
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 20 July 2023
Sec. Biogeography and Macroecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1216567
This article is part of the Research Topic The Ecology, Diversity and Migration Pattern of Aquatic Organisms in a Changing Climate View all 5 articles
Introduction: Taste and odor (T&O) problems have been affecting drinking water safety. As a eutrophicated drinking water reservoir in Tianjin city, the Yuqiao Reservoir was threatened by 2-MIB and geosmin in recent years.
Methods: In this study, quantile regression analysis and metagenome were used to quickly and accurately screen the producers and drivers of 2-MIB and geosmin in this reservoir.
Results: The mean concentrations of 2-MIB and geosmin in the four-year were 103.58 ± 128.13 ng/L and 14.29 ± 27.95 ng/L, respectively. 2-MIB concentrations were higher in summer and autumn, with a bimodal variation throughout the year. Geosmin concentrations showed a decreasing trend from year to year from 2018 to 2021. Metagenome revealed that Pseudanabaena sp. dqh15, Microcoleus pseudautumnalis Ak1609, Pseudanabaena limnetica, and Planktothricoides raciborskii were the 2-MIB-producers, while Streptosporangium caverna and Dolichospermum circinale were the geosmin-producers. Multivariate quantile regression analysis indicated Pseudanabaena sp. and CODMn were the best predictors of 2-MIB concentrations, temperature and CODMn were the most useful parameters for describing geosmin concentration change. 2-MIB concentrations increased with the increase of Pseudanabaena sp. cell density and CODMn. Geosmin concentrations were higher at harsh temperatures and increased with higher CODMn. CODMn was significantly and positively correlated with the biosynthesis of secondary metabolites synthesis and terpenoid backone biosynthesis pathway. Both quantile regression and metagenome results showed that CODMn was an important driver of odor compounds.
Discussion: Metagenome achieved higher resolution of taxonomic annotation than amplicons to identify odor-producers, which helps us to understand the main taxa of odor-producing microorganisms in Chinese water bodies and the genetic basis of odor compounds in microorganisms. Understanding the sources and drivers of odor compounds was useful for improving taste and odor problem management. This is the first time that the main odor-producing microorganisms in water bodies have been resolved by microbial metagenomic functional gene prediction.
Since the 1950s, China has built 87,000 reservoirs and dams to address water shortage and water security, making them an increasingly important source of drinking water (Liu and Yang, 2012; Wang Y. et al., 2021). However, the frequent occurrence of taste and odor (T&O) events in reservoirs recently has posed a huge challenge to tap water treatment plants, while threatening the safety of drinking water resources (Xu et al., 2022). Odor compounds are often misunderstood as an aesthetic issue, but they are also a major indicator of public acceptance of drinking water (Wu et al., 2021b). Due to their low odor thresholds (both < 10 ng/L), 2-methylisocampheol (2-MIB) and trans-1,10-dimethyl-trans-9-decalol (geosmin) are the most common and typical contaminants that affect water quality in reservoirs (Devi et al., 2021). In China, 2-MIB and geosmin are listed as the main control compounds to ensure the safety of drinking water sources (Rong et al., 2018). Given the chemical stability and small molecular weight of 2-MIB and geosmin, it is difficult to remove them through conventional water treatment processes (coagulation, flocculation, filtration, aeration, and disinfection/oxidation by chlorine), and chemical methods of removing odor substances can produce hazardous oxidation by-products (Sagehashi et al., 2005; Xu et al., 2022). Once encountering T&O episodes in drinking water, it is difficult for water treatment to take applicable control measures, resulting in enormous financial damages. It is necessary to identify and monitor the producers of odor substances in advance to ensure the safety of drinking water and reduce economic losses.
As to the producer of 2-MIB and geosmin, there are mainly producers such as cyanobacteria, fungi, myxobacteria and actinomycetes have been reported (Juttner and Watson, 2007), and cyanobacteria was proven as the main source of 2-MIB and geosmin in the aquatic environment (Lee E.S. et al., 2020). Among more than 100 articles published since 1990 on cyanobacteria associated geosmin/2-MIB in drinking water systems, mainly distributed in North America, Australia, Europe, China, Japan, South Korea, Philippines and South Africa (Su et al., 2015; Devi et al., 2021). Cyanobacterial species that have been associated with 2-MIB- or geosmin-related off-flavors include Anabaena, Aphanizomenon, Lyngbya, Oscillatoria, Phormidium, Planktothrix, and Pseudanabaena (Peterson et al., 1995; Sugiura et al., 1997; Zimba et al., 1999; Su et al., 2015; Zhang et al., 2016; Cai et al., 2017; Rong et al., 2018). A total of 132 cyanobacterial strains from 21 genera and 72 cyanobacterial strains from 13 genera produced geosmin and 2-MIB, respectively. The fungi, myxobacteria and actinomycetes that produce geosmin and 2-MIB have been less studied than cyanobacteria (Devi et al., 2021). The higher incidence of geosmin events in the United States, Canada and Australia, and the higher incidence of 2-MIB events in China and Korea suggest that differences in geographical distribution influence the occurrence and frequency of T&O events (Devi et al., 2021). The Yuqiao Reservoir has experienced eutrophication during the last decade, accompanied by the frequent occurrence of bloom events (Huo et al., 2018). The Yuqiao reservoir has been reported to have earthy-musty taste and odor (T&O) issues with a relatively high amount of 2-MIB. Due to the diversity of these microorganisms, it is still necessary to identify the main producer of 2-MIB and geosmin in the specific waterbody.
On the other hand, not all these microorganisms can be cultured in the laboratory, so it becomes critical to use culture-independent molecular tools to track the odor-producers (Cristina Casero et al., 2019). The biosynthesis pathways of 2-MIB and geosmin were elucidated in actinobacteria and cyanobacteria, providing a molecular basis for traceability analysis of odor compounds (Hamano et al., 2002; Cane and Watt, 2003; Ludwig et al., 2007; Komatsu et al., 2008; Wang et al., 2011). In the synthesis of 2-MIB, the generic precursor of monoterpenoids, GPP (geranyl pyrophosphate), is methylated to methyl-GPP, and then 2-MIB synthase cyclizes methyl-GPP to 2-MIB (Oldfield and Lin, 2012; Brock et al., 2013). Geosmin is generated from farnesyl diphosphate (FPP) synthesized by the bifunctional single sesquiterpene cyclase (geosmin synthase) in the presence of Mg2+ (Jiang et al., 2006; Jiang et al., 2007). The geosmin synthase gene and the 2-MIB synthase gene are indicators to investigate the producer of geosmin and 2-MIB, which provided a useful tool to screen the producer of geosmin and 2-MIB. Moreover, high-throughput sequencing is a lower-cost and more efficient sequence determination technology compared to traditional Sanger sequencing, and has played an increasingly important role in the life sciences since its introduction in 2005 (Margulies et al., 2006). Applying high-throughput sequencing to odor compounds biosynthetic genes provides a time-saving, high-resolution tool for the diversity of odor-producing microorganisms in freshwater bodies worldwide. High-throughput sequencing, including amplicon sequencing and metagenomic sequencing, could rapidly detect unculturable low-abundance microorganisms in the environment (Liu et al., 2021). Amplicon sequencing is widely used in the study of microbial diversity, especially for toxic or odor-producing cyanobacteria (Cristina Casero et al., 2019; Qiu et al., 2021). However, the identification of species by amplicon sequencing can only reach a genus-level resolution, and the selection of primers and the determination of the number of PCR cycles during sequencing have a large impact on the sequencing results. Currently, the developed geosmin/2-MIB primers have focused on cyanobacteria, thus quantifying the species composition of geosmin/2-MIB-producing cyanobacteria (Devi et al., 2021). These primers could rapidly identify a lot of geosmin/2-MIB-producing cyanobacteria, such as Anabaena sp. Planktothrix sp., Phormidium sp., Oscillatoria sp., Pseudoanabaena sp (Giglio et al., 2008; Giglio et al., 2011; Tsao et al., 2014). This neglected the contribution of actinomycetes and bacteria to geosmin/2-MIB in the environment. In previous studies, the correlation between the qPCR of geosmin/2-MIB synthase gene and geosmin/2-MIB content was not correlated, low correlated, and highly correlated (Su et al., 2013; Tsao et al., 2014; Chiu et al., 2016; Wang et al., 2016; Huang et al., 2018). When the correlation between gene copy numbers and geosmin/2-MIB concentration is low, it indicates that cyanobacteria contribute only a small fraction of geosmin/2-MIB in water. The presence of odor-producing actinomycetes and bacteria in the water is one of the important factors affecting the correlation value. Metagenomic sequencing is performed by randomly interrupting microbial genomes and assembling small fragments into longer sequences (Liu et al., 2021). Metagenomic sequencing has no primer biases. Metagenomic sequencing can provide not only a more accurate species composition (species or strain level) compared to amplicon sequencing, but also functional gene information (Smits et al., 2017). Nevertheless, the application of metagenomic sequencing technology in odor-producer identification and monitoring is still rare, and more research work is needed.
Besides the above, the synthesis and release of odor compounds were influenced by environmental factors. Temperature and light intensity were the major factors that affect the production of 2-MIB and geosmin (Shen et al., 2022). Unfavorable conditions, such as strong light, low and high temperatures, could increase the potential for geosmin/2-MIB synthesis in cyanobacterial cells (Zimba et al., 1999; Wang et al., 2011; Zhang et al., 2016; Cai et al., 2017). Moreover, nitrogen and phosphorus could affect the production of geosmin and 2-MIB. TN : TP < 29 : 1 favored the generation and accumulation of 2-MIB concentration in the Yangcheng Lake (Wu et al., 2021a). Ammonium was essential to promote the production of 2-MIB and geosmin compounds (Perkins et al., 2019). The microbial communities in waters were diverse, and the production of odor compounds might change under the interaction between different microbial species. Microcystis aeruginosa could obviously promote 2-MIB production of Pseudanabaena sp. (Zhang et al., 2020). Identifying key influencing environmental factors could reduce the risk of T&O outbreaks (Wang et al., 2011; Cai et al., 2017; Oh et al., 2017; Alghanmi et al., 2018) and be useful for adaptive environmental management. Further work is still needed to identify the environmental drivers for geosmin and 2-MIB.
In this study, a long-term field investigation was conducted from 2018 to 2021 in the Yuqiao Reservoir, Tianjin. Macrogenomic approaches were used to investigate the main producers of 2-MIB and geosmin and their related functional genes, while quantile regression was used to find the drivers of 2-MIB and geosmin. The results would be helpful to get insight into the problems of T&O and support drinking water resource management.
The Yuqiao Reservoir (40° 02’ N, 117° 25’ E) is an important source of drinking water for Tianjin and a large storage reservoir for the Luanhe River-Tianjin water diversion project, adjacent to Hebei Province and Beijing. The Yuqiao Reservoir is mainly formed by the confluence of three major tributaries, namely the Shahe River, the Luanhe River, and the Lihe River, with a total storage capacity of 15.59 × 108 m3 and a utilizable storage capacity of 3.85 × 108 m3 and an average water depth of 4.5 m. Samples (0.5 m below the surface) from 1# to 3# were collected in 2018. From 2019, four additional sites (4# to 7#) have been added. The Yuqiao Reservoir and the sampling stations are shown in Figure 1.
During sampling, oxidation-reduction potential (ORP), water temperature (Temp), dissolved oxygen (DO), and pH were measured in situ with a multi-parameter probe (YSI-6600, USA). Transparency (SD) was measured with a Secchi disk (diameter: 20 cm, black and white). Water samples were collected at 0.5 m below the surface water monthly for biological, physical, and chemical analysis from 2018 to 2021. 1000 ml of Subsample was preserved with 1% Lugol’s iodine solution (Hawkins et al., 2005). After 48 hours of sedimentation, the residues were condensed to 50 ml for quantitative phytoplankton analysis (Hu and Wei, 2006). Phytoplankton species were identified and counted by taking 100 ml of the concentrate and placing it in a phytoplankton counting frame under a 400 × magnification optical microscope according to the method in Hu and Wei (2006).
Subsamples for nutrient analysis were kept in ice boxes until transferred to the laboratory’s 4 °C refrigerator. Concentrations of nitrate nitrogen (NO3-N), total nitrogen (TN), total phosphorus (TP), ammonium (NH4-N) and phosphate (PO4-P) were measured according to the general laboratory methods (Clescerl, 1998). The permanganate index (CODMn) was determined by the acidic potassium permanganate method by taking 100 ml of unfiltered subsamples. Chlorophyll a was determined by UV spectrophotometer after 90% acetone extraction (Xu et al., 2010).
Geosmin and 2-MIB concentrations were extracted and absorbed from the water samples by headspace solid-phase microextraction (HSPME) using 65 mm PDMS/DVB fiber (57310-U, Supelco, USA). Then, the extracted fiber was used for gas chromatography-mass spectrometry (GC-MS) analysis (HP 6890 GC-5973 MSD; Agilent Technologies, USA) to quantify geosmin and 2-MIB concentrations (Watson et al., 2000; Li et al., 2007). Water samples from four typical sites were selected for metagenomic sequencing, namely the outlet (1#), the inlet (3#), the center (2#) and the high human activity area (4#) of the reservoir. Water samples from these sites were extracted for DNA. Microbial biomass in the water samples was collected onto 0.22-μm membrane filters (Millipore) and stored in a refrigerator at −80 °C. Total genomic DNA was extracted from the membrane filters by the cetyltrimethylammonium bromide (CTAB) method as described by Xu et al. (2010). The quality and concentration of DNA were determined by measuring the 260 nm and 280 nm absorbance using a spectrophotometer.
The 2 µg DNA samples were mechanically interrupted by ultrasound, and then 300 bp DNA fragments were selected for library construction using NEBNext® UltraTM DNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s instructions. High-throughput sequencing of microbial metagenomic sample libraries was performed using Illumina HiSeq2500. Fastp (v 0.23.1) software was used to filter the raw sequencing data according to its default parameters to obtain high-quality sequencing data (Chen et al., 2018). The filtered high-quality data was used to perform metagenomic assembly using MEGAHIT software in default mode and filtered for contig sequences shorter than 300 bp (Li et al., 2015). The metagenomic assembly results were evaluated using QUAST software (Gurevich et al., 2013). The MetaGeneMark software (v 2.10) was used for gene prediction (Fu et al., 2012). Redundancy removal was performed using the MMseqs2 software with a 95% similarity threshold and a 90% coverage threshold (Mirdita et al., 2019). Non-redundant contigs were compared with the KEGG (Kyoto Encyclopedia of Genes and Genomes) database using BLAST. A contig was identified as the sequence of the functional gene if the BLAST hit (BLASTn, e-value cut-off: 10−5) had a sequence identity of > 90% (Kristiansson et al., 2011). Non-redundant contigs were compared to the Nr (Non-Redundant protein) database and annotated as characteristic sequences of the species based on their best BLASTn hits (nucleotide sequence identity > 90%) with a threshold e-value of 10−5. To calculate the relative abundance of KEGG functional genes and species abundance in metagenomic samples, the raw reads were mapped back to non-redundant contigs using bbmap (v 37.81) and then homogenized using the R package vegan (v 2.5-7) to eliminate the effect of total readings when comparing abundance between samples (Wang C. et al., 2021). Based on the gene functional composition matrix and species composition matrix obtained from Beta diversity analysis, samples were hierarchically clustered by the Unweighted pair-group method with arithmetic mean (UPGMA) through R language tools to determine the similarity of gene functional composition and species composition among samples (Giangreco et al., 2010). The closer the samples were, the shorter the branch lengths were, indicating that the functional composition or species composition was more similar between samples. Spearman correlations between environmental factors and functional genes and species, respectively, were analyzed using the R package psych (v 2.2.5), and correlation heat maps were drawn using the R package pheatmap (v 1.0.12). The nucleotide sequences were deposited in SRA database under accession numbers PRJNA895910.
Before performing quantile regression analysis, all independent variables need to be unit normalized (X = X − Xmin/Xmax − Xmin) due to the wide range of values of the original data. A variance inflation factor (VIF) greater than 10 is generally considered to represent a serious covariance problem in the model (Fornaroli et al., 2016). To reduce multicollinearity, independent variable screening is required, and VIF (VIF < 10) is used to stepwise select variables. Quantile regression was used to study the linear relationship among 2-MIB and geosmin concentrations and environmental variables at 5 quantiles (0.05th, 0.1th, 0.25th, 0.50th, 0.75th, 0.90th, and 0.95th). Quantile regression models can estimate the upper or lower boundaries of 2-MIB and geosmin concentrations to measure limiting factors. The relationships between univariate environmental variables and 2-MIB or geosmin concentrations were fitted as linear, exponential, logarithmic, and quadratic curves. The statistical analyses used the quantreg package in R Project software (Lipsitz et al., 2016). Due to the small sample size (n = 122), we converted Akaike Information Criterion (AIC) to the corrected Akaike Information Criterion (AICc) for every studied quantile. AICc differences (Δi = AICci − minimum AICc) were used to choose the best-fitting model and to calculate a set of Akaike weights (wi) (Johnson and Omland, 2004). We determined the best model for the quantile under study by averaging the wi of each model from all five quantile model selection analyses. In general, variables from univariate models greater than 10% wi were selected for subsequent analyses (Allen and Vaughn, 2010). We then used the variables and shapes from the best-fit univariate model to fit the multivariate quantile regression model.
A total of 103 genera of cyanophyta (20 genera), bacilariophyta (21 genera), chlorophyta (46 genera), cryptophyta (2 genera), chrysophyta (4 genera), pyrrophyta (4 genera), euglenophyta (4 genera), and xanthophyta (2 genera) were identified from 2018 to 2021 (Figure 2A). Cyanophyta (94.62%), bacilariophyta (2.84%) and chlorophyta (1.74%) were the main taxa, whereas the other algal abundances together accounted for only 0.80%. The dominant genera were Pseudanabaena, Cylinderspermopsis, Aphanizonmenon, Microcystis, Panktothrix, Leptolyngbya, Raphidiopsis, Synedra, Merimopedia, and Limnothrix, which represented 43.77, 16.48, 9.67, 6.24,5.49, 4.90, 1.90, 1.63 and 1.50% of the phytoplankton community.
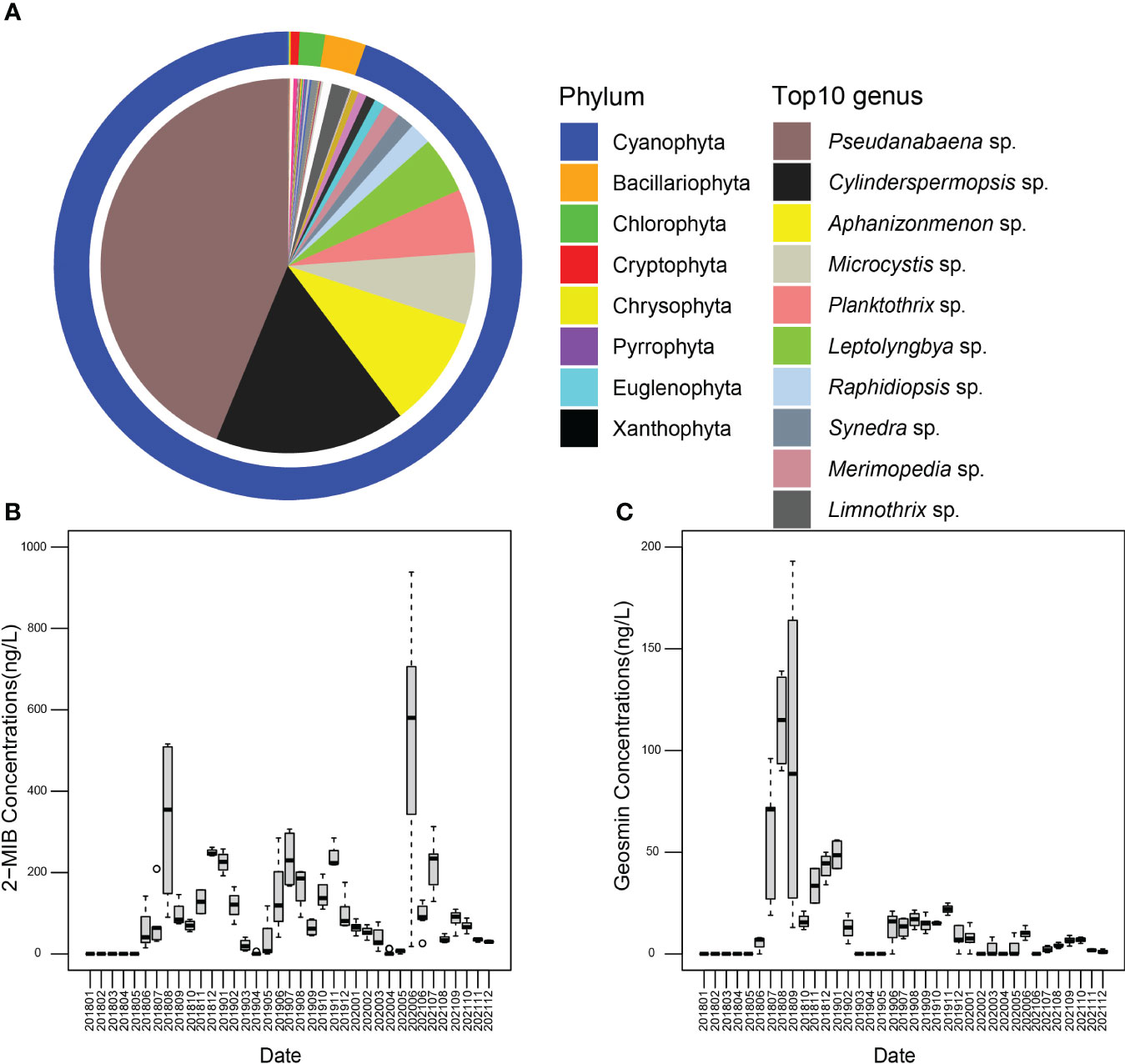
Figure 2 (A) The variations of the phytoplankton community in the Yuqiao Reservoir. (B) Concentrations of 2-MIB from the Yuqiao Reservoir during 2018–2021. (C) Concentrations of geosmin from the Yuqiao Reservoir during 2018–2021.
The variation characteristics of 2-MIB and geosmin concentrations were shown in Figures 2B, C. The concentrations of 2-MIB ranged from 0 to 938.30 ng/L, with an annual average of 103.58 ± 128.13 ng/L. The annual variation of 2-MIB concentrations showed a bimodal pattern. It is noteworthy that 2-MIB concentrations were below the odor threshold in March and April during the survey period. Geosmin concentrations ranged from 0 to 193 ng/L with an average of 14.29 ± 27.95 ng/L. From 2018 to 2021, the concentration of geosmin decreases year by year. Geosmin concentrations reached the maximum in August 2018 and November 2019. Geosmin concentration for 2020 to 2021 was very low with an average of 3.35 ± 3.77 ng/L.
Functional genes of terpenoid backone biosynthesis and biosynthesis of secondary metabolites pathway directly related to 2-MIB and geosmin synthesis at each point have high expression levels in June and July 2021 (Figure 3A). The expression levels of functional genes in these two pathways were lower in November 2021.
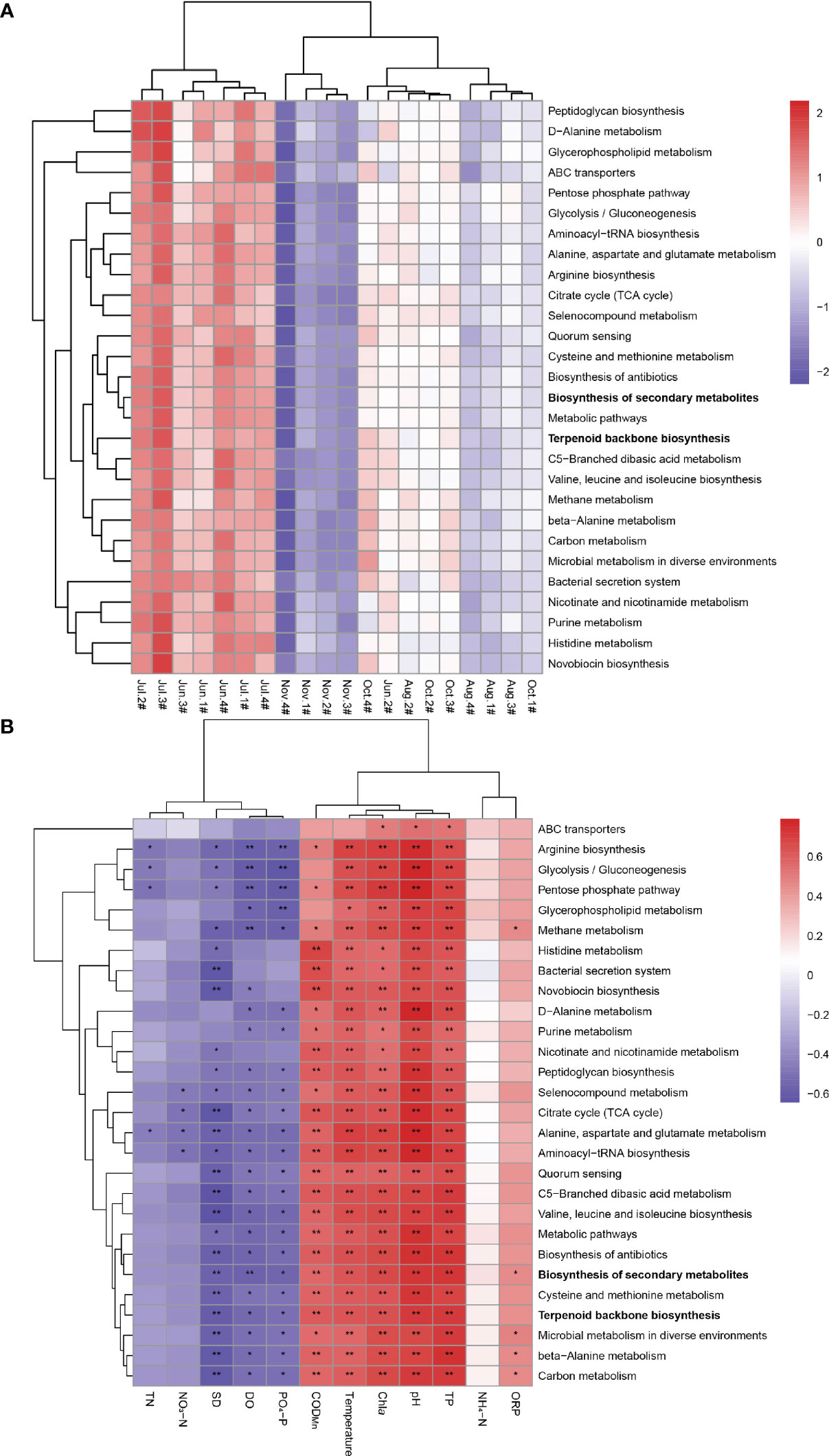
Figure 3 (A) Correlation heatmap for the relationship between environmental samples and functional genes. (B) Correlation heatmap for the relationship between environmental factors and functional genes. * p < 0.05; ** p < 0.01.
Powerful macrogenomic analysis of 2-MIB and geosmin biosynthesis genes was used to explore to identify potential odor-producers and their dynamics in the Yuqiao Reservoir. Based on the four distance matrices obtained from Beta diversity analysis, the samples were hierarchically clustered using UPGMA through R language tools to determine the similarity of species composition among samples (Figure 4A). The results showed that the species composition of samples from the same month clustered together, and the species composition of samples from the same month was more similar. As shown in Figure 4A, Pseudanabaena and Acidimicrobium were the predominant bacterial genera. The relative abundance of Pseudanabaena sp. gradually increased with the increase of months, and the maximum relative abundance of Pseudanabaena sp. was 17.93% in November. The relative abundance of Acidimicrobium sp. ranged from 1.62% to 4.65%, with the maximum value in July and the minimum value in November.
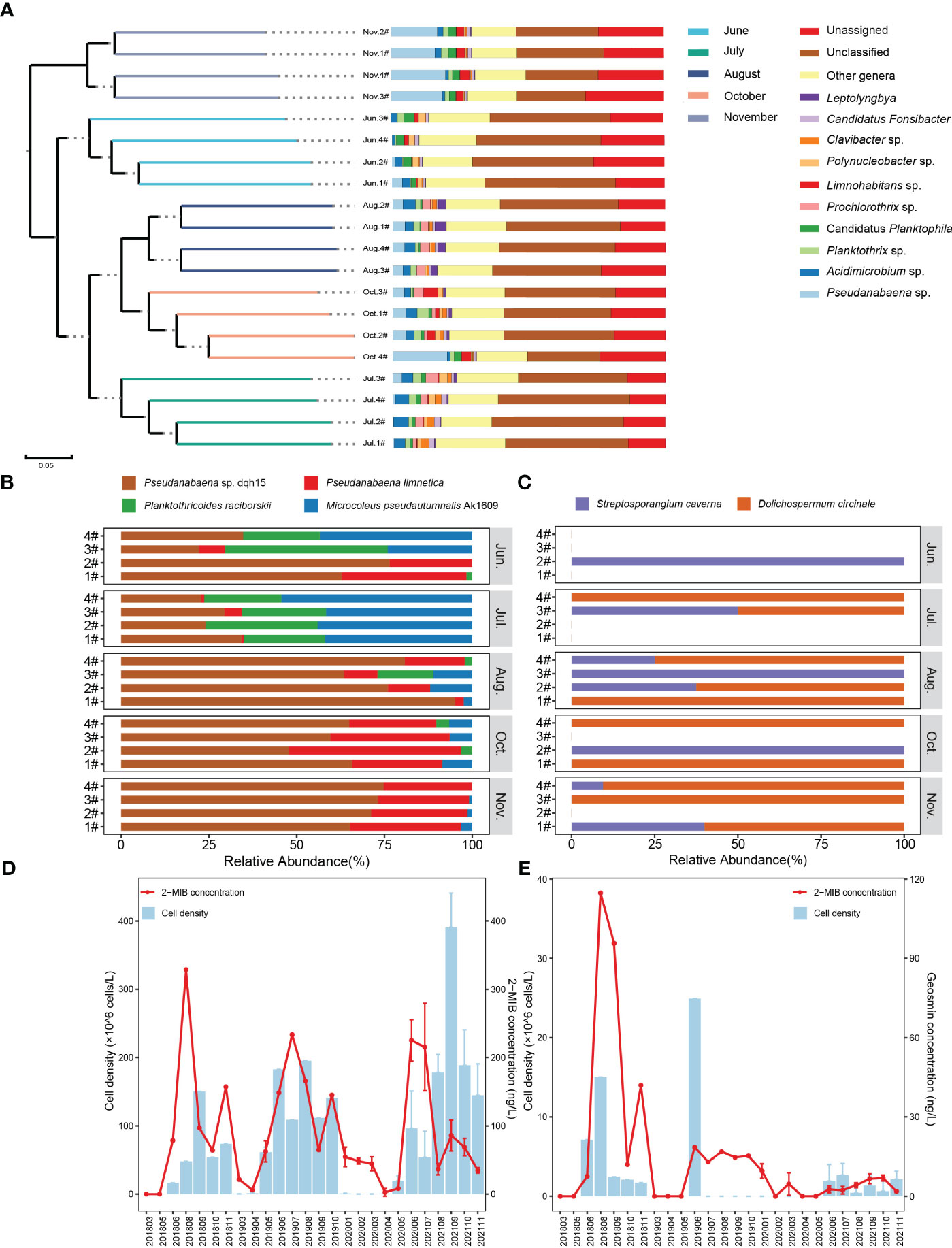
Figure 4 (A) Cluster analysis and microorganism distribution of environmental samples. (B) The variation of 2-MIB-producer by metagenomic sequencing. (C) The variation of geosmin-producer by metagenomic sequencing. (D) Variation trend of odor compounds concentrations and the 2-MIB-producer cell density. (E) Variation trend of odor compounds concentrations and the geosmin-producer cell density.
Geosmin-producers were Streptosporangium caverna and Dolichospermum circinale based on the analysis of geosmin synthase genes (Figure 4B). Streptomyces was found only at 2# in June. No geosmin-producer was found in 1# in July, 2# in July, 3# in October, and 4# in November. The percentages of Dolichospermum circinale were 0%, 75%, 59%, 67%, and 83% from June to November excluding September. The content of geosmin in the reservoir was detected by gas chromatography-mass spectrometry (GC-MS), and geosmin in June was below the detection limit. Geosmin concentrations in the remaining four months were in the following order: October (7.41 ± 0.85 ng/L) > August (3.96 ± 1.26 ng/L) > July (2.47 ± 1.22 ng/L) > November (1.72 ± 0.39 ng/L).
According to the 2-MIB synthesis pathway analysis, 2-MIB-producers were Pseudanabaena sp. dqh15, Microcoleus pseudautumnalis Ak1609, Pseudanabaena limnetica, and Planktothricoides raciborskii (Figure 4C). The relative abundance of Microcoleus pseudautumnalis Ak1609 and Planktothricoides raciborskii tended to increase from June to July and decrease from August to November. Pseudanabaena sp. is the main producer of 2-MIB, with Pseudanabaena sp. dqh15 highly dominating. 2-MIB concentrations in the five months followed the order: July (208.59 ± 87.61 ng/L) > June (77.01 ± 36.61 ng/L) > October (72.43 ± 16.42 ng/L) > August (34.86 ± 10.22 ng/L) > November (33.50 ± 5.10 ng/L). Temperature (R2 = 0.60), transparency (R2 = 0.61), total phosphorus (R2 = 0.43), total nitrogen (R2 = 0.38), ammonia nitrogen (R2 = 0.52), dissolved oxygen (R2 = 0.40), oxidation-reduction potential (R2 = 0.33) and permanganate index (R2 = 0.35) were the significantly key environmental factors affecting odor-producing phytoplankton communities (p < 0.05) (Figure 5). Comparison between the odor-producer cell density and odor compounds concentrations was shown in Figures 4D, E. The variation trends of 2-MIB-producers cell density by microscope and 2-MIB concentration were roughly the same, but the variation trends of geosmin-producers cell density by microscope and geosmin concentration are different.
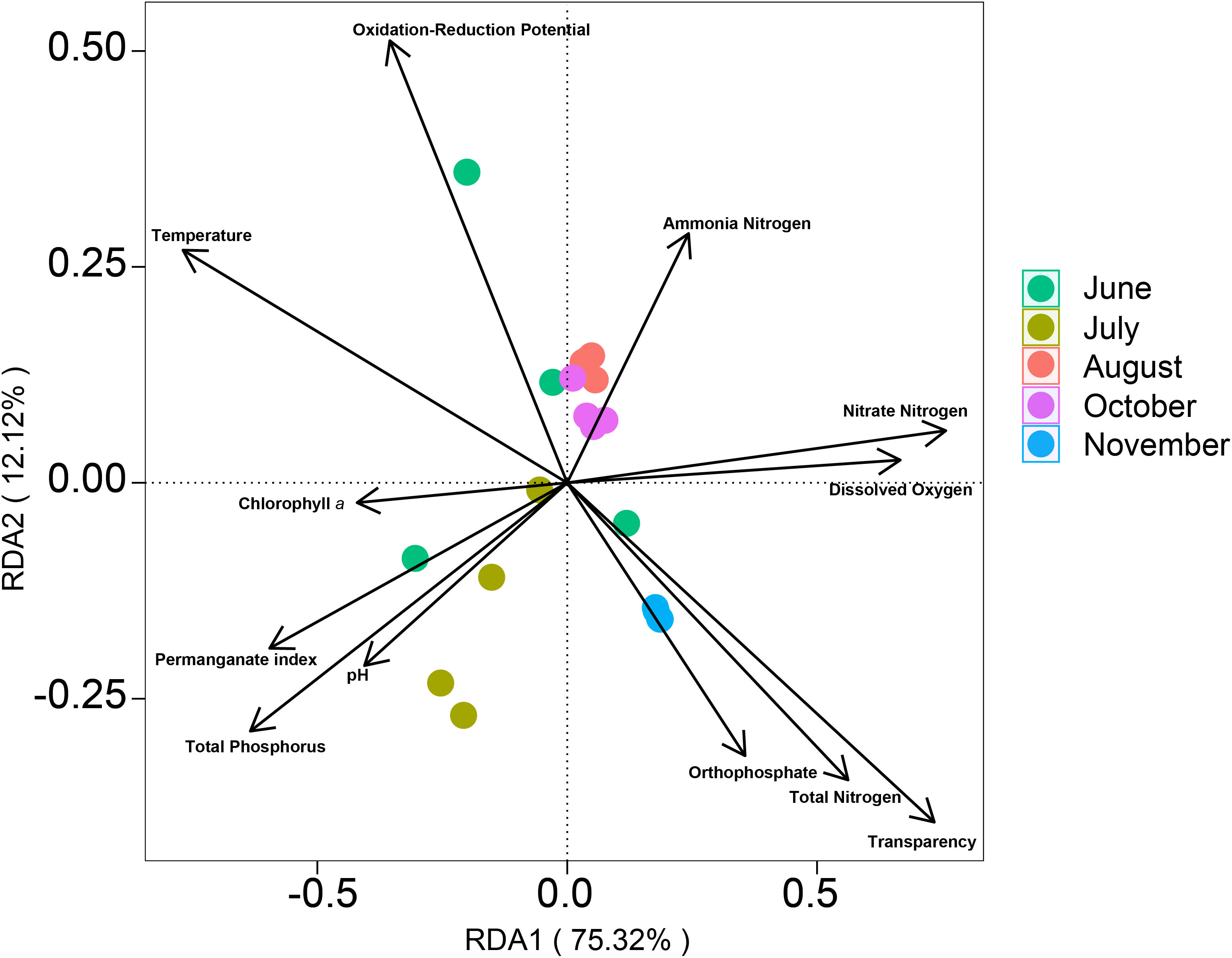
Figure 5 Diagram of RDA analysis between odor-producing phytoplankton communities and environmental factors.
The statistical description of the total 23 environmental variables in the model was shown in Table 1. To reduce multicollinearity among the variables, 21 environmental variables (VIF < 10) were retained, and 2 variables (total algal cell density and cyanobacterial cell density) were removed. These 21 environmental variables were analyzed with 2-MIB or geosmin concentrations separately, and then ranked by Akaike weights.
To better describe 2-MIB and geosmin concentrations, we selected the models with average Akaike weights greater than 10% of the highest one for multiple quantile regression analysis (Table 2). For 2-MIB concentration, the selected univariate model had a quadratic relationship with permanganate index (wi = 0.30), followed by a linear relationship with chlorophyll a (wi = 0.23), a logarithmic relationship with Aphanizomenon cell density (wi = 0.18), a quadratic relationship with Oscillatoria cell density (wi = 0.14), a logarithmic relationship with Pseudanabaena cell density (wi = 0.06) and a logarithmic relationship with temperature (wi = 0.05), respectively. For geosmin concentrations, the selected univariate model was a quadratic relationship with Oscillatoria cell density (wi = 0.44), permanganate index (wi = 0.34), and temperature (wi = 0.11), and a logarithmic relationship with Cylindrospermopsis cell density (wi = 0.10).
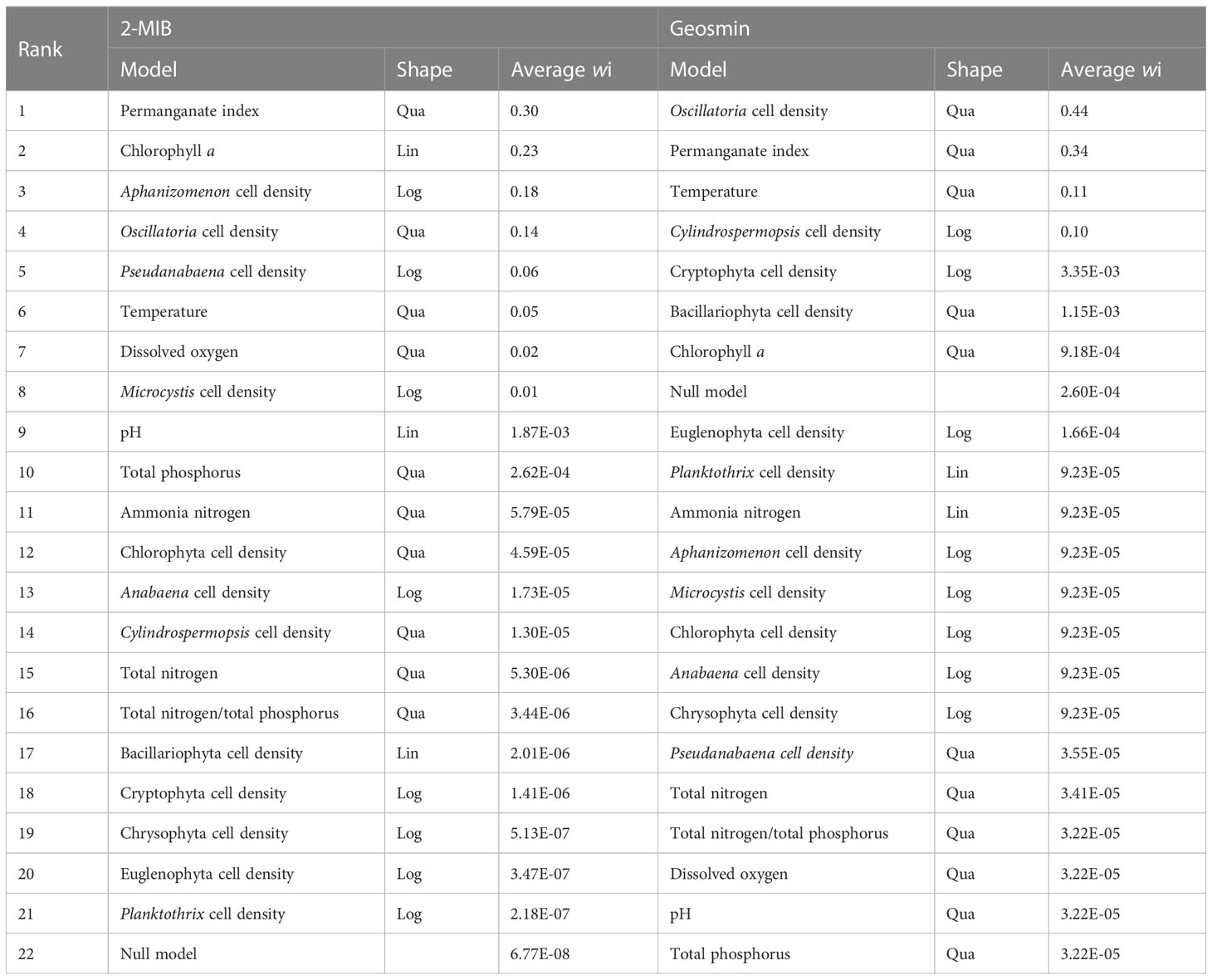
Table 2 The selection of univariate quantile regression models for 2-MIB and geosmin concentrations.
The model considering permanganate index and Pseudanabaena cell density as the independent variables was selected as the best (averaged wi = 0.57) for describing 2-MIB concentrations (Table 3). The best-fit model that considered both permanganate index and temperature explained geosmin concentrations better (average wi = 0.35). For 2-MIB and geosmin concentrations, the 5th quantile is a constant model (y = 0), so the lower boundary is not considered. Only the upper boundary represented by the 95h quantile is used to describe how 2-MIB and geosmin concentrations vary through the two environmental factors. 2-MIB concentrations were generally higher in high permanganate index and increased with increasing Pseudanabaena cell density (Figure 6A). Geosmin concentrations had higher concentrations at lower or higher temperatures and increased with increasing CODMn (Figure 6B). The final model equations of quantile regression are in Supplementary Tables 1–3.
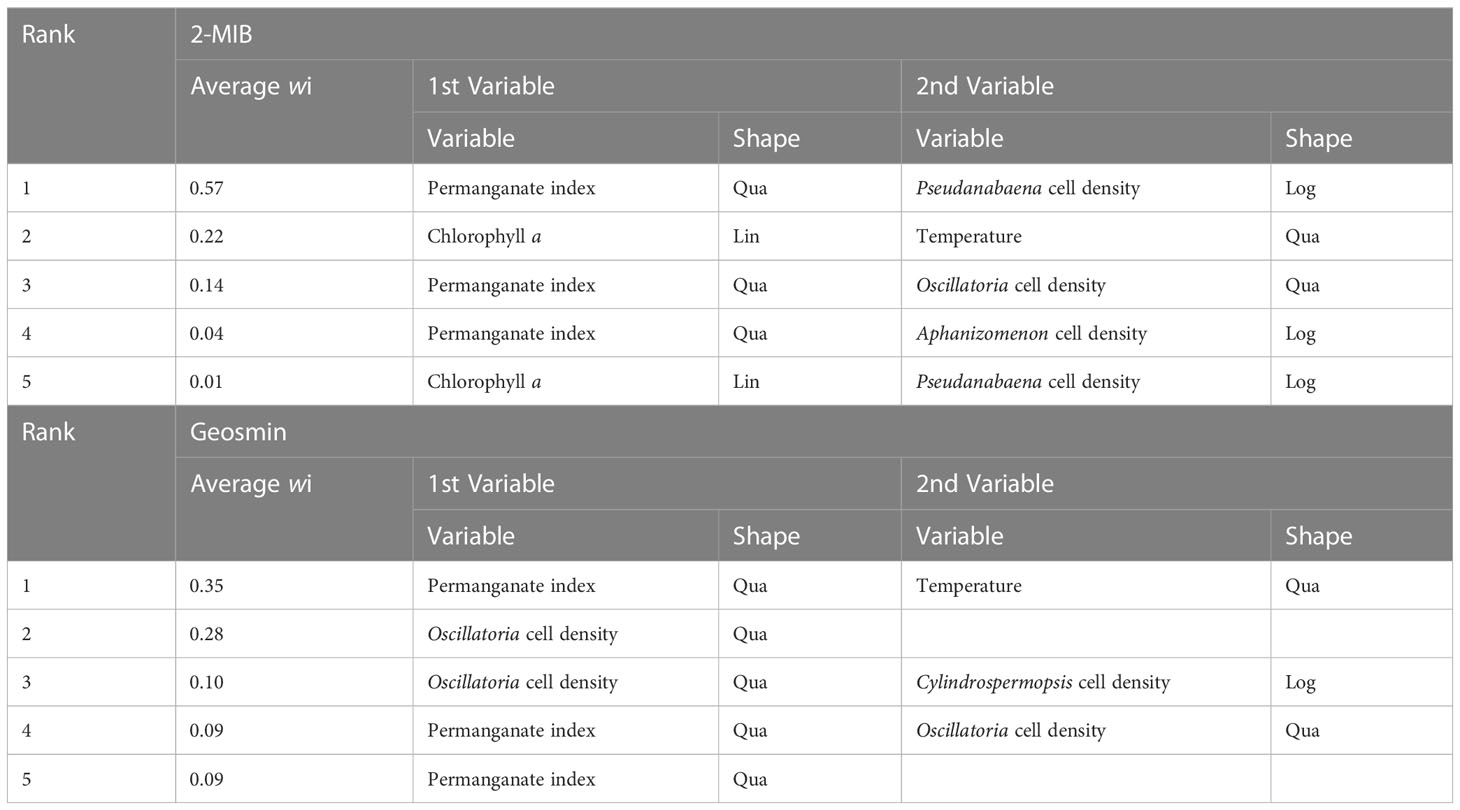
Table 3 The selection of multivariate quantile regression models for 2-MIB and geosmin concentrations.
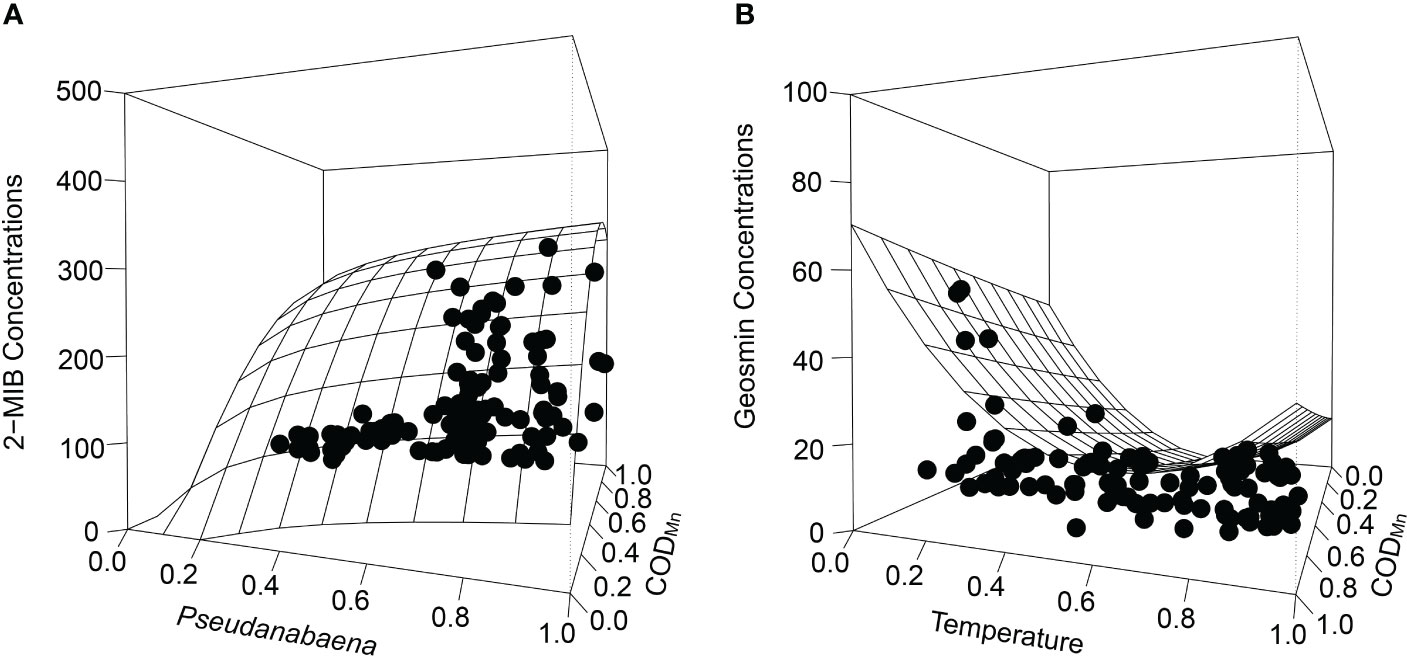
Figure 6 Multivariate quantile regression models for (A) 2-MIB concentrations and (B) geosmin concentrations. Surfaces represent the 95th quantile regression model.
According to the correlation heatmap in Figure 3B, oxidation-reduction potential (p < 0.05), permanganate index (p < 0.01), pH (p < 0.01), chlorophyll a (p < 0.01), temperature (p < 0.01), and total phosphorus (p < 0.01) were positively correlated with Biosynthesis of secondary metabolites synthesis pathways, whereas dissolved oxygen (p < 0.01), transparency (p < 0.01), and phosphate (p < 0.05) were negatively correlated with the pathway. Terpenoid backone biosynthesis was positively correlated with oxidation-reduction potential, permanganate index (p < 0.01), pH (p < 0.01), chlorophyll a (p < 0.01), temperature (p < 0.01), and total phosphorus (p < 0.01), and negatively correlated with dissolved oxygen (p < 0.05), transparency (p < 0.01), and phosphate (p < 0.05).
The Yuqiao Reservoir has experienced eutrophication during the last decade, accompanied by the frequent occurrence of bloom events (Huo et al., 2018). The coexistence of multiple odors and multiple odor-producers has threatened the Yuqiao Reservoir since 2018. Many studies have shown that phytoplankton was important sources of odor compounds with little consideration given to bacteria and actinomycetes, probably because phytoplankton is easier to observe, isolate and culture compared to actinomycetes and bacteria (Wu et al., 2021b). Amplicon sequencing is commonly used to study microbial diversity in the environment, and the main genes marked are 16srDNA, 18S rDNA and internal transcribed spacer (ITS) (Liu et al., 2021). Amplicon sequencing to analyze microbial functional gene diversity is flawed due to the immaturity of comparative databases and primers. Metagenomic sequencing provides more information, not only extending taxonomic resolution to the species or strain level but also providing potential functional information (Xu et al., 2018). However, metagenomic sequencing requires more funding, high sample quality requirements and increased difficulty in data analysis. Streptosporangium sp. is a member of the family Actinomycetaceae (Kemmerling et al., 1993). 2-MIB and geosmin were first identified from the actinomycete and its molecular formula and exact chemical structure were determined successively in the following years (Gerber and Lecheval, 1965; Medsker et al., 1968). Dolichospermum sp., Pseudoanabaena sp., Microcoleu sp., and Planktothricoides sp. have been frequently reported to be odor-producers all over the world (Niiyama et al., 2016; Zhang et al., 2016; Alghanmi et al., 2018; Huang et al., 2018; Kim et al., 2020). Planktothricoides raciborskii and Dolichospermum circinale were not detected by microscopic observation from June to September 2021. Since Planktothricoides raciborskii and Microcoleus pseudautumnalis Ak1609 are benthic cyanobacteria, water samples were collected only 0.5 m below the water surface for fixation (Stielow and Ballantine, 2003; Su et al., 2015). Odor-producing and non-odor-producing strains often coexist in the field (Cai et al., 2017). Changes in the rise and fall of odor-producing and non-odor-producing strains are considered to be the most important factors regulating the concentration of odors in freshwater (Devi et al., 2021). The abundance of Pseudanabaena sp., Microcoleus sp., and Planktothricoides sp. in the microbial community in July was 1.27%, 0.04%, and 0.87%, respectively, while the abundance of Pseudanabaena sp., Microcoleus sp. and Planktothricoides sp. in the 2-MIB producers in the same month was 29.28%, 45.49%, and 25.22%, respectively. It can be speculated that the proportion of 2-MIB-producing taxa was higher in Microcoleus sp. and Planktothricoides sp. than in Pseudanabaena sp. The relationship between odor compounds and microorganisms in water bodies is a widespread concern at home and abroad. The risk of 2-MIB exceeding 15 ng/L in water was as high as 90% when the Planktothrix sp. density was more than 4.0 × 105 cells/L (Su et al., 2015). Phormidium sp. was the 2-MIB-producing microorganism, accounting for 80–95% of the algal cell density, with an estimated 2-MIB production of 0.022 pg/cell (Sun et al., 2013). A strong positive correlation was detected between 2-MIB concentrations and Pseudanabaena sp. cell numbers (Lee et al., 2022). Geosmin concentrations was positively correlated with cyanobacteria (R2 = 0.84, p < 0.0001) and not significantly correlated with actinomycetes (R2 = 0.01, p = 0.709) (Lee J. E. et al., 2020). Significant correlation between odor-producers cell densities and 2-MIB/geosmin concentrations was acquired according to the Pearson correlation test (p < 0.01) (Figure 7). The concentration of geosmin/2-MIB in the reservoir may be controlled not only by the density of odor-producer cells but also by environmental factors (Zhang et al., 2016).
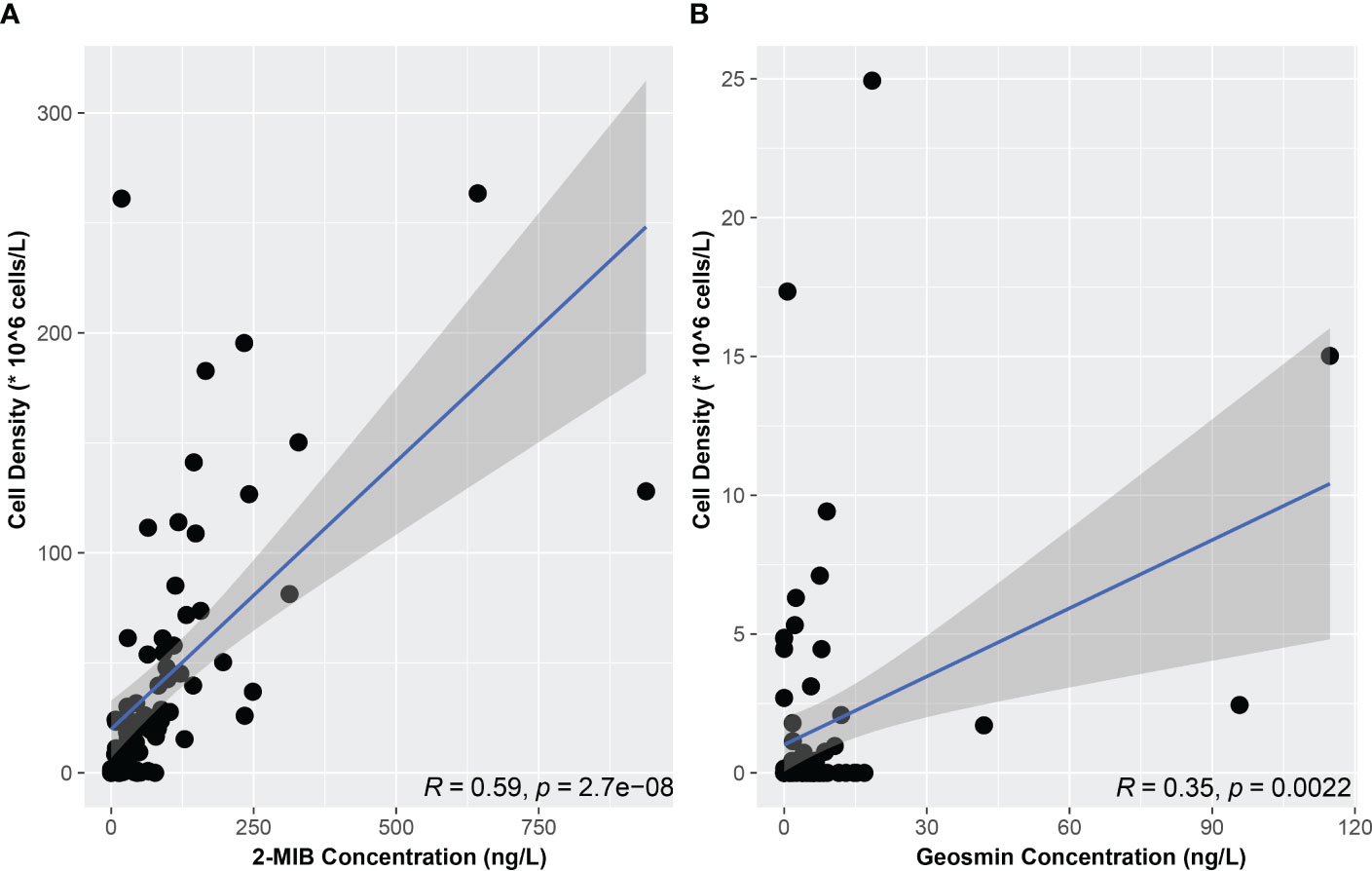
Figure 7 Correlation relationship between odor-producers cell densities and odor compounds concentrations. (A) 2-MIB (B) Geosmin.
Different odor-producing microorganisms vary greatly in their ability to produce odor, and external physicochemical and biological factors may affect growth and odor production (Wang et al., 2011; Wang et al., 2015; Zhang et al., 2020). CODMn was the driver for both 2-MIB and geosmin, and both odors increased with increasing CODMn. Geosmin and 2-MIB are terpenoids produced and secreted by microorganisms (Lovell et al., 1986). In the correlation analysis between functional genes and environmental factors, a significantly positive correlation between CODMn and the biosynthetic pathway of secondary metabolites/terpenoid backbone biosynthesis pathway could be found (Figure 3B). In previous studies, several empirical models have been developed based on the strong correlation between chlorophyll a or COD and odor compounds (Bowmer et al., 1992; Rosen et al., 1992; Panova and Dimkov, 2008; Kim et al., 2015). COD had a significant effect on the T&O prediction model and was positively correlated with α-β-ionone concentrations (Qi et al., 2012). The odor occurrence of 2-MIB and geosmin was effectively predicted and validated using multiple linear regression and artificial neural network techniques with chlorophyll a and COD as explanatory variables (Sugiura et al., 2004). COD is an important indicator to determine the pollution of organic matter in water. The decay of aquatic plants in the Yuqiao Reservoir led to an increase in CODMn, higher CODMn plays a stress factor to phytoplankton and significantly stimulated the synthetize of T&O, and made the increase of odor compounds concentration (Supplementary Figure 1). The three temperature treatments could be arranged in descending order as 10 °C, 35 °C, and 25 °C based on geosmin produced by Dolichospermum ucrainica (Wang and Li, 2015). The production of geosmin was increased with incubation temperature (20–30 °C) by Streptomyces roseoflavus (Tung et al., 2005). Streptomyces tendae cultures incubated at 30 and 45 °C for 48 h produced more geosmin than those incubated at 10 and 20 °C (Dionigi and Ingram, 1994). Many studies found that when external conditions were not adapted to the growth of Cyanobacteria and its growth rate was low, more 2-MIB and geosmin were produced by individual algal cells (Zimba et al., 1999; Wang et al., 2015; Zhang et al., 2016). The direct precursors of geosmin and 2-MIB synthesis, GPP and FPP, are intermediate products of the chlorophyll a synthesis pathway. Some experimental evidence suggests that the biosynthesis of geosmin and 2-MIB in cyanobacteria is associated with chlorophyll a synthesis (Giglio et al., 2008; Zhang et al., 2009; Giglio et al., 2011; Wang et al., 2011). Since geosmin and 2-MIB have common precursors with chlorophyll a synthesis in cyanobacterial cells, the synthesis of earthy-musty odorants and photosynthetic pigments is largely a competition for substrates, i.e., GPP and FPP tend to be converted to 2-MIB and geosmin during the phase of reduced photosynthetic activity and pigment synthesis (Giglio et al., 2008; Zhang et al., 2009; Giglio et al., 2011; Wang et al., 2011; Wang and Li, 2015). At lower or higher temperatures, the production of geosmin by Dolichospermum sp. and Streptomyces sp. is elevated. To our knowledge, our study is one of the first applications of quantile regression in long-term studies of odorant substances in reservoirs, a method that can help managers focus on specific factors, focus future monitoring efforts, and critically provide information.
A four-year survey revealed the distribution of 2-MIB/geosmin and the distribution of odor-producers in the Yuqiao Reservoir, and the following conclusions were drawn. Metagenome unmasked that Pseudanabaena sp. dqh15, Microcoleus pseudautumnalis Ak1609, Pseudanabaena limnetica, and Planktothricoides raciborskii were the 2-MIB-producers, while Streptosporangium caverna and Dolichospermum circinale were the geosmin-producers. Metagenome is a rapid, high-precision and high-throughput method for detecting odor-producers. Quantile regression analysis indicated Pseudanabaena sp. and CODMn were the best predictors of 2-MIB concentrations, temperature and CODMn were the most useful parameters for describing geosmin concentration change. Combining quantile regression and metagenome results showed that CODMn was an important driver of odor compounds. Quantile regression has the advantages of not assuming the existence of moment functions, not being affected by anomalous observations, and not making any distributional assumptions. When accurate and complete data are obtained, quantile regression can quickly find the drivers of odor compounds in the reservoir.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA895910.
PQ: Conceptualization, methodology, software, visualization, formal analysis, writing – original draft, data curation. YZ: Investigation. WM: Project administration. GS: Software, validation. YB: Writing – review & editing, supervision. All authors contributed to the article and approved the submitted version.
This research was Jointly funded by National key R&D plan (No: 2021YFC3200900) and National Natural Science Foundation of China (No: 31971477).
The calculating resource was supported by the Wuhan Branch, Supercomputing Center, Chinese Academy of Sciences. We thank all members in the Analysis and Testing Center at IHB for technical supports.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1216567/full#supplementary-material
Alghanmi H. A., Alkam F. M., Al-Taee M. M. (2018). Effect of light and temperature on new cyanobacteria producers for geosmin and 2-methylisoborneol. J. Appl. Phycology. 30 (1), 319–328. doi: 10.1007/s10811-017-1233-0
Allen D. C., Vaughn C. C. (2010). Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. J. North Am. Benthological Society. 29 (2), 383–394. doi: 10.1899/09-024.1
Bowmer K. H., Padovan A., Oliver R. L., Korth W., Ganf G. G. (1992). Physiology of geosmin production by Anabaena-circinalis isolated from the Murrumaidgee river, Australia. Water Sci. Technology. 25 (2), 259–267. doi: 10.2166/wst.1992.0060
Brock N. L., Ravella S. R., Schulz S., Dickschat J. S. (2013). A detailed view of 2-methylisoborneol biosynthesis. Angewandte Chemie-International Edition. 52 (7), 2100–2104. doi: 10.1002/anie.201209173
Cai F. F., Yu G. L., Zhang K., Chen Y. X., Li Q., Yang Y. M., et al. (2017). Geosmin production and polyphasic characterization of Oscillatoria limosa Agardh ex Gomont isolated from the open canal of a large drinking water system in Tianjin city, China. Harmful Algae. 69, 28–37. doi: 10.1016/j.hal.2017.09.006
Cane D. E., Watt R. M. (2003). Expression and mechanistic analysis of a germacradienol synthase from streptomyces coelicolor implicated in geosmin biosynthesis. Proc. Natl. Acad. Sci. USA 100 (4), 1547–1551. doi: 10.1073/pnas.0337625100
Chen S., Zhou Y., Chen Y., Gu J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34 (17), 884–890. doi: 10.1093/bioinformatics/bty560
Chiu Y.-T., Yen H.-K., Lin T.-F. (2016). An alternative method to quantify 2-MIB producing cyanobacteria in drinking water reservoirs: method development and field applications. Environ. Res. 151, 618–627. doi: 10.1016/j.envres.2016.08.034
Clescerl L. S. (1998). Standard methods for the examination of water and wastewater 20th Ed (Washington, DC: American Public Health Association), 481–486.
Cristina Casero M., Velazquez D., Medina-Cobo M., Quesada A., Cires S. (2019). Unmasking the identity of toxigenic cyanobacteria driving a multi-toxin bloom by high-throughput sequencing of cyanotoxins genes and 16S rRNA metabarcoding. Sci. Total Environment. 665, 367–378. doi: 10.1016/j.scitotenv.2019.02.083
Devi A., Chiu Y. T., Hsueh H. T., Lin T. F. (2021). Quantitative PCR based detection system for cyanobacterial geosmin/2-methylisoborneol (2-MIB) events in drinking water sources: current status and challenges. Water Res. 188, 116478. doi: 10.1016/j.watres.2020.116478
Dionigi C. P., Ingram D. A. (1994). Effects of temperature and oxygen concentrations on geosmin production by Strepttomyces-tendae and Penicillium-expansum. J. Agric. Food Chem. 42 (1), 143–145. doi: 10.1021/jf00037a025
Fornaroli R., Cabrini R., Zaupa S., Bettinetti R., Ciampittiello M., Boggero A. (2016). Quantile regression analysis as a predictive tool for lake macroinvertebrate biodiversity. Ecol. Indicators. 61, 728–738. doi: 10.1016/j.ecolind.2015.10.024
Fu L., Niu B., Zhu Z., Wu S., Li W. (2012). CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 28 (23), 3150–3152. doi: 10.1093/bioinformatics/bts565
Gerber N. N., Lecheval H. A. (1965). Geosmin an earthy-smelly substance isolated from actinomycetes. Appl. Microbiol. 13 (6), 935–&. doi: 10.1128/aem.13.6.935-938.1965
Giangreco I., Nicolotti O., Carotti A., De Carlo F., Gargano G., Bellotti R. (2010). Analysis of X-ray structures of matrix metalloproteinases via chaotic map clustering. BMC Bioinf. 11, 500. doi: 10.1186/1471-2105-11-500
Giglio S., Jiang J., Saint C. P., Cane D. E., Monis P. T. (2008). Isolation and characterization of the gene associated with geosmin production in cyanobacteria. Environ. Sci. Technology. 42 (21), 8027–8032. doi: 10.1021/es801465w
Giglio S., Saint C. P., Monis P. T. (2011). Expression of the geosmin synthase gene in the cyanobacteria anabaena circinalis AWQC318. J. Phycology. 47 (6), 1338–1343. doi: 10.1111/j.1529-8817.2011.01061.x
Gurevich A., Saveliev V., Vyahhi N., Tesler G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics. 29 (8), 1072–1075. doi: 10.1093/bioinformatics/btt086
Hamano Y., Dairi T., Yamamoto M., Kuzuyama T., Itoh N., Seto H. (2002). Growth-phase dependent expression of the mevalonate pathway in a terpenoid antibiotic-producing streptomyces strain. Bioscience Biotechnol. Biochem. 66 (4), 808–819. doi: 10.1271/bbb.66.808
Hawkins P. R., Holliday J., Kathuria A., Bowling L. (2005). Change in cyanobacterial biovolume due to preservation by lugol’s iodine. Harmful Algae. 4 (6), 1033–1043. doi: 10.1016/j.hal.2005.03.001
Hu H. J., Wei Y. X. (2006). The freshwater algae of China-systematics, taxonomy and ecology (Beijing, China.: Science Press), 1–1023.
Huang X., Huang Z., Chen X.-P., Zhang D., Zhou J., Wang X., et al. (2018). The predominant phytoplankton of Pseudoanabaena holding specific biosynthesis gene-derived occurrence of 2-MIB in a drinking water reservoir. Environ. Sci. Pollut. Res. 25 (19), 19134–19142. doi: 10.1007/s11356-018-2086-z
Huo D., Chen Y., Liu P., Li Y., Qiao Z., Li R. (2018). Molecular detection of microbial communities associated with Microcystis vs Synechococcus dominated waters in Tianjin, China. J. Oceanology Limnology. 36 (4), 1145–1156. doi: 10.1007/s00343-018-7182-x
Jiang J. Y., He X. F., Cane D. E. (2006). Geosmin biosynthesis. streptomyces coelicolor germacradienol/germacrene d synthase converts farnesyl diphosphate to geosmin. J. Am. Chem. Society. 128 (25), 8128–8129. doi: 10.1021/ja062669x
Jiang J., He X., Cane D. E. (2007). Biosynthesis of the earthy odorant geosmin by a bifunctional streptomyces coelicolor enzyme. Nat. Chem. Biol. 3 (11), 711–715. doi: 10.1038/nchembio.2007.29
Johnson J. B., Omland K. S. (2004). Model selection in ecology and evolution. Trends Ecol. Evolution. 19 (2), 101–108. doi: 10.1016/j.tree.2003.10.013
Juttner F., Watson S. B. (2007). Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 73 (14), 4395–4406. doi: 10.1128/aem.02250-06
Kemmerling C., Gurtler H., Kroppenstedt R., Toalster R., Stackebrandt E. (1993). Evidence for the phylogenetic heterogeneity of the genus streptosporangium. Systematic Appl. Microbiol. 16 (3), 369–372. doi: 10.1016/s0723-2020(11)80267-5
Kim J., Kim J., Cho Y. (2015). Establishing a predictive model for chlorophyll-a concentration in Lake Daechung, Korea using multilinear statistical techniques. J. Environ. Engineering. 141 (2), 4014061. doi: 10.1061/(asce)ee.1943-7870.0000883
Kim K., Yoon Y., Cho H., Hwang S.-J. (2020). Molecular probes to evaluate the synthesis and production potential of an odorous compound (2-methylisoborneol) in cyanobacteria. Int. J. Environ. Res. Public Health 17 (6), 1933. doi: 10.3390/ijerph17061933
Komatsu M., Tsuda M., Omura S., Oikawa H., Ikeda H. (2008). Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc. Natl. Acad. Sci. USA 105 (21), 7422–7427. doi: 10.1073/pnas.0802312105
Kristiansson E., Fick J., Janzon A., Grabic R., Rutgersson C., Weijdegard B., et al. (2011). Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PloS One 6 (2), e17038. doi: 10.1371/journal.pone.0017038
Lee E. S., Kim Y. N., Kim S. B., Jung J. S., Cha Y. S., Kim B. S. (2020). Distribution and characteristics of geosmin and 2-MIB-producing actinobacteria in the han river, Korea. Water Supply. 20 (5), 1975–1987. doi: 10.2166/ws.2020.110
Lee J. E., Youn S.-J., Byeon M., Yu S.-J. (2020). Occurrence of cyanobacteria, actinomycetes, and geosmin in drinking water reservoir in Korea: a case study from an algal bloom in 2012. Water Supply. 20 (5), 1862–1870. doi: 10.2166/ws.2020.102
Lee J. E., Yu M. N., Yu S., Byeon M. (2022). Occurrence and phylogenetic analysis of pseudanabaena sp. producing 2-methylisoborneol in drinking water source of South Korea. Environ. Microbiol. Rep. 14 (2), 197–202. doi: 10.1111/1758-2229.13031
Li D., Liu C.-M., Luo R., Sadakane K., Lam T.-W. (2015). MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 31 (10), 1674–1676. doi: 10.1093/bioinformatics/btv033
Li L., Wan N., Gan N., Xia B. D., Song L. R. (2007). Annual dynamics and origins of the odorous compounds in the pilot experimental area of Lake Dianchi, China. Water Sci. Technology. 55 (5), 43–50. doi: 10.2166/wst.2007.160
Lipsitz M., Belloni A., Chernozhukov V., Fernandez-Val I. (2016). Quantreg. nonpar: an r package for performing nonparametric series quantile regression. R J. 8 (2), 370–381. doi: 10.32614/RJ-2016-052
Liu Y.-X., Qin Y., Chen T., Lu M., Qian X., Guo X., et al. (2021). A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell. 12 (5), 315–330. doi: 10.32614/RJ-2016-052
Liu J., Yang W. (2012). Water sustainability for China and beyond. Science. 337 (6095), 649–650. doi: 10.1126/science.1219471
Lovell R. T., Lelana I. Y., Boyd C. E., Armstrong M. S. (1986). Geosmin and musty muddy flavors in pond-raised channel catfish. Trans. Am. Fisheries Society. 115 (3), 485–489. doi: 10.1577/1548-8659(1986)115<485:Gamfip>2.0.Co;2
Ludwig F., Medger A., Boernick H., Opitz M., Lang K., Goettfert M., et al. (2007). Identification and expression analyses of putative sesquiterpene synthase genes in phormidium sp and prevalence of geoA-like genes in a drinking water reservoir. Appl. Environ. Microbiol. 73 (21), 6988–6993. doi: 10.1128/aem.01197-07
Margulies M., Egholm M., Altman W. E., Attiya S., Bader J. S., Bemben L. A., et al. (2006). Genome sequencing in microfabricated high-density picolitre reactors (vol 437, pg 376, 2005). Nature. 441 (7089), 120–120. doi: 10.1038/nature04726
Medsker L. L., Jenkins D., Thomas J. F. (1968). Odorous compounds in natural waters. an earthy-smelling compound associated with blue-green algae and actinomycetes. Environ. Sci. Technology. 2 (6), 461–464. doi: 10.1021/es60009a005
Mirdita M., Steinegger M., Soeding J. (2019). MMseqs2 desktop and local web server app for fast, interactive sequence searches. Bioinformatics. 35 (16), 2856–2858. doi: 10.1093/bioinformatics/bty1057
Niiyama Y., Tuji A., Takemoto K., Ichise S. (2016). Pseudanabaena foetida sp nov and p. subfoetida sp nov (Cyanophyta/Cyanobacteria) producing 2-methylisoborneol from Japan. Fottea. 16 (1), 1–11. doi: 10.5507/fot.2016.006
Oh H. S., Lee C. S., Srivastava A., Oh H. M., Ahn C. Y. (2017). Effects of environmental factors on cyanobacterial production of odorous compounds: geosmin and 2-methylisoborneol. J. Microbiol. Biotechnol. 27 (7), 1316–1323. doi: 10.4014/jmb.1702.02069
Oldfield E., Lin F.-Y. (2012). Terpene biosynthesis: modularity rules. Angewandte Chemie-International Edition. 51 (5), 1124–1137. doi: 10.1002/anie.201103110
Panova A., Dimkov R. (2008). Depth distribution of freshwater microorganisms and physicochemical characteristics in iskar reservoir. Biotechnol. Biotechnol. Equipment. 22 (1), 560–565. doi: 10.1080/13102818.2008.10817512
Perkins R. G., Slavin E. I., Andrade T. M. C., Blenkinsopp C., Pearson P., Froggatt T., et al. (2019). Managing taste and odour metabolite production in drinking water reservoirs: the importance of ammonium as a key nutrient trigger. J. Environ. Management. 244, 276–284. doi: 10.1016/j.jenvman.2019.04.123
Peterson H. G., Hrudey S. E., Cantin I. A., Perley T. R., Kenefick S. L. (1995). Physiological toxicity, cell-membrane damage and the release of dissolved organic-carbon and geosmin by aphanizomenon flos-aquae after exposure to water- treatment chemicals. Water Res. 29 (6), 1515–1523. doi: 10.1016/0043-1354(94)00300-v
Qi M., Chen J., Sun X., Deng X., Niu Y., Xie P. (2012). Development of models for predicting the predominant taste and odor compounds in Taihu Lake, China. PloS One 7 (12), e51976. doi: 10.1371/journal.pone.0051976
Qiu P. F., Chen Y. X., Li C. J., Huo D., Bi Y. H., Wang J. B., et al. (2021). Using molecular detection for the diversity and occurrence of cyanobacteria and 2-methylisoborneol-producing cyanobacteria in an eutrophicated reservoir in northern China. Environ. Pollution 288, 117772. doi: 10.1016/j.envpol.2021.117772
Rong C., Liu D. P., Li Y., Yang K., Han X. B., Yu J. W., et al. (2018). Source water odor in one reservoir in hot and humid areas of southern China: occurrence, diagnosis and possible mitigation measures. Environ. Sci. Europe. 30, 45. doi: 10.1186/s12302-018-0175-8
Rosen B. H., Macleod B. W., Simpson M. R. (1992). Accumulation and release of geosmin during the growth phases of Anabaena circinalis (Kutz.) rabenhorst. Water Sci. Technology. 25 (2), 185–190. doi: 10.2166/wst.1992.0051
Sagehashi M., Shiraishi K., Fujita H., Fujii T., Sakoda A. (2005). Adsorptive ozonation of 2-methylisoborneol in natural water with preventing bromate formation. Water Res. 39 (16), 3900–3908. doi: 10.1016/j.watres.2005.06.032
Shen Q., Wang Q., Miao H., Shimada M., Utsumi M., Lei Z., et al. (2022). Temperature affects growth, geosmin/2-methylisoborneol production, and gene expression in two cyanobacterial species. Environ. Sci. Pollut. Res. 29 (8), 12017–12026. doi: 10.1007/s11356-021-16593-5
Smits S. A., Leach J., Sonnenburg E. D., Gonzalez C. G., Lichtman J. S., Reid G., et al. (2017). Seasonal cycling in the gut microbiome of the hadza hunter-gatherers of Tanzania. Science 357 (6353), 802–80+. doi: 10.1126/science.aan4834
Stielow S., Ballantine D. L. (2003). Benthic cyanobacterial, microcoleus lyngbyaceus, blooms in shallow, inshore Puerto Rican seagrass habitats, Caribbean sea. Harmful Algae. 2 (2), 127–133. doi: 10.1016/s1568-9883(03)00007-6
Su M., Gaget V., Giglio S., Burch M., An W., Yang M. (2013). Establishment of quantitative PCR methods for the quantification of geosmin-producing potential and anabaena sp in freshwater systems. Water Res. 47 (10), 3444–3454. doi: 10.1016/j.watres.2013.03.043
Su M., Yu J., Zhang J., Chen H., An W., Vogt R. D., et al. (2015). MIB-producing cyanobacteria (Planktothrix sp.) in a drinking water reservoir: distribution and odor producing potential. Water Res. 68, 444–453. doi: 10.1016/j.watres.2014.09.038
Sugiura N., Nishimura O., Inamori Y., Ouchiyama T., Sudo R. (1997). Grazing characteristics of musty-odor-compound-producing phormidium tenue by a microflagellate, monas guttula. Water Res. 31 (11), 2792–2796. doi: 10.1016/s0043-1354(97)00115-2
Sugiura N., Utsumi M., Wei B., Iwami N., Okano K., Kawauchi Y., et al. (2004). Assessment for the complicated occurrence of nuisance odours from phytoplankton and environmental factors in a eutrophic lake. Lakes & Reservoirs 9 (3–4), 195–201. doi: 10.1111/j.1440-1770.2004.00246.x
Sun D. L., Yu J. W., An W., Yang M., Chen G. G., Zhang S. J. (2013). Identification of causative compounds and microorganisms for musty odor occurrence in the Huangpu River, China. J. Environ. Sci. 25 (3), 460–465. doi: 10.1016/s1001-0742(12)60012-6
Tsao H.-W., Michinaka A., Yen H.-K., Giglio S., Hobson P., Monis P., et al. (2014). Monitoring of geosmin producing anabaena circinalis using quantitative PCR. Water Res. 49, 416–425. doi: 10.1016/j.watres.2013.10.028
Tung S. C., Lin T. F., Tseng I. C., Lin H. M. (2005). Identification of 2-MIB and geosmin producers in Feng-Shen reservoir in south Taiwan. Water Science & Technology Water Supply 6 (2), 55–61. doi: 10.2166/ws.2006.050
Wang C., Hu R., Strong P. J., Zhuang W., Huang W., Luo Z., et al. (2021). Prevalence of antibiotic resistance genes and bacterial pathogens along the soil-mangrove root continuum. J. Hazardous Materials. 408, 124985. doi: 10.1016/j.jhazmat.2020.124985
Wang Z., Li R. (2015). Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Systematics Ecology. 58, 219–226. doi: 10.1016/j.bse.2014.12.013
Wang Y., Li Y., Liang J., Bi Y., Wang S., Shang Y. (2021). Climatic changes and anthropogenic activities driving the increase in nitrogen: evidence from the south-to-North water diversion project. Water 13 (18), 2517. doi: 10.3390/w13182517
Wang Z., Song G., Shao J., Tan W., Li Y., Li R. (2016). Establishment and field applications of real-time PCR methods for the quantification of potential MIB-producing cyanobacteria in aquatic systems. J. Appl. Phycology. 28 (1), 325–333. doi: 10.1007/s10811-015-0529-1
Wang Z., Xiao P., Song G., Li Y., Li R. (2015). Isolation and characterization of a new reported cyanobacterium leptolyngbya bijugata coproducing odorous geosmin and 2-methylisoborneol. Environ. Sci. Pollut. Res. 22 (16), 12133–12140. doi: 10.1007/s11356-015-4470-2
Wang Z., Xu Y., Shao J., Wang J., Li R. (2011). Genes associated with 2-methylisoborneol biosynthesis in cyanobacteria: isolation, characterization, and expression in response to light. PloS One 6 (4), e18665. doi: 10.1371/journal.pone.0018665
Watson S. B., Brownlee B., Satchwill T., Hargesheimer E. E. (2000). Quantitative analysis of trace levels of geosmin and MIB in source and drinking water using headspace SPME. Water Res. 34 (10), 2818–2828. doi: 10.1016/s0043-1354(00)00027-0
Wu A. J., Wang Y. D., Friese K., Zhang L., Han C., Kang D. J., et al. (2021a). Spatial and seasonal distribution of 2-methylisoborneol in a Large Eutrophic Shallow Lake, China. Water Air Soil Pollution. 232 (9), 387. doi: 10.1007/s11270-021-05340-8
Wu T., Zhu G., Zhu M., Xu H., Yang J., Zhao X. (2021b). Effects of algae proliferation and density current on the vertical distribution of odor compounds in drinking water reservoirs in summer. Environ. Pollution. 288. doi: 10.1016/j.envpol.2021.117683
Xu Y., Wang G., Yang W., Li R. (2010). Dynamics of the water bloom-forming microcystis and its relationship with physicochemical factors in Lake Xuanwu (China). Environ. Sci. pollut. Res. 17 (9), 1581–1590. doi: 10.1007/s11356-010-0345-8
Xu H. Z., Zhang J., Wang W. N., Li Y. Z., Pei H. Y. (2022). Moderate pre-ozonation coupled with a post-peroxone process remove filamentous cyanobacteria and 2-MIB efficiently: from bench to pilot-scale study. J. Hazardous Materials. 424. doi: 10.1016/j.jhazmat.2021.127530
Xu J., Zhang Y., Zhang P., Trivedi P., Riera N., Wang Y., et al. (2018). The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 9. doi: 10.1038/s41467-018-07343-2
Zhang T., Li L., Song L., Chen W. (2009). Effects of temperature and light on the growth and geosmin production of lyngbya kuetzingii (Cyanophyta). J. Appl. Phycology. 21 (3), 279–285. doi: 10.1007/s10811-008-9363-z
Zhang K., Pan R., Luo Z., Zhang T., Fan J. (2020). Interspecific competition between microcystis aeruginosa and Pseudanadaena and their production of T&O compounds. Chemosphere. 252, 126509. doi: 10.1016/j.chemosphere.2020.126509
Zhang T., Zheng L., Li L., Song L. (2016). 2-methylisoborneol production characteristics of pseudanabaena sp FACHB 1277 isolated from Xionghe Reservoir, China. J. Appl. Phycology. 28 (6), 3353–3362. doi: 10.1007/s10811-016-0864-x
Keywords: odor producers, drivers, metagenomic sequencing, quantile regression, cyanobaceria
Citation: Qiu P, Zhang Y, Mi W, Song G and Bi Y (2023) Producers and drivers of odor compounds in a large drinking-water source. Front. Ecol. Evol. 11:1216567. doi: 10.3389/fevo.2023.1216567
Received: 04 May 2023; Accepted: 21 June 2023;
Published: 20 July 2023.
Edited by:
M. Belal Hossain, Noakhali Science and Technology University, BangladeshReviewed by:
Cuong Tu Ho, Vietnam Academy of Science and Technology, VietnamCopyright © 2023 Qiu, Zhang, Mi, Song and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Bi, Yml5aEBpaGIuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.