
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 15 February 2023
Sec. Ecophysiology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1120271
This article is part of the Research Topic New Frontiers in the Application of Stable Isotopes to Ecological and Ecophysiological Research View all 16 articles
 Lucas Navarrete1,2
Lucas Navarrete1,2 Nico Lübcker3
Nico Lübcker3 Felipe Alvarez1,2
Felipe Alvarez1,2 Roberto Nespolo2,4,5
Roberto Nespolo2,4,5 Juan Carlos Sanchez-Hernandez6
Juan Carlos Sanchez-Hernandez6 Karin Maldonado7
Karin Maldonado7 Zachary D. Sharp8
Zachary D. Sharp8 John P. Whiteman9
John P. Whiteman9 Seth D. Newsome3
Seth D. Newsome3 Pablo Sabat1,2*
Pablo Sabat1,2*Tracing how free-ranging organisms interact with their environment to maintain water balance is a difficult topic to study for logistical and methodological reasons. We use a novel combination of triple-oxygen stable isotope analyses of water extracted from plasma (δ16O, δ17O, δ18O) and bulk tissue carbon (δ13C) and nitrogen (δ15N) isotopes of feathers and blood to estimate the proportional contribution of marine resources, seawater, and metabolic water used by two species of unique songbirds (genus Cinclodes) to maintain their water balance in a seasonal coastal environment. We also assessed the physiological adjustments that these birds use to maintain their water balance. In agreement with previous work on these species, δ13C and δ15N data show that the coastal resident and invertivore C. nigrofumosus consumes a diet rich in marine resources, while the diet of migratory C. oustaleti shifts seasonally between marine (winter) to freshwater aquatic resources (summer). Triple-oxygen isotope analysis (Δ17O) of blood plasma, basal metabolic rate (BMR), and total evaporative water loss (TEWL) revealed that ~25% of the body water pool of both species originated from metabolic water, while the rest originated from a mix of seawater and fresh water. Δ17O measurements suggest that the contribution of metabolic water tends to increase in summer in C. nigrofumosus, which is coupled with a significant increase in BMR and TEWL. The two species had similar BMR and TEWL during the austral winter when they occur sympatrically in coastal environments. We also found a positive and significant association between the use of marine resources as measured by δ13C and δ15N values and the estimated δ18O values of ingested (pre-formed) water in both species, which indicates that Cinclodes do not directly drink seawater but rather passively ingest when consuming marine invertebrates. Finally, results obtained from physiological parameters and the isotope-based estimates of marine (food and water) resource use are consistent, supporting the use of the triple-oxygen isotopes to quantify the contribution of water sources to the total water balance of free-ranging birds.
Bird species face both predictable and unpredictable changes in environmental conditions that impact food and water availability (Maddocks and Geiser, 2000; Landes et al., 2020). For example, an increase in ambient temperature and a decrease in the availability of freshwater affects several aspects of avian physiology including rates of energy expenditure, body mass, thermal tolerance, thermal conductance, and evaporative water loss, all of which are directly linked to a bird’s ability to maintain their water balance (Carmi et al., 1993; Sabat et al., 2006a; Barceló et al., 2009; Sabat et al., 2009; Gerson and Guglielmo, 2011; Smith et al., 2017; McWhorter et al., 2018). Organisms living in seasonal environments can adjust their morphology and physiology to respond to predictable environmental changes, a phenomenon often referred to as acclimatization, a particular type of phenotypic plasticity. It is increasingly important to explore the adaptive mechanisms behind these adjustments and assess their impact on fitness because of unprecedented shifts in environmental conditions resulting from climate change, which will likely impact the amount and timing of resource availability, especially water (Şekercioğlu et al., 2012; Khaliq et al., 2014; Cooper et al., 2019; Whiteman et al., 2019; Huey and Buckley, 2022).
Deserts and other xeric habitats are among the most challenging environments for maintaining organismal water balance (Paces et al., 2021; Cabello-Vergel et al., 2022). Despite the crucial importance of water to survival, how animals deal with water scarcity has received less attention than the consequences of reduced food availability (McKechnie et al., 2016; Cooper et al., 2019; Gerson et al., 2019; Paces et al., 2021; Cabello-Vergel et al., 2022). An organism’s water balance is a function of the interplay between (1) physical environment and water availability, (2) physiological and behavioral mechanisms for conserving water by reducing the total evaporative water loss (TEWL) and/or thermal conductance, and (3) the production of metabolic water (Bartholomew and Cade, 1963; MacMillen, 1990; Gerson and Guglielmo, 2011; Rutkowska et al., 2016; Albright et al., 2017). For example, some bird species respond to dehydrating conditions by increasing their rates of energy expenditure (e.g., basal metabolic rate, BMR), a response that is commonly assumed to be the cost of living in arid environments and/or regularly consuming salty water (Arad et al., 1987; Gutiérrez et al., 2011; Peña-Villalobos et al., 2013; Sabat et al., 2017). Such increases in metabolic rate could be a mechanism for water production, reducing the need for water conservation and the reliance on (pre-formed) drinking/food water (see Peña-Villalobos et al., 2013; Sabat et al., 2017). This hypothesis is supported by observations in captive rufous-collared sparrows (Zonotrichia capensis), in which mass loss and an increase in the mass-specific metabolic rates were associated with a higher contribution of metabolic water to the body water pool (Navarrete et al., 2021). No studies have examined this hypothesis in wild birds, and only a handful have quantified the contribution of metabolic water to the water budgets of free-ranging individuals (MacMillen, 1990; Williams and Tieleman, 2001; Giulivi and Ramsey, 2015).
Coastal deserts are especially intriguing habitats because they do not support large amounts of terrestrial productivity nor do they have significant sources of freshwater (Polis and Hurd, 1996), but can occur adjacent to very productive nearshore marine ecosystems (Fariña et al., 2008). Terrestrial animals can exploit abundant marine resources at the cost of having to deal with high salt loads (Mahoney and Jehl, 1985; Nyström and Pehrsson, 1988; Fariña et al., 2008). Salty foods can impose significant osmoregulatory challenges to songbirds (Order Passeriformes), which lack functional salt glands (Shoemaker, 1972) and have a reduced ability to concentrate urine (Goldstein and Skadhauge, 2000; Sabat, 2000). Worldwide, there are only a few passerine species (genus Cinclodes) capable of living in arid coastal deserts while consuming significant amounts of salty marine prey, and several of them are endemic to the central and northern coasts of Chile. Using stable isotope analyses and osmometry to study three species of Cinclodes, Sabat and del Río (2005) reported that the osmolality of stomach contents increased as the proportion of marine diet (assessed by stable isotope analysis) became more substantial. Typical salt concentrations in the body fluids of terrestrial and freshwater prey are 100–300 mOsm/kg (Beyenbach, 2016), whereas some coastal Cinclodes consume prey (e.g., mollusks and crustaceans) with salt concentrations of up to 800–1,100 mOsm/kg in their body fluids (Schmidt-Nielsen, 1997).
Here, we use multiple isotope tracers to explore seasonal variation in diet and water balance of two species of endemic, South American coastal passerines from the genus Cinclodes to investigate how seasonal variation in habitat use, ambient temperature, and marine resource use are related to how birds acquire (food/drinking versus metabolic) and conserve water (TEWL), expend energy (BMR), and dissipate heat (thermal conductance). C. nigrofumosus is year-round resident that forages on marine invertebrates in intertidal environments, while its sister species C. oustaleti also consumes invertebrates but migrates seasonally between dry coastal habitats and high elevation freshwater streams (Newsome et al., 2015; Rader et al., 2017; Tapia-Monsalve et al., 2018). We used carbon (δ13C) and nitrogen (δ15N) isotope analysis of feathers and blood collected during the summer and winter to characterize seasonal marine versus terrestrial resource use (Newsome et al., 2007; Martínez del Rio et al., 2009). We then used a novel methodological approach based on the measurements of the three stable isotopes of oxygen in blood plasma to measure the proportion of the body water pool that was derived from metabolic water (Whiteman et al., 2019; Passey and Levin, 2021; Sabat et al., 2021). This method utilizes natural differences in the oxygen isotope composition of preformed water versus atmospheric oxygen, which is the source of oxygen for the formation of metabolic water in the mitochondria (Whiteman et al., 2019). While several studies have focused on the physiological adjustments these species use to reduce water loss, none have identified seasonal shifts in the use of different source(s) of water (pre-formed versus metabolic) that is a critical to understanding water balance in free-ranging birds (Navarro et al., 2018; Smit et al., 2019).
We hypothesized that C. nigrofumosus and C. oustaleti used different strategies to maintain water balance due to variation in their ecological traits. We predicted that seasonal scarcity of freshwater and overall higher consumption of marine resources by C. nigrofumosus would result in higher osmoregulatory costs leading to elevated BMR and a corresponding increase in metabolic water production to maintain their water balance during summer. We also expected that birds in summer would exhibit lower TEWL and higher thermal conductance to reduce water loss associated with evaporative cooling. In winter when the two species occur in sympatry along the coast, we predicted that migratory C. oustaleti would rely less on salty marine resources than C. nigrofumosus and instead consume more terrestrial invertebrates, which provide a source of less salty (food) water. By extension this would reduce the proportional contribution of metabolic water to their body water pool.
Wild C. oustaleti (n = 11) and C. nigrofumosus (n = 9) were collected at Bahia Mansa (32°14′22″S 71°30′54″W) on the central coast of Chile in the austral winter (June 2021) when these species occur in sympatry. We also collected seven individuals of C. nigrofumosus in summer (January 2022) at the same locality. This study site has a Mediterranean climate (mean annual precipitation = 396 mm) characterized by mild dry summers (mean monthly precipitation = 2 mm, Tmin = 13°C; Tmax = 19) and cold rainy winters (mean monthly precipitation = 85 mm; Tmin = 7°C; Tmax = 13) (di Castri and Hajek 1976). We observed no clear signs of reproduction (e.g., brood patch) or active molting in C. nigrofumosus captured during the summer. This species is not sexually dimorphic so we could not determine the sex of individuals we captured. We used mist nets and spring traps to capture birds, which were caged individually in the dark after capture to minimize stress. Biometric parameters, cloacal temperature (Tb), and blood samples were collected from each individual in the field. Blood samples were collected from the humeral vein using heparinized hematocrit capillaries. Blood was maintained in coolers (~4°C) for <2 h and then centrifuged at 10,000 rpm for 10 min to separate plasma from red blood cells. The plasma was then stored frozen until cryogenic distillation followed by oxygen isotope analysis. In addition, a subsample of whole blood was dried on two glass microscope slides and then transferred to microcentrifuge tubes and stored for δ13C and δ15N analysis.
Immediately after capture, birds were transported to Algarrobo, Chile ~15 min from the capture site for captive physiological measurements. While in captivity, birds consumed mealworms and water, which were available ad libitum. We measured BMR (mL O2 h−1) and total evaporative water loss (TEWL) in post-absorptive (fasted for 4-h), resting birds, during their inactive period between 21:00 and 07:00 h using standard flow-through respirometry (Tapia-Monsalve et al., 2018). We removed mealworms from the cages ~4 h before BMR measurements started to ensure a post-absorptive state. Respirometry measurements for BMR were performed on up to three birds per night. Measurements were made at an ambient temperature (Ta) of 30.0 ± 0.5°C, which is within the thermoneutral zone (TNZ), using an infrared O2-CO2 analyzer equipped with a hygrometer (FMS, Sable Systems®). All trials were conducted in metallic metabolic chambers (volume 2,000 mL) that received air free of water and CO2 removed via Drierite and CO2 absorbent, respectively, at a flow of 800 mL/min (±1%). Inside these darkened metabolic chambers, birds perched on a wire-mesh grid that allowed excreta to fall into a tray containing mineral oil, thus trapping the water from this source. Oxygen consumption was calculated according to the equation (Lighton, 2008): VO2 = FR × 60 × (Fi O2 − Fe O2)/ (1 − Fi O2), where FR is the flow rate in mL min−1, and FiO2 and Fe O2 are the fractional concentrations of inflow and outflow O2 in the metabolic chamber, respectively. We calculated absolute humidity (kg/m3) of air entering and leaving the chamber as ρ = P/(T × Rw), where P is water vapor pressure of the air in Pascal, T is the dewpoint temperature in Kelvin and Rw is the gas constant for water vapor (461.5 J/kg K, Lide, 2001). P was determined using the average value of the vapor pressure of the air entering the empty chamber during a baseline period of 15 min before and after each experiment with a dew-point hygrometer located in the FMS. TEWL was calculated as TEWL = (Ve × ρout – Vi × ρin), where TEWL is in mg/mL, ρin and ρout are the absolute humidity in kg/m3 of the inlet air and the outlet air respectively, Vi is the flow rate of the air entering the chamber as given by the mass flow controller (800 mL min-1), and Ve is the flow of exiting air. Ve was calculated following as: Ve = Vi – [VO2 × (1–RQ)] + VH2O. Vin and VO2 (mL min-1) are known, and we assumed a respiratory quotient (RQ) of 0.71 (Sabat et al., 2006a,b). Output from the H2O (kPa) analyzer, the oxygen analyzer (%), and the flow meter were digitalized using a Universal Interface II (Sable Systems, Nevada, United States) and recorded on a personal computer using EXPEDATA data acquisition software (Sable Systems, Nevada, United States). To estimate BMR and TEWL, we averaged O2 concentrations and water vapor pressures of the excurrent air stream over a 20 min period after steady state was reached, which occurs after 3 h in Cinclodes (Sabat et al., 2021). We estimated the metabolic water production (MWP) using the equivalence of 0.567 mL H2O per liter O2 consumed (Schmidt-Nielsen, 1997) and calculated the ratio between metabolic water production and water losses (MWP/ TEWL) for the 20 min period during which steady state was reached. The ratio MWP/TEWL is interpreted as the ability of birds to rely on metabolic water to maintain water balance.
To estimate wet thermal conductance (Cw), we also measured metabolic rates of birds at a Ta below the TNZ, on the day of capture, between 8:00 and 17:00 h, as described above for BMR. Because the “wet” thermal conductance is roughly constant in endotherms below thermoneutrality (Nicol and Andersen, 2007; Rezende and Bacigalupe, 2015; Andreasson et al., 2020), for simplicity and logistic restrictions we measured Cw at 15.0 ± 0.5°C. Cw was calculated as metabolic rate (MR15) measured at 15°C using the equation MR/(Tb-Ta). In this case water and food was available for birds until they were placed in the metabolic chamber. Body mass was measured before the metabolic measurements using an electronic balance (± 0.1 g) and cloacal body temperature (Tb) was recorded with a thin Cole-Palmer copper-constantan thermocouple attached to a Digisense thermometer (Model 92,800–15) within a minute after the birds were removed from metabolic chamber to minimize the effect of manipulation on the temperature measurement (Nord and Folkow, 2019) We considered body temperatures of ≤36°C as hypothermic (Swanson et al., 2012) and all birds were normothermic at the end of 15°C or 30°C exposure trials. After each respirometry measurement, the birds were provided food and water ad libitum until their release.
To estimate the contribution of metabolic water to the body water, we used a method based on the measurement of Δ17O, which is the positive or negative deviation from the tight correlation that naturally exists between values of δ17O and δ18O (Whiteman et al., 2019). The premise of this method is that metabolic water and drinking/food water together provide 80–99% of the body water of most animals (Bryant and Froelich, 1995; Kohn, 1996), with the remaining contribution (1–20%) resulting from condensation reactions that use bound oxygen from dietary nutrients. Here, we ignore this latter contribution and acknowledge that this induces uncertainty (Whiteman et al., 2019). Of the two other sources, metabolic water is assumed to have a Δ17O value of −0.44‰ reflecting that of inhaled atmospheric oxygen (Whiteman et al., 2019; Wostbrock et al., 2020), and drinking/food water is assumed to have a Δ17O value of meteoric water with a mean Δ17O value of ~0.03‰ across a wide variety of potential sources (e.g., lakes, rivers, precipitation; Sharp et al., 2018). While extensive evaporation lowers the Δ17O values of the remaining water (Aron et al., 2021), recent studies have suggested that in many biological applications assuming a fixed value of ~0.03‰ for meteoric water is reasonable (Whiteman et al., 2019; Sabat et al., 2021). Under this assumption, a linear mixing model can be used to calculate the proportional contribution from each source (Whiteman et al., 2019). For example, an animal body water sample with a Δ17O value of −0.44‰ represents pure metabolic water and a sample with a Δ17O value of 0.03‰ represents pure meteoric water. This mixing model is in the form: Δ17OBody Water = FM × (−0.44‰) + (1 – FM) × (0.030‰) where FM represents the fractional contribution to body water from metabolic water, and (1 – Fm) represents the contribution from drinking/food water. In previous studies of captive sparrows (Zonotrichia capensis) and mice (Peromyscus maniculatus) this equation accurately predicted relative changes in Δ17O based on metabolic rate and drinking water intake (Whiteman et al., 2019; Sabat et al., 2021).
We cryogenically distilled body water from blood plasma on a vacuum line. We fluorinated 1.5 μL of the distilled water with BrF5 at 450°C for 15 min under a vacuum to evolve O2, which was purified via a 6 ft. × 1/8″ (1.83 m × 3.2 mm) 60/80 Mol Sieve 13X gas chromatograph (GC) column and analyzed via dual-inlet on a Thermo Scientific™ 253 Plus isotope ratio mass spectrometer (Sharp et al., 2018) against a working reference O2 gas at the University of New Mexico Center for Stable Isotopes (UNM–CSI; Albuquerque). The measured values of δ17O and δ18O were linearized (δ′xO = 1,000 × ln((δxO/1000) + 1); x = 17O or 18O) and then used to calculate Δ′17O (δ′17O – (0.528 × δ′18O); Whiteman et al., 2019). Deviations from the normal linear mass-dependent fractionation between δ′17O and δ′18O that is defined by an arbitrary reference line with a slope (λ) of 0.528, are expressed as Δ′17O. Samples were corrected using a one-point calibration based on intermittent measurements of an in-house standard (NM2) calibrated against VSMOW-2.
In addition to using Δ17O values to understand reliance upon metabolic water, we used the combination of FM values and δ18O values of body water to calculate estimated δ18O values of ingested pre-formed drinking/food water (δ18OD + PF) with the equation δ18ODFW = (δ18OBW - (FM) × (δ18OAir)) / (1-FM) and assumed δ18OAir incorporated via respiration was 19.4‰ due to the fractionation that occurs during absorption of inhaled atmospheric oxygen. This fractionation depends on the efficiency of oxygen absorption (EO2; Epstein and Zeiri, 1988). Although this efficiency was not measured in our study species, previous research suggests that an EO2 of 0.4 is reasonable for small passerines (Clemens, 1988; Arens and Cooper, 2005), which in humans produces a fractionation of ~4.4‰ (Epstein and Zeiri, 1988). The estimated δ18O of ingested water generally changes by <3‰ if you apply the plausible range of fractionation values for absorbed oxygen (2–6‰) to equation 3, which is smaller than much of the naturally-occurring variation in δ18O of potential water and, therefore, unlikely to affect our conclusions.
We used δ13C and δ15N analysis to estimate the relative contribution of marine and terrestrial prey to the diet of Cinclodes species (Martínez del Rio et al., 2009). In general, baseline δ13C and δ15N values are higher in marine than terrestrial food webs (Martínez del Rio et al., 2009). For C. nigrofumosus, seasonal comparisons were made via analysis of whole blood collected in summer and winter, a tissue that integrates dietary resources assimilated during ~1–2 months prior to capture (Martínez del Rio et al., 2009). For C. oustaleti, seasonal comparisons were made by comparing the isotopic composition of two tissues: a primary feather (P1) reflecting dietary resources assimilated during the molting period that occurs during the austral summer in Cinclodes (Bertolero and Zavalaga, 2003), and blood representing the winter during which they were captured. We chose not to correct for tissue-specific discrimination between feathers and whole blood, which in a controlled experiment on passerine Dendroica coronata consuming a diet containing 97% insect were ~ 2.1‰ for δ13C and ca. 0.8 ‰ for δ15N (Pearson et al., 2003); these values are small in comparison to observed differences between potential marine and terrestrial food resources available at the study sites (Martínez del Rio et al., 2009).
Approximately 0.5–0.6 mg of dried whole blood or feather was weighed into tin capsules, and carbon (δ13C) and nitrogen (δ15N) isotope values were measured on a Costech 4,010 elemental analyzer coupled to a Thermo Scientific Delta V Plus isotope ratio mass spectrometer at UNM–CSI. Isotope values are reported using standard delta (δ) notation in parts per thousand or per mil (‰) as: δX = (Rsample/Rstandard–1) where Rsample and Rstandard are the ratios of the heavy to light isotope of the sample (e.g., 15N/14N) and the reference, respectively. The internationally accepted references are Vienna PeeDee Belemnite (VPDB) for δ13C and atmospheric N2 (AIR) for δ15N. Within-run precision (SD) for both δ13C and δ15N was estimated via analysis of three proteinaceous internal reference materials and measured to be ≤0.2‰ for both isotope systems.
Because body mass did not differ between seasons, after checking normality (Shapiro–Wilk) and homoscedasticity (Levene), we used a Student t-test to compare mean BMR, TEWL, MWP/TEWL, and thermal conductance between seasons. Because some isotopic data sets (δ15N) did not meet the assumptions of normality, we decided to use non-parametric test to compare data. A Mann–Whitney U-test was used to compare δ13C and δ15N values between seasons for the same tissue between species and a Wilcoxon W test to compare δ13C and δ15N values of feathers and blood collected from the same species to provide a seasonal comparison. To compare physiological data between species in winter we used ANCOVA with body mass as a covariate. We used a Pearson product–moment correlation matric to explore the relationship between and diet and body water Δ17O using all dataset. JAMOVI software (Version 2.3., Jamovi project 2022) was used for all statistical analyses. Following Muff et al. (2021), in this paper we expressed statistical results in the language of evidence instead of the use of arbitrary value of p thresholds (e.g., p = 0.05). This approach translates approximate ranges of p-values into specific language, although the boundaries of such ranges should not be understood as hard thresholds: p < 0.01 as “strong evidence,” 0.01 < p < 0.05 as “moderate evidence,” and 0.05 < p 0.10 as “weak evidence”; for further details see Muff et al. (2021).
For C. nigrofumosus, we found no evidence that mb differed between seasons (t8 = −1.22, p = 0.243; all results described here are presented in Table 1). We found strong evidence that its summer whole organism BMR was ~19% higher (t8 = −3.03, p = 0.009), and moderate evidence that their TEWL was ~29% higher (t8 = −2.36, p = 0.034), than winter. There was no evidence that estimated water balance at 30°C (MWP/TEWL) differed between seasons in C. nigrofumosus (t8 = 1.26; p = 0.230), but wet thermal conductance increased ~18% in summer compared with winter in this species (t8 = −2.48; p = 0.026). A linear regression of all data from both species collected in winter provided strong evidence that log BMR (F1, 18 = 57.58; r2 = 0.76; p = 0.001) and log TEWL (F1, 18 = 20.54; r2 = 0.53; p < 0.001) were positively correlated with log body mass. After removing the effect of body mass, there was no evidence of difference between species in whole-organismal BMR (F1, 17 = 0.019, p = 0.89) and TEWL (F1, 17 = 1.53, p = 0.232). The ratio MWP/TEWL was also similar between species (t8 = 0.022; p = 0.983). Finally, there was strong evidence that Cw was ~46% higher in C. nigrofumosus than C. oustaleti (t8 = −7.08, p < 0.001).
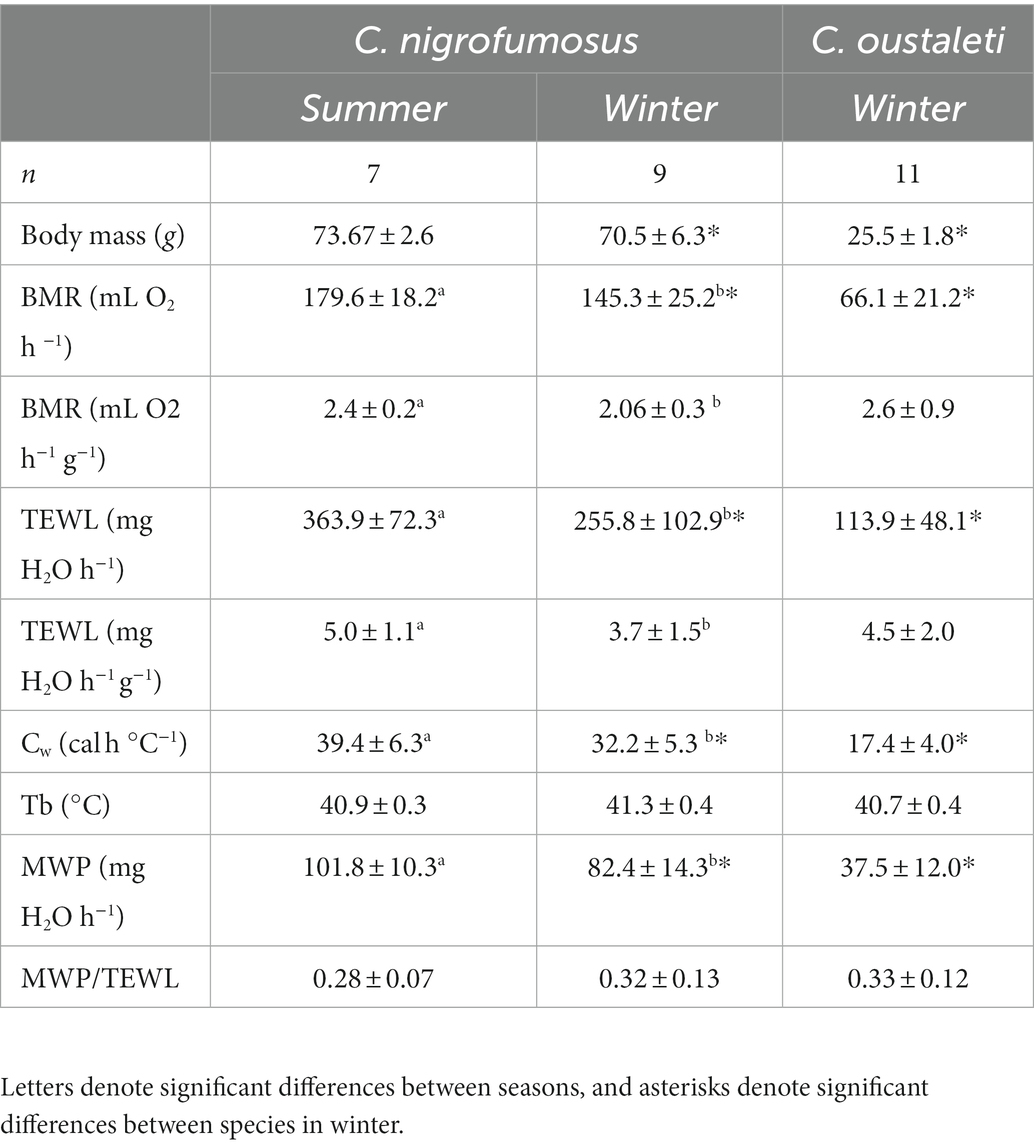
Table 1. Mean (±SD) physiological and biochemical variables measured in C. nigrofumosus captured in summer and winter and for C. oustaleti captured in winter.
There was no evidence of a difference between summer and winter δ13C (Mann–Whitney U = 17, p = 1.0) and δ15N (U = 7.5, p = 0.12) values of C. nigrofumosus (Table 2). In contrast, there was strong to moderate evidence that values of δ13C and δ15N differed between summer and winter in C. oustaleti as feather δ13C values representing summer were 4‰ higher (Wilcoxon W = −28, p = 0.015) and δ15N values were 13‰ higher (W = −28, p = 0.05) than isotope values for blood representing winter (Table 2). There was strong evidence that d13C and d15N values of feathers representing summer foraging differed between species (Mann–Whitney U = 0.0, p < 0.001; Table 2). There was no evidence that δ13C values from blood collected in winter differed between species (Mann–Whitney U = 11.0, p = 0.11), and moderate evidence of a difference for δ15N (U = 6, p = 0.04) (Table 2). There was no evidence that δ13C values from blood collected in winter differed between species (Mann–Whitney U = 11.0, p = 0.11), and moderate evidence of a difference for δ15N (U = 6, p = 0.04) (Table 2).
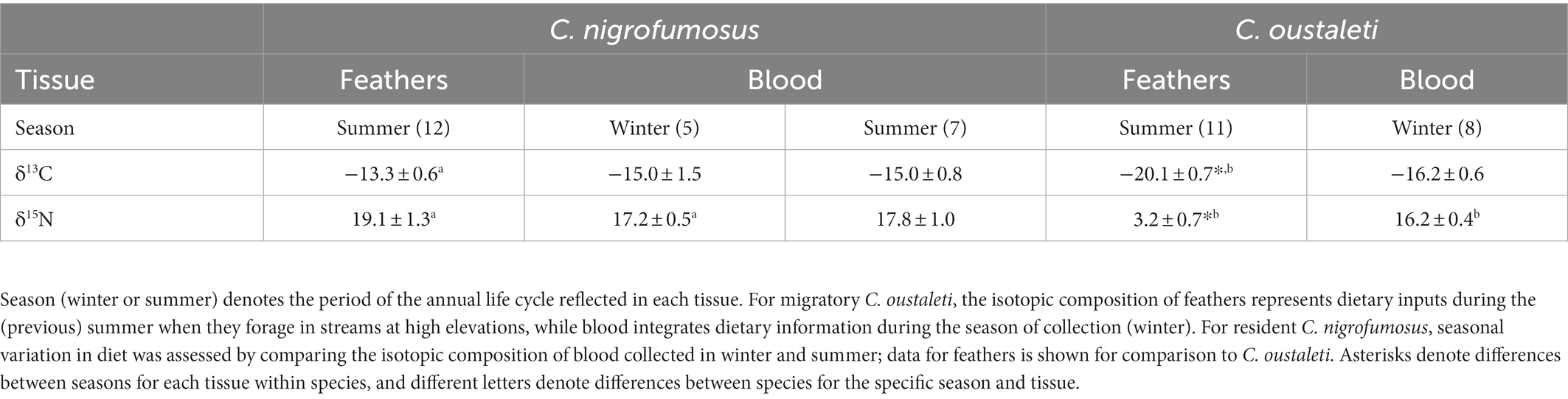
Table 2. Mean (±SD) δ13C and δ15N values of C. nigrofumosus and C. oustaleti tissues collected from a coastal locality from central Chile.
There was little or no evidence that values of Δ17O differed between summer and winter for C. nigrofumosus (t7 = −1.76, p = 0.122, Figure 1). Using equation 2, this difference would translate to seasonal contributions from metabolic water to body water (Fm) of 24.8% in summer versus 20.7% in winter. There was no evidence that values of Δ17O (and hence Fm) differed between species in winter (t12 = 0.652, p = 0.527) (Figure 1). There was also no evidence that the mean δ18O values of plasma differed between winter and summer for C. nigrofumosus (−1.4 ± 0.7‰ and −1.6 ± 0.5‰ respectively; t7 = 0.277, p = 0.790), or between C. oustaleti (−3.5 ± 1.1‰) and C. nigrofumosus (using pooled data from summer and winter: −1.5 ± 1.3‰; t17 = 1.57, p = 0.135). The mean estimated δ18O value of the combined drinking/food water ingested by C. nigrofumosus did not differ between seasons (winter −6.5 ± 2.4‰, summer −8.1 ± 0.8‰; t7 = 1.41, p = 0.203). However, we found moderate evidence that the drinking/food water ingested by C. oustaleti (δ18O = −9.7 ± 2.6‰) was more negative than for C. nigrofumosus in winter (−6.5 ± 2.4‰, t12 = 2.18, p = 0.05) (Figure 2).
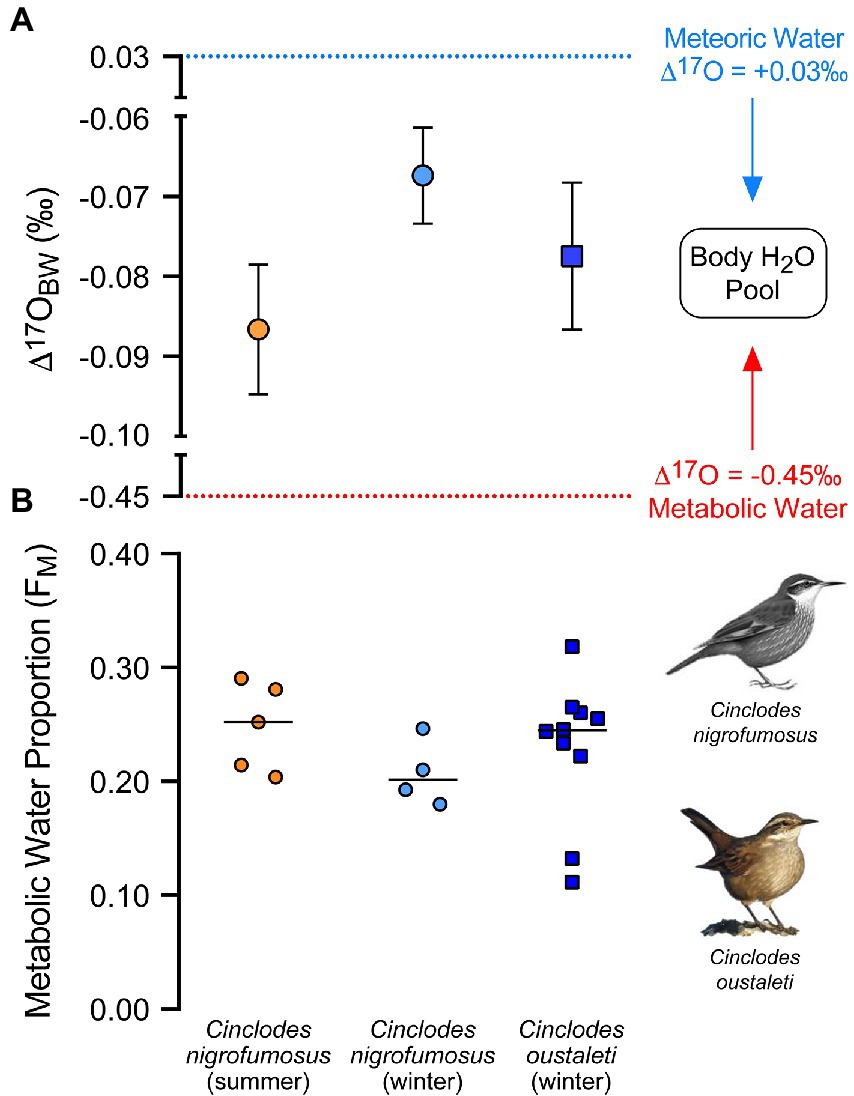
Figure 1. (A) Mean (±SE) Δ’17O values of body water cryogenically distilled from blood plasma and (B) the estimated proportion of metabolic water to the total body water pool in two species of Cinclodes inhabiting a coastal environment in central Chile.
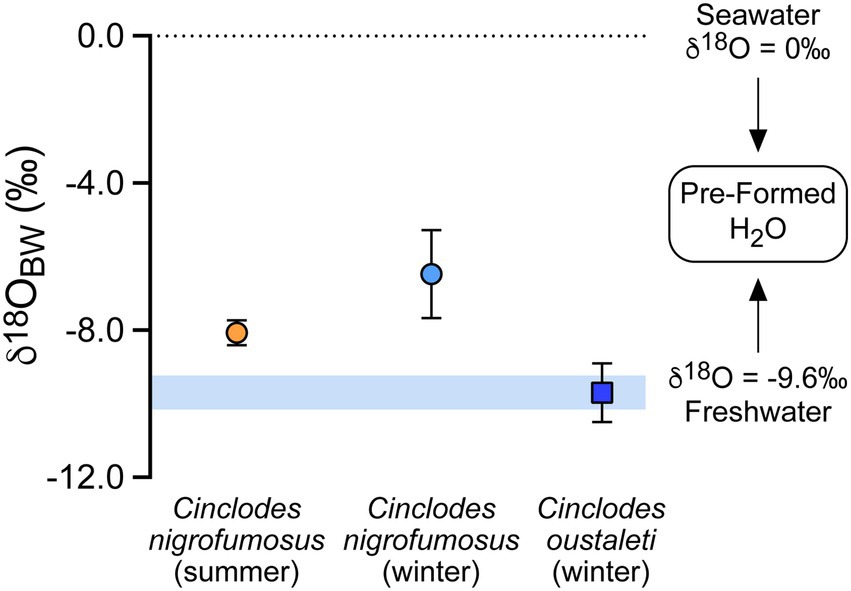
Figure 2. Estimated δ18O values (mean ± SE) of ingested pre-formed (drinking/food) water in two species of Cinclodes inhabiting a coastal environment in central Chile.
Using the whole dataset (i.e., both species and seasons), there was moderate evidence that the estimated isotopic value of drinking water (δ18ODW) was different between species (t17 = 2.18, p = 0.03) and that d18ODW was positively correlated with the δ13C and δ15N values of tissues (Supplementary Table S1 for statistical details). When data were analyzed separately for each species, there was moderate evidence that δ18ODW was positively correlated with blood δ15N in C. oustaleti (r2 = 0.714, p = 0.034) but no evidence of a relationship in C. nigrofumosus (r2 = 0.018 p = 0.7330) (Figure 3). There was no evidence of a relationship between FM and δ15N in either species (r2 = 0.439, p = 0.15 and r2 = 0.02, p = 0.73 for C. oustaleti and C. nigrofumosus respectively). Finally, when we analyzed the whole dataset we found very strong evidence that Δ17O correlated positively with δ18O values of plasma (r2 = 0.54, p < 0.001).”
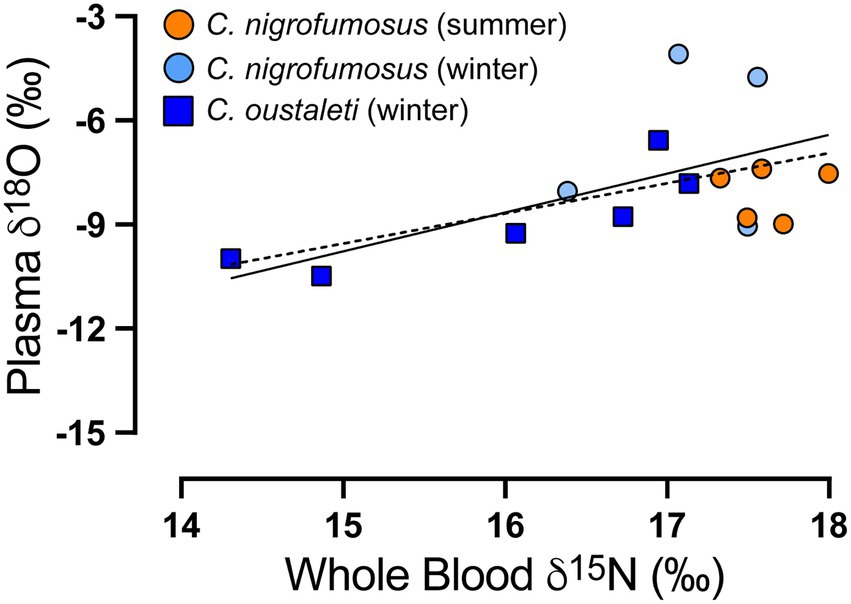
Figure 3. Positive and significant linear correlation between blood δ15N values and the estimated δ18O of ingested water based on Δ17O for two species of Cinclodes inhabiting a coastal environment in central Chile. The dotted line represents the relationship for data pooled across both species, while the solid line represents the relationship for only C. oustaleti.
The main objective of our study was to evaluate the integrated effect of seasonal variation on selected physiological and ecological traits of passerine birds living in a dry coastal environment. We explored whether the interaction between a suite of physiological variables––thermoregulation, osmoregulation, and water balance—varies seasonally in two closely related passerine species that differ in their consumption of marine versus terrestrial resources. Our results suggest that C. nigrofumosus and C. oustaleti vary in their reliance on marine resources (Table 1): δ13C and δ15N values of blood and feathers confirmed the coastal resident C. nigrofumosus consumes a diet rich in marine invertebrates, while the diet of migratory C. oustaleti shifts seasonally between marine (winter) and freshwater/terrestrial (summer) resources indicative of their migration from wintering in marine intertidal habitats to stream habitats at high elevations during the summer in central Chile (Martínez del Rio et al., 2009; Newsome et al., 2015; Tapia-Monsalve et al., 2018). Triple oxygen isotope analysis of blood plasma revealed that a similar proportion of the body water pool of both species originated from metabolic water, and the contribution of metabolic water tended to increase in summer in C. nigrofumosus in concert with increases in BMR and decreases in TEWL and Cw. In the following sections, we explore the causes and consequences of the seasonal variation in physiological variables and the contribution of different water sources to the total water balance of each species.
In passerines, the intake of moderately salty water (~400 mOsm/kg NaCl) tends to increase urine osmolality and BMR (Peña-Villalobos et al., 2014; Sabat et al., 2017). The observed seasonal increase in BMR of C. nigrofumosus, however, does not appear to be associated with an increased osmotic challenge as δ13C and δ15N data show this species consumed a high but similar proportion of marine resources between seasons. Previous studies suggest that the osmoregulatory physiology of Cinclodes is influenced by both ecological (diet composition) and environmental (climate) factors in a complex fashion (Sabat and del Río, 2005; Sabat et al., 2006a,b). For example, isotopic data for C. nigrofumosus from another locality in central Chile (Los Molles, 32°14’22’S 71°30’54’W) suggested a greater consumption of marine resources and an increase in plasma concentration during winter compared to summer; however, urine was more concentrated in the (hot and dry) summer than in the (cold and rainy) winter (Sabat and del Río, 2005). This pattern suggests that the effect of salt intake on osmoregulatory physiology in C. nigrofumosus depends on environmental temperature and availability of meteoric water. Furthermore, the mechanistic link between salt intake, energy expenditure, and the role of metabolic water in maintaining water balance is an intriguing topic that requires further attention.
Several studies have investigated seasonal changes in BMR and other measures of metabolic rate (e.g., RMR or FMR) in response to environment temperature, but results have revealed a noticeable difference in the magnitude of the response to thermal (i.e., seasonal) acclimatization (Arens and Cooper, 2005; Cavieres and Sabat, 2008; Noakes and McKechnie, 2020; Swanson et al., 2020) and the mechanisms for the global pattern of BMR acclimatization is poorly understood. It is generally believed that BMR in free-ranging birds is primarily driven by temperature, although it is possible that other abiotic and biotic factors such as photoperiod, reproduction, and body condition may be important (Daan et al., 1990; Chastel et al., 2003; Vézina and Williams, 2003; Zheng et al., 2008; McNab, 2009; Vezina and Salvante, 2010). Our results reveal considerable flexibility in the thermal physiology of C. nigrofumosus, as we found a ~ 20% decrease in BMR and thermal conductance and a ~ 30% decrease in TEWL in winter relative to summer, but no seasonal change in body mass. Reduced thermal conductance may enable a seasonal decline in BMR because heat is more effectively retained in winter (Speakman and Król, 2010; Rezende and Bacigalupe, 2015; Nord and Nilsson, 2019). This combination of trends suggests that the intake/storage of dietary energy and loss of heat to the environment were reduced in concert (Novoa et al., 1994; Cooper et al., 2019). Under such a scenario, the contribution of metabolic water to the body water pool would decrease. Our results contrast with the typical acclimatization response of birds from higher latitudes (McKechnie et al., 2015; Noakes and McKechnie, 2020; Swanson et al., 2022) and supports the idea that changes in BMR is not related to enhancing cold tolerance in areas where birds face milder winter minimum temperatures and more modest thermoregulatory demands.
Reproduction may also influence BMR and TEWL because behaviors such as nest building, courtship/mating, and parental care in addition to synthesizing eggs are costly and influence energy budgets (Wiersma et al., 2004; Mainwaring and Hartley, 2013; Williams, 2018). The influence of reproduction on resting rates of energy expenditure (including BMR) in free-ranging birds, however, is controversial (Nilsson, 2002; Chastel et al., 2003; Welcker et al., 2015). While seasonal increases in BMR can be explained by reproductive demands, such changes in metabolic activity may also be an adaptive response to increase metabolic water production (MacMillen, 1990; Navarrete et al., 2021). This hypothesis is consistent with results of both field- and lab-based studies that report increases in mass-specific BMR in free-ranging desert birds during the summer (Smit and McKechnie, 2010; McKechnie et al., 2015) and in captive sparrows (Z. capensis) who responded to water restriction by losing mass and increasing their mass-specific BMR. This hypothesis is also supported by the trend reported here showing a seasonal increase in the contribution of metabolic water to the body water pool of C. nigrofumosus in summer (see below). Overall, it is important to note that whole-organism metabolic rate can be affected by essentially any change in morphology or physiology, so changes in traits such as BMR can be consistent with multiple, non-exclusive mechanistic explanations.
Δ17O results suggest that the contribution of metabolic water to the total water budget in C. nigrofumosus was slightly higher in summer (~25%) than in winter (~21%), in agreement with expectations based on differences in BMR between seasons. Both estimates are slightly lower to previously reported 17O-based estimates for C. nigrofumosus (~28%) sampled from another locality ~200 km to the north of our field site (Sabat et al., 2021). For C. oustaleti, estimates of the metabolic water contribution (23%) were nearly identical to those reported for this species from the more northern locality (Sabat et al., 2021). Overall, these 17O-based estimates for the importance of metabolic water in Cinclodes in the field agree with those based on (1) respirometry under controlled conditions in the lab, where MWP/TEWL ratios vary from 28 to 33% (Table 2), and (2) doubly-labeled water (DLW) administered to free-ranging zebra finches (Taeniopygia guttata), an arid-adapted passerine (Cooper et al., 2019).
Given the importance of marine resources for both species in the winter, estimated δ18O values of preformed (drinking/food) water was expected to be close to that of seawater (0‰); however, mean (±SD) values were lower and significantly differed between C. oustaleti (−9.7 ± 2.6‰) and C. nigrofumosus (−6.5 ± 2.4‰). The mean δ18O value for C. oustaleti is nearly identical to that measured in local meteoric and tap waters (−9.7 ± 0.5‰, n = 3). Acknowledging that the end-member δ18O value for local meteoric waters is poorly constrained at present, a two-source mixing model shows that seawater contributes ~0–45% and ~ 24–66% of the total water ingested by C. oustaleti and C. nigrofumosus, respectively. These estimates differ from those obtained from a limited number of C. oustaleti (n = 3) and C. nigrofumosus (n = 3) individuals sampled at a more arid locality 200 km to the north of our study site, where ~48–100% of ingested water was sourced from seawater (Sabat et al., 2021). This difference could reflect lower terrestrial primary productivity at the more northern location, where higher consumption of marine prey would result in increased intake of seawater (Sabat et al., 2006b). Lastly, the positive correlation reported here between tissue δ15N and δ18O of the blood plasma (Figure 3; Supplementary Table S1) supports the hypothesis that Cinclodes do not directly drink seawater, but passively ingest it when consuming intertidal invertebrates (Sabat et al., 2021). Future studies that evaluate the importance of seawater in Cinclodes along a latitudinal gradient in aridity are crucial to establish the relative importance of ecological (resource use) and environmental (temperature and/or humidity) factors that influence water balance in this unique group of passerines.
A recent meta-analysis of field metabolic rate (FMR) and field water flux (FWF) collected from a diverse set of birds reported that seabirds had a higher FMR than terrestrial species, and granivores had a lower FMR than other functional groups (Song and Beissinger, 2020). Similarly, seabirds and terrestrial birds inhabiting regions with higher rainfall had higher FWF. Because the proportion of metabolic water in the total body water pool is dependent on both metabolic rate and water intake, both variables must be considered to understand water balance. Using data for species with both FMR and FWF data (n = 59) and assuming 0.567 mL of metabolic water is produced per liter O2 consumed (Sabat et al., 2021), the average proportion of metabolic water to the body water pool for terrestrial birds and seabirds is 22 and 16%, respectively. The estimated proportion of the total water pool derived from metabolic water for Cinclodes (~21–25%) is slightly higher than terrestrial birds and in the upper range for seabirds. These results suggest that Cinclodes is more dependent on metabolic water than other terrestrial birds but cannot solely rely on seawater to maintain water balance, which likely is the result of limitations imposed by renal function. The maximum concentrating capacity of Cinclodes urine rarely exceeds 100 mOsm/kg, which is the concentration of seawater (Sabat et al., 2004), while seabird salt gland secretions can be more than twice this concentration (Sabat, 2000). Finally, it is important to note that the DLW-based estimates of FMR and FWF for free-ranging birds are typically lower than those for captive birds studied in the laboratory under controlled conditions (Bartholomew and Cade, 1963; MacMillen, 1990). Overall, the 17O-based method agrees with more direct methods using DLW (field) or respirometry (lab), which confirms the usefulness of using triple oxygen isotope measurements to estimate the water balance in the field.
Although our Δ17O-based estimates of the contribution of metabolic water to the total body water pool of Cinclodes are consistent with patterns in other physiological measurements (BMR, TEWL) and previous studies using other methods (DLW), it is important to recognize that Equation 2 is a simplification and includes assumptions that have not yet been fully explored. While meteoric waters collected in a wide variety of environmental contexts have a mean Δ17O (±SD) value of 0.03 ± 0.02‰ (Sharp et al., 2018), a more comprehensive understanding of how Δ17O values of drinking and food water available to animals in different environmental contexts is needed to refine this approach in field-based studies of water balance. For instance, extensive evaporation reduces the Δ’17O values of the residual water (Aron et al., 2021; Passey and Levin, 2021), a process that could impact meteoric waters or organism body water via evaporative water loss in animals living in arid environments. The possibility of fractionation effects on Δ17O could be captured in a flux-based model, rather than the mixing-model approach described by Equation 2. Future studies should consider these complexities and build on the nascent applications of this method (Pack et al., 2013; Whiteman et al., 2019; Sabat et al., 2021).
Our study revealed that the seasonal acclimatization response of C. nigrofumosus is not the typical response of birds from more mesic environments at higher latitudes. The higher BMR observed in summer could be associated with a higher intake of marine prey/seawater or with the energetic costs of reproduction, which may lead to an increase in the contribution of metabolic water to the body water pool. Triple oxygen isotope analysis suggests that the contribution of metabolic water is ~23% of the total water budget in both Cinclodes species, with a slight increase in summer relative to winter for C. nigrofumosus, concomitant with the observed seasonal increase in BMR. These results agree with more direct methods for estimating the proportional contribution of metabolic water to the body water pool based on DLW, confirming the usefulness of Δ17O to examine the water balance of free-ranging birds. Finally, water use strategies also differed between species with seawater contributing 24–66% and 0–45% of the pre-formed water ingested by C. nigrofumosus and C. oustaleti respectively, highlighting the importance of seawater in maintaining water balance in this unique group of passerines.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the animal study was reviewed and all protocols were approved by the institutional Animal Care Committee of the University of Chile (CICUA), and National Research and Development Agency (ANID).
PS, SN, and JW: designed research. LN, NL, FA, and PS: performed research. LN and PS: analyzed data. PS, SN, RN, JS-H, KM, ZS, NL, and JW: wrote the paper. All co-authors edited the paper. All authors contributed to the article and approved the submitted version.
This work was funded by ANID PIA/BASAL FB0002, ANID/CONICYT FONDECYT Regular Nº 1200386, and National Science Foundation grants to SN (IOS-1941903) and JW (IOS-1941853).
We thank to Andrés Sazo and its invaluable fieldwork assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1120271/full#supplementary-material
Albright, T. P., Mutiibwa, D., Gerson, A. R., Smith, E. K., Talbot, W. A., O’Neill, J. J., et al. (2017). Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl. Acad. Sci. U. S. A. 114, 2283–2288. doi: 10.1073/pnas.1613625114
Andreasson, F., Nord, A., and Nilsson, J. Å. (2020). Age differences in night-time metabolic rate and body temperature in a small passerine. J. Comp. Physiol. B 190, 349–359. doi: 10.1007/s00360-020-01266-5
Arad, Z., Gavrieli-Levin, I., Eylath, U., and Marder, J. (1987). Effect of dehydration on cutaneous water evaporation in heat-exposed pigeons (Columba livia). Physiol. Zool. 60, 623–630. doi: 10.1086/physzool.60.6.30159978
Arens, J. R., and Cooper, S. J. (2005). Metabolic and ventilatory acclimatization to cold stress in house sparrows (Passer domesticus). Physiol. Biochem. Zool. 78, 579–589. doi: 10.1086/430235
Aron, P. G., Levin, N. E., Beverly, E. J., Huth, T. E., Passey, B. H., Pelletier, E. M., et al. (2021). Triple oxygen isotopes in the water cycle. Chem. Geol. 565:120026. doi: 10.1016/j.chemgeo.2020.120026
Barceló, G., Salinas, J., Cavieres, G., Canals, M., and Sabat, P. (2009). Thermal history can affect the short-term thermal acclimation of basal metabolic rate in the passerine Zonotrichia capensis. J. Therm. Biol. 34, 415–419. doi: 10.1016/j.jtherbio.2009.06.008
Bartholomew, G. A., and Cade, T. J. (1963). The water economy of land birds. Auk 80, 504–539. doi: 10.2307/4082856
Bertolero, A., and Zavalaga, C. (2003). Observaciones sobre la biometría y la muda del Churrete Marisquero (Cinclodes taczanowskii) en Punta San Juan, costa sur del Perú. Ornitol. Neotrop. 14, 469–475.
Beyenbach, K. W. (2016). The plasticity of extracellular fluid homeostasis in insects. J. Exp. Biol. 219, 2596–2607. doi: 10.1242/jeb.129650
Bryant, D. J., and Froelich, P. N. (1995). A model of oxygen isotope fractionation in body water of large mammals. Geochim. Cosmochim. Acta 59, 4523–4537. doi: 10.1016/0016-7037(95)00250-4
Cabello-Vergel, J., González-Medina, E., Parejo, M., Abad-Gómez, J. M., Playà-Montmany, N., Patón, D., et al. (2022). Heat tolerance limits of Mediterranean songbirds and their current and future vulnerabilities to temperature extremes. J. Exp. Biol. 225:jeb244848. doi: 10.1242/jeb.244848
Carmi, N., Pinshow, B., Horowitz, M., and Bernstein, M. H. (1993). Birds conserve plasma volume during thermal and flight-incurred dehydration. Physiol. Zool. 66, 829–846. doi: 10.1086/physzool.66.5.30163826
Cavieres, G., and Sabat, P. (2008). Geographic variation in the response to thermal acclimation in rufous-collared sparrows: are physiological flexibility and environmental heterogeneity correlated? Funct. Ecol. 22, 509–515. doi: 10.1111/j.1365-2435.2008.01382.x
Chastel, O., Lacroix, A., and Kersten, M. (2003). Pre-breeding energy requirements: thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. J. Avian Biol. 34, 298–306. doi: 10.1034/j.1600-048X.2003.02528.x
Clemens, D. T. (1988). Ventilation and oxygen consumption in rosy finches and house finches at sea level and high altitude. J. Comp. Physiol. B 158, 57–66. doi: 10.1007/BF00692729
Cooper, C. E., Withers, P. C., Hurley, L. L., and Griffith, S. C. (2019). The field metabolic rate, water turnover, and feeding and drinking behavior of a small Avian Desert Granivore during a summer heatwave. Front. Physiol. 10:1405. doi: 10.3389/fphys.2019.01405
Daan, S., Masman, D., and Groenewold, A. (1990). Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 259, R333–R340. doi: 10.1152/ajpregu.1990.259.2.R333
Di Castri, F., and Hajek, E. (1976). Bioclimatología de Chile. (Santiago, Chile: Universidad Católica de Chile.
Epstein, S., and Zeiri, L. (1988). Oxygen and carbon isotopic compositions of gases respired by humans. Proc. Natl. Acad. Sci. 85, 1727–1731. doi: 10.1073/pnas.85.6.1727
Fariña, J. M., Palma, A. T., and Ojeda, F. P. (2008). “Subtidal kelp associated communities off the temperate Chilean coast” in Food Webs Trophic Dynamics of Marine Benthic Ecosystems, eds. G. M. Branch and T. R. McClanahan (New York, USA: Oxford University Press),79–102.
Gerson, A. R., and Guglielmo, C. G. (2011). House sparrows (Passer domesticus) increase protein catabolism in response to water restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R925–R930. doi: 10.1152/ajpregu.00701.2010
Gerson, A. R., McKechnie, A. E., Smit, B., Whitfield, M. C., Smith, E. K., and Talbot, W. A. (2019). The functional significance of facultative hyperthermia varies with body size and phylogeny in birds. Funct. Ecol. 33, 597–607. doi: 10.1111/1365-2435.13274
Giulivi, C., and Ramsey, J. (2015). On fuel choice and water balance during migratory bird flights. Int. Biol. Rev. 2015:58. doi: 10.18103/ibr.v0i1.58
Goldstein, D. L., and Skadhauge, E. (2000). “Renal and Extrarenal regulation of body fluid composition” in Sturkie’s Avian Physiology ed. G. C. Whittow (Amsterdam: Elsevier), 265–297.
Gutiérrez, J. S., Masero, J. A., Abad-Gómez, J. M., Villegas, A., and Sánchez-Guzmán, J. M. (2011). Understanding the energetic costs of living in saline environments: effects of salinity on basal metabolic rate, body mass and daily energy consumption of a long-distance migratory shorebird. J. Exp. Biol. 214, 829–835. doi: 10.1242/jeb.048223
Huey, R. B., and Buckley, L. B. (2022). Designing a seasonal acclimation study presents challenges and opportunities. Integr. Organ. Biol. 4:obac016. doi: 10.1093/iob/obac016
Khaliq, I., Hof, C., Prinzinger, R., Böhning-Gaese, K., and Pfenninger, M. (2014). Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. Royal Soc. B: Biol. Sci. 281:20141097. doi: 10.1098/rspb.2014.1097
Kohn, M. J. (1996). Predicting animal δ18O: accounting for diet and physiological adaptation. Geochim. Cosmochim. Acta 60, 4811–4829. doi: 10.1016/S0016-7037(96)00240-2
Landes, J., Pavard, S., Henry, P. Y., and Terrien, J. (2020). Flexibility is costly: hidden physiological damage from seasonal phenotypic transitions in heterothermic species. Front. Phys. 11:985. doi: 10.3389/fphys.2020.00985
Lighton, J. R. B. (2008). Measuring Metabolic Rates: A Manual for Scientists. New York, NY: Oxford University Press.
MacMillen, R. E. (1990). Water economy of Granivorous birds: a predictive model. Condor 92:379. doi: 10.2307/1368235
Maddocks, T. A., and Geiser, F. (2000). Seasonal variations in thermal energetics of Australian silvereyes (Zosterops lateralis). J. Zool. 252, 327–333. doi: 10.1111/j.1469-7998.2000.tb00627.x
Mahoney, S. A., and Jehl, J. R. (1985). Adaptations of migratory shorebirds to highly saline and alkaline lakes: Wilson’s phalarope and American avocet. Condor 87, 520–527. doi: 10.2307/1367950
Mainwaring, M. C., and Hartley, I. R. (2013). The energetic costs of nest building in birds. Avian Biol. Res. 6, 12–17. doi: 10.3184/175815512X13528994072997
Martínez del Rio, C., Sabat, P., Anderson-Sprecher, R., and Gonzalez, S. P. (2009). Dietary and isotopic specialization: the isotopic niche of three Cinclodes ovenbirds. Oecologia 161, 149–159. doi: 10.1007/s00442-009-1357-2
McKechnie, A. E., Noakes, M. J., and Smit, B. (2015). Global patterns of seasonal acclimatization in avian resting metabolic rates. J. Ornithol. 156, 367–376. doi: 10.1007/s10336-015-1186-5
McKechnie, A. E., Whitfield, M. C., Smit, B., Gerson, A. R., Smith, E. K., Talbot, W. A., et al. (2016). Avian thermoregulation in the heat: efficient evaporative cooling allows for extreme heat tolerance in four southern hemisphere columbids. J. Exp. Biol. 219, 2145–2155. doi: 10.1242/jeb.138776
McNab, B. K. (2009). Ecological factors affect the level and scaling of avian BMR. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 152, 22–45. doi: 10.1016/j.cbpa.2008.08.021
McWhorter, T. J., Gerson, A. R., Talbot, W. A., Smith, E. K., McKechnie, A. E., and Wolf, B. O. (2018). Avian thermoregulation in the heat: evaporative cooling capacity and thermal tolerance in two Australian parrots. J. Exp. Biol. 221:jeb168930. doi: 10.1242/jeb.168930
Muff, S., Nilsen, E. B., O’Hara, R. B., and Nater, C. R. (2021). Rewriting results sections in the language of evidence. Trends Ecol. Evol. 37, 203–210. doi: 10.1016/j.tree.2021.10.009
Navarrete, L., Bozinovic, F., Peña-Villalobos, I., Contreras-Ramos, C., Sanchez-Hernandez, J. C., Newsome, S. D., et al. (2021). Integrative physiological responses to acute dehydration in the rufous-collared sparrow: metabolic, enzymatic, and oxidative traits. Front. Ecol. Evol. 9:767280. doi: 10.3389/fevo.2021.767280
Navarro, R. A., Meijer, H. A. J., Underhill, L. G., and Mullers, R. H. E. (2018). Extreme water efficiency of cape gannet Morus capensis chicks as an adaptation to water scarcity and heat stress in the breeding colony. Mar. Freshw. Behav. Physiol. 51, 30–43. doi: 10.1080/10236244.2018.1442176
Newsome, S. D., Martínez del Rio, C., Bearhop, S., and Phillips, D. L. (2007). A niche for isotopic ecology. Front. Ecol. Environ. 5, 429–436. doi: 10.1890/1540-9295(2007)5[429:ANFIE]2.0.CO;2
Newsome, S. D., Sabat, P., Wolf, N., Rader, J. A., Del Rio, C. M., and Peters, D. P. C. (2015). Multi-tissue δ2H analysis reveals altitudinal migration and tissue-specific discrimination patterns in Cinclodes. Ecosphre. 6:art213. doi: 10.1890/ES15-00086.1
Nicol, S. C., and Andersen, N. A. (2007). Cooling rates and body temperature regulation of hibernating echidnas (Tachyglossus aculeatus). J. Exp. Biol. 210, 586–592. doi: 10.1242/jeb.02701
Nilsson, J. Å. (2002). Metabolic consequences of hard work. Proc. R. Soc. Lond. B Biol. Sci. 269, 1735–1739. doi: 10.1098/rspb.2002.2071
Noakes, M. J., and McKechnie, A. E. (2020). Phenotypic flexibility of metabolic rate and evaporative water loss does not vary across a climatic gradient in an Afrotropical passerine bird. J. Exp. Biol. 223:jeb220137. doi: 10.1242/jeb.220137
Nord, A., and Folkow, L. P. (2019). Ambient temperature effects on stress-induced hyperthermia in Svalbard ptarmigan. Biol. Open 8:bio043497. doi: 10.1242/bio.043497
Nord, A., and Nilsson, J. Å. (2019). Heat dissipation rate constrains reproductive investment in a wild bird. Funct. Ecol. 33, 250–259. doi: 10.1111/1365-2435.13243
Novoa, F. F., Bozinovic, F., and Rosenmann, M. (1994). Seasonal changes of thermal conductance in Zonotrichia capensis (Emberizidae), from Central Chile: the role of plumage. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 107, 297–300. doi: 10.1016/0300-9629(94)90384-0
Nyström, K. K., and Pehrsson, O. (1988). Salinity as a constraint affecting food and habitat choice of mussel-feeding diving ducks. Ibis 130, 94–110. doi: 10.1111/j.1474-919X.1988.tb00960.x
Paces, B., Waringer, B. M., Domer, A., Burns, D., Zvik, Y., Wojciechowski, M. S., et al. (2021). Evaporative water loss and stopover behavior in three passerine bird species during autumn migration. Front. Ecol. Evol. 9:704676. doi: 10.3389/fevo.2021.704676
Pack, A., Gehler, A., and Süssenberger, A. (2013). Exploring the usability of isotopically anomalous oxygen in bones and teeth as paleo-CO2-barometer. Geochim. Cosmochim. Acta 102, 306–317. doi: 10.1016/j.gca.2012.10.017
Passey, B. H., and Levin, N. E. (2021). Triple oxygen isotopes in meteoric waters, carbonates, and biological apatites: implications for continental paleoclimate reconstruction. Rev. Mineral. Geochem. 86, 429–462. doi: 10.2138/rmg.2021.86.13
Pearson, S. F., Levey, D. J., Greenberg, C. H., and Martínez del Rio, C. (2003). Effects of elemental composition on the incorporation of dietary nitrogen and carbon isotopic signatures in an omnivorous songbird. Oecol. 135, 516–523. doi: 10.1007/s00442-003-1221-8
Peña-Villalobos, I., Nuñez-Villegas, M., Bozinovic, F., and Sabat, P. (2014). Metabolic enzymes in seasonally acclimatized and cold acclimated rufous-collared sparrow inhabiting a Chilean Mediterranean environment. Curr. Zool. 60, 338–350. doi: 10.1093/czoolo/60.3.338
Peña-Villalobos, I., Valdés-Ferranty, F., and Sabat, P. (2013). Osmoregulatory and metabolic costs of salt excretion in the rufous-collared sparrow Zonotrichia capensis. Comp. Biochem. physiol., Mol. Part A; Integr. Physiol. 164, 314–318. doi: 10.1016/j.cbpa.2012.10.027
Polis, G. A., and Hurd, S. D. (1996). Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 147, 396–423. doi: 10.1086/285858
Rader, J. A., Newsome, S. D., Sabat, P., Chesser, R. T., Dillon, M. E., and Martínez del Rio, C. (2017). Isotopic niches support the resource breadth hypothesis. J. Anim. Ecol. 86, 405–413. doi: 10.1111/1365-2656.12629
Rezende, E. L., and Bacigalupe, L. D. (2015). Thermoregulation in endotherms: physiological principles and ecological consequences. J. Comp. Physiol. B 185, 709–727. doi: 10.1007/s00360-015-0909-5
Rutkowska, J., Sadowska, E. T., Cichon, M., and Bauchinger, U. (2016). Increased fat catabolism sustains water balance during fasting in zebra finches. J. Exp. Biol. 219, 2623–2628. doi: 10.1242/jeb.138966
Sabat, P. (2000). Birds in marine and saline environments: living in dry habitats. Rev. Chil. Hist. Nat. 73, 401–410. doi: 10.4067/s0716-078x2000000300004
Sabat, P., Cavieres, G., Veloso, C., and Canals, M. (2006b). Water and energy economy of an omnivorous bird: population differences in the rufous-collared sparrow (Zonotrichia capensis). Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 144, 485–490. doi: 10.1016/j.cbpa.2006.04.016
Sabat, P., and del Río, C. M. (2005). Seasonal changes in the use of marine food resources by Cinclodes nigrofumosus (Furnariidae, Aves): Carbon isotopes and osmoregulatory physiology. Rev. Chil. Hist. Nat. 78, 253–260. doi: 10.4067/S0716-078X2005000200009
Sabat, P., Gonzalez-Vejares, S., and Maldonado, K. (2009). Diet and habitat aridity affect osmoregulatory physiology: an intraspecific field study along environmental gradients in the rufous-collared sparrow. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 152, 322–326. doi: 10.1016/j.cbpa.2008.11.003
Sabat, P., Maldonado, K., Canals, M., and Del Rio, C. M. (2006a). Osmoregulation and adaptive radiation in the ovenbird genus Cinclodes (Passeriformes: Furnariidae). Funct. Ecol. 20, 799–805. doi: 10.1111/j.1365-2435.2006.01176.x
Sabat, P., Maldonado, K., Rivera-Hutinel, A., and Farfan, G. (2004). Coping with salt without salt glands: osmoregulatory plasticity in three species of coastal songbirds (ovenbirds) of the genus Cinclodes (Passeriformes: Furnariidae). J. Comp. Physiol. B 174, 415–420. doi: 10.1007/s00360-004-0428-2
Sabat, P., Narváez, C., Peña-Villalobos, I., Contreras, C., Maldonado, K., Sanchez-Hernandez, J. C., et al. (2017). Coping with saltwater habitats: metabolic and oxidative responses to salt intake in the rufous-collared sparrow. Front. Physiol. 8, 1–11. doi: 10.3389/fphys.2017.00654
Sabat, P., Newsome, S. D., Pinochet, S., Nespolo, R., Sanchez-Hernandez, J. C., Maldonado, K., et al. (2021). Triple oxygen isotope measurements (Δ'17O) of body water reflect water intake, metabolism, and δ18O of ingested water in passerines. Front. Physiol. 12:710026. doi: 10.3389/fphys.2021.710026
Schmidt-Nielsen, K. (1997). Animal Physiology: Adaptation and Environment. New York, NY: Cambridge University Press.
Şekercioğlu, Ç. H., Primack, R. B., and Wormworth, J. (2012). The effects of climate change on tropical birds. Biol. Conserv. 148, 1–18. doi: 10.1016/j.biocon.2011.10.019
Sharp, Z. D., Wostbrock, J. A. G., and Pack, A. (2018). Mass-dependent triple oxygen isotope variations in terrestrial materials. Geochem. Perspect. Lett 7, 27–31. doi: 10.7185/geochemlet.1815
Shoemaker, V. (1972). “Osmoregulation and excretion in birds” in Avian Biology. eds. D. S. Farner, J. King, and K. Parkes (New York: Academic Press), 527–574.
Smit, B., and McKechnie, A. E. (2010). Avian seasonal metabolic variation in a subtropical desert: basal metabolic rates are lower in winter than in summer. Funct. Ecol. 24, 330–339. doi: 10.1111/j.1365-2435.2009.01646.x
Smit, B., Woodborne, S., Wolf, B. O., and McKechnie, A. E. (2019). Differences in the use of surface water resources by desert birds are revealed using isotopic tracers. Auk 136:uky005. doi: 10.1093/auk/uky005
Smith, E. K., O'Neill, J. J., Gerson, A. R., McKechnie, A. E., and Wolf, B. O. (2017). Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. J. Exp. Biol. 220, 3290–3300. doi: 10.1242/jeb.161141
Song, S., and Beissinger, S. R. (2020). Environmental and ecological correlates of avian field metabolic rate and water flux. Funct. Ecol. 34, 811–821. doi: 10.1111/1365-2435.13526
Speakman, J. R., and Król, E. (2010). Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746. doi: 10.1111/j.1365-2656.2010.01689.x
Swanson, D. L., Thomas, N. E., Liknes, E. T., and Cooper, S. J. (2012). Intraspecific correlations of basal and maximal metabolic rates in birds and the aerobic capacity model for the evolution of endothermy. PLoS ONE. 7: e34271. doi: 10.1371/journal.pone.0034271
Swanson, D. L., Agin, T. J., Zhang, Y., Oboikovitz, P., and DuBay, S. (2020). Metabolic flexibility in response to within-season temperature variability in house sparrows. Integr. Org. Biol. 2:obaa039. doi: 10.1093/iob/obaa039
Swanson, D. L., Zhang, Y., and Jimenez, A. G. (2022). Skeletal muscle and metabolic flexibility in response to changing energy demands in wild birds. Front. Physiol. 13:13. doi: 10.3389/fphys.2022.961392
Tapia-Monsalve, R., Seth, M., Juan, D. N., Hernandez, C. S., Bozinovic, F., Nespolo, R., et al. (2018). Terrestrial birds in coastal environments: metabolic rate and oxidative status varies with the use of marine resources. Oecol. 188, 65–73. doi: 10.1007/s00442-018-4181-8
The jamovi project (2022). jamovi. (Version 2.3) [Computer Software]. Available at: https://www.jamovi.org. (Accessed January 10, 2023).
Vezina, F., and Salvante, K. G. (2010). Behavioral and physiological flexibility are used by birds to manage energy and support investment in the early stages of reproduction. Curr. Zool. 56, 767–792. doi: 10.1093/czoolo/56.6.767
Vézina, F., and Williams, T. D. (2003). Plasticity in body composition in breeding birds: what drives the metabolic costs of egg production? Physiol. Biochem. Zool. 76, 716–730. doi: 10.1086/376425
Welcker, J., Speakman, J. R., Elliott, K. H., Hatch, S. A., and Kitaysky, A. S. (2015). Resting and daily energy expenditures during reproduction are adjusted in opposite directions in free-living birds. Funct. Ecol. 29, 250–258. doi: 10.1111/1365-2435.12321
Whiteman, J. P., Sharp, Z. D., Gerson, A. R., and Newsome, S. D. (2019). Relating Δ17O values of animal body water to exogenous water inputs and metabolism. BioSci. 69, 658–668. doi: 10.1093/biosci/biz055
Wiersma, P., Selman, C., Speakman, J. R., and Verhulst, S. (2004). Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. Lond. B Biol. Sci. 271, S360–S363. doi: 10.1098/rsbl.2004.0171
Williams, T. D. (2018). Physiology, activity and costs of parental care in birds. J. Exp. Biol. 221:jeb169433. doi: 10.1242/jeb.169433
Williams, J. B., and Tieleman, B. I. (2001). “Physiological ecology and behavior of desert birds” in Current Ornithology (Boston, MA: Springer)
Wostbrock, J. A. G., Cano, E. J., and Sharp, Z. D. (2020). An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chem. Geol. 533:119432. doi: 10.1016/j.chemgeo.2019.119432
Keywords: birds, metabolic water, metabolic rates, stable isotopes, Cinclodes
Citation: Navarrete L, Lübcker N, Alvarez F, Nespolo R, Sanchez-Hernandez JC, Maldonado K, Sharp ZD, Whiteman JP, Newsome SD and Sabat P (2023) A multi-isotope approach reveals seasonal variation in the reliance on marine resources, production of metabolic water, and ingestion of seawater by two species of coastal passerine to maintain water balance. Front. Ecol. Evol. 11:1120271. doi: 10.3389/fevo.2023.1120271
Received: 09 December 2022; Accepted: 30 January 2023;
Published: 15 February 2023.
Edited by:
Jose A. Masero, University of Extremadura, SpainReviewed by:
Luis Gerardo Herrera Montalvo, Universidad Nacional Autónoma de México, MexicoCopyright © 2023 Navarrete, Lübcker, Alvarez, Nespolo, Sanchez-Hernandez, Maldonado, Sharp, Whiteman, Newsome and Sabat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pablo Sabat, ✉ psabat@uchile.cl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.