
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 28 July 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.955053
This article is part of the Research TopicEffects of Non-Random Sources of Alteration on Biodiversity and Ecosystem FunctioningView all 19 articles
Damming usually modifies riverine habitats, which affects various aspects of fish diversity, especially in a reservoir cascade. Their influence on fish assemblage has been studied widely, but a lack of data from the diversity perspective remains. The Gezhouba Reservoir and Three Gorges Reservoir are two of the largest cascaded reservoirs located on the upper Yangtze River. In this study, we investigated the current fish assemblages in 2020∼2021 and retrieved 22 previous investigations in different sections of this cascade system to analyze how fish taxonomic, functional, and phylogenetic alpha- and beta-diversity change with the distance from the dams and the impounding age during 1998∼2021, and all sampling sites are located in the upper section of the dams. The total species richness and phylogenetic diversity increased significantly with the distance from the dams, but the functional diversity did not change substantially. No significant difference was found in the influence of impounding age on the three aspects of fish diversity. We observed a noticeable increase in non-indigenous fish species richness, functional diversity, and phylogenetic diversity over time, these effects were similar in areas at different distances from the dams. The species richness and phylogenetic diversity of lotic fish decreased from the lotic to lentic zones, whereas the functional and phylogenetic diversities decreased significantly with impounding age. The taxonomic beta-diversity was remarkably higher than the functional and phylogenetic beta-diversities. The differences among the three facets of beta-diversity were driven by a lower functional turnover than the taxonomic and phylogenetic turnovers, and their nestedness components were low without exception. The present study suggests that trade-offs should be considered when designing policies to protect fish diversity based on different objectives.
Freshwater ecosystems harbor some of the highest levels of fish diversity on earth but are also the most vulnerable (Tedesco et al., 2017). Dam construction is one of the major factors affecting the freshwater fish diversity worldwide, as it changes different aspects of the ecosystem, such as primary production, hydrological dynamics, and riverine connections (Loures and Pompeu, 2019). A new reservoir usually has the highest productivity and supports high fish abundance after its initial impounding due to the rich nutrient and plant material input from the surrounding areas. Subsequently, it enters a “decrease-stable” process with an increase in impounding age (Agostinho et al., 2008). Impoundment can alter the hydrological dynamics and generate a habitat gradient (comprising the lentic, transitional, and lotic habitats) in a reservoir; usually, these habitats have different physical and chemical characteristics and support different fish assemblage structures and diversity (Vašek et al., 2016). For example, the disruption of riverine habitats usually decreases lotic fish diversity due to destruction of their feeding and reproductive environment (Cheng et al., 2015). There is also the opinion that the lotic-lentic stretches are beneficial in maintaining species richness, as the lentic habitat may favor the establishment and dispersal of non-indigenous species (dos Santos et al., 2018). The interruption of longitudinal connectivity of natural rivers after damming often leads to considerable decline in migratory fish abundance due to loss of access to spawning and nursery habitats (Sá-Oliveira et al., 2015). Understanding the spatial and temporal changes in fish diversity after damming have always been a pressing concern for fish resource management, protection, and restoration.
Most previous studies on the effects of damming have considered fish taxonomic diversity (e.g., species richness) due to its simplicity and convenience; however, effective estimation and conservation efforts require a deeper understanding of other facets of fish diversity, such as functional and phylogenetic diversity (Wong et al., 2018; Su et al., 2021; Wang et al., 2021). Functional diversity quantifies the ranges of unique morphological, physiological, and ecological traits of fish community (Palacios-Salgado et al., 2019); phylogenetic diversity reflects the genetic variability within fish assemblage, which can influence the community’s adaptability in response to environmental variations (Lima-Junior et al., 2021). Previous studies revealed that one fish assemblage can exhibit similar variation patterns of taxonomic, functional, and phylogenetic diversities in response to environmental changes (e.g., Tuya et al., 2018), while further studies suggest that multiple approaches can furnish complementary information (e.g., Wong et al., 2018; Roa-Fuentes et al., 2019; Jiang et al., 2021). In addition, the three facets of diversity usually have different sensitivity to environmental variations, among which the functional diversity seems to be the most vulnerable one (Sanchez-Perez et al., 2020; Lin et al., 2021).
In addition to investigating multiple facets of fish diversity within different fish communities (alpha-diversity), quantifying the spatial and temporal variations across fish community compositions (beta-diversity) have become another important topic of investigation (Anderson et al., 2011). Beta-diversity evaluates dissimilarity among the communities directly, and it is being used more frequently in evaluating the geographic and temporal changes in fish diversity (Villéger et al., 2013; Li et al., 2018; Nakamura et al., 2020). Beta-diversity can be further divided into turnover and nestedness components (Baselga, 2010). The turnover component reflects the taxonomic, functional, and phylogenetic replacement among the communities, whereas the nestedness component implies the difference in taxonomic, functional, and phylogenetic quantity (Villéger et al., 2013). Quantifying the β-diversity of fish assemblages with different components can provide a comprehensive assessment for assembly mechanisms, if we consider the taxonomic, functional, and phylogenetic dimensions (Li et al., 2021). For example, fish communities with a high taxonomic beta-diversity may have low functional or phylogenetic beta-diversities if their respective species are functionally or phylogenetically similar (Jiang et al., 2021). High beta-diversity can result either from a low proportion of shared species (or function, phylogeny) among communities with similar species (or function, phylogeny) quantity, or from a different quantity of species (or function, phylogeny) among communities (Villéger et al., 2013). It is, therefore, crucial to understand the relationship between alpha- and beta-diversities as well as their different components to investigate the ecological processes structuring fish assemblages.
The Yangtze River is the world’s third largest river and its mainstem can be divided into the lower (Shanghai to Hukou, 938 km), middle (Hukou to Yichang, 955 km), and upper reaches (Yichang to headstream, 4,504 km; Xiong et al., 2021). The upper reach has an abundant level of hydropower resource and has the largest cascading reservoir system in China. The Three Gorges Reservoir (TGR) and Gezhouba Reservoir (GZB), both located in the Yichang, Hubei province, are two large reservoirs [i.e., height > 15 m or height 5–15 m and impounding > 3 million m3; International Commission on Large Dams [ICOLD], 2019], forming the lowest part of the reservoir cascade. These reservoirs impound approximately 700 km long stretch of the Yangtze River, and their impact on fish has been extensively investigated (Xu et al., 2021). For example, several researchers evaluated the spatial and temporal changes of the fish assemblage structure (Wu et al., 2007; Gao et al., 2010; Liu et al., 2012; Yang et al., 2012; Zhao et al., 2015; Lin et al., 2019), biomass (Liao et al., 2018), life-history strategies (Perera et al., 2014; Liao et al., 2019), and spawning conditions (Yu et al., 2019; Ma et al., 2020). In contrast, only a few studies have focused on these changes from a fish diversity perspective (e.g., Zhang et al., 2020). How facets of fish biodiversity change at different dimensions, the underlying causes and factors, and the spatial and temporal patterns of these alterations have not been studied in detail.
In this study, we mainly aim to assess the spatial and temporal variations of fish diversity in relation to the construction of the Three Gorges Dam and Gezhouba Dam and to provide scientific rationale for fish resource management and protection. To achieve these objectives, we investigated the current fish assemblages in 2020∼2021 and simultaneously retrieved previous investigations documenting species composition, and formed a total of 27 datasets to evaluate the taxonomic, functional, and phylogenetic alpha- and beta-diversities of fish assemblages across the GZB and TGR. We expected different change patterns over locations and impounding ages among different facets of fish diversity, and we hypothesized that taxonomic diversity decreased less with impounding age, compared to functional and phylogenetic diversities.
The Three Gorges Reservoir is one of the largest reservoirs in the world. It was impounded through three stages, and its final impoundment formed a reservoir of 1,080 km2 with a total length of 667 km in 2009. The Gezhouba Reservoir (GZB), located at about 60 km downstream of the Three Gorges Reservoir (TGR), was constructed in 1988 and formed a reservoir with a total length of 40 km. We based the current study at nine mainstem sites (GZB, Zigui, Wushan, Yunyang, Wanzhou, Zhongxian, Fuling, Banan, and Jiangjin) and three tributary sites (Xiakou, Shuanglong, and Gaoyang) along the two reservoirs. These sampling sites are located in the upper section of the Three Gorges Dam and Gezhouba Dam with different distance from the dam bodies, which represent diverse habitat characteristics (lentic, transitional, and lotic), and were affected by the impoundment of the TGR or GZB at different periods (Table 1 and Figure 1). The upper reach of the Yangtze River runs from the “Three Gorges” (i.e., the TGR and GZB), which refers to reaches between Chongqing and Yichang (Figure 1). All 12 sampling sites were located in these areas, which showed riverine condition and have spatially similar fish assemblage structures before the dam construction. But the background fish assemblage structures were quite different between the upper and middle-lower reaches [Investigation Group of Fishery [IGF], 1975; Fan et al., 2012], so we only sampled fish assemblages above dams.

Table 1. Summary information of data sources, including their sampling sites, sampling period, and respective baselines.
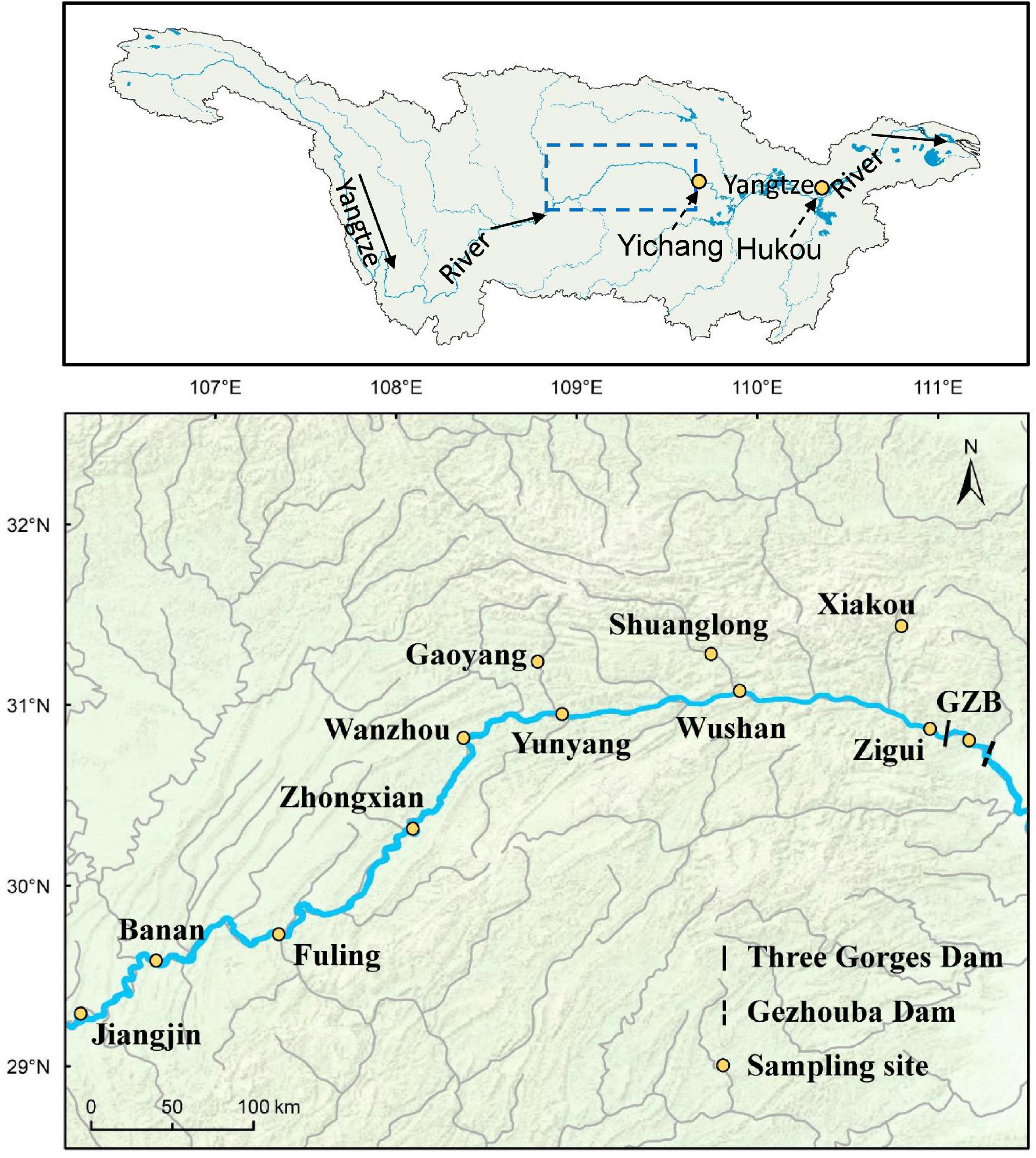
Figure 1. The map above shows the whole Yangtze River and its two demarcation points (Yichang and Hukou) and the study areas; the map below exhibits the Three Gorges Reservoir and Gezhouba Reservoir and all sampling sites. Solid arrows on the top panel represent the flow direction of the Yangtze River.
From April 2020 to July 2021, we sampled fishes using experimental multi-panel gillnets and benthic fyke nets (12 m in length, 0.75 mm, knot to knot) at five sampling sites (Zigui, Xiakou, Wushan, Yunyang, and Zhongxian) of the TGR. To cover the benthic and pelagic water, we used two types of multi-panel gillnets, benthic (2 m in height) and pelagic (5 m in height), having the same length and mesh-size structures, i.e., the total length of each gillnet was 30 m and consisted of 12 different mesh-sizes (10, 16, 20, 25, 31, 39, 48, 58, 70, 86, 110, 125 mm, knot-to-knot). At each sampling site, we randomly selected three locations (≈ 500 m apart) and deployed three benthic gillnets, three pelagic gillnets, and three benthic fyke nets for 12 h (18:00–19:00 to 6:00–7:00) per location. We sampled each site for 2 days per season to increase our sample size.
We also retrieved historical fish presence/absence data from a variety of sources and as many as possible, including published articles, books, and scientific reports; and we recorded the present/absent fish as 1/0, respectively. To evaluate fish diversity in each period and section accurately, we discarded those sources that lack a detailed fish list. Given that the fish species documented in previous studies were based on different classified standards, we checked the Latin names of all species in FishBase dataset and updated them with the currently valid scientific names; and we adjusted the species composition data frame accordingly. We discarded those fish species that were recorded only once, and our final dataset included 27 datasets and 140 fish species.
Previous investigations along the cascade system of the TGR and GZB were conducted during different periods before and after their constructions. To evaluate the response of fish diversity to the filling process, we defined a pristine baseline related to the condition prior to the respective impoundment per sampling site (Table 1). The GZB was first impounded in 1988, followed by the first (2003), second (2006), and third impoundments (2009) of the TGR, which raised the water level to 145 m, 156 m, and 175 m, respectively. The three fillings of the TGR generated respective reservoir areas reaching Fuling, Changshou, and Jiangjin sections. Therefore, we considered 1988 as the baseline for the GZB site, 2003 for the sampling sites between the Three Gorges Dam and Fuling section, 2006 for the sampling sites between Fuling and Changshou sections, and 2009 for the sampling sites between Changshou and Jiangjin sections. These baselines should be appropriate to assess changes in fish assemblages with the impoundments of the TGR and GZB. The impounding age was calculated by the difference in sampling year from the baseline respectively. Simultaneously, aiming to estimate the temporal and spatial changes of beta-diversity, we defined the fish composition of the lotic Banan section, investigated in 1998, as a consistent baseline. This is mainly because Banan is located at the tail of the TGR with a relatively high species richness, which has not yet been affected by the TGR impoundments and maintained its natural state in 1998 (Luo, 1999; Yang et al., 2012; Liao et al., 2018). Lotic and non-indigenous fishes received much attention in previous studies, and we specially selected these fish faunas to analyze their diversity changes (Ba and Chen, 2012; Xiao et al., 2015).
The taxonomic diversity was assessed by species richness (SR), which was defined as the total number of fish species per sampling. We selected eleven functional traits representing the major trait categories of body size, feeding, migration, reproduction, and habitat preference (Supplementary Table 1). We collected the functional trait data from FishBase, published literatures, books, and our measurements. In the cases where a small number of species lacked published/unpublished data and preserved specimens, we replaced their functional data with that of their congeneric species. We used functional richness (FRic; Villéger et al., 2008) and functional dispersion (FDis; Laliberté and Legendre, 2010) to quantify functional diversity of each fish assemblage. We were also interested in and studied these diversity indices of non-indigenous, lotic, and lentic fish faunas.
We used the most common cytochrome b (Cytb) sequence to construct phylogenetic relationships among the 140 fish species that were studied. The FASTA-formatted sequence data were collected from the National Center for Biotechnology Information (NCBI). We imported the sequence data to MEGA software (version 7.0) and used Neighbor-Joining method to build a phylogenetic tree that was used to measure the phylogenetic diversities. We selected Faith’s Phylogenetic Diversity (PD; Faith, 1992) and Phylogenetic Species Variability (PSV; Helmus et al., 2007) to quantify phylogenetic diversity of each fish assemblage. More specifically, the FRic and PD represent absolute diversities and are thought to be related to species richness, whereas the FDis and PSV represent diversity dispersion and are statistically independent of species richness. These indexes are robust metrics reflecting the functional and phylogenetic diversities (Kellar et al., 2015; Rurangwa et al., 2021).
Based on the aforementioned “site × species,” “species × traits,” and “species × phylogenetic” datasets, we measured taxonomic, functional, and phylogenetic beta-diversities (βSPE, βFUN, and βPHY; Villéger et al., 2013). These indices range from 0 to 1 and the higher values indicate greater dissimilarity between samplings. We measured the βSPE, βFUN, and βPHY based on the Jaccard dissimilarity index, and divided each index into its respective turnover component and nestedness component (Baselga, 2010; Villéger et al., 2013). The three facets of beta-diversity and their components can be compared to each other, because their decomposition is based on the same metrics, namely shared and non-shared richness (Villéger et al., 2013).
The spatial and temporal comparisons in diversity indexes were assessed using linear mixed-effects models (LMM, Bates et al., 2015). The indexes of alpha- and beta-diversity were modeled as a function of impounding age, distance from the dam, and their interactions. As no significant interactions were found, we adjusted models by removing interactive terms. We included sampling time and sampling site as a random factor to account for the lack of independence between multiple sampling events conducted at the same site and during the same year (Bates et al., 2015). We also focused specifically on the taxonomic, functional, and phylogenetic diversities of non-indigenous and lotic fish faunas and followed the same LMM analysis process (model habitat and age) as we did for overall α-diversity. Model assumptions were verified and indicated no problems regarding the linearity, normality, and homogeneity of variances (Jacqmin-Gadda et al., 2007). We conducted all analyses in R (v.4.0.2, R Foundation for Statistical Computing, Vienna, Austria), using dbFD function in package FD (Laliberté et al., 2014), pd and psv functions in package picante (Kembel et al., 2020), beta.pair, functional.beta.pair, and phylo.beta.pair functions in package betapart (Baselga and Orme, 2012), check_heteroscedasticity function in package performance (Lüdecke et al., 2021), and lmer function in package lme4 (Bates et al., 2015). Figures were created using package ggplot2 (Wickham, 2016).
A total of 140 species belonging to 10 orders, 23 families, and 88 genera were documented across the GZB and the TGR, from 1998 to 2021. Cypriniformes containing four families and 99 species was the most speciose order, followed by Siluriformes (five families, 19 species). The family Cyprinidae had the most species (77 species), followed by Cobitidae (14 species), and Bagridae (12 species). Among these species, a total of 11 species were recorded at each investigation: Clenopharyngodon idellus, Aristichthys nobilis, Cyprinus carpio, Carassius auratus, Hemiculter leucisculus, Hemiculter bleekeri, Coreius heterodon, Squalidus argentatus, Saurogobio dabryi, Pelteobagrus nitidus, and Silurus asotus; a total of 13 non-indigenous fish species and 56 lotic species were documented.
We found that values of the SR, FRic, and PD decreased with increasing impounding ages, and increased with distance from the dam, but only the influences of distance from the dam on the SR and PD were statistically significant (LMM, p < 0.01; Figure 2 and Table 2). Values of the FDis and PSV changed in a different pattern, i.e., their values increased with impounding ages, but decreased with either decreased or increased distance from the dam, but only the influence of distance on the FDis was significant (p < 0.05; Figure 2 and Table 2). The effects from the interaction of distance and impounding age were non-significant for any index (p > 0.05).
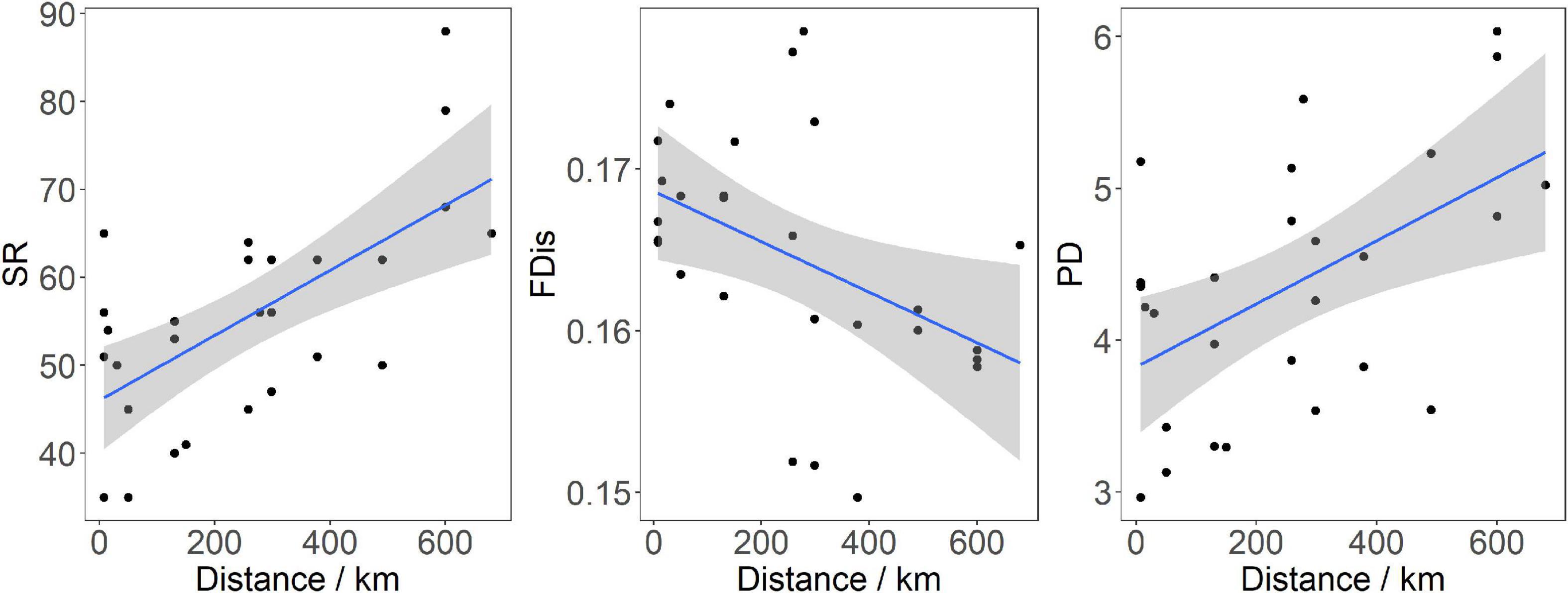
Figure 2. The effect of distance from the dam on species richness (SR), functional dispersion (FDis), and phylogenetic diversity (PD) of fish assemblages of the Three Gorges Reservoir and Gezhouba Reservoir. The gray band represents the 95% confidence interval.
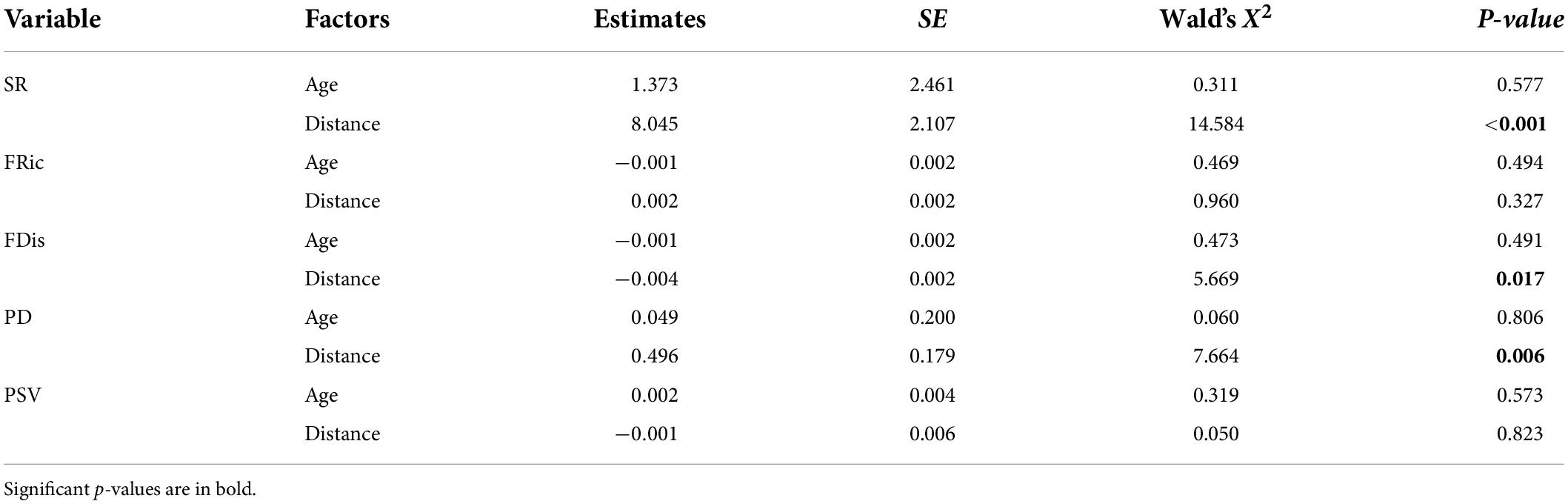
Table 2. Summary of linear mixed-effect models to explain variations in taxonomic, functional, and phylogenetic alpha-diversities as a function of impounding age and distance from the dam.
The alpha-diversities of non-indigenous, lotic, and lentic fish were influenced by damming in different spatial and temporal patterns. Specifically, the SR, FRic, PD, and FDis of the non-indigenous fish increased significantly with impounding ages, except for the PSV, whereas these indexes were not significantly affected neither by the distance from the dam nor by the interactions between age and distance (Table 3). The average species number of non-indigenous fish investigated by 27 studies was 4.3.
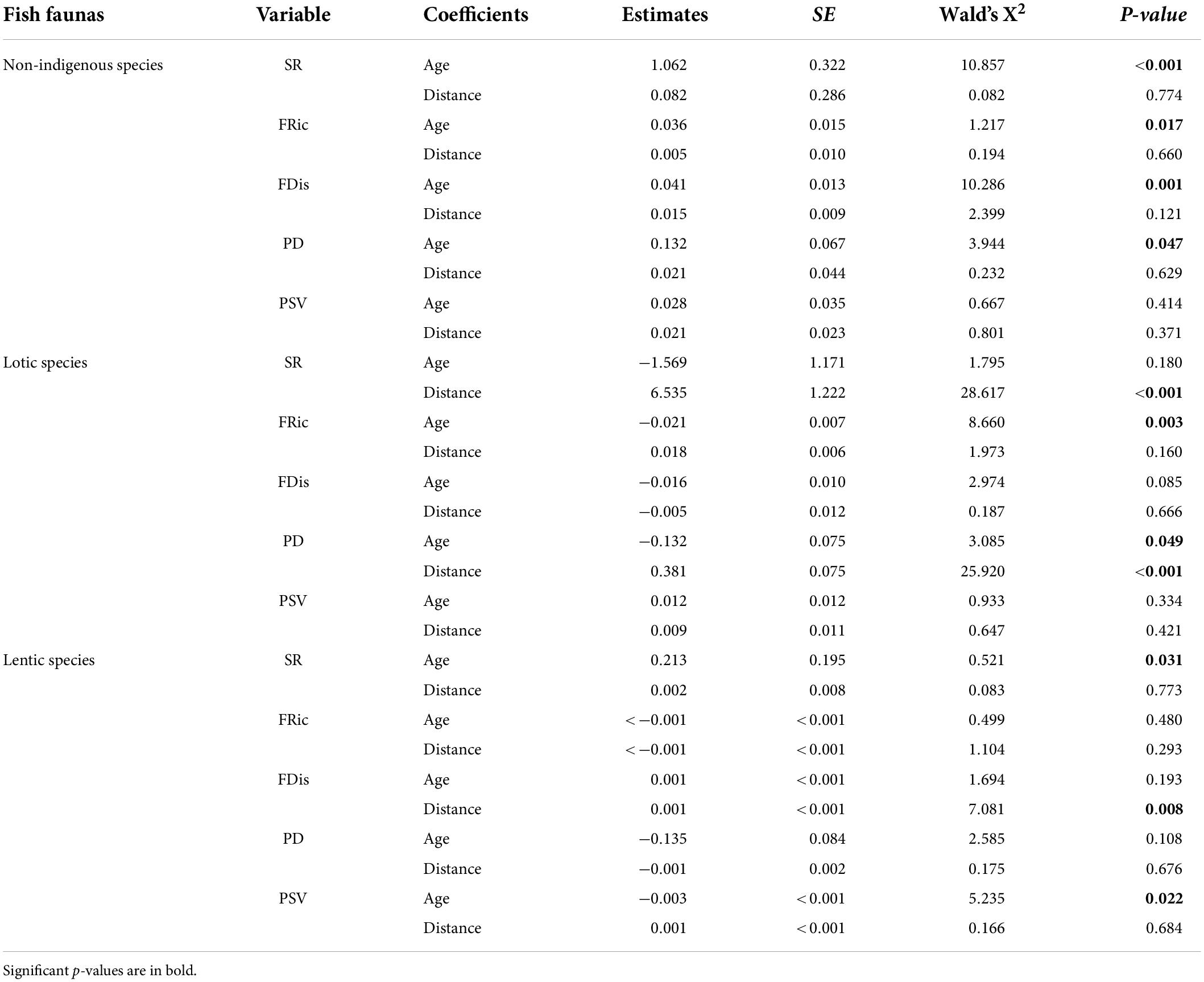
Table 3. Summary of linear mixed-effect models to explain variations in taxonomic, functional, and phylogenetic alpha-diversities of non-indigenous, lotic, and lentic fish species, as a function of impounding age and distance from the dam.
While analyzing lotic species, we observed a significant increase in the SR and PD with increasing distance from the dam, where the FRic was not significantly affected by the distance but was negatively affected by the impounding age. We did not find a significant influence from impounding ages nor a statistical interaction between impounding age and distance for the FDis and PSV. As for lentic species, their SR values were significantly increased with impounding age but were spatially similar. From functional and phylogenetic perspectives, only FDis was positively influenced by distance significantly, and PSV was negatively affected by the impounding age (Table 3).
Mean value of taxonomic beta-diversity (βSPE: 0.59 ± 0.12) was significantly higher than those of functional beta-diversity (βFUN: 0.16 ± 0.03) and phylogenetic beta-diversity (βPHY: 0.38 ± 0.07; ANOVA, F = 212, p < 0.01); and the average βPHY value was higher than that of the βFun (Tukey’s HSD test, p < 0.01). Values of the βSPE, βFUN, and βPHY increased with increasing impounding ages, but only the increase in βFUN was significant (Figure 3 and Table 4). As for spatial variations, over distance from the dam, the decreases in beta-diversities occur in a similar way for the βSPE, βFUN, and βPHY, but the decrease in βFUN was non-significant (LMM, main effect of distance, X2 = 0.05, p = 0.82; Figure 3 and Table 4).
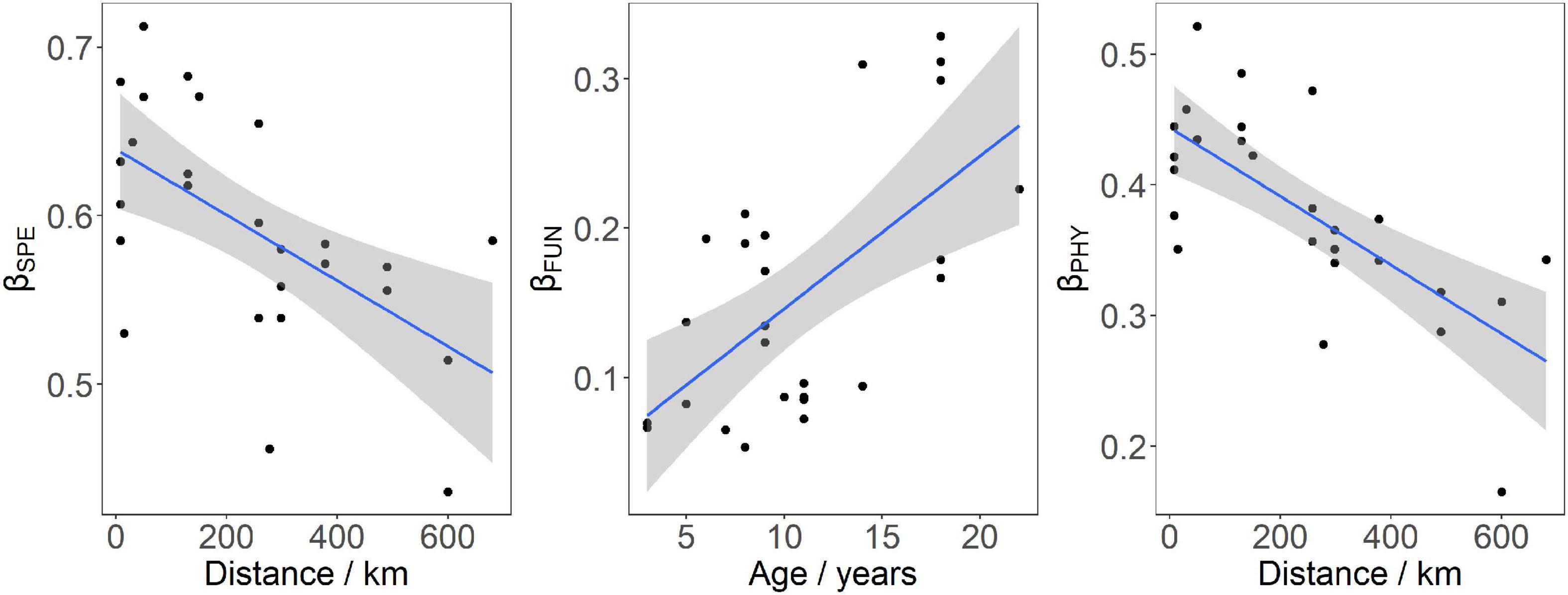
Figure 3. The effect of distance from the dam on taxonomic (βSPE) and phylogenetic beta-diversities (βPHY), and the effect of impounding age on functional beta-diversity (βFUN) of the Three Gorges Reservoir and Gezhouba Reservoir. The gray band represents the 95% confidence interval.
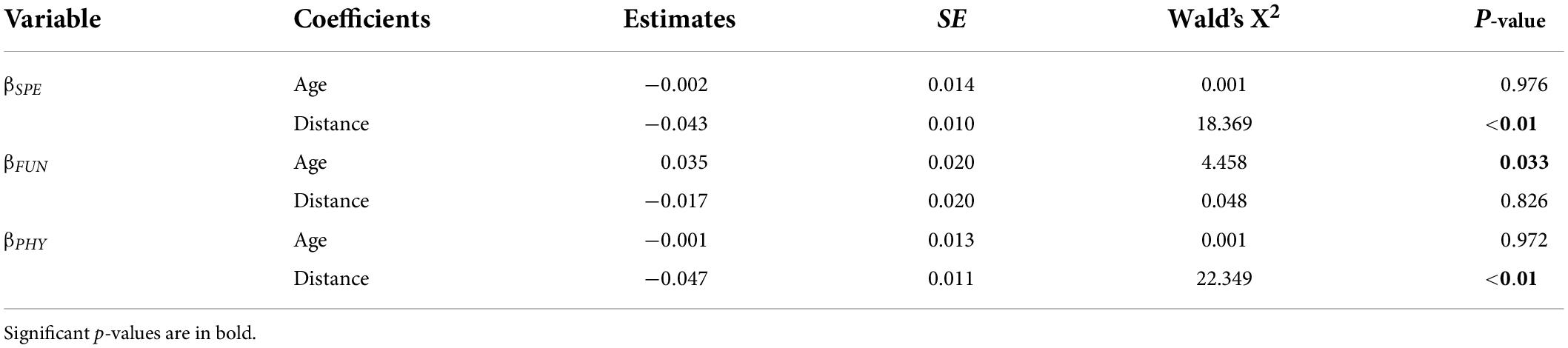
Table 4. Summary of linear mixed-effect models to explain variations in taxonomic, functional, and phylogenetic beta-diversities as a function of impounding age and distance from the dam.
The nestedness component values were similar among the βSPE (0.09), βFUN (0.10), and βPHY (0.06; Kruskal–Wallis test, X2 = 2.68, p = 0.26); whereas turnover component values of the βSPE (0.50) were significantly higher than those of the βFUN (0.06) and βPHY (0.32; X2 = 66.80, P < 0.01; and βFUN vs. βPHY: p < 0.01). The turnover component dominated the βSPE and βPHY, which accounted for 84.75% and 84.21%, respectively. The βFUN was dominated by the nestedness component, which accounted for 62.50%.
Dam construction, coupled with related hydrologic alteration and biological invasions, represents the greatest threat to fish diversities in rivers (Vega-Retter et al., 2020). In general, fish species richness usually decreases once dams are constructed and impounded (e.g., Lima et al., 2016), and this influence is the most serious in lentic habitats and can continue for years at some reservoirs (e.g., Araguari reservoir cascade system, Loures and Pompeu, 2018). Previous studies mainly focused on the response in fish assemblage structures in GZB and TGR and revealed both their rapid (e.g., Gao et al., 2010) and long-term variations (e.g., Liao et al., 2018) after dam construction. Most studies investigating fish alpha-diversity, conducted at different periods and sections, suggested that the fish species richness usually decreased after their constructions (Wu et al., 2007; Yang et al., 2012; Liao et al., 2018; Lin et al., 2019). Here, we pooled 27 investigations and found out that the spatial and temporal differences of the SR were driven mainly by the spatial factor (distance from the dam), and the SR increased from the lentic to lotic zones of the reservoir. This finding demonstrated that the degree of habitat alteration was the main factor affecting fish species richness (Lima et al., 2016).
Damming usually negatively affects species richness through disrupting migration routes and altering habitat, and such influence can last for years, either in the upper stream of the Yangtze River (Yang et al., 2012; Liao et al., 2018) or other reservoir ecosystems (e.g., Loures and Pompeu, 2018, 2019). In the present study, we found some differences, i.e., that the influence of impounding on the SR was non-significant over time. We added functional and phylogenetic diversities as supplements and found consistent results; like the SR, the FRic, PD, FDis, and PSV also did not significantly change with the impounding age. This consistent pattern indicated that fish alpha-diversity gradually turned into a stable level after the dam construction and impoundment, especially at the scale of the entire reservoir. In addition, we found that all of the SR, FRic, and PD of the non-indigenous fish species increased with impounding years, suggesting that non-native fish species more or less offset the richness losses of other species (Liew et al., 2016; Turgeon et al., 2019).
The creation of reservoirs favors the establishment of non-indigenous fish populations, adding threats to native freshwater fish species (Johnson et al., 2008; Casimiro et al., 2017). Although some researchers proposed that dams can hinder fish spreading upward or downward into new water areas (Dana et al., 2011), the dam-driven hydrological alterations and catchments connection can often facilitate fish invasion (Casimiro et al., 2017; Kerr et al., 2021). The consistent increase in taxonomic, functional, and phylogenetic alpha-diversity indices of non-indigenous species with the impounding age confirmed that the construction of the GZB and TGR promoted fish invasion and increased non-indigenous fish alpha-diversity. A reservoir cascade along the Araguari River Basin exhibits a similar phenomenon; the non-native richness increased with reservoir age, and these effects were similar in both lotic and lentic habitats (Loures and Pompeu, 2019). Turgeon et al. (2019) concluded that non-indigenous fish species usually increased after damming in tropical and temperate river ecosystems. Artificial reservoirs provided “stepping-stone” habitats for the fish invasion (Johnson et al., 2008) and provided vacant niches that facilitated non-indigenous fishes colonizing and expanding their populations therein (Turgeon et al., 2019). In 2020∼2021, we sampled a total of ten non-indigenous fish species at five sampling sites, where we investigated five to seven indigenous species per sampling site, suggesting that dam provides an initial blockage and later facilitation of non-indigenous fish.
Damming usually modifies physical and chemical characteristics of rivers, such as flow dynamics, water velocity, water depth, water temperature, and channel geomorphology, and these variations form a longitudinal gradient based on distance from the dam (Vašek et al., 2016). In the present study, we found different responses in taxonomic, functional, and phylogenetic diversities to the longitudinal habitats among different fish faunas. The tail section of the TGR still exhibits lotic habitats and was reported to maintain a higher level of fish species richness (Liao et al., 2018). Our results demonstrated that the SR and PD of total fish assemblages or lotic fish faunas increased with distance from the dam, indicating that the remaining lotic habitats play a crucial role in maintaining species richness and genetic diversity (Loures and Pompeu, 2019). However, the functional diversity did not exhibit spatial gradient for fish assemblages or lotic fish faunas, which indicated that functional diversity seems to be more vulnerable to damming. Lin et al. (2021) also found that congruence is hard to achieve among the three facets of fish diversity, among which functional diversity was the most vulnerable to damming. Sanchez-Perez et al. (2020) also observed an increase in species richness but a decrease in functional richness in the Segura River, southern Spain. These patterns may be due to the functional traits being filtered more seriously by multiple abiotic and biotic factors (Arantes et al., 2019). However, the FRic of non-indigenous fish, consistent with the SR and PD, was non-significantly affected by the distance from the dam, indicating that the impoundment promoted non-indigenous species rapidly spreading to the whole reservoirs by connecting mainstem and distributaries (Júlio Júnior et al., 2009). Therefore, trade-offs should be considered when protecting fish diversity based on multiple facets (Doxa et al., 2020).
Understanding how fish taxonomic, functional, and phylogenetic composition varies spatially and temporally through beta-diversity is crucial (Frota et al., 2021). In the present study, we found that the three-facets of beta-diversity have different values and exhibited different spatial and temporal patterns. Firstly, the taxonomic and phylogenetic beta-diversities decreased significantly with distance from the dam and maintained stability with impounding ages, which further indicated that the taxonomic and phylogenetic structures were altered more severely in lentic habitats compared with the upper lotic habitats of the TGR and that the lotic habitats are not only crucial in conservations of both species and genetic diversities, but also in maintaining of their heterogeneity (Zhang et al., 2018). The functional beta-diversity was lower than taxonomic and phylogenetic beta-diversities, indicating that the current functional structure of fish assemblages is more similar to those during the pre-damming period compared to taxonomic and phylogenetic aspects. Moreover, unlike the taxonomic and phylogenetic beta-diversities, the functional beta-diversity was spatially similar but increased significantly with impounding ages. Some studies proposed that environmental filters and species invasion due to the habitat alteration and elimination of natural barriers contributed the most to the functional homogeneity (e.g., Vitule et al., 2012; Toussaint et al., 2018). Considering these patterns together, we deduced that species introduction and lotic/migratory function simplification should be considered as key factors explaining temporal functional homogeneity in these ecosystems (Santos and Araujo, 2015; Zhang et al., 2018).
Quantifying the multiple components of the three-faceted beta-diversity can further analyze this viewpoint (Villéger et al., 2013). In the present study, the differences among the three-facets of beta-diversity were driven by a lower functional turnover compared to the taxonomic and phylogenetic turnover, while their nestedness components were low without exception, indicating a higher species (phylogeny) replacement between fish assemblages of pre-damming and post-damming periods (Soininen et al., 2018). Damming caused a series of environmental alterations, such as water velocity, food availability, water level fluctuation, and flow dynamics; and these alterations usually caused species replacement, i.e., some sensitive fish species were replaced by eurytopic species and non-indigenous species (Frota et al., 2021). On the other hand, low functional beta-diversity revealed that the frequent species replacements in fish assemblages occurred mainly between fish species that were functionally redundant (Villéger et al., 2013), which also suggested that the GZB and TGR ecosystems may still retain some habitats that determined functional traits and diversities of fish assemblages. Ecologists advise that, for areas where turnover dominates beta diversity, we should protect a larger number of areas to conserve regional diversity; in contrast, we should specially protect one large area with high alpha diversity when nestedness dominates the overall beta diversity (Jiang et al., 2021). From these perspectives, we should protect not only lotic habitats in the tail section of this ecosystem, but also other small habitats in the mainstem and tributaries.
In this study, we analyzed the spatial and temporal changes of the taxonomic, functional, and phylogenetic facets of alpha- and beta-diversities of the GZB and TGR fish communities. We demonstrated that multiple diversity dimensions can add comprehensive information. The findings reveal that even three decades after GZB construction and 12 years after TGR construction, the longitudinal location still plays key role in variations of taxonomic and phylogenetic alpha diversities. With increasing impounding years, taxonomic and phylogenetic alpha diversities gradually became stable, due to the increased diversity of the non-indigenous species. We proposed that the impoundments of the reservoir cascades facilitated expansion of non-indigenous fish. In comparison, the functional diversity did not exhibit obvious spatial differences for overall fish assemblages or lotic fish faunas. Functional diversity seemed to be more vulnerable to damming. Our results regarding beta-diversity and its components also exhibited spatial and temporal patterns, reminding us that trade-offs should be considered when designing policies to protect fish diversity based on different objectives.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Ethics Committee of the Institute of Hydrobiology, Chinese Academy of Sciences. And all experimental methods were performed following the guidelines for the care and use of experimental animals of China (GB/T35892 2018).
CL: investigation, data analysis, writing original draft, and funding acquisition. JW: investigation, resources, and data curation. SY: methodology, resources, and writing—review and editing. WL: methodology and writing—review and editing. SC and TZ: writing—review and editing. JL: writing—review and editing and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was funded by the National Key Research and Development Program of China (2020YFD0900500), the National Natural Science Foundation of China (32102798), the China Postdoctoral Science Foundation (2020M672448), and the Earmarked Fund for China Agriculture Research System (CARS-45). SC was supported by the Forest and Wildlife Research Center of Mississippi State University, United States (MISZ-081700).
We would like to thank the convenience provided by the Agricultural Comprehensive Law Enforcement Unit of Zigui, Xingshan, Wushan, and Yunyang during data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.955053/full#supplementary-material
Agostinho, A. A., Pelicice, F. M., and Gomes, L. C. (2008). Dams and the fish fauna of the neotropical region: impacts and management related to diversity and fisheries. Braz. J. Biol. 68, 1119–1132. doi: 10.1590/S1519-69842008000500019
Anderson, M. J., Crist, T. O., Chase, J. M., Vellend, M., Inouye, B. D., Freestone, A. L., et al. (2011). Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28. doi: 10.1111/j.1461-0248.2010.01552.x
Arantes, C. C., Fitzgerald, D. B., Hoeinghaus, D. J., and Winemiller, K. O. (2019). Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Curr. Opin. Environ. Sustain. 37, 28–40. doi: 10.1016/j.cosust.2019.04.009
Ba, J. W., and Chen, D. Q. (2012). Invasive fishes in three gorges reservoir area and preliminary study on effects of fish invasion owing to impoundment. J. Lake Sci. 24, 185–189. doi: 10.18307/2012.0203
Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Conserv. 19, 134–143. doi: 10.1111/j.1466-8238.2009.00490.x
Baselga, A., and Orme, C. D. L. (2012). betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812. doi: 10.1111/j.2041-210X.2012.00224.x
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Casimiro, A. C. R., Garcia, D. A. Z., Costa, A. D. A., Britton, J. R., and Orsi, M. L. (2017). Impoundments facilitate a biological invasion: dispersal and establishment of non-native armoured catfish Loricariichthys platymetopon (Isbrückler & Nijssen, 1979) in a Neotropical river. Limnologica 62, 34–37. doi: 10.1016/j.limno.2016.11.001
Cheng, F., Li, W., Castello, L., Murphy, B. R., and Xie, S. G. (2015). Potential effects of dam cascade on fish: lessons from the Yangtze River. Rev. Fish Biol. Fisher. 25, 569–585. doi: 10.1007/s11160-015-9395-9
Dana, E. D., García-de-Lomas, J., González, R., and Ortega, F. (2011). Effectiveness of dam construction to contain the invasive crayfish Procambarus clarkii in a Mediterranean mountain stream. Ecol. Eng. 37, 1607–1613. doi: 10.1016/j.ecoleng.2011.06.014
dos Santos, D. A., Hoeinghaus, D. J., and Gomes, L. C. (2018). Spatial scales and the invasion paradox: a test using fish assemblages in a Neotropical floodplain. Hydrobiologia 817, 121–131. doi: 10.1007/s10750-018-3531-1
Doxa, A., Devictor, V., Baumel, A., Pavon, D., Medail, F., and Leriche, A. (2020). Beyond taxonomic diversity: revealing spatial mismatches in phylogenetic and functional diversity facets in Mediterranean tree communities in southern France. For. Ecol. Manage. 474:118318. doi: 10.1016/j.foreco.2020.118318
Faith, D. P. (1992). Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. doi: 10.1016/0006-3207(92)91201-3
Fan, Z. H., Ba, J. W., and Duan, X. B. (2012). Studies on fish resources and species diversity in the middle reaches of the Yangtze River from Yichang to Chenglingji section. Freshw. Fish 42, 20–25. doi: 10.3969/j.issn.1000-6907.2012.04.004
Frota, A., Ganassin, M. J., Pacifico, R., Gomes, L. C., and da Graça, W. J. (2021). Spatial distribution patterns and predictors of fish beta-diversity in a large dam-free tributary from a Neotropical floodplain. Ecohydrology 15:e2376. doi: 10.1002/eco.2376
Gao, X., Zeng, Y., Wang, J. W., and Liu, H. Z. (2010). Immediate impacts of the second impoundment on fish communities in the Three Gorges Reservoir. Environ. Biol. Fish. 87, 163–173. doi: 10.1007/s10641-009-9577-1
Helmus, M. R., Bland, T. J., Williams, C. K., and Ives, A. R. (2007). Phylogenetic measures of biodiversity. Am. Nat. 169, E68–E83. doi: 10.1086/511334
International Commission on Large Dams [ICOLD] (2019). Number of Dams by Country Members. Paris: International Commission on Large Dams.
Investigation Group of Fishery [IGF] (1975). Resource of the Yangtze River in Sichuan Province. Investigation Report of Fishery Resource in Main Stream of Yangtze River. Chengdu: Investigation Group of Fishery, 1–22.
Jacqmin-Gadda, H., Sibillot, S., Proust, C., Molina, J. M., and Thiébaut, R. (2007). Robustness of the linear mixed model to misspecified error distribution. Comput. Stat. Data Anal. 51, 5142–5154. doi: 10.1016/j.csda.2006.05.021
Jiang, X. M., Zheng, P., Cao, L., and Pan, B. Z. (2021). Effects of long-term floodplain disconnection on multiple facets of lake fish biodiversity: decline of alpha diversity leads to a regional differentiation through time. Sci. Total Environ. 763:144177. doi: 10.1016/j.scitotenv.2020.144177
Johnson, P. T., Olden, J. D., and Vander Zanden, M. J. (2008). Dam invaders: impoundments facilitate biological invasions into freshwaters. Front. Ecol. Environ. 6, 357–363. doi: 10.1890/070156
Júlio Júnior, H. F., Tós, C. D., Agostinho, Â. A., and Pavanelli, C. S. (2009). A massive invasion of fish species after eliminating a natural barrier in the upper Rio Paraná basin. Neotrop. Ichthyol. 7, 709–718. doi: 10.1590/S1679-62252009000400021
Kellar, P. R., Ahrendsen, D. L., Aust, S. K., Jones, A. R., and Pires, J. C. (2015). Biodiversity comparison among phylogenetic diversity metrics and between three North American prairies. Appl. Plant Sci. 3:1400108. doi: 10.3732/apps.1400108
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2020). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Kerr, J. R., Vowles, A. S., Crabb, M. C., and Kemp, P. S. (2021). Selective fish passage: restoring habitat connectivity without facilitating the spread of a non-native species. J. Environ. Manage. 279:110908. doi: 10.1016/j.jenvman.2020.110908
Laliberté, E., and Legendre, P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. doi: 10.1890/08-2244.1
Laliberté, E., Legendre, P., and Shipley, B. (2014). Package ‘FD’: Measuring Functional Diversity from Multiple Traits, and other Tools for Functional Ecology. Available Online at: https://CRAN.R-project.org/package=FD (accessed May 02, 2022).
Li, B., Xu, D. D., Wang, Z. J., Yue, X. J., and Zhang, Y. G. (2013). Stable isotope (13C and 15N) analysis of fish food web of the Xiaojiang Bay in three gorges Reservoir. Acta Ecol. Sin. 33, 6704–6711.
Li, F. S., Yan, Y. Z., Zhang, J. N., Zhang, Q., and Niu, J. M. (2021). Taxonomic, functional, and phylogenetic beta diversity in the Inner Mongolia grassland. Glob. Ecol. Conserv. 28:e01634. doi: 10.1016/j.gecco.2021.e01634
Li, Y. R., Tao, J., Chu, L., and Yan, Y. Z. (2018). Effects of anthropogenic disturbances on alpha and beta diversity of fish assemblages and their longitudinal patterns in subtropical streams, China. Ecol. Freshw. Fish 27, 433–441. doi: 10.1111/eff.12358
Lian, Y. X. (2016). Spatio-Temporal Distribution Patterns of Fishes as Related to Ecological Factors in the Three Gorges Reservoir. [Ph.D. thesis]. Beijing: The University of Chinese Academy of Sciences.
Liao, C. S., Chen, S. B., Correa, S. B., Li, W., Zhang, T. L., and Liu, J. S. (2019). Impoundment led to spatial trophic segregation of three closely related catfish species in the Three Gorges Reservoir, China. Mar. Freshw. Res. 71, 750–760. doi: 10.1071/MF19181
Liao, C. S., Chen, S. B., De Silva, S. S., Correa, S. B., Yuan, J., Zhang, T. L., et al. (2018). Spatial changes of fish assemblages in relation to filling stages of the three gorges Reservoir, China. J. Appl. Ichthyol. 34, 1293–1303. doi: 10.1111/jai.13798
Liew, J. H., Tan, H. H., and Yeo, D. C. J. (2016). Dammed rivers: impoundments facilitate fish invasions. Freshw. Biol. 61, 1421–1429. doi: 10.1111/fwb.12781
Lima, A. C., Agostinho, C. S., Sayanda, D., Pelicice, F. M., Soares, A. M. V. M., and Monaghan, K. A. (2016). The rise and fall of fish diversity in a Neotropical river after impoundment. Hydrobiologia 763, 207–221. doi: 10.1007/s10750-015-2377-z
Lima-Junior, D. P., Bellay, S., Hoeinghaus, D. J., Bini, L. M., Lima, L. B., Yotoko, K., et al. (2021). Host diversity, phylogenetic relationships and local environmental factors drive infection patterns of a non-native parasite in tropical floodplain fish assemblages. Hydrobiologia 848, 1041–1057. doi: 10.1007/s10750-020-04509-2
Lin, L., Deng, W., Huang, X., and Kang, B. (2021). Fish taxonomic, functional, and phylogenetic diversity and their vulnerabilities in the largest river in Southeastern China. Ecol. Evol. 11, 11533–11548. doi: 10.1002/ece3.7945
Lin, P. C., Gao, X., Liu, F., Li, M. Z., and Liu, H. Z. (2019). Long-term monitoring revealed fish assemblage zonation in the three gorges Reservoir. J. Oceanol. Limnol. 37, 1258–1267. doi: 10.1007/s00343-019-8165-2
Liu, C. C., Gao, X., Lin, P. C., Yang, S. R., Liu, H. Z., and Cao, W. X. (2012). Fish community structure in Gezhouba reservoir. Resour. Environ. Yangtze Basin 21, 843–849.
Loures, R. C., and Pompeu, P. S. (2018). Long-term study of reservoir cascade in south-eastern Brazil reveals spatio-temporal gradient in fish assemblages. Mar. Freshw. Res. 69, 1983–1994. doi: 10.1071/MF18109
Loures, R. C., and Pompeu, P. S. (2019). Temporal changes in fish diversity in lotic and lentic environments along a reservoir cascade. Freshw. Biol. 64, 1806–1820. doi: 10.1111/fwb.13372
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P., and Mokowski, D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Sour. Softw. 6:3139. doi: 10.21105/joss.03139
Luo, F. (1999). An investigation on the fishes types at Mudong town. J. Ningde Teach. Coll. 11, 31–38.
Ma, C., Xu, R., He, W., and Xia, J. J. (2020). Determining the limiting water level of early flood season by combining multiobjective optimization scheduling and copula joint distribution function: a case study of three gorges reservoir. Sci. Total Environ. 737:139789. doi: 10.1016/j.scitotenv.2020.139789
Mims, M. C., Olden, J. D., Shattuck, Z. R., and Poff, N. L. (2010). Life history trait diversity of native freshwater fishes in North America. Ecol. Freshw. Fish 19, 390–400. doi: 10.1111/j.1600-0633.2010.00422.x
Nakamura, G., Vicentin, W., Súarez, Y. R., and Duarte, L. (2020). A multifaceted approach to analyzing taxonomic, functional, and phylogenetic β-diversity. Ecology 101:e03122. doi: 10.1002/ecy.3122
Palacios-Salgado, D. S., Campos-Dávila, L., Granados-Amores, J., Cruz-Escalona, V. H., Peterson, M. S., Moreno-Sánchez, X. G., et al. (2019). Functional diversity in fish assemblages of the Tropical Eastern Pacific Ocean: a review of two decades of progress in the functional diversity approach. Hidrobiologica 29, 17–40.
Perera, H. A. C. C., Li, Z. J., De Silva, S. S., Zhang, T. L., Yuan, J., Ye, S. W., et al. (2014). Effect of the distance from the dam on river fish community structure and compositional trends, with reference to the Three Gorges Dam, Yangtze River, China. Acta Hydrobiol. Sin. 38, 438–445. doi: 10.7541/2013.62
Perônico, P. B., Agostinho, C. S., Fernandes, R., and Pelicice, F. M. (2020). Community reassembly after river regulation: rapid loss of fish diversity and the emergence of a new state. Hydrobiologia 847, 519–533. doi: 10.1007/s10750-019-04117-9
Roa-Fuentes, C. A., Heino, J., Cianciaruso, M. V., Ferraz, S., Zeni, J. O., and Casatti, L. (2019). Taxonomic, functional, and phylogenetic beta-diversity patterns of stream fish assemblages in tropical agroecosystems. Freshw. Biol. 64, 447–460. doi: 10.1111/fwb.13233
Rurangwa, M. L., Aguirre-Gutiérrez, J., Matthews, T. J., Niyigaba, P., Wayman, J. P., Tobias, J. A., et al. (2021). Effects of land-use change on avian taxonomic, functional and phylogenetic diversity in a tropical montane rainforest. Divers. Distrib. 27, 1732–1746.
Sanchez-Perez, A., Oliva-Paterna, F. J., Colin, N., Torralva, M., and Gorski, K. (2020). Functional response of fish assemblage to multiple stressors in a highly regulated Mediterranean river system. Sci. Total Environ. 730:138989. doi: 10.1016/j.scitotenv.2020.138989
Santos, A. B. I., and Araujo, F. G. (2015). Evidence of morphological differences between Astyanax bimaculatus (Actinopterygii: Characidae) from reaches above and below dams on a tropical river. Environ. Biol. Fish. 98, 183–191. doi: 10.1007/s10641-014-0248-5
Sá-Oliveira, J. C., Hawes, J. E., Isaac-Nahum, V. J., and Peres, C. A. (2015). Upstream and downstream responses of fish assemblages to an eastern Amazonian hydroelectric dam. Freshw. Biol. 60, 2037–2050. doi: 10.1111/fwb.12628
Shaffer, J. A., Munsch, S., and Juanes, F. (2018). Functional diversity responses of a nearshore fish community to restoration driven by large-scale dam removal. Estuar. Coast. Shelf Sci. 213, 245–252. doi: 10.1016/j.ecss.2018.08.030
Soininen, J., Heino, J., and Wang, J. J. (2018). A meta-analyses of nestedness and turnover components of β diversity across organisms and ecosystems. Glob. Ecol. Conserv. 27, 96–109. doi: 10.1111/geb.12660
Su, G. H., Logez, M., Xu, J., Tao, S. L., Villéger, S., and Brosse, S. (2021). Human impacts on global freshwater fish biodiversity. Science 371, 835–838. doi: 10.1126/science.abd3369
Tedesco, P. A., Beauchard, O., Bigorne, R., Blanchet, S., Buisson, L., Conti, L., et al. (2017). A global database on freshwater fish species occurrence in drainage basins. Sci. Data 4:170141. doi: 10.1038/sdata.2017.141
Toussaint, A., Charpin, N., Beauchard, O., Grenouillet, G., Oberdorff, T., Tedesco, P. A., et al. (2018). Non-native species led to marked shifts in functional diversity of the world freshwater fish faunas. Ecol. Lett. 21, 1649–1659. doi: 10.1111/ele.13141
Turgeon, K., Turpin, C., and Gregory-Eaves, I. (2019). Dams have varying impacts on fish communities across latitudes: a quantitative synthesis. Ecol. Lett. 22, 1501–1516. doi: 10.1111/ele.13283
Tuya, F., Herrero-Barrencua, A., Bosch, N. E., Abreu, A. D., and Haroun, R. (2018). Reef fish at a remote tropical island (Principe Island, Gulf of Guinea): disentangling taxonomic, functional and phylogenetic diversity patterns with depth. Mar. Freshw. Res. 69, 395–402. doi: 10.1071/MF17233
Vašek, M., Prchalová, M., Øíha, M., Blabolil, P., Èech, M., Draštík, V., et al. (2016). Fish community response to the longitudinal environmental gradient in Czech deep-valley reservoirs: implications for ecological monitoring and management. Ecol. Indic. 63, 219–230. doi: 10.1016/j.ecolind.2015.11.061
Vega-Retter, C., Muñoz-Rojas, P., Rojas-Hernández, N., Copaja, S., Flores-Prado, L., and Véliz, D. (2020). Dammed river: short-and long-term consequences for fish species inhabiting a river in a Mediterranean climate in central Chile. Aquat. Conserv. 30, 2254–2268. doi: 10.1002/aqc.3425
Villéger, S., Grenouillet, G., and Brosse, S. (2013). Decomposing functional β−diversity reveals that low functional β−diversity is driven by low functional turnover in European fish assemblages. Glob. Ecol. Conserv. 22, 671–681. doi: 10.1111/geb.12021
Villéger, S., Mason, N. W. H., and Mouillot, D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. doi: 10.1890/07-1206.1
Vitule, J. R. S., Skora, F., and Abilhoa, V. (2012). Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Divers. Distrib. 18, 111–120. doi: 10.1111/j.1472-4642.2011.00821.x
Wang, J., Chen, L., Tang, W. J., Heino, J. N., and Jiang, X. M. (2021). Effects of dam construction and fish invasion on the species, functional and phylogenetic diversity of fish assemblages in the Yellow River Basin. J. Environ. Manage. 293:112863. doi: 10.1016/j.jenvman.2021.112863
Wong, J. S., Chan, Y. K., Ng, C. S., Tun, K. P., Darling, E. S., and Huang, D. (2018). Comparing patterns of taxonomic, functional and phylogenetic diversity in reef coral communities. Coral Reefs 37, 737–750. doi: 10.1007/s00338-018-1698-6
Wu, Q., Duan, X. B., Xu, S. Y., Xiong, C. X., and Chen, D. Q. (2007). Studies on fishery resources in the three gorges reservoir of the Yangtze River. Freshw. Fish 37, 70–75. doi: 10.3969/j.issn.1000-6907.2007.02.018
Xiao, Q., Yang, Z., Tang, H. Y., Duan, P. X., Wang, X. Q., Xiao, T. Y., et al. (2015). Species diversity of fish and its conservation in the mainstream of the lower reaches of Wu River. Biodivers. Sci. 23, 499–506. doi: 10.17520/biods.2014270
Xiong, F. Y., Olden, J. D., Lu, Y., Liu, H., Qu, X., Guo, C. B., et al. (2021). Riparian land use and in-channel stressors drive fish community structure in the Yangtze River. Landsc. Ecol. 36, 3079–3095. doi: 10.1007/s10980-021-01278-8
Xu, W., Yang, Z., Yi, R., Yao, J. Z., and Chen, X. J. (2021). Relationship between environmental variables and egg abundance of the four major Chinese carps, downstream of the Three Gorges Reservoir. River Res. Appl. 37, 1191–1200. doi: 10.1002/rra.3750
Yang, F., Yao, W. Z., Deng, H. T., Chen, D. Q., Liu, S. P., and Duan, X. B. (2013). The current situation of fish resources in the Daning River after the impoundment of the Three Gorges Reservoir. Freshw. Fish. 43, 51–57. doi: 10.3969/j.issn.1000-6907.2013.04.010
Yang, S. R., Gao, X., Li, M. Z., Ma, B. S., and Liu, H. Z. (2012). Interannual variations of the fish assemblage in the transitional zone of the Three Gorges Reservoir: persistence and stability. Environ. Biol. Fish. 93, 295–304. doi: 10.1007/s10641-011-9936-6
Yang, Z., Tang, H. Y., Zhu, D., Liu, H. G., Wan, L., Tao, J. P., et al. (2015). Spatiotemporal patterns of fish community structures in the Three Gorges Reservoir and its upstream during the 175-m-deep impoundment. Acta Ecol. Sin. 35, 5064–5075. doi: 10.5846/stxb201309302395 [in Chinese with English Abstract].
Yu, M. X., Yang, D. Q., Liu, X. L., Li, Q. F., and Wang, G. Q. (2019). Potential impact of a large-scale cascade reservoir on the spawning conditions of critical species in the Yangtze River, China. Water 11:2027. doi: 10.3390/w11102027
Zhang, C., Ding, L. Y., Ding, C. Z., Chen, L. Q., Sun, J., and Jiang, X. M. (2018). Responses of species and phylogenetic diversity of fish communities in the Lancang River to hydropower development and exotic invasions. Ecol. Indic. 90, 261–279. doi: 10.1016/j.ecolind.2018.03.004
Zhang, C., Fujiwara, M., Pawluk, M., Liu, H. Z., Cao, W. X., and Gao, X. (2020). Changes in taxonomic and functional diversity of fish communities after catastrophic habitat alteration caused by construction of Three Gorges Dam. Ecol. Evol. 10, 5829–5839. doi: 10.1002/ece3.6320
Zhang, X. Z., Xue, Y., Zhang, C. L., Ren, Y. P., Xu, B. D., and Chen, Y. (2021). Sampling intensity influences the estimation of functional diversity indices of fish communities. Ecol. Indic. 121:107169. doi: 10.1016/j.ecolind.2020.107169
Keywords: reservoir cascade, species richness, functional diversity, phylogenetic diversity, conservation
Citation: Liao C, Wang J, Ye S, Li W, Correa SB, Zhang T and Liu J (2022) Multifaceted fish diversities respond differently to impounding age and longitudinal location along a reservoir cascade. Front. Ecol. Evol. 10:955053. doi: 10.3389/fevo.2022.955053
Received: 28 May 2022; Accepted: 08 July 2022;
Published: 28 July 2022.
Edited by:
Tian Zhao, Key Laboratory of Mountain Ecological Rehabilitation and Biological Resource Utilization, Chengdu Institute of Biology (CAS), ChinaReviewed by:
Sukran Yalcin Ozdilek, Çanakkale Onsekiz Mart University, TurkeyCopyright © 2022 Liao, Wang, Ye, Li, Correa, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiashou Liu, anNsaXVAaWhiLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.