
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 08 September 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.958467
This article is part of the Research TopicFood Webs and Stable Isotopes, volume IIView all 13 articles
 Cristina Andolina1,2
Cristina Andolina1,2 Geraldina Signa1,2*
Geraldina Signa1,2* Giovanna Cilluffo1,2
Giovanna Cilluffo1,2 Simona Iannucci1,3
Simona Iannucci1,3 Antonio Mazzola1,2
Antonio Mazzola1,2 Salvatrice Vizzini1,2
Salvatrice Vizzini1,2Biological invasions are a widespread problem worldwide, as invasive non-indigenous species (NIS) may affect native populations through direct (e. g., predation) or indirect (e.g., competition) trophic interactions, leading to changes in the food web structure. The trophic relationships of the invasive eastern mosquitofish Gambusia holbrooki and the native big-scale sand smelt Atherina boyeri coexisting in three Mediterranean coastal ponds characterized by different trophic statuses (from oligotrophic to hypereutrophic) were assessed in spring through isotopic niche analysis and Bayesian mixing models. The two fish relied on the distinctive trophic pathways in the different ponds, with the evidence of minimal interspecific niche overlap indicating site-specific niche divergence mechanisms. In more detail, under hypereutrophic and mesotrophic conditions, the two species occupied different trophic positions but relying on a single trophic pathway, whereas, under oligotrophic conditions, both occupied a similar trophic position but belonging to distinct trophic pathways. Furthermore, the invaders showed the widest niche breadth while the native species showed a niche compression and displacement in the ponds at a higher trophic status compared to the oligotrophic pond. We argue that this may be the result of an asymmetric competition arising between the two species because of the higher competitive ability of G. holbrooki and may have been further shaped by the trophic status of the ponds, through a conjoint effect of prey availability and habitat complexity. While the high trophic plasticity and adaptability of both species to different environmental features and resource availability may have favored their coexistence through site-specific mechanisms of niche segregation, we provide also empirical evidence of the importance of environmental control in invaded food webs, calling for greater attention to this aspect in future studies.
The invasion of non-indigenous species (NIS) is considered among the major threats for marine ecosystem functioning and services worldwide. The consequences of species invasion and establishment in recipient ecosystems are complex and depend on the interaction between the ecological characteristics of the invader species and the environmental and biological attributes of the recipient ecosystem (Occhipinti-Ambrogi, 2007; Chan and Briski, 2017). In particular, while high dispersal ability and physiological plasticity, fast growth, high feeding rate, and generalist feeding strategies are among the key factors of success for invasive NIS (David et al., 2017 and reference therein), the establishment of self-sustaining NIS populations is further favored in disturbed habitats (Chan and Briski, 2017).
Change in trophic dynamics and structure with severe consequences for native species is one of the most common ecological processes that the establishment of invasive NIS may trigger in recipient ecosystems (Jackson et al., 2012; Britton et al., 2018; Costa-Pereira et al., 2019). Depending on the trophic position of the invader species, direct trophic interactions with native species can exert bottom-up or top-down cascading controls on local food webs (Vander Zanden et al., 1999; Gallardo et al., 2016). On the contrary, interspecific exploitative competition mechanisms may occur when invasive and native species are trophically analogous and may lead to competitive exclusion or coexistence through niche differentiation mechanisms, such as spatial segregation or resource partitioning (David et al., 2017 and references therein). As invasive NIS are known for their high competitive abilities, they may induce changes in habitat or resource use by natives, displacement and/or contraction of their trophic niche, and alteration of trophic interactions and food web structure (Vander Zanden et al., 1999; Carmona-Catot et al., 2013; Tran et al., 2015). Therefore, the study of trophic niche and food web structure in invaded ecosystems can help to reveal the ecological changes driven by the biological invasion, to understand how trophic relationships between invasive and native species promoted their coexistence, and to predict the future evolution of recently invaded ecosystems.
The eastern mosquitofish Gambusia holbrooki has been intentionally introduced from the southern USA to European and Australian freshwaters since the early 1900s with the purpose to control mosquito populations and reduce the risk of spreading mosquito-related diseases. In addition to freshwaters, G. holbrooki thrives in a wide range of habitats, including estuaries and near-shore marine areas, and has been identified as one of the most widespread invasive fish worldwide (Lowe et al., 2000). It is a voracious predator with high feeding rates (Rehage et al., 2005), also known as an omnivore with opportunistic feeding strategies (Blanco et al., 2004; Kalogianni et al., 2014). A large array of terrestrial and aquatic organisms have been identified as common prey, along with macrophyte detritus (Blanco et al., 2004; Rehage et al., 2005; Remon et al., 2016). Besides high physiological adaptability and trophic plasticity and feeding rates, viviparity, high fecundity, and resistance to pollutants provide mosquitofish with a high competitive ability compared to native fish (Pyke, 2005).
Overall, a strong competitive impact of G. holbrooki on native species has been well documented in a variety of ecosystems (Alcaraz et al., 2008; MacDonald et al., 2012; Ruiz-Navarro et al., 2013). In particular, laboratory and field studies demonstrated the influence of abiotic factors in shaping the performance of mosquitofish, including the competitive effects on native species (Rincón et al., 2002; Blanco et al., 2004; Carmona-Catot et al., 2013; Ruiz-Navarro et al., 2013). High frequency of aggressive behaviors and higher feeding rate than native species have been observed at higher water temperature (Rincón et al., 2002; Carmona-Catot et al., 2013) and lower salinity (Alcaraz et al., 2008; Ruiz-Navarro et al., 2013). On the contrary, a limited influence of habitat features (e.g., size and complexity) and water quality (e.g., nutrient concentration and turbidity) on mosquitofish life-history traits and predation has been reported (Blanco et al., 2004; Cano-Rocabayera et al., 2019). Even with the large body of literature existing on the ecological effects of invasive fish, including mosquitofish, in aquatic systems, the trophic aspects have been scantly addressed especially in combination with environmental stressors.
Carbon and nitrogen stable isotope analysis (SIA, δ13C, and δ15N) is a powerful tool to investigate trophic interactions (e.g., Michener and Kaufman, 2008; Mancinelli and Vizzini, 2015; Nielsen et al., 2018) and has been widely used to describe trophic niche features within an isotopic framework (e.g., isotopic niche breadth and overlap) (Chen et al., 2011; Abrantes et al., 2014), ontogenetic diet shifts (Layman et al., 2011; Andolina et al., 2020), and organic matter pathways (Vizzini et al., 2005; Signa et al., 2013b). Furthermore, SIA has the potential to infer the effects of invasive species on aquatic food webs, including resource shift deriving from intraspecific and interspecific competitions (Jackson et al., 2012; Mancinelli et al., 2017; Britton et al., 2018).
Here, we studied the isotopic niche as a proxy for the trophic niche (sensu Bearhop et al., 2004) of the invasive eastern mosquitofish G. holbrooki (Girard, 1859) and the native big-scale sand smelt Atherina boyeri (Risso, 1810), coexisting in a Mediterranean coastal system (Marinello ponds, Italy) featured by several small, shallow, and brackish ponds with different marine influences, geomorphological features, and trophic conditions (from oligotrophic to hypereutrophic) due to the external subsidies of gull guano (Signa et al., 2012). The big-scale sand smelt is a small euryhaline fish that inhabits the littoral zones of the Eastern Atlantic and the Mediterranean Sea, where it is frequently found in large schools both along the coasts and in lagoons and coastal lakes (Kara and Quignard, 2019). Alongside physiological adaptability, the big-scale sand smelt shows also high trophic plasticity and a generalist trophic behavior (Vizzini and Mazzola, 2002, 2005).
We hypothesize that generalist feeding behavior and trophic plasticity of both G. holbrooki and A. boyeri represent strategies which promote the coexistence of the two species through resource partitioning, therefore resulting in the separation of the trophic niche and different isotopic niche features (e.g., breadth, overlap). We also hypothesize that the contrasting trophic conditions may modulate this process by shaping the trophic niches according to prey availability and habitat complexity.
The study was carried out in the coastal system of Marinello located along the north-eastern coast of Sicily (Italy, Mediterranean Sea) (Figure 1), consisting of five small (1–4 ha), shallow (max depth: 2–4 m), and brackish (mean salinity: 26–34 PSU) ponds (Verde, Fondo Porto, Porto Vecchio, Mergolo, and Marinello), separated from the adjacent sea by littoral bars and lacking direct freshwater input (Mazzola et al., 2010). The present research focused on three of the five ponds (i.e., VE, Verde; ME, Mergolo; and FP, Fondo Porto), on which several studies were conducted and found high inter-pond variability in terms of trophic status and primary production (Signa et al., 2012), contamination level (Signa et al., 2013a,b), macrobenthic communities (Signa et al., 2015), and trophic structure (Vizzini et al., 2016). These differences were attributed to the guano-derived fertilization induced by the colony of the yellow-legged gull Larus michahellis (Naumann, 1840) resident in the cliff next to the pond VE alongside the variability in geomorphological features of the ponds. The deeper landward ponds, VE, and ME (max depth, respectively, 3 and 3.5 m, Mazzola et al., 2010), are characterized by seabird-induced hypereutrophication (i.e., guanotrophication) and mesotrophic conditions, respectively (mean Chl-a: 44.7 and 8.8 mg m−3; mean TSICHL (Trophic State Index, sensu Acquavita et al., 2015): 65 and 41 mg m−3), resulting in high (although seasonally fluctuating) phytoplanktonic production and high internal variability (littoral vs. deeper area) (Signa et al., 2012, 2015). In contrast, the smaller (1.3 ha) and shallower (1.2 m deep, Mazzola et al., 2010) seaward pond FP is oligotrophic (mean Chl-a: 3.3 mg m−3; mean TSICHL: 35 mg m−3) and features higher water transparency and a macrophyte-covered seabed (Signa et al., 2012, 2015). Accordingly, biotic communities and trophic pathways vary among ponds (Vizzini et al., 2016), with benthic assemblages partially mirroring the strong environmental gradients, namely showing not only decreasing abundances from FP to VE but also the highest structural and functional diversity in ME (Signa et al., 2015). As regards fish, comparable assemblages characterize the three Marinello ponds, with the native A. boyeri and the invasive G. holbrooki among the most abundant species in the three ponds, where they coexist throughout the year, with the highest abundance in spring (Vizzini et al., 2016).
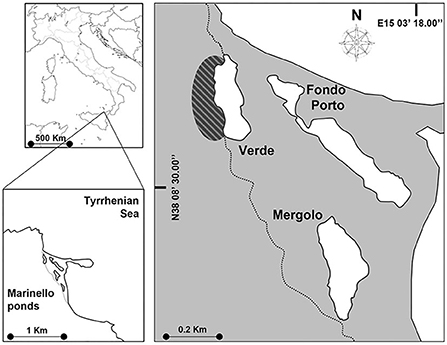
Figure 1. Map of the Marinello coastal system. The ponds studied were Verde (VE), Mergolo (ME), and Fondo Porto (FP) at increasing distance from the gull colony indicated by the striped oval. The dotted line indicates the edge of the Tindari promontory.
Atherina boyeri and Gambusia holbrooki specimens were collected using a small beach seine (4 m length, 3 mm mesh) in the three ponds Verde (VE), Mergolo (ME), and Fondo Porto (FP) in spring 2012 when the ponds host the highest diversity and abundances of fish, invertebrates, and organic matter sources (Signa et al., 2015). The hauls were performed at each pond in triplicate by dragging the seine on a perpendicular line (10 m) from a depth of about 1.5 m up to the shoreline. In addition, the percent coverage of macrophytes (macroalgae and seagrasses) was estimated by visual census along the shores and the central area of each pond and ranked according to the marine version of the Braun-Blanquet score developed by Kenworthy et al. (1993) as a proxy for habitat complexity.
After collection, fish were kept cool and in the dark upon arrival at the laboratory, where they were identified, subjected to biometric measurements (standard length SL, wet weight WW), and grouped per size class: small (SL <30 mm) and large (SL > 30 mm), according to previous studies carried out in Mediterranean lagoons (Vizzini and Mazzola, 2002; Blanco et al., 2004). Both size classes of the two species were found in all the ponds, except for small A. boyeri, which was not found in FP. Total fish abundance was calculated for both species and size class by pooling data obtained from the three hauls and expressed as individuals per 100 m2. Moreover, a minimum of five and a maximum of ten specimens per size class were randomly taken for each species from each pond (Table 1) and processed for isotopic analyses, with this sample size being sufficient to ensure reliable isotopic niche determination through the specific statistical package SIBER (Jackson et al., 2011; see Section Data analysis for details). Dorsal muscle was dissected, dried at 60°C to constant weight, and ground to a fine homogeneous powder using a mortar and pestle. Stable isotope analysis was performed through an isotope ratio mass spectrometer (Thermo Delta Plus XP) connected to an elemental analyzer (Thermo Flash EA 1112). Stable isotopes were expressed in standard delta (δ) notation as parts per thousand (‰):
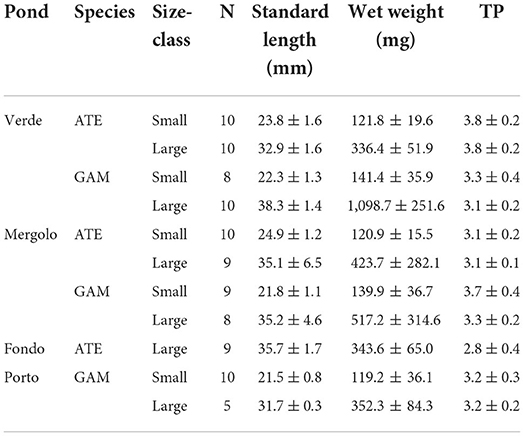
Table 1. Sample size (N), biometric measures (Mean ± SD), and trophic position (TP) of small and large specimens of A. boyeri (ATE) and G. holbrooki (GAM) from the three ponds of the Marinello coastal system.
where R is the ratio 13C:12C or 15N:14N. The results were reported relative to Vienna Pee Dee Belemnite (VPDB) for δ13C and atmospheric air for δ15N. Analytical precision based on the standard deviation of replicates of internal standards (International Atomic Energy Agency IAEA-CH-6 for δ13C and IAEA-NO-3 for δ15N) was 0.1‰ for δ13C and 0.2‰ for δ15N.
To assess isotopic niche breadth and overlap of the populations of the two species (Atherina boyeri and Gambusia holbrooki) across size classes in the three ponds, δ13C and δ15N data were first corrected to avoid any potential bias given by differences in basal resources of the three ponds (Olsson et al., 2009).
The surface-grazing snail Hydrobia ventrosa was used as baseline, according to Vizzini et al. (2016), to correct both δ13C and δ15N. In more detail, the corrected δ13C was calculated following the equation by Olsson et al. (2009):
where δ13Cf is the carbon isotopic value of the fish, δ13Cbmean is the mean carbon isotopic value of the baseline (δ13C mean value ± standard deviation: −17.4 ± 0.7‰ in VE, −16.4 ± 0.5‰ in ME, and −17.4 ± 0.2‰ in FP), and CRb is the carbon range (δ13Cmax-δ13Cmin) of the baseline (1.3‰ in VE, 1.0‰ in ME, and 0.3‰ in FP) (data from Vizzini et al., 2016).
The same baseline was used to correct δ15N, i.e., estimating the fish trophic position (TP) following the equation by Post (2002):
with δ15Nf being the nitrogen isotopic value of the fish, δ15Nb being the mean nitrogen isotopic value of the baseline (δ15N mean value ± standard deviation: 13.4 ± 0.4‰ in VE, 6.9 ± 0.7‰ in ME, and 10.9 ± 0.1‰ in FP), Δn being the expected enrichment in δ15N per trophic level (3.4‰ according to Post, 2002), and λ being the trophic level of the species used as a baseline that was set as 2.
Corrected δ13C and δ15N data were then used to estimate the standard ellipse area corrected for small sample size (SEAc), the SEAc overlap, and the Bayesian standard ellipse area (SEAb) (Jackson et al., 2011) using the SIBER package v 2.1.5 (Stable Isotope Bayesian Ellipses in R) (Jackson et al., 2011) in R v. 4.0.2 (R Core Team, 2020). The SEAc encompassed 40% of the data and was expressed as single values; the SEAb was derived from 100,000 posterior iterations and was expressed as a range of probable values reported in posterior density plots reflecting estimation uncertainty (Jackson et al., 2011). Differences in TP and SEAb among ponds, fish species, and size classes were tested, respectively, with non-parametric permutational analysis of variance (PERMANOVA, PRIMER-E Ltd., Plymouth, UK; Anderson, 2017) and ANOVA (R v. 4.0.2; R Core Team, 2020) followed by pairwise comparisons.
In addition, to estimate the main trophic pathways sustaining the two species' population in the ponds, Bayesian mixing models were run using MixSIAR v 3.1.11 (Stock et al., 2018) in R (R Core Team, 2020). Carbon and nitrogen stable isotope data (not corrected) of all possible basal sources of organic matter in each pond were taken from Vizzini et al. (2016). Sources included in the models were seagrasses, macroalgae, suspended particulate organic matter (SPOM), and sedimentary organic matter (SOM) (see Supplementary Table S1 for species list and isotopic values). Trophic enrichment factors (TEFs) used in the model were, respectively, 0.4 ± 1.3‰ for δ13C and 3.4 ± 1.0‰ for δ15N, according to Post (2002), which were doubled as these fish are secondary consumers/omnivores. Whenever more than one species belonged to the seagrass or macroalgae source categories, the mixing model output was reported as the a posteriori sum of the contribution of each species (see Supplementary Table S1).
The three ponds were characterized by different habitat complexity in terms of macrophyte cover and marine Braun-Blanquet score. High internal variability characterized both ponds at a higher trophic status, Verde (VE) andMergolo (ME), in contrast to what was observed in the oligotrophic pond Fondo Porto (FP). In particular, the shores of both VE and ME were covered with patches of seagrasses and macroalgae interspersed with bare bottom ranging from 0 to 50% for seagrasses in both ponds and from 0 to 100% and to 75% for macroalgae in VE and ME, respectively (Table 2). In contrast, the shores of FP were entirely covered by macrophytes with the percentage ranging between 5 and 75% for both seagrasses and macroalgae (Table 2). The greatest variability among the ponds was found in the central areas, with no macrophytes recorded in VE, patchy macrophyte cover in ME (25–100 and 0–50% for seagrasses and macroalgae, respectively), and higher and more homogeneous coverage of both seagrasses (25–50%) and macroalgae (25–100%) in FP. Accordingly, the marine Braun-Blanquet score varied from 0 to 5 in both VE and ME and only from 2 to 5 in FP (Table 2).
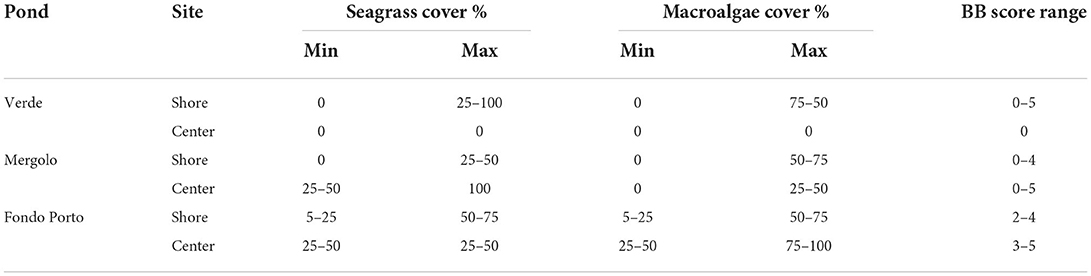
Table 2. Macroalgae and seagrass percentage cover of the three ponds of the Marinello coastal system (shores and central area) ranked according to the marine version of the Braun-Blanquet score (Kenworthy et al., 1993).
Overall, the native big-scale sand smelt A. boyeri outnumbered the invasive eastern mosquitofish G. holbrooki (Figure 2). Small specimens predominated over large specimens in each pond, except for A. boyeri in FP where no small fish were found and were more abundant in ME than in the other ponds.
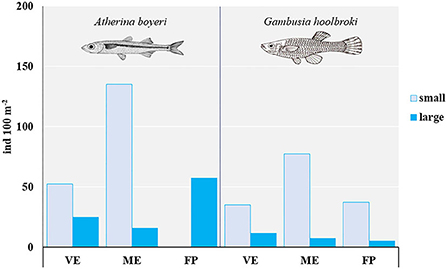
Figure 2. Total abundance (ind. 100 m−2) of small and large specimens of A. boyeri and G. holbrooki from the three ponds of the Marinello coastal system: VE, Verde; ME, Mergolo; FP, Fondo Porto.
δ13C and δ15N of fish varied among the three ponds according to their trophic status: values were overall more depleted in both carbon and nitrogen in the mesotrophic ME, intermediate in carbon and most enriched in nitrogen in the guanotrophic VE, and most enriched in carbon and intermediate in nitrogen in the oligotrophic FP (Figure 3A). Similarly, the standard ellipse area (SEAc), representing the “core” isotopic niche of the two fish species, showed a clear grouping by pond within the corrected isotopic space (δ13Ccorr–TP bi-plot) (Figure 3B). The niche of both species collected in ME showed a more carbon-depleted position, followed by the niches in VE and FP, which showed the most carbon-enriched position. Within each pond, the niches of the two fish across size classes were positioned in different ways: both in VE and ME, the niches of the two species showed a similar positioning along the δ13Ccorr axis, while they clustered apart along the TP axis, with A. boyeri at higher TP values than G. holbrooki in VE and conversely in ME (Figure 3B). In contrast, in FP, the niches of the two species differed mainly in δ13Ccorr values, with more 13Ccorr-enriched values for G. holbrooki than A. boyeri (Figure 3B). In line with these patterns, the niche overlap between the two species at all size classes was negligible in all the ponds (range: 0–12%, Table 3). High intraspecific SEAc overlap was observed for A. boyeri: 87 and 98% of the niche of large specimens overlapped with that of small specimens in VE and ME, respectively (Figure 3B, Table 3). In contrast, only a partial overlap was observed between the two size classes of G. holbrooki in all the ponds (38, 33, and 24%, respectively, in VE, ME, and FP; Figure 3B, Table 3).
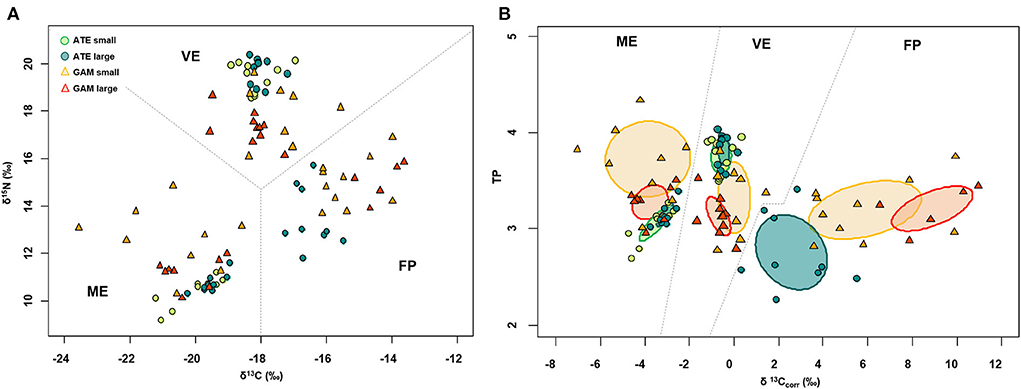
Figure 3. Bi-plots showing (A) raw and (B) baseline corrected δ13C (δ13Ccorr) and δ15N (TP) of small and large specimens of A. boyeri (ATE) and G. holbrooki (GAM) from the three ponds of the Marinello coastal system: VE, Verde; ME, Mergolo; FP, Fondo Porto. In (B) is reported standard ellipse area corrected for small sample size (SEAc ‰2) representing the isotopic niches.
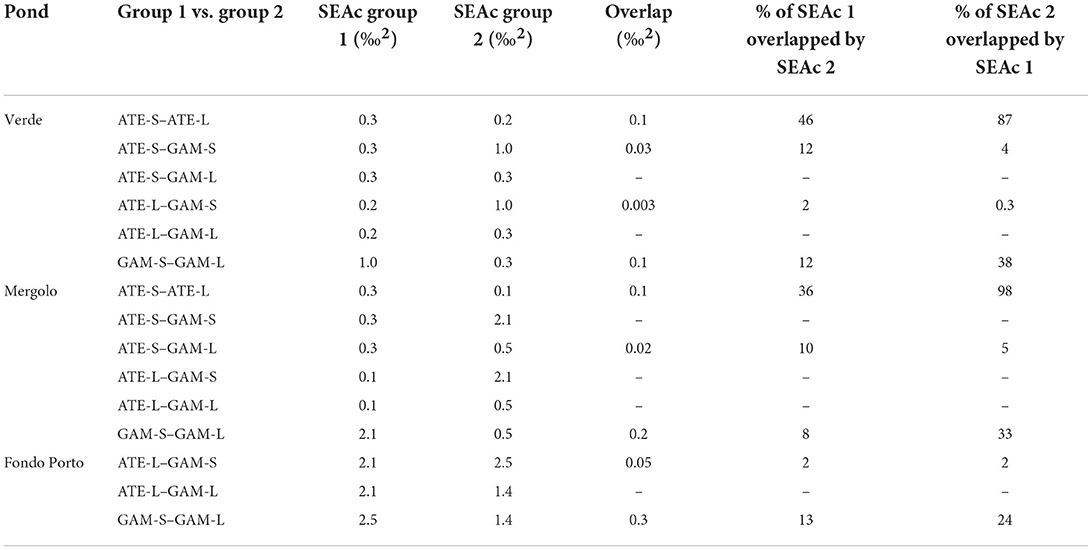
Table 3. Pairwise comparisons of SEAc (based on corrected stable isotope data) and overlap values (‰2) estimated between small (S) and large (L) specimens of A. boyeri (ATE) and G. holbrooki (GAM) from the three ponds of the Marinello coastal system.
The isotopic niche breadth of the two species, expressed as SEAb, showed overall wider niches for small mosquitofish than all the other fish and wider niche in FP than in the other ponds (Figure 4). Moreover, in the three ponds, the niche breadth of both mosquitofish and sand smelt was significantly wider in small than large specimens (p < 0.001), but only in VE and ME, the niche breadth of sand smelt was smaller than that of both mosquitofish size classes (Figure 4). At the same time, comparing ponds, both small and large G. holbrooki showed significant decreasing niche breadth from FP to ME and VE, while large A. boyeri showed larger niche in FP than both ME and VE (p < 0.001). Similar to the large specimens, the niche of small A. boyeri was rather narrow and comparable between VE and ME (Figure 4).
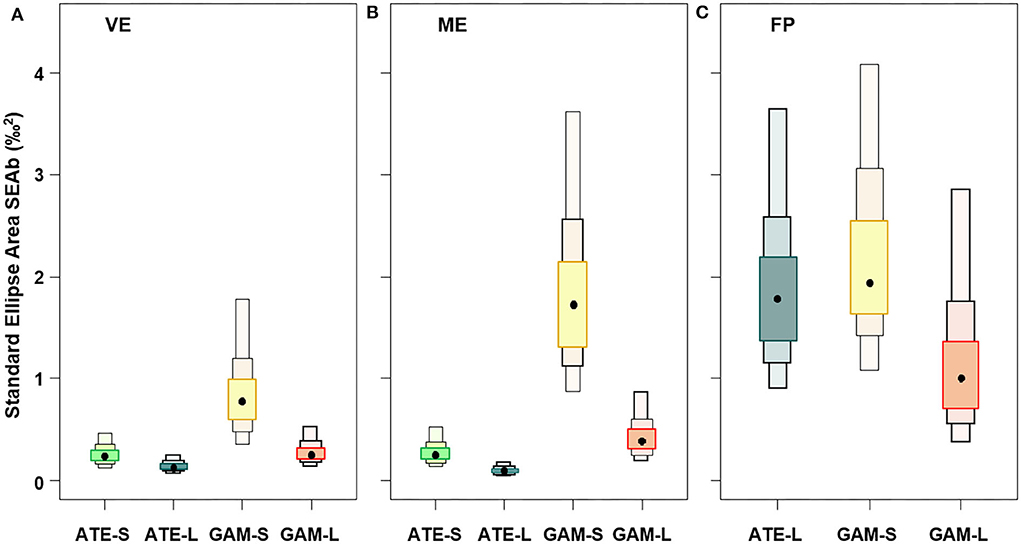
Figure 4. Box plots representing the niche breadth expressed as standard ellipse area (SEAb‰2, based on baseline corrected stable isotope data to trophic position and corrected carbon) of small (S) and large (L) specimens of A. boyeri (ATE) and G. holbrooki (GAM) from the three ponds of the Marinello coastal system: (A) Verde (VE), (B) Mergolo (ME), and (C) Fondo Porto (FP). Boxes present relative credible intervals of 95% (light color), 75% (intermediate color), and 50% (dark color) and mode (black circle).
The trophic position (TP) estimated showed that the two species broadly occupy a trophic level comprised between 3 and 4 while varying among ponds, species, and size classes (Table 1, Supplementary Table S2). Moreover, in the eutrophic pond VE, A. boyeri showed a significantly higher TP than G. holbrooki, while the opposite trend emerged in the other two ponds, where G. holbrooki showed higher TP than A. boyeri. Moreover, A. boyeri showed significantly increasing TP values from FP to ME and VE and G. holbrooki showed higher TP only in ME than in the other ponds. As regards size classes, only mosquitofish showed significant differences with higher values in small than large specimens (Table 1, Supplementary Table S2).
Mixing models revealed that the basal organic matter sources provided a different proportional contribution to the trophic pathways sustaining the two fish species in the different ponds (Figure 5, Supplementary Table S1 for details). In VE, sedimentary organic matter (SOM) was the dominant basal source underlying the diet of all fish, with the exception of large mosquitofish, for which the proportional contribution of SOM decreased in favor of macroalgae. A clear different pattern was found in ME, where the suspended particulate organic matter (SPOM) was the prevailing basal resource in the trophic pathways supporting the diet of all fish (Figure 5, Supplementary Table S1). Lastly, in FP, all the basal resources contributed in similar proportions to the pathways underlying the diet of large sand smelts, while macroalgae and seagrasses prevailed for mosquitofish (Figure 5, Supplementary Table S1).
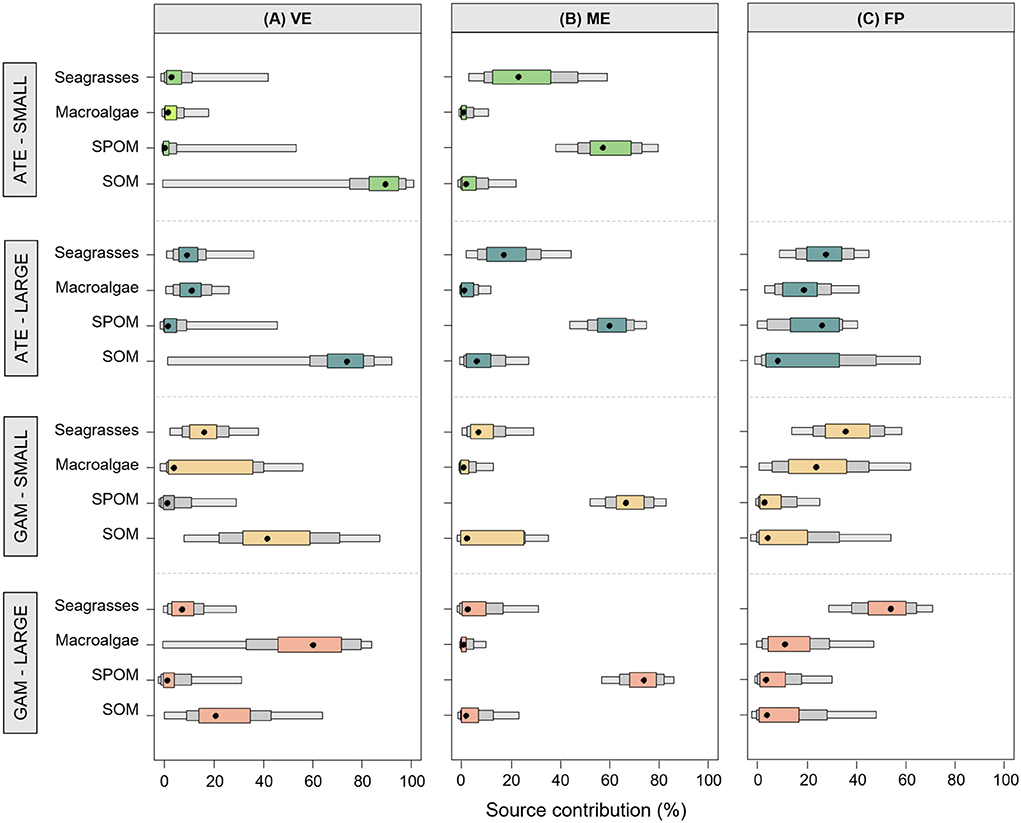
Figure 5. Percentage contribution of different basal resource categories to the diet of small (S) and large (L) specimens of A. boyeri (ATE) and G. holbrooki (GAM) from the three ponds of the Marinello coastal system: (A) Verde (VE), (B) Mergolo (ME), and (C) Fondo Porto (FP). Boxes present relative credible intervals of 95% (light color), 75% (intermediate color), and 50% (dark color) and mode (black circle). Details of the Bayesian isotopic mixing model output are presented in Supplementary Table S1.
This study analyses the trophic relationships between the invasive eastern mosquitofish G. holbrooki and the native big-scale sand smelt A. boyeri co-occurring in the brackish ponds of the Marinello coastal system at the beginning of the productive season (i.e., spring). The results obtained highlighted a clear differentiation in the fish isotopic values and niche position, consistent with the distinct trophic background of the coastal ponds and the reliance of fish on different trophic pathways.
The nutrient subsidies arising from the gull colony (L. michahellis) strongly influence the neighboring Verde pond (VE), but only to a limited extent the nearby Fondo Porto pond (FP) and the farthest Mergolo pond (ME) (Signa et al., 2012; Vizzini et al., 2016). δ15N is acknowledged as a powerful proxy for the ornithogenic input in terrestrial and coastal areas, due to the high values of bird guano (Mizutani and Wada, 1988; Signa et al., 2021 and references therein). Accordingly, the different positioning of the fish populations along the vertical axis of the isotopic bi-plot found in this study mirrors the gradual decrease in the incorporation of guano-derived nutrients into local food webs (Vizzini et al., 2016), with the highest influence in the closest pond VE and the lowest in the farthest ME. On the contrary, we found that the different positioning along the δ13C axis mirrors the reliance of fish on distinct organic matter pathways. The overlap between the δ13C values of guano (δ13C = −20.2 ± 2.5 ‰, Vizzini et al., 2016) and those of both sedimentary organic matter (SOM) (−19.5 ± 1.1‰, Supplementary Table S1) and macroalgae (−20.0 ± 0.8‰, Supplementary Table S1) in VE indicates, in fact, a clear accumulation of seabird-derived organic matter in the system and its incorporation in basal sources (Vizzini et al., 2016). Moreover, Bayesian mixing models revealed that the avian-subsidized SOM and the opportunistic macroalgae (e.g., Cladophora sp.), which are particularly abundant in the littoral zone forming dense patches, were the main basal sources supporting the trophic pathway leading to both fish species in VE. In contrast, we observed a single planktonic pathway based on suspended particulate organic matter (SPOM) dominating in the mesotrophic ME and a mixed benthic pathway characterized by a high contribution of macrophytes dominating in the seaward and oligotrophic FP. This is consistent with previous findings that showed a dominance of benthic pathways in VE in spring (Vizzini et al., 2016) and inter-pond variability in resource availability and habitat complexity that reflects on benthic abundance and diversity (Signa et al., 2015), confirming, therefore, that the observed differences in trophic pathways are driven in part by the differing seabird subsidies. Alongside the reliance on different organic matter pathways in the three ponds, the patterns observed within each pond in terms of isotopic niche positioning and trophic level suggest that the coexistence of the two species may have been promoted by site-specific mechanisms of niche differentiation. First, the negligible niche overlap between the two species throughout the ponds indicates a clear resource partitioning, consistent with previous studies of invasive fish, including mosquitofish. In particular, both in Australian wetlands and Mediterranean lagoons, mosquitofish coexists with other endemic fish, including sand smelt, with limited niche sharing (Gisbert et al., 1996; Stoffels and Humphries, 2003; MacDonald et al., 2012). Niche divergence, rather than convergence, is suggested, indeed, as a general pattern within invaded fish communities, except in cases of high invader abundance (Tran et al., 2015; Britton et al., 2018), a condition not observed in this study. Second, the different niche positioning in the three ponds indicated that the two species have developed different trophic, and maybe behavioral, strategies to coexist, depending on different trophic conditions, presumably mediated by different resource availability (e.g., habitat complexity and prey diversity and abundance). It is not new that resource availability can change significantly over space and time due to the presence of ecological gradients, such as seasonality and productivity, and that, in turn, this can affect species niches and competitive interactions (Abbey-Lee et al., 2013; Costa-Pereira et al., 2019). Here, we found that, in the oligotrophic shallow pond (FP), characterized by mixed macrophyte-covered bottoms and a high abundance of deposit feeders, the two fish species belonged to distinct benthic pathways, with SOM as the main basal carbon source for sand smelts, and macroalgae and seagrasses for mosquitofish. While habitat complexity would provide different niches that are partitioned by the two species (Beisel et al., 2000; MacDonald et al., 2012), the high abundance of food resources throughout the pond may have facilitated the integration of mosquitofish into the native food web by being able to exploit unused resources and thus avoid competitive interactions (Britton et al., 2018).
In contrast, in the ponds characterized by higher trophic state and lower habitat complexity (VE and ME), the Bayesian mixing models revealed that the two coexisting fish tended to rely on the same trophic pathway, rather than exploit distinct pathways, probably as an effect of the limited range of exploitable pathways under mesotrophic and hypereutrophic conditions. At the same time, the change in trophic positions and the reduction of the niche breadth (SEAb) observed for the native big-scale sand smelt from the oligotrophic to both mesotrophic and hypereutrophic ponds helped to recall the classical niche theories and found confirmation in several empirical studies. It has been postulated, indeed, that invasive species tend to out-compete native species through asymmetrical competitive mechanisms, such as by contracting and/or displacing the native's niche to lower or higher trophic positions due to their higher competitive ability (Vander Zanden et al., 1999; Jackson et al., 2012; Britton et al., 2018). Under this framework, the different patterns observed in the two ponds at higher trophic state may find justification in the different levels of biodiversity that characterizes them. While the harsh conditions of the guanotrophic VE support low-diversity communities featured by only benthic deposit feeders (i.e., chironomids, amphipods, and small gastropods) and epifaunal carnivorous palaemonid shrimps, the mesotrophic ME is characterized by high-diversity communities with benthic filter feeders and deposit feeders, as well as carnivorous polychaetes (Signa et al., 2015). Therefore, we suggest that, in the less diverse and harsher pond VE, the mosquitofish included plant materials in their diet to balance the decrease in animal prey abundance (Blanco et al., 2004; Kalogianni et al., 2014) and restricted the access of sand smelts to only a few high-order consumers, such as the juveniles of the palaemonid shrimps that thrive in the pond. In contrast, the higher structural and functional biodiversity of ME favored the invader's niche expansion, particularly evident in small specimens, consistent with the “resource diversity hypothesis” (MacArthur, 1969), and constrained the native species' diet to a few low-order consumers, such as epifaunal filter/deposit feeders.
Looking at the intraspecific level, the narrow niche breadth (SEAb), together with the high niche overlap (>90%) of small- and big-sized sand smelts from ME and VE, indicates a specialist diet across size classes, contrary to what was observed in FP where the wider niche of large specimens may indicate release from intraspecific competition (Britton et al., 2018). This clear spatial pattern of SEAb also indicates lower trophic diversity and variety of both exploited resources and trophic levels for both small and large specimens from VE and ME than FP. A. boyeri can shift between small hyperbenthic and epifaunal prey (e.g., isopods, amphipods, mysids, polychaetes, gastropods, and bivalves) in shallow vegetated coastal areas, because of its high trophic plasticity (Vizzini and Mazzola, 2002, 2005; Chrisafi et al., 2007). Such flexible feeding habits, coupled with the high abundance, may have represented the winning strategy that allowed the sand smelt to accommodate a certain degree of niche contraction rather than its suppression.
In contrast, the mosquitofish G. holbrooki exhibited a low intraspecific niche overlap (~20%) between size classes in all ponds and a clear niche narrowing from small to larger specimens, suggesting ontogenetic dietary specialization. The high niche breadth of the small mosquitofish indicated a clear generalist feeding behavior, as well as high trophic diversity and omnivory degree. Omnivory plays an important stabilizing role in spatially compressed food webs, alleviating the strong destabilizing force of top-down pressures potentially exerted by top predators (McCann et al., 2005). At the same time, the large among-individual variability may also indicate that individual mosquitofish use different foraging tactics feeding on only a subset of the available resources (Abbey-Lee et al., 2013) from different trophic pathways and levels, as a mechanism for reducing intraspecific competition (Matthews and Mazumder, 2004; Abbey-Lee et al., 2013) and ensuring population growth (Blanco et al., 2004). The eastern mosquitofish is acknowledged, indeed, not only as an opportunistic predator with a very wide prey spectrum, including zooplankton, insects, benthic invertebrates, fish, and amphibian larvae and eggs (Specziár, 2004; Pyke, 2005), but also as an omnivore able to ingest large amounts of algae and vegetal detritus in turbid and shallow estuaries and lakes (Blanco et al., 2004; Franco et al., 2008).
Lastly, assuming that the two species occupy a similar fundamental niche (i.e., the multidimensional environmental conditions within which a species can live in the absence of competitors, sensu Hutchinson, 1957), we infer that the native sand smelt has been induced to undergo different mechanisms of trophic displacement and/or contraction of its realized niche to coexist with the invasive mosquitofish. While this is consistent with the general adaptative response of native species subject to asymmetric competition with invaders exhibiting superior competitive abilities (Byers, 2000; Carey and Wahl, 2010; Tran et al., 2015), we provided evidence for the occurrence of site-specific environmental control on invaded trophic niches as a result of the combined effect of differing resource availability and habitat complexity.
We used a combination of isotopic niche analysis and Bayesian mixing models to reveal complex site-specific trophic relationships between the invasive eastern mosquitofish Gambusia holbrooki and the sympatric big-scale sand smelt Atherina boyeri co-occurring in shallow coastal ponds with different environmental features. The interplay of the trophic status and geomorphological features of the ponds influenced the availability of resources, in terms of prey diversity and habitat complexity, leading to site-specific mechanisms of trophic niche divergence. Moreover, under oligotrophic conditions, the high habitat complexity and abundances of benthic prey provided different niches that were partitioned by the two species. In contrast, under higher trophic state and lower habitat complexity, an asymmetric competition between the two species might have arisen due to the competitive superiority of mosquitofish, leading to a clear displacement and contraction of the sand smelt niche. At the same time, the broadening of the invader's niche, especially marked for small specimens, may have been driven by a high prey diversity level, consistent with the “resource diversity hypothesis” (MacArthur, 1969). Although a large body of literature exists on the ecological effects of invasive fish in coastal systems, trophic aspects have been scantly addressed especially in combination with environmental stressors. This research gives new insights into the mechanisms that promote the coexistence of invasive and native species in shallow and highly variable marine coastal systems. However, our study is limited by the short temporal context serving as a snapshot of the trophic relationships between invasive and native species under the trophic gradient that occurs during the productive spring season. Given the highly variable nature of coastal ponds, we did not exclude seasonal changes of the trophic relationships of the two fish species according to resource availability, which ensures the success of their long coexistence. Lastly, while we demonstrated the great potential of isotopic niche analysis for detecting complex ecosystem responses to invasion by NIS, additional studies are advocated to further understand the interaction between environmental stressors and fish resource partitioning on a larger temporal scale.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because Samplings were conducted with permits from the Authority of the Laghetti di Marinello Nature Reserve (permit # 28599). No other permits were needed. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
GS, SV conceived, designed, and supervised data integration and interpretation. CA performed the mixing models and isotopic niche analysis. CA, GS, and GC performed data analysis and interpretation. AM, SV funded the study. All authors contributed to the manuscript drafting and revision. All authors read and approved the submitted version.
This study was funded by the University of Palermo and CoNISMa-Marine Strategy.
The authors acknowledge the Director and the Staff of the Nature Reserve Laghetti di Marinello for the permission to work and the logistic support to access the study sites. The authors are also grateful to Andrea Savona for assistance during field activities and Elisa A. Aleo for help with laboratory analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.958467/full#supplementary-material
Abbey-Lee, R. N., Gaiser, E. E., and Trexler, J. C. (2013). Relative roles of dispersal dynamics and competition in determining the isotopic niche breadth of a wetland fish. Freshw. Biol. 58, 780–792. doi: 10.1111/fwb.12084
Abrantes, K. G., Barnett, A., and Bouillon, S. (2014). Stable isotope-based community metrics as a tool to identify patterns in food web structure in east African estuaries. Funct. Ecol. 28, 270–282. doi: 10.1111/1365-2435.12155
Acquavita, A., Aleffi, I. F., Benci, C., Bettoso, N., Crevatin, E., Milani, L., et al. (2015). Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: the marano and grado lagoon (northern Adriatic Sea). Reg. Stud. Mar. Sci. 2, 132–144. doi: 10.1016/j.rsma.2015.08.017
Alcaraz, C., Bisazza, A., and García-Berthou, E. (2008). Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia 155, 205–213. doi: 10.1007/s00442-007-0899-4
Anderson, M. J. (2017). “Permutational multivariate analysis of variance (PERMANOVA),” in ***Wiley StatsRef: Statistics Reference Online (Hoboken, NJ: Wiley), 1–15. doi: 10.1002/9781118445112.stat07841
Andolina, C., Franzoi, P., Jackson, A. L., Mazzola, A., and Vizzini, S. (2020). Vegetated habitats trophically support early development stages of a Marine migrant fish in a coastal lagoon. Estuar. Coasts 43, 424–437. doi: 10.1007/s12237-019-00683-2
Bearhop, S., Adams, C. E., Waldron, S., Fuller, R. A., and Macleod, H. (2004). Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012. doi: 10.1111/j.0021-8790.2004.00861.x
Beisel, J. N., Usseglio-Polatera, P., and Moreteau, J. C. (2000). The spatial heterogeneity of a river bottom: a key factor determining macroinvertebrate communities. Hydrobiologia 422–423, 163–171. doi: 10.1023/A:1017094606335
Blanco, S., Romo, S., and Villena, M. J. (2004). Experimental study on the diet of mosquitofish (Gambusia holbrooki) under different ecological conditions in a shallow lake. Int. Rev. Hydrobiol. 89, 250–262. doi: 10.1002/iroh.200310684
Britton, J. R., Ruiz-Navarro, A., Verreycken, H., and Amat-Trigo, F. (2018). Trophic consequences of introduced species: comparative impacts of increased interspecific vs. intraspecific competitive interactions. Funct. Ecol. 32, 486–495. doi: 10.1111/1365-2435.12978
Byers, J. E. (2000). Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81, 1225–1239. doi: 10.1890/0012-9658(2000)081[1225:CBTESI]2.0.CO;2
Cano-Rocabayera, O., de Sostoa, A., Coll, L., and Maceda-Veiga, A. (2019). Managing small, highly prolific invasive aquatic species: exploring an ecosystem approach for the eastern mosquitofish (Gambusia holbrooki). Sci. Total Environ. 673, 594–604. doi: 10.1016/j.scitotenv.2019.02.460
Carey, M. P., and Wahl, D. H. (2010). Native fish diversity alters the effects of an invasive species on food webs. Ecology 91, 2965–2974. doi: 10.1890/09-1213.1
Carmona-Catot, G., Magellan, K., and García-Berthou, E. (2013). Temperature-specific competition between invasive mosquitofish and an endangered cyprinodontid fish. PLoS ONE 8, e54734. doi: 10.1371/journal.pone.0054734
Chan, F. T., and Briski, E. (2017). An overview of recent research in marine biological invasions. Mar. Biol. 164, 121. doi: 10.1007/s00227-017-3155-4
Chen, G., Wu, Z., Gu, B., Liu, D., Li, X., and Wang, Y. (2011). Isotopic niche overlap of two planktivorous fish in southern China. Limnology 12, 151–155. doi: 10.1007/s10201-010-0332-2
Chrisafi, E., Kaspiris, P., and Katselis, G. (2007). Feeding habits of sand smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). J. Appl. Ichthyol. 23, 209–214. doi: 10.1111/j.1439-0426.2006.00824.x
Costa-Pereira, R., Araújo, M. S., Souza, F. L., and Ingram, T. (2019). Competition and resource breadth shape niche variation and overlap in multiple trophic dimensions. Proc. R. Soc. B Biol. Sci. 286, 1–9. doi: 10.1098/rspb.2019.0369
David, P., Thébault, E., Anneville, O., Duyck, P. F., Chapuis, E., and Loeuille, N. (2017). Impacts of invasive species on food webs: a review of empirical data. Adv. Ecol. Res. 56, 1–60. doi: 10.1016/bs.aecr.2016.10.001
Franco, A., Elliott, M., Franzoi, P., and Torricelli, P. (2008). Life strategies of fishes in European estuaries: the functional guild approach. Mar. Ecol. Prog. Ser. 354, 219–228. doi: 10.3354/meps07203
Gallardo, B., Clavero, M., Sánchez, M. I., and Vilà, M. (2016). Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 22, 151–163. doi: 10.1111/gcb.13004
Gisbert, E., Cardona, L., and Castelló, F. (1996). Resource partitioning among planktivorous fish larvae and fry in a Mediterranean coastal lagoon. Estuar. Coast. Shelf Sci. 43, 723–735. doi: 10.1006/ecss.1996.0099
Hutchinson, G. E. (1957). Concluding remarks, coldspring harbor symposium. Quant. Biol. 22, 415–427. doi: 10.1101/SQB.1957.022.01.039
Jackson, A. L., Inger, R., Parnell, A. C., and Bearhop, S. (2011). Comparing isotopic niche widths among and within communities: SIBER—stable isotope bayesian ellipses. R. J. Anim. Ecol. 80, 595–602. doi: 10.1111/j.1365-2656.2011.01806.x
Jackson, M. C., Donohue, I., Jackson, A. L., Britton, J. R., Harper, D. M., and Grey, J. (2012). Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 7, 1–12. doi: 10.1371/journal.pone.0031757
Kalogianni, E., Giakoumi, S., Andriopoulou, A., and Chatzinikolaou, Y. (2014). Prey utilisation and trophic overlap between the non native mosquitofish and a native fish in two Mediterranean rivers. Mediterr. Mar. Sci. 15, 287–301. doi: 10.12681/mms.609
Kara, M. H., and Quignard, J. P. (2019). Fishes in Lagoons and Estuaries in the Mediterranean 2: Sedentary Fish. Hoboken, NJ: John Wiley and Sons, Inc. doi: 10.1002/9781119452768
Kenworthy, W. J., Durako, M. J., Fatemy, S. M. R., Valavi, H., and Thayer, G. W. (1993). Ecology of seagrasses in northeastern Saudi Arabia 1 year after the Gulf War oil spill. Mar. Pollut. Bull. 27, 213–222. doi: 10.1016/0025-326X(93)90027-H
Layman, C. A., Hammerschlag-peyer, C. M., Yeager, L. A., and Araújo, M. S. (2011). A hypothesis-testing framework for studies investigating ontogenetic niche shifts using stable isotope ratios. PLoS ONE 6, e27104. doi: 10.1371/journal.pone.0027104
Lowe, S., Browne, M., Boudjelas, S., and De Poorter, M. (2000). 100 of the World's worst invasive alien species: a selection from the global invasive species database. Encycl. Biol. Invas. 12, 159. doi: 10.1525/9780520948433-159
MacArthur, R. H. (1969). Patterns of communities in the tropics. Biol. J. Linn. Soc. 1, 19–30. doi: 10.1111/j.1095-8312.1969.tb01809.x
MacDonald, J. I., Tonkin, Z. D., Ramsey, D. S. L., Kaus, A. K., King, A. K., and Crook, D. A. (2012). Do invasive eastern gambusia (Gambusia holbrooki) shape wetland fish assemblage structure in south-eastern Australia? Mar. Freshw. Res. 63, 659–671. doi: 10.1071/MF12019
Mancinelli, G., Guerra, M. T., Alujević, K., Raho, D., Zotti, M., and Vizzini, S. (2017). Trophic flexibility of the Atlantic blue crab Callinectes sapidus in invaded coastal systems of the Apulia region (SE Italy): a stable isotope analysis. Estuar. Coast. Shelf Sci. 198, 421–431. doi: 10.1016/j.ecss.2017.03.013
Mancinelli, G., and Vizzini, S. (2015). Assessing anthropogenic pressures on coastal marine ecosystems using stable CNS isotopes: state of the art, knowledge gaps, and community-scale perspectives. Estuar. Coast. Shelf Sci. 156, 195–204. doi: 10.1016/j.ecss.2014.11.030
Matthews, B., and Mazumder, A. (2004). A critical evaluation of intrapopulation variation of δ13C and isotopic evidence of individual specialization. Oecologia 140, 361–371. doi: 10.1007/s00442-004-1579-2
Mazzola, A., Bergamasco, A., Calvo, S., Caruso, G., Chemello, R., Colombo, F., et al. (2010). Sicilian transitional waters: current status and future development. Chem. Ecol. 26, 267–283. doi: 10.1080/02757541003627704
McCann, K. S., Rasmussen, J. B., and Umbanhowar, J. (2005). The dynamics of spatially coupled food webs. Ecol. Lett. 8, 513–523. doi: 10.1111/j.1461-0248.2005.00742.x
Michener, R. H., and Kaufman, L. (2008). “Stable isotope ratios as tracers in marine food webs: an update,” in Stable Isotopes in Ecology and Environmental Science, 2nd Edn, eds R. Michener, and K. Lajtha (Hoboken, NJ: Blackwell Publishing Ltd), 238–282. doi: 10.1002/9780470691854.ch9
Mizutani, H., and Wada, E. (1988). Nitrogen and carbon isotope ratios in seabird rookeries and their ecological implications. Ecology 69, 340–349. doi: 10.2307/1940432
Nielsen, J. M., Clare, E. L., Hayden, B., Brett, M. T., and Kratina, P. (2018). Diet tracing in ecology: method comparison and selection. Methods Ecol. Evol. 9, 278–291. doi: 10.1111/2041-210X.12869
Occhipinti-Ambrogi, A. (2007). Global change and marine communities: alien species and climate change. Mar. Pollut. Bull. 55, 342–352. doi: 10.1016/j.marpolbul.2006.11.014
Olsson, K., Stenroth, P., Nyström, P., and Granéli, W. (2009). Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshw. Biol. 54, 1731–1740. doi: 10.1111/j.1365-2427.2009.02221.x
Post, D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Pyke, G. H. (2005). A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fish. 15, 339–365. doi: 10.1007/s11160-006-6394-x
R Core Team. (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Rehage, J. S., Barnett, B. K., and Sih, A. (2005). Foraging behaviour and invasiveness: do invasive Gambusia exhibit higher feeding rates and broader diets than their noninvasive relatives? Ecol. Freshw. Fish 14, 352–360. doi: 10.1111/j.1600-0633.2005.00109.x
Remon, J., Bower, D. S., Gaston, T. F., Clulow, J., and Mahony, M. J. (2016). Stable isotope analyses reveal predation on amphibians by a globally invasive fish (Gambusia holbrooki). Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 724–735. doi: 10.1002/aqc.2631
Rincón, P. A., Correas, A. M., Morcillo, F., Risueño, P., Lobón-Cerviá, J., Risueno, P., et al. (2002). Interaction between the introduced eastern mosquitofish and two autochthonous Spanish toothcarps. J. Fish Biol. 61, 1560–1585. doi: 10.1111/j.1095-8649.2002.tb02498.x
Ruiz-Navarro, A., Torralva, M., and Oliva-Paterna, F. J. (2013). Trophic overlap between cohabiting populations of invasive mosquitofish and an endangered toothcarp at changing salinity conditions. Aquat. Biol. 19, 1–11. doi: 10.3354/ab00512
Signa, G., Mazzola, A., Costa, V., and Vizzini, S. (2015). Bottom-up control of macrobenthic communities in a guanotrophic coastal system. PLoS ONE 10, e0117544. doi: 10.1371/journal.pone.0117544
Signa, G., Mazzola, A., Tramati, C. D., and Vizzini, S. (2013a). Gull-derived trace elements trigger small-scale contamination in a remote Mediterranean nature reserve. Mar. Pollut. Bull. 74, 237–243. doi: 10.1016/j.marpolbul.2013.06.051
Signa, G., Mazzola, A., and Vizzini, S. (2012). Effects of a small seagull colony on trophic status and primary production in a Mediterranean coastal system (Marinello ponds, Italy). Estuar. Coast. Shelf Sci. 111, 27–34. doi: 10.1016/j.ecss.2012.06.008
Signa, G., Mazzola, A., and Vizzini, S. (2021). Seabird influence on ecological processes in coastal marine ecosystems: an overlooked role? A critical review. Estuar. Coast. Shelf Sci. 250, 107164. doi: 10.1016/j.ecss.2020.107164
Signa, G., Tramati, C. D., and Vizzini, S. (2013b). Contamination by trace metals and their trophic transfer to the biota in a Mediterranean coastal system affected by gull guano. Mar. Ecol. Prog. Ser. 479, 13–24. doi: 10.3354/meps10210
Specziár, A. (2004). Life history pattern and feeding ecology of the introduced eastern mosquitofish, Gambusia holbrooki, in a thermal spa under temperate climate, of Lake Hévíz, Hungary. Hydrobiologia 522, 249–260. doi: 10.1023/B:HYDR.0000029978.46013.d1
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X. (2018). Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6, e5096. doi: 10.7717/peerj.5096
Stoffels, R. J., and Humphries, P. (2003). Ontogenetic variation in the diurnal food and habitat associations of an endemic and an exotic fish in floodplain ponds: consequences for niche partitioning. Environ. Biol. Fishes 66, 293–305. doi: 10.1023/A:1023918420927
Tran, T. N. Q., Jackson, M. C., Sheath, D., Verreycken, H., and Britton, J. R. (2015). Patterns of trophic niche divergence between invasive and native fishes in wild communities are predictable from mesocosm studies. J. Anim. Ecol. 84, 1071–1080. doi: 10.1111/1365-2656.12360
Vander Zanden, M. J., Casselman, J. M., and Rasmussen, J. B. (1999). Food web consequences of species invasions in lakes. Nature 401, 464–467. doi: 10.1038/46762
Vizzini, S., and Mazzola, A. (2002). Stable carbon and nitrogen ratios in the sand smelt from a Mediterranean coastal area: feeding habits and effect of season and size. J. Fish Biol. 60, 1498–1510. doi: 10.1111/j.1095-8649.2002.tb02443.x
Vizzini, S., and Mazzola, A. (2005). Feeding ecology of the sand smelt Atherina boyeri (Risso 1810) (Osteichthyes, Atherinidae) in the western Mediterranean: evidence for spatial variability based on stable carbon and nitrogen isotopes. Environ. Biol. Fishes 72, 259–266. doi: 10.1007/s10641-004-2586-1
Vizzini, S., Savona, B., Thang, D. C., and Mazzola, A. (2005). Spatial variability of stable carbon and nitrogen isotope ratios in a Mediterranean coastal lagoon. Hydrobiologia 550, 73–82. doi: 10.1007/s10750-005-4364-2
Keywords: biological invasion, alien species, stable isotopes, mosquitofish, sand smelt, coastal ponds
Citation: Andolina C, Signa G, Cilluffo G, Iannucci S, Mazzola A and Vizzini S (2022) Coexisting with the alien: Evidence for environmental control on trophic interactions between a native (Atherina boyeri) and a non-indigenous fish species (Gambusia holbrooki) in a Mediterranean coastal ecosystem. Front. Ecol. Evol. 10:958467. doi: 10.3389/fevo.2022.958467
Received: 31 May 2022; Accepted: 12 August 2022;
Published: 08 September 2022.
Edited by:
Rona A. R. McGill, University of Glasgow, United KingdomReviewed by:
Jessica Rettig, Denison University, United StatesCopyright © 2022 Andolina, Signa, Cilluffo, Iannucci, Mazzola and Vizzini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geraldina Signa, Z2VyYWxkaW5hLnNpZ25hQHVuaXBhLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.