- 1College of Life Science, Shihezi University, Shihezi, China
- 2Xinjiang Production and Construction Corps Key Laboratory of Oasis Town and Mountain-Basin System Ecology, Shihezi, China
Rapid monitoring and early elimination are important measures to control the spread of invasive plants. Ambrosia artemisiifolia is a globally distributed harmful invasive weed. The aim of this study was to clarify the invasion habitat preferences of A. artemisiifolia and the interspecific associations or phylogenetic relationships between this and native species in the Yili River Valley of Xinjiang, China. We identified the preferred habitat types of A. artemisiifolia, and investigated the composition and distribution of native species at the early stage of invasion by targeted sampling at 186 sites. By comparing the associations and phylogenetic distance between A. artemisiifolia and native species with those in Xinjiang and worldwide, we assessed the feasibility of using native species as indicators for rapid monitoring of A. artemisiifolia. A. artemisiifolia displayed an obvious invasive preference for semi-arid areas, particularly road margins (27.96%), forest (21.51%), farmland (19.35%), wasteland (12.37%), residential areas (10.75%), and grassland (8.06%). The composition and distribution of native species were similar across habitats, with more than 50% co-occurrence of A. artemisiifolia with Setaria viridis, Poa annua, Arrhenatherum elatius, Artemisia annua, Artemisia vulgaris, Artemisia leucophylla, Cannabis sativa, and Chenopodium album. A. artemisiifolia was more likely to show co-occurrence with closely related species. Overall, 53.85% of the above indicator native species with high co-occurrence were widely distributed in the potential suitable areas for A. artemisiifolia in Xinjiang. Globally, the species with the highest occurrence belonged to the genera Chenopodium (58%), Bromus, Poa, Setaria, and Trifolium (>40%). Therefore, native species with the strong association and phylogenetic distant relationship to A. artemisiifolia can be employed as indicators for rapid and accurate monitoring in semi-arid areas.
Introduction
Alien plant invasion can be roughly divided into three stages: introduction, colonization, and naturalization (Radosevich, 2007). Accurate monitoring at an early stage is essential for immediate detection and effective prevention and control of invasive plants. At present, the monitoring of alien invasive plants relies mainly on inefficient manual large-scale screening (Richter et al., 2013). Although hyperspectral data monitoring has been proposed as an alternative, the corresponding results are strongly affected by source data and model accuracy, on top of elevated operational costs (Thamaga and Dube, 2018; Al-Lami et al., 2021). Because the early invasive population is difficult to spot due to low density and patchy or sporadic distribution, attempts should focus on rapid, efficient, and low-cost invasive species monitoring technology in the early stage.
The growth and distribution of invasive plants depend on the environment. Understanding habitat requirements and species distribution is essential for the successful monitoring and effective control of alien species (Hauser and McCarthy, 2009; Giljohann et al., 2011). Although the introduction and diffusion of alien species are random and the habitats they colonize are very diverse, interactions with abiotic and biotic factors will define a preference for certain habitats, especially during establishment and population expansion (Hejda et al., 2015; Andelkovic et al., 2022). At a local scale, habitat type is the best predictor of plant invasion, trumping the importance of propagule pressure and climate (Chytrý et al., 2008a,b, 2009). Therefore, it is crucial to identify suitable or preferred habitat characteristics of alien species, and conduct a census in these habitats to enable rapid monitoring.
Interaction with native species plays an important role for individual survival and population growth of invasive plants, overcoming the constraints of abiotic factors and propagule pressure (Thomaz and Michelan, 2011; Waller et al., 2016). On the one hand, alien species usually show positive interspecific associations with widespread native species in specific habitats (Fridley et al., 2007; Lei et al., 2018). On the other hand, Darwin noted how the relationship between alien and native species affected the successful naturalization of the former (Darwin, 1929). According to this simple methodological framework, the relationship between alien and native species can be used to predict which species are prone to invasion in which ecosystems (Procheş et al., 2010).
Furthermore, regular changes to interspecific associations and kinship between invasive and native species could potentially define some general rules governing specific invasion trends, leading to common indicator species. These could be employed as search tools for alien species in targeted rapid monitoring.
The invasive annual herbaceous species Ambrosia artemisiifolia has become a global problem, particularly in Europe and China. Crop yields are reduced in the invaded areas, and the large amount of pollen produced is harmful to human health (Essl et al., 2015; Hamaoui-Laguel, 2018). A. artemisiifolia is strongly competitive. When exposed to water and nitrogen stress, it responds by adjusting biomass allocation and by making other phenotypic plasticity changes, which makes it highly adaptable to habitats likely to be invaded (Leskovšek et al., 2012a,b). This species, thus, has a wide range of suitable habitat types, including croplands, transportation corridors, wastelands, and riparian areas (Montagnani et al., 2017). Therefore, it is necessary to identify the preferred habitats of A. artemisiifolia and then use indicator species to rapidly and effectively monitor these habitats to carry out prevention and control as early as possible.
Ambrosia artemisiifolia is widely distributed in northeast, north, and south China (Feng et al., 2012; Xing, 2012; Liu, 2019). Previously, we found that Yili Valley, located in the arid and semi-arid Xinjiang Province of China, was first invaded by A. artemisiifolia in 2010 (Dong et al., 2020). In this study, we investigated the community invaded by A. artemisiifolia in the Yili Valley to identify the preferred habitat of this species in semi-arid regions. Moreover, we evaluated the interspecific associations between A. artemisiifolia and native species in each invasive habitat, as well as the corresponding phylogenetic relationship. Finally, we used this information to determine whether the indicator species of Yili Valley could be employed as universal predictors for all of Xinjiang and global distribution areas of A. artemisiifolia. As an overall objective, this study is expected to aid the rapid monitoring of A. artemisiifolia in semi-arid areas.
Materials and methods
Study area
The Yili Valley (42°14′–44°53′N, 80°09′–84°56′E) lies in the westernmost part of the Tianshan Mountain Range of Xinjiang. The Valley comprises 56,400 km2, and has a continental temperate arid climate. The region has an average annual temperature of 10.4°C and precipitation of 417.6 mm. In grassland habitats, which represent the wettest area in Yili River, precipitation can reach 500 mm annually. The Yili Valley, with its rich plant diversity and extensive seed dispersal via canals, cattle, sheep, and tourists, provides favorable conditions for the invasion and rapid spread of alien species (Jia et al., 2011).
Experimental design and statistical analysis
Habitat investigation and sample setup of Ambrosia artemisiifolia invasion
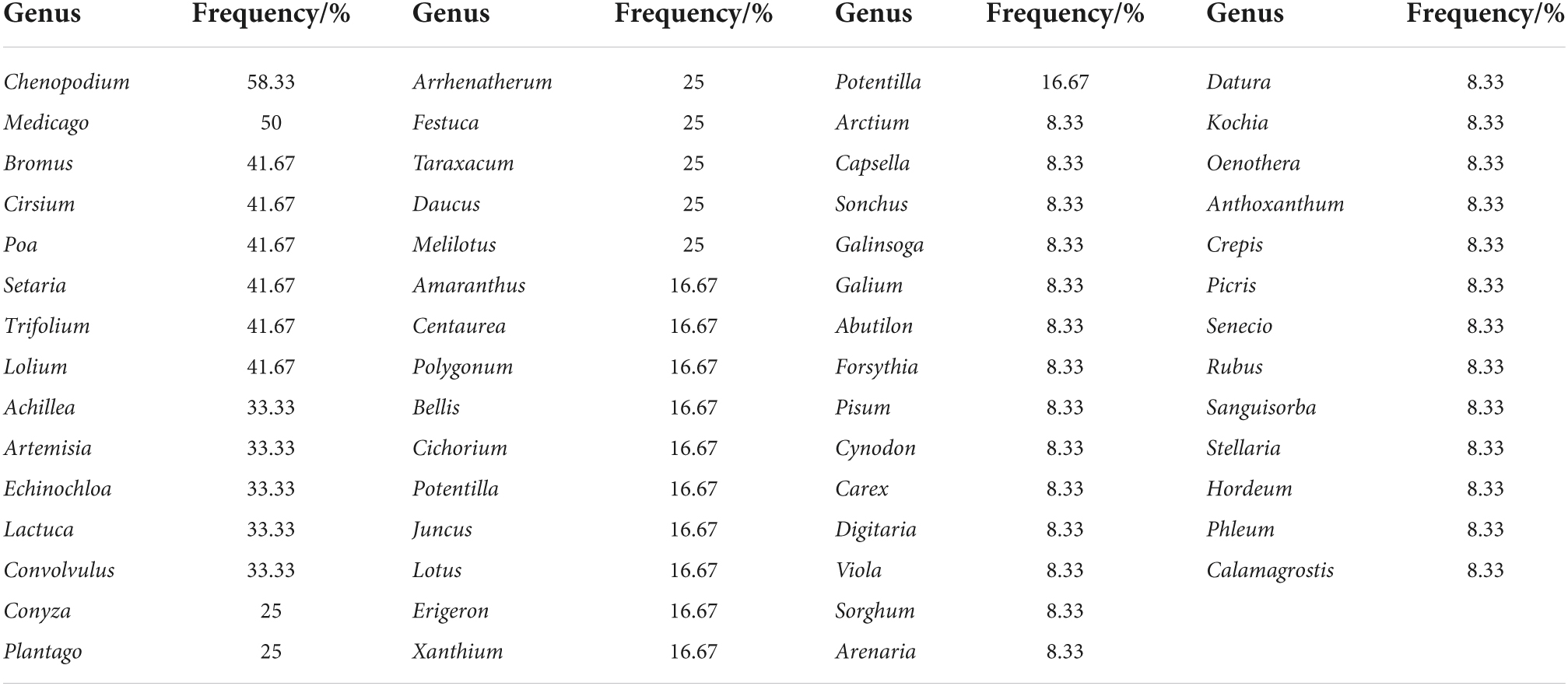
In August 2020, all communities at the initial stage of invasion by A. artemisiifolia (≤20% population coverage) were surveyed (Fenesi et al., 2015). Each invasive community was considered an invasion point, which was demarcated by latitude and longitude based on GPS data, as well as by habitat type (Figure 1).
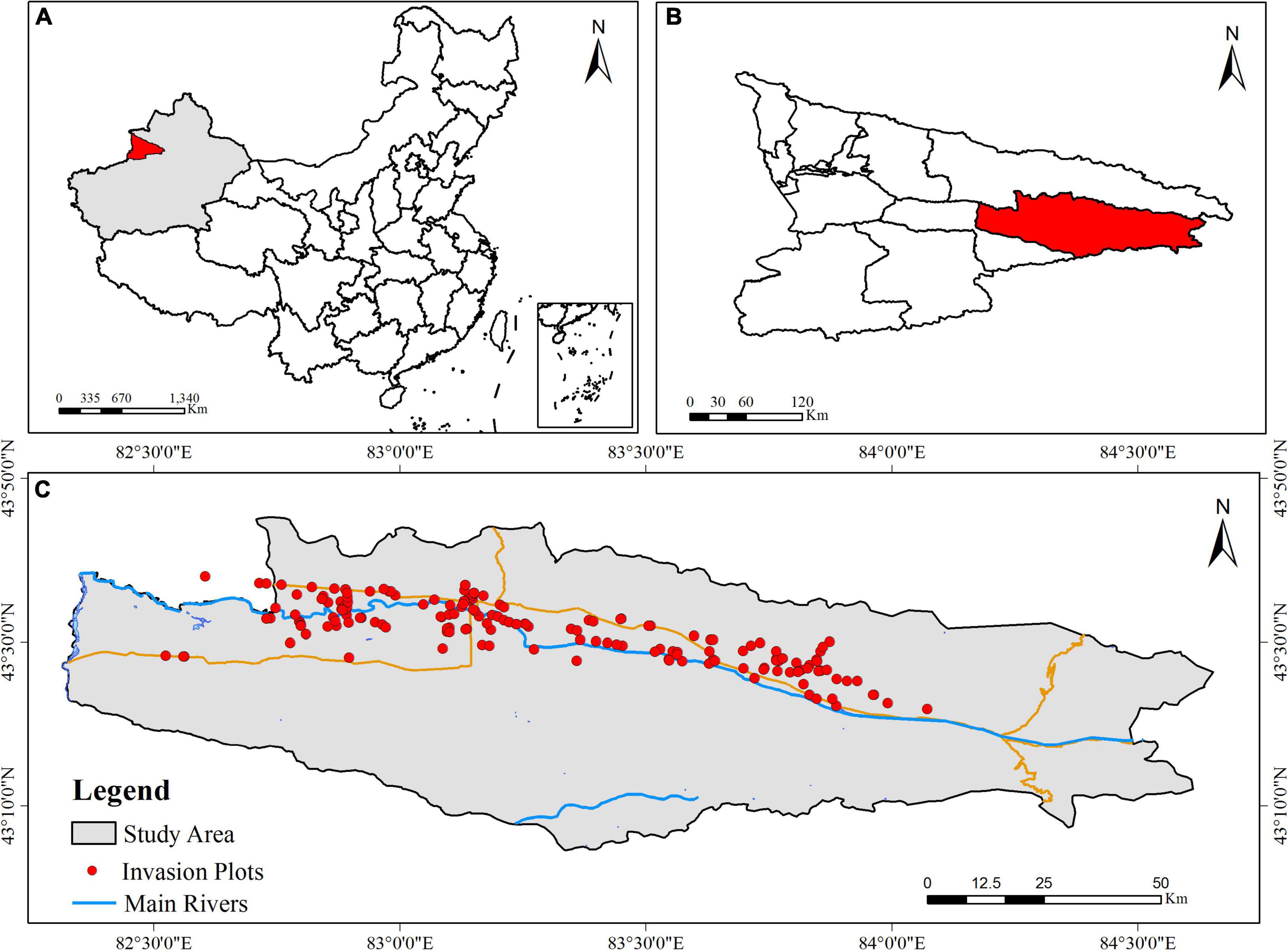
Figure 1. Distribution points of Ambrosia artemisiifolia invasion communities in the study area. (A) The semi-arid regions Xinjiang is located in northwest of China. (B) The Yili Valley is located in northwest of Xinjiang. (C) Study area in the Yili Valley.
Observation plots were set up in the areas of A. artemisiifolia distribution within each habitat. Continuous or discontinuous 2 m × 2 m squares were used to cover the distribution of A. artemisiifolia. Species frequency was defined as the number of squares with a particular species/total number of squares × 100%. After the species frequency calculation of a certain habitat samples was completed, the species frequency of all samples in the habitat were regarded as repeated, and the mean and standard deviation were calculated as the species frequency of the habitat type.
There were some alien species (such as Conyza canadensis) that, according to our observations, were successfully naturalized in the study area. These species did not cause harm, and their distribution was stable and common. Therefore, we considered these as native species during our statistical analysis.
For species that could not be accurately identified in the field, plants were collected and identified in the laboratory. Growth forms and species taxonomy were identified according to descriptions in the Flora of China (Chinese Academy of Sciences Flora of China Editorial, 1998), Flora of Xinjiang (Editorial Committee of Xinjiang Flora, 1992–2011).
Interspecific associations between Ambrosia artemisiifolia and native species in the Yili Valley
Interspecific association reflects the coexistence of two species. A strong interspecific association indicates that ecological demand and habitat selection of A. artemisiifolia and native species have strong convergence and divergence. The χ2 statistic, association coefficient (AC), percentage of co-occurrence (PC), Ochiai index (OI), and dice index (DI) were used to determine the associations and co-occurrence probability between A. artemisiifolia and native species. To avoid bias introduced by rare species, taxa with a frequency < 10% were excluded from the analysis.
A 2 × 2 contingency table was constructed to show the coexistence interspecific associations between A. artemisiifolia and native species. The significance of interspecific associations were assessed by chi-square tests with Yates correction as follows:
Each A. artemisiifolia invasion point was taken as the unit of calculation, with N representing the total number of A. artemisiifolia invasion points, a the number of points in which two species co-occurred, b and c the number of points in which only one species occurred, and d the number of points in which none of the species occurred. For n = 1 degrees of freedom, the interspecific association of each species pair was non-significant if χ2 < 3.841 (P > 0.05), significant if 3.841 < χ2 < 6.635 (0.01 < P < 0.05), and highly significant if χ2 > 6.635 (P < 0.01). A positive association was indicated if ad > bc, and a negative association was indicated if ad < bc. The χ2 statistic could objectively and accurately reflect the significance of association between species pairs, but it could not quantify the closeness of this association.
The AC can further reflect the degree of interspecific associations, but it tends to exaggerate the significance of such associations in the absence of both species. The AC is calculated as follows:
The AC range is [−1, 1], and the closer it is to 1, the stronger is the positive association between species; whereas a value close to −1 indicates a strong negative association. AC = 0 indicates complete independence between species.
The PC reflects the degree of positive association between species, but tends to exaggerate the role of a, b, and c in the determination of association. The formula is PC = a/(a+b+c). The PC range is [0, 1]; the closer the value is to 1, the higher is the degree of positive connection, and the more likely two species are to appear or not together. Consequently, the ecological habitats and environmental requirements of the two species become more consistent.
The OI and DI can accurately reflect the probability and degree of association between different species pairs, and overcome the deviation caused by the large influence of d on the point AC. They are calculated as follows:
The range of these two indices is [0, 1]; the closer either of them is to 1, the higher is the degree of positive association between species pairs, and the higher is the probability of co-occurrence.
Phylogenetic relationship between Ambrosia artemisiifolia and native species in the Yili Valley
The PhyloMaker package in R was used to generate a phylogenetic tree framework, with the phylogenetic clade based on the APG classification system. The latter was done after the list of all families and species obtained from the survey was corrected in R 4.1.3 The phylogenetic distance (PD) between the invader and resident species in a recipient community was used as a metrics to represent their phylogenetic relatedness (Feng and Fu, 2008; Tretyakova et al., 2021). PD values were calculated uniformly for all indigenous species, regardless of habitat, using the picante package in R.
Distribution and representativeness of indicator species from the Yili Valley in potential suitable areas of Xinjiang
The classification of potential suitable areas of A. artemisiifolia plays an important role in early warning and effective monitoring. Through the Maxent model, Ma et al. (2020) used environmental factors, including climate data (precipitation and temperature); soil data; land use data; and altitude, slope, and aspect data, to identify the 13 areas listed in this study that are potentially suitable for A. artemisiifolia. The Flora of Xinjiang (Editorial Committee of Xinjiang Flora, 1992–2011) was consulted to check whether indicator species from the Yili Valley were distributed in all potential suitable areas for A. artemisiifolia in Xinjiang, and their proportion was calculated. Determining whether the main habitat types of the species recorded in the Flora were similar to those of indicator species could attest to the universality and representativeness of such species across Xinjiang.
Prevalence and representation of indicator and native species of the Yili Valley in major invasive areas of Ambrosia artemisiifolia across the world
To assess the difference and similarity of species composition between indicator species in the Yili Valley and native species in the main worldwide invasion areas of A. artemisiifolia, we search the literatures of associated species in the world’s recorded A. artemisiifolia distribution areas and count the native species present in the literatures (Igrc et al., 1995; Song and Prots, 1998; Makra et al., 2005; Brandes and Nitzsche, 2006; Fumanal et al., 2006; Essl et al., 2009; Gajnik and Peternel, 2009; Galzina et al., 2010; Patracchini and Ferrero, 2011; Puc and Wolski, 2013; Csontos et al., 2015; Gentili et al., 2016; Romain et al., 2016; Abramova, 2018; Chadaeva et al., 2018; Mang et al., 2018; Gusev, 2019; Petrova, 2019; Pinke et al., 2019).
To prevent discrepancies arising from different classification methods, the genera of recorded species were used to calculate the number of references for the occurrence of a species (i.e., the occurrence frequency of the species relative to the total number of references, which was 19). By arranging the frequency of each species, the similarity between the identified indicator species and the associated species of A. artemisiifolia was determined. This calculation defined the universality and representativeness of the species in present global distribution areas of A. artemisiifolia.
Results
Habitat types and species composition of Ambrosia artemisiifolia in the Yili Valley
A total of 186 invasion sites of A. artemisiifolia in the Yili Valley were cataloged. The invasive communities encompassed six habitat types: road margins (52), forest (40), farmland (36), wasteland (23), residential area (20), and grassland (15). A. artemisiifolia could not be found in other habitats, suggesting clear habitat preference for invasion.
Composition and distribution frequency of native species were similar across all habitats (Table 1). Specifically, 17 species were common in all habitats; they accounted for 60.71% of grassland species, 53.13% of farmland species, 56.67% of forest species, 60.71% of road margins species, 77.27% of residential area species, and 80.95% of wasteland species.
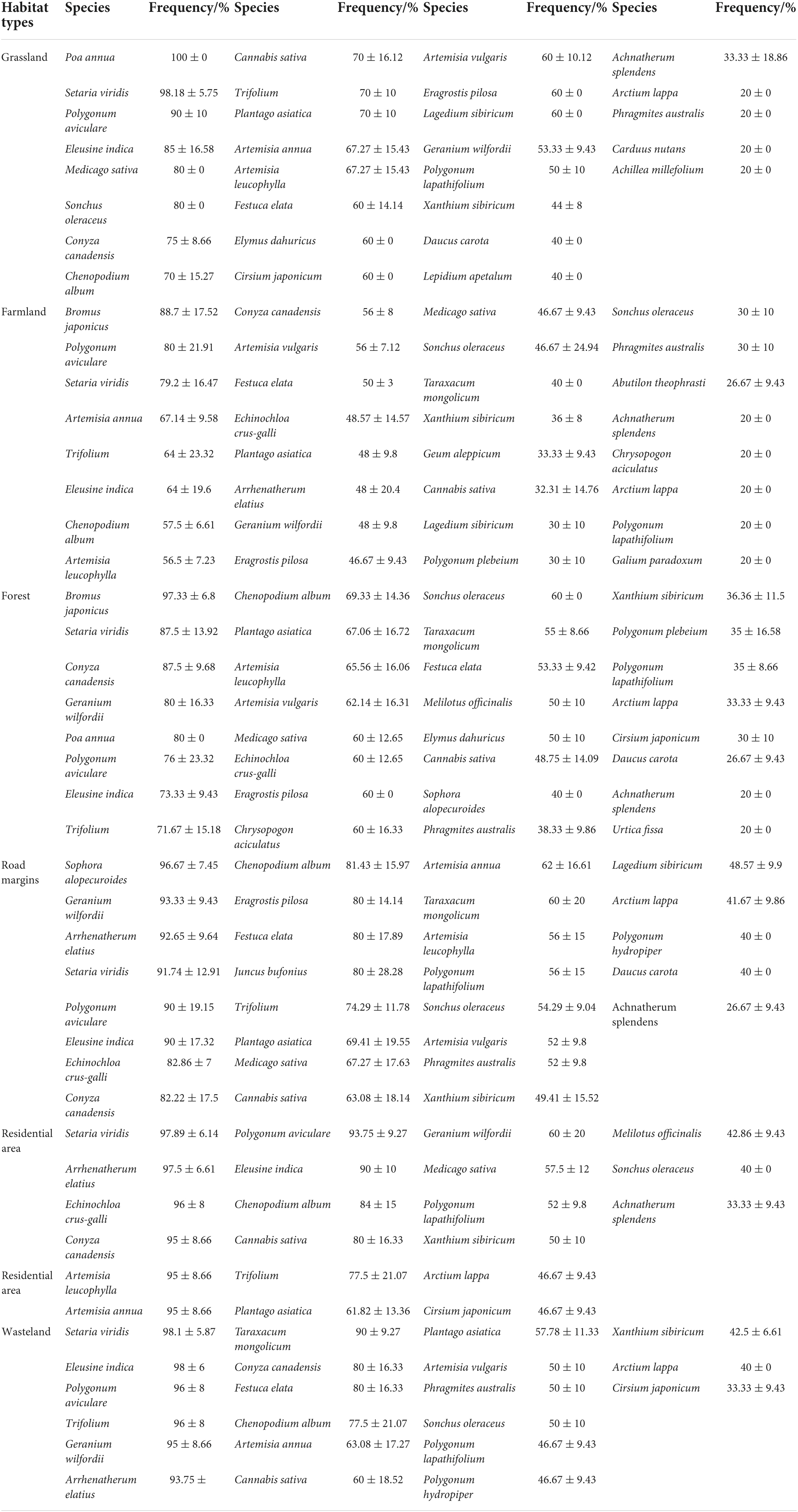
Table 1. Distribution frequency (Mean ± SD) of native species in Ambrosia artemisiifolia invasive communities in the Yili Valley.
The most frequent grass species were Setaria viridis, Poa annua, Bromus spp., Arrhenatherum elatius, Echinochloa crus-galli, Eleusine indica, and some Artemisia species. Other common species were Chenopodium album, Plantago asiatica, C. canadensis, Cannabis sativa, and Trifolium spp. These results pointed to similar species composition and distribution in the habitats invaded by A. artemisiifolia (Table 1).
Interspecific associations between Ambrosia artemisiifolia and native species
No significant interspecific association between A. artemisiifolia and native species could be detected in all habitats. S. viridis, P. annua, and Bromus spp. correlated positively with A. artemisiifolia in all habitats; whereas A. elatius did so only in road margins, residential areas, and wasteland. Artemisia annua, Artemisia vulgaris, and Artemisia leucophylla showed similar association characteristics and were positively associated with A. artemisiifolia in all habitats. Instead, C. sativa exhibited positive association with A. artemisiifolia in farmland and grassland, but a negative association in other habitats. A negative association was observed also between C. album and A. artemisiifolia in all habitats (Table 2).
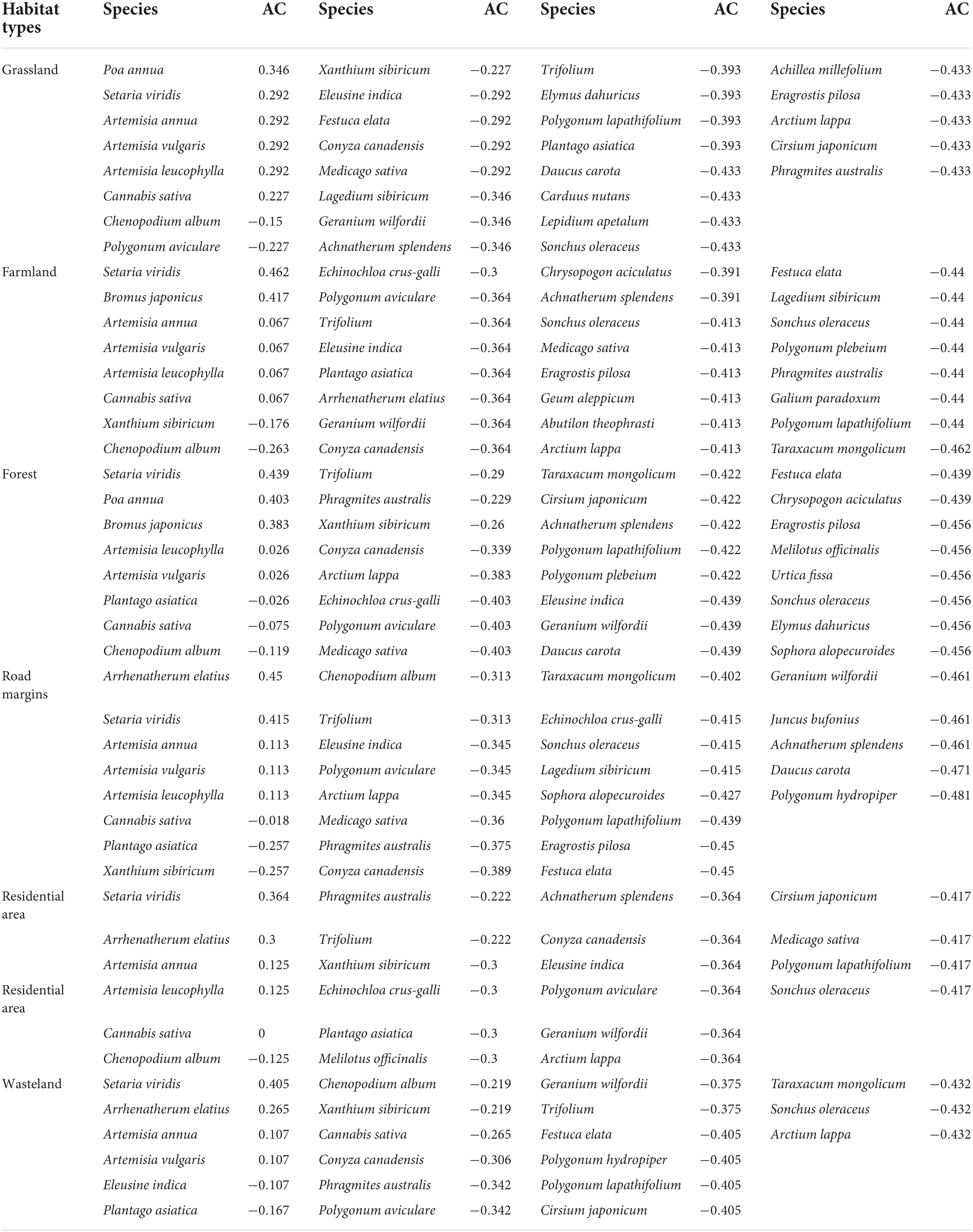
Table 2. Association coefficient (AC) between native species and Ambrosia artemisiifolia invasive habitat community in the Yili Valley.
The PC of A. artemisiifolia and native species in different habitats was above 50% and reached up to 96.2% for S. viridis, P. annua, Bromus spp., A. annua, A. vulgaris, and A. leucophylla in all habitats. The PC of A. elatius in road margin, residential area, and wasteland habitats were above 60%, with a peak of 95.2%. Except for wasteland, the OI and DI values of C. sativa surpassed 60% in all habitats; whereas those of C. album were greater than 50% in all habitats (Table 3).
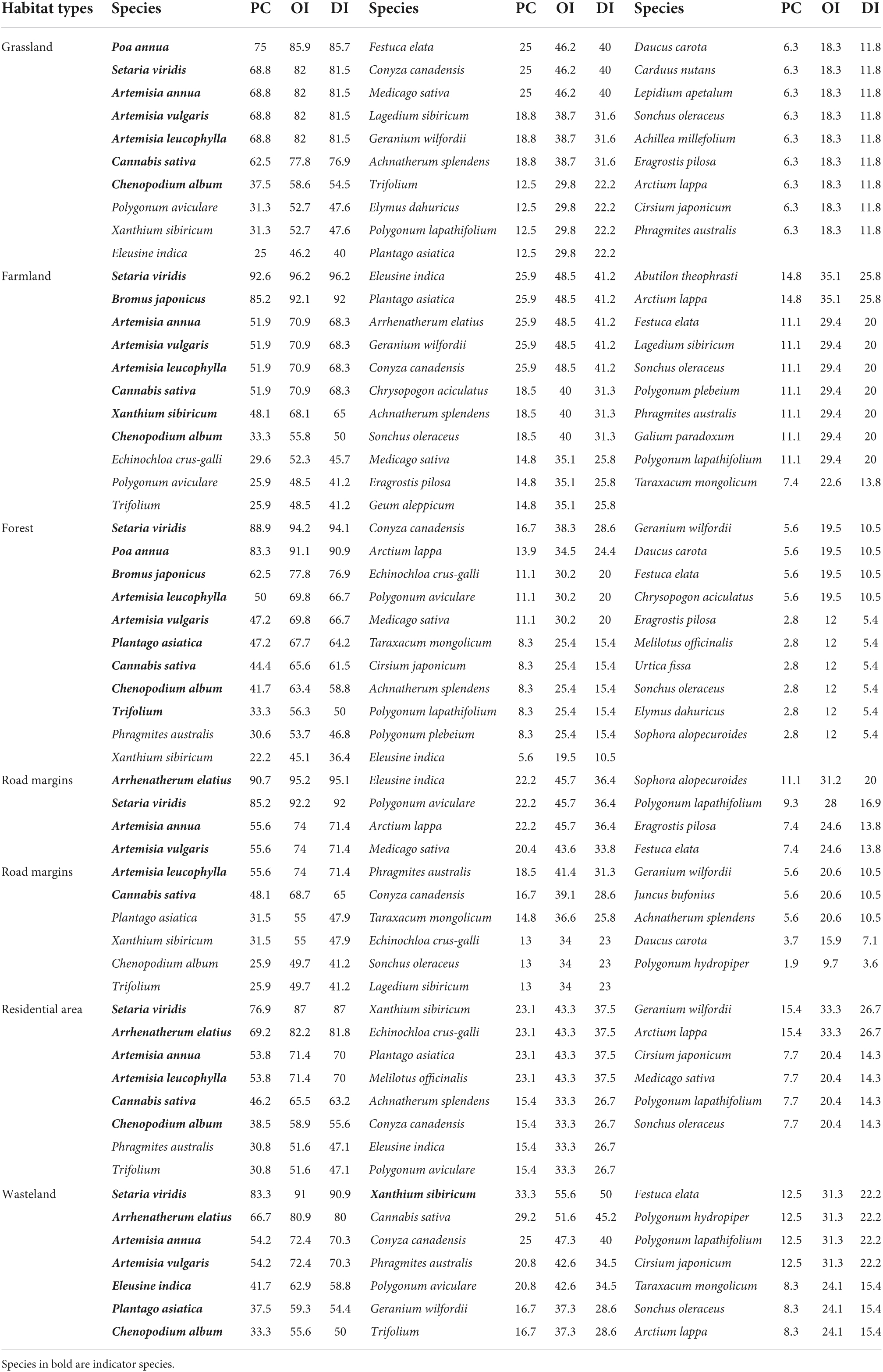
Table 3. Common percentages of native species and Ambrosia artemisiifolia invasive habitat communities in the Yili Valley.
Various native species and A. artemisiifolia showed different PC due to different habitats (Table 3). The PC of A. artemisiifolia and Xanthium sibiricum in farmland reached 68.1%, that of Bromus spp. in forest reached 77.8%, that of Trifolium reached 56.3%, that of E. indica in wasteland reached 68.1%, and that of P. asiatica reached 59.3%.
Phylogenetic relationship between Ambrosia artemisiifolia and its native companion species
The greatest PD (376.57 MA) was observed between A. artemisiifolia and species of the Gramineae family with elevated PC, such as S. viridis, P. annua, and Bromus spp. The PD between A. artemisiifolia and C. album, C. sativa, and Trifolium spp. increased over time, with the proportion of species showing a distant relationship being greater in the early stages of A. artemisiifolia invasion (Figure 2).
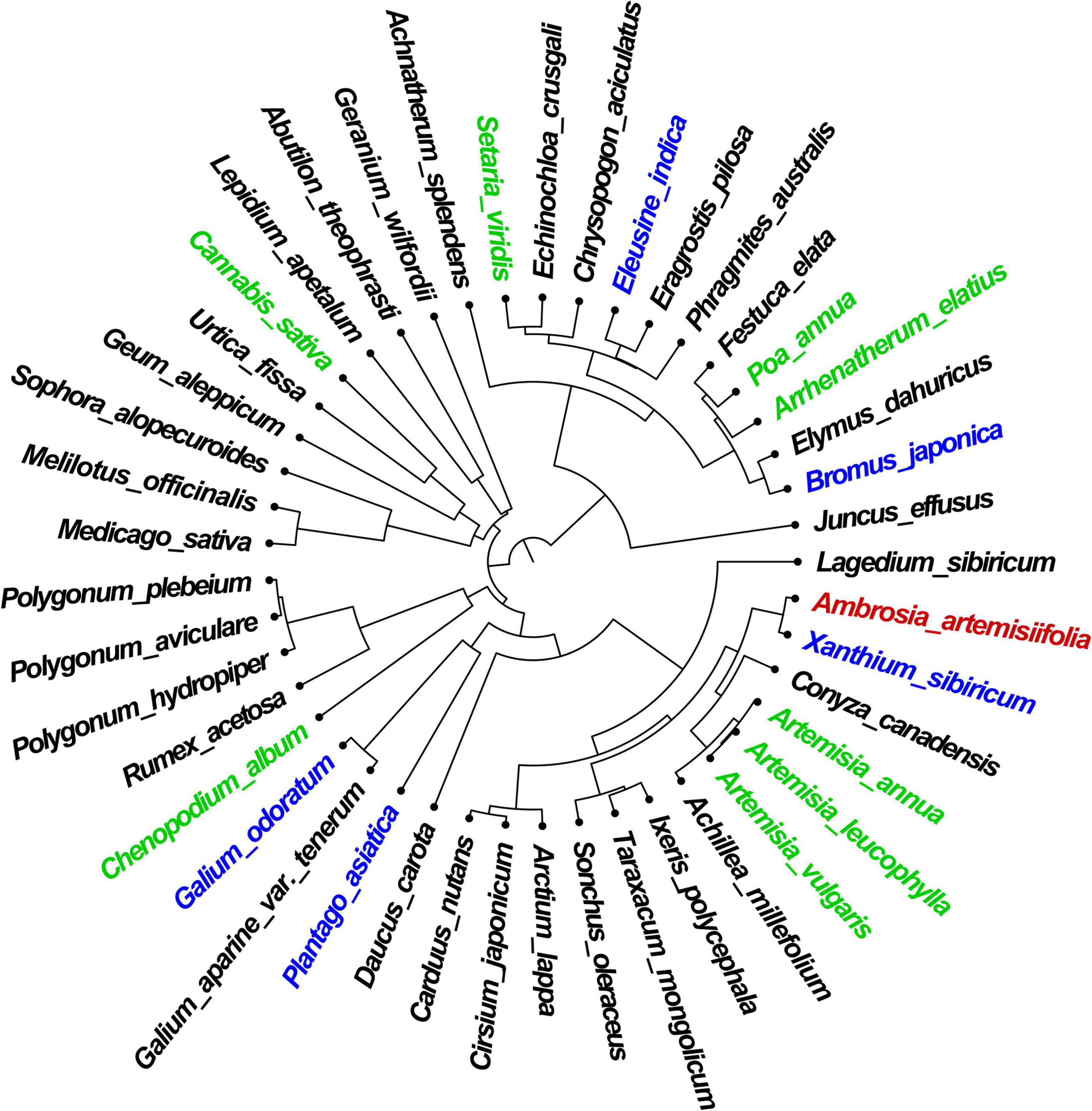
Figure 2. Phylogenetic distance between Ambrosia artemisiifolia (red-colored) and native species in the study area. Green-colored species are the indicator species present in all habitat types. Blue-colored species are indicator species that occur only in a particular habitat type.
Indicator species in the potential habitat of Ambrosia artemisiifolia in Xinjiang
Poa annua, C. album, Trifolium spp., and A. elatius are widespread in Xinjiang, and their distribution area covered 100% of the potential suitable areas of A. artemisiifolia in this region. The distribution of A. annua showed a 46.15% overlap with potential suitable areas of A. artemisiifolia in Xinjiang, including severe, moderate, and mildly suitable areas. The degree of overlap between A. artemisiifolia, Bromus spp., S. viridis, A. leucophylla, and P. asiatica was only 30.77%, decreasing further to 15.38% for C. sativa, X. sibiricum, and E. indica. Notably, the habitats defined by each indicator species were similar to the main habitat types in the suitable area, especially in farmland, forest, road margins, and wasteland (Table 4).
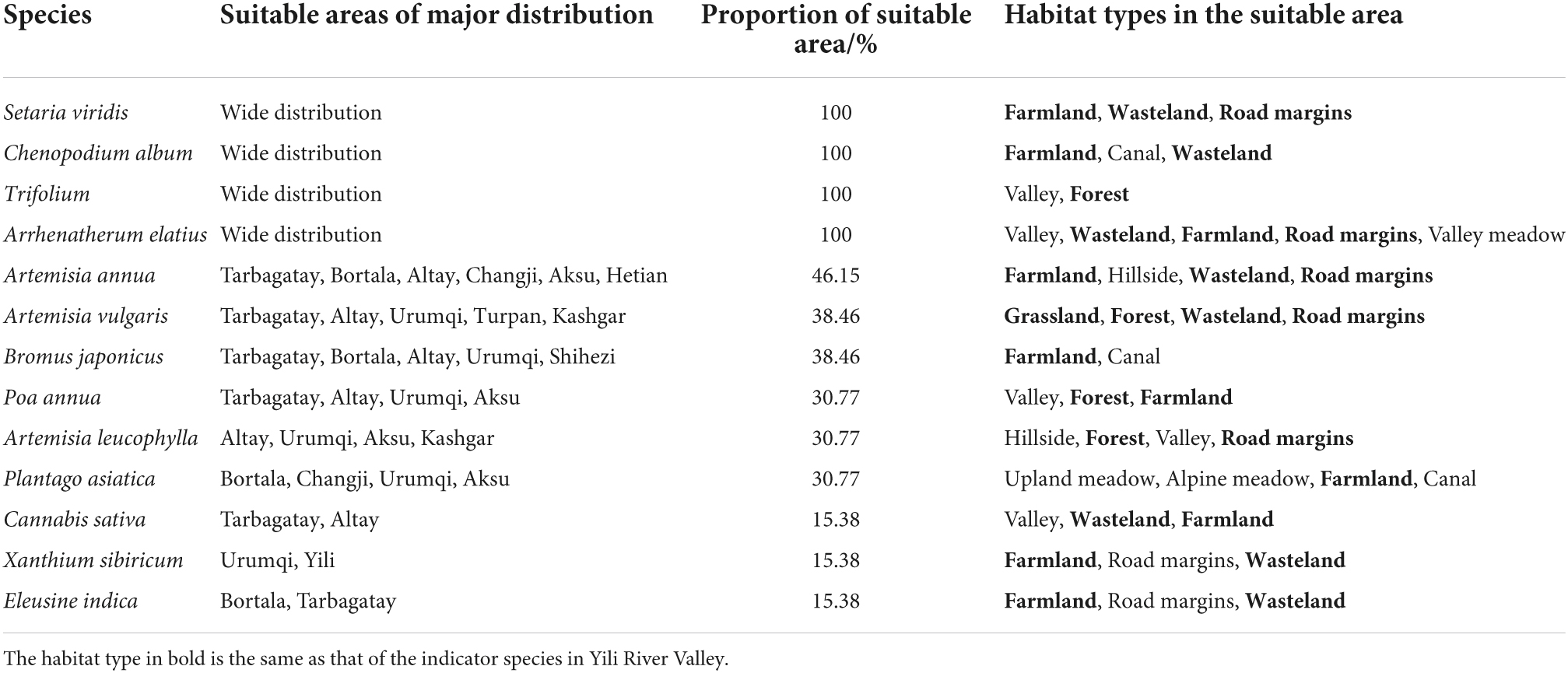
Table 4. Distribution of indicator species in potential suitable areas of Ambrosia artemisiifolia in Xinjiang.
Representation of indicator species from the Yili Valley in global distribution areas of Ambrosia artemisiifolia
In terms of species composition, 13 families (72.22%), 31 genera (61.76%), and 22 species (39.29%) were shared between the native species of the Yili Valley and those of areas currently infested by A. artemisiifolia across the world (Table 5). Among them, species belonging to the Gramineae and Compositae families accounted for the largest share. These common species had a high frequency of distribution in the Yili Valley.
Among all indicator species, C. album had the highest frequency of occurrence (58%); whereas species belonging to the Bromus, Poa, Setaria, and Trifolium genera appeared more than 40% of the time (Table 5).
Discussion
Ambrosia artemisiifolia shows obvious habitat preference when invading a semi-arid area
The successful establishment of alien invasive species is determined by the fluctuation of abiotic environmental factors, propagule pressure, and the interaction between species (Pysek and Chytry, 2014). Habitat conditions play a fundamental role by influencing the invasion process and the composition of native species, thus defining the relationship between the latter and the invaders, as well as the spread and harm caused by these (Catford et al., 2012; Hejda et al., 2015).
Water is an important factor affecting species distribution. Indeed, precipitation contributes more than 50% to the potential distribution of A. artemisiifolia (Ma et al., 2020; Liu et al., 2021). Precipitation above 280 mm promoting growth and propagation of A. artemisiifolia. Precipitation in most areas of The Yili Valley is above 280 mm; while the average precipitation in Xinyuan County, where A. artemisiifolia is particularly abundant, is 417 mm (Dong et al., 2020). Therefore, in low-lying areas, such as grassland, farmland, and road margins, the water supply can meet the needs for germination, growth, and reproduction of A. artemisiifolia seeds, thereby ensuring successful settlement and population expansion. By simulating the effect of different precipitation levels on the growth of A. artemisiifolia, found that A. artemisiifolia was highly adaptable to drought (Leiblein and Lösch, 2011; Leskovšek et al., 2012a), explaining its widespread distribution in a habitat with little water such as wasteland. Temperature had no significant effect on the growth and distribution of A. artemisiifolia in the Yili Valley (Dong et al., 2020).
Although the habitats of A. artemisiifolia across the world are not exactly the same as those in this study, priority targeting of habitats preferentially invaded by A. artemisiifolia is the basis for rapid surveillance (Epanchin-Niell and Hastings, 2010). This study describes the habitats that may be preferred for invasion by A. artemisiifolia throughout the world.
The probability of co-occurrence between Ambrosia artemisiifolia and species with strong positive correlation and distant relationship is higher at the neighborhood scale
In this study, the invasive community of A. artemisiifolia was at the stage from establishment to population growth, and the association between A. artemisiifolia and other species were weak (Table 2). The lack of any significant association on the whole indicated that the current invasive community of A. artemisiifolia was in a dynamic succession process and had not yet stabilized (Liu et al., 2017). At this stage, interspecific competition was weak, meaning that native species ecologically similar to A. artemisiifolia, including S. viridis, P. annua, A. elatius, A. annua, A. vulgaris, A. leucophylla, C. sativa, and C. album, as well as other dominant native plants in the community, did not compete intensely for resources and exhibited a high PC (Lei et al., 2018).
At the same time, species related to A. artemisiifolia were detected in all habitats, indicating similar habitat selection and adaptation (Ozaslan et al., 2016). However, at the neighborhood scale (i.e., within the habitat sample in this study), the PC of A. artemisiifolia and other local related species, such as X. sibiricum., Arctium lappa, and C. canadensis, was below 50% and as low as 6.3% (Table 3). Only Artemisia species presented higher PC. All Asteraceae species accounted for a very small proportion of indicator species.
Darwin’s naturalization and pre-adaptation hypotheses need not be mutually exclusive. Phylogenetic similarity may be both close and distant in the same system, as it may vary across spatial scales and at different stages of invasion (Diez et al., 2008; Procheş et al., 2010; Cadotte et al., 2018; Tretyakova et al., 2021). At fine spatial scales (in relation to plant size), one can expect closely related organisms to exist in mutually exclusive patterns due to competitive interactions. Species less closely related to the local community are more likely to coexist by minimizing competitive exclusion (Maitner et al., 2021). Li et al. (2015) found that the probability of invader establishment declined with increasing PD between the invader and residents; whereas the average size of surviving invader individuals increased with PD. Because of their adaptability to environmental conditions, successfully established A. artemisiifolia became more closely related to the community during the invasion stage, but grew phylogenetically more distant over time, as they were striving to replace closely related native plants (Ma et al., 2016). These studies suggest that the Darwin’s pre-adaptation hypothesis is more applicable to large scales and early stages of establishment, while Darwin’s naturalization hypothesis is applicable to neighborhood scales and late growth stages, which is similar to our results.
Indicator species are universal and representative
This study found a similar species composition of invasive communities across different habitats (Table 1). Native species were present in all invasive habitats except for some species, such as Achillea millefolium, Sophora alopecuroides, and Melilotus spp., in residential areas, grassland, and forest. Among widely distributed native species, S. viridis, P. annua, A. elatius, A. annua, A. vulgaris, A. leucophylla, C. sativa, and C. album exhibited positive correlation with A. artemisiifolia in all habitats and a high PC (Tables 2, 3). These indicator species accounted for 33.33% of native species, whose distribution frequency was more than 50% in the grassland, 81.82% in the farmland, 42.86% in the forest, 26.09% in the road margins, 37.5% in the residential areas, and 37.5% in the wasteland. These species not only reflect the co-occurrence with A. artemisiifolia, but are also representative and universal in various habitats, as well as easy to locate and identify. In the same way, X. sibiricum in the farmland, Bromus in the forest, Trifolium in the wasteland, and P. asiatica are good indicators and representative of their respective habitats.
By comparing similarities between the distribution of indicator species in potential suitable areas of A. artemisiifolia in the Yili Valley and the main habitat types, we found that indicator species grew in all such areas. In Tarbagatay and Bortala, more than 80% of indicator species were present in each habitat. The Maxent model predicted that precipitation in these two areas could meet the demand of A. artemisiifolia (Ma et al., 2020). Grassland was the main habitat type in the potential distribution area within the Yili Valley. This suggests that the distribution of indicator species from the Yili Valley points to potential suitable areas of A. artemisiifolia throughout Xinjiang. Therefore, it is feasible to rapidly monitor A. artemisiifolia by targeting indicator species in preferred invasive habitats of semi-arid regions.
In this study, native species associated with A. artemisiifolia across the world were counted by genus, limiting the influence of taxonomic differences and distribution habitat heterogeneity on the results. Except for C. sativa, which appeared only in the Yili River Valley, other species were distributed in all areas invaded by A. artemisiifolia. Setaria, Bromus, Elytrigia, Artemisia, and other species presented a wide distribution range. These results provide guidance and a reference for the worldwide rapid monitoring of A. artemisiifolia invasion.
At the time of monitoring, the worldwide distribution of A. artemisiifolia, and the composition and distribution of native species varied across habitats. Additionally, habitats with indicator species may not necessarily contain A. artemisiifolia because of non-dispersal or unsuccessful establishment. However, our study provides a reference key for finding common dominant native species as monitoring clues for the preferred habitat of A. artemisiifolia invasion. This is particularly true of Chenopodium spp., whose PC was 58% in the presence of A. artemisiifolia and reached up to 63.4% in a forest habitat (Table 3). Such examples improve dramatically the surveillance at an early stage of the invasion process, thereby facilitating prevention and control efforts.
Conclusion
In semi-arid areas, the preferred habitat of A. artemisiifolia and the transmission channel to surrounding areas can be accurately monitored by looking at the indicator species, i.e., dominant native species with strong correlation and distant phylogenetic relationship to A. artemisiifolia. Building on the potential suitable areas for A. artemisiifolia predicted by the Maxent model, this study provides clues for improved monitoring of this invasive species, thus reducing costs. All A. artemisiifolia found during monitoring should be removed in a timely manner to prevent the species from quickly forming dense populations and causing further harm.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WZ conceived this study, performed data analyses, and wrote the manuscript. MS, HW, XL, and PS collected data of this study. TL led and coordinated the project. All authors read and approved the final manuscript.
Funding
This study was supported by Key Science and Technology Project of Xinjiang Production and Construction Corps (No. 2022AB010) and National Natural Science Foundation of China (No. 31770461).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abramova, L. M. (2018). Distribution of invasive species of Ambrosia L. genus in the south urals (Republic of Bashkortostan). Russ. J. Biol. Invasions 9, 1–8. doi: 10.1134/S2075111718010022
Al-Lami, A. K., Abbood, R. A., Maliki, A., and Al-Ansari, N. (2021). Using vegetation indices for monitoring the spread of nile rose plant in the tigris river within wasit province, Iraq. Remote Sensing Appl. Soc. Environ. 22:100471. doi: 10.1016/j.rsase.2021.100471
Andelkovic, A., Pavlovic, D., Marisavljevic, D., Zivkovic, M., Novkovic, M., Popovic, S., et al. (2022). Plant invasions in riparian areas of the middle danube basin in serbia. NeoBiota 71, 23–48. doi: 10.3897/neobiota.71.69716
Brandes, D., and Nitzsche, J. (2006). Biology, introduction, dispersal, and distribution of common ragweed (Ambrosia artemisiifolia L.) with special regard to Germany Biologie, Einschleppung, Ausbreitung und Verbreitung der Beifublttrigen Ambrosie (Ambrosia artemisiifolia L.) unter besonderer. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 58, 286–291.
Cadotte, M. W., Campbell, S. E., Li, S. P., Sodhi, D. S., and Mandrak, N. E. (2018). Preadaptation and naturalization of nonnative species: darwin’s two fundamental insights into species invasion. Annu. Rev. Plant Biol. 69, 661–684. doi: 10.1146/annurev-arplant-042817-040339
Catford, J. A., Vesk, P. A., Richardson, D. M., and Pyšek, P. (2012). Quantifying levels of biological invasion: towards the objective classification of invaded and invasible ecosystems. Global Change Biol. 18, 44–62. doi: 10.1111/j.1365-2486.2011.02549.x
Chadaeva, V. A., Shhagapsoeva, K. A., Tsepkova, N. L., and Shhagapsoev, S. H. (2018). Monitoring of ambrosia artemisiifolia l. distribution in meadow phytocenoses of kabardino-balkarian republic (Central Caucasus). Russ. J. Biol. Invasions 9, 195–203. doi: 10.1134/S2075111718020030
Chinese Academy of Sciences Flora of China Editorial (1998). Flora of China. Beijing: Science Press.
Chytrý, M., Jarošík, V., Pyšek, P., Hájek, O., Knollová, I., Tichý, L., et al. (2008a). Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 89, 1541–1553. doi: 10.1890/07-0682.1
Chytrý, M., Maskell, L. C., Pino, J., Pyšek, P., Vilà, M., Font, X., et al. (2008b). Habitat invasions by alien plants: a quantitative comparison among mediterranean, subcontinental and oceanic regions of Europe. J. Appl. Ecol. 45, 448–458. doi: 10.1111/j.1365-2664.2007.01398.x
Chytrý, M., Pyšek, P., Wild, J., Pino, J., Maskell, L. C., and Vilà, M. (2009). European map of alien plant invasions based on the quantitative assessment across habitats. Diversity Distribut. 15, 98–107. doi: 10.1111/j.1472-4642.2008.00515.x
Csontos, P., Angyal, Z., Chmura, D., Nagy, J., and Tamás, J. (2015). New stand of invasive neophyte Ambrosia artemisiifolia L. and its potential reproduction. Polish J. Ecol. 63, 453–459. doi: 10.3161/15052249PJE2015.63.3.015
Diez, J. M., Sullivan, J. J., Hulme, P. E., Edwards, G., and Duncan, R. P. (2008). Darwin’s naturalization conundrum: dissecting taxonomic patterns of species invasions. Ecol. Lett. 11, 674–681. doi: 10.1111/j.1461-0248.2008.01178.x
Dong, H., Song, Z., Liu, T., Liu, Z., Liu, Y., Chen, B., et al. (2020). Causes of differences in the distribution of the invasive plants Ambrosia artemisiifolia and Ambrosia trifida in the Yili Valley, China. Ecol. Evol. 10, 13122–13133. doi: 10.1002/ece3.6902
Epanchin-Niell, R. S., and Hastings, A. (2010). Controlling established invaders: integrating economics and spread dynamics to determine optimal management. Ecol. Lett. 13, 528–541. doi: 10.1111/j.1461-0248.2010.01440.x
Essl, F., Biró, K., Brandes, D., Broennimann, O., Bullock, J. M., Chapman, D. S., et al. (2015). Biological flora of the british isles: Ambrosia artemisiifolia. J. Ecol. 103, 1069–1098. doi: 10.1111/1365-2745.12424
Essl, F., Dullinger, S., and Kleinbauer, I. (2009). Changes in the spatio-temporal patterns and habitat preferences of Ambrosia artemisiifolia during its invasion of Austria. Preslia 81, 119–133.
Fenesi, A., Vágási, C., Beldean, M., Földesi, R., Kolcsár, L., Shapiro, J. T., et al. (2015). Solidago canadensis impacts on native plant and pollinator communities in different-aged old fields. Basic Appl. Ecol. 16, 335–346. doi: 10.1016/j.baae.2015.03.003
Feng, L., Yue, M. F., Tian, X. S., Qi, G. J., and Lv, L. H. (2012). Distribution and growth characteristics of common ragweed (Ambrosia artemisiifolia L.) in Guangdong Province, China. Chinese J. Biosafety 21, 210–215.
Feng, Y., and Fu, G. (2008). Nitrogen allocation, partitioning and use efficiency in three invasive plant species in comparison with their native congeners. Biol. Invasions 10, 891–902. doi: 10.1007/s10530-008-9240-3
Fridley, J. D., Stachowicz, J. J., Naeem, S., Sax, D. F., Seabloom, E. W., Smith, M., et al. (2007). The invasion paradox: reconciling pattern and process in species invasions. Ecology 88, 3–17. doi: 10.1890/0012-9658(2007)88[3:TIPRPA]2.0.CO;2
Fumanal, B., Plenchette, C., Chauvel, B., and Bretagnolle, F. (2006). Which role can arbuscular mycorrhizal fungi play in the facilitation of Ambrosia artemisiifolia L. invasion in France? Mycorrhiza 17, 25–35. doi: 10.1007/s00572-006-0078-1
Gajnik, D., and Peternel, R. (2009). Methods of intervention in the control of ragweed spread (Ambrosia artemisiifolia L.) in the area of Zagreb county and the city of zagreb. Collegium Antropologicum 33, 1289–1294.
Galzina, N., Barić, K., Šćepanović, M., Goršić, M., and Ostojić, Z. (2010). Distribution of invasive weed Ambrosia artemisiifolia L. in croatia. Agriculturae Conspectus Sci. 75, 59–65.
Gentili, R., Gilardelli, F., Bona, E., Prosser, F., and Citterio, S. (2016). Distribution map of Ambrosia artemisiifolia L. (Asteraceae) in Italy. Plant Biosystems Int. J. Dealing Aspects Plant Biol. 151, 381–386. doi: 10.1080/11263504.2016.1176966
Giljohann, K. M., Hauser, C. E., Williams, N. S. G., and Moore, J. L. (2011). Optimizing invasive species control across space: willow invasion management in the Australian Alps. J. Appl. Ecol. 48, 1286–1294. doi: 10.1111/j.1365-2664.2011.02016.x
Gusev, A. P. (2019). Invasion of Ambrosia artemisifolia L. into the landscapes of the Southeastern Belarus. Russ. J. Biol. Invasions 10, 129–135. doi: 10.1134/S2075111719020061
Hamaoui-Laguel, L. (2018). Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Climate Change 5, 766–771. doi: 10.1038/nclimate2652
Hauser, C. E., and McCarthy, M. A. (2009). Streamlining ‘search and destroy’: cost-effective surveillance for invasive species management. Ecol. Lett. 12, 683–692. doi: 10.1111/j.1461-0248.2009.01323.x
Hejda, M., Chytry, M., Pergl, J., Pyšek, P., and Diez, J. (2015). Native range habitats of invasive plants: are they similar to invaded range habitats and do they differ according to the geographical direction of invasion? Diversity Distrib. 21, 312–321. doi: 10.1111/ddi.12269
Igrc, J., Deloach, C. J., and Zlof, V. (1995). Release and establishment of Zygogramma suturalis F (Coleoptera: Chrysomelidae) in croatia for control of common ragweed (Ambrosia artemisiifolia L.). Biol. Control 5, 203–208. doi: 10.1006/bcon.1995.1025
Jia, F. Q., Han, N. E., Zhang, X. F., Zhang, W., and Zhao, Y. (2011). Age structures of components of Leymus chinensis population in different habitats in the Yili River Valley Area, China. Chinese J. Grassland 33, 95–99.
Lei, Y., Wu, Z.-L., Wu, L.-Z., Shi, H.-L., Bai, H. T., Fu, W., et al. (2018). Interspecific correlation between exotic and native plants under artificial wetland forests on the Dianchi lakeside, south-west China. Mar. Freshwater Res. 69, 669–676. doi: 10.1071/MF17177
Leiblein, M. C., and Lösch, R. (2011). Biomass development and CO2 gas exchange of Ambrosia artemisiifolia L. under different soil moisture conditions. Flora 206, 511–516. doi: 10.1016/j.flora.2010.09.011
Leskovšek, R., Eler, K., Batič, F., and Simončič, A. (2012a). The influence of nitrogen, water and competition on the vegetative and reproductive growth of common ragweed (Ambrosia artemisiifolia L.). Plant Ecol. 213, 769–781. doi: 10.1007/s11258-012-0040-6
Leskovšek, R., Datta, A., Knezevic, S. Z., and Simončič, A. (2012b). Common ragweed (Ambrosia artemisiifolia) dry matter allocation and partitioning under different nitrogen and density levels. Weed Biol. Manag. 12, 98–108. doi: 10.1111/j.1445-6664.2012.00439.x
Li, S. P., Guo, T., Cadotte, M. W., Chen, Y. J., Kuang, J. L., Hua, Z. S., et al. (2015). Contrasting effects of phylogenetic relatedness on plant invader success in experimental grassland communities. J. Appl. Ecol. 52, 89–99. doi: 10.1111/1365-2664.12365
Liu, L., Wang, X., Wen, Q., Jia, Q., and Liu, Q. (2017). Interspecific associations of plant populations in rare earth mining wasteland in southern China. Int. Biodeterioration Biodegradation 118, 82–88. doi: 10.1016/j.ibiod.2017.01.011
Liu, X. L., Li, H. Q., Wang, J. H., Sun, X. P., and Xing, L. G. (2021). The current and future potential geographical distribution of common ragweed, Ambrosia artemisiifolia in China. Pakistan J. Botany 53, 167–172. doi: 10.30848/PJB2021-1(18)
Liu, Y. (2019). Study on Occurrencen, Spread and Control of Ambrosia artemisiifolia L. in Henan Province. Master’s thesis, Zhengzhou (Henan Province): Agricultural University of Henan.
Ma, C., Li, S. P., Pu, Z., Tan, J., Liu, M., Zhou, J., et al. (2016). Different effects of invader–native phylogenetic relatedness on invasion success and impact: a meta-analysis of Darwin’s naturalization hypothesis. Proc. Biol. Sci. 283:20160663. doi: 10.1098/rspb.2016.0663
Ma, Q. Q., Liu, T., Dong, H. G., Zhao, W. X., Wang, H. Y., and Wang, R. L. (2020). Predicting the potential distribution of ragweed in Xinjiang using multi-scale data source. J. Arid Land Resources Environ. 34, 188–193.
Maitner, B. S., Park, D. S., Enquist, B. J., and Dlugosch, K. M. (2021). Where we’ve been and where we’re going: the importance of source communities in predicting establishment success from phylogenetic relationships. Ecography 2022:e05406. doi: 10.1111/ecog.05406
Makra, L., Juhász, M., and Béczi, R. (2005). The history and impacts of airborne Ambrosia (Asteraceae) pollen in Hungary. Grana 44, 57–64. doi: 10.1080/00173130510010558
Mang, T., Essl, F., Moser, D., and Dullinger, S. (2018). Climate warming drives invasion history of Ambrosia artemisiifolia in central Europe. Preslia 90, 59–81. doi: 10.23855/preslia.2018.059
Montagnani, C., Gentili, R., Smith, M., Guarino, M. F., and Citterio, S. (2017). The worldwide spread, success, and impact of ragweed (Ambrosia spp.). Critical Rev. Plant Sci. 36, 139–178. doi: 10.1080/07352689.2017.1360112
Ozaslan, C., Onen, H., Farooq, S., Gunal, H., and Akyol, N. (2016). Common ragweed: an emerging threat for sunflower production and human health in Turkey. Weed Biol. Manag. 16, 42–55. doi: 10.1111/wbm.12093
Patracchini, C., and Ferrero, V. A. (2011). Common ragweed (Ambrosia artemisiifolia) growth as affected by plant density and clipping. Weed Technol. 25, 268–276. doi: 10.1614/WT-D-09-00070.1
Petrova, S. E. (2019). Development of invasive weeds Ambrosia artemisiifolia L. and A. trifida L. (Asteraceae) in Moscow Oblast. Russ. J. Biol. Invasions 10, 370–381. doi: 10.1134/S2075111719040088
Pinke, G., Kolejanisz, T., Vér, A., Nagy, K., and Czúcz, B. (2019). Drivers of Ambrosia artemisiifolia abundance in arable fields along the Austrian-Hungarian border. Preslia 91, 369–389. doi: 10.23855/preslia.2019.369
Procheş, Ş, Wilson, J., Richardson, D. M., and Rejmánek, M. (2010). Searching for phylogenetic pattern in biological invasions. Global Ecol. Biogeography 17, 5–10.
Puc, M., and Wolski, T. (2013). Forecasting of the selected features of Poaceae (R. Br.) Barnh., Artemisia L. and Ambrosia L. pollen season in Szczecin, north-western Poland, using Gumbel’s distribution. Annals Agricultural Environ. Med. AAEM 20:36.
Pysek, P., and Chytry, M. (2014). Habitat invasion research: where vegetation science and invasion ecology meet. J. Veg. Sci. 25, 1181–1187. doi: 10.1111/jvs.12146
Radosevich, S. R., Holt, J. S., and Ghersa, C. M. (2007). Ecology of Weeds and Invasive Plants: Relationship to Agriculture and Natural Resource Management. Hoboken, NJ: John Wiley & Sons.
Richter, R., Dullinger, S., Essl, F., Leitner, M., and Vogl, G. (2013). How to account for habitat suitability in weed management programmes? Biol. Invasions 15, 657–669. doi: 10.1007/s10530-012-0316-8
Romain, S., Andreas, L., Edita, T., Anna-Karin, K., Sanda, R., Lars, A., et al. (2016). Phenological variation in Ambrosia artemisiifolia l. facilitates near future establishment at northern latitudes. PLoS One 11:e0166510. doi: 10.1371/journal.pone.0166510
Song, J. S., and Prots, B. (1998). Invasion of Ambrosia artemisiifolia L.(Compositae) in the ukrainian carpathians Mts. and the transcarpathian plain (central Europe). Korean J. Biol. Sci. 2, 209–216. doi: 10.1080/12265071.1998.9647409
Thamaga, K. H., and Dube, T. (2018). Testing two methods for mapping water hyacinth (Eichhornia crassipes) in the Greater Letaba river system, South Africa: discrimination and mapping potential of the polar-orbiting Sentinel-2 MSI and Landsat 8 OLI sensors. Int. J. Remote sensing 39, 8041–8059. doi: 10.1080/01431161.2018.1479796
Thomaz, S. M., and Michelan, T. S. (2011). Associations between a highly invasive species and native macrophytes differ across spatial scales. Biol. Invasions 13, 1881–1891. doi: 10.1007/s10530-011-0008-9
Tretyakova, A. S., Yakimov, B. N., Kondratkov, P. V., Grudanov, N. Y., and Cadotte, M. W. (2021). Phylogenetic diversity of urban floras in the central urals. Front. Ecol. Evol. 9:663244. doi: 10.3389/fevo.2021.663244
Waller, D. M., Mudrak, E. L., Amatangelo, K. L., Klionsky, S. M., and Rogers, D. A. (2016). Do associations between native and invasive plants provide signals of invasive impacts? Biol. Invasions 18, 3465–3480. doi: 10.1007/s10530-016-1238-7
Keywords: Ambrosia artemisiifolia, invasive species, interspecific association, Darwin’s naturalization hypothesis, Darwin’s pre-adaptation hypothesis, early stage
Citation: Zhao W, Liu T, Sun M, Wang H, Liu X and Su P (2022) Rapid monitoring of Ambrosia artemisiifolia in semi-arid regions based on ecological convergence and phylogenetic relationships. Front. Ecol. Evol. 10:926990. doi: 10.3389/fevo.2022.926990
Received: 23 April 2022; Accepted: 06 July 2022;
Published: 22 July 2022.
Edited by:
Qinfeng Guo, United States Forest Service (USDA), United StatesReviewed by:
Barış Özüdoğru, Hacettepe University, TurkeyAleksandra Savic, Institute for Plant Protection and Environment (IZBIS), Serbia
Copyright © 2022 Zhao, Liu, Sun, Wang, Liu and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong Liu, NDY5MDA0NTA5QHFxLmNvbQ==
 Wenxuan Zhao
Wenxuan Zhao Tong Liu
Tong Liu Mingming Sun1,2
Mingming Sun1,2 Hanyue Wang
Hanyue Wang