
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Ecol. Evol., 23 August 2023
Sec. Population, Community, and Ecosystem Dynamics
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1212879
This article is part of the Research TopicNeotropical Dung Beetle Diversity: Ecological, Historical, and Anthropogenic PerspectivesView all 11 articles
A correction has been applied to this article in:
Corrigendum: The relationships between dung beetles and monkeys in the Neotropical region
 Gonzalo Halffter†‡
Gonzalo Halffter†‡ Mario E. Favila*†
Mario E. Favila*†The relationship between dung beetles and arboreal mammals has been scarcely studied, and many of the reports refer to observations without a standardized methodology. The accelerated loss of tropical forests urges us to understand this mutualistic association. Using our studies on arboreal dung beetles in the Palenque Archaeological Zone-National Park, Mexico, as a baseline, we analyzed the information on arboreal dung beetles in Neotropical forests in Mexico and around the world. Canthon euryscelis Bates, 1867, Canthon angustatus Harold, 1867, Canthon subhyalinus Harold, 1867, and Canthon femoralis (Chevrolat, 1834) are the main species collected in trees of Palenque, Onthophagus maya Zunino, 1981 and other non-Scarabaeinae species were occasionally collected from trees in Palenque. The small Canthon species are skilled fliers strongly relationship with monkeys in Palenque and other tropical regions of Mexico and Central America. In South America, arboreal dung beetles are more diverse and include these and other dung beetle species associated with monkeys. Several dung beetle species of the genus Onthophagus have been reported in association with African monkeys. In India, several studies report a wide variety of dung beetle species associated with monkeys. In Australia and New Guinea, only some species of Macropocopris are described as being associated with arboreal marsupials, but in Borneo, several dung beetle species have been observed associated with arboreal marsupials, mostly in managed forests. In Madagascar, Arachnoides gandi is the only beetle species reported in trees. We need to formulate a systematic and comparative methodology to understand better how arboreal beetles search for food, where the food is located, and how brood balls are made, how male-female pairs meet and nest, and how they contribute to arboreal dung recycling.
The dung beetles of the subfamily Scarabaeinae are essentially a terrestrial group, considered part of the soil fauna (Hanski and Cambefort, 1991). However, aspects of the ecology and behavior of Scarabaeinae species that spend much time of their life in canopy of trees in different tropical forests have been described worldwide (Davis et al., 1997; Rivera-Cervantes and Halffter, 1999; Larsen et al., 2006; Vulinec et al., 2007; Noriega, 2015). Studies addressing this line of research have been discontinuous, and much of the current information has been reported as anecdotic or incidental observations, sometimes recorded during studies on mammal behavior (Tirado-Herrera et al., 2002; Jacobs et al., 2008). Recirculating the dung of monkeys living on trees by “aerial” dung beetles is not a marginal but an essential aspect for tropical forests to survive (Halffter and Halffter, 2009). Systematic studies focused on the eco-ethology of this group of canopy beetles are scarce. Compiling and categorizing the data available in the literature and elsewhere will allow us to assess the current state of knowledge, the topics investigated, the aspects that remain unknown, and the methodological approaches used to study Scarabaeinae species inhabiting the canopy in tropical forests.
In tropical rainforests, many vertebrates live in the upper forest canopy and some dung beetle species have been observed foraging or perching in the upper canopy and different vegetation layers of forests (Davis et al., 1997). The number of species and the species that forage or perch on plants or tree branches of tropical forests differ between the Neotropical region and other regions of the world (Cambefort and Walter, 1991; Gill, 1991). However, the association between dung beetles and the vertebrate fauna that lives in the forest canopy has not been studied to the same extent as the one between ground dung beetles and vertebrates that live in this stratum.
We aimed to evaluate the current knowledge of the relationship between monkeys and dung beetles and propose a systematic methodology for studying these relationships in the Neotropical region and other tropical forests of the world. In addition to the Scarabaeinae, other coleopterans and insects are associated with monkey dung.
Our research questions were the following:
1. What has been investigated about the Scarabaeinae species inhabiting the arboreal layer of tropical forests, and what questions do they suggest? Why do the prevailing species in the forest canopy belong to the genus Canthon in America but to the genus Onthophagus in Africa? What are the methodological approaches for studying beetles living in the canopy, and how can they be improved to obtain comparable results?
2. What are the foraging and perching behaviors of arboreal dung beetles? Is there evidence of Scarabaeinae species being closely associated with certain species of arboreal mammals? How does the resource relocation behavior take place in the canopy? How do beetles of different sexes meet? How do they make the ball? How do they roll it?
3. What is the vertical distribution of Scarabaeinae species in tropical forests of southern Mexico and other tropical regions? Do the observed patterns match the vertical temperature and humidity gradients or the behavior of monkeys and other arboreal mammals?
The Palenque Archaeological Zone-National Park stretches across 1771 hectares. It is located in the northern limit of the Lacandona forest in Chiapas. Palenque was one of the great Mayan cities during the classic period (250-900 BC). It reached peak development between 600 BC and 800 BC. After different conflicts and invasions, this and other Mayan towns declined and were almost completely abandoned (Mathews, 2007). Nearly 600 hectares of high evergreen forest still remain, altered but maintaining its ecological structure thanks to the local authorities in charge of preserving the archaeological zone. The monkeys thriving in the area that are a food source for dung beetles are the black howler or howler monkey, Alouatta pigra Lawrence, 1933, which is relatively abundant, and the spider monkey, Ateles geoffroyi (Kuhl, 1820).
We collected dung beetles for 24 h on 21 June 1993. Traps were set at 5 m, 10 m, 15 m, and 20 m high onto six large trees separated by almost two hundred meters between them in the forest, far from the archaeological zone. Each trap consisted of a 9 cm × 9 cm × 8 cm plastic box with a triangular opening in the lid, filled with a 2 cm-thick layer of soil and baited with 4 g of human feces or carrion (fish) (for further details, refer to Halffter and Favila, 1993). The baits remained active for 24 hours. The results reflect the total number of beetles collected in 25 traps, six baited with fish carrion and 19 with human feces (Table 1).
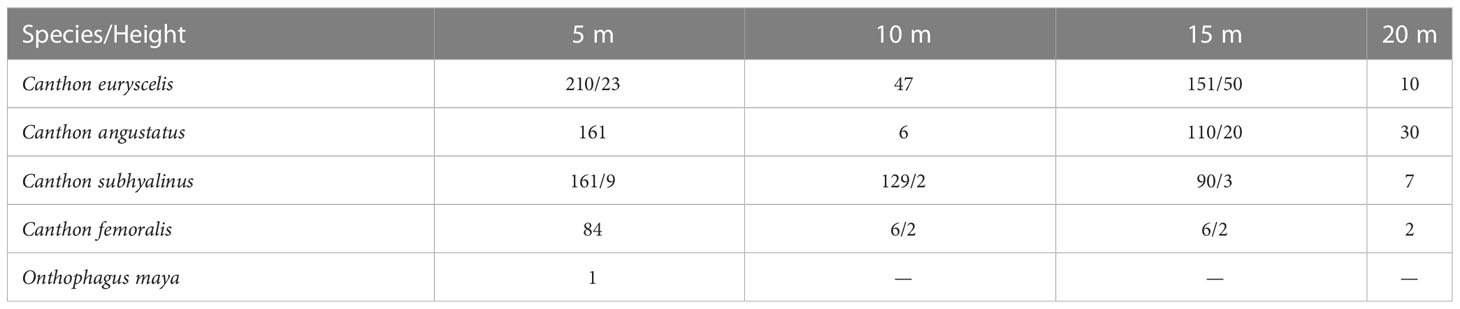
Table 1 Dung beetle species caught in 18 copro traps (3 per tree)/and 6 necro traps (one per tree) affixed to 6 trees separated by at least 100 m between them in the tropical forest of the Archaeological zone in Palenque, Chiapas, Mexico.
We constructed a database conducting a systematized search of peer-reviewed papers on the Web of Science (WoS) to collect the studies about the species of dung beetles associated with monkeys in the Neotropical region and other regions of the world. The search was conducted during the first semester of 2023, including articles published from 1980 to 2023. We search the next terms in the title, abstract, and keywords for each paper: “arboreal dung beetle*” AND “arboreal carrion beetles” AND “monkeys and dung beetles” OR “vertical stratification dung beetles” OR “perching dung beetles” OR “dung beetles and trees”. In addition, we used other search strategies to broaden the scope of the review, such as directly contacting some authors.
The WoS search retrieved 277 articles, of which we included only those meeting the following criteria: the study should analyze arboreal, perching, and/or flying behaviors of dung beetles associated with monkeys, food location, or search of mates in tropical forests. After reviewing the titles and abstracts of the full WoS search results, we selected 12 articles for a full review, which were those related to the monkeys and arboreal dung beetles, perching behavior, and dung beetles in trees. As the systematic search did not capture articles or chapters that were published before 1980 or the journal or book lacked a digital repository, we used other search strategies to broaden the scope of the review such as directly contacting some authors and including articles from the personal library of the authors.
We extracted the following data from the selected literature: a) the taxonomic identity of each dung beetles species studied (current names of species were used in case of synonyms or new combinations; b) study location, including locality and country; the environment and known food resources of each species c) flying behavior observed in different dung beetle species, including duration, location of the food or mate; d) average duration of flying behavior observed in different dung beetle species; f) nest characteristics in the trees (structure, complexity, and location relative to the base of the tree); g) male/female nesting behavior h) male/female searching behavior, perching heights.
The studies conducted to analyze the vertical distribution patterns of the species of the subfamily Scarabaeinae in Neotropical and other tropical forests have used arboreal pitfall traps with different baits (human feces, fruits, and carrion) and positioned at different heights; only a single article mentions having conducted direct observations (Howden and Young, 1981). There is a comprehensive review by Noriega and Vulinec (2021) about dung beetle perching and their vertical stratification in the understory, which does not include canopy beetles.
The most frequent species collected on trees was Canthon euryscelis Bates, 1867 (418 individuals), followed by Canthon subhyalinus Harold, 1867 (387 individuals), Canthon angustatus Harold, 1867 (307 individuals), and Canthon femoralis (Chevrolat, 1834) (102 individuals) (Table 1). All these Canthon species are small and skilled fliers, suggesting a strong relationship with monkeys, but maybe also with other mammals and reptiles living on trees. Canthon euryscelis and Canthon angustatus were mostly collected at 5 m high (50.23% and 52.4%, respectively, of individuals collected in aerial traps) and 15 m high (36.12% and 35.8% respectively); however, C. euryscelis was collected more frequently at 10 m than at 20 m (11.2% and 2.4%, respectively), and the opposite occurred for C. angustatus (1.9% and 9.7%, respectively). More Canthon subhyallinus individuals were collected a 5 m (41.6%), then at 10 m (33.3%) and 15 m (23.3%) high, but were rarely caught at 20 m high (1.8%). Only a single individual of Onthophagus maya Zunino, 1981, was collected at 5 m high (Table 1).
This section outlines the information gathered about arboreal dung beetles inhabiting the tropical forests of México. This information shows that the association of a certain beetle species with monkey dung is common in a given location but varies significantly between sites. The relationship between beetles and monkey dung is strong and determines the abundance of some species in the canopy, being substantially reduced at ground level. In the tropical forests of southern Mexico, four species of Scarabaeinae (Canthon spp.) show this relationship with the monkeys Alouatta palliata (Gray, 1849), A. pigra Lawrence, 1933, and probably with Ateles geoffroyi Gray, 1866.
In Mexico Canthon angustatus is the most abundant species associated with monkeys in Mexico and shows the strongest and most constant relationship. It is distributed from the ground level up to 30 m high (with up to 50 specimens caught per trap, Halffter personal observations). In the state of Chiapas, C. angustatus has been collected in the Montes Azules Biosphere Reserve (Howden and Young, 1981; Solís and Kohlmann, 2002; Chamé-Vázquez and Gómez-Gómez, 2005; Navarrete and Halffter, 2008) and Palenque (this work). In Veracruz, C. angustatus has been collected in Las Choapas (Sánchez-Huerta et al., 2019). This species is also found in Campeche, Quintana Roo, and Tabasco (Sánchez-Huerta et al., 2019). Besides, it has been described from Nicaragua and cited from Belize, Costa Rica, El Salvador, Honduras, Nicaragua, Guatemala, Panama, Colombia, Ecuador, and Peru (Sánchez-Huerta et al., 2019), always strongly associated with dung of Alouatta palliata and Allouatta pigra. Howden and Young (1981) pointed out that C. angustulus inhabiting Barro Colorado Island is a diurnal species with two peaks of activity, one from 06:00 to 10:00 hours and the other from 15:00 to 18:00 hours, coinciding with the periods of monkey defecation. Food balls are made on the foliage of trees. Canthon angustatus and Canthon subhyalinus have been observed at more than 20 m high in the forest canopy (Howden and Young, 1981). These beetles first separate a fragment of dung that then falls to the ground with the beetles clinging to it, resembling a rain of dung balls and beetles (personal observations). Once on the ground, the beetle reshapes the ball and rolls it like other roller beetles. In Panama, C. angustatus is only associated with howler monkeys in lowland moist forests. It probably does not compete with C. subhyalinus, which prefers high-canopy areas with a flat terrain where C. angustatus is uncommon (Howden and Young, 1981). However, this observation remains to be confirmed in future research on Neotropical arboreal dung beetles.
Canthon subhyalinus is the second arboreal dung beetle species associated with monkeys in Mexico and Central America. Like the other Canthon species, it is small and an excellent flyer. Halffter and collaborators conducted an intense collection of dung beetles in the Palenque forest in 1965 using pitfall traps placed on the ground. Only a single individual of C. subhyalinus was caught in these traps, among other species; in contrast, in traps set at 15 m high, they caught over 222 individuals (see Rivera-Cervantes and Halffter, 1999). These authors reported that in 1993 (this study) in the same zone, no C. subhyalinus specimens were caught in any of 22 traps placed at ground level; by contrast, many specimens were captured in three traps affixed at 5 m, 10 m, 15 m, and 20 m high (Table 1). These observations suggest that C. subhyalinus spends more time in the forest canopy, closely related to Alouatta monkeys. The abundant catches of C. subhyalinus in trees of other tropical forests of Chiapas (Boca de Chajul) and Los Tuxtlas, Veracruz, confirm the above (Estrada et al., 1993; Navarrete and Halffter, 2008).
The dispersal center of C. subhyalinus appears to be the intermontane valleys of Colombia (Rivera-Cervantes and Halffter, 1999; Figure 1A). It is located on the Pacific slope of Ecuador and expands southward to Peru and Bolivia. Along the Caribbean coast, it reaches Venezuela and French Guiana. To the north, C. subhyalinus has been found in Panama, Costa Rica, and in some tropical forest areas of southern Mexico (Lacandona forest, Chiapas, and Quintana Roo). The known northernmost distribution limit is Los Tuxtlas, Veracruz (Rivera-Cervantes and Halffter, 1999).
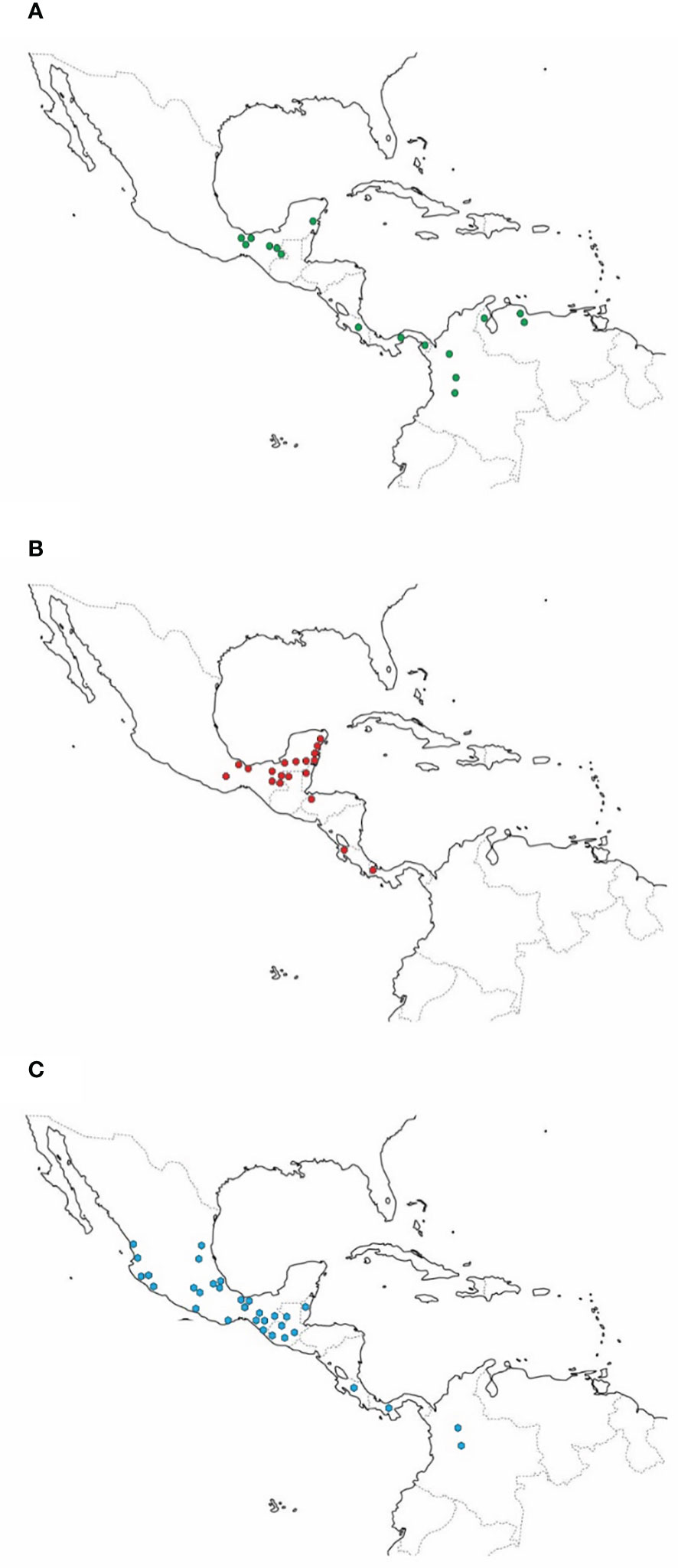
Figure 1 (A) Distribution of Canthon subhyalinus; (B) Distribution of Canthon euryscelis, (C) Distribution of Canthon femoralis.
Canthon euryscelis is also commonly associated with monkeys. This species thrives in evergreen forests and is highly abundant in sites inhabited by Alouatta monkeys, although, unlike the species described above, it can be captured in partially cleared patches (Rivera-Cervantes and Halffter, 1999). The behavior of C. euryscelis is similar to that of C. (Gl.) subhyalinus. In 1993, an average of 2.2 specimens per trap were caught in traps placed on the ground (Rivera-Cervantes and Halffter, 1999), but was quite abundant at 5 m and 15 m high (Table 1). Canthon euryscelis has been caught in tropical forests of southeastern Mexico (Campeche, Chiapas, Quintana Roo, Oaxaca, Tabasco, and Veracruz). In Central America, it has been observed in Belize, Guatemala, Honduras, Costa Rica, and Panama (Rivera-Cervantes and Halffter, 1999, Figure 1B).
Canthon femoralis is also a species commonly associated with monkeys in tropical forests of southeastern Mexico. It is widespread in tropical Mexico, Central America and northwestern South America (Figure 1C). Canthon femoralis has a strong predilection for monkey dung in tropical rainforests. However, this species can be considered the least obligatory height specialist of the four dung-beetle Canthon species since it is also found in cow dung on the ground in partially cleared forest patches (Rivera-Cervantes and Halffter, 1999; Amézquita and Favila, 2010). At the Tropical Biological Station in Los Tuxtlas, Mexico, C. femoralis has been abundantly collected on the ground (Favila and Díaz, 1997), although more than 25 individuals per trap have been caught in traps affixed at 20 m and 27 m high (Estrada et al., 1993). In other tropical forests, this species has been observed flying between 5 m and 15 m or higher (height not specified by the authors) and perching on leaves between 0.3 m and 1.5 m high (Morón, 1979; Rivera-Cervantes and Halffter, 1999). In Palenque, Chiapas, C. femoralis was observed by the first author in 1965 rolling small fragments of monkey dung on leaves.
In Palenque, Chiapas, traps placed at ground level caught 1 to 9 specimens of C. femoralis per trap. This species has also been captured at ground level and 6 m high in Jalcomulco, Veracruz, in a disturbed tropical deciduous forest at an altitude of 400 m asl (Rivera-Cervantes and Halffter, 1999). The presence, and especially the abundance, of C. femoralis is highly variable geographically. It may be the most abundant dung roller beetle associated with monkeys in a given location, while being rare in other similar places. This species probably also uses the dung of other arboreal mammals, like the coati (Nasua narica, Estrada et al., 1993). Canthon femoralis has been caught at elevations ranging from sea level to 1600 m asl (Rivera-Cervantes and Halffter, 1999).
Canthon femoralis makes unique brood balls (Figure 2), which are left abandoned on the ground after being finalized. For its elaboration, the ball is first separated from the upper center or upper surface of a dung mass; the male, the female, or a bisexual pair initiates this operation. Afterward, the male pushes the food ball forward, with the female traveling on top of it. As a result, the ball is gradually coated with a layer of soil (4 mm thick). It has an opening and a “neck” that ends in a well-defined aeration channel (Figure 2). The egg chamber is located at the base of the channel. Nest balls are abandoned on the ground or in a slightly excavated crater (Rivera-Cervantes and Halffter, 1999).
The relationship between dung beetles and monkeys observed in Mexico also occurs in Central America. In Panama, Howden and Young (1981) reported Canthon angustatus Harold and Canthon subyhalinus Harold caught in traps at more than 20 m high within forested areas. Canthon subyhalinus, was found on the leaves among fresh monkey dung masses. The Canthon species fall to the ground clinging to the ball, where the usual rolling process continues. For its part, Gill (1991) observed Canthon aequinoctiale Harold, 1869, at 8 m high eating monkey dung deposited on the leaves but has not been seen building food balls. This author reports highly abundant Canthon (Glaphyrocanthon) sp. associated with Callicebus monkey dung at 10 m high, along with some individuals of Sylvicanthon foveivenre (Schmit, 1920) plus a few specimens of other two Scarabaeinae species. Gill (1991) reported Canthon angustatus in a tropical forest of Panama making balls of monkey dung in the forest canopy.
For reasons not yet explained, there is a remarkable change in the dung beetle-monkey relationship in forests of southwestern Mexico, Central America, and South America. In the tropical forests of Mexico and Central America, there is a clear relationship of Canthon species with monkeys, mostly of the genus Alouatta, with differences in abundances between sites. The perching behavior is common in these Canthon species. According to Gill (1991), the two foraging behaviors observed in dung beetles in the Neotropical region are perching and flying, and differences in both behaviors may influence the dispersal ability of the species. Fast fliers are also widely distributed across South America and Central America. Comparing the geographic range of perching versus flying species during foraging will help to determine whether these two foraging behavior strategies are related.
Noriega (2011) collected Canthon smaragdulus (Fabricius, 1781) in Colombia using a new model of elevated pitfall traps baited with Alouatta seniculus dung. Later, in a study carried out in the same locality, Noriega (2012) compared the assemblages of dung beetles that reached the feces of A. seniculus and Lagothrix lagotricha (Humboldt, 1812), finding 32 species and marked differences in the species composition of beetles attracted by each dung type. Subsequently, Noriega et al. (2020) collected 369 specimens of 21 dung beetle species perching on plants. These included three species of Onthophagus, seven Canthidium, two Dichotomius, two Eurysternus, two Phanaeini (Oxyternon conscipillatum (Weber, 1801) and Phanaeus chalcomelas (Perty, 1830) and five Deltochilini, including Sylvicanthon aequinoctialis (Harold, 1869). Undoubtedly, this assemblage of beetles associated with monkeys is markedly different from the ones collected in Mexico and Central America. Finally, Noriega and Vulinec (2021) summarized the information on dung beetles perching on leaves worldwide.
In the tropical forests of Peru, the relationship of dung beetles with tamarin monkeys (Seguinus myxtrax Spix and Seguinus fuscicollis Spix) was studied by Culot et al. (2011). They followed a tamarin group and collected their feces immediately after defecation. Traps baited with this dung were buried in the ground for 24 hours. These authors collected 330 beetles of 25 species of Scarabaeinae. The medium-sized-to-large species included two Canthidium, two Dichotomius, and numerous species of rollers (Deltochilini). Also in Peru, Larsen et al. (2006) captured Canthon brunneus, C. femoralis bimaculatus, C. sp., and C. subyhalinus in the canopy of a primary terra firma or a floodplain forest. In the French Guiana, Feer (2000; 2015) noted that a severe reduction of the arboreal mammal fauna may also reduce the Scarabaeinae fauna (refer to Nichols et al., 2009).
Vulinec (2002) examined beetles associated with monkeys in Peru, the Amazonas, and Rondonia (Brazil). She estimated that primates eat between 25% and 40% of the biomass produced by the forest, being they an essential element in the food chain. When a part of the forest is cleared, the populations of Scarabaeinae beetles also decrease. Alouatta palliata is a diurnal monkey frequently observed on fig trees (Gill, 1991). Its feces attract multiple species, including hundreds of individuals of C. angustatus, which form a dense cloud that covers the area where monkeys thrive. The stinking, greenish-yellow dung is defecated in the forest canopy, falling onto the vegetation as a cascade. This excrement is transported by beetles as firm balls that are rolled in the canopy until they fall to the ground with the beetles clinging to them. Once on the ground, beetles form male-female pairs that keep rolling the food ball.
Halffter and Matthews (1966) published the first review on the ecology and behavior of the Scarabaeinae, including several of the first publications addressing the relationship between dung beetles and monkeys (or, where appropriate, other mammals). Thus, according these authors Luederwaldt (1922) reported individuals of Canthon (Glaphyroncanthon) quadrigutatus (Olivier) in Tapajos, Brazil, on both monkey feces and a recently hunted monkey, with about ten specimens around the anus. The monkey was reported as corresponding to Alouatta sp by Pereira and Martínez (1956). According to these authors, C. subhyalinus was collected from another monkey, Callicebus brunnens Wager, in Guapó, Brazil.
Vaz de Mello and Louzada (1997) reported the capture in Viçosa, Minas Gerais, Brazil, of 22 specimens of Sylvicanthon foveiventris (Schmidt) (a Canthonina beetle) in traps baited with human feces at 10 m high in the forest, in addition to Canthon (Glaphyrocanthon) sp., Parahyboma furcatum (Laporte, 1840), and Canthidum sp. The forest is home to a rich fauna of Scarabaeinae, including the beetles that thrive on the ground. Although illustrative, the publications reviewed for South America are punctual and in quantity and quality. Some of the beetle species associated with monkeys in Mexico are also found in South America, but other arboreal beetle species in this region are larger than Canthon species (Vaz de Mello and Louzada, 1997).
Published works on African dung beetles are scarce. Walter (1983) studied the beetle-monkey relationship in Gabon, specifically in the Mpassa forest. This was the first study addressing the Scarabaeinae in the canopy of African forests. Traps were placed at 3 m, 5 m, 10 m, and 21 m high in dense, humid, evergreen forests. The traps were wooden squares (50 cm × 50 cm) affixed horizontally at the heights indicated. The lid had a central orifice, which attracted the specimens into the baited trap. Walter collected five species at different heights (Table 2). Similar to our sampling in Mexico, he observed few abundant species. The three most common species were collected mainly at 5 m and 10 m high. All the beetle species caught are small. Sisyphus does not exceed 5 mm in length and Onthophagus is 3.5 mm in length; the exception was O. mpassa, which is 7.2 mm in length. Onthophagus ahenominas (4 specimens) and O. mppasa were the rarest species. The other three beetle species were abundant. Arboreal Sisyphus species make the ball in the canopy, and before leaving it, the ball remains attached to a leaf or a branch, not falling to the ground. Onthophagus species cannot bury their eggs in trees. Thus, eggs are scattered among the dung in the canopy. The association of these beetle species with monkeys and the canopy has made them lose the nesting habit throughout evolution, returning to a highly primitive form, similar to the most primitive Scarabaeinae. Walter (1981) described that Sisyphus arboreus makes balls and rolls them in the canopy of forest trees. In addition, four species of Onthophagus use dung excreted in situ. These fascinating data from Walter are the first to report an Onthophagus species as a roller of an elaborate ball.
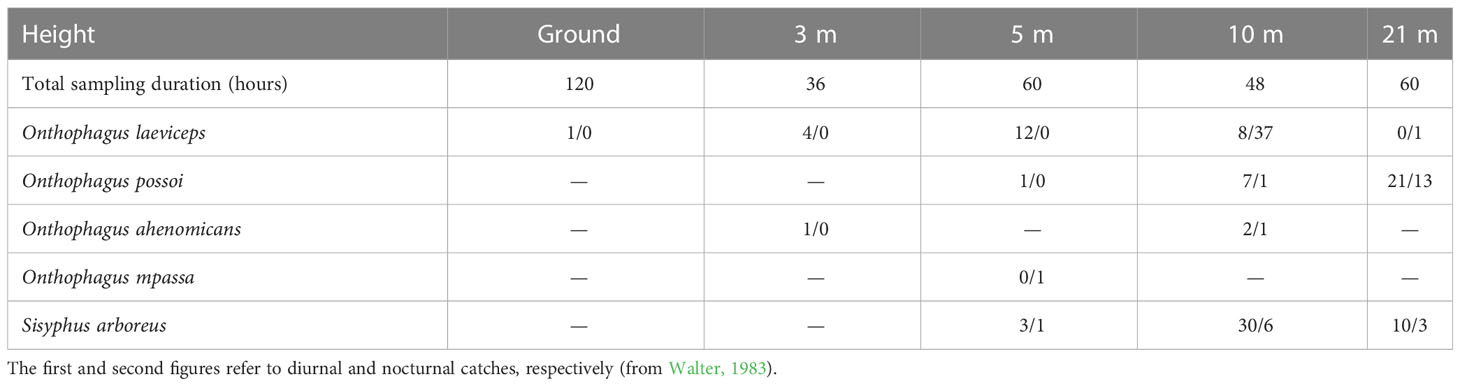
Table 2 Dung beetle species caught by Walter (1983) in the Mpassa forest.
The comments by Hanski and Cambefort (1991) on the Scarabaeinae-monkey relationship in Africa do not fully coincide with those by Walter (1985). The information reported by Hanski and Cambefort (1991) was recorded from the Makukon forests and referred to three species collected between 10 m and 20 m high. A Sisyphus individual was observed rolling a dung ball in the canopy. They also reported that Onthophagus, unable to dig in the canopy, accumulates dung and litter to form a mass that protects the eggs.
The relationship between beetles and monkeys in Africa mentioned above is consistent with our observations in Mexico: very few important beetle species are highly abundant at different heights in forest trees. These findings contrast markedly with the reports for South America and the cases mentioned below, where multiple species are associated with monkey dung.
In the Madagascar forest, the small roller beetle Arachnodes gandi was observed making nests at ground level and 50 cm high (Vadon, 1947). According to Vadon (1947), A. gandi makes a dung ball in the canopy, which falls to the ground, and the beetle continues rolling it as described for other rollers.
This region hosts multiple species of dung beetles, some of which are very large, associated with monkeys of many different taxa. By contrast, in Mexico and Central America this role is played by the Canthonini, which are also abundant in other areas of the Neotropics.
In the Western Ghats, a global biodiversity hotspot in southwestern India, Sabu and Nithya (2016) collected Caccobius gallinus Arrow, Caccobius meridionalis Boucomont, Onthophagus vladimiri Frey, Onthophagus furcillifer Bates, and Onthophagus centricornis (Fabricius) in pitfall traps set at approximate 15 m height attached to a rope. These authors consider that the lower abundance of arboreal primates in this region explains the low abundance of arboreal dung beetles. However, the abundance and diversity of arboreal dung beetles are higher in dry forests because the rainy season is very short and, as a consequence, dung is available in the canopy for longer periods than in the wet forest (Sabu and Nithya, 2016). These authors also proposed that arboreal dung beetles are a more recent group than beetles that forage and reproduce on the ground. Phylogenetic studies are necessary to test this hypothesis.
We have relatively abundant information from the tropical forests of Borneo, which are home to populations of 10 primate species, including colobins, orangutans, gibbons, and two macaque species. Davis et al. (1997) reported having collected 2378 beetles in traps at 5 m and 10 m high. A curious phenomenon has been observed in Borneo, contrasting with observations in other tropical forests: arboreal beetles have not been mostly collected in the primary (undisturbed) forest. Instead, they are collected in high abundances in managed forests or plantations (refer to Davis and Sutton, 1998). According to these authors, the percentage of beetle species caught is distributed as follows: 1.72% in primary forests, 22.32% in managed forests, and 75.96% in forest plantations. According to Davis and Sutton (1998), arboreal beetles are absent on the ground but abundant at 5 m and up to 20 meters high, with some individuals being spotted at 25 m high. This unusual importance of managed forests and plantations was again mentioned by Davis et al. (2000) for Sabah-Borneo. The beetles considered “forest specialists” are primarily found in plantations. In fact, 14 of the 40 species of dung beetles found in plantations are endemic to Borneo.
The importance of the edge of tropical forests in Borneo is the opposite of their role in other tropical forests. Borneo is relatively well studied, and the beetles that dominate in its forests and plantations are taxonomically different from those in other areas. In Borneo, Deltochilini species are not rollers; instead, dung beetle assemblages are dominated by groups that are typically diggers. Davis et al. (2000) highlighted this remarkable shift in behavior.
Davis (1993) pointed out that the use of dung excreted by monkeys (or other arboreal mammals) and carrion by the Scarabaeinae is possible when these food types are available in the canopy in sufficient amounts and with continued supply. This, along with the existence of tall trees, favors the presence of arboreal dung beetles. Given the competitive nature between species that is common in tropical forests, the existence of arboreal beetles highlights the competition for one of their main resources: monkey feces. Borneo is characterized by the diversity of the resources exploited by beetles (Abdul Rahman et al., 2021). The genus Onthophagus, which comprises most beetle species (632) in Sabah Malasyan, Borneo, includes six fruit-eating species, among them one that consumes fruits while these still are hanging from tree branches (the other fruit-eating beetles consume fruits that have fallen to the ground) (Davis and Sutton, 1997). Perching, which occurs in many species, is a food-searching strategy and a thermoregulation mechanism (Davis, 1993). Onthophagus displays the same type of association described by Walter in Africa. When Andrew J. David worked on the subject from 1990 to 1993, he shared with one of us (Halffter) original information (notes and drawings) on the Scarabaeinae inhabiting trees in Borneo from a collecting campaign in Sabah (Borneo, Malay) in 1990. He found three arboreal Onthophagus species associated with primates. Onthophagus nanus was described as the most abundant arboreal beetle species, and it was observed rolling — a truly exceptional behavior for a burrower like Onthophagus. A pair of beetles roll a “ball” (a piece of dung) in the way typically reported for many rollers, such as Canthon species: the male pushes the “ball” forward with his hind legs while the female remains on top of it, serving as a counterweight and favoring the rolling of the food ball.
Two articles Tregidgo et al. (2010), from Malaysia, and Rahman et al. (2021), from Singapore), confirm several of the findings just mentioned: the importance of Onthophagus and Caccobius as arboreal beetles (especially in secondary forests). Both species show the morphological adaptations mentioned above: very long metatarsals, spurs on the metatarsals, and tarsi of the hind legs adapted to hold the foliage better. Among Onthophagus, O. deliencis is particularly abundant, making and rolling dung balls in the forest canopy.
Several species of Macropocopris Arrow (an Onthophagini genus from Australia) have modified tarsal nails that allow them to attach to hair, especially in the anal region (cited by Halffter and Matthews, 1966: 43-44; Hanski and Cambefort, 1991). When a feces pellet is defecated, the beetle falls with it to the ground, where it is used to build the nest.
At the beginning of our systematic information gathering on the Scarabaeinae-monkey relationships in tropical forests around the world, we expected significant differences between regions. The plant composition of these forests differs according to the region, and the arboreal Scarabaeinae fauna is also different (Davis et al., 1997). However, we did not expect to find functional differences as important as those observed. In this chapter, we have examined the most important differences and the questions that arise from them.
In the tropical forest of southern Mexico, arboreal Scarabaeinae species are scarce: four almost permanent and abundant species and one that is rarely observed. The frequent species are small Canthonina, which are excellent rollers. These species can make nest balls in the canopy, then drop the ball while clinging to it, continue rolling it on the ground, and ultimately bury the ball in the usual way described by Halffter and Matthews (1966). The structure and composition of arboreal dung beetle assemblages are similar in Central America, although there may be some additional distinctive species.
In South America, there are far more species of monkeys and beetles. Their number, but also their taxonomic diversity, is much greater. The species are much more taxonomically varied, although the type of association remains relatively unchanged. Interesting, some arboreal species in South America are large species, not yet found in tropical forest of México and Central America.
Information about this relationship obtained in Africa is scarce, but the data available suggest that it differs markedly from the relationship observed in South America. In Africa, the Scarabaeinae associated with monkeys are mostly from genus Sisyphus and Onthophagus. An exceptional feature is that these beetles nest in the canopy. In the case of Sisyphus, the ball made in the tree canopy remains attached to a branch. For its part, the case of Onthophagus is extraordinary. This abundant genus of burrowing species that make a nest on the ground displays a unique behavior in its relationship with the African monkeys: it builds its nests in the canopy using the dung that accumulates between the leaves.
In India, the Scarabaeinae-monkey association comprises numerous species of beetles from different taxonomic groups, including several large species. Further studies in this region will broaden our understanding of arboreal dung beetle habits.
In Borneo, the relationship is radically different. There are multiple arboreal species, but they are mainly found in forest edges and plantations not in undisturbed forests. The opposite of Mexico and Central America, where very few arboreal dung beetle species thrive under these conditions.
An essential aspect detected from comparing all the works focusing on arboreal beetles and their association with primates is the lack of a standardized sampling methodology. There are many types of traps, and no single model is used consistently in different study areas. Different types of traps may yield different results and attract or capture other species (see Bacc et al., 2023).
In 1993 we propose a methodology for the studies of dung beetles as an insect focal group for analyzing the effects of human activity on the biodiversity (Halffter and Favila, 1993). Here we suggest using a standardized methodology to compare the arboreal diversity found at different heights or layers of the tropical forest. We suggest selecting a fixed number of trees, 20 or much better 30, each one separated approach 100 m longtwo, a distance that has been considered adequate to ensure independence between samples. However, we have to investigate which is the minimal distance between traps to this goal. In each tree, we suggest putting two traps hung at 5, 10, 15, 20 and 25 meters high if it is possible. We suggest using the arboreal dung traps proposed by Noriega (2011). The same number of traps must be placed in each selected tree. To avoid bait interference between types of baits, we suggest using a type of bait in each tree (dung, carrion, or fruits). The review of the traps must be made every hour or two hours. If that it is not possible, one in the morning, the other at noon, and the last in the afternoon. We can rebait at the afternoon the traps to collect nocturnal arboreal dung beetles and pick up the traps in the morning (6:00 AM). In an extreme case, we can hang the traps in the trees in the morning until the afternoon (6:00 PM), rebait the traps, and leave them the night picking up them in the morning. We can repeat our sampling protocol for five days. In all cases, the traps must be protected with a cover (a plastic plate) to prevent the traps from flooding, if rains.
We suggest conducting comparative studies on arboreal dung beetles in different regions using the same methodology according to the research objectives. This review opens new areas of research into the role and dynamics of arboreal dung betel in the tropical region.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
With affection and gratitude, GH dedicates this work to Violeta Halffter and Mario Enrique Favila, who were his colleagues in Palenque and lifelong collaborators. With serious difficulties due to illness, the assistance of Virginia Vázquez was essential to write the text and in many other aspects of the edition process. Thanks also to Juan Carlos Serio and Jorge Ramos for the series of photographs (of the howler monkey and its feces), and to Alberto González for his valuable contribution. Thank all of you so much. GH also appreciates the help of Dr. Sara Lariza Rivera-Gasperín, Fernando Escobar, and Victoria Capello for their valuable assistance in the literature survey. Thanks to Dr. Jorge Ari Noriega for reviewing the manuscript; his comments and suggestions were very valuable. María Elena Sánchez reviewed and edited the original version in English; her observations and comments were also very useful. Comments by two reviewers greatly improved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdul Rahman I. L., Yap S., Goh T. G., Toh K. X., Yee Q. H., Puniamoorthy N. (2021). Vertical stratification of dung beetles in young secondary forests of Singapure. Biotropica. doi: 10.1111/btp.13000
Amézquita S., Favila M. E. (2010). Removal rates of native and exotic dung by dung beetles (Scarabaeidae: scarabaeinae) in a fragmented tropical rain forest. Environ. Entomol. 39, 328–336. doi: 10.1603/EN09182
Bacc A., Mateus L. A. F., Peres C. A., Haugaasen T., Louzada J., Hawes J. E., et al. (2023). Bait attractiveness changes community metrics in dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Ecol. Evol. doi: 10.1002/ece3.9975
Cambefort I., Walter P. (1991). “Dung beetles in Tropical Forests in Africa” in Dung Beetle Ecology, eds. Hanski I., Cambefort I. (Princeton University Press), 198–210.
Chamé-Vázquez E. R., Gómez-Gómez B. (2005). Primer registro de Canthon angustatus Harold 1867. En México (Coleoptera: Scarabaeoidea). Acta Zool. Mex. (n.s.) 21, 159–160. doi: 10.21829/azm.2005.2131981
Culot L. D. J., Mamn F. J. J., Muñoz Lazo M., Hynen C., Hewmann E. W. (2011). Tamarins and dung beetles: An efficient diplochrons dispersal system in the Peruanian Amazonian. Biotropica 43 (1), 84–92. doi: 10.1111/j.1744-7429.2010.00655.x
Davis A. L. V. (1993). The Ecology and Behaviour of Rainforest Dung Beetle in Northern Borneo (Ph.D. Thesis University of Leeds), 147.
Davis A. J., Huijbregat H., Krilelacen J. (2000). The role of local and regional processes in shaping dung beetles communities in Tropical forest plantations in Borneo. Glob. Ecol. Biogeogr. 9, 281–292. doi: 10.1046/j.1365-2699.2000.00189.x
Davis A. J., Huijbregts J. M., Spriggs A. M., Krikken, Sutton S. (1997). “The ecology and behavior of arboreal dung beetles in Borneo,” in Canopy Arthrops. Eds. Stork N. E., Adis J., Didham R. K. (Chapman & Hall), 417–432.
Davis A. J., Sutton S. L. (1997). A dung beetle that feeds on fig: Implications for the measurement of species rarity. J. Trop. Ecol. 13, 759–766. doi: 10.1017/S0266467400010919
Davis A. J., Sutton S. L. (1998). The effects of rainforest canopy loss on arboreal dung beetles in Borneo: implications for the measument of biodiversity in derived tropical ecosystems. Divers. Distrib. 4, 167–173. doi: 10.1046/j.1472-4642.1998.00017.x
Estrada A., Halffter G., Coates-Estrada R., Meritt D. A. (1993). Dung beetles attracted to mamMalian herbivore (Alouatta palliata) and omnivore (Nasua narica) dung in the tropical rain forest of Los Tuxtlas, México. J. Trop. Ecol. 9, 45–54. doi: 10.1017/S0266467400006933
Favila M. E., Díaz A. (1997). “Escarabajos coprófagos y necrófagos,” in Historia Natural de Los Tuxtlas. Eds. Soriano E. G., Voght R. D. y R. (Universidad Nacional Autónoma de México), 383–384.
Feer F. (2000). Les Coléopteres coprophages et nécrophages (Scarabaeidae s. str. Et Aphodiinae) de la forét de Guyane francaise: composition spécifique et estructure des peuplements. Ann. Soc Entomol. 36, 29–43.
Feer F. (2015). Les modalités du percher dans un assemblage de Scarabaeinae de la forét de Guyane francaise. Ann. Soc Entomol. 51 (4), 331–340. doi: 10.1080/00379271.2016.1146633
Gill B. D. (1991). “Dung beetles in tropical American forests,” in Dung beetle Ecology. Ed. Hanski I.y. C. (Priceton University Press), 211–229.
Halffter G., Favila M. E. (1993). The Scarabaeinae (Insecta: Coleoptera) an animal group for analyzing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biol. Inter. 27, 15–21.
Halffter G., Halffter V. (2009). Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits of vegetable detritus. Boletin S, E.
Halffter G., Matthews E. G. (1966). The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Fol. Entomol. Mex. 12-14), 1–312.
Howden H. F., Young O. P. (1981). Panamanian scarabaeinae: taxonomy, distribution and habits. Contr. Am. Entomol. 18 (1), 1–204.
Jacobs J., Nole I., Palminteri S., Ratcliffe B. (2008). First come, first serve: "sit and wait" behavior in dung beetles at the source of primate dung. Neotrop. Entomol. 37 (6), 641–645. doi: 10.1590/S1519-566X2008000600003
Larsen T. H., Lopera A., Forsyth A. (2006). Extreme Trophic and habitat specialization by Peruvian dung beetles (Coleoptera, Scarabaeidae, Scarabaeinae). Col. Bull. 60 (9), 315–324. doi: 10.1649/0010-065X(2006)60[315:ETAHSB]2.0.CO;2
Luederwaldt H. (1922). Ein Canthon (Coleopt. Lamellicornidae, coprinae) and Affen. Deutecher Vercin, Weissenschaff and Knnst. São Paulo, Brazil.
Mathews (2007). “Palenque arqueology: an introduction,” in Palenque. Recent Investigations at the classic Maya Center. Ed. Marken D. B. (AltaMira Press, USA), 3–16.
Morón M. A. (1979). Fauna de coleópteros lamelicornios en de la Estación de Biología Tropical “Los Tuxtlas”, Veracruz, México. An. Inst. Biol. UNAM. 50 Ser. Zoología 1), 375–454.
Navarrete D., Halffter G. (2008). Dung Beetle (Coleoptera, Scarabaeidae: Scarabaeinae) diversity in continuos forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: The effects of antrophogenic changes. Biodivers. Conserv. 17, 2869–2892. doi: 10.1007/s10531-008-9402-8
Nichols E., Gardner T. A., Perez C. A., Spector S. (2009). Co-declining mammals and dung factors an impeding ecological cascade. Oikos 118, 481–487. doi: 10.1111/j.1600-0706.2009.17268.x
Noriega J. A. (2012). Dung beetles (Coleoptera: Scarabaeinae) attracted to Lagothrix lagotricha (Humboldt) and Alouatta seniculus (Linnaeus) (Primates: Atelidae) dung in a Colombian Amazon Forest. Psyche 437589, 1–6. doi: 10.1155/2012/437589
Noriega J. A. (2015). “How a locality can have so many species? A case study with dung beetles (Coleoptera: Scarabaeinae) in a tropical rain forest in Colombia,” in Beetles: Biodiversity, Ecology and Role in the Environment. Ed. Stack C. (New York, USA: Nova Science Publishers, Inc), 175–204.
Noriega J. A., March-Salas M., Pertierra L. R., Vulinec K. (2020). Spatial partitioning of perching on plants by tropical dung beetles depends on body size and leaf characteristics: a sit-and-wait strategy for food location. Ecol. Entomol. 45 (5), 1108–1120. doi: 10.1111/een.12898
Noriega J. A., Vulinec K. (2021). Perching behavior by dung beetles (Coleoptera: Scarabaeidae): A spatial segregation mechanism to dilute interspecific competition in highly rich assamblages? Ann. Entomol. Soc Am. 114 (1), 17–26. doi: 10.1093/aesa/saaa040
Pereira I. S., Martínez A. (1956). Os genero de Canthonini Americanos (Col. Scarabaeidae). Rev. Bras. Ent. 6, 91–192.
Rahman I. L. A., Yap S., Goh T. G., Toh K. X., Yee Q. Q. H., Puniamoorthy N. (2021). Vertical stratification of dung beetles in young secondary forests of Singapore. Biotropica 53, 1522–1534. doi: 10.1111/btp.13000
Rivera-Cervantes L. E., Halffter G. (1999). Monografía de las especies mexicanas de Canthon del subgénero Glaphyrocanthon (Coleoptera: Scarabaeidae, Scarabaeinae). Acta Zool. Mex. 77), 23–150. doi: 10.21829/azm.1999.77771693
Sabu T. K., Nithya S. (2016). Comparison of the arboreal dung beetles (Coleoptera: scarabaeidae: scarabaeinae) of the wor and dry forests of western India. Coleopt. Bull. 70 (1), 144–148. doi: 10.1649/072-070.0121
Sánchez-Huerta J. L., Moctezuma V., Halffter G. (2019). Nuevo registro de distribución de Canthon angustatus. Harold en Veracruz. Southwest. Entomol. 44 (1), 353–355. doi: 10.3958/059.044.0143
Solís A., Kohlmann B. (2002). El género Canthon (Coleoptera, Scarabaeidae) en Costa Rica. Gio. Ital. Entomol. 10, 1–67.
Tirado-Herrea E. R., Vulinec K., Knogge C., Heymann E. W. (2002). Sit and wait at the source of dung-an unusual strategy of dung beetles. Ecotropica 8, 87–888.
Tregidgo D. J., Qir L., Barlow J., Sodhi N. S., Lee-Hong Linn S. (2010). Vertical stratification responses of an arboreal dung beetle species to tropical forest fragmentation in Malasya. Biotropica 42 (5), 521–525. doi: 10.1111/j.1744-7429.2010.00649.x
Vadon J. (1947). Les epilissiens de Madagascar (Coleoptera, Scarabaeidae, Canthonini) II Bulletin de l´Academie Malagache 26, 173–174.
Vaz de Mello F. Z., Louzada J. N. C. (1997). Consideracoes sobre forrageo arbóreo por Scarabaeiadae (Coleoptera, Scarabaoidea), e dados sua ocorrencia em lloresta tropical do Brasil. Acta Zool. Mex. (n.s.) 72, 55–61. doi: 10.21829/azm.1997.72721737
Vulinec K. (2002). Dung beetle communities and seed dispersal in primary forest and disturbed land in Amazonia. Biotropica 34 (2), 299–309. doi: 10.1111/j.1744-7429.2002.tb00541.x
Vulinec K., Mellow D. J., Fonseca C. R. V. (2007). Arboreal foraing heigth in a common neotropical dung beetle, Canthon subhyalinus Harold (Coleoptera: Scarabaeidae). Col. Bull. 61 (1), 75–81. doi: 10.1649/915.1
Walter P. (1981). Aphodiinae et Trogidae du plateu Bateke zairois (col.), Nouv. Rev. Ent. 11 (4), 343–349. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCALZOOLINEINRA83X0096558
Walter P. (1983). Contribution a la connaissance des Scarabéides coprophages du Gabon. 2. Présence de populations dans la canopée de la foret Gabonaise. Bull. Soc Entomol. 88, 514–521. doi: 10.3406/bsef.1983.18085
Keywords: Scarabaeinae, arboreal, trees, tropical forests, dung
Citation: Halffter G and Favila ME (2023) The relationships between dung beetles and monkeys in the Neotropical region. Front. Ecol. Evol. 11:1212879. doi: 10.3389/fevo.2023.1212879
Received: 27 April 2023; Accepted: 02 August 2023;
Published: 23 August 2023.
Edited by:
William Wyatt Hoback, Oklahoma State University, United StatesReviewed by:
Alfredo Ramírez-Hernández, Instituto Potosino de Investigación Científica y Tecnológica (IPICYT), MexicoCopyright © 2023 Halffter and Favila. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario E. Favila, bWFyaW8uZmF2aWxhQGluZWNvbC5teA==
†These authors have contributed equally to the work
‡Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.