
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 03 April 2023
Sec. Urban Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1020982
This article is part of the Research TopicEffects of Noise on Organisms: From Mechanisms to Ecological ConsequencesView all 8 articles
Steadily increasing human population is changing the environment in many ways. One of the most disturbing impacts is the development of anthropogenic noise pollution connected to ever-growing traffic intensity. The road network can have both positive and negative effects on biodiversity and populations. Many bird species use acoustic communication to establish and maintain their territories and for intra-pair and adult–young communication. Noise pollution can impact negatively on breeding success and biorhythm if this communication is masked by noise and the individuals must adjust their singing activity. Yellowhammer (Emberiza citrinella) is a common bird species of agricultural landscapes whose population is declining due to agricultural intensification. It is found also in habitats near highways with forest steppe-like characteristics, where it is affected by the high levels of anthropogenic noise pollution. This study aimed to determine how this species adapts to noise from highway traffic by adjusting its singing activity. The influence of locality type, immediate and long-term impact of traffic noise on the average and total length of song sequences in the birdsong, and influence on the total number of recorded song sequences during the second hour after sunrise were evaluated in this study. Our results showed that Yellowhammer’s singing activity changed in localities close to highways compared to agricultural landscape. With increasing long-term traffic intensity on highways, song duration of the Yellowhammer song was decreasing. The present traffic intensity led to later onset of dawn chorus and decreasing strophe length with increasing number of passing vehicles. Furthermore, in the agricultural landscape, Yellowhammer’s song duration increased with increasing distance from the nearest road.
Increase in the human population is linked inevitably to a growing rate of the planet’s urbanization. This results in ever new changes in the environment, often occurring as disturbing anthropogenic influences on organisms (Lukanov and Naumov, 2019). Animal populations in cities exhibit some of the highest rates of phenotypic change (Alberti et al., 2017) and urbanization elicits diverse plastic behavioral responses in animals, which may lead to rapid evolutionary changes (Caspi et al., 2022). Phenotypic plasticity facilitates adaptation to urban environments, and the drivers behind novel nesting behaviors are complex and multifaceted (Bressler et al., 2020). One of the most disturbing impacts is the development of anthropogenic noise pollution (Huffeldt and Dabelsteen, 2013), which is to a large extent linked to the development of road and motorway networks and increase in traffic. The onset of such changes is very abrupt compared to the speed of evolution. Therefore, these changes may become limiting factors for some species, as some species using acoustic communication are not able to cope with the newly established conditions (Partecke et al., 2006). As a consequence, animals living in areas exposed to anthropogenic noise may suffer reduced reproductive success and ultimately that may lead to the exclusion of species from otherwise suitable habitats (Slabbekoorn and Peet, 2003).
The effects of increasing road network development can have both positive and negative impacts on organisms. Morelli et al. (2014) found that roads, railways, and several associated structures commonly related to the decline of biodiversity may also have positive effects on certain bird species or may influence bird diversity. In suburban and periurban areas dominated by road networks, bird communities’ richness was greater compared to that in rural areas, indicating that several of these characteristic features of urban landscapes are attractive to numerous bird species. On the other hand, habitats along the road edges seem to act as ecological traps, where passerines are attracted to the vegetative diversity of periphery habitats but experience greater mortality caused by the road and traffic. This can have an especially significant impact on rare species populations (Kuitunen et al., 2003).
The traffic noise of a big city, as well as of the landscape around highways, is characterized by sounds with high volume at low frequencies (Slabbekoorn and Ripmeester, 2008), which is substantially different from the sounds of natural habitats. Despite the heterogeneity of urban landscapes, human modifications of the environment in cities have some common characteristics likely to affect communication, such as elevated noise levels, abundant large flat surfaces, and altered sound channels (Warren et al., 2006). These characteristics have parallels in natural systems, such as streams, canyons, and windy environments (Katti and Warren, 2004). Industry and road traffic have become the largest sources of anthropogenic noise. Growing with great intensity, noise pollution is one of the most important but also still not thoroughly studied factors affecting the environment (Fuller et al., 2007; Nakamura-Garcia and Ríos-Chelén, 2007). Also present in areas around motorways, railways, and airports, it creates an increasingly dense network penetrating out of towns into wooded areas (Forman and Alexander, 1998; Klingbeil et al., 2020).
Traffic noise could interfere with the acoustic communication upon which birds depend for the establishment and maintenance of territories and for intra-pair and adult–young communication (Rheindt, 2003). It may limit the ability to detect a signal or lead to signal overlap (Ryan and Brenowitz, 1985). The singing activity of most bird species corresponds to certain seasonal and daily patterns. Prolonged photoperiod in the spring causes heightened testosterone secretion, which leads to increased vocalization (Dawson et al., 2001). The singing activity of birds reaches its maximum during the nesting season. Throughout the day, it is most intense in the morning at dawn and is slightly less intense in the evening at dusk (Catchpole and Slater, 1995). The noise level in urban areas changes during the day at predictable intervals, reaching its lowest values during night-time when people are least active (Slabbekoorn and Ripmeester, 2008).
Birds respond to noisy conditions in several ways. Some songbird species seem to compensate for excess daytime noise by vocalizing at night. However, this shift costs individuals a large amount of energy that is expended at the cost of sleep (Fuller et al., 2007). Grunst et al. (2021) found a negative effect of traffic noise on the sleep behavior of a free-living songbird, reducing its sleep duration, proportion, and bout length while inducing birds to exit nest boxes earlier in the morning. Traffic noise is ceaselessly present in big cities around main roads. City road networks have become suitable study sites for assessing the impact of traffic noise on birds, which to date has been assessed mainly in big cities but not near highways outside of cities. Bermúdez-Cuamatzin et al. (2020) have shown conclusively that Great Tits (Parus major) in urban and rural areas differ in the timing of their singing activity: the dawn chorus starts earlier in the city, and thereafter there is less singing activity in urban streets than in rural woodland. This confirms the early rise in cities, as reported for many bird species and sometimes attributed to traffic noise during daytime, to artificial light at night, or both. Another strategy to avoid masking of the signal is to sing at higher frequencies, as reported by Slabbekoorn and Peet (2003) and Nemeth et al. (2013) in the case of traffic-noise conditions or by Sebastianelli et al. (2021) in relation to natural low-frequency noise along ocean coast. Both studies showed that the dominant frequency in the song increased the closer the birds were to the source of the noise. Increased level of vigilance at the expense of feeding time has been another adjustment observed in animals as a response to noisy conditions (Rabin et al., 2006; Leveau, 2020). For example, Common Chaffinch (Fringilla coelebs), devotes less time to feeding at artificially elevated noise levels. Research has shown that birds do not change their alertness to new stimuli but rather rely on visual assessment of the environment and monitoring of impending danger from predators when the detection of auditory stimuli is limited by noise. Anthropogenic noise disrupts not only the detection of individuals of their own species but also of predators. The risk of predation can have a negative effect on food intake and can lead to a reduced chance of survival while also decreasing reproductive success (Quinn et al., 2006).
Other aspects influencing the varying onset and course of singing activity of birds can be of natural origin. The timing of sunrise and prevailing weather conditions including cloud cover, temperature, wind, precipitation, and atmospheric pressure (Kreithen and Keeton, 1974), and the attenuation and degradation of acoustic signals during transmission through the atmosphere impose limits on acoustic communication (Wiley and Richards, 1978). Atmospheric turbulence from wind is the primary determinant of the intensity of irregular amplitude fluctuations (Richards and Wiley, 1980). Morton (1975) hypothesized that information transfer of avian sounds may be based on temporal aspects of the signal since these are less distorted in temperature- and wind-speed-stratified open environments. The timing of bird activity seems to be also temperature-dependent, although there the findings vary. For instance, Bruni et al. (2014) reported that several species of songbirds began to sing earlier when the temperatures were warmer, on the contrary, Da Silva et al. (2016) did not find any effect of temperature for several other songbird species. Cloudy conditions typically delay the onset of dawn song due to reduced sky radiance (Bruni et al., 2014), and under cool conditions, the song activity is likely to be decreased (Nordt and Klenke, 2013).
Many studies, including some of the aforementioned, have focused on changes in frequency or shift in start of birds’ dawn vocal activity, but no studies have been made in relation to Yellowhammer’s singing activity under traffic noise. Furthermore, the impact of anthropogenic noise on the singing activity of songbirds has been assessed mainly in big cities (Slabbekoorn and Boer-Visser, 2007; Nordt and Klenke, 2013; Moseley et al., 2018; Gómez, 2022) but not so often near highways outside of cities. This leads us to the subject of this study for which we chose Yellowhammer (Emberiza citrinella) as a model species. The aim was to compare how singing activity differs in males nesting in the immediate vicinity of highway and males who defend their territories in traditional agricultural landscape further from the highway, as this aspect has not been determined yet in other studies. We also compared the differences between days with high and low traffic intensity and took into account the current weather conditions, in order to control for a possible effect of weather on the Yellowhammer’s singing activity not captured by the random effect of locality.
Yellowhammer (Emberiza citrinella) is a common Palearctic passerine inhabiting a wide range spanning from Spain to Central Asia (del Hoyo et al., 2011). It is a common bird species of agricultural landscapes, forest edges, and forest clearings. It frequently inhabits also bushes along roads, railways and flowing waterways. Nevertheless, its population is declining due to agricultural intensification (BirdLife Intrenational, 2020). Yellowhammer is also to be found in habitats near highways with forest steppe-like characteristics that can supply what is a shrinking natural habitat (Hladík, 2021). The bird has become a model species for singing activity research, especially with a focus on its dialects (Diblíková et al., 2019). Although relatively much attention is given to the singing of Yellowhammer, the influence of traffic noise on its singing has not yet been studied.
Through 6 consecutive weeks, we recorded the voice activity of male Yellowhammer using Sony ICD-PX312 digital recorders. Recordings were made in the period from 16 April 2016 to 30 May 2016, which was during the most intense vocalization period within the breeding season. Each week, recordings were taken from Saturday afternoon to Monday evening, except on Sunday 8 May and Monday 9 May when the recordings did not take place due to bad weather conditions.
Each week, two localities, 30–50 km distant from Prague, Czech Republic and occupied by Yellowhammer populations, were identified. One of them, regarded as “noise-polluted” locality, was located close to one of the D6, D7, and D8 highways, in sections without any permanent sources of artificial light – e.g. illuminated intersections, petrol stations, illuminated traffic signs or advertising boards. The selected highways have relatively intensive traffic (average 13,000–33,000 cars per day) and are surrounded by agricultural landscape with scattered green vegetation. Approximately 2–5 km distant from this noise-polluted locality, a corresponding locality, regarded as “without noise pollution,” was determined within agricultural landscape, 1 to 5.9 km distant from the closest highway and 100–920 m from the nearest road. The structure of the greenery at both types of localities was comparable, and there were no retaining walls or other measures present which could affect the sound propagation. Because a new pair of noise-polluted and without-noise-pollution localities were selected each of the 6 weeks, we had 12 localities in total (see Figure 1, Table 1). The altitude of the localities ranged from 168 to 411 meters.
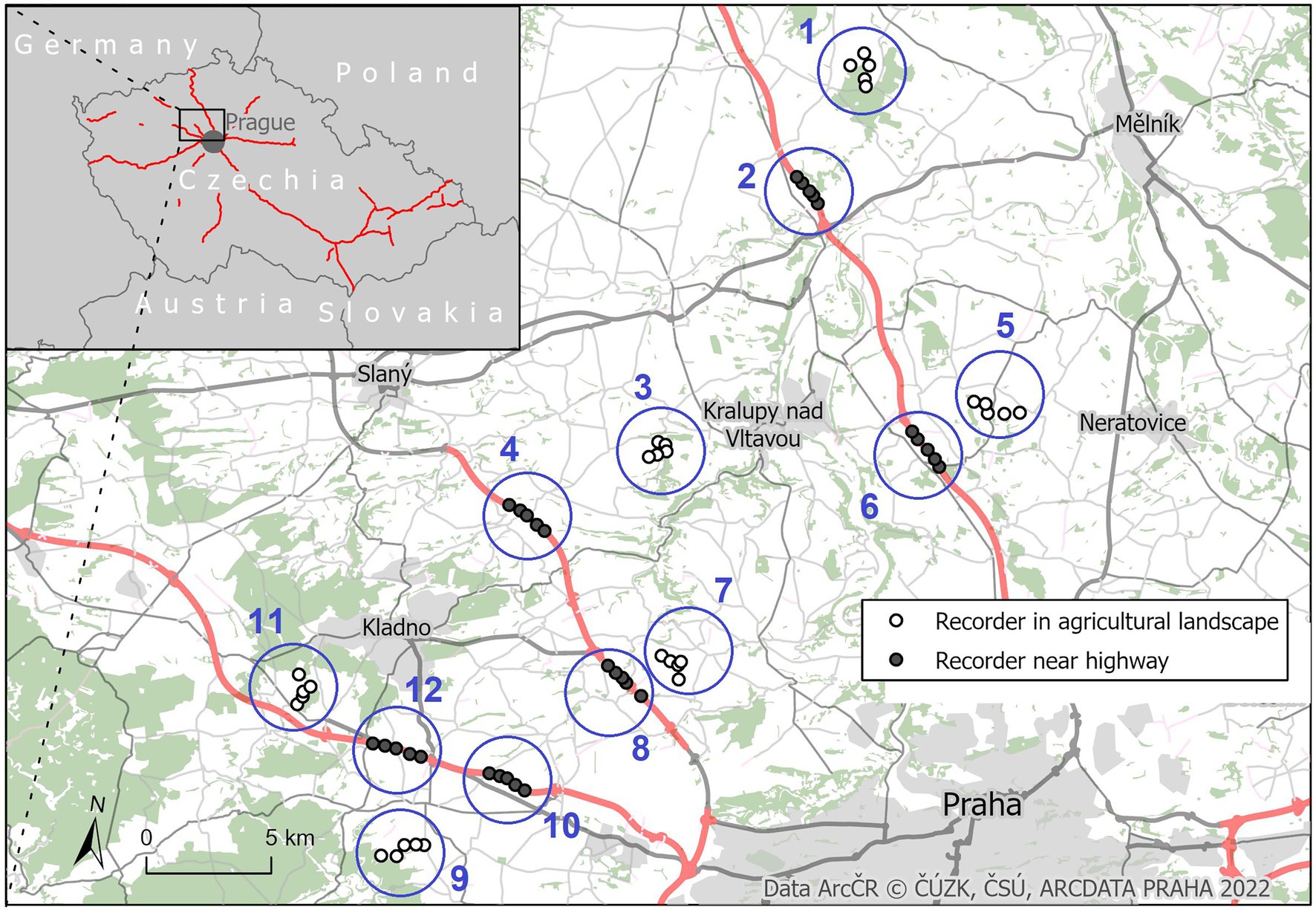
Figure 1. Location of recorders of Yellowhammer’s singing near highways and in the agricultural landscape. In both maps, red lines represent highway network of the Czech Republic.
In each locality, five individual Yellowhammer territories (herein referred as “sites”) were identified based on the observation of the individual’s movement, resulting in 12 × 5 = 60 individual territories. A recorder was placed at 1–1.5 m height in the middle of each territory, 200–350 m apart from one another in order to completely rule out the possibility that a single male Yellowhammer could be recorded on several devices. One male Yellowhammer was always recorded on one recorder and if there was another male on the recording vocalizing in the distance, its singing activity was not analyzed. In the course of the recording, the birds were not observed, only while placing and collecting the recording devices. The upper part of a PET drink bottle was used as a cover for the digital recorder, protecting it against weather, theft, and eliminating risk of accidental displacement, movement, or covering of the recorder.
For further analysis, we selected and processed the period 60 min long from 60 to 120 min after sunrise, when the birds have maximum activity (Keast, 1994; Bruni et al., 2014; Kułaga and Budka, 2020) and at the same time the noise from the highway is more intense. Because at each site, the recording was made both on Sunday and Monday morning, we could thus compare the singing activity on a weekend day and a working day. Yellohammer’s singing consists of well-separated individual songs called strophes (Catchpole and Slater, 2010). Each strophe has a characteristic melodic structure and is separated by a silent period, which makes it easy to manually distinguish and count the individual syllables from the whole chorus. To detect individual songs, the recordings were processed using Avisoft SASLab Pro software (Raimund Specht, Berlin, Germany). A finite impulse response (time domain filter FIR) low-pass filter of 2.5 kHz and a high-pass filter of 6 kHz were used for better detection of singing and background noise reduction. For every individual song in the analyzed period, its start and end times were marked, regardless of the number of strophes they contained. Only those with well recognizable beginnings and ends, however, were used for further analysis. Each morning, we further identified the time when the bird started its first song (usually during the last 30 min before sunrise). In summary, we identified the following variables for each recording day and site: locality type (highway versus agriculture landscape), day (Sunday versus Monday), song duration (seconds), average strophe length (seconds), number of strophes, and onset of singing (seconds relative to sunrise).
Some recordings had to be discarded due to bad weather conditions with heavy rain making it impossible to detect the singing. Furthermore, some recordings wherein no male singing activity was detected were also discarded, as were those where the background noise was too high, making it impossible to detect the singing. A total of 11 recordings were discarded for these reasons and from the original 120 h of hour-long recordings obtained, 109 were used for further statistical analysis. This corresponded to 58 Yellowhammer individuals (see Table 1) and 8,468 individual strophes.
We assessed the noise by the actual traffic intensity at a given recording time, which was measured by counting passing cars from the recordings, as in the spectrogram the sound of a motor vehicle is displayed as a significant vertical curve. The long-term traffic conditions at a given recording place were assessed using data on annual average traffic intensity for the corresponding sections of motorways as provided by the Directorate of Roads and Motorways from the official national traffic census in 2016 (Directorate of Roads and Motorways, 2022). Furthermore, weather data such as air temperature (° C), wind speed (km/h), humidity (%), cloudiness (intesity on scale from 1 to 10; 1 meaning clear sky without any clouds, and 10 meaning completely cloudy) and air pressure (hPa) were provided by the Czech Hydrometeorological Institute (unpublished data) for the nearest meteorological stations within the national network of meteorological stations. These were located 4–10 km from the study locations.
We evaluated the effects of traffic noise on the Yellowhammer singing activity using linear mixed-effect models fitted by minimizing the restricted maximum likelihood (REML; package lme4 for R; Bates et al., 2015). We used the following variable as response variables: (i) song duration (s), (ii) average strophe length (s), and (iii) onset of singing (i.e., the start of singing relative to sunrise, in minutes). Because of the high correlation with song duration (r = 99%), the variable number of strophes was not analyzed (the correlation between song duration and average strophe length was only about 60%). Each response variable was analyzed by fitting a separate model for it. Because the recordings made at the same locality might have been subject to common specific conditions (local weather conditions, acoustic conditions specific for the time and location, etc.), we considered a random-intercept effect of locality. Further, because recordings made at the same site on Sunday and Monday correspond to the same individual, we also included a random-intercept effect of individual, nested within the effect of locality. To control for a possible effect of weather conditions on the Yellowhammer’s singing not captured by the random effect of locality, we included the following five weather variables as predictors into all our models: air temperature (temp), air pressure (press), wind speed (wind), cloudiness (cloud), and air humidity (humid). Before performing the model selection, we checked for possible multicollinearity by computing the variance inflation factor (VIF; function vif() from the R package car; Fox and Weisberg, 2019) on a model without interactions. We found air humidity to be highly explainable by the other weather variables (VIF > 8). We therefore excluded this variable from the models, which led to the reduction of VIF to acceptable levels (VIF < = 2).
We performed three different types of analyses. First, we compared the singing activity between highways and agricultural landscapes (variable loc), and between Sunday and Monday (variable day). The full models (one for each response variable) included all possible interactions among locality type, day, and meteorological variables (but not the interactions among meteorological variables themselves). In this way we considered a possible varying effect of locality type depending on the day (and vice versa), as well as varying effect of day resp. locality type depending on weather conditions. The full models were hence structured as follows:
Second, to further investigate whether Yellowhammer’s vocal activity is affected by the level of traffic noise even in the generally calm (compared to highways) agricultural landscapes, we evaluated the effect of distance to the nearest road in the agricultural landscapes (variable dist_road) on the Yellowhammer’s singing. The full models included interactions of each of the continuous predictors (i.e., the meteorological variables and distance to the nearest road) with day, because we might expect their effect depending on the actual level of the traffic noise. These models were only fitted on the data from the localities in the agricultural landscapes, and the structure of the full models was as follows:
Third, we further investigated what drives the singing activity under high traffic noise on highways, i.e., whether it is rather the instant traffic intensity (number of cars per hour, variable vehicles) or long-term traffic intensity (annual average of the number of cars per day for a given week day and highway segment, variable traff_int) on the Yellohammer’s singing. This part of analysis was only done with the data from the highway localities. The full models were structured as follows:
For each model, we performed a two-step model selection. First, we evaluated the significance of the random effect terms by REML-likelihood ratio test (function ranova() from the package lmerTest for R; Kuznetsova et al., 2017). Second, after identifying the random effect structure by dropping the insignificant terms, we proceeded by performing AIC-based stepwise backward selection of the fixed effect terms while following the principle of marginality (i.e., evaluating the higher interaction terms before the lower ones). For this procedure, all models were refitted by maximum likelihood. In each step, we dropped the term associated with the highest reduction of AIC, and then we selected the model with the minimum AIC over all models. Although this often resulted in models including insignificant terms, we only interpreted the significant ones. The significance of the fixed effect terms in the final models was assessed using Wald chi-squared test (function Anova from the R package car; Fox and Weisberg, 2019). The final model’s goodness-of-fit was evaluated by the generalized R-squared proposed by Nakagawa et al. (2017) and implemented in the R package MuMIn (Bartoń, 2022). All statistical analyses were done in the R/RStudio statistical software (R Core Team, 2021; RStudio Team, 2022). For data manipulation and graphics, we used the collection of R packages from the tidyverse meta-package (Wickham, 2020). The complete R code of the statistical analysis is available at https://github.com/vojta-bartak/Yellowhammer.git
We found that Yellowhammer’s song duration differed by locality type (Table 2, Figure 2). Whereas in the agricultural landscape, the average song duration was around 180 s, the average song duration in the localities near highways was only around 50 s. There was also a significant effect of wind speed and air pressure (Table 2), suggesting that the weather variables could also have an impact, although not affecting the magnitude of the effect of the locality type (i.e., there is no significant interaction between locality type and these weather variables).
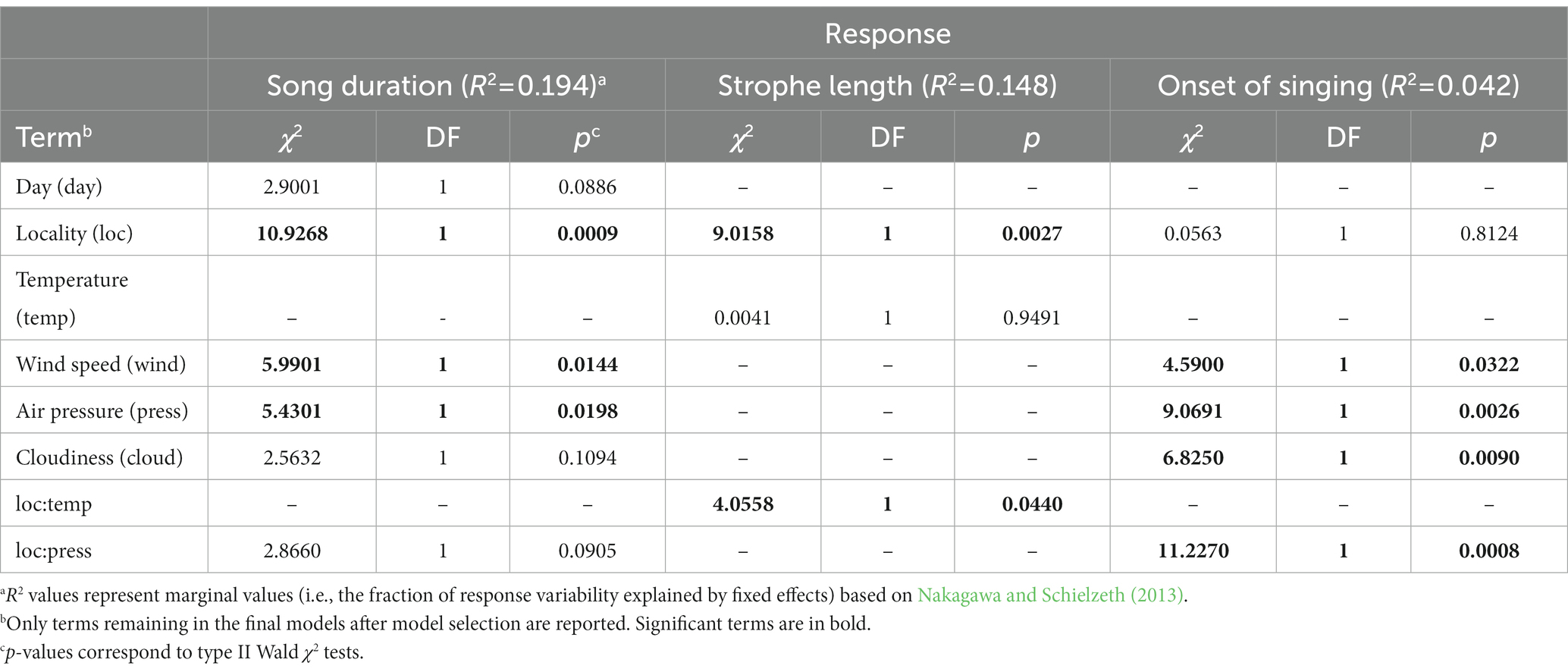
Table 2. Analysis of deviance for linear mixed models comparing Yellowhammer singing activity in agricultural landscapes and near highways.
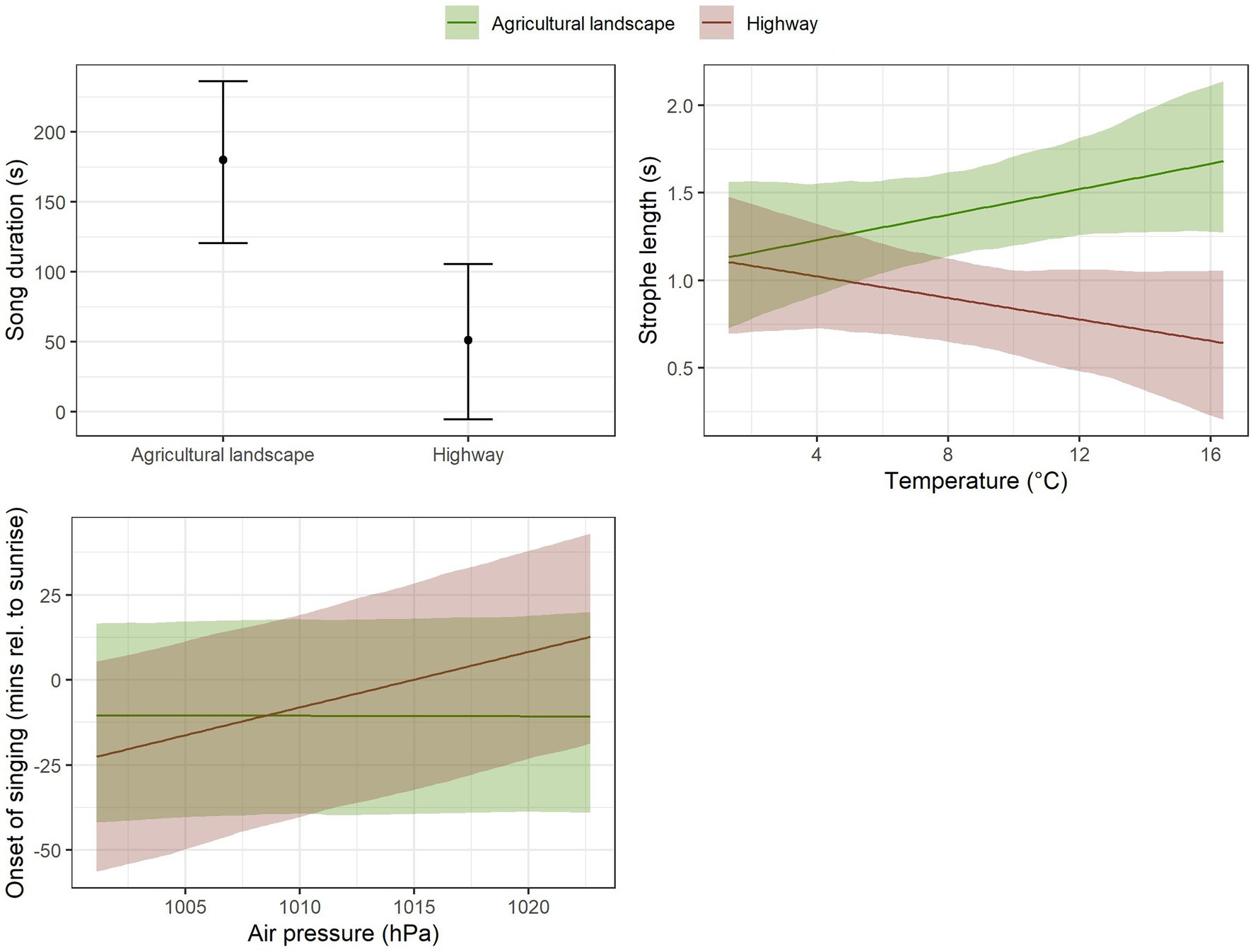
Figure 2. Impact of locality type (agricultural landscape versus highway) on the total length of Yellowhammer’s singing, impact of locality type in interaction with temperature on mean duration of a Yellowhammer song strophe, and impact of locality in interaction with air pressure on the start of Yellowhammer’s dawn chorus from 60 to 120 min after sunrise as estimated by linear mixed model. The displayed effects are for Monday and for mean observed cloudiness. The confidence bands are based on parametric bootstrap and reflect the uncertainty in random effect parameters.
The strophe length was affected by locality type in interaction with temperature (Table 2, Figure 2, Supplementary Table S1). Under low temperatures around 2°C, there was practically no difference in strophe length between the agricultural landscape and highways. But with increasing temperature, this difference grew to ca 1.5 s longer strophes in the agricultural landscape compared to highways (Figure 2).
We found a positive effect of air pressure on the onset of Yellowhammer’s singing at localities close to the highways, but not in the agricultural landscape (Figure 2). There was, however, relatively high uncertainty associated with this effect (see the wide confidence bands in the corresponding plot in Figure 2).
In the agricultural landscape, we found that Yellowhammer’s song duration increased with increasing distance from the nearest road (Table 3, Figure 3, Supplementary Table S2). Although those roads were by far not so highly frequented as highways, they nevertheless had non-negligible traffic inasmuch as they often lead to the highway access road. The total song duration at sites directly next to the nearest road was only around 20 s and it was increasing by an additional ca 100 s with each 250 m distance. The effect of day was not significant (Figure 3).
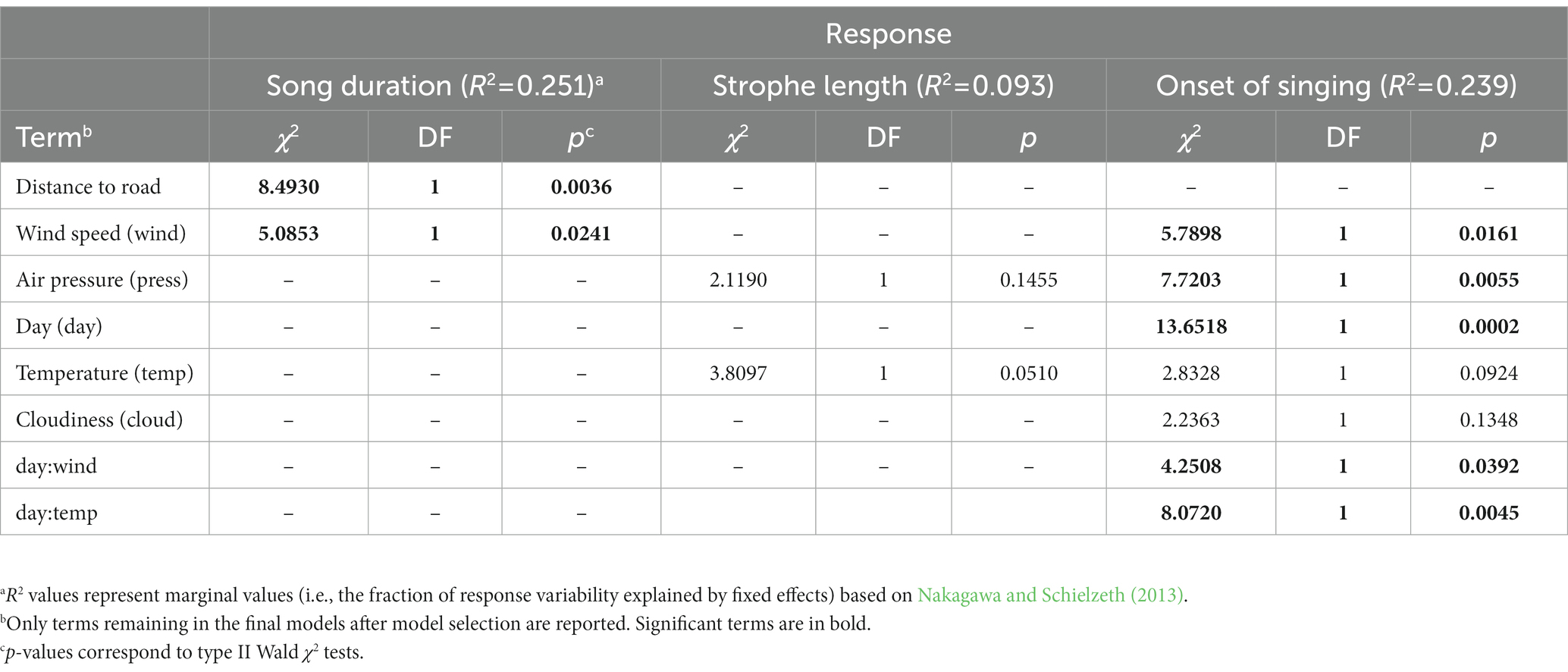
Table 3. Analysis of deviance tables for linear mixed models investigating effect of distance to nearest road on Yellowhammer singing activity.
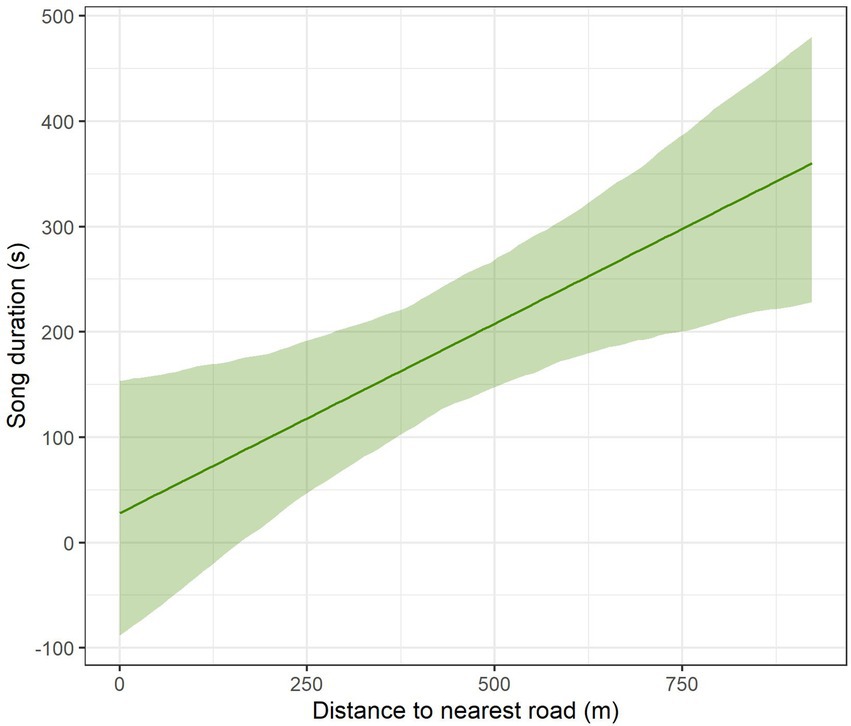
Figure 3. Impact of distance to the nearest road on total song duration for Yellowhammer in the agricultural landscape, estimated by linear mixed models. The displayed effect was generated while setting the wind speed to its average observed value. The confidence bands are based on parametric bootstrap and reflect the uncertainty in random effect parameters.
We found there to be an impact of both present and long-term highway traffic intensity on the different aspects of Yellowhammers’ singing activity (Table 4, Figure 4, Supplementary Table S3). With increasing long-term traffic intensity on highways, measured as the yearly average daily number of cars per day, the song duration of the Yellowhammer song was decreasing. For average 20,000 passing cars per day, the song duration was ca 80 s; with average 40,000 passing cars per day, the song duration was only around 30 s. For the impact of the present traffic intensity, measured as the number of cars per hour, the strophe length was decreasing with increasing number of passing vehicles. With 180 passing vehicles per hour, the strophe length was ca 1 s; with 600 passing vehicles per hour, the strophe length was only ca 0.5 s. Lastly, we found a later onset of singing relative to sunrise. With 200 passing vehicles, the onset of Yellowhammer’s singing was ca 14 min before sunrise; with 600 passing vehicles it was ca 1 min after sunrise (Figure 4).
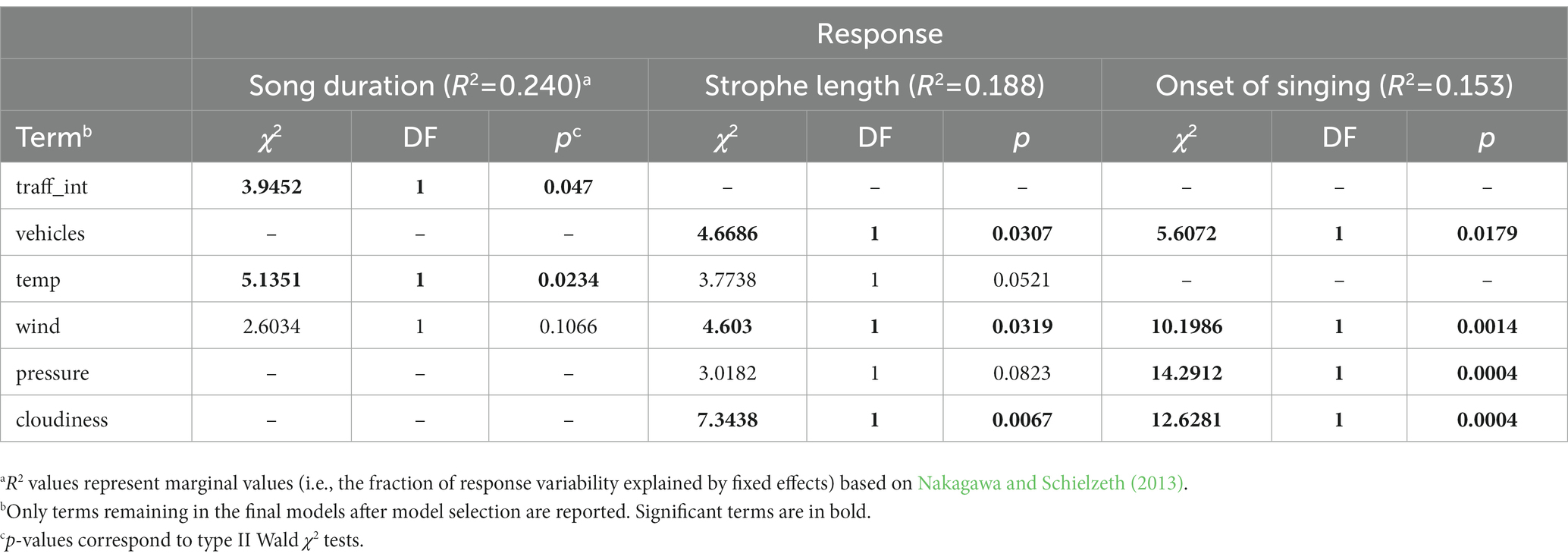
Table 4. Analysis of deviance tables for linear mixed models investigating effect of present-time and long-term traffic intensity on Yellowhammer singing activity.
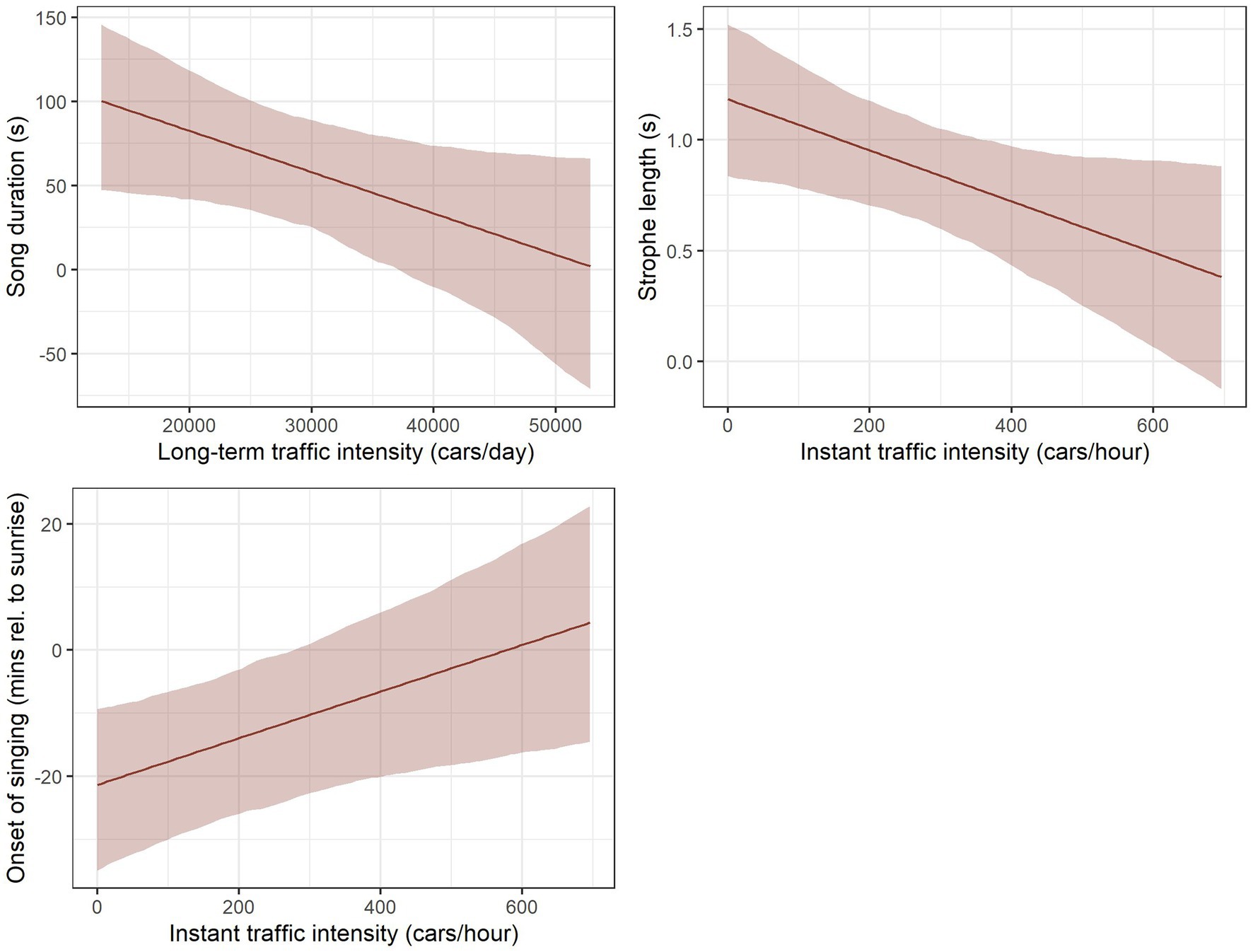
Figure 4. Impact of long-term and present traffic intensity on singing activity of Yellowhammer in the localities near highways, estimated by linear mixed models. The displayed effects were assessed while setting the values of other predictors (meteorological variables) to their average observed values. The confidence bands are based on parametric bootstrap and reflect the uncertainty in random effect parameters.
Examining the influence of highway noise on Yellowhammer’s song duration and strophe length, near highways versus agricultural landscape, we found specific dependencies between the observed singing characteristics and noise pollution. The typical song of the Yellowhammer consists of 1 to 3 parts. In this study it was not distinguished how many parts each strophe had, but shorter strophes usually have only 1 or 2 parts. Further study of strophe structures could be a subject of future studies.
Assessment of the singing activity of the Yellowhammer at noise-polluted areas near highways and at control areas in agricultural landscape showed significant difference between the two locality types. The total song duration was significantly longer at localities in the agricultural landscape than near highways. The total sum of the lengths of the individual singing for the whole 6 weeks of monitoring was 60.5% shorter at the noise-polluted localities (total sum 7.404.709 s for the 6 weeks recorded in agricultural landscape localities versus 2.928.161 s at localities near the highway). Therefore, we can state that Yellowhammer shortens the length of the song sequences sung in noisy places. A similar phenomenon was observed in Great Tit by Slabbekoorn and Boer-Visser (2007), reducing the length of individual strophes in urban areas. In contrast, Hamao et al. (2011) and Díaz et al. (2011), reported that birds in a noisier area did just the opposite and increased the proportion of time spent singing.
It must be acknowledged, of course, that to analyze singing in noisy localities was made more difficult by the high level of background noise caused by passing vehicles. It is therefore likely that not all the singing activity of the Yellowhammer was recorded, and it is necessary to take this into account. On the other hand, we can state that the number of individual strophes at the noisy areas was lower by 56% in terms of recorded sequences than at the control locations, which is a relatively large difference, and more correctly counting all the sequences probably would not have changed the result by much. Potvin et al. (2011) also found a decreased strophe rate in Silvereyes (Zosterops lateralis) in urban environments.
Many bird species have been observed to increase the minimum frequencies of their songs in response to noise (Ríos-Chelén et al., 2012; Nemeth et al., 2013; Kunc and Schmidt, 2020). The findings of Moseley et al. (2018) provide strong experimental support for cultural selection as a potential mechanism for acoustic adaptation to environments with anthropogenic noise. Klingbeil et al. (2020) reanalyzed how passerine song predicted exposure to noise using a more comprehensive dataset of acoustic song measurements and showed that it is large frequency bandwidths, rather than diverse strophes, that predict the exposure of species to noise pollution. Given that larger bandwidths often encompass higher maximum frequencies, which are less masked by anthropogenic noise, their result suggests that tolerance to noise pollution might depend mostly upon having the high-frequency parts of a song little masked by noise, thus preventing acoustic communication from going entirely unnoticed at long distances. The shift in the minimum frequency was not a subject of our study, but we found that Yellowhammer shortened the average duration of the strophe at localities near highways. This is in contrary to the findings of Sánchez et al. (2022) who found that Lincoln’s sparrow (Melospiza lincolnii) increases its singing rate in areas with chronic industrial noise. Assuming that the birds living in localities affected by noise pollution have higher stress hormone levels (Partecke et al., 2006 ex. Wingfield and Ramenofsky, 1999), Yellowhammer males seem to tend toward shortening their singing effort by leaving out the third syllable and mainly singing only one to two syllables (Supplementary Figures S1,S2). However, the amount of syllables was not assessed in this manuscript and could be examined in future studies.
Yellowhammer generally responds to noise by shortening the duration of its song. In the specific case of present-time noise caused by passing vehicles, we found a significant shortening of strophe length with increasing present traffic intensity. Statistical evaluation of long-term traffic noise showed a significant effect on the song duration. The variation in traffic noise intensity was also assessed in this study. Therefore, the recording took place every week on Sundays and Mondays. On Sunday mornings, the traffic can be expected to be calmer, whereas on Monday mornings, people commute to work and the traffic is much heavier. Based on statistics of the Directorate of Roads and Motorways (2022), however, we found that although the traffic is very heavy on Mondays, the intensity is even higher during the week, during Tuesdays to Thursdays. The main role in the traffic noise intensity is played by the total number of trucks, which on average was up to 4 times higher on Mondays than on Sundays. The numbers of passenger cars were quite similar on Sundays and Mondays. The results from statistical evaluation as to the effect of day on vocal activity of Yellowhammer showed no significant change in the duration of the total vocalization but significant change in the mean strophe length. We also found Yellowhammer responding to the day of the week by reducing its singing activity on Mondays, as 33% fewer strophes were recorded than on Sundays (Supplementary Figure S1).
McLaughlin and Kunc (2013) studied different intensities of noise and were the first to confirm the effect of different noise pollution levels on voice activity in European Robin (Erithacus rubecula). Under artificial conditions with increasing noise intensity, male robins were found to shorten the length of their song sequence or to leave the location. In natural conditions, robins manifested no significant change in their behavior. Lee and Park (2019) found that eastern Great Tit males in noisier locations sang with higher maximum and minimum frequencies and higher frequency bandwidth than did birds in quieter locations. Although there was a relatively small but significant difference in note and strophe length between the two groups, overall song components measured showed a significant difference between low-noise and high-noise groups. This implies that eastern Great Tits keep their strophes shorter to counteract the masking effects of noise and defend their territories (Dhondt and Lambrechts, 1991). Longer strophe length has been shown to be related to good habitat quality and high breeding success (Lambrechts and Dhondt, 1986). One can speculate that more successful males dominate where there is good habitat quality at low noise locations as opposed to in the poor habitat nearest roads at high noise locations. A study by Liu et al. (2020) showed that Zebra Finches (Taeniopygia guttata) show spatial avoidance of near- but not far-distance traffic noise. The birds avoided space with near-distance, high amplitude traffic noise when given a choice at no cost in terms of reduced access to food or increased risk of predation.
We found that with increasing average long-term traffic intensity Yellowhammer’s song duration was decreasing. Gallardo et al. (2021) suggest that helicopter noise induces a temporal shift in songbird vocalizations, but this shift only occurs in areas with very loud and frequent helicopter traffic. Gentry et al. (2017) also found that Nuttall’s White-crowned Sparrow (Zonotrichia leucophrys nuttalli) males at urban locations exhibited immediate signaling flexibility in response to fluctuations in background noise, whereas males at rural locations did not. Urban males decreased their maximum frequency and bandwidth with experimental noise. That, in turn, increased tonality, an adjustment that can improve signal detectability and discrimination in noisy environments. Ríos-Chelén et al. (2015) found that males in noisier places produced songs with fewer syllables and slower repeat rate of elements in some components (rattles). Birds may also improve the efficacy of communication in noise by increasing usage of other signaling modalities. Red-winged blackbirds also perform a visual display in different intensities while singing. Sierro et al. (2017) found that European Blackbirds (Turdus merula) exposed to aircraft noise began singing their chorus earlier, modified their song, and spent more time singing. Those findings suggest adaptive changes to the local noise environment. Blackbirds living near a large airport sang songs more often without twitter, only motif notes, and when they did include a twitter part in their songs, the twitter proportion was smaller. This may suggest that, in an effort to reduce masking by noise, airport blackbirds emphasized the loudest part of their songs (motifs) while disregarding the fainter part (twitter). The airport blackbirds did not, however, use higher frequencies than did those in the control population. Interesting findings were done by Brumm et al. (2004) on acoustic communication in noise in a New World monkey common marmosets (Callithrix jacchus) as the first evidence of such mechanism of vocal plasticity in an animal communication system. The studied marmosets increased the sound level of their spontaneous calls and the duration of the call syllabels in response to increased levels of white noise broadcast to them.
A thorough analysis of calls and acoustic conditions across a traffic road noise gradient by Barrero et al. (2020) revealed that Little Bustard (Tetrax tetrax) males showed some capacity for vocal adjustment by modifying the rate and duration of their calls – increasing their call rate when exposed to high levels of traffic noise and shortening the call duration with higher noise levels. This is similar to our findings as to the impact of present-time traffic on the average strophe length, which was decreasing with an increasing number of passing vehicles. We assume the individuals were trying to overcome the masking effect of the traffic noise by prolonging the strophe length. Also interesting are results from Phillips et al. (2020), which are the first to show a relationship between vocal performance, a physically limited song trait, and territory quality, thereby confirming that territory quality predicts avian vocal performance across an urban–rural gradient. These findings link fundamental aspects of sexual selection inasmuch as habitat quality and the quality of sexually selected signals appear to be associated: males with the highest performing songs are defending territories of the highest quality.
We also evaluated the influence of air temperature, cloudiness, wind speed, humidity, and air pressure on the total length of Yellowhammer song sequences in order to control for a possible effect of weather on the Yellowhammer’s singing activity not captured by the random effect of locality. An interesting finding was the impact of temperature on the Yellowhammer’s strophe lentgh. With increasing temperature, the strophes were getting longer in the agricultural landscape and even slightly shorter at localities near highways. Not many papers were examining the impact of weather factors on the singing activity of birds. According to Hannah (2007), weather factors including wind and temperature gradients, influence sound propagation. When the wind is blowing in the same direction as the sound, the sound is refracted toward the ground: the conditions are, therefore, favorable for sound propagation. This could be used by the male Yellowhammers to increase the sound propagation in a certain direction. Temperature gradients also influence the propagation of sound waves over long distances. Indeed, temperature influences the density of the air, which in turn influences the speed of sound. For air, which is considered a perfect gas, the lower the temperature, the higher the density and the lower the velocity. This decrease in speed is accompanied by a change in the trajectory of the sound waves: they are refracted. The refraction of sound waves is similar to the refraction of light (Hannah, 2007). There is not much known about how air pressure modulates sounds and thus birdsong, but it is known that with decreasing air pressure also the oxygen partial pressure decreases, that is, the lower the air pressure, the less oxygen in the air. Considering the lower oxygen partial pressure in the air, one might hypothesize that birds experiencing low air pressure would have a simpler song to ensure oxygen supply (Schäfer et al., 2017). According to a study by Schäfer et al. (2017), the influence of weather parameters (air pressure, atmospheric humidity, air and soil temperatures) on song traits of urban Great Tit, Eurasian Blue Tit (Cyanistes caeruleu), and European Blackbird (Turdus merula) was highly significant. The song trait variability of the investigated species was affected more by weather conditions than by urban characteristics in the city. However, the three species differed in their reactions to specific weather parameters. Smaller species seemed to be more affected by weather than larger species. With increasing humidity, the elements within a verse of Great Tit’s song became longer. Blue Tit had a narrower bandwidth of the element with the maximum bandwidth within a verse when the air pressure was low. It seems that different species show different song adaptations, as Brumm (2004) found no effect of environmental influences on song variables of the Common Nightingale (Luscinia megarhynchos) when considering air temperature and atmospheric humidity. Also O’Connor and Hicks (1980) found different influence of weather conditions for respective species on the detection of birds during common birds census fieldwork.
We found no difference in the onset of Yellowhammer singing activity at the localities in the agricultural landscape and near highways as measured by minutes before or after sunrise. This is in contrast to the results of Gil et al. (2015), who evaluated the impact of airport traffic on changes in the vocalization of bird populations living near airports and found that birds responded to different intensities of aircraft noise with a change in the timing of the beginning of their vocalization. They vocalized earlier in the morning to avoid overlapping with the noise pollution from the aircraft. The weather factors, on the other hand, showed a high level of significance in our analysis.
Areas near highways are not only affected by the noise pollution from cars, but also by the light pollution from the car headlights. In some papers it is not possible to distinguish the impact of light and noise pollution. Miller (2006) found that individuals of migratory thrush inhabiting localities with large amounts of artificial light started vocalizing earlier in the morning than did individuals in habitats with small amounts of artificial light. Hasan (2010) also studied the influence of human-made environmental noise and light pollution on the beginning of vocalizing in blackbirds at dawn. Artificial light was not found to be a determining factor to initiate the beginning of singing activity in that study. Significantly higher was the impact of noise pollution. A study published by Mendes et al. (2011) revealed changes in total frequency and frequency of sequences related to changes in ambient noise in rural areas versus urban areas and the time shift of vocalization in morning caused by anthropogenic impacts. Bergen and Abs (1997) found that Great Tit, Blue Tit, and Common Chaffinch started vocalizing earlier at dawn in areas with the presence of traffic noise than in quiet locations. The study by Hennigar et al. (2019) showed that birds in boreal forest are attracted to traffic noise but not to artificial lighting and that Yellow-rumped Warbler (Setophaga coronate) also began singing earlier in the presence of noise.
Dominoni et al. (2019) showed that artificial light at night and noise interact and produce complex effects on activity patterns of Great Tits. On the one hand, a presence of light at night may override a daytime effect of noise, whereas, on the other hand, continuous noise exposure may enhance the effect of light during the night as well as around dusk and dawn to an extent that is more than the simple addition of the two single effects of these stimuli. This finding is contrary to previous findings on this and similar species living near other anthropogenic sources of noise (Slabbekoorn and Peet, 2003).
In conclusion, we found an impact of traffic noise on singing activity of Yellowhammer. Our results show statistically significant impact of locality type, temperature and air pressure on the singing activity of this species. Furthermore, at localities in the agricultural landscape, we found a statistically significant impact of distance to the nearest road on the song duration. With increasing distance, the song duration was also increasing. In relation to the dawn, we found no difference in the singing activity of the Yellowhammer at localities in the agricultural landscape and near highways as measured in minutes before or after sunrise. For the impact of the present traffic intensity, measured as the number of cars per hour, we found a later onset of singing relative to sunrise with increasing number of passing vehicles.
Nevertheless, it is necessary to take into account other possible factors influencing the singing activity of Yellowhammer and which would possibly be the subject of future studies. These might include, for example, nesting season, light pollution, population density, and presence of other bird species (although Yellowhammer is usually relatively indifferent to other species). Also, localities close to other motorways might be evaluated.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material. The complete R code of the statistical analysis is available at https://github.com/vojta-bartak/Yellowhammer.git.
Ethical review and approval was not required for the animal study because during the processing of the study, there was no catchment of individuals in the wild, and there were no restrictions or threats to the studied individuals. Only a recording of their singing was made, using a sound recorder placed in the territory of the studied individual.
PZ conceived the experiment. LH and KM conducted the experiment. VB and AR-R analyzed the results. AR-R wrote the manuscript. VB and PZ reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Internal grant agency – Faculty of Environmental Sciences, Czech University of Life Sciences, Prague, grant number: 2023B0034.
The authors thank Marek Platil for supporting us in the collection of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1020982/full#supplementary-material
Alberti, M., Correa, C., Marzluff, J. M., Hendry, A. P., Palkovacs, E. P., Gotanda, K. M., et al. (2017). Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl. Acad. Sci. U. S. A. 114, 8951–8956. doi: 10.1073/pnas.1606034114
Barrero, A., Llusia, D., Traba, J., Iglesias-Merchan, C., and Morales, M. B. (2020). Vocal response to traffic noise in a non-passerine bird: the little bustard Tetrax tetrax. Ardeola 68, 143–162. doi: 10.13157/arla.68.1.2021.ra8
Bartoń, K. (2022). MuMIn: Multi-Model Inference. R package version 1.46.0. Available at: https://CRAN.R-project.org/package=MuMIn
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bergen, F., and Abs, M. (1997). Etho-ecological study of the singing activity of the blue tit (Parus caeruleus), great tit (Parus major) and chaffinch (Fringilla coelebs). J. fuer Ornithologie. 138, 451–467. doi: 10.1007/BF01651380
Bermúdez-Cuamatzin, E., Delamore, Z., Verbeek, L., Kremer, C., and Slabbekoorn, H. W. (2020). Variation in diurnal patterns of singing activity between urban and rural great tits. Front. Ecol. Evol. 8:246. doi: 10.3389/fevo.2020.00246
BirdLife Intrenational (2020). European Bird Census Council European Bird Populations: Estimates and Trends, Cambridge, UK: BirdLife International (BirdLife Conservation Series No. 10).
Bressler, S. A., Diamant, E. S., Tingley, M. W., and Yeh, P. J. (2020). Nests in the cities: adaptive and non-adaptive phenotypic plasticity and convergence in an urban bird: adaptive plasticity and convergence. Proc. R. Soc. B Biol. Sci. 287:20202122. doi: 10.1098/rspb.2020.2122
Brumm, H. (2004). The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440. doi: 10.1111/j.0021-8790.2004.00814.x
Brumm, H., Voss, K., Köllmer, I., and Todt, D. (2004). Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 207, 443–448. doi: 10.1242/jeb.00768
Bruni, A., Mennill, D. J., and Foote, J. R. (2014). Dawn chorus start time variation in a temperate bird community: relationships with seasonality, weather, and ambient light. J. Ornithol. 155, 877–890. doi: 10.1007/s10336-014-1071-7
Caspi, T., Johnson, J. R., Lambert, M. R., Schell, C. J., and Sih, A. (2022). Behavioral plasticity can facilitate evolution in urban environments. Trends Ecol. Evol. 37, 1092–1103. doi: 10.1016/j.tree.2022.08.002
Catchpole, C. K., and Slater, P. J. B. (1995). Bird Song: Biological Themes and Variations. Cambridge University Press, Cambridge.
Catchpole, C. K., and Slater, P. J. B. (2010). The Study of Bird Song. Cambridge University Press.Cambridge.
Da Silva, A., Valcu, M., and Kempenaers, B. (2016). Behavioural plasticity in the onset of dawn song under intermittent experimental night lighting. Anim. Behav. 117, 155–165. doi: 10.1016/j.anbehav.2016.05.001
Dawson, A., King, V. M., Bentley, G. E., and Ball, G. F. (2001). Photoperiodic control of seasonality in birds. J. Biol. Rhythm. 16, 365–380. doi: 10.1177/074873001129002079
del Hoyo, J., Elliott, A., and Christie, D. (2011). Handbook of the Birds of the World: Weavers to New World Warblers. Barcelona, Spain. Lynx Edicions. 15.
Dhondt, A. A., and Lambrechts, M. M. (1991). The many meanings of great tit song. Belg. J. Zool. 121, 247–256.
Díaz, M., Parra, A., and Gallardo, C. (2011). Serins respond to anthropogenic noise by increasing vocal activity. Behav. Ecol. 22, 332–336. doi: 10.1093/beheco/arq210
Diblíková, L., Pipek, P., Petrusek, A., Svoboda, J., Bílková, J., Vermouzek, Z., et al. (2019). Detailed large-scale mapping of geographical variation of yellowhammer Emberiza citrinella song dialects in a citizen science project. Ibis 161, 401–414. doi: 10.1111/ibi.12621
Directorate of Roads and Motorways. (2022). Nationwide traffic census 2016. Available at: http://scitani2016.rsd.cz/pages/informations/default.aspx (Accessed May 25, 2022).
Dominoni, D., Smit, J., Visser, M., and Halfwerk, W. (2019). Multisensory pollution: artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major). Environ. Pollut. 256:113314. doi: 10.1016/j.envpol.2019.113314
Forman, R. T. T., and Alexander, L. E. (1998). Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 29, 207–231. doi: 10.1146/annurev.ecolsys.29.1.207
Fox, J., and Weisberg, S. (2019). An {R} Companion to Applied Regression, 3rd. Thousand Oaks CA: Sage.
Fuller, R. A., Warren, P. H., and Gaston, K. J. (2007). Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370. doi: 10.1098/rsbl.2007.0134
Gallardo, K., Paxton, K., and Hart, P. (2021). Temporal changes in songbird vocalizations associated with helicopter noise in Hawai'i's protected natural areas. Landsc. Ecol. 36, 829–843. doi: 10.1007/s10980-020-01179-2
Gentry, K. E., Derryberry, E. P., Danner, R. M., Danner, J. E., and Luther, D. A. (2017). Immediate signaling flexibility in response to experimental noise in urban, but not rural, white-crowned sparrows. Ecosphere 8:e01916. doi: 10.1002/ecs2.1916
Gil, D., Honarmand, M., Pascual, J., Pérez-Mena, E., and Macías Garcia, C. (2015). Birds living near airports advance their dawn chorus and reduce overlap with aircraft noise. Behav. Ecol. 26, 435–443. doi: 10.1093/beheco/aru207
Gómez, O. H. M. (2022). Artificial light at night drives earlier singing in a Neotropical bird. Animals 12, 1–10. doi: 10.3390/ani12081015
Grunst, M. L., Grunst, A. S., Pinxten, R., and Eens, M. (2021). Variable and consistent traffic noise negatively affect the sleep behavior of a free-living songbird. Sci. Total Environ. 778:146338. ISSN 0048-9697,. doi: 10.1016/j.scitotenv.2021.146338
Hamao, S., Watanabe, M., and Mori, Y. (2011). Urban noise and male density affect songs in the great tit (Parus major). Ethol. Ecol. Evol. 23, 111–119. doi: 10.1080/03949370.2011.554881
Hannah, L. (2007). Wind and temperature effects on sound propagation. New Zealand Acoustics 20:2. https://www.acoustics.org.nz/sites/www.acoustics.org.nz/files/journal/pdfs/Hannah,_L_NZA2007_(a).pdf
Hasan, N. M. (2010). The effect of environmental conditions on the start of dawn singing of blackbirds (Turdus merula) and bulbuls (Pycnonotidae). Jordan J. Biol. Sci. 3, 13–16.
Hennigar, B., Ethier, J. P., and Wilson, D. R. (2019). Experimental traffic noise attracts birds during the breeding season. Behav. Ecol. 30, 1591–1601. doi: 10.1093/beheco/arz123
Hladík, Š. (2021). The Highway Impact on Bird Communities. [diploma thesis] Department of Ecology, Czech University of Life Sicences in Prague. Czechia.
Huffeldt, N. P., and Dabelsteen, T. (2013). Impact of a noise-polluted urban environment on the song frequencies of a cosmopolitan songbird, the great tit (Parus major), in Denmark. Ornis Fennica 90, 94–102.
Katti, M., and Warren, P. S. (2004). Tits, noise and urban bioacoustics. Trends Ecol. Evol. 19, 109–110. doi: 10.1016/j.tree.2003.12.006
Keast, A. (1994). Temporal vocalisation patterns in members of a eucalypt Forest bird community: the effects of weather on song production. Emu 94, 172–180. doi: 10.1071/MU9940172
Klingbeil, B. T., La Sorte, F. A., Lepczyk, C. A., Fink, D., and Flather, C. H. (2020). Geographical associations with anthropogenic noise pollution for north American breeding birds. Glob. Ecol. Biogeogr. 29, 148–158. doi: 10.1111/geb.13016
Kreithen, M. L., and Keeton, W. T. (1974). Detection of changes in atmospheric pressure by the homing pigeon, Columba livia. J. Comp. Physiol. 89, 73–82. doi: 10.1007/BF00696164
Kuitunen, M., Viljanen, J., Rossi, E., and Sternroos, A. (2003). Impact of busy roads on breeding success in pied flycatchers Ficedula hypoleuca. Environ. Manag. 31, 79–0085. doi: 10.1007/s00267-002-2694-7
Kułaga, K., and Budka, M. (2020). Nocturnal singing by diurnal birds in a temperate region of Central Europe. J. Ornithol. 161, 1143–1152. doi: 10.1007/s10336-020-01794-5
Kunc, H., and Schmidt, R. (2020). Species sensitivities to a global pollutant: a meta-analysis on acoustic signals in response to anthropogenic noise. Glob. Chang. Biol. 27, 675–688. doi: 10.1111/gcb.15428
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Lambrechts, M. M., and Dhondt, A. A. (1986). Male quality, reproduction, and survival in the great tit (Parus major). Behav. Ecol. Sociobiol. 19, 57–63. doi: 10.1007/BF00303843
Lee, C., and Park, C. R. (2019). An increase in song pitch of eastern great tits (Parus minor) in response to urban noise at Seoul, Korea. Urban Ecosyst. 22, 227–233. doi: 10.1007/s11252-018-0809-z
Liu, Q., Slabbekoorn, H. W., and Riebel, K. (2020). Zebra finches show spatial avoidance of near but not far distance traffic noise. Behaviour 157, 333–362. doi: 10.1163/1568539X-bja10004
Lukanov, S., and Naumov, B. (2019). Effect of anthropogenic noise on call parameters of Hyla arborea (Anura: Hylidae). Ecol. Quest. 30, 1–60. doi: 10.12775/EQ.2019.006
McLaughlin, K. E., and Kunc, H. P. (2013). Experimentally increased noise levels change spatial and singing behaviour. Biol. Lett. 9:1. doi: 10.1098/rsbl.2012.0771
Mendes, S., Colino-Rabanal, V. J., and Peris, S. J. (2011). Bird song variations along an urban gradient: the case of the European blackbird (Turdus merula). Landsc. Urban Plan. 99, 51–57. doi: 10.1016/j.landurbplan.2010.08.013
Miller, M. W. (2006). Apparent effects of light pollution on singing behavior of American robins. Condor 108, 130–139. doi: 10.1093/condor/108.1.130
Morelli, F., Beim, M., Jerzak, L., Jones, D., and Tryjanowski, P. (2014). Can roads, railways and related structures have positive effects on birds? - a review. Transp. Res. D Transp. Environ. 30, 21–31. doi: 10.1016/j.trd.2014.05.006
Morton, E. S. (1975). “Ecological Sources of Selection on Avian Sounds,” in The American Naturalist. Vol 109, 17–34. doi: 10.1086/282971
Moseley, D. L., Derryberry, G. E., Phillips, J. N., Danner, J. E., Danner, R. M., Luther, D. A., et al. (2018). Acoustic adaptation to city noise through vocal learning by a songbird. Proc. R. Soc. B 285:20181356. doi: 10.1098/rspb.2018.1356
Nakagawa, S., Johnson, P. C. D., and Schielzeth, H. (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14:20170213. doi: 10.1098/rsif.2017.0213
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4:133–142. doi: 10.1111/J.2041-210x.2012.00261.X
Nakamura-Garcia, M. T., and Ríos-Chelén, A. A. (2007). More than noise: light, moon phase, and singing behavior in a passerine. Urban Ecosyst. 25, 291–303. doi: 10.1007/s11252-021-01142-2
Nemeth, E., Pieretti, N., Zollinger, S. A., Geberzahn, N., Partecke, J., Miranda, A. C., et al. (2013). Bird song and anthropogenic noise: vocal constraints may explain why birds sing higher-frequency songs in cities. Proc. R. Soc. B Biol. Sci. 280, –20122798. doi: 10.1098/rspb.2012.2798
Nordt, A., and Klenke, R. (2013). Sleepless in town - drivers of the temporal shift in Dawn song in urban European blackbirds. PLoS One 8, 1–10. doi: 10.1371/journal.pone.0071476
O’Connor, R. J., and Hicks, R. K. (1980). The influence of weather conditions on the detection of birds during common birds census fieldwork. Bird Study 27, 137–151. doi: 10.1080/00063658009476672
Partecke, J., Schwabl, I., and Gwinner, E. (2006). Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952. doi: 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2
Phillips, J. N., Cooper, W. J., Luther, D. A., and Derryberry, E. P. (2020). Territory quality predicts avian vocal performance across an urban-rural gradient. Front. Ecol. Evol. 8:455. doi: 10.3389/fevo.2020.587120
Potvin, D. A., Parris, K. M., and Mulder, R. A. (2011). Geographically pervasive effects of urban noise on frequency and strophe rate of songs and calls in silvereyes (Zosterops lateralis). Proc. R. Soc. B Biol. Sci. 278, 2464–2469. doi: 10.1098/rspb.2010.2296
Quinn, J. L., Whittingham, M. J., Butler, S. J., and Cresswell, W. (2006). Noise, predation risk compensation and vigilance in the chaffinch Fringilla coelebs. J. Avian Biol. 37, 601–608. doi: 10.1111/j.2006.0908-8857.03781.x
R Core Team (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria.
Rabin, L. A., Coss, R. G., and Owings, D. H. (2006). The effects of wind turbines on antipredator behavior in California ground squirrels (Spermophilus beecheyi). Biol. Conserv. 131, 410–420. doi: 10.1016/j.biocon.2006.02.016
Rheindt, F. E. (2003). The impact of roads on birds: does song frequency play a role in determining susceptibility to noise pollution? J. fur Ornithologie 144, 295–306. doi: 10.1007/BF0246562
Richards, D. G., and Wiley, R. H. (1980). Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am. Nat. 115, 381–399. doi: 10.1086/283568
Ríos-Chelén, A. A., Lee, G. C., and Patricelli, G. L. (2015). Anthropogenic noise is associated with changes in acoustic but not visual signals in redwinged blackbirds. Behav. Ecol. Sociobiol. 69, 1139–1151. doi: 10.1007/s00265-015-1928-7
Ríos-Chelén, A. A., Salaberria, C., Barbosa, I., Macías Garcia, C., and Gil, D. (2012). The learning advantage: bird species that learn their song show a tighter adjustment of song to noisy environments than those that do not learn. J. Evol. Biol. 25, 2171–2180. doi: 10.1111/j.1420-9101.2012.02597.x
Ryan, M. J., and Brenowitz, E. A. (1985). The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am. Nat. 126, 87–100. doi: 10.1086/284398
Sánchez, N. V., Bayne, E. M., and Hilje, B. (2022). Lincoln’s sparrow (Melospiza lincolnii) increases singing rate in areas with chronic industrial noise. Ibis. doi: 10.1111/ibi.13174
Schäfer, J., Janocha, M., Klaus, S., and Tietze, T. (2017). How weather instead of urbanity measures affects song trait variability in three European passerine bird species. Ecol. Evol. 7, 4868–4880. doi: 10.1002/ece3.3032
Sebastianelli, M., Blumstein, D. T., and Kirschel, A. N. G. (2021). Higher-pitched bird song towards the coast supports a role for selection in ocean noise avoidance. Bioacoustics 31, 41–58. doi: 10.1080/09524622.2021.1879680
Sierro, J., Schloesing, E., Pavón, I., and Gil, D. (2017). European blackbirds exposed to aircraft noise advance their chorus, modify their song and spend more time singing. Front. Ecol. Evol. 5:68. doi: 10.3389/fevo.2017.00068
Slabbekoorn, H., and Boer-Visser, A. (2007). Cities change the songs of birds. Curr. Biol. 16, 2326–2331. doi: 10.1016/j.cub.2006.10.008
Slabbekoorn, H., and Peet, M. (2003). Ecology: birds sing at a higher pitch in urban noise. Nature 424:267. doi: 10.1038/424267a
Slabbekoorn, H., and Ripmeester, E. A. P. (2008). Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83. doi: 10.1111/j.1365-294X.2007.03487.x
Warren, P. S., Katti, M., Ermann, M., and Brazel, A. (2006). Urban bioacoustics: It’s not just noise. Anim. Behav. 71, 491–502. doi: 10.1016/j.anbehav.2005.07.014
Wickham, H. (2020). Tidyr: Tidy messy data. R package version 1.1.2. Available at: https://CRAN.R-project.org/package=tidyr
Wiley, R. H., and Richards, D. G. (1978). Physical constraints on acoustic communication in the atmosphere: Implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3:69–94. doi: 10.1007/BF00300047
Keywords: noise pollution, songbird, urbanization, vocal activity, road ecology, weather conditions
Citation: Ritz-Radlinská A, Barták V, Hodačová L, Maidlová K and Zasadil P (2023) The singing activity of the Yellowhammer (Emberiza citrinella) under traffic noise around highways. Front. Ecol. Evol. 11:1020982. doi: 10.3389/fevo.2023.1020982
Received: 28 November 2022; Accepted: 09 March 2023;
Published: 03 April 2023.
Edited by:
David Andrew Luther, George Mason University, United StatesReviewed by:
Felipe N. Moreno-Gómez, Universidad Católica del Maule, ChileCopyright © 2023 Ritz-Radlinská, Barták, Hodačová, Maidlová and Zasadil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petr Zasadil, emFzYWRpbEBmenAuY3p1LmN6
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.