
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 30 September 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.982590
This article is part of the Research Topic Adaptive Evolution of Organs Size in Cold-Blooded Animals View all 6 articles
The brain is among the most energetically costly organs in the vertebrate body, while the size of the brain varies within species. The expensive-tissue hypothesis (ETH) predicts that increasing the size of another costly organ, such as the gut, should compensate for the cost of a small brain. Here, the ETH was tested by analyzing the relationship between brain size variation and digestive tract length in a Swelled-vented frog (Feirana quadranus). A total of 125 individuals across 10 populations ranging from 586 to 1,702 m a.s.l. from the Qinling-Daba Mountains were sampled. With the increase in altitude, the brain size decreases and the digestive tract length increases. Different brain regions do not change their relative size in a consistent manner. The sizes of telencephalon and cerebellum decrease with the increase in altitude, while the olfactory nerve increases its size at high altitudes. However, the olfactory bulb and optic tectum have no significant relationship with altitude. After controlling for snout-vent length (SVL), a significant negative correlation could be found between brain size and digestive tract length in F. quadranus. Therefore, the intraspecific variation of brain size follows the general patterns of ETH in this species. The results suggest that annual mean temperature and annual precipitation are environmental factors influencing the adaptive evolution of brain size and digestive tract length. This study also suggests that food composition, activity times, and habitat complexity are the potential reasons driving the adaptive evolution of brain size and digestive tract length.
The brain has always been of interest to almost every field of biology and ecology dealing with animals as it is the central system of learning, memory, cognition, and information processing (Barton and Harvey, 2000; Gonda et al., 2013). Although the direct relationship between brain size and its functional capacity remains controversial, brain size has generally been taken as an important trait that reflects the number of neurons and cognition (Marhounová et al., 2019). The evolution of brain size could have profound effects on important ecological and evolutionary processes, such as selection pressures in the environment, thereby contributing to an enhanced understanding of the ecological fitness of species (Sayol et al., 2016a). The brains of higher vertebrates show great variation in size at interspecific and intraspecific levels (Striedter, 2006; Gonda et al., 2013), in absolute and relative items of the overall size of the brain or even the size of the main brain parts. Understanding the ultimate cause of brain size evolution has become a challenging task for evolutionary biologists (Minias and Podlaszczuk, 2017).
Considerable evidence showed that ecological, social, and sexual selection pressures influence brain size changes in species (Pitnick et al., 2006; Dunbar and Shultz, 2007; Barton and Capellini, 2011). Moreover, the brain is a metabolically costly organ (Mink et al., 1981), and the large amount of energy required for its metabolism limits the evolution of brain size (Striedter, 2006). Therefore, the brain size of an organism is predominantly determined by the tradeoff between the “selective advantage of increasing cognition” and the “energy expenditure of maintaining a larger brain” (Kotrschal et al., 2013a,b; Liu et al., 2022).
The expensive-tissue hypothesis (ETH) predicts that increasing brain size could inevitably reduce the size of other metabolically costly tissues, such as the gut (Aiello and Wheeler, 1995). The ETH was first used to explain the negative correlation between brain size and digestive tract length in primates and human evolution (Aiello and Wheeler, 1995). Later on, it was used to explain the negative correlation between the bat brain and testicular weight (Pitnick et al., 2006). However, the applicability and accuracy of this hypothesis have always been controversial. Currently, studies have verified the evolutionary relationship between brain size and digestive tract length in some animals. Some studies have shown a significant negative correlation between brain size and digestive tract length, thus supporting the ETH (Kaufman, 2003; Jin et al., 2015; Tsuboi et al., 2015; Liao et al., 2016). On the contrary, other studies have found no such relationship or even the opposite (Lemaître et al., 2009; Barrickman and Lin, 2010; Navarrete et al., 2011; Liu et al., 2018). The study of Opsanus tau shows that increased investment in one structure does not necessarily drive a loss of mass in one or more organs (Dornburg et al., 2018). Studies in mice have shown that evolutionary increase in cognitive abilities was initially associated with brain plasticity and fueled by an enlarged gut, which was not traded off for brain size, as the ETH posits (Konarzewski et al., 2020).
Although the ETH is based on primates (Aiello and Wheeler, 1995), the best evidence supporting it comes from poikilotherms (Kaufman, 2003; Kotrschal et al., 2013a; Tsuboi et al., 2015). As ectotherms, amphibians are good models or subjects for brain research. The relative brain size of Rana omeimontis was significantly negatively correlated with the relative digestive tract length, and energy restriction could explain the change in brain size, thus supporting the ETH (Jin et al., 2015). However, some studies suggested that the relative brain size was not significantly correlated with the relative digestive tract length, and the change in brain size could not be explained by energy restriction, which did not support the ETH (Liao et al., 2016; Liu et al., 2018). Besides, the study found no negative correlation between brain mass and intestinal length, and even no negative correlation was discovered between brain mass and the mass of other organs in Bufo gargarizans (Mi and Liao, 2021).
Phenotypic plasticity in morphological and physiological traits is a universal phenomenon in animal groups (Balciauskas et al., 2020; Huang et al., 2021; Liang et al., 2021; Zamora–camacho, 2021; Zedda et al., 2021; Giacomini et al., 2022; Hinds et al., 2022). The phenotypic plasticity theory states that organisms change their morphology and physiological functions as an adaptive response to environmental conditions (Clifton et al., 2020). Therefore, changes in brain size are an evolutionary adaptation to environmental changes. Environmental conditions are crucial factors affecting the evolution of brain size adaptation. Nearly half a century ago, evolutionary biologists have carried out considerable research on the adaptive evolution of the brain size of warm-blooded animals. The results showed that in different environments, the types of food, habitat types, seasonality, activity time, breeding investment, and the difference in life history and the brain the size of the organism could change due to the differences in altitude gradient (Aiello and Wheeler, 1995; Barton and Harvey, 2000; Reader and Laland, 2002; Sol et al., 2005; van Woerden et al., 2011; Sayol et al., 2016b; Minias and Podlaszczuk, 2017; Baldwin et al., 2022). Changes in altitude, latitude, and longitude act on organisms primarily by affecting the temperature and rainfall of their habitat. In general, biodiversity and environmental complexity are higher where temperature and rainfall are higher (Carnaval et al., 2014). Therefore, the intrinsic mechanism of ETH could be explored by studying the relationship between the average annual temperature and rainfall and the brain and intestine.
In recent years, the adaptive evolution of brain size in amphibians and other poikilotherms has also received more attention (Liao et al., 2015, 2016, 2018; Luo et al., 2017; Yu et al., 2018; Huang et al., 2020). Research uncovered that amphibians’ brain size evolution is closely related to environmental factors; for instance, the seasonal variation in temperature could explain the variabilities of the relative brain size and the size of the optic tectum (TEC) in 30 amphibian species (Luo et al., 2017), and the toad’s relative brain size decreased with the increase in altitude (Yao et al., 2021).
As the “backbone of China,” the Qinling Mountains have unique topographic characteristics, and the regional response to climate change is more typical and representative. The Qinling Mountains stretch across the central part of China, which is a transitional region of north–south climate change in this country. Obvious spatial differences could be observed in temperature and annual precipitation changes (Xia et al., 2019). These advantaged environmental conditions provide convenience for studying the influence of environmental conditions on brain size. The Chinese endemic frog species swelled-vented frog (Feirana quadranus) is widely distributed in the Qinling-Daba Mountains. F. quadranus generally lives in mountain streams and near areas with an altitude range of 335–1,830 m a.s.l. in the Qinling-Daba Mountains. This species mainly preys on various insects and sometimes some other small animals. It is a common species in the Qinling-Daba Mountains with a high population density, thus especially suitable for studying intraspecific variation (Wang et al., 2019). The current declining population of F. quadranus requires urgent conservation-related research, and the present study could provide scientific advice that could better predict the species’ endangerment and guide local biodiversity conservation measures (Gonzalez-Voyer et al., 2016).
The life history of amphibian anurans requires experiencing both aquatic and terrestrial environments, and the habitat is more complex compared to other taxa. Environmental factors may have different effects on different tissues and organs, and the difference in energy consumption of different tissues and organs may be more obvious. Therefore, we studied intraspecific size variation in brain size, the size of the brain region, and digestive tract length of F. quadranus along an altitudinal gradient. In particular, the ETH related to brain size evolution was tested using this system. To gain insight into the physiological adaptability of this species in response to environmental changes, we also tested whether brain size and digestive tract length were correlated with annual mean temperature and annual precipitation.
A total of 125 individuals, including 50 males and 75 females, were collected from 10 sample sites during the breeding season in 2021 (Figure 1 and Table 1). The main locations were in the Qinling-Daba Mountain area, with an elevation range of 586–1,702 m. The specimen for this study was approved by the Animal Ethics Committee at China West Normal University.
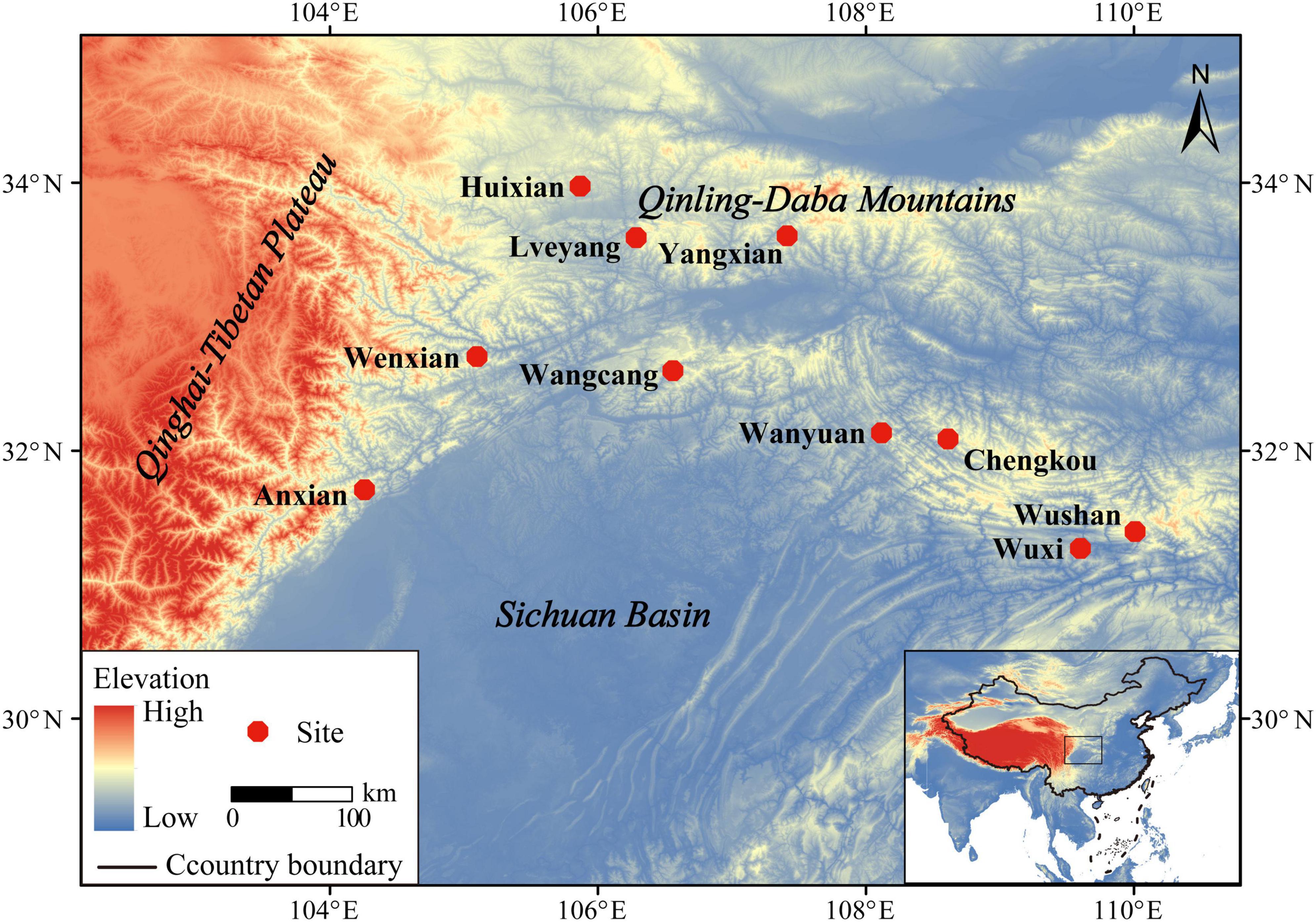
Figure 1. Topographic maps showing the sampling locations of 10 populations in Feirana quadranus in China.
All specimens were taken to the laboratory, kept individually in rectangular tanks (0.5 m × 0.4 m × 0.4 m) before being anesthetized with benzocaine, and preserved in 4 % buffered formalin in a phosphate buffer. After 2 months of preservation, snout-vent SVL was measured to the nearest 0.01 mm with calipers. Brains were dissected out and weighed (to the nearest 0.1 mg) with an electronic balance (Jiang et al., 2015). The complete brain regions taken out in this study mainly included the olfactory nerve (OLF-N), olfactory bulb (OLF-B), telencephalon (TEL), optic tectum (OPT), and cerebellum (CER), a total of five parts (Jiang et al., 2015).
A digital camera was used to take photographs of these brains from dorsal, lateral, and ventral views (Figure 2). The coronal and sagittal planes were parallel to the camera sensor, and a caliper with scale was placed in all photographs as a reference. Finally, the brain size was measured three times with the tpsDig software (Huber et al., 1997). All the measurements were conducted by the same investigator (YF) to eliminate interobserver variability (Burns et al., 2009). The length (L), width (W), and height (H) of each brain and the total volume of fluid displacements were used to obtain the ellipsoid model brain volume (V) as follows: V = (L*W*H)*π/(6*1.43). This formula was adopted to calculate the total brain volume and the volume of different brain parts for each individual OLF-N, OLF-B, TEL, OPT, and CER. For paired structures, only the right hemisphere was measured and the volume was doubled (Jiang et al., 2015).
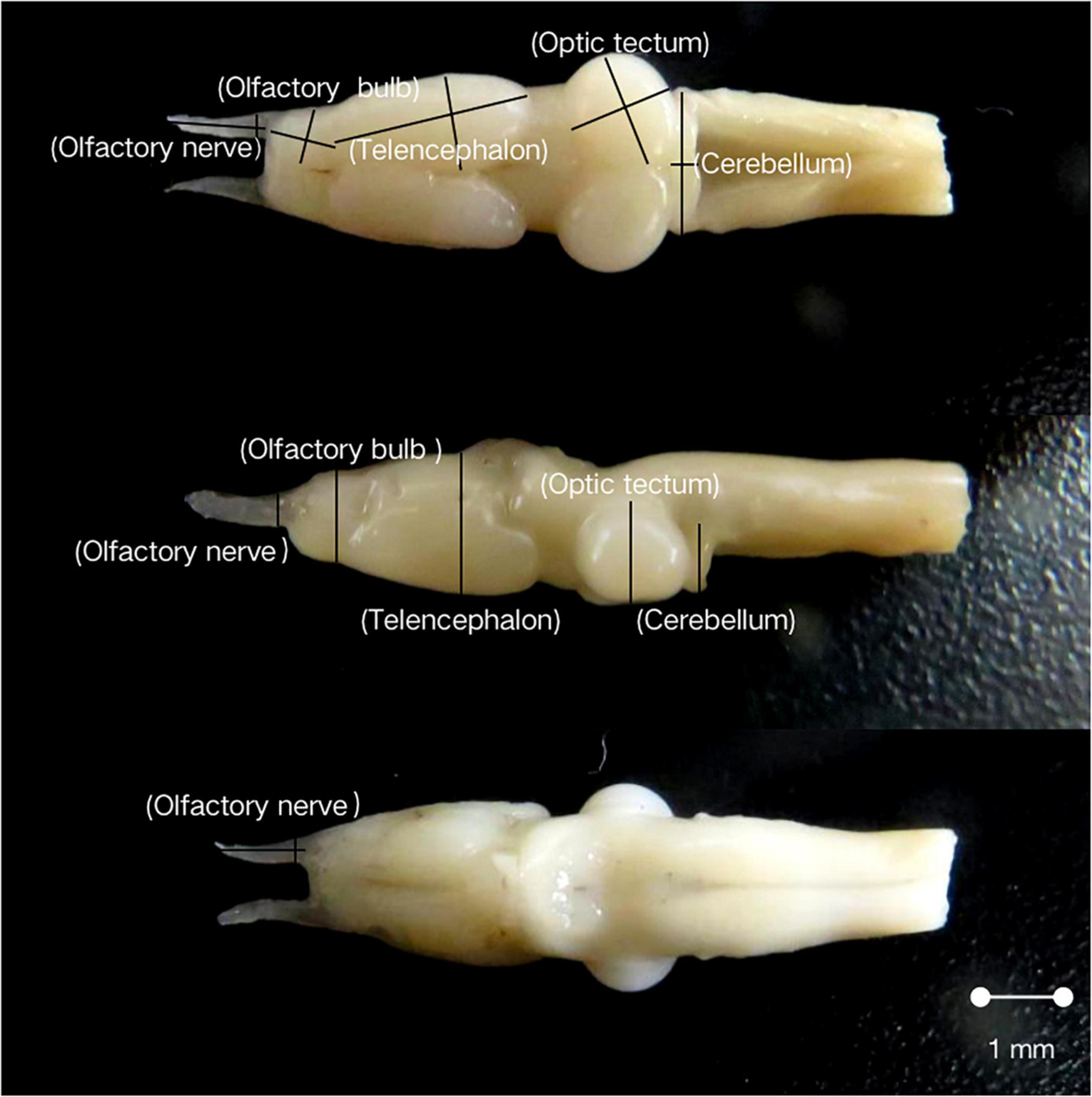
Figure 2. Dorsal, lateral, and ventral views of the brain in Feirana quadranus. Length, width, and height measures for each of the five brain structures (olfactory nerve, olfactory bulb, telencephalon, optic tectum, and cerebellum) are shown. Further details are found in the “materials and methods” section.
Similarly, the digestive tract was dissected out, and the digestive tract was placed on a foam board and secured with a pin to avoid overlap and bumps affecting its length measurement. Then, a digital camera was used to take images of the digestive tract with a caliper. Finally, the tpsDig software was used to measure the length of the digestive tract.
All continuous variables were log10 transformed to meet the assumption of normality and enhance the homogeneity of variances (type III sums of squares tests conducted with SPSS v.22.0). To analyze the differences in brain volume and digestive tract length among populations, we used one-way analyses of variance (ANOVA) for brain volume and digestive tract length separately, controlling for SVL as a covariate. Next, we ran a general linear model (GLM) with brain volume as a dependent variable, digestive tract length as fixed effects, and SVL as a covariate to test the original ETH. Correlations between organs were analyzed using the residuals from the log--log regressions of the brain and digestive tract length on SVL as relative values to offset the effect of SVL on organs. Then, we used multiple linear regression model (MLRM) to assess the effect of geographical gradients (e.g., latitude, longitude, and altitude) on relative brain volume, the relative size of brain regions, and relative digestive tract length. Finally, we analyzed the relationship between relative brain volume and relative digestive tract length and environmental parameters (e.g., annual mean temperature and annual precipitation) of each site among all populations. We also analyzed the relationship between the relative size of brain regions and environmental parameters. The annual mean temperature and annual precipitation in this study were obtained from the World Climate Database.1
One-way ANOVA revealed a significant difference in brain volume (F9,115 = 4.583, P < 0.001) and digestive tract length (F9,115 = 2.803, P = 0.005) among populations. We found that brain volume was significantly negative correlated with digestive tract length when controlling for SVL (Figure 3; digestive tract length, F44,79 = 1.923, P = 0.006; SVL, F1,112 = 12.559, P = 0.001).
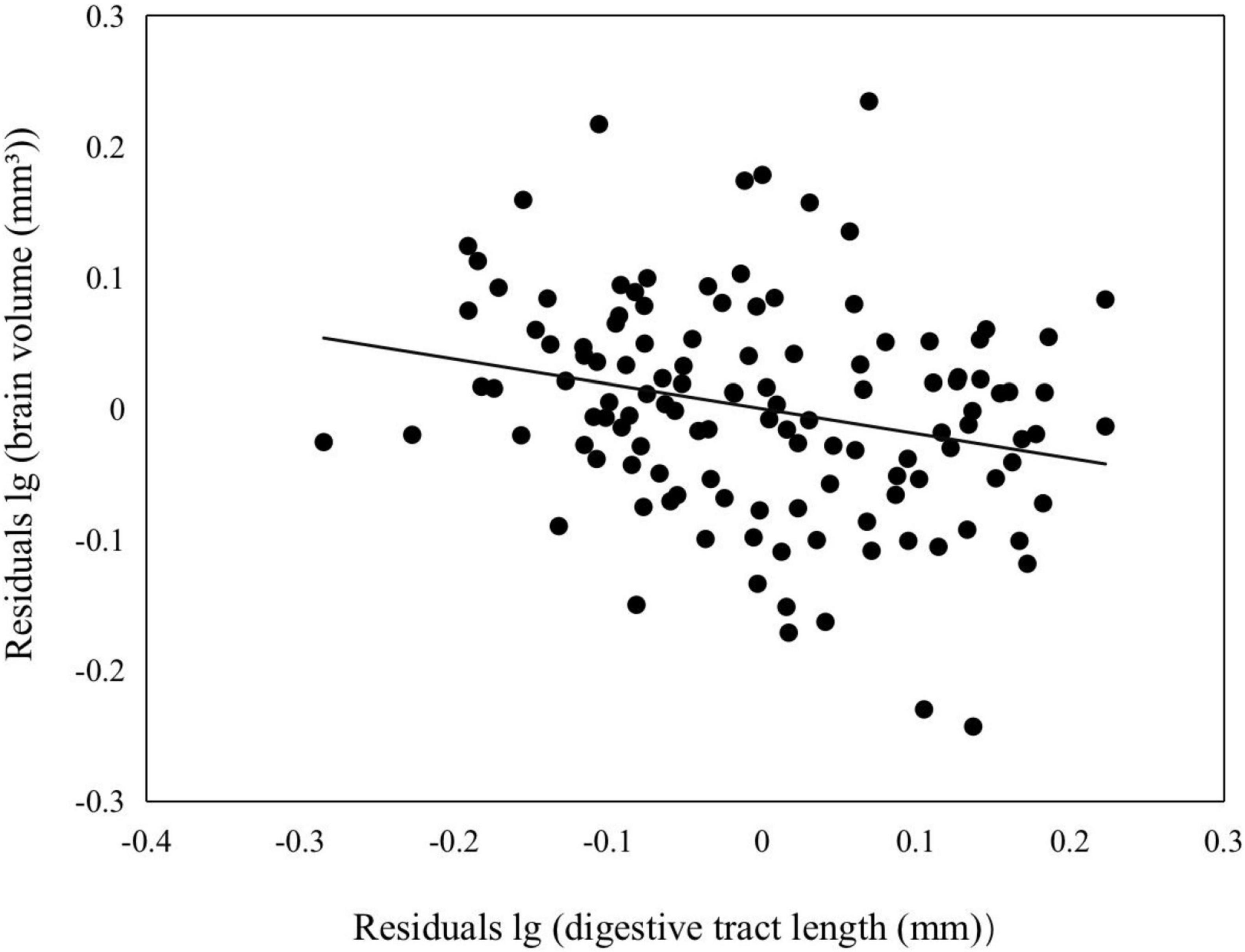
Figure 3. Correlations between relative brain volume and relative digestive tract length in Feirana quadranus.
We further examined the variation in the geographic gradients of organ size at the population level based. The relative brain volume was negatively correlated with altitude and latitude, but did not vary significantly with longitude (Table 2). The relative digestive tract length was positively correlated with altitude and longitude, but did not vary significantly with latitude (Table 2). The relative volume of OLF-N enlarged with the increase in altitudes (F3,118 = 2.145, t = 2.304, P = 0.023). Meanwhile, the relative volume of TEL and CER reduced with the increase in altitudes (TEL, F3,118 = 2.445, t = −2.334, P = 0.021; CER, F3,118 = 7.009, t = −2.403, P = 0.018) and the relative volume of OPT reduced with the increase in latitude (F3,118 = 8.837, t = −3.977, P < 0.001). However, the relative sizes of OLF-B had no significant relationship with geographical gradients (all P > 0.05). The results of MLRM show that the relative brain size was positive correlated with annual mean temperature (F2,121 = 20.641, t = 5.611, P < 0.001), while relative digestive tract length was negative correlated with annual mean temperature (F2,121 = 5.107, t = −3.118, P = 0.002). The MLRM showed that the relative TEL volume and relative OPT volume was positively correlated with annual mean temperature, while relative OLF-N volume was negatively correlated with annual mean temperature, and relative OPT volume size and relative CER volume size were positively correlated with annual precipitation (Table 3). No significant correlation was found between the other relative brain regions’ size and environmental factors (Table 3).
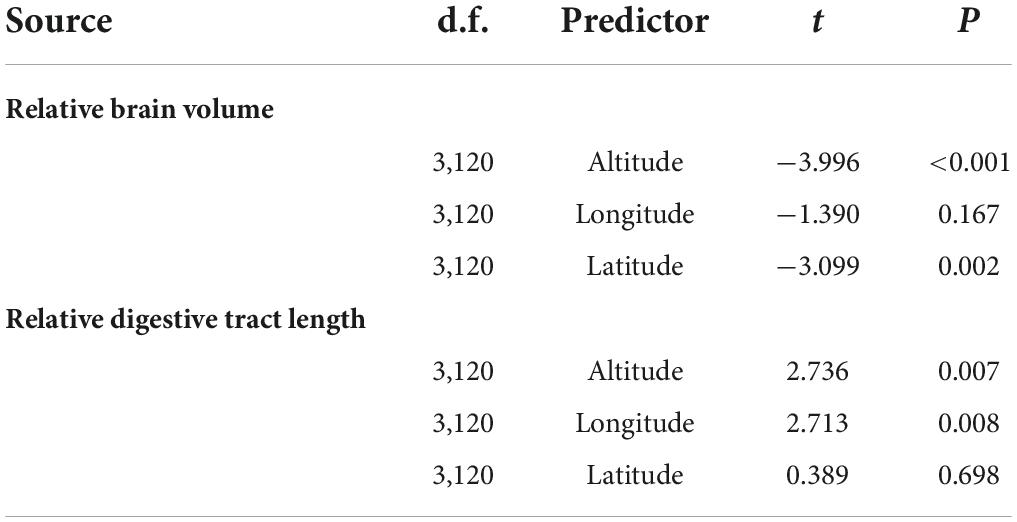
Table 2. The effects of geographical gradients on variation in relative brain volume and relative digestive tract length of the Feirana quadranus using multiple linear regression model (MLRM).
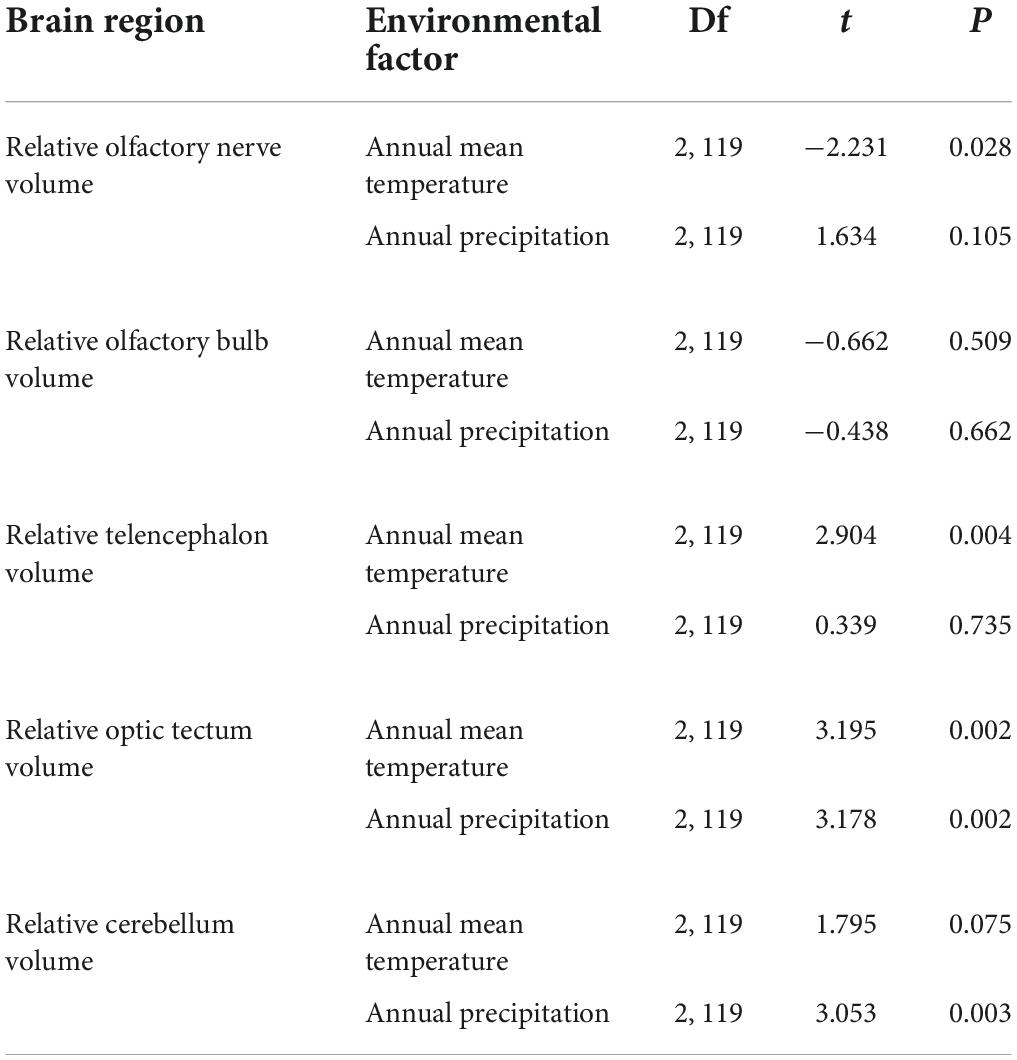
Table 3. The effects of annual mean temperature and annual precipitation on variation in the relative volume of brain region of the Feirana quadranus using multiple linear regression model (MLRM).
The results uncovered a negative correlation between brain size and digestive tract length after controlling for SVL in F. quadranus, supporting the original “brain versus gut” prediction arising from the ETH. The brain size of F. quadranus increased and the digestive tract length decreased with the increase in annual mean temperature. Thus, this study supports the existence of energetic constraints as important factors influencing the patterns of brain size diversification among frogs.
After controlling for SVL, a negative correlation between brain size and digestive tract length was observed in this species. Although the applicability and accuracy of the ETH have always been controversial, the brain–gut relationship in this species typically conforms to ETH, consistent with some previous studies. For example, the relationship between brain size and other energy-consuming organs in 30 anuran species was consistent with the ETH (Liao et al., 2016). The ETH was originally proposed to explain why the brain of several anthropoid primates and humans accounts for 1–2% of body weight (Aiello and Wheeler, 1995; Striedter, 2006). Ectotherms were believed to have small brains and be less likely to produce energy tradeoffs due to energy constraints in the evolutionary process, but this study and other studies demonstrated that the brain and digestive tract were negatively correlated in anuran species (Jin et al., 2015; Liao et al., 2016). These studies indicated that energy constraints and tradeoffs are prevalent in the evolution of the brain and digestive tract in vertebrates, especially in ectotherms.
Populations live in different environments with different altitudes, latitudes, and longitudes, and climate conditions. These environmental differences could be expressed in temperature, rainfall, ultraviolet light, oxygen pressure, and even indirectly related factors, such as food (Naya et al., 2009; Lou et al., 2013). When altitude was included in the statistical analysis, brain size was found to be decreased with the increase in altitudes, whereas a trend of digestive tract length increase with increased altitudes was observed. The altitudinal gradient is one of the most commonly used ecological differences to reveal biodiversity and its evolutionary mechanisms (Hodkinson, 2005; Keller et al., 2013). High-altitude environments are characterized by hypoxia, low temperature, high ultraviolet radiation, high climatic diurnal, and seasonal variability (Storz et al., 2010). Therefore, high altitude represents strong directional selection (Yao et al., 2021). Altitude, temperature, and rainfall are closely related to environmental factors. The combination of dehydration and cold temperatures in high-altitude areas leads to shorter periods of activity in the diurnal and seasonal cycles, thus also limiting energy supplies. Organisms at high altitudes must allocate the limited energy for all their functional organs to sustain basic life activities. With altitude increase, the energy investment in the brain decreases, whereas in the digestive tract increases. This could be an important reason for causing the negative correlation between brain size and digestive tract length.
Normally, the temperature is extremely important in shaping the characteristics of an organism. Temperature and rainfall could affect activity time, food abundance, natural enemy pressure, and habitat complexity. Therefore, this study not only focused on the relationship between brains and guts; it could also help gain fresh and deeper insights into how these environmental factors drive the evolution of the brain and high energy-consuming tissues. Three possible explanations could help understand the underlying mechanism of the evolution of brain size and digestive tract length in F. quadranus.
At first, changes in food composition in different environments may lead to a tradeoff between the brain size and the digestive tract length. The mean temperature of the environment often determines food availability. In general, areas with low average annual temperature and rainfall have low biodiversity and environmental complexity (Connell and Orias, 1964). The adult F. quadranus mainly feeds on insects; however, insect mortality is closely related to annual average temperature (Fitt, 1989; Morecroft et al., 2002; Savage et al., 2004; Staley et al., 2007; Wen and Zhang, 2010; Shi et al., 2011). There is evidence supporting that, in such harsh environments with high altitudes, decreased temperature may result in decreasing animal-based foods and increasing plant-based foods in anuran species (Naya et al., 2009; Lou et al., 2013; Wang et al., 2017). To better digest the nutrients in the food and improve survival in high altitudes, an increase in indigestible materials may drive the increased relative size of the digestive tract in these individuals (Wang et al., 2017). As a result, the amount of energy allocated to the brain reduces and the size of the brain decreases.
Under different environmental conditions, the variation of individual activity time is also one of the main reasons affecting the evolution of brain size. Shorter activity times may result in smaller brain size and increased digestive tract length. For Fejervarya limnocharis, individuals foraging at higher altitudes with shorter activity times have increased digestive tract lengths (Wang et al., 2017). A study in Bufo andrewsi revealed that the brain became smaller due to the decrease in its activity time at a high latitude with low temperature. In addition, populations of B. andrewsi with longer activity times developed larger optic tectum than those with shorter activity times, which may be an adaptation to a life of high risk at lower latitudes (Naya et al., 2009; Lou et al., 2013; Wang et al., 2017).
The habitat complexity including the difference in habitat type and predation risk is also an important factor driving intraspecific brain size evolution in different environments (Striedter, 2006; Liao et al., 2022). Direct experimental evidence showed that brain size evolution is intimately linked to the evolution of neuron number and cognition (Marhounová et al., 2019). Recent studies suggested that environmental factors have a significant influence on the evolution of some brain regions in amphibians. For example, habitat type could explain the change in telencephalon size, and natural enemy pressure could explain the change in bulbus olfactorius and optic tecta size in 43 amphibious species (Liao et al., 2015). Studies have shown that the increase in brain size is closely related to the recognition of predators (Liao et al., 2015; van der Bijl et al., 2015). With the increase in altitude, the changes in annual mean temperature and annual precipitation can not only lead to fewer food resources but also fewer types and numbers of natural predators. In such a simpler environment, the reduced cognitive demands drive the evolution of decreased brain size correspondingly, providing excess energy to develop the digestive tract. Meanwhile, places with high annual average temperature and high rainfall have high biodiversity and complex environment. The factors mentioned above could encourage individuals living in these environments to increase their brain size and cognitive abilities to cope with more complex environments and predation stress, thereby increasing their chances of survival. Besides, although the overall brain size decreases with altitude, the olfactory nerve increases its size along the altitudinal gradients. The function of the olfactory nerve is mainly to perceive odor information, which is essential for locating predators and finding and capturing prey. This finding suggested that F. quadranus at high altitudes, cold temperature, and dry conditions likely faces additional cognitive challenges, which demand an enlarged olfactory nerve to cope with it.
In conclusion, the findings suggested that the ETH is supported in F. quadranus because brain size and digestive tract length are inversely related. By influencing food availability, activity times, and habitat complexity, environmental factors such as annual mean temperature and annual precipitation are the potential mechanisms driving the adaptive evolution of brain size and digestive tract length.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Animal Ethics Committee at China West Normal University.
YF and YH conceived, wrote, and edited the manuscript. YH secured funding. YF, YS, CY, XL, and YL participated in laboratory work and collected the data. YF conducted data analysis and visual representation of the data. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (32270461 and 31901234) and the Key Fund Project of the Sichuan Provincial Department of Education (18ZA0473).
The authors wish to thank Shuang Huang for her fieldwork. The reported experiments comply with the current laws of China concerning animal experimentation, and permission to collect amphibians was received from the Ethical Committee for Animal Experiments in China Council on Animal Care guidelines. The sacrifice of animals was approved by the Animal Ethics Committee at China West Normal University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aiello, L. C., and Wheeler, P. (1995). The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221.
Balciauskas, L., Amshokova, A., Balciauskiene, L., Benedek, A. M., Cichocki, J., Csanády, A., et al. (2020). Geographical clines in the size of the herb field mouse (Apodemus uralensis). Integr. Zool. 15, 55–68. doi: 10.1111/1749-4877.12407
Baldwin, J. W., Garcia-Porta, J., and Botero, C. A. (2022). Phenotypic responses to climate change are significantly dampened in big-brained birds. Ecol. Lett. 25, 939–947. doi: 10.1111/ele.13971
Barrickman, N. L., and Lin, M. J. (2010). Encephalization, expensive tissues, and energetics: an examination of the relative costs of brain size in strepsirrhines. Am. J. Phys. Anthropol. 143, 579–590. doi: 10.1002/ajpa.21354
Barton, R. A., and Capellini, I. (2011). Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl. Acad. Sci. U.S.A. 108, 6169–6174. doi: 10.1073/pnas.1019140108
Barton, R. A., and Harvey, P. H. (2000). Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058.
Burns, J. G., Saravanan, A., and Rodd, F. H. (2009). Rearing environment affects the brain size of guppies: Lab-reared guppies have smaller brains than wild-caught guppies. Ethology 115, 122–133. doi: 10.1111/j.1439-0310.2008.01585.x
Carnaval, A. C., Waltari, E., Rodrigues, M. T., Rosauer, D., VanDerWal, J., Damasceno, R., et al. (2014). Prediction of phylogeographic endemism in an environmentally complex biome. Proc. R. Soc. B Biol. Sci. 281:20141461. doi: 10.1098/rspb.2014.1461
Clifton, I. T., Chamberlain, J. D., and Gifford, M. E. (2020). Role of phenotypic plasticity in morphological differentiation between water snake populations. Integr. Zool. 15, 329–337. doi: 10.1111/1749-4877.12431
Connell, J. H., and Orias, E. (1964). The ecological regulation of species diversity. Am. Nat. 98, 399–414.
Dornburg, A., Warren, D. L., Zapfe, K. L., Morris, R., Iglesias, T. L., Lamb, A., et al. (2018). Testing ontogenetic patterns of sexual size dimorphism against expectations of the expensive tissue hypothesis, an intraspecific example using oyster toadfish (Opsanus tau). Ecol. Evol. 8, 3609–3616. doi: 10.1002/ece3.3835
Dunbar, R. I., and Shultz, S. (2007). Evolution in the social brain. Science 317, 1344–1347. doi: 10.1126/science.1145463
Fitt, G. P. (1989). The ecology of Heliothis species in relation to agroeco systems. Ann. Rev. Entomol. 34, 17–53.
Giacomini, G., Herrel, A., Chaverri, G., Brown, R. P., Russo, D., Scaravelli, D., et al. (2022). Functional correlates of skull shape in Chiroptera: feeding and echolocation adaptations. Integr. Zool. 17, 430–442.
Gonda, A., Herczeg, G., and Merilä, J. (2013). Evolutionary ecology of intraspecific brain size variation: a review. Ecol. Evol. 3, 2751–2764. doi: 10.1002/ece3.627
Gonzalez-Voyer, A., González-Suárez, M., Vilà, C., and Revilla, E. (2016). Larger brain size indirectly increases vulnerability to extinction in mammals. Evolution 70, 1364–1375. doi: 10.1111/evo.12943
Hinds, L. A., Henry, S., Van de Weyer, N., Robinson, F., Ruscoe, W. A., and Brown, P. R. (2022). Acute oral toxicity of zincphosphide: an assessment for wild house mice (Mus musculus). Integr. Zool. [Online ahead of print] doi: 10.1111/1749-4877.12666
Hodkinson, I. D. (2005). Terrestrial insects along elevation gradients: species and community responses to altitude. Biol. Rev. 80, 489–513. doi: 10.1017/S1464793105006767
Huang, S. L., Li, G. L., Pan, Y. L., Song, M., Zhao, J., Wan, X., et al. (2021). Density–induced social stress alters oxytocin and vasopressin activities in the brain of a small rodent species. Integr. Zool. 16, 149–159. doi: 10.1111/1749-4877.12467
Huang, Y., Mai, C. L., Liao, W. B., and Kotrschal, A. (2020). Body mass variation is negatively associated with brain size: Evidence for the fat-brain trade-off in anurans. Evolution 74, 1551–1557. doi: 10.1111/evo.13991
Huber, R., van Staaden, M. J., Kaufman, L. S., and Liem, K. F. (1997). Microhabitat use, trophic patterns, and the evolution of brain structure in African cichlids. Brain Behav. Evol. 50, 167–182. doi: 10.1159/000113330
Jiang, A., Zhong, M. J., Xie, M., Lou, S. L., Jin, L., Robert, J., et al. (2015). Seasonality and age is positively related to brain size in Andrew’s toad (Bufo andrewsi). Evol. Biol. 42, 339–348. doi: 10.1007/s11692-015-9329-4
Jin, L., Zhao, L., Liu, W. C., Zeng, Y., and Liao, W. B. (2015). Evidence for the expensive-tissue hypothesis in the Omei Wood Frog (Rana omeimontis). Herpetol. J. 25, 127–130.
Kaufman, J. A. (2003). On the expensive-tissue hypothesis: independent support from highly encephalized fish. Curr. Anthropol. 44, 705–707.
Keller, I., Alexander, J. M., Holderegger, R., and Edwards, P. J. (2013). Widespread phenotypic and genetic divergence along altitudinal gradients in animals. J. Evol. Biol. 26, 2527–2543. doi: 10.1111/jeb.12255
Konarzewski, M., Goncerzewicz, A., Knapska, E., Dzik, J., and Górkiewicz, T. (2020). Energetic costs of cognitive abilities: Testing the expensive tissue hypothesis. Authorea [Online ahead of print] doi: 10.22541/au.159069206.66900218
Kotrschal, A., Rogell, B., Bundsen, A., Svensson, B., Zajitschek, S., Brännström, I., et al. (2013a). Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. doi: 10.1016/j.cub.2012.11.058
Kotrschal, A., Rogell, B., Bundsen, A., Svensson, B., Zajitschek, S., Brännström, I., et al. (2013b). The benefit of evolving a larger brain: big-brained guppies perform better in a cognitive task. Anim. Behav. 86, e4–e6. doi: 10.1016/j.anbehav.2013.07.011
Lemaître, J. F., Ramm, S. A., Barton, R., and Stockley, P. (2009). Sperm competition and brain size evolution in mammals. J. Evol. Biol. 22, 2215–2221. doi: 10.1111/j.1420-9101.2009.01837.x
Liang, T., Meiri, S., and Shi, L. (2021). Sexual size dimorphism in lizards: Rensch’s rule, reproductive mode, clutch size, and line fitting method effects. Integr. Zool. [Online ahead of print] doi: 10.1111/1749-4877.12569
Liao, W. B., Huang, Y., Zeng, Y., Zhong, M. J., Luo, Y., and Lüpold, S. (2018). Ejaculate evolution in external fertilizers: Influenced by sperm competition or sperm limitation? Evolution 72, 4–17. doi: 10.1111/evo.13400
Liao, W. B., Jiang, Y., Li, D. Y., Jin, L., Zhong, M. J., Qi, Y., et al. (2022). Cognition contra camouflage: how the brain mediates predator-driven crypsis evolution. Sci. Adv. 8:eabq1878. doi: 10.1126/sciadv.abq1878
Liao, W. B., Lou, S. L., Zeng, Y., and Kotrschal, A. (2016). Large brains, small guts: The expensive tissue hypothesis supported within Anurans. Am. Nat. 188, 693–700. doi: 10.1086/688894
Liao, W. B., Lou, S. L., Zeng, Y., and Merila, J. (2015). Evolution of anuran brains: disentangling ecological and phylogenetic sources of variation. J. Evol. Biol. 28, 1986–1996. doi: 10.1111/jeb.12714
Liu, Y. T., Luo, Y., Gu, J., Jiang, S., and Liao, W. B. (2018). The relationship between brain size and digestive tract length do not support expensive-tissue hypothesis in Hylarana guentheri. Acta Herpetol. 13, 141–146. doi: 10.13128/Acta_Herpetol-20920
Liu, Y. T., Wu, Z. J., and Liao, W. B. (2022). Large-brained birds display lower extra-pair paternity. Integr. Zool. [Online ahead of print] doi: 10.1111/1749-4877.12636
Lou, S., Li, Y., Jin, L., Mi, Z., Liu, W., and Liao, W. (2013). Altitudinal variation in digestive tract length in Yunnan pond frog (Pelophylax pleuraden). Asian Herpetol. Res. 4, 263–267. doi: 10.3724/SP.J.1245.2013.00263
Luo, Y., Zhong, M. J., Huang, Y., Li, F., Liao, W. B., and Kotrschal, A. (2017). Seasonality and brain size are negatively associated in frogs: evidence for the expensive brain framework. Sci. Rep. 7, 1–9. doi: 10.1038/s41598-017-16921-1
Marhounová, L., Kotrschal, A., Kverková, K., Kolm, N., and Němec, P. (2019). Artificial selection on brain size leads to matching changes in overall number of neurons. Evolution 73, 2003–2012. doi: 10.1111/evo.13805
Mi, Z. P., and Liao, W. B. (2021). No evidence for the expensive-tissue hypothesis in the Asiatic toad (Bufo gargarizans). North Western J. Zool. 17, 73–76.
Minias, P., and Podlaszczuk, P. (2017). Longevity is associated with relative brain size in birds. Ecol. Evol. 7, 3558–3566. doi: 10.1002/ece3.2961
Mink, J. W., Blumenschine, R. J., and Adams, D. B. (1981). Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 241, R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203
Morecroft, M., Bealey, C., Howells, O., Rennie, S., and Woiwod, I. (2002). Effects of drought on contrasting insect and plant species in the UK in the mid-1990s. Glob. Ecol. Biogeogr. 11, 7–22. doi: 10.1046/j.1466-822X.2002.00174.x
Navarrete, A., Van Schaik, C. P., and Isler, K. (2011). Energetics and the evolution of human brain size. Nature 480, 91–93. doi: 10.5167/uzh-57326
Naya, D. E., Veloso, C., and Bozinovic, F. (2009). Gut size variation among Bufo spinulosus populations along an altitudinal (and dietary) gradient. Fin. Zool. Bot. Publ. Board 46, 16–20. doi: 10.5735/086.046.0102
Pitnick, S., Jones, K. E., and Wilkinson, G. S. (2006). Mating system and brain size in bats. Proc. R. Soc. B Biol. Sci. 273, 719–724. doi: 10.1098/rspb.2005.3367
Reader, S. M., and Laland, K. N. (2002). Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl. Acad. Sci. 99, 4436–4441. doi: 10.1073/pnas.062041299
Savage, V. M., Gillooly, J. F., Brown, J. H., West, G. B., and Charnov, E. L. (2004). Effects of SVL and temperature on population growth. Am. Nat. 163, 429–441.
Sayol, F., Lefebvre, L., and Sol, D. (2016a). Relative brain size and its relation with the associative pallium in birds. Brain Behav.Evol. 87, 69–77. doi: 10.1159/000444670
Sayol, F., Maspons, J., Lapiedra, O., Iwaniuk, A. N., Székely, T., and Sol, D. (2016b). Environmental variation and the evolution of large brains in birds. Nat. Commun. 7, 1–8. doi: 10.1038/ncomms13971
Shi, P., Ikemoto, T., and Ge, F. (2011). Development and application of models for describing the effects of temperature on insects’ growth and development. Chin. J. Appl. Entomol. 48, 1149–1160.
Sol, D., Duncan, R. P., Blackburn, T. M., Cassey, P., and Lefebvre, L. (2005). Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl. Acad. Sci. U.S.A. 102, 5460–5465. doi: 10.1073/pnas.0408145102
Staley, J. T., Mortimer, S. R., Morecroft, M. D., Brown, V. K., and Masters, G. J. (2007). Summer drought alters plant-mediated competition between foliar-and root-feeding insects. Glob. Change Biol. 13, 866–877. doi: 10.1111/j.1365-2486.2007.01338.x
Storz, J. F., Scott, G. R., and Cheviron, Z. A. (2010). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125–4136. doi: 10.1242/jeb.048181
Striedter, G. F. (2006). Précis of principles of brain evolution. Behav. Brain Sci. 29, 1–12. doi: 10.1017/S0140525X06009010
Tsuboi, M., Husby, A., Kotrschal, A., Hayward, A., Buechel, S. D., Zidar, J., et al. (2015). Comparative support for the expensive tissue hypothesis: big brains are correlated with smaller gut and greater parental investment in Lake Tanganyika cichlids. Evolution 69, 190–200. doi: 10.1111/evo.12556
van der Bijl, W., Thyselius, M., Kotrschal, A., and Kolm, N. (2015). Brain size affects the behavioural response to predators in female guppies (Poecilia reticulata). Proc. R. Soc. B Biol. Sci. 282:20151132. doi: 10.1098/rspb.2015.1132
van Woerden, J. T., Willems, E. P., van Schaik, C. P., and Isler, K. (2011). Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199. doi: 10.1111/j.1558-5646.2011.01434.x
Wang, W. Y., Zhang, R., Yin, Q. X., Zhang, S. P., Li, W. Q., and Mi, Z. P. (2017). Digestive tract length is positively correlated with altitude across Fejervarya limnocharis populations. Anim. Biol. 67, 227–237.
Wang, X., Huang, Y., Zhong, M., Yang, S., Yang, X., Jiang, J., et al. (2019). Environmental stress shapes life-history variation in the swelled-vented frog (Feirana quadranus). Evol. Ecol. 33, 435–448. doi: 10.1007/s10682-019-09980-5
Wen, L., and Zhang, Y. (2010). Modelling of the relationship between the frequency of large-scale outbreak of the beet army worm, Spodoptera exigua (Lepidoptera: Noctuidae) and the wide-area temperature and rainfall trends in China. Acta Entomol. Sin. 53, 1367–1381.
Xia, H., Qin, Y., Feng, G., Meng, Q., Cui, Y., Song, H., et al. (2019). Forest phenology dynamics to climate change and topography in a geographic and climate transition zone: the Qinling mountains in Central China. Forests 10:1007. doi: 10.3390/f10111007
Yao, Z., Qi, Y., Yue, B., and Fu, J. (2021). Brain size variation along altitudinal gradients in the Asiatic Toad (Bufo gargarizans). Ecol. Evol. 11, 3015–3027. doi: 10.1002/ece3.7192
Yu, X., Zhong, M. J., Li, D. Y., Jin, L., Liao, W. B., and Kotrschal, A. (2018). Large-brained frogs mature later and live longer. Evolution 72, 1174–1183. doi: 10.1111/evo.13478
Zamora–camacho, F. J. (2021). Sex and habitat differences in size and coloration of an amphibian’s poison glands match differential predator pressures. Integr. Zool. [Online ahead of print] doi: 10.1111/1749-4877.12597
Keywords: brain size, energetic constraints, expensive-tissue hypothesis, environmental factors, Feirana quadranus
Citation: Fu Y, Song Y, Yang C, Liu X, Liu Y and Huang Y (2022) Relationship between brain size and digestive tract length support the expensive-tissue hypothesis in Feirana quadranus. Front. Ecol. Evol. 10:982590. doi: 10.3389/fevo.2022.982590
Received: 30 June 2022; Accepted: 13 September 2022;
Published: 30 September 2022.
Edited by:
Jianping Jiang, Chengdu Institute of Biology (CAS), ChinaReviewed by:
Yossi Yovel, Tel Aviv University, IsraelCopyright © 2022 Fu, Song, Yang, Liu, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Huang, c3VuZmxvd2VyLWh5QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.