
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 18 July 2022
Sec. Paleontology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.927537
This article is part of the Research TopicA Fossil View of Insect Evolution: Integrating Paleontological Evidence to Explore the Origins of Insect BiodiversityView all 10 articles
This study describes a new genus Simplicivenius gen. nov. of Reduviinae with two new species, Simplicivenius tuberculosus sp. nov. and Simplicivenius rectidorsius sp. nov. from the Yixian Formation in northeastern China. The diagnosis of the genus includes the width of the scutellum about 2/3 of the width of the pronotum and the absence of longitudinal veins around the exterior cell of the membrane. This is the oldest fossil record of Reduviinae and indeed Reduviidae at present, dating back to the Early Cretaceous. This implies that Reduviinae existed before the Late Cretaceous biological mass extinction, hinting that Reduviinae may be a basal taxon within the Higher Reduviidae. Moreover, the fossils prove the correctness of the previous speculations on the ancestral state of the stridulitrum and the fossula spongiosa of the assassin bugs. The fossula spongiosa on the mid leg and fore leg are subequal in size and both occupy 1/3 of the tibia is considered the primitive type of fossula spongiosa.
Reduviidae is the second-largest family within Heteroptera, including about 25 subfamilies and more than 7,000 described species at present (Weirauch et al., 2014). Among them, Reduviinae is a cosmopolitan group, which is most abundant in tropical and subtropical regions. It is the second-largest subfamily of Reduviidae, including 141 genera and more than 1,070 species (Shah et al., 2022). Species of this subfamily are predatory and prey on other animals for their food (Hwang and Weirauch, 2012). Most of them live under dead bark, sometimes near human dwellings, in bushes, and in arid environments such as deserts, where their habitats are more sophisticated and varied (Sahayaraj, 2007). The first extant species of this subfamily, Reduvius personatus, was described in 1758 by Linnaeus (Javahery, 2013).
There are only 33 genera and 51 species of fossils that have been described in Reduviidae, which is quite different from the present situation in that it is the second-largest family of Heteroptera. Among these 51 species, there are 18 species whose subfamilies are undetermined, and the remaining 33 species are classified into eight subfamilies: Reduviinae, Holoptilinae, Emesinae, Centrocnemidinae, Triatominae, Phymatinae, Harpactorinae, and Ectrichodiinae. Germar et al. (1856) found a fossil of assassin bugs in Eocene amber from the Baltic (Germar et al., 1856), which is the first report of Reduviidae fossils. A large number of Reduviidae fossils had been found and reported in the 20th century, but the geological age is still in Cenozoic. Poinar (2019) discovered a primitive Triatominae fossil in early Late Cretaceous amber from northern Myanmar, this is the earliest record of fossil specimens of Reduviidae and the first report of Mesozoic assassin bugs fossil (Bush, 2021). To sum up, the current fossil records of Reduviidae are mostly Cenozoic, only a few fossils have been reported in Mesozoic, and the geological period stays in the early Late Cretaceous.
A new genus and two new species of Reduviinae are described in this article, which can be traced back to the Early Cretaceous and is the earliest known fossil record of Reduviidae in the world. The discovery of these fossils will enrich the diversity of the fossil species of Reduviidae to a certain extent, and provide direct fossil evidence for the subsequent interpretation of the evolution and origin of Reduviidae.
The research materials involved in this study are three impression fossils, all of which were excavated from the Yixian Formation of Liaoning Province, Northeast China. The geological period of the Yixian Formation is suggested to be Early Cretaceous Aptian-Barremian (Barrett, 2000; He et al., 2008; Chang et al., 2017). Two of them are fossil pairs of plates. All are preserved in the Fossil Herbarium, Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (Curator: Yunzhi Yao). The morphological aspects were mainly observed under a Nikon SMZ25 microscope, which was connected in real-time to a Nikon DS-Ri2 digital photographic system. The morphological terminology used in this study is mainly referenced by Lent and Wygodzinsky (1979), Weirauch (2008), and Zhang and Weirauch (2011). All units of dimensions are in millimeters (mm).
Order Hemiptera Linnaeus, 1758
Suborder Heteroptera Latreille, 1810
Infraorder Cimicomorpha Leston, Pendergrast & Southwood, 1954
Family Reduviidae Latreille, 1807
Subfamily Reduviinae Amyot & Serville, 1843
Genus Simplicivenius Zhang, Liu, and Yao, gen. nov.
Type Species. Simplicivenius tuberculosus sp. nov. (Figure 1).
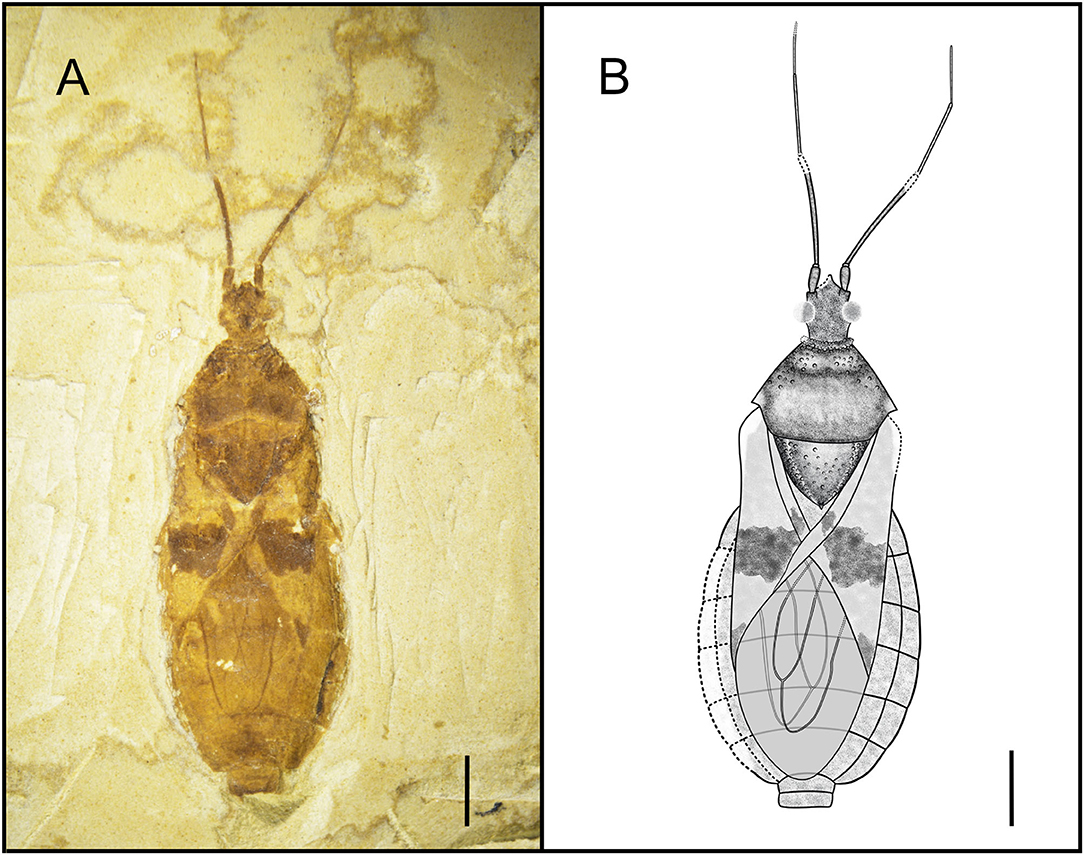
Figure 1. Dorsal habitus of Simplicivenius tuberculosus gen. et sp. nov., holotype CNU-HET-LB2022001 (A) Photograph. (B) Line drawing. Scale bars = 2 mm.
Etymology. The name of this new genus is a combination of “Simpl-” (meaning “simple”) and “Civenius” (meaning “vein”). Gender masculine. The reason for this name is that the veins of this new genus are relatively simple, with only two large wing cells on the membrane and no small longitudinal veins protruding from the outer edge of the cell formed by the M and Cu veins.
Diagnosis. Medium-sized, with a long oval shape. Anteocular region slightly longer and wider than postocular region, postocular region with obvious lateral constriction; antennae divided into four segments, with scape thickest and pedicel longest, pedicel with no obvious pseudosegmentation; anterior lobe of pronotum obviously shorter than posterior lobe, margin of posterior lobe prominent or nearly straight, with spines at humeral angle and slightly variable in shape; scutellum triangular, width obviously larger than length, apical scutellar process upturned, width of scutellum about 2/3 of width of pronotum; apical portion of corium with the macula, membrane with two large parallel cells, exterior cell slightly wider than the inner cell, without longitudinal vein extending from the outer margin of the exterior cell.
Comparison. This new fossil genus shares the following characteristics with the Reduviinae of the type genus Reduvius: both fore leg and mid leg straight and hemelytron without discoidal cell. However, it can be distinguished from the latter genus by the following characteristics: anterior lobe of pronotum significantly shorter than the posterior lobe. Other than that, the two new fossil species appear at first glance to be very similar to Acanthaspis quinquespinosa Fabricius, 1781 in the genus Acanthaspis, both of which have the following features: scape thickest and pedicel longest; anterior lobe of pronotum shorter than posterior lobe; scutellum triangular; apical scutellar process upturned and macula at sub-apical part of the corium. Nevertheless, further observations revealed the following unique features of the new fossil genus: width of scutellum approximately 2/3 of width of pronotum; no longitudinal vein in exterior cell of membrane.
Simplicivenius tuberculosus sp. nov. (Figure 1).
Holotype. CNU-HET-LB2022001, adult male.
Etymology. The name of this species comes from the Latin word “tuberculosus” (meaning “tuberculate”), referring to the multiple tubercles on the side of the pronotum and the scutellum.
Locality and Horizon. Early Cretaceous, Aptian-Barremian, Yixian, Liaoning Province, Northeast China.
Diagnosis. Conspicuous tubercles distributed on collar; posterior lobe of pronotum twice as long as anterior lobe, anterior lobe of pronotum and lateral area of scutellum, posterior margin of pronotum convex; hemelytron with a large macula on sub-apical portion of corium, a small sub-triangular macula on apical portion, a small rectangular macula on clavus; two elongated large cells consisting of M, Cu and Pcu veins on membrane.
Description. Head medium-sized, 1/8 of body length, anteocular region about 1.32 times of postocular region, postocular region short and cylindrical; eye large, dorsal length of almost 1/3 of head, dorsal width of greater than 1/2 of interocular space; interocellar space greater than single ocellar width, interocular space about 3.4 times of interocellar space; antenna divided into four segments, width of four segments tapered, scape and pedicel rod-shaped, width of scape twice width of pedicel, basiflagellomere and distiflagellomere obviously slender, distiflagellomere thinnest, its width 1/5 of the width of scape, pedicel longest, its length about four times that of scape; labium not preserved.
Collar incompletely preserved, edges of collar have small tubercles of different sizes; anterior margin of anterior lobe of pronotum slightly concave inward, anterior lobe has small tubercles symmetrical from left to right, lateral part of subanterior margin of anterior lobe predominant, closer area to transverse sulcus of pronotum fewer tubercles, median longitudinal groove faint, pronotal transverse sulcus with a small number of small tubercles on sides, humeral angle with slightly shorter and thicker spine-like process, apex of process is slightly pendulous, lateral angle rounded and blunt, posterior margin convex; scutellum triangular, about 1.48 times as wide as long, width almost as long as distance between two humeral angles, length about 1/8 of body length, sides of scutellum with symmetrical and dense tubercles, tubercles on middle part relatively sparse.
Hemelytra large macula on corium, which contrasts with other colors around corium, its size about 1/3 of corium, whereas macula on middle of clavus and apex of corium much smaller; abdomen elliptical and basically well preserved, intersegmental sutures also well preserved.
Legs not preserved.
Although the key characteristics of male genitalia are not preserved, it can be seen that the apex of the abdomen is very flat. Female external genitalia are generally more pointed, so it is difficult to press into such a shape and then combined with its size, this specimen is more likely to be male.
Dimensions (mm). Body length: 14.22; maximum abdominal width: 6.07; head length: 1.80; length of anteocular region: 0.7; length of postocular region: 0.53; width of interocular space: 0.75; width of interocellar space: 0.22; antennal segment lengths I-IV: 0.72, 2.96, 2.14, 1.42; length of anterior lobe of pronotum: 0.78; length of posterior lobe of pronotum: 1.75; width of pronotum: 3.92; length of scutellum: 1.79; width of scutellum: 2.65.
Simplicivenius rectidorsius sp. nov. (Figures 2, 3).
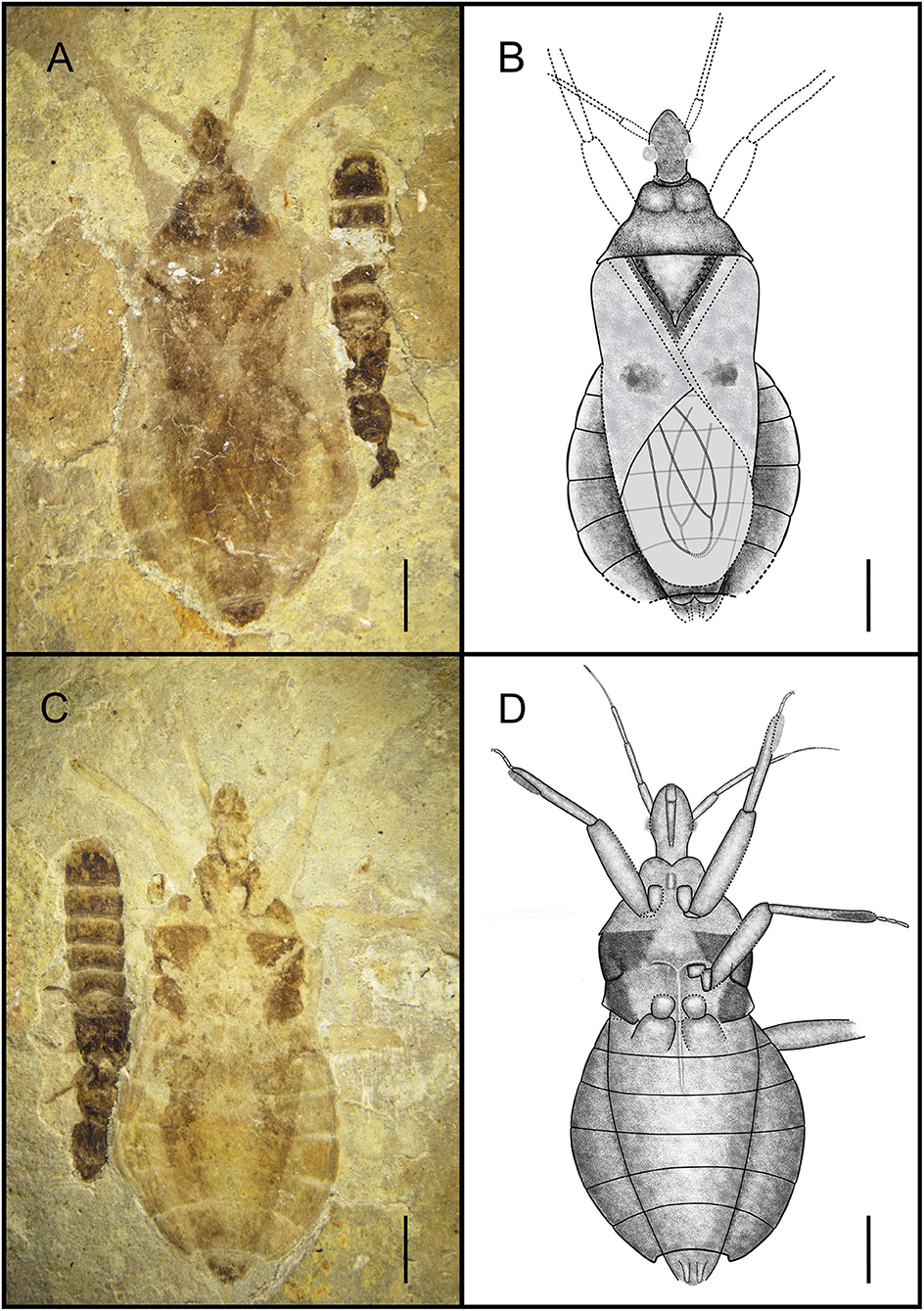
Figure 2. Habitus of Simplicivenius rectidorsius gen. et sp. nov., holotype. CNU-HET-LB2022002 (A) Photograph in dorsal view. (B) Line drawing in dorsal view. (C) Photograph in ventral view. (D) Line drawing in ventral view. Scale bars = 2 mm.
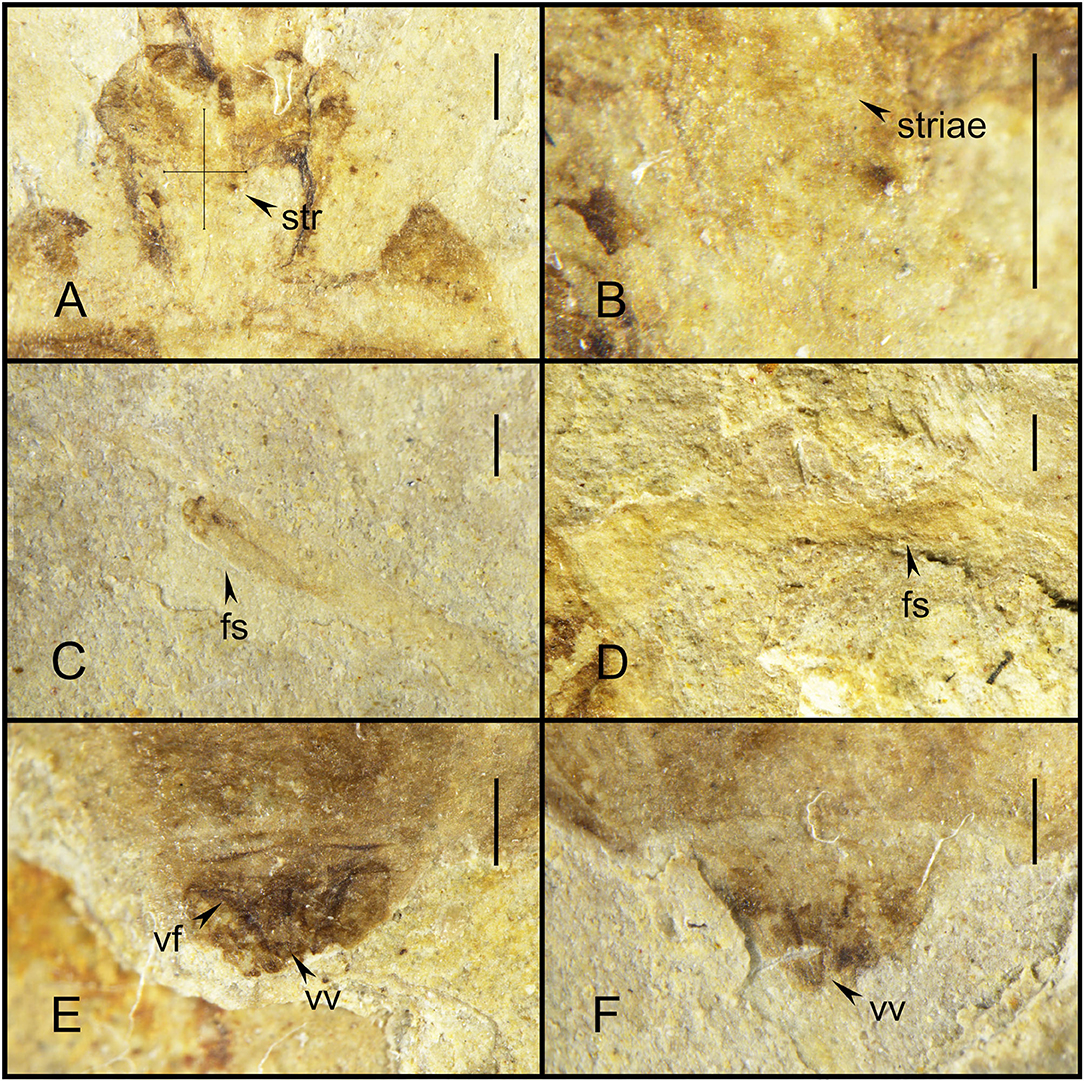
Figure 3. Simplicivenius rectidorsius gen. et sp. nov. photographs with morphological details, holotype. CNU-HET-LB2022002. (A) Conservation range of stridulitrum. (B) Structures of striae. (C) Fossula spongiosa on fore tibia. (D) Fossula spongiosa on mid tibia. (E) Female genitalia in dorsal view. (F) Female genitalia in ventral view. Scale bars = 0.5 mm. str, stridulitrum; fs, fossula spongiosa; vf, valvifer; vv, valvula.
Holotype. CNU-HET-LB2022002, part, and counterpart, adult female.
Etymology. The species name is a combination of the Latin words “rect-” (meaning “straight”) and “dorsalis” (meaning “dorsal”), referring to the nearly straight posterior margin of the pronotum.
Locality and Horizon. Early Cretaceous, Aptian-Barremian, Yixian, Liaoning Province, Northeast China.
Diagnosis. Collar well-developed, collar process slightly uplifted; ratio of length of anterior and posterior lobes of pronotum about 2:3, posterior margin of pronotum nearly straight with position of humeral angle about the same level; lateral part of scutellum with symmetrical tubercles; stridulitrum long and wide; sub-apical portion of corium with irregular small macula, membrane with two elongated large cell, exterior cell wider than inner cell; fossula spongiosa on fore leg and mid leg occupying about 1/3 of tibial ventral surface, tarsus of fore and mid legs three-segmented, hind coxae close together.
Description. Head medium-sized, 1/8 of body length, anteocular region measures 1.32 times that of postocular region, postocular region short and cylindrical; eye large, dorsal length almost 1/3 of head, dorsal width more than 1/2 of interocular space; interocellar space more than single ocellar width, interocular space is about 3.4 times that of interocellar space; antenna divided into four segments, width of four segments tapered, scape and pedicel rod-shaped, width of scape twice width of pedicel, basiflagellomere and distiflagellomere obviously slender, distiflagellomere thinnest, its width 1/5 of width of scape, pedicel longest, and its length about four times that of scape; labium is not preserved.
Collar well-developed, collar process slightly elevated; posterior lobe of pronotum approximately 1.62 times longer than anterior lobe, anterior margin of pronotum slightly depressed inward, median longitudinal groove faint, humeral angle on posterior lobe elongated spine, posterior margin of pronotum at same level as base of spines at humeral angle, nearly flat; scutellum triangular, 1/8 of body length and width, about 1.17 times its length, with small projections on lateral margins, a thicker base of apical portion of scutellum, tip of scutellar process sharp and upturned; stridulitrum incompletely preserved, but can be identified as long and wide under the microscope (Figures 3A,B). Middle of mesostethium and metastethium with a longitudinal ridge.
Femur and tibia of right fore leg well preserved, fossula spongiosa impressions on fore tibia, which well preserved, coxa and trochanter of left fore leg largely intact, fore coxae elongated, distance between fore coxae is greater than width of one coxa, fore femur robuster than fore tibia, femur elliptical columnar, middle wider than both ends, widest point 0.91 mm, tibia cylindrical, width basically equal everywhere, width about 0.32 mm, fossula spongiosa relatively large, occupying about 1/3 of whole tibia (Figure 3C), tarsus three-segmented, first segment shortest and third longest; right mid leg not preserved; outer edges of coxa and trochanter of left mid leg largely preserved, fossula spongiosa impression on mid tibia (Figure 3D) (preserved on the fossil is the ventral view of fossula spongiosa, probably caused by the turning of the tibial segment), and mid tarsus well preserved, mid coxae nearly triangular, fossula spongiosa also occupies about 1/3 of tibia, mid tarsus three-segmented, first and second segments basically equal in length, third segment longest; only coxae of hind leg is preserved, sub-circular hind coxae close to each other, distance between hind coxae smaller than width of one coxa.
Abdomen well preserved, elliptical, intersegmental sutures also basically well preserved. Connexivum well preserved, paranotum very broad. Female genitalia can be observed with valvifers and valvulae (Figures 3E,F).
Dimensions (mm). Body length: 14.92; maximum abdominal width: 6.72; head length: 2.25; length of anteocular region: 0.95; length of postocular region: 0.71; width of interocular space: 0.56; width of interocellar space: 0.25; antennal segment lengths I-IV: 0.91, 1.31, 1.16, 0.50; length of anterior lobe of pronotum: 0.74; length of posterior lobe of pronotum: 1.20; width of pronotum: 4.20; length of scutellum: 1.86; width of scutellum: 2.71; length of fore femur: 2.88; length of fore tibia: 3.33; length of fossula spongiosa of fore leg: 1.10; length of total fore tarsus: 0.94; length of mid tibia: 3.14; length of fossula spongiosa of mid leg: 1.10; length of total mid tarsus: 1.05.
Associated Specimen. In addition to the assassin bug, this fossil also has a rove beetle larva next to it, which belongs to Coleoptera, and although it is documented that the assassin bugs prey on Coleoptera as well (Youssef and Abd-Elgayed, 2015; Loko et al., 2022), there is no direct evidence that this assassin bug was preying on the rove beetle larva in this fossil, but the leg of the assassin bug was in contact with the carapace of the larva. Coincidentally, the assassin bug fossil documented by Swanson also shows Coleoptera (Swanson et al., 2021), and the leg of the assassin bug is also in contact with the body of the beetle.
The present fossil genus can be identified as Reduviidae on the basis of the following characteristics:membrane with two large cells, prosternal sulcus with stridulitrum present, head necklike behind eyes (Schuh and Weirauch, 2020). According to the zoogeographic classification of the world by Olson et al. (2001), the fossils of this study were recovered from northeastern China, which falls within the Palaearctic boundary that encompasses mainly the Eurasian and northern African regions. Within Reduviidae, Emesinae, Ectrichodiinae, Harpactorinae, Holoptilinae, Peiratinae, Stenopodainae, and Reduviinae are distributed in this lineage, so the fossils in this study are mainly compared to these seven subfamilies (Weirauch et al., 2014). Emesinae has a slender body which differs greatly from the oval body of the fossils in this study, and therefore, this subfamily is not considered in the first place (Wygodzinsky, 1966). The main difference between Ectrichodiinae and other subfamily assassin bugs is the presence of at least two apical scutellar processes, unlike the present fossils where only one apical scutellar process is present (Forthman et al., 2016). The key identifying character of Peiratinae is that the anterior lobe of the pronotum is significantly longer than the posterior lobe, which is the opposite of all the fossils in this study (Liu et al., 2020). In addition, the fossils in this study are clearly observed two large parallel cells present only at the membrane of hemelytron, whereas Harpactorinae has a square cell at the corium and Stenopodainae has a pentagonal or hexagonal cell at the corium (Wygodzinsky and Giacchi, 1994; Forero et al., 2004; Chen et al., 2020), the fossils are fundamentally different from the distribution of cells in these two subfamilies. Holoptilinae is missing the fossula spongiosa on the tibiae and has long setae on the body and each leg (Malipatil, 1985); the fossils in this study do not reveal the preservation of a large number of setae, although it is not certain whether the taxa represented by the fossils did not have setae covering the whole body itself or whether the setal structure was not preserved because the fossils are too old, the fossula spongiosa can be clearly observed in Simplicivenius rectidorsius gen. et sp. nov. Reduviinae is identified by the presence of the ocellus, three-segmented tarsus, two closed cells on the membrane of the hemelytron, and the fossula spongiosa on the tibia of fore and mid legs. In addition, Reduviinae is usually identified by the lack of specific morphological features unique to other subfamilies (Weirauch, 2014). The fossils in this study are highly overlapping with Reduviinae in terms of identifying features. Therefore, after a comprehensive comparison, the two fossils were finally classified as Reduviinae.
Hwang and Weirauch (2012) estimated the divergence dating of key evolutionary nodes within Reduviidae based on BEAST analysis using a relaxed-clock model and 11 fossil calibrations points. It is shown that the diversification of Higher Reduviidae began at 97 Ma (81–113 Ma) in the Late Cretaceous, coinciding with two global changes in the timing of angiosperm and phytophagous insect radiation. The analysis also showed that all lineages of Reduviinae are located in the branches of Higher Reduviidae, and the same results were shown in the cladistic analysis of Reduviidae based on morphological characters (Weirauch, 2008), the molecular phylogeny within the assassin bugs obtained from mitochondrial and nuclear ribosomal genes (Weirauch and Munro, 2009), and in the phylogenetic analysis based on transcriptomic and ribosomal DNA data (Zhang et al., 2016). The fossils in this study are geologically dated to the Early Cretaceous, and there is ample evidence to support their classification as Reduviinae, which is the earliest known Reduviinae fossil in the world. This result suggests that the Reduviinae emerged well before the Late Cretaceous biological mass extinction (Newell, 1965) survived the Late Cretaceous biological mass extinction, and then gradually flourished with the radiation of angiosperms and phytophagous insects. Such an early geological age also allows the onset of diversification of the Higher Reduviidae from the Late Cretaceous at 97 Ma (81–113 Ma) to at least the Early Cretaceous, indicating that Reduviinae may have been a basal taxon within the Higher Reduviidae. Poinar (2019) suggested that the fossil species Paleotriatoma metaxytaxa Triatominae from the early Late Cretaceous (100 Ma) evolved from a clade of Reduviinae based on the presence of ocelli, three-segmented tarsi, two closed cells on the forewing membrane, slight bend in the labium and fossula spongiosa on the fore and mid tibias. This also seems to further imply that Reduviinae may be a basal taxon along with Peiratinae. But it is not sufficient to rely on the geological history period of fossil records to draw such a conclusion, so we will also discuss below from the perspective of morphological characters.
The oldest fossil of Simplicivenius rectidorsius gen. et sp. nov. also provides the most direct fossil evidence for previous studies on the stridulitrum and fossula spongiosa of Reduviidae. The evolution of the stridulitrum of Reduviid ae was investigated by Cai et al. (1994), which classified the stridulitrum of adult Reduviidae into nine terminal types and suggested that the subwide total-striate stridulitrum or wide total-striate stridulitrum probably represented the ancestral status of Reduviidae. However, this conclusion remains tentative, and direct evidence to support it is not available. The striae of the stridulitrum are preserved in the fossil species Simplicivenius rectidorsius gen. et sp. nov. Although the complete appearance of the stridulitrum is not preserved as the fossil is too old, it is not difficult to conclude that the stridulitrum of this fossil species is at least the subwide total-striate type, judging from the distribution range of the striae on the prosternum of the fossil. Besides, Zhang et al. (2016) reconstructed the ancestral state of the raptorial leg of the assassin bugs based on transcriptomic and ribosomal DNA data. The fossula spongiosa were present in the ancestors of assassin bugs, as reported by Zhang et al. (2016). And auspiciously, the fossula spongiosa is also clearly preserved in Simplicivenius rectidorsius gen. et sp. nov. The fossula spongiosa of the fore leg can be seen, and the horizontal plate impressions that evolved only within Reduviidae to separate the fossula spongiosa cavity from the medial of the tibia are also preserved in the mid leg, and the fossula spongiosa impressions of the fore leg and mid leg are preserved simultaneously and both occupy 1/3 of the tibia (Weirauch, 2007). Another fossil species of Triatominae Paleotriatoma metaxytaxa found in the early Late Cretaceous (100 Ma) also has fossula spongiosa on both the fore leg and mid leg that occupy 1/3 of the tibia (Poinar, 2019). The fossula spongiosa were found to be present in both fore and mid legs in most species, and the fossula spongiosa of fore leg were usually larger than the fossula spongiosa of mid leg, while only a few taxa have fossula spongiosa on the fore leg only (Weirauch, 2007). From the above, it can be seen that there are three types of the fossula spongiosa of mid leg: first, the fossula spongiosa on the fore leg and mid leg being subequal in size; second, the fossula spongiosa on the fore leg being significantly larger than that on the mid leg; third, the fossula spongiosa on the mid leg is absent. The type I is relatively more developed and emerged earlier than the other two types. The evolution of fossula spongiosa throughout the Reduviidae showing a degenerative trend as revealed from the study by Zhang et al. (2016). The law of irreversibility states that an organ that has degenerated or disappeared completely during the evolutionary process will never reappear in subsequent evolutionary processes. If an organ that has disappeared in response to environmental changes is needed again, the organism can only produce another organ to perform the role of the original organ (Gould, 1970). The developmental priority also assumes that the earlier features in individual development are plesiomorphic and the later features are apomorphic (Hennig, 1999). Therefore, the type I represented by Simplicivenius rectidorsius gen. et sp. nov and Paleotriatoma metaxytaxa are presumed to be the ancestral states in assassin bugs and the remaining two types emerged subsequently. This also implies that the lack of fossula spongiosa in most species of mid leg which play an auxiliary role in fixing the prey seems to be eliminated firstly during the degradation of the fossula spongiosa. This may be caused by changes in the predatory behavior of Reduviidae due to changes in the predator (Haridass and Ananthakrishnan, 1980; Weirauch and Cassis, 2006; Zhang and Weirauch, 2014; Wang and Liang, 2015; Castro-Huertas et al., 2019). The most primitive types of fossula spongiosa and stridulitrum in Reduviidae are found in the fossils of Reduviinae in this study, which further indicates that Reduviinae is probably the basal taxon in the Higher Reduviidae, rather than the branching taxon suggested in previous studies (Weirauch, 2008; Weirauch and Munro, 2009; Hwang and Weirauch, 2012; Zhang et al., 2016). The results of Weirauch (2008) based on morphological characters showed that Harpactorinae that lack fossula spongiosa in most species as a basal taxon within the Higher Reduviidae, which may need to be reconsidered.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conceptualization, methodology, and formal analysis: PZ and YL. Validation: PZ, DR, and YY. Investigation and writing—original draft preparation: PZ. Resources, project administration, and funding acquisition: DR and YY. Data curation: YY. Writing—review and editing: YL, DR, and YY. Visualization and supervision: YL. All authors have read and agreed to the published version of the manuscript.
This project is supported by the National Natural Science Foundation of China [Nos. 31970436, 31730087, 42288201, and 32020103006] and the Beijing Municipal Natural Science Foundation and Beijing Municipal Education Commission [KZ201810028046].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to the editor and reviewers for constructive criticism and valuable comments on the manuscript.
Barrett, P. M. (2000). Evolutionary consequences of dating the Yixian Formation. Trends Ecol. Evol. 15, 99–103. doi: 10.1016/S0169-5347(99)01782-6
Bush, T. N. (2021). Systematics of Ectrichodiella Fracker and Bruner, 1924 with a description of the first fossil millipede assassin bug (Insecta: Heteroptera: Reduviidae: Ectrichodiinae). UC Riverside: University Honors. Available onlilne at: https://escholarship.org/uc/item/0zx7m0dj
Cai, W., Chou, Y., and Lu, J. (1994). The morphology, postembryonic development and evolution of stridulitra in Reduviidae with special reference to their taxonomic importance (Heteroptera: Reduvioidea). Insect Sci. 1, 1–16. doi: 10.1111/j.1744-7917.1994.tb00190.x
Castro-Huertas, V., Forero, D., and Grazia, J. (2019). Comparative morphology of the raptorial leg in thread-legged bugs of the tribe Metapterini Stål, 1859 (Hemiptera, Heteroptera, Reduviidae, Emesinae). Zoomorpholog. 138, 97–116. doi: 10.1007/s00435-019-00431-x
Chang, S. C., Gao, K. Q., Zhou, C. F., and Jourdan, F. (2017). New chronostratigraphic constraints on the Yixian Formation with implications for the Jehol Biota. Palaeogeogr. Palaeoclimatol. Palaeoecol. 487:399–406. doi: 10.1016/j.palaeo.2017.09.026
Chen, Z., Liu, Y., and Cai, W. (2020). Notes on the genus Enoplocephala Miller (Hemiptera: Reduviidae: Stenopodainae), with the description of a new species from Borneo. Raffles. B. Zool. 68. doi: 10.1080/00379271.2020.1844048
Forero, D., Weirauch, C., and Baena, M. (2004). Synonymy of the reduviid (Hemiptera: Heteroptera) genus Torrealbaia (Triatominae) with Amphibolus (Harpactorinae), with notes on Amphibolus venator (Klug, 1830). Zootaxa. 670, 1–12. doi: 10.11646/zootaxa.670.1.1
Forthman, M., Chłond, D., and Weirauch, C. (2016). Taxonomic monograph of the endemic millipede assassin bug fauna of Madagascar (Hemiptera: Reduviidae: Ectrichodiinae). Bull. Am. Mus. Nat. Hist. 2016, 1–152. doi: 10.1206/amnb-928-00-01.1
Germar, D. F., Berendt, G. C., and Hagen, H. (1856). Die im bernstein befindlichen Hemipteren und Orthopteren der Vorwelt. Berlin: Nicolaische Buchhandlung.
Gould, S. J. (1970). Dollo on Dollo's law: Irreversibility and the status of evolutionary laws. J. Hist. Biol. 3, 189–212. doi: 10.1007/BF00137351
Haridass, E. T., and Ananthakrishnan, T. N. (1980). Functional morphology of the fossula spongiosa in some reduviids (Insecta-Heteroptera-Reduviidae). Proceedings: Animal Sciences. 89, 457–466. doi: 10.1007/BF03179132
He, H., Pan, Y., Tauxe, L., Qin, H., and Zhu, R. (2008). Toward age determination of the M0r (Barremian–Aptian boundary) of the Early Cretaceous. Phys. Earth Planet. 169, 41–48. doi: 10.1016/j.pepi.2008.07.014
Hwang, W. S., and Weirauch, C. (2012). Evolutionary history of assassin bugs (Insecta: Hemiptera: Reduviidae): insights from divergence dating and ancestral state reconstruction. PLoS ONE. 7, e45523. doi: 10.1371/journal.pone.0045523
Javahery, M. (2013). Natural history of Reduvius personatus Linnaeus (Hemiptera: Heteroptera: Reduviidae) in North America. Mun. Ent. Zool. 8, 685–703.
Lent, H., and Wygodzinsky, P. (1979). Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bull. Am. Mus. Nat Hist. 163, 123–520.
Liu, Y., Chen, Z., Webb, M. D., and Cai, W. (2020). Oblongiala zimbabwensis, a new assassin bug genus, and species from Zimbabwe, with a key to the Afrotropical genera of Peiratinae (Hemiptera: Heteroptera: Reduviidae). Acta Ent. Mus. Nat. Pra. 60, 659–665. doi: 10.37520/aemnp.2020.047
Loko, Y. L. E., Toffa, J., Gavoedo, D. M., Kitherian, S., Orobiyi, A., and Tamò, M. (2022). Effect of population density on oviposition, development, and survival of Alloeocranum biannulipes (Hemiptera: Reduviidae) preying on Dinoderus porcellus (Coleoptera: Bostrichidae). JoBAZ. 83, 1–8. doi: 10.1186/s41936-022-00267-w
Malipatil, M. B. (1985). Revision of Australian holoptilinae (Reduviidae: Heteroptera). Aus. J. Zool. 33, 283–299. doi: 10.1071/ZO9850283
Newell, N. D. (1965). Mass extinctions at the end of the Cretaceous period. Science. 149, 922–924. doi: 10.1126/science.149.3687.922
Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V., Underwood, E. C., et al. (2001). Terrestrial ecoregions of the world: a new map of life on EarthA new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience. 51, 933–938. doi: 10.1641/0006-3568(2001)051(0933:TEOTWA)2.0.CO;2
Poinar, G. (2019). A primitive triatomine bug, Paleotriatoma metaxytaxa gen. et sp. nov. (Hemiptera: Reduviidae: Triatominae), in mid-Cretaceous amber from northern Myanmar. Cretac. Res. 93:90–97. doi: 10.1016/j.cretres.2018.09.004
Sahayaraj, K. (2007). Ecotypic variation in the biology of Acanthaspis quinquespinosa Fabricius 1781 (Hemiptera: Reduviidae: Reduviinae) from peninsular India. Egypt. J. Biol. 9.
Schuh, R. T., and Weirauch, C. (2020). True bugs of the World (Hemiptera: Heteroptera) Classification and Natural history, 2nd ed. Manchester, UK: Siri Scientific Press.
Shah, S. I. A., Ahmad, A., and Cai, W. (2022). Notes on Acanthaspis quinquespinosa Complex (Hemiptera: Reduviidae: Reduviinae) with Description of a New Species from Pakistan. Pakistan J. Zool. pp. 1–13. doi: 10.17582/journal.pjz/20210712100720
Swanson, D. R., Heads, S. W., Taylor, S. J., and Wang, Y. (2021). A new remarkably preserved fossil assassin bug (Insecta, Heteroptera, Reduviidae) from the Eocene Green River Formation of Colorado. Pap. Palaeontol. 7, 1459–1478. doi: 10.1002/spp2.1349
Wang, J., and Liang, A. P. (2015). Ultrastructure of the fossula spongiosa and pretarsus in Haematoloecha nigrorufa (Stål) (Hemiptera: Heteroptera: Reduviidae: Ectrichodinae). Zootaxa. 3963, 230. doi: 10.11646/zootaxa.3963.2.4
Weirauch, C. (2007). Hairy attachment structures in Reduviidae (Cimicomorpha, Heteroptera), with observations on the fossula spongiosa in some other Cimicomorpha. Zool. Anz. 246, 155–175. doi: 10.1016/j.jcz.2007.03.003
Weirauch, C. (2008). Cladistic analysis of Reduviidae (Heteroptera: Cimicomorpha) based on morphological characters. Syst. Entomol. 33, 229–274. doi: 10.1111/j.1365-3113.2007.00417.x
Weirauch, C., Bérenger, J. M., Berniker, L., Forero, D., Forthman, M., Frankenberg, S., et al. (2014). An illustrated identification key to assassin bug subfamilies and tribes (Hemiptera: Reduviidae). Can. J. Arthropod Identif. 26, 1–115.
Weirauch, C., and Cassis, G. (2006). Attracting ants: the trichome and novel glandular areas on the sternum of Ptilocnemus lemur (Heteroptera: Reduviidae: Holoptilinae). Entomol. Am-ny. 114, 28–37. doi: 10.1664/0028-7199(2006)114(28:AATTAN)2.0.CO;2
Weirauch, C., and Munro, J. B. (2009). Molecular phylogeny of the assassin bugs (Hemiptera: Reduviidae), based on mitochondrial and nuclear ribosomal genes. Mol. Phylogenet. Evol. 53, 287–299. doi: 10.1016/j.ympev.2009.05.039
Wygodzinsky, P., and Giacchi, J. C. (1994). Key to the genera of Stenopodainae of the new world (Insecta, Heteroptera, Reduviidae). Physis. 49: 5–9.
Wygodzinsky, P. W. (1966). A monograph of the Emesinae (Reduviidae, Hemiptera). Bull. Am. Mus. Nat. Hist. 133, 1–616.
Youssef, N. A., and Abd-Elgayed, A. A. (2015). Biological parameters of the predator, Amphibolus venator Klug (Hemiptera: Reduviidae) preying on larvae of Tribolium confusum Duv. (Coleoptera: Tenebrionidae). Ann. Agric. Sci. 60, 41–46. doi: 10.1016/j.aoas.2015.01.002
Zhang, G., and Weirauch, C. (2011). Matching dimorphic sexes and immature stages with adults: resolving the systematics of the Bekilya group of Malagasy assassin bugs (Hemiptera: Reduviidae: Peiratinae). Syst. Entomol. 36, 115–138. doi: 10.1111/j.1365-3113.2010.00551.x
Zhang, G., and Weirauch, C. (2014). Molecular phylogeny of Harpactorini (Insecta: Reduviidae): correlation of novel predation strategy with accelerated evolution of predatory leg morphology. Cladistics. 30, 339–351. doi: 10.1111/cla.12049
Keywords: Reduviinae, fossil, new genus, stridulitrum, fossula spongiosa, Early Cretaceous
Citation: Zhang P, Liu Y, Ren D and Yao Y (2022) The Oldest Fossils From China Provide the Most Direct Evidence for the Ancestral State of Fossula Spongiosa and Stridulitrum of Reduviidae. Front. Ecol. Evol. 10:927537. doi: 10.3389/fevo.2022.927537
Received: 24 April 2022; Accepted: 24 May 2022;
Published: 18 July 2022.
Edited by:
Chenyang Cai, Nanjing Institute of Geology and Paleontology (CAS), ChinaReviewed by:
Dominik Chłond, University of Silesia in Katowice, PolandCopyright © 2022 Zhang, Liu, Ren and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunzhi Yao, eWFveXoxMDBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.