
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 27 September 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1199438
This article is part of the Research Topic Proceedings of INTECOL 2022: LOOK UP! CONSERVATION AND RESTORATION View all 6 articles
It is now largely recognized that pollinators are threatened in agricultural habitats. Cities are thus seen as potential refuges for pollinators, if suitable green spaces are available, because they present favorable abiotic conditions for many pollinator species. However, data on resources used by bees in urban habitats are scarce. Moreover, promoting indigenous meadows in urban green spaces could help pollinator’s survival. In this study, Apis mellifera was taken as a model to investigate potential difference in plant diversity used in agricultural and urban habitat. Pollen loads were sampled in 15 hives in both habitat types, using pollen traps. Then, the attractiveness of a melliferous meadow on wild bees was tested. To that end, a new seed mix (BF) including 35 indigenous plants producing nectar and/or pollen harvested by bees was developed and its attractiveness was compared to a seed mix widely used in Geneva (PFG). For most of the season, quantity and diversity of the pollen sampled was not significantly different between agricultural and urban habitats. Nevertheless, honey bees used different species in both habitats, probably because different plant communities are present. Sixty-one wild bee species were observed foraging in the new BF seed mix compared to only 47 species in the PFG. Likewise, more plants species were used in the BF seed mix than in the PFG. These results show that urban zones can be interesting for pollinators because they display diverse and abundant plant communities. Additionally, it shows that urban parks are species rich habitats, and that pollinator communities respond immediately to additional resources when available.
The use of pesticides, the homogenization of landscapes and the loss of natural habitats have been identified as the main causes for the decline of pollinator diversity in agricultural land (Brown and Paxton, 2009; Potts et al., 2010; Goulson et al., 2015). This is especially concerning as pollinators are essential to 75% of the worldwide crop production (Klein et al., 2007). Several authors suggested that, at moderate level of urbanization, cities could stand as refuges for pollinator populations (McKinney, 2008; Gunnarsson and Federsel, 2014; Hall et al., 2017; Baldock, 2020). Indeed, many cities include resource rich green spaces, displaying suitable abiotic conditions (i.e. warm and dry) for many pollinator species, especially in the wild bee group (Apoidea). Indeed, most of wild bee species is thermophilic and beneficiates from flower-rich, well-exposed meadows (Westrich, 2019), which corresponds to what can often be found in urban green spaces (Lahr et al., 2018). Besides, pesticides are less used in urban green spaces than in agricultural habitats (Muratet and Fontaine, 2015).
For several years, governments deploy strategies in favor of Biodiversity, including actions aiming at maintaining and improving their hosting potential for biodiversity in cities (Federal Office for the Environment (FOEN), 2017). For example, the use of insecticides is banished from urban public areas since 2017 in Geneva city and a biodiversity strategy plan has been implemented by Geneva region authorities since 2018 (Etat de Genève, 2018). One of the most efficient action favoring biodiversity, appears to be improving resource availability in urban areas (Bretzel et al., 2016; Dylewski et al., 2019; Wilson and Jamieson, 2019; Wenzel et al., 2020). However, the way urban green spaces are maintained is often not in favor of wild bee populations. For example, frequent mowing maintaining tidy lawns prevents many valuable plant species from flowering, consequently decreasing resources available for bees (Larson et al., 2013; Shwartz et al., 2013). This means that cities can be largely different from one another in their potential for hosting pollinator populations, depending on factors such as resources they provide, their maintenance policy for green spaces, the size and the connectivity between green spaces or the percentage of impervious surface (Gunnarsson and Federsel, 2014; Beninde et al., 2015; Turo and Gardiner, 2019; Fauviau et al., 2022).
Thus, more data are needed to determine what measures cities can implement for biodiversity, whether cities can really function as an asset for pollinator conservation and if more efforts in bringing floral resources is truly efficient to increase wild bee abundance and diversity in urban areas (Beninde et al., 2015; Bretzel et al., 2016; Ayers and Rehan, 2021).
We focused our study on wild and domestic bees, as they are known to be the most efficient pollinators (Ollerton, 2017).
In this study, we first investigated what resources are available for bees in urban areas, taking domestic bees as a model. We aimed at testing whether a difference in resource availability exists for bees between urban and agricultural habitats in Geneva region. The following questions were addressed: Are the plant species used by bees in both habitats the same? Is there a difference in the quantity of resource available for bees between urban and agricultural habitats? We hypothesized that Geneva is a relatively species-rich city, hosting many indigenous and exotic plant species, and providing enough trophic resources to maintain healthy colonies within urban habitats.
Secondly, we wanted to investigate whether developing a seed mix, especially designed to fulfill wild bee species food requirements would increase resource availability in urban parks. We think that bringing more resources into cities, for example by adding indigenous meadows in urban parks, can benefit domestic and wild bees. This should be especially true in the summer months, when the cycles of mass-flowering plants, such as oilseed rape (Brassica napus) are completed. We hypothesized that a seed mix essentially composed of plants species known to be used by bees can perform equally or even better with fewer plant species than mixes already commercialized, composed of many plant species that are not all used by bees.
In order to compare resources used by bees between urban and agricultural habitat, we sampled pollen load in 10 urban and 5 agricultural hives in the region of Geneva (Figure 1), using pollen traps (Topitzhofer et al., 2021). These traps were set off collecting pollen during 24 hours every second week, between May and October 2016, for a total of 11 sampling dates. Pollen loads were brought back to the lab the same day and stored at −20°C. Each sample was then weighted and a subsample of 350 pollen loads was randomly selected. These 350 pollen loads were sorted according to color and the number of pollen loads for each color was counted. We also weighted approximately thirty pollen loads subsamples, where the exact number of loads were known, in order to assess the mean weight of a single pollen load, which enabled us to calculate the contribution of each plant group within the whole sample. Usually, domestic bees collect one plant species at a time (Percival, 1947; Louveaux, 1990; Topitzhofer et al., 2021) which enable us to make one microscopic preparation for each color of pollen load. Because several plant species can display very similar colors, we used three to five pollen loads among a color, for each microscopic preparation. Pollen could then be identified to the furthest taxonomical level using morphological criteria under a light microscope (Moore et al., 1991; Kirk, 1994; Karpovich et al., 2015).
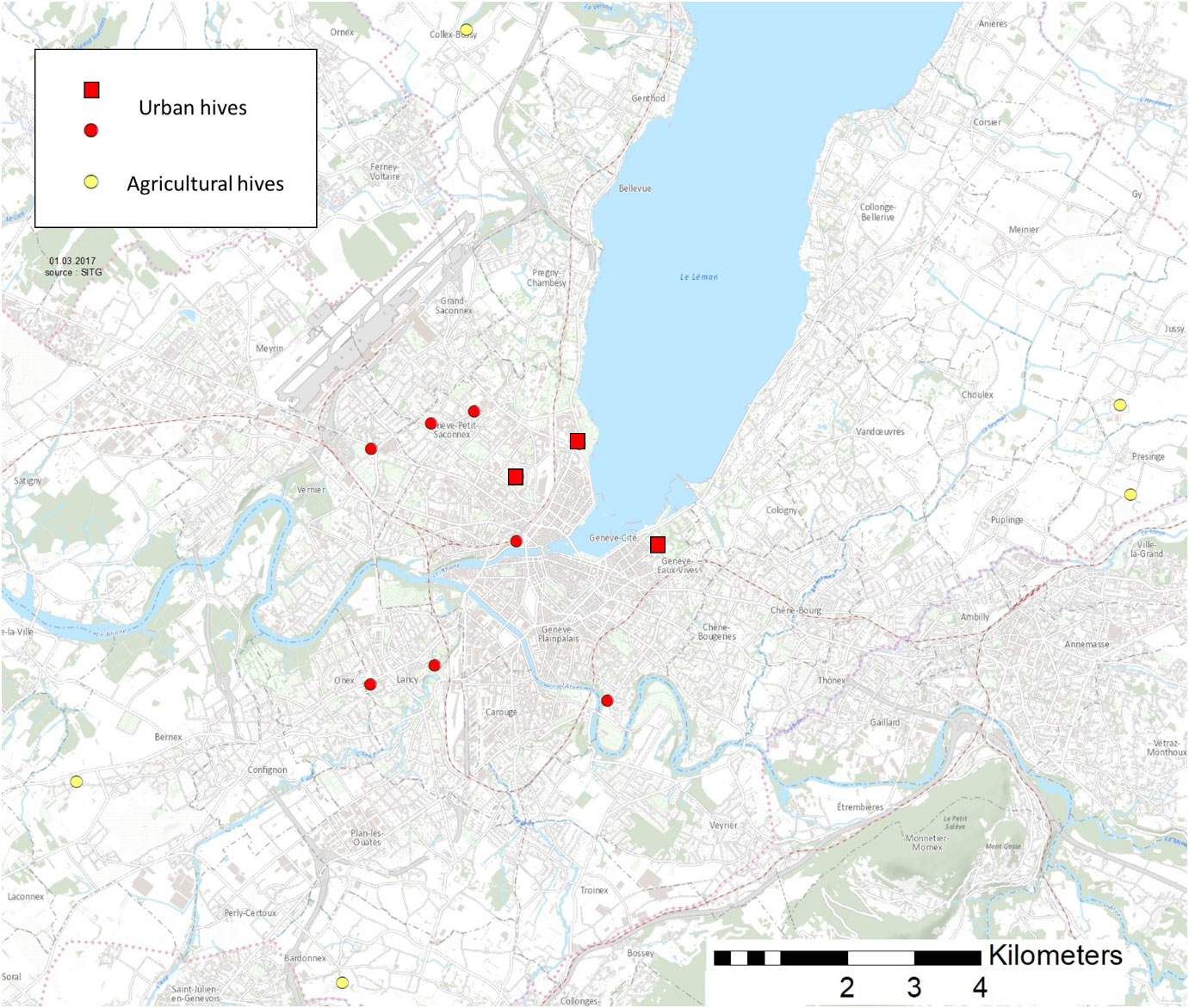
Figure 1 Position of the 15 hives sampled around Geneva region. Yellow marks represent agricultural hives, red marks represent urban hives. Red squares represent the three urban parks were the comparison of seed mixes was implemented.
Based on the list of plant taxa we found to be collected by domestic bees in both habitats and on the literature search as well as expert recommendations (Bieri, personal communication; Pritsch, 2007; Westrich, 2019), we elaborated a new seed mix designed to be sown in urban parks. Three criteria were applied to select the plant species included in the BF seed mix: (1) the different plant species must be used by bees (for pollen, nectar or both), (2) they must be ecologically adapted to environmental conditions in target parks, and (3) together, their flowering phenology must span throughout the whole season. This specific mixture of seed targets a nectariferous and polliniferous semi-dry meadow (Mesobromion) from central European regions. Because we know that wild bee species might use different plant families depending on specific traits (i.e. morphology or level of specialization) (Danforth et al., 2019; Westrich, 2019) and that plant from the same family are likely to display similar pollen nutritional content (Jeannerod et al., 2022), we tried to maximize the number of plant families included in the seed mix so as to maximize the attractiveness of the mix for a large number of wild bee species. The initial theoretical composition of this central European Mesobromion includes 50 species displaying an optimum level of development within this community. The list of high-frequency species was extracted from the database compiled by R. Pantke, used as support for the Phytosuisse project (Prunier et al., 2014; Prunier et al., 2019). Protected, non-native or regionally vulnerable species (Mombrial et al., 2020) were excluded. Polliniferous and nectariferous production and the flowering period were considered using a four-scale level (Pritsch, 2007). A selection of nectariferous and polliniferous species, specific to the pioneer communities of the Dauco-Melilotion was added in order to facilitate the initiation of the plant succession. The final list considers the availability of seeds from local suppliers certifying local origin for the region, according to the Swiss green list (infoflora, 2023). Two Poaceae (Bromus erectus and Festuca rubra) were added in order to help structuring the plant community at the early stage of the succession. Finally, this mix called Beeflora (BF), includes 37 indigenous plants species within 17 families, all of them being used by bees for pollen, nectar or both, except from the two grass species (Appendix 1).
The attractiveness of the BF seed mix was compared to a commercial seed mix called “Prairie fleurie genevoise” (PFG), which is already largely sown in Geneva region. The PFG seed mix is composed of 45 indigenous plant species, including 15 grass species. Both seed mixes were sown in three urban parks in the city of Geneva in autumn 2017 (LaGrange, MonRepos and Beaulieu) on 16 to 25 m2 plots (Figure 1). The attractiveness of the seed mixes was monitored from April to August 2019, for a total of 9 sampling dates. On each date, we followed the attractiveness of the two seed mixes using one square meter quadrat, placed according to the presence of flowering plants. For each quadrat, we first estimated the covering percentage of every plant species that displayed flowers ready to be visited by bees. We then observed the quadrat for two minutes and recorded every bee visit along with the plant species it was visiting. When possible, we identified bees directly in the field. The rest of the species was collected, brought back to the lab and prepared for identification. Bees that could not be captured were identified to the furthest taxonomical level directly in the field. Wild bees collected were identified to the species level using Amiet et al. (2001; 2004; 2007; 2010; 2014; 2017). For each sampling date, park and seed mix, we used four quadrats of one square meter.
In order to compare abundance and diversity of pollen collected in agricultural and urban habitats at each sampling date, we first used a Wilcoxon test, testing the difference in pollen total weight between agricultural and urban colonies for each sampling date separately. To test the influence of the type of habitat at each sampling date on pollen taxa richness, we also used a Wilcoxon test. Then, we sorted pollinic resources according to plant traits, grouping trees and bushes on the one hand and herbaceous plants in the other hand and we tested whether the proportion of each plant type changed during the season using Qi square, in urban and agricultural habitats separately. In order to detect whether domestic bees had collected more quantity of pollen from herbaceous or from ligneous plants at each sampling date, we then applied a Wilcoxon test, comparing quantities of pollen loads collected from herbaceous or ligneous plants in urban and agricultural habitats separately. For the sake of clarity, we converted the number of pollen loads in grams on figures that illustrate the comparison of pollen quantity collected between ligneous and herbaceous vegetation.
To compare abundance and diversity of bees between BF and PFG mixes throughout the season, we first cumulated all the visits for a given park and a given mix in a month. We then used a general linear model with mixed effect to test whether the number of visits and the number of species visiting mixes were different between mixes for each months of the season. Wild bee total abundance was log-transformed in order to reach normality. We also tested the total number of visits of wild bee species on spontaneous plants compared (i.e. from the seedbank) to the number of visits on plant species sawn for both mixes, using linear model. We repeated the same analysis for the number of visits for domestic bees.
For this analysis, we cumulated all the visits of wild bees or domestic bees for a given category of plant (from the seedbank vs from a seed mix) throughout the hole season for a given park. The number of visits of domestic bees was log-transformed to reach normality.
In order to compare the resource availability between seed mixes, we took the covering percentage of flowering plants cumulated over the whole season for a given park, sorted plants according to their origin (from the seedbank vs from a seed mix) and compared the quantity of flowering plants between the two mixes using a linear model, taking the origin of the plant species as a factor.
All statistical analyses were performed using R software (R Core Team, 2010).
In total, we collected nearly 2 Kg of pollen, representing around 290,000 loads. One hundred and twenty-nine plant taxa of different taxonomical resolutions were observed, 115 in urban and 92 in agricultural habitat. These taxa belonged to 60 different families, among them 27 trees and bushes, 31 herbaceous plant families and two species of climbing plants. The Wilcoxon tests comparing pollen quantities collected between agricultural and urban habitats throughout the season showed that there was no difference in abundance of pollen collected in both habitats for all sampling dates except mid-May, where agricultural colonies collected significantly more pollen than urban colonies (on average respectively 53.04g ± 2.37 and 38.4g ± 23.97 for agricultural and urban colonies; p = 0.013, w = 45; Figure 2). Likewise, the number of plant taxa collected by urban and agricultural colonies were not different along the season for all sampling dates, except in the beginning of May where the agricultural colonies collected significantly more taxa than urban colonies (on average respectively 9.2 ± 1.2 taxa and 6.11 ± 0.54 taxa for agricultural and urban colonies; p = 0.012, w = 40; Figure 3). The Qi square tests, comparing proportion of pollen between ligneous and herbaceous vegetation throughout the season showed differences in proportion of plant types during the season for agricultural and urban colonies (p < 0.001, X2 = 61,694.45 and p < 0.001, X2 = 35,935.84 for urban and agricultural colonies respectively). The comparisons of the quantity of pollen collected from ligneous or herbaceous vegetation for each sampling date showed that there is significantly more trees and bushes used by urban colonies in the two first sampling dates (p = 0.024, w = 66 and p = 0.006, w = 85, for the beginning of May and mid-May respectively) and significantly more trees and bushes than herbaceous plants collected in mid-May by agricultural colonies (p = 0.009, w = 25). On average, urban colonies collected 11.66g ± 3.33 of pollen from trees and bushes and only 2.63g ± 1.29 of pollen from herbaceous vegetation in the beginning of May and 37.06g ± 23.93 of pollen from trees and bushes and only 0.64g ± 0.16 of pollen from herbaceous vegetation in mid-May. Agricultural colonies collected on average, 43.52g ± 5.26 of pollen from trees and bushes and only 8.94g ± 4.58 of pollen from herbaceous vegetation in mid-May (Figures 4A, B).
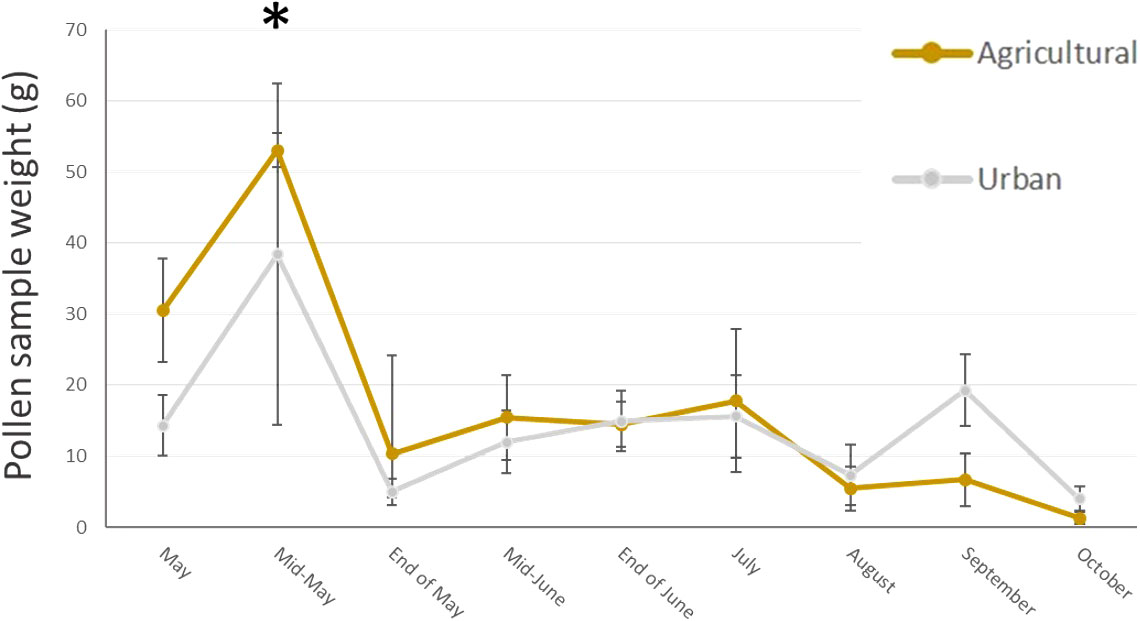
Figure 2 Mean pollen quantity (g) collected by urban (grey line) and agricultural (brown line) colonies at each sampling date. Error bars represent standard error, stars represent significant differences: *<0.05.
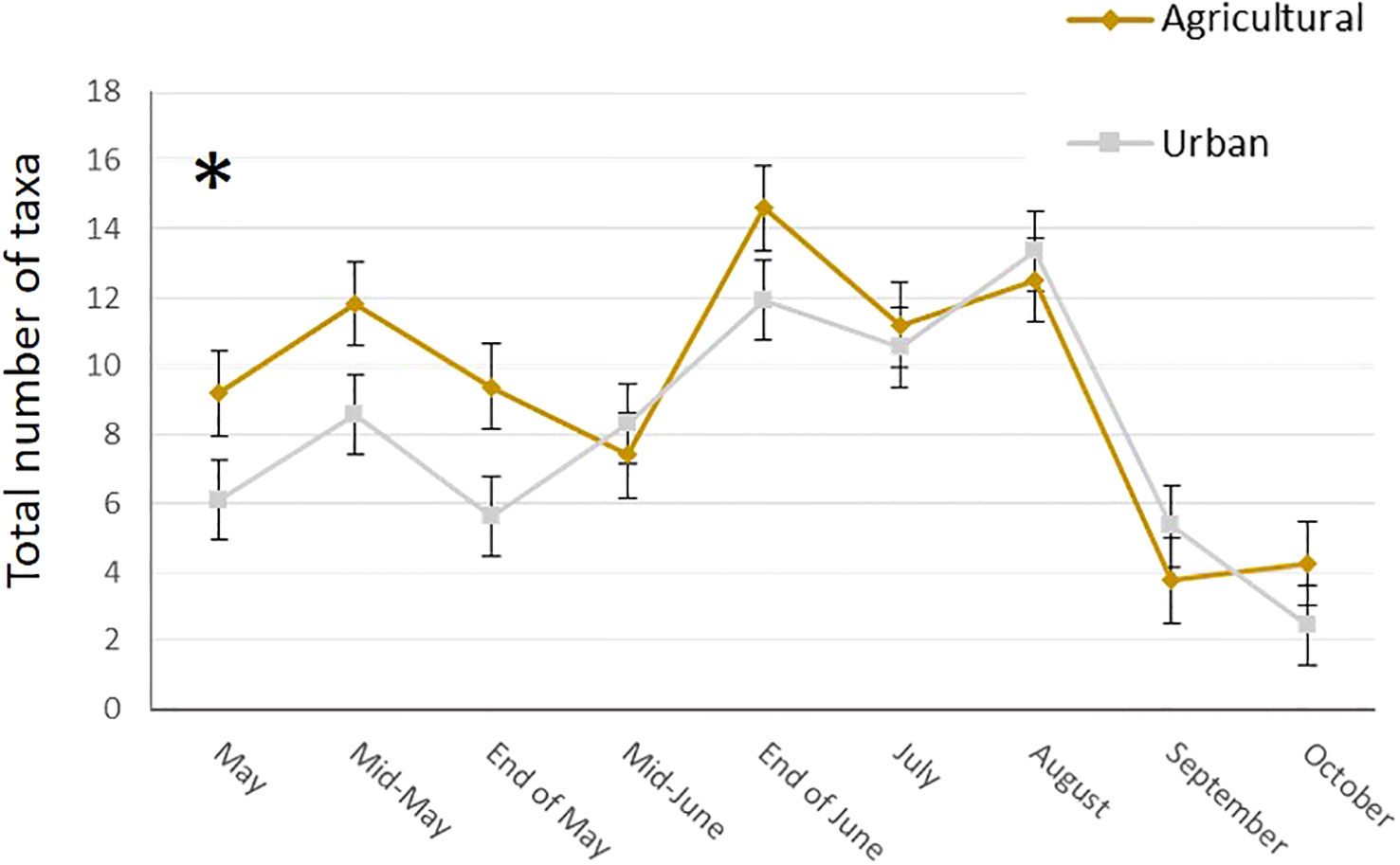
Figure 3 Mean number of taxa collected by urban (grey line) and agricultural (brown line) colonies at each sampling date. Error bars represent standard error, stars represent significant differences: *<0.05.
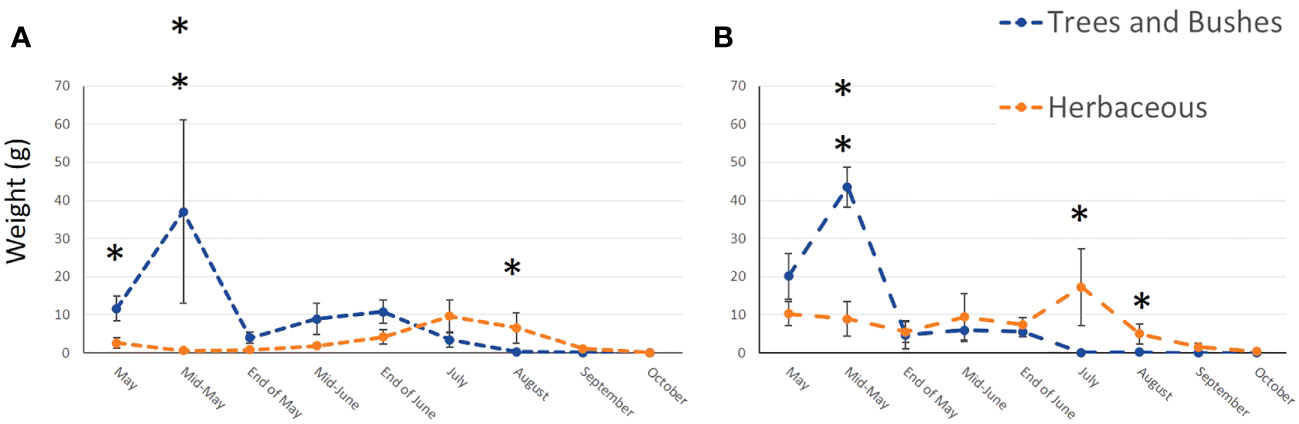
Figure 4 Mean weight of pollen collected from ligneous (blue line) and herbaceous (orange line) vegetation throughout the season by urban (A) and agricultural (B) colonies. Error bars represent standard error, stars represent significant differences: *<0.05; **<0.01.
We observed the opposite trend at the end of the season, where herbaceous vegetation was more used than ligneous one by agricultural and urban colonies. However, our results showed that agricultural colonies use significantly more herbaceous than ligneous vegetation in July and August (p = 0.009, w = 0 and p = 0.029, w = 0, for July and August respectively), whereas urban colonies use significantly more herbaceous than ligneous vegetation only in August (p = 0.01, w = 2). On average, agricultural colonies collected 17.25g ± 10.01 of pollen from herbaceous vegetation and only 0.04g ± 0.03 of pollen from trees and bushes in July and 4.97g ± 2.64 of pollen from herbaceous vegetation and only 0.13g ± 0.04 of pollen from trees and bushes in August. Urban colonies collected on average, 6.54g ± 3.97 of pollen from herbaceous vegetation and only 0.32g ± 0.19 of pollen from trees and bushes in August (Figures 4A, B).
Indeed, the comparison between agricultural and urban colonies for the top 5 most abundant plant taxa collected at each sampling date shows that urban colonies mostly harvest pollen from ligneous vegetation in the three first sampling dates (ligneous Rosaceae, Acer pseudoplatanus, Aesculus hippocastanum and Acer campestre in May; Acer pseudoplatanus, Acer platanoides, Aesculus hippocastanum, Acer campestre and ligneous Rosaceae in mid-May and Cornus sanguinea, Gleditsia sp. Liriodendron tulipifera and ligneous Rosaceae in the end of May) whereas for agricultural colonies, several herbaceous plant species belong to the top 5 most abundant plants collected already at the beginning of the season (Pisum sp. and Brassica napus in May, Trifolium pratense in mid-May, Centaurea cyanus, Papaver rhoeas, and Brassica napus in the end of May). In the end of June, urban colonies still used a majority of ligneous plant species (Tilia sp., Ligustrum vulgare, Rubus sp. and Castanea sativa), whereas the plant species mostly used by agricultural colonies only included Castanea sativa and Rubus sp. The rest of the top 5 most used plant species were Centaurea cyanus and species from the Poaceae family. At the end of June, agricultural colonies also collected important quantities of fungi spores. In July, agricultural colonies mostly collected pollen from herbaceous species (Poaceae family, Plantago lanceolata, Helianthus annuus, Trifolium repens and herbaceous Rosaceae), whereas urban colonies still used important quantity of ligneous species (Ligustrum vulgare and Magnolia sp.). In August, urban and agricultural colonies mostly used herbaceous plant species (Taraxacum officinale aggr., Plantago lanceolata, herbaceous Rosaceae and herbaceous Fabaceae for urban colonies and Poaceae and Asteraceae for agricultural colonies). In September and October, urban and agricultural colonies mostly used Hedera helix, which represented 99% and 72% of the pollen collected by urban and agricultural colonies respectively. Finally, we selected several herbaceous plant species, such as Centaurea species or Cichorium intybus from the Asteraceae family, Echium vulgare or species from the Fabaceae family, that were especially appreciated by domestic bees, to elaborate our new BF seed mix.
In total, we observed 893 plant–bee couples during the monitoring of the Prairie fleurie genevoise (PFG) and the Beeflora (BF) seed mixes in 2019. Four-hundred and ninety-one couples were observed in the BF mix and 402 were observed in the PFG mix. Four hundred and ninety-four wild bees were captured and identified to the species level, 269 were identified in situ including 222 Apis mellifera, and 130 wild bees could not be captured or identified. Sixty-nine wild bee species were identified in total, 61 species were observed in the BF mix and 47 in the PFG mix. Twenty-two wild bee species were strictly observed in the BF mix, whereas only 8 species were strictly observed in the PFG mix. These 8 species were observed visiting plants that are also present in the BF seed mix, two of them being spontaneous (Taraxacum officinale aggr. and Veronica filiformis), whereas 9 wild bee species strictly observed in the BF seed mix were visiting sawn plants that are absent from the PFG seed mix (Cichorium intybus, Echium vulgare, Lotus corniculatus, Malva moschata, Melilotus officinalis and M. albus). Thirty-nine wild bee species were observed in both seed mixes. Fifty-one plant species were visited by bees, 33 plant species in the BF mix (including 21 sawn species, 64%) and 31 in the PFG mix (including 17 sawn species, 55%) (Appendix 1). Although we found more species and individuals visiting plants in the BF than in the PFG seed mix in total, the models testing the effect of the mix and the sampling month on the abundance and richness of wild bees showed that there is, on average, no difference between the BF and the PFG seed mix (p = 0.4, df = 18 and p = 0.56, df = 18, for abundance and richness of wild bees respectively). Nevertheless, the models showed that the month of sampling had a significant effect on abundance and diversity of wild bees (p < 0.001, df = 4 for abundance and diversity). Indeed, there was more species and individuals visiting both mixes in June and July than in all other sampling dates (Figures 5A, B). Additionally, the model testing the effects of seed mixes and of the origin of the plant visited (spontaneous vs sawn) on the number of wild bee visits showed that wild bees used significantly more often the plant species originated from the mix than spontaneous plant species in both seed mixes (p < 0.01, df = 1; Figure 6). Likewise, domestic bees also used more often plants originated from the mix than spontaneous species (p < 0.001, df = 1), but they also visited significantly more often the BF than the PFG mix (p < 0.05, df = 1; Figure 7). In the same way, the model testing the effect of the seed mix and the origin of the plant (spontaneous vs sawn) on the quantity of flowering plants showed that there are significantly more flowering plants originated from the mix than spontaneous flowering plants in the BF mix (p < 0.01, df = 1), whereas in the PFG mix, both spontaneous and sawn flowering plants showed similar total quantity (p = 0.35, df = 1; Figure 8). This is in line with the observation of the most visited plants in both seed mixes. Indeed, within the 9 most used plants in the PFG mix (Leucanthemum vulgare aggr., Achillea millefolium, Salvia pratensis, Daucus carota, Reseda lutea and Centaurea jacea), accounting for 85% of the visits, 3 were spontaneous (Taraxacum officinale aggr., Geranium molle and Veronica filiformis), whereas in the BF seed mix, only one spontaneous plant (Veronica filiformis) belongs to the 9 most used plants, accounting for 77% of the visits (Malva moschata, Echium vulgare, Melilotus albus, M. officinalis, Daucus carota, Salvia pratensis, Centaurea jacea and Reseda lutea) (Appendix 2).
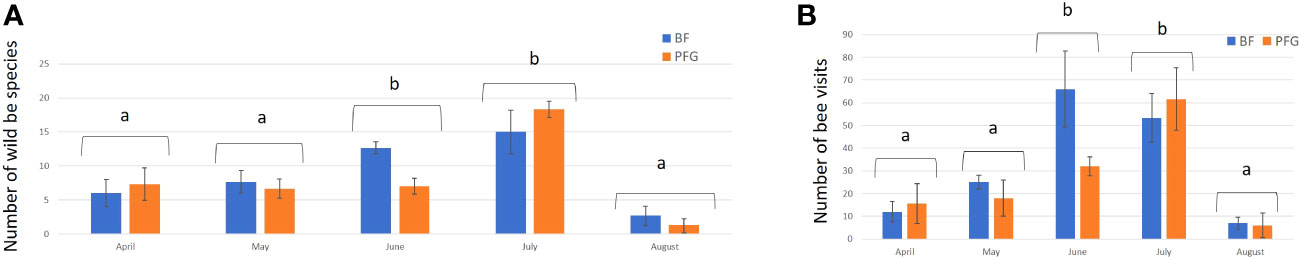
Figure 5 Mean number of wild bee species (A) and mean number of bee visits (B) in the Beeflora (BF) seed mix (blue bars) and the Prairie Fleurie Genevoise (PFG) seed mix (orange bars) for each sampling month. Error bars represent standard deviation, letters indicate significant differences.
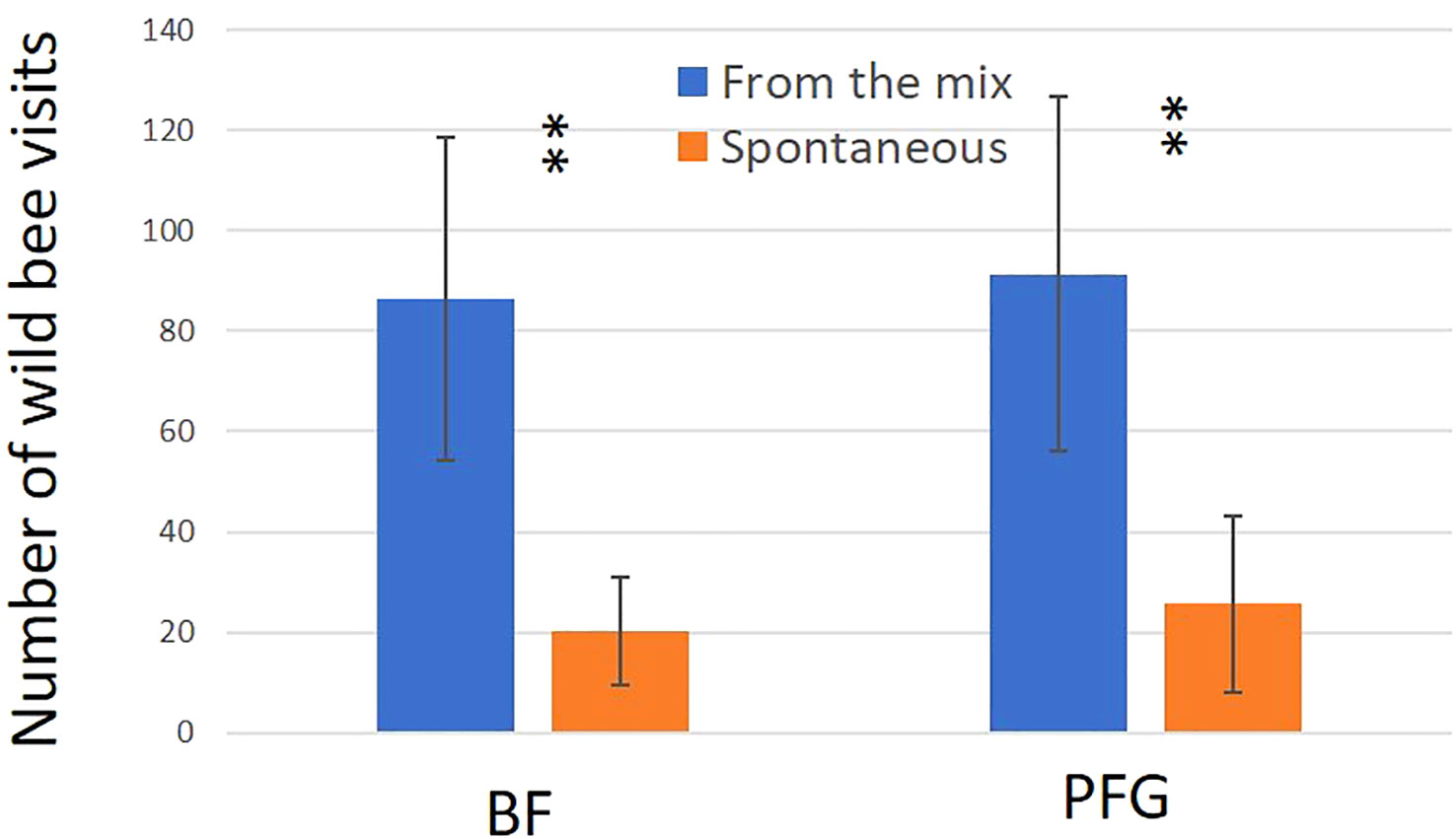
Figure 6 Mean number of wild bee visits for the Beeflora (BF) seed mix (right hand side) and the Prairie Fleurie Genevoise (PFG) seed mix (left hand side). Blue bars represent sawn species (From the mix) and orange bars represent species from the seedbank (Spontaneous). Error bars represent standard deviation, stars represent significant differences: **<0.01.
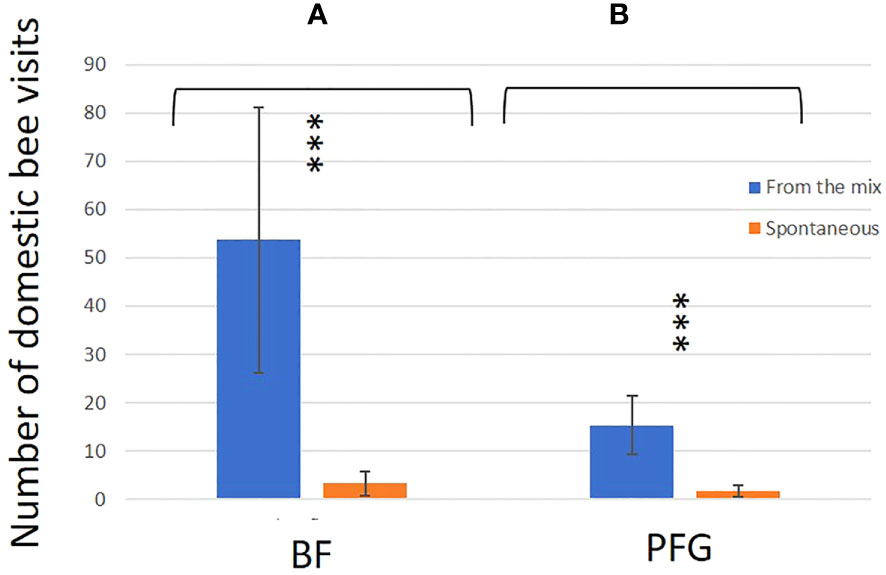
Figure 7 Mean number of domestic bee visits for the BF seed mix (right hand side) and PFG seed mix (left hand side). Blue bars represent the sawn species (From the mix) and orange barres represent species from the seedbank (Spontaneous). Error bars represent standard deviation, stars and letters represent significant differences: ***<0.001.
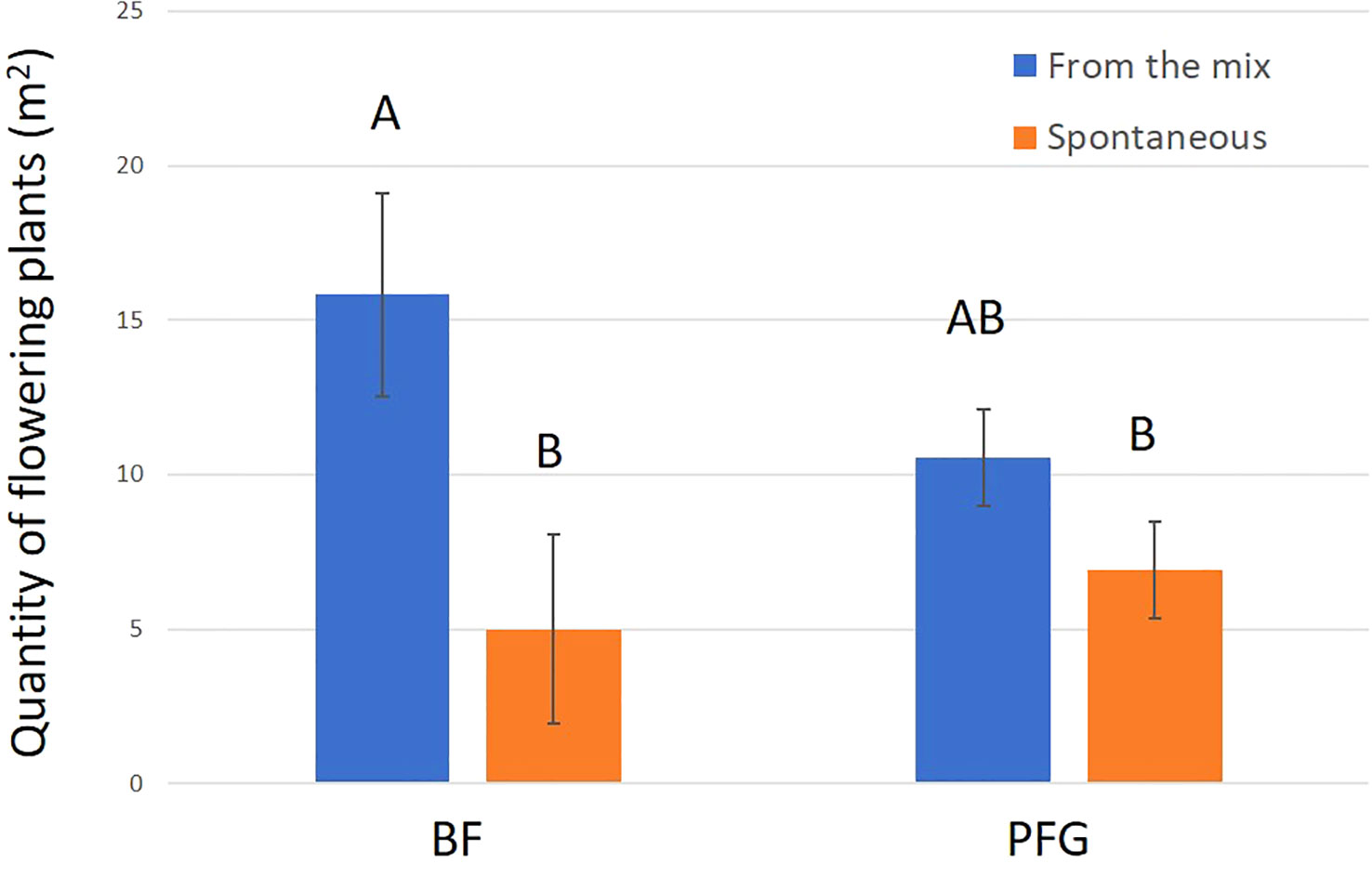
Figure 8 Mean quantity (m2) of flowering plants for the BF seed mix (right hand side) and PFG seed mix (left hand side). Blue bars represent the sawn species (From the mix) and orange bars represent species from the seedbank (Spontaneous). Error bars represent standard deviation, letters represent significant differences.
We showed that, in Geneva, resources available in urban habitats are similar to resources available in agricultural habitats in terms of quantity and richness. Indeed, the quantity and richness of pollen collected by domestic bees in urban habitat were not different from agricultural land for all sampling dates, except in May were agricultural colonies collected more quantity of pollen (mid-May) from more different taxa (beginning of May) than urban colonies. Facilitated access to cultivated and wild ligneous Rosaceae for bees in agricultural land probably explains this result, as the vast majority of the pollen collected by bees in agricultural habitat in the beginning of May came from ligneous vegetation. However agricultural colonies still collected a certain quantity of pollen from herbaceous plants in the beginning of May, while urban colonies concentrated their pollen collection on ligneous vegetation and collected almost no pollen from herbaceous plants. This might explain why they also collected fewer taxa than agricultural colonies. Except the two first sampling dates, quantity and richness of the pollen collected by agricultural and urban colonies were similar. This means that, for most of the season, urban habitats release enough trophic resources for a successful beekeeping production. However, we showed that urban and agricultural colonies did not use the same plant species throughout the season. Indeed, urban colonies relied longer on resources from ligneous vegetation than agricultural colonies. We know that domestic bees particularly appreciate plants that produce large nectar flows (Percival, 1947; Louveaux, 1990), because they found important resource quantity at the same place. Many ligneous plants produce a large number of flowers ready to be visited at the same time, which explains why trees and bushes are also largely appreciated by domestic bees (Donkersley, 2019). Urban habitat includes non-native tree species such as Gleditsia or Liriodendron that flower later in the season than native tree species such as Acer or Prunus. This explains why urban colonies use pollen released by ligneous vegetation longer in the season, as nectar flows lasts longer thanks to non-native tree species. For agricultural and urban colonies, the contribution of trees and bushes in providing pollen resources is important, especially in the beginning of the season. In the summer, domestic bees found resources provided by herbaceous vegetation, which was less abundant, but relatively more diverse. Indeed, the quantity of pollen collected from trees and bushes nearly reached zero in July for agricultural colonies and equaled zero in August for urban and agricultural colonies. Additionally, from July on, agricultural colonies started collecting pollen from unusual sources. Indeed, we observed that a relatively important part of the pollen used in the summer by agricultural colonies were collected from Poaceae species (mainly from Zea mays) or even from fungi spores. While fungi spores have been reported to be a valuable food source for bees (Parish et al., 2020), Zea mays seems to be less suitable than many dicotyledon plant species, providing poor quantity of essential nutrients (Wood et al., 2022). This suggests that domestic bees are not able to find more attractive pollinic resource at that time in agricultural land, consequently collecting any resources they find because they need to store important quantities of pollen for their brood to survive winter (Pound et al., 2022). We did not observe the same trend in urban colonies, that seem to continue finding resources from typically melliferous species, such as Trifolium or Potentilla, even in the summer months. This result is probably explained by the fact that such common herbaceous plant species are tremendously abundant in urban green spaces (especially when no pesticides is used to maintain lawns) and that citizen owning hives in their backyard are also conscious that this kind of plant species need to be preserved in order to raise healthy colonies. Indeed, in this study, healthy urban hives were either close to an urban park or in residential zone displaying private gardens. Urban hives that were not in such situation, for example on roof tops, were largely unsuccessful, which also explain the larger error bars on abundance data of urban colonies. The so-called “hungry gap”, well known among beekeepers (Percival, 1947) seems to be more intense in agricultural than in urban habitats.
Summer is thus the time of the year when domestic bees mostly need additional resources under temperate continental climate. Consequently, it is also when we can have an impact on resource availability by creating indigenous meadows. That is why we elaborated the Beeflora seed mix so as to display its maximum richness and resource abundance in the summer months. Altogether, we found 69 wild bee species foraging in our experimental meadows. This represents almost 20% of all species present in Geneva region (Amiet et al., 2001; Amiet et al., 2004; Amiet et al., 2007; Amiet et al., 2010; Amiet et al., 2014; Amiet et al., 2017). Knowing that these species were observed in only six relatively small sized plots in only three different urban parks, it suggests that urban green spaces in Geneva are suitable for hosting a large wild bee diversity. Moreover, it shows that enhancing plant species attractive to bees can have positive effects even with small scales meadows (Simao et al., 2018; Griffiths-Lee et al., 2022). This confirms previous works on urban diversity in Geneva, which suggested that pollinator diversity might be surprisingly large. Indeed, Pétremand et al. (2022) observed 73 species of syrphids in four urban parks in the city of Geneva.
Our results showed, on average, no difference in diversity or abundance of wild bee species between the Beeflora (BF) and the Praire fleurie genevoise (PFG) seed mixes, which could mean that this new BF seed mix does not perform better at attracting bees than an already commercialized seed mix. However, the BF meadow was able to attract 14 more species in total, including 8 species that visited plants exclusively present in the BF seed mix. Moreover, the BF seed mix attracted on average the same number of wild bee individuals as the PFG mix with fewer plant species sawn. This is probably because fewer grass species were included in the BF mix and because several melliferous plants sawn, such as Melilotus sp or Echium vulgare are particularly appreciated by bees, as they produce a large number of flowers at the same time and because their blossoming lasts for several weeks. Many spontaneous plants were also visited by bees, outlining the important role of common plant species, such as Taraxacum or Veronica, in providing resources availability for bees in cities. Although Taraxacum and species from the Asteraceae family in general are known to be poor quality food source for bees (Wood et al., 2022), they might still provide bee species with additional resources when other plant species in flower become scarce, which could be helpful in balance with other more nutritive food sources (Splitt et al., 2021). Additionally, bees might use such sources of pollen to help avoid predation by kleptoparasites (Splitt et al., 2021), that are not able to develop on nutrient poor pollen or might be sensitive to poisonous secondary metabolite they might contain. The interdiction of pesticide use in public areas probably helps common plant species to fully develop and flower which in turn beneficiate wild bees. Nevertheless, for both mixes, the plants sawn were more often visited by wild bees than plants from the seedbank. This was also true for domestic bees, that visited spontaneous plants relatively less often than wild bees. Sawing indigenous meadow is thus an efficient tool impacting resource availability for wild bees in urban green spaces. Besides, domestic bees visited, on average, the BF mix more often than the PFG mix. We suppose that this is explained by the presence of plants in the BF mix that displayed a large number of flowers at the same time, such as Melilotus or Echium, thus corresponding to the nectar flows they particularly appreciate. No such plant was present in the PFG mix. Including plant species that honey bees especially appreciate probably helps lowering competition between domestic and wild bees as domestic bees would concentrate their efforts on working a few efficient plants producing abundant nectar and therefore keeping the more modest plant species, such as Reseda lutea or Daucus carota, available to be worked by wild bee species. For both mixes, a large part of the visits (respectively 77% and 85% for the BF and PFG mixes) were concentrated on a few plant species, revealing that the attractiveness of wildflower meadows essentially relies on key plant species (Garbuzov and Ratnieks, 2014; Warzecha et al., 2018).
Even though both mixes received more bee visits on sawn species than on species from the seedbank, we showed that the PFG mix displayed as much spontaneous as sawn cover of plants in flower. This was not the case for the BF mix, which displayed more sawn than spontaneous cover of plants in flower. This indicates that seed mix expression was more successful for the BF than for the PFG mix, implying that less effort is needed to grow the Beeflora meadow for a better result in terms of quantity of resources added compared to the Prairie fleurie genevoise seed mix.
This study revealed differences in the trophic resources available for pollinators between urban and agricultural habitats. The contribution of ligneous vegetation in providing food for bees is of great importance, especially in the beginning of the season and it lasts longer in urban than in agricultural habitats. However, data are still needed to investigate if wild bee species also use ligneous vegetation and to what extent (Donkersley, 2019). Indeed, honey bee food requirement is probably different from the requirement of wild bees as their larvae consume the product of the workers gland (Chauvin, 1968) and not the pollen and nectar directly available from the plant as wild bee species do. We know that some well-studied wild bee species, such as Osmia bicornis, use tree species as pollen source (Splitt et al., 2021; Casanelles-Abella et al., 2022), but the vast majority of wild bee species diet still needs more insights, i.e. what plant species are used as pollen and/or nectar source (Ayers and Rehan, 2021). Several studies recently investigated wild bee communities at the tree canopy and revealed that diversity could be larger than previously thought (Allen and Davies, 2023; Cunningham-Minnick et al., 2023; Urban-Mead et al., 2023). However, those studies focus on natural and semi-natural habitats, and no such research has yet been conducted in urban areas, to our knowledge. Besides, direct observations of bees visiting tree flowers should be implemented to fully understand whether wild bees actually use trees as food sources or if they were captured at the canopy, flying over tree tops for other reasons (Cunningham-Minnick et al., 2023). Nevertheless, urban trees were found to be potential valuable food sources (Somme et al., 2016). Indeed, several tree species produce nectar (Somme et al., 2016) and specific tree species such as Acer pseudoplatanus have been reported to enhance wild bee abundance at the canopy level (Allen and Davies, 2023).
Urban green spaces can be species-rich habitat, provided that suitable and sufficient trophic resources are available, which relies on several key plant species. In this context, bringing more food sources by growing indigenous meadows, even at small scales, is an efficient tool to support diversity and abundance of wild bees in urban green spaces. Besides, several tree species are valuable food source for honey bees and are likely to also provide resources for many wild bee species. Hence, these tree species must be preserved and planted when the context allows it.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
SR elaborated the project and obtained financial support to implement it. PP synthesized data on melliferous plants and elaborated the composition of the Beeflora seed mix. SR and PP contributed to write the manuscript. CH implemented the collection of data in the field, identified the pollen samples and the wild bee species, implemented the statistical analyses and wrote the manuscript. All authors contributed to the article and approved the submitted version.
We want to thank: Gaël Petremand, Gaëlle Renaudineau, Anouk Athanasiades, Pierrick Buri, Isaline Quartat, Robin Perriard, Clothilde Viard, Marie Bessat, and Jane O’Rourke, who helped collecting data in the field and preparing pollen samples and bee specimens; Katharina Bieri for her expertise in pollen identification and for sharing her knowledge on melliferous plant species that contributed to elaborate the plant list for the Beeflora seed mix; Christophe Praz, who checked the wild bee identifications and provided access to his collection, which greatly helped the identifications of wild bees; Adrien Favre for his valuable help to improve the manuscript; The Gelbert Fondation for its financial support; the beekeeper who allowed us to collect pollen loads in their hives; and the two reviewers, whose comments and suggestions helped improve the quality of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1199438/full#supplementary-material
Allen G., Davies R. G. (2023). Canopy sampling reveals hidden potential value of woodland trees for wild bee assemblages. Insect Conserv. Diversity 16, 33–46. doi: 10.1111/icad.12606
Amiet F., Herrman M., Müller A., Neumeyer R. (2001). Apidae 3 – halictus, lasioglossum (Neuchâtel: CSCF & SEG).
Amiet F., Herrman M., Müller A., Neumeyer R. (2010). Apidae 6 - andrena, melliturga, panuginus, panurgus (Neuchâtel: CSCF & SEG).
Amiet F., Müller A., Neumeyer R. (2014). Apidae 2 - colletes, dufourea, hylaeus, nomia, nomioides, rhophitoides, rophites, sphecodes, systropha (Neuchâtel: CSCF & SEG).
Amiet F., Müller A., Praz C. (2017). Apidae 1 - allgemeiner teil, gattungen, apis, bombus info fauna (Neuchâtel: CSCF & SEG).
Ayers A. C., Rehan S. M. (2021). Supporting bees in cities: how bees are influenced by local and landscape features. Insects 12, 18. doi: 10.3390/insects12020128
Baldock K. C. R. (2020). Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 38, 63–71. doi: 10.1016/j.cois.2020.01.006
Beninde J., Veith M., Hochkirch A. (2015). Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 18, 581–592. doi: 10.1111/ele.12427
Bretzel F., Vannucchi F., Romano D., Malorgio F., Benvenuti S., Pezzarossa B. (2016). Wildflowers: From conserving biodiversity to urban greening A review. Urban. Forestry. Urban. Greening. 20, 428–436. doi: 10.1016/j.ufug.2016.10.008
Brown M. J. F., Paxton R. J. (2009). The conservation of bees: a global perspective. Apidologie 40, 410–416. doi: 10.1051/apido/2009019
Casanelles-Abella J., Muller S., Keller A., Aleixo C., Orti M. A., Chiron F., et al. (2022). How wild bees find a way in European cities: Pollen metabarcoding unravels multiple feeding strategies and their effects on distribution patterns in four wild bee species. J. Appl. Ecol. 59, 457–470. doi: 10.1111/1365-2664.14063
Chauvin R. (1968). Traité de Biologie de l'abeille: Biologie et Physiologie générale (Paris: Masson et Cie).
Cunningham-Minnick M. J., Milam J., Kane B., Roberts H. P., King D. I. (2023). Abundant, distinct, and seasonally dynamic bee community in the canopy-aerosphere interface above a temperate forest. Ecol. Evol. 13, 14. doi: 10.1002/ece3.9739
Danforth B. N., R., Neffe J., Fawcett F. (2019). The solitary bees: biology, evolution, conservation (Oxford: Princeton University Press).
Donkersley P. (2019). Trees for bees. Agric. Ecosyst. Environ. 270, 79–83. doi: 10.1016/j.agee.2018.10.024
Dylewski L., Mackowiak L., Banaszak-Cibicka W. (2019). Are all urban green spaces a favourable habitat for pollinator communities? Bees, butterflies and hoverflies in different urban green areas. Ecol. Entomol. 44, 678–689. doi: 10.1111/een.12744
Fauviau A., Baude M., Bazin N., Fiordaliso W., Fisogni A., Fortel L., et al. (2022). A large-scale dataset reveals taxonomic and functional specificities of wild bee communities in urban habitats of Western Europe. Sci. Rep. 12, 15. doi: 10.1038/s41598-022-21512-w
Federal Office for the Environment (FOEN). (2017). Action Plan for the Swiss Biodiversity Strategy. Bern: FOEN.
Garbuzov M., Ratnieks F. L. W. (2014). Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct. Ecol. 28, 364–374. doi: 10.1111/1365-2435.12178
Goulson D., Nicholls E., Botias C., Rotheray E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 10. doi: 10.1126/science.1255957
Griffiths-Lee J., Nicholls E., Goulson D. (2022). Sown mini-meadows increase pollinator diversity in gardens. J. Insect Conserv. 26, 299–314. doi: 10.1007/s10841-022-00387-2
Gunnarsson B., Federsel L. M. (2014). Bumblebees in the city: abundance, species richness and diversity in two urban habitats. J. Insect Conserv. 18, 1185–1191. doi: 10.1007/s10841-014-9729-2
Hall D. M., Camilo G. R., Tonietto R. K., Ollerton J., Ahrne K., Arduser M., et al. (2017). The city as a refuge for insect pollinators. Conserv. Biol. 31, 24–29. doi: 10.1111/cobi.12840
infoflora. (2023). Liste verte. infoflora. Available at: https://www.infoflora.ch/fr/conservation-des-especes/semences-plantes-sauvages.html#liste-verte.
Jeannerod L., Carlier A., Schatz B., Daise C., Richel A., Agnan Y., et al. (2022). Some bee-pollinated plants provide nutritionally incomplete pollen amino acid resources to their pollinators. PloS One 17, 13. doi: 10.1371/journal.pone.0269992
Karpovich I., Drebezgina E., Elovikova E. (2015). Атлас пыльцевых эёрен = Pollen atlas. Уральский рабочий, Екатеринбург [Ekaterinbourg.
Kirk W. D. (1994). A colour guide to pollen loads of the honey bee (Staffordshire, UK: Department of biological sciences, Keele University).
Klein A. M., Vaissiere B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B-Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Lahr E. C., Dunn R. R., Frank S. D. (2018). Getting ahead of the curve: cities as surrogates for global change. Proc. R. Soc. B-Biol. Sci. 285, 9. doi: 10.1098/rspb.2018.0643
Larson J. L., Redmond C. T., Potter D. A. (2013). Assessing insecticide hazard to bumble bees foraging on flowering weeds in treated lawns. PloS One 8, 7. doi: 10.1371/journal.pone.0066375
Louveaux J. (1990). Les relations abeilles-pollens. Bull. la Société. Bot. France. Actualités. Botaniques. 137, 121–131. doi: 10.1080/01811789.1990.10827009
McKinney M. L. (2008). Effects of urbanization on species richness: A review of plants and animals. Urban. Ecosyst. 11, 161–176. doi: 10.1007/s11252-007-0045-4
Mombrial F. C., M., Favre E., Lacroix A., Sandoz E., Sandoz F., Tribot S. (2020). Liste rouge des plantes vasculaires du canton de Genève (Geneva: Conservatoire et Jardin Botanique de la Ville de Genève, Genève).
Moore P. D., Collison M. E., Collinson M. E., Webb J. A. (1991). Pollen analysis. 2nd ed. (Oxford: Blackwell).
Muratet A., Fontaine B. (2015). Contrasting impacts of pesticides on butterflies and bumblebees in private gardens in France. Biol. Conserv. 182, 148–154. doi: 10.1016/j.biocon.2014.11.045
Ollerton J. (2017). “Pollinator diversity: distribution, ecological function, and conservation,” in Annual review of ecology, evolution, and systematics, vol. 48 . Ed. Futuyma D. J. (Annual Reviews, Palo Alto), 353–376.
Parish J. B., Scott E. S., Hogendoorn K. (2020). Nutritional benefit of fungal spores for honey bee workers. Sci. Rep. 10, 8. doi: 10.1038/s41598-020-72758-1
Percival M. (1947). Pollen collection by apis-mellifera. New Phytol. 46, 142–173. doi: 10.1111/j.1469-8137.1947.tb05076.x
Pétremand G. R. S., Heiniger C., Speight M. (2022). “Les syrphes des milieux urbains,” in Genève sous la loupe: les syrphes du canton. Ed. genève F. (Genève: Diptera, Syrphidae), 96–105.
Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Pound M. J., Vinkenoog R., Hornby S., Benn J., Goldberg S., Keating B., et al. (2022). Determining if honey bees (Apis mellifera) collect pollen from anemophilous plants in the UK. Palynology 47 (2), 2154867. doi: 10.1080/01916122.2022.2154867
Pritsch G. (2007). Bienenweide: 200 Trachtpflanzen erkennen und bewerten (Stuttgart: Franckh-Kosmos Verlags-GmbH).
Prunier P. G. F., Béguin D., Boissezon A., Delarze R., Hegg O., et al. (2014). Un référentiel pour les associations végétales de Suisse: PhytoSuisse Vol. 3 (Document phtosociologique), 403–411.
Prunier P.G. F., Béguin D., Boissezon A., Delarze R., Hegg O., Klölzi F., et al. (2019). Phytosuisse: un référentiel pour les associations végétales de Suisse. Available at: http://www.infoflora.ch/fr/milieux/phytosuisse/.
R Core Team (2010). R: a language and environment for statistical computing (Vienna: R Foundation for Statistical Computing).
Shwartz A., Muratet A., Simon L., Julliard R. (2013). Local and management variables outweigh landscape effects in enhancing the diversity of different taxa in a big metropolis. Biol. Conserv. 157, 285–292. doi: 10.1016/j.biocon.2012.09.009
Simao M. C. M., Matthijs J., Perfecto I. (2018). Experimental small-scale flower patches increase species density but not abundance of small urban bees. J. Appl. Ecol. 55, 1759–1768. doi: 10.1111/1365-2664.13085
Somme L., Moquet L., Quinet M., Vanderplanck M., Michez D., Lognay G., et al. (2016). Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban. Ecosyst. 19, 1149–1161. doi: 10.1007/s11252-016-0555-z
Splitt A., Skorka P., Strachecka A., Boranski M., Teper D. (2021). Keep trees for bees: Pollen collection by Osmia bicornis along the urbanization gradient. Urban. Forestry. Urban. Greening. 64, 10. doi: 10.1016/j.ufug.2021.127250
Topitzhofer E., Lucas H., Carlson E., Chakrabarti P., Sagili R. (2021). Collection and identification of pollen from honey bee colonies. Jove-J. Visualized. Experiments. 167, e62064. doi: 10.3791/62064-v
Turo K. J., Gardiner M. M. (2019). From potential to practical: conserving bees in urban public green spaces. Front. Ecol. Environ. 17, 167–175. doi: 10.1002/fee.2015
Urban-Mead K. R., Van Dyke M., Muniz P., Young A. D., Danforth B. N., McArt S. H. (2023). Early spring orchard pollinators spill over from resource-rich adjacent forest patches. J. Appl. Ecol. 60, 553–564. doi: 10.1111/1365-2664.14350
Warzecha D., Diekotter T., Wolters V., Jauker F. (2018). Attractiveness of wildflower mixtures for wild bees and hoverflies depends on some key plant species. Insect Conserv. Diversity 11, 32–41. doi: 10.1111/icad.12264
Wenzel A., Grass I., Belavadi V. V., Tscharntke T. (2020). How urbanization is driving pollinator diversity and pollination – A systematic review. Biol. Conserv. 241, 15. doi: 10.1016/j.biocon.2019.108321
Westrich P. (2019). Die Wildbienen Deutschlands. 2., aktualisierte Auflage edition (Stuttgart (Hohenheim: Ulmer).
Wilson C. J., Jamieson M. A. (2019). The effects of urbanization on bee communities depends on floral resource availability and bee functional traits. PloS One 14, 18. doi: 10.1371/journal.pone.0225852
Keywords: pollinator, urban habitat, floral resources, melissopalynology, indigenous meadow
Citation: Heiniger C, Rochefort S and Prunier P (2023) Floral resources used by bees in urban areas: the case of Geneva, Switzerland. Front. Ecol. Evol. 11:1199438. doi: 10.3389/fevo.2023.1199438
Received: 03 April 2023; Accepted: 04 September 2023;
Published: 27 September 2023.
Edited by:
Anthony Lehmann, University of Geneva, SwitzerlandReviewed by:
Michał Filipiak, Jagiellonian University, PolandCopyright © 2023 Heiniger, Rochefort and Prunier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlène Heiniger, Y2hhcmxlbmUuaGVpbmlnZXJAaGVzZ2UuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.