- 1Department of Biological Sciences, Ohio University, Athens, OH, United States
- 2Department of Biomedical Sciences, Ohio University Heritage College of Osteopathic Medicine, Athens, OH, United States
- 3Ohio Center for Ecological and Evolutionary Studies, Ohio University, Athens, OH, United States
- 4Buraiga Chimpanzee Project, Bigodi, Uganda
- 5Department of Ecology Evolution, and Environmental Biology, Columbia University, New York City, NY, United States
- 6Uganda Wildlife Authority, Kampala, Uganda
- 7Museum of Natural History, University of Colorado, Boulder, CO, United States
Data on space-use patterns are essential for understanding species ecology and conservation. Individual chimpanzee communities are known to vary in home range size and habitat use dynamics, reflecting site-specific strategies to differences in resource availability on different landscapes. Here we present home range estimates for the Buraiga chimpanzees of Kibale National Park, Uganda, a community of eastern chimpanzees (Pan troglodytes schweinfurthii) living within the largest remaining population fragment in Uganda. The Buraiga chimpanzees are currently undergoing habituation for research and tourism under the direction of the Uganda Wildlife Authority (UWA). We analyzed 15 months of GPS data (August 2019 – March 2020, and January – July 2022), calculating overall and seasonal home range and core area estimates with two methods, minimum convex polygon (MCP) and kernel density estimates (KDE). Home range was estimated to cover an area of 15.77 km2 (95% KDE), and 24.90 km2 (100% MCP). Additionally, we found that 15.82% of the Buraiga chimpanzee’s home range overlaps with community-managed land, primarily the Kanyanchu Swamp corridor and adjacent agricultural land. Seasonally, we found that Buraiga chimpanzees used a larger area during dry season months, compared with rainy season months. Documenting how great ape populations utilize increasingly anthropogenically influenced landscapes is important in order to facilitate long-term survival in the face of climate change, habitat fragmentation, and other ongoing threats.
1 Introduction
Among primates, ecological factors (i.e., seasonality of food availability) and social factors (i.e., population density, number of males) often serve as primary predictors of home range size (Wrangham, 1987; Dunbar, 1988; Herbinger et al., 2001), the area in which an animal forages for food, search for mates, and raise offspring (Burt, 1943). Although chimpanzees spend much of their time in small parties, groups of chimpanzees share a common home range, and are therefore considered to be members of a single community, a pattern which is called fission-fusion (Goodall, 1986; Wrangham, 1987). The home range size of chimpanzee communities can vary over time and across populations, but home range sizes are considered large for primates and generally greater than 10 km2, often increasing with the number of individuals in the community (Wrangham, 1987; Herbinger et al., 2001). The smallest documented home range is Taï Middle Community, Côte d'Ivoire at approximately 3.1 km2 (Herbinger et al., 2001), and the largest is Semuliki, Uganda at 72 km2 (Samson and Hunt, 2012). For chimpanzees, ecological factors (i.e., seasonality of food availability) and social factors (i.e., the size of the community, number of males in the community) have been found to be primary predictors of range size (Wrangham, 1987; Dunbar, 1988; Herbinger et al., 2001; Newton-Fisher, 2003). Various reports of intercommunity encounters have shown that chimpanzees will aggressively defend their core areas and also patrol far reaching peripheral regions of home ranges (Herbinger et al., 2001).
Individual home ranges may appear similar for members of a community; however, each individual may use habitats differently (Hasegawa, 1990). For example, Chapman and Wrangham (1993) found that males in Kanyawara, Uganda used an area approximately 1.5 to 2 times larger than females. Furthermore, males tended to remain within boundary areas approximately 4 times as often as did females (Chapman and Wrangham, 1993). In Taï National Forest, Côte d’Ivoire, however, researchers documented females using the entire home range occupied by their community, and that they travelled in groups with a greater number of females (Boesch, 1991). Unlike the Kanyawara community, chimpanzees in Taï also form female-female coalitions, potentially explaining differences in space use patterns (Chapman and Wrangham, 1993).
In addition to a host of ecological and social predictors, home ranges may also be influenced by anthropogenic factors such as human presence and habitat alterations. For example, anthropogenic landscapes often contain an abundance of food items which may be a draw for crop feeding, thus impacting habitat use and range size (e.g. Hockings et al., 2009; Bessa et al., 2015; Bryson-Morrison et al., 2017; Couturier et al., 2022). Additionally, other high risk anthropogenic areas such as roads (e.g., Cibot et al., 2015; McLennan and Asiimwe, 2016) may also impact movement patterns. Different chimpanzee communities throughout their range experience a range of human impacts with some inhabiting the interior of protected areas with little human impacts [e.g., Ngogo: Amsler (2009), Fongoli: Pruetz (2006)]. Others inhabit forest fragments with high human impacts [e.g., Bossou: Matsuzawa and Humle (2011), Bulindi: McLennan (McLennan, 2010)]. There are, however, fewer documented chimpanzee communities living at edges of protected areas adjacent to human-dominated landscapes (see Sebitoli: Krief et al., 2014).
The Buraiga-Kisongi chimpanzee community represents a newly habituated chimpanzee community in Kibale National Park (KNP), Uganda, an area of high chimpanzee and human density. Under the direction of the Uganda Wildlife Authority (UWA), this community has been undergoing the process of habituation for tourism in earnest since 2016 (Guma et al., 2019). As such, there is little data on space use and individual and community home ranges. Our goal was to document how Buraiga-Kisongi home range and territory interface with neighboring chimpanzee communities, and whether Buraiga-Kisongi chimpanzees’ home range was exclusively within the boundaries of KNP. Given that KNP is surrounded by densely settled rural landscapes, little suitable chimpanzee habitat remains outside park boundaries. Yet farmers around KNP have reported chimpanzees foraging on crops, such as eucalyptus, banana pith, and sugar cane (Krief et al., 2014). Documenting the home range and core areas of the Buraiga community is part of a greater initiative by UWA to understand the movements of chimpanzee communities in relation to park boundaries, and how chimpanzees interact with neighboring chimpanzee and human communities. These data provide essential space use information for Buraiga conservation efforts, beginning to paint a picture of spatial ecology and behavior within and outside of KNP. As great ape populations are increasingly limited to habitat fragments and protected areas (McCarthy et al., 2015; McLennan et al., 2020a) it is important to document landscape use in real time to mitigate conflict and promote sustainable coexistence.
The objectives of this study are to:
1. Document Buraiga-Kisongi chimpanzee movement and space use patterns, and compare these results with other published home range data,
2. Estimate individual and community home range and core area sizes and spatial locations in reference to KNP boundary, and
3. Estimate seasonal and monthly, as well as sex-specific, variation in space use.
2 Materials and methods
2.1 Study area and subjects
This study was conducted in Kibale National Park (KNP) in southwestern Uganda (0° 12’ – 0` 40’ N and 30° 35’ E; Figure 1). KNP is a nationally protected area encompassing 795 km2, situated near the Rwenzori Mountains (Uganda Wildlife Authority, 2015). The park is a mid-altitude rainforest, composed of moist evergreen and semideciduous forest at 1,100 to 1,590 m elevation (Johns, 1996; Struhsaker, 1999). KNP has a tropical climate with two rainy seasons from March to May and September to November, with an annual rainfall of between 1,100 and 1,700 mm (Uganda Wildlife Authority, 2015). The area adjacent to KNP near the study area primarily consists of agricultural fields and a narrow riparian forest fragment at the location of the Kanyanchu river where it exits KNP. Subjects of this study were members of the Buraiga chimpanzee community from the Kisongi subgroup (hereafter Buraiga-Kisongi) (Guma et al., 2019; Edwards et al., 2023).
2.2 Data collection
We collected geographic coordinates with a handheld GPS unit carried by a human observer (Garmin GPSMap 64s and 66s) during daily continuous focal samples on adult Buraiga-Kisongi individuals. Individuals were selected ad libitum due to variable habituation levels across the community (N = 1557.33 hours; N = 377 focal observations), data collection took place between 7 am and 7 pm. The GPS unit was set to track mode, recording a point every 30 seconds during each focal follow. This high-frequency sampling regime was selected to mitigate the potential for a high failure rate given the forest cover density and frequent cloud cover. Following Great Ape Tourism best practices (Macfie and Williamson, 2010) and the Uganda Wildlife Authority’s Standard Operating Procedures, human observers maintained a distance of no less than 7 m from the nearest chimpanzee.
We collected data during two field seasons, August 2019 – March 2020, and January – July 2022. We alternated between male and female focal individuals on consecutive days (when possible). Of the 377 focal samples, we analyzed space use patterns for 265 focal follows of adult males (N = 1,166 hours) and 96 focal follows of adult females (N = 389 hours). Just 2.83 hours were from individuals of unknown sex, reflecting harder to see (i.e., situated high in trees) and/or less habituated individuals.
We supplemented direct focal follow data with indirect ad libitum GPS data for the following traces on days when we were unable to collect direct observational data: fresh (less than 24 hours-old) knuckle prints, feces, and feeding traces. Points were also taken for all new nests (less than 24 hours-old). Chimpanzee feces are distinguishable from those of other primate species by color, form, and size (McLennan, 2013; Bessa et al., 2015). We collected GPS data on feeding traces when we were certain that they were left by chimpanzees, such as when we knew that an individual or party had been in the area recently, or the traces were species-specific (i.e. fruit wadges) (Morgan and Sanz, 2006; Pruetz, 2006; McLennan, 2010). When multiple points of indirect data were found, we marked a single GPS location to avoid over-estimation (McLennan, 2013).
2.3 Spatial and temporal data analysis
To calculate home range estimates, we used two complementary methods, minimum convex polygons (MCP) (Getz and Wilmers, 2004) and kernel density estimates (Worton, 1989), using the R package adehabitatHR (Calenge, 2006) in R 4.0.2 (R Core Team, 2020). MCP entails constructing the smallest possible convex polygon around all points representing the presence of the study animal, or a pre-selected percentage of those points (e.g., 95% MCP, which excludes outlier locations). Although this method is the simplest measure of home range, it tends to grossly overestimate home range size, particularly when a given home range is non-convex (Burgman and Fox, 2003). Additionally, MCP’s focus on peripheral locations provides no insight into range structure. However, MCP is useful for drawing comparisons with previous studies of chimpanzee home ranges (see Chapman & Wrangham, 1993). Kernel density estimation (KDE) methods, in contrast, have been shown to better estimate home range (Seaman and Powell, 1996). KDE calculates the probability of finding a given individual in a specific area, by weighing each observation’s location by the time the focal animal spent in a particular area (relative to neighboring locations). We used the reference bandwidth in all KDE home range calculations.
We subsampled the GPS relocations to one point every 30 min to reduce spatial and temporal point autocorrelation (N = 3139 relocations) prior to HR calculation. To estimate HR, we calculated the following estimates: minimum convex polygon (95 and 100% MCP), and kernel density estimate (50, 90, 95% KDE) from the subsampled relocations and indirect observation points collectively (N = 3486 relocations). We define 50% KDE as the core area (CA) and 95% KDE as the home range (HR). We compared these home range estimates with published estimates from 16 other chimpanzee sites using student’s t-tests.
We further subsampled the data to test for seasonal and monthly changes in CA and HR, and differences in home range use by males and females overall and seasonally (rainy and dry seasons). Additionally, we estimated individual home ranges for three males, RTW, BLK, and SUN. These individuals were selected because they were the individuals from whom we had the greatest amount of data, distributed evenly across the study period. Due to variations in habituation levels across individuals and fluctuations in daily group composition, it was not possible to get an even sample from specific individual chimpanzees throughout the duration of the study.
3 Results
3.1 Home range and core area estimates
During two field seasons, August 2019 – March 2020, and January 2022 – July 2022, we collected data on adult chimpanzees totaling 1,557.33 hours of focal observational data from 377 focal samples. Of those, 265 focal follows were conducted on adult males (N = 1,166 hours on 23 identified individuals and 41 focal observations of unidentified individuals) and 96 focal follows of adult females (N = 389 hours on 14 identified individuals and 57 focal observations on unidentified adult female individuals).
In sum, 3,486 spatial data points were used to estimate community home range during the study period. Of those, 2, 399 data points were from males, 713 from females, and 24 from individuals of unknown sex. Indirect observations (i.e., feeding traces, fresh feces, nests) comprised 350 spatial data points. Buraiga-Kisongi individuals used an area extending northwest from Fort Portal-Kamwenge Road and the Dura River as far southeast as the boundary of the park (Figure 2). The MCP estimates for 95% and 100% of relocations corresponded to 16.45 km2 and 24.90 km2, respectively (Table 1). The kernel density method estimated a 95% KDE HR = 15.77 km2, and a core area (50% KDE) CA = 3.32 km2. During the study period, core areas were located near the boundary of the park, with a second area located approximately 2.39 km from the park boundary. We found that 15.82% of the 95% KDE home range extended outside of the national park.

Figure 2 Map of community home range estimates for Buraiga-Kisongi chimpanzees across two field seasons (August 2019 – March 2020, and January – July 2022). Home range estimates (50, 90, 95% KDE isopleths, and 95% MCP) overlaid on satellite image of Kibale National Park (park boundary yellow dotted line) with the Dura, Kanyanchu, and Kanyandungu Rivers (light blue line), and Fort Portal-Kamwenge Road (white dotted line).

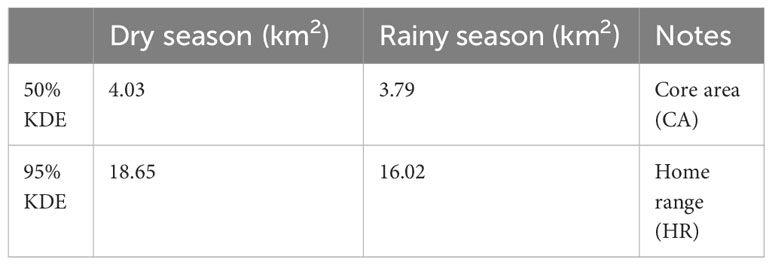
Table 1 Community home range areas of the Buraiga chimpanzees, using kernel density estimates (KDE) and minimum convex polygon (MCP).
We compared results of community home range analyses for Buraiga-Kisongi chimpanzees with home range estimates from 16 other chimpanzee communities across Africa (Table 2). Mean home range size from available 100% MCP estimates is 24.46 km2 (range = 6.8 – 59.0 km2, N = 14 chimpanzee communities). Buraiga-Kisongi chimpanzees exhibited a slightly larger home range at 24.90 km2, not significantly different from mean home ranges of other chimpanzee communities reported in the literature (t = -0.13, df = 11, p = 0.90). The 95% isopleth KDE for Buraiga-Kisongi is 15.77 km2, close to the mean of 15.31 (range = 3.1 – 35.7 km2) calculated for the 10 other communities used in this comparison (t = 2.14, df = 11, p-value = 0.06).
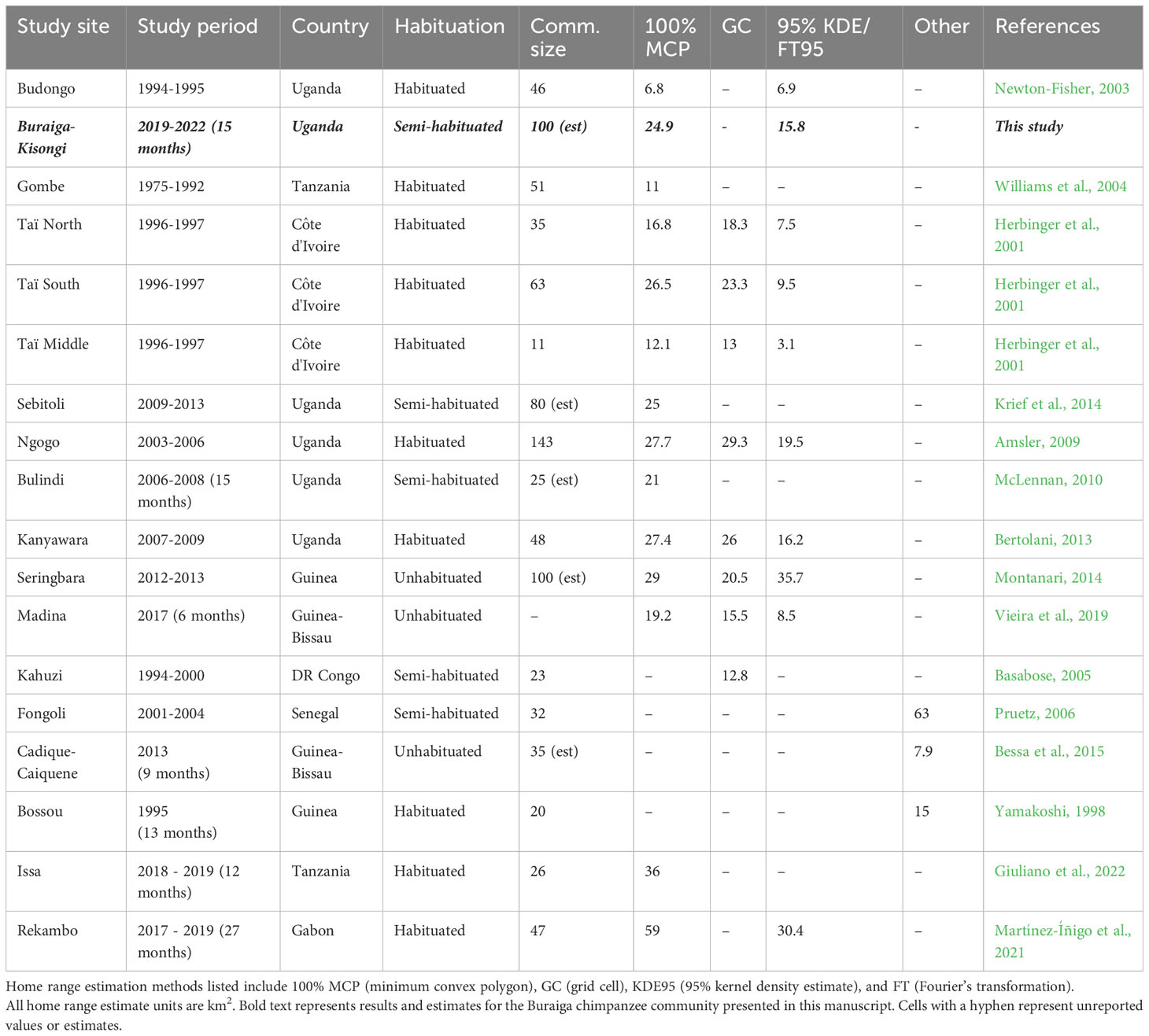
Table 2 Comparisons of home range estimates from 17 chimpanzee communities (Adapted from Vieira et al. (2019).
3.2 Seasonal home range variation
Home range areas varied by season with Buraiga individuals occupying 16.02 km2 (95% KDE) during rainy season months (March – May and September – November) and 18.65 km2 (95% KDE) during dry season months (June – August and December – February) (Figure 3; Table 3). Core areas, represented by 50% KDE, varied slightly by season, with chimpanzees using 3.79 km2 during rainy season months and 4.03 km2 during dry season months (see Supplmentary Information for monthly home range and core area estimates).
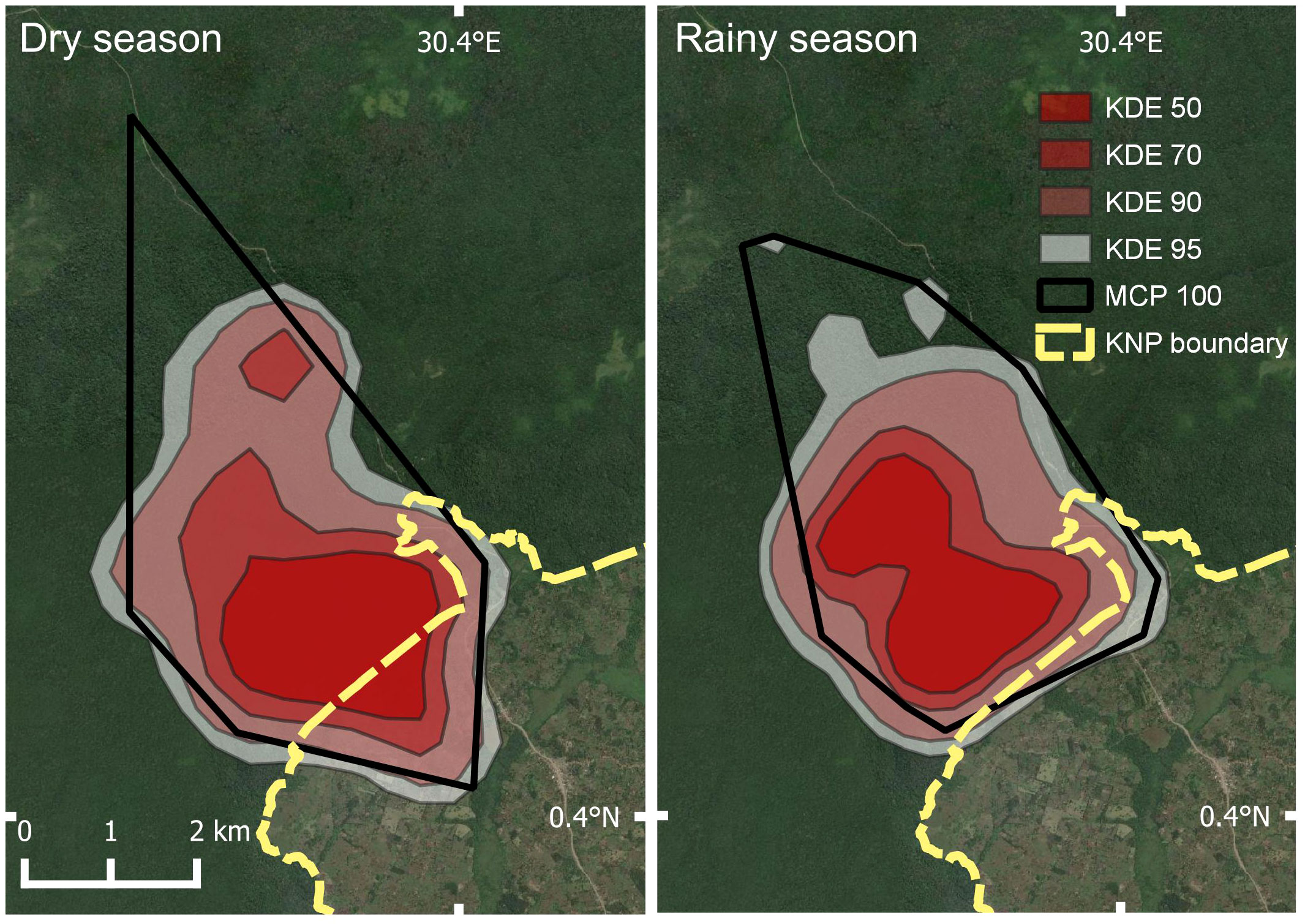
Figure 3 Home range estimation by season [dry season (left), and rainy season (right)] of Buraiga-Kisongi chimpanzees, across two field seasons (August 2019 – March 2020, and January – July 2022).
3.3 Home range variation by sex
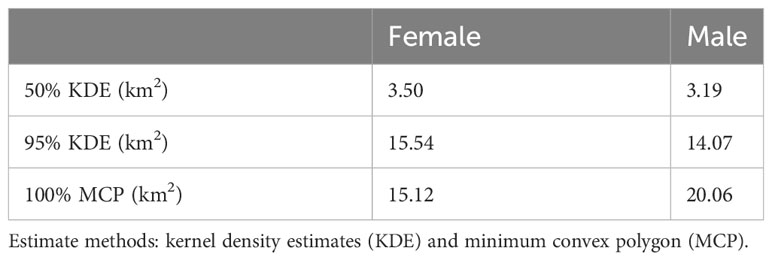
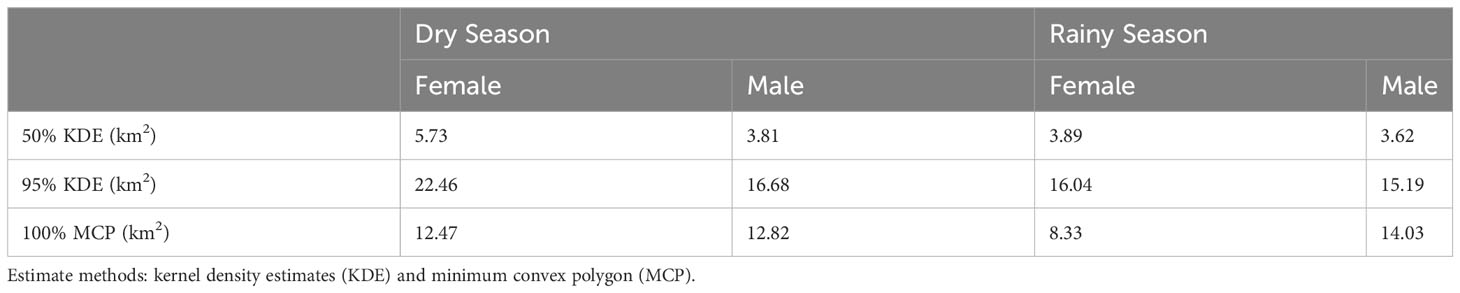
Home range estimates differed significantly between males and females only for 100% MCP estimates (Males: 20.06 km2; females 15.54 km2; Figure 4; Table 4). Based on the KDE, females used a slightly larger area than males. Seasonal differences in range size revealed opposite trends, although details varied depending upon the method used (Figure 5; Table 5). For example, using 95% isopleth KDE, rainy season home ranges did not differ significantly between males and females. However, during dry season months, the 95% isopleth KDE home ranges were substantially larger for females (Males: 16.68 km2; Females: 22.46 km2).
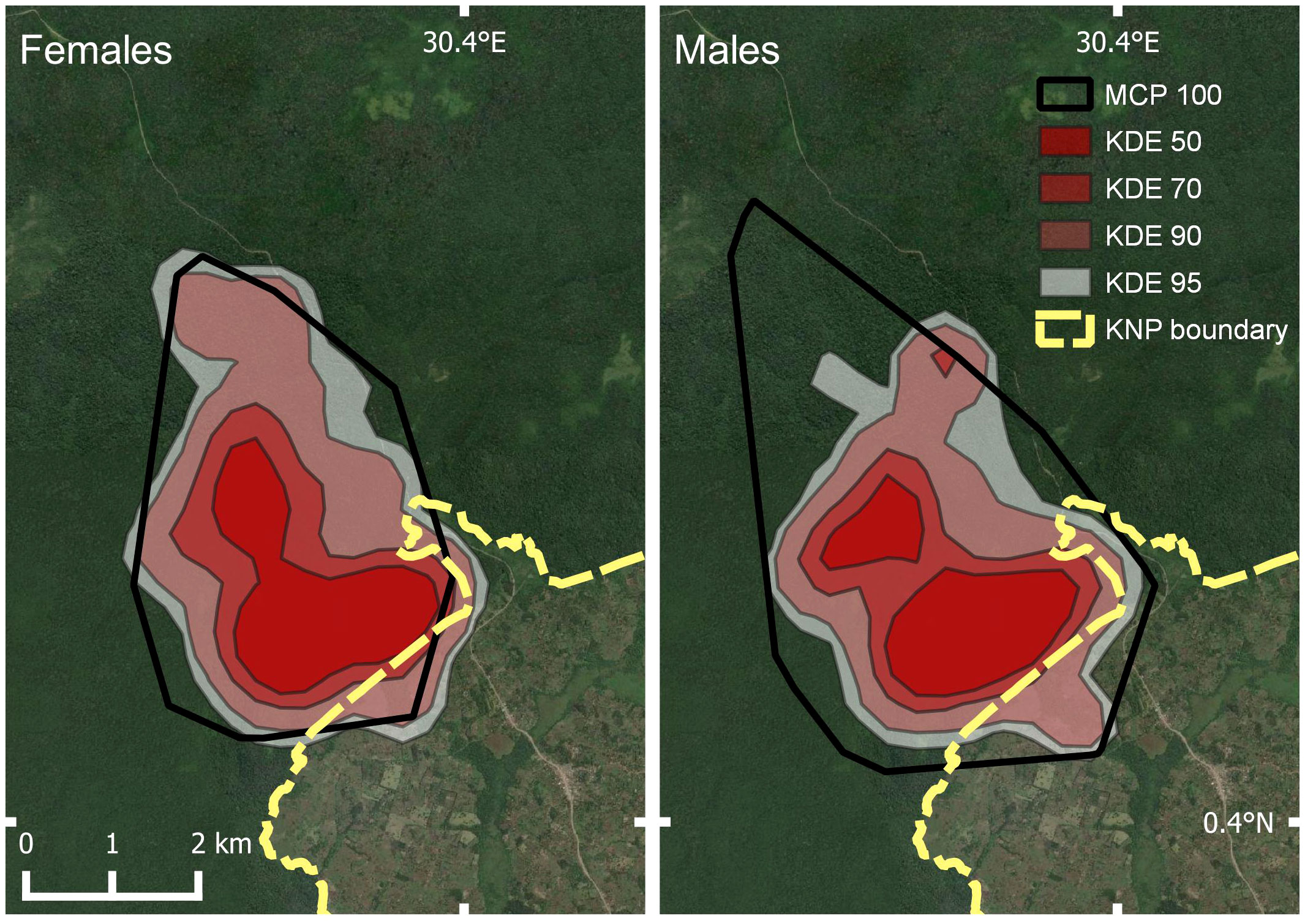
Figure 4 Map of female (left) and male (right) Buraiga-Kisongi chimpanzee home range estimates, across two field seasons (August 2019 – March 2020, and January – July 2022).
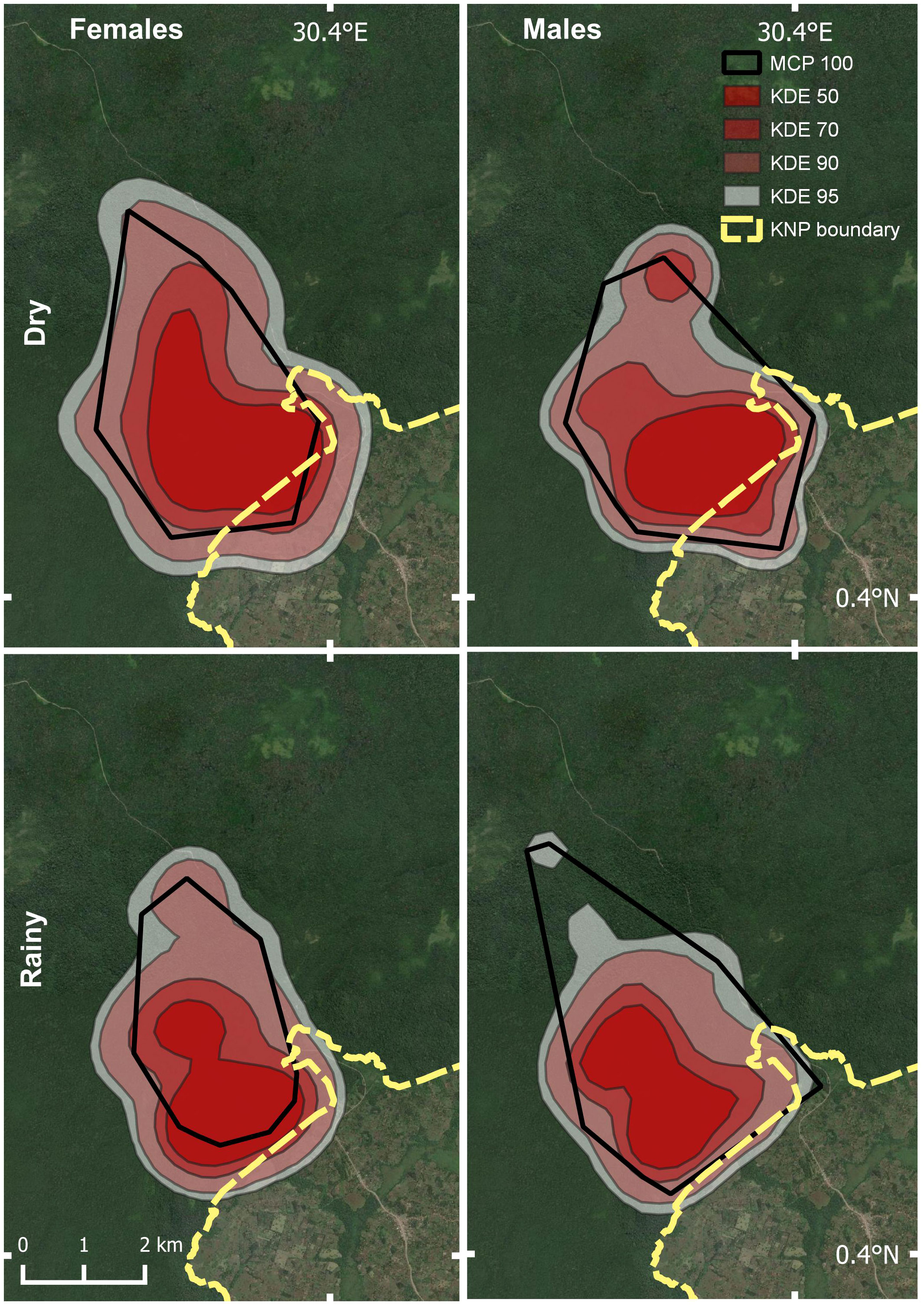
Figure 5 Map of Buraiga-Kisongi chimpanzees’ seasonal home range variation by sex, across two field seasons (August 2019 – March 2020, and January – July 2022).
3.4 Individual home range variation
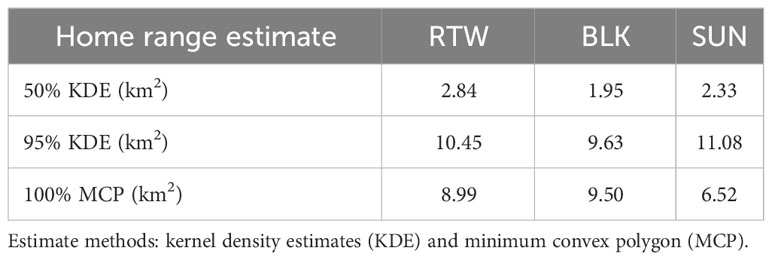
Individual home range ranges varied little among three adult male individuals (Figure 6; Table 6). We sampled BLK for 101.93 hours across 12 months, RTW for 170.95 hours across 14 months, and SUN for 80.25 hours across 12 months. SUN had the largest 95% KDE home range (11.08 km2), with a core area more centrally located in the park, compared with other individual male home ranges located closer to park boundaries (Figure 6). BLK had the smallest core area based on 50% KDE (1.95 km2).
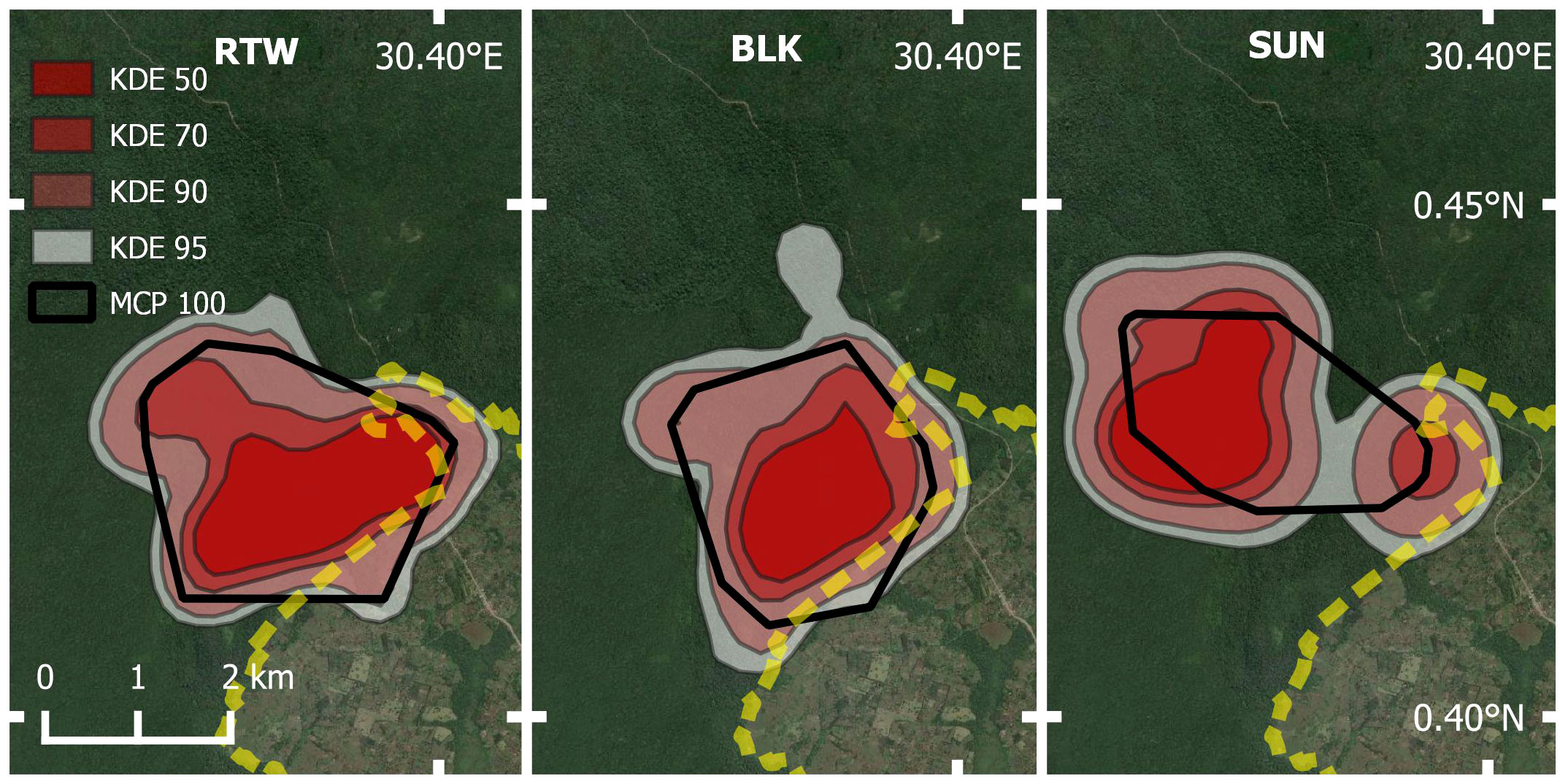
Figure 6 Map of Buraiga-Kisongi chimpanzees’ individual home range estimates (n = 3 adult males), across two field seasons (August 2019 – March 2020, and January – July 2022).
4 Discussion
Estimated at 24.9 km2 (100% MCP) or 15.8 km2 (95% KDE), Buraiga-Kisongi chimpanzees’ home range is average-sized, when compared with other published chimpanzee home range estimates. Within KNP, chimpanzee community home ranges are similar in size, with Ngogo having the largest home range based on 95% KDE. Home range size in chimpanzees has been found to be influenced by a range of factors, including presence of neighboring chimpanzee communities, variations in resource availability and abundance, and presence of predators (Wrangham, 1987; Dunbar, 1988; Herbinger et al., 2001; Newton-Fisher, 2003). Although no distinctive reason has been provided for the larger home range size at Ngogo, Ngogo’s higher chimpanzee density has been attributed to wider and more abundant distribution of high yield fruiting trees throughout their home range, compared with lower resource availability across the home range of Kanyawara chimpanzees (Potts, 2008). Moreover, other studies have linked larger home range size with a larger community population, which may explain the variation seen at these two sites. Further research will be necessary to parse the influences of home range size at Buraiga-Kisongi, but it is interesting that all documented home range estimates available for chimpanzee communities living in KNP are similar. Although research has shown that home range estimation methods may be vulnerable to differences in sample size, Amsler (2009) found that range size reach an asymptote after 2,500 locations, under which range estimations were underestimated. The present estimation of Buraiga-Kisongi home range size, based on 15 months of data and 3,486 geospatial data points, should produce a reliable estimate.
Our space-use analysis suggests that Buraiga-Kisongi home range is bounded by the Dura River, Fort Portal-Kamwenge Road, and the KNP boundary, and that incursions into community-owned agricultural land via the Kanyanchu Swamp corridor are not uncommon. Additionally, Buraiga-Kisongi individuals occasionally forage and nest west of the Dura River, north of the Fort Portal-Kamwenge Road, and in community owned agricultural lands (Figure 2). Core area estimates showed that Buraiga chimpanzees use landscapes near the border of the national park and the interior of their range, although notably 15.82% of the home range is situated outside of the national park in community managed and owned land. Seasonal availability of ripe fruit, figs, and crops both inside and outside the park likely influences space use behavior, as chimpanzees used the Kanyanchu Swamp corridor and adjacent agricultural land exclusively during dry season months.
Interestingly, Buraiga-Kisongi individuals often use the Kanyanchu River swamp perhaps to exit the park without being seen by humans. They appear to use this corridor in a similar manner as they use habitats within KNP, engaging in activities such as feeding, grooming, playing, resting, and nesting in groups between 1 and 20 individuals (W.I. Edwards, unpubl. data). However, they also travel through this corridor to access agricultural plots adjacent to the swamp, foraging there for wild foods and agricultural foods, which can lead to risky encounters with humans. Crop-foraging has been documented from other chimpanzee communities, most commonly, though not exclusively, in highly disturbed forest-farm mosaics such as Bossou, Guinea and Bulindi, Uganda (Hockings and McLennan, 2012). Chimpanzees have been found to adopt different strategies to mitigate and limit human encounters, such as bringing crops into forest patches (Bossou, Guinea; Hockings et al., 2007), and foraging at night (Sebitoli, Uganda; Krief et al., 2014). Outside of KNP, Buraiga-Kisongi chimpanzees feed on both wild and cultivated resources, for example, elephant grass pith (Pennisetum purpureum), papyrus pith (Cyperus papyrus), Ficus vallis-choudae, Psuedospondias microcarpa, Phoenix reclinata fruit, sugar cane (Saccharum officinarum), eucalyptus bark (Eucalyptus spp.), and banana pith (Mussa spp.) (W.I. Edwards, unpubl. data). This may reflect diet selection choices, because these are also the months when fewer ripe fruits are available inside park boundaries. It should also be noted that of 218 night nests recorded, 62 were located outside of KNP, indicating that Buraiga-Kisongi chimpanzees not only forage outside of the park, but also sleep there.
Seasonally, Buraiga-Kisongi chimpanzees used a larger area during dry season months. During this season, chimpanzees used the Kanyanchu Swamp corridor and intensified use in northwestern interior portions of the park, adjacent to the Dura River. This may reflect patterns of resource availability within and outside of KNP, with chimpanzees traveling larger distances between food patches during seasons of lower resource availability, as seen at other chimpanzee sites (Basabose, 2005; Moore et al., 2018). Significantly, dry season months are also the only time when the swamp is not completely flooded and difficult to navigate, hence this may factor into habitat choice for the Buraiga-Kisongi chimpanzees.
Interestingly, we found that regardless of season, Buraiga-Kisongi females used a larger area than males. In most previous studies at other long-term research sites, males have been documented to utilize larger areas than females, particularly during times when large groups of males patrol the periphery of their range (“border patrols”, see Hasegawa, 1990). The trend documented here of females routinely using larger areas than males in the Buraiga-Kisongi site may reflect sampling differences in our study. Of the 358 identified focal observations, 257 were conducted on males, and 101 on females. The number of focal hours collected on female members of the Buraiga-Kisongi community represented 26.7% of total focal hours. As the number of focal hours is tied to the number of GPS relocations, disparity in home range size between males and females may reflect differences in GPS relocations, as subsampling regimes resulting in smaller GPS relocations can result in a smaller MCP and larger KDE home range estimates, as seen in the Buraiga-Kisongi results (see Martínez-Íñigo et al., 2021). Alternately, larger home range size documented for female Buraiga-Kisongi individuals may reflect real differences in habitat use, with females more widely dispersed across the Buraiga-Kisongi home range. Additional long-term data as more Buraiga-Kisongi females become fully habituated will help to clarify these relationships.
Individual home range data for three adult males, RTW, SUN, and BLK, did not reveal substantial home range size differences, although SUN’s core area was more centrally located within the park than those of the other two males. This is interesting because SUN is younger and lower ranking than the other two individuals. BLK and RTW also tended to spend a lot of time together, explaining the large overlap between their ranges. Although additional data are needed to better understand individual space use dynamics, differences in range use may reflect differences in rank and daily social groupings, with higher ranking males spending more time near the park boundary than lower ranking males. Home ranges of individual chimpanzees within a community are not often reported in the literature, although the few that do report male home ranges also find a correlation between higher rank and larger home ranges (Chapman and Wrangham, 1993; Newton-Fisher, 2003).
There has been little human incursion into KNP itself over the past 30 years (Hartter and Southworth, 2009), and indeed, most chimpanzees in Uganda live in and around protected areas (Plumptre and Cox, 2006). Nevertheless, land use changes outside parks place anthropogenic pressures upon chimpanzee communities. This is not unique to Buraiga; human and chimpanzee communities overlap in many places, such as Bossou, Bulindi, Sebitoli, and Semuliki (see review Hockings & McLennan, 2012). We documented mixed-sex groups of 1-20 individuals outside of the park engaging in routine behaviors such as grooming, resting, playing, and feeding, indicating that Buraiga-Kisongi chimpanzees are not leaving the park only to crop raid. Data presented here indicate that areas outside the park are routinely used as part of Buraiga-Kisongi home range, despite threats of human intervention, and for a broad range of activity types, as seen the other sites where chimpanzees and humans live in shared landscapes (Onderdonk and Chapman, 2000; Hockings and McLennan, 2012; McLennan et al., 2020b). Buraiga-Kisongi chimpanzees’ use of the Kanyanchu Swamp corridor, in a country where over 60% of forests and an unknown number of wetlands are unprotected (Hartter and Southworth, 2009), further emphasizes their utility in chimpanzee livelihood.
Due to the difficulty of following and documenting chimpanzees along an uneven and flooded landscape, it is likely that Buraiga-Kisongi individuals use the swamp corridor and adjacent land even more intensely than is described by data presented herein. Indeed, local community members often commented that they observed chimpanzees on their agricultural land even during times that we were following other individuals within KNP, or in other areas outside the park. Additional long-term data with a larger team of trackers are needed to clarify relationships among how different subgroups utilize habitats over time.
A robust literature on chimpanzee spatial ecology documents that individual communities vary widely in terms of home range size and habitat use dynamics, reflecting site-specific strategies for variation in resource availability on different landscapes. Habitat fragmentation and landscape alteration make it possible - and timely - to record how species respond to these changes even on short time scales. Baseline data in this study provide essential space use information for Buraiga conservation efforts, painting a picture of dynamic space use behaviors both within and outside of KNP and raising additional questions about how chimpanzee communities navigate spatial considerations across seasons and in relation to neighboring chimpanzee and human populations. Although the Buraiga-Kisongi chimpanzees primarily focused on habitats within KNP, our data show that they regularly used areas outside the protected area as part of their home range. In a time of ever-increasing habitat change, it is important to document populations on anthropogenically influenced landscapes to offer a richer and deeper understanding of ecological flexibility and resilience among endangered great apes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee in the Ohio University Office of Research and Compliance (protocol #19-O-010). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WE: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. VP: Formal Analysis, Methodology, Writing – review & editing. NG: Conceptualization, Writing – review & editing. HA: Conceptualization, Writing – review & editing. GB: Conceptualization, Writing – review & editing. LN: Writing – review & editing. NS: Conceptualization, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the Ohio University Heritage College of Osteopathic Medicine (NS and WE), and WE further acknowledges funding from Primate Conservation, Inc. (PCI #1739), the Ohio Center for Ecology and Evolutionary Studies, the Ohio University Student Enhancement Award, the Ohio University Graduate Student Senate, the Ohio University College of Arts and Sciences, and the Ohio University Department of Biological Sciences.
Acknowledgments
The Uganda Wildlife Authority and the Uganda National Council for Science and Technology granted permission for this study. Protocol IACUC #19-O-010 was approved by the Institutional Animal Care and Use Committee in the Ohio University Office of Research Compliance. WIE would like to thank Drs. Susan Williams, Sabrina Curran, and Jessica Rothman for their guidance and insights. We thank the following individuals for their valuable counselling and cooperation: George Owoyesigire and Daniel Akaruhanga. This work would not be possible without the support of Martin Ainebyona, Martin Amanya, Christopher Amanyire, Christopher Kaijabwangu, David Kato, Vincent Kule, Landus Mumbere, Shadil Mubiru, Vincent Tulyashemererwa, and Sebastiano Tusingwire. We thank John Mwesige for daily logistical assistance. WIE extends special thanks to her host, Harriet Kemigisha, and their staff, Stephen Ahabwomugisha, Yoweri Bahati, Alex Kamanyire, Winstone Nahabwe, Suzan Namata, Deneson Niwamanya, and Amon Nkurunziza.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1267688/full#supplementary-material
References
Amsler S. J. (2009). Ranging behavior and territoriality in chimpanzees at Ngogo, Kibale National Park, Uganda. [dissertation]. [Ann Arbor (MI)]: University of Michigan.
Basabose A. K. (2005). Ranging patterns of chimpanzees in a montane forest of Kahuzi, Democratic Republic of Congo. Int. J. Primatol. 26, 33–54. doi: 10.1007/s10764-005-0722-1
Bertolani M. P. (2013). Ranging and travelling patterns of wild chimpanzees at Kibale, Uganda: GIS approach. [dissertation] [Cambridge (UK)]: University of Michigan.
Bessa J., Sousa C., Hockings K. J. (2015). Feeding ecology of chimpanzees (Pan troglodytes verus) inhabiting a forest-mangrove-savanna-agricultural matrix at Caiquene-Cadique, Cantanhez National Park, Guinea-Bissau. Am. J. Primatol. 77, 651–665. doi: 10.1002/ajp.22388
Boesch C. (1991). The effects of leopard predation on grouping patterns in forest chimpanzees. Bahviour 3–4, 220–242. doi: 10.1163/156853991X00544
Bryson-Morrison N., Tzanopoulos J., Matsuzawa T., Humle T. (2017). Activity and Habitat Use of Chimpanzees (Pan troglodytes verus) in the Anthropogenic Landscape of Bossou, Guinea, West Africa. Int. J. Primatol. 38, 282–302. doi: 10.1007/s10764-016-9947-4
Burgman M. A., Fox J. C. (2003). Bias in species range estimates from minimum convex polygons: Implications for conservation and options for improved planning. Anim. Conserv. 6, 19–28. doi: 10.1017/S1367943003003044
Burt W. H. (1943). Territoriality and home range concepts as applied to mammals. J. Mammal. 24, 346–352. doi: 10.2307/1374834
Calenge C. (2006). The package adehabitat for the R software: a tool for the analysis of space and habitat use by animal. Ecol. Model 197, 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
Chapman C. A., Wrangham R. W. (1993). Range use of the forest chimpanzees of kibale: implications for the understanding of chimpanzee social organization. Am. J. Primatol. 31, 263–273. doi: 10.1002/ajp.1350310403
Cibot M., Bortolamiol S., Seguya A., Krief S. (2015). Chimpanzees facing a dangerous situation: A high-traffic asphalted road in the sebitoli area of Kibale National Park, Uganda. Am. J. Primatol. 900, 890–900. doi: 10.1002/ajp.22417
Couturier C., Bortolamiol S., Ortmann S., Okimat J. P., Asalu E., Krief S. (2022). All-you-can-eat: influence of proximity to maize gardens on the wild diet and the forest activities of the sebitoli chimpanzee community in Kibale National Park. Animals 12 (7), 806–628. doi: 10.3390/ani12070806
Edwards W. I., Guma N., Agaba H., Balyesiima G., Asalu E., Rothman J., et al. (2023). Leaf sponge tool use by Buraiga chimpanzees, Pan troglodytes schweinfurthii, in Kibale National Park, Uganda. Afr. J. Ecol. 00, 1–5. doi: 10.1111/aje.13163
Getz W. M., Wilmers C. C. (2004). A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography (Cop). 4, 489–505. doi: 10.1111/j.0906-7590.2004.03835.x
Giuliano C., Stewart F. A., Piel A. K. (2022). Chimpanzee (Pan troglodytes schweinfurthii) grouping patterns in an open and dry savanna landscape, Issa Valley, western Tanzania. J. Hum. Evol. 163, 103137. doi: 10.1016/j.jhevol.2021.103137
Goodall J. (1986). The Chimpanzees of Gombe: patterns of behavior (Cambridge, MA: Belknap Press of Harvard University Press).).
Guma N., Balyesiima G., Agaba H. (2019). “Application of SMART Soultions to habituation of Buraiga chimpanzee Community: Kibale National Park Experience, Uganda,” in 2nd African Primatological Society Conference: Primate Conservation Challenge in Africa - Challenges and Opportunities, vol. 1. (Entebbe).
Hartter J., Southworth J. (2009). Dwindling resources and fragmentation of landscapes around parks: Wetlands and forest patches around Kibale National Park, Uganda. Landsc. Ecol. 24, 643–656. doi: 10.1007/s10980-009-9339-7
Hasegawa T. (1990). “Sex differences in ranging patterns,” in The Chimpanzees of the Mahale Mountains: Sexual and Life History Strategies. Ed. Nishida T. (Tokyo: University of Tokyo Press), 99–114.
Herbinger I., Boesch C., Rothe H. (2001). Territory characteristics among three neighboring chimpanzee communities in the Taï National Park, Côte d’Ivoire. Int. J. Primatol. 22, 143–167. doi: 10.1023/A:1005663212997
Hockings K. J., Anderson J. R., Matsuzawa T. (2009). Use of wild and cultivated foods by chimpanzees at Bossou, Republic of Guinea: Feeding dynamics in a human-influenced environment. Am. J. Primatol. 71, 636–646. doi: 10.1002/ajp.20698
Hockings K. J., Humle T., Anderson J. R., Biro D., Sousa C., Ohashi G., et al. (2007). Chimpanzees share forbidden fruit. PloS One 2, 1–4. doi: 10.1371/journal.pone.0000886
Hockings K. J., McLennan M. R. (2012). From forest to farm: systematic review of cultivar feeding by chimpanzees - management implications for wildlife in antropogenic landscapes. PloS One 7, e33391. doi: 10.1371/journal.pone.0033391
Johns B. G. (1996). Responses of chimpanzees to habituation and tourism in the Kibale Forest, Uganda. Biol. Conserv. 78, 257–262. doi: 10.1016/S0006-3207(96)00044-4
Krief S., Cibot M., Bortolamiol S., Seguya A., Krief J., Masi S. (2014). Wild chimpanzees on the edge: nocturnal activities in croplands. PloS One 9, e109925. doi: 10.1371/journal.pone.0109925
Macfie E. J., Williamson E. A. (2010). Best Practice Guidelines for Great Ape Tourism (Gland, Switzerland: IUCN/SSC Primate Specialist Group (PSG).
Martínez-Íñigo L., Baas P., Klein H., Pika S., Deschner T. (2021). Home range size in central chimpanzees (Pan troglodytes troglodytes) from Loango National Park, Gabon. Primates 62, 723–734. doi: 10.1007/s10329-021-00921-x
Matsuzawa T., Humle T. (2011). The Chimpanzees of Bossou and Nimba. Ed. Sugiyama Y. (Tokyo: Springer Japan).
McCarthy M. S., Lester J. D., Howe E. J., Arandjelovic M., Stanford C. B., Vigilant L. (2015). Genetic censusing identifies an unexpectedly sizeable population of an endangered large mammal in a fragmented forest landscape. BMC Ecol. 15, 1–15. doi: 10.1186/s12898-015-0052-x
McLennan M. (2010). Chimpanzee ecology and interactions with people in an unprotected human-dominated landscape at Bulindi, western Uganda. [dissertation]. [Oxford]: Oxford Brookes University.
McLennan M. R. (2013). Diet and Feeding Ecology of Chimpanzees (Pan troglodytes) in Bulindi, Uganda: Foraging Strategies at the Forest-Farm Interface. Int. J. Primatol. 34, 585–614. doi: 10.1007/s10764-013-9683-y
McLennan M. R., Asiimwe C. (2016). Cars kill chimpanzees: case report of a wild chimpanzee killed on a road at Bulindi, Uganda. Primates 57, 377–388. doi: 10.1007/s10329-016-0528-0
McLennan M. R., Hintz B., Kiiza V., Rohen J., Lorenti G. A., Hockings K. J. (2020a). Surviving at the extreme: Chimpanzee ranging is not restricted in a deforested human-dominated landscape in Uganda. Afr. J. Ecol. 59, 17–28. doi: 10.1111/aje.12803
McLennan M. R., Lorenti G. A., Sabiiti T., Bardi M. (2020b). Forest fragments become farmland: Dietary Response of wild chimpanzees (Pan troglodytes) to fast-changing anthropogenic landscapes. Am. J. Primatol. 82, 1–18. doi: 10.1002/ajp.23090
Montanari D. (2014). Ranging patterns of Seringbara chimpanzees: methodological insights. [master's thesis] [Utrect]: Universiteit Utrect.
Moore J. F., Masozera M. K., Niyigaba P., Ndikubwimana I., Cipolletta C. (2018). Shifting through the forest: home range, movement patterns, and diet of the eastern chimpanzee (Pan troglodytes schweinfurthii) in Nyungwe National Park, Rwanda. Am. J. Primatol. 80, e22897. doi: 10.1002/ajp.22897
Morgan D., Sanz C. (2006). “Chimpanzee feeding ecology and comparisons with sympatric gorillas in the Goualougo Triangle, Republic of Congo,” in Feeding Ecology in Apes and Other Primates. Ecological, Physical and Behavioural Aspects. Eds. Hohmann G., Robbins M. M., Boesch C. (Cambridge, UK: Cambridge University Press), 97–122.
Newton-Fisher N. E. (2003). The home range of the Sonso community of chimpanzees from the Budongo Forest, Uganda. Afr. J. Ecol. 41, 150–156. doi: 10.1046/j.1365-2028.2003.00408.x
Onderdonk D. A., Chapman C. A. (2000). Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int. J. Primatol. 21, 587–611. doi: 10.1023/A:1005509119693
Plumptre A. J., Cox D. (2006). Counting primates for conservation: Primate surveys in Uganda. Primates 47, 65–73. doi: 10.1007/s10329-005-0146-8
Potts K. B. (2008). Habitat Heterogeneity on Multiple Spatial Scales in Kibale National Park, Uganda: Implications for Chimpanzee Population Ecology and Grouping Patterns [dissertation]. [New Haven (CT)]: Yale University.
Pruetz J. D. (2006). “Feeding ecology of savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal,” in Feeding ecology in apes and other primates. Eds. Hohmann G., Robbins M., Boesch C. (Cambridge, UK: Cambridge University Press), 326–364.
R Core Team (2020). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Samson D. R., Hunt K. D. (2012). A Thermodynamic Comparison of Arboreal and Terrestrial Sleeping Sites for Dry-Habitat Chimpanzees (Pan troglodytes schweinfurthii) at the Toro-Semliki Wildlife Reserve, Uganda. Am. J. Primatol. 74, 811–818. doi: 10.1002/ajp.22031
Seaman D. E., Powell R. A. (1996). An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 77, 2075–2085. doi: 10.2307/2265701
Struhsaker T. T. (1999). Ecology of an African Rain Forest: Logging in Kibale and the Conflict Between Conservation and Exploitation (Gainesville, FL: University Press of Florida).
Uganda Wildlife Authority (2015). Kibale National Park - General Management Plan (2015 - 2025). Available at: https://www.ugand awild life.org/ wildl ife- a- conse rvati on- 2/resea rcher s- corne r/gener al- manag ement - plans ?downl oad=15:kibale_natio nal_park_gmp.
Vieira W. F., Kerry C., Hockings K. J. (2019). A comparison of methods to determine chimpanzee home-range size in a forest–farm mosaic at Madina in Cantanhez National Park, Guinea-Bissau. Primates 60, 355–365. doi: 10.1007/s10329-019-00724-1
Williams J. M., Oehlert G. W., Carlis J. V., Pusey A. E. (2004). Why do male chimpanzees defend a group range? Anim. Behav. 68, 523–532. doi: 10.1016/j.anbehav.2003.09.015
Worton B. J. (1989). Kernel methods for estimating the utilization distribution in home- range studies. Ecology 70, 164–168. doi: 10.2307/1938423
Wrangham R. (1987). “Ecology and social relationships in two species of chimpanzee,” in Ecological Aspects of Social Evolution: Birds and Mammals. Eds. Rubenstein D., Wrangham R. (Princeton: Princeton University Press), 352–378.
Keywords: spatial ecology, habitat mosaic, corridor, Buraiga, Kibale National Park, home range, kernel density estimate, Pan troglodytes
Citation: Edwards WI, Popescu VD, Guma N, Agaba H, Balyesiima G, Nakami L and Stevens NJ (2023) Living at the edge: home range patterns of the Buraiga Chimpanzee Community, Kibale National Park, Uganda. Front. Ecol. Evol. 11:1267688. doi: 10.3389/fevo.2023.1267688
Received: 26 July 2023; Accepted: 16 October 2023;
Published: 30 October 2023.
Edited by:
Isabel Marques, University of Lisbon, PortugalReviewed by:
Moreen Uwimbabazi, National Forestry Resources Research Institute (NaFORRI), UgandaElizabeth Brunton, University of the Sunshine Coast, Australia
Copyright © 2023 Edwards, Popescu, Guma, Agaba, Balyesiima, Nakami and Stevens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wren I. Edwards, d3JlbmVkd2FyZHNAZ21haWwuY29t
†Present address: Nancy J. Stevens, Department of Anthropology, University of Colorado, Boulder, CO, United States
 Wren I. Edwards
Wren I. Edwards Viorel D. Popescu
Viorel D. Popescu Nelson Guma6
Nelson Guma6 Nancy J. Stevens
Nancy J. Stevens