
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 August 2023
Sec. Chemical Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1233655
This article is part of the Research Topic Recent Advances in the Chemical Ecology of Parasitic Hymenoptera View all 7 articles
Female egg parasitoids must optimize their ability to find a suitable host for reproduction in a limited foraging time. Odorant cues associated with the plant–host complex play an essential role in guiding females toward the host. However, parasitoid response is not always identical within the same genotype, and it could be influenced by the environment. This phenotypic plasticity affects parasitoid behavior and morphology and is directly linked to rearing conditions. Yet, how plasticity influences olfactory responses of egg parasitoids toward plant–host odors is largely unexplored. Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) is an effective biocontrol agent of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). Laboratory no-choice and choice tests showed T. japonicus potential to develop in eggs of non-target Pentatomidae. In Y-tube olfactometer we evaluated the olfactory responses of T. japonicus reared on different hosts toward plant–host derived volatiles associated with H. halys and two other stink bug species. Parasitoids reared on the main host H. halys positively responded only to odors from V. faba–H. halys complex. When reared on alternative hosts, T. japonicus was smaller and did not exhibit attraction to any stimuli, although egg load was only partially affected. Host-induced phenotypic plasticity should be considered when evaluating parasitoids for classical biological control.
Entomophagous insects, such as egg parasitoids, rely on odor cues for finding a suitable oviposition site (Fatouros et al., 2008; Greenberg et al., 2023). Therefore, for optimal foraging behavior, it is fundamental that host associated cues are easily detectable and reliable (Vet and Dicke, 1992; Steidle and van Loon, 2002). Plants attacked by herbivorous insects emit volatile compounds that attract parasitoids in a very specific way (de Moraes et al., 1998; Dicke, 1999; Chiappini et al., 2012). In addition, odors from the herbivore itself can be informative, for foraging parasitoid females, of the presence of suitable host stages (Afsheen et al., 2008; Ayelo et al., 2022). One key aspect of parasitoid behavior is that it is influenced by exogenous factors (Boivin, 2010). Parasitoids have the capacity to adapt to changes in the environmental conditions to maximize their fitness (Whitman and Agrawal, 2009; Boivin, 2010). The ability of a single genotype to produce distinct phenotypes when exposed to different environments is referred as phenotypic plasticity (West-Eberhard, 1989). Morphological, physiological, and behavioral traits have been well documented to be affected by phenotypic plasticity in parasitoids (Harvey, 2005; Boivin, 2010). For instance, adult size depends on host size, and, when in relation with other parameters such as egg load, longevity, and fecundity, it can be used as a proxy for parasitoid fitness (Roitberg et al., 2001). Indeed, differences in body size can determine parasitoid behavior, such as dispersal ability and searching efficiency within patches, with consequences on the parasitoid foraging activity (Visser, 1994; Ellers et al., 1998). Unexpectedly, the effect of phenotypic plasticity induced by parasitoid rearing conditions to the olfactory responses of foraging egg parasitoids is largely unexplored.
The brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), is native to northeastern Asia, and it was accidentally introduced in several areas of the US, Canada, Europe, and Chile, where it established and now is considered a serious agricultural pest (Lee et al., 2013; Leskey and Nielsen, 2018). The egg parasitoid Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) is the most promising biocontrol agent of H. halys. In 2020, a biocontrol program utilizing T. japonicus was started in Italian regions invaded by the stink bug (Zapponi et al., 2021). No-choice and choice laboratory tests and analysis of field collected egg masses have revealed, however, that T. japonicus can successfully develop also in stink bug species other than H. halys (Zhang et al., 2017; Lara et al., 2019; Haye et al., 2020; Sabbatini-Peverieri et al., 2021). Interestingly, such parasitization on alternative hosts eventually resulted in the emergence of different phenotypes of the parasitoid (Botch and Delfosse, 2018). Here, we conducted a series of Y-tube olfactomer bioassays to evaluate behavioral responses of T. japonicus phenotypes, reared on the main host, H. halys, or on alternative non-target stink bug hosts, toward odors from plant–host complexes. Previous research has revealed that T. japonicus originating from H. halys positively responds to olfactory cues from Solanum lycopersicum L.–H. halys complex (Bertoldi et al., 2019). Here, we hypothesized that behavioral changes toward host-associated cues may occur because of the species of parasitoid rearing host.
Adult H. halys, Dolycoris baccarum L. (both herbivorous species), and Arma custos (F.) (a predatory species) (all Hemiptera: Pentatomidae) were initially collected in natural and agricultural ecosystems located in north and central Italy. These non-target stink bugs are present both in Europe and Asia (Panizzi, 2013; Zhao et al., 2018) and were selected because of the higher rates of parasitization by T. japonicus displayed in no-choice host suitability tests (Haye et al., 2020; Sabbatini-Peverieri et al., 2021). Moreover, A. custos is a well-known predator of several herbivorous pests within Lepidoptera, Coleoptera, Hymenoptera, and Hemiptera (Fan et al., 2020). Laboratory colonies of the stink bugs were reared in a climatic chamber maintained in controlled environmental conditions (25 ± 1°C, 60 ± 5% relative humidity, 16/8-h light/dark). Approximatively, 60 males and females of H. halys, D. baccarum, and A. custos were reared in mesh cages (Kweekkooi, 40 cm × 40 cm × 60 cm, Vermandel, Hulst, The Netherlands). A diet consisting of carrots (Daucus carota L.), apples (Malus domestica Borkh), pears (Pyrus communis L.), hazelnuts (Corylus avellana L.), and sunflower seeds (Helianthus annus L.) was supplied three times a week to H. halys and D. baccarum, whereas Tenebrio molitor (L.) (Coleoptera: Tenebrionidae) pupae were offered to A. custos adults. Water was provided with an upside-down cotton jar sealed on a Petri dish. On the bottom of all the cages, a paper towel served as substrate for food and as supplementary oviposition substrate. Egg masses were daily collected and used for stink bug rearing maintenance or frozen at −20°C for parasitoid rearing. Four physogastric females (about 2 weeks old) of each stink bug species were taken from the colonies and used for the bioassays.
Adult T. japonicus were reared in 50ml Falcon plastic tubes and maintained in a climatic chamber (Corning, Italy) at controlled conditions (25 ± 1°C, 85 ± 5 relative humidity, 16/8-h light/dark). A diet consisting of droplets of pure honey was provided on a piece (2 cm × 2 cm) of laboratory film (Parafilm®, Bemis, USA). The original strain of T. japonicus was provided by United States Department of Agriculture (USDA) with kind permission of K.A. Hoelmer (USDA-ARS Beneficial Insects Introductions Research Unit, in Newark, DE, USA) (Sabbatini-Peverieri et al., 2021). Three different colonies of the parasitoid were established and maintained on eggs of H. halys, D. baccarum, and A. custos. Each colony was reared in separate tubes. In each parasitoid tube, a stink bug egg mass of a given species was offered to one mated T. japonicus female (approximately 1 week old), which originated from the same colony. Newly emerged parasitoids were allowed to mate before being used in bioassays. Tested females belonged to the fourth to eighth generation and were approximatively 8 days old. In addition, for each parasitoid colony, 30 females were randomly collected for body size assessment. Females were killed at −20°C, and their head width and height as well as right hind femur and tibia length were measured by microscopic inspection and analyzed using ImageJ (Rasband, 1997). Furthermore, the egg load of T. japonicus reared on all the three host species was specifically recorded at 4, 8, 12, and 20 days after emergence. For each combination of timing and host species, 9–10 females were killed in 96% ethanol, and, then, they were individually transferred in a drop of water on a glass slide placed under a stereo microscope (Gaudreau et al., 2022). Female head and thorax were removed, and the abdomen was gently opened using two fine tweezers. Mature eggs were stained with 0.025% Evan’s Blue and counted (similarly to Rondoni et al., 2017; Sabbatini-Peverieri et al., 2021; Gaudreau et al., 2022; Giovannini et al., 2022). Egg pictures were taken with a digital camera mounted on the top of the microscope. The dimension of 200 mature eggs for each host species was measured using ImageJ (Rasband, 1997).
Broad bean seeds (Vicia faba L. cv Aguadulce Supersimonia, Fabaceae) were allowed to soak for 24 h before being sown in plastic pots (9 cm × 9 cm × 13 cm) filled with peat (Traysubstrat, Klasmann-Deilmann GmbH). Plants were grown and maintained in a climatic chamber (24 ± 2°C, 70 ± 5% relative humidity, 12/12-h light/dark) and irrigated three times per week. Vicia faba plants with seven to eight fully expanded leaves were caged in a sleeve cage of 25 cm × 55 cm made of a 4-mm-diameter mesh net. Single potted plants were exposed, for a maximum of 3 days, to two mated females of each species of stink bug, allowing them to feed and eventually oviposit on the plants (as in Rondoni et al., 2018). Approximately, 10 mg of sunflower seeds (for H. halys and D. baccarum) or six T. molitor pupae (for A. custos) were added in the cage as additional nourishment. Plants were checked daily at 8:00 am for the presence of egg masses. In case of oviposition, plants were removed from the cage and used for bioassays on the following day. Plants with an egg mass with lower than 10 elements were eventually discarded and not used for the bioassays.
Behavioral responses of T. japonicus females toward plant–host complex volatile cues associated to stink bug species were tested in a Y-tube olfactometer. According to the species of parasitoid rearing host, T. japonicus from H. halys was tested against odors from attacked plants or from females of all the three stink bug species (Rondoni et al., 2022), Trissolcus japonicus from D. baccarum was tested against odors from H. halys or D. baccarum, whereas T. japonicus from A. custos was assayed against odors from H. halys or A. custos. Two different treatments were tested as odor sources:
a) Plant with eggs: a V. faba plant bearing an egg mass of H. halys, D. baccarum, or A. custos;
b) Females: odor from four physogastric females of H. halys, D. baccarum, or A. custos.
A clean plant, i.e., a V. faba plant not exposed to insects (CLEAN PLANT) or clean air (AIR), was used as control for treatments a) and b), respectively.
The olfactometer consisted in a Y-tube carved in a plexiglass plate (200 mm (length) × 190 mm (width) × 10 mm (depth)) and sandwiched between two glass plates (each: 200 mm (length) × 150 mm (width) × 5 mm (depth)). The common stem of the Y-tube was 90 mm in length. Each arm was 80 mm long, and a 100° angle separated the two arms. The internal section of the Y-tube was 15 mm × 10 mm. Four identical olfactometers were simultaneously used to test the behavior of four parasitoids at the same time. An airstream, consisting in medical-grade clean air (N2:O2 = 80:20), was provided from a pressurized tank. The airstream was conveyed in two routes, each consisting of a Dreschel bottle (250 ml) containing distilled water to humidify the air, a flowmeter, and a glass chamber [52 cm (height) × 12 cm (internal diameter)] containing the odor source (treatment or control). The airflow exiting the glass chamber was subsequently divided into four streams and directed to one of the two arms of each olfactometer. Silicone tubes (6 mm internal diameter) conveyed the airflow from the tank to the glass chambers and to each olfactometer (Rondoni et al., 2022). Plastic opened screw caps and “Y” tubing connectors (Kartell Spa, Noviglo, Italy) were used to connect the different parts. The flow rate entering each arm of the olfactometer (~ 0.2 L/min) was measured using a digital flowmeter (model GFM17, Aalborg, New York, USA). All the setup was surrounded by a black fabric curtain to prevent external visual cues, and two 36-W cool white fluorescent tubes were placed above to illuminate the device. The bioassay room was maintained at 25°C. Stink bugs, plants, and parasitoids were moved to the experimental room 30 min before bioassays for acclimatization. A single female parasitoid was introduced in the central stem of the Y-tube and allowed to freely move for 10 min. After four bioassays, the positions of the treatment and control were switched. Glass plates and plexiglass parts were cleaned with a laboratory detergent (2% solution of Cleanilab LM1; Kartell Spa, Noviglo, Italy) and rinsed with tap water. Glass parts were eventually rinsed with acetone and placed in a drying oven at 180°C for few hours. The presence of each parasitoid in the control or test arm and in the central stem of the olfactometer was recorded using JWatcher 1.0 (Blumstein et al., 2006). Each egg parasitoid female was tested once. In total, for each stimulus (plant with an egg mass or stink bug females), three to four different sets of the odor source were tested (Supplementary Tables 1, 2).
For each treatment, the residence time, i.e., the time spent by the female in each arm of the olfactometer, was used to describe the parasitoid behavior. Those females that, during the 10 min of bioassay, did not choose either arm were considered not responding and were discarded from the analysis (similar to Rondoni et al., 2022). Depending on the T. japonicus origin, 50 to 55 females were eventually evaluated for each treatment involving plants, whereas 49 to 55 T. japonicus females were evaluated against stink bug females. For the analysis, data were transformed to ensure that only one measurement per individual parasitoid was later analyzed. In details, the adopted transformation foresaw the calculation of the log ratio of the residence time in the test arm versus the control arm. For each combination of odor sources, linear models (LMs) were fitted to test differences of treatment versus control. Egg volume was calculated assuming a fusiform shape and was estimated as 2/3 × π × r2 × h, where r = egg width/2 and h = egg length/2 (Ameri et al., 2014). Parasitoid dimension, egg volume, and egg load, i.e., the number of mature eggs at each timing, were analyzed by means of LMs followed by multiple comparisons procedure. For egg load, the timing since parasitoid emergence (log transformed) was included as covariate. Analyses were conducted in the R statistical environment, version 4.3.0 (R Core Team, 2023), packages “MASS” (Venables and Ripley, 2002) and “emmeans” (Lenth, 2022).
Trissolcus japonicus females originating from H. halys exhibited a positive response toward volatiles emitted by V. faba plants with H. halys eggs, as demonstrated by the higher residence time in treatment vs. control (LM, P = 0.01) (Figure 1). Conversely, when exposed to odors from D. baccarum or A. custos, parasitoid females did not exhibit any preference (LM, P > 0.05 for all the tested comparisons). When reared on the two alternative hosts, D. baccarum or A. custos, T. japonicus females were not attracted to any of the odor stimuli (LM, P > 0.05 for all the tested comparisons). When parasitoids were exposed to odors from female stink bugs, regardless of the nature of the rearing host, they did not exhibit any response toward the different stimuli (LM, P > 0.05 for all the tested comparisons) (Figure 2).
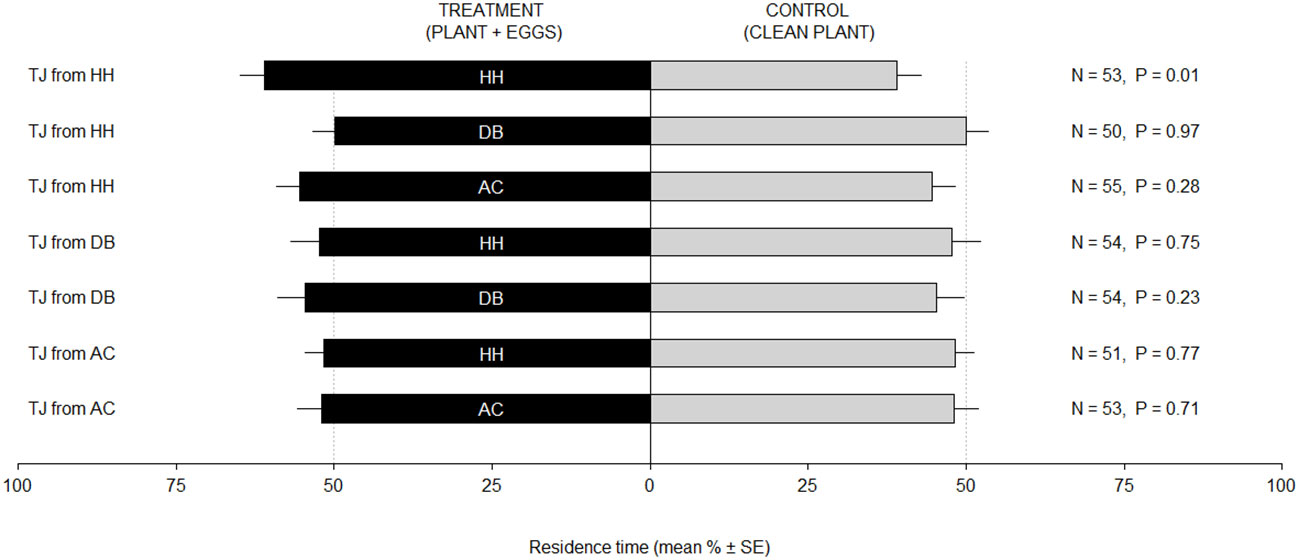
Figure 1 Residence time (mean % ± SE) of Trissolcus japonicus (TJ) females in the test and control arms of a Y-tube olfactometer. Bioassayed parasitoids were reared on Halyomorpha halys (TJ from HH), Dolycoris baccarum (TJ from DB), or Arma custos (TJ from AC). Treatments consisted in a Vicia faba plant bearing an egg mass of H. halys (HH), D. baccarum (DB), or A. custos (AC). Control consisted of a clean V. faba plant. Data were analyzed by means of linear models.
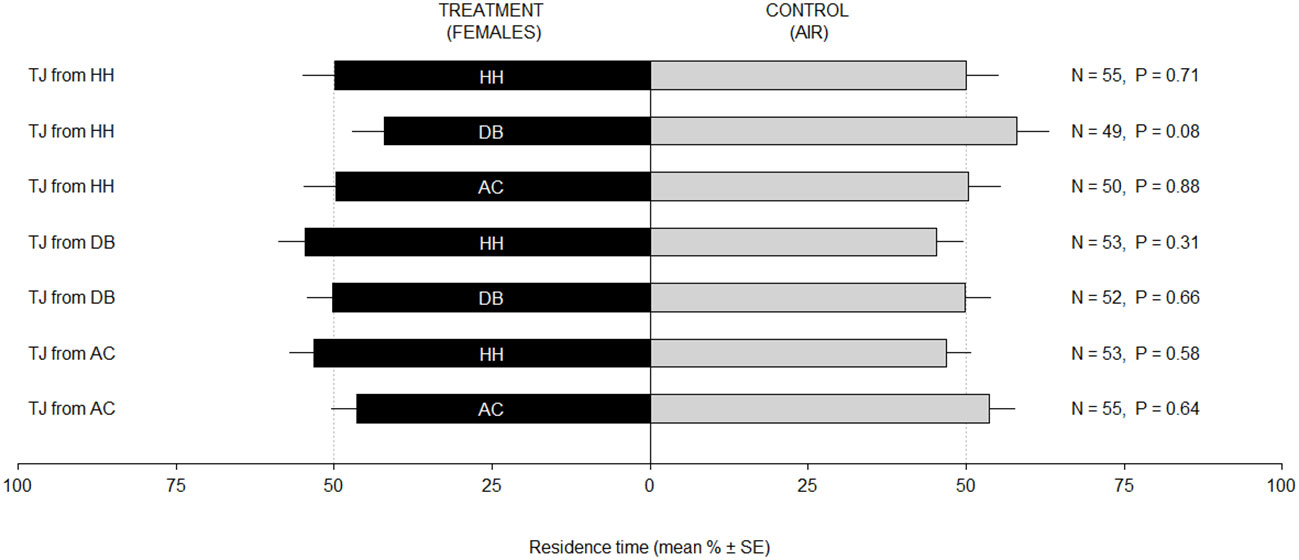
Figure 2 Residence time (mean % ± SE) of Trissolcus japonicus (TJ) females in the test and control arms of a Y-tube olfactometer. Bioassayed parasitoids were reared on Halyomorpha halys (TJ from HH), Dolycoris baccarum (TJ from DB), or Arma custos (TJ from AC). Treatments consisted in four physogastric females of H. halys (HH), D. baccarum (DB), or A. custos (AC). Control consisted of clean air. Data were analyzed by means of linear models.
Parasitoid size varied depending on the rearing host (Table 1). Trissolcus japonicus reared on D. baccarum exhibited shorter head length, head width, femur length, and tibia length compared with A. custos or H. halys. Parasitoids from A. custos were smaller compared with those from H. halys. The egg load increased with the time since parasitoid emergence (regression coefficient in LM = 6.15, t-value = 6.15, P < 0.0001) and was higher for T. japonicus reared on A. custos or H. halys compared with those on D. baccarum (Figure 3A). The egg volume differed among T. japonicus reared on the three host species, as it showed a reduction from H. halys, A. custos, and D. baccarum used as rearing host, respectively (Figure 3B).
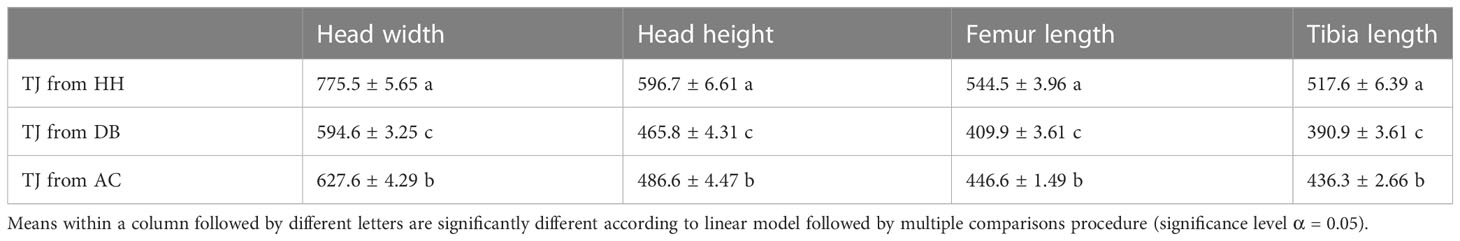
Table 1 Morphological variation (mean ± SE) of 30 Trissolcus japonicus females reared on Halyomorpha halys (TJ from HH), Dolycoris baccarum (TJ from DB), or Arma custos (TJ from AC).
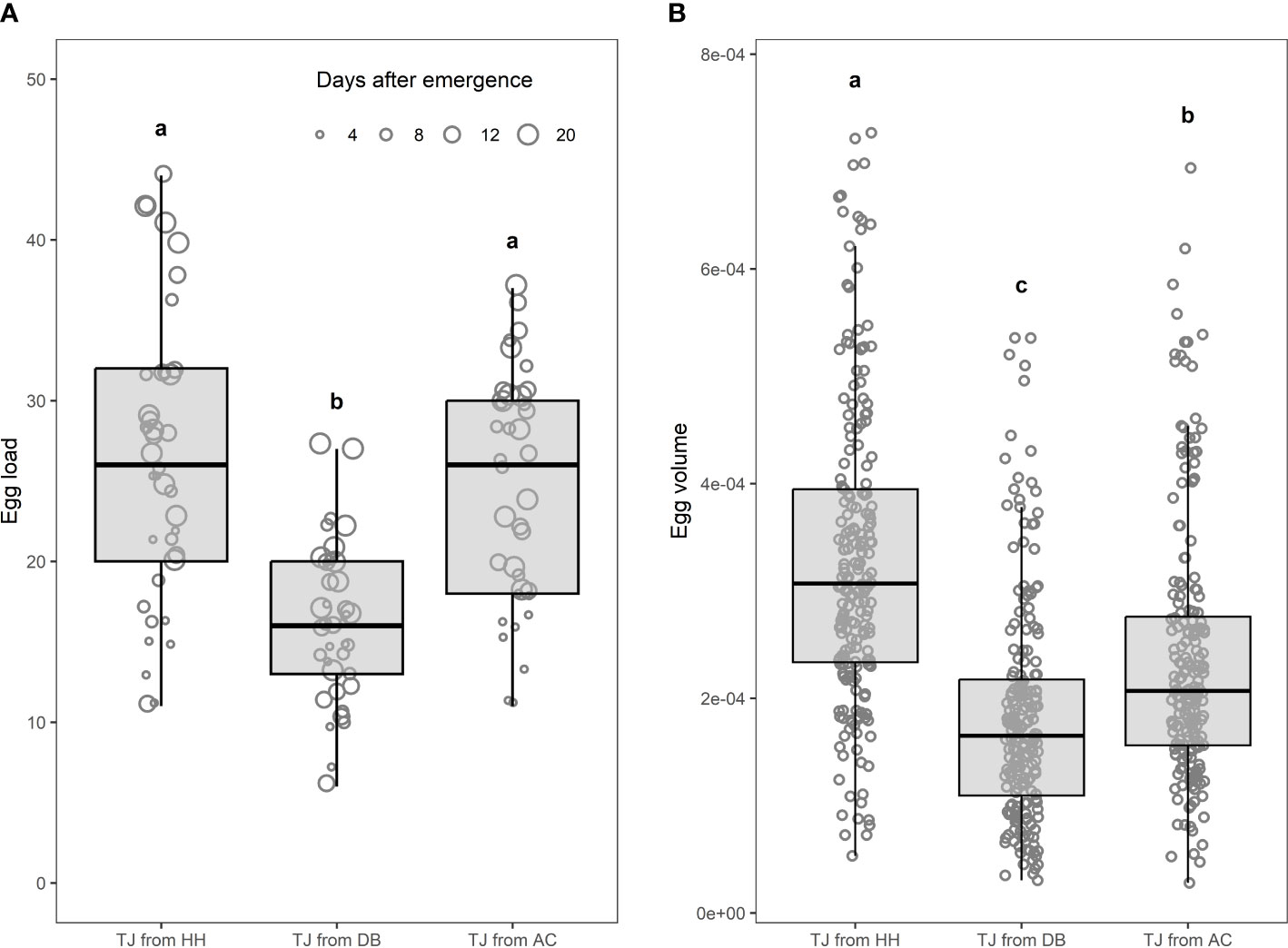
Figure 3 Egg load (n) (A) and egg volume (mm3) (B) from Trissolcus japonicus (TJ) females reared on Halyomorpha halys (TJ from HH), Dolycoris baccarum (TJ from DB), or Arma custos (TJ from AC). Different letters are significantly different according to linear model followed by multiple comparisons procedure (significance level α = 0.05). In addition, egg load increased with time (days) since parasitoid emergence (regression coefficient = 6.15, t-value = 6.15, P < 0.0001).
Our results showed, for the first time, that T. japonicus females emerging from H. halys eggs were attracted to V. faba plants bearing an egg mass of the main host, H. halys, and not to plants with eggs of the native stink bug species. In addition, parasitoids that were reared on the alternative hosts, D. baccarum and A. custos, did not respond to plant volatiles induced by H. halys. This change in the parasitoid behavior is also linked to a smaller parasitoid size, suggesting the existence of a phenotypic plasticity in T. japonicus.
Plants bearing naturally laid stink bug eggs emit oviposition-induced volatiles that can be exploited by egg parasitoids in the host searching behavior (Colazza et al., 2004; Manzano et al., 2022). For example, T. japonicus is attracted to tomato plants exposed to oviposition by H. halys and not to odors emitted directly by eggs or by plants exposed only to stink bug feeding (Bertoldi et al., 2019). The stink bug Nezara viridula L., when feeds and oviposits on plants, elicits specific compounds attracting the co-evolved egg parasitoid Trissolcus basalis Woll (Hymenoptera: Scelionidae) (Colazza et al., 2004). The scelionid parasitoid Trissolcus mitsukurii (Ashmead) exploits plant chemical cues associated with the co-evolved H. halys (Rondoni et al., 2022; Scala et al., 2022). In stink bugs, oviposition is always associated with feeding punctures on the plant (Peiffer and Felton, 2014). Consistently, although there are exceptions (Blassioli Moraes et al., 2005; Michereff et al., 2011), several studies underlined that feeding alone does not attract egg parasitoids, but oviposition is also needed (Conti and Colazza, 2012; Hilker and Fatouros, 2015). Indeed, the blend of volatile compounds emitted by plants was shown to differ after exposure to feeding alone or to feeding and oviposition by H. halys and such difference may explain parasitoid attraction (Akotsen-Mensah et al., 2021; Peterson et al., 2022). Although a chemical analysis of the volatile profiles emitted by attacked plants would be needed, we can deduce that the positive response of T. japonicus toward plants with eggs of H. halys is a further confirmation that the parasitoid is capable to exploit long-range cues associated with the target host. This is consistent with the remarkable levels of parasitism shown in the field both in native and non-native areas (Yang et al., 2009; Zhang et al., 2017; Zapponi et al., 2021; Haye et al., 2023). Remarkably, we found that, when T. japonicus was provided with a different rearing host, this induced a phenotypic plasticity in behavioral and morphological traits of the parasitoid progeny. Recognition of essential semiochemicals, such as host plant cues, can be impaired when natural enemies emerge from alternative hosts (van Lenteren and Bigler, 2009). Boyle et al. (2020) noticed a different behavior of T. japonicus when this was reared on its main host, H. halys, or on the alternative host, Podisus maculiventris (Say) (Hemiptera: Pentatomidae). Notably, T. japonicus positively responded to H. halys contact kairomones only when the parasitoid was reared on that specific host species and not when reared on the alternative host (Boyle et al., 2020). In a different system, when the generalist parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae) was reared on the suitable host Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae), it exhibited an improved host recognition for this aphid, compared with the less suitable Sitobion avenae F. In contrast, when the parasitoid was reared on S. avenae, the preference for A. pisum disappeared (Daza-Bustamante et al., 2003). Similarly, in our case, the observed reduced preference by T. japonicus, reared on alternative hosts, toward H. halys could be related to the low suitability of the rearing eggs. In fact, females reared on alternative hosts exhibited not only a small body size but also a lower egg dimension and egg load (but only with D. baccarum) compared with individuals reared on H. halys (Sabbatini-Peverieri et al., 2021). Parasitoid size and plasticity in egg load are a proxy for fitness and may determine the foraging behavior of parasitoids (Pak et al., 1985; Collins and Dixon, 1986; Fletcher et al., 1994). It is expected that smaller parasitoids are more time-limited because they are more likely to be affected by environmental conditions compared with the larger ones (e.g., Olson and Andow, 1998; Jervis et al., 2008). In this sense, synovigenic parasitoids, as T. japonicus (Haye et al., 2020; Bittau et al., 2021), in case of sub-optimal ovarian development may opt to search for food, rather than for host (Jervis et al., 2008). Hence, it can be hypothesized that the altered olfactory response of T. japonicus strains emerging from D. baccarum may lead to a lower specificity in oviposition decision. Some examples in the literature have shown a strict link between egg load or parasitoid size with foraging behavior, although general conclusions have yet to be drawn (Jervis et al., 2008). The intensity of searching behavior and walking speed are positively correlated with the egg load in several parasitoid species (reviewed by Minkenberg et al., 1992). For instance, in Telenomus podisi Ashmead (Hymenoptera: Scelionidae), body size, oviposition, and marking rates were all positively correlated (Abram et al., 2016). In Dirhinus giffardii Silvestri (Hymenoptera: Chalcididae), female body size was positively correlated with foraging ability (Wang and Messing, 2004). Contrary to expectations, the egg load produced by T. japonicus reared on A. custos did not differ from that of parasitoids reared on H. halys, despite the significantly smaller egg dimensions. Because parasitoids reared on A. custos were of intermediate dimension between H. halys and D. baccarum, we can hypothesize that the negative effect of alternative host for parasitoid rearing on the egg load only occurs below a certain parasitoid body size. However, egg load is a complex trait that is also under the influence of the quality of the environment, parasitoid life expectancy, and the perceived possibility to further disperse and explore new habitats (Ellers et al., 2000; Ellers and Jervis, 2003). Additional investigation is, therefore, needed to understand the trade-off between reproduction and survival during adult life span and its effect on the parasitoid behavior.
The parasitoid did not respond to plants with an egg mass of the non-target species: D. baccarum or A. custos. Such results agree with previous laboratory studies (Haye et al., 2020; Sabbatini-Peverieri et al., 2021). In a no-choice test, the host acceptance by T. japonicus was significantly lower for D. baccarum compared with that for H. halys (Haye et al., 2020). In the same study, host acceptance was similar for A. custos and H. halys, but, in a two-choice test, parasitization was remarkably higher in the latter species (Haye et al., 2020). We, therefore, hypothesize that T. japonicus is not naturally able to locate eggs of these stink bugs at distance. However, in those habitats where H. halys is present at a high density, the parasitoid can be attracted from distance by cues from the main host and fortuitously oviposit on alternative species that may encounter (Zhang et al., 2017). Recent surveys conducted in northern Italian ecosystems did not reveal emergence of T. japonicus from naturally laid eggs of A. custos or D. baccarum (Falagiarda et al., 2023; Haye et al., 2023).
Furthermore, our research demonstrated that the parasitoids do not respond in the olfactometer to female stink bugs regardless of the rearing host. Similarly, other studies failed to demonstrate attractiveness of H. halys females for T. japonicus or other parasitoids when tested in a standard setting of Y-tube olfactometer (Bertoldi et al., 2019; Rondoni et al., 2022). In fact, such cues can essentially be exploited only when they are provided at very short distance (Bertoldi et al., 2019; Malek et al., 2021).
The demonstrated phenotypic plasticity of T. japonicus behavior may have implications on the preservation of parasitoid populations in the nature. It can be expected that, in case of reduction of H. halys population in invaded areas, more individuals of T. japonicus can switch hosts, because of higher random encounters with native stink bugs. Theoretically, this would lead to an increase in abundance of low fitness parasitoids, which are characterized by low foraging abilities, hence low probability to produce progeny (Robertson et al., 2013). Hence, the presence of the parasitoid strictly depends on its main host, with positive implications for preventing the spread of the exotic biocontrol agent in areas where H. halys is not present. A better understanding of how the reduced body size influences the parasitoid fitness is, therefore, essential. Long-term adaptation of the parasitoid on the alternative hosts must be also investigated. For instance, although the generalist parasitoid A. ervi exhibited an initial reduction in fitness due to rearing on an alternative (less suitable) host, this was followed by an adaptive recovery after many continuous generations (Henry et al., 2008).
Some aspects of our study must be considered at parasitoid, plant, and herbivore levels for future investigations. Using the olfactometer, we simulated the long-range diffusion of plant volatiles (Vet and Dicke, 1992). Although the active volatile compounds were not identified in this study, we provided evidence that the parasitoid ability to exploit such stimulus is strictly linked to its main host. However, some of the stimuli from host species in different life stages can be exploited by parasitoids at short range (Fatouros et al., 2005; Conti and Colazza, 2012; Bertoldi et al., 2021). For instance, T. japonicus recognizes tracks of H. halys females in arena bioassays (Boyle et al., 2020; Malek et al., 2021). Furthermore, laboratory assays are a simplified condition compared to the complexity of natural habitats, where multiple odor blends from different plants or other insects are present (Wäschke et al., 2013). Considering the polyphagous nature of H. halys, additional plants could be further considered (Bariselli et al., 2016; Kriticos et al., 2017). Although stink bugs do not injure the plant with oviposition (Michereff et al., 2016), we cannot exclude that the size of the naturally laid egg mass affects the plant response and, consequently, the parasitoid behavior (Griese et al., 2017). Although the size of an egg mass is rather constant for H. halys, this is more variable for the other two species (Giovannini et al., 2022; Wu et al., 2022). Finally, it is recognized that herbivorous insects originating from different countries may exhibit diverse behavior [e.g., the stem borers Sesamia nonagrioides Lefèbvre (Lepidoptera: Noctuidae) (Moyal et al., 2011; Glaser et al., 2015) and Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) (Dhillon et al., 2021)]. In the invaded areas, H. halys is present with multiple genotypes originating from several parts of the world, possibly, because of multiple independent invasions (Valentin et al., 2017; Cesari et al., 2018; Yan et al., 2021). Whether the different genotypes of H. halys induce the same plant response remains unknown.
Because the efficacy of the parasitoid could vary depending on rearing host, including fitness evaluation of parasitoid strains in risk-assessment protocols is crucial for better understanding the value of a candidate biocontrol agent.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
ECh, GS-P, GR, and ECo contributed to conception and design of the study. ECh collected the data. ECh and GR performed the statistical analysis. ECh, GR, and ECo interpreted the results. ECh and GR wrote the first draft of the manuscript. ECh, GS-P, GR, and ECo edited the manuscript. GS-P, PFR, and ECo provided funding. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the Ministero per le Politiche Agricole, Alimentari e Forestali (MiPAAF), projects “Proteggo 1.3” (MiPAAF DISR-05-0155972 6/04/2021) and “Proteggo 1.4” (MiPAAF DISR-05-0001837-04/01/2022), and by the Regional Phytosanitary Service of Umbria Region. GR was funded by H2020-MSCA-IF, Grant agreement ID: 101026399.
We thank Daniela Fortini, Vito Antonio Giannuzzi, Andrea Luchetti, Adriana Poccia, and Valeria Rossi for help with insect collection, rearing, and data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1233655/full#supplementary-material
Abram P. K., Parent J.-P., Brodeur J., Boivin G. (2016). Size-induced phenotypic reaction norms in a parasitoid wasp: an examination of life-history and behavioural traits. Biol. J. Linn. Soc. 117, 620–632. doi: 10.1111/bij.12658
Afsheen S., Xia W., Ran L., Zhu C. S., Lou Y. G. (2008). Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect. Sci. 15, 381–397. doi: 10.1111/j.1744-7917.2008.00225.x
Akotsen-Mensah C., Blaauw B. R., Rivera M. J., Rodriguez-Saona C., Nielsen A. L. (2021). Behavioral response of Halyomorpha halys (Hemiptera: Pentatomidae) and its egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to host plant odors. Front. Ecol. Evol. 9, 696814. doi: 10.3389/fevo.2021.696814
Ameri M., Rasekh A., Michaud J. P. (2014). Body size affects host defensive behavior and progeny fitness in a parasitoid wasp, Lysiphlebus fabarum. Entomol. Exp. Appl. 150, 259–268. doi: 10.1111/eea.12158
Ayelo P. M., Yusuf A. A., Chailleux A., Mohamed S. A., Pirk C. W. W., Deletre E. (2022). Chemical cues from honeydew and cuticular extracts of Trialeurodes vaporariorum serve as kairomones for the parasitoid Encarsia formosa. J. Chem. Ecol. 48, 370–383. doi: 10.1007/s10886-022-01354-6
Bariselli M., Bugiani R., Maistrello L. (2016). Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 46, 332–334. doi: 10.1111/epp.12289
Bertoldi V., Rondoni G., Brodeur J., Conti E. (2019). An egg parasitoid efficiently exploits cues from a coevolved host but not those from a novel host. Front. Physiol. 10, 746. doi: 10.3389/fphys.2019.00746
Bertoldi V., Rondoni G., Peri E., Conti E., Brodeur J. (2021). Learning can be detrimental for a parasitic wasp. PloS One 16, e0238336. doi: 10.1371/journal.pone.0238336
Bittau B., Dindo M. L., Burgio G., Sabbatini-Peverieri G., Hoelmer K. A., Roversi P. F., et al. (2021). Implementing mass rearing of Trissolcus japonicus (Hymenoptera: Scelionidae) on cold-stored host eggs. Insects 12, 840. doi: 10.3390/insects12090840
Blassioli Moraes M. C., Laumann R., Sujii E. R., Pires C., Borges M. (2005). Induced volatiles in soybean and pigeon pea plants artificially infested with the neotropical brown stink bug, Euschistus heros, and their effect on the egg parasitoid, Telenomus podisi. Entomol. Exp. Appl. 115, 227–237. doi: 10.1111/j.1570-7458.2005.00290.x
Blumstein D. T., Daniel J. C., Evans C. S. (2006). JWatcher 1.0: An introductory user’s guide 211. Available at: http://www.jwatcher.ucla.edu/.
Boivin G. (2010). Phenotypic plasticity and fitness in egg parasitoids. Neotrop. Entomol. 39, 457–463. doi: 10.1590/s1519-566x2010000400001
Botch P. S., Delfosse E. S. (2018). Host-acceptance behavior of Trissolcus japonicus (Hymenoptera: Scelionidae) reared on the invasive Halyomorpha halys (Heteroptera: Pentatomidae) and nontarget species. Environ. Entomol. 47, 403–411. doi: 10.1093/ee/nvy014
Boyle S. M., Weber D. C., Hough-Goldstein J., Hoelmer K. A. (2020). Parental host species affects behavior and parasitism by the pentatomid egg parasitoid, Trissolcus japonicus (Hymenoptera: Scelionidae). Biol. Control 149, 104324. doi: 10.1016/j.biocontrol.2020.104324
Cesari M., Maistrello L., Piemontese L., Bonini R., Dioli P., Lee W., et al. (2018). Genetic diversity of the brown marmorated stink bug Halyomorpha halys in the invaded territories of Europe and its patterns of diffusion in Italy. Biol. Invasions 20, 1073–1092. doi: 10.1007/s10530-017-1611-1
Chiappini E., Salerno G., Berzolla A., Iacovone A., Cristina Reguzzi M., Conti E. (2012). Role of volatile semiochemicals in host location by the egg parasitoid Anagrus breviphragma. Entomol Exp. Appl. 144, 311–316. doi: 10.1111/j.1570-7458.2012.01290.x
Colazza S., Fucarino A., Peri E., Salerno G., Conti E., Bin F. (2004). Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 207, 47–53. doi: 10.1242/jeb.00732
Collins M. D., Dixon A. F. G. (1986). The effect of egg depletion on the foraging behaviour of an aphid parasitoid. J. Appl. Entomol. 102, 342–352. doi: 10.1111/j.1439-0418.1986.tb00932.x
Conti E., Colazza S. (2012). Chemical ecology of egg parasitoids associated with true bugs. Psyche (London) 2012, 651015. doi: 10.1155/2012/651015
Daza-Bustamante P., Fuentes-Contreras E., Niemeyer H. M. (2003). Acceptance and suitability of Acyrthosiphon pisum and Sitobion avenae as hosts of the aphid parasitoid Aphidius ervi (Hymenoptera: Braconidae). Eur. J. Entomol. 101, 49–53. doi: 10.14411/eje.2003.010
de Moraes C., Pare P., Tumlinson J. (1998). Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573. doi: 10.1038/31219
Dhillon M. K., Tanwar A. K., Kumar S., Hasan F., Sharma S., Jaba J., et al. (2021). Biological and biochemical diversity in different biotypes of spotted stem borer, Chilo partellus (Swinhoe) in India. Sci. Rep. 11, 5735. doi: 10.1038/s41598-021-85457-2
Dicke M. (1999). Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl. 91, 131–142. doi: 10.1046/j.1570-7458.1999.00475.x
Ellers J., Jervis M. (2003). Body size and the timing of egg production in parasitoid wasps. Oikos 102, 164–172. doi: 10.1034/j.1600-0706.2003.12285.x
Ellers J., Sevenster J. G., Driessen G. (2000). Egg load evolution in parasitoids. Am. Nat. 156, 650–665. doi: 10.1086/316990
Ellers J., Van Alphen J. J. M., Sevenster J. G. (1998). A field study of size-fitness relationships in the parasitoid Asobara tabida. J. Anim. Ecol. 67, 318–324. doi: 10.1046/j.1365-2656.1998.00195.x
Falagiarda M., Carnio V., Chiesa S. G., Pignalosa A., Anfora G., Angeli G., et al. (2023). Factors influencing short-term parasitoid establishment and efficacy for the biological control of Halyomorpha halys with the samurai wasp Trissolcus japonicus. Pest Manage. Sci. 79, 2397-2414. doi: 10.1002/ps.7423
Fan S., Chen C., Zhao Q., Wei J., Zhang H. (2020). Identifying potentially climatic suitability areas for Arma custos (Hemiptera: Pentatomidae) in China under climate change. Insects 11, 1–14. doi: 10.3390/insects11100674
Fatouros N. E., Bukovinszkine-Kiss G., Kalkers L. A., Gamborena R. S., Dicke M., Hilker M. (2005). Oviposition-induced plant cues: Do they arrest Trichogramma wasps during host location? Entomol. Exp. Appl. 115, 207–215. doi: 10.1111/j.1570-7458.2005.00245.x
Fatouros N. E., Dicke M., Mumm R., Meiners T., Hilker M. (2008). Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 19, 677–689. doi: 10.1093/beheco/arn011
Fletcher J. P., Hughes J. P., Harvey I. F. (1994). Life expectancy and egg load affect oviposition decisions of a solitary parasitoid. Proc. R. Soc Lond. B. 258, 163–167. doi: 10.1098/rspb.1994.0157
Gaudreau M., Brodeur J., Abram P. K. (2022). Adult female exposure to mild ultraviolet radiation reduces longevity but not egg load in two parasitoid wasps. Entomol. Exp. Appl. 170, 965–972. doi: 10.1111/eea.13225
Giovannini L., Sabbatini-Peverieri G., Marianelli L., Rondoni G., Conti E., Roversi P. F. (2022). Physiological host range of Trissolcus mitsukurii, a candidate biological control agent of Halyomorpha halys in Europe. J. Pest Sci. 95, 605–618. doi: 10.1007/s10340-021-01415-x
Glaser N., Gallot A., Legeai F., Harry M., Kaiser L., Le Ru B., et al. (2015). Differential expression of the chemosensory transcriptome in two populations of the stemborer Sesamia nonagrioides. Insect Biochem. Mol. Biol. 65, 28–34. doi: 10.1016/j.ibmb.2015.07.008
Greenberg L. O., Huigens M. E., Groot A. T., Cusumano A., Fatouros N. E. (2023). Finding an egg in a haystack: variation in chemical cue use by egg parasitoids of herbivorous insects. Curr. Opin. Insect Sci. 55, 101002. doi: 10.1016/j.cois.2022.101002
Griese E., Dicke M., Hilker M., Fatouros N. E. (2017). Plant response to butterfly eggs: inducibility, severity and success of egg-killing leaf necrosis depends on plant genotype and egg clustering. Sci. Rep. 7, 7316. doi: 10.1038/s41598-017-06704-z
Harvey J. A. (2005). Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol. Exp. Appl. 117, 1–13. doi: 10.1111/j.1570-7458.2005.00348.x
Haye T., Moraglio S. T., Stahl J., Visentin S., Gregorio T., Tavella L. (2020). Fundamental host range of Trissolcus japonicus in Europe. J. Pest. Sci. 93, 171–182. doi: 10.1007/s10340-019-01127-3
Haye T., Moraglio S. T., Tortorici F., Marazzi C., Gariepy T. D., Tavella L. (2023). Does the fundamental host range of Trissolcus japonicus match its realized host range in Europe? J. Pest Sci. doi: 10.1007/s10340-023-01638-0
Henry L. M., Roitberg B. D., Gillespie D. R. (2008). Host-range evolution in Aphidius parasitoids: Fidelity, virulence and fitness trade-offs on an ancestral host. Evol.; Int. J. Org. Evol. 62, 689–699. doi: 10.1111/j.1558-5646.2007.00316.x
Hilker M., Fatouros N. E. (2015). Plant responses to insect egg deposition. Annu. Rev. Entomol. 60, 493–515. doi: 10.1146/annurev-ento-010814-020620
Jervis M. A., Ellers J., Harvey J. A. (2008). Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 53, 361–385. doi: 10.1146/annurev.ento.53.103106.093433
Kriticos D. J., Kean J. M., Phillips C. B., Senay S. D., Acosta H., Haye T. (2017). The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest. Sci. 90, 1033–1043. doi: 10.1007/s10340-017-0869-5
Lara J. R., Pickett C. H., Kamiyama M. T., Figueroa S., Romo M., Cabanas C., et al. (2019). Physiological host range of Trissolcus japonicus in relation to Halyomorpha halys and other pentatomids from California. BioControl 64, 513–528. doi: 10.1007/s10526-019-09950-4
Lee D. H., Short B. D., Joseph S. V., Bergh J. C., Leskey T. C. (2013). Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 42, 627–641. doi: 10.1603/EN13006
Lenth R. emmeans: Estimated Marginal Means, Aka Least-Squares, R Package Version 1.8.2. 2022. Available at: https://CRAN.R-project.org/package=emmeans (Accessed 27 January 2023).
Leskey T. C., Nielsen A. L. (2018). Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 63, 599–618. doi: 10.1146/annurev-ento-020117-043226
Malek R., Kaser J. M., Anfora G., Ciolli M., Khrimian A., Weber D. C., et al. (2021). Trissolcus japonicus foraging behavior: Implications for host preference and classical biological control. Biol. Contr. 161, 104700. doi: 10.1016/j.biocontrol.2021.104700
Manzano C., Fernandez P. C., Hill J. G., Luft Albarracin E., Virla E. G., Aráoz M. V. C. (2022). Chemical ecology of the host searching behavior in an egg parasitoid: Are common chemical cues exploited to locate hosts in taxonomically distant plant species? J. Chem. Ecol. 48, 650–659. doi: 10.1007/s10886-022-01373-3
Michereff M. F. F., Borges M., Aquino M. F. S., Laumann R. A., Gomes A. M., Blassioli-Moraes M. C. (2016). The influence of volatile semiochemicals from stink bug eggs and oviposition-damaged plants on the foraging behaviour of the egg parasitoid Telenomus podisi. Bull. Entomol. Res. 106, 663–671. doi: 10.1017/S0007485316000419
Michereff M. F. F., Laumann R. A., Borges M., Michereff-Filho M., Diniz I. R., Neto A. L. F., et al. (2011). Volatiles mediating a plant-herbivore-natural enemy interaction in resistant and susceptible soybean cultivars. J. Chem. Ecol. 37, 273–285. doi: 10.1007/s10886-011-9917-4
Minkenberg O. P. J. M., Tatar M., Rosenheim J. A. (1992). Egg load as a major source of variability in insect foraging and oviposition behavior. Oikos 65, 134–114. doi: 10.2307/3544896
Moyal P., Tokro P., Bayram A., Savopoulou-Soultani M., Conti E., Eizaguirre M., et al. (2011). Origin and taxonomic status of the Palearctic population of the stem borer Sesamia nonagrioides (Lefèbvre) (Lepidoptera: Noctuidae). Biol. J. Linn. Soc 103, 904–922. doi: 10.1111/j.1095-8312.2011.01666.x
Olson D. M., Andow D. A. (1998). Larval crowding and adult nutrition effects on longevity and fecundity of female Trichogramma nubilale Ertle & Davis (Hymenoptera: Trichogrammatidae). Environ. Entomol. 27, 508–514. doi: 10.1093/ee/27.2.508
Pak G., Van Halder I., Lindeboom R., Stroet J. G. (1985). Ovarian egg supply, female age and plant spacing as factors influencing searching activity in the egg parasite Trichogramma sp. Mededelingen van Faculteit landbouwwetenschappen. Rijksuniversiteit Gent 50, 369–378.
Panizzi A. R. (2013). History and contemporary perspectives of the integrated pest management of soybean in Brazil. Neotrop. Entomol. 42, 119–127. doi: 10.1007/s13744-013-0111-y
Peiffer M., Felton G. W. (2014). Insights into the saliva of the brown marmorated stink bug Halyomorpha halys (Hemiptera: Pentatomidae). PloS One 9, e88483. doi: 10.1371/journal.pone.0088483
Peterson H. M., Ray S., Ali J. G., Krawczyk G. (2022). Feeding and oviposition by the brown marmorated stink bug, Halyomorpha halys (Stål) induce direct and systemic changes in volatile compound emissions from potted peach and tree of heaven. Arth. Plant Interact. 16, 227–247. doi: 10.1007/s11829-022-09893-1
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing 2023). Available at: https://www.R-project.Org.
Robertson B. A., Rehage J. S., Sih A. (2013). Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 28, 552–560. doi: 10.1016/j.tree.2013.04.004
Roitberg B. D., Boivin G., Vet L. E. M. (2001). Fitness, parasitoids, and biological control: An opinion. Can. Entomol. 133, 429–438. doi: 10.4039/Ent133429-3
Rondoni G., Bertoldi V., Malek R., Djelouah K., Moretti C., Buonaurio R., et al. (2018). Vicia faba plants respond to oviposition by invasive Halyomorpha halys activating direct defences against offspring. J. Pest Sci. 91, 671–679. doi: 10.1007/s10340-018-0955-3
Rondoni G., Chierici E., Giovannini L., Sabbatini-Peverieri G., Roversi P. F., Conti E. (2022). Olfactory responses of Trissolcus mitsukurii to plants attacked by target and non-target stink bugs suggest low risk for biological control. Sci. Rep. 12, 1880. doi: 10.1038/s41598-022-05873-w
Rondoni G., Ielo F., Ricci C., Conti E. (2017). Behavioural and physiological responses to prey-related cues reflect higher competitiveness of invasive vs. native ladybirds. Sci. Rep. 7, 3716. doi: 10.1038/s41598-017-03471-9
Sabbatini-Peverieri G., Boncompagni L., Mazza G., Paoli F., Dapporto L., Giovannini L., et al. (2021). Combining physiological host range, behavior and host characteristics for predictive risk analysis of Trissolcus japonicus. J. Pest Sci. 94, 1003–1016. doi: 10.1007/s10340-020-01311-w
Scala M., Fouani J. M., Zapponi L., Mazzoni V., Wells K. E., Biondi A., et al. (2022). Attraction of egg parasitoids Trissolcus mitsukurii and Trissolcus japonicus to the chemical cues of Halyomorpha halys and Nezara viridula. Insects 13, 439. doi: 10.3390/insects13050439
Steidle J. L. M., van Loon J. J. A. (2002). “Chemoecology of parasitoid and predator oviposition behaviour,” in Chemoecology of insect eggs and egg deposition. Eds. Hilker M., Meiners T. (Berlin: Blackwell), 291–317.
Valentin R. E., Nielsen A. L., Wiman N. G., Lee D. H., Fonseca D. M. (2017). Global invasion network of the brown marmorated stink bug, Halyomorpha halys. Sci. Rep. 7, 9866. doi: 10.1038/s41598-017-10315-z
van Lenteren J. C., Bigler F. (2009). “Quality Control of Mass Reared Egg Parasitoids,” in Egg parasitoids in agroecosystems with emphasis on Trichogramma. Eds. Consoli F., Parra J., Zucchi R. (Dordrecht: Springer Netherlands), 315–340. doi: 10.1007/978-1-4020-9110-0_12
Venables W. N., Ripley B. D. (2002). Modern applied statistics with S. 4th ed (New York, NY: Springer).
Vet L. E. M., Dicke M. (1992). Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37, 141–172. doi: 10.1146/annurev.en.37.010192.001041
Visser M. E. (1994). The importance of being large: the relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J. Anim. Ecol. 63, 963–978. doi: 10.2307/5273
Wang X. G., Messing R. H. (2004). Fitness consequences of body-size-dependent host species selection in a generalist ectoparasitoid. Behav. Ecol. Sociobiol. 56, 513–522. doi: 10.1007/s00265-004-0829-y
Wäschke N., Meiners T., Rostás M. (2013). “Foraging strategies of parasitoids in complex chemical environments,” in Chemical ecology of insect parasitoids. Eds. Wajnberg E., Colazza S. (Chichester, UK: John Wiley & Sons, Ltd). doi: 10.1002/9781118409589.ch3
West-Eberhard M. J. (1989). Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278. doi: 10.1146/annurev.es.20.110189.001341
Whitman D. W., Agrawal A. A. (2009). “What is phenotypic plasticity and why is it important?,” in Phenotypic plasticity of insects: Mechanisms and consequences. Eds. Whitman D. W., Ananthakrishnan T. N. (Enfield, USA: Science Publishers, Inc), 1–63. doi: 10.1201/b10201-2
Wu S., Zeng W., Deng W., Li J., Li M., Tan L., et al. (2022). Parental sex and not kinship determines egg cannibalism in Arma custos Fallou (Hemiptera: Pentatomidae: Asopinae). Front. Ecol. Evol. 10, 758587. doi: 10.3389/fevo.2022.758587
Yan J., Vétek G., Pal C., Zhang J., Gmati R., Fan Q. H., et al. (2021). ddRAD sequencing: an emerging technology added to the biosecurity toolbox for tracing the origin of brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). BMC Genomics 22, 355. doi: 10.1186/s12864-021-07678-z
Yang Z. Q., Yao Y. X., Qiu L. F., Li Z. X. (2009). A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann. Entomol. Soc Am. 102, 39–47. doi: 10.1603/008.102.0104
Zapponi L., Tortorici F., Anfora G., Bardella S., Bariselli M., Benvenuto L., et al. (2021). Assessing the distribution of exotic egg parasitoids of Halyomorpha halys in europe with a large-scale monitoring program. Insects 12, 316. doi: 10.3390/insects12040316
Zhang J., Zhang F., Gariepy T., Mason P., Gillespie D., Talamas E., et al. (2017). Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 90, 1127–1141. doi: 10.1007/s10340-017-0863-y
Keywords: alternative host, Arma custos, biocontrol, Dolycoris baccarum, egg load, Halyomorpha halys, invasive species, Trissolcus japonicus
Citation: Chierici E, Sabbatini-Peverieri G, Roversi PF, Rondoni G and Conti E (2023) Phenotypic plasticity in an egg parasitoid affects olfactory response to odors from the plant–host complex. Front. Ecol. Evol. 11:1233655. doi: 10.3389/fevo.2023.1233655
Received: 02 June 2023; Accepted: 24 July 2023;
Published: 16 August 2023.
Edited by:
Joachim Ruther, University of Regensburg, GermanyReviewed by:
Benjamin Fürstenau, Julius Kühn-Institut, GermanyCopyright © 2023 Chierici, Sabbatini-Peverieri, Roversi, Rondoni and Conti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriele Rondoni, Z2FicmllbGUucm9uZG9uaUB1bmlwZy5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.