
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 26 June 2023
Sec. Behavioral and Evolutionary Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1164310
Web-building spiders that build detritus-based bell-shaped cobwebs are model organisms for studies on behavioral plasticity because their web architecture components are easily quantified and behavioral investments are clearly separated. We investigated the web architectures and behavioral investments of the cobwebs built by Campanicola campanulata under different weight (heavy, medium, and light) detritus to research its cobweb architecture variation and analyzed the investment trade-off between foraging and defense. The results showed that spiders could actively choose lighter detritus to build retreats to reduce material and energy cost. There was a clear trade-off between defense and foraging investment of spiders choosing different weight detritus for their webs. The total length of gumfooted lines (foraging investment) was longer for the spiders that chose lighter detritus, but the energy expenditure during web-building (defense investment) was higher for the spiders that chose heavier detritus.
Building behavior in sit-and-wait predators is a widespread mechanism because animal structures are extended phenotypic traits that include structures such as spider webs and ant-lion pits, mediating a number of fitness-related processes (e.g., foraging, mating, and defense) (Doucet and Montgomerie, 2003; Pinter-Wollman, 2015; Smith et al., 2015; Wolff et al., 2018). Although the overall significance of animal buildings is broadly recognized (Hansell, 2005; Tso et al., 2007; Korb, 2010), there is little understanding of the economy and plasticity of building behavior. The behavioral flexibility of orb spiders has been extensively studied, while the relationship between the structure constructed and behavioral investment of theridiids has been poorly studied because there are so few such model organisms (Herberstein, 2011; Scharf et al., 2011; Hesselberg, 2015). Web-building spiders are excellent silk craftsmen because they can use various types of silk to build a series of dazzling structures, from simple silk to the retreat for molting and protecting the egg sac to spider webs (Foelix, 2011). At present, there are more than 10,000 known species of web-building spiders (World Spider Catalog, 2023), which are ubiquitous predators in most terrestrial ecosystems and are a common subject of behavioral and ecological research (Herberstein and Wignall, 2011).
Spider web may be effective in defending against predators and capturing prey, but the material and energy cost are high. Therefore, spiders may reduce various costs during web evolution in different ways. Most notably, the evolution of spiders from cribellate silk to viscid silk drastically reduced the total cost of web production. The captured threads produced by orb web spiders in the family Araneidae is more viscous per unit volume, and the speed and material availability are significantly improved (Kawamoto and Japyassu, 2008; Opell et al., 2008; Opell and Schwend, 2009; Sahni et al., 2010). Different spiders improve the effectiveness of their web-building behavior in different ways. For example, the orb-web spiders can prey on a large number of flying insects by using the aerial orb web constructed by relatively sparse silk, and the spiders can effectively save silk protein by recycling silk (Janetos, 1982; Riechert and Gillespie, 1986). Some spiders usually use the webs of other individuals as structural support when building webs. For example, it has been reported that a part of Nephila web is routinely used by Leucauge as the anchor point of a single orb web (Blackledge, 2011). Individuals entering a certain habitat can use the web of the settled spider as an anchor or support silk, thus saving silk (Jakob, 1991; Lloyd and Elgar, 2006).
Optimal foraging theory predicts that sit-and-wait predators can make decisions based on external conditions and the costs and benefits of adopting specific behaviors to maximize fitness (Scharf et al., 2011). There has been a lot of research on the flexibility of the web-building behavior of orb web spiders; however, so far, no studies have explicitly tested the plasticity of theridiids web-building behavior to save resources and energy by actively choosing materials for web or retreat construction. There are overlapping resource requirements between competitive behaviors; web-building spiders must allocate resource investment among different behaviors to maximize fitness. Therefore, spiders choosing different materials to build webs or retreats may have trade-offs between different behavioral investments during web-building.
The cobwebs of spiders in the family theridiidae is monophyletic and derived from an orb web, and the modification of web construction behavior may have occurred many times independently (Benjamin and Zschokke, 2003; Eberhard et al., 2008). Theridiid web shows a high degree of evolutionary flexibility, and the evolutionary trend seems to be from extensive to reduced amounts of viscid silk in webs, and finally to total absence (Benjamin and Zschokke, 2003; Zschokke et al., 2006). Such an adaptation might have, by relieving the spider of the necessity to produce viscid silk, thereby increase its fitness by conserving silk protein (Benjamin and Zschokke, 2003; Zschokke et al., 2006). Theridiids rest during the day and their position there seem likely to be the result of selection to avoid being preyed upon by visually orienting predators. Many theridiids have also evolved the characteristic of using small pieces of detritus to construct an inverted cone or cup in which the spider rests (Eberhard, 1986; Eberhard et al., 2008). The behavior of cobweb-building spiders that build detritus-based, bell-shaped cobweb has attracted the attention of researchers in recent years (Li et al., 2021). Previous studies have shown that the spider’s behavior is highly plastic and susceptible to internal factors such as development stage and feeding (Zhang et al., 2022a; Zhang et al., 2022b). However, whether external factors such as prey or predator cues and detritus material weight will affect the cobweb-building behavior of those spiders is still unknown. The weight and volume of the bell-shaped retreat are the real reflection of different behavioral investments (Zhang et al., 2022a; Zhang et al., 2022b); therefore, it is highly possible that this spider will actively choose detritus to build web, which may provide a new perspective for the study of web-building behavior. The cobweb architecture of this spider is easy to measure and the behavioral investment is easy to quantify, which makes it an ideal model for studying the trade-offs between different behavioral investments (Zhang et al., 2022a; Zhang et al., 2022b). The construction of a more defensive retreat may require higher energy costs because it requires transporting heavier detritus, while building a capture web with higher foraging functions may only require more silk protein to build more gumfooted lines. In this study, we asked the following: (1) Do mature female spiders choose lighter detritus to build webs to save energy? (2) Would the web architecture and behavioral investments increase with the weight of detritus? (3) Is there a trade-off between the foraging investment and defense investment of spiders that choose different weights of detritus to build detritus-based, bell-shaped cobwebs?
Campanicola campanulata (Araneae: Theridiidae) is a cobweb-building spider that mainly feeds on ants and is widely distributed in Hubei, Zhejiang, and Guizhou provinces of China (Chen, 1993). It only builds a web in cool but rainproof places in forests, farmland, hillsides, or other habitats. It usually lives in aggregation but build webs solitarily. The web of C. campanulata is composed of three parts: anchoring silk (anchoring component), a bell-shaped retreat (defense component), and a capture web (foraging component) consisting of dozens of gumfooted lines (Figure 1A). Anchor silks are usually made of strands of silk suspended from concave walls or tree roots above ground. These structures prevent damage to the web caused by winds. Upon entering a new habitat, the spider will wander around the ground and attach silk to detritus suitable for building retreat. Then, it will construct anchor silk and secure it to the substrate. The spider uses the fourth pair of legs and silk to transport detritus up into the air until it forms a retreat that matches its size. The construction of retreat is energetically costly because its weight is usually 30–40 times the body weight of the spider. As a foraging tool, gumfooted lines emanate from the retreat edge and are fastened to coarse sand particles or fixed substrata (Zhang et al., 2022b). When ants are intercepted by sticky gumfooted lines, the lines will fall off the substrate and transmit vibration to the spider, which will eventually pull the prey off the ground, and the spider would capture it and take it to the retreat and consume it (Zhang et al., 2022b). The configuration of suspending the retreat can significantly reduce the vulnerability of spiders to the enemy because there are silken tunnels inside the retreat (Henschel and Jocqué, 1994), which the dangerous prey and predators cannot invade.
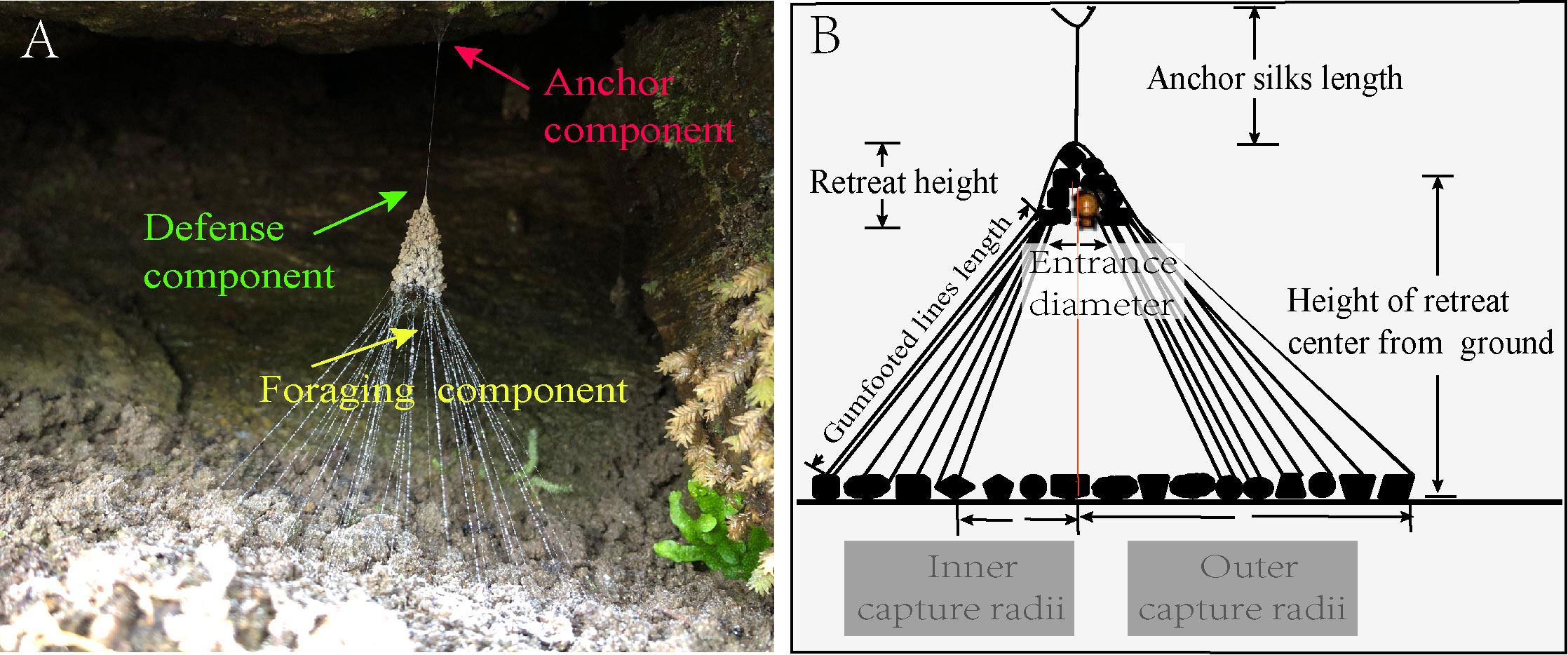
Figure 1 Web morphology and diagrammatic sketch of web architectures of spider C. campanulata. (A) Web components; (B) main parameters of web architectures measured in this study.
From 25 to 28 March 2022, we collected sub-adult (i.e., 1 molt before adulthood, ~2.4 mm in body length) C. campanulata from Hubei Dabie Mountain National Nature Reserve in Huanggang, Hubei Province, China (31°5′N, 115°48′E), and raised them to adulthood in the laboratory. The spiders were maintained individually in a plastic box (length × width × height: 10 cm × 5 cm × 10 cm) with bamboo sticks attached to their inner sides to allow them to build webs. The front and back sides of the box were made of removable transparent Perspex glass. All spiders were kept in the laboratory with controlled environmental conditions (temperature: 25 ± 1°C; relative humidity: 80 ± 5%; photoperiod: 14 h:10 h L:D). The bottom of the box was covered with a layer of sand for the spider to collect to build a retreat, and a piece of sponge with absorbed water was placed at the bottom of the box to provide water for the spider. We fed each spider with 2 ants (Monomorium sp., ~2.2 mm in body length) every 2 days, and the developmental status (molting) of each spider was checked twice daily (09:00 and 21:00).
We selected 60 newly matured female spiders (~3 days after maturity) that were well-fed for 3 days and randomly divided into three treatments of the same amount and different weight of detritus: heavy treatment (HT)—1,000 grains of sand, with a total weight of approximately 5.02 g; medium treatment (MT)—500 grains of sand and 500 particles of soil, with a total weight of approximately 4.32 g; and light treatment (LT)—1,000 particles of soil, with a total weight of approximately 2.61 g; and the spiders were given 24 h to allow them to build webs. All the detritus used in the experiment were collected in the wild habitat and screened with multi-layer standard screens to ensure consistent volume. The particle diameter of all experimental materials was between 0.6 and 0.8 mm. The detritus of the medium treatment was fully mixed before the experiment. We measured the body size (carapace width, body length, and body weight) of each spider before the experiment.
We measured nine types of web architecture parameters in each web, including the length of anchor silk (ASL), the height of retreat (RH), the number of gumfooted lines (GLN), the height of the retreat center from the ground (CH), the diameter of retreat entrance (ED), the length of each gumfooted line (GL), the lengths of inner and outer capture radii (CRI and CRO), and the weight of the retreat (RW) (Figure 1B). We used (CRI and CRO)/2 to calculate the average radius (CR) to estimate the capture area (CA). The weight of the retreat was measured after the other eight web architecture parameters were measured. We cut the retreat off from the web and measured its weight to the nearest 0.01 mg using an electronic balance (FA1004N type, HANGPING). Because the capture area is an important indicator for prey capture and the volume of retreat is an important indicator of predator avoidance, spiders with a bigger capture area may have higher foraging success and spiders with larger retreats may offer better protection from predation (Manicom et al., 2008); we used the same formula in previous studies to calculate the capture area and retreat volume (Zhang et al., 2022a; Zhang et al., 2022b) and also quantify the foraging investment (i.e., total length of gumfooted lines, GTL and lateral area of retreats, RLA) and defense investment (DI) during web-building (Zhang et al., 2022a; Zhang et al., 2022b). For each web of the medium treatment, we also analyzed the proportion of soil particles, the weight of soil particles, the weight of retreat, and defense investment in the detritus in each finished web. To test whether the spider would actively choose the detritus, we also calculated the theoretical weight of the retreat based on the initial detritus ratio, the amount of detritus, and the average weight per grain of detritus in the medium treatment. We also calculated the theoretical results of the defense investment based on the weight of retreat and the actual height of retreat from the ground. We selected the GTL to quantify foraging investment because gumfooted lines are the primary foraging tool of cobweb spiders (DiRienzo and Montiglio, 2016).
We use Kruskal–Wallis rank sum test to compare the differences in web architecture and behavioral investments between treatments because the results of the Shapiro–Wilk test for normality of data and the Levene test for homogeneity of variance showed that the data were not normally distributed. The paired comparative analysis is used to compare the results between any two groups if there are significant differences. We use Wilcoxon’s signed-rank test for matched pairs to compare the differences between the theoretical and the actual results in the medium treatment in the ratio and weight of soil particles, retreat weight, and defense investment. We performed all statistical analyses using R 4.2.1 (R Core Team, 2022). All tests were two-tailed, and the p-value for significance was set at <0.05.
There were no significant differences between the body size (carapace width, body length, and body weight) of the spiders among the three treatments (Table 1). All tested spiders built an intact web.
There were significant differences among the three treatments in the length of anchor silk (F2, 57 = 4.248, df = 2, p = 0.019, Table 2), the diameter of the retreat entrance (F2, 57 = 11.390, df = 2, p < 0.001, Table 2), the weight of the retreat (F2, 57 = 8.856, df = 2, p < 0.001, Table 2), the number of gumfooted lines (F2, 57 = 11.259, df = 2, p < 0.001, Table 2; Figure 2A), the volume of the retreat (F2, 57 = 7.377, df = 2, p < 0.001, Table 2; Figure 2B), and the capture area of the web (F2, 57 = 27.605, df = 2, p < 0.001, Table 2; Figure 2C), indicating that the weight of the detritus significantly affected the web-building behavior of spider. There was no significant difference in all measured web architecture parameters between medium and lighter treatment, which indicates that spiders in the medium treatment selected the light detritus to build web. However, there were no significant difference between the three treatments in the height of the retreat (F2, 57 = 2.536, df = 2, p = 0.088, Table 2) and the height of the retreat from the ground (F2, 57 = 2.342, df = 2, p = 0.105, Table 2).
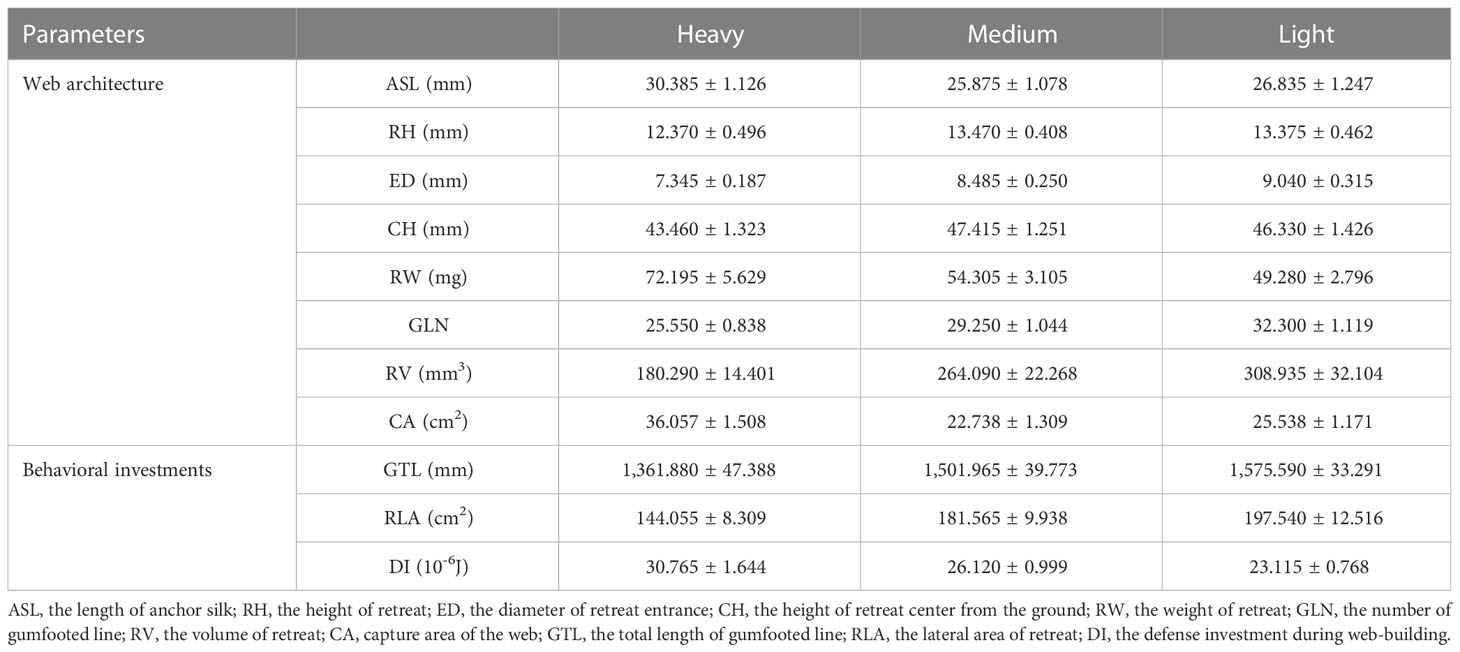
Table 2 Mean ( ± SE) of web architectures and behavioral investments measured between treatments (N = 20 in each treatment).
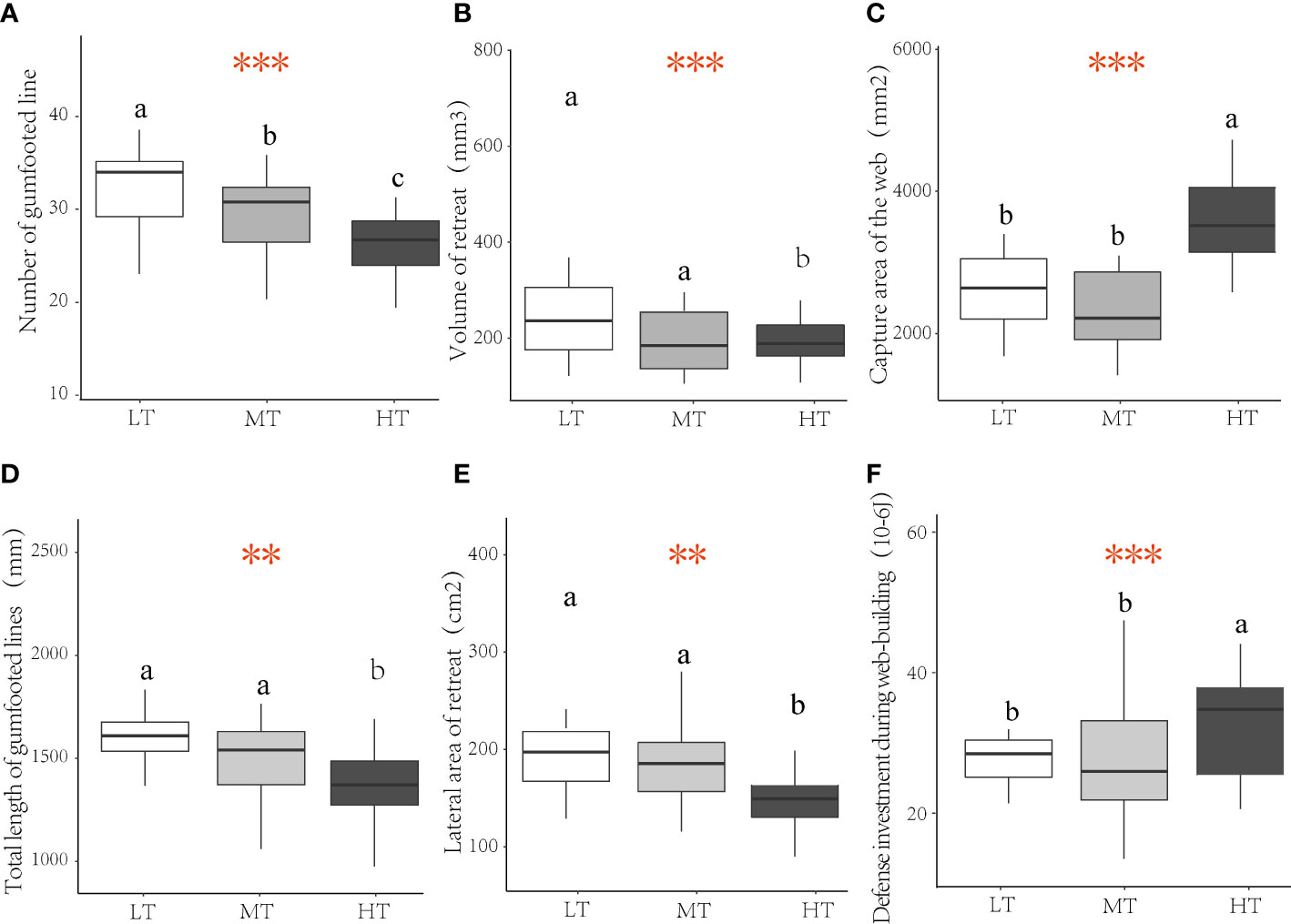
Figure 2 Boxplots of the web architecture and behavioral investments for female C. campanulata in different treatments: (A) number of gumfooted line (GLN); (B) volume of retreat (RV); (C) capture area of the web (CA); (D) total length of gumfooted lines (GTL); (E) lateral area of retreat (RLA); (F) defense investment during web-building (DI). Boxplots show the median (central line), first and third quartiles (box), and different lowercase letters indicate significant difference between treatments. **p < 0.01; ***p < 0.001. HT, heavy treatment; MT, medium treatment; LT, light treatment.
There were significant differences among all the behavioral investment parameters among the three treatments (GTL: F2, 57 = 7.164, df = 2, p = 0.002, Table 2; Figure 2D; RLA: F2, 57 = 6.970, df = 2, p = 0.002, Table 2; Figure 2E; DI: F2, 57 = 10.382, df = 2, p < 0.001, Table 2; Figure 2F), indicating that the weight of the detritus significantly affected the behavioral investments of spider. There was no significant difference in all measured behavioral investments between medium and light treatment, which indicates that spiders in the medium treatment selected the light detritus to build web to reduce the behavioral investment during web-building. However, GTL and RLA were significantly increased in light treatment compared with that in heavy and medium treatment (Figures 2D, E), and DI was significantly increased in heavy treatment compared with that in light and medium treatment (Figure 2F). These results suggested that the weight of detritus can significantly affect the investment of spiders in different behaviors.
There were significant differences between the theoretical and the actual value of the ratio of soil particles (V = 210, p < 0.001, Table 3; Figure 3A) and the weight (V = 0, p < 0.001, Table 3; Figure 3B) in the web-building materials of medium-treated spiders. The weight of the retreat (V = 0, p < 0.001, Table 3; Figure 3C) and the defense investment during web-building (V = 0, p < 0.001, Table 3; Figure 3D) also have significant differences between the theoretical and the actual value. The proportion of soil particles in the detritus of the retreats constructed by the medium-treated spiders reached 84% on average, far higher than the initial proportion of detritus provided by the experiment, which shows that spiders have actively selected the lighter weight detritus to build web to reduce the defense investment during web-building.
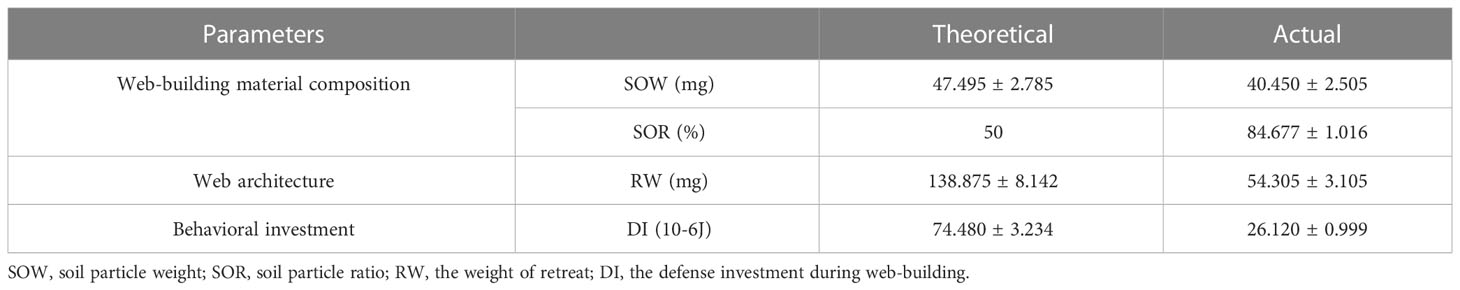
Table 3 Mean ( ± SE) of theoretical and actual values of the web-building materials composition, web architecture, and behavioral investment in medium treatment (N = 20).
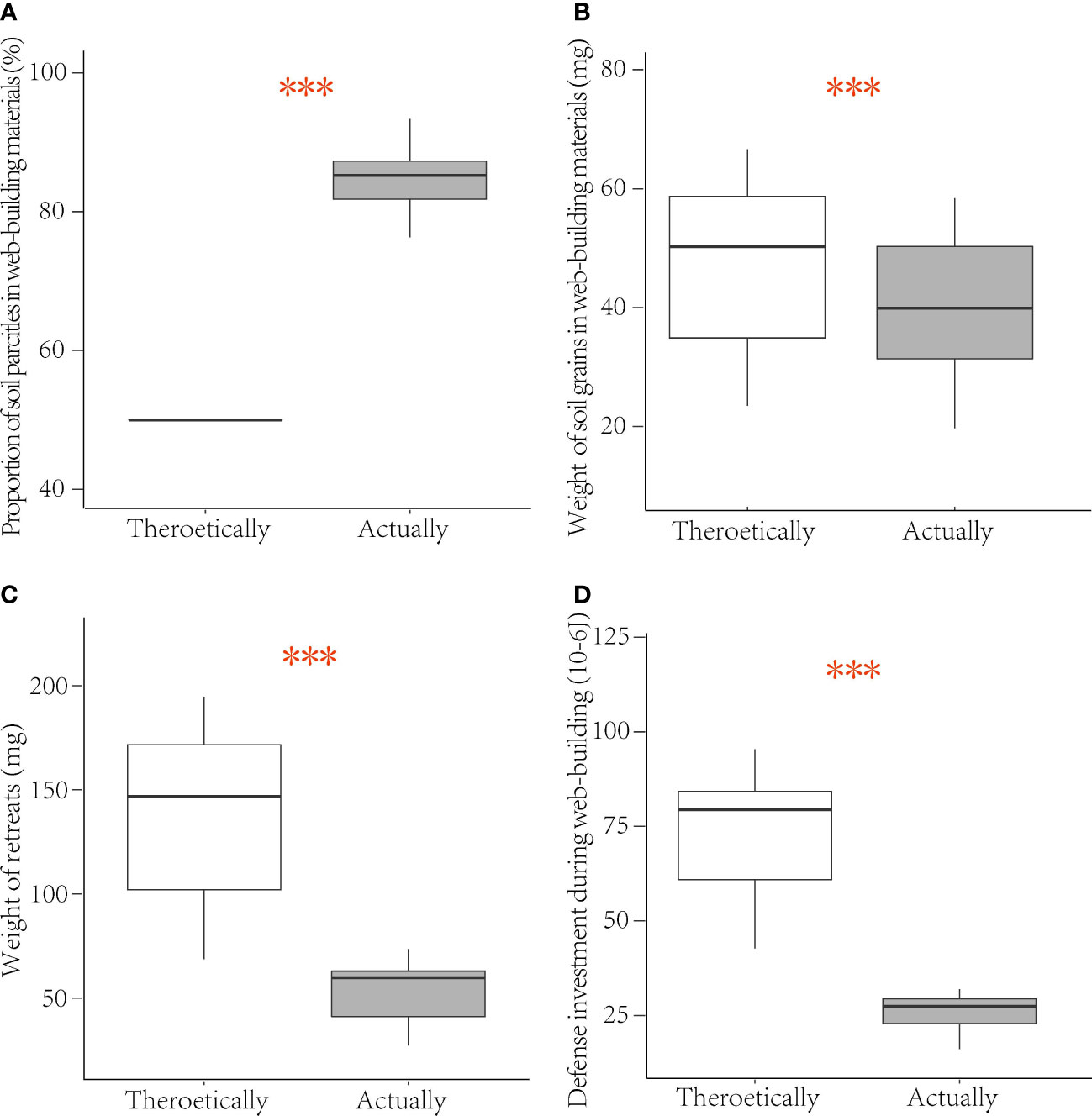
Figure 3 Boxplots of theoretical and actual value of web-building materials composition, retreat weight, and defense investment of female C. campanulata in the medium treatment: (A) proportion of soil particles in web-building materials (SOR); (B) weight of soil particles in web-building materials (SOW); (C) weight of the retreat (RW); (D) defense investment during web-building (DI). Boxplots show the median (central line), first and third quartiles (box). ***p < 0.001.
Our results confirmed that C. campanulata selected lighter detritus to construct detritus-based bell-shaped cobwebs under conditions of varying detritus in order to reduce web-building costs and maximize fitness. Unlike other studies that focus on spiders recycling silk and using the web of settled spiders as anchor or support silk, our research has for the first time revealed the energy-saving web-building strategy of spiders by selecting materials. Orb web spiders ingest silk when clearing their webs every day and recycle it in the subsequent webs, thus significantly improving the economy of web construction (Breed et al., 1964; Peakall, 1971; Opell, 1999). However, the web-building behavior of C. campanulata is costly because the total time of detritus transport is only 2 h during the 20 h of web-building, suggesting that the spider needs a long rest to recover its energy in order to complete the detritus transport (H. Zhang, personal observation).
The results of the plasticity of web architecture and behavioral investment induced by the different weight of detritus showed that these external factors had a significant impact on the web-building behavior of C. campanulata. Specifically, spiders that choose lighter detritus to build retreats invest more in the construction of web structures for foraging, such as more and longer gumfooted lines because these spiders may not need to consume much silk protein when building retreats. While spiders that choose heavier detritus invest more in the construction of web structures for defense because they not only need to consume more energy to transport heavier detritus, but also need more silk resources to wrap web-building materials. Therefore, these spiders have higher energy costs and material costs when building retreats. This result showed that the parameters of the cobweb architecture were good models to distinguish the spider’s “investment” when there are different detritus in the habitat. In addition, this result is also consistent with previous reports that spider web-building behavior was considered to be a combination of plastic responses to environmental factors (Herberstein and Wignall, 2011; Blamires, 2010).
Trap-building predators make decisions based on the external conditions and the costs and benefits of adopting specific behavior to maximize fitness according to the optimal foraging theory (Scharf et al., 2011). In our study, the spider showed different web-building strategies according to different detritus in the habitat. Spiders with lighter detritus build webs with more gumfooted lines and larger retreats to increase the chance of catching and retaining prey while avoiding being bitten by dangerous prey, such as some species of ants (Líznarová and Pekár, 2013). In contrast, spiders with heavy detritus avoid predation (Manicom et al., 2008) by building a retreat with high energy consumption and a larger capture area with sparse gumfooted line to increase the chance of capturing prey to supplement the resource consumption during web-building as soon as possible.
Trial spiders in this study were all newly mature females, for which the main physiological transitions are reproduction, including copulation, production of the egg sac, and parental care. All of these activities require a safe place (Beaulieu et al., 2016; Mikát et al., 2021), so C. campanulata that were treated with heavy detritus will construct such retreats even though the defense investment is so high. Thus, adult females treated with heavy detritus choose to allocate more energy to defense than those treated with light detritus to maximize reproductive success. We have observed in the wild that a few matured females construct cobwebs with little or even no gumfooted lines, which could reduce the possibility that spiderlings will face predators, such as their most common, but most dangerous prey (i.e., ants). Most euryphagous spiders seem to avoid preying on ants because they have strong spines, mandibles, and formic acid for effective group attacks (Pekár and Toft, 2015). Our results are consistent with previous studies showing that egg production has significant effects on cobweb (black widow web) architecture (Dirienzo and Aonuma, 2018; Zhang et al., 2022a).
The cobwebs of heavily detritus-treated spiders had a higher defense investment and a lower foraging investment. These phenomena indicate that females are likely to actively construct retreats for foraging, mating, and breeding to increase reproductive success when they mature, regardless of gathering food. This is consistent with other studies, which demonstrated that production of both egg sacs and webs is energetically costly (Ford, 1977; Jakob, 1991), and thus females may make trade-offs by investing more in aspects that provide greater reproductive fitness benefits (defense) and less in others (foraging). This line of reasoning is reinforced by the fact that animals should structurally increase defensive efforts when they have offspring (Dirienzo and Aonuma, 2018). The lightly detritus-treated spiders had a higher foraging investment and a lower defense investment in their cobwebs despite the fact that they have larger retreats. These findings suggest that the light detritus spiders expend less energy during web-building though we actually did not measure specific energy consumption. Another possible explanation is that the resource consumption of the lightly detritus-treated spiders did not reach the threshold adjusted for defense investment because all the test spiders satiated prior to the experiment. As such, there is an obvious trade-off between foraging investment and defense investment in the selection of different weights of detritus for C. campanulata to construct detritus-based, bell-shaped cobwebs.
In summary, we found that the weight of detritus had a significant effect on the web architecture and behavioral investment during web-building of C. campanulata. In order to reduce material and energy costs, C. campanulata could choose lighter detritus to build web. There was a clear trade-off between foraging and defense investment for those that chose different weights of detritus. These findings may provide a new insight into the behavior of web-building spiders because this study is the first to reveal the economic strategy of active selection of web-building materials by cobweb spiders. Future studies should consider sampling at larger population scales to determine whether the patterns documented here would be true for other web-building spiders.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.5061/dryad.g1jwstqvb.
HZ conceived and designed the experiments. HZ and ZL collected and raised spiders. HZ conducted experiments and performed data analysis. HZ and CL wrote the manuscript. LW and CL provided supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the Outstanding young and middle-aged science and technology innovation team project in colleges and universities of Hubei Province (T2022030).
We thank Zhongwei Deng for help with field work. We also thank Long Yu for help in data processing and analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Beaulieu M., Bischofberger I., Lorenz I., Scheelen L., Fischer K. (2016). Reproducing butterflies do not increase intake of antioxidants when they could benefit from them. Biol. Lett. 12, 20150941. doi: 10.1098/rsbl.2015.0941
Benjamin S. P., Zschokke S. (2003). Webs of theridiid spiders: construction, structure and evolution. Biol. J. Linn. Soc 78 (3), 293–305. doi: 10.1046/j.1095-8312.2003.00110.x
Blackledge T. A. (2011). Prey capture in orb weaving spiders: are we using the best metric? J. Arachnol. 39 (2), 205–210. doi: 10.1636/Chi10-52.1
Blamires S. J. (2010). Plasticity in extended phenotypes: orb web architectural responses to variations in prey parameters. J. Exp. Biol. 213 (Pt18), 3207–3212. doi: 10.1242/jeb.045583
Breed A. L., Levine V. D., Peakall D. B., Witt P. N. (1964). The fate of the intact orb web of the spider Araneus diadematus cl. Behaviour 23, 43–60. doi: 10.1163/156853964X00085
Chen Z. F. (1993). A new species of the genus Achaearanea from zhejiang province (Araneae: theridiidae). Acta Zootax. Sini. 18 (1), 36–38.
Dirienzo N., Aonuma H. (2018). Plasticity in extended phenotype increases offspring defence despite individual variation in web structure and behaviour. Anim. Behav. 138, 9–17. doi: 10.1016/j.anbehav.2018.01.022
DiRienzo N., Montiglio P. O. (2016). Linking consistent individual differences in web structure and behavior in black widow spiders. Behav. Ecol. 27 (5), 1424–1431. doi: 10.1093/beheco/arw048
Doucet S. M., Montgomerie R. (2003). Multiple sexual ornaments in satin bowerbirds: ultraviolet plumage and bowers signal different aspects of male quality. Behav. Ecol. 14 (4), 503–509. doi: 10.1093/beheco/arg035
Eberhard W. G. (1986). “Effects of orb web geometry on prey interception and retention,” in Spiders webs, behavior, and evolution. Ed. Shear W. A. (Stanford CA: Stanford University Press), 70–100.
Eberhard W. G., Agnarsson I., Levi H. W. (2008). Web forms and the phylogeny of theridiid spiders (Araneae: theridiidae): chaos from order. Syst. Biodivers. 6 (04), 415–475. doi: 10.1017/S1477200008002855
Ford M. J. (1977). Energy costs of the predation strategy of the web-spinning spider Lepthyphantes zimmermanni bertkau (Linyphiidae). Oecologia 28 (4), 341–349, 28(4). doi: 10.1007/BF00345989
Henschel J. R., Jocqué R. (1994). Bauble spiders: a new species of achaearanea (Araneae: theridiidae) with ingenious spiral retreats. J. Nat. Hist. 28 (6), 1287–1295. doi: 10.1080/00222939400770651
Herberstein M. E. (2011). Spider behaviour: flexibility and versatility. Ed. Herberstein M. E. (Cambridge: Cambridge University Press).
Herberstein M. E., Wignall A. (2011). Introduction: spider biology. In Spider behaviour: flexibility and versatility. (Cambridge University Press) pp. 1–30. doi: 10.1017/CBO9780511974496.002
Hesselberg T. (2015). Exploration behaviour and behavioural flexibility in orb-web spiders: a review. Curr. Zool. 61 (2), 313–327. doi: 10.1093/czoolo/61.2.313
Jakob E. M. (1991). Costs and benefits of group living for pholcid spiderlings: losing food, saving silk. Anim. Behav. 41 (4), 711–722. doi: 10.1016/S0003-3472(05)80908-X
Janetos A. C. (1982). Foraging tactics of two guilds of web-spinning spiders. Behav. Ecol. Sociobiol. 10, 19–27. doi: 10.1007/BF00296392
Kawamoto T. H., Japyassu H. F. (2008). Tenacity and silk investment of two orb weavers: considerations about diversification of the araneoidea. J. Arachnol. 36 (2), 418–424. doi: 10.1636/CA07-129.1
Korb J. (2010). “Termite mound architecture, from function to construction,” in Biology of termites: a modern synthesis (Dordrecht: Springer).
Li Z. C., Agnarsson I., Peng Y., Liu J. (2021). Eight cobweb spider species from China building detritus based, bell-shaped retreats (Araneae, theridiidae). ZooKeys 1055, 95–121. doi: 10.3897/zookeys.1055.67620
Líznarová E., Pekár S. (2013). Dangerous prey is associated with a type 4 functional response in spiders. Anim. Behav. 85 (6), 1183–1190. doi: 10.1016/j.anbehav.2013.03.004
Lloyd N. J., Elgar M. A. (2006). Costs and benefits of facultative aggregating behaviour in the orb-spinning spider Gasteracantha minax thorell (Araneae: araneidae). Aust. J. Ecol. 22 (3), 256–261. doi: 10.1111/j.1442-9993.1997.tb00670.x
Manicom C., Schwarzkopf L., Alford R. A., Schoener T. W. (2008). Self-made shelters protect spiders from predation. Proc. Nat. Acad. Sci. U.S.A. 105 (39), 14903–14907. doi: 10.1073/pnas.0807107105
Mikát M., Matoušková E., Straka J. (2021). Nesting of Ceratina nigrolabiata, a biparental bee. Sci. Rep. 11 (1), 5026. doi: 10.1038/s41598-021-83940-4
Opell B. D. (1999). Changes in spinning anatomy and thread stickiness associated with the origin of orb-weaving spiders. Biol. J. Linn. Soc. 68, 593–612. doi: 10.1111/j.1095-8312.1999.tb01190.x
Opell B. D., Markley B. J., Hannum C. D., Hendricks M. L. (2008). The contribution of axial fiber extensibility to the adhesion of viscous capture threads spun by orb-weaving spiders. J. Exp. Biol. 211 (14), 2243–2251. doi: 10.1242/jeb.016147
Opell B. D., Schwend H. S. (2009). Adhesive efficiency of spider prey capture threads. Zoology 112 (1), 16–26. doi: 10.1016/j.zool.2008.04.002
Peakall D. B. (1971). Conservation of web proteins in the spider, Araneus diadematus. J. Exp. Zool. 176 (3), 257–264. doi: 10.1002/JEZ.1401760302
Pekár S., Toft S. (2015). Trophic specialisation in a predatory group: the case of prey-specialised spiders (Araneae). Biol. Rev. 90 (3), 744–761. doi: 10.1111/brv.12133
Pinter-Wollman N. (2015). Nest architecture shapes the collective behaviour of harvester ants. Biol. Lett. 11 (10), 20150695. doi: 10.1098/rsbl.2015.0695
R Core Team (2022). R: a language and environment for statistical computing (Vienna: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Riechert S. E., Gillespie R. G. (1986). “Habitat choice and utilization in web-building spiders,” in Spiders, webs, behavior, and evolution;. Ed. Shear W. A. (California: Stanford University Press).
Sahni V., Blackledge T. A., Dhinojwala A. (2010). Viscoelastic solids explain spider web stickiness. Nat. Commun. 1, 19. doi: 10.1038/ncomms1019
Scharf I., Lubin Y., Ovadia O. (2011). Foraging decisions and behavioral flexibility in trap-building predators: a review. Biol. Rev. 86 (3), 626–639. doi: 10.1111/j.1469-185X.2010.00163.x
Smith M. L., Ostwald M. M., Seeley T. D. (2015). Adaptive tuning of an extended phenotype: honeybees seasonally shift their honey storage to optimize male production. Anim. Behav. 103, 29–33. doi: 10.1016/j.anbehav.2015.01.035
Tso I. M., Chiang S. Y., Blackledge T. A. (2007). Does the giant wood spider Nephila pilipes respond to prey variation by altering web or silk properties? Ethology 113 (4), 324–333. doi: 10.1111/j.1439-0310.2007.01318.x
Wolff J. O., Jones B., Herberstein M. E. (2018). Plastic material investment in load-bearing silk attachments in spiders. Zoology 131, 45–47. doi: 10.1016/j.zool.2018.05.002
World Spider Catalog (2023) World spider catalog. version 24 (Natural History Museum Bern). Available at: http://wsc.nmbe.ch (Accessed 2023-03-16).
Zhang H. X., Li G., Li C. C., Chen J., Zhao Z. Y., Zhang S. C., et al. (2022b). Feeding mediated web-building plasticity in a cobweb spider. Curr. Zool. XX, 1–10. doi: 10.1093/cz/zoac077
Zhang H. X., Zhong R., Yu L., Chen J., Agnarsson I., Liu J. (2022a). Safety is increasingly important in cobweb spiders based on life history. Integr. Zool. 00, 1–10. doi: 10.1111/1749-4877.12682
Keywords: detritus-based, bell-shaped cobweb, behavioral plasticity, Campanicola campanulata, defense, foraging, trade-off
Citation: Zhang H, Wen L, Li Z and Li C (2023) Economic web-building behavior and behavioral investment trade-offs in a cobweb spider. Front. Ecol. Evol. 11:1164310. doi: 10.3389/fevo.2023.1164310
Received: 12 February 2023; Accepted: 05 June 2023;
Published: 26 June 2023.
Edited by:
Gang Li, Soochow University, ChinaReviewed by:
Thomas Hesselberg, University of Oxford, United KingdomCopyright © 2023 Zhang, Wen, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changchun Li, bGNjMzg2QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.