
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Ecol. Evol. , 14 August 2023
Sec. Chemical Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1267179
This article is part of the Research Topic Methods in Chemical Ecology: 2022/23 View all 8 articles
Editorial on the Research Topic
Methods in Chemical Ecology: 2022/23
Chemical ecology, i.e. the interdisciplinary study of chemically mediated organismic interactions, has its origin in the 19th century (Hartmann, 2008), but got most popular in the second half of the 20th century (Meinwald and Eisner, 2008). Since then, hundreds of studies are published every year on this topic (Figure 1), with the aim to better understand the chemical mechanisms of interactions within and among plants, animals, and microorganisms. One reason for the success of this field of research is that all organisms release, detect, and respond to chemicals, making the number and kinds of interactions to potentially study extremely high (Meinwald and Eisner, 2008). Another reason is that there are continuous advances in the methods available, further increasing the number of possible research questions tackled (Mori and Noge, 2021). This Research Topic aimed to highlight studies that developed or reviewed experimental techniques and methods used to investigate fundamental questions in chemical ecology research. It brings together four articles that describe methodological developments in chemical analytics, in identifying compounds detected by insects, in investigating the functions of chemicals, and in collecting and analysing volatile organic compounds. One study introduced a model system appropriate to teach chemical ecology in practical classes, another one evaluated methods to identify cryptic and potentially endangered ecotpyes of an orchid, and the seventh study of this Research Topic reviewed approaches used to test for olfactory learning in insects.
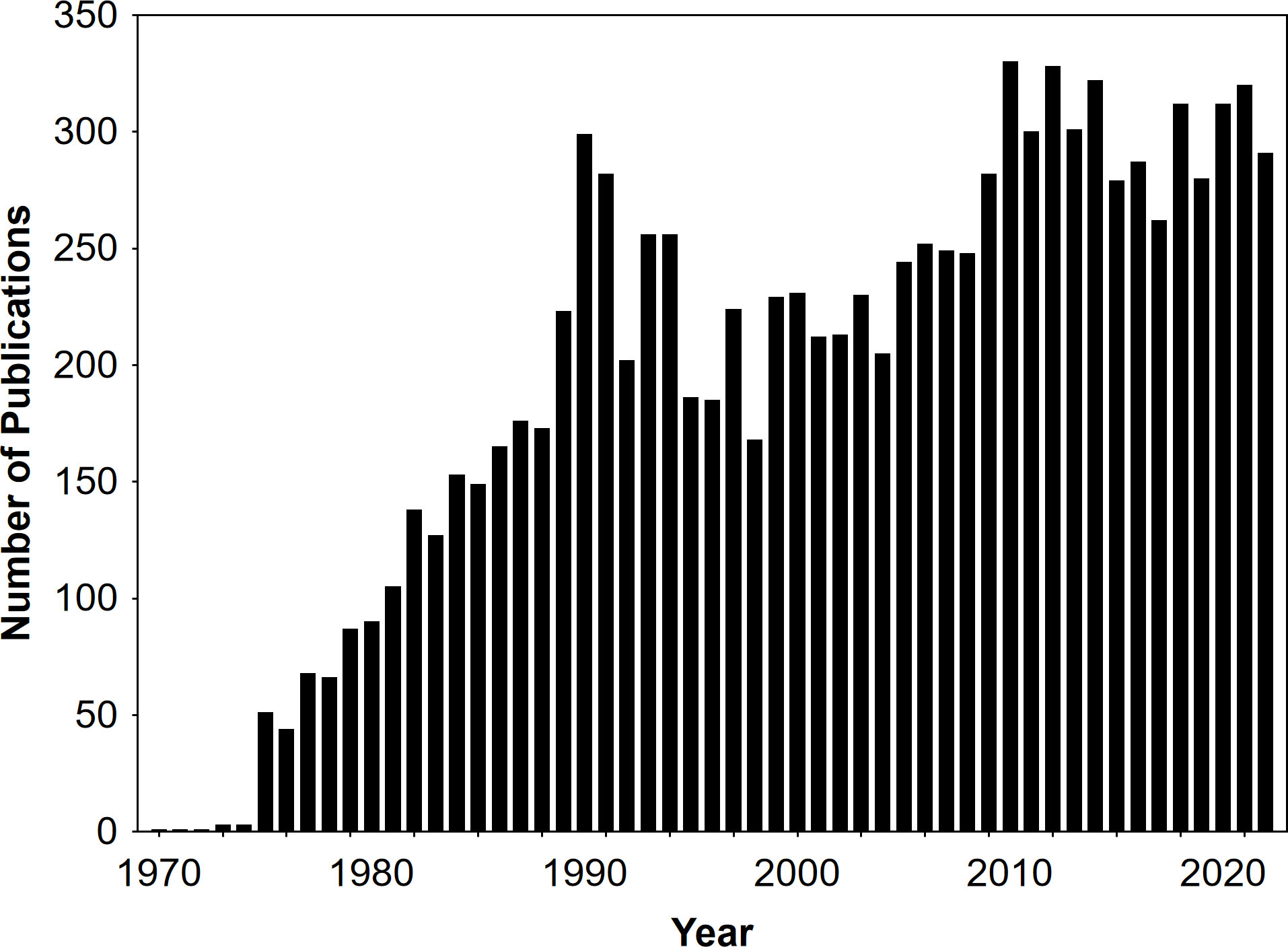
Figure 1 Number of studies published per year (1970-2022) on “chemical ecology” according to Web of Science™.
Floral scents are key for the interaction between flowering plants and their pollinators (Raguso, 2008), with the number of studies and scientists working on floral scents continuously increasing. Eisen et al. present a set of best practices for floral scent research by outlining methods for data collection (from experimental designs to the chemical identification of compounds) and the analysis of the obtained data. The authors also created the R package bouquet, which provides a data analysis pipeline.
Plants often release a complex mixture of scent compounds, with only a subset thereof being perceived by animal interaction partners (Gfrerer et al., 2021; Gfrerer et al., 2022). Gas chromatography coupled to electroantennographic detection (GC/EAD) is a tool often used to identify physiologically active compounds in the antennae of insects, and thereby narrowing down the number of compounds potentially involved in the communication (Schiestl and Marion-Poll, 2001). Shuttleworth and Johnson introduce a method that integrates a GC, an EAD setup, a flame ionization detector (FID) and a mass spectrometer (MS). This setup resolves some of the challenges associated with dividing the GC effluent between a detector at atmospheric pressure (an antenna) and a detector under vacuum (the MS). It is especially useful for samples that do not allow multiple injections [thermal desorption, solid-phase microextraction (SPME)].
Behavioral assays are critical steps in identifying volatile organic compounds involved in the communication among organisms (Haynes and Millar, 2012), and Huber and Schiestl describe a simple and cheap method for offering the volatiles to the organism(s) of interest. Volatiles are at first applied as a solvent/volatile solution to a silicone septum, before allowing the solvent to evaporate. Afterwards, the volatiles are emitted at repeatable rates that can be fine-tuned to the desired emission rate.
Pollen is an important reward for various flower visitors, such as flies and bees (Waser and Ollerton, 2006). Recently, it was demonstrated for bees that the protein-to-lipid ratio as well as the contents and ratios of fatty acids in pollen are strongly related to bee foraging decisions and development (Arien et al., 2020; Ruedenauer et al., 2020). Villagómez et al. propose a common protocol for the extraction of lipids from pollen and their analysis via GC/FID and/or GC/MS to obtain reliable and comparable results of pollen fatty acid profiles.
Chemical ecological experiments are often time-consuming and require elaborate equipment, and thus, are less appropriate for student classes, especially for less advanced students. Mamin et al. introduce cotton (Gossypium hirsutum) as a model for teaching plants chemical ecology. This plant species stores defensive compounds in glands as a constitutive defense, whereas the density and the chemical filling (size) of these glands increase systematically in leaves in response to herbivory (Opitz et al., 2008). As cotton gland induction can be readily visualized under modest magnification, cotton is highly suited to teach chemical ecology and aspects of plant defense theory in practical classes.
At least three different ecotypes, one thereof potentially endangered, were recently described from sexually deceptive Drakaea livida orchids using chemical analyses of the labella of unpollinated flowers and pollinator choice trials (Weinstein et al., 2022). As flowers will not be pollinated when collecting labella from unpollinated flowers and pollinators are not always available, Weinstein et al. tested whether identifying ecotypes could be successfully applied by sampling the labella from pollinated flowers, thereby not affecting the reproductive success of the plants. Indeed, they evidenced that the use of volatiles from the labellum of recently pollinated flowers is an effective way to determine the ecotype of unknown individuals.
Many insects do not only innately respond to olfactory cues but are also capable of learning (Papaj and Prokopy, 1989). In their review, Adam et al. discuss the scope and limits of olfactory learning in insects and raise the question whether currently used learning paradigms in artificial laboratory settings are appropriate to answer all ecologically relevant questions. They believe that many insects are able to learn olfactory information in as little as one trial. To evidence one trial olfactory learning, refining the experimental set-ups in the laboratory as well as designing research studies in an ecologically relevant framework is needed.
SD: Conceptualization, Writing – original draft. LC: Writing – review & editing. PW: Writing – review & editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arien Y., Dag A., Yona S., Tietel Z., Cohen T. L., Shafir S. (2020). Effect of diet lipids and omega-6:3 ratio on honey bee brood development, adult survival and body composition. J. Insect Physiol. 124, 104074. doi: 10.1016/j.jinsphys.2020.104074
Gfrerer E., Laina D., Gibernau M., Fuchs R., Happ M., Tolasch T., et al. (2021). Floral scents of a deceptive plant are hyperdiverse and under population-specific phenotypic selection. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.719092
Gfrerer E., Laina D., Wagner R., Gibernau M., Hörger A. C., Comes H. P., et al. (2022). Antennae of psychodid and sphaerocerid flies respond to a high variety of floral scent compounds of deceptive Arum maculatum L. Sci. Rep. 12 (1), 5086. doi: 10.1038/s41598-022-08196-y
Hartmann T. (2008). The lost origin of chemical ecology in the late 19th century. Proc. Natl. Acad. Sci. United States America 105 (12), 4541–4546. doi: 10.1073/pnas.0709231105
Haynes K. F., Millar J. G. (2012). Methods in Chemical Ecology Volume 2: Bioassay Methods (US: Springer).
Meinwald J., Eisner T. (2008). Chemical ecology in retrospect and prospect. Proc. Natl. Acad. Sci. United States America 105 (12), 4539–4540. doi: 10.1073/pnas.0800649105
Mori N., Noge K. (2021). Recent advances in chemical ecology: complex interactions mediated by molecules. Biosci Biotechnol Biochem. 85 (1), 33–41. doi: 10.1093/bbb/zbaa034
Opitz S., Kunert G., Gershenzon J. (2008). Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J. Chem. Ecol. 34 (4), 508–522. doi: 10.1007/s10886-008-9453-z
Papaj D. R., Prokopy R. J. (1989). Ecological and evolutionary aspects of learning in phytophagous insects. Annu. Rev. Entomol 34, 315–350. doi: 10.1146/annurev.en.34.010189.001531
Raguso R. A. (2008). Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol Evol Syst 39, 549–569. doi: 10.1146/annurev.ecolsys.38.091206.095601
Ruedenauer F. A., Raubenheimer D., Kessner-Beierlein D., Grund-Mueller N., Noack L., Spaethe J., et al. (2020). Best be(e) on low fat: linking nutrient perception, regulation and fitness. Ecol. Lett. 23 (3), 545–554. doi: 10.1111/ele.13454
Schiestl F. P., Marion-Poll F. (2001). “"Detection of physiologically active flower volatiles using gas chromatography coupled with electroantennography,",” in Molecular methods of plant analysis, 21: Analysis of taste and aroma. Eds. Jackson J. F., Linskens H. F., Inman R. B. (Berlin: Springer), 173–198.
Waser N. M., Ollerton J. (2006). Plant-Pollinator Interactions: From Specialization to Generalization (Chicago: University of Chicago Press).
Keywords: gas chromatography/mass spectrometry (GC/MS), flower scent analysis, olfactory learning, gas chromatography/electroantennographic detection, behavioral assays, pollen lipids, plant defense
Citation: Dötterl S, Chen L and Wen P (2023) Editorial: Methods in Chemical Ecology: 2022/23. Front. Ecol. Evol. 11:1267179. doi: 10.3389/fevo.2023.1267179
Received: 26 July 2023; Accepted: 26 July 2023;
Published: 14 August 2023.
Edited and Reviewed by:
Stefano Colazza, University of Palermo, ItalyCopyright © 2023 Dötterl, Chen and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Dötterl, c3RlZmFuLmRvZXR0ZXJsQHBsdXMuYWMuYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.