
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 06 February 2023
Sec. Chemical Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1107597
Plant metabolism is an important functional trait, and its metabolites have physiological and ecological functions to adapt to the growth environment. However, the physiological and ecological functions of metabolites from different provinces of the same plant species are still unclear. Therefore, this study aimed to determine whether metabolites from different provinces of Masson pine (Pinus massoniana Lamb.) have the corresponding metabolic traits. The gas chromatography–mass spectrometry technique and metabonomic analysis methods were used to characterize 35 Masson pine half-sib families from two provinces. A total of 116 metabolites were putatively identified in 35 families of Masson pine, among which the average content of organic acids was the highest, followed by saccharides and alcohols, and phosphoric acids. Comparative analysis of metabolite groups showed that organic acids, amines, and others were significantly different between the Masson pine families from Guangxi and Guizhou provinces. Six differential metabolites were found between the provinces from Guizhou and Guangxi, namely caffeic acid, L-ascorbic acid, gentiobiose, xylitol, d-pinitol, and β-sitosterol. The most significantly enriched pathways among differentially expressed metabolites between the two provinces were steroid biosynthesis, phenylpropanoid biosynthesis, glutathione metabolism, pentose and glucuronate interconversions. Overall, the results showed that Masson pine half-sib families from different geographical provinces have different metabolite profiles and their metabolites are affected by geographical provenance and growth environment adaptability. This study revealed that the breeding of Masson pine families from different provinces changed the metabolite profiles, providing a reference for the multipurpose breeding of Masson pine.
Masson pine (Pinus massoniana Lamb.) is a tree species native to China, and it grows throughout its southern, central, and northern subtropical climate zones (Xu et al., 2022). It is widely distributed throughout 17 Chinese provinces and is the most prevalent tree within the country’s coniferous forests. Among all conifers in China, Masson pine is the most widely distributed pine species over the largest area, with the highest comprehensive whole tree utilization ratio (Yang et al., 2020; Ni et al., 2021), and it provides considerable economic benefits. From the 1970s to the 1980s, studies on Masson pine families were conducted in Guizhou, Guangxi and other southern provinces of China. These studies identified a series of high-yield Masson pine families based on wood and oleoresin indicators. Also, extensive phenotypic differences have been found between families from different provinces based on the evaluation considering tree height, diameter at breast height (DBH), volume and other growth indicators (Liu et al., 2020; Zhang et al., 2022). Previous studies have focused more on the metabolism of Masson pine under different stress conditions (Shi et al., 2022; Wang et al., 2022). However, an in-depth analysis of the metabolic differences among Masson pine families of different provinces is lacking.
Metabolites are intermediates and end products of cellular metabolic processes that play an important role in plant growth, evolution and adaptation to climate change (Carreno-Quintero et al., 2013; Deng et al., 2020). Plant functional traits are major drivers mediating the mechanism by which plants adapt to a changing environment (Xu et al., 2018), and plants mainly respond to variable habitat conditions by adjusting their metabolism (Pappas et al., 2016; Li et al., 2021). The primary and secondary metabolites are key components of metabolic pathways. Many studies have investigated the involvement of secondary metabolites in plant stress resistance, and they have been found to have an important role in seed production and development (Desmedt et al., 2020). Primary plant metabolites are involved in basic life functions, such as cell growth, development, and reproduction, through their roles in molecular signal pathways to trigger defense responses (Zaynab et al., 2019). They also exert broad antiviral effects (Liu et al., 2013) and have antioxidant, antiaging, hypoglycemic, immune-enhancing, and anticancer activities (Chen et al., 2014). Although masson pine displays its own stable metabolite profile; few studies have focused on profiling the metabolites among the families from different provinces. In recent years, plant metabonomics has been used to study the yield and quality traits of crop varieties (Zhang et al., 2020; Chen et al., 2022; Yang et al., 2022). The purpose of this study is to develop effective technology and methods to identify resources of Masson pine families from different provinces, analyze the differential metabolites of Masson pine families from different provinces, and obtain basic data for the conservation of Masson pine families.
Metabolites are the final products of metabolism that reflect changes in plant phenotype and function. However, the metabolic mechanism of Masson pine families from different provinces in the same plantation is still unclear. Thus, another aim of this study was to identify the main components and differential metabolites of Masson pine families from two provinces. The results of this study improved our understanding of metabolites in Masson pine families from different provinces and will facilitate further research on these differential metabolites.
The study area (Figures 1A,B) was a large area of Masson pine artificial pure forest located at an altitude of 820–1,000 m above sea level (a.s.l) within Taijiang County, Guizhou Province, China (26°50′51.85″N, 108°17′32.67″E; Figures 1A,B). The area has a humid subtropical monsoon climate. The annual extreme maximum and minimum temperatures of the Masson pine test area are 37.10°C and −10.30°C, respectively, the annual average temperature is 15.70°C, the frost-free period is 286 days, and the annual precipitation is 1,050–1,400 mm.
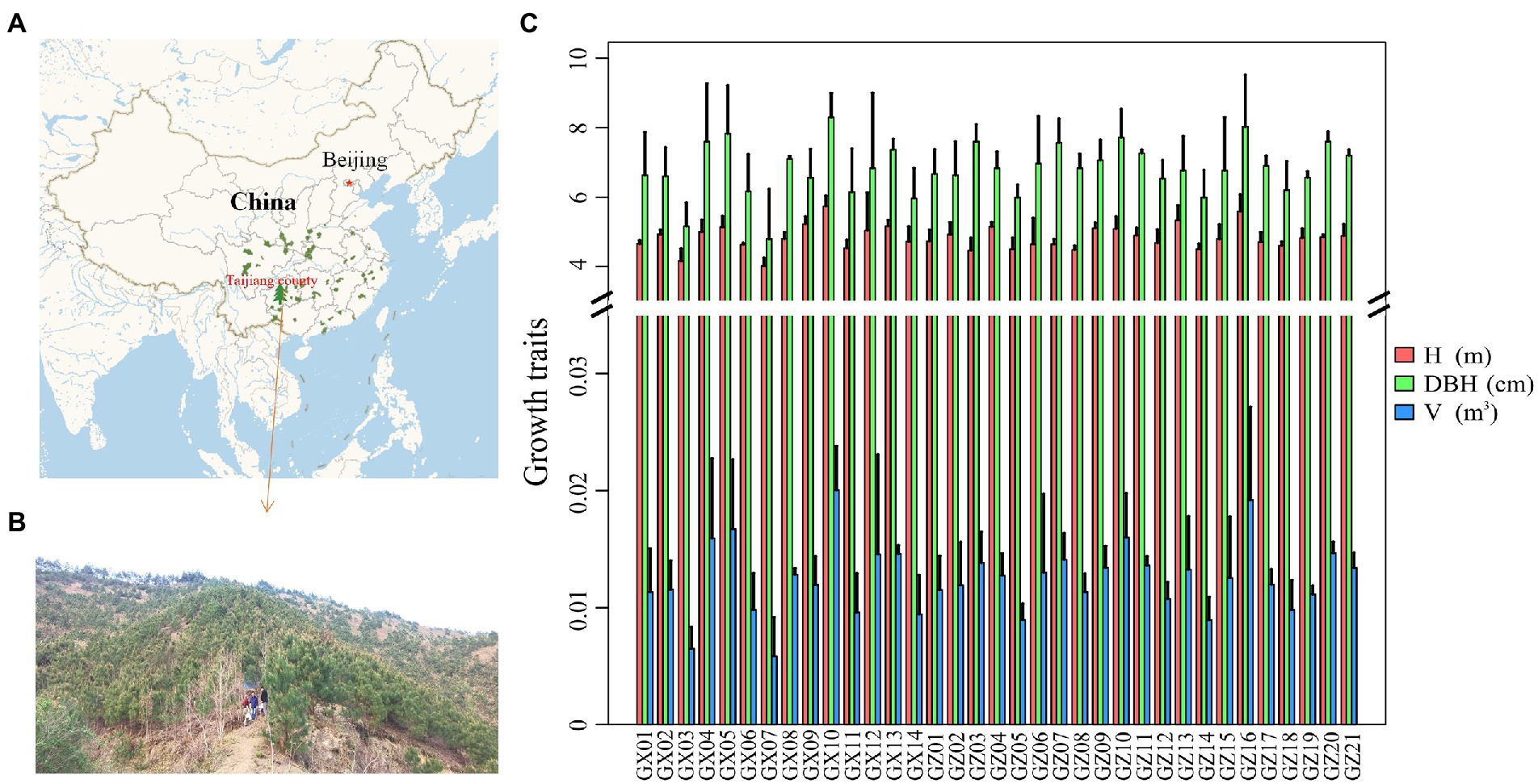
Figure 1. Distribution of Masson pine resources (A), study area and plot setting (B), and growth status (C).
In the spring of 2014, an area within the barren mountains at the experimental site was burned, and a hole-shaped area of land was used for this study. Thirty-five half-sib Masson pine families were planted from left to right using green belt afforestation (Figure 1B). Fourteen of the families were of Guangxi province and named GX01 to GX14, and 20 families were of Guizhou province and were named GZ01 to GZ21. One-year-old seedlings from these 35 families were planted for afforestation. The planting holes measured 40 cm × 30 cm × 30 cm, and the plant row spacing was 2.0 m × 2.5 m. The same management method, weeding by hand and no fertilization, was used for all families.
In the spring of 2021, the needles of the 35 families were collected to analyze the different metabolites. The needles from four directions of the same tree canopy were pooled into one sample, three trees from each family were collected as samples, and the fresh samples were stored in deep freezer at −80°C until further metabolome analysis. The tree height (H, in m) was measured with a measuring rod. The DBH (in cm) was measured with a diameter tape. The tree height and DBH of the selected families were measured in 2021. Tree volume (V, in m3) was calculated based on the formula for the local living stand volume of the Masson pine. The changes in DBH and tree volume were relatively consistent among families, and the tree height of the families of Guizhou province was greater than those of Guangxi province on average. GX10, GZ16, and GZ13 had the largest average tree height; GX10, GZ16, and GX05 had the largest DBH; and GX10, GZ16, and GX05 had the largest average standing volume (Figure 1C).
The test sample, consisting of 50 mg of needles cut into small pieces, was placed in a 10-mL plastic centrifuge tube containing 30 μl of mixed internal standard (adipic acid 10.04 mg/ml, phenyl glucoside 8.04 mg/ml, positive valine 4.9 mg/ml, and methanol and water, 1:1) and 3 ml of extraction solvent mix (methanol:chloroform:water = 2.5:1:1). After homogenizing for 1 min, the sample was extracted, sonicated at 4°C for 20 min, and centrifuged at 3,000–5,000 rpm for 5 min. Afterwards, a 150-μl aliquot of the supernatant extract was added to a 1.5-ml centrifuge tube, dried with nitrogen, and set aside.
Subsequently, 40 μl of 25 mg/ml methoxyamine hydrochloride was added to the supernatant extract (the methanol-water phase), sealed with parafilm, vortexed-mixed for 5 min, and incubated in a constant temperature water bath at 40°C for 120 min (briefly vortexing once at 60 min for approximately 30 s). After cooling to 25°C, briefly centrifuging, and adding 200 μl of bis(trimethylsilyl)trifluoroacetamide (trimethylchlorosilane 1%), the tube was sealed with parafilm, vortex-mixed for 30 s, and reacted in a thermostatic water bath at 81°C for 120 min (briefly vortexing at 60 min for 10 s). Subsequently, after cooling the reaction mixture to room temperature, vortexing for 2 min, and centrifuging at 12,000 rpm for 5 min, the supernatant was then injected into a linear tube. The methanol–water phase was derivatized and analyzed by GC–MS.
GC-MS analysis was performed on an Agilent 7890A GC system coupled with an Agilent 5975C mass spectrometer detector, equipped with an HP-5 MS (60 m × 250 μm × 0.25 μm) capillary column (Agilent Technologies Inc., Santa Clara, CA, United States), using the following conditions: inlet temperature of 280°C, injection volume of 2 μl, split ratio of 20:1, and column flow rate of 1.0 ml/min. The heating program was run for a total of 72 min, and the following temperatures and times were used: 60°C for 2 min, ramping at 5°C/min to 230°C for 5 min, and 8°C/min to 290°C for 23.5 min.
The MS conditions were as follows: ion source temperature of 230°C, quadrupole temperature of 150°C, ionization energy of 70 eV, transmission line temperature of 280°C, full scan mass range of 45–600 aum, solvent delay of 11.90 min, and the acquisition mode was full-scan acquisition mode.
Metabolites were identified using the GC–MS spectral libraries (NIST08 and Willy08 libraries). The chemicals used were qualitative and quantitative with ions, the information obtained was organized, and the specific metabolites of the different Masson pine families were characterized. The metabolites were classified using standard methods, and referencing the associated literature (Zhang et al., 2020). The statistical analyses were performed using the R (version 4.0.0) software (Kanehisa and Goto, 2000; Chong and Xia, 2018).
The STAMP (version 2.1.3) software (Parks et al., 2014) with Welch’s t-test was used to determine the difference in metabolite profiles between the Masson pine families from the two geographical provenances at α = 0.05, and log10 (x + 1) was used to convert the analysis data before analysis. This analysis was performed using the R (version 4.0.0) software (R Core Team, 2019), in particular, the “tidyverse” and “ggplot2” (Wickham, 2016) packages were used for the visualization of the results.
Principal component analysis (PCA): Unsupervised PCA was performed using the statistics function prcomp within the R (version 4.0.0.) software. The data were scaled to unit variance before unsupervised PCA (Ren et al., 2022).
Identification of differential metabolites: significantly differentially regulated metabolites between the Masson pine families from two provenances were determined by variable importance in project (VIP) ≥ 1 and absolute log2FC (fold change) ≥ 1. The VIP values were extracted from the orthogonal partial least squares discriminant analysis (OPLS-DA) results, and were determined using the R package MetaboAnalystR: OPLSR. Anal in the R software. To avoid overfitting, a permutation test (200 permutations) was performed. The metabolic group data were analyzed according to the OPLS-DA model, the score chart of each group was drawn, and the differences between each group were further determined (Thévenot et al., 2015). The prediction parameters of the evaluation model included R2X, R2Y, and Q2, where R2X and R2Y represent the interpretation rate of the model to the X and Y matrices, respectively, and Q2 represents the predictive ability of the model. The closer these three indicators are to 1, the more stable and reliable the model is. When Q2 > 0.5, it can be considered as an effective model, and when Q2 > 0.9, it is an excellent model.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation and pathway enrichment analysis: the identified differential metabolites were annotated using the KEGG Compound database,1 and the annotated metabolites were then mapped to the KEGG Pathway database.2 Pathways with significantly differentially regulated metabolites were mapped and then input into the Metabolite Set Enrichment Analysis (MSEA) and enrichment was assessed using the MSEA Metabolic Pathway library.
In total, 116 metabolites were identified and grouped into 10 groups, and the main chemical groups were saccharides and alcohols, amino acids and derivatives, organic acids, amines, lipids, phosphoric acids, phenolic acids, nucleotides and derivatives, sterols, etc. There were 43 saccharides and alcohols, 23 amino acids and derivatives, and 22 organic acids (Figure 2A). These metabolites are predominantly the products or intermediates of Masson pine photosynthesis, which is the main physiological function of needles. The average content of organic acid was the highest (102,649 μg/g), followed by that of saccharides and alcohols (92,242 μg/g) and phosphoric acids (2,945 μg/g), while the content of other groups was lower than 2,000 μg/g (Figure 2B).
The contents of organic acids, amines, and others groups were significantly different between the Masson pine from Guangxi and Guizhou provinces (p < 0.05), and other groups showed no significant difference between Masson pine from the two provinces (p > 0.05). The content of organic acids of Masson pine from Guangxi province is significantly higher than that of Masson pine from Guizhou province (p < 0.05), but the content of other groups is lower than that of Masson pine from Guizhou province, among which the content of amines and others are significantly lower than that of Masson pine from Guizhou province (Figure 3).
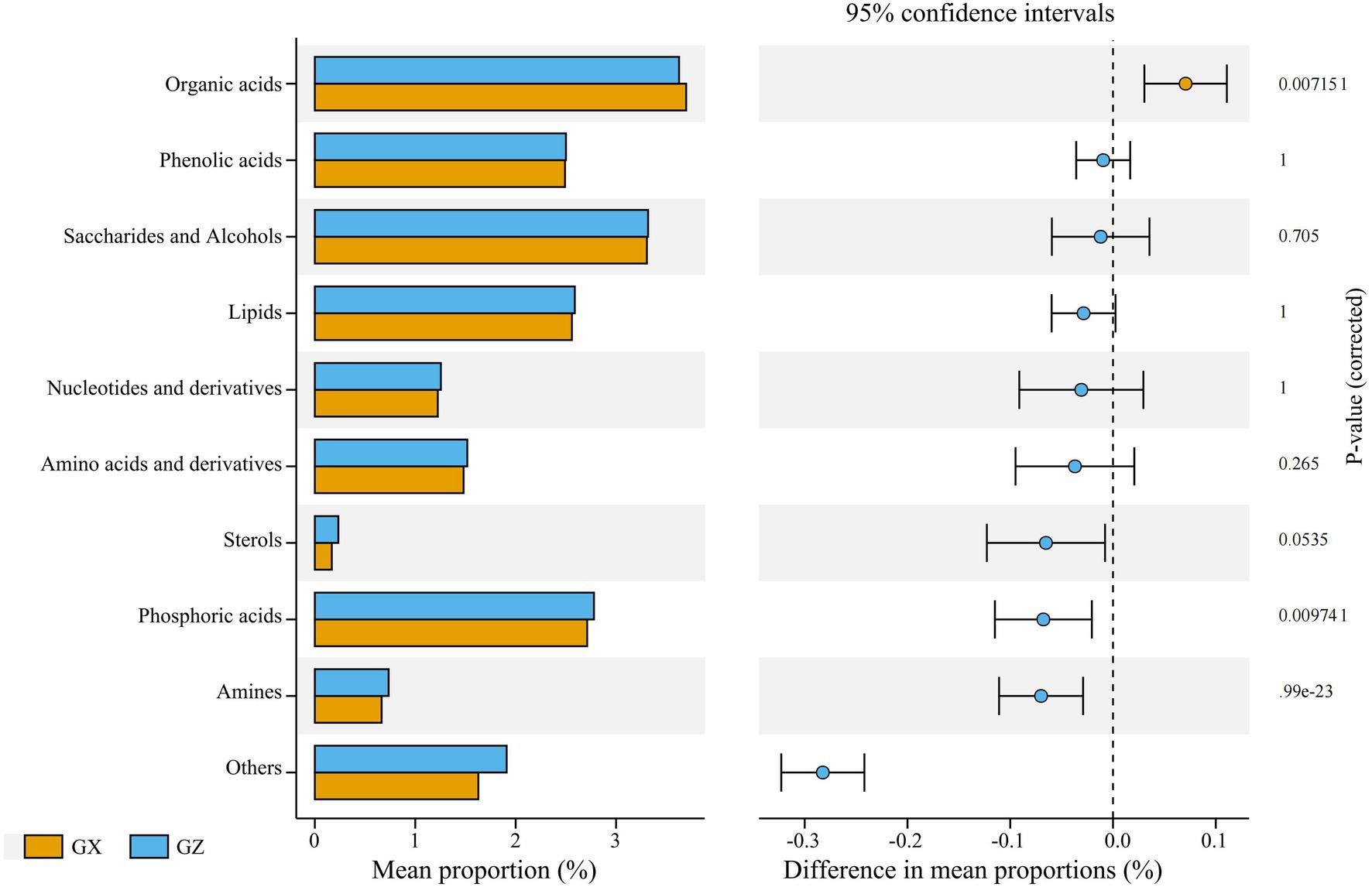
Figure 3. Extended error bar plot indicating the Masson pine families from the two provinces. Welch’s t-test at α = 0.05. GX represents the Masson pine families from the Guangxi province; GZ represents the Masson pine families from the Guizhou provinces.
PCA can provide a preliminary understanding of the overall metabolic differences between Masson pine families from various provenances and the variability between families within each province. To better determine the differences between the two provinces, we used both PCA and OPLS-DA methods. The results showed that there was an obvious separation between the Masson pine families from Guangxi and Guizhou provinces (Figure 4A), and three principal components explained 34.79% of the sample information in total (Figure 4B).
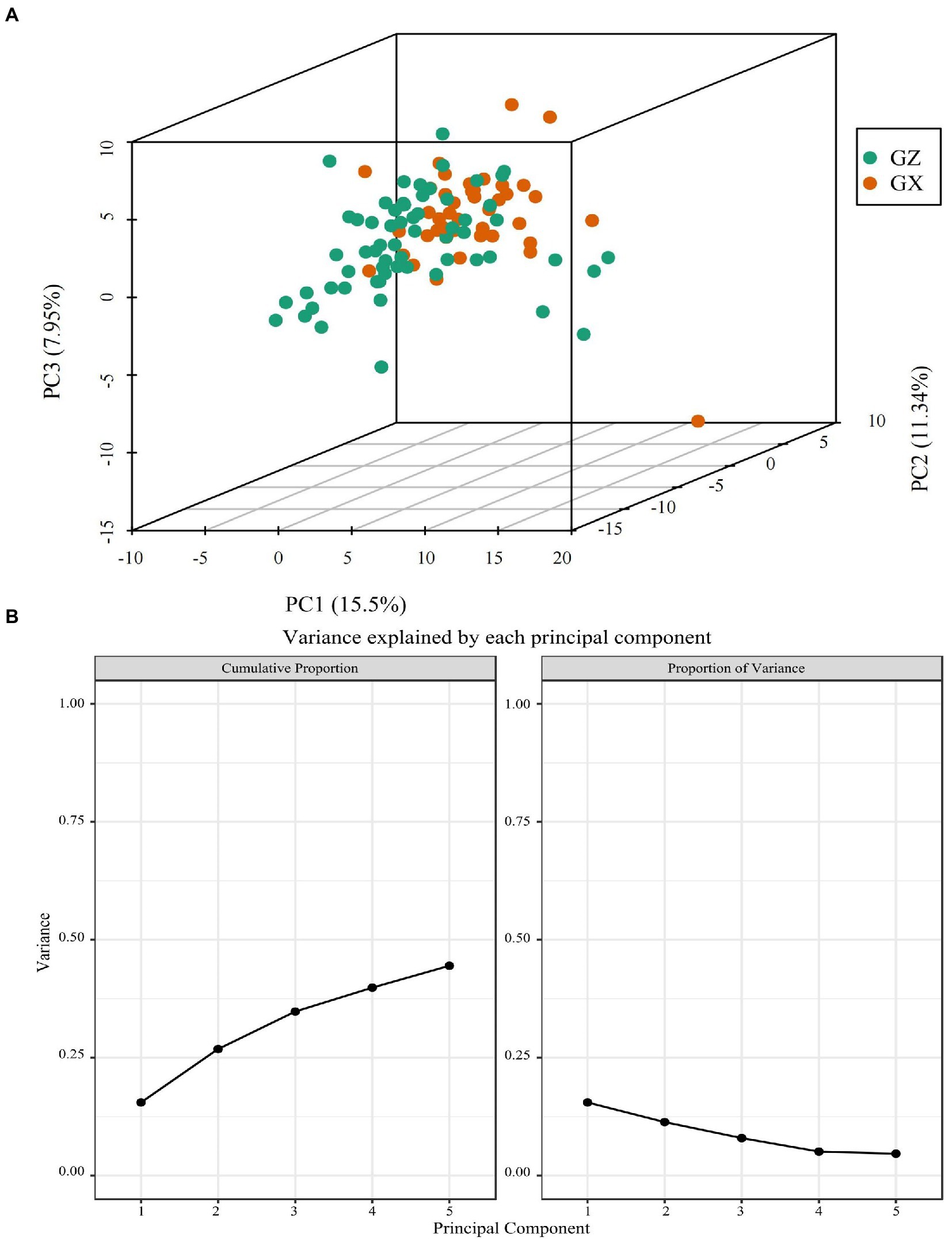
Figure 4. The three-dimensional (3D) PCA (A) and variation explained by each principal component (B).
OPLS-DA can discriminate the differences between the two groups and improve the effectiveness and analysis ability of the model. Therefore, we used R2X, R2Y, and Q2 of the OPLS-DA model to verify its reliability. For the OPLS-DA model obtained from the data of the Masson pine families from the two provinces, these three parameters were 0.267, 0.878 and 0.75, respectively (Figure 5A). In addition, we used random permutation and combination experiments to evaluate the accuracy of the OPLS-DA model. The R2 and Q2 values on the left side were less than the initial values, indicating that the model was reliable (Thévenot et al., 2015). The OPLS-DA scores showed a clear separation between the Masson pine families from the two provinces (Figure 5B). The metabolites with VIP ≥ 1 were determined by OPLS-DA S-plot (Figure 5C). The top 10 upregulated and downregulated metabolites of GZ vs. GX are shown in Figure 5D.
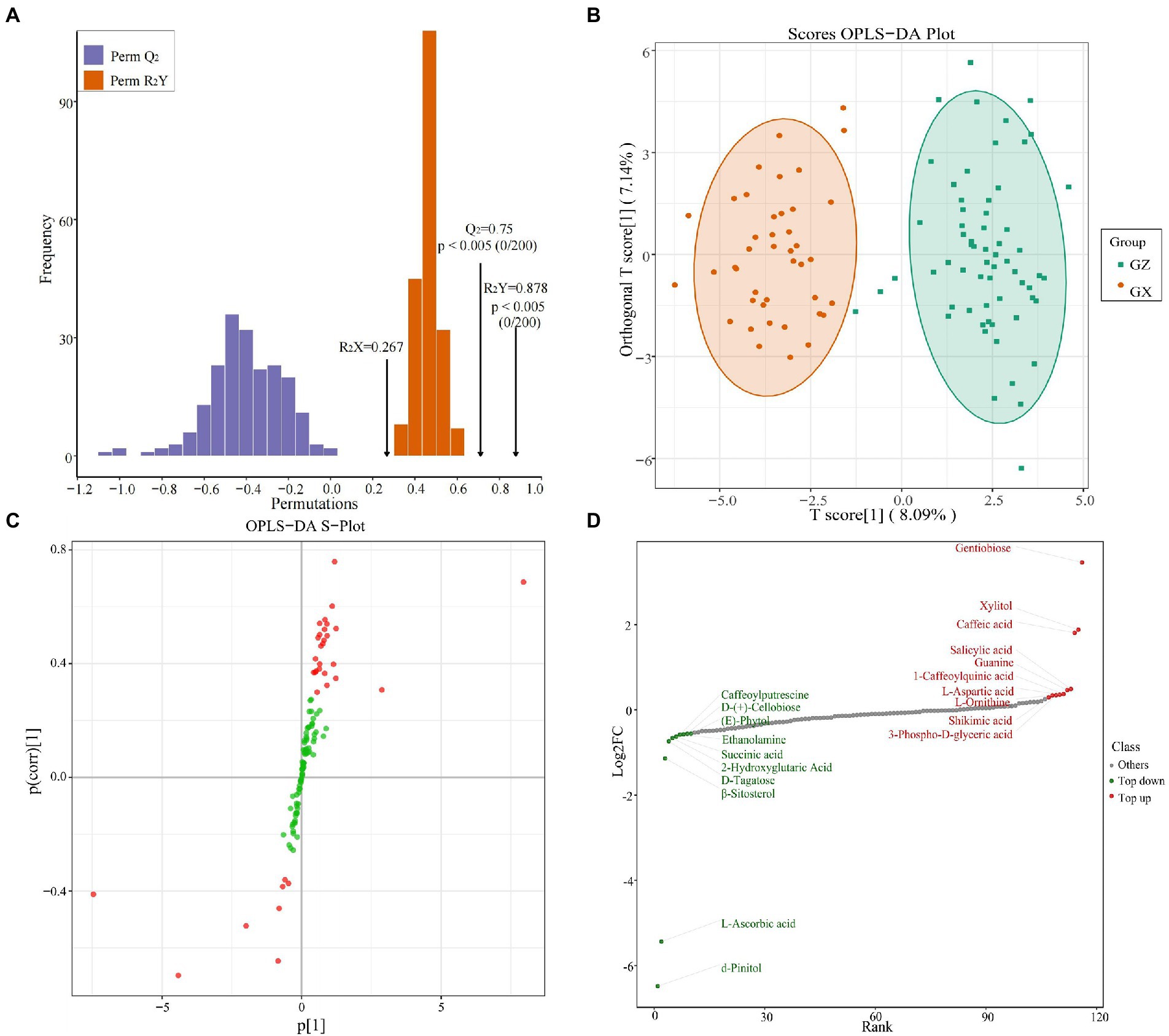
Figure 5. Validation of OPLS-DA model (A), scores OPLS-DA (B), s-plot of OPLS-DA (C), and top 10 upregulated and downregulated metabolites of GZ vs. GX provinces (D).
With the threshold value of VIP > 1.0 and absolute log2FC ≥ 1, we identified six differential metabolites between Masson pine families from Guizhou and Guangxi provinces, including three upregulated and three downregulated metabolites (Figure 6A). According to the VIP value, the differential metabolites were caffeic acid, L-ascorbic acid, gentiobiose, xylitol, d-pinitol, and β-sitosterol (Figure 6B). These differential metabolites play an important role in distinguishing the provenance of Masson pine families.
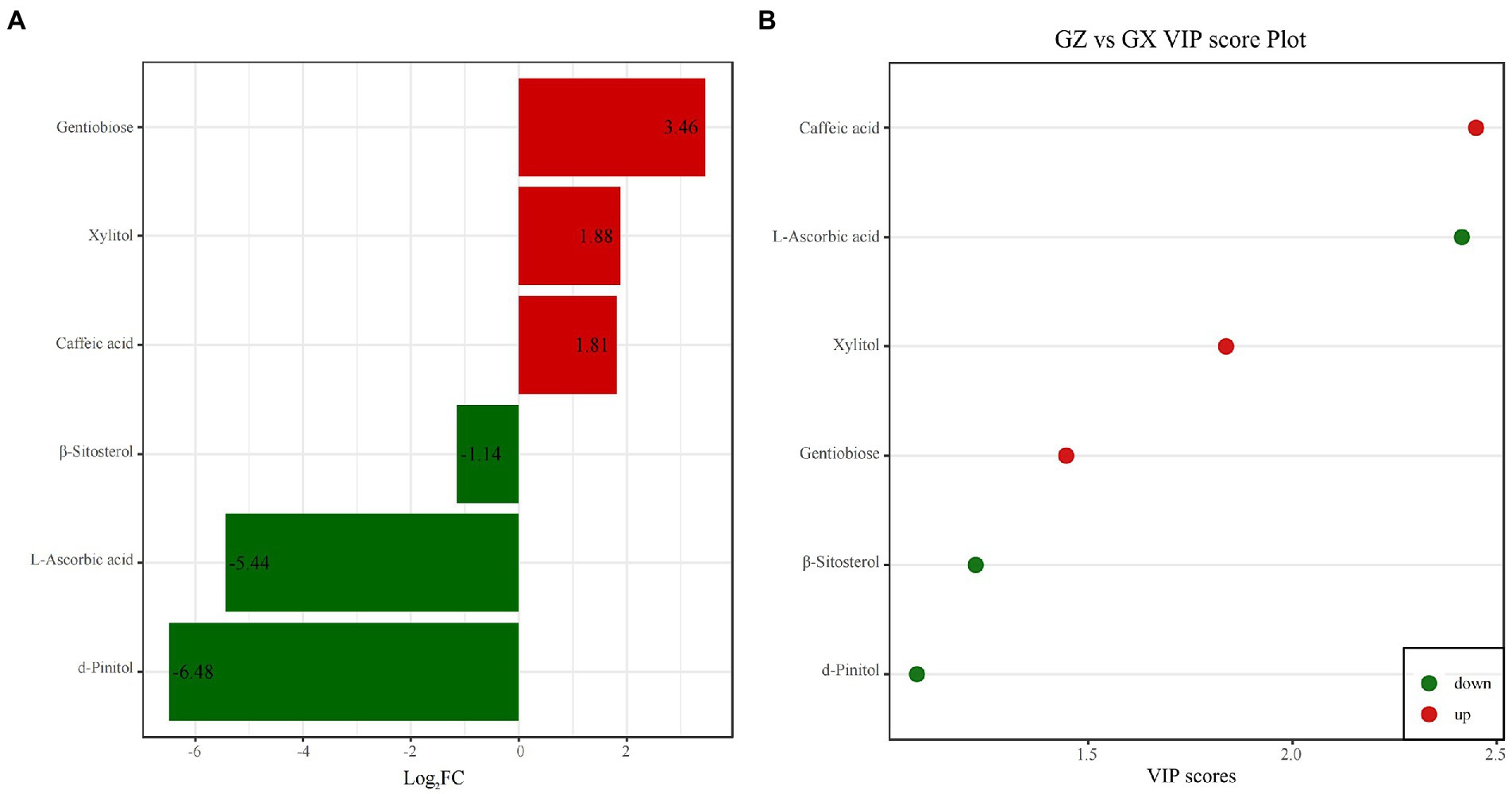
Figure 6. Difference multiple histogram (A) and VIP score plot (B) of different metabolites of Masson pine families from two provinces.
To examine the relationship between different metabolites and metabolic pathways among Masson pine families from different provenances, we searched the Human Metabolome Database (HMDB) or KEGG database for four significantly differentially regulated metabolites, namely caffeic acid, L-ascorbic acid, xylitol, and β-sitosterol. We used the KEGG database to annotate and display the differentially regulated metabolites (Kanehisa and Goto, 2000), and metabolism and environmental information processing were the two main metabolic pathways enriched in the differentially regulated metabolites. Among them, metabolism includes metabolic pathways, biosynthesis of secondary metabolites, steroid biosynthesis, phenylpropanoid biosynthesis, pentose and glucuronate interconversions, glutathione metabolism, biosynthesis of cofactors, ascorbate and aldarate metabolism, while environmental information processing includes ABC transporters (Figure 7A). These metabolites enriched in these pathways may be the most important metabolites determining potential provenance differences. In addition, the KEGG pathways enrichment analysis of the differentially regulated metabolites showed that the pathways most significantly enriched in differentially regulated metabolites between the Masson pine families from the two provenances were steroid biosynthesis, phenylpropanoid biosynthesis, glutathione metabolism, pentose and glucuronate interconversions (Figure 7B).

Figure 7. KEGG classification (A) and pathway enrichment analysis (B) of deferentially regulated metabolites.
The study of plant metabolic components in different Masson pine tree families is of great significance for the identification of different Masson pine families from various provinces. Genetic information, tree height, volume, branch growth, nitrogen and phosphorus nutrient efficiency differ considerably among the various Masson pine families (Hu et al., 2016; Ye et al., 2020; Ni et al., 2021; Shi et al., 2021). However, little attention has been paid to the metabolic differences of different Masson pine families from various provinces.
All Masson pine families have the same characteristics metabolite profile, and the main chemical groups were saccharides and alcohols, amino acids and derivatives, organic acids, amines, lipids, phosphoric acids, phenolic acids, nucleotides and derivatives, sterols, and others. Remarkably, the results of this study showed that the Masson pine families from two provinces showed different metabolite characteristics. The content of organic acids of Masson pine families from the Guangxi province was higher than that of Masson pine families from the Guizhou province, but the other groups were lower than those of Masson pine families from the Guizhou province. The Masson pine families from the Guizhou and Guangxi provinces could be clearly separated by PCA based on differentially regulated metabolites. These results suggested that some key differentially regulated metabolites were formed during the domestication of Masson pine trees from different provinces, indicating that they played an important role in such process. Therefore, our study once again verified that the profile of metabolites in all pines was determined by the interaction of genetic background and the abiotic and biotic factors, and the content of metabolites in the Masson pine families was affected by the geographical environment.
Plants synthesize hundreds of thousands of metabolites, and different species and genera produce different types of primary and secondary metabolites (Allwood et al., 2008). Both primary and secondary metabolites play important roles in plant stress resistance (Zaynab et al., 2018; Zhang et al., 2020). In this study, only the methanol–water phase was derivatized and analyzed by GC-MS, as a result mainly primary metabolites were identified in this study. A total of 116 metabolites were identified, which were mainly primary metabolites. Primary metabolites are present in nearly every part of the plant species and are synthesized through the same or nearly the same biochemical pathways (Canarini et al., 2019), and these metabolites are critical for plant growth, development, and reproduction (Abdelrahman et al., 2019). These metabolites are produced during the growth phase and function as signaling molecules that trigger defense responses through signal transduction and pathogen-recognition pathways (Kachroo and Robin, 2013), and their induction patterns are similar to those of secondary metabolites (Devoto et al., 2005). For example, proline plays an important role in plant defense mechanisms and stress responses, and biotic and abiotic stresses usually lead to proline accumulation in plants, which serves as a potential indicator of plant stress resistance (Maroli et al., 2016). The needles of Masson pine trees are useful for metabolite research as they are a storage organ and contain metabolites with functional traits. Masson pine terpenoids contribute to drought resistance (Quan and Ding, 2017), and they defend the plant against herbivores (Chen et al., 2019). We identified many kinds of amino acids and organic acids in Masson pine families, and these components are associated with plant growth traits and stress resistance.
This study showed that the content of many metabolites was significantly different between Masson pine families from two provinces, indicating that they participated in the process of domestication and breeding of Masson pine families and may be involved in some metabolic regulation. We found that six deferentially regulated metabolites contributed the most to the metabolic differences of Masson pine families of GZ vs. GX provinces, and these metabolites are the key metabolites for the ecological adaptation of plants (Fernández de Simón et al., 2017; Mitiouchkina et al., 2020; Sánchez-Hidalgo et al., 2021; He et al., 2022). In the genus Pinus, there is great potential for incorporating phytochemical metabolism into conifer breeding programs (Nantongo et al., 2021), and several studies have also directly or indirectly linked primary metabolites to biotic stress responses (Nantongo et al., 2022). Chemical analysis of Pinus has shown that volatile substances in needles differ considerably between species (Ioannou et al., 2014). The results of this study indicate that the d-pinitol in the Masson pine families from Guizhou province is associated with a high accumulation of metabolites during the breeding process, and this provides a direction for the future development of high value-added natural Masson pine products. The inositol ether d-pinitol is a naturally occurring compound found in Pinaceae that has positive effects on human hypoglycemia and cardiovascular protection (Moreira et al., 2018), stimulates insect spawning (Honda et al., 2012), and is associated with hypoglycemic, cardiovascular, antiviral, and larvicidal activities. Xylitol is a naturally occurring alcohol found in most plant materials, including many fruits and vegetables (Cheng et al., 2009), which may be extracted from the hardwood species containing xylan (Ahuja et al., 2020). β-Sitosterol is a sterol found in almost all plants, which as the main component of many plants and vegetables has a variety of biological effects (Liu et al., 2019). β-Sitosterol is also found in some medicinal plants and is widely used in the pharmaceutical industry (Babu and Jayaraman, 2020).
Caffeic acid is found in all plants as it is a key intermediate in the biosynthesis of lignin (Reuter et al., 2020). Previous studies have shown that an increase in caffeic acid content in trees will lead to an increase in lignin content (Bubna et al., 2011; Zhao et al., 2022). Caffeic acid is a common phenolic compound widely distributed in plants, which has broad antibacterial and antiviral activities, as well as a variety of pharmacological properties, such as anti-inflammatory, anti-anxiety, and anti-depressive activities (Deguchi and Ito, 2020; Dukie et al., 2020; Dziedzinski et al., 2020; Grabska-Kobylecka et al., 2020). L-ascorbate acid and β-gentiobiose are commonly used antioxidants, which serve as important physiological indicators of plant resistance to stress and have important significance for plant identification and breeding (Takahashi et al., 2014; Yang et al., 2018; Shah et al., 2022). L-ascorbic acid is an important metabolite that is crucial to plant physiological and biochemical processes, which besides being involved in the synthesis of ethylene, gibberellin, anthocyanin and other molecules involved in various metabolic pathways in plants, it can also improve the anti-stress and antioxidant capacity of crops (Anjum et al., 2014; Noctor et al., 2014; Akram et al., 2017). β-Gentiobiose is a rare disaccharide that accumulates abundantly in plants and is involved in various aspects of plant development, such as fruit ripening and dormancy release of bud and growth dormancy (Dumville and Fry, 2003; Takahashi et al., 2018). In this study, the Masson pine families with high contents of L-ascorbate acid and β-gentiobiose showed stronger stress resistance.
The results of this study show that the composition of the major metabolites of Masson pine families from different provinces is consistent, and there are 116 metabolites from 35 families. The groups of metabolites were mainly organic acids, saccharides and alcohols, and phosphoric acids. The selection and domestication of families resulted in the differences in the metabolism of Masson pine trees from different provinces, and the chemical groups showed that the contents of organic acids, amines and other groups were significantly different between Masson pine families from Guangxi and Guizhou provinces. Six deferentially regulated metabolites were identified between the Masson pine families from the Guizhou and Guangxi provinces. The deferentially regulated metabolites were caffeic acid, L-ascorbic acid, β-gentiobiose, xylitol, d-pinitol, and β-sitosterol. Further analysis showed that the pathways most significantly enriched in deferentially regulated metabolites between Masson pine plants from the two provinces were steroid biosynthesis, phenylpropanoid biosynthesis, glutathione metabolism, pentose and glucuronate interconversions. Our results also showed that Masson pine families from different provinces have different metabolite characteristics, and that their metabolites are affected by the geographical provenance and growth environment adaptability. Moreover, further studies should focus on the analysis of the chloroform phase to determine whether the secondary metabolites content is different among the Masson pine families from the two provinces.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
WQ: methodology, investigation, software, and writing (original draft). XZ: investigation and writing (original draft). CZ: investigation. HD: validation and editing. GD: experiment design, conceptualization, supervision, and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Technology Planning Project of Guizhou Province (QKHPTRC[2018]5261 and QKHPTRC[2019]5102), the National Key Research and Development Program of China (2017YFD0600302), the National Natural Science Foundation of China (31960301), and the Postdoctoral Research Foundation of China (2020M673583XB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1107597/full#supplementary-material
Abdelrahman, M., Hirata, S., Sawada, Y., Hirai, M. Y., Sato, S., Hirakawa, H., et al. (2019). Widely targeted metabolome and transcriptome landscapes of Allium fistulosum–a. cepa chromosome addition lines revealed a flavonoid hot spot on chromosome 5A. Sci. Rep. 9:3541. doi: 10.1038/s41598-019-39856-1
Ahuja, V., Macho, M., Ewe, D., Singh, M., Saha, S., and Saurav, K. (2020). Biological and pharmacological potential of xylitol: a molecular insight of unique metabolism. Foods 9:1592. doi: 10.3390/foods9111592
Akram, N. A., Shafiq, F., and Ashraf, M. (2017). Ascorbic acid–a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 8:613. doi: 10.3389/fpls.2017.00613
Allwood, J. W., Ellis, D. I., and Goodacre, R. (2008). Metabolomic technologies and their application to the study of plants and plant–host interactions. Physiol. Plant. 132, 117–135. doi: 10.1111/j.1399-3054.2007.01001.x
Anjum, N. A., Gill, S., Gill, R., Hasanuzzaman, M., Duarte, A. C., Tuteja, N., et al. (2014). Metal/metalloid stress tolerance in plants: role of ascorbate, its redox couple, and associated enzymes. Protoplasma 251, 1265–1283. doi: 10.1007/s00709-014-0636-x
Babu, S., and Jayaraman, S. (2020). An update on β-sitosterol: a potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 131:110702. doi: 10.1016/j.biopha.2020.110702
Bubna, G. A., Lima, R. B., Zanardo, D. Y., Dos Santos, W. D., Ferrarese Mde, L., and Ferrarese-Filho, O. (2011). Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 168, 1627–1633. doi: 10.1016/j.jplph.2011.03.005
Canarini, A., Kaiser, C., Merchant, A., Richter, A., and Wanek, W. (2019). Corrigendum: root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 10:420. doi: 10.3389/fpls.2019.00420
Carreno-Quintero, N., Bouwmeester, H. J., and Keurentjes, J. J. B. (2013). Genetic analysis of metabolome–phenotype interactions: from model to crop species. Trends Genet. 29, 41–50. doi: 10.1016/j.tig.2012.09.006
Chen, X. Q., Fang, Y. P., Nishinari, K., We, H., Sun, C. C., Li, J. R., et al. (2014). Physicochemical characteristics of polysaccharide conjugates prepared from fresh tea leaves and their improving impaired glucose tolerance. Carbohydr. Polym. 112, 77–84. doi: 10.1016/j.carbpol.2014.05.030
Chen, R. X., He, X. Y., Chen, J., Gu, T. Z., Liu, P. C., Xu, T., et al. (2019). Traumatic resin duct development, terpenoid formation, and related synthase gene expression in Pinus massoniana under feeding pressure of Monochamus alternatus. J. Plant Growth Regul. 38, 897–908. doi: 10.1007/s00344-018-9900-1
Chen, D., Sun, Z., Gao, J. J., Peng, J. K., Wang, Z., Zhao, Y. N., et al. (2022). Metabolomics combined with proteomics provides a novel interpretation of the compound differences among Chinese tea cultivars (Camellia sinensis var. sinensis) with different manufacturing suitabilities. Food Chem. 377:131976. doi: 10.1016/j.foodchem.2021.131976
Cheng, K. K., Zhang, J. A., Ling, H. Z., Ping, W. X., Huang, W., Ge, J. P., et al. (2009). Optimization of pH and acetic acid concentration for bioconversion of hemicellulose from corncobs to xylitol by Candida tropicalis. Biochem. Eng. J. 43, 203–207. doi: 10.1016/j.bej.2008.09.012
Chong, J., and Xia, J. (2018). MetaboAnalystR: an R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 34, 4313–4314. doi: 10.1093/bioinformatics/bty528
Deguchi, Y., and Ito, M. (2020). Caffeic acid and rosmarinic acid contents in genus Perilla. J. Nat. Med. 74, 834–839. doi: 10.1007/s11418-020-01418-5
Deng, M., Zhang, X., Luo, J., Liu, H., Wen, W., Luo, H., et al. (2020). Metabolomic analysis reveals differences in evolution between maize and rice. Plant J. 103, 1710–1722. doi: 10.1111/tpj.14856
Desmedt, W., Mangelinckx, S., Kyndt, T., and Vanholme, B. (2020). A phytochemical perspective on plant defense against nematodes. Front. Plant Sci. 11:602079. doi: 10.3389/fpls.2020.602079
Devoto, A., Ellis, C., Magusin, A., Chang, H. S., Chilcott, C., Zhu, T., et al. (2005). Expression profiling reveals COI1 to be a key regulator of genes involved in woundand methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 58, 497–513. doi: 10.1007/s11103-005-7306-5
Dukie, S., Davey, A. K., Rao, C. M., and Arora, D. (2020). Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J. Funct. Foods 64:103638. doi: 10.1016/j.jff.2019.103638
Dumville, J. C., and Fry, S. C. (2003). Gentiobiose: a novel oligosaccharin in ripening tomato fruit. Planta 217, 346–348. doi: 10.1007/s00425-003-1018-3
Dziedzinski, M., Kobus-Cisowska, J., Szymanowska, D., Stuper-Szablewska, K., and Baranowska, M. (2020). Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules 25:3527. doi: 10.3390/molecules25153527
Fernández de Simón, B., Sanz, M., Cervera, M. T., Pinto, E., Aranda, I., and Cadahía, E. (2017). Leaf metabolic response to water deficit in Pinus pinaster Ait. Relies upon ontogeny and genotype. Environ. Exp. Bot. 140, 41–55. doi: 10.1016/j.envexpbot.2017.05.017
Grabska-Kobylecka, I., Kaczmarek-Bak, J., Figlus, M., Prymont-Przyminska, A., Zwolinska, A., Sarniak, A., et al. (2020). The presence of caffeic acid in cerebrospinal fluid: evidence that dietary polyphenols can cross the blood-brain barrier in humans. Nutrients 12:1531. doi: 10.3390/nu12051531
He, Z., Wang, Y., Yan, Y., Qin, S., He, H., Mao, R., et al. (2022). Dynamic analysis of physiological indices and transcriptome profiling revealing the mechanisms of the allelopathic effects of phenolic acids on Pinellia ternata. Front. Plant Sci. 13:1039507. doi: 10.3389/fpls.2022.1039507
Honda, K., Minematsu, H., Muta, K., Ômura, H., and Nishii, W. (2012). D-Pinitol as a key oviposition stimulant for sulfur butterfly, Colias erate: chemical basis for female acceptance of host-and non-host plants. Chemoecology 22, 55–63. doi: 10.1007/s00049-011-0098-y
Hu, C. Y., Zhao, L., Zhou, Z. C., Dong, G. Q., and Zhang, Y. (2016). Genetic variations and correlation analysis of N and P traits in Pinus massoniana under combined conditions of N deposition and P deficiency. Trees 30, 1341–1350. doi: 10.1007/s00468-016-1370-0
Ioannou, E., Koutsaviti, A., Tzakou, O., and Roussis, V. (2014). The genus Pinus: a comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 13, 741–768. doi: 10.1007/s11101-014-9338-4
Kachroo, A., and Robin, G. P. (2013). Systemic signaling during plant defense. Curr. Opin. Plant Biol. 16, 527–533. doi: 10.1016/j.pbi.2013.06.019
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Li, J. L., Wu, H. Y., Wang, L., Huang, Y. J., and Wang, L. B. (2021). Key taste components in two wild edible boletus mushrooms using widely targeted metabolomics. Biochem. Syst. Ecol. 96:104268. doi: 10.1016/j.bse.2021.104268
Liu, C. M., Chen, H. J., Chen, K., Gao, Y. F., Gao, S., Liu, X. F., et al. (2013). Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Int. J. Biol. Macromol. 52, 21–24. doi: 10.1016/j.ijbiomac.2012.09.020
Liu, R., Hao, D., Xu, W., Li, J., Li, X., Shen, D., et al. (2019). β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 57, 161–168. doi: 10.1080/13880209.2019.1577461
Liu, Q. H., Xie, Y. N., Liu, B., Yin, H. H., Zhou, Z. C., Feng, Z. P., et al. (2020). A transcriptomic variation map provides insights into the genetic basis of Pinus massoniana lamb. Evolution and the association with oleoresin yield. BMC Plant Biol. 20:375. doi: 10.1186/s12870-020-02577-z
Maroli, A. S., Nandula, V. K., Duke, S. O., and Tharayil, N. (2016). Stable isotope resolved metabolomics reveals the role of anabolic and catabolic processes in glyphosate-induced amino acid accumulation in amaranthus palmeri biotypes. J. Agric. Food Chem. 64, 7040–7048. doi: 10.1021/acs.jafc.6b02196
Mitiouchkina, T., Mishin, A. S., Somermeyer, L. G., Markina, N. M., Chepurnyh, T. V., Guglya, E. B., et al. (2020). Plants with genetically encoded autoluminescence. Nat. Biotechnol. 38, 944–946. doi: 10.1038/s41587-020-0500-9
Moreira, L. N., Silva, J. F., Silva, G. C., Lemos, V. S., and Cortes, S. F. (2018). Activation of eNOS by d-pinitol induces an endothelium-dependent vasodilatation in mouse mesenteric artery. Front. Pharmacol. 9:528. doi: 10.3389/fphar.2018.00528
Nantongo, J. S., Potts, B. M., Davies, N. W., Fitzgerald, H., Rodemann, T., and O’Reilly-Wapstra, J. M. (2021). Additive genetic variation in Pinus radiata bark chemistry and the chemical traits associated with variation in mammalian bark stripping. Heredity 127, 498–509. doi: 10.1038/s41437-021-00476-z
Nantongo, J. S., Potts, B. M., Frickey, T., Telfer, E., Dungey, H., Fitzgerald, H., et al. (2022). Analysis of the transcriptome of the needles and bark of Pinus radiata induced by bark stripping and methyl jasmonate. BMC Genomics 23:52. doi: 10.1186/s12864-021-08231-8
Ni, Z. X., Zhou, P. Y., Xin, Y., Xu, M., and Xu, L. A. (2021). Parent-offspring variation transmission in full-sib families revealed predominantly paternal inheritance of chloroplast DNA in Pinus massoniana (Pinaceae). Tree Genet. Genomes 17:36. doi: 10.1007/s11295-021-01519-6
Noctor, G., Mhamdi, A., and Foyer, C. H. (2014). The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 164, 1636–1648. doi: 10.1104/pp.113.233478
Pappas, C., Fatichi, S., and Burlando, P. (2016). Modeling terrestrial carbon and water dynamics across climatic gradients: does plant trait diversity matter? New Phytol. 209, 137–151. doi: 10.1111/nph.13590
Parks, D. H., Tyson, G. W., Hugenholtz, P., and Beiko, R. G. (2014). STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. doi: 10.1093/bioinformatics/btu494
Quan, W. X., and Ding, G. J. (2017). Root tip structure and volatile organic compound responses to drought stress in Masson pine (Pinus massoniana lamb.). Acta Physiol. Plant. 39:258. doi: 10.1007/s11738-017-2558-7
Ren, S., Zha, M. R., Li, Y. J., Song, K., He, J., Liu, S. M., et al. (2022). Comparative metabolomic study of three clones of Eucommia ulmoides wood. J. Wood Chem. Technol. 42, 318–329. doi: 10.1080/02773813.2022.2088794
Reuter, D. N., Stewart, C. N., and Lenaghan, S. C. (2020). Lighting the way: advances in engineering autoluminescent plants. Trends Plant Sci. 25, 1176–1179. doi: 10.1016/j.tplants.2020.08.004
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Core Team. doi: 10.1108/eb003648
Sánchez-Hidalgo, M., León-González, A. J., Gálvez-Peralta, M., González-Mauraza, N. H., and Martin-Cordero, C. (2021). D-Pinitol: a cyclitol with versatile biological and pharmacological activities. Phytochem. Rev. 20, 211–224. doi: 10.1007/s11101-020-09677-6
Shah, A. A., Riaz, L., Siddiqui, M. H., Nazar, R., Ahmed, S., Yasin, N. A. A., et al. (2022). Spermine-mediated polyamine metabolism enhances arsenic-stress tolerance in Phaseolus vulgaris by expression of zinc-finger proteins related genes and modulation of mineral nutrient homeostasis and antioxidative system. Environ. Pollut. 300:118941. doi: 10.1016/j.envpol.2022.118941
Shi, L. L., Chen, J. L., Zhang, Q., and Bai, Q. S. (2021). TMT-based comparative proteomic analysis reveals regulatory pathways and protein targets associated with resin biosynthesis in Pinus massoniana. Ind. Crop. Prod. 172:114077. doi: 10.1016/j.indcrop.2021.114077
Shi, Z., Deng, X. X., Zeng, L. X., Shi, S. Q., Lei, L., and Xiao, W. F. (2022). Acclimation strategy of Masson pine (Pinus massoniana) by limiting flavonoid and terpenoid production under low light and drought. Int. J. Mol. Sci. 23:8441. doi: 10.3390/ijms23158441
Takahashi, H., Imamura, T., Konno, N., Takeda, T., Fujita, K., Konishi, T., et al. (2014). The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous Perennial gentiana. Plant Cell 26, 3949–3963. doi: 10.1105/tpc.114.131631
Takahashi, H., Kikuchi-Fujisaki, S., Yoshida, C., Yamada, H., Yamashita, T., Konno, N., et al. (2018). Gtgen3A, a novel plant GH3 β-glucosidase, modulates gentio-oligosaccharide metabolism in Gentiana. Biochem. J. 475, 1309–1322. doi: 10.1042/BCJ20170866
Thévenot, E. A., Roux, A., Xu, Y., Ezan, E., and Junot, C. (2015). Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Prot. Res. 14, 3322–3335. doi: 10.1021/acs.jproteome.5b00354
Wang, P., Zhou, S. J., Li, A., and Xie, L. B. (2022). Influence of aluminum at low pH on the rhizosphere processes of Masson pine (Pinus massoniana lamb). Plant Growth Regul. 97, 499–510. doi: 10.1007/s10725-022-00816-x
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer. Available at: https://ggplot2. tidyverse.org
Xu, J. S., Chai, Y. F., Wang, M., Dang, H., Guo, Y. X., Chen, Y., et al. (2018). Shifts in plant community assembly processes across growth forms along a habitat severity gradient: a test of the plant functional trait approach. Front. Plant Sci. 9:180. doi: 10.3389/fpls.2018.00180
Xu, Y. W., Ge, X. G., Zhou, B. Z., Lei, L., and Xiao, W. F. (2022). Variations in rhizosphere soil total phosphorus and bioavailable phosphorus with respect to the stand age in Pinus massoniana lamb. Front. Plant Sci. 13:939683. doi: 10.3389/fpls.2022.939683
Yang, M., Chuan, Y. C., Guo, C. W., Liao, J. J., Xu, Y. G., Mei, X. Y., et al. (2018). Panax notoginseng root cell death caused by the autotoxic ginsenoside Rg1 is due to over-accumulation of ROS, as revealed by transcriptomic and cellular approaches. Front. Plant Sci. 9:264. doi: 10.3389/fpls.2018.00264
Yang, C. K., Shen, S. Q., Zhou, S., Li, Y. F., Mao, Y. Y., Zhou, J. J., et al. (2022). Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 15, 258–275. doi: 10.1016/j.molp.2021.10.005
Yang, Z. Q., Xia, H., Tan, J. H., Feng, Y. H., and Huang, Y. L. (2020). Selection of superior families of Pinus massoniana in southern China for large-diameter construction timber. J. For. Res. 31, 475–484. doi: 10.1007/s11676-018-0815-2
Ye, Y. J., Wang, J. W., Ni, Z. X., Meng, X., Feng, Y. H., Yang, Z. Q., et al. (2020). Small RNA and degradome sequencing reveal roles of miRNAs in strobilus development in masson pine (Pinus massoniana). Ind. Crop. Prod. 154:112724. doi: 10.1016/j.indcrop.2020.112724
Zaynab, M., Fatima, M., Abbas, S., Sharif, Y., Umair, M., Zafar, M. H., et al. (2018). Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 124, 198–202. doi: 10.1016/j.micpath.2018.08.034
Zaynab, M., Fatima, M., Sharif, Y., Zafar, M. H., and Khan, K. A. (2019). Role of primary metabolites in plant defense against pathogens. Microb. Pathog. 137:103728. doi: 10.1016/j.micpath.2019.103728
Zhang, Q. H., Pei, X. N., Lu, X. B., Zhao, C. L., Dong, G. L., Shi, W. L., et al. (2022). Variations in growth traits and wood physicochemical properties among Pinus koraiensis families in Northeast China. J. For. Res. 33, 1637–1648. doi: 10.1007/s11676-022-01455-8
Zhang, L. Y., Yu, Y. B., and Yu, R. Z. (2020). Analysis of metabolites and metabolic pathways in three maize (Zea mays L.) varieties from the same origin using GC–MS. Sci. Rep. 10:17990. doi: 10.1038/s41598-020-73041-z
Keywords: Masson pine, growth traits, metabolomics, biochemistry, GC-MS
Citation: Quan W, Zhao X, Zhao C, Duan H and Ding G (2023) Characterization of 35 Masson pine (Pinus massoniana) half-sib families from two provinces based on metabolite properties. Front. Ecol. Evol. 11:1107597. doi: 10.3389/fevo.2023.1107597
Received: 25 November 2022; Accepted: 16 January 2023;
Published: 06 February 2023.
Edited by:
Ernesto Mollo, National Research Council (CNR), ItalyReviewed by:
Panos Petrakis, Hellenic Agricultural Organization – ELGO, GreeceCopyright © 2023 Quan, Zhao, Zhao, Duan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guijie Ding, ✉ Z2pkaW5nQGd6dS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.