
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 21 October 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.999488
This article is part of the Research Topic Neotropical Dung Beetle Diversity: Ecological, Historical, and Anthropogenic Perspectives View all 11 articles
Ecological studies with Scarabaeinae dung beetles have increased exponentially over the past 30 years, using lethal pitfall traps baited with mammal feces or carrion as the preferred sampling method. Different studies have determined the distance between pitfall traps for effective sampling, but the number of traps is often subjective, leading to excessive or poor sampling. This study provides quantitative guidelines for establishing the sample size for optimal completeness of dung beetle diversity by systematically reviewing the relationship between sampling intensity and sampling coverage, habitat type, and the journal impact factor in peer-reviewed research. We gathered 94 studies covering a range from México to Argentina. Sampling was conducted mainly in forested habitats, followed by treeless agriculture and agroforestry systems, with a median value of 50 pitfall traps per sampled habitat. Sampling completeness was above 0.9 in 95% of the studies. Oversampling ranged from 1 to more than 96,000 individuals, and sampling deficit varied between 2 and 3,300 specimens. Sampling intensity and the journal impact factor were significantly and positively correlated with oversampling, but these variables did not explain the sampling deficit. The positive correlation between journal impact factor and oversampling may reflect a publication bias where high-impact journals and researchers seek more generalizable information obtained with a higher sampling intensity. Dung beetle oversampling was not homogeneous between habitats, being highest in old-growth forests and lowest in disturbed habitats such as pastures and forest edges. Our results show that the collection intensity used in dung beetle studies should be reconsidered carefully. By incorporating ethical principles used in animal science, we suggest sampling guidelines for a robust sampling scheme of dung beetle diversity, which would also prevent oversampling. Consciously reducing sampling intensity will make resource use more cost-effective. We suggest increasing the number of independent sampling units rather than intensifying subsampling, thereby increasing the predictive power of statistical models to obtain more robust evidence of the phenomena under study.
Scarabaeinae dung beetles are among the most studied and best-known insect groups (Nichols et al., 2007; Fuzessy et al., 2021). Although globally distributed, they are most abundant in the tropics (Gill, 1991). Dung beetles provide vital ecosystem functions, including nutrient recycling, soil removal, secondary seed dispersal, and control of livestock parasites (Nichols et al., 2008). Environmental disturbances that affect mammalian communities — the primary resource suppliers for dung beetles — rapidly cause alterations in dung beetle communities (Nichols et al., 2009; Bogoni et al., 2019). Microclimatic changes in humidity, temperature, and soil conditions may also negatively affect dung beetles (Giménez Gómez et al., 2020; Pessôa et al., 2021). Besides, dung beetles are highly effective biological indicators of habitat quality, given their stable taxonomy and quick response to habitat disturbances, in addition to our deep understanding of their ecology (Favila and Halffter, 1997; Nichols et al., 2007; Tarasov and Dimitrov, 2016; Fuzessy et al., 2021).
The ease and relatively inexpensive collection of dung beetles make them an extremely popular model group in ecology (Gardner et al., 2008). Ecological and biodiversity studies with dung beetles have increased exponentially over the past 30 years (Figure 1). Although several methods have been proposed for the systematic collection of dung beetles, such as NTP-80 (sensu Morón and Terrón-S, 1984) and flight interception traps (Davis et al., 2001), pitfall traps baited with mammal feces or carrion are the most popular sampling method (Price and Feer, 2012). Pitfall traps consist of a plastic container buried flush with the ground, usually filled up to one-third of its capacity with an aqueous solution that prevents dung beetles from escaping while preserving the specimens fresh (Iannuzzi et al., 2020).
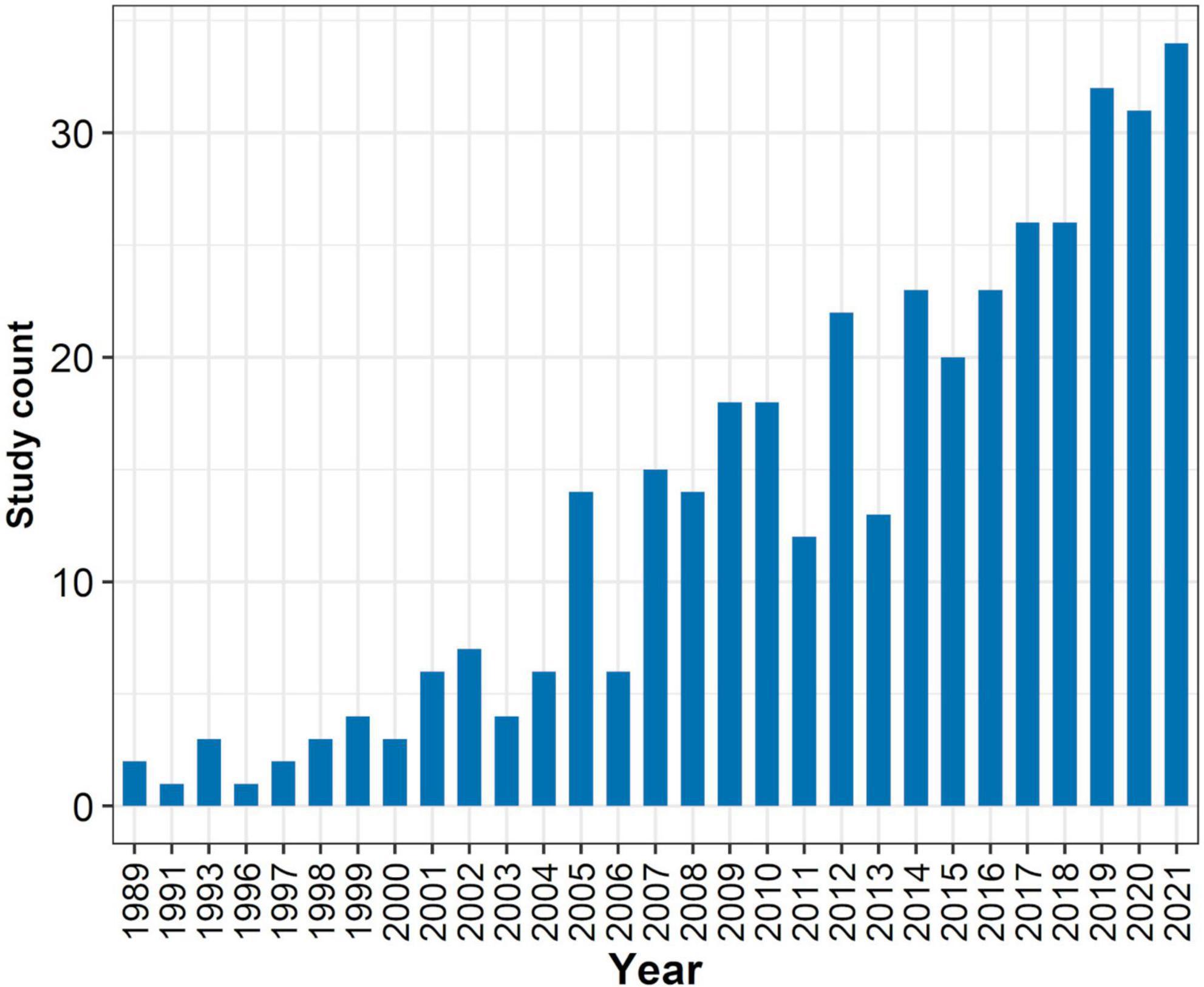
Figure 1. Ecological studies addressing dung beetles throughout the years. Data gathered from a search on Web of Science using the following terms: ((“Dung Beetle*” OR Scarabaeinae) AND Tropic* AND (Disturb* OR “Land-use change” OR modific* OR fragmenta* OR Ecolog*)).
Several studies have evaluated the factors involved in conducting a comprehensive and statistically rigorous sampling of dung beetle communities using pitfall traps. The effective sampling area of pitfall traps in tropical habitats is well documented (Larsen and Forsyth, 2005; da Silva and Hernández, 2015). The effectiveness of different bait types (Filgueiras et al., 2009; Whipple and Hoback, 2012; Marsh et al., 2013; Bogoni et al., 2014), the effective activity time for pitfall traps according to bait type (Flechtmann et al., 2009; Price and Feer, 2012), and the efficacy of different liquid preservatives (Aristophanous, 2010) have also been evaluated. However, much remains to be understood regarding the sampling effort (e.g., the number of traps) needed to obtain a representative sample of dung beetle diversity. Some authors recommend a minimum of 30 pitfall traps per habitat type, distributed in two or three linear transects (Villarreal et al., 2004); others proposed using seven or ten pitfall traps per sampling site (Larsen and Forsyth, 2005; Krell, 2007), whereas Feer (2000) suggest that the number of traps is not as significant as the sampling time. These suggestions are based on empirical field experience. While the few systematic approaches for establishing an appropriate number of traps are very valuable (i.e., Price and Feer, 2012; Ferrer-Paris et al., 2013; Tocco et al., 2017), these derive from local and highly contextual studies, making it difficult to generalize their results.
A method to assess and compare diversity through sampling coverage instead of sampling size was proposed by Chao and Jost (2012). Coverage estimates the proportion of individuals in a community that belongs to the species observed in the sample. As completeness increases, the proportion of individuals of undetected species in the community decreases. Comparing samples robustly without discarding information through the rarefaction process helps design sampling schemes that ensure a representative community sample (Bonar et al., 2011; Montes et al., 2021; Roswell et al., 2021). Insufficient species sampling restrains effective diversity comparisons between communities, while oversampling is less pragmatic as it wastes time and money and leads to the unnecessary population extraction of hundreds to thousands of specimens, including non-targeted ones (Tocco et al., 2017). A substantial decline in species abundance in animal communities can ultimately lead to impaired ecosystem functioning (see Gaston et al., 2018).
Recent studies have shown the accelerated decline of terrestrial insects due to habitat loss and climate change (Sánchez-Bayo and Wyckhuys, 2019; Wagner et al., 2021). These environmental pressures are arguably more intense on organisms susceptible to habitat disturbances, such as Scarabaeinae dung beetles, characterized by their relatively low reproductive and growth rates, making this group more vulnerable to extinction (Horgan and Fuentes, 2005; Larsen et al., 2005). Nevertheless, we expect an increasing demand for field data on dung beetles for future ecological studies, given their proven effectiveness as an ecological model (Brischoux and Angelier, 2015). Given the discouraging environmental scenario for insect populations and the continuous need for dung beetle field data, our main objective is to provide quantitative guidelines that establish the sample size for optimal completeness of dung beetle diversity. To this end, we have systematically reviewed and analyzed the relationship between sampling effort and the degree of coverage completeness of species richness, the journal impact factor, and the habitats surveyed in ecological studies of Neotropical dung beetles. Our guidelines aim to lead to more practical, cost-effective, sustainable, and ethical dung beetle sampling without under- or oversampling individuals and species.
To construct the database, we systematically searched published literature on the Web of Science website (WoS)1. The search covered articles published from 1980 to 2021. We employed the search terms ((“Dung Beetle*” OR Scarabaeinae) AND (“Disturbance gradient*” OR “Habitat disturbance*” OR “Land-use change” OR Anthro* OR Modification OR Fragmentation OR Agriculture OR Pasture*) AND (“Species richness” OR Diversity OR Abundance*) AND (Communit* OR Assemblage*) AND (“Tropical forest” OR Tropic*)).
We included only those articles that met the following criteria: (1) the study should address the ecology and diversity of Scarabaeinae; (2) the study should be conducted within the Neotropics (sensu Morrone et al., 2022); (3) the study should report the abundance of collected dung beetles; (4) abundance data should be reported separately for each species, habitat, or locality; (5) each dataset should be unique, i.e., not having been used previously in a different publication.
From each selected article, we extracted the number of individuals collected by species, habitat type, and number of replicate samples collected in each habitat (n); the Scopus impact factor of the journal where and when each paper was published; the species collection method; the total number of traps per habitat; the bait type; geographic information regarding the sampling sites, including the locality, municipality, and country; the climatic season when samples were collected; and the Neotropical dominion zone (sensu Morrone et al., 2022) where the study was carried out (Supplementary Tables 1, 2). Dominions are part of a hierarchical system that categorizes geographic regions according to their extant biota (Morrone, 2014). We omitted biogeographic provinces — a spatially finer biogeographic division in Morrone’s scheme (2022) — because the poor representativeness of some provinces would have created a significant imbalance between categories.
Considering the heterogeneity of habitat classifications in each paper, we decided to recategorize them into broader land-use types, pooling those habitats with similar characteristics (Table 1). Our new classification scheme could not include some habitat types because of their unique characteristics, low representativeness, or location in transition zones between Neotropical and Nearctic ecosystems. Such categories in our new classification scheme were altitudinal gradients (n = 8), landscape types (n = 6), Nearctic/tropical transition zones (n = 5), shrublands (n = 3), and pine forests (n = 2).
All analyses were performed using the statistical environment R v.4.1.1 (R Core Team, 2021). We determined the sampling coverage and the abundance needed to reach 99% of sampling completeness based on the number of individuals collected per species and habitat type in each study with Chao and Jost’s (2012) coverage estimator using the “iNEXT” package in R (Hsieh et al., 2016). We selected 99% completeness to perform a more conservative assessment of the abundance needed to achieve a near-complete sampling of species richness in the habitats sampled in each study. We also quantified the number of individuals exceeding (oversampling) or required (sampling deficit) to achieve 99% coverage. Oversampling and sampling deficit were represented by positive and negative values, respectively.
Linear mixed models were used to evaluate the correlation of sampling intensity and the journal impact factor with dung beetle oversampling and sampling deficit. To control for potential confounding factors caused by variations in the dung beetle trapping efficiency observed with different traps (Ong et al., 2022; Supplementary Table 2 and Supplementary Figure 1), we restricted the analysis to only those studies that used pitfall trapping as the primary collection method. We did not control for sampling season (SS) and bait type (BT) as linear mixed models showed no significant relationships between these independent factors and dung beetle sampling (SS: F = 1.09, P = 0.34; BT: F = 1.82, P = 0.15; Supplementary Table 3).
Sampling intensity was represented by the number of pitfall traps used in each habitat of each study. We adjusted the number of traps to the number of resamplings conducted at each study site (Samplingintensity = No. ofpitfalltraps*No. ofresamplings) to obtain a less biased value of sampling intensity. We defined resampling as the number of times the researcher sampled a particular site during each study. Due to the high heterogeneity observed between response and predictor variables, the data were log-transformed to normalize the distribution of trap numbers and dung beetle oversampling. Thus, we modeled sampling deficit as log-transformed positive values. The identity of each study and the biogeographic dominion were employed as nested random variables (Biogeographic dominion/study ID) to control for the lack of independence of the predictor factor derived from the intrinsic characteristics of each study (researcher, sampling site, and design) and environmental similarities within biogeographic dominions. We eliminated dominions whose data did not significantly correlate with dung beetle oversampling to increase model fit. Model simplification was supported by significantly lower Akaike information criterion values (Δ AIC > 2; Supplementary Tables 4A,B; Burnham and Anderson, 2002).
Exploratory analysis models showed no significant differences in dung beetle oversampling patterns between biogeographic dominions (F = 0.54; P = 0.80, Supplementary Table 3). Therefore, we pooled the data to model how sampling intensity determines dung beetle oversampling in each habitat (Table 1) using the study identity and its biogeographic dominions as random variables. All linear mixed models were constructed with the lme4 R package (Bates et al., 2015). Model fit and the assumptions of residuals normality, variance homoscedasticity, and independence between the response variables were checked with the Performance R-package (Lüdecke et al., 2021). The predicted parameters of the linear mixed models were obtained with the “ggeffects” package in R (Lüdecke, 2018).
Our search recovered 272 published papers, from which we selected 87 after applying the exclusion criteria mentioned above. We included seven additional articles from the authors’ collection not captured by the systemized search (Supplementary Table 1; Study ID: 17, 29, 35, 55, 70, 81, 82). The studies covered ten countries: 32 in Mexico, seven in Central America, and 55 in South America; of these, 38 were conducted in Brazil (Figure 2 and Supplementary Table 1). Sixty percent of the studies were located in the Mesoamerican, Pacific and Parana dominions (30.9, 17, and 17%, respectively), followed by the Boreal Brazilian, and South Brazilian dominions (Supplementary Table 2). The Southeastern Amazonian and Chacoan dominions were the least represented, comprising 10% of the study sites (Supplementary Table 2). Most sample sites belonged to forest habitats under varying degrees of disturbance (60%; see Table 1), followed by treeless agriculture systems (24%) and agroforestry systems, which were the less represented habitat types (13%; Table 1).
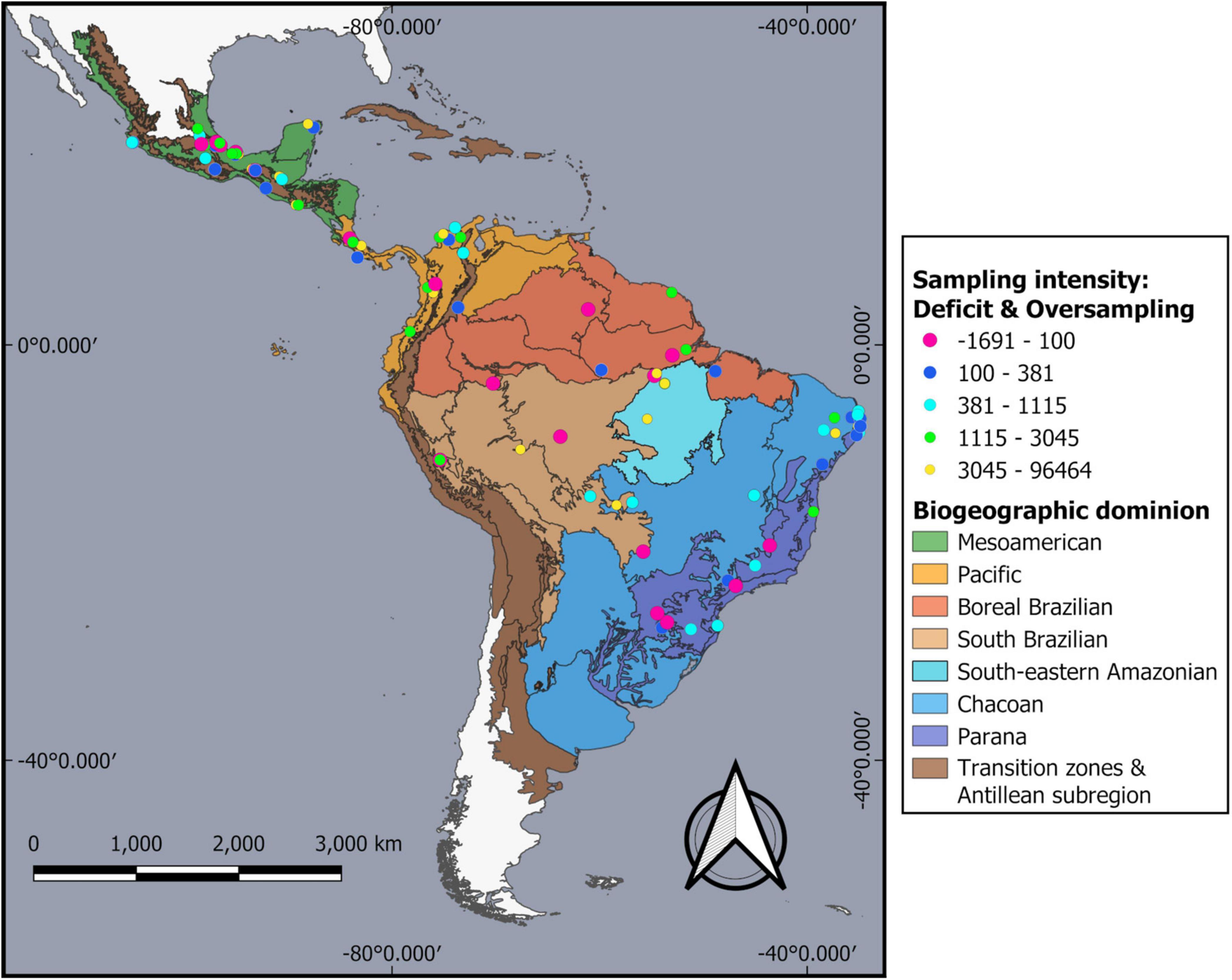
Figure 2. Geographic distribution of the sites studied. Sampling intensity is reported for each study site. The sampling deficit shows those sites where the sample did not reach 99% completeness, with negative values representing the effective abundance deficit per sampling site. Oversampling shows those sites where the sample exceeded 99% completeness, indicating the excess abundance per sampling site. Vector image from Morrone et al. (2022).
Because of the high heterogeneity and extreme outliers found in abundance and pitfall numbers, the data were described with median and mean values. We found a median of 52 traps and a mean of 247 traps per sampled habitat; sampling intensity ranged from four to 12,600 traps (Supplementary Table 2). Regarding studies with pitfall traps, 268 sampled habitats (73%) achieved 99% sampling coverage, 67 (18%) between 98 and 95%, and 33 (9%) showed a sampling coverage below 95%. The mean and median sampling coverage values per habitat and study were 98 and 99%, respectively; the lowest recorded value was 33%. Oversampling ranged from 1 to 96,464 individuals, with a mean of 2,928 dung beetle specimens and a median of 630. Sampling deficits varied between 2 and 3,329 dung beetles, with mean and median values of 248 and 103 dung beetles, respectively (Supplementary Table 2).
Dung beetle oversampling was significantly explained by sampling intensity and the journal impact factor (Figure 3). The total explanatory power of the linear mixed model was 0.71 (conditional R2), of which 0.41 was due to the fixed effects alone (marginal R2). According to our model parameters, oversampling increased by 0.98% and 0.55% for every 1% increase in trap number and journal impact factor, respectively (Supplementary Table 4B).
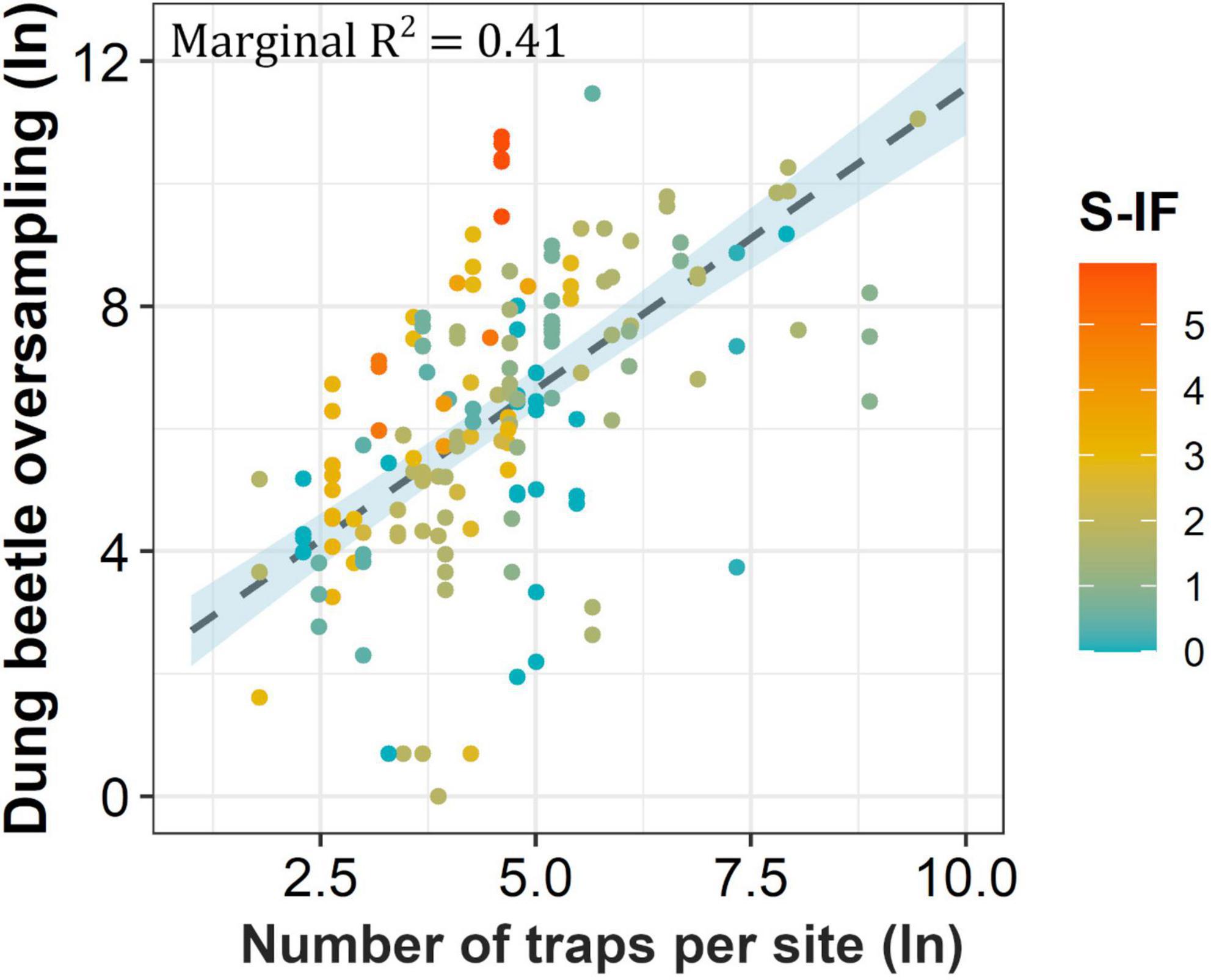
Figure 3. Correlation between log transformed (ln) number of traps per sampling site and dung beetle oversampling. Bar colors indicate the Scopus impact factor (S-IF) of the journal where the study was published. The complete model results are shown in Supplementary Table 4B. Model fit and assumptions are shown in Supplementary Figure 2.
The transformed predicted values from our model show that oversampling increased from tens to hundreds of dung beetle individuals per site, in line with the number of pitfall traps placed (Table 2 and Supplementary Table 4C). For instance, ten pitfall traps led to an excess of 54 dung beetles (min 22 and max 130), 50 traps to 265 (134 min, 518 max), and 300 traps to 1,033 (534 min, 2018 max) per site. The sampling deficit of dung beetles was not significantly explained by sampling intensity and the journal impact factor, and the model explanatory power was low (conditional R2 = 0.12, marginal R2 = 0.02; Supplementary Table 4D).
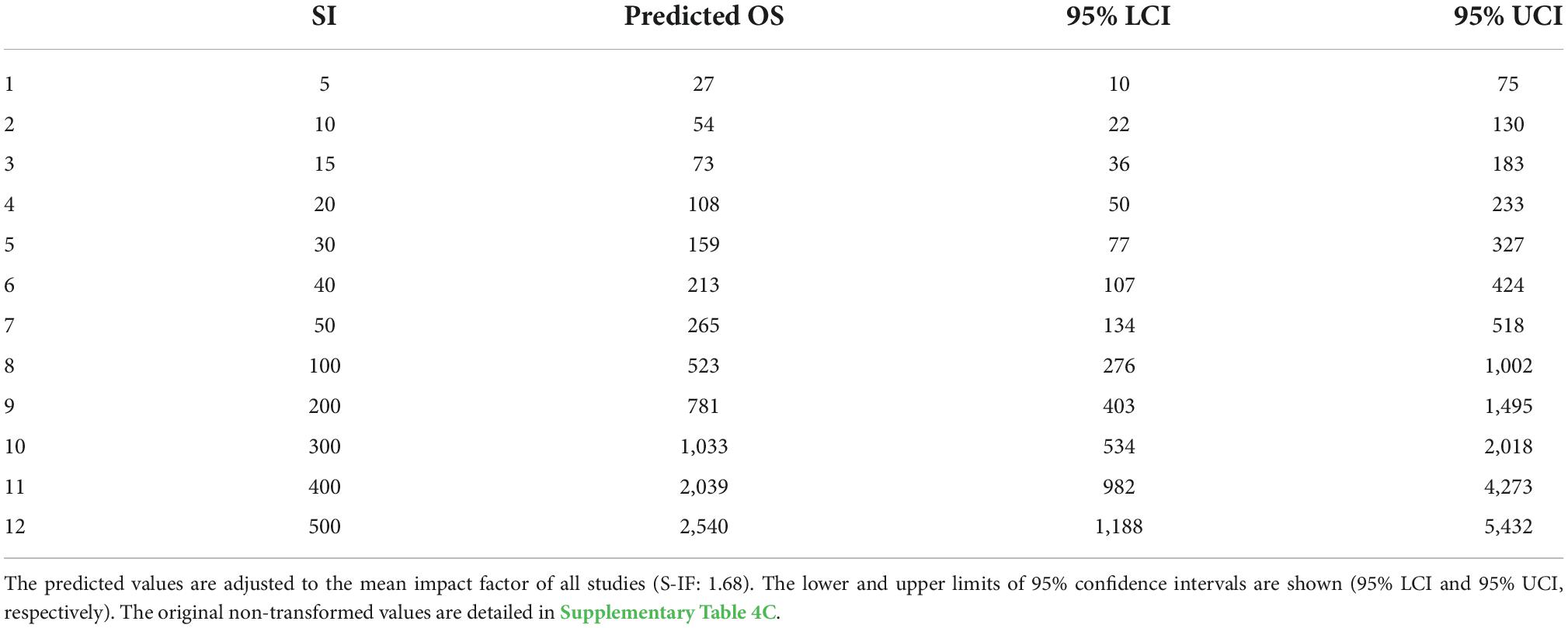
Table 2. Predicted dung beetle oversampling values (Predicted OS) on their original scale (i.e., natural log exponential) as a function of sampling intensity (SI, number of traps).
Dung beetle oversampling was significantly explained by sampling intensity in most habitats (Figure 4), except for the lowly-shaded agroforestry systems and crops (Supplementary Table 5A). The models based on forest edges and cloud forests showed the best fit (marginal R2 = 0.90 and 0.74, respectively), followed by the shaded agroforestry systems (marginal R2 = 0.66). Tropical deciduous and old-growth forest models showed an intermediate fit (marginal R2 = 0.32 and 0.31, respectively), whereas the lowest fit values were obtained for the second-growth forest, pasture, and forest fragment models (marginal R2 = 0.22–0.18; Figure 4). Dung beetle oversampling was not homogeneous between habitats. Old-growth forests showed the highest oversampling rates, followed by forest fragments (Table 3 and Supplementary Table 5B). In comparison, oversampling rates were low in more disturbed habitats, such as shaded agroforestry systems, second-growth forests, pastures, and forest edges (Table 3 and Supplementary Table 5B). Oversampling rates of cloud forests and tropical deciduous forests were intermediate between those of forest fragments and shaded agroforestry systems (Table 3 and Supplementary Table 5B).
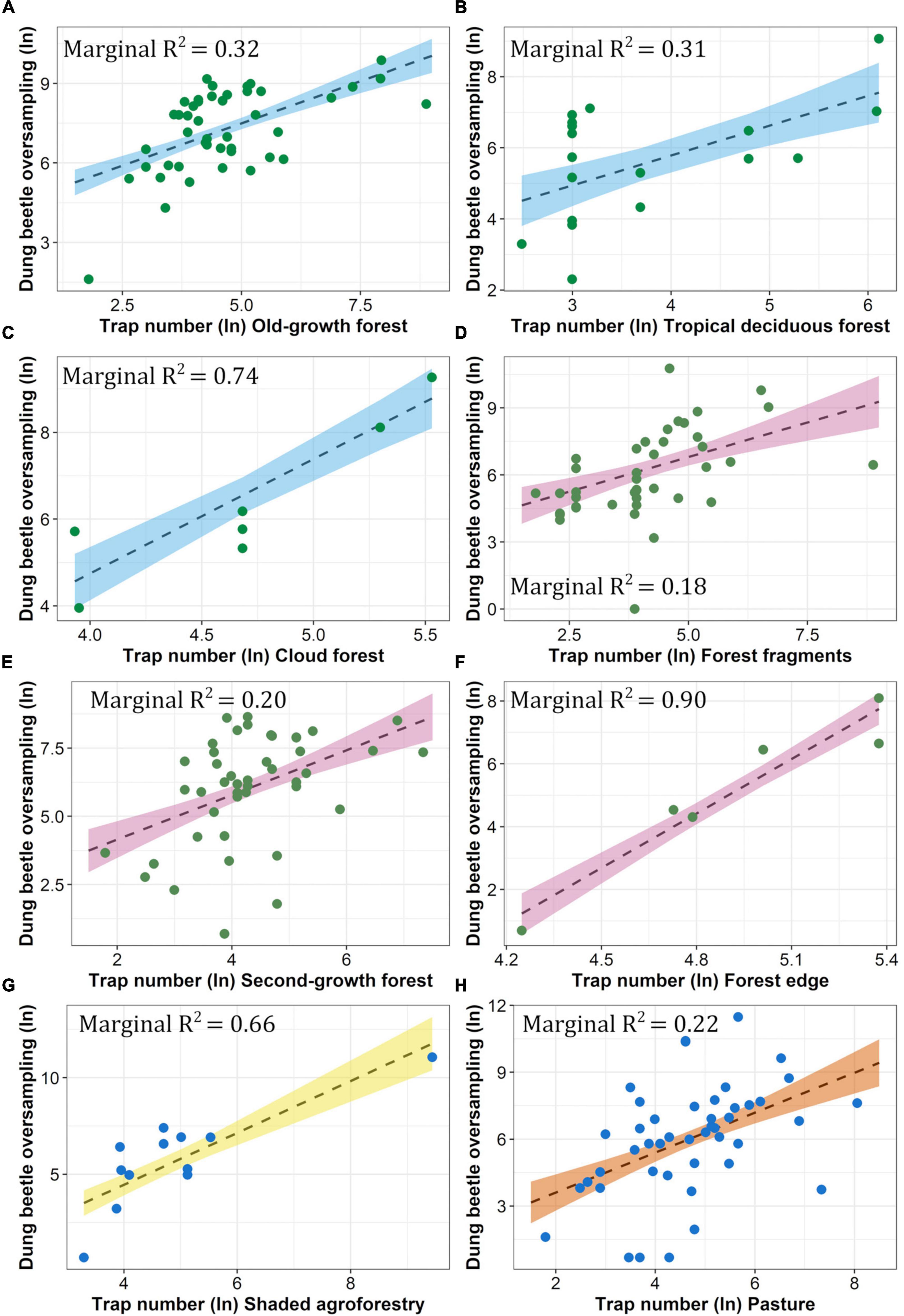
Figure 4. Correlation between the log-transformed (ln) number of traps and dung beetle oversampling across habitat categories. (A) Old-growth forest, (B) tropical deciduous forest, (C) cloud forest, (D) forest fragments, (E) second-growth forest, (F) forest edge, (G) shaded agroforestry, and (H) pasture. The complete model results are shown in Supplementary Table 5A. Model fit and assumptions are shown in Supplementary Figure 3.
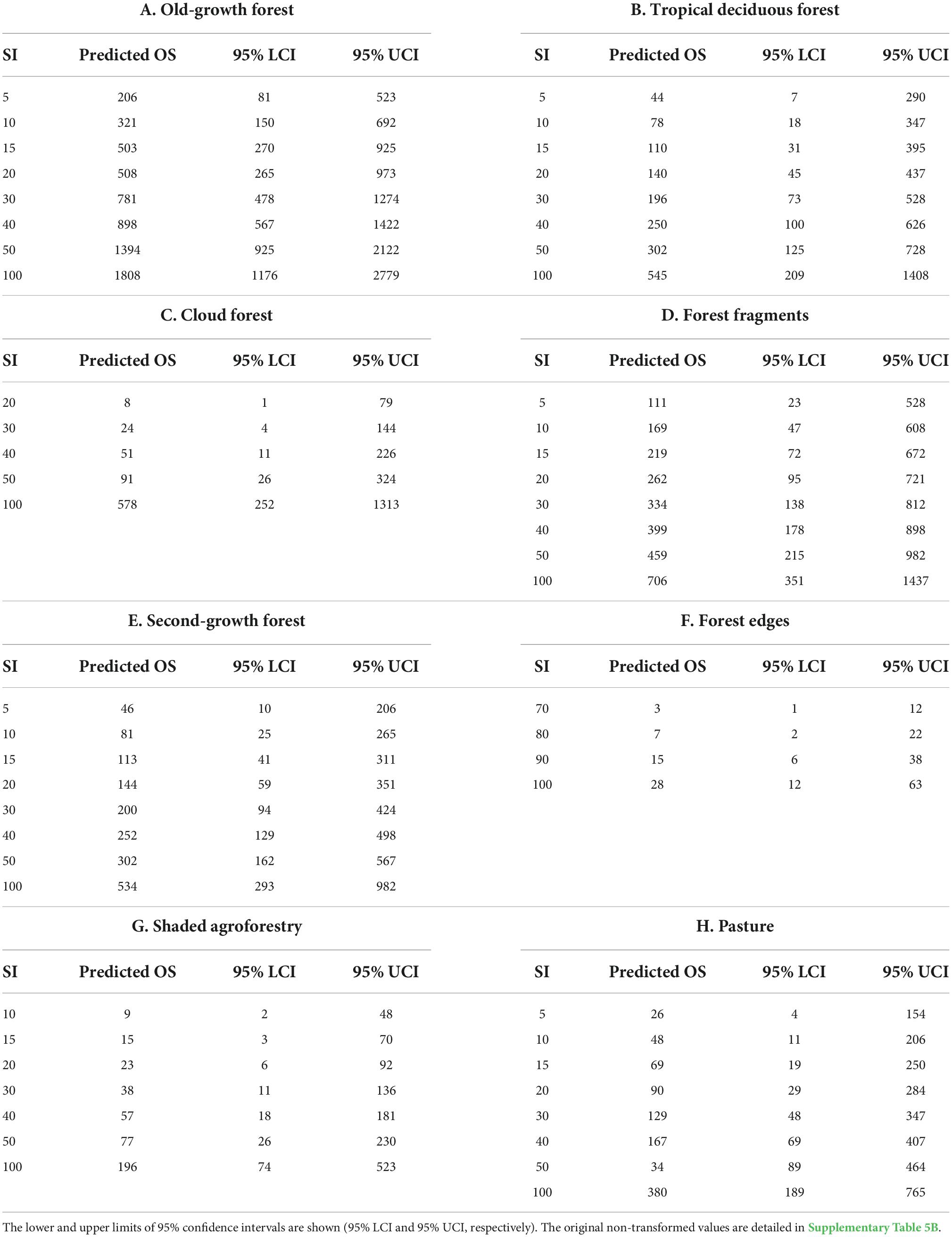
Table 3. Predicted dung beetle oversampling values (predicted OS) on their original scale (i.e., natural log exponential) as a function of sampling intensity (SI, number of traps) in different habitat types.
Researchers are interested in practical, cost-effective, but statistically rigorous sampling methods when constructing biodiversity inventories. Robust sampling is especially critical when biodiversity monitoring is used for making management decisions such as terminating an allegedly harmful mining project or assessing the impact of a hydropower plant (Hayward et al., 2015; Kühl et al., 2020). Therefore, data accuracy and precision are essential. However, biological diversity cannot be accurately measured because the observed number of species is always a downward-biased estimator of the true species richness (Gotelli and Colwell, 2011). An appropriate sampling effort can help reduce such measurement errors and facilitate achieving asymptotic estimates of diversity (Bonar et al., 2011). Our data showed that 95% of the reviewed studies were effective at measuring dung beetle diversity (SC > 90%). The remaining studies obtained a sampling coverage between 88 and 33%. Therefore, the likelihood of undersampling dung beetle diversity through pitfall traps is low.
Sampling intensity correlated significantly and positively with dung beetle oversampling. Although the relationship between sampling intensity and completeness is similar to that of the species-area (Hill et al., 1994), very few traps were needed to obtain (or exceed) the abundance required to achieve 99% species coverage. Sampling coverage above 90% using five pitfall traps was achieved in most cases, and studies with 20 or more pitfall traps per habitat reached 99.99% sampling coverage. Such a sampling scheme could lead to a less cost-effective use of research funds since there is a high possibility that additional sampling will only add dominant specimens rather than increase species richness (see Chao et al., 2014). The surprisingly low number of pitfall traps needed to obtain a representative sample of dung beetle diversity can be explained by the extremely high effectiveness of these baited traps in attracting and capturing dung beetles (Ong et al., 2022). For instance, studies involving several collecting methods and different Coleoptera families have consistently shown significantly higher capture rates and abundances for Scarabaeinae dung beetles (e.g., Caballero and León-Cortés, 2012; Ramírez-Ponce et al., 2019; Quinto et al., 2021).
Oversampling rates were also significantly and positively correlated with the impact factor of peer-reviewed journals. High-impact factor journals aim for generalizable ecological evidence that can be extrapolated and replicated to other locations (Barto and Rillig, 2012). Such data may require a high sampling intensity across extensive areas or over several years (Hughes et al., 2017), ultimately leading to oversampling, as shown by our models. The correlation between the journal impact factor and dung beetle oversampling may also be an indirect outcome of studies intended for publication in high-impact factor journals, which likely influences the overall research design and sampling intensity. The sampling deficit of dung beetle diversity was not explained by sampling intensity or the journal impact factor. Deforestation and land-use change possibly explain the poor explanatory power of the sampling deficit since these anthropogenic disturbances cause a significant decline in dung beetle diversity and abundance (Nichols et al., 2007; Fuzessy et al., 2021). As fewer dung beetles are present in a given habitat due to anthropogenic disturbances, the capture rate of pitfall traps will be reduced, hence increasing the likelihood of undersampling. Our results also suggest that no minimum effective number of traps could lead to incomplete sampling of dung beetle diversity. That is, as long as no environmental factor significantly affects dung beetle abundance and diversity, pitfall traps will likely capture a sample of reasonably good completeness (i.e., SC ≥ 90%).
Oversampling was lower in agroforestry systems and pastures than in forested habitats, including forest fragments and second-growth forests. The population dynamics of dung beetle assemblages differ significantly between forest inner areas and pastures (Horgan, 2008; Silva et al., 2017). Pasture habitats are typically diversity-poor because of their more extreme microclimatic conditions, which act as a natural barrier preventing the entry and establishment of the most susceptible species (Giménez Gómez et al., 2020; Rivera et al., 2022). Dung beetle populations may also be smaller in pasture systems than in forests due to more hostile environmental conditions that prevail in these systems, as suggested by differences in capture between the two habitats (e.g., Quintero and Roslin, 2005; Braga et al., 2013; Rivera et al., 2020; Salomão et al., 2020). Therefore, the asymptote of species richness is reached more rapidly in pastures than in forested habitats, while the supposedly small populations of pastures can also favor low oversampling rates.
Forest habitats, particularly old-growth forests, had high oversampling rates even with a relatively low sampling intensity. Old-growth forests possess more niches and resources for Neotropical dung beetle species, as most species in this region evolve within forested habitats (Halffter and Matthews, 1966; Gill, 1991). Also, dung beetle populations may grow faster under undisturbed conditions (Beiroz et al., 2017; Fuzessy et al., 2021), making it easier to obtain a large sample size with less effort. On the other hand, forest edges require more intensive sampling to exceed the abundance needed to achieve 99% completeness. This finding suggests that the sampling effort in this habitat type may need to be high. Forest edges are likely low-quality habitats for many dung beetle species, especially if the contrast between contiguous habitats is high (Spector and Ayzama, 2003; Martello et al., 2016; Villada-Bedoya et al., 2016; Martínez-Falcón et al., 2018). Besides, forest edges may be subject to continuous changes due to traditional land-use dynamics such as crop rotation and abandonment, preventing beetle populations from reaching a more stable state (Barnes et al., 2014).
The hypothesis that extracting individuals from their natural environment may adversely impact populations has been little studied in vertebrates (McCay and Komoroski, 2004; Sullivan and Sullivan, 2013; Poe and Armijo, 2014; Hope et al., 2018), but much less in invertebrates (Gezon et al., 2015). Nevertheless, the consensus is that the impact of scientific collections on animal populations is minimal (Rocha et al., 2014, but see Delibes et al., 2011; Minteer et al., 2014). Gezon et al. (2015) argued that removing invertebrates during scientific sampling may liberate ecological niches and reduce competition, leading to population growth. However, new niches can be colonized by new individuals or species as long as populations are not fragmented or spatially isolated (Thomas, 2000; Ricketts, 2001), which today is increasingly challenging because forest remnants are becoming more isolated from each other due to deforestation (Laurance et al., 2012). In addition, according to Gezon et al. (2015), lethal sampling probably exerts no effect if the individuals sampled have already reproduced. According to our systematic research, most studies collect dung beetles during the rainy season (49% rainy, 39% dry and rainy; see Supplementary Table 2) — the period of their highest activity rate (Correa et al., 2021) —, enabling efficient sampling of these insects. However, most Neotropical dung beetle species emerge, feed, and reproduce during the rainy season (Halffter and Edmonds, 1982), so it is challenging to assume that all the collected individuals have already reproduced. Finally, Gezon et al. (2015) focused on bee taxa, which includes multiple families, and collected 14,000 bees over five years of intensive sampling. Our database shows that with sufficient sampling effort, it is possible to collect and exceed 14,000 individuals of tropical Scarabaeinae in less than three months (Supplementary Table 2). Therefore, although Gezon’s criteria are valuable, a more careful approach is needed for dung beetles because these criteria are not entirely applicable to them.
It is worth mentioning that we are not against using or collecting dung beetles in research since scientific collections represent a valuable register of biodiversity, whose importance for conservation has been reviewed in depth by several authors (Patterson, 2002; Suarez and Tsutsui, 2004; Rocha et al., 2014). Instead, we advocate a thorough discussion of the collection methods used for dung beetles, recalling the five Rs and Precautionary Principles. The R principles, proposed by Russel and Burch (1959), suggest that scientific research with animals should be guided by refinement, reduction, and replacement. We acknowledge the difficulty in refining or replacing lethal collection practices because identifying live dung beetles is highly challenging. Many species are sympatric and morphologically indistinguishable (Larsen and Forsyth, 2005), thus requiring specimen collection for correct identification. However, we can apply the reduction principle effectively because, as demonstrated in the present study, few pitfall traps are needed to obtain a representative and robust sample of dung beetle diversity. Two additional R principles — respect and responsibility — were proposed by Crespi-Abril and Rubilar (2021). These ethical-based epistemological practices highlight the importance of researchers respecting and showing empathy for life, recognizing its value regardless of its complexity, and taking responsibility for their actions, as animals are no longer a means but also an end for conservation.
Although growing evidence shows the decline of tropical insect populations in the Anthropocene (Lister and Garcia, 2018; Wagner, 2020), there is still no proof that oversampling affects dung beetle populations. However, “the absence of evidence is not evidence of absence” (Crespi-Abril and Rubilar, 2021). In this sense, we can also apply the precautionary principle, which aims to prevent or reduce damage even if the evidence is insufficient to determine the magnitude or probability of occurrence (Kriebel et al., 2001). Ethical sampling that consciously reduces the number of pitfall traps in each independent sampling unit following the Rs and Precautionary principles will improve the cost-efficiency of resource use in research while preventing specimen oversampling. Researchers can focus instead on increasing the number of independent sampling units using a smaller number of traps, thereby increasing the predictive power of statistical models and obtaining more robust evidence of the phenomenon under study (see Gotelli and Elllison, 2004).
Our models showed that a representative sampling of dung beetle diversity (i.e., SC >90%) could be achieved with no more than ten pitfall traps. Therefore, we recommend placing up to six pitfall traps per independent sampling unit when using only a single bait type (dung or carrion) and up to eight pitfall traps when using both bait types. We do not consider traps baited with fruit as the beetle capture rate is significantly low. If the research addresses forest habitats solely, the number of pitfall traps may be smaller, e.g., three to five traps per sampling unit (see Price and Feer, 2012). These recommendations can also apply to landscape-scale studies (see Arroyo-Rodríguez and Fahrig, 2014). For example, if a landscape-site design is used, six to ten traps can be distributed around the centroid of the landscape. In landscape-scale designs, pitfall traps can be distributed in five or six groups of three to four pitfall traps each. The number of pitfall traps in each independent sampling unit can be further reduced for longitudinal studies in which the same site is sampled several times. A presampling protocol may be the best way to assess the optimum number of traps per site, considering our suggestions as a starting point. In conclusion, a sampling scheme guided by ethical guidelines will make the research more economical, time-effective, statistically robust, and friendlier to dung beetle biodiversity.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
JR and MF contributed to the conception and design of the study, and organized the database. JR performed the statistical analysis and wrote the first draft of the manuscript. Both authors contributed to manuscript revision, read, and approved the submitted version.
We thank Claudia Gómez Falcón and Citlaly Itzel Reyes García for their invaluable help with data extraction. Special thanks to Javier Alexis Ascanio Lárraga for his assistance in processing the geospatial information. We also thank Alejandra Guzman Luna whose valuable comments helped to improve our manuscript significantly. JR thanks the Consejo Nacional de Ciencia y Tecnología (CONACYT) for Ph.D. grant # 70982. María Elena Sánchez-Salazar edited the manuscript in English.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.999488/full#supplementary-material
Aristophanous, M. (2010). Does your preservative preserve? A comparison of the efficacy of some pitfall trap solutions in preserving the internal reproductive organs of dung beetles. Zookeys 34, 1–16. doi: 10.3897/zookeys.34.215
Arroyo-Rodríguez, V., and Fahrig, L. (2014). Why is a landscape perspective important in studies of primates? Am. J. Primatol. 76, 901–909. doi: 10.1002/ajp.22282
Barnes, A. D., Emberson, R. M., Chapman, H. M., Krell, F.-T. T., and Didham, R. K. (2014). Matrix habitat restoration alters dung beetle species responses across tropical forest edges. Biol. Conserv. 170, 28–37. doi: 10.1016/j.biocon.2013.12.006
Barto, E. K., and Rillig, M. C. (2012). Dissemination biases in ecology: Effect sizes matter more than quality. Oikos 121, 228–235. doi: 10.1111/j.1600-0706.2011.19401.x
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beiroz, W., Slade, E. M., Barlow, J., Silveira, J. M., Louzada, J., and Sayer, E. (2017). Dung beetle community dynamics in undisturbed tropical forests: Implications for ecological evaluations of land-use change. Insect Conserv. Divers. 10, 94–106. doi: 10.1111/icad.12206
Bogoni, J. A., da Silva, P. G., and Peres, C. A. (2019). Co-declining mammal–dung beetle faunas throughout the Atlantic Forest biome of South America. Ecography (Cop.) 42, 1803–1818. doi: 10.1111/ecog.04670
Bogoni, J. A., Hernández, M. I. M., and Preisser, E. (2014). Attractiveness of Native Mammal’s feces of different trophic guilds to dung beetles (Coleoptera: Scarabaeinae). J. Insect Sci. 14:299. doi: 10.1093/jisesa/ieu161
Bonar, S. A., Fehmi, J. S., and Mercado-Silva, N. (2011). “An overview of sampling issues in species diversity and abundance surveys,” in Biological diversity: Frontiers in measurement and assessment, eds A. E. Magurran and B. J. McGill (Oxford: Oxford University Press), 11–24.
Braga, R. F., Korasaki, V., Andresen, E., and Louzada, J. (2013). Dung beetle community and functions along a habitat-disturbance gradient in the amazon: A rapid assessment of ecological functions associated to biodiversity. PLoS One 8:e57786. doi: 10.1371/journal.pone.0057786
Brischoux, F., and Angelier, F. (2015). Academia’s never-ending selection for productivity. Scientometrics 103, 333–336. doi: 10.1007/s11192-015-1534-5
Burnham, K. P., and Anderson, D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach. New York, NY: Springer.
Caballero, U., and León-Cortés, J. L. (2012). High diversity beetle assemblages attracted to carrion and dung in threatened tropical oak forests in Southern Mexico. J. Insect Conserv. 16, 537–547. doi: 10.1007/s10841-011-9439-y
Chao, A., and Jost, L. (2012). Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 93, 2533–2547. doi: 10.1890/11-1952.1
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Colwell, R. K., and Elllison, A. M. (2014). Rarefaction and extrapolation with hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Correa, C. M. A., da Silva, P. G., Puker, A., Gil, R. L., and Ferreira, K. R. (2021). Rainfall seasonality drives the spatiotemporal patterns of dung beetles in Amazonian forests in the arc of deforestation. J. Insect Conserv. 25, 453–463. doi: 10.1007/s10841-021-00313-y
Crespi-Abril, A.-C., and Rubilar, T. (2021). Moving forward in the ethical consideration of invertebrates in experimentation: Beyond the Three R’s Principle. Rev. Biol. Trop. 69, S346–S357. doi: 10.15517/rbt.v69isuppl.1.46366
da Silva, P. G., and Hernández, M. I. M. (2015). Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS One 10:e0126112. doi: 10.1371/journal.pone.0126112
Davis, A. J., Holloway, J. D., Huijbregts, H., Krikken, J., Kirk-Spriggs, A. H., and Sutton, S. L. (2001). Dung beetles as indicators of change in the forests of northern Borneo. J. Appl. Ecol. 38, 593–616. doi: 10.1046/j.1365-2664.2001.00619.x
Delibes, M., de Carmen Blázquez, M., Soriano, L., Revilla, E., and Godoy, J. A. (2011). High antipredatory efficiency of insular lizards: A warning signal of excessive specimen collection? PLoS One 6:e29312. doi: 10.1371/journal.pone.0029312
Favila, M. E., and Halffter, G. (1997). The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zool. Mex. Nueva Ser. 0, 1–25.
Feer, F. (2000). Dung and carrion beetles (Scarabaeidae s. str. et Aphodiidae) of the rain forest of French Guiana: Species composition and structure of populations. Ann. la Société Entomol. Fr. 36, 29–43.
Ferrer-Paris, J. R., Sánchez-Mercado, A., and Rodríguez, J. P. (2013). Optimización del muestreo de invertebrados tropicales: Un ejemplo con escarabajos coprófagos (Coleoptera: Scarabaeinae) en Venezuela. Rev. Biol. Trop. 61, 89–110. doi: 10.15517/rbt.v61i1.10941
Filgueiras, B. K. C., Liberal, C. N., Aguiar, C. D. M., Medina Hernandez, M. I., and Iannuzzi, L. (2009). Attractivity of omnivore, carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera. Scarabaeidae) in a tropical Atlantic rainforest remnant. Rev. Bras. Entomol. 53, 422–427. doi: 10.1590/S0085-56262009000300017
Flechtmann, C. A. H., Tabet, V. G., and Quintero, I. (2009). Influence of carrion smell and rebaiting time on the efficiency of pitfall traps to dung beetle sampling. Entomol. Exp. Appl. 132, 211–217. doi: 10.1111/j.1570-7458.2009.00885.x
Fuzessy, L. F., Benítez-López, A., Slade, E. M., Bufalo, F. S., Magro-de-Souza, G. C., Pereira, L. A., et al. (2021). Identifying the anthropogenic drivers of declines in tropical dung beetle communities and functions. Biol. Conserv. 256, 7–17. doi: 10.1016/j.biocon.2021.109063
Gardner, T. A., Barlow, J., Araujo, I. S., Ávila-Pires, T. C., Bonaldo, A. B., Costa, J. E., et al. (2008). The cost-effectiveness of biodiversity surveys in tropical forests. Ecol. Lett. 11, 139–150. doi: 10.1111/j.1461-0248.2007.01133.x
Gaston, K. J., Cox, D. T. C., Canavelli, S. B., García, D., Hughes, B., Maas, B., et al. (2018). Population abundance and ecosystem service provision: The case of birds. Bioscience 68, 264–272. doi: 10.1093/biosci/biy005
Gezon, Z. J., Wyman, E. S., Ascher, J. S., Inouye, D. W., and Irwin, R. E. (2015). The effect of repeated, lethal sampling on wild bee abundance and diversity. Methods Ecol. Evol. 6, 1044–1054. doi: 10.1111/2041-210X.12375
Gill, B. D. (1991). “Dung beetles in tropical american forests,” in Dung beetle ecology, eds I. Hanski and Y. Cambefort (Princeton, NJ: Princeton University Press), 211–229. doi: 10.1371/journal.pone.0075819
Giménez Gómez, V. C., Verdú, J. R., and Zurita, G. A. (2020). Thermal niche helps to explain the ability of dung beetles to exploit disturbed habitats. Sci. Rep. 10, 1–14. doi: 10.1038/s41598-020-70284-8
Gotelli, N. J., and Elllison, A. M. (2004). A primer of ecological statistics. Sunderland, MA: Sinauer Associates, Inc.
Gotelli, N., and Colwell, R. (2011). “Estimating species richness,” in biological diversity. frontiers in measurement and assessment, eds A. E. Magurran and B. J. McGill (New York, NY: Oxford University Press), 39–54. doi: 10.2307/3547060
Halffter, G., and Edmonds, W. (1982). The nesting behavior of dung beetles (Scarabaeinae). An ecological and evolutive approach. Xalapa: Instituto de Ecología, A.C.
Halffter, G., and Matthews, E. G. (1966). The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomol. Mex. 12, 1–312.
Hayward, M. W., Boitani, L., Burrows, N. D., Funston, P. J., Karanth, K. U., Mackenzie, D. I., et al. (2015). Ecologists need robust survey designs, sampling and analytical methods. J. Appl. Ecol. 52, 286–290. doi: 10.1111/1365-2664.12408
Hill, J. L., Curran, P. J., and Foody, G. M. (1994). The effect of sampling on the species-area curve. Glob. Ecol. Biogeogr. Lett. 4, 97–106.
Hope, A. G., Sandercock, B. K., and Malaney, J. L. (2018). Collection of scientific specimens: Benefits for biodiversity sciences and limited impacts on communities of small mammals. Bioscience 68, 35–42. doi: 10.1093/biosci/bix141
Horgan, F. G. (2008). Dung beetle assemblages in forests and pastures of El Salvador: A functional comparison. Biodivers. Conserv. 17, 2961–2978. doi: 10.1007/s10531-008-9408-2
Horgan, F. G., and Fuentes, R. C. (2005). Asymmetrical competition between Neotropical dung beetles and its consequences for assemblage structure. Ecol. Entomol. 30, 182–193. doi: 10.1111/j.0307-6946.2005.00673.x
Hsieh, T. C., Ma, K. H., and Chao, A. (2016). iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456. doi: 10.1111/2041-210X.12613
Hughes, B. B., Beas-Luna, R., Barner, A. K., Brewitt, K., Brumbaugh, D. R., Cerny-Chipman, E. B., et al. (2017). Long-Term studies contribute disproportionately to ecology and policy. Bioscience 67, 271–278. doi: 10.1093/biosci/biw185
Iannuzzi, L., Liberal, C. N., de Souza, T. B., Pellegrini, T. G., da Cunha, J. C. S., Koroiva, R., et al. (2020). “Sampling methods for Beetles (Coleoptera),” in Measuring Arthropod Biodiversity, eds J. C. Santos and G. W. Fernandes (Cham: Springer), 125–185. doi: 10.1007/978-3-030-53226-0_6
Kriebel, D., Tickner, J., Epstein, P., Lemons, J., Levins, R., Loechler, E. L., et al. (2001). The precautionary principle in environmental science. Environ. Health Perspect. 109, 871–876. doi: 10.1289/ehp.01109871
Kühl, H. S., Bowler, D. E., Bösch, L., Bruelheide, H., Dauber, J., Eichenberg, D., et al. (2020). Effective biodiversity monitoring needs a culture of integration. One Earth 3, 462–474. doi: 10.1016/j.oneear.2020.09.010
Larsen, T. H., and Forsyth, A. (2005). Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 37, 322–325. doi: 10.1111/j.1744-7429.2005.00042.x
Larsen, T. H., Williams, N. M., and Kremen, C. (2005). Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 8, 538–547. doi: 10.1111/j.1461-0248.2005.00749.x
Laurance, W. F., Carolina Useche, D., Rendeiro, J., Kalka, M., Bradshaw, C. J. A., Sloan, S. P., et al. (2012). Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–293. doi: 10.1038/nature11318
Lister, B. C., and Garcia, A. (2018). Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. U.S.A. 115, E10397–E10406. doi: 10.1073/pnas.1722477115
Lüdecke, D. (2018). ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 3:772. doi: 10.21105/joss.00772
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P., and Makowski, D. (2021). An r package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6:3139. doi: 10.21105/joss.03139
Marsh, C. J., Louzada, J., Beiroz, W., and Ewers, R. M. (2013). Optimising bait for pitfall trapping of amazonian dung beetles (Coleoptera: Scarabaeinae). PLoS One 8:e73147. doi: 10.1371/journal.pone.0073147
Martello, F., Andriolli, F., de Souza, T. B., Dodonov, P., and Ribeiro, M. C. (2016). Edge and land use effects on dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in Brazilian cerrado vegetation. J. Insect Conserv. 20, 957–970. doi: 10.1007/s10841-016-9928-0
Martínez-Falcón, A. P., Zurita, G. A., Ortega-Martínez, I. J., and Moreno, C. E. (2018). Populations and assemblages living on the edge: Dung beetles responses to forests-pasture ecotones. PeerJ 6:e6148. doi: 10.7717/peerj.6148
McCay, T. S., and Komoroski, M. J. (2004). Demographic responses of shrews to removal of coarse woody debris in a managed pine forest. For. Ecol. Manage. 189, 387–395. doi: 10.1016/j.foreco.2003.09.005
Minteer, B. A., Collins, J. P., Love, K. E., and Puschendorf, R. (2014). Avoiding (re)extinction. Science 344, 260–261. doi: 10.1126/science.1250953
Montes, B. E., Lefcheck, J. S., Guerra-castro, E., Klein, E., Simoes, N., Macaya, E. C., et al. (2021). Optimizing large-scale biodiversity sampling effort: Toward an unbalances survey desing. Oceanography 34, 80–91.
Morón, M. Á, and Terrón-S, R. A. (1984). Distribucion altitudinal y estacional de los insectos necrofilos en la sierra norte de Hidalgo, México. Acta Zool. Mex. Nueva Ser 3, 1–47.
Morrone, J. J. (2014). Biogeographical regionalisation of the neotropical region. Zootaxa 3782, 1–110. doi: 10.11646/zootaxa.3782.1.1
Morrone, J. J., Escalante, T., Rodriguez-Tapia, G., Carmona, A., Arana, M., and Mercado-Gomez, J. D. (2022). Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Cienc. 94, 1–5. doi: 10.1590/0001-3765202220211167
Nichols, E., Gardner, T. A., Peres, C. A., Spector, S., and Network, S. R., and The Scarabaeinae Research Network. (2009). Co-declining mammals and dung beetles: An impending ecological cascade. Oikos 118, 481–487. doi: 10.1111/j.1600-0706.2009.17268.x
Nichols, E., Larsen, T., Spector, S., Davis, A. L., Escobar, F., Favila, M., et al. (2007). Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biol. Conserv. 137, 1–19. doi: 10.1016/j.biocon.2007.01.023
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M. E., et al. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 141, 1461–1474. doi: 10.1016/j.biocon.2008.04.011
Ong, X. R., Hemprich-Bennett, D., Gray, C. L., Kemp, V., Chung, A. Y. C., and Slade, E. M. (2022). Trap type affects dung beetle taxonomic and functional diversity in Bornean tropical forests. Austral Ecol. 47, 68–78. doi: 10.1111/aec.13124
Patterson, B. D. (2002). On the continuing need for scientific collecting of mammals. J. Neotrop. Mamm. 9, 253–262.
Pessôa, M. B., Alves-Martins, F., De Marco Júnior, P., and Hortal, J. (2021). Unveiling the drivers of local dung beetle species richness in the Neotropics. J. Biogeogr. 48, 861–871. doi: 10.1111/jbi.14043
Poe, S., and Armijo, B. (2014). Lack of effect of herpetological collecting on the population structure of a community of Anolis (Squamata: Dactyloidae) in a disturbed habitat. Herpetol. Notes 7, 153–157.
Price, D. L., and Feer, F. (2012). Are there pitfalls to pitfalls? Dung beetle sampling in French Guiana. Org. Divers. Evol. 12, 325–331. doi: 10.1007/s13127-012-0106-2
Quintero, I., and Roslin, T. (2005). Rapid recovery of dung beetle communities following habitat fragmentation in central amazonia. Ecology 86, 3303–3311.
Quinto, J., Martínez-Falcón, A. P., Murillo-Pacheco, J. I., Abdala-Roberts, L., and Parra-Tabla, V. (2021). Diversity patterns of tropical epigeal beetle assemblages associated with monoculture and polyculture plantations with big-leaf mahogany. Neotrop. Entomol. 50, 551–561. doi: 10.1007/s13744-021-00870-6
Ramírez-Ponce, A., Calderón-Patrón, J. M., Vásquez, H. M. G., and Moreno, C. E. (2019). Biotic heterogeneity among scarab beetle communities in an anthropized landscape in the Central Valleys of Oaxaca. Mexico. J. Insect Conserv. 23, 765–776. doi: 10.1007/s10841-019-00169-3
Ricketts, T. H. (2001). The matrix matters: Effective isolation in fragmented landscapes. Am. Nat. 158, 87–99. doi: 10.1086/320863
Rivera, J. D., Espinosa de los Monteros, A., da Silva, P. G., and Favila, M. E. (2022). Dung beetles maintain phylogenetic divergence but functional convergence across a highly fragmented tropical landscape. J. Appl. Ecol 59, 1781–1791. doi: 10.1111/1365-2664.14185
Rivera, J. D., Gómez, B., Navarrete-Gutiérrez, D. A., Ruíz-Montoya, L., Delgado, L., and Favila, M. E. (2020). Mechanisms of diversity maintenance in dung beetle assemblages in a heterogeneous tropical landscape. PeerJ 8, 1–24. doi: 10.7717/peerj.9860
Rocha, L. A., Aleixo, A., Allen, G., Almeda, F., Baldwin, C. C., Barclay, M. V. L., et al. (2014). Specimen collection: An essential tool. Science. 344, 814–815. doi: 10.1126/science.344.6186.814
Roswell, M., Dushoff, J., and Winfree, R. (2021). A conceptual guide to measuring species diversity. Oikos 130, 321–338. doi: 10.1111/oik.05876
Russel, W. M. S., and Burch, R. L. (1959). The principles of humane experimental technique. London: Methuen & Co LTD.
Salomão, R. P., Favila, M. E., and González-Tokman, D. (2020). Spatial and temporal changes in the dung beetle diversity of a protected, but fragmented, landscape of the northernmost Neotropical rainforest. Ecol. Indic. 111:105968. doi: 10.1016/j.ecolind.2019.105968
Sánchez-Bayo, F., and Wyckhuys, K. A. G. (2019). Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27. doi: 10.1016/j.biocon.2019.01.020
Silva, R. J., Pelissari, T. D., Krinski, D., Canale, G., and Vaz-de-Mello, F. Z. (2017). Abrupt species loss of the Amazonian dung beetle in pastures adjacent to species-rich forests. J. Insect Conserv. 21, 487–494. doi: 10.1007/s10841-017-9988-9
Spector, S., and Ayzama, S. (2003). Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian Neotropical forest-savanna ecotone. Biotropica 35, 394–404. doi: 10.1111/j.1744-7429.2003.tb00593.x
Suarez, A. V., and Tsutsui, N. D. (2004). The value of museum collections for research and society. Bioscience 54, 66–74. doi: 10.1641/0006-35682004054[0066:TVOMCF]2.0.CO;2
Sullivan, T. P., and Sullivan, D. S. (2013). Influence of removal sampling of small mammals on abundance and diversity attributes: Scientific implications. Human Wildlife Interact. 7, 85–98.
Tarasov, S., and Dimitrov, D. (2016). Multigene phylogenetic analysis redefines dung beetles relationships and classification (Coleoptera: Scarabaeidae: Scarabaeinae). BMC Evol. Biol. 16:257. doi: 10.1186/s12862-016-0822-x
Thomas, C. D. (2000). Dispersal and extinction in fragmented landscapes. Proc. R. Soc. B Biol. Sci. 267, 139–145. doi: 10.1098/rspb.2000.0978
Tocco, C., Quinn, D. E. A., Midgley, J. M., and Villet, M. H. (2017). Optimising design and effort for environmental surveys using dung beetles (Coleoptera: Scarabaeidae). Can. Entomol. 149, 214–226. doi: 10.4039/tce.2016.48
Villada-Bedoya, S., Cultid-Medina, C. A., Escobar, F., Guevara, R., and Zurita, G. (2016). Edge effects on dung beetle assemblages in an Andean mosaic of forest and coffee plantations. Biotropica 0, 1–11. doi: 10.1111/btp.12373
Villarreal, H., lvarez, M., Córdoba, S., Escobar, F., Fagua, G., Gast, F., et al. (2004). Manual de métodos para el desarrollo de inventarios de biodiversidad. Instituto de investigación de recursos biológicos Alexander von Humboldt. Bogotá DC: Ramos López Editorial, 236.
Wagner, D. L. (2020). Insect declines in the anthropocene. Annu. Rev. Entomol. 65, 457–480. doi: 10.1146/annurev-ento-011019-025151
Wagner, D. L., Grames, E. M., Forister, M. L., Berenbaum, M. R., and Stopak, D. (2021). Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. U. S. A. 118, 1–10. doi: 10.1073/PNAS.2023989118
Keywords: sampling effectiveness, Neotropics, animal ethics, cost-effective sampling, precautionary principle, R’ principles
Citation: Rivera JD and Favila ME (2022) Good news! Sampling intensity needed for accurate assessments of dung beetle diversity may be lower in the Neotropics. Front. Ecol. Evol. 10:999488. doi: 10.3389/fevo.2022.999488
Received: 21 July 2022; Accepted: 28 September 2022;
Published: 21 October 2022.
Edited by:
Davide Valenti, University of Palermo, ItalyReviewed by:
Dana Price, Salisbury University, United StatesCopyright © 2022 Rivera and Favila. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose D. Rivera, amRyNDk1QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.