- 1Graduate Group in Animal Behavior, University of California, Davis, Davis, CA, United States
- 2California National Primate Research Center, University of California, Davis, Davis, CA, United States
- 3Department of Psychology, University of California, Davis, Davis, CA, United States
- 4Department of Neurobiology, Physiology, and Behavior, University of California, Davis, Davis, CA, United States
Some paired primates use complex, coordinated vocal signals to communicate within and between family groups. The information encoded within those signals is not well understood, nor is the intricacy of individuals’ behavioral and physiological responses to these signals. Considering the conspicuous nature of these vocal signals, it is a priority to better understand paired primates’ responses to conspecific calls. Pair-bonded titi monkeys (Plecturocebus cupreus) sing duets comprised of the male and female’s long call. Here, we use a playback study to assess female titi monkeys’ responses to different vocal stimuli based on the subject’s pairing status. Six adult female titi monkeys participated in the study at two timepoints—pre-pairing and post-pairing. At each timepoint, subjects underwent three distinct playbacks—control recording, male solo vocalization, and pair duet. Behaviors such as locomotion and vocalizations were scored during and after the playback, and cortisol and androgen values were assessed via a plasma blood sample. Female titi monkeys attended more to social signals compared to the control, regardless of pairing status. However, in the time immediately following any playback type, female titi monkeys trilled more and spent a greater proportion of time locomoting during pre-pairing timepoints (compared to post-pairing). Female titi monkeys’ behavioral responses to social audio stimuli, combined with subjects’ increases in cortisol and androgens as paired individuals, imply female titi monkeys attend and respond to social signals territorially.
Introduction
Many social animals use vocal signals to communicate with conspecifics (Silk, 2007). Frequently, research studies quantify the variation of and identify the mechanisms underlying social vocal signals (Fishbein et al., 2021). However, listeners’ perception and interpretation of these vocal signals represents fertile grounds for additional study to illuminate what aspects of vocal variation are meaningful to conspecifics. Primates across the order frequently use vocal communication and display wide variety in call structure, meaning, and function (Bradbury and Vehrencamp, 1998, pp. 658–665). Combined with contextual knowledge, such as relationship with the caller or listener, vocal variation can be understood by conspecifics and can simplify social interactions by, for instance, communicating the motivational state (i.e., aggressiveness or passiveness) of another (Cheney and Seyfarth, 2018). Generally, primates have a highly adaptive ability to produce vocal variation within a wide range of social situations (Cheney and Seyfarth, 2018).
Playback studies—in which researchers broadcast pre-recorded vocal signals—have been used to identify what information vocalizations signal to conspecifics based upon the behavioral responses of listening individuals (Fischer et al., 2013). While playback studies have been used across the animal kingdom, we focus here on the non-human primate literature, to better place our study in context. For example, calls can encode information about physiological and emotional states during social conflict (e.g., male chacma baboons (Papio ursinus) and chimpanzees (Pan troglodytes; Fischer et al., 2013). Many species use calls to evoke intended behavioral responses from group members, such as red-fronted lemurs (Eulemur fulvus rufus) and white sifakas (Propithecus verreauxi) using alarm calls to initiate group fleeing behavior in the presence of predators (Fischer et al., 2013). In wild rhesus macaques (Macaca mulatta), wild Japanese macaques (Macaca fuscata), captive cotton-top tamarins (Saguinus oedipus), and captive coppery titi monkeys (Plecturocebus cupreus) vocalizations can provide cues about sex, reproductive status, and group membership (Ghazanfar and Hauser, 2001; Clink et al., 2019). Female rhesus macaques use vocalizations to distinguish kin from non-kin, as shown by the greater amount of time spent orienting toward vocalizations made by kin (Ghazanfar and Hauser, 2001). Overall, playback studies demonstrate the wide range of information that can be communicated via vocal signals in primates.
Primates live in a wide range of social groupings, spanning from solitary to multi-level fission/fusion societies (Terborgh and Janson, 1986; Kappeler and van Schaik, 2002). These varying social groups have emerged across primate evolution due to a combination of ecological, social, and physiological pressures that provide rich opportunity to investigate sociality (Kappeler and van Schaik, 2002; Dunbar and Shultz, 2021). Amongst this wide variety, pair living remains one of the least common social groupings in mammals, especially primates (Kleiman, 1977; Lukas and Clutton-Brock, 2013), and one that necessitates unique communication (Singletary and Tecot, 2020). Some or all species of gibbons (Marshall and Marshall, 1976; Palombit, 1994; Geissmann, 2002), siamangs (Geissmann, 2002), titi monkeys (Robinson, 1979a,b), owl monkeys (Depeine et al., 2008), and tarsiers (Nietsch, 1999; Clink et al., 2022) live in adult female/male pairs and communicate using specialized vocal signals (Singletary and Tecot, 2020). Given the rarity of this social organization, it is of considerable importance to better understand the communication processes reinforcing pair living.
Playback studies have been used to investigate the meaning of vocal signals in some pair-living primates (Robinson, 1981; Fichtel and Hilgartner, 2013; Caselli et al., 2015; Garcia de la Chica et al., 2021). For example, researchers played back calls of unknown, single owl monkeys (Aotus azarae) to owl monkey pairs and found the location of the playback did not influence resulting behaviors, but paired owl monkeys—both the adult male and female—reacted more to unfamiliar male calls than female calls with greater movement toward the playback and more vocalizations, revealing the tendency for both mates to defend their partner (Garcia de la Chica et al., 2021). Beyond owl monkeys, the remaining pair-living primates (e.g., gibbons, siamangs, titi monkeys, and tarsiers) all participate in highly coordinated vocal interactions—often called duets—in which the adult female and male emit sex-specific vocal contributions (Marshall and Marshall, 1976; Robinson, 1979a,b; Nietsch, 1999; De Gregorio et al., 2022). However, the fine-scale social behaviors of these species are difficult to study in the wild (Bossuyt, 2002; Caselli et al., 2014, 2015).
Copious studies of titi monkeys—both in the wild and in captivity—have illuminated the strong and selective pair bonds that mated adult titi monkeys (Plecturocebus spp.) form with each other (Bales et al., 2017). Pair bonds are enduring socio-emotional attachments characterized by a suite of behaviors including preference for one’s mate over an opposite-sex stranger, proximity maintenance, and separation distress; for an extensive definition and review, see Bales et al. (2021). Titi monkeys duet every morning in species-typical, stereotyped duets, communicating social information both with their mate and with conspecifics. Functionally, titi monkey’s duets serve as territorial signals, allowing groups to claim occupancy and reinforce boundaries when threatened (Robinson, 1979b, 1981). Titi monkeys approach neighboring groups when conspecifics duet near territorial boundaries, providing evidence of joint territorial defense by the adult female and male (Plecturocebus cupreus; Robinson, 1979b, 1981; Callicebus nigrifrons; Caselli et al., 2014, 2015). Titi monkeys mate guard and display agonistic behaviors toward strangers (Fernandez-Duque et al., 2000). Coppery titi monkey (Plecturocebus cupreus; previously classified as Callicebus moloch, then Callicebus cupreus, and ultimately Plecturocebus cupreus and hereafter referred to as “titi monkeys”) pairs duet together as early as the first day of pairing (Müller and Anzenberger, 2002). The aforementioned behaviors—territorial defense, mate guarding, agonism toward strangers, and vocal duetting—represent a suite of behaviors commonly attributed to titi monkeys’ general territorial defense (Robinson, 1981; Caselli et al., 2015; Mercier et al., 2020).
Titi monkeys’ expansive vocal repertoires have been studied in multiple species and in multiple contexts (observation and experimentation, both in wild and captive settings). Trills are often used in the context of separation wherein individuals cannot access a group member (Moynihan, 1966). Additionally, infants most commonly use trills as a generalized vocalization that elicits care from parents (Robinson, 1979a; Lau et al., 2020). Peeps are commonly used as a proximity-seeking call and are used by subadult and adult individuals (Arias del Razo et al., 2022a). Titi monkey long calls (referred to as a “long call” when vocalized alone, but a “duet contribution” when two titi monkeys coordinate their long calls to form a “duet”) are one of the most conspicuous and species-typical behaviors performed by titi monkeys. Titi monkey duets, in particular, are understudied both in captivity and in the wild. What is known about this particularly conspicuous behavior indicates wide variation in titi monkey duet features and the potential for these duets to carry information about caller identity. In the wild, titi monkey duets were longer in duration during inter-group encounters as opposed to spontaneous duets (Dolotovskaya and Heymann, 2022). Adult male and female’s contributions to the duet are individually identifiable (Lau et al., 2020). However, cross-sectional evidence shows that titi duet contributions do have a degree of plasticity, as pair mates converge with their partner in their note rate over time (Clink et al., 2019). Within that plasticity, there are also limited impacts of heritability in titi monkey’s duet contributions (Clink et al., 2022). While most studies of coppery titi monkey vocal variance have occurred in captivity, audibly and visually, titi monkey duets from captivity are indistinguishable from those in the wild (Robinson, 1979a; Lau et al., 2020). This previous work demonstrates the ability of titi monkey calls to carry a wide range of information that is both statistically identifiable and, to some extent, behaviorally relevant to titi monkeys. The present study aims to investigate another element of titi monkey communication by observing responses to social playbacks in a controlled, captive setting. This project serves the secondary function of validating the retention and use of previously observed wild titi monkey behavior in a captive population for the first time.
Physiologically, multiple hormones may be involved in social and territorial behaviors in this species. Cortisol, a steroid hormone produced by the adrenal gland, plays an important role in social behavior. In closely related, pair-living owl monkeys (Aotus azarae), females’ cortisol levels are high during gestation, and both males and females have lower cortisol during periods of intensive infant care (Corley et al., 2021). The activational effects of cortisol serve a variety of functions and the nuance of cortisol’s affects has recently reframed the importance of interpreting cortisol results with full consideration for the context in which it is investigated (Epel et al., 2018). Titi monkeys display robust responses to dexamethasone challenge of the adrenocortical system (Mendoza and Moberg, 1985). Additionally, titi monkeys are quite responsive to novelty in that titi monkeys require far less novelty than closely related squirrel monkeys (Saimiri sciureus) to evoke a cortisol response (Hennessy et al., 1995). Titi monkey infants show increased cortisol when separated from their parents (Hoffman et al., 1995) and in adulthood, titi monkeys’ cortisol levels are higher when separated from one’s mate compared to a non-separation period of identical duration (Arias del Razo et al., 2022a). In addition to the impacts of cortisol, androgens in titi monkeys have been studied in the social contexts of puberty (Arias del Razo et al., 2020), jealousy (Maninger et al., 2017), and, importantly for the present study, separation from one’s pair mate (Arias del Razo et al., 2022a). The previous research of the role of both cortisol and androgens in titi monkey social behavior indicates that many titi monkey social situations will likely involve activation of adrenocortical and androgen systems. Given what little is known about the role of cortisol and androgens in the social vocalizations of this species, we aimed to investigate the physiological impacts of social vocal communication within the present study.
While previous work has identified the variance and function of titi monkey duet contributions, few studies to date have assessed how titi monkeys’ behavioral and physiological responses to vocal stimuli vary. In black-fronted titi monkeys, three pairs responded with vocal and approach responses to all conspecific playback stimuli (male solo, female solo, and duet), but not to the control (Caselli et al., 2015). Additionally, black-fronted titi monkeys did not respond differentially to solos and duets and appeared to use a joint territorial defense approach in responding to any conspecific vocalizations (Caselli et al., 2015). While we expect similar behavioral responses in coppery titi monkeys, the present study expands on Caselli and colleagues’ previous work and expands our knowledge into the captive setting. Due to funding limitations and the focus on female pair-bonding behavior in the Bales Laboratory, we chose to focus our attention on female titi monkeys for this project. In this study, we assessed titi monkey females’ responses to unfamiliar male solo vocalizations and duets of unfamiliar female/male pairs. Titi monkeys’ social behaviors are fairly subtle, and individuals display species-wide neophobia—both of which make studying intricate social responses in the field quite challenging (Bossuyt, 2002). To date, no study has looked at titi monkeys’ responses to playbacks in captivity. It is unknown what information titi monkeys receive when listening to social vocalizations. For this study, we utilized the breeding colony of coppery titi monkeys (Plecturocebus cupreus) at the California National Primate Research Center (CNPRC) in Davis, California. The CNPRC facility allowed us to perform playback studies with experimental control and fine-scale observations that are impossible in the field. We chose to focus on female titi monkeys based upon limited resources and the unique role of the female titi monkey in parenting and maintaining pair proximity (Dolotovskaya et al., 2020b).
While this study was inherently investigatory in nature, we did pose a hypothesis and a few corresponding predictions prior to the study. First, we hypothesized that captive female titi monkeys’ behavioral and physiological states when hearing playbacks reflect the known territorial responses of titis based upon pairing status. We predicted female titi monkeys would have higher cortisol levels during social stimuli playbacks compared to the control, regardless of pairing status. We also predicted female titi monkeys would have higher androgens during post-pairing duet stimulus playback and solo stimulus playback compared to the post-pairing control playback and all pre-pairing stimuli. Behaviorally, we predicted a greater number of vocalizations, locomotion, and time spent orienting to the stimuli in response to duet and solo playbacks compared to the control, regardless of pairing status. We expected all behavioral and physiological changes in social playback responses compared to the control playback to be higher for duet playbacks than solo playbacks.
Methods
Subjects
All coppery titi monkeys (Plecturocebus cupreus) used for this project were captive born at the CNPRC. The titi monkeys were housed indoors in stainless steel enclosures measuring 1.2 × 1.2 × 2.1 m (volume = 3.024 m3) or 1.2 × 1.2 × 1.8 m (volume = 2.592 m3). Cage height depended upon their location in the CNPRC. All rooms were maintained at 21°C on a 12-h light cycle with lights on from 06:00 to 18:00. Subjects were fed a diet of monkey chow, carrots, bananas, apples, and rice cereal twice a day. Subjects were offered one Spanish peanut during daily health checks as a reward for presenting their abdomen and digits for inspection. Water was available ad libitum and additional oat foraging enrichment was provided twice daily. Subjects were housed in natal family groups that varied in composition during the pre-pairing portion of the study. During the post-pairing portion of the study, subjects lived in female/male pairs. All groups were in acoustic contact with other titi monkey pairs both within their room and with animals in other rooms but had minimal visual contact with animals outside their own cage. This housing situation is the same as described in previous studies of this colony (Mendoza and Mason, 1986a; Tardif et al., 2006).
For this study, we chose our focal subjects (n = 6 females) from available, unpaired females living with either one parent, one same-sex sibling, or both parents and a sibling. At the start of the playback study, females ages ranged from 1.89–3.64 years of age, for a mean ± SD age of 2.64 ± 0.74 years.
Study design
Testing occurred at two testing timepoints: once approximately 1 month before the focal subject was paired with their pair mate, and again approximately 8 months post-pairing (Figure 1). The post-pairing timepoint was originally scheduled at 6 months post-pairing because 4–6 months post-pairing is the timeframe in which a strong behavioral preference, as well as associated neurological changes, are displayed towards one’s partner (Rothwell et al., 2020; Arias del Razo et al., 2022b). However, COVID-19 pandemic-related delays necessitated the delay of the post-pairing sessions. As such, females were tested 8 months post-pairing.
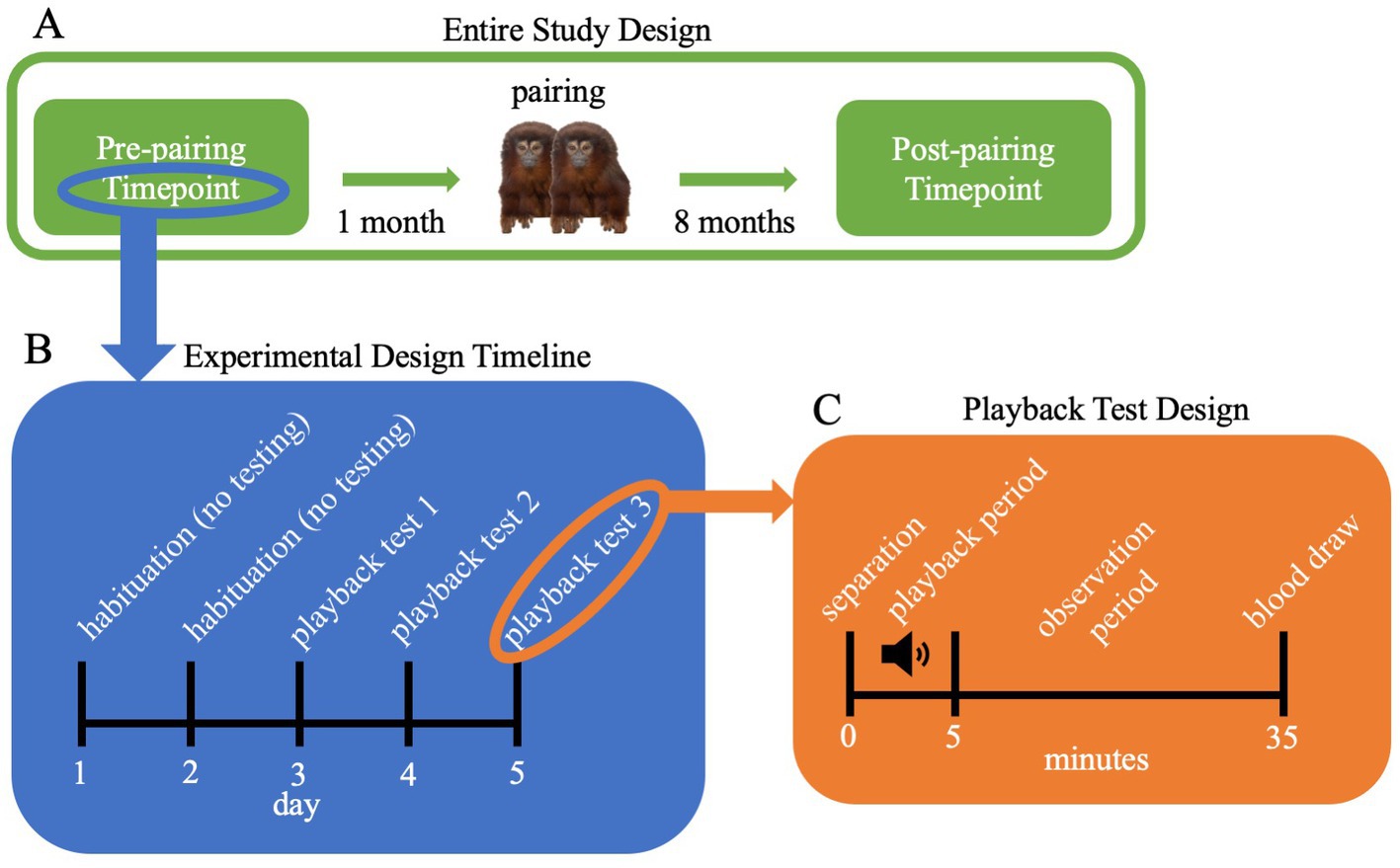
Figure 1. Schematic of the playback study design. (A) Entire Study Design: adult female titi monkey subjects underwent the pre-pairing playback timepoint while still living with their family. One month after the pre-pairing timepoint, female titi monkey subjects underwent pairing with an unfamiliar adult male. Eight months following pairing with one’s mate, female subjects underwent the same playback study at the post-pairing timepoint. (B) Pairing Timepoint Design: during each testing timepoint, titi monkey subjects were given 2 days to habituate to the testing room, followed by 3 days of playback tests. (C) Playback Test Design: for each playback test, females were first separated from their cage mates (family or pair mate depending on the timepoint). The 5-min playback period then commenced, followed by a 30-min observation period of silence. At the end of the 35-min test, subjects were handled for a blood draw.
Prior to testing, we relocated females to a private testing room to eliminate acoustic contact with other animals. Female subjects were accompanied to the testing room with either their family (pre-pairing timepoint) or their pair mate (post-pairing timepoint). Following the relocation, we gave subjects two undisturbed days to habituate to the testing room (Bales et al., 2017). Following the habituation period, testing occurred over three days during which the three playback tests were counter-balanced across subjects: (1) control recording of monkey room ambient acoustics (no animal vocalizations), (2) unfamiliar, unpaired male solo vocalization, (3) unfamiliar, paired male and female duet (Figure 1). We chose the control recording, which included the sound of hoses and the air conditioning system, as these sounds were familiar, but recorded in a room that did not have titi monkeys in it. Thus, this control recording represented a familiar sound type, but a novel recording of it. Similarly, we chose solo male recordings and duet recordings as all titi monkeys are familiar with these social signals. However, we chose recordings from unfamiliar animals to assess responses to the type of call, not the individual who was calling. We focused on male solo vocalizations and duets as they represent two distinct pairing statuses (unpaired and paired) and are signals that an opposite sex individual (our focal female subjects) pays specific attention to either when seeking a mate or defending their territory (Robinson, 1981).
Male solos and pair duets were recorded at the CNPRC. We confirmed stimuli were unknown to test subjects by ensuring recordings were either (1) recorded before the focal subject was born or (2) recorded in a room other than the room in which the focal subject lived. We used male solo recordings from monkeys who were an average age of 14.8 years old ± standard deviation of 4.3 years (range 11.7–20.9 years old). These male monkeys were not paired and living alone at the time of recording. They vocalized solos alone, without any other monkeys vocalizing. We did not edit the monkeys’ calls. We used duet recordings from pairs that had been together for 6.6 ± 6.1 years (range 1.9–15.2 years). There is evidence that titi monkeys’ duet contributions change with age—individuals’ pulse note rate of repetition decreases with age and the total duration of the pulse note duet contribution increases in overall duration (Clink et al., 2019). Additionally, there is evidence that individuals’ pulse duration decreases with increasing pair tenure, and that pair mates become more similar to each other in their pulse rate with pair tenure (Clink et al., 2019). As such, we aimed to capture a wide range of ages and pair tenure in the various stimulus recordings used. However, we were limited by the availability of solo males and the need to ensure stimulus individuals were strangers to our focal subjects.
All playbacks were broadcast at species-typical amplitude, measured by sound pressure level (SPL) meter (approximately 110 dB). We pre-recorded playback vocalizations from titi monkey individuals both unrelated to our subject females and from different housing areas, ensuring the playback stimuli were unfamiliar. Each subject heard a unique exemplar for each playback type at each timepoint to avoid pseudo-replication and ensure appropriate sampling of the colony population (Kroodsma, 1989, 1990).
At the beginning of each test, we removed all family members (during the pre-pairing timepoint) or the pair mate (during the post-pairing timepoint) from the cage, leaving the female subject alone. We separated the focal subject from her home social setting to ensure all responses to the playback stimuli were individually driven and not impacted by the behavior of other animals in the group. While the separation paradigm itself does introduce a degree of social distress (Arias del Razo et al., 2022a), separation occurred before all playback stimulus types. As such, the control stimulus serves as the reference level for the solo and duet stimuli and provides a comparison with which to observe the behavioral and physiological impacts of social playbacks beyond the impacts of separation alone. The playback recording was broadcast for 5 min—the average duration of indoor-housed coppery titi monkey duets—followed by an additional 30-min observation period. In total, each playback test lasted 35 min. The methods for the separation paradigm and subsequent blood draw (detailed below) followed temporary separation protocols developed for previous projects in this lab with the addition of the acoustic playback (Figure 1; Arias del Razo et al., 2022a).
Female cycling
Our hormonal outcome measures, androgens and cortisol, can vary based on the levels of circulating estrogen and progesterone (Van Goozen et al., 1997). To assess reproductive status, we collected urine samples three times weekly during the first morning urination (0530–0600 h), with a maximal interval of 3 days between collection of successive samples for any given individual while our female monkeys were participating in this study. Urine sample collection began 2 weeks prior to the start of the playback study. We collected an average of 13.14 ± standard error of 0.88 samples per individual (range: 9–15). Following collection, samples were aliquoted into 2 ml cryo tubes and stored at −80°C until assay. Titi monkey reproductive cycles are, on average, 17 days long (Valeggia et al., 1999). As such, we assayed 1 month’s worth of samples per subject for urinary estrogen (E1C) and pregnanediol (PdG) conjugates to identify reproductive cycling (or lack thereof). E1C and PdG were assayed at the UC Davis Endocrinology Laboratory using an enzyme-immunoassay described in detail elsewhere (Valeggia et al., 1999; Conley et al., 2022). Inter-assay Coefficients of Variation (CVs) were 0.88% and intra-assay CVs were 3.73%.
Ovulation was assumed to have occurred if PdG concentrations were > 100 ng/mg Cr in two consecutive samples that together totaled >400 ng/mg Cr, and were defined as luteal phases (Conley et al., 2022). Given recent evidence that female titi monkeys begin regularly reproductively cycling around 2.5 years of age but can have intermittent cycles earlier—and can begin cycling while in the natal family group or once paired—cycling information was included in all behavioral and physiological variables’ initial models (Conley et al., 2022).
For inclusion in our models, females were coded either as non-cycling (based on urinary assay), cycling (based on urinary assay or a previous pregnancy), or pregnant (based on a positive ultrasound). The three reproductive statuses—non-cycling, cycling, or pregnant—were coded 0, 1, and 2, respectively.
Hormonal response to playbacks
All playback experiments occurred at the same time of day (1,330 h) to eliminate the potential confounding effects of circadian cortisol and androgen rhythms (Place and Nichols, 1991; Smith and French, 1997). At the end of each 35-min test, a 0.5 ml blood sample was collected via femoral venipuncture to assess androgen and cortisol levels. Samples were collected 41.10 ± 0.32 min (mean ± standard error) from the start of the audio playback and 3.52 ± 0.30 min from the start time of handling. Blood samples were immediately placed on ice and, within 5 min, centrifuged at 4°C for 15 min. We extracted plasma and stored samples at −80°C. Plasma cortisol and androgens were assayed at the UC Davis Endocrinology Laboratory using an enzyme immunoassay previously validated both chemically and biologically for titi monkeys and described in detail elsewhere (Witczak et al., 2021; Conley et al., 2022; Arias del Razo et al., 2022a). Inter-assay CV was 2.5% for cortisol (intra-assays CVs were 9.3 and 9.4% for the two plates) and the intra-assay CV was 13.6% for the single androgen plate. All hormone measures were natural log-transformed prior to all analyses so that the data met the assumptions of normality.
Behavioral scoring
Behavioral measures were recorded to assess the female’s response to each playback type (Mendoza and Mason, 1986a; Fernandez-Duque et al., 2000) during two periods: (1) the playback period in which the females listened to an audio stimulus and (2) the observation period in which females were observed for 30 min immediately after the audio stimulus. We separated all behavioral analyses into these two periods (playback and observation) to illuminate the immediate and following impacts of the playback on behavior. The 35-min test was filmed to enable later behavioral scoring. The percent of time locomoting was scored from video recordings of each test using the DVRecorder module of Behavior Tracker.1 Orientation to the stimulus was scored in real time using the Recorder module of Behavior Tracker (see footnote 1).
We recorded the subjects’ vocalizations during and after the playback using a Marantz PND 660 recorder and a Marantz directional condenser microphone (Marantz, Kanagawa, Japan) to enable accurate classification of quickly repeated, intricate calls. After testing, calls were identified and scored from spectrograms using Raven Pro 1.6 Sound Analysis Software (K. Lisa Yang Center for Conservation Bioacoustics at the Cornell Lab of Ornithology, 2022, Ithaca, NY). We generated spectrograms with a 512-point (11.6 ms) Hann window (3 dB bandwidth = 124 Hz), with 75% overlap, and a 1,024-point discrete Fourier transform, resulting in time and frequency measurement precision of 2.9 ms and 43.1 Hz (Lau et al., 2020). We did not down-sample the original sound files. One observer (ARL) scored all the subjects’ vocal output, which included peeps, trills, alarm calls, long call introduction notes, long calls, and the latency to vocalize (Figure 2).
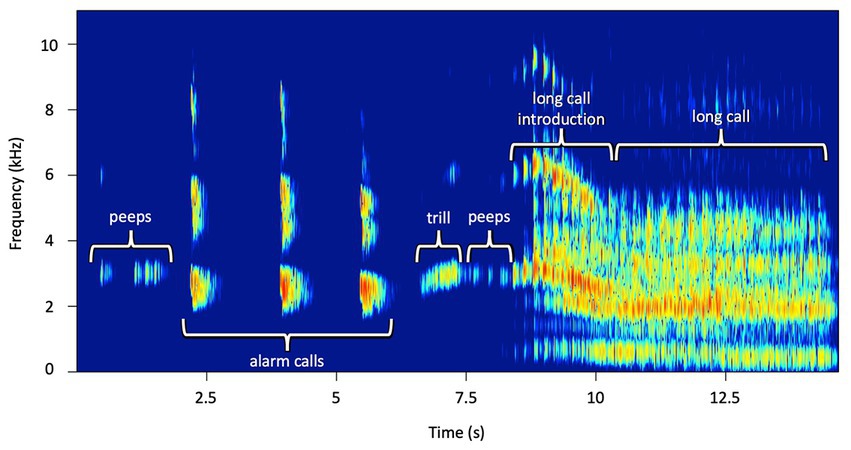
Figure 2. Representative spectrogram of female titi monkey vocal responses during the playback experiment. Peeps, alarm calls, trills, long call introductions, and long calls are displayed within one spectrogram.
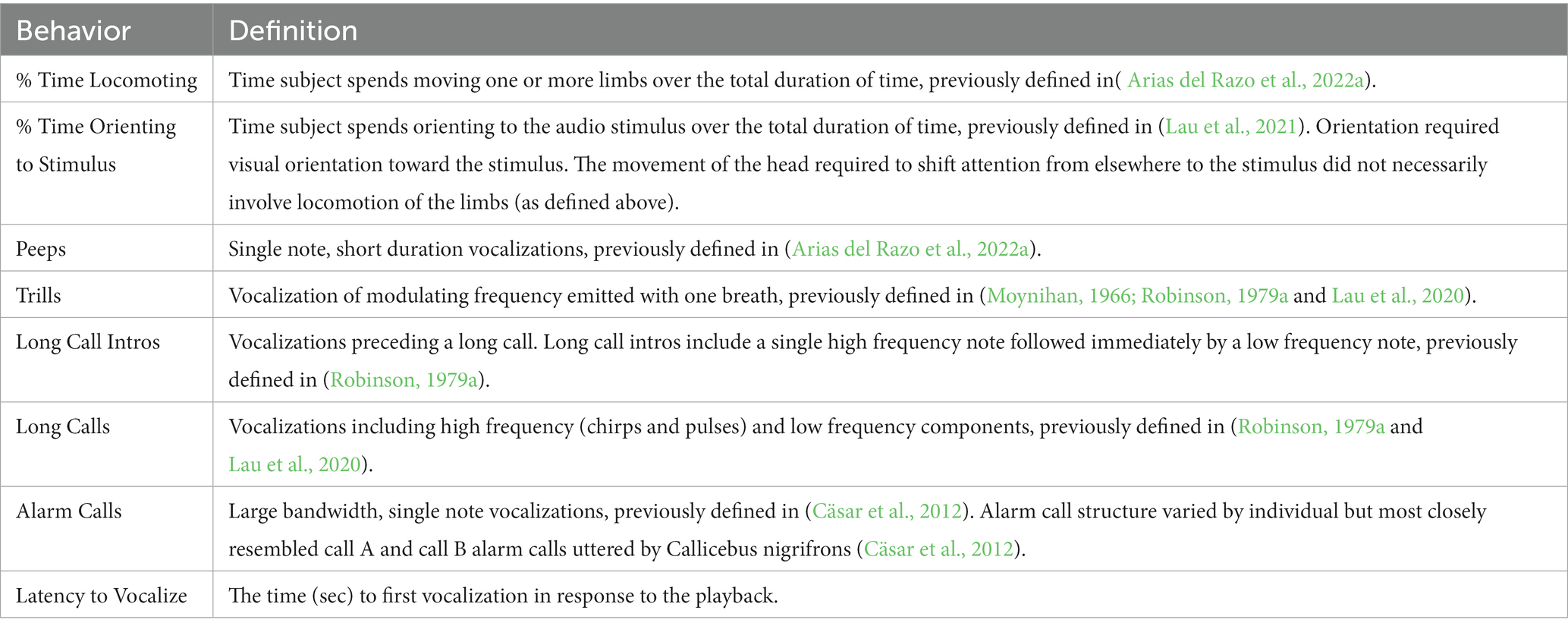
For the full ethogram and descriptive statistics of all outcome variables, see Table 1 and Table 2, respectively.
Model selection
We conducted all data analyses using R programming language and environment (R Core Team, 2022). We used backwards model selection of linear mixed effects models (lmm function) from the nlme package (Pinheiro, 2009; Pinheiro et al., 2017) to assess how pairing status (unpaired or paired), reproductive status (not cycling, cycling, or pregnant), and playback type (ambient control, male solo, or duet) predicted behavioral and physiological measures (cortisol, androgens, percent time orienting to the stimulus, percent time locomoting, peeps, and trills). Subject served as a random effect for all models due to known variability in titi monkey behavior (vocal behavior: Lau et al., 2020; pair affiliation: Rothwell et al., 2020; parenting behavior: Karaskiewicz et al., 2021).
We built each initial model with our three fixed effects and random effect included. As we worked through backwards model selection, we removed each fixed effect one at a time and compared each model to the initial model using the anova function (R Core Team, 2022). We used the resulting log likelihood ratio and p value to assess model fit, using a standard threshold of p ≤ 0.05 as our criteria for retaining or excluding fixed effects.
Regardless of how much they contributed to each model, pairing status and playback type were retained as predictors in all final models to fully account for the experimental paradigm of the study. Reproductive status remained as a fixed effect in all models in which reproductive status contributed significantly to the final model. Given the known variation in titi monkey vocal behavior based upon female reproductive status (Dolotovskaya and Heymann, 2022), we retained reproductive status in all vocal behavior models. Regardless of the random effect’s contribution to overall variance, we retained the random effect of subject in all models. We examined a quantile-quantile plot of the residuals of each final model to assess goodness of fit. We report the results of the final model for all behavioral and physiological outcome measures.
For the outcome variable latency to vocalize, we used a survival/event time model because one subject (during the post-pairing, solo stimulus playback) did not vocalize during entire the 35-min test. We fitted two Cox Proportional Hazards regression models using the coxph function of the survival library (Therneau, 2019). The first was a null model—the second model added fixed effects of reproductive status, stimulus, and pairing status. We used Akaike’s Information Criterion to compare the second model to the first.
All figures presented below were created in R programming language and environment (R Core Team, 2022) using the ggplot2 (Wickham et al., 2016) and cowplot (Wilke et al., 2019) packages.
Post-hoc comparisons
Following backwards model selection, we chose to run contrast comparisons to determine the difference between the three levels of predictor variables that had three levels (reproductive status and stimulus type) and that were statistically significant predictors in the respective model. We used the glht function from the multcomp package (Hothorn et al., 2016) to perform Tukey’s Honest Significant Difference (HSD) test, allowing us to compare the means of each level for our three-level predictor variables.
Results
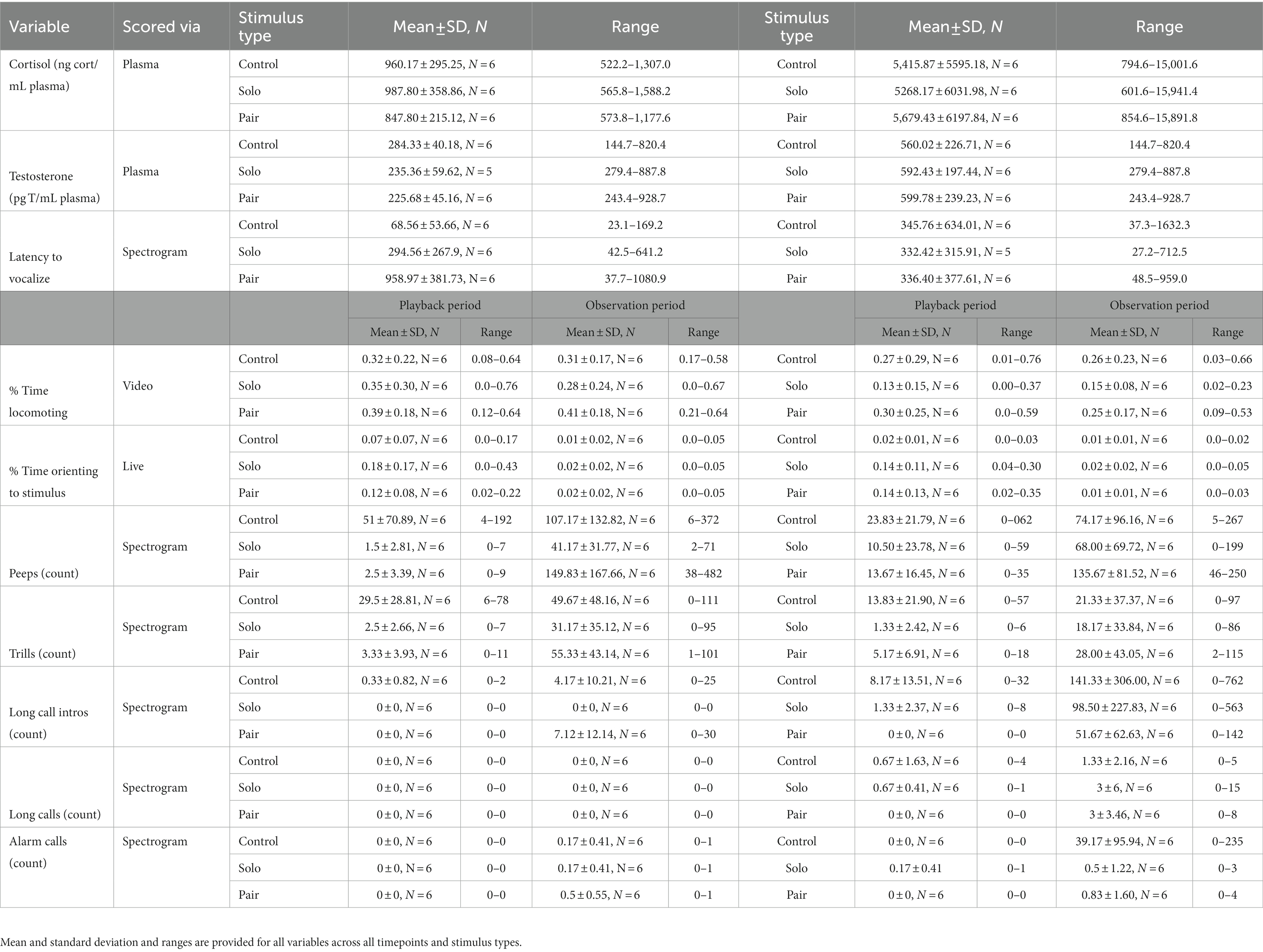
Reproductive status was retained in the final models for all vocal behaviors (see Supplementary material for reproductive status results). All final models included subject as a random effect, regardless of how much subject contributed to the model fit. All final model results are presented in Table 3. All significant behavioral and physiological outcome measures are included in the text as boxplot visualizations; all others are available upon request. The descriptive statistics for all variables are displayed by predictor in Table 2. Model results are presented in Table 3.
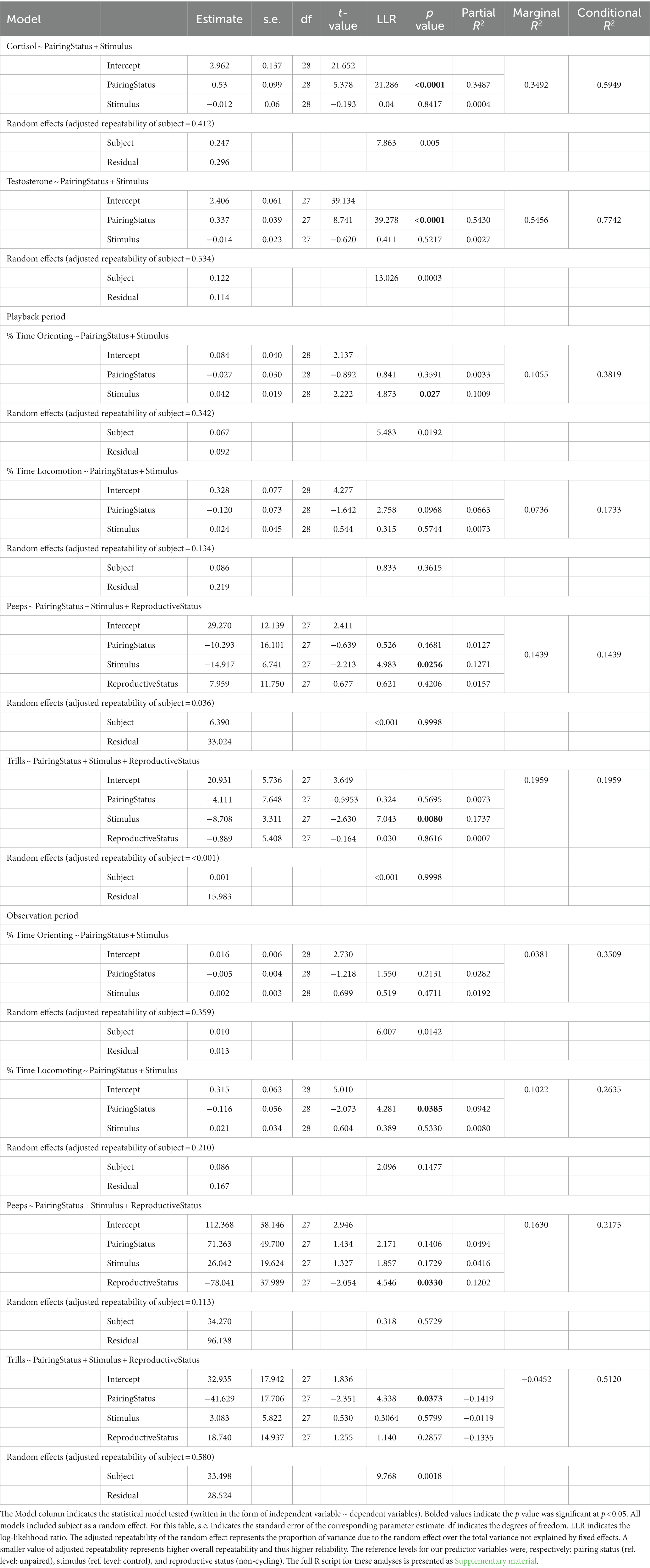
Table 3. Results of the linear mixed-effects models assessing physiological and behavioral responses to different stimulus types during two different pairing statuses.
We ran four post-hoc Tukey’s HSD tests across the entire project. We only performed Tukey’s HSD on models for which a three-level predictor variable contributed significantly to the overall model variance. In three of our final models, playback stimulus type contributed to total variance (models for percent time orienting during the playback period, peeps during the playback period, and trills during the playback period). In one of our final models, reproductive status contributed to the total variance (model for peeps during the observation period). Full results of post-hoc comparisons are presented below.
Physiological responses
We successfully collected plasma blood samples from all subjects at all timepoints. However, for one test timepoint, there was enough volume to assay for cortisol, but not androgens. As such, results for androgens represent 35 samples while results for cortisol represent a full 36 samples. Female subjects had higher cortisol (conditional R2 = 0.3487, t(28) = 5.378, p < 0.0001; Figure 3A) and androgens (partial R2 = 0. 0.5430, t(27) = 8.741, p < 0.0001; Figure 3B) in the post-pairing timepoints than in the pre-pairing timepoints, regardless of stimulus type (Table 3).
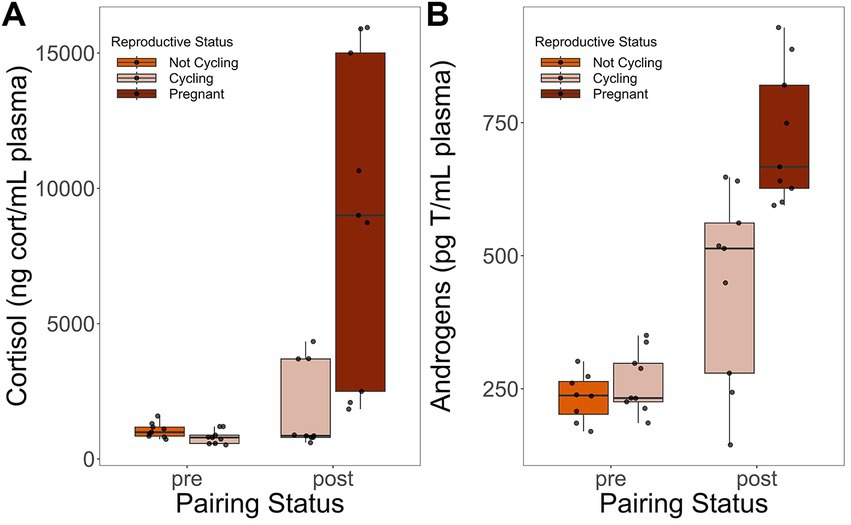
Figure 3. Cortisol (A) and androgen (B) boxplots of female titi monkey plasma hormone levels according to pairing status and reproductive status. Each box delineates the 1st and 3rd interquartile (25% and 75%) with the median as the 2nd interquartile (50%). The whiskers represent the data “range.” Data points above and below the whiskers are outliers. For both cortisol (A) and androgens (B), females had higher values post-pairing than pre-pairing. This effect existed regardless of stimulus type or reproductive status. For descriptive statistics, see Table 2. For model results, see Table 3.
Behavioral responses
We successfully captured the behavioral responses of all 6 subjects, across 2 pairing statuses, in response to three playback stimulus types. This resulted in a total of 36 observations per outcome variable across the study.
During the playback period, titi monkeys’ percent time orienting to the direction of the audio stimuli varied based on playback stimulus type (partial R2 = 0.1009, t(28) = 2.222, p = 0.0270; Table 3). Our Tukey’s HSD post-hoc test indicated that our subjects spent a lower percent of time orienting to the stimulus during the control playback period compared to the solo (p < 0.001) or duet (p = 0.0276) conditions; Figure 4A]. However, there was no significant difference in the means for the solo and paired playbacks [(p = 0.5353), nor were there differences based upon pairing status (partial R2 = 0.0033, t(28) = −0.892, p = 0.3591).
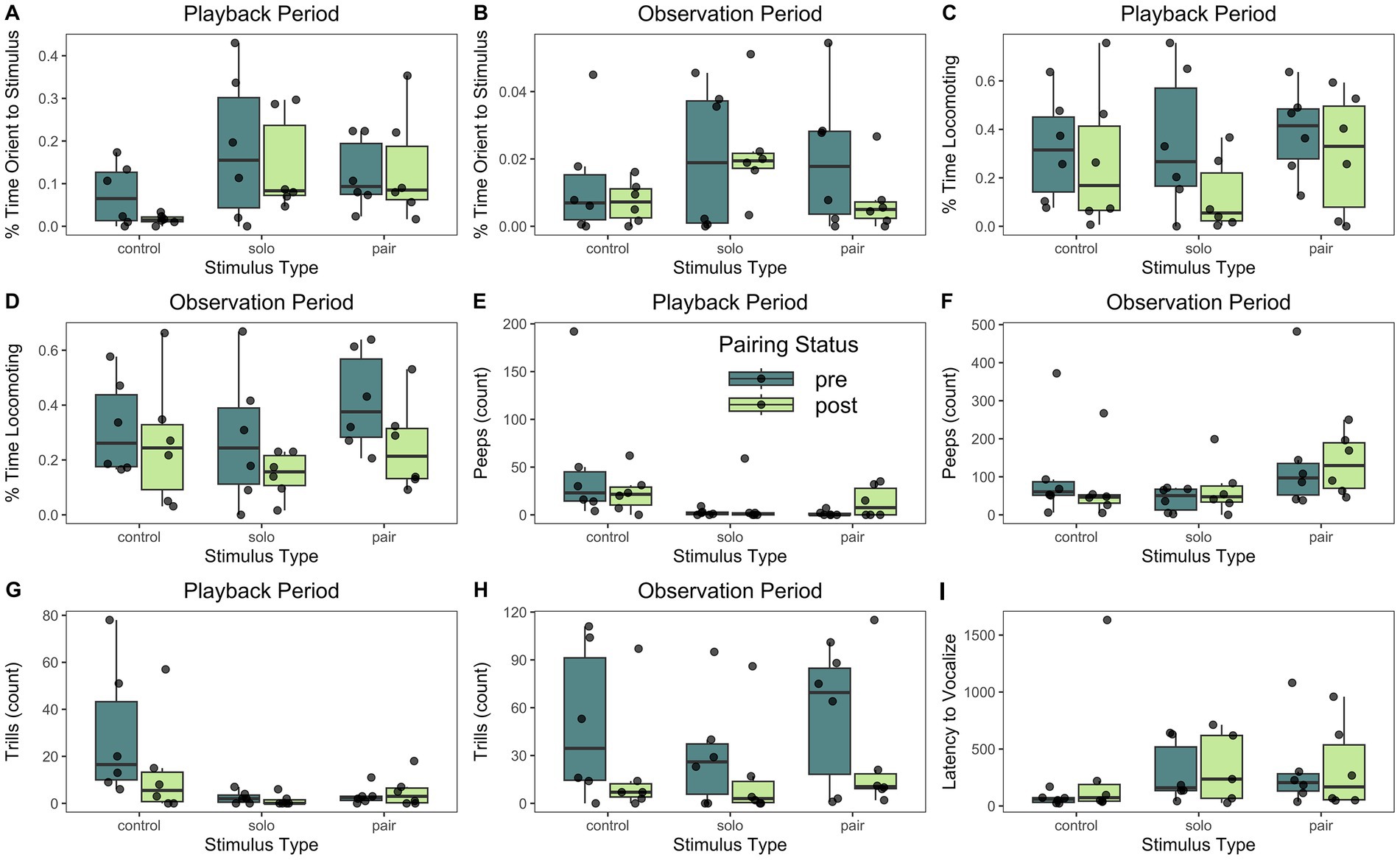
Figure 4. Boxplots of all behavioral outcomes modeled in this study, according to pairing status and stimulus type. Each box delineates the 1st and 3rd interquartile (25% and 75%) with the median as the 2nd interquartile (50%). The whiskers represent the data “range.” Data points above and below the whiskers are outliers. (A) During the playback period, percent time spent orienting to the stimulus varied based upon playback stimulus type. (B) During the observation period, percent time orienting did not vary significantly based upon any of our predictor variables. (C,D) The percent time spent locomoting did not vary based upon any of our predictor variables for either the playback period (C) or the observation period (D). (E,F) Playback stimulus type predicted the number of peeps emitted during the playback period (E), but not during the observation period (F). (G) The number of trills vocalized during the playback period varied based on stimulus type in that female titi monkeys vocalized more trills during the control playback compared to the two social playbacks. (H) During the observation period, the number of trills vocalized was predicted by pairing status in that titi monkey females vocalized more trills pre-pairing compared to post-pairing. (I) The latency to vocalize was not predicted by any of our predictors. For descriptive statistics, see Table 2. For model results, see Table 3.
Additionally, during the observation period, our subjects’ percent time spent orienting to the stimuli did not vary based on pairing status (partial R2 = 0.0282, t(28) = −1.218, p = 0.2131) or playback stimulus type (partial R2 = 0.0192, t(28) = 0.699, p = 0.4711) (Figure 4B).
Within the playback period, subjects’ percent time locomoting was not strongly predicted by pairing status (partial R2 = 0.0663, t(28) = −1.642, p = 0.0968) or stimulus type (R2 = 0.0073, t(28) = 0.315, p = 0.5744) (Figure 4C). However, during the observation period, titi monkeys spent a greater proportion of time locomoting during all the pre-pairing tests as compared to the post-pairing tests (R2 = 0.0942, t(28) = −2.073, p = 0.0385; Figure 4D), but playback stimulus did not predict locomotor behavior (partial R2 = 0.0080, t(28) = 0.604, p = 0.5330).
Vocal behaviors we scored included peeps, trills, alarm calls, long call introduction notes, and long calls (Table 1; Figure 2; Robinson, 1979a,b). Due to highly skewed data and few individuals vocalizing some vocal types, we were unable to run models for alarm calls, long call introduction notes, and long calls. However, descriptive statistics of these outcome variables are available in Table 2, along with the raw data in our Supplementary material.
During the playback period, the number of peeps vocalized was predicted by playback stimulus type (partial R2 = 0.1271, t(28) = −2.213, p = 0.0256), but not reproductive status (partial R2 = 0.0157, t(28) = 0.677, p = 0.4206) or pairing status (partial R2 = 0.0127, t(28) = −0.639, p = 0.4681). Our Tukey’s HSD post-hoc test indicated that our subjects peeped more in response to the control playback compared to the solo playback (p = 0.0333) and the duet playback (p = 0.0421; Figure 4E). However, there was not a significant difference in the number of peeps in response to the solo and pair playbacks (p = 0.9958).
Additionally, during the playback period, the number of trills was also predicted by playback stimulus type (partial R2 = 0.1737, t(28) = −2.630, p = 0.0080) but not pairing status (partial R2 = 0.0073, t(28) = −0.5953, p = 0.5695) or reproductive status (partial R2 = 0.0007, t(28) = −0.164, p = 0.8616). Our Tukey’s HSD post-hoc test indicated that our subjects trilled more in response to the control playback compared to the solo playback (p < 0.001) and the duet playback (p = 0.0029; Figure 4G), but there was not a significant difference in the number of trills in response to the solo and duet playback types (p = 0.8985).
In the observation period, pairing status predicted subjects’ number of trills, in that subjects vocalized more trills pre-pairing compared to post-pairing (partial R2 = −0.1419, t(28) = −2.351, p = 0.0373; Figure 4H). Stimulus type (partial R2 = −0.0119, t(28) = 0.530, p = 0.5799) nor reproductive status (partial R2 = −0.1335, t(28) = 1.255, p = 0.2857) predicted trill behavior.
Reproductive status predicted only one behavior in this study: number of peeps during the observation period (partial R2 = 0.1202, t(28) = −2.054, p = 0.0330) (Figure 5). Pairing status (partial R2 = 0.0494, t(28) = 1.434, p = 0.1406) nor playback stimulus type (partial R2 = 0.0416, t(28) = −1.327, p = 0.1729) predicted peep behavior in the observation period (Figure 4F). Our Tukey’s HSD post-hoc test indicated our subjects vocalized more peeps when reproductively cycling (p = 0.0217) or pregnant (p = 0.0433) compared to non-cycling. However, there was not a significant difference in the number of trills vocalized between cycling and pregnant females (p = 0.6520).
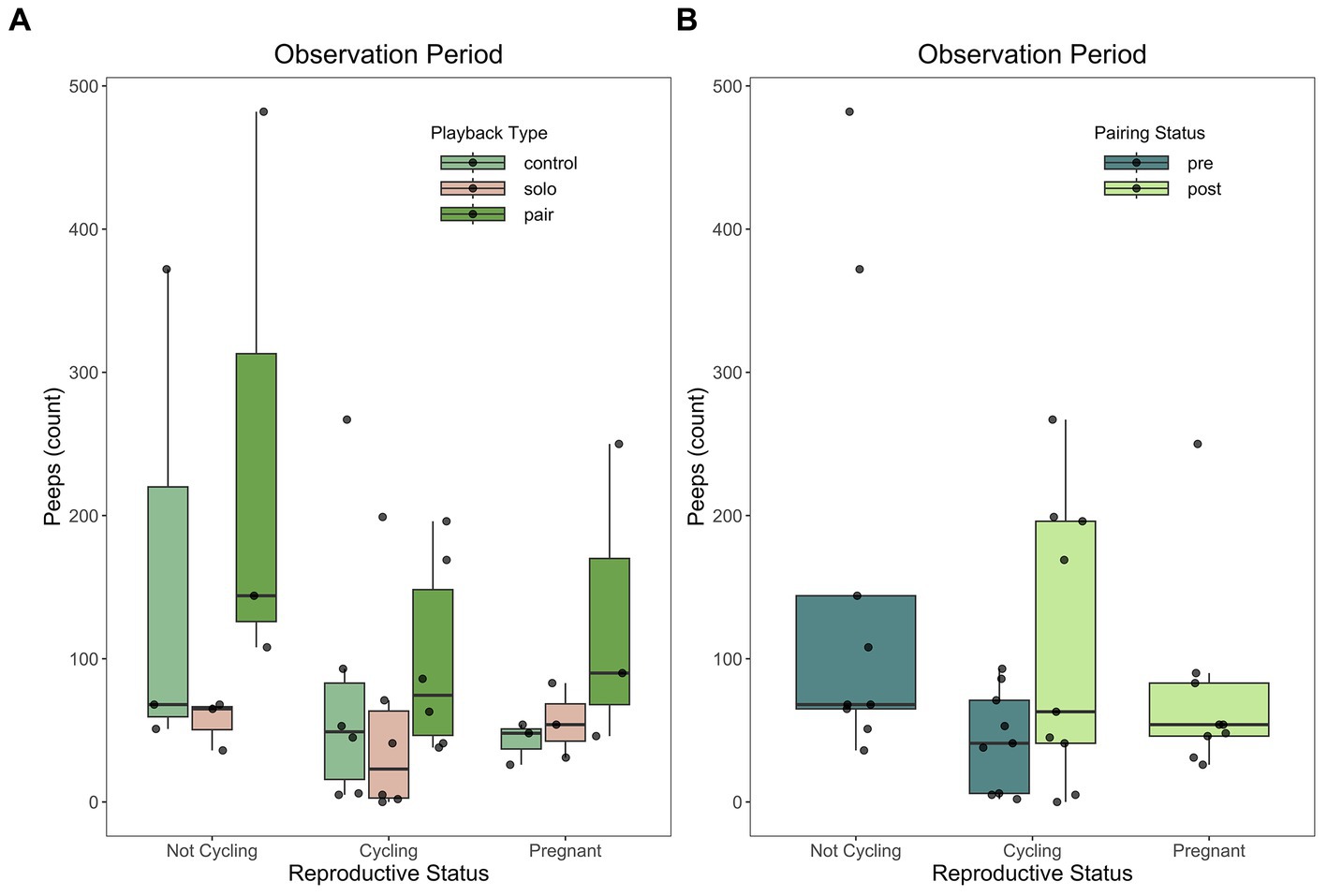
Figure 5. The number of peeps was predicted by reproductive status during the observation period. Female titi monkeys vocalized more peeps when not reproductively cycling as compared to reproductively cycling or pregnant females. This effect held regardless of playback stimulus type (A) or pairing status (B). For descriptive statistics, see Table 2. For model results, see Table 3.
Finally, for latency to vocalize, one of our 6 females did not vocalize during the post-pairing, solo playback stimulus test, resulting in a total of 35 latencies to vocalize and one censored observation. Our Cox Proportional Hazards models’ AIC values for our first (null) model and second model were 191.44 and 192.60, respectively, indicating that our null model had a slightly better fit. However, the difference in AIC values was relatively small (1.16), suggesting that both models may provide a reasonable fit to the data. Broadly speaking, reproductive status, playback type, nor pairing status influenced female titi monkeys’ latency to vocalize (Figure 4I).
Discussion
Generally, the findings of the present study are consistent with what is currently known about titi monkey social behavior. This project is the first to validate the use of vocal playbacks in the captive setting, providing evidence that titi monkeys do respond to social acoustic stimuli in a manner consistent with expectations for their species and social status. Across all outcome variables, the response to solo and duet playback stimuli did not differ significantly. As such, we focus on differences between control and social (solo and duet) playback stimuli.
Notably, a few findings emerged from this project peripheral to our initial predictions. We will first discuss the physiological responses to playbacks, behavioral responses to the playbacks, and then report interesting side notes, before discussing the limitations of and future recommendations following this study.
Physiological responses to playbacks
Female titi monkeys had higher androgen and cortisol levels post-pairing compared to pre-pairing. This difference existed irrespective of female reproductive status (removed from the final model) or playback type (included in the final model). Though reproductive status was removed from the final model due to backwards model selection, graphs of the cortisol values do indicate that pregnancy and cycling generally increase cortisol levels (Figure 3). However, the fact that cortisol and androgens are higher at post-pairing timepoints compared to pre-pairing timepoints may indicate a territorial response to the playback paradigm. Given the titi monkey’s unique parental care system in which the father contributes significantly to infant care (Mendoza and Mason, 1986b), combined with the fact that female titi monkeys actively maintain proximity with their mates (Dolotovskaya et al., 2020b), the higher androgen levels observed in post-pairing females in this study may reflect a reversal of traditional sex roles in this species. Or, at the very least, an equivalent contribution of both male and female titi monkeys to territorial responses. Female titi monkeys may respond behaviorally (Robinson, 1981; present study) and endocrinologically (present study) to territorial threats in a manner similar to male individuals of other species (Ord, 2021). The findings of this study are supported by the behavioral results of a simulated intruder test in which paired adult male and female titi monkeys responded with agonistic behaviors when viewing themselves in a mirror (Mercier et al., 2020). This study provided evidence of territorial behavior in female titi monkeys, including back arching and tail-lashing (Mercier et al., 2020). Future studies should investigate the role of androgens in both male and female titi monkeys’ responses to territorial intrusions.
Behavioral responses to playbacks
The playback stimulus type (control vs. solo vs. duet) predicted behavior during the playback period (5-min playback), but not the observation period (30 min following the playback). Specifically, female titi monkeys vocalized a greater number of trills and peeps during the control playbacks as opposed to the social playbacks (solo and duet). Additionally, subjects spent a greater proportion of time orienting to the direction of the playback audio during the social playbacks (solo and duet) as opposed to the control stimuli. Together, these results imply titi monkey females are actively listening (not vocalizing as much) and assessing (looking in the direction of) social signals (solo and duet) as compared to the control playback. Alternatively, or in conjunction, female titi monkeys may be vocalizing more in response to the control playback due to a lack of acoustic competition (i.e., if no other monkeys are vocalizing, the subject may vocalize more). Subjects’ vocal responses during the control playback correspond with typical titi monkey responses to separation from their mate or family members in previous separation paradigms (Mendoza and Mason, 1986a; Hoffman et al., 1995; Arias del Razo et al., 2022a). These results partially support our initial hypothesis that titi monkey females would respond differently to control playbacks versus social playbacks, but the lack of a distinctly different response to the male solos or pair duets does not allow us to speculate on what information titi monkey females do or do not perceive within these unfamiliar calls. This result may be a reflection of titi monkeys’ generalized neophobic responses to unfamiliar stimuli, as seen previously in neophobia (Hennessy et al., 1995) and novel object presentation studies (Lau et al., 2021).
Pairing status (pre-pairing vs. post-pairing) predicted vocal and locomotor behavior during the observation period (30 min following the playback). Titi monkeys trilled more in the pre-pairing conditions than the post-pairing conditions. Trill vocalizations are typically uttered by infant and juvenile titi monkeys more often than adults and are commonly thought of as “infant” vocalizations (Lau et al., 2020; Savidge and Bales, 2020) as trill vocalizations typically elicit reunion behaviors from parents (Hoffman et al., 1995). Based upon the younger age of our females during their pre-pairing timepoint and status as unpaired females within their natal groups, the larger number of trills pre-pairing compared to post-pairing fits the pre-existing knowledge of titi vocal behavior at different developmental stages and social situations.
In addition to their vocal responses, titi monkey females also spent more time locomoting in the pre-pairing observation periods as compared to the post-pairing observation periods. In the wild, unpaired titi monkeys occupy either their parents’ territories or exist as a floater without a territory prior to finding a mate (Dolotovskaya et al., 2020a). As such, a withdraw response (locomotion) as opposed to defense via long calling (Robinson, 1981) is consistent with pre-pairing females’ lack of a territory that is theirs to defend. Given the laboratory nature of this study, titi monkeys are unable to show species-typical withdrawal or fleeing behavior that would likely occur in a wild setting.
Taken together, female titi monkeys’ response following a playback (during the observation period) is determined by females’ pairing status more so than the content of the individual playbacks.
Notes of interest
Reproductive status was excluded from all models except one. The final model for number of peeps during the observation period retained reproductive status as a fixed effect. In this study, titi monkey females vocalized more peeps during the observation period while non-cycling compared to cycling or pregnant females. Peeps are used primarily as contact calls or general arousal signals (Robinson, 1979a,b; Arias del Razo et al., 2022a) While this single result alone is not enough to fully assess the impacts of reproductive status on titi monkey vocal behavior, the greater number of peeps uttered by non-cycling females may suggest that cycling and pregnant females spend more time attending to the environment while non-cycling females may employ a strategy of soliciting their family group. However, the limited sample size of 6 individuals does not allow for any truly conclusive assertions about titi monkey vocal behavior regarding reproductive status.
Limitations and future directions
While this project was originally designed with a target sample size of 9 individuals, we were restricted to only 6 individuals due to COVID-19 pandemic-related issues. We recommend additional experiments to bolster the findings presented here. This study was also limited to female animals as part of a larger project assessing female pair bonding. Projects that include male titi monkeys will allow for comparisons between the sexes.
Additionally, while this study was conducted at a consistent time of day to control for daily hormone fluctuations, future studies may find interesting behavioral variation in response to playbacks at different times of day. Temporal fluctuations of behavior in this species have not yet been investigated.
The duet playbacks used in this study were broadcast from one speaker. Previous work in avian studies indicates that multi-speaker playbacks simulate a more realistic duet playback (Douglas and Mennill, 2010). Separating each sound source from a titi monkey duet recording is very difficult given substantial overlap between male and female contributions. However, it would be possible to artificially create a duet by broadcasting two solo songs simultaneously in a stereo playback design (I.e., male song from speaker A and female song from speaker B). To make the playback realistic, each song would have to be edited to ensure accurate coordination of male and female song phrases when triggering the playback. This method would constitute an ideal, unfamiliar duet stimulus. Future studies should attempt this method.
One possible confounding factor is the nested separation study occurring within this playback study. Adult titi monkeys’ attention and anxiety-related behaviors are impacted by the removal of a pair mate from the enclosure (Savidge and Bales, 2020). While a separation from the subject’s family or mate (depending on pairing status) occurred for all playback tests, the overall impacts of separation cannot be disentangled from the impacts of each playback stimulus type. The results found here may have been stronger if separation did not occur, as the separation paradigm induces physiological and behavioral arousal (Arias del Razo et al., 2022a). However, by separating females from their family/mate, we were able to ensure that the results found here were not confounded by idiosyncratic behavior of the family/mate and were individually driven. The results presented here suggest that beyond the effects of separation, social playbacks do alter behavior and physiology of the listener. Future studies should aim to replicate this study and compare individuals’ responses to those of paired males and females listening to playbacks together as the joint pair response to playbacks will further illuminate social communication patterns in this species.
Conclusion
In summary, we found evidence that female titi monkeys attend to social signals by vocalizing less and orienting more in the direction of the playback than control recordings while the playback is occurring regardless of pairing status. However, in the time immediately following any playback type, female’s pairing status predicts vocal and locomotor responses irrespective of playback type. Namely, female titi monkeys trill more pre-pairing and long call more post-pairing, as well as spend a greater proportion of time locomoting at pre-pairing timepoints. Future studies should aim to understand male titi monkeys’ responses to different acoustic signals as well as those of paired monkeys listening to playbacks in tandem.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the IACUC of the University of California, Davis. This study met all legal requirements of the United States as well as guidelines set by the American Society of Primatologists for the ethical treatment of non-human primates. This study was carried out in compliance with the ARRIVE guidelines.
Author contributions
AL and KB designed the study. AL carried out data collection. AL and AC processed the data, ran all statistical analyses, and composed the first draft of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institutes of Health (grant numbers HD092055 and OD011107), the Good Nature Institute, and the American Society of Primatologists Small Grant.
Acknowledgments
We gratefully acknowledge National Institutes of Health grant HD092055 and OD011107, as well as the Good Nature Institute. We thank the American Society of Primatologists for awarding the Small Grant to AL in support of this project. Many thanks to Rebecca Cotterman and Al Conley for their expertise in endocrinology. We thank the husbandry staff at the CNPRC for providing excellent care to all subjects. We thank Damien Caillaud and Gail Patricelli for early insight on the design of this study. We thank many individuals from the Bales Lab who helped with data collection, including Lynea Witczak, Madison Dufek, Amber Wright, Chloe Karaskiewicz, John Paulus, Alexander Baxter, Logan Savidge, and Mathieu Legrand. We appreciate comments on early drafts of this manuscript from Chloe Karaskiewicz and help with Figure 1 from Danny Lau. Our thanks go to the titi monkeys who participated in this study, and our final thanks go to Chuck Snowdon for his years of support and enthusiasm for this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1145205/full#supplementary-material
Footnotes
References
Arias del Razo, R., Berger, T., Conley, A. J., Freeman, S. M., Goetze, L. R., Jacob, S., et al. (2020). Effects of chronic intranasal oxytocin on behavior and cerebral glucose uptake in juvenile titi monkeys. Psychoneuroendocrinology 113:104494. doi: 10.1016/j.psyneuen.2019.104494
Arias del Razo, R., Vazquez, M. D. L. V., Turcanu, P., Legrand, M., Floch, M., Weinstein, T. A., et al. (2022b). Long term effects of chronic intranasal oxytocin on adult pair bonding behavior and brain glucose uptake in titi monkeys (Plecturocebus cupreus). Horm. Behav. 140:105126. doi: 10.1016/j.yhbeh.2022.105126
Arias del Razo, R., Vazquez, M. D. L. V., Turcanu, P., Legrand, M., Lau, A. R., Weinstein, T. A., et al. (2022a). Effects of chronic and acute intranasal oxytocin treatments on temporary social separation in adult Titi monkeys (Plecturocebus cupreus). Front. Behav. Neurosci. 16:877631 doi: 10.3389/fnbeh.2022.877631
Bales, K. L., Ardekani, C. S., Baxter, A., Karaskiewicz, C. L., Kuske, J. X., Lau, A. R., et al. (2021). What is a pair bond? Horm. Behav. 136:105062. doi: 10.1016/j.yhbeh.2021.105062
Bales, K. L., Del Razo, R. A., Conklin, Q. A., Hartman, S., Mayer, H. S., Rogers, F. D., et al. (2017). Focus: comparative medicine: Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. Yale J. Biol. Med. 90, 373–387.
Bossuyt, F. (2002). Natal dispersal of titi monkeys (Callicebus moloch) at Cocha Cashu, Manu National Park, Peru. Am. J. Phys. Anthropol. 34:47.
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles of animal communication. Sinauer Associates Inc, Sunderland, MA.
Cäsar, C., Byrne, R., Young, R. J., and Zuberbühler, K. (2012). The alarm call system of wild black-fronted titi monkeys, Callicebus nigrifrons. Behav. Ecol. Sociobiol. 66, 653–667. doi: 10.1007/s00265-011-1313-0
Caselli, C. B., Mennill, D. J., Bicca-Marques, J. C., and Setz, E. Z. (2014). Vocal behavior of black-fronted titi monkeys (Callicebus nigrifrons): acoustic properties and behavioral contexts of loud calls. Am. J. Primatol. 76, 788–800. doi: 10.1002/ajp.22270
Caselli, C. B., Mennill, D. J., Gestich, C. C., Setz, E. Z., and Bicca-Marques, J. C. (2015). Playback responses of socially monogamous black-fronted titi monkeys to simulated solitary and paired intruders. Am. J. Primatol. 77, 1135–1142. doi: 10.1002/ajp.22447
Cheney, D. L., and Seyfarth, R. M. (2018). Flexible usage and social function in primate vocalizations. Proc. Natl. Acad. Sci. 115, 1974–1979. doi: 10.1073/pnas.1717572115
Clink, D. J., Comella, I. A., Tasirin, J. S., and Klinck, H. (2022). Tarsier islands: exploring patterns of variation in tarsier duets from offshore islands of North Sulawesi. Am. J. Primatol. 76:e23410. doi: 10.1002/ajp.23410
Clink, D. J., Lau, A. R., and Bales, K. L. (2019). Age-related changes and vocal convergence in titi monkey duet pulses. Behaviour 156, 1471–1494. doi: 10.1163/1568539X-00003575
Clink, D. J., Lau, A. R., Kanthaswamy, S., Johnson, L. M., and Bales, K. L. (2022). Moderate evidence for heritability in the duet contributions of a south American primate. J. Evol. Biol. 35, 51–63. doi: 10.1111/jeb.13962
Conley, A. J., Berger, T., Del Razo, R. A., Cotterman, R. F., Sahagún, E., Goetze, L. R., et al. (2022). The onset of puberty in colony-housed male and female titi monkeys (Plecturocebus cupreus): possible effects of oxytocin treatment during peri-adolescent development. Horm. Behav. 142:105157. doi: 10.1016/j.yhbeh.2022.105157
Corley, M., Perea-Rodriguez, J. P., Valeggia, C., and Fernandez-Duque, E. (2021). Associations between fecal cortisol and biparental care in a pair-living primate. Am. J. Phys. Anthropol. 176, 295–307. doi: 10.1002/ajpa.24368
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. 34, 205–219. doi: 10.1080/03949370.2021.2015451
Depeine, C. C., Rotundo, M., Juárez, C. P., and Fernandez-Duque, E. (2008). Hoot calling in owl monkeys (Aotus azarai) of Argentina: sex differences and function. Am. J. Primatol. 70:69.
Dolotovskaya, S., and Heymann, E. W. (2022). Coordinated singing in coppery Titi monkeys (Plecturocebus cupreus): resource or mate defense? Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.898509
Dolotovskaya, S., Roos, C., and Heymann, E. W. (2020a). Genetic monogamy and mate choice in a pair-living primate. Sci. Rep. 10, 1–13. doi: 10.1038/s41598-020-77132-9
Dolotovskaya, S., Walker, S., and Heymann, E. W. (2020b). What makes a pair bond in a Neotropical primate: female and male contributions. R. Soc. Open Sci. 7:191489. doi: 10.1098/rsos.191489
Douglas, S. B., and Mennill, D. J. (2010). A review of acoustic playback techniques for studying avian vocal duets. J. Field Ornithol. 81, 115–129. doi: 10.1111/j.1557-9263.2010.00268.x
Dunbar, R. I., and Shultz, S. (2021). Social complexity and the fractal structure of group size in primate social evolution. Biol. Rev. 96, 1889–1906. doi: 10.1111/brv.12730
Epel, E. S., Crosswell, A. D., Mayer, S. E., Prather, A. A., Slavich, G. M., Puterman, E., et al. (2018). More than a feeling: a unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169. doi: 10.1016/j.yfrne.2018.03.001
Fernandez-Duque, E., Valeggia, C. R., and Mason, W. A. (2000). Effects of pair-bond and social context on male–female interactions in captive titi monkeys (Callicebus moloch, primates: Cebidae). Ethology 106, 1067–1082. doi: 10.1046/j.1439-0310.2000.00629.x
Fichtel, C., and Hilgartner, R. (2013). “Noises in the dark: vocal communication in Lepilemur ruficaudatus and other nocturnal pair-living primates” in Leaping ahead: Advances in prosimian biology. eds. J. Master, M. Gamba, and F. Génin (New York: Springer), 297–304. doi: 10.1007/978-1-4614-4511-1_33
Fischer, J., Noser, R., and Hammerschmidt, K. (2013). Bioacoustic field research: a primer to acoustic analyses and playback experiments with primates. Am. J. Primatol. 75, 643–663. doi: 10.1002/ajp.22153
Fishbein, A. R., Prior, N. H., Brown, J. A., Ball, G. F., and Dooling, R. J. (2021). Discrimination of natural acoustic variation in vocal signals. Sci. Rep. 11:916. doi: 10.1038/s41598-020-79641-z
Garcia de la Chica, A., Wood, D. B., Rotundo, M., and Fernandez-Duque, E. (2021). Responses of a pair-living, sexually monogamous primate to the simulated presence of solitary individuals: a field playback experiment. Ethology 127, 1002–1018. doi: 10.1111/eth.13222
Geissmann, T. (2002). Duet-splitting and the evolution of gibbon songs. Biol. Rev. 77, 57–76. doi: 10.1017/S1464793101005826
Ghazanfar, A. A., and Hauser, M. D. (2001). The auditory behaviour of primates: a neuroethological perspective. Curr. Opin. Neurobiol. 11, 712–720. doi: 10.1016/s0959-4388(01)00274-4
Hennessy, M. B., Mendoza, S. P., Mason, W. A., and Moberg, G. P. (1995). Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: species differences in characteristic modes of responding to the environment. Physiol. Behav. 57, 331–338. doi: 10.1016/0031-9384(94)00250-9
Hoffman, K. A., Mendoza, S. P., Hennessy, M. B., and Mason, W. A. (1995). Responses of infant titi monkeys, Callicebus moloch, to removal of one or both parents: evidence for paternal attachment. Dev. Psychobiol. 28, 399–407. doi: 10.1002/dev.420280705
Hothorn, T., Bretz, F., Westfall, P., Heiberger, R. M., Schuetzenmeister, A., Scheibe, S., et al. (2016). “Package ‘multcomp’.” Simultaneous inference in general parametric models. Project for statistical computing, Vienna, Austria.
K. Lisa Yang Center for Conservation Bioacoustics at the Cornell Lab of Ornithology . (2022). Raven pro: Interactive sound analysis software (version 1.6.3) [computer software]. Ithaca, NY: The Cornell Lab of Ornithology.
Kappeler, P. M., and van Schaik, C. P. (2002). Evolution of primate social systems. Int. J. Primatol. 23, 707–740. doi: 10.1023/A:1015520830318
Karaskiewicz, C. L., Witczak, L. R., Lau, A. R., Dufek, M. E., and Bales, K. L. (2021). Parenting costs time: changes in pair bond maintenance across pregnancy and infant rearing in a monogamous primate (Plecturocebus cupreus). New Dir. Child Adolesc. Dev. 2021, 21–42. doi: 10.1002/cad.20438
Kroodsma, D. E. (1989). Suggested experimental designs for song playbacks. Anim. Behav. 37, 600–609. doi: 10.1016/0003-3472(89)90039-0
Kroodsma, D. E. (1990). Using appropriate experimental designs for intended hypotheses in ‘song’ playbacks, with examples for testing effects of song repertoire sizes. Anim. Behav. 40, 1138–1150. doi: 10.1016/S0003-3472(05)80180-0
Lau, A. R., Clink, D. J., and Bales, K. L. (2020). Individuality in the vocalizations of infant and adult coppery titi monkeys (Plecturocebus cupreus). Am. J. Primatol. 82:e23134. doi: 10.1002/ajp.23134
Lau, A. R., Grote, M. N., Dufek, M. E., Franzetti, T. J., Bales, K. L., and Isbell, L. A. (2021). Titi monkey neophobia and visual abilities allow for fast responses to novel stimuli. Sci. Rep. 11, 1–9. doi: 10.1038/s41598-021-82116-4
Lukas, D., and Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677
Maninger, N., Mendoza, S. P., Williams, D. R., Mason, W. A., Cherry, S. R., Rowland, D. J., et al. (2017). Imaging, behavior and endocrine analysis of “jealousy” in a monogamous primate. Front. Ecol. Evol. 5:119. doi: 10.3389/fevo.2017.00119
Marshall, J. T. Jr., and Marshall, E. R. (1976). Gibbons and their territorial songs. Science 193, 235–237. doi: 10.1126/science.193.4249.235
Mendoza, S. P., and Mason, W. A. (1986a). Contrasting responses to intruders and to involuntary separation by monogamous and polygynous new world monkeys. Physiol. Behav. 38, 795–801. doi: 10.1016/0031-9384(86)90045-4
Mendoza, S. P., and Mason, W. A. (1986b). Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch). Anim. Behav. 34, 1336–1347. doi: 10.1016/S0003-3472(86)80205-6
Mendoza, S. P., and Moberg, G. P. (1985). Species differences in adrenocortical activity of New World primates: response to dexamethasone suppression. Am. J. Primatol. 8, 215–224. doi: 10.1002/ajp.1350080304
Mercier, F., Witczak, L. R., and Bales, K. L. (2020). Coppery titi monkey (Plecturocebus cupreus) pairs display coordinated behaviors in response to a simulated intruder. Am. J. Primatol. 82:e23141. doi: 10.1002/ajp.23141
Moynihan, M. (1966). Communication in the titi monkey, Callicebus. J. Zool. 150, 77–127. doi: 10.1111/j.1469-7998.1966.tb02999.x
Müller, A. E., and Anzenberger, G. (2002). Duetting in the titi monkey Callicebus cupreus: structure, pair specificity and development of duets. Folia Primatol. 73, 104–115. doi: 10.1159/000064788
Nietsch, A. (1999). Duet vocalizations among different populations of Sulawesi tarsiers. Int. J. Primatol. 20, 567–583. doi: 10.1023/A:1020342807709
Ord, T. J. (2021). Costs of territoriality: a review of hypotheses, meta-analysis, and field study. Oecologia 197, 615–631. doi: 10.1007/s00442-021-05068-6
Palombit, R. (1994). Dynamic pair bonds in hylobatids: implications regarding monogamous social systems. Behaviour 128, 65–101. doi: 10.1163/156853994X00055
Pinheiro, J. (2009). Nlme: linear and nonlinear mixed effects models. R package version 3. 1-96. Available at: http://cran.r-project.org/web/packages/nlme/.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Heisterkamp, S., Van Willigen, B., et al. (2017). Package ‘nlme’. Linear and nonlinear mixed effects models, version, 3(1).
Place, V. A., and Nichols, K. C. (1991). Transdermal delivery of testosterone with TESTODERM™ to provide a Normal circadian pattern of testosterone. Ann. N. Y. Acad. Sci. 618, 441–449. doi: 10.1111/j.1749-6632.1991.tb27263.x
R Core Team (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Robinson, J. G. (1979a). An analysis of the organization of vocal communication in the titi monkey Callicebus moloch. Z. Tierpsychol. 49, 381–405.
Robinson, J. G. (1979b). Vocal regulation of use of space by groups of titi monkeys Callicebus moloch. Behav. Ecol. Sociobiol. 5, 1–15. doi: 10.1007/BF00302691
Robinson, J. G. (1981). Vocal regulation of inter-and intragroup spacing during boundary encounters in the titi monkey, Callicebus moloch. Primates 22, 161–172. doi: 10.1007/BF02382607
Rothwell, E. S., Carp, S. B., Savidge, L. E., Mendoza, S. P., and Bales, K. L. (2020). Relationship tenure differentially influences pair-bond behavior in male and female socially monogamous titi monkeys (Callicebus cupreus). Am. J. Primatol. 82:e23181. doi: 10.1002/ajp.23181
Savidge, L. E., and Bales, K. L. (2020). An animal model for mammalian attachment: infant titi monkey (Plecturocebus cupreus) attachment behavior is associated with their social behavior as adults. Front. Psychol. 11:25. doi: 10.3389/fpsyg.2020.00025
Silk, J. B. (2007). The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. B 362, 539–559. doi: 10.1098/rstb.2006.1994
Singletary, B., and Tecot, S. (2020). Multimodal pair-bond maintenance: a review of signaling across modalities in pair-bonded nonhuman primates. Am. J. Primatol. 82:e23105. doi: 10.1002/ajp.23105
Smith, T. E., and French, J. A. (1997). Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiol. Behav. 62, 225–232. doi: 10.1016/S0031-9384(97)00103-0
Tardif, S., Bales, K., Williams, L., Moeller, E. L., Abbott, D., Schultz-Darken, N., et al. (2006). Preparing New World monkeys for laboratory research. ILAR J. 47, 307–315. doi: 10.1093/ilar.47.4.307
Terborgh, J., and Janson, C. H. (1986). The socioecology of primate groups. Annu. Rev. Ecol. Syst. 17, 111–136. doi: 10.1146/annurev.es.17.110186.000551
Valeggia, C. R., Mendoza, S. P., Fernandez-Duque, E., Mason, W. A., and Lasley, B. (1999). Reproductive biology of female titi monkeys (Callicebus moloch) in captivity. Am. J. Primatol. 47, 183–195. doi: 10.1002/(SICI)1098-2345(1999)47:3<183::AID-AJP1>3.0.CO;2-J
Van Goozen, S. H., Wiegant, V. M., Endert, E., Helmond, F. A., and Van de Poll, N. E. (1997). Psychoendocrinological assessment of the menstrual cycle: the relationship between hormones, sexuality, and mood. Arch. Sex. Behav. 26, 359–382. doi: 10.1023/A:1024587217927
Wickham, H., Chang, W., and Wickham, M. H. (2016). Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version, 2(1), 1–189.
Wilke, C. O., Wickham, H., and Wilke, M. C. O. (2019). Package ‘cowplot’. Streamlined plot theme and plot annotations for ‘ggplot2.
Keywords: long call, duet, pair bonding, hormones, titi monkey
Citation: Lau AR, Cunningham AD and Bales KL (2023) Pairing status and stimulus type predict responses to audio playbacks in female titi monkeys. Front. Ecol. Evol. 11:1145205. doi: 10.3389/fevo.2023.1145205
Edited by:
Patrice Adret, Universidad Autónoma Gabriel René Moreno, BoliviaReviewed by:
Alba Garcia De La Chica, University of Buenos Aires, Argentina Valeria Torti, University of Turin, ItalyCopyright © 2023 Lau, Cunningham and Bales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allison R. Lau, YWxsam9uZXNAdWNkYXZpcy5lZHU=
 Allison R. Lau
Allison R. Lau Ashley D. Cunningham3
Ashley D. Cunningham3 Karen L. Bales
Karen L. Bales