- 1Ecology Laboratory, Department of Animal Biology, Faculty of Science and Technology, Cheikh Anta Diop University, Dakar, Senegal
- 2Department of Anthropology, Texas State University, San Marcos, TX, United States
This work focuses on the nesting behavior of the West African chimpanzee (Pan troglodytes verus) in the anthropized habitats of the village of Tikankali and its surroundings. Studies on chimpanzee nesting behavior are carried out at several sites of Senegal but never in Tikankaly. Thus, proximity with the Niokolo Koba National Park and the presence of a gold mining industry mean that data on chimpanzee nesting behavior and anthropogenic impacts in their habitats for decisions-making about chimpanzee conservation in this area. We recorded a total of 213 chimpanzee nests during two surveys over a distance of 47.81 km (i.e., 47.81 km x 2). Data were collected in October 2020 and October 2021. The majority of nests (63%) were found in wooded savannah, 19% in bamboo savannah, 09% in gallery forest and 07% in open forest. The results showed that 22 plant species belonging to 08 families are used for chimpanzee nests in and around Tikankali. However, half of the nests were in Pterocarpus erinaceus (53%); followed by Hexalobus monopetalus (8%); Diospyros mespiliformis (6%), Piliostigma thonningii (6%), Lannea acida (6%); and Grewia bicolor (4%). The average height of trees used as chimpanzee nest supports was 9.88 m (SD=3.60) and the average height of nests was 7.46 m (SD=3.23). Linear regression analysis (r=0.84; N=213; p< 0.05) suggested a preference for nesting at a particular height but also that nest height is a function of the supporting tree’s height. The current study contributes to the knowledge of chimpanzee nesting behavior in Tikankali, the anthropogenic disruption and will help in the implementation of a good chimpanzee management and conservation strategy in Senegal.
Introduction
The West African chimpanzee, Pan troglodytes verus, was classified as “endangered” on the IUCN list of threatened species due to the decline of its numbers in all their habitats (Oates et al., 2008) and then “critically endangered” since 2016 (Humle et al., 2016; Kühl et al., 2017). Thus, to strengthen its protection status, it is also listed in Appendix 1 of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, 2018) and Appendices I and II of the Convention on Migratory Species of Wild Animals (CMS) (UNEP/CMS/COP12, 2017; Ndiaye et al., 2020). Agricultural threats combined with gold mining and other anthropogenic activities have caused western chimpanzee populations to decline by 80% between 1990 and 2014 in West Africa (Kühl et al., 2017). Based on species distribution modeling, it was estimated that approximately 52,800 individuals remain (Heinicke et al., 2019). Habitat loss is one of the most significant threats to global biodiversity. Primates are particularly vulnerable, as tropical forests are being deforested at an alarming rate (Campbell et al., 2008; Kühl et al., 2017; Schwitzer et al., 2019; Diallo et al., 2022). In total, countries with primate populations lose about 125,000 km2 of forest per year (Chapman and Peres, 2021). Agricultural expansion and mining exploitation are responsible for nearly 90% of deforestation in the world (FAO/COP26, 2021). According to FAO, this loss of forest is estimated at more than 420 million hectares worldwide since 1990.
Senegal is characterized by the transformation of forests and other chimpanzee habitats into agricultural land and a development of gold mining exploitation (Ndiaye et al., 2018a). In the commune of Tomboronkoto (Kedougou/Senegal), including the Tikankali area, the gradual felling of trees important to chimpanzee nesting and feeding areas has been observed (Badji, 2019). Overall, the conversion of chimpanzee habitat to agricultural land is becoming more and more frequent in this area and thus poses a real threat to chimpanzees. If these activities continue, this could lead to impacts in nesting behavior and result in the displacement of chimpanzees to other habitable areas. Chimpanzees play an important role in the regeneration of forest and wooded ecosystems (Badji, 2019). This global observation on habitat loss and ecosystem destruction in Senegal is far from reaching its limits due to population growth, overexploitation of forest resources, and economic demands (Pruetz et al., 2002; Pruetz, 2006; Badji, 2013; Ndiaye et al., 2018a).
Agricultural expansion with systematic felling of trees and slash-and-burn systems is impacting chimpanzees’ home range for both nesting and feeding (Badji, 2019). Chimpanzees are known to build sleeping or resting platforms called nests using the branches and/or leaves of these trees (Tutin and Fernandez, 1984; Goodall, 1962). However, other parameters such as anthropogenic factors like gold mining, agricultural activities, pastoralism, and poaching can also influence site selection and nesting heights in chimpanzees (Potts et al., 2011).
Chimpanzee nest building has continually been an important aspect of primate ecology research (Goodall, 1962; Reynollds, 1965; Baldwin et al., 1981; Anderson et al., 1983; Fruth and Hohmann, 1994). These tree nests allow chimpanzees to protect themselves from environmental challenges and predators (Stewart, 2011; Stewart and Pruetz, 2013). Sometimes chimpanzees build nests on the ground Ham (1998). This ground nesting behavior was first described by Matsuzawa and Yamakoshi (1996) and confirmed by Koops et al. (2007). In Senegal, this ground nesting behavior was described by Pruetz et al. (2008) and Badji et al. (2018) respectively in Fongoli and Bagnomba. Ndiaye Papa Ibnou and Ndiaye Yaya Hamady (unpublished data) also observed this behavior in chimpanzees living outside protected areas, more precisely in the Randgold mining permit in Massawa in 2020.
Preferences for nest-supporting plant species have been noted, as well as choice of nest-building heights (Wrogemann, 1992; Fleury-Brugière, 2001; Badji et al., 2018; Badji, 2019). Tree selection for nesting by chimpanzees has been attributed to the physical properties of trees, including tree diameter, tree height, and height of the lowest branch (Hakizimana et al., 2015); Hernandez-Aguilar et al., 2013) and the type of plant species (Brownlow et al., 2001; Sanz et al., 2007; Stanford and O’Malley, 2008). In addition to tree selection, chimpanzees also select nesting areas within forests, particularly with a preference for forest cover (Koops et al., 2012; Bryson-Morrison et al., 2017), high altitude (Koops et al., 2012; Barca et al., 2018), steep slopes (Dutton et al., 2017; Abwe et al., 2018; Kamgang et al., 2018) and a closed canopy (Abwe et al., 2018).
The main objective of this study is to improve data about chimpanzee nesting behavior in Senegal and the understanding of environmental factors affecting chimpanzee nesting behavior in this country. Thus, we aim to provide information about the plant species bearing chimpanzees nests, nest heights, nesting habitats and the impact of human activities, particularly gold mining on chimpanzee nesting behavior in Tikankali. The results of this study will be very useful for decisions-making and the management of natural resources and space for the conservation of the West African chimpanzee in this municipality.
Methods
Study area
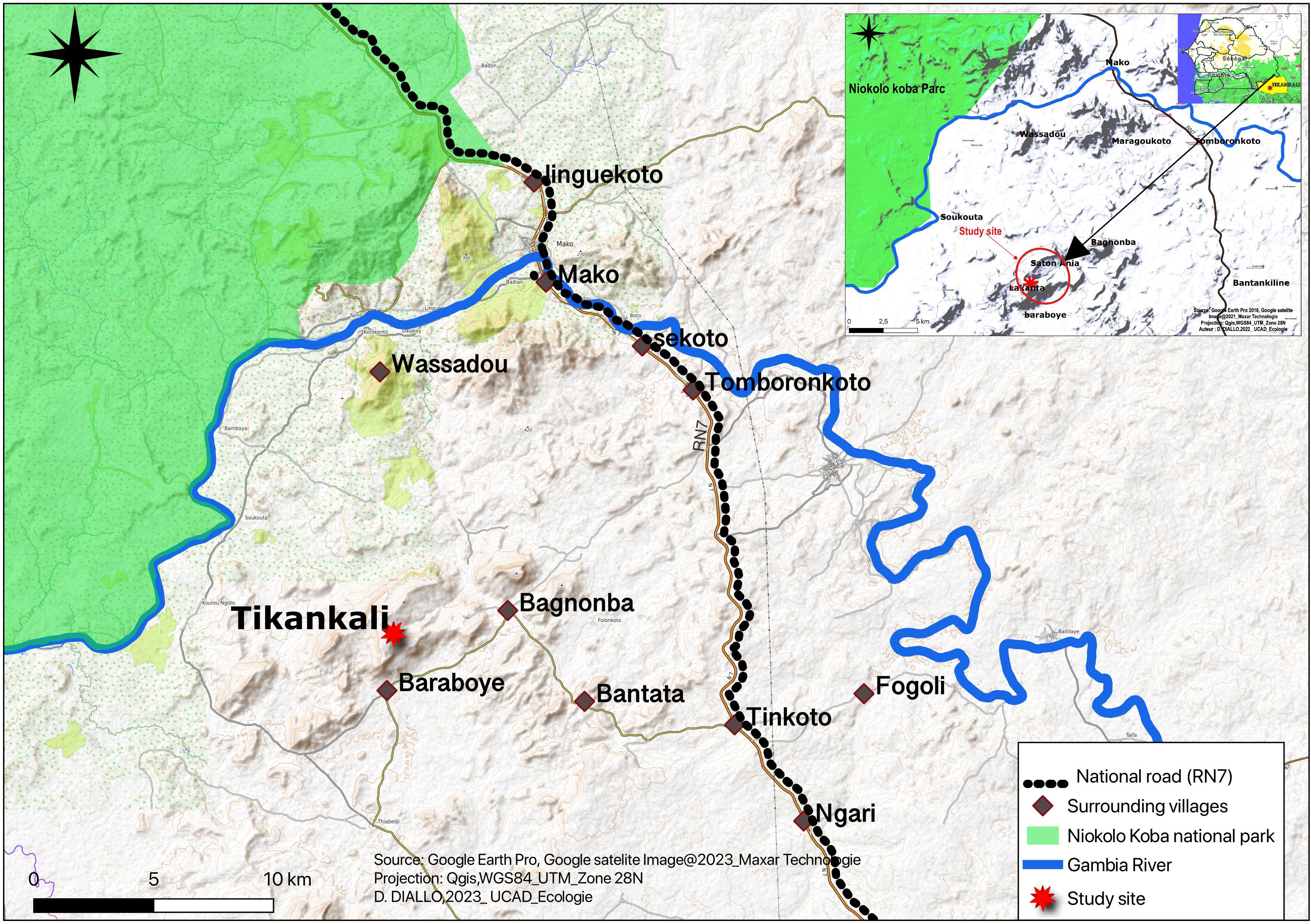
The study was carried out in the area of the village of Tikankali in the southeastern part of Senegal (12°.70N; 12°.40W), in the department of Kedougou, district of Bandafassi, and in the commune of Tomboronkoto. Tikankali is located 12 km from the boundaries of Niokolo Koba National Park (PNNK) and about 10 km from the Gambia River (Figure 1). The surveyed area in Tikankali is in the north of the hilly village of Baraboye and west of the village of Bagnomba. Next to the village of Bagnomba, there is a special biotope, a 423 m high hill used as a refuge for chimpanzees (Ndiaye et al., 2015; Badji et al., 2018). The vegetation of this rugged area consists largely of Pterocarpus erinaceus, Diospyros mespiliformis, Hexalobus monopetalus, and various Combretaceae. The climate is Sudano-Guinean with an annual rainfall of 1200 to 1300 mm (Arbonnier, 2009). However, the recent growing of artisanal mining exploitation is associated with the immigration of people disposed to hunt and eat chimpanzees. For this, they need to work closely with local people and wildlife conservation authorities for bring up a good strategy for the conservation of chimpanzees and their habitats in this area.
Data collection
The data for this study were collected during a total of 41 field days distributed between two expeditions: the first one in October 2020 and the second in October 2021. We used the reconnaissance transect method to collect data on chimpanzees’ presence. This active survey method involves walking in a predetermined direction along a transect. It allows for deviation from the pre-established central direction to walk along areas where the potential to see evidence of chimpanzee presence is more frequent (Ndiaye et al., 2018a). Thus, we walked a total of 95.62 km (47.81 km x 2) of transects distributed proportionally over the different types of habitats (wooded savannah, forest gallery, light forest, shrub savannah, bamboo savannah). We also noted that the same transects with the same distances were walked in October 2020 and October 2021. Taking into account that previous studies have shown that the chimpanzees’ habitats in this area are particularly forest galleries and galleries on the edges of plateaus or hills, we focused our surveys for this study along all habitats likely to support chimpanzees to subsequently analyze chimpanzee habitats occupation. Surveys are done on foot from early morning (from 06 h at the latest) to late afternoon, between 16 h and 17 h. Sometimes we took breaks of about 10 minutes to rest. Our walking speed was about 1km/h on average because of the hilly configuration of the terrain (hills and rocks).
If evidence of chimpanzee presence was observed, we recorded the geographic coordinates of the location of the observed nests, the time of observation, the number of nests per tree, the supporting tree of each nest, the height of the nest from the ground, the height of the supporting tree and the age of the nest and anthropogenic impacts that threaten chimpanzee survival in this environment. The age of the nest (fresh, recent, old, decomposed) is determined according to the classification of Tutin and Fernandez (1984) (Table 1). For the measurement of the height of the nests and the supporting tree, we used a rangefinder (Bushnell YARDAGE PRO 450). During the surveys, we also recorded any threats to the chimpanzee’s survival (deforestation, agriculture, pastoralism).
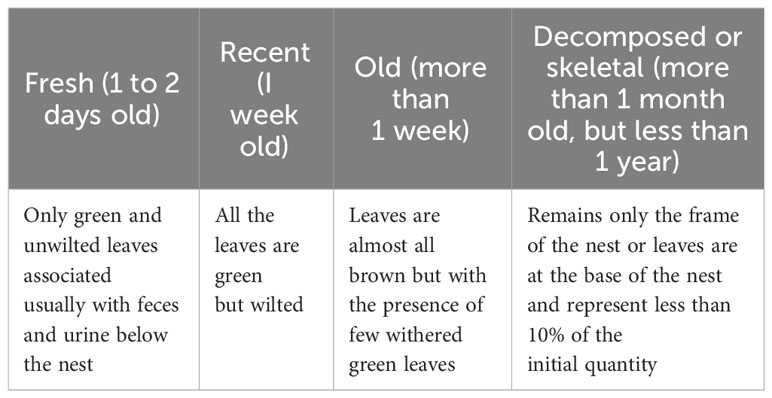
Table 1 Determination of the nest age according to Tutin and Fernandez (1984).
Data analysis
The data analysis was done using different software. We entered all the data from the fieldwork in an Excel spreadsheet version (16.61.1). Then, we proceeded to the analysis of the distribution of the nests according to the supporting plant species and the distribution according to the height and the type of habitat by using the dynamic cross table. We used the data in the spreadsheet to make index projections on maps, statistical tests, and graphs with Qgis version (3.20.2-Odense) and Statistical Package for the Social Sciences (SPSS) version (28.0.1.1), respectively.
We mentioned all the indices of anthropogenic activities observed Durant the field work and we took pictures. Thus, mapped all this information for identify the overlaps of this activities and chimpanzee’s nesting sites. According to Hunt and McGrew (2002), in Tikankali vegetation type is characterized particularly by:
● Arboreal savannah: Arboreal savannah comprises large trees with a very sparse canopy. Shrubs and grasses may be present in the middle canopy and ground layer, as the canopy leaves sufficient light;
● Shrub savannah: this type of savannah has no canopy and is dominated by shrubs and a continuous expanse of grass;
● Wooded savannah: this savannah ecosystem is characterized by a mosaic of scattered trees and shrubs forming a clear canopy. This habitat remains the dominant vegetation formation in the area, and is often interspersed with dense vegetation, sometimes interspersed with bamboo (Oxytenanthera abyssinica), known as bamboo savannah. In this study, we have considered the two vegetation compositions as separate habitats (wooded savannah and bamboo savannah) and
● Gallery forest (mostly evergreen and often closed canopy) grows from steep-sided watercourses in narrow, alluvial valleys cut by erosion through laterite pans. The woody vegetation is a multi-layer mix of lianas, shrubs, and trees.
We used linear regression analysis and the Fischer test for identify relationships between tree height and nest height in this site
Results
Distribution of nests
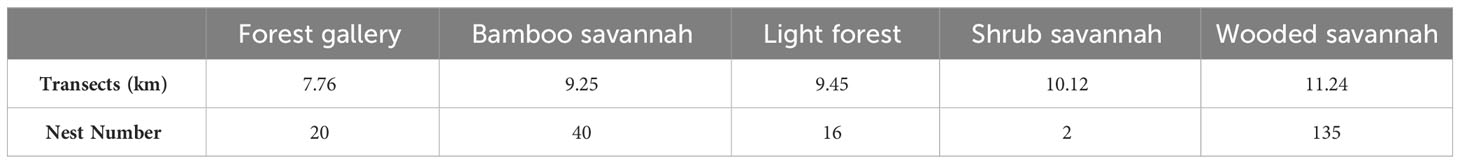
We recorded a total of 213 chimpanzee nests on the total length of walked transects (95.62 km) distributed proportionally over the different types of habitats of the study site (Table 2).
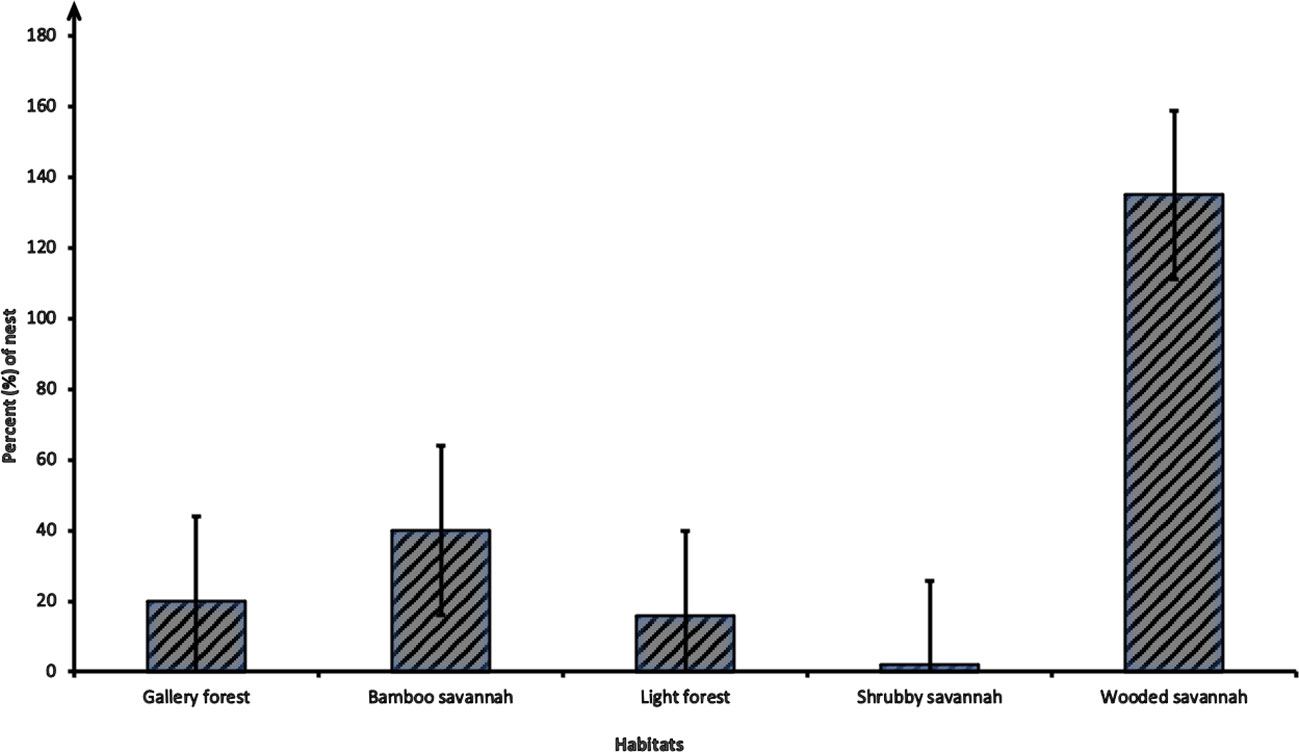
The distribution of nests by habitat showed that chimpanzees used some habitats much more than others. The largest number of nests was found in woodland savannah (135 nests), followed by bamboo savannah (40 nests), gallery forest (20 nests), open or Light Forest (16 nests), and shrubby savannah (2 nests), which accounted for only 1% of observations (Figure 2).
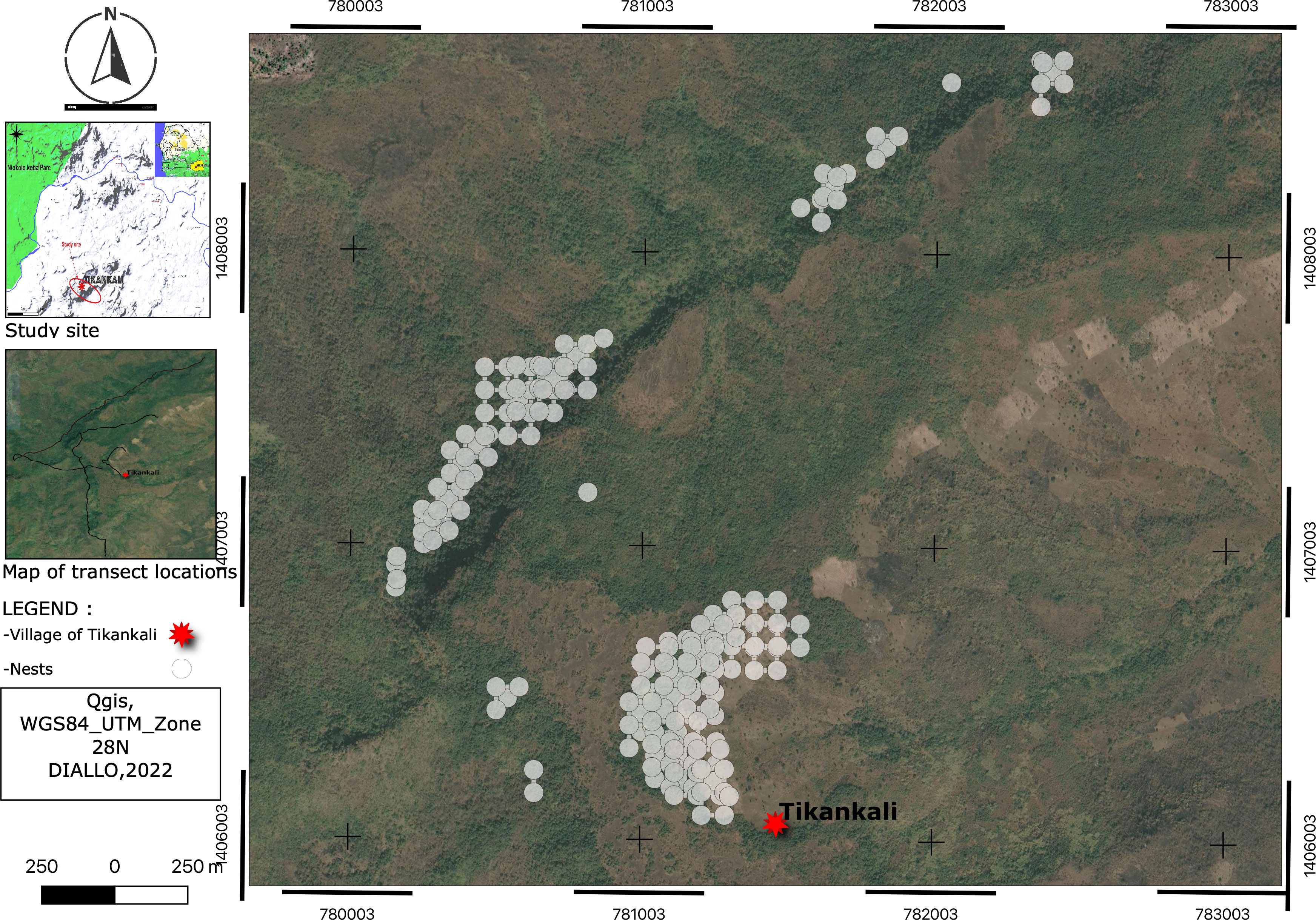
We found that most nests are concentrated on hills edges or in galleries forest, which is often difficult for humans to access. The data also revealed fragmented habitat use by chimpanzees (Figure 3).
To avoid overlapping indices on the spatial nest distribution map, we projected the coordinates of the nests and then in the symbology menu on Qgis, use the cluster mode with point displacement to avoid the superposition of the clues. In the case of index overlay, there is a central point and a grid of points.
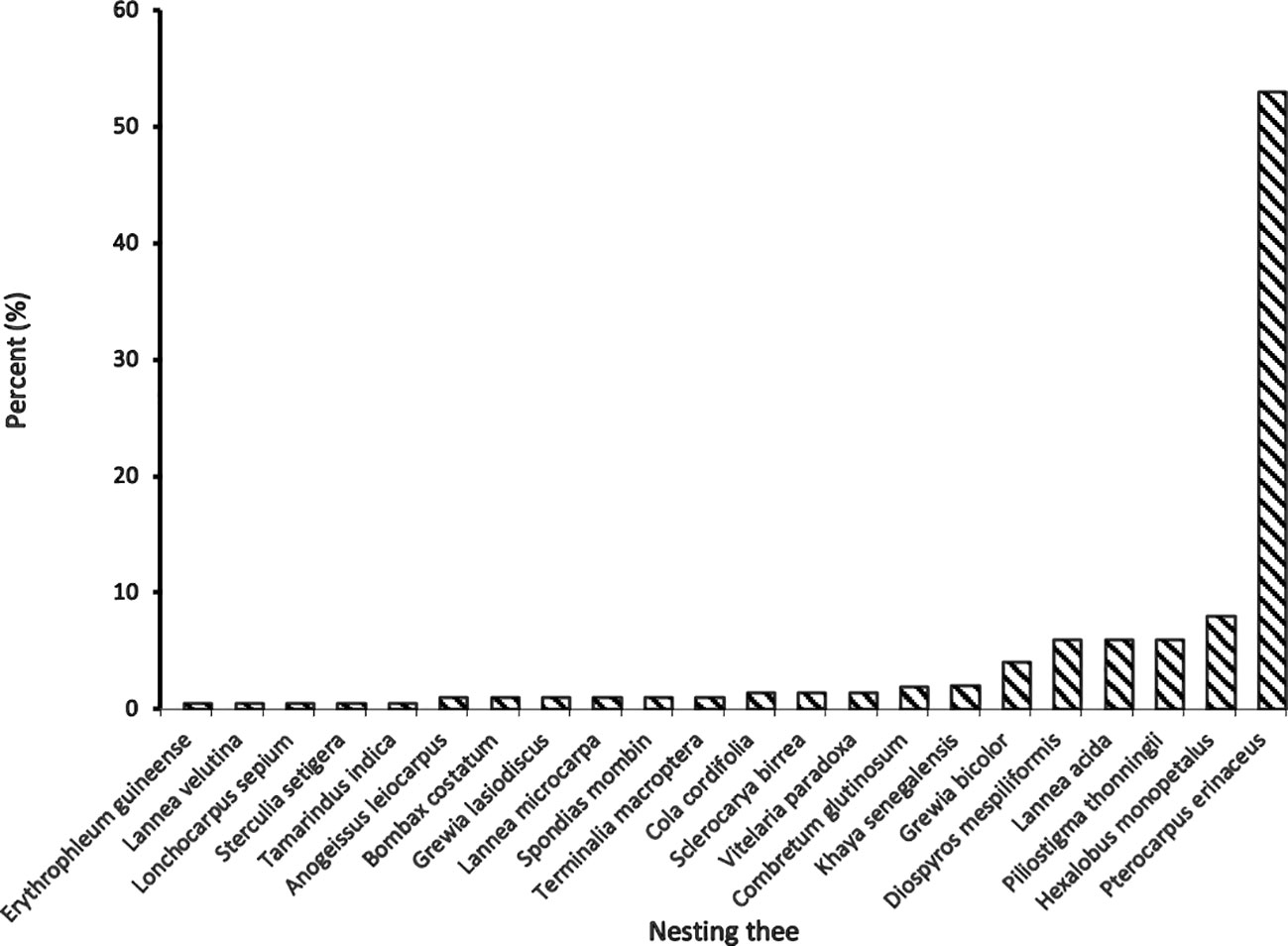
At least 22 plant species belonging to 8 families carried chimpanzee nests in and around Tikankali (Figure 4). Analysis of the data revealed a highly disproportionate distribution of nests by nest-supporting plant species.
Half of the nests were in Pterocarpus erinaceus (N=113; 53%); followed by Hexalobus monopetalus (N = 17; 8%); Diospyros mespiliformis, Piliostigma thonningii, Lannea acida (N=13; 6%); and Grewia bicolor (N = 9; 4%). We also found that Khaya senegalensis, Cola cordifolia, and Anogeissus leiocarpus are weakly involved in chimpanzees’ nest building in this area.
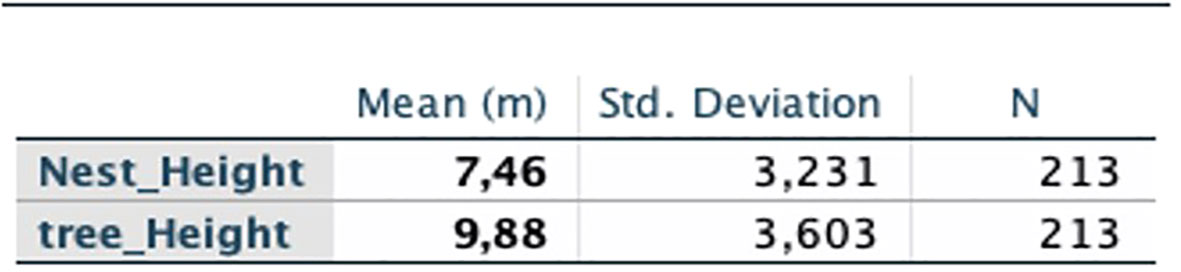
The measurements taken on the supporting tree with the rangefinder allowed us to calculate the average tree height and the average nest height in the study area (Figure 5).
Of the 213 nests identified, only 0.93% of the nests had a height greater than 14 m, 11.2% were at heights between 12 and 14 m, 16.43% were between 10 and 12 m, 24.88% at heights between 8 and 10m, 16.46% at heights between 6 and 8m, 15.49% between 4 and 6m and 14.54% were at a height of less than 4m.
The histogram data show that most of the plant species carried that nest construction were between 6.00 m and 14.00 m high, and the majority of the chimpanzees’ nests were between 4.00 m and 12.00 m high (Figure 6). Thus, it can be seen that chimpanzees did not preferentially nest at heights given the high cumulative number of nests between 2 and 8 m (69.01%). The results in Figure 4 showed that chimpanzees did not build nests at heights above 15 m, yet they nested on trees taller than 15 m.
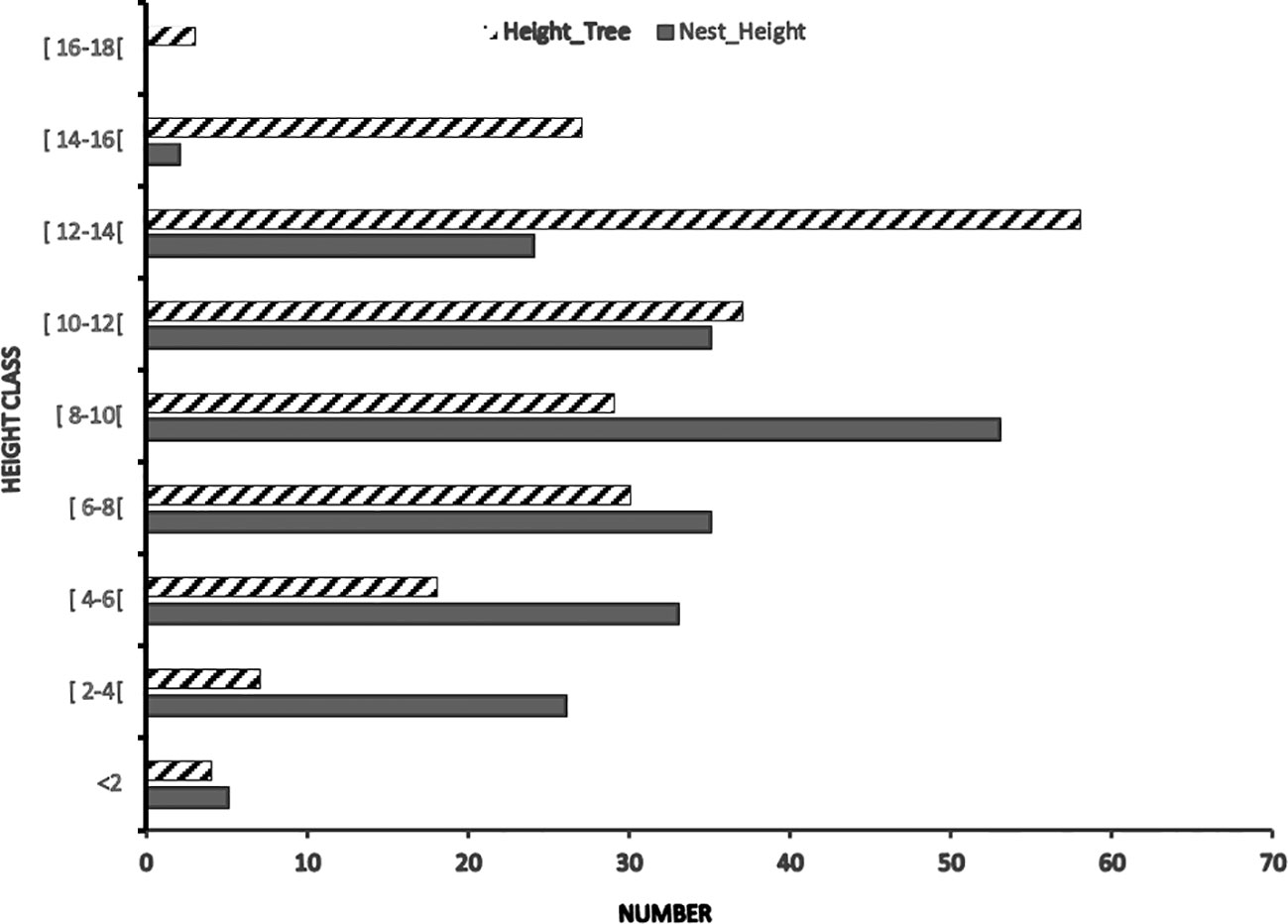
Figure 6 Distribution of the height of nests (HN) of chimpanzees and support trees (HT) according to 2-meter size classes.
Statistical tests
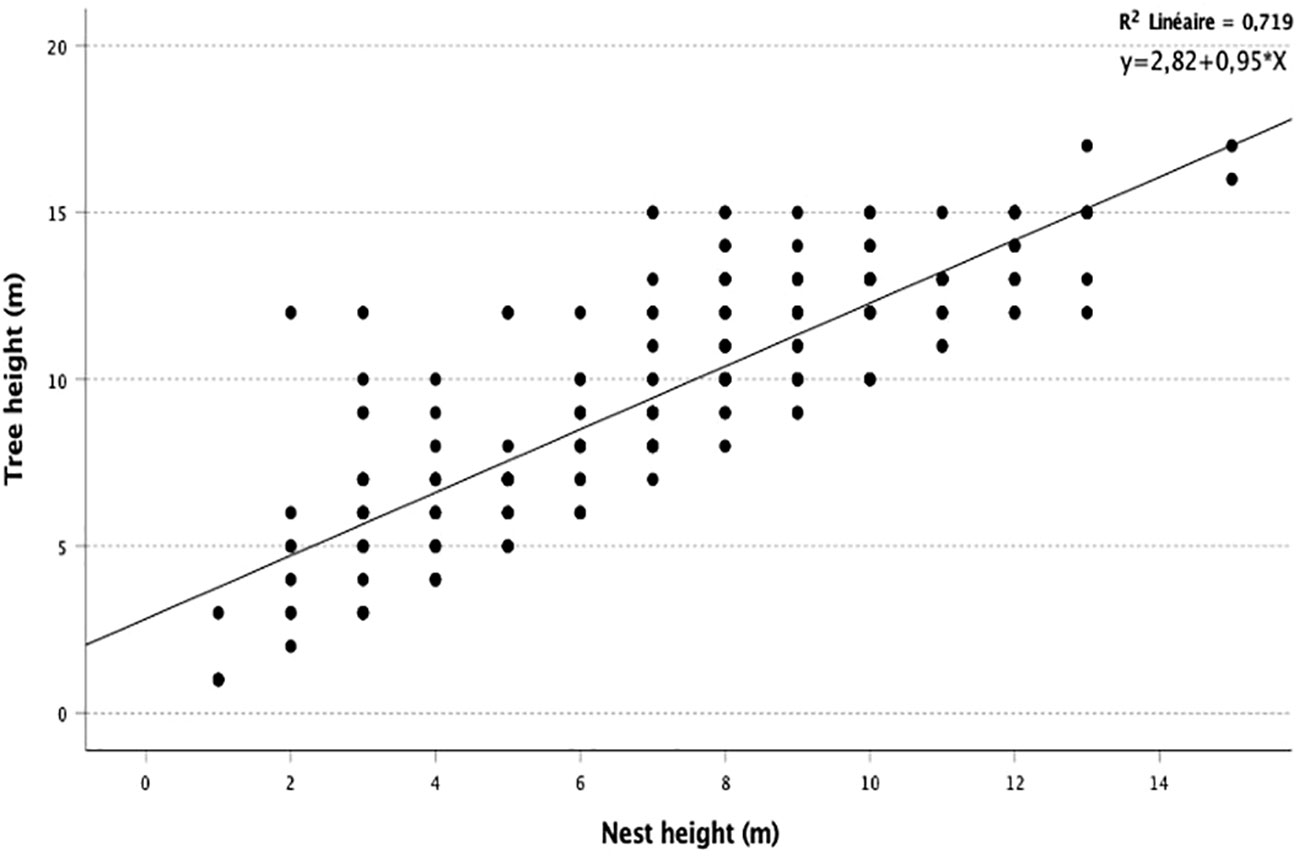
Linear regression analysis shows that there was a strong relationship between tree height and nest height. This correlation is statistically significant, and 71.9% of the variability (r-two) of the sample is explained by regression (R = 0.84; N = 213; P < 0.05) (Figure 7). The results allowed us to confirm the hypothesis that chimpanzees tend to nest at higher elevations as tree height increases. In contrast, the reduced rate of nests found between 14 and 16 m suggests that this increase in tree height is not the primary determinant of increased nesting height.
The significance of the Fischer test is less than 0.01. This means that the relationship between the two variables (HN and HT) is highly significant. Given the sign of the coefficient and the probability obtained, we could say that tree height has a positive significant effect on the nest height at the 1% threshold.
Threats
Threats come in many distinct forms and orders. They range from degradation to elimination of chimpanzee habitats. Most of the threats recorded were related to human activities (agriculture/pastoralism). These threats are assessed in terms of impacts and evidence of the presence in the study area. In Tikankali, the lack of arable land remains a real problem for indigenous populations. The only area suitable for agricultural activities is mainly found in the flat parts of the valleys. People walk a rough path of 2 to 3 km before arriving at suitable areas for agriculture, in proximity to the nesting grounds of chimpanzees. The scarcity of arable land because of the rugged terrain means that these populations benefit at most from the few places favorable to agriculture. This growing need for arable land is often synonymous with large-scale clearing and alteration of the natural landscape.
Overall, the major threats to chimpanzees are clearing vegetation for agriculture, ‘transhumance’ (transient pastoralist communities explore the area and prune branches of an assortment of tree species to be used as fodder for their livestock), fires, and logging in specific places.
This image shows how woodlands are being assaulted and transformed into farmland in the Tikankali area (Figure 8). In addition to being a source of deforestation, this conversion of forest into farmland may in the future be a source of conflict between humans and wildlife due to cohabitation.
Discussion
In Tikankali, we recorded chimpanzee nests in 22 plant species. Among these species carrying nests, we recorded predominantly 6 species: Pterocarpus erinaceus, Hexalobus monopetalus, Diospyros mespiliformis, Piliostigma thonningii, Lannea acida and Grewia bicolor accounted for 83% of nests. Except for Lannea acida, Grewia bicolor and Hexalobus monopetalus all other plant species involved in nesting in Tikankali remain almost the same compared to studies conducted in nearby Bagnomba (Badji et al., 2018), Bantankiline (Diallo, 2018; Diallo et al., 2022) and elsewhere in the Kedougou region (Ndiaye et al., 2013; Ndiaye et al., 2018b; Sylla et al., 2022). These authors have described a preference on tree bearing chimpanzees nests that can be explained by several essential and extrinsic parameters (age of the individual, sex, social position, physiological state, habitat type, vegetation, climate, presence or absence of predators, etc.) (Baldwin, 1979; Baldwin et al., 1982; Groves and Sabater, 1985; Sept et al., 1992; Lehman and Fleagle, 2006; Pruetz et al., 2008; Kamilar, 2009; Badji et al., 2018; Badji, 2019; Diallo et al., 2022). In the present study, this choice would seem to be related to the abundance and physical properties of the support species because we found that the majority of plant species involved in nest construction are known for the hardness of their wood (Ndiaye et al., 2013). Nevertheless, a further study of the abundance and physical characteristics of these species and the vegetation availability would be necessary to confirm these observations on selection.
Analysis of chimpanzees’ nest distribution across space shows a highly disproportionate use of habitats.
Chimpanzees in and around Tikankali had a patchy use of space and nested much more in wooded savannah and bamboo savannah habitats than other habitats (82.16%) nests (Figure 3). These results are consistent with the observations of Pruetz et al. (2002); Hernandez-Aguilar (2009); Boyer (2011); and Ndiaye et al. (2018a); Ndiaye et al. (2018b). The most frequently used habitat remains the wooded savannah with 63.38% of nests. This observation corroborates with the results of the study by Pruetz et al. (2008) in Fongoli and Diallo et al. (2022) in Bantankiline with (67%) and (47.71%) of nests, respectively located in the wooded savannah. In contrast, the considerable number of nests found in the bamboo savannah (18.76%) is higher than the number of nests found in Fongoli and Assirik areas 3% of nests (Pruetz et al., 2008). This increased nesting in our study at this open bamboo savannah habitat may be due to anthropogenic pressure on chimpanzee habitat that has been reported by other authors (Pruetz, 2002; Pruetz, 2006; Badji, 2013; Ndiaye et al., 2013; Badji et al., 2018; Diallo et al., 2022). These open habitats are difficult to access and are frequently found in rugged areas with very little frequency by humans, thus more isolated and safer for chimpanzees. In sum, the disproportionality in the distribution of nests in different habitats could be caused by the availability of plant species in these habitats or by their stability.
Topographically, our study area is more rugged than the Fongoli and Bantankilin areas, which are characterized by flat areas with wooded savannah as the dominant habitat (Pruetz et al., 2002; Diallo et al., 2022). Authors such as Furuichi and Hashimoto (2004) and Ndimuligo (2007) have reported that the topography of the environment can also influence the natural distribution of chimpanzees.
In this study, gallery forests generated only 9.39% of nests. This low nesting rate in the galleries was also observed in the Fongoli area with 9% of nests (Pruetz and Bertolani, 2007). This observation may be due to the scarcity of such habitat types (Tappan, 2010). We know from Baldwin et al. (1981) and Pruetz et al. (2008) that chimpanzees in Senegal nest preferentially live in closed habitats such as gallery forests. At Mount Assirik, for example, 54% of nests are in gallery forests (Pruetz et al., 2008) Similarly, Baldwin et al. (1981) showed that chimpanzees living in the Niokolo Koba National Park, and more specifically on Mount Assirik, nested more on forest galleries than on other types of habitat. The use of gallery forests as nesting habitat is also seasonally dependent because according to Ndiaye et al. (2013) during the dry season, chimpanzees nested more frequently in gallery forests, likely due to the availability of drinking water, food, and dense vegetation. Conversely, flooding of valleys during the rainy season and decreasing availability of nesting trees may encourage chimpanzees to move to higher areas (hills and gallery forests at the edge of the hill) or to nest higher up on the gallery according to Baldwin (1981). The lower nesting rate in gallery forests in our study area may be related to the low availability of this habitat and by anthropization and human presence in this particular biotope (Badji, 2013; Badji, 2019). This observation remains the same as those of several other authors (Lehman and Fleagle, 2006; Kamilar, 2009; Potts et al., 2011) who reported that the distribution of chimpanzee nests can be strongly influenced by human presence.
In this study, the average nesting height (NH) is 7.46 m and the average tree height (TH) is 9.88 m. Given the large number of nests built at heights below 9m (71.33%) and the average nest heights, Tikankali chimpanzees did not appear to nest at heights reported for other Senegalese chimpanzees (Pruetz et al., 2008; Badji et al., 2018; Badji, 2019; Diallo et al., 2022; Sylla et al., 2022) where the average heights were 8.33 m a Fongoli; 7.90 m in Bagnomba, 10.50 m in Tomboronkoto, 8.7 m in Niokolo Koba National Park and 9.91 m in Bantankilin, respectively. This difference in nesting height between these different sites can be explained by the configuration (topography) and floristic composition of the ecosystems sheltering these chimpanzees or to the presence of predators, which is why Baldwin et al. (1981) say that the height at which nests are built is also influenced by the presence of predators such as panthers. To describe this behavior, the author tried to compare the nesting behavior of chimpanzees living on Mount Assirik with that of another community living in Equatorial Guinea. chimpanzees in Equatorial Guinea, while there were more large trees in Guinea than in Senegal, but the existence of chimpanzee predators was greater on Mount Assirik than in any other area in the chimpanzee’s range.
We suggest that chimpanzees have diverse behaviors at different nesting sites, even within the same general region. Thus, depending on the location and habitat, the layout of nests varies in height, and authors such as Goodall (1962) have stated that nests can be built at any height above 15 feet above the ground. Nevertheless, similarities in nesting behavior have been noted at some sites with ecological similarities such as Tikankali (NH=7.46m) and Bagnomba (NH=7.90m); Bantankiline (NH=9.91 m) and Fongoli (NH=8.83 m). At these sites, we found that chimpanzees have virtually the same nesting habits in terms of average nest height.
In terms of threat, the transformation of forests into arable land is the most common phenomenon in the study area. This deforestation activity results in a reduction and fragmentation of chimpanzee habitats. Because cropland is extremely close to chimpanzee habitats, their expansion encroaches on the chimpanzee home range, which can create a dangerous social dynamic between farmers and non-human primates (Oates et al., 2007; Mittermeier et al., 2009). This conversion of forests to arable land would reduce the space occupied by chimpanzees and thus cause a change in their nesting behavior or push chimpanzees to take refuge in isolated areas for greater safety (Badji, 2019). These behavioral changes could encourage chimpanzees to rob people’s crops and thus create a situation of human and chimpanzee conflict. This could increase the constraints on the survival of this species. Gold mining, which was identified as the main threat in Bantankiline (Diallo et al., 2022) is relatively low in Tikankali.
Conclusion
This work shows the continued presence of chimpanzees in the habitats of Tikankali despite the alarming presence of threats from the transformation of forests into cropland. Chimpanzee nests are mainly found in wooded savannah, bamboo savannah, and forest gallery habitats. The most commonly used plant species for bearing nests in Tikankali are Pterocarpus erinaceus, Hexalobus monopetalus, Diospyros mespiliformis, Piliostigma thonningii, Lannea acida, and Grewia bicolor (83% of nests). We have observed a risk of impacts of this ecosystem by anthropogenic factors characterized essentially by excessive deforestation related to the expansion of crop fields. Chimpanzee nesting height is generally between 4 and 15 m with an average height of 7.46 m. This variation in average nesting height may be related to predators, the height of available plant species, or the topography of the nesting site. The information from this work will help to highlight the situation of the chimpanzees and the threats to their survival in this area. This information will also be very useful to feed into action plans and management of natural resources in this area and decision-making by administrative authorities and natural resource managers for the implementation of good biodiversity conservation policies, especially for the chimpanzee and their habitats.
Data availability statement
The datasets presented in this article are not readily available because the data are from a collaboration between the university and the mining company Petowal mining and can only be used upon agreement of both parties. Requests to access the datasets should be directed to Dame Diallo, ZGFtZS5kaWFsbG9AdWNhZC5lZHUuc24=.
Ethics statement
The animal study was approved by Comité d’Ethique de la Recherche (CER)/UCAD. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
This work results from a close and fruitful collaboration between the authors from the work’s planning to the manuscript’s writing. The authors were each involved according to their abilities, availability, and possibilities. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. DD was granted aid by Petowal Mining Company S.A. (PMC) for his doctoral thesis.
Acknowledgments
The authors of this work sincerely thank the administrative and academic authorities of the Cheikh Anta Diop Universities of Dakar (Senegal), and Texas State University (United States). They confound the technical and financial partners, respectively the Direction des Eaux et Forêts, Chasse et conservation des sols the Direction des Parcs Nationaux and Petowal Mining Company SA, without their support this work could not have been carried out. A special mention is reserved for the local populations of Tikankali, especially to our field guide Mamadou Camara for his generosity and availability. Data were collected during the biodiversity survey organized by the Department of Environment of Petowal Mining Company S.A. (PMC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abwe E. E., Morgan J. B., Tchiengue B., Kentatchime F., Doudja R., Ketchen M. E., et al. (2018). Habitat differentiation among three Nigeria-Cameroon chimpanzees (Pan troglodytes ellioti). Ecol. Evol. 9, 1489–1500. doi: 10.1002/ece3.4871
Anderson J. R., Williamson E. A., Carter J. (1983). Chimpanzees of Sapo forest, Liberia: Density nests, tools and meat-eating. Primates 24, 594–601. doi: 10.1007/BF02381692
Arbonnier M. (2009). “Trees, shrubs, and lianas of the dry zones of West Africa Third Edition Quae,” in National Museum of Natural History, EAN13 eBook (PDF): 9782759206742, 574p.
Badji L. (2013). Contribution à l’étude du régime alimentaire du Chimpanzé, Pan troglodytes verus à Fongoli (Kédougou, Sénégal) (Sénégal: Mémoire de master Université Cheikh Anta Diop de Dakar), 35p.
Badji L. (2019). Éléments d’écologie du chimpanzé d’Afrique de l’Ouest (Pan troglodytes verus) dans la commune de Tomboronkoto (Kédougou, Sénégal): implication pour sa conservation (Sénégal: Thèse de doctorat unique d’Écologie et Gestion des Écosystèmes, Faculté des sciences et techniques, université Cheikh Anta Diop), 112p.
Badji L., Ndiaye P. I., Lindshield S. M., Ba C. T., Pruetz J. D. (2018). Savanna chimpanzee (Pan troglodytes verus) nesting ecology at Bagnomba (Kedougou, Senegal). Primates 59, 235–241. doi: 10.1007/s10329-017-0647-2
Baldwin P. (1979). The natural history of the chimpanzee (Pan troglodytes verus) at Mt. Assirik, Senegal (USA: University of Stirling, Stirling), 284p.
Baldwin P., McGrew W., Tutin C. (1982). Wide ranging chimpanzees at mt. assirik, senegal. Int. J. Primatology 3, 367–385.
Baldwin P., Sabater P., McGrew W., Tutin C. (1981). Comparisons of nests made by different populations of chimpanzees (Pan troglodytes). Primates 22, 474–486. doi: 10.1007/BF02381239
Barca B., Turay B. S., Kanneh B. A., Tayleur C. (2018). Nest ecology and conservation of western chimpanzees (Pan troglodytes verus) in Gola Rainforest National Park, Sierra Leone. Primate Conserv. 32, 1–7.
Boyer K. M. (2011). Chimpanzee conservation in light of impending iron ore mining project in SE senegal (Master thesis of Iowa State University), 75.
Brownlow A., Plumptre A. J., Reynolds V., Ward R. (2001). Sources of variation in the nesting behavior of chimpanzees (Pan Troglodytes schweinfurthii) in the Budongo Forest, Uganda. Am. J. Primatology 55 (1), 49–55. doi: 10.1002/ajp.1038
Bryson-Morrison N., Tzanopoulos J., Matsuzawa T., Humle T. (2017). Activity and habitat use of chimpanzees (Pan troglodytes verus) in the anthropogenic landscape of Bossou, Guinea, West Africa. Int. J. Primatology 38 (2), 282–302. doi: 10.1007/s10764-016-9947-4
Campbell G., Kuehl H., N’Goran P. K., Boesch C. (2008). Alarming decline of West African chimpanzees in Côte d’Ivoire. Curr. Biol. 18, R903–R904. doi: 10.1016/j.cub.2008.08.015
Chapman C. A., Peres C. (2021). Primate conservation: Lessons learned in the last 20 years can guide future efforts. Evolutionary Anthropology 30 (5), 345–361. doi: 10.1002/evan.21920
CITES (2018). “Convention on international trades in endangered species of wild fauna and flora 2018. great apes (Hominidae spp.),” in Report of the secretariat. seventieth meeting of the standing committee rosa khutor, sochi (Russian Federation), 10.
Diallo B. (2018). Étude du comportement de nidification du chimpanzé de l’Afrique de l’Ouest, Pan troglodytes verus, à Bantankiline (Kédougou, Sénégal) (Sénégal: mmémoire de master en Écologie et Gestion des Écosystèmes, département de biologie animale/FST), 34pp.
Diallo D., Ndiaye P. I., Badji L., Micheletti K., Diallo B., Pruetz J. D. (2022). Nidification du chimpanzé de savane (Pan troglodytes verus) dans la zone non protégée de Bantankiline (Kédougou, Sénégal). Bull. Societe Royale Sci. Liege 91 (1), 84–104. doi: 10.25518/0037-956.10926
Dutton P., Moltchanova E., Chapman H. (2017). Nesting ecology of a small montane population of the Nigerian/Cameroon chimpanzee (Pan troglodytes ellioti) in Nigeria. Folia Primatologica 87 (6), 361–374. doi: 10.1159/000454921
FAO/COP26 (2021). La déforestation est l’affectation de terrains forestiers à d’autres usages, comme l’agriculture et les infrastructures. Food and Agriculture Organisation of the United Nations, Glasgow (United Kingdom).
Fleury-Brugière M. (2001). Estimation préliminaire de la population de chimpanzés de la Zone Intégralement Protegée-Mafou du Parc National du Haut Niger (République de Guinée. Rapport de Projet de la Composante Parc National du Haut Niger du Programme AG), 83 pp.
Fruth B., Hohmann G. (1994). “Comparative analyses of nest building behavior in bonobos and chimpanzees,” in Chimpanzee Cultures. Eds. McGrew W. C., de Waal F. B. M., Wrangham R. W., Heltne P. (Cambridge: Harvard University Press), 109–128.
Furuichi T., Hashimoto C. (2004). Botanical and topographical factors influencing nesting-site selection by chimpanzees in Kalinzu forest, Uganda. Int. J. Primatology 25, 755–765. doi: 10.1023/B:IJOP.0000029121.25284.7f
Goodall J. (1962). Nest building behavior in the free-ranging chimpanzee. Ann. New York Acad. Sci. 102, 455–467. doi: 10.1111/j.1749-6632.1962.tb13652.x
Groves C., Sabater P. (1985). From ape's nest to human fix-point. Man 20, 22–47. doi: 10.2307/2802220
Hakizimana D., Buckers A., Brotcorne F., Huynen M. (2015). Characterization of nest sites of chimpanzees (Pan troglodytes schweinfurthii) in Kibira National Park, Burundi. Afr. Primates 10, 1–12.
Ham R. (1998). Nationwide chimpanzee survey and large mammal survey, Republic of Guinea (Guinea-Conakry: European Communion), 115p. Unpublished.
Heinicke S., Mundry R., Boesch C., Hockings H. J., Kormos R., Ndiaye P. I., et al. (2019). Towards systematic and evidence-based conservation planning for western chimpanzees. Am. J. Primatology 2019, 81:e23042. doi: 10.1002/ajp.23042
Hernandez-Aguilar R., Moore J., Stanford C. (2013). Chimpanzee nesting patterns in savanna habitat: environmental influences and preferences. Am. J. Primatology 75, 979–994. doi: 10.1002/ajp.22163
Hernandez-Aguilar R. A. (2009). Chimpanzee nest distribution and site reuse in a dry habitat : Implications for early hominin ranging. J. Hum. Evol. 57, 350–364.
Humle T., Boesch C., Campbell G., Junker J., Koops K., Kuehl H., et al. (2016). Pan troglodytes (errata version published in 2018). IUCN Red List Threatened Species 2016, e.T15933A129038584. doi: 10.2305/IUCN.UK.2016-2.RLTS.T15933A17964454.en
Hunt K. D., McGrew W. C. (2002). “Chimpanzees in the dry habitats of assirik, senegal and semliki wildlife reserve, uganda,” in Behavioural diversity in chimpanzees and bonobos. Eds. Boesch C., Hohmann G., Marchant L. F. (, New York: Cambridge University Press), 35–51.
Kamgang S. A., Bobo K. S., Maisels F., Ambahe R. D. D., Ongono D. E. A. (2018). The relationship between the abundance of the Nigeria-Cameroon chimpanzee (Pan troglodytes ellioti) and its habitat: A conservation concern in Mbam-Djerem National Park, Cameroon. BMC Ecology 18 (40). doi: 10.1186/s12898-018-0199-3
Kamilar J. (2009). Environmental and geographic correlates of the taxonomic structure of primate communities. Am. J. Phys. Anthropology 139, 382–393.
Koops K., Humle T., Sterck E., Matsuzawa T. (2007). Ground-nesting by the chimpanzees of the Nimba Mountains, Guinea: environmentally or socially determined? Am. J. Primatology 69, 407–419. doi: 10.1002/ajp.20358
Koops K., McGrew W., De Vries H., Matsuzawa T. (2012). Nest-Building by Chimpanzees (Pan troglodytes verus) at Seringbara, Nimba Mountains: Antipredation, Thermoregulation, and Antivector Hypotheses. Int. J. Primatology 33, 356–380. doi: 10.1007/s10764-012-9585-4
Kühl H. S., Sop T., Williamson E. A., Mundry R., Brugiere D., Campbell G., et al. (2017). The Critically Endangered western chimpanzee declines by 80%. Am. J. Primatology, 1–15. doi: 10.1002/ajp.22681
Lehman S., Fleagle J. (2006)Biogeography and primates: A review. In: Primate biogeography (US: Springer). Available at: http://link.springer.com/10.1007/0-387-31710-4_1 (Accessed July 31, 2018).
Matsuzawa T., Yamakoshi G. (1996). “Comparison of chimpanzee material culture between bossou and nimba, west africa,” in Reaching into thought: The minds of the great apes. Eds. Russon A. E., Bard K. A., Parker S. T. (Cambridge University Press), 211–232.
Mittermeier R., Wallis J., Rylands A., Ganzhorn J., Oates J., Williamson E., et al. (2009). ). Primates in peril: the world’s 25 most endangered primates 2008–2010: Primate Conservation. Conserv. Int. 24 (1), 1–57. doi: 10.1896/052.024.0101
Ndiaye P. I., Diedhiou Y., Dotras L., Lindshield S. M., Diouck D., Sonko A., et al. (2020). “Action plan for conservation of chimpanzee in senegal: importance of the involvement of local actors,” in IPS congres.
Ndiaye P. I., Badji L., Lindshield S. M., Bâ C. T., Pruetz J. D. (2015). Détermination du statut du Chimpanzé, Pan troglodytes verus, dans une zone non protégée de savane boisée à Bagnomba, Sénégal: Implication pour sa conservation (France: Colloque de la Société Francophone de Primatologie à strasbourg).
Ndiaye P. I., Badji L., Lindshield S. M., Pruetz J. D. (2018b). Nest-building behaviour by chimpanzees (Pan troglodytes verus) in the non-protected area of diaguiri (Kedougou, Senegal): implications for conservation. Folia Primatologica 89 (5), 316–326. doi: 10.1159/000490945
Ndiaye P. I., Galat G., Galat-Luong A., Nizinski G. (2013). Note on the seasonal use of lowland and highland habitats by the west African chimpanzee pan troglodytes verus (Schwarz 1934) (Primates: hominidae): implications for its conservation. J. Threatened Taxa 5 (17), 3696–3700. doi: 10.11609/JoTT.03229.3697-700
Ndiaye P. I., Lindshield S. M., Badji L., Pacheco L., Wessling E. G., Boyer K. M., et al. (2018a). Survey of chimpanzees (Pan troglodytes verus) outside protected areas in southeastern Senegal. Afr. J. Wildlife Res. 48 (1), 1–14. doi: 10.3957/056.048.013007
Ndimuligo S. (2007). “Assessment of chimpanzee (Pan troglodytes) population and habitat in kwitanga forest, western Tanzania,” in Master of Science in Resource Conservation Biology (Johannesburg, South Africa: University of Witwatersrand), 76.
Oates J. F., Tutin C. E. G., Humle T., Wilston M. L., Baillie J. E. M., Balmforth Z., et al. (2008) Pan troglodytes In IUCN 2012 (IUCN Red List of Threatened Species). Available at: www.iucnredlist.org (Accessed February 2017).
Oates T. W., Valderrama P., Bischof M., Nedir R., Jones A., Simpson J., et al. (2007). Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. Int. J. Oral. Maxillofac. Implants. 22 (5), 755–760.
Potts K., Watts D., Wrangham R. (2011). Comparative feeding ecology of two communities of chimpanzees (Pan troglodytes) in Kibale National Park, Uganda. Int. J. Primatology 32, 669–690. doi: 10.1007/s10764-011-9494-y
Pruetz J. D., Bertolani P. (2007). Savanna chimpanzees, Pan troglodytes verus, hunt with tools. Curr. Biol. 17 (5), 412–417. doi: 10.1016/j.cub.2006.12.042
Pruetz J. D. (2002). Competition between chimpanzees (Pan troglodytes verus) and humans in southeastern senegal. Am. J. Physiol. Anthropology, 128.
Pruetz J. D., Fulton S. J., Marchant L. F., McGrew W. C., Schiel M., Waller M. (2008). Arboreal nesting as anti-predator adaptation by Savanna Chimpanzees (Pan troglodytes verus) in southeastern Senegal. Am. J. Primatology 70, 393–401. doi: 10.1002/ajp.20508
Pruetz J. D., Marchant L. F., Arno J., McGrew W. C. (2002). Survey of savanna chimpanzees (Pan troglodytes verus) in southeastern Senegal. Am. J. Primatology 58 (1), 35–43. doi: 10.1002/ajp.10035
Reynollds V. (1965). Some behavioural comparisons between the chimpanzee and the montain in gorila in the wild. Am. Anthropologist 67, 691–706. doi: 10.1525/aa.1965.67.3.02a00050
Sanz C., Morgan D., Strindberg S., Onononga J. (2007). Distinguer les nids des chimpanzés et gorilles sympatriques. J. Appl. Ecol. 44, 263–272. doi: 10.1111/j.1365-2664.2007.01278.x
Schwitzer C., Mittermeier R. A., Rylands A. B., Chiozza F., Williamson E. A., Macfie E. J., et al. (eds.). (2017). Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. (Arlington, VA: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society), 99pp.
Sept J. M., King B. J., McGrew W. C., Moore J., Paterson J. D., Strier K. B., et al. (1992). Was there no place like home? a new perspective on early hominid archaeological sites from the mapping of chimpanzee nests. Curr. Anthropology 33, 187–207. doi: 10.1086/204050
Stanford C., O’Malley R. (2008). Sleeping tree choice by Bwindi chimpanzees. Am. J. Primatology 70, 642–649. doi: 10.1002/ajp.20539
Stewart F. (2011). Brief communication: Why sleep in a nest? empirical testing of the function of simple shelters made by wild chimpanzees. Am. J. Phys. Anthropology 146, 313–318. doi: 10.1002/ajpa.21580
Stewart F., Pruetz J. D. (2013). Do chimpanzee nests serve an anti-predatory function? Anti-predatory nest function. Am. J. Primatology 75, 593–604. doi: 10.1002/apj.22138
Sylla S. F., Ndiaye P. I., Lindshield S. M., Bogart S. L., Pruetz J. D. (2022). the western chimpanzee (pan troglodytes verus) in the antenna zone (Niokolo Koba national park, Senegal): nesting ecology and sympatrics with other mammals: applied ecology and environmental research. Applied Ecology and Environmental Research 20, 3, 2663–2681. doi: 10.15666/aeer/2003_26632681
Tappan G. (2010). Carte de l’occupation des terres de la région de Kédougou (Kédougou, Sénégal). USGS Publication, South Dakota.
Tutin C. E. G., Fernandez M. (1984). Nationwide census of gorilla and chimpanzee population in Gabon. Am. J. Primatology 6, 313–336. doi: 10.1002/ajp.1350060403
UNEP/CMS/COP12 (2017). “United nations environment Programme/Convention on the conservation of migratory species of wild animals/ the twelfth session of the conference of the parties 2017. proposal for a concerted action for the nut-cracking chimpanzees of west africa (Pan troglodytes verus) already listed on appendices i and II of the convention,” in 12th meeting of the conference of the parties, Manila, Philippines, Doc. 25.1.1.
Keywords: anthropogenic impacts, chimpanzee, nesting behavior, tree species bearing nest, Senegal
Citation: Diallo D, Ndiaye PI, Badji L and Pruetz JD (2024) Savannah chimpanzee (Pan troglodytes verus) nesting behavior in the unprotected area of Tikankali near to a mining exploitation and the Niokolo Koba National Park in Senegal. Front. Ecol. Evol. 12:1228373. doi: 10.3389/fevo.2024.1228373
Received: 24 May 2023; Accepted: 18 January 2024;
Published: 09 February 2024.
Edited by:
Alex Kenneth Piel, University College London, United KingdomReviewed by:
Inza Koné, Swiss Centre for Scientific Research, Côte d’IvoireTetsuro Matsuzawa, California Institute of Technology, United States
Copyright © 2024 Diallo, Ndiaye, Badji and Pruetz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dame Diallo, ZGFtZS5kaWFsbG9AdWNhZC5lZHUuc24=
 Dame Diallo
Dame Diallo Papa Ibnou Ndiaye
Papa Ibnou Ndiaye Landing Badji1
Landing Badji1