
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 02 March 2023
Sec. Ecophysiology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1083045
This article is part of the Research Topic Behavioral and Physiological Adaptations of Mammals and Birds to Anthropogenic Disturbances View all 11 articles
 Aldin Selimovic1*
Aldin Selimovic1* Mathilde L. Tissier2
Mathilde L. Tissier2 Gabrielle Stalder1
Gabrielle Stalder1 Johanna Painer-Gigler1
Johanna Painer-Gigler1 Anna Haw1
Anna Haw1 Hanna Rauch1
Hanna Rauch1 Walter Arnold1
Walter Arnold1Large plots of maize in agricultural landscapes are associated with reduced reproductive output of females and impaired population development of free-living populations of European brown hares (Lepus europaeus, Pallas 1778). We investigated in captive brown hares experimentally whether these effects could result from an undersupply of niacin due to a suspected maize-biased diet in such areas. We repeatedly mated adult female hares, fed ad libitum either with a niacin deficient pellet mainly consisting of maize plant parts, or with the same pellet enriched with niacin to meet physiological requirements. We measured effects of the experimental feeding on body mass of females, reproductive output, growth and survival of leverets. We found significantly higher body mass of females fed the control pellet, and faster body mass gain of their leverets until standardized weaning. We found no significant difference between niacin deficient and control diet groups in reproductive output of females and survival rates of leverets. Our results show that even a diet severely depauperate of niacin affects reproductive success of female brown hares only slightly, presumably because of sufficient conversion of tryptophan to niacin, or of additional supply of niacin by caecal microorganism.
Maize (Zea mays) is not only the most produced crop in the world (Nuss and Tanumihardjo, 2010), but also one of the main energy crops cultivated in Europe (Klenke et al., 2017). High energy yield per area unit and high economical profit will make maize the most cultivated plant in Europe in the future (Nielsen and Oleskowicz-Popiel, 2008). Economic advantages come, however, with a high price regarding ecological aspects of maize cultivation. Intensive maize cultivation is blamed for increasing threats to biodiversity, with particularly negative effects on farmland animals (Fargione et al., 2009; Klenke et al., 2017). One of these species is the European brown hare (Lepus europaeus, Pallas 1778). Brown hare populations show drastic declines in many European countries since the 1960s (Edwards et al., 2000). Hunting bag records, reliably reflecting population trends (Langbein et al., 1999), demonstrate this decline in several European countries (Poland: Pielowski and Raczynski (1976), Denmark: Madsen et al. (1996); Wincentz Jensen (2009), parts of Croatia: Pintur et al. (2006); Popović et al. (2008), Serbia: Ristić et al. (2021), Germany, Austria, Bulgaria, Luxembourg, Netherlands, Slovakia, Switzerland: Mary and Trouvilliez (1995), United Kingdom: Smith et al. (2005)).
Several studies searched for causes of the hare decline. Reported main driving factors are changes in climate (Hackländer et al., 2002a; Smith et al., 2005; van Wieren et al., 2006), and intensification of agricultural practices resulting in a loss of set asides, grass stripes and habitat heterogeneity (Vaughan et al., 2003; Jennings et al., 2006; Schai-Braun et al., 2020; Johann and Arnold, 2021). Particularly maize affects hare populations negatively when cultivated in large monoculture (Meriggi and Alieri, 1989; Smith et al., 2005; Sliwinski et al., 2019). Due to the loss of habitat heterogeneity and plant biodiversity, hares are unable to meet their nutritional requirements there, because these picky herbivores search for plants rich in fat and protein (Schai-Braun et al., 2015). Left with no better choice, hares select green parts of maize plants as well as grains throughout the year in lower Austria (Steineck, 1978; Reichlin et al., 2006). Consequently, large areas under maize have negative effects on population development, i.e., the difference between autumn and spring population densities, and reproductive output of females, likely mediated by impaired supply of niacin and tryptophan (trp) (Selimovic et al., 2022). Both maize seeds and leaves are poor in niacin and trp, an essential amino acid (Hogan et al., 1955; Henderson et al., 1959; Goss, 1968; Mawson and Jacobs, 1978). The maize plant is not only poor in niacin and trp. In addition to such deficiencies, up to 90% of niacin is present as niacytin in mature maize grains, i.e., bound up in a complex with hemicellulose which renders it unavailable to vertebrates (Ammerman et al., 1995; Ball, 2005; Baker, 2008).
Nicotinic acid and nicotinamide are products of niacin decomposition, and are essential for in vivo synthesis of nicotinamide adenine dinucleotide (NAD) (Wan et al., 2010). Too low supply of trp and niacin with the diet can lead to severe health issues like dementia, skin rashes, diarrhea, or aggressiveness and growth retardation (Krehl et al., 1945; Kantak et al., 1980; Hegyi et al., 2004; Wan et al., 2010; Walz et al., 2013; Tissier et al., 2017). Tissier et al. (2017) further demonstrated in European hamsters (Cricetus cricetus, Linnaeus 1758), that niacin deficiency, resulting from a maize dominated diet, severely reduces reproductive output mainly due to increased infanticide by mothers.
We investigated in this study experimentally in captive brown hares the effects of solely feeding pellets mainly consisting of maize plant parts, but containing sufficient amounts of trp. Control diet groups received the same pellet further enriched with niacin to meet physiological requirements. We tested for effects of the diet poor in niacin on survival, body mass, reproductive success, and on growth of leverets. We expected severe health issues and high mortality in both mothers and leverets receiving the experimental pellet, or milk from mothers on the niacin depauperate diet.
All procedures were carried out in accordance with the Austrian legislation as assessed and approved by the institutional ethics commission of the University of Veterinary Medicine Vienna (GZ: BMBWF-68.205/0080-V/3b/2019).
The study was conducted during 2021. Experimental hares were kept in a large barn with open sidewalls at natural ambient temperatures. Twenty adult females, 2.5–4.5 years old, were used for experimental feeding and reproduction twice, once at begin of the annual reproductive season (Mai-April), and once at the end (August–September). Four of the experimental does were primiparous during the first experimental period. Daily mean air temperature in the barn was during the first experimental period 12.3°C (min = 6.2°C and max = 21.4°C), during the second 18.0°C (min = 11.6°C and max = 26.7°C). Each doe was kept in a fenced 6 m*2 m enclosure with two wooden boxes as cover (size 60 cm*60 cm*100 cm). The ground was filled and regularly re-filled with wood chips. Additionally, each enclosure contained as enrichment two 50 cm*40 cm wooden frames, height 4 cm, regularly filled with fresh soil. Further, fresh branches were provided once a week. In order to exclude possible effects of consummation of wood chips and branches on our results, these were tested for niacin in our lab and tested samples lacked any detectable niacin concentrations. Food and water was available ad libitum in two bowls, cleaned and refilled daily. Does were mated by releasing an adult male into an enclosure for two consecutive days.
For both experimental periods, females were randomly assigned to an experimental and control diet group containing 10 individuals each. Both groups were fed pelleted food, a food source readily accepted by brown hares as known from our long time experience of keeping and breeding brown hares (Hackländer et al., 2002a,b; Valencak and Ruf, 2009; Schai-Braun et al., 2021). During an experimental feeding period, each doe was weighed twice at begin and end to the nearest g. Leverets were weighed to the nearest g every day, beginning with day one after birth. At each weighing, the health status of an individual was checked by a veterinarian. Five leverets reaching our animal welfare endpoint criteria during the experiment (body mass < 20% than the mean body mass of other leverets of the same age, or severe disease symptoms like diarrhea, or neurological disorders), were sacrificed by several blunt blows to the neck. Body mass data from two sacrificed and one other leveret with diagnosed coccidiosis at the age of 18 days were omitted from analyzes because of obviously impaired growth. We kept the body mass data from three sacrificed animals in our dataset, because growth curves of these individuals showed no visible deviation from those of other leverets in the experiment.
For designing an experimental food pellet mirroring a maize biased diet of free-living hares, we used as primary material commercially available pellets made purely from air dried maize plant parts (Mais cobs™, Agrobs GmbH Degerndorf, Germany), and pellets made from air dried alfalfa (Luzerne cobs™, Agrobs GmbH Degerndorf, Germany). Regarding the content of macro-and micronutrients, we aimed at a composition of the experimental food pellet similar to the standard pellet used for many years of successfully keeping and breeding European hares at our institute (Hasenpellet, Garant Pöchlarn, Austria). In order to meet the protein and fat content of the standard pellet, we added pea protein isolate (Erbsenproteinpulver, Erdschwalbe GmbH, Neu-Ulm, Germany), and commercial sunflower oil (Sonnenblumenöl, Delikatessa GesmbH, Wiener Neustadt, Austria). Addition of the pea protein isolate also compensated for the low trp content of maize. As binding material, we used molasses (3% Melasse Königshofer GmbH, Ebergassing, Austria). Vitamin D3 was added in form of cholecalciferol (25 μg/kg pellet). Minerals were added in form of calcium carbonate, calcium dihydrogen phosphate, sodium chloride, magnesium oxide, trace minerals as zinc oxide, copper sulfate, potassium iodide, and sodium selenite. As an additional precaution against unwanted trp deficiency, we supplemented the niacin deficient experimental pellet with 0.109 g L-trp/kg during the first experimental period. Because of the surprisingly low effects of feeding the niacin deficient diet, evident already during the first experimental period, we dispensed L-trp supplementation of the experimental pellet fed during the second experimental period. The control diet group received during both experimental periods the experimental pellet supplemented with niacin to meet a dietary supply as recommended for rabbits (Weisbroth et al., 1974). We chose nicotinamide as source, because this form of niacin is well tolerated, without unwanted side effects known for other forms of niacin. For a full description of the ingredients of the fed pellets, see Table 1.
Food pellets were produced in 15 kg batches. We grounded the maize and alfalfa pellets with an electric grinder (Maximilian Fuchs & Co, Vienna, Austria) with a 3 mm sieve. For each batch, we created a pre-mix by adding all supplements as well as the pea protein isolate, and the sunflower oil to 2 kg of grounded plant components. This pre-mix was then added to the rest of plant components and mixed thoroughly with an electrical mixer (TX-MX1200E™, Einhell Landau/Isar, Germany). This mixture was pelleted with an electric pellet press (Magic PTO™, Ceccato Olindo San Giorgio delle Pertiche, Italy). After cooling and drying overnight, pellets were stored in sun proof plastic containers.
Does were slowly familiarized to consume the experimental pellets during 5 weeks before mating by gradually replacing our standard food pellet with the experimental pellet, either niacin deficient or control. After 5 weeks, does only received experimental pellets and were mated thereafter. Each feeding period with solely experimental pellets lasted for 28 days after removal of the male when a female did not give birth, or for 28 days after giving birth. We selected this time period, because it corresponds to the approximate age of leverets at weaning in free-living hares (Broekhuizen and Maaskamp, 1980; Zörner, 1981).
We calculated linear mixed effects models (lme) using the “nlme” package (Pinheiro et al., 2021) of the statistical package R (R Core Team, 2021) to test for possible effects and interactive effects of diet group and experimental period. We accounted for a possible effect of ambient temperature by including the daily mean air temperature in the stable as a covariate in each model. In the models analyzing body mass of leverets, we further adjusted for possible effects of litter size, mother’s age, and mother’s body mass at mating, or weaning, respectively. Litter size was analyzed with and without failures to litter. In both models, we adjusted for mother’s body mass and age at mating. Further, we analyzed with a binomial test whether diet group influenced failure to litter. When investigating effects on the body mass of mothers, we adjusted for age and initial body mass. In all models, we accounted for repeated measurements of individuals by including a random effect individual ID. For the analysis of growth of juveniles, we nested the random factor “leveret ID” within “mother ID” to further account for potential, but unknown differences in maternal care between mothers. Differences between slopes of regressions were tested with type III Anova. We checked with diagnostic plots the distribution of model residuals, and, if necessary, Box-Cox-transformed response variables to avoid deviation from normality. We used the “visreg” package (Breheny et al., 2020) for plotting results from lme models. In order to test and plot the effect of experimental feeding on survival of leverets, we used the function “survdiff” from package “survival” (Therneau, 2022) which uses tests from the G-rho family and Kaplan–Meier estimates of survival.
The gradual replacement of our standard hare pellet with experimental pellets during the 5 weeks before mating did not result in significant changes of body mass of females at mating, although there was a tendency of lower body mass of females receiving the niacin deficient diet [Table 2, effect of diet type F(1,15) = 3.0, p = 0.106]. The 40 mating events resulted in 16 litters, with 17 leverets born in April–May and 13 in August–September. Body mass of mothers at mating, leverets at birth, litter size, and failures of giving birth were similar in both the niacin deficient and control diet group (Table 2, all tests for effects of diet group and experimental period, n.s.). However, leveret body mass at birth differed significantly according to diet group and experimental period, with lower body mass at birth during the second experimental period in offspring born to mothers fed the niacin deficient diet [Table 2, interaction F(1,14) = 6.4, p = 0.024].
During both experimental periods, body mass of mothers increased during lactation [F(1,89) = 8.1, p = 0.006], was higher during the late summer experimental period [F(1,89) = 19.2, p < 0.001], but lower in both experimental periods of individuals receiving the niacin deficient diet [F(1,89) = 11.3, p = 0.001, Figure 1]. Independent of these seasonal and diet group difference, mothers’ seemed to gain more body mass during the nursing period at higher daily mean ambient temperatures [F(1,89) = 3.7, p = 0.058].
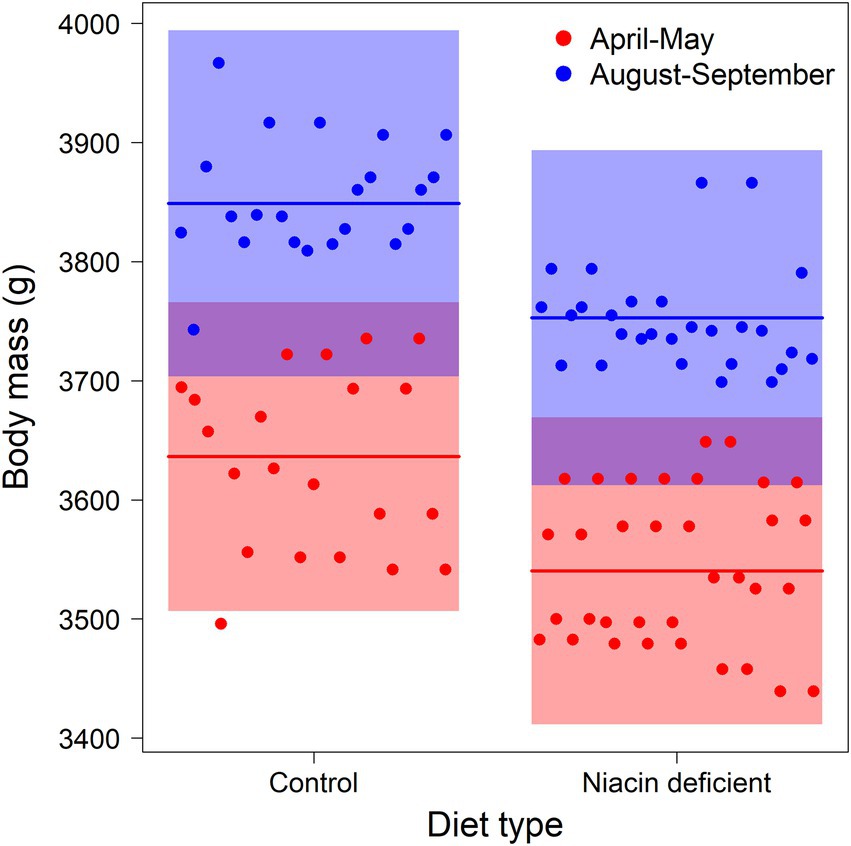
Figure 1. Body masses of European brown hare mothers measured during lactation in April–May (red circles) and August–September (blue circles). Animals were fed ad libitum exclusively with a niacin deficient pellet, or a niacin supplemented control pellet (horizontal lines indicate group means, shaded areas 95% confidence intervals of the means).
Leverets increased their body mass after birth rapidly, but slightly slower when nursed by a mother receiving the niacin deficient diet [Figure 2, interaction of leveret’s age and diet type fed to mother on leveret body mass, F(1,589) = 64.3, p < 0.001]. Leverets were during the second lactation period in August – September heavier [F(1,10) = 6.8, p = 0.028]. Litter size had a borderline significant negative effect on body mass development of leverets [F(1,10) = 4.2, p = 0.068]. Independent of season, there was a strong negative correlation between body mass of leverets and daily mean ambient temperature in the stable [F(1,589) = 64.9, p < 0.001].
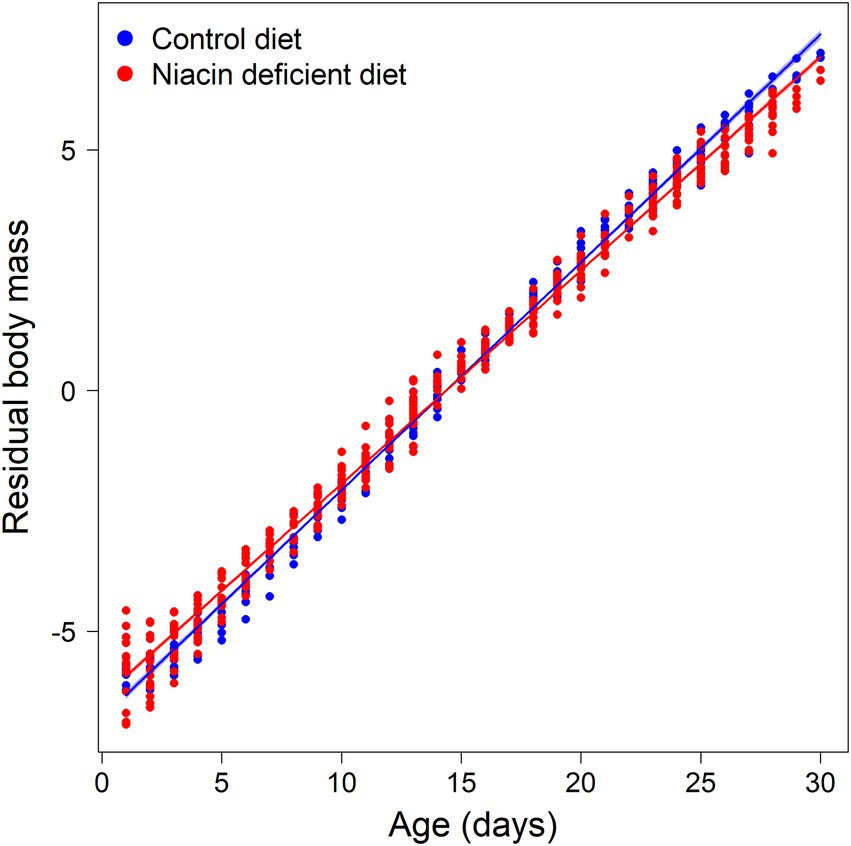
Figure 2. Residuals of Box-Cox-transformed body masses of leverets measured during the period of nursing (shaded areas indicate 95% confidence intervals of the estimated regression lines). Leverets had, like their mothers, access ad libitum to either a niacin deficient pellet, or a niacin supplemented control pellet.
Of the 30 leverets born during the two experimental periods, two leveret from the niacin deficient diet group and three from the control diet group were sacrificed because they had reached our animal welfare endpoint criteria. Four had severe infectious diseases of the gastrointestinal tract, and one showed severe neurological disorders. For analyzing the effect of diet type on leveret survival, we considered these five individuals as having died during the experiment. However, there was no statistically detectable difference in the survival probabilities of leverets from both diet type groups (CHI2 = 0.7, p = 0.4; Figure 3). A similar survival analysis for does was not possible, because all 20 experimental does survived the experiment.
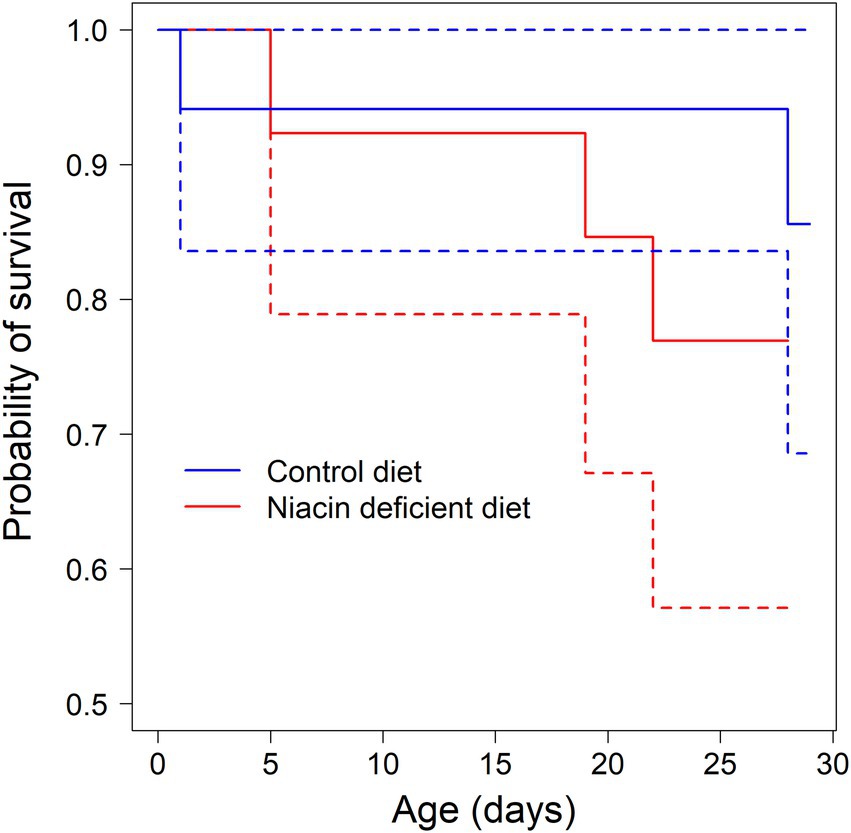
Figure 3. Probability of survival of leverets during the nursing period from birth to forced weaning at the age of 28 days (solid lines, estimated survival probability, dashed lines 95% confidence intervals of survival estimates). Leverets had, like their mothers, access ad libitum to either a niacin deficient pellet, or a niacin supplemented control pellet.
Survival and reproduction is impaired in hare populations living in areas characterized by maize monoculture. Niacin undersupply due to a maize biased nutrition was suspected to contribute to this phenomenon (Selimovic et al., 2022). We conducted this study to evaluate experimentally effects of dietary niacin deficiency on health and reproductive success of European brown hares. In contrast to our expectation, poor supply of niacin, as a result of feeding exclusively a niacin deficient pellet, did not increase mortality, or cause any severe health issues, neither in the experimental does nor in their offspring. The minimum amount of dietary supply of niacin and trp is unknown for European hares. The closest related species for which such information is available is the rabbit. The amount of niacin provided with our experimental pellet was only 13.1% of the required concentration of niacin per kg food (Weisbroth et al., 1974). Conversely, the pellet contained a sufficient concentration of trp (100% of the recommended amount during the first experimental period, 93% during the second when trp supplementation was suspended; cf. Table 1; Adamson and Fisher, 1973).
Reasons for the lack of pathologies in brown hares, well known for other species to result from a dietary undersupply of niacin, could be (i) compensatory synthesis of niacin from trp (Krehl et al., 1945; Wooley, 1947; Hegyi et al., 2004; Terakata et al., 2012; Tissier et al., 2017). The conversion of trp to niacin occurs at maximum rate when trp requirements are covered (Olcese et al., 1949), a condition fulfilled with our experimental pellet. Although this conversion is inefficient (67:1, Olcese et al., 1949; Goldsmith, 1965), it could supply enough niacin when trp rich food is available ad libitum, as it was the case in our experiment. However, as maize tissues are not only deficient in niacin but also in trp, this pathway may be limited in the wild. Further, the conversion of trp to niacin becomes more effective during late pregnancy (Fukuwatari et al., 2004), another possible contribution why our experimental females remained healthy despite a severe nutritional undersupply with niacin, (ii) Despite the widely accepted inability of vertebrates to hydrolyze niacytin to make it bioavailable, hares may be able to accomplish this at least to a small degree as reported for rats (Mason and Kodicek, 1970), and (iii) Niacin could be delivered in sufficient amount by caecal microorganism (McBee, 1971; Hunt and Harrington, 1974; Halls, 2010). The caecum is in many small herbivorous mammals the principal site of microbial activity (Hume, 1989). Some of these caecum fermenters excrete and re-ingest fermented caecal material as so-called “soft feces” or “caeotrophs.” Caecotrophs are rich in nutrients and vitamins, particularly of the B-group, including niacin (Halls, 2010). Caecotrophy is considered most sophisticated in leporids (Hirakawa, 2001). This may explain why a maize-biased diet produced more detrimental effects in European hamsters (Tissier et al., 2017) compared to European brown hares, the subject of our study. Even among leporids, there seem to be differences. Rabbits also use niacin produced by ceacum microorganism (Kulwich et al., 1953; Hunt and Harrington, 1974), but compensation of a dietary undersupply is apparently less efficient than in the European hare, considering the relatively high niacin concentration recommend for rabbit nutrition (Weisbroth et al., 1974). This surprising difference calls for a comparison of the nutrient contents of soft feces and the caecum microbiomes of European hares and rabbits. According to our results, substantial differences may exist.
Despite the lack of increased mortality or health issues when feeding the experimental pellet, we found significant effects on body mass at birth and on growth of leverets. Leveret body mass at birth was significantly lower in the niacin deficient diet group in August–September. An undersupply of trp, and therefore impaired conversion of trp to niacin, could have been responsible for this difference. Although still 93% of the recommended trp supply was provided with the experimental pellet fed during August–September, this difference may have been crucial, because trp plays an important role in fetal development (Badawy, 2015). During the nursing period, offspring of mothers fed the niacin deficient pellet gained mass at a lower rate. A shortfall of niacin availability is known to negatively affect the fat content of milk (Havlin et al., 2017). Apparently, this holds true for European brown hare mothers as well. Although we have no information about the fat content of milk produced by the does in our experiment, a too low fat content of milk most likely explains retarded growth of offspring from does fed the niacin deficient pellet. The effects of dietary niacin deficiency on growth of leverets occurred on top of a strong effect of ambient temperature. The negative effects of higher daily mean ambient temperature on body mass gain could result from impaired dissipation of heat produced as a byproduct of the high metabolic rate of rapidly growing juveniles (Valencak and Ruf, 2009).
Notwithstanding that, an experimentally induced undersupply with niacin had astonishingly little negative effects. However, it has been shown that maize monoculture, probably increasing the consumption of maize by herbivores (MacGowan et al., 2006), impairs the development of free-living European brown hare populations (Selimovic et al., 2022). The maize plant is not only poor in niacin but also in trp. Therefore, detrimental effects of a maize biased diet on European brown hare populations may to a large degree be mediated more by trp deficiency than by niacin undernutrition. Nevertheless, the weak effects of low niacin concentration in the food, found in this study under buffered environmental conditions, could translate into increased mortality under natural conditions. Free ranging leverets are in spring frequently exposed to not only much lower ambient temperatures, but also to wind and precipitation (Hackländer et al., 2001). Such conditions increase the basal metabolic rate of leverets (Hackländer et al., 2002a). In addition, free-living leverets have no shelter and thus have most likely higher energy requirements than leverets in our study, at least in spring. When mothers are unable to deliver enough energy with milk because of malnutrition caused by maize monoculture in their home ranges during lactation, or the fattening period preceding the reproductive season, growth of juveniles may well be much more impaired as found under our experimental conditions. As a result, juvenile mortality may become substantial under natural conditions, considering that weak juveniles are also more likely to fall victim to predators. High juvenile mortality negatively affects population development particularly when spring litters suffer, because early born leverets are most important for population growth (Olesen and Asferg, 2006).
Altogether, our study showed that a niacin deficient diet negatively affects growth of brown hare leverets. Combined with low plant biodiversity due to monoculture, and increasingly unfavorable climatic conditions, a maize biased nutrition seem to be an additional, so far neglected factor contributing to the decline of brown hares throughout Europe.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by all procedures were carried out in accordance with the Austrian legislation as assessed and approved by the institutional ethics commission of the University of Veterinary Medicine Vienna (GZ: BMBWF-68.205/0080-V/3b/2019).
AS, WA, and MT conceived the study. AS and WA analyzed data and wrote the manuscript. GS and JP-G contributed to designing the experiment. AS, AH, and HR collected data. AH and HR provided veterinary care. All authors contributed to the article and approved the submitted version.
The authors would like to thank Peter Steiger and Viola Kaiser for taking care of the hares, helping with daily controls, and production of pellets. The authors further thank Manuela Habe and Christian Bachl for help and instruction to familiarize experimental animals to handling, Renate Hengsberger for manuscript formatting. The authors thank the Austrian Federal Ministry of Education, Science and Culture, the Austrian hunting associations, the City of Vienna, the province of Lower Austria, and the Academy for the Protection of Zoo Animals and Wildlife for financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamson, I., and Fisher, H. (1973). Amino acid requirement of the growing rabbit: an estimate of quantitative needs. J. Nutr. 103, 1306–1310. doi: 10.1093/jn/103.9.1306
Ammerman, C. B., Baker, D. H., and Lewis, A.J. (1995). Bioavailability of Nutrients for Animals. Amino acids, Minerals, and Vitamins. San Diego: Academic Press. 441.
Badawy, A. A. B. (2015). Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep. 35:e00261. doi: 10.1042/BSR20150197
Baker, D. H. (2008). Animal models in nutrition research. J. Nutr. 138, 391–396. doi: 10.1177/011542659200700137
Ball, G. F. M. (2005). Vitamins in Foods. Analysis, Bioavailability, and Stability. CRC Press. Florida. 824.
Breheny, P., Burchett, W., and Breheny, M. P. (2020). Package ‘visreg’ (R package version 2.7). Available at: https://cran.r-project.org/web/packages/visreg/index.html
Broekhuizen, S., and Maaskamp, F. (1980). Behaviour of does and leverets of the European hare (Lepus europaeus) whilst nursing. J. Zool. (Lond.) 191, 487–501. doi: 10.1111/j.1469-7998.1980.tb01480.x
Edwards, P. J., Fletcher, M. R., and Berny, P. (2000). Review of the factors affecting the decline of the European brown hare, Lepus europaeus (Pallas, 1778) and the use of wildlife incident data to evaluate the significance of paraquat. Agric. Ecosyst. Environ. 79, 95–103. doi: 10.1016/S0167-8809(99)00153-X
Fargione, J. E., Cooper, T. R., Flaspohler, D. J., Hill, J., Lehman, C., McCoy, T., et al. (2009). Bioenergy and wildlife: threats and opportunities for grassland conservation. Bioscience 59, 767–777. doi: 10.1525/bio.2009.59.9.8
Fukuwatari, T., Murakami, M., Ohta, M., Kimura, N., Jin-No, Y., Sasaki, R., et al. (2004). Changes in the urinary excretion of the metabolites of the tryptopan-niacin pathway during pregnancy in Japanese women and rats. J. Nutr. Sci. Vitaminol. 50, 392–398. doi: 10.3177/jnsv.50.392
Goldsmith, G. A. (1965). Niacin: Antipellagra factor, hypocholesterolemic agent. Model of nutrition research yesterday and today. JAMA 194, 167–173. doi: 10.1001/jama.1965.03090150059014
Goss, J. A. (1968). Development, physiology, and biochemistry of corn and wheat pollen. Bot. Rev. 34, 333–359. doi: 10.1007/BF02985391
Hackländer, K., Arnold, W., and Ruf, T. (2002a). Postnatal development and thermoregulation in the precocial European hare (Lepus europaeus). J. Comp. Physiol. B. 172, 183–190. doi: 10.1007/s00360-001-0243-y
Hackländer, K., Frisch, C., Klansek, E., Steineck, T., and Ruf, T. (2001). Die Fruchtbarkeit weiblicher Feldhasen (Lepus europaeus) aus Revieren mit unterschiedlicher Populationsdichte. Z. Jagdwiss. 47, 100–110. doi: 10.1007/BF02239822
Hackländer, K., Tataruch, F., and Ruf, T. (2002b). The effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus). Physiol. Biochem. Zool. 75, 19–28. doi: 10.1086/324770
Halls, A. E. (2010). Nutritional Requirements for Rabbits. 10 Available at: https://docplayer.net/21068707-Nutritional-requirements-for-rabbits-oct-2010-amy-e-halls-m-sc-monogastric-nutritionist-shur-gain-nutreco-canada-inc.html
Havlin, J. M., Robinson, P. H., and Garrett, J. E. (2017). Niacin feeding to fresh dairy cows: immediate effects on health and milk production. Anim. Prod. Sci. 57, 1069–1078. doi: 10.1071/AN15419
Hegyi, J., Schwartz, R. A., and Hegyi, V. (2004). Pellagra: dermatitis, dementia, and diarrhea. Int. J. Dermatol. 43, 1–5. doi: 10.1111/j.1365-4632.2004.01959.x
Henderson, L. M., Someroski, J. F., Rao, D. R., Wu, P. H. L., Griffith, T., and Byerrum, R. U. (1959). Lack of a tryptophan-niacin relationship in corn and tobacco. J. Biol. Chem. 234, 93–95. doi: 10.1016/S0021-9258(18)70341-4
Hirakawa, H. (2001). Coprophagy in leporids and other mammalian herbivores. Mammal Rev. 31, 61–80. doi: 10.1046/j.1365-2907.2001.00079.x
Hogan, A. G., Gillespie, G. T., Koçtürk, O., O'Dell, B. L., and Flynn, L. M. (1955). The percentage of protein in corn and its nutritional properties. J. Nutr. 57, 225–239. doi: 10.1093/jn/57.2.225
Hume, I. D. (1989). Optimal digestive strategies in mammalian herbivores. Physiol. Zool. 62, 1145–1163. doi: 10.1086/physzool.62.6.30156206
Hunt, C. E., and Harrington, D. D. (1974). “Nutrition and nutritional diseases of the rabbit” in The Biology of the Laboratory Rabbit. eds. S. H. Weisbroth, R. E. Flatt, and A. L. Kraus. 1st ed (New York, San Francisco, London: Academic Press), 403–433.
Jennings, N. V., Smith, R. K., Hackländer, K., Harris, S., and White, P. C. L. (2006). Variation in demography, condition and dietary quality of hares Lepus europaeus from high-density and low-density populations. Wildl. Biol. 12, 179–189. doi: 10.2981/0909-6396(2006)12[179:VIDCAD]2.0.CO;2
Johann, F., and Arnold, J. (2021). Scattered woody vegetation promotes European brown hare population. Basic Appl. Ecol. 56, 322–334. doi: 10.1016/j.baae.2021.08.012
Kantak, K. M., Hegstrand, L. R., and Eichelman, B. (1980). Dietary tryptophan modulation and aggressive-behavior in mice. Pharmacol. Biochem. Behav. 12, 675–679. doi: 10.1016/0091-3057(80)90147-1
Klenke, R., Frey, B., and Zarzycka, A. (2017). “Case study 5: the effects of increased rape and maize cropping on agricultural biodiversity” in Service Contract to Support Follow-up Actions to the Mid-term Review of the EU Biodiversity Strategy to 2020 in Relation to Target 3A – Agriculture. Report to the European Commission. eds. G. Siriwardena and G. Tucker (London: European Commission, Institute for European Environmental Policy), 147–183. doi: 10.2779/981605
Krehl, W. A., Teply, L. J., Sarma, P. S., and Elvehjem, C. A. (1945). Growth-retarding effect of corn in nicotinic acid-low rations and its counteraction by tryptophane. Science 101, 489–490. doi: 10.1126/science.101.2628.489
Kulwich, R., Struglia, L., and Pearson, P. B. (1953). The effect of coprophagy on the excretion of B vitamins by the rabbit. J. Nutr. 49, 639–645. doi: 10.1093/jn/49.4.639
Langbein, J., Hutchings, M. R., Harris, S., Stoate, C., Tapper, S. C., and Wray, S. (1999). Techniques for assessing the abundance of Brown Hares Lepus europaeus. Mammal Rev. 29, 93–116. doi: 10.1046/j.1365-2907.1999.00040.x
MacGowan, B., Humberg, L., Beasley, J., DeVault, T., Retamosa, M., and Rhodes, O. E. (2006). Corn and Soybean Crop Depredation by Wildlife FNR-265-W. Purdue University. West Lafayette, IN.
Madsen, J., Asferg, T., Clausager, I., and Noer, H. (1996). Status og jagttider for danske vildtarter. TEMA-rapport fra DMU. D. Miljøundersøgelser. 112. Available at: http://www.dmu.dk/1_viden/2_Publikationer/3_miljobib/rapporter/MB03.pdf
Mary, C., and Trouvilliez, J. (1995). Special lièvre d’Europe. Bulletin Mensuel de l’Office National de la Chasse 204:96.
Mason, J. B., and Kodicek, E. (1970). The metabolism of niacytin in the rat. Studies of the excretion of nicotinic acid metabolites. Biochem. J. 120, 509–513. doi: 10.1042/bj1200509
Mawson, A. R., and Jacobs, K. W. (1978). Corn consumption, tryptophan, and cross-national homicide rates. J. Orthomol. Psychiatry 7, 227–230. Available at http://orthomolecular.org/library/jom/1978/pdf/1978-v07n04-p227.pdf
McBee, R. H. (1971). Significance of intestinal microflora in herbivory. Annu. Rev. Ecol. Syst. 2, 165–176. doi: 10.1146/annurev.es.02.110171.001121
Meriggi, A., and Alieri, R. (1989). Factors affecting brown hare density in northern Italy. Ethol. Ecol. Evol. 1, 255–264. doi: 10.1080/08927014.1989.9525515
Nielsen, J. B. H., and Oleskowicz-Popiel, P. (2008). “Biogas – A promising renewable energy source for Europe” in AEBIOM Workshop-European Parliament (Brussels)
Nuss, E. T., and Tanumihardjo, S. A. (2010). Maize: a paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 9, 417–436. doi: 10.1111/j.1541-4337.2010.00117.x
Olcese, O., Pearson, P. B., and Sparks, P. (1949). Intestinal synthesis of niacin and the metabolic interrelationship of tryptophan and niacin in the rabbit. J. Nutr. 39, 93–105. doi: 10.1093/jn/39.1.93
Olesen, C. R., and Asferg, T. (2006). Assessing potential causes for the population decline of European brown hare in the agricultural landscape of Europe-review of the current knowledge. NERI Technical Report. 31. Available at: http://www.dmu.dk/Pub/FR600.pdf
Pielowski, Z., and Raczynski, J. (1976). “Ecological conditions and rational management of hare populations” in Ecology and Management of European Hare Populations. eds. Z. Pielowski and Z. Pucek (Warszawa: Polish Hunting Association), 269–286.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Eispack Heisterkamp, S., Van Willigen, B., et al. (2021). Nlme: Linear and nonlinear mixed effects models (R Package Version 3.1-158). Available at: https://CRAN.R-project.org/package=nlme
Pintur, K., Popović, N., Alegro, A., Severin, K., Slavica, A., and Kolić, E. (2006). Selected indicators of brown hare (Lepus europaeus Pallas, 1778) population dynamics in northwestern Croatia. Vet. Arhiv. 76, S199–S209. Available at: https://www.bib.irb.hr/230033
Popović, N., Pintur, K., Alegro, A., Slavica, A., Lacković, M., and Sertić, D. (2008). Temporal changes in the status of the European hare (Lepus europaeus Pallas, 1778) population of Međimurje, Croatia. Natura Croatica 17, 247–257. Available at: https://hrcak.srce.hr/33971
R Core Team (2021). R: A language and environment for statistical computing Available at: http://www.R-project.org/
Reichlin, T., Klansek, E., and Hackländer, K. (2006). Diet selection by hares (Lepus europaeus) in arable land and its implications for habitat management. Eur. J. Wildl. Res. 52, 109–118. doi: 10.1007/s10344-005-0013-3
Ristić, Z., Ponjiger, I., Matejević, M., Kovačević, M., Ristić, N., and Marković, V. (2021). Effects of factors associated with the decline of brown hare abundance in the Vojvodina region (Serbia). Hystrix 32, 67–71. doi: 10.4404/hystrix-00334-2020
Schai-Braun, S. C., Reichlin, T. S., Ruf, T., Klansek, E., Tataruch, F., Arnold, W., et al. (2015). The European hare (Lepus europaeus): a picky herbivore searching for plant parts rich in fat. PLoS One 10:e0134278. doi: 10.1371/journal.pone.0134278
Schai-Braun, S. C., Ruf, T., Klansek, E., Arnold, W., and Hackländer, K. (2020). Positive effects of set-asides on European hare (Lepus europaeus) populations: leverets benefit from an enhanced survival rate. Biol. Conserv. 244:108518. doi: 10.1016/j.biocon.2020.108518
Schai-Braun, S. C., Steiger, P., Ruf, T., Arnold, W., and Hackländer, K. (2021). Maternal effects on reproduction in the precocial European hare (Lepus europaeus). PLoS One 16:e0247174. doi: 10.1371/journal.pone.0247174
Selimovic, A., Tissier, M. L., and Arnold, W. (2022). Maize monoculture causes niacin deficiency in free-living European brown hares and impairs local population development. Front. Ecol. Evol. 10:1017691. doi: 10.3389/fevo.2022.1017691
Sliwinski, K., Ronnenberg, K., Jung, K., Strauß, E., and Siebert, U. (2019). Habitat requirements of the European brown hare (Lepus europaeus Pallas 1778) in an intensively used agriculture region (Lower Saxony, Germany). BMC Ecol. 19:31. doi: 10.1186/s12898-019-0247-7
Smith, R. K., Jennings, N. V., and Harris, S. (2005). A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mammal Rev. 35, 1–24. doi: 10.1111/j.1365-2907.2005.00057.x
Steineck, T. (1978). Die Botanische Zusammensetzung des Mageninhaltes bei Feldhasen (Lepus europaeus P.). Dr. med. vet. Dissertation, Vienna: University of Veterinary Medicine.
Terakata, M., Fukuwatari, T., Sano, M., Nakao, N., Sasaki, R., Fukuoka, S.-I., et al. (2012). Establishment of true niacin deficiency in quinolinic acid Phosphoribosyltransferase knockout mice. J. Nutr. 142, 2148–2153. doi: 10.3945/jn.112.167569
Therneau, T. M. (2022). A package for survival analysis in R (R package version 3.4-0). Available at: https://CRAN.R-project.org/package=survival.
Tissier, M. L., Handrich, Y., Dallongeville, O., Robin, J.-P., and Habold, C. (2017). Diets derived from maize monoculture cause maternal infanticides in the endangered European hamster due to a vitamin B3 deficiency. Proc. R. Soc. B 284:20162168. doi: 10.1098/rspb.2016.2168
Valencak, T., and Ruf, T. (2009). Energy turnover in European hares is centrally limited during early, but not during peak lactation. J. Comp. Physiol. B. 179, 933–943. doi: 10.1007/s00360-009-0376-y
van Wieren, S. E., Wiersma, M., and Prins, H. H. T. (2006). Climatic factors affecting a brown hare (Lepus europaeus) population. Lutra 49, 103–110. Available at: https://www.zoogdiervereniging.nl/publicaties/2006/lutra-492van-wieren-et-al2006
Vaughan, N., Lucas, E.-A., Harris, S., and White, P. C. L. (2003). Habitat associations of European hares Lepus europaeus in England and Wales: implications for farmland management. J. Appl. Ecol. 40, 163–175. doi: 10.1046/j.1365-2664.2003.00784.x
Walz, J. C., Stertz, L., Fijtman, A., dos Santos, B. T. M. Q., and de Almeida, R. M. M. (2013). Tryptophan diet reduces aggressive behavior in male mice. Psychol. Neurosci. 6, 397–401. doi: 10.3922/j.psns.2013.3.18
Wan, P., Moat, S., and Anstey, A. (2010). Pellagra: a review with emphasis on photosensitivity. Br. J. Dermatol. 164, 1188–1200. doi: 10.1111/j.1365-2133.2010.10163.x
Weisbroth, S. H., Flatt, R. E., and Kraus, A. L. (1974). The Biology of the Laboratory Rabbit. New York: Academic Press. 1–496 p.
Wincentz Jensen, T.-L. (2009). Identifying Causes for Population Decline of the Brown Hare (Lepus Europaeus) in Agricultural Landscapes in Denmark. Aarhus University. Denmark.
Wooley, J. G. (1947). Niacin deficiency in rabbits and response to tryptophane and to niacin. Proc. Soc. Exp. Biol. Med. 65, 315–317. doi: 10.3181/00379727-65-15946
Keywords: maize biased diet, growth, survival, pellagra, nicotinamide, tryptophan, Lepus europaeus, niacin
Citation: Selimovic A, Tissier ML, Stalder G, Painer-Gigler J, Haw A, Rauch H and Arnold W (2023) The effect of dietary niacin deficiency on reproduction of European brown hares: An experimental study. Front. Ecol. Evol. 11:1083045. doi: 10.3389/fevo.2023.1083045
Received: 28 October 2022; Accepted: 09 February 2023;
Published: 02 March 2023.
Edited by:
Maria K. Oosthuizen, University of Pretoria, South AfricaReviewed by:
Mitsue Sano, University of Shiga Prefecture, JapanCopyright © 2023 Selimovic, Tissier, Stalder, Painer-Gigler, Haw, Rauch and Arnold. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aldin Selimovic, QWxkaW4uU2VsaW1vdmljQHZldG1lZHVuaS5hYy5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.