- 1Donghu Experimental Station of Lake Ecosystems, State Key Laboratory of Freshwater Ecology and Biotechnology of China, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China
- 2College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing, China
- 3College of Fisheries, Huazhong Agricultural University, Engineering Research Center of Green Development for Conventional Aquatic Biological Industry in the Yangtze River Economic Belt, Ministry of Education, Hubei Provincial Engineering Laboratory for Pond Aquaculture, Wuhan, China
Aquatic gastropods are important integral components of the macroinvertebrate community in freshwater ecosystems and play critical roles in freshwater ecosystems by contributing to biodiversity, nutrient cycling, and water quality. However, the variation of aquatic gastropods’ community structure under the combined effects of warming and nutrient enrichment remains largely unknown. To investigate this question, we performed an outdoor mesocosm experiment examining the interaction of warming (a 4.5 °C increase in mean temperature above ambient conditions) and nutrient enrichment (phosphorus addition) on the aquatic gastropods’ community and dominant population (Bellamya aeruginosa). We analyzed the changes in community dynamics (abundance and biomass), size structure, and stoichiometric traits (only B. aeruginosa). Results showed that phosphorus enrichment alone had a positive effect on the total abundance and biomass of gastropods, as well as the abundance and biomass of B. aeruginosa. Warming alone only produced a positive effect on total abundance. However, the combined effects of warming and phosphorus enrichment negatively affected the biomass and abundance of the whole gastropod community and the dominant gastropod population. The body mass of B. aeruginosa increased because of warming, whereas the body mass of the gastropod community negatively responded to warming. Phosphorus enrichment alone had no remarkable effects on body mass. The combined effects of warming and phosphorus enrichment negatively affected the whole community’s body mass but had no substantial effect on the body mass of B. aeruginosa. For body stoichiometric traits, warming or phosphorus enrichment alone produced positive effects on the nitrogen and phosphorus contents of B. aeruginosa. The combined effects caused adverse effects on the contents of the two elements. The effect of warming alone only decreased the ratio of nitrogen to phosphorus. Results suggested that the response levels in size structure between the gastropod community and the dominant population differed remarkably. Composition species shift was the main cause of the decrease in aquatic gastropods’ community size structure. The shift in species composition at the whole gastropod community level caused by warming and phosphorus enrichment may result in more complex and unpredicted consequences through cascade effects on the structure and function of freshwater ecosystems.
Introduction
Body size is an important ecological characteristic of organisms (Brown et al., 2004). Body size is correlated with many physiological and ecological processes, such as metabolism, fecundity, competition, predation and energy use (Woodward et al., 2005). Hence, body size variations can cause potentially profound effects on the aquatic ecosystem across multiple scales of organisation. The responses of organisms’ body size are worth investigating because global warming trend has not been halted (Ranasinghe et al., 2021) and eutrophication is still a significant water quality issue in global aquatic ecosystems (Smith and Schindler, 2009).
The temperature-size rule states that temperature and the body size of ectotherm organisms are closely related (Atkinson, 1994). Studies suggested that warming could alter the community size structure of aquatic benthic invertebrate organisms (Dossena et al., 2012; Nelson et al., 2017). The responses of aquatic benthic invertebrate communities to warming are related to nutrition conditions and seasons (Dossena et al., 2012, Nelson et al., 2017). Recent findings suggested that if nutrient supply increases with warming or primary production enhanced by warming could satisfy higher trophic levels of community energy demand, the energy would be sufficient to support a larger consumer body size (Nelson et al., 2017; O’Gorman et al., 2017). In addition, warming may alter community body size structure by a shift in the relative abundances of differently sized species, also known as the ‘species shift’ or ‘composition shift’ hypothesis (Daufresne et al., 2009; Ohlberger, 2013).
Aquatic gastropods are important components of macroinvertebrate communities in many freshwater ecosystems and play key roles in food web structure and biodiversity, material circulation, energy flow, and water quality (Brönmark and Weisner, 1996; Covich et al., 1999; Reed and Janzen, 1999; Guo et al., 2022). However, variations in the size structure of aquatic gastropods to warming are rarely studied. Warming could increase the proportion of larger and warm-adapted species (e.g., gastropods) compared with small and cold-adapted species under sufficient resource conditions (Nelson et al., 2017). However, we do not know what is happening within this community of aquatic gastropods when facing warming.
Ecological stoichiometry is a conceptual framework that investigating the relationship between key elements (e.g., nitrogen, phosphorus) and organisms in ecological systems. It has been widely and successfully used in freshwater pelagic ecosystems and has great application potential in benthic ecosystems (Sterner and Elser, 2002; Cross et al., 2005; Frost et al., 2005). Phosphorus is a key element for the growth of aquatic organisms and has a close relationship with the animal body size and phylogeny (Sterner, 2009). Pieces of evidence from zooplankton showed that, organisms need to increase phosphorus allocation to rRNA synthesis to raise the protein synthesis rate to maintain a rapid growth rate under high-temperature conditions (Elser et al., 2000; Persson et al., 2011). Woods et al. (2003) indicated that the phosphorus content of poikilotherms declines with increased temperature through a literature survey study, because warming increases phosphorus use efficiency. These contradictory results show that the response of phosphorus to warming still needs to be explored. In addition, studies have yet to investigate how the stoichiometric traits of aquatic gastropods respond to warming and phosphorus enrichment.
Here, we present a well-replicated, factorial outdoor mesocosm experiment to investigate the effects of warming and phosphorus enrichment on aquatic gastropods’ community dynamics (variation in abundance and biomass), size structure and ecological stoichiometric traits (i.e., nitrogen, phosphorus, and nitrogen-phosphorus ratio [N: P ratio]). Firstly, we hypothesised that aquatic gastropods’ community abundance and biomass would change under different experimental treatment conditions (Figure 1A). Warming may alter the phenology of gastropods (Villeneuve et al., 2021), and phosphorus enrichment may alter resource conditions (McCormick et al., 2001). These effects would ultimately act on the biomass and abundance of aquatic gastropods. Secondly, we hypothesised that the aquatic gastropod community’s body size would tend to be smaller because of the warming effects. The decrease in body size might be caused by the direct effects of warming or by composition shift (Figure 1B). Thirdly, we hypothesised that the effects of warming on gastropods’ phosphorus content would be context specific (Figure 1C) because evidence from other biota showed that warming increases phosphorus demand (Main et al., 1997; Persson et al., 2011) and phosphorus use efficiency (Sievers et al., 2004; Toseland et al., 2013) simultaneously.

Figure 1. Possible consequences of experimental treatments on the aquatic gastropods. (A) Experimental treatments alter the abundance and biomass of gastropods. (B) Warming decreases the gastropods’ body size via direct effect or composition shift. (C) Warming increases phosphorus demand and phosphorus use efficiency, the direction of the phosphorus changes depends on the particular condition.
Materials and methods
Mesocosm establishment
The climate change experimental mesocosm system is situated at Huazhong Agricultural University in Wuhan City, Central China (30°29′N, 114°22′E). It consisted of 48 insulated cylindrical polyethylene tanks (diameter = 1.5 m, height = 1.4 m), and each tank was supplied with aquarium heating devices and temperature sensors (water temperatures were measured every second). Water temperature in each mesocosm was continuously monitored and individually adjusted according to the corresponding treatment conditions. In addition, ambient temperature was measured in the controls using an automatic temperature control system (Wang et al., 2020).
In late December 2013, sediment was collected with a Peterson grab sampler (approximately 30 cm depth) from a pelagic area in Lake Liangzihu (30°11′3″N, 114°37′59″E). The water body’s total nitrogen (TN) and total phosphorus (TP) contents were 0.432 and 0.023 mg L−1, respectively (Li et al., 2018). In addition, it is a mesotrophic lake (Dong et al., 2016). The sediment was thoroughly mixed in a cleaned container and then transferred to the experimental tanks. Each tank had a 10 cm-deep, un-sieved, well-mixed bed layer of lake sediment and was filled with mixed water to a depth of 1 m. Mixed water, including tap water (87.5%) and water collected from Lake Nanhu (12.5%, near our mesocosm system), was used to simulate mesotrophic lakes (TN and TP concentrations in Lake Nanhu were 3.250 and 0.198 mg L−1, respectively). Lake water from Lake Nanhu was flushed through a 20 μm mesh to eliminate large particle residues, fish, and other uncontrolled aquatic gastropods.
The tanks were randomly assigned to one of four experimental treatments in a fully factorial design (two factors: warming and phosphorus addition with two levels), each replicated six times: (C) control tanks, which were unheated and received no phosphorus addition for mimicking the current state of Lake Liangzihu; (T) increased temperature by 4.5°C compared with the control for mimicking the projected average regional temperature by the century’s end under high-emission scenario RCP 8.5 (IPCC, 2013); (E) phosphorus enrichment without warming, comprising the addition of 50 μg L−1 phosphate (KH2PO4) every 2 weeks to mimic the phosphorus loading of the lake; and (T + E), which is a combination of warming and phosphorus addition treatments to investigate the interaction effects between them. Phosphorus enrichment is often regarded as the key driver of eutrophication (Correll, 1998). Some studies suggested phosphorus control is more critical than nitrogen for mitigating eutrophication (Carpenter, 2008; Wang and Wang, 2009).
Before the start of the experiment, about 1 month was left for de-chlorination and the establishment of the aquatic community. Our study was conducted for 338 days between the 26th of January and the 29th of December 2014. Warming and phosphorus addition were started on the first day. During this period, evaporation loss from the mesocosms was supplied with tap water when not compensated for by rainfall. Freshwater gastropods, including Bellamya aeruginosa, Parafossarulus striatulus, Alocinma longicornis, Radix swinhoei, and Gyraulus convexiusculus (species information could be found in Supplementary files), originated from the sediment. No fish was present during the experiment. Other macroinvertebrates, such as oligochaetes, also could originate from the sediment.
Sampling and analysis
Water samples were collected with a Plexiglas tube (diameter = 70 mm, length = 1 m) from 31st March 2014. Three replicate water samples from each mesocosm were collected and thoroughly mixed, then used for the analysis of TP (Jin and Tu, 1990) with a spectrophotometer (UV-2800, Unico, China). We monitored gastropods from 3rd April 2014 to 25th December 2014. A board (40 × 50 cm2) was attached to the wall of the mesocosm and was 0.3 m below the water surface. We used a fine benthic diddle net (425 μm) to take out the boards and collected all gastropods. Gastropods were cleaned carefully, blotted dry, counted, and weighed with a 0.1 mg electronic analytical balance, then gastropods were released back into the corresponding mesocosms. The sampling frequency was every 2 weeks in winter and every week during the other seasons. Nitrogen and phosphorus contents of B. aeruginosa were detected in each season. The sampling number of B. aeruginosa in the C, E, T, and T + E groups was 51, 48, 60, and 26, respectively. The gastropods were left in clear water for 24 h to eliminate the effects of intestinal contents and then their shells were removed. Only muscle tissues were preserved and analysed (Liess and Hillebrand, 2005). According to Liess and Hillebrand (2005), the stoichiometric traits (e.g., N: P) between the whole gastropods (including shells) and only muscle tissue were not statistically different. Specimens were freeze-dried and then ground to powder. Nitrogen content was analyzed by an elemental analyzer (Flash EA 1112, CE Instruments, Italy). Phosphorus content was analyzed as PO4 after hot-acid hydrolysis with potassium persulfate (Grasshoff et al., 1983; Jin and Tu, 1990).
Statistics
Repeated measures data, including the water TP content, total abundance and biomass of the gastropod community, the abundance and biomass of B. aeruginosa, the body size of the gastropod community, and the body size of B. aeruginosa were analysed using generalized mixed models (GLMMs) with the “lmer” or “glmer” functions from the “lme4” R package (Bates et al., 2015). The nitrogen content, phosphorus content, and N: P ratio was also analysed by GLMMs. Before the analyses, variables were transformed using square root or log transformation as necessary. The normality of variance was checked by residual normal QQ plots (Kozak and Piepho, 2017). The abundance of gastropods was analysed using a Poisson distribution to fit count data. The model included the main effects of warming and phosphorus addition with their mutual interactions as fixed effects. The sampling date was regarded as random effects for analysis on TP content, gastropod abundance, biomass, and body size; and the weight data of gastropods and the sampling date were regarded as random effects for analysis on stoichiometric traits. The post hoc pairwise comparisons amongst treatment levels were assessed via Tukey’s test with the “lsmeans” R package (Lenth, 2016). We used the “ggpredict” function available in the “ggeffects” package (Lüdecke, 2018) to plot predictions from each statistical model. We presented the variation of gastropod body mass across time using the loess smoothing method implemented in the “ggplot2” package. All of the analyses were performed at a 0.05 statistical significance level using R software (ver. 3.5.1; R Development Core Team, 2014).
Results
Water conditions in the experimental tanks
Water temperature in the experimental tanks followed the desired experimental design, in which warming groups (T and T + E) were always approximately 4.5°C higher than ambient temperature groups (C and E). The average daily water temperature ranged from 3.7°C to 33.6°C in ambient temperature groups, and ranged from 5.8°C to 37.9°C in warming groups (Figure 2A).
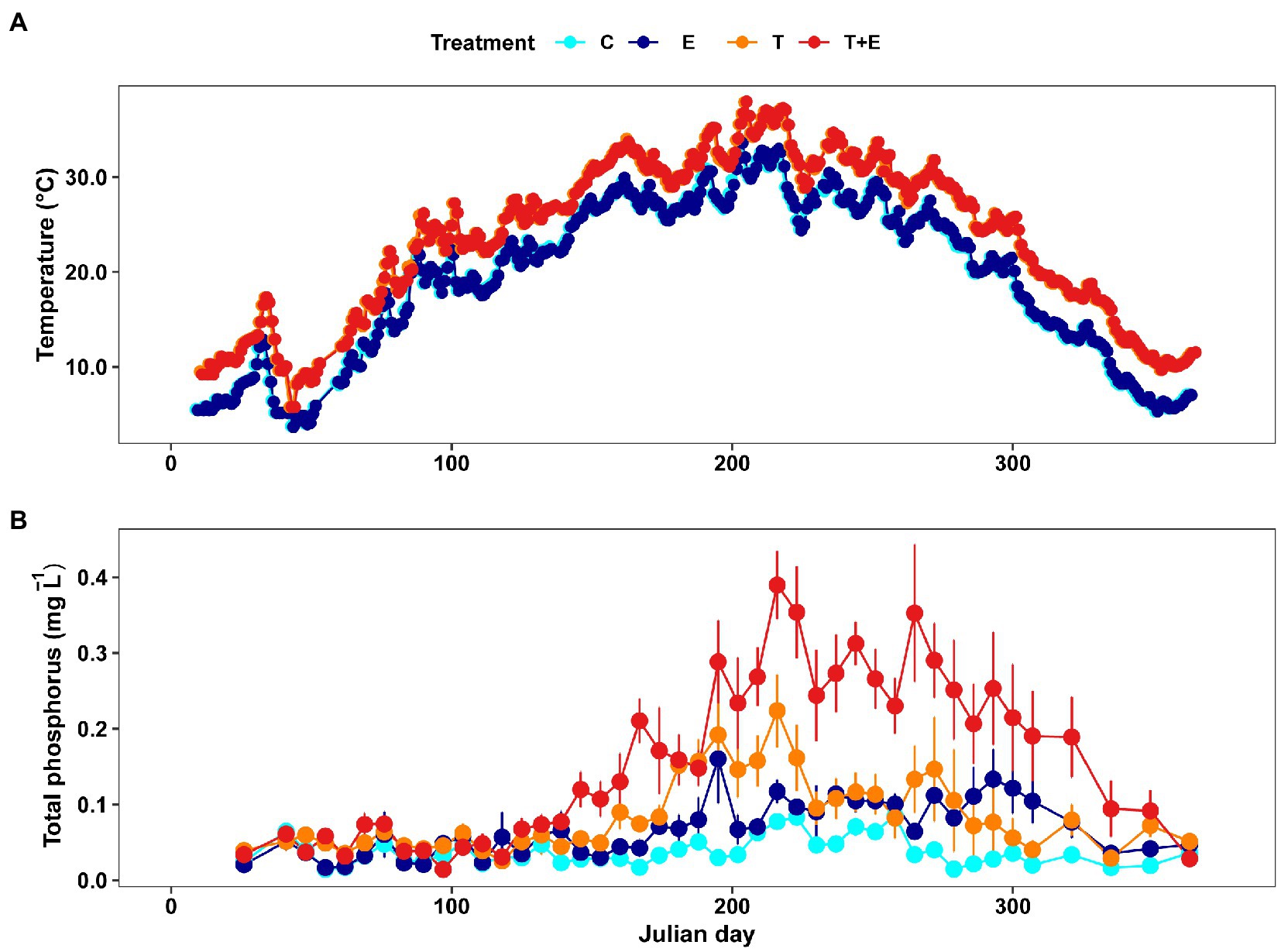
Figure 2. Mean daily water temperature (A) and TP (B) in the four treatments (C represents controls, E represents phosphorus addition, T represents warming, T + E represents warming and phosphorus addition; n = 6 per treatment) during the whole experimental period.
All treatments registered significantly higher TP concentrations than the controls (GLMMs: Z = 7.296, p < 0.001 for warming; Z = 4.682, p < 0.001 for phosphorus addition; Z = 4.792, p < 0.001 for the interaction between the two factors; Figure 2B). The middle stages of the experiment showed a significantly higher TP concentration in the T + E group than in the C, T, and E groups (Tukey, p < 0.001; Figure 2B). The TP concentration in the T group was higher than in the E group (Tukey, p = 0.045). Group E also had a higher TP concentration than the C group (Tukey, p < 0.001).
Gastropod community dynamic variation
Five gastropod species appeared during the experimental period. B. aeruginosa, R. swinhoei and A. longicornis were present in all groups, P. striatulus was only present in the phosphorus addition groups (E and T + E), and G. convexiusculus was only present in the E and T groups. For gastropod biomass, B. aeruginosa was the dominant gastropod species in all groups (Figure 3). For gastropod abundance, B. aeruginosa was also the dominant gastropod species in the C, E, and T groups. The combined effects of warming and phosphorus addition induced composition shifts in gastropod community. B. aeruginosa and R. swinhoei were the abundant gastropod species in the T + E group (Figure 3).
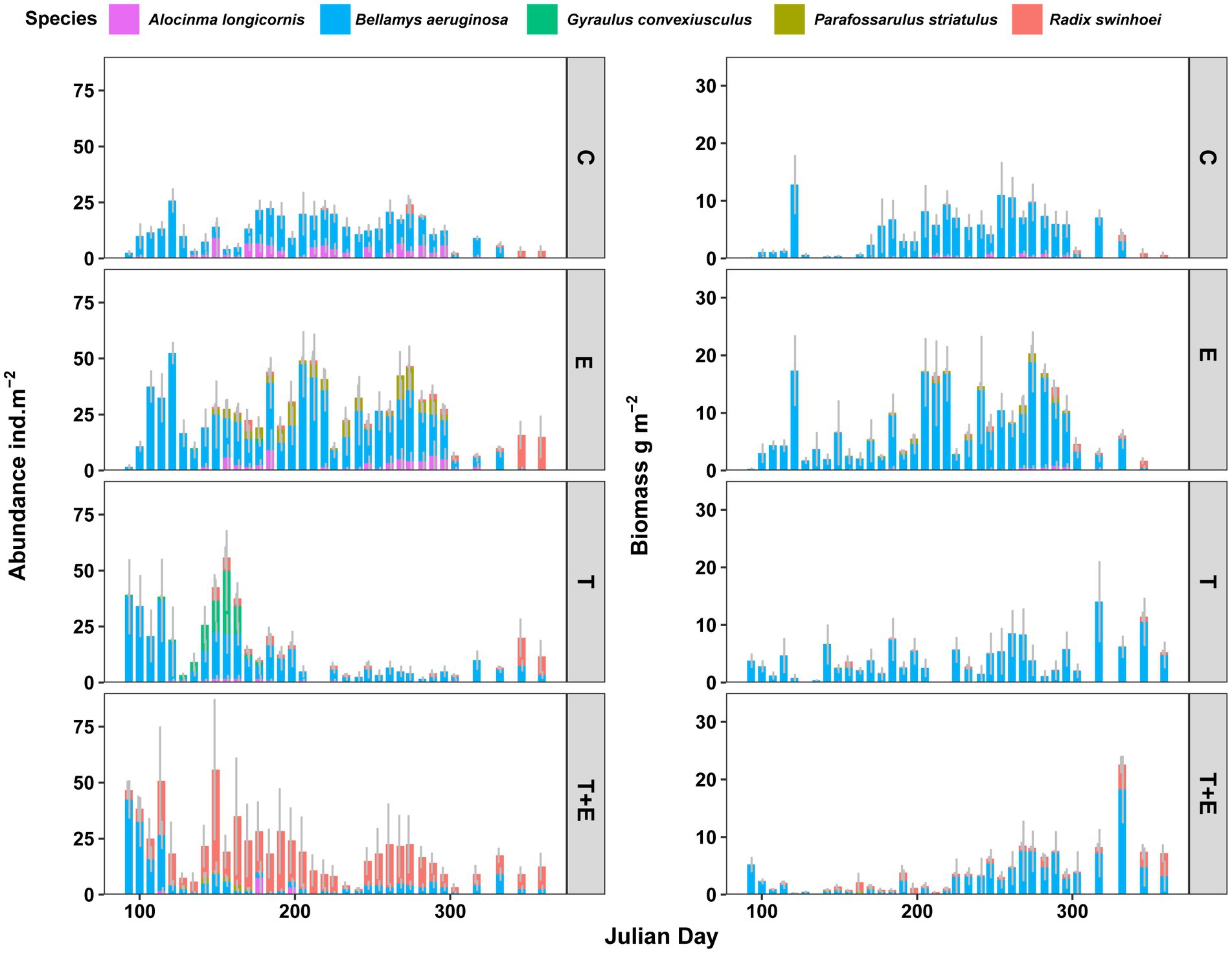
Figure 3. Time series of gastropods’ abundance (left) and biomass (right) for different groups (mean ± se; n = 6). C represents controls, E represents phosphorus addition, T represents warming, and T + E represents warming and phosphorus addition.
Experimental treatments changed gastropod community dynamics, and the total abundance and biomass variation over time in the E, T, and T + E groups were different compared with those in the C (Figure 3). Warming or phosphorus enrichment alone produced positive effects on the total abundance of gastropods, but the interaction between these two factors caused negative effects (Figure 4; Table 1). For B. aeruginosa abundance, remarkable effects were caused by phosphorus addition alone. The interaction between warming and phosphorus addition, and as well as warming alone, did not produce substantial effects on the abundance of B. aeruginosa (Table 1).
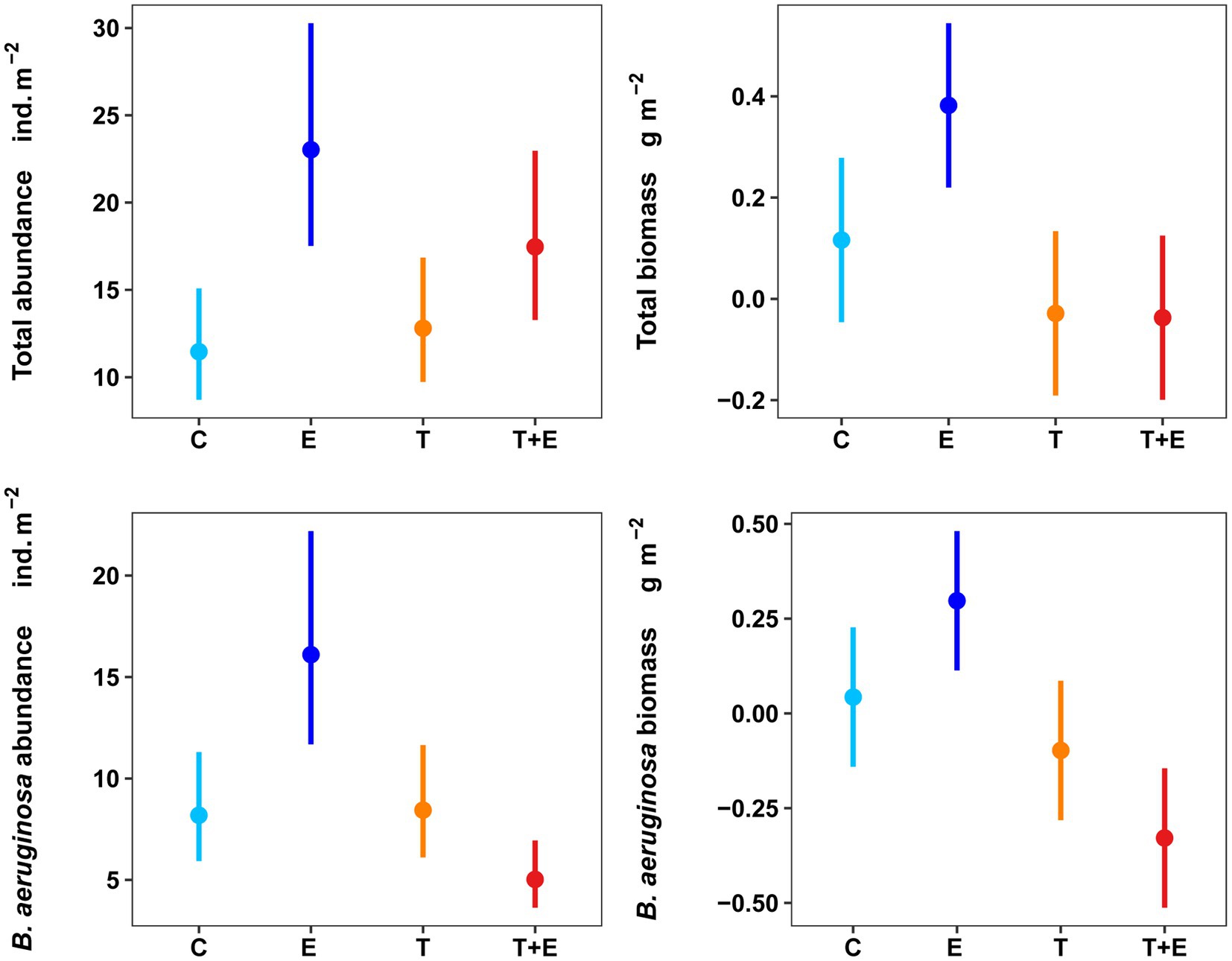
Figure 4. Effects of warming and phosphorus enrichment on aquatic gastropods’ total abundance (A), total biomass (B), and B. aeruginosa’s abundance (C), and biomass (D). Data represent predicted (fitted with the “ggpredict” function) values to warming, phosphorus addition, and their interaction. Biomass data were transformed by log (x + 0.1) to a Gaussian distribution. C represents controls, E represents phosphorus addition, T represents warming, and T + E represents warming and phosphorus addition.
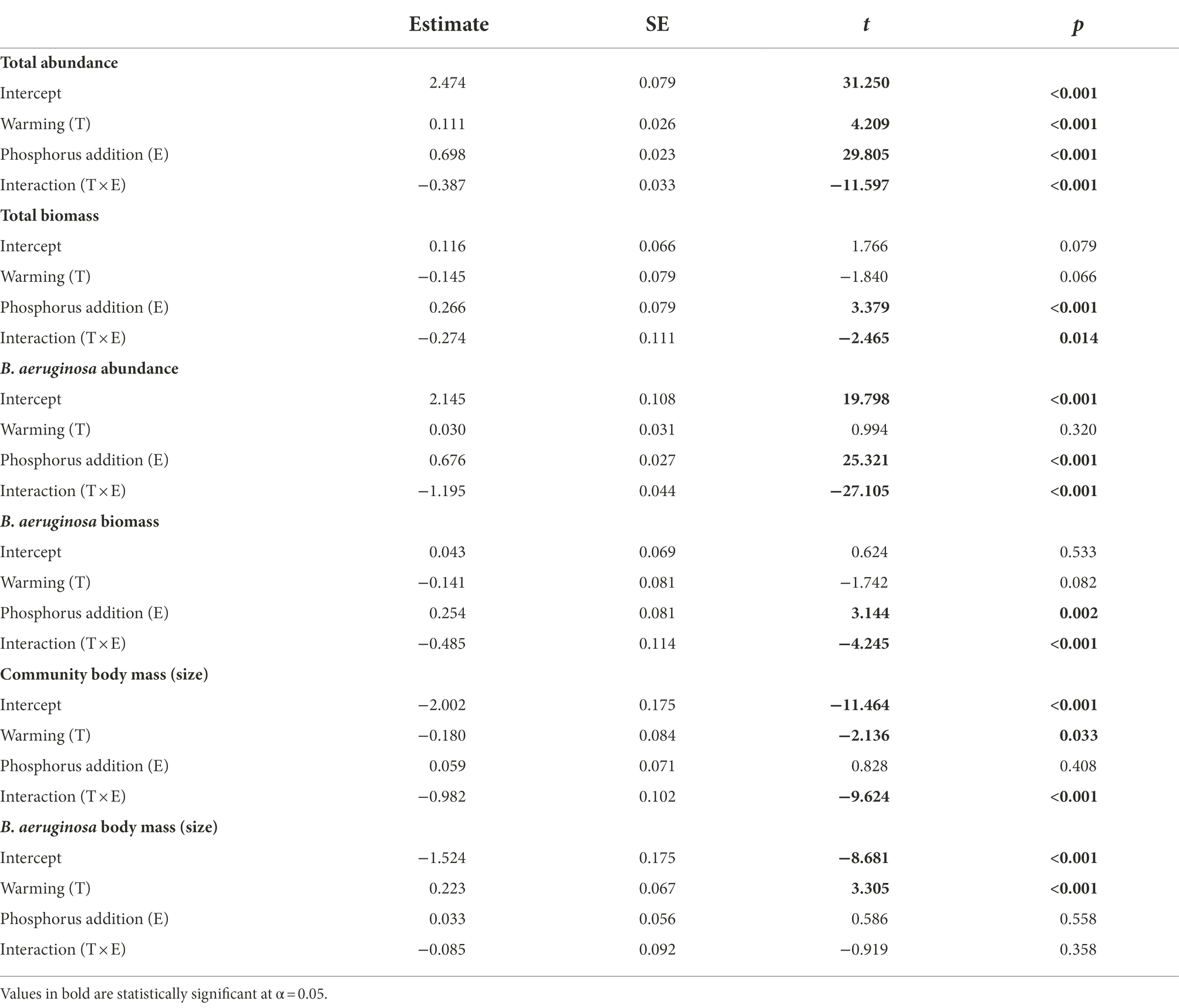
Table 1. Results of the generalized mixed models (GLMMs) testing the effects of warming and phosphorus addition on gastropods’ abundance, biomass, and body size. Bold numbers indicate p < 0.05.
Phosphorus addition alone had a remarkably positive effect on the total biomass of gastropods, but the interaction between warming and phosphorus addition produced adverse effects. Warming caused a marginal effect (p = 0.066) on total biomass (Figure 4; Table 1). For B. aeruginosa biomass, similar effects were caused by phosphorus addition alone and the interaction between warming and phosphorus addition, whereas warming also caused a marginal effect (p = 0.082) on B. aeruginosa biomass (Figure 4; Table 1).
Body mass
The gastropod size structure changed with treatments (Figure 5). Warming alone caused a negative effect on the body mass of the gastropod community (Figure 5A; Table 1). The T group had smaller gastropods in the early experimental stage compared with the ambient temperature groups (Figure 5A). Phosphorus addition did not cause a remarkable effect on the body mass of the gastropod community. The interaction between warming and phosphorus addition also negatively affected the gastropod community’s body mass (Figure 5A; Table 1). The trend of the gastropod community’s body mass across time in the T + E group was very different compared with those in the other groups (Figure 5A).

Figure 5. Time series of aquatic gastropods’ body mass (log-transformed; A) and time series of B. aeruginosa’s body mass (log-transformed; B) for different treatments. Smoothed curves were fitted with loess smoothing (span = 0.8). C represents controls, E represents phosphorus addition, T represents warming, and T + E represents warming and phosphorus addition.
For the dominant species, B. aeruginosa, warming alone considerably increased its body mass. Phosphorus addition or its interaction with warming did not produce any substantial effect (Figure 5B; Table 1).
Body stoichiometry of Bellamya aeruginosa
Warming alone, phosphorus alone, and the interaction between these two factors increased the nitrogen and phosphorus contents of B. aeruginosa (Figures 6A,B; Table 2). Warming alone caused a remarkable decrease in N: P ratio in the warming groups (Figure 6C; Table 2).
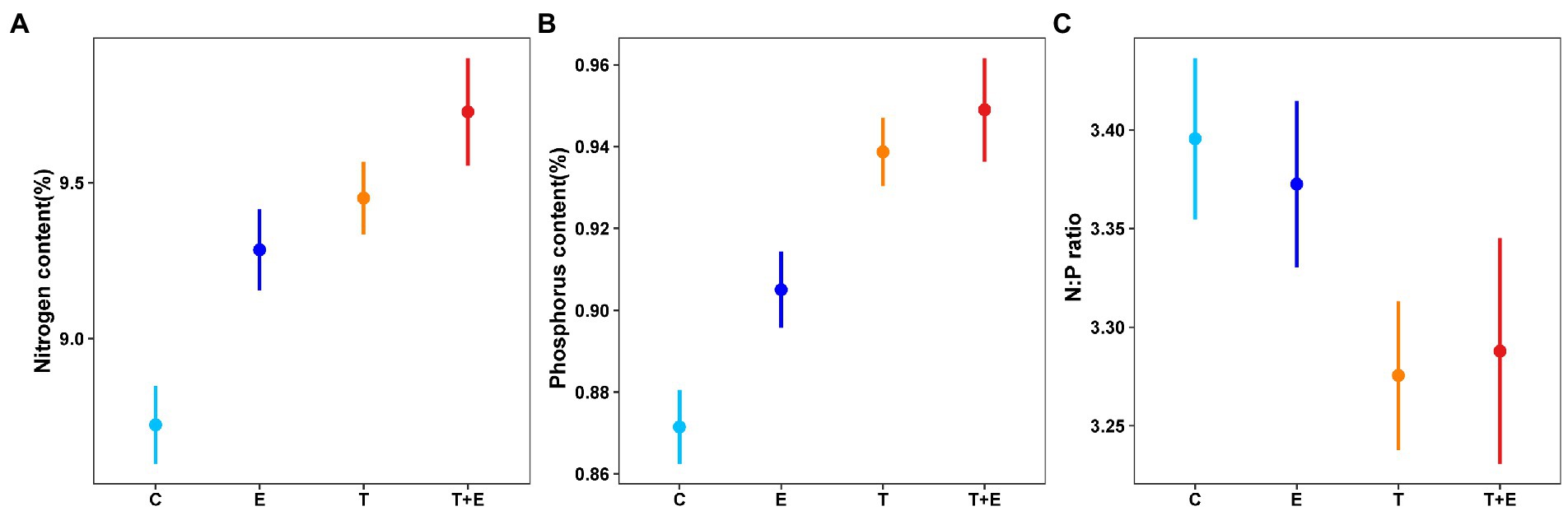
Figure 6. Effects of treatments on nitrogen (A) and phosphorus contents (B), and N: P ratio (C) of B. aeruginosa. Data represent predicted (fitted with the “ggpredict” function) values for the response of stoichiometry of B. aeruginosa to warming, phosphorus addition, and their interaction. The phosphorus content and the N: P ratio was transformed by square roots to increase normality. C represents controls, E represents phosphorus addition, T represents warming, and T + E represents warming and phosphorus addition.
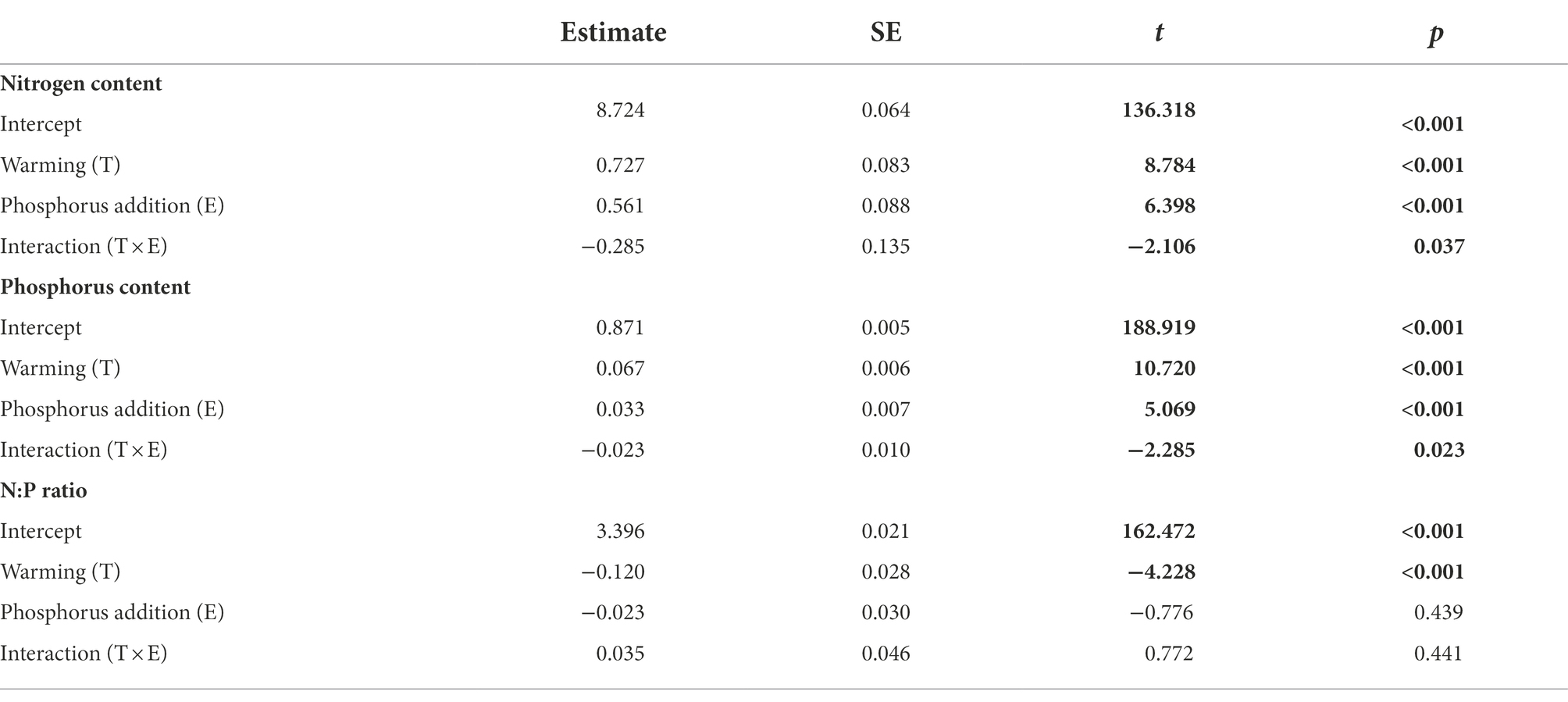
Table 2. Results of the generalized mixed models (GLMMs) testing the effects of warming and phosphorus addition on the contents of nitrogen and phosphorus, and N: P ratio of B. aeruginosa. Bold numbers indicate p < 0.05
Discussion
Climate warming and eutrophication are two major coexisting global stressors in freshwater ecosystems and often have combined effects, inducing more severe and complex consequences that are difficult and costly to manage and remediate (Moss et al., 2011). We know very little about the responses of aquatic gastropods to these two stressors, even though this compartment is an important component of the macroinvertebrate community. The aquatic gastropod community dynamics remarkably varied over time due to the effects of warming and phosphorus enrichment treatments. Warming increased the total abundance of gastropods possibly because more species with smaller size (e.g., G. convexiusculus and R. swinhoei) appeared in warming treatments, rather than being dominated by phenology changes. Phosphorus enrichment increased abundance and biomass via increased resource availability. The negative effects of the interaction between these two factors may be related to the dominant species shift. Changes in the gastropod community’s dynamics may interact with other aquatic biotic components (e.g., trophic mismatch) and cause more complex ecological consequences (Edwards and Richardson, 2004; Zhang et al., 2021).
As shown by our results, warming decreased the body size of the whole gastropod community, even in the phosphorus enrichment condition because of composition shifts. In agreement with results from other ectothermic aquatic organisms (e.g., bacteria, phytoplankton, zooplankton and fish), small species increased in communities due to warming (Hiddink and Hofstede, 2007; Daufresne et al., 2009; Winder et al., 2009). In the T group, the species with a small body size (G. convexiusculus) that appeared in the early stage of the experiment may be responsible for the decline in community size. G. convexiusculus gradually disappeared over time. In the T + E group, R. swinhoei maintained a certain amount of abundance throughout the experiment. The gastropods’ composition shift may have been due to species’ tolerance. Previous studies suggested that the peak abundance or biomass of B. aeruginosa appeared in warm and cool seasons in some subtropical shallow lakes (Yan et al., 2000; Gong et al., 2009). Therefore, B. aeruginosa may have a high thermal tolerance, and warming alone could not alter its dominance in the gastropod community in our study. R. swinhoei accounted for a large proportion of the abundance composition at the end of our experiment (winter) and may have strong adaptability to low temperatures. A previous study documented that Radis sp. has a wide distribution and can even survive in extreme boreal climates or lakes with ice cover periods of more than half a year (Taft et al., 2012). Phosphorus addition alone did not induce the alteration of dominant gastropod species, which might be related to B. aeruginosa having a certain tolerance to nutrition enrichment (Wang et al., 2011).
For the dominant gastropod species, the body size of B. aeruginosa was increased by warming possibly because of the resource conditions. Organisms’ metabolic rates are directly affected by temperature (Gillooly et al., 2001). Increasing metabolic demand associated with climate warming will be limited and therefore reduce the body size of ectotherms if the nutrient supply level remains unchanged (Sheridan and Bickford, 2011). Natural warming experiments have provided evidence that larger consumers at higher trophic levels will be sustained if nutrient supply increases with temperature to offset the rising metabolic demand of primary producers (O’Gorman et al., 2017). In our study, the combined effects of warming and phosphorus addition remarkably increased the water TP content, and warming alone also had a positive effect on the water TP content. Sufficient resources are available for primary producers. Warming and phosphorus enrichment increased the abundance of planktonic algae and macrophytes (Li et al., 2018; Yu et al., 2018; Supplementary Figure S1), accelerated litter decomposition by warming (Pan et al., 2021) or nutrient enrichment (Ferreira et al., 2015), and might provide more foods to consumers (e.g., gastropod) through detritivorous and herbivorous food web pathways (Ardon et al., 2021).
Our results showed that B. aeruginosa’s nitrogen and phosphorus contents increased under warmer conditions. Research about zooplankton (Calanoid copepods) has reported an inverse relationship between body size and element content (e.g., body phosphorus content has a positive relationship with growth rate but a negative relationship with body size) (Carrillo et al., 2001). A low N: P ratio may be associated with a faster development rate (Beck et al., 2021), inducing a higher body size (Walters and Hassall, 2006). Hence, more phosphorus and nitrogen would be needed to sustain a higher rate of protein synthesis if the phosphorus and nitrogen utilisation efficiency improved by warming cannot meet the growth and development requirements of organisms.
The interaction between warming and phosphorus enrichment will significantly influence the aquatic gastropod community and freshwater ecosystems. For instance, B. aeruginosa feeds on organic detritus and algae (Liu et al., 1993) and rarely grazes macrophytes (Li et al., 2019), whereas R. swinhoei feeds extensively on submerged macrophytes when it has a high density (Li et al., 2009) apart from detritus and algae. Dietary differences may change primary producers’ structure and function through top-down effects. The direct grazing effects of herbivorous gastropods might interact with eutrophication and increase the chance of macrophyte collapse (Liu et al., 2021). In addition, B. aeruginosa has a larger maximum size than R. swinhoei and a higher shell toughness than Radix sp. (Zhu et al., 2013), and shell toughness is closely related to anti-predation (Avery and Etter, 2006). The differences in prey composition and anti-predation ability may influence secondary or even higher-level predators’ behaviors and some functional traits through bottom-up effects (Navarrete and Manzur, 2008). Hence, the cascade effect of aquatic gastropod community changes is important for future studies to consider, especially because freshwater biodiversity is facing an unprecedented decline (Rosset and Oertli, 2011; Reid et al., 2018).
Conclusion
In conclusion, our results suggest that climate warming and phosphorus enrichment could alter aquatic gastropods’ community dynamics and size structure. These changes could be further investigated in a future study by considering the cascading effects amongst trophic levels. Warming and phosphorus enrichment caused different effects on body size at the dominant species’ population and the whole gastropod community levels. The body size of B. aeruginosa was increased by warming and was associated with stoichiometric traits, whereas the whole gastropod community’s body size decreased because of the composition shift. Hence, we predict that a smaller aquatic gastropod community body size will appear in aquatic ecosystems with warming and phosphorus enrichment, which shifting the dominant gastropod species and altering their abundance and biomass. Species shifts at the gastropod community level would cause more complex and unpredicted consequences in freshwater ecosystem structure and function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JX, MZ, and XS designed the study. MZ and XS conducted field and laboratory measurements. TW, PZ, HZ, HW, MZ, and JX analyzed the data and wrote the manuscript. TW, PZ, MZ, and JX revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Basic and Applied Basic Research Foundation of Guangdong Province, China (No. 2019B1515120065), the National Key R&D Program of China (Grant No. 2018YFD0900904), and the Water Pollution Control and Management Project of China (Grant No. 2018ZX07208005). JX acknowledges the support received from the International Cooperation Project of the Chinese Academy of Sciences (Grant No. 152342KYSB20190025) and the National Natural Science Foundations of China (Grant No. 31872687).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.979378/full#supplementary-material
References
Ardon, M., Zeglin, L. H., Utz, R. M., Cooper, S. D., Dodds, W. K., Bixby, R. J., et al. (2021). Experimental nitrogen and phosphorus enrichment stimulates multiple trophic levels of algal and detrital-based food webs: a global meta-analysis from streams and rivers. Biol. Rev. 96, 692–715. doi: 10.1111/brv.12673
Atkinson, D. (1994). Temperature and organism size: a biological law for ectotherms? Adv. Ecol. Res. 25, 1–58. doi: 10.1016/S0065-2504(08)60212-3
Avery, R., and Etter, R. J. (2006). Microstructural differences in the reinforcement of a gastropod shell against predation. Mar. Ecol. Prog. Ser. 323, 159–170. doi: 10.3354/meps323159
Bates, D., Machler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme 4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beck, M., Mondy, C. P., Danger, M., Billoir, E., and Usseglio-Polatera, P. (2021). Extending the growth rate hypothesis to species development: can stoichiometric traits help to explain the composition of macroinvertebrate communities? Oikos 130, 879–892. doi: 10.1111/oik.08090
Brönmark, C., and Weisner, S. E. B. (1996). Decoupling of cascading trophic interactions in a freshwater, benthic food chain. Oecologia 108, 534–541. doi: 10.1007/BF00333731
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. doi: 10.1890/03-9000
Carpenter, S. R. (2008). Phosphorus control is critical to mitigating eutrophication. Proc. Natl. Acad. Sci. U. S. A. 105, 11039–11040. doi: 10.1073/pnas.0806112105
Carrillo, P., Villar-argaiz, M., and Medina-sánchez, J. M. (2001). Relationship between N: P ratio and growth rate during the life cycle of Calanoid copepods: an in situ measurement. J. Plankton Res. 23, 537–547. doi: 10.1093/plankt/23.5.537
Correll, D. L. (1998). The role of phosphorus in the eutrophication of receiving waters: a review. J. Environ. Qual. 27, 261–266. doi: 10.2134/jeq1998.00472425002700020004x
Covich, A. P., Palmer, M. A., and Crowl, T. A. (1999). The role of benthic invertebrate species in freshwater eosystems: zoobenthic species influence energy flows and nutrient cycling. Bioscience 49, 119–127. doi: 10.2307/1313537
Cross, W. F., Benstead, J. P., Frost, P. C., and Thomas, S. A. (2005). Ecological stoichiometry in freshwater benthic systems: recent progress and perspectives. Freshw. Biol. 50, 1895–1912. doi: 10.1111/j.1365-2427.2005.01458.x
Daufresne, M., Lengfellner, K., and Sommer, U. (2009). Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. U. S. A. 106, 12788–12793. doi: 10.1073/pnas.0902080106
Dong, W., Min, S., Tang, L., Zeng, Q., Yang, J., and Yao, M. (2016). Research of eutrophication of Honghu Lake and Liangzi Lake. Environ. Protect. Sci. 42, 66–70. (In Chinese with English abstract)
Dossena, M., Yvon-Durocher, G., Grey, J., Montoya, J. M., Perkins, D. M., et al. (2012). Warming alters community size structure and ecosystem functioning. Proc. R. Soc. B Biol. Sci. 279, 3011–3019. doi: 10.1098/rspb.2012.0394
Edwards, M., and Richardson, A. J. (2004). Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884. doi: 10.1038/nature02808
Elser, J. J., O’Brien, W. J., Dobberfuhl, D. R., and Dowling, T. E. (2000). The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J. Evol. Biol. 13, 845–853. doi: 10.1046/j.1420-9101.2000.00215.x
Ferreira, V., Castagneyrol, B., Koricheva, J., Gulis, V., Chauvet, E., and Graca, M. A. (2015). A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol. Rev. Camb. Philos. Soc. 90, 669–688. doi: 10.1111/brv.12125
Frost, P. C., Cross, W. F., and Benstead, J. P. (2005). Ecological stoichiometry in freshwater benthic ecosystems: an introduction. Freshw. Biol. 50, 1781–1785. doi: 10.1111/j.1365-2427.2005.01457.x
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M., and Charnov, E. L. (2001). Effects of size and temperature on metabolic rate. Science 293, 2248–2251. doi: 10.1126/science.1061967
Gong, Z., Li, Y., and Xie, P. (2009). Population dynamics and production of Bellamya aeruginosa (reeve)(Mollusca: Viviparidae) in Lake Dong Hu, Wuhan. J. Lake Sci. 21, 401–407.
Grasshoff, K., Kremling, K., and Ehrhardt, M.. (1983). Methods of seawater analysis. 2nd edn. Weinheim, Germany: Verlag Chemie.
Guo, Y., Zhang, P., Chen, J., and Xu, J. (2022). Freshwater snail and shrimp differentially affect water turbidity and benthic primary producers. Water Biol. Secur. 1:100004. doi: 10.1016/j.watbs.2021.100004
Hiddink, J. G., and Hofstede, R. T. (2007). Climate induced increases in species richness of marine fishes. Glob. Chang. Biol. 14, 453–460. doi: 10.1111/j.1365-2486.2007.01518.x
IPCC (2013). “Climate change (2013): the physical science basis” in Working Group I Contribution of to the Fifth Assessment. Report of the Intergovernmental Panel on Climate Change. eds. T. F. Stocker, D. H. Qin, G.-K. Plattner, T. MMB, S. K. Allen, and J. Boschung, et al. (Cambridge, UK, New York, NY, USA: Cambridge University Press)
Jin, X. C., and Tu, Q. Y.. (1990). Investigation specifications for lake eutrophication, 2nd ed. China Environmental Science Press, Beijing, China.
Kozak, M., and Piepho, H.-P. (2017). What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J. Agron. Crop Sci. 204, 86–98. doi: 10.1111/jac.12220
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Li, W., Li, Y. J., Nie, W. H., Gao, G. Q., Fan, H. B., Zhong, J. Y., et al. (2019). Bellamya aeruginosa (reeve) promote the growth of Elodea nuttallii (planch.) H. St John in high nutrient environment. J. Freshw. Ecol. 34, 81–89. doi: 10.1080/02705060.2018.1549597
Li, K. Y., Liu, Z. W., Hu, Y. H., and Yang, H. W. (2009). Snail herbivory on submerged macrophytes and nutrient release: implications for macrophyte management. Ecol. Eng. 35, 1664–1667. doi: 10.1016/j.ecoleng.2008.05.009
Li, C., Wang, T., Zhang, M., and Xu, J. (2018). Maternal environment effect of warming and eutrophication on the emergence of curled pondweed, Potamogeton crispus L. Water 10:1285. doi: 10.3390/w10091285
Liess, A., and Hillebrand, H. (2005). Stoichiometric variation in C: N, C: P, and N: P ratios of littoral benthic invertebrates. J. N. Am. Benthol. Soc. 24, 256–269. doi: 10.1899/04-015.1
Liu, Y., He, L., Hilt, S., Wang, R., Zhang, H., and Ge, G. (2021). Shallow lakes at risk: nutrient enrichment enhances top-down control of macrophytes by invasive herbivorous snails. Freshw. Biol. 66, 436–446. doi: 10.1111/fwb.13649
Liu, Y. Y., Zhang, W. Z., and Wang, Y. X.. (1993). Medical malacology. China Ocean Press, Beijing, China.
Lüdecke, D. (2018). Ggeffects: tidy data frames of marginal effects from regression models. J. Open Source Softw. 3:772. doi: 10.21105/joss.00772
Main, T. M., Dobberfuhl, D. R., and Elser, J. J. (1997). N: P stoichiometry and ontogeny of crustacean zooplankton: a test of the growth rate hypothesis. Limnol. Oceanogr. 42, 1474–1478. doi: 10.4319/lo.1997.42.6.1474
McCormick, P. V., O’Dell, M. B., Shuford, R. B. E., Backus, J. G., and Kennedy, W. C. (2001). Periphyton responses to experimental phosphorus enrichment in a subtropical wetland. Aquat. Bot. 71, 119–139. doi: 10.1016/S0304-3770(01)00175-9
Moss, B., Kosten, S., Meerhoff, M., Battarbee, R. W., Jeppesen, E., Mazzeo, N., et al. (2011). Allied attack: climate change and eutrophication. Inland Wat. 1, 101–105. doi: 10.5268/IW-1.2.359
Navarrete, S. A., and Manzur, T. (2008). Individual- and population-level responses of a keystone predator to geographic variation in prey. Ecology 89, 2005–2018. doi: 10.1890/07-1231.1
Nelson, D., Benstead, J. P., Huryn, A. D., Cross, W. F., Hood, J. M., Johnson, P. W., et al. (2017). Experimental whole-stream warming alters community size structure. Glob. Chang. Biol. 23, 2618–2628. doi: 10.1111/gcb.13574
O’Gorman, E. J., Zhao, L., Pichler, D. E., Adams, G., Friberg, N., Rall, B. C., et al. (2017). Unexpected changes in community size structure in a natural warming experiment. Nat. Clim. Chang. 7, 659–663. doi: 10.1038/nclimate3368
Ohlberger, J. (2013). Climate warming and ectotherm body size - from individual physiology to community ecology. Funct. Ecol. 27, 991–1001. doi: 10.1111/1365-2435.12098
Pan, M., Wang, T., Hu, B., Shi, P., Xu, J., and Zhang, M. (2021). Mesocosm experiments reveal global warming accelerates macrophytes litter decomposition and alters decomposition-related bacteria community structure. Water 13:1940. doi: 10.3390/w13141940
Persson, J., Wojewodzic, M. W., Hessen, D. O., and Andersen, T. (2011). Increased risk of phosphorus limitation at higher temperatures for Daphnia magna. Oecologia 165, 123–129. doi: 10.1007/s00442-010-1756-4
Ranasinghe, R., Ruane, A. C., Vautard, R., Arnell, N., Coppola, E., Cruz, F. A., et al. (2021). Climate Change Information for Regional Impact and for Risk Assessment. Cambridge: Cambridge University Press.
Reed, W. L., and Janzen, F. J. (1999). Natural selection by avian predators on size and colour of a freshwater snail (Pomacea flagellata). Biol. J. Linn. Soc. 67, 331–342. doi: 10.1006/bijl.1998.0305
Reid, A. J., Carlson, A. K., Creed, I. F., Eliason, E. J., Gell, P. A., Johnson, P. T. J., et al. (2018). Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94, 849–873. doi: 10.1111/brv.12480
Rosset, V., and Oertli, B. (2011). Freshwater biodiversity under climate warming pressure: identifying the winners and losers in temperate standing waterbodies. Biol. Conserv. 144, 2311–2319. doi: 10.1016/j.biocon.2011.06.009
R Development Core Team (2014). R: A language and environment for statistical computing. in Version 3.1.2. R Foundation for Statistical Computing. Available at. https://www.R-project.org
Sheridan, J. A., and Bickford, D. (2011). Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 1, 401–406. doi: 10.1038/nclimate1259
Sievers, A., Beringer, M., Rodnina, M. V., and Wolfenden, R. (2004). The ribosome as an entropy trap. Proc. Natl. Acad. Sci. U. S. A. 101, 7897–7901. doi: 10.1073/pnas.0402488101
Smith, V. H., and Schindler, D. W. (2009). Eutrophication science: where do we go from here? Trends Ecol. Evol. 24, 201–207. doi: 10.1016/j.tree.2008.11.009
Sterner, R. W., and Elser, J. J.. (2002). Ecological stoichiometry: The biology of elements from molecules to the biosphere. Princeton University Press, Princeton.
Taft, L., Wiechert, U., Riedel, F., Weynell, M., and Zhang, H. C. (2012). Sub-seasonal oxygen and carbon isotope variations in shells of modern radix sp (Gastropoda) from the Tibetan plateau: potential of a new archive for palaeoclimatic studies. Quat. Sci. Rev. 34, 44–56. doi: 10.1016/j.quascirev.2011.12.006
Toseland, A., Daines, S. J., Clark, J. R., Kirkham, A., Strauss, J., Uhlig, C., et al. (2013). The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 3, 979–984. doi: 10.1038/NCLIMATE1989
Villeneuve, A. R., Komoroske, L. M., and Cheng, B. S. (2021). Environment and phenology shape local adaptation in thermal performance. Proc. R. Soc. B Biol. Sci. 288:20210741. doi: 10.1098/rspb.2021.0741
Walters, R. J., and Hassall, M. (2006). The temperature-size rule in ectotherms: may a general explanation exist after all? Am. Nat. 167, 510–523. doi: 10.1086/501029
Wang, Y., Han, S., Song, W., Zhou, Q., and Huang, J. (2011). The purification of eutrophic water by floatingbed of plants and Bellamya aeruginosa (reeve). Jiangsu J. Agric. Sci. 27, 295–300. (In Chinese with English abstract)
Wang, H. J., and Wang, H. Z. (2009). Mitigation of lake eutrophication: loosen nitrogen control and focus on phosphorus abatement. Prog. Nat. Sci. 19, 1445–1451. doi: 10.1016/j.pnsc.2009.03.009
Wang, T., Xu, J., Molinos, J. G., Li, C., Hu, B., Pan, M., et al. (2020). A dynamic temperature difference control recording system in shallow lake mesocosm. Methods 7:100930. doi: 10.1016/j.mex.2020.100930
Winder, M., Reuter, J. E., and Schladow, S. G. (2009). Lake warming favours small-sized planktonic diatom species. Proc. R. Soc. Biol. Sci. 276, 427–435. doi: 10.1098/rspb.2008.1200
Woods, H. A., Makino, W., Cotner, J. B., Hobbie, S. E., Harrison, J. F., Acharya, K., et al. (2003). Temperature and the chemical composition of poikilothermic organisms. Funct. Ecol. 17, 237–245. doi: 10.1046/j.1365-2435.2003.00724.x
Woodward, G., Ebenman, B., Emmerson, M., Montoya, J. M., Olesen, J. M., Valido, A., et al. (2005). Body size in ecological networks. Trends Ecol. Evol. 20, 402–409. doi: 10.1016/j.tree.2005.04.005
Yan, Y., Liang, Y., and Wang, H. (2000). Annual production of Bellamya aeruginosa in Houhu Lake, Wuhan. J. Lake Sci. 12, 68–72. (In Chinese with English abstract)
Yu, C., Li, C., Wang, T., Zhang, M., and Xu, J. (2018). Combined effects of experimental warming and eutrophication on phytoplankton dynamics and nitrogen uptake. Water 10:1057. doi: 10.3390/w10081057
Zhang, H., Zhang, P. Y., Wang, H., Molinos, J. G., Hansson, L. A., He, L., et al. (2021). Synergistic effects of warming and eutrophication alert zooplankton predator-prey interactions along the benthic-pelagic interface. Glob. Chang. Biol. 27, 5907–5919. doi: 10.1111/gcb.15838
Keywords: climate change, eutrophication, mesocosm, abundance, biomass, body mass, nitrogen, phosphorus
Citation: Wang T, Zhang P, Zhang H, Wang H, Su X, Zhang M and Xu J (2022) Warming and phosphorus enrichment alter the size structure and body stoichiometry of aquatic gastropods. Front. Ecol. Evol. 10:979378. doi: 10.3389/fevo.2022.979378
Edited by:
Christian Henri Nozais, Université du Québec à Rimouski, CanadaReviewed by:
Yu Dan, Wuhan University, ChinaYixin Zhang, Soochow University, China
Gergely Boros, Balaton Limnological Research Institute, Hungary
Copyright © 2022 Wang, Zhang, Zhang, Wang, Su, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peiyu Zhang, emhhbmdwZWl5dUBpaGIuYWMuY24=
 Tao Wang
Tao Wang Peiyu Zhang
Peiyu Zhang Huan Zhang
Huan Zhang Huan Wang
Huan Wang Xiyang Su3
Xiyang Su3 Min Zhang
Min Zhang Jun Xu
Jun Xu