
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol., 10 January 2024
Sec. Population, Community, and Ecosystem Dynamics
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1303867
This article is part of the Research TopicThe Role of Soil Mesofauna as Indicators of Sustainable Ecosystem Management PlansView all 5 articles
Soil is a very fragile ecosystem, often subject to many threats. Wildfires can affect edaphic communities depending on the intensity and seasonality of the fire. Different groups of soil fauna tend to respond differently to this type of disturbance, but the lack of data prevents to fully analyze the impact of fire. Soil microarthropods show a particular sensitivity to disturbances of different nature, making them excellent biological indicators. That is why in recent years many biotic indices to assess soil quality, like QBS-ar (Soil Biological Quality based on arthropods), have been developed. The objective of this study was to evaluate whether there might be a significant difference between areas affected or not by fire in the locality of Andonno (Piedmont, Italy) in terms of QBS-ar values, 18 years after this disturbance, and whether the reforestation intervention is having a positive effect on soil quality. Two sampling sites were selected within the sampling area and in each, six soil samples were taken. Microarthropods were extracted with a Berlese-Tüllgren extractor and soil biological quality was calculated using the QBS-ar index. No significant difference in QBS-ar values were found between the fire burned and unburned areas (p=0.37). The number of biological and euedaphic forms in the two sites was similar. It appears that microarthropod communities manage to recover in a short time, indicating that in the study area soil fauna has shown a fast recovery after extreme events like wildfires.
Soil is a very fragile ecosystem and often subject to many threats (Stolte et al., 2016), which risk causing a decline in the biodiversity of edaphic fauna, resulting in the loss of soil fertility and inability of soil to perform its functions (Orgiazzi et al., 2016; Coyle et al., 2017). Some of the main threats to soil fauna are listed by Jeffery et al. (2010) and Gardi et al. (2013), and include intensive agriculture (Beare et al., 1992; Baker, 1998; Dittmer and Schrader, 2000; Ayuke et al., 2011; Menta et al., 2011; Iordache, 2012), pollution (Cortet et al., 1999; Coyle et al., 2011; Kapusta et al., 2011), climate change (Meehan et al., 2010; McKenzie et al., 2013; Nielsen and Wall, 2013), and desertification (Pflug and Wolters, 2001; Tsiafouli et al., 2005; Maraldo et al., 2009). The lack of previous data regarding edaphic communities makes it difficult to assess their changes over time and thus to identify threats to soil biodiversity (Peng et al., 2022). Human activities often cause soil degradation that leads to reduced biodiversity and simplified edaphic communities (Jie et al., 2002; Ferreira et al., 2022).
Wildfires are a type of threat that primarily impacts terrestrial animals and plant communities, but they can also affect edaphic communities depending on the intensity and seasonality of the fire (Lisa et al., 2015), since they lead to physical, chemical, and biological changes (Callaham et al., 2012). It appears that some microarthropods may decrease in abundance in the months following fire (Jeffery et al., 2010), as most taxa are unable to take refuge in the deeper layers of soil (Coleman and Rieske, 2006). In general, different groups of edaphic organisms tend to respond differently to this type of disturbance (Fattorini, 2010; Radea et al., 2010; Menta and Remelli, 2020); particularly fungal biomass, microbial biomass carbon (C), soil respiration, autotrophic respiration, and C acquisition enzymes decrease in response to fire (Hu et al., 2023), but still much work has to be done to fully understand the impact of fire (Sgardelis and Margaris, 1993; Zaitsev et al., 2016; Turco et al., 2018; Hu et al., 2023). It has been speculated that often the observed changes in edaphic communities do not depend on the disturbance itself, but on the changes that occur to plant communities during succession (Callaham et al., 2003) and the alteration of the nutrient cycle. Soil arthropods and fungi seem to respond particularly to fire of different intensity and temperature (Bezkorovainay et al., 2007; Rutigliano et al., 2013), but their analysis can be long and complex to be performed, due to the richness in species and the need to identify all of them for a thorough assessment (Schatz et al., 2021).
Edaphic microarthropods show a particular sensitivity to disturbances of different nature, making them excellent biological indicators. That is why in recent years many indices that use these organisms to assess soil quality have been developed (Paoletti and Hassall, 1999; Parisi et al., 2005; Nuria et al., 2011).
The QBS-ar (Soil Biological Quality based on arthropods) index was introduced by Parisi (2001) and Parisi et al. (2005) and uses the degree of soil adaptation of different groups of edaphic arthropods to assess soil biological quality. The main advantage of this index is that it does not require recognition at the species level, but only at the order or class level, making it possible to simplify and speed up the process of assigning a soil quality score. It is not necessary, therefore, to count individuals, since for the QBS-ar index just the presence/absence is sufficient to represent the adaptability of that group. This index has become very popular in recent years, partly due to its easy application, robustness, and affordability, and is used for different types of monitoring in different parts of the world (Menta et al., 2018; Venanzi et al., 2019; Galli et al., 2021; Hernández-Tirado et al., 2022; Latterini et al., 2022; Çakır et al., 2023; Kurniawan et al., 2023; Latterini et al., 2023; Reilly et al., 2023). Despite this, it is still scarcely used to assess the impact of fire on soil quality, and the first studies on this are still pretty local and partial (Lisa et al., 2015; Mantoni et al., 2020; Certini et al., 2021; Galli et al., 2021; Lisa et al., 2022).
The objective of this study was to evaluate whether there might be a significant difference between the burned and unburned sites in the Andonno area in terms of QBS-ar values. Our hypothesis is that there are significant differences in the composition of microarthropods at the two study sites and that the QBS-ar index is able to detect these differences even 18 years after the fire. Although the present work represents a restricted case study, this can contribute to the discussion on understanding how soil microarthropod communities react long after such strong disturbances and whether the reforestation intervention is having a positive effect on soil quality. Case studies can be very useful in forestry because they represent the basis for further deeper investigation by meta-analysis.
For this study, an area in the Southern Piedmont region, near Andonno (CN), was selected. This area was affected by a fire in 2003 that destroyed about 1,076 hectares of forest in the Valle Gesso, leaving an open area where reforestation has been ongoing since 2013. Two sampling sites in the study area were chosen, located, respectively, in the burned and unburned areas within the Special Area of Conservation (SAC) IT1160056 Alpi Marittime (Figure 1). Samplings took place in June 2020 and July 2021.
There is a different vegetation composition within the two areas. The unburned area is characterized by a mixed coniferous forest of Larix decidua Miller, 1768 and Abies alba Miller 1758, while the burned area has a predominance of herbaceous species with the presence of maples, downy oaks and birches that are part of the reforestation program.
In each sampling site (80 m2), six random samples of soil measuring 10x10x10 cm were taken using a steel square corer and placed in plastic bags sealed and marked with a tag so that they could be brought to the laboratory. For each sampling area, different data were recorded in order to characterize the different sites (Table 1). Tree, shrub and herbaceous coverage were recorded in the field as a percentage, slope was measured in degrees, and rockiness was estimated using three levels (low, medium, high). Soil samples were transferred to the laboratory where microarthropods were extracted with a Berlese-Tüllgren extractor for 12 days and stored in containers filled with 2/3 alcohol and 1/3 glycerol. Next, they were then divided to order level using a stereo microscope and soil quality was calculated using the QBS-ar index (Parisi et al., 2005). Soil organisms were separated into biological forms, and each was assigned an EMI (Eco-Morphological Index) score ranging from 1 to 20 based on the degree of adaptation to edaphic conditions. The QBS-ar value is obtained by summing the EMI scores of each group, and if there are several scores for a single group, the highest is chosen.
A Student’s t-test was carried out to assess whether there is a significant difference in soil quality between the burned and unburned areas. Before carrying out the analysis, a Shapiro-Wilk test was conducted to evaluate the normality of distribution and an F-test to test if the variances are equal. The data was normally distributed (p> 0.05), and the variances were equal (p>0.05). Tests were performed using Rstudio version 1.3.1093 (R Development Core Team, 2021).
The results are summarized in Table 2; QBS-ar values obtained in the unburned site span between 179 and 227, with a high number of both overall biological forms (range between 14 and 17) and euedaphic forms (between 7 and 10). The maximum QBS value was obtained in the burned site (253), where in general the range of values is wider compared to the unburned site (range between 118 and 253). The number of biological and euedaphic forms in the burned site were similar to the ones of the unburned site, varying from 10 to 19 for the former and between 5 and 10 for the latter.
There was no significant difference in QBS-ar values between the fire-affected and non-fire-affected areas (p=0.37; Figure 2).
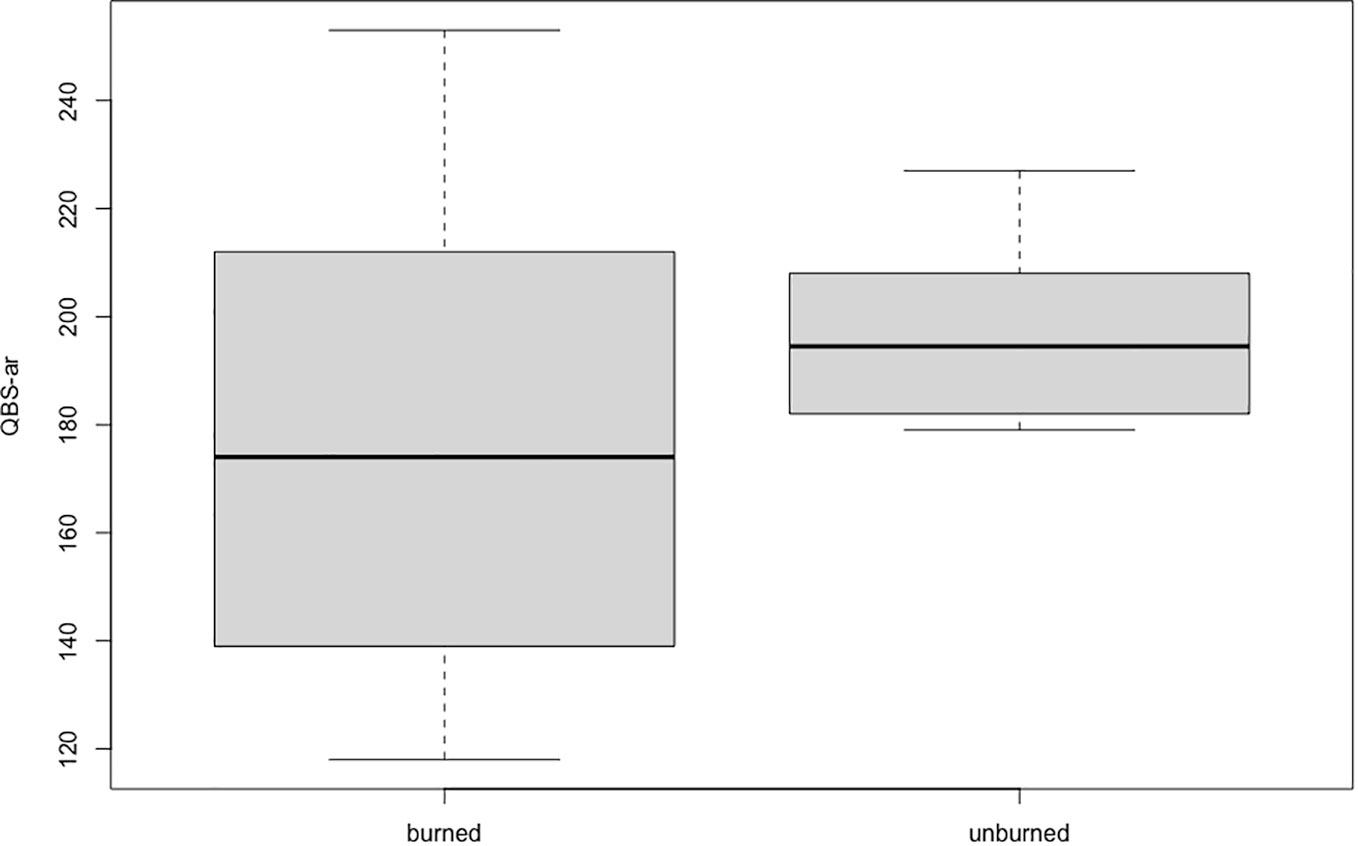
Figure 2 QBS-ar values recorded in burned (AND-I) and unburned (AND-NI) sites. The central box represents the interquartile range, the line in the center represents the median value, the upper and lower whiskers represent values outside the middle 50%.
The results obtained in this research differ from other studies conducted shortly after fires (Mantoni et al., 2020). Most soil microarthropod groups are present even though the area in question has undergone major changes in vegetation. Fires can have a great impact on edaphic fauna both directly and indirectly. Heat waves can cause the death of soil organisms but also change the availability of organic matter, nutrients, and pH (De Marco et al., 2005; Capogna et al., 2009; Pellegrini et al., 2022; Arunrat et al., 2023; Zhang et al., 2023). There are three types of strategies soil organisms can adopt to survive forest fires: flee, hide, and protect. Soil invertebrates usually have low mobility and are therefore more susceptible to fires (Jeffery et al., 2010). Since 2013, in the study area, reforestation has begun with the planting of about 11,000 trees, including birch, oak, rowan, and lodgepole pine. In general, areas where reforestation is underway tend to have low diversity, biomass, and density of soil fauna in the early years, due to the difficulty of dispersal of many of these groups (Petersen, 1995; Lindberg and Bengtsson, 2006). For example, it has been reckoned that, in the absence of disturb, euedaphic collembola need about 30 years to move about 30 m (Ojala and Huhta, 2001). Despite these limitations, it seems that microarthropods manage to newly colonize the affected area in a few decades (Blasi et al., 2013; Galli et al., 2021). In the case study, it appears that the microarthropods are at an advanced stage of recovery in a very short time, less than 20 years, suggesting that here, soil fauna has a fast recovery even after extreme events such as a fire. Importantly, no significant difference was found between the QBS-ar values obtained in the burned and the unburned areas (a mixed coniferous forest), similar to what Galli et al. (2021) pointed out in their study of native forests of secondary growth after fires. If we examine the QBS-ar values obtained in the present study, however, we see that in the burned area these show a great variability, ranging between 118 and 253, probably due to the very large diversity of microhabitats in this area after reforestation. In the unburned area, the values obtained are less variable, between 179 and 227, indicating that this area has a more stable microarthropod community, typical of healthy forest soils (Parisi et al., 2005; Blasi et al., 2013; Galli et al., 2014; Galli et al., 2015; Menta et al., 2017; Menta et al., 2018; Galli et al., 2021; Mantoni et al., 2020; Szigeti et al., 2022). Euedaphic microarthropods (EMI = 20) such as Pauropoda, Symphyla, and Diplura were found in almost all replicates. Diplopoda and Chilopoda are less common but well represented in the samples. Various biological forms of Coleoptera were found in both the burned and unburned sites, including euedaphic forms such as Leptotyphlinae. The presence of most of the euedaphic microarthropods in both sites shows that the burned area is at a good point of restoration regarding soil communities. It might be interesting to continue to monitor changes in the microarthropod presence in this area, in order to delineate transitions to a stable community during reforestation.
Our initial hypothesis that there would be a different microarthropod composition and different QBS-ar values in the two study sites was not confirmed. It would therefore seem likely that the study area is either recovering from the damage caused by the 2003 fire or alternatively the QBS-ar index is not sensitive enough to show a difference after 18 years. Mantoni et al. (2020) have shown how the index can be sensitive to this type of impact after a short period of time, but it is likely that these differences become less noticeable over the years. It must be considered that the QBS-ar gives absolute values based on the presence and absence and biological form of certain groups of microarthropods, but does not consider the species composition, which is likely to change in response to certain types of disturbance. In the present case study, the vegetational transition from mature mixed coniferous forest to a more open reforestation area may have led to changes in the edaphic community that cannot be appreciated using the QBS-ar index.
In conclusion, the QBS-ar index could be a good method for assessing changes in soil quality after events such as fires and other disturbances, but it is probably much more sensitive shortly after the disturbance. It would be useful to assess changes in soil microarthropod communities over the various years so that we can understand how they recover their function over time. It has already been seen in other studies (Blasi et al., 2013; Menta et al., 2018) how the QBS-ar index is sensitive to disturbances of different types, but it would also be useful to assess over time whether beneficial events such as reforestation succeeded in restoring the soil to good health. This study shows that correct management activities can bring fast benefits for restoration of soil microarthropod composition and be a useful tool for the conservation of this important environment.
Case studies like this can be very useful to understand whether certain methodologies can be applicable in different situations, but they also have many limitations as they are restricted studies. Long-term monitoring and the application of other indices, both biological and chemical, would be needed to get a more complete picture of the situation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
TF: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. LF: Data curation, Investigation, Methodology, Writing – review & editing. FC: Methodology, Writing – review & editing, Investigation. CJ: Methodology, Writing – review & editing, Investigation. AG: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Investigation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The results presented in this paper were partially funded by the “Ente di gestione delle aree protette delle Alpi Cozie” in the framework of the project Interreg V Italia-Francia ALCOTRA 2014-2020 – Piano integrato tematico (PITEM) BIODIVALP; specifically, these results are part of the subproject: Studio sulla biodiversità della pedofauna nel territorio delle ZSC It1160056 “Alpi Marittime” e It1160057 “Alte Valli Pesio e Tanaro”. The authors also acknowledge the support of NBFC to University of Roma Tre, funded by the Italian Ministry of University and Research, PNRR, Missione 4 Componente 2, “Dalla ricerca all’impresa”, Investimento 1.4, Project CN00000033.
We would like to thank Giuseppe Canavese, Fabiano Sartirana, Laura Martinelli and Cati Caballo from the Parco Naturale Alpi Marittime.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arunrat N., Sereenonchai S., Kongsurakan P., Iwai C. B., Yuttitham M., Hatano R. (2023). Post-fire recovery of soil organic carbon, soil total nitrogen, soil nutrients, and soil erodibility in rotational shifting cultivation in Northern Thailand. Front. Environ. Sci. 11 (March). doi: 10.3389/fenvs.2023.1117427
Ayuke F. O., Brussaard L., Vanlauwe B., Six J., Lelei D. K., Kibunja C. N., et al. (2011). Soil fertility management: Impacts on soil macrofauna, soil aggregation and soil organic matter allocation. Appl. Soil Ecol. 48 (1), 53–62. doi: 10.1016/j.apsoil.2011.02.001
Baker G. H. (1998). Recognising and responding to the influences of agriculture and other land-use practices on soil fauna in Australia. Appl. Soil Ecol. 9 (1–3), 303–310. doi: 10.1016/S0929-1393(98)00081-X
Beare M. H., Parmelee R. W., Hendrix P. F., Cheng W., Coleman D. C., Crossley D. A. J. (1992). Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecol. Monogr. 62 (4), 569–591. doi: 10.2307/2937317
Bezkorovainay I., Krasnoshchekova E., Ivanova G. (2007). Transformation of soil invertebrate complex after surface fires of different intensity. Biol. Bull. 34, 517–522. doi: 10.1134/S1062359007050159
Blasi S., Menta C., Balducci L., Conti F. D., Petrini E., Piovesan G. (2013). Soil microarthropod communities from Mediterranean forest ecosystems in Central Italy under different disturbances. Environ. Monit. Assess. 185 (2), 1637–1655. doi: 10.1007/s10661-012-2657-2
Çakır M., Akburak S., Makineci E., Bolat F. (2023). Recovery of soil biological quality (QBS-ar) and soil microarthropod abundance following a prescribed fire in the Quercus frainetto forest. Appl. Soil Ecol. 184 (May 2022), 104768. doi: 10.1016/j.apsoil.2022.104768
Callaham M. A., Blair J. M., Todd T. C., Kitchen D. J., Whiles M. R. (2003). Macroinvertebrates in North American tallgrass prairie soils: Effects of fire, mowing, and fertilization on density and biomass. Soil Biol. Biochem. 35 (8), 1079–1093. doi: 10.1016/S0038-0717(03)00153-6
Callaham M. A., Scott D. A., O’Brien J. J., Stanturf J. A. (2012). Cumulative effects of fuel management on the soils of eastern U.S. watersheds. In: Lafayette, R., Brooks, M. T., Potyondy, J.P., Audin, L., Krieger, S.L., Trettin, C.C., eds. Cumulative watershed effects of fuel management in the eastern United States. Gen. Tech. Rep. SRS-161. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station: 202–228.
Capogna F., Persiane A. M., Maggi O., Dowgiallo G., Puppi G., Manes F. (2009). Effects of different fire intensities on chemical and biological soil components and related feedbacks on a mediterranean shrub (Phillyrea angustifolia L.). Plant Ecol. 204 (2), 155–171. doi: 10.1007/s11258-009-9579-2
Certini G., Moya D., Lucas-Borja M. E., Mastrolonardo G. (2021). The impact of fire on soil-dwelling biota: A review. For. Ecol. Manage. 488, 118989. doi: 10.1016/j.foreco.2021.118989
Coleman T. W., Rieske L. K. (2006). Arthropod response to prescription burning at the soil-litter interface in oak-pine forests. For. Ecol. Manage. 233 (1), 52–60. doi: 10.1016/j.foreco.2006.06.001
Cortet J., Gomot-De Vauflery A., Poinsot-Balaguer N., Gomot L., Texier C., Cluzeau D. (1999). The use of invertebrate soil fauna in monitoring pollutant effects. Eur. J. Soil Biol. 35 (3), 115–134. doi: 10.1016/S1164-5563(00)00116-3
Coyle D. R., Nagendra U. J., Taylor M. K., Campbell J. H., Cunard C. E., Joslin A. H., et al. (2017). Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biol. Biochem. 110, 116–133. doi: 10.1016/j.soilbio.2017.03.008
Coyle D. R., Zalesny J. A. Jr., Zalesny R. S., Wiese A. H. (2011). Irrigating poplar energy crops with landfill leachate negatively affects soil micro- and meso-fauna. Int. J. Phytoremediation 13 (9), 845–858. doi: 10.1080/15226514.2011.552927
De Marco A., Gentile A., Arena C., Virzo De Santo A. (2005). Organic matter, nutrient content and biological activity in burned and unburned soils of a Mediterranean maquis area of southern Italy. Int. J.Wild.Fire 14, 365–377. doi: 10.1071/WF05030
Dittmer S., Schrader S. (2000). Longterm effects of soil compaction and tillage on Collembola and straw decomposition in arable soil. Pedobiologia 44 (3–4), 527–538. doi: 10.1078/S0031-4056(04)70069-4
Fattorini S. (2010). Effects of fire on tenebrionid communities of a Pinus pinea plantation: A case study in a Mediterranean site. Biodivers. Conserv. 19 (5), 1237–1250. doi: 10.1007/s10531-009-9749-5
Ferreira C. S. S., Seifollahi-Aghmiuni S., Destouni G., Ghajarnia N., Kalantari Z. (2022). Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 805, 150106. doi: 10.1016/j.scitotenv.2021.150106
Galli L., Bonacchi A., Capurro M., Conti I., Crovetto F., Ferrari C., et al. (2015). Assessment of the impact of trampling on soil Arthropoda in a Mediterranean habitat. Acta Societatis Zool. Bohemicae, 79, 1–2. doi: 10.13140/RG.2.1.2113.5122
Galli L., Capurro M., Menta C., Rellini I. (2014). Is the QBS-ar index a good tool to detect the soil quality in Mediterranean areas? A cork tree Quercus suber L. (Fagaceae) wood as a case of study. Ital. J. Zool. 81 (1), 126–135. doi: 10.1080/11250003.2013.875601
Galli L., Lanza E., Rellini I. (2021). First application of the QBS-ar Index in South America for the assessment of the biological quality of soils in Chile. Soil Sci. Annu. 72 (2), 1–15. doi: 10.37501/soilsa/135990
Gardi C., Jeffery S., Saltelli A. (2013). An estimate of potential threats levels to soil biodiversity in EU. Global Change Biol. 2013) 1538–1548. doi: 10.1111/gcb.12159
Hernández-Tirado A., Castaño-Meneses G., Ibáñez-Huerta A., Ramos-Chávez D. E., Aguirre-Plata L., Pérez-Velázquez D., et al. (2022). Artrópodos edáficos en diferentes usos de suelo de la Sierra Nevada, Tepetlaoxtoc, Estado de México, México. Rev. Colombiana Entomología 48 (1). doi: 10.25100/socolen.v48i1.11632
Hu M., Wang. J., Lu L., Gui H., Wan S. (2023). Global recovery patterns of soil microbes after fire. Soil Biol. Biochem. 183, 109057. doi: 10.1016/j.soilbio.2023.109057
Iordache M. (2012). Abundance of earthworms under fertilization with organo-mineral fertilizers in a chernozem from west of Romania. J. Food Agric. Environ. 10 (3–4), 1103–1105.
Jeffery S., Gardi C., Jones A., Montanarella A., Marmo L., Miko L., et al. (2010). European Atlas of Soil Biodiversity European Commission, Publications Office of the European Union, Luxembourg. doi: 10.2788/94222
Jie C., Jing-zhang C., Man-zhi T., Zi-tong G. (2002). Soil degradation: a global problem endangering sustainable development. J. Geogr. Sci. 12 (2), 243–252. doi: 10.1007/bf02837480
Kapusta P., Szarek-ŁUkaszewska G., Stefanowicz A. M. (2011). Direct and indirect effects of metal contamination on soil biota in a Zn-Pb post-mining and smelting area (S Poland). Environ. pollut. 159 (6), 1516–1522. doi: 10.1016/j.envpol.2011.03.015
Kurniawan I. D., Kinasih I., Akbar M. T. R., Chaidir L., Iqbal S., Pamungkas B., et al. (2023). Arthropod community structure indicating soil quality recovery in the organic agroecosystem of mount ciremai national park’s buffer zone. Caraka Tani: J. Sustain. Agric. 38 (2), 229–243. doi: 10.20961/carakatani.v38i2.69384
Latterini F., Venanzi R., Picchio R., Jagodziński A. M. (2023). Short-term physicochemical and biological impacts on soil after forest logging in Mediterranean broadleaf forests: 15 years of field studies summarized by a data synthesis under the meta-analytic framework. Forest: Int. J. For. Res. 96 (4), 547–560. doi: 10.1093/forestry/cpac060
Latterini F., Venanzi R., Tocci D., Picchio R. (2022). Depth-to-water maps to identify soil areas that are potentially sensitive to logging disturbance: initial evaluations in the mediterranean forest context. Land 11 (5), 709. doi: 10.3390/land11050709
Lindberg N., Bengtsson J. (2006). Recovery of forest soil fauna diversity and composition after repeated summer droughts. Oikos 114 (3), 494–506. doi: 10.1111/j.2006.0030-1299.14396.x
Lisa C., Paffetti D., Marchi E., Nocentini S., Travaglini D. (2022). Use of an edaphic microarthropod index for monitoring wildfire impact on soil in mediterranean pine forests. Front. For. Glob. Change 5. doi: 10.3389/ffgc.2022.900247
Lisa C., Paffetti D., Nocentini S., Marchi E., Bottalico F., Fiorentini S., et al. (2015). Impact of wildfire on the edaphic microarthropod community in a Pinus pinaster forest in central Italy. IForest 8 (Dec2015), 874–883. doi: 10.3832/ifor1404-008
Mantoni C., Di Musciano M., Fattorini S. (2020). Use of microarthropods to evaluate the impact of fire on soil biological quality. J. Environ. Manage. 266 (April), 110624. doi: 10.1016/j.jenvman.2020.110624
Maraldo K., Ravn H. W., Slotsbo S., Holmstrup M. (2009). Responses to acute and chronic desiccation stress in Enchytraeus (Oligochaeta: Enchytraeidae). J. Comp. Physiol. B: Biochem. System. Environ. Physiol. 179 (2), 113–123. doi: 10.1007/s00360-008-0305-5
McKenzie S. W., Hentley W. T., Hails R. S., Jones T. H., Vanbergen A. J., Johnson S. N. (2013). Global climate change and above-belowground insect herbivore interactions. Front. Plant Sci. 4 (OCT). doi: 10.3389/fpls.2013.00412
Meehan T. D., Crossley M. S., Lindroth R. L. (2010). Impacts of elevated CO2 and O3 on aspen leaf litter chemistry and earthworm and springtail productivity. Soil Biol. Biochem. 42 (7), 1132–1137. doi: 10.1016/j.soilbio.2010.03.019
Menta C., Conti F. D., Pinto S. (2017). Microarthropods biodiversity in natural, seminatural and cultivated soils—QBS-ar approach. Appl. Soil Ecol. 123 (June 2017), 740–743. doi: 10.1016/j.apsoil.2017.05.020
Menta C., Conti F. D., Pinto S., Bodini A. (2018). Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 85 (June 2017), 773–780. doi: 10.1016/j.ecolind.2017.11.030
Menta C., Leoni A., Gardi C., Delia Conti F. (2011). Are grasslands important habitats for soil microarthropod conservation? Biodivers. Conserv. 20 (5), 1073–1087. doi: 10.1007/s10531-011-0017-0
Menta C., Remelli S. (2020). Soil health and arthropods: From complex system to worthwhile investigation. Insects 11 (1), 54. doi: 10.3390/insects11010054
Nielsen U. N., Wall D. H. (2013). The future of soil invertebrate communities in polar regions: Different climate change responses in the Arctic and Antarctic? Ecol. Lett. 16 (3), 409–419. doi: 10.1111/ele.12058
Nuria R., Jérôme M., Léonide C., Christine R., Gérard H., Etienne I., et al. (2011). IBQS: A synthetic index of soil quality based on soil macro-invertebrate communities. Soil Biol. Biochem. 43 (10), 2032–2045. doi: 10.1016/j.soilbio.2011.05.019
Ojala R., Huhta V. (2001). Dispersal of microarthropods in forest soil. Pedobiologia 45 (5), 443–450. doi: 10.1078/0031-4056-00098
Orgiazzi A., Panagos P., Yigini Y., Dunbar M. B., Gardi C., Montanarella L., et al. (2016). A knowledge-based approach to estimating the magnitude and spatial patterns of potential threats to soil biodiversity. Sci. Total Environ. 545-546, 11–20. doi: 10.1016/j.scitotenv.2015.12.092
Paoletti M. G., Hassall M. (1999). Woodlice (Isopoda: Oniscidea): Their potential for assessing sustainability and use as bioindicators. Agricult. Ecosyst. Environ. 74 (1–3), 157–165. doi: 10.1016/S0167-8809(99)00035-3
Parisi V. (2001). La qualità biologica del suolo. Un metodo basato sui microartropodi. Acta Naturalia L’Ateneo Parmense 37, 105–114.
Parisi V., Menta C., Gardi C., Jacomini C., Mozzanica E. (2005). Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agricult. Ecosyst. Environ. 105 (1–2), 323–333. doi: 10.1016/j.agee.2004.02.002
Pellegrini A. F. A., Harden J., Georgiou K., Hemes K. S., Malhotra A., Nolan C. J., et al. (2022). Fire effects on the persistence of soil organic matter and long-term carbon storage. Nat. Geosci. 15 (1), 5–13. doi: 10.1038/s41561-021-00867-1
Peng Y., Peñuelas J., Vesterdal L., Yue K., Peguero G., Fornara D. A., et al. (2022). Responses of soil fauna communities to the individual and combined effects of multiple global change factors. Ecol. Lett. 25 (9), 1961–1973. doi: 10.1111/ele.14068
Petersen H. (1995). Temporal and spatial dynamics of soil Collembola during secondary succession in Danish heathland. Acta Zool. Fennica 196, 190–194.
Pflug A., Wolters V. (2001). Influence of drought and litter age on Collembola communities. Eur. J. Soil Biol. 37 (4), 305–308. doi: 10.1016/S1164-5563(01)01101-3
Radea C., Kazanis D., Arianoutsou M. (2010). Effects of fire history upon soil macroarthropod communities in Pinus halepensis stands in Attica, Greece. Israel J. Ecol. Evol. 56 (2), 165–179. doi: 10.1560/IJEE.56.2.165
R Development Core Team (2021). R: a language and environ-ment for statistical computing (Vienna, Austria: R Foundation for Statisti-cal Computing). Available at: http://www.R-project.org/.
Reilly K., Cavigelli M., Szlavecz K. (2023). Agricultural management practices impact soil properties more than soil microarthropods. Eur. J. Soil Biol. 117, 103516. doi: 10.1016/j.ejsobi.2023.103516
Rutigliano F. A., Migliorini M., Maggi O., D’Ascoli R., Fanciulli P. P., Persiani A. M. (2013). Dynamics of fungi and fungivorous microarthropods in a Mediterranean maquis soil affected by experimental fire. Eur. J. Soil Biol. 56 (2013), 33e43. doi: 10.1016/j.ejsobi.2013.02.006
Schatz H., Fortini L., Fusco T., Casale F., Jacomini C., Giulio A. D. (2021). Oribatid mites (Acari, Oribatida) from Parco Naturale delle Alpi Marittime (Piedmont, Italy). Zootaxa 5082 (6), 501–540. doi: 10.11646/zootaxa.5082.6.1
Sgardelis S. P., Margaris N. S. (1993). Effects of fire on soil microarthropods of a phryganic ecosystem. Pedobiologia 37 (2), 83–94.
Stolte J., Tesfai M., Øygarden L., Kværnø S., Keizer J., Verheijen F., et al. (2016). Soil threats in Europe, EUR 27607 ENdoi:10.2788/828742 (online) European Commission, Publications Office of the European Union: Luxembourg. doi: 10.2788/488054
Szigeti N., Berki I., Vityi A., Winkler D. (2022). Soil mesofauna and herbaceous vegetation patterns in an agroforestry landscape. Agroforestry Syst. 96 (4), 773–786. doi: 10.1007/s10457-022-00739-6
Tsiafouli M. A., Kallimanis A. S., Katana E., Stamou G. P., Sgardelis S. P. (2005). Responses of soil microarthropods to experimental short-term manipulations of soil moisture. Appl. Soil Ecol. 29 (1), 17–26. doi: 10.1016/j.apsoil.2004.10.002
Turco M., Rosa-Cánovas J. R., Bedia J., Jerez S., Montávez J. P., Llasat M. C., et al. (2018). Exacerbated fires in Mediterranean Europe due to anthropogenic warming projected with nonstationary climate-fire models. Nat. Commun. 9, 3821. doi: 10.1038/s41467-018-06358-z
Venanzi R., Picchio R., Grigolato S., Latterini F. (2019). Soil and forest regeneration after different extraction methods in coppice forests. For. Ecol. Manage. 454, 117666. doi: 10.1016/j.foreco.2019.117666
Zaitsev A. S., Gongalsky K. B., Malmström A., Persson T., Bengtsson J. (2016). Why are forest fires generally neglected in soil fauna research? A mini-review. Appl. Soil Ecol. 98, 261–271. doi: 10.1016/j.apsoil.2015.10.012
Keywords: edaphic fauna, mesofauna, soil monitoring, soil quality, biological index, forest management, forest fire
Citation: Fusco T, Fortini L, Casale F, Jacomini C and Di Giulio A (2024) Fast soil recovery after a fire: case study in Maritime Alps (Piedmont, Italy) using microarthropods and QBS-ar index. Front. Ecol. Evol. 11:1303867. doi: 10.3389/fevo.2023.1303867
Received: 28 September 2023; Accepted: 22 December 2023;
Published: 10 January 2024.
Edited by:
Luís Carlos Iunes Oliveira Filho, Universidade do Estado de Santa Catarina, BrazilReviewed by:
Alberto Masoni, University of Florence, ItalyCopyright © 2024 Fusco, Fortini, Casale, Jacomini and Di Giulio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Di Giulio, YW5kcmVhLmRpZ2l1bGlvQHVuaXJvbWEzLml0
†ORCID: Tommaso Fusco, orcid.org/0000-0003-0279-904X
Lorenzo Fortini, orcid.org/0000-0003-3324-9278
Francesca Casale, orcid.org/0000-0002-8642-6461
Carlo Jacomini, orcid.org/0000-0002-9983-830X
Andrea Di Giulio, orcid.org/0000-0003-0508-0751
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.