
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol., 15 December 2023
Sec. Ecophysiology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1320745
This article is part of the Research TopicThe Adaptation, Plasticity and Extinction of Forest Plants to Climate Change: Mechanisms behind the Morphological, Physiological, Phenological and Ecological TraitsView all 8 articles
Plasticity is vital for plants to rapidly acclimate to environmental changes, especially under the climate change. Global warming could advance bud break and extend the growing season, but it also increases the risk of frost damage to developing leaves. In this study, we explored the phenological plasticity of bud burst of half-sib family sugar maple (Acer saccharum Marsh.) seedlings from 11 seed origins in two common gardens at the center and the northern edge of the species distribution in Quebec, Canada. Results showed that the phenological plasticity of sugar maple originating from inland was significantly higher than those from coastal areas at the beginning of leaf development. This discrepancy may result from the long-term frost change frequency of seed origins. Our study suggests that in the context of climate warming, the higher plasticity observed in sugar maple originating from inland areas may benefit from the phenological adaptation of sugar maple and the survival of local populations. It also suggests that inland populations may have a higher potential regarding to assisted migration, but this needs to be confirmed for other functional traits than phenology.
Global climate change has affected tree growth significantly, and trees have to adapt to the environmental changes in-situ or migrate to new growth conditions to survive (Aitken et al., 2008). Phenotypic plasticity, determined as the ability to alter phenotype quickly in response to environmental changes (Nicotra et al., 2010; Matesanz and Valladares, 2014; Merilä and Hendry, 2014), is faster than genotypic adaptation and plays a crucial role in survival in various environments (Chevin et al., 2013; Franks et al., 2014). However, to achieve high phenotypic plasticity, plants need to invest resources to perceive environmental information and produce a series of allocational, anatomical, or morphological traits adjustments to adapt to changing conditions (DeWitt, 1998; Van Kleunen and Fischer, 2005; Auld et al., 2010). Multiple phenotypes exhibit a wide range of variability, which increase the chance to adapt to weather events and gain a high competitive ability (Gomulkiewicz and Kirkpatrick, 1992; Hufford and Gomulkiewicz, 1999; Holloway, 2002), while others may inevitably mismatch the environmental conditions, leading to maladaptation (Ghalambor et al., 2007). Thus, plants with higher plasticity could develop a better competitive ability under climate change. However, the environmental conditions vary within the species range, which create the potential for local adaptation and a variety of amplitudes in the expression of plasticity due to species origins (Vitasse et al., 2013; Valladares et al., 2014).
Phenotypic plasticity could enable genotypes to develop specific reaction norms under environmental changes, thus buffering the rapid growth condition changes and facilitating future genetic variation (Holloway, 2002; Sultan, 2004; Nicotra et al., 2010). The degree of plasticity depends on the variability of environmental conditions (Churkina et al., 2005; Vitasse et al., 2009a; Vitasse et al., 2009b). Wide inter-annual variability in environmental conditions may promote higher plasticity at the individual level to cope with the unstable conditions and produce higher intra-specific trait variations at the population level (Rubio de Casas et al., 2008; Kumordzi et al., 2019; Cardou et al., 2022). However, under stable conditions, there is a tendency for populations to exhibit reduced plasticity (Chevin et al., 2010; Matesanz et al., 2010; Valladares et al., 2014). Compared to the center of the distribution, marginal populations are generally more isolated and show higher plasticity (Valladares et al., 2014). This divergent plasticity shows a deviating sensitivity to environmental factors, indicating a higher ability of marginal populations to cope with changing environmental conditions.
Plant phenology is the study of periodically recurring patterns of growth and development during the year, such as the start (leaf unfolding) and end (leaf coloring) dates of growing seasons (Lieth, 2013; Piao et al., 2019; Inouye, 2022). Numerous efforts have been made to incorporate phenology into climate change models to better understand the response of trees (Chuine and Beaubien, 2001; Badeck et al., 2004; Cleland et al., 2007; Rosbakh et al., 2021). For example, climate warming leads to earlier sprouting and longer growing season, potentially increases net carbon uptake of the ecosystem (Keenan et al., 2014). Also, the temperature has been demonstrated to be a driving factor of ecotypic variation (Silvestro et al., 2019). The developmental phase from dormancy to activity period is one of the most critical transitions in the bud tissues (Jewaria et al., 2021). The breaking of endodormancy in spring requires efficient accumulation of cold temperature in autumn and winter (chilling) (Singh et al., 2016). After that, heat in spring (forcing) and photoperiod could strongly affect the further reactivation of bud break and growth (Chen et al., 2018; Huang et al., 2020). Understanding the adaptive capacity of phenological responses to climate fluctuations, particularly changes in chilling and forcing factors, is crucial for predicting the prospects of species in the context of climate change.
As an important economic species in eastern North America, sugar maple (Acer saccharum Marsh.) has been studied to predict the response to climate change (Putnam and Reich, 2017). A previous study showed that phenotypic plasticity plays a dominant role in regulating the bud phenology of sugar maple populations (Guo et al., 2023). However, the geographical pattern of plasticity and the possible climatic drivers are less known. In this study, we examined the intra-specific climatic variation and phenological plasticity of sugar maple seedlings from 11 Canadian origins, which were planted in two common gardens at the central and northern edges of their distribution range. We expect that sugar maple originating from the inland have higher phenotypic plasticity for bud break than those from coastal areas because of higher frost change frequency.
This study examined open-pollinated progenies of sugar maple from 11 seed origins across the species’ range in Canada (Figure 1; one half-sib family per seed origin). This area is included in the bioclimatic domains of deciduous and mixed forests of the northern temperate zone, which is dominated by both broadleaves and conifers (mainly Acer saccharum Marsh., Acer rubrum L., Betula alleghaniensis Britt., Abies balsamea (L.) Mill.).
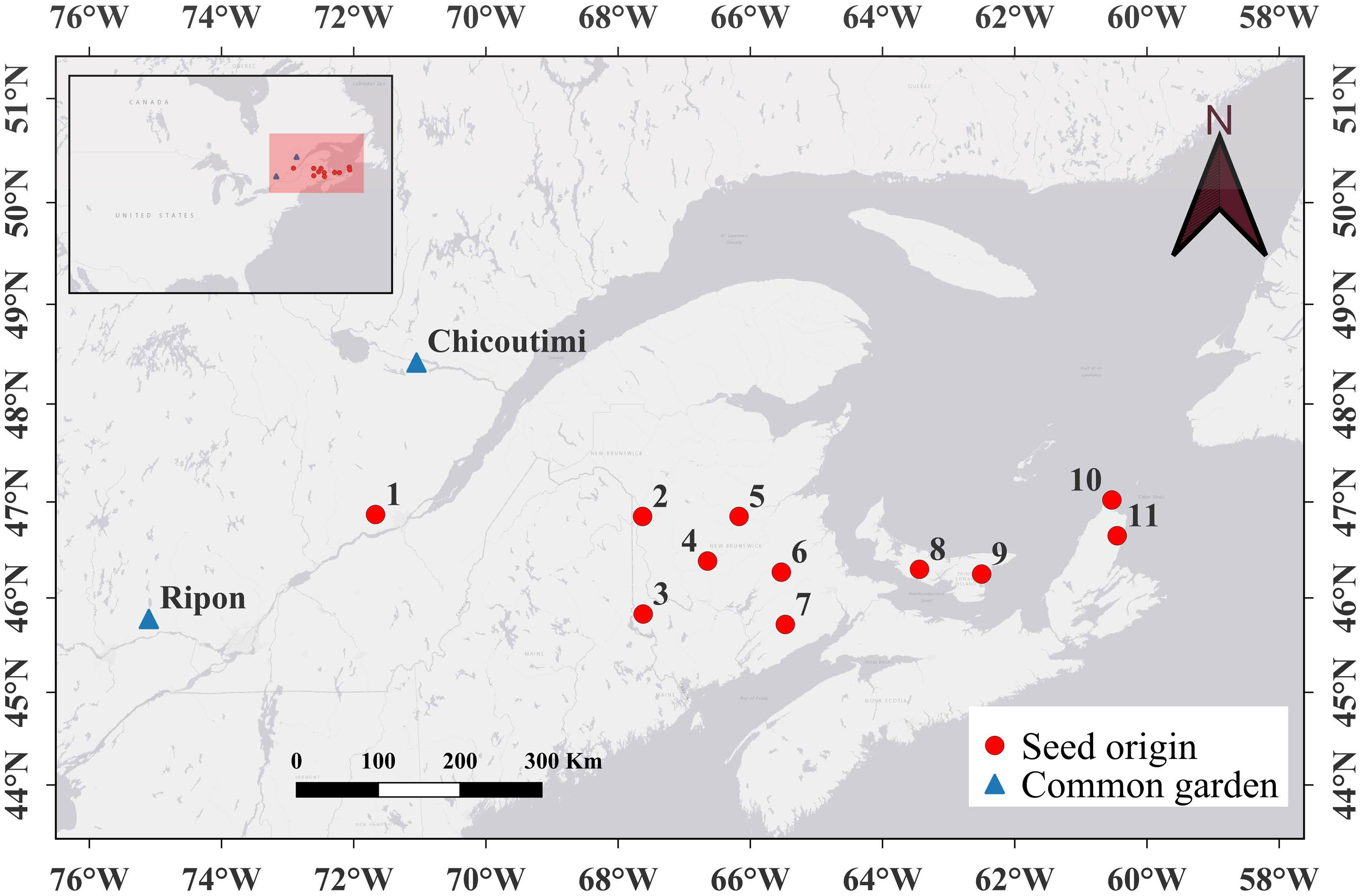
Figure 1 Locations of the 11 seed origins (represented by circles) of sugar maple. The triangles mark the locations of the two common gardens in Chicoutimi and Ripon.
The mean annual temperature among the seed origins ranged between 3.1 and 5.7°C, with the lowest recorded at seed origin 1 and the highest at seed origin 8 (Supplementary Table 1). Seed origin 1 represented the coldest location, with a minimum mean annual temperature of -10.9°C. Conversely, seed origin 6 exhibited the warmest conditions, with a minimum mean annual temperature of -9.0°C and a maximum mean annual temperature of 20.0°C (Supplementary Table 1). The range of mean annual temperature across the seed origins was 2.2°C. Additionally, it is worth noting that the mean annual temperature in Ripon was 4.5°C, and 3.2°C in Chicoutimi. Annual precipitation ranged from 1144 to 3385 mm, increasing from west towards the east.
In April 2018, we used seed material from sugar maple populations to cultivate one-year-old seedlings in greenhouses. Subsequently, in the spring of 2019, these seedlings were transplanted into two common gardens located in Chicoutimi and Ripon (Quebec, Canada, Figure 1). Chicoutimi and Ripon are located at the central and northern edge of the natural distribution of sugar maple, respectively. The different growing conditions of two common gardens provided an experimental design that helped to study the effects of genetic variations and phenological plasticity in bud phenology. The trials were set up using a single-tree plot layout with a spacing of 3 m × 3.5 m. On average, between 6 and 10 seedlings from each seed origin were planted, resulting in a total of 145 and 173 seedlings in Chicoutimi and Ripon, respectively.
During mid-April to mid-June in 2020, we meticulously monitored bud and leaf phenology for all seedlings twice a week. Generally, the observed two-year-old seedings had five buds. To ensure methodological consistency and reduce the influence of confounding factors in subsequent analyses, we deliberately selected and closely observed the apical bud, which is widely accepted and established practice in the field (Rosique-Esplugas et al., 2021). We recorded the progression of bud and leaf development, divided them into eight distinct phases, following the methodology outlined by Skinner and Parker (1994): (1) bud swell, characterized by reddish scales and an enlarging bud; (2) bud elongation, displaying a yellowish hue between the scales; (3) green tip stage, with light green tips and the area between the scales but the bud still closed; (4) bud break, featuring loosened scales but barely visible expanding leaf tips; (5) extended bud break, where the leaf bundle expanded beyond the scales but without separated leaves; (6) initial leaf emergence, marked by leaves starting to expand perpendicularly to the base of the bud; (7) initial leaf expansion, showcasing light green, small, and wrinkled leaves; and (8) full leaf expansion, with entirely flattened and expanded leaves. The onset of each phenological phase was determined as the first day (DOY, day of the year) when that specific phase was observed in each individual.
The daily minimum, maximum, and mean temperature for the year 2020 were obtained by extracting data based on the coordinates of the common gardens from the ERA5 dataset using Google Earth Engine (Gorelick et al., 2017). Climatologies at high resolution for the earth’s land surface areas (CHELSA) climate data include monthly temperature, precipitation, and derived parameter estimates at a spatial resolution of 30 arcsec from 1979-2013 (Karger et al., 2017). Four climatic variables, including frost change frequency (the number of events in which the minimum temperature or maximum temperature goes above or below 0°C), growing degree days (the annual cumulative sum of temperatures exceeding 0°C), growing season length (the number of days with temperatures exceeding 5°C, without snow cover and soil water available), and number of frost days (the annual count of days when the daily minimum temperature is less than 0°C) were downloaded from CHELSA to describe the annual trend and extreme conditions across seed origins and between common gardens. A Principal Component Analysis (PCA) was then performed to determine the contribution of each climatic variable to the total variance. All seed origins were classified into different groups using four climatic variables (frost change frequency, growing degree days, growing season length, and number of frost days) according to the results of the Partitioning Around Medoids clustering algorithm, the extension of the k-means clustering algorithm (Kaufman and Rousseeuw, 1987).
For each common garden, we calculated the average bud phenology for all individuals of the same seed origin, and we used the difference in leaf development between the two common gardens to illustrate population-level plasticity. The analysis of variance (ANOVA) was used to test the difference in plasticity among groups of seed origins based on the abovementioned cluster results. All statistical analyses were performed using R software (R Core Team, 2021).
The average frost change frequency among the seed origins was 67 occurrences, ranging from the highest of 79 occurrences in seed origin 7 to the lowest of 50 occurrences in seed origin 10. The average growing season length was 176 days, with the longest and shortest also recorded in seed origin 7 and 10 (186 and 161 days, respectively). The mean growing degree days were 1570 days, with the longest days recorded in seed origin 7 at 1772 days and the shortest recorded in seed origin 11 at 1330 days. The average number of frost days was 154 days, ranging from a maximum of 171 days in seed origin 2 to a minimum of 135 days in seed origin 10 (Supplementary Table 2). PCA extracted two main principal components (PC), explaining 77.2% and 14.5% of the variability in climatic parameters for seed origins for PC1 and PC2, respectively. Frost change frequency was most strongly correlated with PC1 (-0.94) and had the highest contribution at 68.33%, indicating that frost change frequency was the primary influencing factor (Figure 2; Supplementary Table 3).
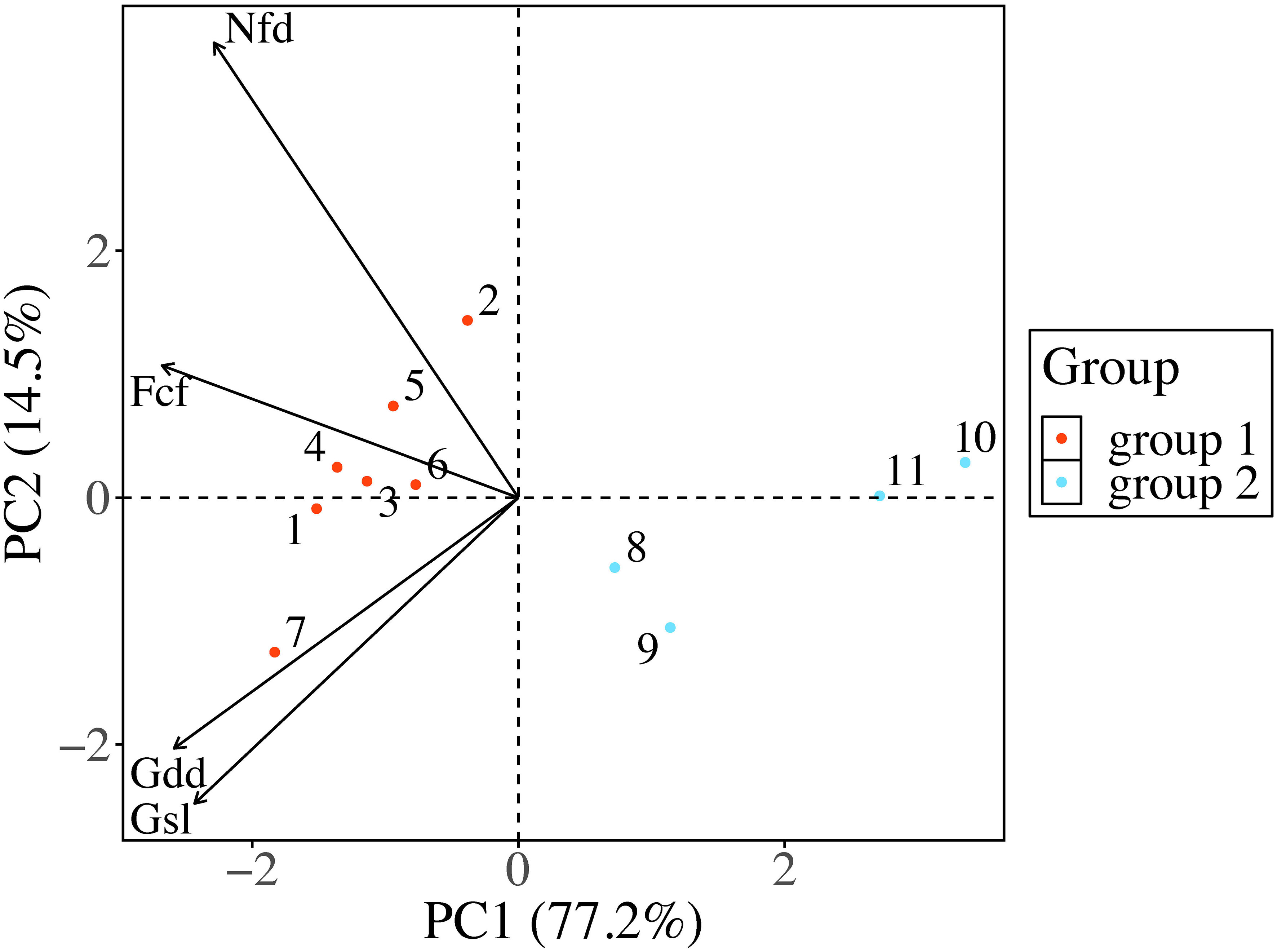
Figure 2 Principal component analysis and cluster results of the climatic variability among seed origins. Group 1 includes seed origins 1-7, while group 2 includes 8-11. (Fcf, frost change frequency; Gdd, growing degree days; Gsl, growing season length; Nfd, number of frost days).
According to the K-medoids clustering results (Figure 2; Supplementary Figure 1), the seed origins 1-7 were classified as group 1, and the seed origins 8-11 were classified as group 2. Seed origins in group 1 had longer growing season length, higher number of frost days, and higher frost change frequency than those in group 2 close to the Gulf of Saint Lawrence River.
Bud and leaf phenology occurred earlier in Ripon compared to Chicoutimi (Figure 3). Phases 1-3 occurred on DOY 121, 124, and 127 in Ripon, 10 days before Chicoutimi (DOY 131, 134, and 137). The differences between sites for the phases 4-6 were 9, 6, and 3 days, respectively. Phases 7 and 8 differed by 1-4 days between the two common gardens. The differences in bud phenology between the two sites gradually diminished as the phases progressed. Overall, the leafing period in Ripon spanned 30 days, whereas it covered 23 days in Chicoutimi.
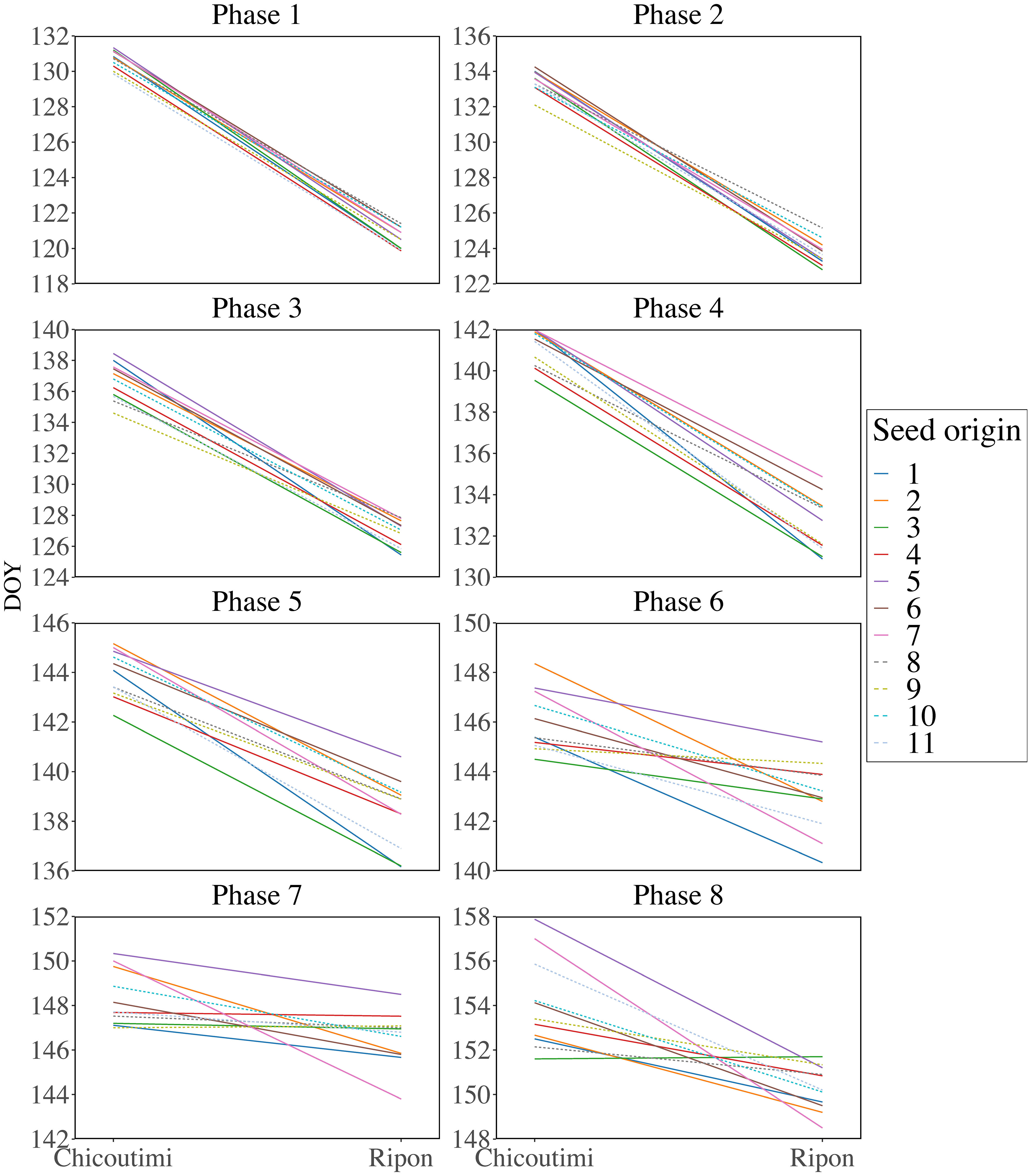
Figure 3 Reaction norm of phenological plasticity of sugar maple in the two common gardens. The solid line represents group 1 (seed origins 1-7), while the dashed line represents group 2 (seed origins 8-11).
The seed origins with the highest or lowest phenological plasticity varied across the eight developmental phases. Seed origin 3 exhibited the highest variation for phases 1 and 2, with an 11-day difference between the two common gardens (Supplementary Table 4). In phases 3-5, seed origin 1 showed the most significant difference of 8-13 days between the two common gardens, while seed origin 7 showed the most significant difference in phases 6-8, with a 6-9-day gap. Additionally, seed origin 10 demonstrated the lowest variation in phase 1, with a 9-day difference between the common gardens. For phases 2-4, seed origin 8 exhibited the least variation, with a 7-8-day difference between the gardens. In phases 5-7, seed origin 9 had the smallest difference, ranging from 0-4 days. Seed origin 3 showed the lowest variation in phase 8, with no difference between the common gardens. Almost for all phases, the phenological variation of seed origins in group 1 was higher than that in group 2 (Figure 4). However, the difference was significant only for phases 1-3 (p < 0.05; Figure 4; Supplementary Table 5).
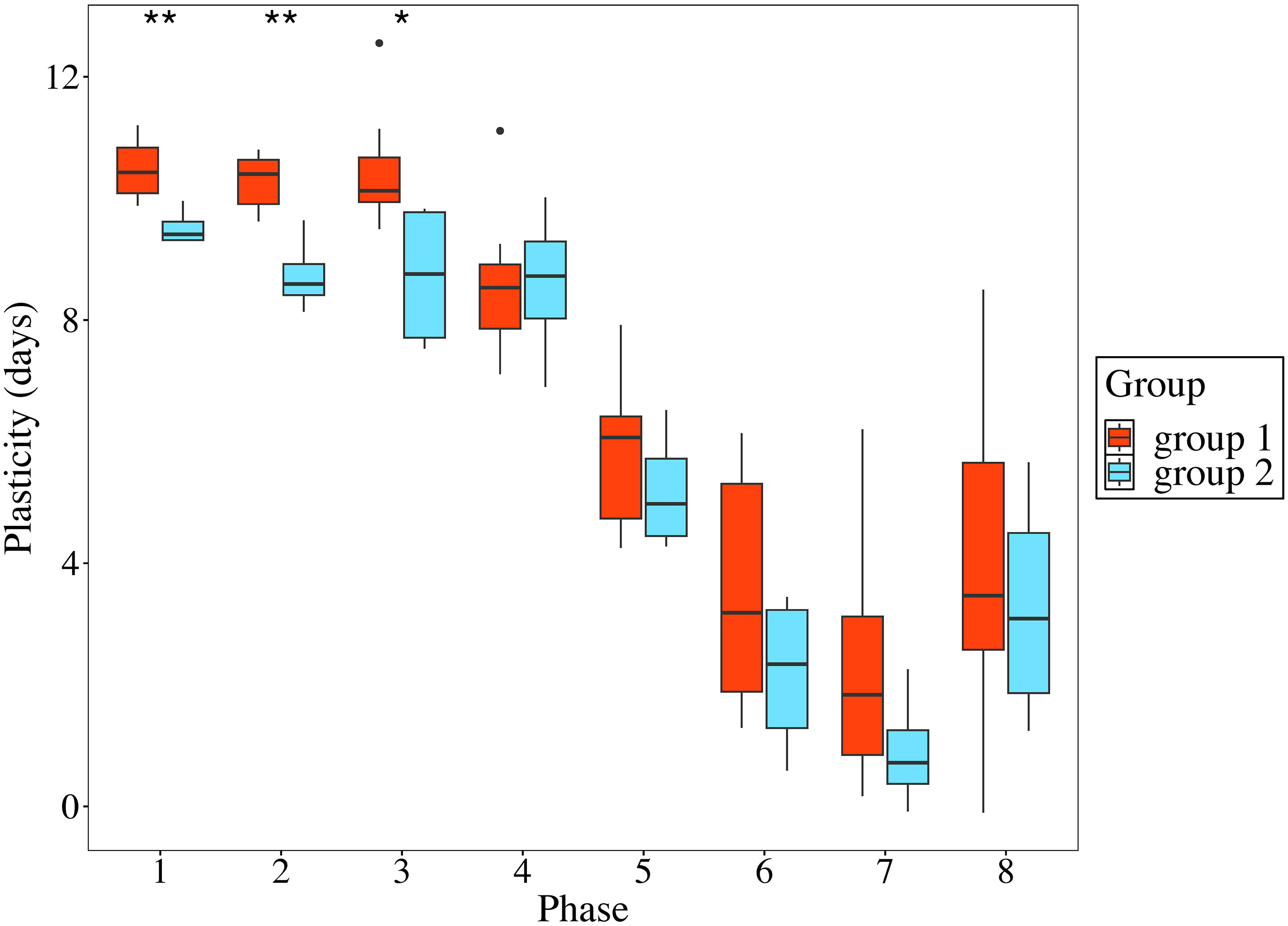
Figure 4 The phenological variations of sugar maple seed origins between two groups. Significance levels are *< 0.05, and **< 0.01.
Buds of sugar maple occured earlier in the southern common garden (Ripon). In our study, the mean spring temperature (April-May) in 2020 in Ripon was 4.6 °C, higher than that in Chicoutimi (2.4 °C). Under warmer spring conditions, sugar maple may be more effective in accumulating heat to achieve the threshold of forcing condition, leading to an advanced break of dormancy (Myking and Heide, 1995; Fu et al., 2013). Our results are consistent with previous studies conducted in temperate and boreal ecosystems (Fu et al., 2014; Zani et al., 2020; Vitasse et al., 2022). For example, the timings of leaf unfolding of Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. advanced by 5.7 days for each additional degree Celsius, as observed across five common gardens (Vitasse et al., 2010). Similarly, the buds of Populus fremontii in Arizona flushed earlier when the trees grow in the common gardens located in warmer regions (Cooper et al., 2019; Gao et al., 2023).
The variability of DOY between Ripon and Chicoutimi decreased for the later leafing stages (7 and 8). The difference in photoperiod is an essential factor for spring phenology. Day length during bud burst (phase 1) was 15.0 h in Chicoutimi, which was longer than that recorded in Ripon (14.1 h) due to the different latitudes and timings of growth reactivation between common gardens (Guo et al., 2022). A more extended photoperiod could have facilitated bud development (Way and Montgomery, 2014), thus resulting in similar timings of full leaf expansion. A previous study demonstrated that sugar maple leafing benefits from a longer day length, and photoperiod can outweigh the delaying effects of colder springs (Ren et al., 2020). However, the similar ending of leaf expansion in the two common gardens remains partially unexplained and could result from the heat damage events occurring during the studied year. Thus, a better understanding of the impact of current weather on leaf development is needed and requires long-term monitoring of bud phenology in the two common gardens.
We found that inland sugar maple showed a higher plasticity at the beginning of leaf development compared to those in coastal regions. In our study conducted under continental climatic conditions, sugar maples were exposed to a wide inter-annual weather variability, mainly for temperature. This temperature variation is a critical limiting factor influencing bud phenology during the spring season (Guo et al., 2020). Also, with a higher number of frost days and frost change frequency, sugar maple originating from inland areas may face an increasing risk of frost damage. In order to mitigate the risk of frost damage, enhancing phenological plasticity becomes crucial. This adaptation allows for a rapid response to weather fluctuations and extends the leafing period, thereby improving the available carbon fixation period. (Körner and Basler, 2010; Vitasse et al., 2014). Previous studies have demonstrated that plasticity correlates with climatic variability, and species experiencing a wider climatic fluctuation also exhibit higher plasticity (Van Buskirk, 2008). For example, plasticity for plant performance strongly correlates with inter-annual precipitation variability of original sites (Pratt and Mooney, 2013). Also, phenotypic plasticity in final shoot height and maximum biomass per shoot increases towards the higher latitudes due to the range of climatic variability increases with latitude (Ren et al., 2020). Our study demonstrated that plants from the inland areas exhibit a higher plasticity, which may help them to respond suitably to rapid changes in climate.
Climate might affect the phenology of different seed origins to varying degrees. It was proved that under climate change, instead of the assumption of homogeneously plasticity across a species’ range, the model given the factor of population differentiation could increase the forecasts of species range shifts (Valladares et al., 2014). In our study, the higher plasticity was observed in inland areas, which showed a higher variability in a given environmental condition. Compared with coastal areas, sugar maple originating from inland areas may have more advantages to cope with the changing climate. Therefore, future studies on the effects of simulated climate change on species phenology should consider specifically the different degrees of phenological plasticity within the same species.
Phenotypic plasticity plays a vital role in the response of plants to the environment, whose changes are critical for the survival of individuals and local populations. In this study, we demonstrated that due to the higher frost change frequency, sugar maple originating from inland areas showed higher plasticity of bud burst than those from coastal areas at the beginning of leaf development. We predict that in an assisted migration context, sugar maple originating from inland may rapidly acclimate its phenology to the local environment. Thus, quantifying the climatic conditions especially the frost change frequency experienced by specific population, may increase the predictive accuracy of its acclimatation potential, and help to define its potential in assisted migration efforts.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YZ: Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. CB: Writing – review & editing. XG: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing. VM: Formal analysis, Validation, Writing – review & editing. MK: Formal analysis, Validation, Visualization, Writing – review & editing. SD: Data curation, Investigation, Writing – review & editing. SR: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by Ministère des Forêts, de la Faune et des Parcs du Québec, Natural Sciences and Engineering Research Council of Canada (Alliance Grants and Engage Grants), Fonds de Recherche Nature et Technologies Québec (Établissement de nouveaux chercheurs), Observatoire régional de recherche en forêt boréale, National Natural Science Foundation of China (32201543), Guangxi Science and Technology Base and Talent Project (No. 2021AC19325).
The authors thank D. Alano, P. Benoît, F. Gagnon, C. Mura, and P. Ren for technical support, Y. Gobeil for permitting the study on his property, A. Garside for checking the English text, and the reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1320745/full#supplementary-material
Aitken S. N., Yeaman S., Holliday J. A., Wang T., Curtis-McLane S. (2008). Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Appl. 1 (1), 95–1115. doi: 10.1111/j.1752-4571.2007.00013.x
Auld J. R., Agrawal A. A., Relyea R. A. (2010). Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B: Biol. Sci. 277 (1681), 503–511. doi: 10.1098/rspb.2009.1355
Badeck F.-W., Bondeau A., Böttcher K., Doktor D., Lucht W., Schaber Jörg, et al. (2004). Responses of spring phenology to climate change. New Phytol. 162 (2), 295–3095. doi: 10.1111/j.1469-8137.2004.01059.x
Cardou Françoise, Munson A. D., Boisvert-Marsh L., Anand M., Arsenault André, Bell F.W., et al. (2022). Above- and belowground drivers of intraspecific trait variability across subcontinental gradients for five ubiquitous forest plants in North America. J. Ecol. 110 (7), 1590–16055. doi: 10.1111/1365-2745.13894
Chen L., Huang J.-G., Ma Q., Hänninen H., Tremblay F., Bergeron Y. (2018). Long-term changes in the impacts of global warming on leaf phenology of four temperate tree species. Global Change Biol 25 (3), 997–1004. doi: 10.1111/gcb.14496
Chevin L.-M., Collins Sinéad, Lefèvre François (2013). Phenotypic plasticity and evolutionary demographic responses to climate change: taking theory out to the field. Funct. Ecol. 27 (4), 967–9795. doi: 10.1111/j.1365-2435.2012.02043.x
Chevin L.-M., Lande R., Mace G. M. (2010). Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PloS Biol. 8 (4), e10003575. doi: 10.1371/journal.pbio.1000357
Chuine I., Beaubien E. G. (2001). Phenology is a major determinant of tree species range. Ecol. Lett 4 (5), 500–510. doi: 10.1046/j.1461-0248.2001.00261.x
Churkina G., Schimel D., Braswell B. H., Xiao X. (2005). Spatial analysis of growing season length control over net ecosystem exchange. Global Change Biol 11 (10), 1777–1787. doi: 10.1111/j.1365-2486.2005.001012.x
Cleland E. E., Chuine I., Menzel A., Mooney H. A., Schwartz M. D. (2007). Shifting plant phenology in response to global change. Trends Ecol. Evol. 22 (7), 357–3655. doi: 10.1016/j.tree.2007.04.003
Cooper H. F., Grady K. C., Cowan J. A., Best R. J., Allan G. J., Whitham T. G. (2019). Genotypic variation in phenological plasticity: Reciprocal common gardens reveal adaptive responses to warmer springs but not to fall frost. Global Change Biol. 25 (1), 187–2005. doi: 10.1111/gcb.14494
DeWitt T. J. (1998). Costs and limits of phenotypic plasticity: Tests with predator-induced morphology and life history in a freshwater snail. J. Evolutionary Biol. 11 (4), 465–480. doi: 10.1046/j.1420-9101.1998.11040465.x
Franks S. J., Weber J. J., Aitken S. N. (2014). Evolutionary and plastic responses to climate change in terrestrial plant populations. Evolutionary Appl. 7 (1), 123–1395. doi: 10.1111/eva.12112
Fu Y. H., Campioli M., Deckmyn G., Janssens I. A. (2013). Sensitivity of leaf unfolding to experimental warming in three temperate tree species. Agric. For. Meteorology 181, 125–132. doi: 10.1016/j.agrformet.2013.07.016
Fu Y. H., Campioli M., Vitasse Y., De Boeck H. J., Van den Berge J., AbdElgawad H., et al. (2014). Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. 111 (20), 7355–73605. doi: 10.1073/pnas.1321727111
Gao J., Yang B., Mura C., Boucher Y., Rossi S. (2023). Increased inter-annual variability in budburst dates towards the northern range edge of black spruce. Agric. For. Meteorology 333, 109410. doi: 10.1016/j.agrformet.2023.109410
Ghalambor C. K., McKay J. K., Carroll S. P., Reznick D. N. (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol 21 (3), 394–407. doi: 10.1111/j.1365-2435.2007.01283.x
Gomulkiewicz R., Kirkpatrick M. (1992). Quantitative genetics and the evolution of reaction norms. Evolution 46 (2), 390–4115. doi: 10.1111/j.1558-5646.1992.tb02047.x
Gorelick N., Hancher M., Dixon M., Ilyushchenko S., Thau D., Moore R. (2017). Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environment 202, 18–27. doi: 10.1016/j.rse.2017.06.031
Guo X., Buttò V., Mohytych V., Klisz M., Surget-Groba Y., Huang J., et al. (2023). Plasticity plays a dominant role in regulating the phenological variations of sugar maple populations in Canada. Front. Ecol. Evol. 11. doi: 10.3389/fevo.2023.1217871
Guo X., Khare S., Silvestro R., Huang J., Sylvain J.-D., Delagrange S., et al. (2020). Minimum spring temperatures at the provenance origin drive leaf phenology in sugar maple populations. Tree Physiol 40 (12), 1639–1647. doi: 10.1093/treephys/tpaa096
Guo X., Klisz M., Puchałka Radosław, Silvestro R., Faubert P., Belien E., et al. (2022). Common-garden experiment reveals clinal trends of bud phenology in black spruce populations from a latitudinal gradient in the boreal forest. J. Ecol. 110 (5), 1043–10535. doi: 10.1111/1365-2745.13582
Holloway G. J. (2002). Phenotypic plasticity: beyond nature and nurture. Heredity 89 (6), 410–410. doi: 10.1038/sj.hdy.6800153
Huang J.-G., Ma Q., Rossi S., Biondi F., Deslauriers A., Fonti P., et al. (2020). Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. U.S.A 117 (34), 20645–20652. doi: 10.1073/pnas.2007058117
Hufford L., Gomulkiewicz R. (1999). Review of phenotypic evolution: A reaction norm perspective., Carl D. Schlichting, Massimo pigliucci. Systematic Bot. 24 (4), 686–688. doi: 10.2307/2419653
Inouye D. W. (2022). Climate change and phenology. WIREs Climate Change 13 (3), e764. doi: 10.1002/wcc.764
Jewaria P. K., Hänninen H., Li X., Bhalerao R. P., Zhang R. (2021). A hundred years after: endodormancy and the chilling requirement in subtropical trees. New Phytol 231 (2), 565–570. doi: 10.1111/nph.17382
Karger D. N., Conrad O., Böhner Jürgen, Kawohl T., Kreft H., Soria-Auza R. W., et al. (2017). Climatologies at high resolution for the earth's land surface areas. Sci. Data 4, 170122. doi: 10.1038/sdata.2017.122
Kaufman L., Rousseeuw P. J. (1987). Clustering by means of Medoids. Statistical data analysis based on the L1–norm and related methods. Ed. Dodge Y. (North-Holland: Faculty of Mathematics and Informatics).
Keenan T. F., Gray J., Friedl M. A., Toomey M., Bohrer G., Hollinger D. Y., et al. (2014). Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Climate Change 4, 598–604. doi: 10.1038/nclimate2253
Körner C., Basler D. (2010). Phenology under global warming. Science 327 (5972), 1461–1462. doi: 10.1126/science.1186473
Kumordzi B. B., Aubin I., Cardou Françoise, Shipley B., Violle C., Johnstone J., et al. (2019). Geographic scale and disturbance influence intraspecific trait variability in leaves and roots of North American understorey plants. Funct. Ecol. 33 (9), 1771–17845. doi: 10.1111/1365-2435.13402
Lieth H. (2013). Phenology and seasonality modeling Vol. 8 (Springer Berlin, Heidelberg: Springer Science & Business Media).
Matesanz S., Gianoli E., Valladares F. (2010). Global change and the evolution of phenotypic plasticity in plants. Ann. New York Acad. Sci. 1206 (1), 35–55. doi: 10.1111/j.1749-6632.2010.05704.x
Matesanz S., Valladares F. (2014). Ecological and evolutionary responses of Mediterranean plants to global change. Environ. Exp. Bot. 103, 53–67. doi: 10.1016/j.envexpbot.2013.09.004
Merilä J., Hendry A. P. (2014). Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evolutionary Appl. 7 (1), 1–145. doi: 10.1111/eva.12137
Myking T., Heide O. M. (1995). Dormancy release and chilling requirement of buds of latitudinal ecotypes of Betula pendula and B. pubescens. Tree Physiol 15 (11), 697–704. doi: 10.1093/treephys/15.11.697
Nicotra A. B., Atkin O. K., Bonser S. P., Davidson A. M., Finnegan E. J., Mathesius U., et al. (2010). Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 15 (12), 684–692. doi: 10.1016/j.tplants.2010.09.008
Piao S., Liu Q., Chen A., Janssens I. A., Fu Y., Dai J., et al. (2019). Plant phenology and global climate change: Current progresses and challenges. Global Change Biol. 25 (6), 1922–19405. doi: 10.1111/gcb.14619
Pratt J. D., Mooney K. A. (2013). Clinal adaptation and adaptive plasticity in Artemisia californica: implications for the response of a foundation species to predicted climate change. Global Change Biol 19 (8), 2454–2466. doi: 10.1111/gcb.12199
Putnam R. C., Reich P. B. (2017). Climate and competition affect growth and survival of transplanted sugar maple seedlings along a 1700-km gradient. Ecol. Monogr. 87 (1), 130–1575. doi: 10.1002/ecm.1237
R Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Ren L., Guo X., Liu S., Yu T., Guo W., Wang R., et al. (2020). Intraspecific variation in Phragmites australis: Clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. J. Ecol 108 (6), 2531–2543. doi: 10.1111/1365-2745.13401
Rosbakh S., Hartig F., Sandanov D. V., Bukharova E. V., Miller T. K., Primack R. B. (2021). Siberian plants shift their phenology in response to climate change. Global Change Biol. 27 (18), 4435–44485. doi: 10.1111/gcb.15744
Rosique-Esplugas C., Cottrell J. E., Cavers S., Whittet R., Ennos R. A. (2021). Clinal genetic variation and phenotypic plasticity in leaf phenology, growth and stem form in common ash (Fraxinus excelsior L.). Forestry. doi: 10.1093/forestry/cpab026
Rubio de Casas R., Vargas P., Pérez-Corona E., Cano E., Manrique E., García-Verdugo C., et al. (2008). Variation in sclerophylly among Iberian populations of Quercus coccifera L. @ is associated with genetic differentiation across contrasting environments. Plant Biol 11 (3), 464–472. doi: 10.1111/j.1438-8677.2008.00128.x
Silvestro R., Rossi S., Zhang S., Froment I., Guo Huang J., Saracino A. (2019). From phenology to forest management: Ecotypes selection can avoid early or late frosts, but not both. For. Ecol. Manage 436, 21–26. doi: 10.1016/j.foreco.2019.01.005
Singh R. K., Svystun T., AlDahmash B., Jönsson A. M., Bhalerao R. P. (2016). Photoperiod- and temperature-mediated control of phenology in trees - a molecular perspective. New Phytol 213 (2), 511–524. doi: 10.1111/nph.14346
Skinner M., Parker B. L. (1994). Field guide for monitoring sugar maple bud development (USA: Research report (Vermont Agricultural Experiment Station). no. 8.
Sultan S. E. (2004). Promising directions in plant phenotypic plasticity. Perspect. Plant Ecology Evol. Systematics 6 (4), 227–233. doi: 10.1078/1433-8319-00082
Valladares F., Matesanz S., Guilhaumon François, Araújo M. B., Balaguer L., Benito-Garzón M., et al. (2014). The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17 (11), 1351–13645. doi: 10.1111/ele.12348
Van Buskirk J. (2008). A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am. Nat 160 (1), 87–102. doi: 10.1086/340599
Van Kleunen M., Fischer M. (2005). Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 166 (1), 49–605. doi: 10.1111/j.1469-8137.2004.01296.x
Vitasse Y., Baumgarten F., Zohner C. M., Rutishauser T., Pietragalla B., Gehrig R., et al. (2022). The great acceleration of plant phenological shifts. Nat. Climate Change 12 (4), 300–302. doi: 10.1038/s41558-022-01283-y
Vitasse Y., Bresson C. C., Kremer A., Michalet R., Delzon S. (2010). Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 24 (6), 1211–12185. doi: 10.1111/j.1365-2435.2010.01748.x
Vitasse Y., Delzon S., Bresson C. C., Michalet R., Kremer A. (2009a). Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res 39 (7), 1259–1269. doi: 10.1139/x09-054
Vitasse Y., Hoch Günter, Randin C. F., Lenz A., Kollas C., Scheepens J. F., et al. (2013). Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171 (3), 663–6785. doi: 10.1007/s00442-012-2580-9
Vitasse Y., Lenz A., Körner C. (2014). The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Science, 5. doi: 10.3389/fpls.2014.00541
Vitasse Y., Porté A. Josée, Kremer A., Michalet R., Delzon S. (2009b). Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia 161, 187–198. doi: 10.1007/s00442-009-1363-4
Way D. A., Montgomery R. A. (2014). Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ 38 (9), 1725–1736. doi: 10.1111/pce.12431
Keywords: Acer saccharum, bud burst, common garden, climate change, temperature
Citation: Zhou Y, Bai C, Guo X, Mohytych V, Klisz M, Delagrange S and Rossi S (2023) Inland populations of sugar maple manifest higher phenological plasticity than coastal populations. Front. Ecol. Evol. 11:1320745. doi: 10.3389/fevo.2023.1320745
Received: 12 October 2023; Accepted: 22 November 2023;
Published: 15 December 2023.
Edited by:
Peijian Shi, Nanjing Forestry University, ChinaReviewed by:
Tao Yan, Lanzhou University, ChinaCopyright © 2023 Zhou, Bai, Guo, Mohytych, Klisz, Delagrange and Rossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiali Guo, Z3VveGw2NjY2QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.