- Laboratory of Forest Protection, Graduate School of Bioagricultural Sciences, Nagoya University, Nagoya, Japan
Bambusoideae are well-known for their large-scale flowering, seeding, and death events that occur after a vegetative phase that can often last for decades. These events are a valuable resource for many animals. Their mast seeding, in particular, affects rodent populations although the causal relationship between them is speculative. Moreover, the species of animals that utilize this resource also remains unclear. Therefore, this study aimed to examine the interaction between mast seeding plants and animals by conducting a feeding test using the seeds of a dwarf bamboo species (Sasa borealis) at different seasons and in different forest types. The results revealed that two mouse species, Apodemus speciosus and A. argenteus, utilized the seeds. They both predated as well as removed and sometimes buried the seeds, exhibiting small-seed caching behavior. Furthermore, A. speciosus was found to exhibit higher seed predation at covered sites and switching to removal behavior at the more exposed, open sites, while A. argenteus showed no distinguishable trends. Additionally, A. speciosus utilized the seeds less frequently in fall than in summer, suggesting a shift in diet towards acorns when available. Collectively, the results of this study provide novel insights into the interaction between mast seeding plants and the animals that utilize them.
1 Introduction
Certain plants produce large seed crops, often in the form of masting, which in turn leads to an increase in the population of the animals that utilize them (Ogawa et al., 2017). These seed-utilizing animals are known to both predate and cache seeds for later use. Such interactions between seed predators and seeding plants have been widely studied in trees with masting seeds, such as acorns, at intervals of several years (Jensen and Nielsen, 1986; den Ouden et al., 2005; Saitoh et al., 2007). However, for plants that require decades or even centuries to produce seeds, such research has inevitably stalled. Moreover, the fauna that utilizes the seeds of such plants also remains largely unknown. For example, the mast seeding of Bambusoideae is associated with increased populations of several rodent species (Gonzalez et al., 2000; Bovendorp et al., 2020; Suzuki et al., 2022), but the causal relationship between them is speculative. Thus, the interaction between the rodents and the seeds (Klinger and Rejmánek, 2009), and whether Bambusoideae show a functional response to the rodents remain unknown. If the rodents act as both seed predators and seed dispersers, they may have a positive impact on the Bambusoideae. In contrast, the expansion of certain bamboo species such as the dwarf bamboo, Sasa borealis, seed dispersal could threaten other plants, since its leaves cover the forest floor and prevent the regeneration of other species (Nakashizuka, 1987), resulting in changes to the original vegetation and ecosystems over time. To elucidate these issues, we conducted a feeding test on S. borealis seeds, after the seeding event that occurred in plants that have been established in the site for 120 years. During the test period, the behaviors of animals that interacted with the seeds were recorded using trail cameras to observe how they handled the seeds, and to look out for novel behaviors such as seed removal and small-seed caching (Vander Wall, 2003). The forest floor conditions, and season are related factors that are known to alter seed foraging behavior in mice (Kikuzawa, 1988; Haugaasen et al., 2010; Best et al., 2022). By conducting the study in areas with different forest floor conditions and in different seasons, we aimed to determine whether this pattern of plant–animal interactions occurs with small seeds such as S. borealis. The findings from this experiment on the use patterns of S. borealis seeds by animals can provide a first step towards understanding the ecological role of Bambusoideae seeds supplied through interval masting over longer timescales.
2 Materials and methods
2.1 Study site
The study was conducted in Takatokke district of Nagoya University Forest (in the Inabu Field affiliated to the Graduate School of Bioagricultural Sciences, Nagoya University), located in the northeastern part of Aichi Prefecture, central Japan. The elevation was 1,075 m asl. In 2020, the annual precipitation was 2,334 mm and the average annual temperature was 9.5°C. At and around this site, the mast flowering and seeding of S. borealis occurred in 2016 and 2017. In 2021, two plots were set up: Plot-BF (broad-leaved forest, 35°13’04” N, 137°34’28” E), in a secondary forest comprising deciduous broad-leaved trees, mainly Quercus crispula, Cerasus jamasakura, and Lindera triloba, and Plot-CF (coniferous forest, 35°12’53” N, 137°34’20” E), in a forest comprising 59-year-old Larix kaempferi with L. triloba in the shrub layer. In each plot, two test points were prepared outside (OUT) and inside (IN) the dead culm cluster of S. borealis, approximately 10 m apart.
2.2 Feeding test
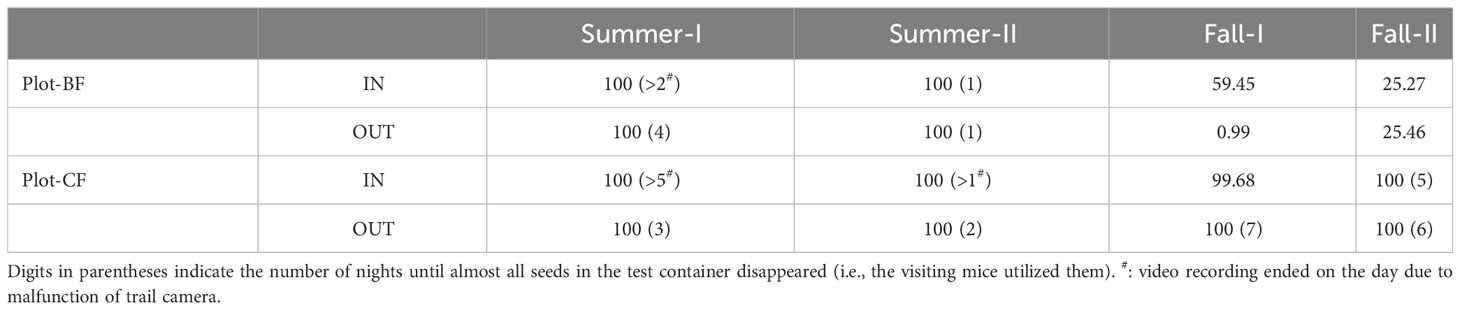
At each test point, a “food station” was established by placing a shallow metal container (22 cm in diameter) on the ground. Sasa borealis seeds (26.8 ± 4.8 mg/seed, n = 50), which are much smaller in size than acorns (Q. crispula, 2.8 ± 0.7 g/acorn, n = 112), were harvested in 2017 in Dando National Forest, Aichi prefecture. The seed husks were removed before being stored at room temperature (about 20 – 25°C) in our laboratory until further testing. Twenty grams (approximately 750 grains) of S. borealis seeds were placed at each food station. After one week, the remaining seeds were collected and weighed; thus, the weight of seeds utilized by the visiting animals was calculated by subtracting the weight of the remaining seeds from the initial weight (20 g). Seed utilization rate (%) was defined as the percentage of lost weight to initial weight. A trail camera (HYKE HCSP2, Hyke Inc., Hokkaido, Japan) was placed approximately 50 cm from each container to monitor its contents and the surroundings. The seeds and cameras were set at all food stations within an hour on the first day of each trial when recording commenced. The camera was set to high sensor sensitivity to record a video after the sensor response. Based on the videos, the species of visiting animals, their seed utilization behaviors (predation or removal), and foraging time at each point were examined. Even if removal occurred after predation, the data included it as “predation”. Behavioral data observed after the number of remaining seed became too low (less than 10 seeds) and when two or more animals simultaneously visited were excluded, as they could affect their behavior. A series of feeding tests were conducted from August 25 – September 1 (Summer-I), September 1 – 8 (Summer-II), September 30 – October 7 (Fall-I), and October 7 –14 (Fall-II), 2021.
2.3 Statistical analyses
To analyze the effects of foraging season and location on the utilization rate of S. borealis, a generalized linear mixed model (GLMM) was used. The season, plot, test point, and their interactions were included as explanatory variables, and foraging time was explored as a covariate (random factor). The GLMM analysis excluded data from tests in which video recording ended early due to malfunction of the trail camera. To analyze the effects on the animal’s behavior (predation or removal) on the seeds, a generalized linear model (GLM) was used, with the season, plot, test point, and their interactions included as explanatory variables. Differences in the mean foraging time between predation and removal at each point were compared using Welch’s T test or Mann–Whitney U test. All analysis programs were run in R (v4.1.2; R Core Team, 2021).
3 Results
3.1 Visiting animals
In 16 trials (four test points multiplied by four times), two mouse species utilized S. borealis seeds: the large Japanese field mouse [Apodemus speciosus, body weight = 20–60 g (Kaneko, 2005)] and small Japanese field mouse [Apodemus argenteus, body weight = 10–20 g (Kaneko, 2005)]. On one occasion, a raccoon dog (Nyctereutes procyonoides) approached the food container but did not touch the seeds. No other animals were recorded at all. However, near the container, there were some hoofprints of deer (Cervus nippon).
3.2 Seed utilization rate
Quantitative data was calculated from the decrease in weight of seeds in each container after one week (Table 1). In summer, the utilization rates were 100% at all test points. In fall, they dropped in Plot-BF, especially outside the dead culm cluster (Fall-I-OUT), although they were almost 100% in the coniferous forest plot (Plot-CF). The number of nights until all seeds disappeared tended to be shorter in summer than in fall. GLMM analysis showed that the season (estimate = −57.6, p < 0.01), and interaction between season and plot (estimate = 86.8, p < 0.005) were significantly related to the utilization rate (Table S1).
3.3 Mouse behavior
Mice frequently predated seeds by first peeling off the chaff (episperm) (Video S1). They sometimes removed seeds to another location singly or in batches (two or more), taking them in their mouth. Single removal often occurred in fall. Removal after predation was also observed. When mice carried seeds out of the container, surprisingly, they soon buried the seeds in the ground (Video S2). This caching behavior was recorded 7 and 5 times in summer and fall, respectively.
3.4 Predation or removal by mouse species
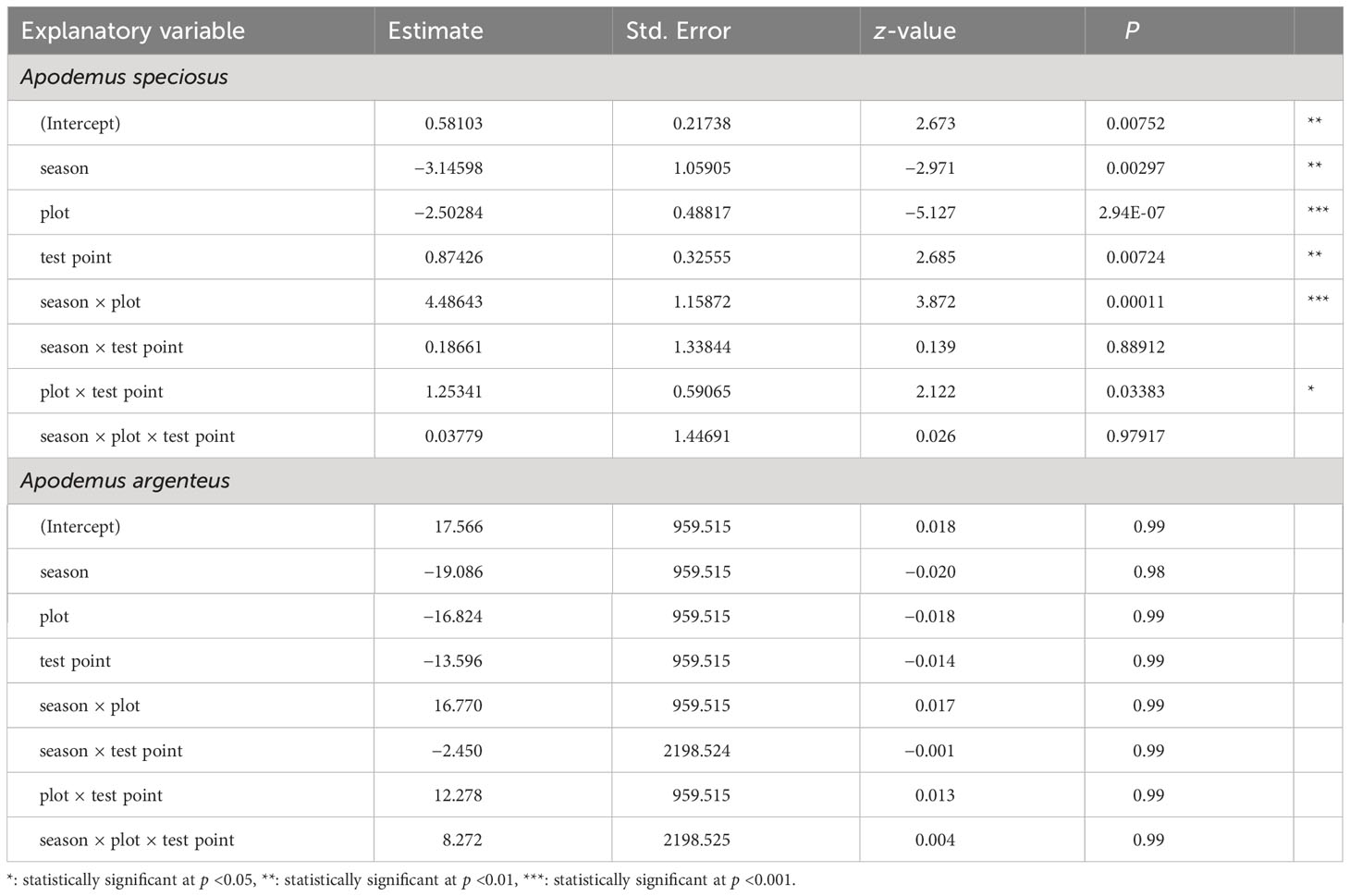
Figure 1 shows the ratio of frequency in the two seed handling patterns exhibited by the mice. For A. speciosus, in most trials, the predation rate was higher in the IN than OUT group in both plots, and conversely, the removal rate was higher in OUT than IN. The frequency of mouse visits (“n”) decreased in Plot-BF from summer to fall, but increased in Plot-CF. The trend of A. argenteus was less clear than that of A. speciosus. The “n” values of the OUT group tended to exceed those of the IN group in summer and vice versa in fall, except for Plot-CF of Fall-I. In Plot-BF, the “n” values of A. speciosus significantly decreased in fall while those of A. argenteus increased, particularly in the IN group. GLM analysis revealed that the season, plot, test point, interaction between season and plot, and interaction between plot and test point were significantly related to mouse behavior (Table 2). However, in A. argenteus, none of the explanatory variables showed such a significant relationship.
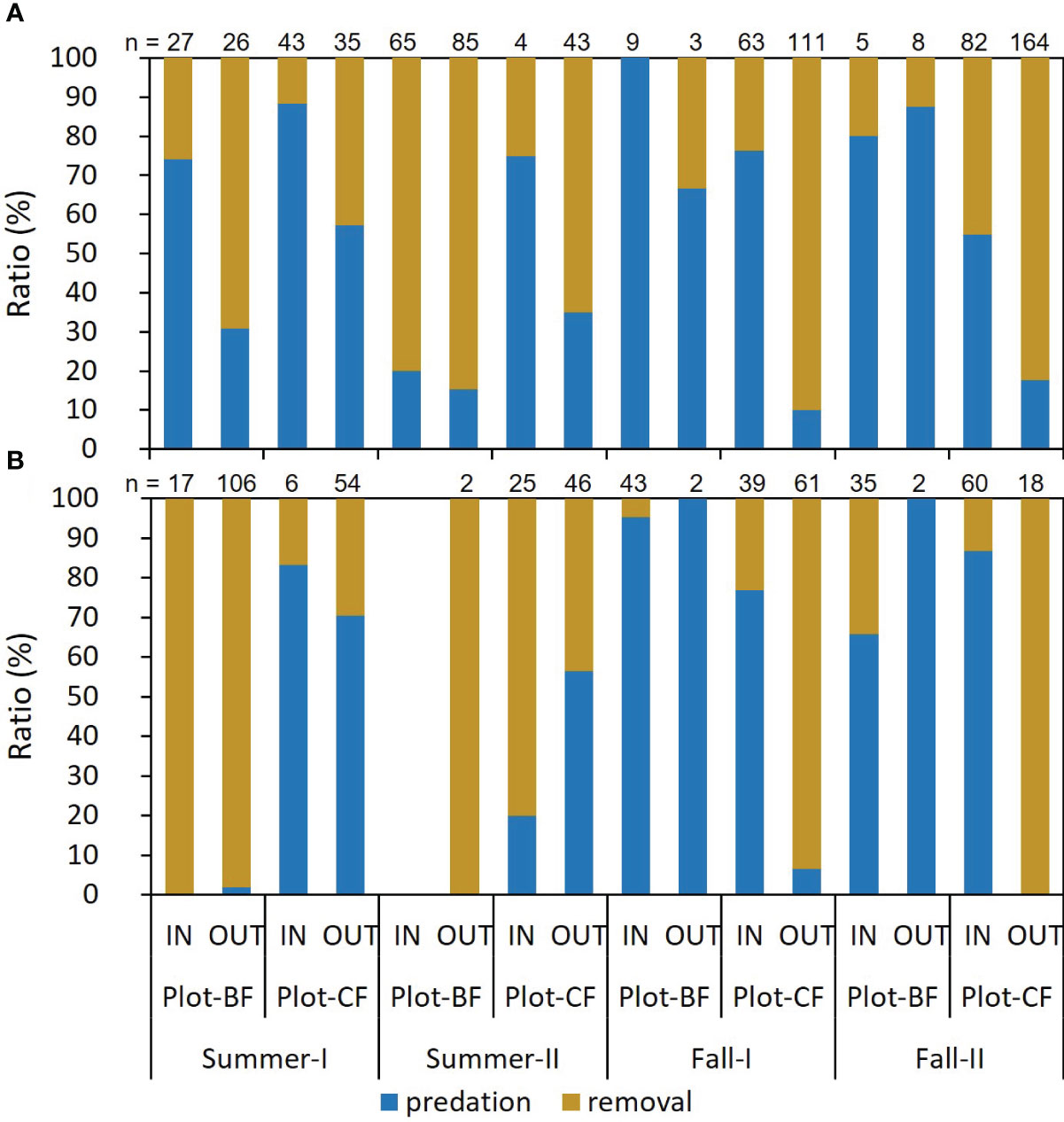
Figure 1 Ratio of seed predation or removal by mice. (A) Apodemus speciosus (B) Apodemus argenteus. n: Number of recordings.
3.5 Foraging time
In both species, predation took significantly longer than removal at some points, regardless of the season, plot, and IN or OUT variables (Figure 2). In fall, the average time was longer in IN than in OUT, especially for A. argenteus. Removal time decreased overall from summer to fall.
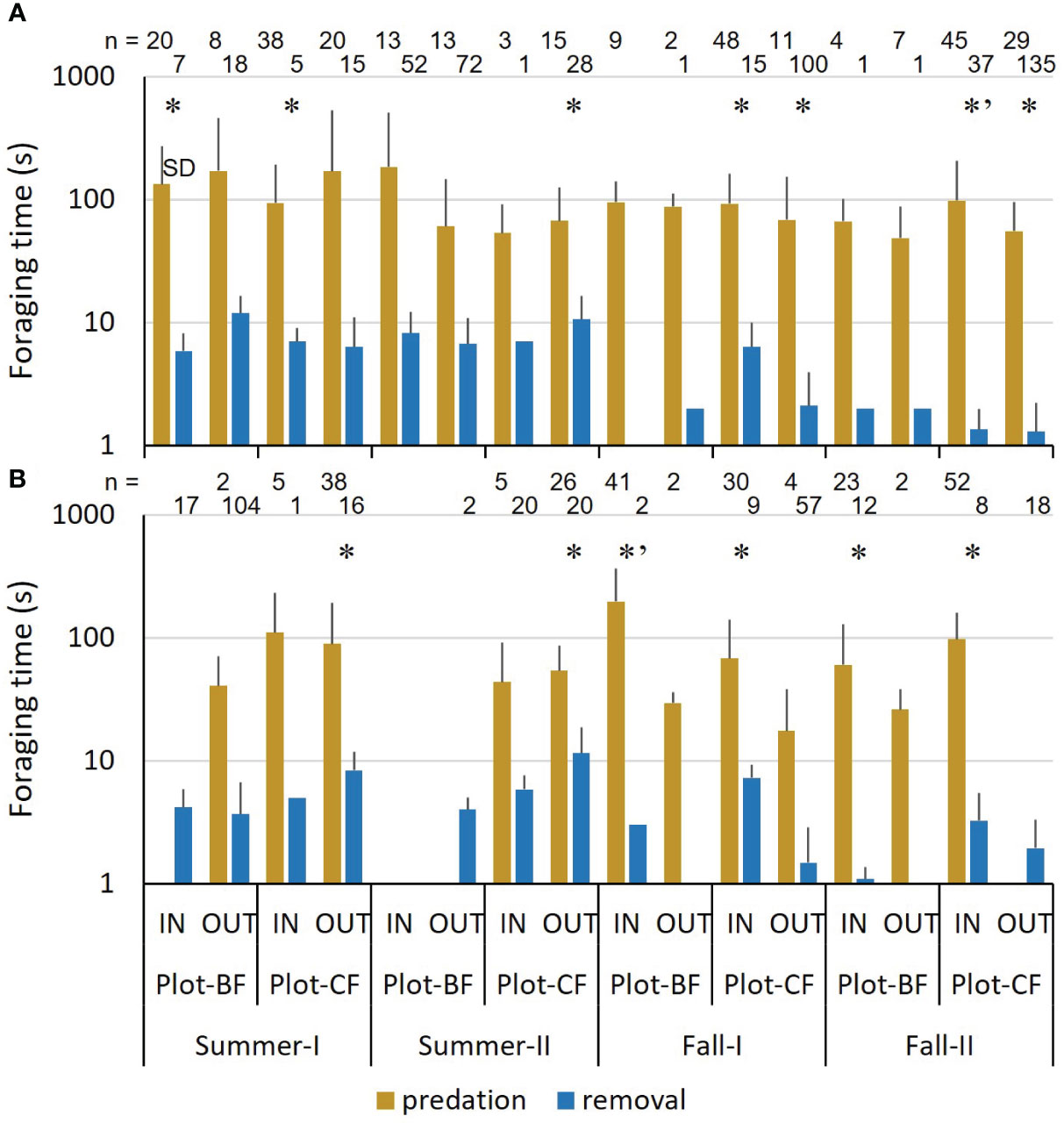
Figure 2 Mean foraging time of mice. (A) Apodemus speciosus (B) Apodemus argenteus. * (Welch’s T test) and *’ (Mann–Whitney U test) indicate significant differences between predation and removal at each test point. n: Number of recordings.
4 Discussion
This study revealed that the only animals that utilized S. borealis seeds, which were supplied in large quantities at the same time as a masting phenomenon, in central Japan were two species of Japanese mice. A study based in Chiloé, Chile, revealed that Bambusoideae seeds were consumed by rodents as well as birds (finch-like species and the large pigeon) (Willson and Armesto, 1996). Yet, we did not observe any squirrels and granivorous birds utilizing the seeds in our study. However, N. procyonoides was observed approaching and the possibility of foraging cannot be ruled out.
The seed utilization rate was found to change with season and the interaction between season and plot. Forget (1992) and Shimada (2001) showed experimentally that the behavior of rodents, whether or not they forage for the seeds, depends on the presence other foods at the same time. Therefore, the lack of other seeds to forage in summer may be the reason for the high utilization rate of S. borealis in both our plots. In fall, other seeds such as acorns of Q. crispula were potentially supplied inside and outside Plot-BF, resulting in the low utilization rate of S. borealis. Meanwhile, in Plot-CF, a coniferous forest, there was no such supply, and the high rate may be unchanged from summer.
Camera capture using trail cameras confirmed that mice not only predated and/or removed seeds with a high frequency but also often buried or cached them in the ground, thus providing novel evidence for small seeds being targeted for caching. Previous studies such as Vander Wall (2003) used trials on rodents as large as A. speciosus and revealed that small seeds (mean 8.7 mg) were predated upon but large seeds (>55 mg) were cached. Similarly, Wang et al. (2012) also reported that experiments conducted on the habitat of Apodemus sp. showed that larger seeds (mean 335 mg) were cached but smaller seeds (<28 mg) of several species were only predated upon. Thus, our study provides new insights that contradict the patterns of predation and caching observed in these previous studies and show that mice can cache small seeds and may have a role as a disperser of small S. borealis seeds.
Regarding seed handling patterns, A. specisus tended to have a higher percentage of predation at IN and removal at OUT. This is in accordance with general rodent behavior, which is the need to avoid natural enemies during feeding, and thus, their predation rate increases in safe places with adequate cover (Phelan and Baker, 1992; Kollmann and Buschor, 2003). The dead S. borealis culm cover in the IN group can function to reduce the predation risk on rodents compared with OUT. Furthermore, the seed predation time of the mouse was longer in IN than in OUT, suggesting that A. speciosus were eating the seeds without fear of predators in IN. In contrast, A. argenteus showed no clear trends in the results. The main reason for the lack of distinct trends may be because A. argenteus is smaller than A. speciosus in body size (Oka, 1992), and therefore cannot act freely to avoid A. speciosus in the coexisting habitat. In addition, based on the number of recordings, A. argenteus barely visited the Plot-BF in Summer-II, and yet this site was concurrently used freely by A. speciosus, suggesting the existence of interspecific competition between the two species. Moreover, the large difference in habitat use by both species in fall may be explained by their differential preference for Q. crispula acorns. Acorns are too large in size to be taken away by A. argenteus, and contain tannins, which act as a protective substance in A. argenteus but not in A. speciosus (Shimada et al., 2004; Saitoh et al., 2007). Therefore, A. speciosus appeared to change its primary food from S. borealis seeds to the available Q. crispula acorns, whereas A. argenteus maintained its prioritization of S. borealis seeds.
In conclusion, this study revealed that the small S. borealis seeds were consumed by mice and were a target for caching, and that the utilization pattern varied according to mouse species, location, and season. In relationships between plants, particularly acorns, and their animal predators, the animal plays an important role in seed dispersal (Klinger and Rejmánek, 2009). This contributes to forest regeneration, which is an important focus area in ecology and evolution. Our study reports on a novel research concept that integrates a very long-period masting event in dwarf bamboo, which produces very small seeds, with the plant’s relationships with other species. Therefore, further research is required to clarify how seeds produced by masting in species such as S. borealis may affect forest ecosystems, while considering other plant taxa in the ecosystem. In this context, future research should examine animal seed preferences among S. borealis and other plant taxa.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/s/7ac7a1e2bf34a12aeabd.
Ethics statement
Ethical review and approval was not required for the animal study because the study is a contactless observation of the behavior of wild animals in the field.
Author contributions
HS and HK conceived the ideas and designed the study. HS collected field data, analyzed the data, and wrote the first draft of the manuscript. HK substantially contributed to revising the manuscript and supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the “Nagoya University Interdisciplinary Frontier Fellowship” [Grant Number JPMJFS2120], awarded to HS.
Acknowledgments
We would like to thank Saitoh Tomoyuki for providing S. borealis seeds. We would also like to thank Norio Yamaguchi and Naoki Takabe for their help in field surveys, and all the members of the Forest Protection Laboratory, Nagoya University for their valuable comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1124393/full#supplementary-material
References
Best I. N., Shaner P. L., Pei K. J., Kuo C. (2022). Of mice and cats: interspecific variation in prey responses to direct and indirect predator cues. Behav. Ecol. Sociobiol. 77, 3. doi: 10.1007/s00265-022-03277-4
Bovendorp R. S., Heming N. M., Percequillo A. R. (2020). Bottom-up effect: a rodent outbreak following the bamboo blooming in a Neotropical rainforest. Mammal Res. 65 (3), 535–543. doi: 10.1007/s13364-020-00505-y
den Ouden J., Jansen P. A., Smit R. (2005). “Jays, mice and oaks: predation and dispersal of Quercus robur and Q. petraea in North-western Europe,” in Seed fate: Predation, Dispersal and Seedling Establishment. Eds. Lambert J. E., Hulme P. E., Vander Wall S. B. (Wallingford, Oxfordshire, UK: CABI), 223–239.
Forget P. M. (1992). Seed removal and seed fate in Gustavia superba (Lecythidaceae). Biotropica 24 (3), 408–414. doi: 10.2307/2388611
Gonzalez L. A., Murua R., Jofre C. (2000). Habitat utilization of two muroid species in relation to population outbreaks in southern temperate forests of Chile. Rev. Chil. Hist. Nat. 73, 489–495. doi: 10.4067/s0716-078x2000000300012
Haugaasen J. M. T., Haugaasen T., Peres C. A., Gribel R., Wegge P. (2010). Seed dispersal of the Brazil nut tree (Bertholletia excelsa) by scatter-hoarding rodents in a central Amazonian forest. J. Trop. Ecol. 26, 251–262. doi: 10.1017/S0266467410000027
Jensen T. S., Nielsen O. F. (1986). Rodents as seed dispersers in a heath—oak wood succession. Oecologia 70, 214–221. doi: 10.1007/bf00379242
Kaneko Y. (2005). “Muridae,” in A Guide to the Mammals of Japan, Revised. Ed. Abe H. (Kanagawa: Tokai University Press), 126–144.
Kikuzawa K. (1988). Dispersal of Quercus mongolica acorns in a broadleaved deciduous forest. For. Ecol. Manage. 25, 1–8. doi: 10.1016/0378-1127(88)90129-6
Klinger R., Rejmánek M. (2009). The numerical and functional responses of a granivorous rodent and the fate of Neotropical tree seeds. Ecology 90, 1549–1563. doi: 10.1890/07-2146.1
Kollmann J., Buschor M. (2003). Edges effects on seed predation by rodents in deciduous forests of northern Switzerland. Plant Ecol. 164, 249–261. doi: 10.1023/A:1021225728493
Nakashizuka T. (1987). Regeneration dynamics of beech forests in Japan. Vegetatio 69, 169–175. doi: 10.1007/bf00038698
Ogawa R., Mortelliti A., Witham J. W., Hunter M. L. (2017). Demographic mechanisms linking tree seeds and rodent population fluctuations: insights from a 33-year study. J. Mammal. 98, 419–427. doi: 10.1093/jmammal/gyw200
Oka T. (1992). Home range and mating system of two sympatric field mouse species, Apodemus speciosus and Apodemus argenteus. Ecol. Res. 7 (2), 163–169. doi: 10.1007/bf02348495
Phelan J. P., Baker R. H. (1992). Optimal foraging in Peromyscus polionotus: the influence of item-size and predation risk. Behaviour 121, 95–109. doi: 10.1163/156853992x00453
R Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Saitoh T., Osawa J., Takanishi T., Hayakashi S., Ohmori M., Morita T., et al. (2007). Effects of acorn masting on population dynamics of three forest-dwelling rodent species in Hokkaido, Japan. Popul. Ecol. 49, 249–256. doi: 10.1007/s10144-007-0041-9
Shimada T. (2001). Hoarding behaviors of two wood mouse species: Different preference for acorns of two Fagaceae species. Ecol. Res. 16 (1), 127–133. doi: 10.1046/j.1440-1703.2001.00378.x
Shimada T., Saitoh T., Matsui T. (2004). Does acclimation reduce the negative effects of acorn tannins in the wood mouse Apodemus speciosus? Acta Theriol. 49, 203–214. doi: 10.1007/bf03192521
Suzuki H., Kashiwagi H., Kajimura H. (2022). How does the 120-year cycle mast seeding of dwarf bamboo affect the rodent population? Ecol. Process. 11, 1–10. doi: 10.1186/s13717-022-00385-x
Vander Wall S. B. (2003). Effects of seed size of wind-dispersed pines (Pinus) on secondary seed dispersal and the caching behavior of rodents. Oikos 100, 25–34. doi: 10.1034/j.1600-0706.2003.11973.x
Wang B., Wang G., Chen J. (2012). Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol. 213, 1329–1336. doi: 10.1007/s11258-012-0091-8
Keywords: caching behavior, dwarf bamboo, masting, rodent, seed predation
Citation: Suzuki H and Kajimura H (2023) Utilization of Sasa borealis seeds by Japanese field mouse: discovery of small-seed caching. Front. Ecol. Evol. 11:1124393. doi: 10.3389/fevo.2023.1124393
Received: 15 December 2022; Accepted: 10 August 2023;
Published: 23 August 2023.
Edited by:
Ann Valerie Hedrick, University of California, Davis, United StatesReviewed by:
Richard T Corlett, Chinese Academy of Sciences (CAS), ChinaXiuqing Nie, Chinese Academy of Forestry, China
Copyright © 2023 Suzuki and Kajimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanami Suzuki, c3V6dWtpLmhhbmFtaS50NkBzLm1haWwubmFnb3lhLXUuYWMuanA=; Hisashi Kajimura, a2FqaW11cmFAYWdyLm5hZ295YS11LmFjLmpw
 Hanami Suzuki
Hanami Suzuki Hisashi Kajimura
Hisashi Kajimura