
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 18 August 2023
Sec. Population, Community, and Ecosystem Dynamics
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1235558
Fall armyworm (FAW), Spodoptera frugiperda, is a highly polyphagous pest that recently invaded Kenya, among other African countries. Information on the pest’s genetic diversity and mechanisms conferring insecticide resistance, in addition to farmers’ knowledge and perceptions on the applicable mitigation measures, inform the development of sustainable management strategies. Therefore, this study collected cross-sectional data from 800 farmers in Kenya and documented their knowledge and perceptions on FAW and factors influencing their choice of FAW control methods. Additionally, we identified the strains present in 8 counties in Kenya using the mitochondrial Cytochrome Oxidase sub-unit I (mt COI) gene, and correlated pesticide use to gut microbiome diversity via 16S rRNA metagenomics to investigate the probable contribution of gut bacteria towards insecticide resistance evolution. All farmers reported FAW infestations, and 24% reported limited and total non-response of FAW to insecticides. Fall armyworm rice-strain and corn-strain were detected ravaging corn fields. However, the corn-strain revealed higher microbial diversity than the rice-strain. Furthermore, pathogenic bacterial genera were elevated in the insect gut in both corn and rice strains after chemical-treatments. Insecticide–endosymbiont interactions should be further explored, and farmers’ training on effective alternative pest control methods is recommended.
Insect pests, diseases, harsh climatic conditions, and the rapidly increasing world population are constant threats to global food security (Stevenson et al., 2017) with fall armyworm (FAW), S. frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) being one of the significant pre-harvest pests of economic concern in Sub-Saharan Africa (SSA) (Kumela et al., 2019). Fall armyworm is highly polyphagous and destructive in its larval phase, which if left unmitigated leads to death of the infested plants (Hardke et al., 2015), causing up to 80% yield losses (Khan et al., 2014). The pest is destructive on corn, wheat, rice, millet, sorghum and, cotton (Ríos-Díez and Saldamando-Benjumea, 2011; Chimweta et al., 2020; Tepa-yotto et al., 2021). In SSA, FAW causes at least 21% loss of corn yields which translates to approximately US$ 2.48 to 6.19 billion loss annually (Kenis et al., 2023). As of 2018 (one year after its official report in Kenya), FAW was already an established economically significant pest, as 83% of corn farmers reported high infestation(s) and yield losses ranging from 30% to 54% due to the pest (De Groote et al., 2020).
Numerous control measures have been developed for FAW management, including synthetic chemical insecticides, biological control methods (predatory and parasitic organisms), botanical pesticides (plant extracts), and cultural practices (Midega et al., 2018; Kumela et al., 2019; Kassie et al., 2020). However, though expensive and potentially harmful, chemical-insecticide use remains consistently common (Baudron et al., 2019; Gutiérrez-moreno et al., 2019; Kumela et al., 2019; Kassie et al., 2020), followed by cultural and botanical control measures (Kansiime et al., 2019; Houngbo et al., 2020). The dominance of chemical pest control measures is worrisome as insecticide residues have been detected in food and water (Rehman and Saggu, 2016; Togola et al., 2018), thereby posing credible risk to human and animal health, aquatic organisms, and fostering rampant evolution of resistance (Siyum et al., 2022). Insecticide-resistant FAW have been widely reported (Ríos-Díez and Saldamando-Benjumea, 2011; Carvalho et al., 2013; Huang et al., 2014; Jakka et al., 2016; Gutiérrez-moreno et al., 2019). Therefore, inter-disciplinary efforts, farmer inclusion and intensive awareness creation of the alternative pest control approaches are requisite for effective management of FAW and avoidance of resistance evolution in the pest (Nkamleu and Adesina, 2000; Despotović et al., 2019). Numerous dynamics such as cost, associated risks, effectiveness of practices/products, effort required, time, education level, tax implication, expected economic and social rewards, and farmers’ attitude have been reported to significantly affect various farmers’ decisions such as whether to (or not to) adopt environmental conservative farming measures, agricultural policies and mitigation measures (Sattler and Nagel, 2010; Wachenheim et al., 2014; Zhang et al., 2015; Trujillo-Barrera et al., 2016). However, no studies have been conducted to investigate how various aspects impede or motivate the choice of chemical and alternative pest control measures among smallholder maize farmers in Kenya. Furthermore, information on farmers’ knowledge about FAW and perceptions regarding the various available control methods is also limited.
Information on genetic variations among FAW populations is important in the development of successful IPM strategies and inference of the population structure to understand haplotype distribution (phylogeographic patterns) and the probable origin of invasive pests. Fall armyworm exists in two main strains: the rice-strain (RS) and the corn-strain (CS). The strains are morphologically identical but can physiologically and genetically be differentiated (Nagoshi and Meagher, 2020). They contrast in host preference/associations, pheromone composition and mating behavior (Pashley and Martin, 1987), growth rates, pupal weights (Pashley, 2018), and also exhibit differential tolerance and resistance development towards control agents (Ríos-Díez and Saldamando-Benjumea, 2011). For instance, the FAW RS is highly susceptible to Bacillus thuringiensis (Bt) and some synthetic insecticides like diazinon and carbaryl, while less susceptible to carbofuran (Pashley et al., 1987). Fall armyworm records high genetic diversity (Nayyar et al., 2021), which is correlated with variability in physiological processes among populations (Veenstra et al., 1995; Pashley, 1988). Characterization of FAW strains and their genetic diversity is therefore important in deciphering crop preference (guided by the strain–host associations and the relative distribution of the strains) and potential insecticide resistance development in the pest populations.
In a bid to unveil the mechanisms behind insecticide resistance evolution in pests, studies have shown that gut microbiota influences the efficacy of both organic and synthetic pest control agents (Russell et al., 2011). For example, Bacillus thuringiensis (Bt)- susceptible populations are characterized by a gut microbial community that is significantly low in diversity and richness indices (Paddock et al., 2021). Additionally, bacterial genera such as Microbacterium, Enterobacter, Arthrobacter, and Pseudomonas (Tehara and Keasling, 2003; Cabrera et al., 2010) have shown the capacity to degrade chlorpyrifos, deltamethrin and lambda-cyhalothrin (Mallick et al., 1999; Singh et al., 2004; Khalid et al., 2016), with rates as high as 50mg within 18 hours (Xu et al., 2007). To determine the effect of the commonly applied chemical insecticides in Kenya on the gut microbiota structure of FAW and to assess the possible contribution of gut microbiota towards resistance evolution, we profiled the FAW larvae gut microbiome before and after Bt and synthetic chemical insecticide treatment.
This study, therefore, provides insights into smallholder farmers’ knowledge on FAW, their perceptions regarding the effectiveness of the commonly applied control measures, and the factors influencing the choice of FAW control methods. Furthermore, insights into the strain distribution, genetic diversity within the FAW populations in Kenya five years post its invasion and effects of insecticides on the FAW gut biodiversity were assessed. This knowledge is critical to understand the pest population structure and biology/mechanisms of insecticide resistance for the development of sustainable targeted FAW management approaches in Kenya.
The study applied a multi-stage sampling technique in eight maize-growing counties in Kenya (Figure 1), randomly sampled based on the distribution along low, mid, and high altitudes agroecological zones: dry mid-altitude (Kajiado), moist mid-altitude (Nandi and Murang’a), dry transitional (Machakos), moist transitional (Bungoma and Kirinyaga), and high tropic (Nyamira and Nyeri). To gather farmers’ perspectives, a questionnaire was used to acquire information, and this was carried out through face-to-face interviews in at least two wards per county. The study employed a systematic random sampling whereby every fifth maize-producing household was interviewed. One hundred households were selected from each county with the assistance of ward agricultural extension officers in the areas. Where possible, the household heads were preferred during the interviews as they are responsible for making the central and critical decisions in their respective households (Antman, 2014). The survey questionnaire covered various aspects of the farmers’ demographic information, farm characteristics, FAW infestation-frequencies, management practices and their relative effectiveness. Farmers were also asked to make estimates of FAW-associated yield losses by paralleling yields before and after the invasion of FAW and insecticides’ efficiency in FAW management using a three-point Likert-scale (Larkin et al., 2007) labeled: 0 – not effective, 1 – moderately effective, and 2 – very effective. The study was conducted between November and December 2020.
The survey data were analyzed using SPSS (version 22) and Stata (version 14.1). Descriptive statistics such as frequencies, means, and standard deviations were calculated correspondingly for the various variables in the survey instrument (questionnaire). Comparative statistical tools such as Chi-square tests and analysis of variance (ANOVA) were used to investigate the statistical differences across the counties in the various assessed variables. The significant difference was tested at a 95% confidence interval. Factors influencing farmers’ choice of FAW control methods were estimated using a Bivariate Probit (BVP) regression model, which investigated the causal effect of the explanatory variables upon the dependent variables (control methods).
Two control methods: synthetic chemical insecticides and cultural control methods were dominant and extensively applied in all counties. Thus, to investigate the influencers of Kenyan smallholder maize farmers’ choice between the two control methods, the Bi-Variate Probit (BVP) regression model was applied. Since the dependent variable was a choice variable that is dichotomous in nature and the Spearman’s rho of non-parametric correlations revealed that the choice variables were highly correlated (χ2 = −0.715, P< 0.01) (Supplementary Table 1), the BVP regression model was considered appropriate to generate unbiased and efficient estimates of the parameters. A correlated nature of the dependent variables (in this case, the two control methods) is characterized by an overall correlation and a stochastic dependency of the error terms (Nkamleu and Adesina, 2000). The BVP model applies the maximum likelihood procedures to estimate and fit the model (Adejumo et al., 2014). The empirical specification of the model is as below:
where and represent the cultural and chemical control methods employed by the ith farmer, respectively; and are vectors of socio-economic and institutional factors affecting the choice of FAW control method, and are vectors of parameters to be estimated, while . and are the error terms of the respective control methods.
Estimates of the BVP model were made by normalizing the first category of each discrete explanatory variable which is statistically termed as a “base category” or the “reference state.” Before estimating the model, the independent variables were tested for multicollinearity and heteroskedasticity as the rule of thumb dictates (Dormann et al., 2013). Dependent and independent variables used in the model are described in Supplementary Table 2.
Fall armyworm larvae samples were collected from vegetative corn plants in seven counties (Kilifi, Bungoma, Busia, Kiambu, Kirinyaga, Makueni, and Taita Taveta) in Kenya for molecular identification, strain characterization and gut microbiome biodiversity analyses. The regions were selected as they vary in cropping systems and ecological parameters such as annual rainfall, temperature, and relative humidity. These randomly sampled counties are distributed along low, mid, and high altitudes agroecological zones: dry mid-altitude (Taita Taveta), moist mid-altitude (Kiambu and Kirinyaga), dry transitional (Makueni), moist transitional (Busia), high tropic (Bungoma) and low coastal (Kilifi). Despite the geographical and agroecological variations, the selected regions had an ongoing corn-growing season at the time of the study, and incidences of FAW infestations had been previously reported (De Groote et al., 2020). Twenty insect specimens were collected per county by random sampling on infested plants. The field-collected FAW samples were preserved in absolute ethanol for transportation to the laboratory, where they were stored at −20°C until DNA extractions and downstream analyses were done.
Cytochrome oxidase subunit I (mt COI) locus by locus haplotype profiling was performed for molecular identification of the pest species and strain characterization of S. frugiperda using 15 samples per county. The specimens (n = 105) were washed in 3% sodium hypochlorite for three seconds and rinsed thrice with distilled water for surface sterilization. The thorax segment of the sterilized FAW larvae samples were excised and transferred individually into 1.5ml Eppendorf tubes. Genomic DNA extraction was done using the Bioline Isolate II DNA extraction kit (Bioline, London, UK) following the manufacturer’s instructions. A fragment of the COI gene was amplified using the Lep primer pair; LepF1 (ATTCAACCAACATAAAGATATTGG) and LepR1 (TAAACTTCTGGATGTCCAAAAAATCA) (Kuate et al., 2019). Each 10µL reaction mix contained 5.675µL of PCR water, 2 µL of reaction buffer, 0.5 µL of the forward and reverse primers, 0.2 µL of 5mM magnesium chloride, 0.125 µL of MyTaq DNA polymerase enzyme and 1 µL of DNA template (21 to 99 ng/µL). Cycling conditions were set as follows; 95°C for 2 minutes followed by 40 cycles [95°C (30 sec), 52 °C (40 sec), and 72 °C (1 minute)], and final elongation at 72 °C for 10 minutes. Bi-directional sequencing of 54 samples was done by Macrogen, Inc, South Korea. Sequence cleaning and editing were done in Geneious v8.1.8 software (http://www.geneious.com) (Banerjee et al., 2017). The clean sequences (n = 46) were queried at GenBank, hosted by National Centre for Biotechnology Information (NCBI), for similarity searches via the Basic Local Alignment Search Tool (BLASTN). The final analysis involved 112 publicly available S. frugiperda sequences from GenBank and 46 high-quality sequences from this study. Sequence alignment was performed using Multiple Sequence Comparison by Log-Expectation (MUSCLE). The Maximum Likelihood tree was constructed using MEGA v. 11.0.9 based on p-distances under the Kimura-2 parameter model with 1,000 bootstrap replicates. The FAW strains in this study were characterized using a defining locus as described by Nagoshi et al. (2019). The ancestral American rice (R-) and corn (C-) strains contained the reported segregating sites and were therefore used as indicators of strain-specific haplotypes (maternal lineages) among FAW populations.
For biogeographical patterns and the associations analysis between the putative Kenyan FAW species and other populations, sequences with indeterminate origins were excluded from the analysis. Intra- and inter-populational diversity assessments were conducted in DnaSP v. 6.12.03 (Rozas et al., 2003). The number of haplotypes (h), haplotype diversity (Hd), nucleotide diversity (π), number of polymorphic and monomorphic sites were determined.
The chemical insecticides: spinetoram, chlorantraniliprole + lambda-cyhalothrin, acephate, emamectin benzoate, lambda-cyhalothrin and a commercial Bt insecticide recorded during the survey were selected for laboratory assays to evaluate the effects of the treatments on the gut microbial biodiversity in FAW larvae. Early third-instar FAW larvae were obtained from the International Centre of Insect Physiology and Ecology (icipe)’s Animal Rearing and Containment Unit (ARCU) for the laboratory bioassays, and insecticides were prepared by dilution using distilled water to the recommended dosage following the manufacturer’s instructions. In each experimental setup, corn leaves were exposed to the respective treatment via topical/surface contamination whereby, vegetative corn leaves were cut into approximately 8 cm long stretches which were then dipped into insecticide solutions before being placed in sterile petri-dishes for larvae to feed on. To avoid cannibalism and to ensure that the mortality scores recorded were absolutely as a result of insecticide intoxication, only a single early third-instar S. frugiperda larva was introduced in each petri-dish containing the contaminated leaves. The experiments were randomized and comprised of three replicates (30 larvae per replicate) and a control group which was exposed to distilled water. Samples for 16S rRNA metagenomics analysis were collected 72 hours post-exposure while those that survived the insecticide- treatment were reared to the first generation.
To assess the effect of chemical insecticides on the gut microbial profiles of FAW larvae, specimens were surface sterilized in 3% sodium hypochlorite for three seconds, rinsed in 70% ethanol for three seconds and rinsed three times with distilled water to remove topical microbes. The insects were then dissected using sterile blades, and the guts picked using a pair of sterile forceps. DNA was extracted from the gut contents of the specimen using the Isolate II Genomic DNA kit (Bioline, London, United Kingdom). Two biological replicates were analyzed per treatment where, the total DNA from three samples per treatment were pooled and analyzed as a single replicate. Therefore, 36 larvae (6 larvae per treatment) that were exposed to chemical insecticides were processed. Furthermore, S. frugiperda exhibited tolerance to two insecticides (lambda-cyhalothrin and Bt insecticide) and survived up to the first generation (F1) after exposure to the treatments. Hence, twelve F1 larvae were also processed (6 larvae per treatment). Spodoptera frugiperda rice strain (RS) and corn strain (CS) samples from the various sampled counties were also processed separately to inform on microbial diversity between the RS and CS in the Kenyan FAW population. DNA extracts were checked for quality and concentration using a Nanodrop 2000/2000c Spectrophotometer before sequencing on an Oxford Nanopore Technologies MK1C device using FLO-MIN 106D (R9.4) flow cells.
The cumulative read counts from the samples for each treatment and county were shown in a stacked bar plot to allow for direct quantitative comparison of abundances at the genus level. A minimum abundance cut-off of 1% was used to select the abundant taxa in each sample; hence taxa with cumulative read counts below 1% were collapsed. The bacterial diversity in each county and treatment population was assessed using Shannon-Wiener index, evenness. Bray-Curtis dissimilarity index (Beals, 1984) was used to estimate the interpopulation diversity. Inter-population distances were computed using principal coordinate analyses (PCoA) in R software v3.5.3. The WIMP-ARMA workflow in EPI2ME (https://epi2me.nanoporetech.com/) was used to identify ARGs within the microbiome of the samples.
The mean age of the farmers was 44 years, with 68% being male. The majority of farmers (87%) had farm sizes ≤5 acres, while 52% had less than 10 years of farming experience. Open-field farming was extensively practiced (98.34%), with 63% of the farmers cultivating maize for subsistence consumption. Inter-cropping (45%) and mixed cropping (43%) were the most common cropping systems and they majorly involved maize and edible legumes (Figure 2).
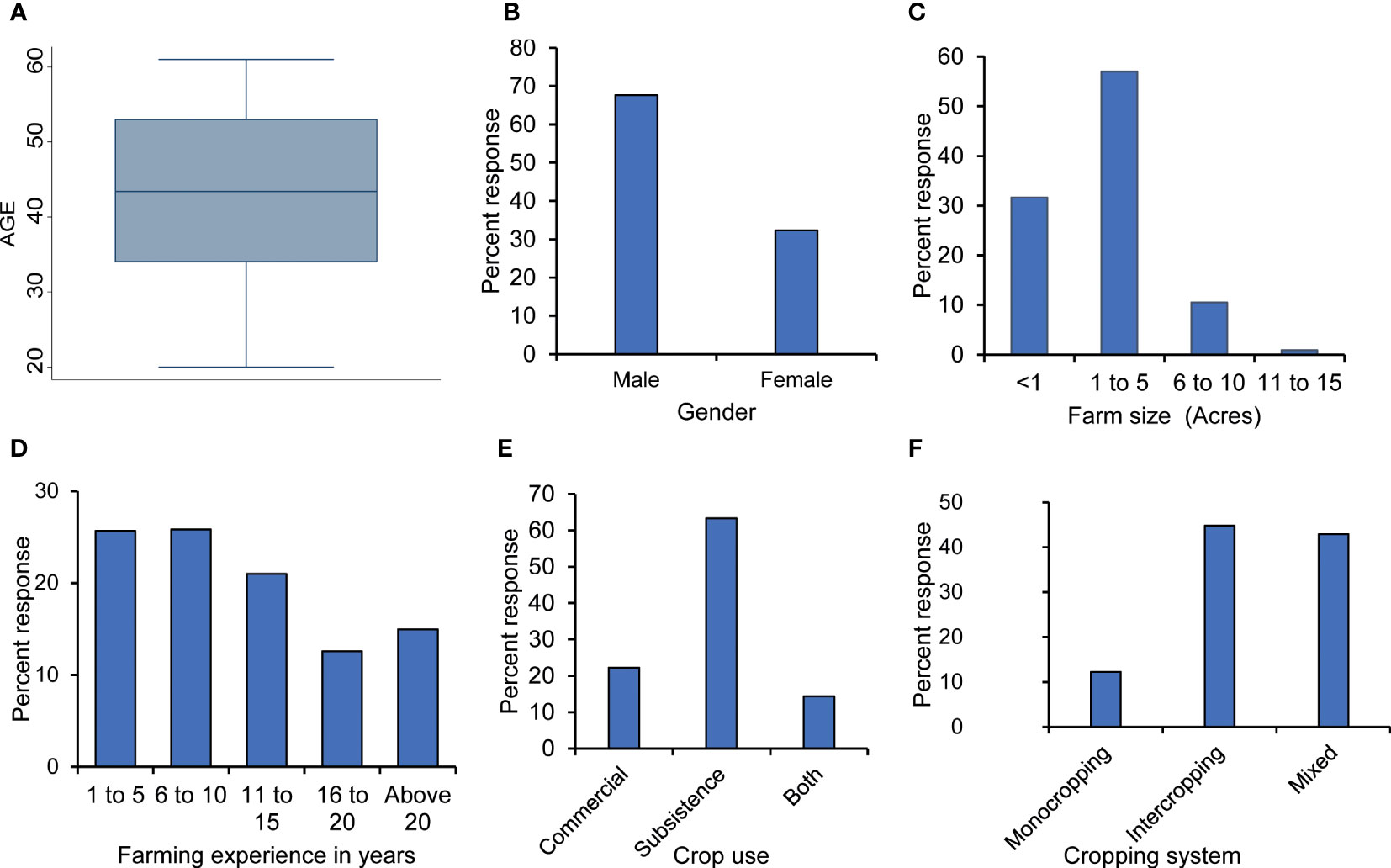
Figure 2 Demographic and farm characteristics of maize farmers in Kenya: (A) mean age, (B) gender, (C) farm size owned, (D) farming experience, (E) intended crop use, and (F) cropping system.
Fall armyworm occurrences were reported in all the counties with 64% of the farmers experiencing infestations in every planting season. Furthermore, only 9% of the respondents experienced FAW infestation once. Nearly two-thirds of the farmers (62%) described FAW population pressure as decreasing rather than increasing over time, and the pest pressure in the season immediately preceding our study as low (41.78%). Sixty-five percent of the participants estimated FAW-associated losses at 10% to 20%, with 53% of Kirinyaga respondents estimating the yield losses at 21% to 50%, while 28% of farmers from Nandi (a maize catchment area in Kenya) estimated FAW-associated losses above 50% (Supplementary Table 2).
Chemical control methods were extensively used in all the surveyed regions (64%), while 23% of the farmers exclusively applied cultural FAW control methods (Figure 3). Twelve percent combined chemical and cultural techniques to manage FAW. Two biological extracts were reported: (i) pepper was mixed with soil, wood ash, wood chippings or fine sand before application at the whorl of corn plants, and (ii) tobacco was diluted with water and sprayed on the vegetative parts of the plants. The choice of control methods significantly differed amongst the counties (χ2 = 417.53, p< 0.01). The top five commonly applied insecticides were diazinon (organophosphate), lambda-cyhalothrin (pyrethroid), alpha-cypermethrin (pyrethroid), emamectin-benzoate (neonicotinoid) and thiamethoxam (neonicotinoid) (Figure 4A). Two of the five; diazinon and lambda-cyhalothrin, had incidences of reduced or total non-response during FAW management (Figure 4B, Supplementary Table 3). The emamectin-benzoate-based insecticide was reported as the most effective FAW control agent. However, the counts of the most effective and least effective insecticides were very low (Figures 4B, C), as most farmers perceived all insecticides to be equally effective. The lambda-cyhalothrin insecticide results were inconclusive as it featured among the top commonly applied, most effective and least effective insecticides. It was therefore selected for laboratory bioassays.
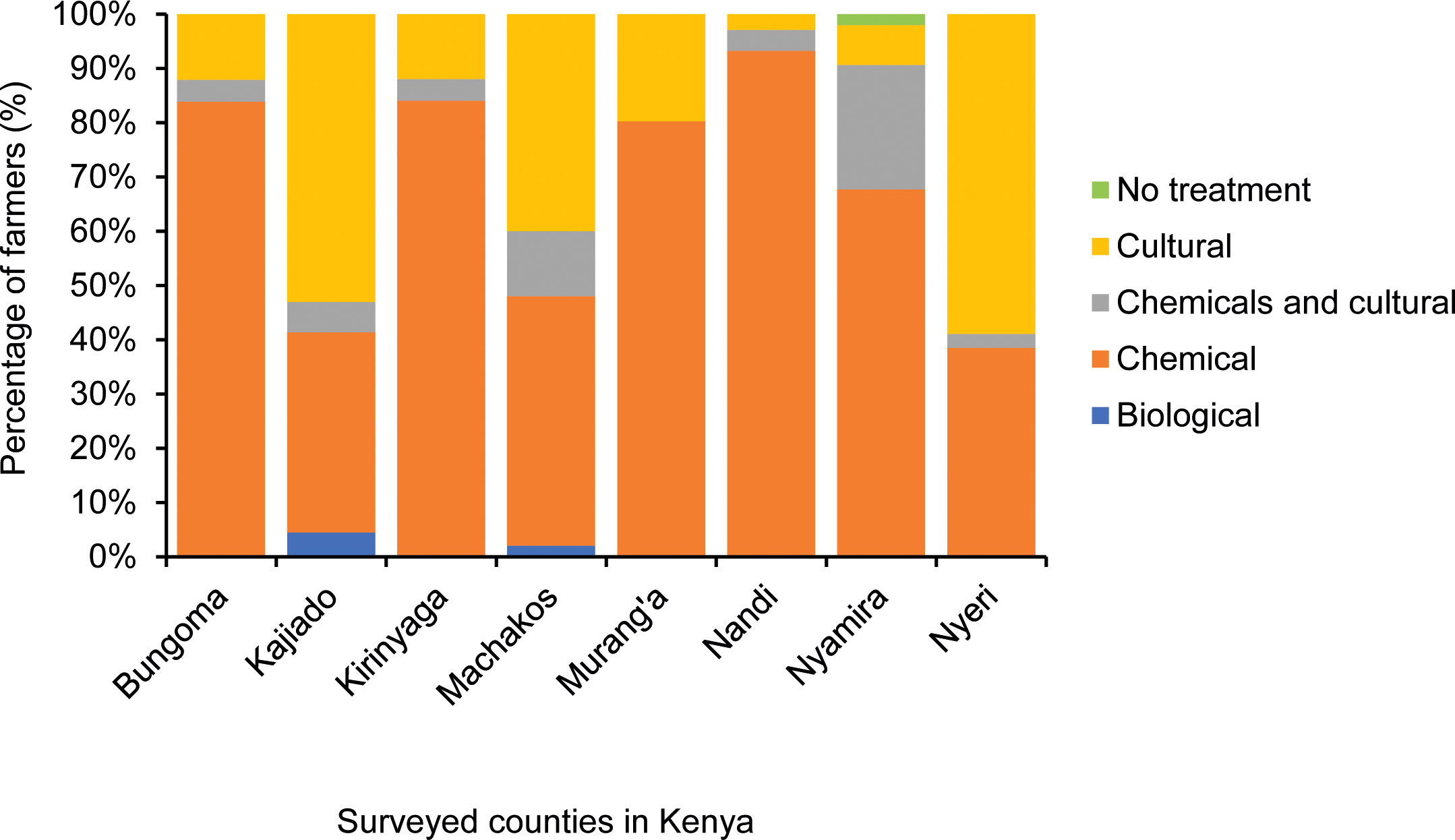
Figure 3 Relative adoption rates of the various pest control methods across different counties in Kenya.
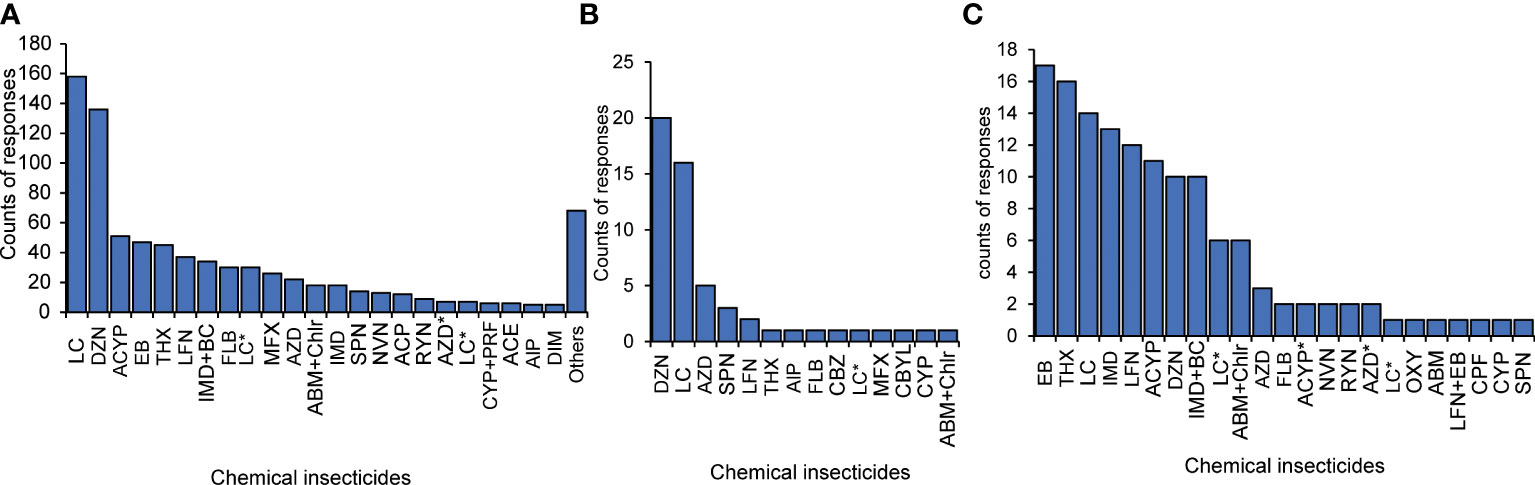
Figure 4 Counts of insecticides that (A) are commonly applied, (B) have field-observed FAW non-response and (C) are reported as most effective. ABM, Abamectin; ABM+Chlr, Abamectin+Chlorantraniliprole; ACP, Acephate; ACE, Acetochlor; ACYP, Alpha-cypermethrin; AlP, Aluminium phosphide; AZD, Neem; CBZ, Carbendazim; CBYL, Cabaryl; CPF, Chlorpyrifos; CYP, Cypermethrin; CYP+PRF, Cypermethrin+Profenofos; DIM, Dimethoate; DZN, Diazinon; EB, Emamectin-Benzoate; FLB, Flubendiamide; IMD, Imidacloprid; IMD+BC, Imidacloprid+beta-cyfluthrin; LFN, Lufeneron; LC, Lambda cyhalothrin; LFN+EB, Lufenuron+Emmamectin benzoate; MFX, Mefenoxam; NVN, biopesticide; OXY, Oxyflurofen; RYN, Rynaxypyr; THX, Thiamethoxam. * denotes different brands of the same pesticide.
Sixty percent of the farmers perceived all insecticides to be equally effective, while 83% were not satisfied with their performance in FAW control (Supplementary Table 4). Seventy-seven and five percent of the farmers described insecticides as moderate and non-effective agents in FAW management, respectively. Twenty four percent of the respondents observed reduced response and total non-response of FAW to insecticide(s) in their farms. Furthermore, pest management-related information was mostly acquired from fellow farmers (40.49%) and agrovets (37.29%) (Supplementary Table 4).
The estimates of the direction of effect of various parameters (farmer-based characteristics and FAW risk-perceptions) on the choice of cultural and chemical FAW control methods as evaluated using the BVP regression model, revealed that low farming experience (< 10 years) indicated a positive inclination towards the choice of chemical control methods (Supplementary Table 5). Farmers with farms bigger than 1 acre and those with high FAW risk-perceptions (> 50% FAW-associated yield losses and high FAW population pressure) indicated a positive likelihood for the adoption of chemical control methods and a negative preference for cultural control methods at 1% significance level (Supplementary Table 5). Age negatively influenced the adoption of chemical methods while, access to extension services negatively influenced the adoption of cultural FAW control measures and positively influenced the adoption of chemical methods at 1% significance level (Supplementary Table 5).
A 652bp fragment of the COI gene was amplified and sequenced in this study. Multiple sequence alignment (MSA) of the processed sequences revealed that only 11 of the 652 sites (loci) were polymorphic (Supplementary Figure 1). Phylogenetic analyses involving 112 publicly available S. frugiperda sequences mined from GenBank identified our samples (n = 46) as S. frugiperda. The S. frugiperda sequences (from this study and sequences from GenBank) formed two broad haplotypes that were shared between Africa, America, and Asia (Figure 5). We found the rice strain to be predominant (52%) in Kenya.
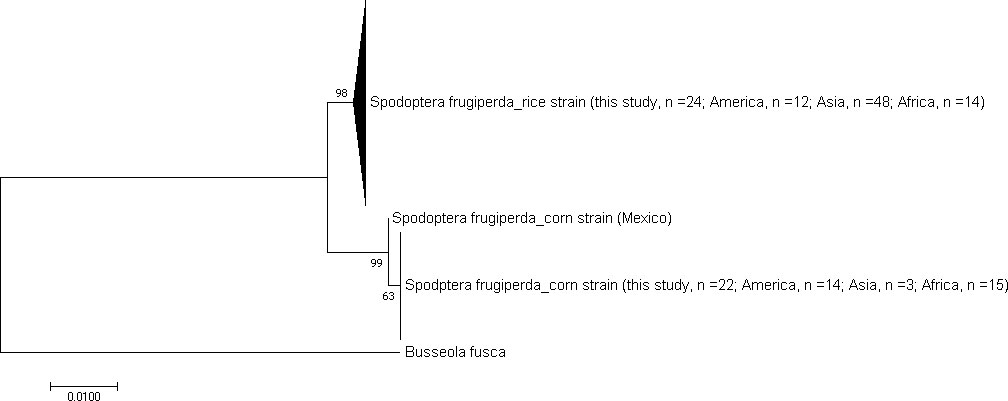
Figure 5 Condensed phylogenetic tree for species identification and FAW strain characterization. The phylogenetic tree contains 46 sequences from this study and 112 sequences from GenBank. The phylogenetic tree was edited in MEGA 11 software and constructed by Maximum Likelihood method, with 1000 bootstrap replicates under the Kimura-2 parameter-model.
This study’s samples indicated a close genealogical relationship with populations from America (Canada, Roraima, Honduras, Puerto Rico, and Mexico), Asia (China, Japan, and India), and other parts of Africa (Ghana, and Sao Tome and Principe) (Supplementary doc B). African, American, and Asian FAW species shared at least eight variable sites and revealed the absence of fixed mutation site(s) among the populations (Supplementary Table 6). Asia indicated the highest variability based on the within-continent mean distance (23%), while Africa and America indicated 1% within-group variability.
The genetic divergence between the Kenyan RS- and CS-variants ranged from 1.37% to 1.55%. (Supplementary doc B). The RS and CS variants revealed limited diversity and allele-heterozygosity as each constituted one haplotype. Significant genetic differentiation between the variants was observed (χ2 = 46.00, p< 0.001, df = 1). Polymorphism and diversity analysis in the Kenyan FAW population indicated that all the surveyed counties revealed high sequence conservation (C = 0.984, p< 0.01) and two probable haplotype variants with low haplotype-diversity (Hd = 0.51 ± 0.0003) and nucleotide-diversity indices (π = 0.0074) (Supplementary Table 7).
The analyses of the gut microbiota of the field-collected FAW samples showed Bacillus, Acinetobacter, and Klebsiella to be present in all the counties though in varying proportions (Supplementary Figure 2). Pathogenic genera: Staphylococcus, Enterobacter, Salmonella, Bacillus, Pseudomonas, and Enterobacter were abundant, especially in Makueni, Kiambu, and Taita-Taveta FAW samples. Enterococcus was among the dominant bacteria genera in samples collected in all the counties except Bungoma. Grouping the samples into strains revealed that the CS had richer microbial diversity than RS (Figure 6A). The three most abundant bacterial genera in both strains were Enterococcus, Acinetobacter, and Klebsiella. Bacillus was also among the dominant genera in both strains but was more abundant in the RS (6.98%), while Escherichia was more abundant in the CS (6.75%) than RS (5.1%). There was significant variation between RS and CS gut microbial profiles within and among counties when the corn and rice strains were profiled. Furthermore, the CS had a higher relative abundance of pathogenic bacterial genera than the RS (Figure 6B). Under laboratory conditions, after exposure to pesticides, there were elevated levels of microbial genera and their respective abundances in the guts of FAW larvae compared to the unexposed/control and the first generation (F1) populations (Figure 6C). The predominant Phyla that inhabited the gut of FAW larvae were Proteobacteria (63%) and Firmicutes (21%). Bacillus, Proteus, Enterococcus, Salmonella, Vibrio, Edwardsiella, Pectobacterium, Acinetobacter, Klebsiella, Escherichia, Enterobacter, and Pseudomonas were the most dominant and elevated bacteria genera in the insecticide-exposed FAW larvae. However, their elevations varied among the treatments (Figure 6C). Acinetobacter was highly abundant (28.21%) in the chlorantraniliprole + lambda-cyhalothrin-exposed population while subtly expressed (< 1%) in the other treatments, including the control/unexposed populations. Edwardsiella and Ethanoliginens were abundant following emamectin benzoate treatment, while Pectobacterium was dominant in the spinetoram-exposed larvae. Pseudomonas and Vibrio were abundant in spinetoram- and lambda-cyhalothrin-exposed larvae, respectively. Enterobacter was abundant in all the chemical-exposed/treated larvae but showed reduced abundance in the Bt-tolerant larvae. Halobacteriovorax was the most abundant genera in the lambda-cyhalothrin_F1 and the unexposed larvae but was less abundant in all other treatments, including Bt_F1. The first generation of the survivors of insecticide exposures, Lambda-cyhalothrin_F1 and Bt_F1, had reduced abundance of the dominant microbial genera in their parental generation (Figure 6C). However, the F1s microbial profiles varied in that they harbored numerous unique genera that were absent in the control and insecticide-treated larvae. The genera included Intestimonas, Steroidobacter, Anaerocolumna, and Devriseae. The Bt_F1 sample also contained Sporomusa, Dongshaea, and Devosia, while the Lambda-cyhalothrin_F1 profile had Halobacteriovorax, Lawsonia, Halobacterium, Burkholhedria, Persephonella, Halothiobacillus, and Bordetella.
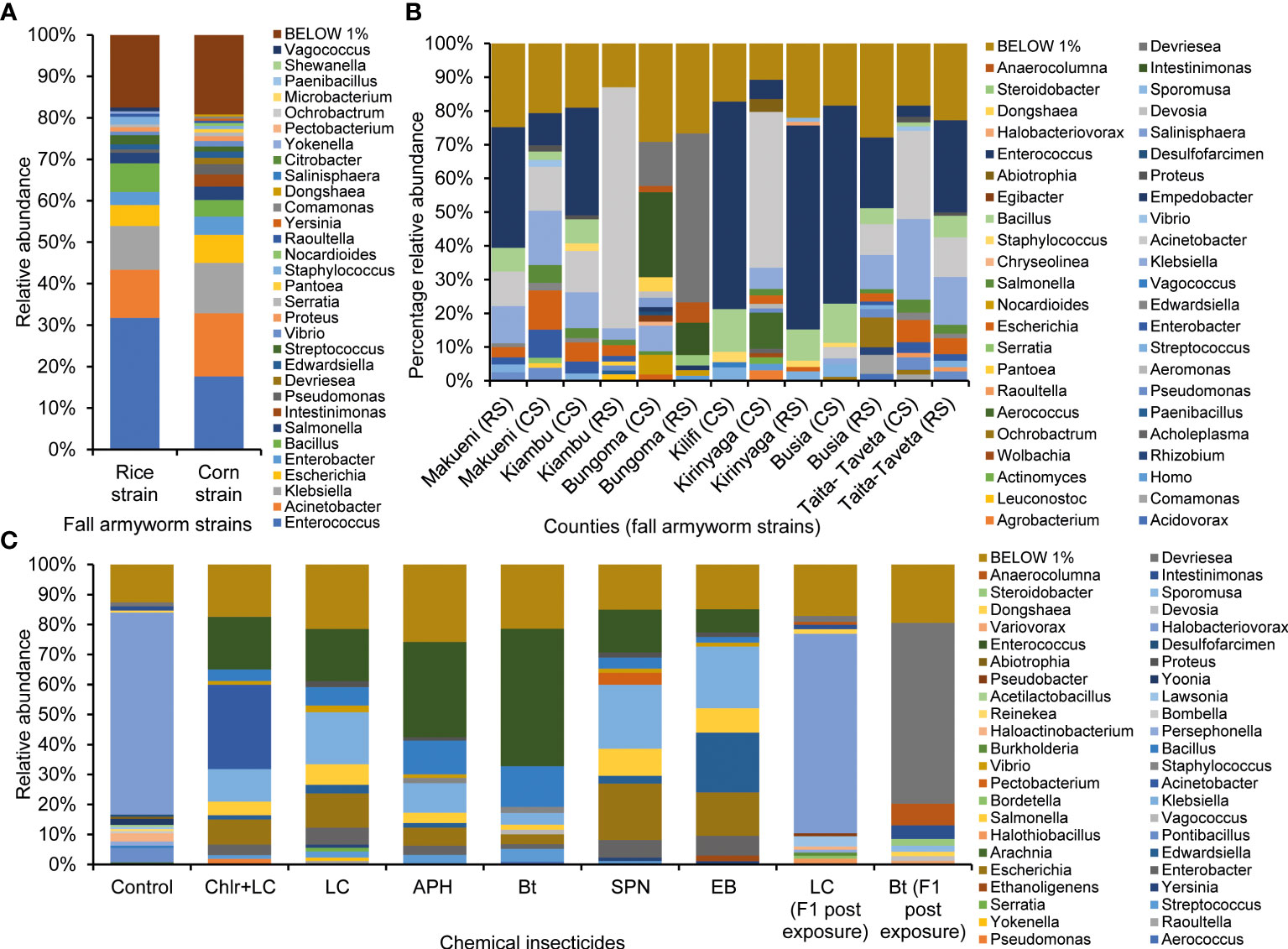
Figure 6 (A) Overall relative microbial diversity and abundance in the rice-strain (RS) and corn-strain (CS) of FAW in Kenya. (B) Relative microbial diversity and abundance among RS and CS of FAW from various counties in Kenya. (C) Microbial abundance in guts of FAW larvae after chemical-insecticide(s) exposure. APH, Acephate; Bt, commercial Bacillus thuringiensis-based insecticide; Chlr+LC, Chlorantraniliprole + Lambda-cyhalothrin; LC, Lambda-cyhalothrin; SPN, Spinetoram.
The richness index in both strains mostly ranged between 300 to 600 species. Makueni CS, Kiambu RS, and Bungoma CS showed the highest species richness values: 1070, 849, and 738, respectively (Figure 7A). Bungoma and Busia CS had the highest evenness indices while Kiambu and Kilifi CS had the lowest (Figure 7B). True-Shannon diversity index ranged between 1.7 to 3.7, with Kilifi, Kiambu, and Kirinyaga CS having the lowest values, while Busia and Kirinyaga RS indicated the highest indices (Figure 7C). Furthermore, in the insecticide-treated larvae, chlorantraniliprole + lambda-cyhalothrin, emamectin benzoate, and acephate indicated the highest species richness indices (935, 847 and 730, respectively), while the first generations (F1) of lambda-cyhalothrin showed the lowest microbial richness values (48) (Figure 8A). Bt-insecticide and the control group indicated the lowest evenness indices (0.46 and 0.40, respectively), while acephate and lambda-cyhalothrin had the highest values (0.51 and 0.54, respectively) (Figure 8B). The True- Shannon diversity index showed that acephate, emamectin benzoate and lambda-cyhalothrin treated larvae had the highest diversity of bacterial genera, while the control group and the F1s had the lowest diversity indices (0.89 and 1.5, respectively) (Figure 8C).
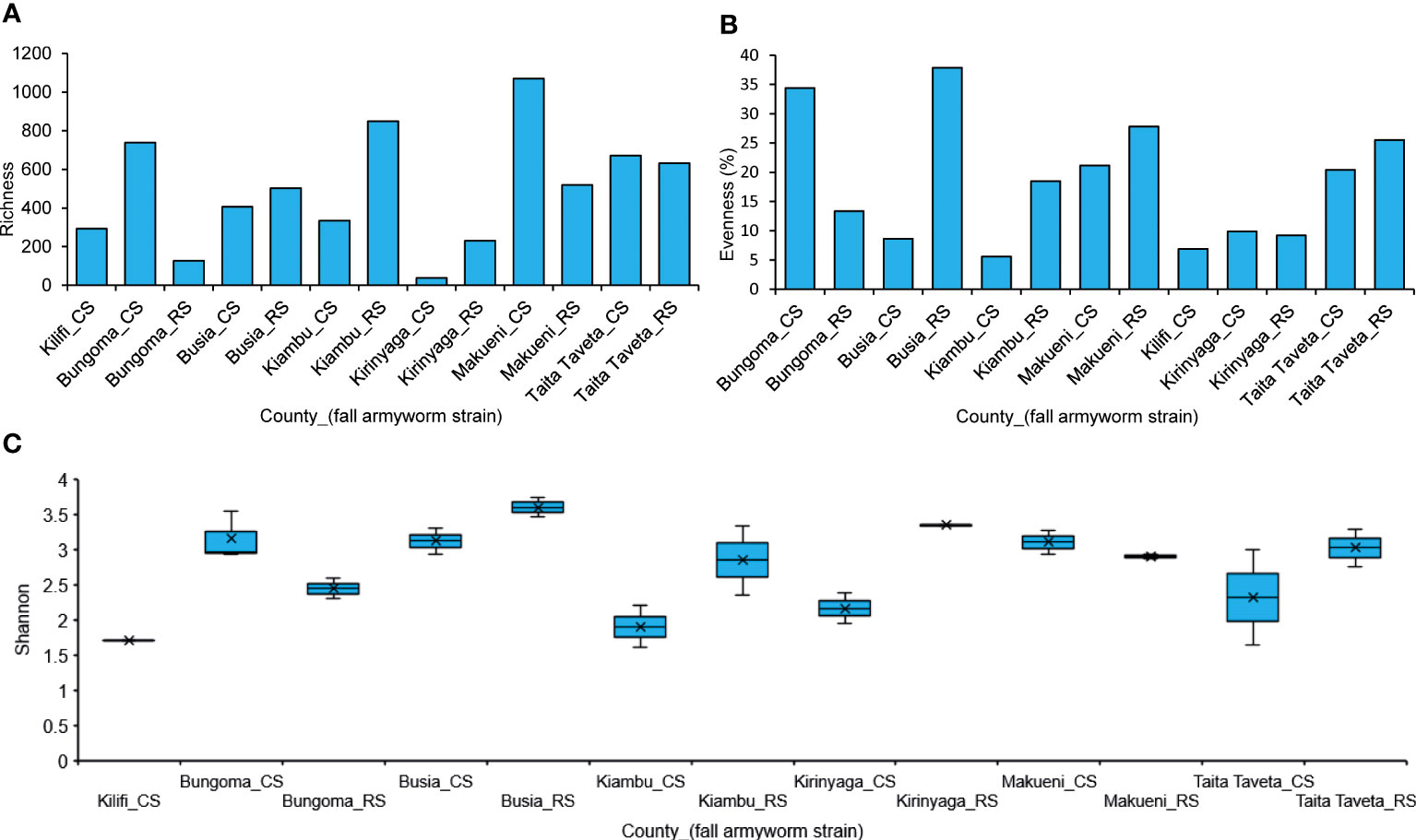
Figure 7 (A) Richness, (B) Evenness, and (C) True-Shannon diversity in field-collected FAW strains per county. CS, Corn strain; RS, Rice strain.
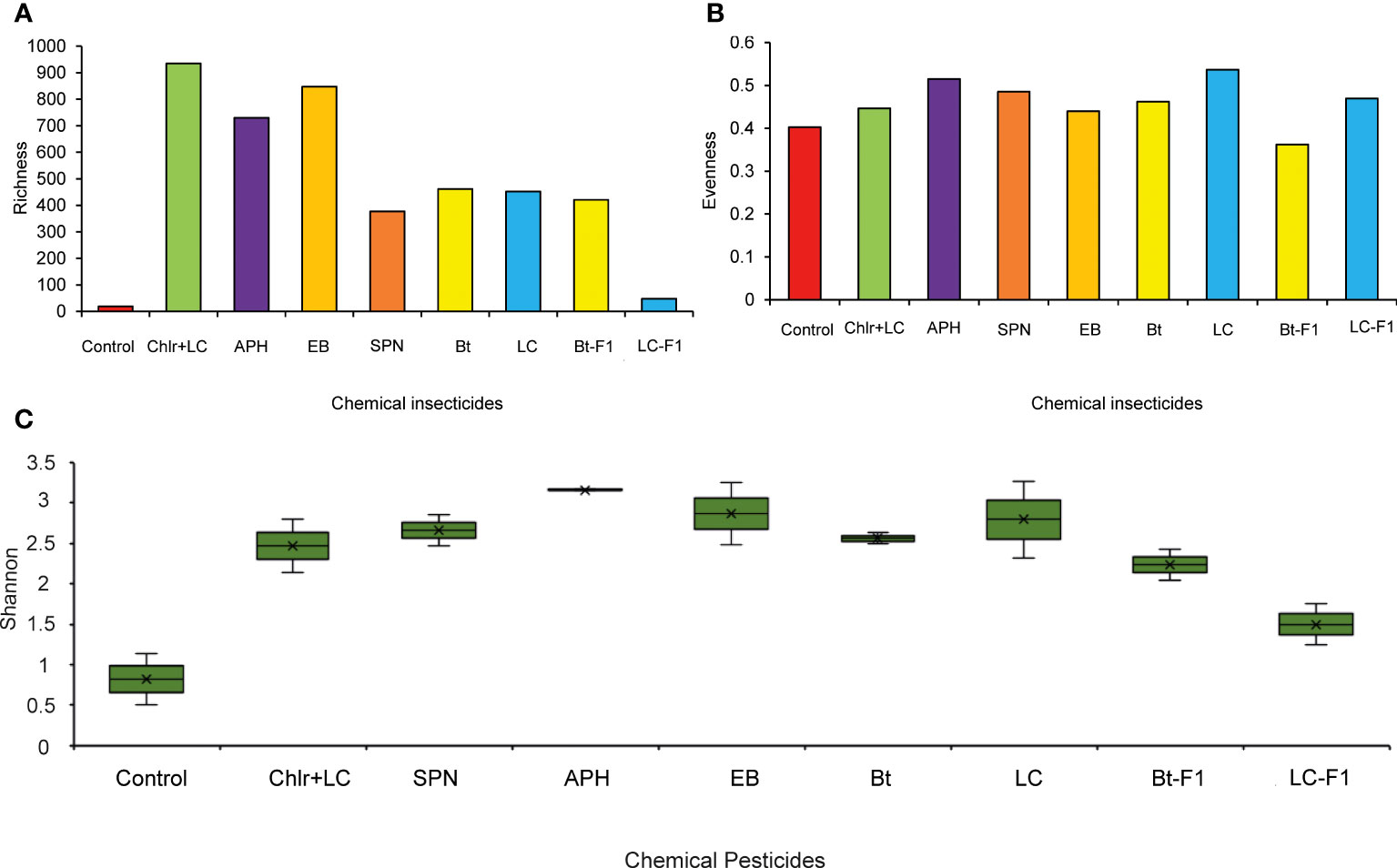
Figure 8 (A) Richness, (B) Evenness, and (C) True-Shannon diversity in FAW larvae after chemical-insecticide(s) exposure. APH, Acephate; Bt, commercial Bacillus thuringiensis-based insecticide; Chlr+LC, Chlorantraniliprole + Lambda-cyhalothrin; LC, Lambda-cyhalothrin; SPN, Spinetoram.
Forty-eight (48) genes were generated by the WIMP-ARMA workflow from the six insecticide treatments (Supplementary Table 8). The genes conferred resistance to 16 identified antibiotic compounds: spectinomycin, neomycin, kanamycin A, tetracycline, tobramycin, kanamycin, streptomycin, amikacin, viomycin, gentamicin, gentamicin C, hygromycin B, paromomycin, eideine, and g418, which belong to different classes of antibiotics (Supplementary Figure 3).
This study provides evidence of the spread of FAW to regions with diverse biotic, abiotic, ecological, and environmental factors in Kenya. The predictors of choice of control methods among smallholder maize farmers and the status of genetic diversity in the Kenyan FAW populations four years after its invasion in the country are also presented. Furthermore, we report the effect of insecticides on the gut microbial diversity among FAW larval populations.
Our survey findings concurred with Denkyirah et al. (Denkyirah et al., 2016), who reported socio-capital (interpersonal networks; farmer to farmer) as a principal channel for information flow, as pest management-related information was majorly acquired from fellow farmers and agrovets. Most respondents who practiced either intercropping or mixed cropping of maize (Zea mays) and edible legumes [soybeans (Glycine max) and common beans (Phaseolus vulgaris)] estimated FAW-associated losses lower than we expected, 30% to 54%, based on previous studies (De Groote et al., 2020). However, Davies et al. (2007) also observed reduced FAW-associated yield losses in fields where intercropping of maize with edible legumes was practiced. Thus, the low pest populations and FAW-associated losses reported in this study could be ascribed to the cropping practices applied by the farmers. Such systems can be incorporated among FAW’s integrated pest management (IPM) options. Our study showed chemical control method as the established/dominant FAW management measure in the general surveyed population. However, the majority of the respondents were unsatisfied with the insecticides’ performance/efficacy, which is in agreement with a report by Kumela et al. (2019). The increasing concerns about food quality and safety are prompting for biological, botanical, and cultural control practices to become more desirable and adoptable (Despotović et al., 2019; Misango et al., 2022). Almost a quarter of our respondents exclusively applied synthetic insecticide-free methods to manage FAW in their farms. Our findings also suggest that cultural orientations and beliefs influence farmers’ choice of pest control methods. Two regions in our study, Kajiado and Nyeri, are among Kenya’s most culturally conserved counties and a majority of the respondents from these counties exclusively practiced cultural pest control measures, while most participants from the other areas used cultural control measures to augment chemical methods. Furthermore, positive motivation for the adoption of chemical control methods among farmers with low farming experience, as was observed in this study, has been previously reported (Nayga, 1996). More experienced farmers are open to alternative control methods, probably due to prior observed/experienced adverse effects from insecticide use (Denkyirah et al., 2016). In agreement with Danso-Abbeam and Baiyegunhi (2017), farmers with large farms preferred chemical control methods. This is probably because of the labor-intensive nature of cultural and alternative management practices (Kinuthia, 2019). Limited knowledge on alternative pest control methods (Razzaghi Borkhani et al., 2011) is also a plausible explanation for their minimal adoption (Hashemi and Damalas, 2011). Access to agricultural extension services positively influenced the adoption of chemical methods. Denkyirah et al. (2016) and Tizale (2007) also reported a positive influence of extension officer services on the adoption of new technologies. Ultimately the adoption of environmental and human-friendly pest control measures such as cultural practices, biological control and IPMs is low probably because of the long periods required for their establishment, high labor demands and their knowledge complexity (Hailu et al., 2018).
Knowledge on species distribution, geographical patterning, and genetic diversity of FAW is pivotal for its successful management and projection of its origin and evolutionary trajectory (Nayyar et al., 2021). Though all our samples were collected from corn fields at the vegetative crop stage, both the RS and CS were detected in all the surveyed counties. The discordancy between FAW strains and the host-plants they infest as observed in this study was similar to the findings reported by Nagoshi et al (2017; 2019). This inconsistency is indicative of the high flexibility of FAW in regard to host preference, thus the rice strain can infest and thrive on corn until its preferred host is available. Therefore, we emphasize the significance of molecular identification of FAW strains and reiterate the ceasing of assumption of FAW strains based on the host plants during sample collection. The Kenyan FAW populations were considered panmictic four years post the pest’s invasion into the country as both the rice- and corn-strains showed limited allele-heterozygosity (diversity) as they constituted a single haplotype each. Isolation by genetic distance and region-specific haplotypes were also not observed, suggesting that: (i) there could be high gene flow across a large geographical range in the species, or (ii) once the species’ genetically homogenized populations expanded, there wasn’t enough time for the population structure’s markers to reach detectable limits. These probable hypotheses are also predicated upon the insect’s physiological and behavioral characteristics. High gene flow across a large geographical range is plausible as FAW is a strong and long-distance flier (Hardke et al., 2015). Fall armyworm’s swift adaptability to diverse climatic environments and its highly migratory nature (Auteri et al., 2018) relieve it of geographical constraints, making rapid gene transfer across a wide geographical range feasible. Population contraction followed by rapid expansion causes a bottleneck effect on the population structure (Puillandre et al., 2008) and is accompanied by a loss of variability in the population (Rozas et al., 2003). Therefore, it is probable that signatures of the Kenyan FAW population structure are yet to attain detectable limits as the pest is newly-invasive in the country (Day et al., 2017). Our findings also indicate a close genealogical relationship between Asian and African FAW populations, as reported by Nayyar et al. (2021), suggesting that the continents probably share a common origin of this invasive pest.
The identification of the resistance mechanisms is essential for combating resistance development and controlling resistant pest populations (Hemingway and Ranson, 1997; Ffrench-Constant et al., 2000). Heightened microbial levels have been reported to convey insecticide tolerance/resistance (Hernández-Martínez et al., 2010). In our study, increased bacterial loads were observed in the insecticide-exposed populations, and economically significant microbes were identified following the profiling of their gut microbiota composition. We also observed that exposure of FAW larvae to chemical insecticides elevated the expression of pathogenic and opportunistic bacterial genera: Enterococcus, Bacillus, Vibrio, Salmonella, Enterobacter, and Streptococcus. Enterococcus and Enterobacter genera have been reported to exhibit xenobiotic-degrading capacity, thereby enhancing insecticide resistance in insects (Xia et al., 2018). Paddock et al. (2021) and Onwona-kwakye et al. (2020) also reported an increased abundance of pathogenic genera in the Western corn rootworm [Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae)] and contaminated soils following insecticide-treatment. In our study, Enterobacter expression rate/abundance was high in all treatments, but its abundance was reduced by about threefold in the Bt-tolerant larvae. This finding is in line with Broderick et al (2006; 2009), who reported that Enterobacter is significant in facilitating Bt-induced mortality of gypsy moth larvae among other Lepidoptera. Acinetobacter, a bacterial genus reported to contain a high esterase activity that confers resistance to cypermethrin (pyrethroid-based insecticide) (Akbar et al., 2015), was highly expressed in the chlorantraniliprole + lambda-cyhalothrin-exposed larvae but was subtly expressed in other chemical treatments. Therefore, we postulated that Acinetobacter’s elevated levels following chlorantraniliprole + lambda-cyhalothrin-exposure were in an attempt to degrade or detoxify lambda-cyhalothrin, a pyrethroid-based active ingredient constituting the insecticide. However, further studies need to be conducted to assess this hypothesis. Serratia sp., which has been reported to degrade 50mg of chlorpyrifos, an organophosphate, within 18 hours (Xu et al., 2007), was also elevated in lambda-cyhalothrin exposed population. The interaction between spinetoram and Pectinobacterium should also be investigated, as the genus was highly abundant in the spinetoram- exposed larvae but less abundant in other chemical treatments. Furthermore, Ethanoliginens was highly expressed in emamectin benzoate-treated larvae. Members of this bacterial genus have been reported to convert carbohydrates into hydrogen gas (H2 (g)) and ethanol (Xing et al., 2008). However, their interaction with insecticides remains unexplored. Edwardsiella also exhibited an interesting interaction with emamectin benzoate as it was at least triple-expressed in the emamectin benzoate-exposed larvae compared to other treatments. Edwardsiella and Klebsiella were abundant in the treated larvae but were undetected in the unexposed/control groups (the F1s and the control). They are therefore potential bacterial candidates for further research to be explored in FAW management approaches. Anaerocolumna and Intestimonas were significantly abundant in the F1 generations, especially in the Bt_F1 larvae. Anaerocolumna is reported to thrive in uranium-contaminated ecologies and to degrade lignin (Tang et al., 2021; Xu et al., 2021). However, its interaction with insecticides is still unexplored. Devriseae, a bacterial genus that harbors Devriseae actinobacterium, associated with dermatitis and recovered in organ lesions of agamid lizards (Xing et al., 2008), was the most abundant in Bt_F1. The interaction/association between Bt, and Devriseae is still unexplored and further studies are warranted to establish its role in FAW. Persephonella, a chemosynthetic microbe crucial in the carbon, sulphur, and nitrogen gas cycles (Mino et al., 2013), was also introduced and significantly expressed alongside Burkholhedria in lambda-cyhalothrin_F1 larvae. Previous studies have reported the degradation of an organophosphate insecticide, Fenitrothion, by Burkholderia (Tago et al., 2015). We also noted that although the diversity of the field-collected samples exhibited remarkable similarity to those of insecticide-treated larvae in the laboratory, as they indicated high abundance and species richness, the composition was relatively different. This could be due to the effect of the pesticide on the gut microbiomes of the lab-reared and field FAW larvae. Environmental factors have been shown to result in similarities in the diversity of gut microbiomes of Aedes albopictus but not the microbiome composition (Baltar et al., 2023). The pathogenic bacterial genera were also among the most abundant and dominant genera, a phenomenon which is common in organisms post-chemical insecticides exposure (Hernández-Martínez et al., 2010). This further affirms that chemical control methods are the established FAW control measures in the general population. Furthermore, the presence of AMR genes in the microbiome of insects is a cause for concern as they may serve as pools for resistance genes which can be transferred to host plants, other pathogens, and animals (Salyers et al., 2004; Marshall et al., 2009).
Spodoptera frugiperda is indeed an economically significant pest in Kenya, and chemical insecticides are the most dominant FAW control methods among corn farmers in Kenya. However, the shift to clean agriculture is gradual but evident currently. It is also distinct that FAW management in invaded areas is not only hazardous to the ecology by prompting the introduction of chemical agents but also contributes to threatening food safety as it predisposes host plants to higher loads of pathogenic microbial genera following insecticide application. In addition, FAW strain identification should be based on molecular characterization approaches, as the pest is highly versatile with regard to the strain-host association. Finally, insect’s guts are potential tools for the discovery of microbes and other novel biomolecules for biotechnological application; because they harbor a wide range of microbes exhibiting insecticide degrading/metabolizing capacity that could be harnessed for bioremediation. However, further studies should be conducted to decipher the probable contribution of gut endosymbionts towards insecticide resistance development. Also, farmers’ training on efficient alternative pest control methods is recommended and paramount in response to insecticide tolerance complaints and the global food safety concerns. We acknowledge the constraint in the sample size and the reduced geographical coverage of our work and therefore, we recommend that further studies should cover larger geographical and time scales as seasons differ with varying agroecological zones.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by icipe Institutional Biosafety Committee (IBC).
Conceptualization: KA and FK, Funding acquisition: KA, Experimental design: KN and FK, Software: KN, IA, and FK, Investigation: KN, Visualization: KN, IA, and FK, Resources: KA and FK, Supervision: FK, KM and KA, Project administration: KA, Writing original draft preparation: KN, Writing, review and editing: KN, IA, KM, FK and KA. All authors have read and approved the manuscript.
This work received financial support from the UK’s Foreign, Commonwealth and Development Office (FCDO) (B2329A-FCDO-FAW and B2291A-FCDO-BIOPESTICIDE) through the International Centre of Insect Physiology and Ecology (icipe).
The authors gratefully acknowledge the icipe core funding provided by the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The first author was supported through the Dissertation and Research Internship Program (DRIP) of icipe under the FCDO Fall Armyworm Biopesticide project. We are also thankful to Fidelis L. Ombura, Maureen Ong’onge and Evalyne Ndotono for their technical assistance and Afrika Okello for her support in the survey. The views expressed herein do not necessarily reflect the official opinion of the donors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1235558/full#supplementary-material
Adejumo O. A., Ojoko E. A., Yusuf S. A. (2014). Factors influencing choice of pesticides used by grain farmers in Southwest Nigeria. J. Biol. Agric. Healthcare. 4 (28), 31–38.
Akbar S., Sultan S., Kertesz M. (2015). Determination of cypermethrin degradation potential of soil bacteria along with plant growth-promoting characteristics. Curr. Microbiol. 70 (1), 75–84. doi: 10.1007/s00284-014-0684-7
Antman F. M. (2014). Spousal employment and intra-household bargaining power. Appl. Econ. Lett. 21 (8), 560–563. doi: 10.1080/13504851.2013.875101
Auteri M., La Russa F., Blanda V., Torina A. (2018). Insecticide resistance associated with kdr mutations in Aedes albopictus: An update on worldwide evidences. Biomed. Res. Int. 2018, 3098575. doi: 10.1155/2018/3098575
Baltar J. M. C., Pavan M. G., Corrêa-Antônio J., Couto-Lima D., Maciel-de-Freitas R., David M. R. (2023). Gut Bacterial Diversity of Field and Laboratory-Reared Aedes albopictus Populations of Rio de Janeiro, Brazil. Viruses 15 (6), 1309. doi: 10.3390/v15061309
Banerjee R., Hasler J., Meagher R., Nagoshi R., Hietala L., Huang F., et al. (2017). Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci. Rep. 7 (1), 1–10. doi: 10.1038/s41598-017-09866-y
Baudron F., Zaman-Allah M. A., Chaipa I., Chari N., Chinwada P. (2019). Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 120, 141–150. doi: 10.1016/j.cropro.2019.01.028
Beals E. W. (1984). Bray-curtis ordination: An effective strategy for analysis of multivariate ecological data. Adv. Ecol. Res. 14 (C), 1–55. doi: 10.1016/S0065-2504(08)60168-3
Broderick N. A., Raffa K. F., Handelsman J. (2006). Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. United. States America 103 (41), 15196–15199. doi: 10.1073/pnas.0604865103
Broderick N. A., Robinson C. J., McMahon M. D., Holt J., Handelsman J., Raffa K. F. (2009). Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 7, 1–9. doi: 10.1186/1741-7007-7-11
Cabrera J. A., Kurtz A., Sikora R. A., Schouten A. (2010). Isolation and characterization of fenamiphos degrading bacteria. Biodegradation 21 (6), 1017–1027. doi: 10.1007/s10532-010-9362-z
Carvalho R. A., Omoto C., Field L. M., Williamson M. S., Bass C. (2013). Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PloS One 8 (4), e62268. doi: 10.1371/journal.pone.0062268
Chimweta M., Nyakudya W. I., Jimu L., Mashingaidze A. B. (2020). Fall armyworm [Spodoptera frugiperda (J.E. Smith)] damage in maize: management options for flood-recession cropping smallholder farmers. Int. J. Pest Manage. 66 (2), 142–154. doi: 10.1080/09670874.2019.1577514
Danso-Abbeam G., Baiyegunhi L. J. S. (2017). Adoption of agrochemical management practices among smallholder cocoa farmers in Ghana. Afr. J. Sci. Technol. Innovation Dev. 9 (6), 717–728. doi: 10.1080/20421338.2017.1380358
Davies T. G. E., Field L. M., Usherwood P. N. R., Williamson M. S. (2007). DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 59 (3), 151–162. doi: 10.1080/15216540701352042
Day R., Abrahams P., Bateman M., Beale T., Clottey V., Cock M., et al. (2017). Fall armyworm: Impacts and implications for Africa. Outlooks. Pest Manage. 28 (5), 196–201. doi: 10.1564/v28_oct_02
De Groote H., Kimenju S. C., Munyua B., Palmas S., Kassie M., Bruce A. (2020). Spread and impact of fall armyworm (Spodoptera frugiperda J.E. Smith) in maize production areas of Kenya. Agricult. Ecosyst. Environ. 292 (December 2019), 106804. doi: 10.1016/j.agee.2019.106804
Denkyirah E. K., Okoffo E. D., Adu D. T., Aziz A. A., Ofori A., Denkyirah E. K. (2016). Modeling Ghanaian cocoa farmers’ decision to use pesticide and frequency of application: the case of Brong Ahafo Region. SpringerPlus 5 (1), 1113. doi: 10.1186/s40064-016-2779-z
Despotović J., Rodić V., Caracciolo F. (2019). Factors affecting farmers’ adoption of integrated pest management in Serbia: An application of the theory of planned behavior. J. Cleaner. Production. 228, 1196–1205. doi: 10.1016/j.jclepro.2019.04.149
Dormann C. F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., et al. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36 (1), 27–46. doi: 10.1111/j.1600-0587.2012.07348
Ffrench-Constant R. H., Anthony N., Aronstein K., Rocheleau T., Stilwell G. (2000). Cyclodiene insecticide resistance: From molecular to population genetics. Annu. Rev. Entomol. 45 (June 2014), 449–466. doi: 10.1146/annurev.ento.45.1.449
Gutiérrez-moreno R., Mota-Sanchez D., Blanco C. A., Whalon M. E., Teran-Santofimio H., Rodrigurez-Maciel J. C., et al. (2019). Field-evolved resistance of the fall armyworm ( Lepidoptera : Noctuidae ) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 112 (2), 792–802. doi: 10.1093/jee/toy372
Hailu G., Niasy S., Zeyaur K. R., Ochatum N., Subramanian S. (2018). Maize–legume intercropping and push–pull for management of fall armyworm, stemborers, and striga in Uganda. Agron. J. 110 (6), 2513–2522. doi: 10.2134/agronj2018.02.0110
Hardke J. T., Lorenz G. M., Leonard B. R. (2015). Fall armyworm (Lepidoptera: Noctuidae) ecology in Southeastern cotton. J. Integrated. Pest Manage. 6 (1), 1–8. doi: 10.1093/jipm/pmv009
Hashemi S. M., Damalas C. A. (2011). Farmers’ perceptions of pesticide efficacy: Reflections on the importance of pest management practices adoption. J. Sustain. Agric. 35 (1), 69–85. doi: 10.1080/10440046.2011.530511
Hemingway J., Ranson H. (1997). Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45, 79–102. doi: 10.1146/annurev.ento.45.1.371
Hernández-Martínez P., Naseri B., Navarro-Cerrillo G., Escriche B., Ferré J., Herrero S. (2010). Increase in midgut microbiota load induces an apparent immune priming and increases tolerance to Bacillus thuringiensis. Environ. Microbiol. 12 (10), 2730–2737. doi: 10.1111/j.1462-2920.2010.02241
Houngbo S., Zannou A., Aoudji A., Sossou H. C., Sinzogan A., Sikirou R., et al. (2020). ‘Farmers’ knowledge and management practices of fall armyworm, spodoptera frugiperda (J.E. Smith) in Benin, West Africa. Agric. (Switzerland). 10 (10), 1–15. doi: 10.3390/agriculture10100430
Huang F., Qureshi J. A., Meagher R. L. Jr., Reisig D. D., Head G. P., Andow D. A., et al. (2014). Cry1F resistance in fall armyworm Spodoptera frugiperda : Single gene versus pyramided Bt maize. PloS One 9 (11), e112958. doi: 10.1371/journal.pone.0112958
Jakka S., Gong L., Hasler J., Banerjee R., Sheets J. J., Narva K., et al. (2016). Field-evolved mode 1 resistance of the fall armyworm to transgenic Cry1Fa-expressing corn associated with reduced Cry1Fa toxin binding and midgut alkaline phosphatase expression. Am. Soc. Microbiol. 82 (4), 1023–1034. doi: 10.1128/AEM.02871-15
Kansiime M., Mugambi, Rwomushana I., Nunda W., Lamontagne-Godwin J., Rware H., et al. (2019). Farmer perception of fall armyworm (Spodoptera frugiderda J.E. Smith) and farm-level management practices in Zambia. Pest Manage. Sci. 75 (10), 2840–2850. doi: 10.1002/ps.5504
Kassie M., Wossen T., De Groote H., Tefera T., Sevgan S., Balew S. (2020). Economic impacts of fall armyworm and its management strategies: Evidence from southern Ethiopia. Eur. Rev. Agric. Econ. 47 (4), 1473–1501. doi: 10.1093/erae/jbz048
Kenis M., Benelli G., Biondi A., Calatayud P., Day R., Desneux N., et al. (2023). Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Generalis. 43 (2), 187–241. doi: 10.1127/entomologia/2022/1659
Khalid S., Hashmi I., Khan S. J. (2016). Bacterial assisted degradation of chlorpyrifos: The key role of environmental conditions, trace metals and organic solvents. J. Environ. Manage. 168, 1–9. doi: 10.1016/j.jenvman.2015.11.030
Khan Z., Midega C., Pittchar J., Murage A., Birkett M., Bruce T., et al. (2014). Achieving food security for one million sub-Saharan African poor through push-pull innovation by 2020. Philos. Trans. R. Soc. B.: Biol. Sci. 369 (1639), 20120284. doi: 10.1098/rstb.2012.0284
Kinuthia C. W. (2019). Determinants of pesticide use and uptake of alternative pest control methods among small scale tomato farmers in Nakuru county, Kenya (Kenya: Egerton University).
Kuate A., Hanna R., Doumtsop Fotio A. R. P., Abang A. F., Nanga S. N., Ngatat S., et al. (2019). Correction: Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Cameroon: Case study on its distribution, damage, pesticide use, genetic differentiation and host plants. PloS One 14 (4), e0215749. doi: 10.1371/journal.pone.0215749
Kumela T., Simiyu J., Sisay B., Likhayo P., Mendesil E., Gohole L., et al. (2019). Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int. J. Pest Manage. 65 (1), 1–9. doi: 10.1080/09670874.2017.1423129
Larkin M., Blackshields G., Brown N. P., Chenna R., Mcgettigan P. A., McWilliam H., et al. (2007). Clustal W and clustal X version 2.0. Bioinformatics 23 (21), 2947–2948. doi: 10.1093/bioinformatics/btm404
Mallick K., Bharati K., Banerji A., Shakil N. A., Sethunathan N. (1999). Bacterial degradation of chlorpyrifos in pure cultures and in soil. Bull. Environ. Contamination. Toxicol. 62 (1), 48–54. doi: 10.1007/s001289900840
Marshall B., Ochieng D. J., Levy S. B. (2009). Commensals: Underappreciated reservoir of antibiotic resistance. Microbe 4 (5), 231–238. doi: 10.1128/microbe.4.231.1
Midega C., Pittchar J., Pickett J., Hailu G., Khan Z. (2018). A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J E Smith), in maize in East Africa’. Crop Prot. 105 (November 2017), 10–15. doi: 10.1016/j.cropro.2017.11.003
Mino S., Makita H., Toki T., Miyazaki J., Kato S., Watanabe H., et al. (2013). Biogeography of Persephonella in deep-sea hydrothermal vents of the Western Pacific. Front. Microbiol. 4 (APR). doi: 10.3389/fmicb.2013.00107
Misango V., Nzuma J. M., Irungu P., Kassie M. (2022). Intensity of adoption of integrated pest management practices in Rwanda: A fractional logit approach. Heliyon 8 (1), e08735. doi: 10.1016/j.heliyon.2022.e08735
Nagoshi R. N., Meagher R. L. (2008). Review of fall armyworm (Lepidoptera: Noctuidae) genetic complexity and migration. Florida Entomologist 91 (4), 546–554. doi: 10.1653/0015-4040-91.4.546
Nagoshi R., Dhanani I., Asokan R., Mahadevaswamy H., Kalleshwaraswamy C., Sharanabasappa, et al. (2019). Genetic characterization of fall armyworm infesting South Africa and India indicate recent introduction from a common source population. PloS One 14 (5), 1–16. doi: 10.1371/journal.pone.0217755
Nagoshi R., Koffi D., Agboka K., Tounou K., Banerjee R., Jurat-fuentes J., et al. (2017). Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. PLOS ONE 12 (7), e0181982. doi: 10.1371/journal.pone.0181982
Nayga R. M. (1996). Sociodemographic influences on consumer concern for food safety: The case of irradiation, antibiotics, hormones, and pesticides. Rev. Agricu. Econ. Agricul. App. Econ. Assoc. 18 (3), 467–475.
Nayyar N., Gracy R., Ashika T., Mohan G., Swathi R., Mohan M., et al. (2021). Population structure and genetic diversity of invasive Fall Armyworm after 2 years of introduction in India. Sci. Rep. 11 (1), 1–12. doi: 10.1038/s41598-021-87414-5
Nkamleu G., Adesina A. (2000). Determinants of chemical input use in peri-urban lowland systems: Bivariate probit analysis in Cameroon. Agric. Syst. 63 (2), 111–121. doi: 10.1016/S0308-521X(99)00074-8
Onwona-kwakye M., Plants-paris K., Keita K., Lee J., Brink P. J. V., Hogarh J. N., et al. (2020). Pesticides decrease bacterial diversity and abundance of irrigated rice fields. Microorganisms 8 (3), 1–13. doi: 10.3390/microorganisms8030318
Paddock K., Pereira A., Finke D., Ericsson A., Hibbard B., Shelby K. (2021). Host resistance to Bacillus thuringiensis is linked to altered bacterial community within a specialist insect herbivore. Mol. Ecol. 30 (21), 5438–5453. doi: 10.1111/mec.15875
Pashley D. (2018). Current status of fall armyworm host strains. Florida Entomol. Soc. Stable. 71 (3), 227–234.
Pashley D., Martin J. (1987). Reproductive incompatibility between host strains of the fall armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. America 80 (6), 731–733. doi: 10.1093/aesa/80.6.731
Pashley D., Quisenberry S., Jamjanya T. (1987). Impact of fall armyworm (Lepidoptera: noctuidae) host strains on the evaluation of Bermuda grass resistance1. J. Econ. Entomol. 80 (6), 1127–1130. doi: 10.1093/jee/80.6.1127
Pashley D. P. (1988). Quantitative genetics, development and physiological adaptation in host strains of fall armyworm. Evolution. 42 (1), 93–102. doi: 10.1111/j.1558-5646.1988.tb04110.x
Puillandre N., Dupas S., Dangles O., Zeddam J., Capdevielle-Dulac C., Barbin K., et al. (2008). Genetic bottleneck in invasive species: The potato tuber moth adds to the list. Biol. Invasions. 10 (3), 319–333. doi: 10.1007/s10530-007-9132-y
Razzaghi Borkhani F., Shabanali Fami H., Rezvanfar A., Pouratashi M. (2011). Application of IPM practices by paddy farmers in Sari county of Mazandaran province, Iran. Afr. J. Agric. Res. 6 (21), 4884–4892. doi: 10.5897/AJAR11.154
Rehman H., Saggu S. (2016). Situation of pesticide consumption and poisoning in Saudi Arabia. Toxicol. Lett. 258 (3), S217. doi: 10.1016/j.toxlet.2016.06.1785
Ríos-Díez J. D., Saldamando-Benjumea C. I. (2011). Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) strains from central Colombia to two insecticides, methomyl and lambda-cyhalothrin: A study of the genetic basis of resistance. J. Econ. Entomol. 104 (5), 1698–1705. doi: 10.1603/EC11079
Rozas J., Sánchez-DelBarrio J., Messeguer X., Rozas R. (2003). DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 (18), 2496–2497. doi: 10.1093/bioinformatics/btg359
Russell R., Scott C., Jackson C., Pandey R., Pandey G., Taylor M., et al. (2011). The evolution of new enzyme function: Lessons from xenobiotic metabolizing bacteria versus insecticide-resistant insects. Evol. Appl. 4 (2), 225–248. doi: 10.1111/j.1752-4571.2010.00175.x
Salyers A., Gupta A., Wang Y. (2004). Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 12 (9), 412–416. doi: 10.1016/j.tim.2004.07.004
Sattler C., Nagel U. J. (2010). Factors affecting farmers’ acceptance of conservation measures-A case study from north-eastern Germany. Land. Use Policy 27 (1), 70–77. doi: 10.1016/j.landusepol.2008.02.002
Singh B. K., Walker A., Morgan J. A., Wright D. J. (2004). Biodegradation of chlorpyrifos by Enterobacter strain B-14 and its use in bioremediation of contaminated soils. Appl. Environ. Microbiol. 70 (8), 4855–4863. doi: 10.1128/AEM.70.8.4855-4863.2004
Siyum N., Giziew A., Abebe A. (2022). Factors influencing adoption of improved bread wheat technologies in Ethiopia: empirical evidence from Meket district. Heliyon 8 (2), e08876. doi: 10.1016/j.heliyon.2022.e08876
Stevenson P. C., Isman M. B., Belmain S. R. (2017). Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crops Products. 110 (August), 2–9. doi: 10.1016/j.indcrop.2017.08.034
Tago K., Okubo T., Itoh H., Kikuchi Y., Hori T., Sato Y., et al. (2015). Insecticide-degrading Burkholderia symbionts of the stinkbug naturally occupy various environments of sugarcane fields in a southeast island of Japan. Microbes Environments. 30 (1), 29–36. doi: 10.1264/jsme2.ME14124
Tang C., Zhong J., Lv Y., Liu X., Li Y., Zhang M., et al. (2021). Response and dynamic change of microbial community during bioremediation of uranium tailings by bacillus sp. Minerals 11 (9), 1–14. doi: 10.3390/min11090967
Tehara S. K., Keasling J. D. (2003). Gene cloning, purification, and characterization of a phosphodiesterase from Delftia acidovorans. Appl. Environ. Microbiol. 69 (1), 504–508. doi: 10.1128/AEM.69.1.504-508.2003
Tepa-yotto G., Tonnang H. E. Z., Goergen G., Subramanian S., Kimathi E., Abdel-rahman E. M., et al. (2021). Global habitat suitability of Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae): Key parasitoids considered for its biological control. Insects. 12 (4), 273. doi: 10.3390/insects12040273
Tizale C. Y. (2007). The dynamics of soil degradation and incentives for optimal management in the Central Highlands of Ethiopia. University of Pretoria (PhD Thesis).
Togola A., Meseka S., Menkir A., Badu-Apraku B., Boukar O., Tamò M., et al. (2018). Measurement of pesticide residues from chemical control of the invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a maize experimental field in Mokwa, Nigeria. Int. J. Environ. Res. Public Health 15 (5), 849. doi: 10.3390/ijerph15050849
Trujillo-Barrera A., Pennings J. M. E., Hofenk D. (2016). Understanding producers’ motives for adopting sustainable practices: The role of expected rewards, risk perception and risk tolerance. Eur. Rev. Agric. Econ. 43 (3), 359–382. doi: 10.1093/erae/jbv038
Veenstra K. H., Pashley D. P., Ottea J. A. (1995). Host-plant adaptation in fall armyworm host strains: Comparison of food consumption, utilization, and detoxication enzyme activities. Ann. Entomol. Soc. America 88 (1), 80–91. doi: 10.1093/aesa/88.1.80
Wachenheim C. J., Lesch W. C., Dhingra N. (2014). The conservation reserve program: a literature review. Agribusiness. Appl. Econ. Rep. 723), 22. doi: 10.22004/ag.econ.164829
Xia X., Sun B., Gurr G. M., Vasseur L., Xue M., You M. (2018). Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 9 (JAN). doi: 10.3389/fmicb.2018.00025
Xing D., Ren N., Rittmann B. E. (2008). Genetic diversity of hydrogen-producing bacteria in an acidophilic ethanol-H2-coproducing system, analyzed using the [Fe]-hydrogenase gene. Appl. Environ. Microbiol. 74 (4), 1232–1239. doi: 10.1128/AEM.01946-07
Xu G., Li Y., Zheng W., Peng X., Li W., Yan Y. (2007). Mineralization of chlorpyrifos by co-culture of Serratia and Trichosporon spp. Biotechnol. Lett. 29 (10), 1469–1473. doi: 10.1007/s10529-007-9444-0
Xu C., Su X., Wang J., Zhang F., Shen G., Yuan Y., et al. (2021). Characteristics and functional bacteria in a microbial consortium for rice straw lignin-degrading. Bioresour. Technol. 331 (March), 125066. doi: 10.1016/j.biortech.2021.125066
Keywords: smallholder farmer, fall armyworm, pest control, insecticides, gut microbiome
Citation: Ndung’u KE, Khamis FM, Ajene IJ, Mbogo KO and Akutse KS (2023) Spodoptera frugiperda population structure and influence of farmers’ practices on gut biodiversity for sustainable management of the pest in Kenya. Front. Ecol. Evol. 11:1235558. doi: 10.3389/fevo.2023.1235558
Received: 05 July 2023; Accepted: 31 July 2023;
Published: 18 August 2023.
Edited by:
Andrea Sciarretta, University of Molise, ItalyReviewed by:
Johnnie Van Den Berg, North-West University, South AfricaCopyright © 2023 Ndung’u, Khamis, Ajene, Mbogo and Akutse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fathiya M. Khamis, ZmtoYW1pc0BpY2lwZS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.