- 1Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Nay Pyi Taw, Myanmar, Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Behavioral and Experimental Economics Research Center, Statistic and Mathematics College, Yunnan University of Finance and Economics, Kunming, China
- 4Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Mengla, China
To avoid risks, organisms must recognize threatening heterospecies from non-threatening ones via acoustic cues from a distance. With land-use change, humans have encroached considerably into natural areas. Therefore, it is beneficial to animals to use acoustic cues to discriminate between different levels of threats posed by humans. Our study aims at testing this discriminatory ability in Asian elephants (Elephas maximus), animals that have been for long history subjected to human interaction. We tested whether eighteen semi-captive elephants could discriminate between voices of their own mahouts (i.e., who take care of the elephants exclusively) and of other mahouts (unfamiliar individuals). The results showed that elephants responded successfully to the commands from their own mahouts, with an average response rate as high as 78.8%. The more years the mahouts had been as their caretakers, the more the elephant showed active responses toward the commands. Female elephants responded to the commands more frequently and faster than males. Also younger elephants responded more frequently and faster than older elephants. We argue that Asian elephants can discriminate between familiar and unfamiliar humans by acoustic cues alone. Proximity with humans may be a factor, as fundamental as domestication, for animals to develop heterospecies discriminatory ability.
Introduction
By interacting with the environment, organisms develop ways to avoid dangers and recognize predators or non-mutualistic species such as parasites (Deecke et al., 2002; Marzluff et al., 2010; Papworth et al., 2013). Accruing the ability to collect information from the ecosystem and other peers ultimately increases the species’ fitness (Tuttle and Ryan, 1981). Encoding individual vocal identity should be adaptive for species above all living in large and complex societies (Rhebergen et al., 2015). For instance, from a distance, herbivores can identify the scents and sounds from predators and flee them before they attack; or birds recognize and react to alarm calls from other prey species to avoid predators (Fallow and Magrath, 2010).
With the impinging development of our society, animals interact with humans more and more frequently. Different people may pose different levels of threats (or benefits) to inhabiting animals (Lee et al., 2011; Chen et al., 2016). This is also the case with animal husbandry, where domesticated animals interact frequently with handlers. Here domestication is defined as evolutionary process forced by human influence (Price, 1984). Domestication selection may have therefore allowed the formation of artificial forces selecting in favor of animals able to discriminate their owners from unfamiliar individuals (Topál et al., 2005). Consequently, differentiating threatening humans from beneficial ones could have become a requisite for both domesticated and wild animals to cope in the “Anthropocene” (Bond et al., 2020).
Many kinds of research on discrimination ability of animals on human individuals used domesticated animals as study subjects, such as the domestic cat (Felis catus; using acoustic cues; Saito and Shinozuka, 2013), the dog (Canis familiaris; using visual cues and acoustic cues; Adachi et al., 2007), the pig (Sus scrofa domesticus; using visual signals and olfactory signals; Koba and Tanida, 2001), the cattle (Bos taurus; using individual humans as cues; Taylor and Davis, 1998), and the lamb (Ovis aries; using individual humans as cues; Boivin et al., 1997). As a result, some argue that artificial selection occurring during domestication could be an evolutionary force influencing animals’ ability to discriminate among human individuals (Topál et al., 2005). However, other studies reveal that some wild animals show some ability to recognize humans on an individual level, and that this ability develops by merely interacting with human beings. For instance, the wild magpie (Pica pica) is aggressive only toward those specific humans who pose a threat to their nests (using human individuals as a cue; Lee et al., 2011). Mockingbirds (Mimus polyglottos; Levey et al., 2009) and American crows (Corvus brachyrhynchos; Marzluff et al., 2010) display similar discrimination abilities. These studies in domesticated and non-domesticated animals indicate that they can learn and apply their knowledge to discriminate against different human individuals while interacting with them. Instead of domestication, the mere proximity to humans could be enough to develop discrimination ability on human individuals for these animals.
This type of research customarily resorts to visual stimuli, such as the images of humans or their behaviors within a short distance (Levey et al., 2009; Marzluff et al., 2010; Papworth et al., 2013). To avoid dangers, some species could use more efficient ways such as acoustic cues (i.e., human voices) to assess threats at an extended range, such as from humans out of sight (Fallow and Magrath, 2010). However, only a few studies have explored if non-domesticated animals can recognize human individuals using these acoustic cues (carrion crow, Corvus corone; Wascher et al., 2012; cheetah, Acinonyx jubatus; Leroux et al., 2018). Comparative research on phylogenetically distant species can promote the further understanding of evolutionary drivers on the animals’ discrimination ability.
Elephants are social animals with advanced cognitive abilities to group living and group cooperation (self-recognition, Plotnik et al., 2006; empathy, Plotnik and de Waal, 2014; cooperation, Plotnik et al., 2011; Li et al., 2021). Furthermore, elephants excel at learning and at reproducing acoustic cues (Heffner and Heffner, 1982; Payne et al., 1986; McComb et al., 2003; Poole et al., 2005). For instance, it was reported that Asian elephants (Elephas maximus) can imitate human speech so accurately that local people cannot tell apart the animal imitation from human voices (Stoeger et al., 2012). The Asian elephant can use various vocalizations for communication between social groups and within the same group, among group members, including trumpets, chirps, roars, and rumbles of different types (Nair et al., 2009). And when perceiving disturbance, Asian elephants can modulate their vocalizations to alert conspecifics (Sharma et al., 2020). Humans have a long history of taming Asian elephants for logging and the carrying of goods in some countries, such as Myanmar and Thailand (Sukumar, 2003). During these long-lasting interactions, it has been reported that the Asian elephant is able to understand some human behavioral cues (Ketchaisri et al., 2019).
Furthermore, with humans encroaching and fragmenting the natural habitats, and with land-use change, there is an increasing overlapped distribution and interaction between humans and elephants (White and Ward, 2011; Liu et al., 2017). Wild elephants near human residences often raid crops, destroy properties, and can sometimes cause human casualties (Chen et al., 2016). In this case, different residents may apply different methods to keep elephants from human settlements (Aziz et al., 2016; Wijayagunawardane et al., 2016). During human-elephant interactions, elephants may develop the ability to discriminate between threatening individuals to non-threatening individuals, and they have excellent memory capacity (Rensch, 1957; Hart et al., 2008). It is valuable to test if Asian elephants can discriminate against humans on an individual level.
Research using two Asian elephants as study subjects at a zoo revealed that elephants showed no significant behavioral difference after hearing acoustic playback of familiar and unfamiliar humans (Polla et al., 2018). However, Asian elephants mainly live in forest habitats, where acoustic signals could be critical for detecting danger. For instance, a previous study shows that Asian elephants can discriminate the tiger growl (threatening to elephants) from the leopard growl (not threatening to elephants; Thuppil and Coss, 2013). Therefore, we predict Asian elephants to be good at acoustic recognition, and the results of the previous study might be consequential to the small sample size and the housing conditions.
To explore if the Asian elephant can recognize familiar humans on an individual level by acoustic cues alone, we studied semi-captive Asian elephants (semi-captive here is defined as captive elephants that can range and forage freely in forest) in Myanmar. In the Indian subcontinent and Southeast Asia, mahouts (or “oozies”; i.e., whose job is to take care of elephant exclusively) used to work with these animals for a living. Today, mahouts continue to be solely in charge of the daily care of the elephants. In particular, we tested if these semi-captive animals could discriminate between their own mahouts and other mahouts (unfamiliar individuals), when there was only an acoustic stimulus given to them. As mentioned, the aim was to test their ability to discriminate between familiar and non-familiar mahouts. We predicted that Asian elephants could be able to recognize the voice from their own mahouts in an accurate manner.
Materials and methods
Study subjects
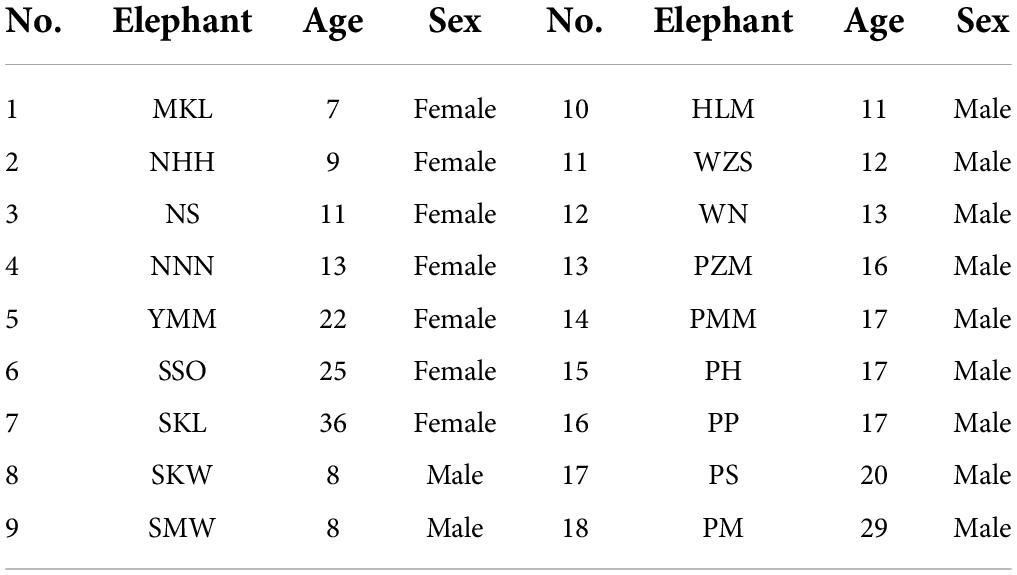
There were 18 semi-captive Asian elephants tested in our study, aged from 7 to 36 years old. Seven of them were females, and 11 were males (Table 1). They were from Myaing Hay Wun elephant camp in Taikkyi, Yangon, Myanmar (21°55′20″ N, 101°16′33″ E), owned by the Myanma Timber Enterprise (MTE). The elephants used to work in logging to carry the wood. Yet since the logging ban in Myanmar in 2016, these elephants no longer need to work in the forests. They were tamed at the age of 5 to follow commands from their assigned mahouts (i.e., each mahout belongs to one elephant exclusively). A mahout usually collects his own elephant in the morning for a health check and takes the elephant to bath, then releases it back to the forest, so that these elephants can range and forage freely in the surrounding forest. Mahouts sometimes feed their elephants with fruits and tamarinds. This routine guarantees that elephants interact with their matching mahouts (the mahout who is assigned to take care of the specific elephant) on a daily basis, making them familiar individuals. Elephants can see, smell, or hear other mahouts, but the interaction between is rare, making other mahouts unfamiliar (or less familiar) individuals. According to the experience from mahouts, elephants only follow commands from their own mahouts most of the time. In addition, care time from a mahout is not necessarily correlated with an elephant’s age, because an elephant could be assigned to another mahout, if the previous mahout becomes unavailable for this job.
Semi-captive elephants from Myaing Hay Wun elephant camp are free-ranging in the surrounding forest, with approximately 20 wild elephants co-occurring in this area. The semi-captive elephants interact with wild elephants from time to time. We have observed these interactions near the elephant camp. No aberrant stereotypical behaviors (i.e., repeatedly performing some behaviors with no clear function and less variant in form; Latham and Mason, 2008) were observed among them, which makes them ideal subjects for most behavioral studies. We selected the 18 elephants based on the following criteria: (1) mahout used “come” effectively (pronunciation in Burmese: 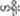 ) as a command to ask his elephant to come near him (here we used “come” command because the experiment set up required target elephant to “come” to the finish line, which was coded as “right response”), (2) those elephants with no history of hurting humans (for the safety of the experimenters and the mahouts), (3) those elephants who followed commands only from his/her own mahout (based on mahout and mahout leader’s experience, where mahout leader is the one who leads mahouts and teaches them about health care and management of elephants), (4) if female, elephants who were not pregnant or not fostering an infant, (5) elephants that were only familiar with one assigned mahout, and, (6) if male, elephants who were not in musth (i.e., a state when a male becomes very aggressive, attributed to increased testosterone levels; Duer et al., 2016).
) as a command to ask his elephant to come near him (here we used “come” command because the experiment set up required target elephant to “come” to the finish line, which was coded as “right response”), (2) those elephants with no history of hurting humans (for the safety of the experimenters and the mahouts), (3) those elephants who followed commands only from his/her own mahout (based on mahout and mahout leader’s experience, where mahout leader is the one who leads mahouts and teaches them about health care and management of elephants), (4) if female, elephants who were not pregnant or not fostering an infant, (5) elephants that were only familiar with one assigned mahout, and, (6) if male, elephants who were not in musth (i.e., a state when a male becomes very aggressive, attributed to increased testosterone levels; Duer et al., 2016).
Experiment set up
We first set up two fences 2 m high, where three mahouts, one mahout leader and two researchers could hide behind (Figure 1). In this way, the elephants were not able to visually identify their mahouts. Second, to avoid that the elephants could smell the mahouts, we randomly constituted six groups with three out of the total 18 elephants. When a group of three mahouts stayed together behind the fence, their scents were mixed, so elephants could not determine the source of commands by scents. With this setup, elephants were only able to recognize and follow the “come” command from a mahout by a mere vocal cue. In this experiment, we did not use playback recording due to equipment and electricity limitation in the remote elephant camp.
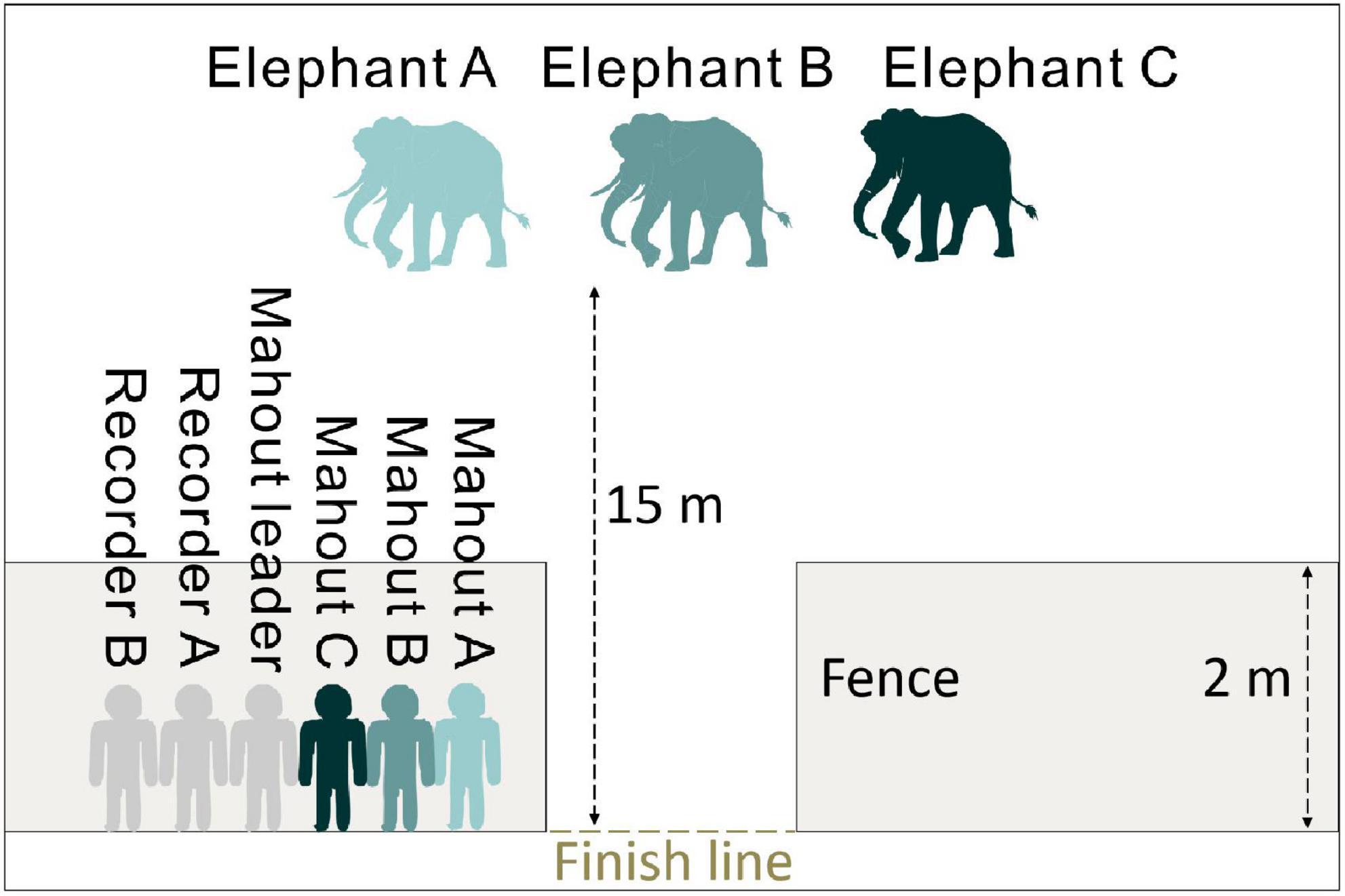
Figure 1. Experiment set up for voice recognition in Asian elephant. Same color represents the matching elephants and mahouts.
Experimental procedure
This experiment was routinely conducted in the early morning or late afternoon to avoid direct sunlight. First, a group of three elephants was introduced to the location 15 m distant from the fence (Figure 1). Then, the three matching mahouts would come behind the fence. Each mahout would give the same vocal command, “come,” one by one. The calling sequence was decided randomly by researcher A, hiding behind the fence, using a random number generator.1 Mahouts gave their calls consistently at around 50 dB, tested by Noise Detector application (version 2.3.102, China). Each session contains three trials. In the first trial, the first mahout would send out commands in succession. If the matching elephant followed the command and came to pass the finish line, the first mahout would lead the elephant to exit the field. If the matching elephant did not come within 2 min, the mahout would lead his elephant to leave the area to allow the experiment to proceed. During this process, if either one of the other two elephants in the group responded to the first mahout and came to the finish line, the other two mahouts would lead the animals back to the initial place, thereby ending the trial. At that moment, the mahouts would come back behind the fence, and the second trial started with a call given by the second mahout. We would repeat this procedure until three mahouts called once (three trials). When all the elephants of the first group left the field, the first session of the first group ended, and the second group of elephants would be introduced to the same place to have the same procedure repeated. Only one session was run for each group in a day. Each group was given 18–20 sessions of the test in total, conducted from October to November 2019. We set two video cameras (SONY HDR-PJ760) up to record the experiment from the viewpoints of the mahouts and the elephants. We coded an elephant coming to the finish line as an “active response,” and an elephant not coming to the finish line as “no response.” And in the “active response,” we coded responses to their own mahouts (matching mahouts) as “right response” and responses to other mahouts (non-matching mahouts) as “wrong response.” It was impossible to record data blind because our study involved focal animals.
To explore what factors may influence the ability of elephants to recognize the vocal signature of their own mahouts, we also recorded the age and sex of each elephant, if the elephant was wild-caught or captive-born (i.e., their mothers were captive or semi-captive elephants), and how long they had been together with their own mahouts (i.e., care time).
This study was approved by Myanma Timber Enterprise [protocol no. 4527/MTE/AA(K)18]. To our best knowledge, there is no animal ethics committee in Myanmar. This study was for this reason reviewed and approved by the Biomedical Ethics Expert Committee of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (protocol no. XTBG-2020-11).
Data analysis
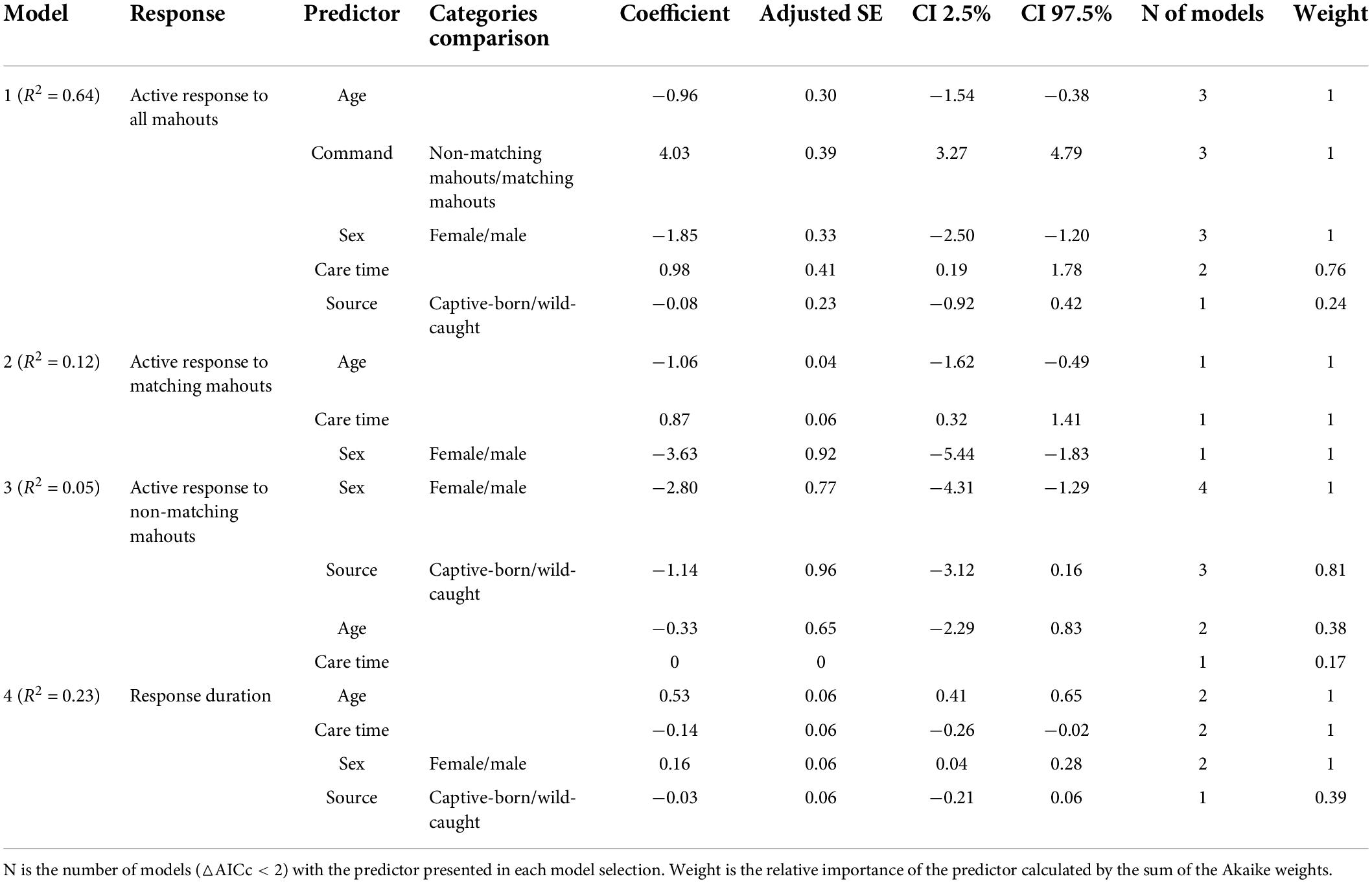
We first calculated each elephant’s successful response rate (i.e., the number of sessions in which an elephant responded only to the matching mahout divided by the number of total sessions). For the overall analysis, the commands from the mahouts were treated as independent repeats. We first fitted generalized linear models (GLM) with the binary reaction (i.e., 1 represented elephant successfully responding to the mahouts, 0 represented elephant not responding to the command from mahout) as a response variable. We set the voice source (commands from matching mahouts or non-matching mahouts) and individual traits (including the age of the elephants, the sex of the elephants, the care time of the mahouts, and whether the elephant was wild-caught or captive-born) as predictors to understand whether the elephants recognize the commands from the matching mahouts and which individual traits are cofactors to their discrimination ability. With this same approach, we analyzed the active response to the “command from matching mahout” and the “command not from matching mahout” separately, to understand: (a) which traits attributed to active responses when elephants were called by a matching mahout (i.e., right response) and (b) which traits attributed to the active responses of the elephants when elephants were called by non-matching mahout (i.e., wrong response). We also considered that different traits could affect an elephant’s response duration (actively responding to the matching mahout). Therefore, we fitted linear models (LMs) with the response duration as a continuous variable and various individual traits as predictors (including the age of elephants, the sex of elephants, the care time of the mahout, and whether the elephant was wild-caught or captive-born). Collinearity was not detected in any model, as all the variance inflation factors (VIF) were <5 (Akinwande et al., 2015; calculated by using the “vif” function with the R package “car”; Fox and Weisberg, 2019). Variance function-based R-squares were calculated for the GLMs by using the “rsq” function with the R package “rsq” (Zhang, 2018).
The model selection was conducted following an information-theoretic approach (Burnham and Anderson, 2002), using the R package “MuMIn” (Bartoń, 2019). For each analysis, we kept models with delta corrected Akaike Information Criteria (△AICc) <2 (e.g., Rayner et al., 2007) to calculate the standardized fully averaged coefficient at 95% confidence interval. The relative importance of predictors in each model was calculated by the sum of the Akaike weights (Burnham and Anderson, 2002). The predictor with coefficient furthest away from 0 is the most robust explanatory variable, where 95% confidence intervals determine the confidence in the direction of the variable’s effect (Grueber et al., 2011). All statistical analyses were computed in R 3.6.3 (R Core Team, 2020).
Results
The elephants’ age in this study ranged from 7 to 36 years (Table 1). Ten of them were captive-born, and eight of them were wild-caught. On average, the time our mahouts spent with the elephants ranged from 6 months to 16 years. Overall, the elephants responded more to the command from their own mahouts (familiar humans), regardless of whether they were captive-born or wild-caught (Figure 2; see parameters of Model 1 in Table 2). The female elephants responded more than the males to the commands, and the younger elephants responded more than the old elephants. Most importantly, we found that with more extended proximity (care time from the mahouts), the elephants showed more active responses (Figure 2; see parameters of Model 1 in Table 2).
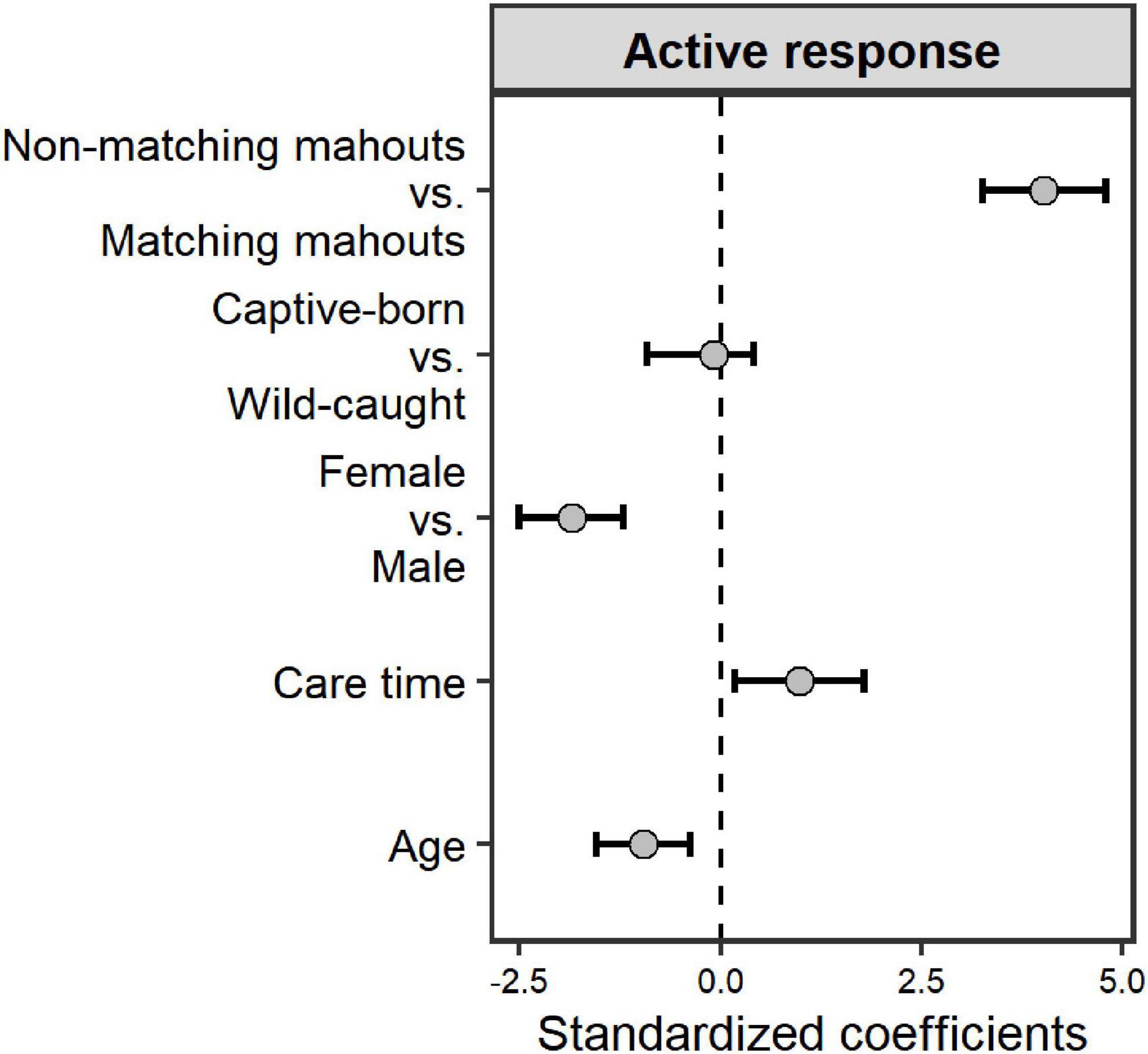
Figure 2. The impact of the predictors (voice source and elephant individual traits) on the active response of the elephants. Grey dots and black whiskers represent the standardized coefficient with 95% confidence interval.
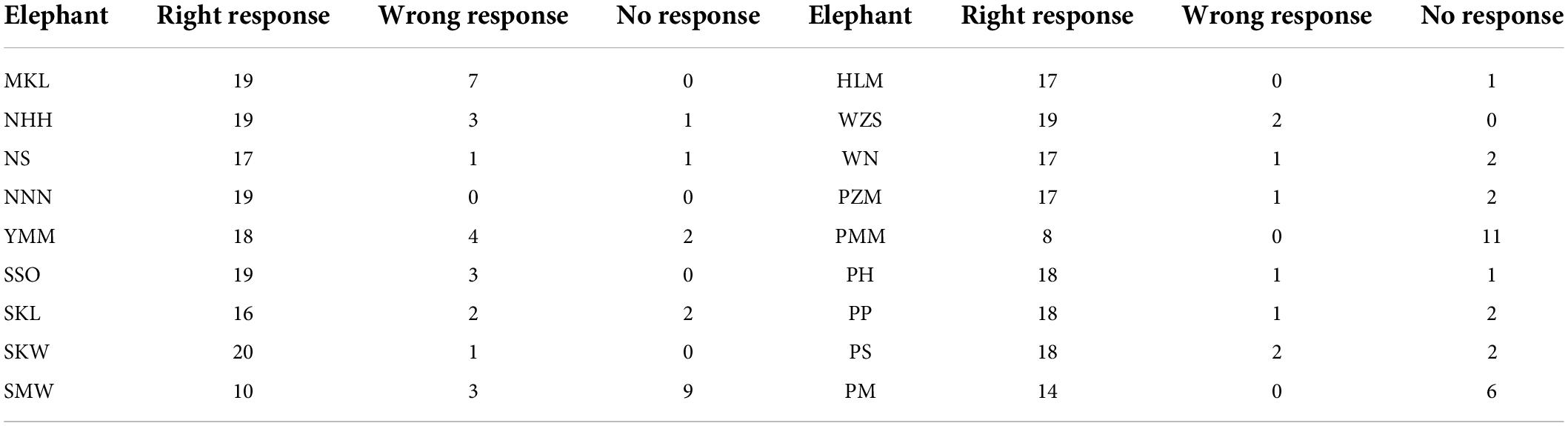
The average successful response (i.e., the elephant responded only to the matching mahout and did not respond to the commands from non-matching mahouts) rate was 78.8% (i.e., the total number of successful responses/numbers of total tests). Among all the 18 elephants, one elephant called NNN, a female elephant, responded 100% correctly to her own mahout and never responded to the commands from non-matching mahouts (see Table 3 for the performance of each elephant in the test). No matter whether the matching mahout or non-matching mahouts gave commands, the female elephants showed significantly more active responses than the male elephants (Figure 3; see parameters of Model 2 and Model 3 in Table 2).
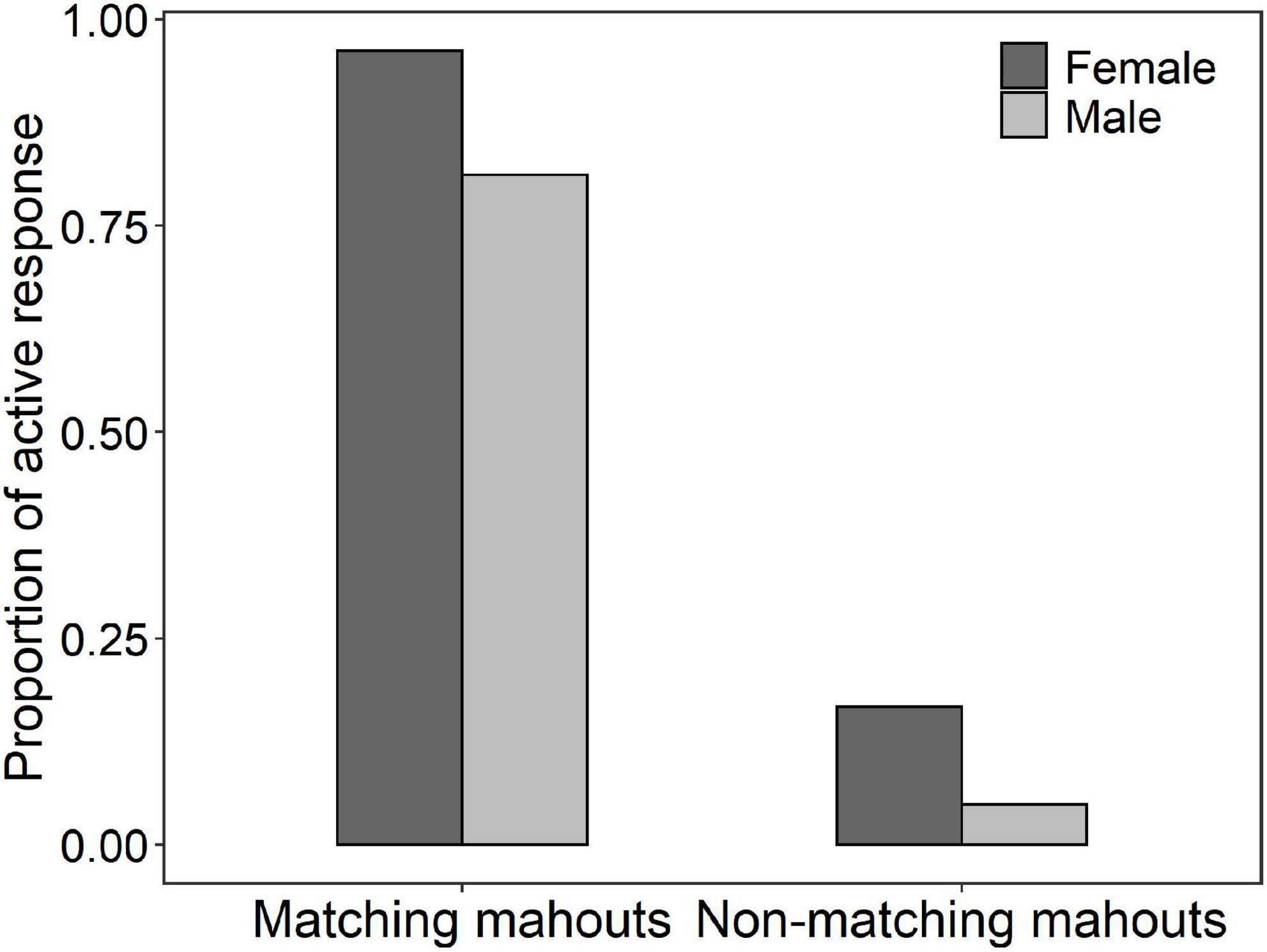
Figure 3. The proportion of active responses of female and male elephants responding to the commands of their matching mahouts or non-matching mahouts (for the full model 2 and 3, see Table 2).
When it was the matching mahout giving out the command, the elephants who had lived longer together with their mahouts (i.e., longer care time of their mahouts, or longer proximity with mahouts in general) showed significantly more active responses toward the commands from the matching mahouts (Figure 4; see parameters of Model 2 in Table 2). Younger elephants showed significantly more active responses to matching mahouts than older elephants when it was the matching mahout giving out the command. Yet, when non-matching mahouts were giving the commands, only the sex of the elephants had an impact on their responses, with the females responding more often than the males (Figure 4, see parameters of Model 3 in Table 2).
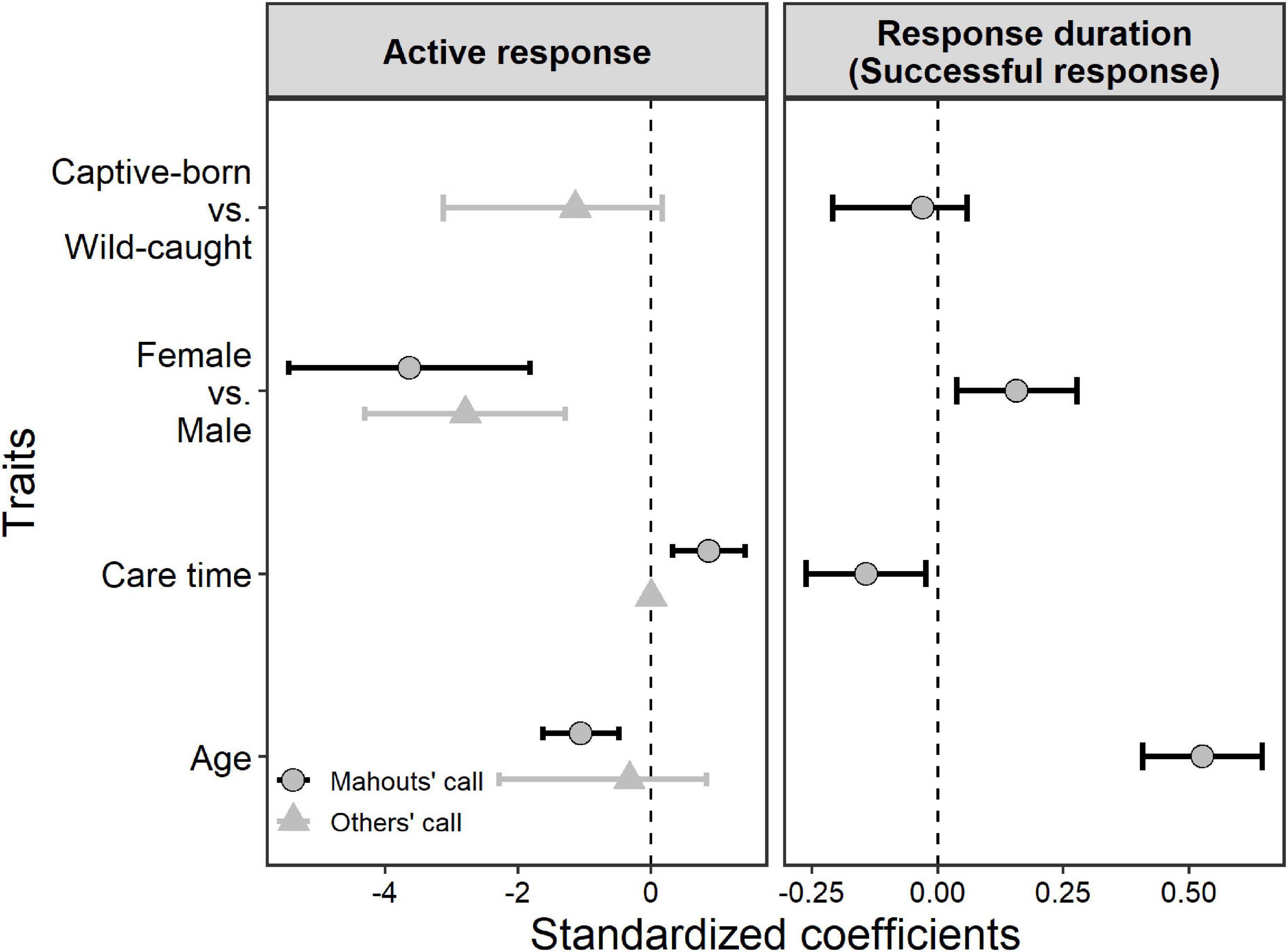
Figure 4. The individual traits’ influence on the active responses of the elephants when their matching mahouts commanded (right response) or when non-matching mahouts commanded (wrong response), and on the response duration when their matching mahouts commanded (right response). Whiskers represent the 95% confidence interval of the coefficient of each predictor.
The response duration of the elephants when their own mahouts gave out the commands revealed similar patterns (Figure 4). Overall, whether the elephants were captive-born or wild-caught had no influence on the elephants’ response duration. Here, female elephants responded more quickly (i.e., shorter response duration) than male elephants (Figure 4; see parameters of Model 4 in Table 2). Most importantly, the elephants responded faster when their mahouts spent more time with them in the past (i.e., care time of mahouts or proximity with mahouts; Figure 4; see parameters of Model 4 in Table 2). Furthermore, younger elephants responded more quickly than older elephants when it was the matching mahout commanding (Figure 4; see parameters of Model 4 in Table 2).
Discussion
Our study finds out that Asian elephants can unambiguously recognize the commands from individual mahouts by acoustic cues (Figure 2). Asian elephants who have been taken care for an extended time can develop a clear ability to discriminate between familiar and unfamiliar humans, regardless of whether they are captive-born or wild-caught (Figures 2, 4). Although ten of the semi-captive elephants in this study are captive-born, they might not go through an evolutionary process of domestication. We therefore argue that, at least for the Asian elephant, domestication is not instrumental in developing this discriminatory ability, and that possessing a familiarity with their handlers is sufficient.
Polla et al. (2018) reported that Asian elephants did not show any significant difference in their trunk-reaching frequency and bout duration when triggered by the voices of familiar and unfamiliar humans. However, with a small sample size of two zoo-housed elephant individuals tested over a limited number of human voices, the auditory discrimination rate they report is hardly conclusive (Jennions and Møller, 2003; Button et al., 2013). Our study used 18 elephants to look for a more general pattern. Furthermore, we used a command pertaining to locomotion, which implied an objective judgment of the animals.
In our discrimination test, the female elephants showed more frequent and faster responses than the male elephants (Figure 4). This may be attributable to the fact that female elephants live in social groups for all their lives (Vidya and Sukumar, 2005; Nandini et al., 2017). This was indeed the case of the elephants tested here. During this time in the elephants’ lives, the females are expected to develop more cooperative behaviors than the male elephants (such as taking care of each other’s calves; Sukumar, 2003). In addition, the younger elephants responded more frequently and faster to their matching mahouts, which may imply that younger individuals have better learning abilities than older elephants (Mader and Price, 1980; Head et al., 1995) or that older elephants are habituated to the commands from their matching mahouts. It is less likely that this pattern is caused by younger elephants being more docile or tamed than older ones, in which case the younger elephants may have responded more even when it was up to the non-matching mahout to give out the command. Yet, younger elephants and older elephants perform the same when they hear the command from unfamiliar mahouts (Figure 4). Further research is needed before drawing conclusions about this age factor. Most importantly, when mahouts spent more time with the matching elephants in the past (longer care time), elephants responded more and faster to the matching mahouts (Figure 4). This result indicates that the human acoustic cue discrimination may be a learning process, an adaptation to the extended exposure and received stimuli from humans become very familiar.
As our study assigned three elephants to a group, there might be a behavioral dependency effect between them. Yet, during the experiment, we only recorded 7 interaction events between elephants (playing with each other; i.e., using trunk to push or tangle with each other), and 28 events of one elephant following another elephant to the finish line, out of 351 trials. Most of the time, they stood still in the initial place or engaged in recreation with sand most of the time. In addition, because of equipment and electricity limitation in the elephant camp, we assigned three mahouts in a group, to avoid elephants using olfactory cues to discriminate among mahouts. Yet a better experiment design using playback experiment is strongly recommended in the future study, to remove the effect of olfactory cues.
Like for all the other wild megafauna, the main threat for Asian elephants is now from human beings (Desai and Riddle, 2015). Human-elephant interactions frequently happen in elephant-range countries (Chen et al., 2016), where local residents use firecrackers or other sounds, like the playback of felid growls, to deter elephants from raiding their crops (Thuppil and Coss, 2016). However, despite this artificial change in the acoustical structure of the animals’ environment, elephants have given proof to quickly adapt to these harmless dissuasive techniques (Joshi, 2013). With large brains, Asian elephants can adapt to degraded environments (Sol et al., 2008), by remembering the link between sounds and context. In Kenya, using acoustic cues only, wild African elephants can recognize subgroups of people from different ethnicities who pose threats or no threats to them (McComb et al., 2014). Our study provides evidence that semi-captive Asian elephants can recognize human voices at an individual level accurately. These elements may point toward why elephants are adapting to living near human residences, making human-elephant conflicts a wicked problem. By living in group and interacting with humans, social learning abilities of elephants may have likely promoted the ability to discriminate among humans both on a subgroup and on an individual level.
To conclude, this study demonstrates that the Asian elephant is able to discriminate between familiar and unfamiliar humans by means of acoustic cues alone. Females and younger individuals perform better in the discrimination test. The longer the elephants have interacted with the matching mahouts, the better they perform in human discrimination. Further comparative research is needed for the assessment of this ability in other non-domesticated animals, to understand the adaptation and evolutionary drivers of novel, functional skills under human-dominant circumstances.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Biomedical Ethics Expert Committee of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (protocol no. XTBG-2020-11).
Author contributions
L-LL, R-CQ, and RP designed the study and wrote the manuscript. L-LL conducted the experiments. RH and L-LL analyzed the data and conducted graphing. All authors read and approved the final manuscript.
Funding
This work was funded by the Transboundary Cooperation on Biodiversity Research and Conservation in Gaoligong Mountains (No. E1ZK251), Major Science and Technique Programs in Yunnan Province (202102AA310055), the Biodiversity Investigation, Observation and Assessment Program (2019-2023) of Ministry of Ecology and Environment of China (Grant No. 8-2-3-4-5), and CAS 135 program (Grant No. 2017 XTBG-F03).
Acknowledgments
We are grateful of Myanma Timber Enterprise for providing us with the permission to conduct this experiment [protocol no. 4527/MTE/AA(K)18], and the staff of the Myaing Hay Wun elephant camp who helped in our study. We especially thank U Mg Mg Chay, Phyo Thu Aung, U Win Ko, and Zin Nwe Soe for acting as interpreters and discussing the experiment. We also thank Francis Commercon for improving the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Adachi, I., Kuwahata, H., and Fujita, K. (2007). Dogs recall their owner’s face upon hearing the owner’s voice. Anim. Cogn. 10, 17–21. doi: 10.1007/s10071-006-0025-8
Akinwande, M. O., Dikko, H. G., and Samson, A. (2015). Variance inflation factor: as a condition for the inclusion of suppressor variable (s) in regression analysis. Open J. Stat. 5:754. doi: 10.4236/ojs.2015.57075
Aziz, M. A., Shamsuddoha, M., Maniruddin, M., Morshed, H. M., Sarker, R., and Islam, M. A. (2016). Elephants, border fence and human-elephant conflict in Northern Bangladesh: Implications for bilateral collaboration towards elephant conservation. Gajah 45, 12–19.
Bartoń, K. (2019). Package ‘MuMIn’: Multi-Model inference, Version 1.43. 6. Available online at: https://cran.r-project.org/web/packages/MuMIn/ (accessed April 9, 2019).
Boivin, X., Nowak, R., Despres, G., Tournadre, H., and Le Neindre, P. (1997). Discrimination between shepherds by lambs reared under artificial conditions. J. Anim. Sci. 75, 2892–2898. doi: 10.2527/1997.75112892x
Bond, M. L., König, B., Lee, D. E., Ozgul, A., and Farine, D. R. (2020). Proximity to humans affects local social structure in a giraffe metapopulation. J. Anim. Ecol. 90, 212–221. doi: 10.1111/1365-2656.13247
Burnham, K. P., and Anderson, D. R. (2002). A Practical Information-Theoretic Approach. Model Selection and Multimodel Inference, 2nd Edn. New York, NY: Springer.
Button, K. S., Ioannidis, J. P., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S., et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Chen, Y., Marino, J., Chen, Y., Tao, Q., Sullivan, C. D., Shi, K., et al. (2016). Predicting hotspots of human-elephant conflict to inform mitigation strategies in Xishuangbanna, Southwest China. PLoS One 11:e0162035. doi: 10.1371/journal.pone.0162035
Deecke, V. B., Slater, P. J., and Ford, J. K. (2002). Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420, 171–173. doi: 10.1038/nature01030
Duer, C., Tomasi, T., and Abramson, C. I. (2016). Reproductive endocrinology and musth indicators in a captive Asian elephant (Elephas maximus). Psychol. Rep. 119, 839–860. doi: 10.1177/0033294116667092
Fallow, P. M., and Magrath, R. D. (2010). Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim. Behav. 79, 411–417. doi: 10.1016/j.anbehav.2009.11.018
Fox, J., and Weisberg, S. (2019). An R Companion to Applied Regression (Third). Thousand Oaks CA: Sage.
Grueber, C. E., Nakagawa, S., Laws, R. J., and Jamieson, I. G. (2011). Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711. doi: 10.1111/j.1420-9101.2010.02210.x
Hart, B. L., Hart, L. A., and Pinter-Wollman, N. (2008). Large brains and cognition: where do elephants fit in? Neurosci. Biobehav. Rev. 32, 86–98. doi: 10.1016/j.neubiorev.2007.05.012
Head, E., Mehta, R., Hartley, J., Kameka, M., Cummings, B., Cotman, C., et al. (1995). Spatial learning and memory as a function of age in the dog. Behav. Neurosci. 109:851. doi: 10.1037/0735-7044.109.5.851
Heffner, R. S., and Heffner, H. E. (1982). Hearing in the elephant (Elephas maximus): absolute sensitivity, frequency discrimination, and sound localization. J. Comp. Physiol. Psychol. 96:926. doi: 10.1037/0735-7036.96.6.926
Jennions, M. D., and Møller, A. P. (2003). A survey of the statistical power of research in behavioral ecology and animal behavior. Behav. Ecol. 14, 438–445. doi: 10.1093/beheco/14.3.438
Joshi, R. (2013). Does escalating conflict and conservation challenges allow the Asian elephants to co-exist with humans in north India? Int. J. Environ. Sustain Dev. 12, 205–221.
Ketchaisri, O., Siripunkaw, C., and Plotnik, J. M. (2019). The use of a human’s location and social cues by Asian elephants in an object-choice task. Anim. Cogn. 22, 907–915. doi: 10.1007/s10071-019-01283-0
Koba, Y., and Tanida, H. (2001). How do miniature pigs discriminate between people?: Discrimination between people wearing coveralls of the same colour. Appl. Anim. Behav. Sci. 73, 45–58. doi: 10.1016/S0168-1591(01)00106-X
Latham, N. R., and Mason, G. J. (2008). Maternal deprivation and the development of stereotypic behaviour. Appl. Anim. Behav. Sci. 110, 84–108. doi: 10.1016/j.applanim.2007.03.026
Lee, W. Y., Choe, J. C., and Jablonski, P. G. (2011). Wild birds recognize individual humans: experiments on magpies Pica pica. Anim. Cogn. 14, 817–825. doi: 10.1007/s10071-011-0415-4
Leroux, M., Hetem, R. S., Hausberger, M., and Lemasson, A. (2018). Cheetahs discriminate familiar and unfamiliar human voices. Sci. Rep. 8, 1–6. doi: 10.1038/s41598-018-33971-1
Levey, D. J., Londoño, G. A., Ungvari-Martin, J., Hiersoux, M. R., Jankowski, J. E., Poulsen, J. R., et al. (2009). Urban mockingbirds quickly learn to identify individual humans. Proc. Natl. Acad. Sci. U.S.A. 106, 8959–8962. doi: 10.1073/pnas.0811422106
Li, L.-L., Plotnik, J. M., Xia, S.-W., Meaux, E., and Quan, R.-C. (2021). Cooperating elephants mitigate competition until the stakes get too high. PLoS Biol. 19:e3001391. doi: 10.1371/journal.pbio.3001391
Liu, P., Wen, H., Harich, F. K., He, C., Wang, L., Guo, X., et al. (2017). Conflict between conservation and development: cash forest encroachment in Asian elephant distributions. Sci. Rep. 7:6404. doi: 10.1038/s41598-017-06751-6
Mader, D., and Price, E. (1980). Discrimination learning in horses: effects of breed, age and social dominance. J. Anim. Sci. 50, 962–965. doi: 10.2527/jas1980.505962x
Marzluff, J. M., Walls, J., Cornell, H. N., Withey, J. C., and Craig, D. P. (2010). Lasting recognition of threatening people by wild American crows. Anim. Behav. 79, 699–707. doi: 10.1016/j.anbehav.2009.12.022
McComb, K., Reby, D., Baker, L., Moss, C., and Sayialel, S. (2003). Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 65, 317–329. doi: 10.1006/anbe.2003.2047
McComb, K., Shannon, G., Sayialel, K. N., and Moss, C. (2014). Elephants can determine ethnicity, gender, and age from acoustic cues in human voices. Proc. Natl. Acad. Sci. U.S.A. 111, 5433–5438. doi: 10.1073/pnas.1321543111
Nair, S., Balakrishnan, R., Seelamantula, C. S., and Sukumar, R. (2009). Vocalizations of wild Asian elephants (Elephas maximus): structural classification and social context. J. Acoust. Soc. Am. 126, 2768–2778. doi: 10.1121/1.3224717
Nandini, S., Keerthipriya, P., and Vidya, T. (2017). Seasonal variation in female Asian elephant social structure in Nagarahole-Bandipur, southern India. Anim. Behav. 134, 135–145. doi: 10.1016/j.anbehav.2017.10.012
Papworth, S., Milner-Gulland, E., and Slocombe, K. (2013). Hunted woolly monkeys (Lagothrix poeppigii) show threat-sensitive responses to human presence. PLoS One 8:e62000. doi: 10.1371/journal.pone.0062000
Payne, K. B., Langbauer, W. R., and Thomas, E. M. (1986). Infrasonic calls of the Asian elephant (Elephas maximus). Behav. Ecol. Sociobiol. 18, 297–301. doi: 10.1007/BF00300007
Plotnik, J. M., and de Waal, F. B. (2014). Asian elephants (Elephas maximus) reassure others in distress. PeerJ 2:e278. doi: 10.7717/peerj.278
Plotnik, J. M., de Waal, F. B., and Reiss, D. (2006). Self-recognition in an Asian elephant. Proc. Natl. Acad. Sci. U.S.A. 103, 17053–17057. doi: 10.1073/pnas.0608062103
Plotnik, J. M., Lair, R., Suphachoksahakun, W., and de Waal, F. B. (2011). Elephants know when they need a helping trunk in a cooperative task. Proc. Natl. Acad. Sci. U.S.A. 108, 5116–5121. doi: 10.1073/pnas.1101765108
Polla, E., Grueter, C., and Smith, C. (2018). Asian elephants (Elephas maximus) discriminate between familiar and unfamiliar human visual and olfactory cues. Anim. Behav. Cogn. 5, 279–291. doi: 10.26451/abc.05.03.03.2018
Poole, J. H., Tyack, P. L., Stoeger-Horwath, A. S., and Watwood, S. (2005). Elephants are capable of vocal learning. Nature 434, 455–456. doi: 10.1038/434455a
Price, E. O. (1984). Behaviour aspects of animal domestication. Q. Rev. Biol. 59, 1–32. doi: 10.1086/413673
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rayner, M. J., Clout, M. N., Stamp, R. K., Imber, M. J., Brunton, D. H., and Hauber, M. E. (2007). Predictive habitat modelling for the population census of a burrowing seabird: a study of the endangered Cook’s petrel. Biol. Conserv. 138, 235–247. doi: 10.1016/j.biocon.2007.04.021
Rhebergen, F., Taylor, R. C., Ryan, M. H., Page, R. A., and Halfwerk, W. (2015). Multimodal cues improve prey localization under complex environmental conditions. Proc. R. Soc. Lond. B Biol. Sci. 282:20151403. doi: 10.1098/rspb.2015.1403
Saito, A., and Shinozuka, K. (2013). Vocal recognition of owners by domestic cats (Felis catus). Anim. Cogn. 16, 685–690. doi: 10.1007/s10071-013-0620-4
Sharma, N., Prakash, S. V., Kohshima, S., and Sukumar, R. (2020). Asian elephants modulate their vocalizations when disturbed. Anim. Behav. 160, 99–111. doi: 10.1016/j.anbehav.2019.12.004
Sol, D., Bacher, S., Reader, S. M., and Lefebvre, L. (2008). Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 172, S63–S71. doi: 10.1086/588304
Stoeger, A. S., Mietchen, D., Oh, S., de Silva, S., Herbst, C. T., Kwon, S., et al. (2012). An Asian elephant imitates human speech. Curr. Biol. 22, 2144–2148. doi: 10.1016/j.cub.2012.09.022
Sukumar, R. (2003). The Living Elephants: Evolutionary Ecology, Behaviour, and Conservation. Oxford: Oxford University Press.
Taylor, A. A., and Davis, H. (1998). Individual humans as discriminative stimuli for cattle (Bos taurus). Appl. Anim. Behav. Sci. 58, 13–21. doi: 10.1016/S0168-1591(97)00061-0
Thuppil, V., and Coss, R. G. (2013). Wild Asian elephants distinguish aggressive tiger and leopard growls according to perceived danger. Biol. Lett. 9:20130518. doi: 10.1098/rsbl.2013.0518
Thuppil, V., and Coss, R. G. (2016). Playback of felid growls mitigates crop-raiding by elephants Elephas maximus in southern India. Oryx 50, 329–335. doi: 10.1017/S0030605314000635
Topál, J., Gácsi, M., Miklósi, Á, Virányi, Z., Kubinyi, E., and Csányi, V. (2005). Attachment to humans: a comparative study on hand-reared wolves and differently socialized dog puppies. Anim. Behav. 70, 1367–1375. doi: 10.1016/j.anbehav.2005.03.025
Tuttle, M. D., and Ryan, M. J. (1981). Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214, 677–678. doi: 10.1126/science.214.4521.677
Vidya, T., and Sukumar, R. (2005). Social organization of the Asian elephant (Elephas maximus) in southern India inferred from microsatellite DNA. J. Ethol. 23, 205–210. doi: 10.1007/s10164-005-0144-8
Wascher, C. A., Szipl, G., Boeckle, M., and Wilkinson, A. (2012). You sound familiar: carrion crows can differentiate between the calls of known and unknown heterospecifics. Anim. Cogn. 15, 1015–1019. doi: 10.1007/s10071-012-0508-8
White, P. C., and Ward, A. I. (2011). Interdisciplinary approaches for the management of existing and emerging human–wildlife conflicts. Wildl. Res. 37, 623–629. doi: 10.1071/WR10191
Wijayagunawardane, M. P., Short, R. V., Samarakone, T. S., Nishany, K. M., Harrington, H., Perera, B., et al. (2016). The use of audio playback to deter crop-raiding Asian elephants. Wildl. Soc. Bull. 40, 375–379. doi: 10.1002/wsb.652
Zhang, D. (2018). rsq: R-Squared and Related Measures. R Package Version 2.1. Available online at: http://CRAN.R-project.org/package=rsq (accessed November 30, 2020).
Keywords: acoustic cues, Elephas maximus, heterospecies recognition, human-elephant interaction, proximity
Citation: Li L-L, He R, Pansini R and Quan R-C (2022) Prolonged proximity to humans ensures better performance of semi-captive Asian elephants at discriminating between human individuals by voice. Front. Ecol. Evol. 10:963052. doi: 10.3389/fevo.2022.963052
Received: 01 July 2022; Accepted: 28 July 2022;
Published: 11 August 2022.
Edited by:
Carla Mucignat, University of Padua, ItalyReviewed by:
Emmanuel Paul Gilissen, Royal Museum for Central Africa, BelgiumSanjeeta Sharma Pokharel, Smithsonian Conservation Biology Institute (SI), United States
Copyright © 2022 Li, He, Pansini and Quan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Chang Quan, cXVhbnJjQHh0YmcuYWMuY24=
 Li-Li Li
Li-Li Li Ruchuan He
Ruchuan He Riccardo Pansini
Riccardo Pansini Rui-Chang Quan
Rui-Chang Quan