhu S-Y and Pu B (2024) Diversity and distribution patterns of soil macroarthropod communities in the Nianchu River Basin, Tibet, China. Front. Ecol. Evol. 12:1297609. doi: 10.3389/fevo.2024.1297609
Received: 12 October 2023; Accepted: 10 January 2024;
Published: 25 January 2024.
Edited by:
Manuela Branco, University of Lisbon, PortugalReviewed by:
Paulo A. V. Borges, University of the Azores, PortugalIgnacio Castro, Simón Rodriguez National Experimental University, Venezuela
Copyright © 2024 Wang, Zhang, He, Zhu and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bu Pu, cHVyYnV6ZEAxNjMuY29t
 Zhuang-Zhuang Wang
Zhuang-Zhuang Wang Peng Zhang1
Peng Zhang1