- 1Laboratory of Animal Behavior and Conservation, College of Biology and the Environment, Nanjing Forestry University, Nanjing, China
- 2Division of Ecoscience, Ewha Womans University, Seoul, South Korea
- 3Javan Gibbon Research and Conservation Project, Bogor, Indonesia
- 4Interdisciplinary Program of Ecocreative, Ewha Womans University, Seoul, South Korea
- 5Department of Human Behavior, Ecology and Culture, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany
- 6Yayasan Konservasi Ekosistem Alam Nusantara (KIARA), Bogor, Indonesia
- 7Department of Forest Resources Conservation and Ecotourism, Faculty of Forestry and Environment, IPB University, Bogor, Indonesia
While the vocalizations of non-human primates were thought to be innate, recent studies have revealed highly flexible vocalizations in immatures. This behavior suggests that social influences have an important role in developing vocalizations. Yet not much is known about how non-human primate vocalization develop and how the vocalizations of immature animals differ between sexes. Here, we analyzed 95 cases of co-singing between mothers and offspring out of 240 female songs from three groups of wild Javan gibbon (Hylobates moloch) in Gunung Halimun-Salak National Park, Indonesia, between 2009 and 2021. Hylobates moloch is one of only two gibbon species with pairs that do not duet. Instead, they produce sex-specific solo songs. We found that both offspring female and male H. moloch follow their mothers’ female-specific songs, similar to other duetting gibbon species. Immatures started co-singing with their mothers from 7 months old, but with an average starting age of about 24 months. As female offspring grew older, they co-sung with mothers more often while male offspring did not. After 7 years of age, both sexes stopped co-singing with their mothers and started singing alone, following their own sex-specific vocalizations. We did not find any relation between male offspring co-singing and territorial functions (e.g., co-singing more during intergroup encounters or closer to home range borders). Our results suggest that mothers’ songs may trigger male offspring and females to practice singing, but not specifically for males to defend territories. We highlight that despite the absence of duets, H. moloch develop their vocalizations from early infancy and throughout their maturation while co-singing with mothers. However, the level of co-singing varies depending on the sexes. Our study is the first to elucidate the sex-specific trajectories of vocal development in H. moloch across years, indicating that offspring in non-duetting gibbons co-sing with mothers like in duetting species.
Introduction
Humans socially learn speech coordination, vocalization usage, and comprehension early in their development (Bruner, 1975). In contrast, the vocalizations of non-human primates were thought to be primarily innate (Winter et al., 1973; Pistorio et al., 2006; Tomasello, 2010). However, recent studies have revealed highly variable and flexible patterns of immature vocalizations in non-human primates. Those developmental changes in vocalization can be influenced by physical maturation, experience, and social feedback on vocal development (Lemasson et al., 2011; Chow et al., 2015; Gultekin and Hage, 2017; Takahashi et al., 2017; Zhang and Ghazanfar, 2018; Gultekin et al., 2021). Many non-human primates exhibit sex differences in their vocalizing behavior. Those sex differences are call types (gibbons: Geissmann, 2002; indris: Giacoma et al., 2010), acoustic features (baboons: Ey et al., 2007; owl monkeys: Garcia de la Chica et al., 2020; titi monkeys: Clink et al., 2019; tamarins: Masataka, 1987), and vocalization usage such as occurrence rates and contexts (colobus monkeys: Bene and Zuberbueler, 2009; macaques: Bernstein and Ehardt, 1985; spider monkeys: Dubreuil et al., 2015). Moreover, many primates exhibit sex differences in vocal ontogeny of immatures (Green, 1981; Tomaszycki et al., 2001; Pistorio et al., 2006; Ey et al., 2007), which might reflect sex differences in adult vocalizations. For instance, adult indris (Indri indri) have a sex-specific song repertoire, and the female and male juveniles differ in their temporal song parameters (De Gregorio et al., 2022). However, little is known about the sex-specific development of primates’ vocalization.
Gibbons (Hylobatidae) are excellent models to study the ontogeny and evolution of vocalizations because of their loud and elaborate songs and the phylogenetic position between great apes and monkeys (Raemaekers et al., 1984). Their songs are innate and species- and sex-specific, and these characteristics are inherited (Brockelman and Schilling, 1984). The most remarkable trait of Hylobatidae is duetting in pairs. These duets mainly consist of antiphonal or simultaneous emissions of stereotyped female great calls consisting of a series of notes with increasing tempo and pitch (Marshall and Marshall, 1976), and variable short-notes of males (Geissmann, 2002). However, Hylobates moloch, our study subject, is one of the only two non-duetting gibbon species together with Kloss’s gibbons (Hylobates klossii), and females and males produce only solo songs (Tenaza, 1976; Kappeler, 1984). Lar gibbons (Hylobates lar) males and H. moloch females in captivity have been recorded to produce highly coordinated duets, the loss of duet in H. moloch is likely derived secondarily from duetting characteristics (Geissmann, 2002). Similarly, a loss of duet might be a derived trait from common ancestors (i.e., synapomorphy) shared by H. moloch and H. klossii (Chan et al., 2010; Roos, 2016; but see Gani et al., 2021). Moreover, H. moloch males rarely sing solos as well compared to females regularly sing solos (Kappeler, 1984; Geissmann and Nijman, 2006). Since the pattern of sexual dimorphism in the adult vocalization is different in duetting and non-duetting species, this may influence immature vocal development in non-duetting species differently from those in duetting species. Yet, little is known about the development of vocalization in gibbons.
Immature female gibbons develop their ability to perform great calls (i.e., vocal control and acoustic structures) by co-singing with their mothers, in several gibbon species (H. lar; Reichard, 2003, H. klossii; Tilson, 1981; Whitten, 1982, Hylobates agilis; Koda et al., 2013, and yellow-cheeked gibbons; Nomascus gabriellae; Merker and Cox, 1999). Co-singing can indicate the strength of bonding between mother and daughter (Koda et al., 2013; Koda, 2016). Furthermore, gibbon mothers may trigger and tutor their daughters by adjusting their vocal structures according to their daughters’ responses (Koda et al., 2013). Immature male gibbons also sing female-specific parts when co-singing with their mothers. This behavior has been reported in duetting gibbons such as H. lar and agile gibbons (H. agilis; Koda et al., 2014), and N. gabriellae, northern white-cheeked gibbon (Nomascus leucogenys), and black crested gibbons (Nomascus concolor; Schilling, 1984; Hradec et al., 2016, 2017, 2021). With physical and sexual maturation, young males of N. gabriellae transitioned from female-like songs to a mix of both male and female-like song parts, to male calls only (Hradec et al., 2021). Similar to immature female gibbons, immature males may also co-sing with their mothers to practice vocalizing.
Adult male H. moloch sing solos extremely rare, for example only one single male song was heard during 130 full day survey in West Java (Kappeler, 1984) and male solos were 8.5% of all songs heard in Central Java (Geissmann and Nijman, 2006). However, we observed immature H. moloch males co-singing with their mothers’ song. Male immatures’ co-singing with mothers in non-duetting H. moloch may relate to territorial defense like the adult female songs or duets (Mitani, 1985; Geissmann and Orgeldinger, 2000; Fan et al., 2009; Ham et al., 2017). Primate vocalizations, especially duets, are often used to defend their territory by advertising the territorial border or their physical condition (i.e., fighting ability; Mitani, 1987; Barelli et al., 2013). For example, adult female H. moloch produced songs more often in the area of their home range that overlapped with neighboring groups than expected based on time spent in the overlapping area versus interior area (Ham et al., 2017; Yi et al., 2020). This suggested that songs of adult female H. moloch function for territorial defense by advertising territorial boundaries (Ham et al., 2017), like other primate species (southern brown howler monkeys: da Cunha and Jalles-Filho, 2007; titi monkeys: Robinson, 1979). Thus, H. moloch immature males may be triggered to co-sing with their mothers more frequently in similar contexts.
This study aims to understand the development of sexually dimorphic vocalization in Javan gibbons (H. moloch). To understand how the vocal ontogeny of H. moloch differs between sexes, we investigated vocal development in H. moloch offspring in Gunung Halimun-Salak National Park, Indonesia, between 2009 and 2021. First, we tested for sex differences in vocal development in offspring H. moloch. Since H. moloch adult females produce solo songs more frequently than adult males while there is no duetting, we hypothesized that the differences between adult female and adult male vocalizations should be reflected in co-singing with adult females during vocal ontogeny of offspring as well. We specifically predicted that (1) female offspring will start co-singing with their mothers at an earlier age than male offspring, (2) females will co-sing with their mothers more often than male offspring, and (3) the frequency of co-singing will increase as female offspring get older, in opposition with the co-singing activity of male offspring. Then, we investigated why male offspring H. moloch co-sing with their mothers despite the absence of duets and the rarity of adult male solo songs. We hypothesized that male offspring co-singing in H. moloch is used for territorial defense similar to that of mothers. Specifically, we predicted that male offspring would co-sing with their mothers more often (1) during intergroup encounters compared to the non-intergroup encounter context and (2) close to their home range border compared to the center of their home ranges. Finally, we reported opportunistically collected cases of adult male and offspring solos.
Methods and materials
Data collection
We have been habituating and following wild H. moloch in Citalahab Forest, Gunung Halimun-Salak National Park, West Java, Indonesia (S 6°44′19″E 106°31′45″), as part of a long-term project called the Javan Gibbon Research and Conservation Project (Kim et al., 2012; Ham et al., 2017; Oktaviani et al., 2018; Yi et al., 2020; Jang et al., 2021). We collected data from three habituated gibbon groups and each gibbon group consists of one adult female, one adult male and 2–3 offspring (Table 1). During the study period, we observed 13 offspring from the three gibbon groups (male; N = 9, female; N = 4). They were distributed in four age categories (infant: 0–2 year, juvenile: 2–5 year, adolescent: 5–8 year, subadult: >8 year; definitions following Brockelman et al., 1998). Whenever there were adult female or male vocal events, we recorded the presence of any offspring co-singing and the Global Positioning System (GPS) coordinates. We defined co-singing as two individuals (mother and her immature offspring in our study) vocalizing simultaneously (Koda et al., 2013; Hradec et al., 2021), while duetting is usually defined as coordinated vocalization between a female and male pair in gibbons (Marshall and Marshall, 1976). When an offspring co-sang with an adult, we also recorded the age of the offspring. We calculated the age of offspring from the date of birth. For the individuals without exact birth information (Noffspring = 5), we calculated the inter-birth interval of the study population between 2010 and 2021 (Noffspring = 10; N within−groupinter−birthinterval = 7; mean ± SD = 1,295 ± 242 days) and subtracted that from the date of birth of the next-born individual with exact birth information in the same group. We compared the body size and behaviors (e.g., spatial distance with other group members) of the individuals with putative age with those of the individuals with known exact age. Then we confirmed no obvious mismatch between the putative age and the body size/behaviors. We also recorded whether co-singing occurred during the intergroup encounters. We excluded the data for which the intergroup encounter context could not be determined for the analyses. We collected GPS coordinates of adult gibbons at 15-min intervals from 2014 to 2019 and 10-min from 2020 to 2021. We followed a gibbon group from a sleeping tree to the next between 0600 and 1700 h.
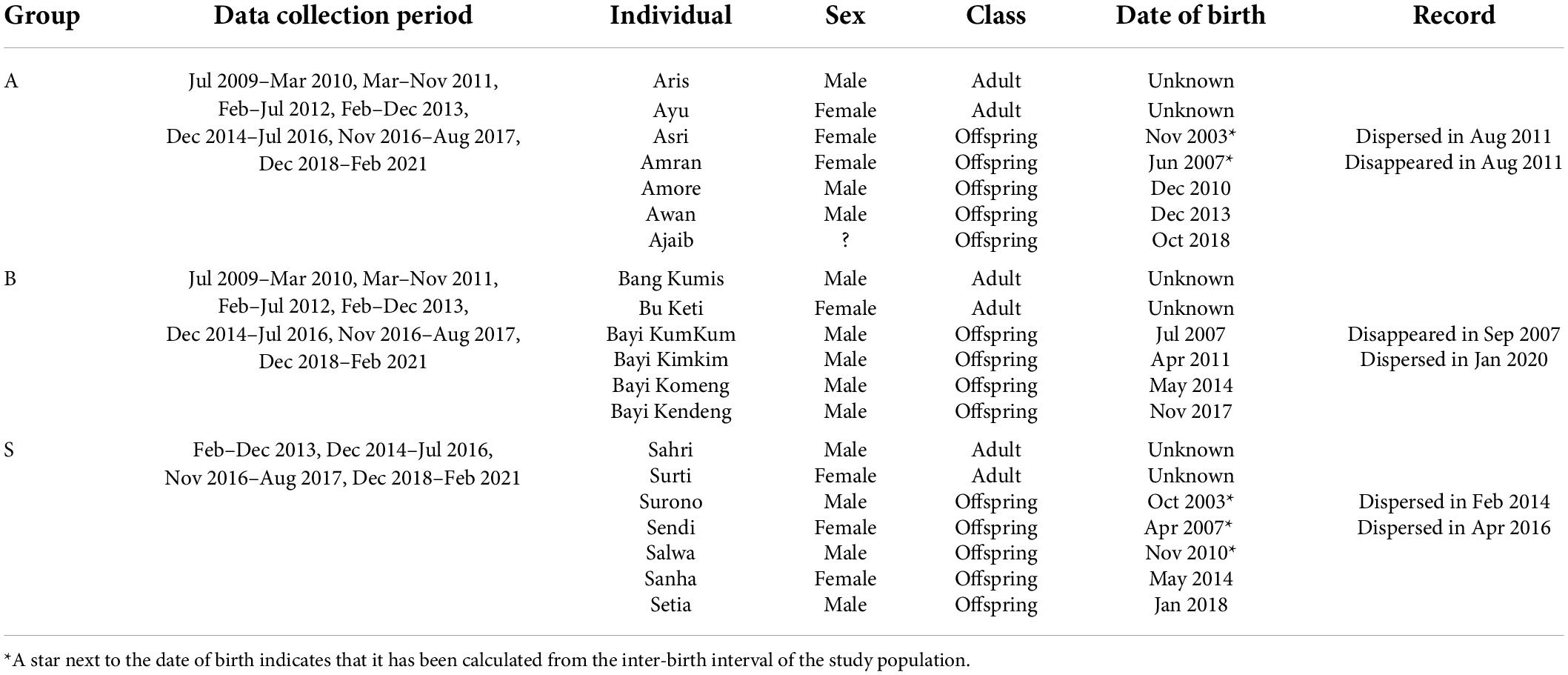
Table 1. The life history of the study subjects and data collection period for each gibbon group in Gunung Halimun-Salak National Park, Indonesia between 2009 and 2021.
In addition, we opportunistically observed fourteen cases of offspring vocalization without an adult female vocalization prior. We also recorded 51 male solos from both the focal and the neighboring groups during the study period. Among these, we could confirm the identity of the singers for 41 cases (22 cases from the focal groups and 19 cases from non-focal groups with visual contact).
Data analyses
First, we checked the age at which offspring males and females start co-singing with their mothers. To do so, we investigated the first record of co-singing between offspring and adult females. Additionally, we calculated the mean age of the first five co-singing events. We chose five events to overcome the potential observation bias of describing only the first event as we may have missed some co-singing events, for instance, while not following the focal group. Following this protocol, we included sufficient data to calculate mean values (Noffspring = 5), while restricting the range of age used to describe the starting age of co-singing. For those with assumed age calculated with inter-birth interval, we did not include them in the calculation in the first or first five co-singing events of co-singing.
To determine the co-singing rates of offspring with mothers, we created a list of female songs (Nfemale song = 240) for each offspring individual per day (Ntotal = 556). When adult females stopped singing more than 5 mins before starting to sing again, we defined it as two different song bouts (hereafter “song” Geissmann, 2002). For example, if an offspring co-sang with at least one female song during the day, we marked the day as an offspring co-singing present. For example, if an adult female sang twice a day, and one of the songs was followed by a juvenile but not by an infant of the focal group, we had a line for each immature for that day, one marked with the juvenile co-singing present and the other marked with the infant co-singing absent. Adult females typically sang one song bouts a day and a maximum of up to three bouts. We analyzed our data on a daily base to compensate for the potential of missing data.
We ran a binomial Generalized Linear Mixed Model (GLMM) with the presence of offspring co-singing with the female songs as the response variable (Ntotal = 532), and the offspring age, sex, and the interaction between the offspring age and sex as test predictors, and interaction between the offspring sex and season (dry, semidry, and wet) to control for a potential seasonal effect. We included the gibbon group ID, offspring ID, and each adult female vocalization event ID as random effects. We added the offspring age and season within gibbon group and offspring ID as random slopes.
We estimated the annual home range of each focal group using 95% kernel density estimates (Worton, 1989) to investigate the effect of the location of female vocalizations and the intergroup encounter on the probability of co-singing in offspring males. We calculated the Euclidean distance between the location of female vocalization and the nearest home range border. If a female sang outside the estimated annual home range, we marked the distance to the closest border with a negative value to differentiate the coordinates inside and outside the home range. The distance was divided by the size of the annual home range (ha) to control the potential effect of the home range size difference between groups. We conducted the spatial analyses using ArcGIS Pro (version 2.8.5; Environmental Systems Research Institute, 2022). Then we ran the second binomial GLMM with the presence of offspring males co-singing to the female songs as the response variable. This has been calculated the same way as co-singing rates of offspring with mothers, we created a list of female songs of which the GPS location was identified (Nfemale song = 110) for each male offspring individual per day (Ntotal = 242). We included the distance from the home range border divided by the home range size and the context (intergroup encounter/non-intergroup encounter) as test predictors. Then, we included the group ID and offspring ID as random effects, and the two predictors (i.e., the distance from home range border divided by the home range size, and the intergroup encounter context) within gibbon group and offspring ID as random slopes.
Before running the two binomial GLMMs, we visually checked the homogeneity of variance and z-transformed all covariates in the models. We also checked for multicollinearity using the R package car (Fox and Weisberg, 2019) and did not find a problem since the variance inflation factor (VIF) values ranged between 1.68 and 2.79. We presented the results only when the full-null model comparison was statistically significant, while the null model was only with the random effect and slopes included. All data were analyzed using R (version 4.1.0; R Core Team, 2021).
Results
We recorded 240 female songs, and among these, 95 cases of co-singing between offspring and mothers, and 51 male solos over 82 months of surveys between 2009 and 2021 (Figure 1). The age of the first recording of offspring co-singing with their mothers was 23.8 ± 18.8 months (mean ± SD; range: 7.0–60.0; Noffspring = 8; Figure 2). The age of the first five recordings of offspring co-singing with their mothers was 42.4 ± 16.3 months (mean ± SD; range: 17.2–62.4; Noffspring = 5; Figure 2). Since we had first recordings of only one female offspring, we could not compare the age of first recordings between sexes directly. Although not included in the study period, we observed a 2-month-old infant (born in November 2021) of unknown sex co-singing with the mother in group A, one of our focal gibbon groups, in February 2022 (Supplementary Audio 1). This is the earliest record of infant gibbon co-singing.
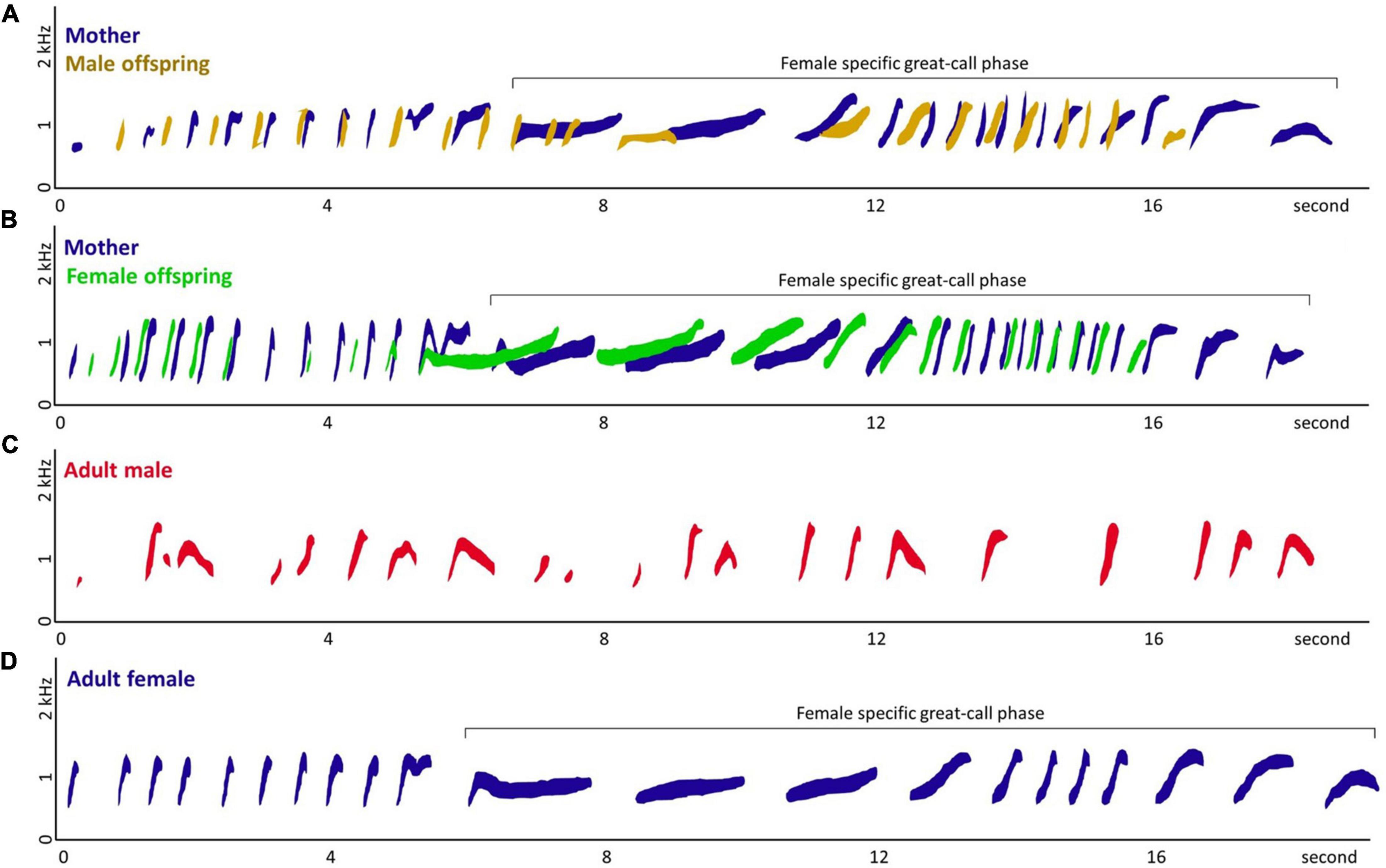
Figure 1. Spectrograms of (A) co-singing between mother and male offspring, (B) co-singing between mother and female offspring, (C) adult male solo, and (D) adult female solo of Hylobates moloch. Panel (A) was recorded from gibbon group S, Surti (mother), and Salwa (male offspring; subadult) in September 2019. Panel (B) was recorded from gibbon group A, Ayu (mother), and Asri (female offspring; adolescent) in September 2009. Panel (C) was recorded from the Javan Gibbon Center, Gunung Gede-Pangrango, Indonesia, in September 2009, due to the difficulty of recording rare adult male solos in the wild. Panel (D) was recorded from the gibbon group B, Bu Keti (adult female) in April 2011. Except (C) adult male solo, all were recorded from the focal gibbon groups in the study area, Gunung Halimun-Salak National Park, Indonesia. The spectrograms were extracted from the recordings using Raven Lite (Version 2.0.3; Yang, 2022) and we manually traced the spectrograms to specify the callers.
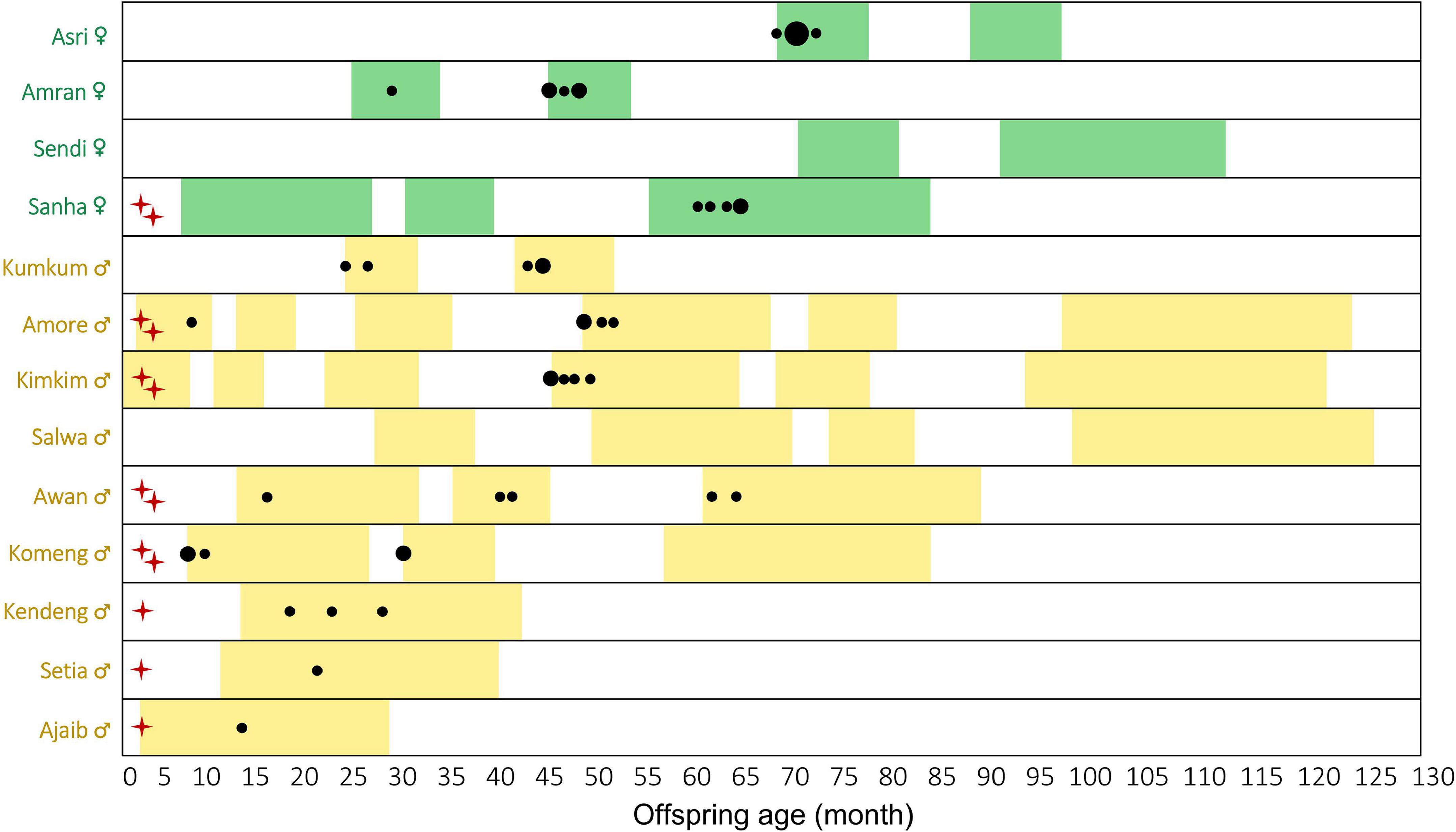
Figure 2. The age of the earliest (maximum) five recordings of each offspring Hylobates moloch co-singing with mothers in Gunung Halimun-Salak National Park, Indonesia, between 2009 and 2011. The colored bar indicates the data collection period covered in this study. The circle size represents the number of co-singing events (1–3 cases). Red crosses close to the individual name indicate that we included the individuals (Noffspring = 8) in calculating the age of the first co-singing record, and two crosses indicate that we included the individuals (Noffspring = 5) in calculating the age of the first five co-singing records. Individuals without crosses were not included in the first co-singing records because our study period does not cover their early infancy and likely missed the first records.
In general, the rate of each offspring co-singing with their mothers’ songs (i.e., number of female songs that offspring co-sung with/total number of female songs produced) was 0.159 ± 0.153 (mean ± SD; Noffspring = 13). Probability of co-singing with mothers changed across developmental stages in both female and male offspring (infancy: 0.091 ± 0.101, juvenility: 0.160 ± 0.141, adolescence: 0.234 ± 0.195, subadult period: 0; Supplementary Figure 1).
We found that the interaction between offspring age and sex and between offspring sex and season significantly affected the presence of offspring co-singing with their mother songs (full-null model comparison: χ2 = 6.912, df = 2, p = 0.032; Table 2 and Supplementary Table 1). The results indicate that as female offspring become older, the probability of female offspring co-singing with their mothers increased while that of male offspring did not (Figure 3). The full model testing the effect of distance from the home range border and the intergroup encounter on co-singing between male offspring and mothers did not fit significantly better than the null model (full-null model comparison: χ2 = 2.059, df = 2, p = 0.357).
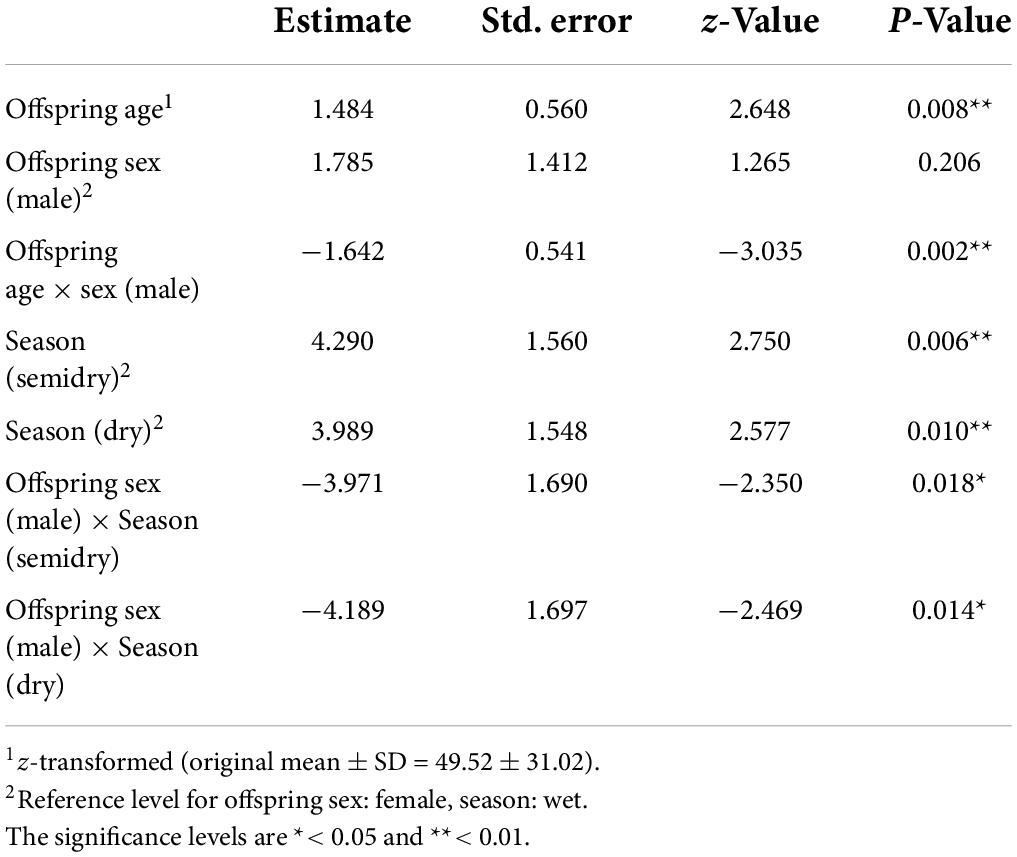
Table 2. The result of the binomial Generalized Linear Mixed Model (GLMM) investigating the effect of offspring age, sex, and season on the offspring Hylobates moloch co-singing with their mothers in Gunung Halimun-Salak National Park, Indonesia from 2009 to 2021.
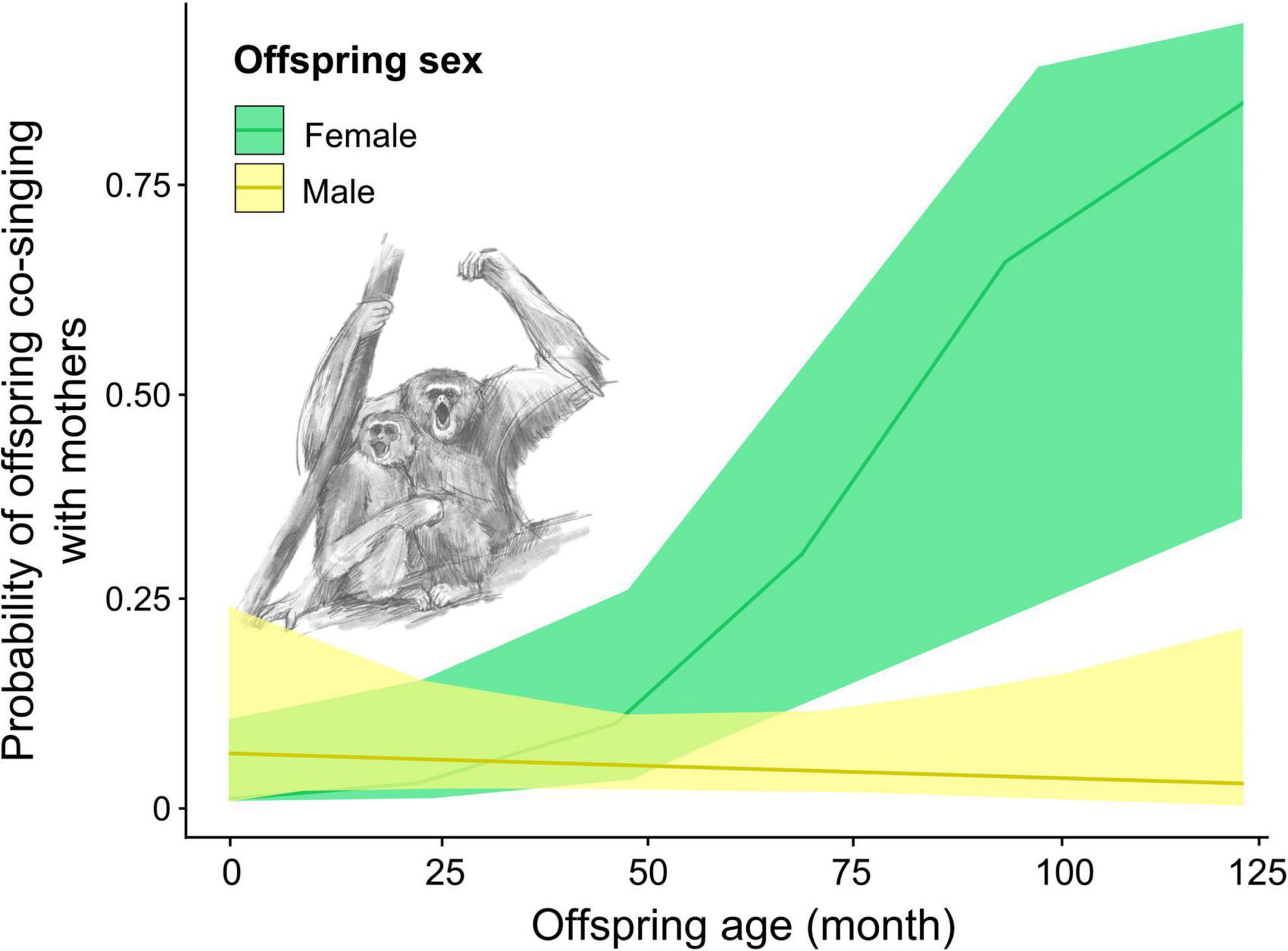
Figure 3. The probability of offspring Hylobates moloch co-singing with their mothers throughout the ages in Gunung Halimun-Salak National Park, Indonesia, between 2009 and 2021.
We found that a female offspring and two male offspring produced vocalizations without the presence of adult vocalization (Nvocalization = 12). While female offspring produced vocalizations similar to female great calls even without adult female singing, male offspring produced adult male-like sounds which were not observed when they co-sung with their mothers. The average age of sex-specific vocalization of offspring without adults is 90 months (Noffspring = 1) for a female offspring and 98 ± 2.8 (mean ± SD; Noffspring = 2) months for male offspring. 75% of these vocalizations were observed during intergroup encounters (Nvocalization = 9). All vocalizations of focal subadult males occurred during intergroup encounters (Nvocalization = 7). Subadult males from two encountering groups simultaneously vocalized before the other group members arrived at the encounter location and after the other members of the groups left. Male offspring also vocalized when the adult male of the neighboring group produced vocalizations. However, we have not heard any offspring co-singing with their fathers, yet we are not able to confirm whether there was co-singing before we started daily follows at 0600, given that male gibbons produce solos at dawn before 0600 h (Raemaekers and Raemaekers, 1985; Geissmann and Nijman, 2006).
Discussion
To our knowledge, this study is the first to record the vocal developmental trajectories in female and male gibbons, covering the entire immature and mature period of offspring. We found considerable differences in vocal development between female and male offspring in non-duetting H. moloch. Both immature female and male H. moloch started co-singing with their mothers during infancy, which began around 7 months, and started co-singing more stably during early juvenility. However, surprisingly, we also observed a 2-month-old infant co-singing with its mother outside of our study period. At this point, we are not able to firmly conclude which sex starts co-singing with their mothers at an earlier age, since the sex ratio of our study subject is highly biased toward males for the first recordings (male; N = 7, female; N = 1). In another pair-living and duetting primate species, I. indri, immature females started participating in choruses earlier than immature males, which might be related to the earlier maturation of females than males in this species (De Gregorio et al., 2022). To clarify the sex difference in the emergence of co-singing in gibbons, an intensive investigation of their early infancy for both sexes would be needed.
As infant gibbons grew older, however, they exhibited a clear sex difference in the rate of co-singing with mothers, supporting our prediction. On average, H. moloch female offspring co-sang with their mothers 2.4 times more often than male offspring. Moreover, female offspring co-sang with their mothers more often as they became older. These results support our predictions regarding the difference in vocal development between female and male offspring. This pattern is consistent with N. gabriellae females (Merker and Cox, 1999). In contrast, male offspring’s engagement in co-singing stayed low throughout development. This sex difference in H. moloch seems to be expressed around 2 or 3 years of age, which is still early in their development, given that gibbons wean around 2 years of age (Treesucon, 1984). Co-singing probability with their mothers increased continuously until the female offspring H. moloch reached the subadult stage at 8 years of age. From this point on, they were no longer observed co-singing with their mothers, similarly to H. lar females (Reichard, 2003). The cessation of co-singing may indicate that female offspring have already acquired adult-level vocalization skills and social independence from mothers (Reichard, 2003; Koda et al., 2013).
In our study, H. moloch male offspring also completely stopped co-singing with their mothers at around 7 years of age despite a lower co-singing rate compared to female offspring. In addition, we observed that young males, after 8 years of age, vocalized alone without other group members. Remarkably, while they always produced only female-like great calls when they co-sang with their mothers before 7 years of age, young males produced male-like vocalizations of simple “wa” notes by themselves after 8 years of age. Nomascus gabriellae young males also stopped co-singing with their mothers around 7 years old, while gradually switching to adult male songs after producing both female and male type vocalizations between the age of 5–7 years (Hradec et al., 2021). We speculate that these sudden changes in H. moloch young male vocalizations might result from hormones related to age and sex, which also may influence the larynx growth and acoustic structures (Newman et al., 2000; Barelli et al., 2013). Further studies should investigate the ontogeny of vocalization with detailed acoustic analyses together with hormonal analyses.
We did not find any relationship between male offspring co-singing with their mothers and territorial defense. Unlike what we predicted, male offspring did not co-sing with their mothers more often during intergroup encounters compared to the non-intergroup encounter context, or close to their home range border compared to the center. During the study period, we recorded only six cases of vocalizations produced in intergroup encounters from the focal adult males over the 82 months of the study period (e.g., 132 female songs produced in intergroup encounters in the same period). Considering H. moloch adult females defend territories by singing solos at the overlapping area with neighbors and advertising the borders (Ham et al., 2017), our finding implies that H. moloch adult males rarely produce solos for the same purpose of female’s territorial defense. However, since H. moloch adult males participate in intergroup encounters mostly by chasing (Yi et al., 2020), we speculate that male offspring also do not relate vocalization or co-singing with adult females for territorial defense.
Then what could be the evolutionary function of co-singing in H. moloch male offspring? Male offspring may co-sing with their mothers to avoid competition with fathers by producing female-like great calls, and thus by relaying information about their immature status (Hradec et al., 2021). However, this is unlikely because in both N. gabriellae (Hradec et al., 2021) and H. moloch (present study), co-singing episodes between young males and mothers were no longer observed after 8 years of age. The competition with fathers should peak around the subadult stage (i.e., >8 year). As Geissmann (2002) suggested, the duetting behavior of H. moloch and H. klossii was likely lost secondarily. We speculate that in duetting ancestors, H. moloch male offspring co-singing with their mothers once had the evolutionarily benefit of facilitating the improvement of their vocalization. However, despite the lower benefits of co-singing compared to duetting ancestors, H. moloch male offspring might benefit from practicing to achieve varieties of adult male songs and strengthen their bonds with their mothers through co-singing.
Finally, we found that H. moloch males rarely but still considerably produced solo songs after 0630 h. We assume that H. moloch males vocalize more often than we report here from our opportunistic data, since male gibbons produce solos at dawn (Raemaekers and Raemaekers, 1985; Geissmann and Nijman, 2006). While previous studies mainly focused on the pre-dawn male chorus, there is no detailed description of post-dawn male songs. Despite their rarity, we found that male vocalizations occurred throughout a day between 0630 and 1700 h, and some male vocalizations lasted more than 40 min. Further studies on adult male vocalization will shed light on the evolution of young male vocalization in H. moloch.
Our study has limitations in several aspects. First, because of data collection methods (i.e., following one focal group a day), we could have missed co-singing between adults and offspring and offspring solos, even though we tried to overcome this limitation by counting the first five co-singing records. Also, we lack observations in the early dawn, in which adult males might produce solos more. Second, we do not know the exact ages of some offspring born before our research period. Even though we relied on the individuals with exact birth records in the first co-singing events and considered body size and behaviors as other proxies, there may be a slight difference from their actual age. Moreover, we have the biased sex ratio in the focal groups resulting in a small sample size for female offspring. Lastly, we could not compare vocal structures between female and male offspring to examine the similarity with their mothers since we did not have good quality recordings. Instead, we focus more on their behavioral ecology than detailed acoustic analyses. We recommend that future studies investigate the ontogeny of vocal structures in both female and male offspring.
Vocalizations of non-human primates have received great attention due to their phylogenetic closeness to humans. However, there is a dearth of data on the vocal development of non-human primates, probably because of prolonged immature periods, long lifespan, and different methodologies and parameters for various vocal patterns (Harvey and Clutton-Brock, 1985; van Schaik and Isler, 2012). Despite the limitations of our study, our results highlight that H. moloch develop their vocalization from early infancy throughout their development while interacting with their mothers. Given their elaborated song structures, co-singing with their mother would help young female H. moloch acquire adult-level communication skills. Even though young males co-sing with their mothers less, still, co-singing can help achieve a variety of adult male songs despite potentially lower benefits than their duetting ancestors. Our findings contribute to understanding the non-duetting gibbon’s vocal development and emphasize further interdisciplinary and longitudinal studies encompassing social systems, life history, behaviors, and physiology.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Indonesian Ministry of Research and Technology (RISTEK), the Indonesian Ministry of Forestry’s Department for the Protection and Conservation of Nature (PHKA), and the Gunung Halimun-Salak National Park.
Author contributions
YY and AC conceptualized the initial idea and drafted the manuscript and performed and interpreted the statistical analyses. YY, AC, SL, SH, RO, and HJ collected the behavioral data. YY, AC, SL, SH, RO, HJ, AM, and JC revised, contributed to the article, and confirmed the final version of the manuscript.
Funding
This research was partly supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A3A03039709) to YY and Amore Pacific Academic and Cultural Foundation (AACF). YY was supported by the Foreign Youth Talent Program (QN2021014010L) from the Ministry of Science and Technology and Research Grants (2021K310C) from the Department of Human Resources and Social Security of Jiangsu Province of the People’s Republic of China. This work was also supported by National Research Foundation of Korea, Posco International Corporation, and Ewha Womans University. This project was conducted in collaboration with the Department of Forest Resources Conservation and Ecotourism, Faculty of Forestry and Environment at the IPB University. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We thank the Indonesian Ministry of Research and Technology (RISTEK), the Indonesian Ministry of Forestry and Environment, and the Gunung Halimun-Salak National Park (GHSNP) for the research permissions. We thank Rinekso Soekmadi, Mirza Kusrini, and GHSNP staff for their assistance and cooperation. We also thank our field assistants, Aris, Muhammad Nur, Ri Rudini, Isra Kurnia, Iyan Sopian, Nandar Pratama, Indra Lesmana, Muhammad A. Ajis, and Alan for their hard work in the field. We are grateful to Sangmi Lee for the gibbon illustration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.910260/full#supplementary-material
References
Barelli, C., Mundry, R., Heistermann, M., and Hammerschmidt, K. (2013). Cues to androgens and quality in male gibbon songs. PLoS One 8:e82748. doi: 10.1371/journal.pone.0082748
Bene, J., and Zuberbueler, K. (2009). Sex differences in the use of vocalizations in wild olive Colobus monkeys. Eur. J. Sci. Res. 25, 266–279.
Bernstein, I. S., and Ehardt, C. L. (1985). Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. J. Comp. Psychol. 99:115. doi: 10.1037/0735-7036.99.2.115
Brockelman, W. Y., and Schilling, D. (1984). Inheritance of stereotyped gibbon calls. Nature 312, 634–636. doi: 10.1038/312634a0
Brockelman, W. Y., Reichard, U., Treesucon, U., and Raemaekers, J. J. (1998). Dispersal, pair formation and social structure in gibbons (Hylobates lar). Behav. Ecol. Sociobiol. 42, 329–339. doi: 10.1007/s002650050445
Bruner, J. S. (1975). The ontogenesis of speech acts. J. Child Lang. 2, 1–19. doi: 10.1016/j.biosystems.2020.104264
Chan, Y.-C., Roos, C., Inoue-Murayama, M., Inoue, E., Shih, C.-C., Pei, K. J.-C., et al. (2010). Mitochondrial genome sequences effectively reveal the phylogeny of Hylobates gibbons. PLoS One 5:e14419. doi: 10.1371/journal.pone.0014419
Chow, C. P., Mitchell, J. F., and Miller, C. T. (2015). Vocal turn-taking in a non-human primate is learned during ontogeny. Proc. R. Soc. B Biol. Sci. 282:20150069. doi: 10.1098/rspb.2015.0069
Clink, D. J., Lau, A. R., and Bales, K. L. (2019). Age-related changes and vocal convergence in titi monkey duet pulses. Behaviour 156, 1471–1494. doi: 10.1163/1568539X-00003575
da Cunha, R. G. T., and Jalles-Filho, E. (2007). The roaring of southern brown howler monkeys (Alouatta guariba clamitans) as a mechanism of active defence of borders. Folia Primatol. 78, 259–271. doi: 10.1159/000105545
De Gregorio, C., Carugati, F., Estienne, V., Valente, D., Raimondi, T., Torti, V., et al. (2022). Born to sing! Song development in a singing primate. Curr. Zool. 67, 585–596. doi: 10.1093/cz/zoab018
Dubreuil, C., Notman, H., and Pavelka, M. S. (2015). Sex differences in the use of whinny vocalizations in spider monkeys (Ateles geoffroyi). Int. J. Primatol. 36, 412–428. doi: 10.1007/s10764-015-9832-6
Environmental Systems Research Institute (2022). ArcGIS Pro. (Version 2.8.5). Available online at: https://www.esri.com/en-us/home
Ey, E., Hammerschmidt, K., Seyfarth, R. M., and Fischer, J. (2007). Age-and sex-related variations in clear calls of Papio ursinus. Int. J. Primatol. 28, 947–960. doi: 10.1007/s10764-007-9139-3
Fan, P. F., Xiao, W., Huo, S., and Jiang, X. L. (2009). Singing behavior and singing functions of black-crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. Am. J. Primatol. 71, 539–547.
Gani, M., Rovie-Ryan, J. J., Sitam, F. T., Kulaimi, N. A. M., Zheng, C. C., Atiqah, A. N., et al. (2021). Taxonomic and genetic assessment of captive White-Handed Gibbons (Hylobates lar) in Peninsular Malaysia with implications towards conservation translocation and reintroduction programmes. ZooKeys 1076:25. doi: 10.3897/zookeys.1076.73262
Garcia de la Chica, A., Huck, M., Depeine, C., Rotundo, M., Adret, P., and Fernandez-Duque, E. (2020). Sexual dimorphism in the loud calls of Azara’s owl monkeys (Aotus azarae): Evidence of sexual selection? Primates 61, 309–319. doi: 10.1007/s10329-019-00773-6
Geissmann, T. (2002). Duet-splitting and the evolution of gibbon songs. Biol. Rev. 77, 57–76. doi: 10.1017/s1464793101005826
Geissmann, T., and Nijman, V. (2006). Calling in wild silvery gibbons (Hylobates moloch) in Java (Indonesia): Behavior, phylogeny, and conservation. Am. J. Primatol. 68, 1–19. doi: 10.1002/ajp.20203
Geissmann, T., and Orgeldinger, M. (2000). The relationship between duet songs and pair bonds in siamangs, Hylobates syndactylus. Anim. Behav. 60, 805–809. doi: 10.1006/anbe.2000.1540
Giacoma, C., Sorrentino, V., Rabarivola, C., and Gamba, M. (2010). Sex differences in the song of Indri indri. Int. J. Primatol. 31, 539–551. doi: 10.1007/s10764-010-9412-8
Green, S. M. (1981). Sex differences and age gradations in vocalizations of Japanese and lion-tailed monkeys (Macaca fuscata and Macaca silenus). Am. Zool. 21, 165–183. doi: 10.1093/icb/21.1.165
Gultekin, Y. B., and Hage, S. R. (2017). Limiting parental feedback disrupts vocal development in marmoset monkeys. Nat. Commun. 8:14046. doi: 10.1038/ncomms14046
Gultekin, Y. B., Hildebrand, D. G., Hammerschmidt, K., and Hage, S. R. (2021). High plasticity in marmoset monkey vocal development from infancy to adulthood. Sci. Adv. 7:eabf2938. doi: 10.1126/sciadv.abf2938
Ham, S., Lappan, S., Hedwig, D., and Choe, J. C. (2017). Female songs of the nonduetting Javan gibbons (Hylobates moloch) function for territorial defense. Int. J. Primatol. 38, 533–552. doi: 10.1007/s10764-017-9964-y
Harvey, P. H., and Clutton-Brock, T. H. (1985). Life history variation in primates. Evolution 39, 559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x
Hradec, M., Bolechová, P., and Svobodová, I. (2016). Production of a female-specific great call in an immature male gibbon, the Nomascus genus. Primates 57, 445–448. doi: 10.1007/s10329-016-0569-4
Hradec, M., Illmann, G., Bartoš, L., and Bolechová, P. (2021). The transition from the female-like great calls to male calls during ontogeny in southern yellow-cheeked gibbon males (Nomascus gabriellae). Sci. Rep. 11, 1–9. doi: 10.1038/s41598-021-01648-x
Hradec, M., Linhart, P., Bartoš, L., and Bolechová, P. (2017). The traits of the great calls in the juvenile and adolescent gibbon males Nomascus gabriellae. PLoS One 12:e0173959. doi: 10.1371/journal.pone.0173959
Jang, H., Oktaviani, R., Kim, S., Mardiastuti, A., and Choe, J. C. (2021). Do Javan gibbons (Hylobates moloch) use fruiting synchrony as a foraging strategy? Am. J. Primatol. 83:e23319. doi: 10.1002/ajp.23319
Kappeler, M. (1984). “Vocal bouts and territorial maintenance in the moloch gibbon,” in The lesser apes : Evolutionary and behavioural biology, eds H. Preuschoft, D. J. Chivers, W. Y. Brockelman, and N. Creel (Edinburgh: Edinburgh University Press), 376–389.
Kim, S., Lappan, S., and Choe, J. C. (2012). Responses of Javan Gibbon (Hylobates moloch) groups in submontane forest to monthly variation in food availability: Evidence for variation on a fine spatial scale. Am. J. Primatol. 74, 1154–1167. doi: 10.1002/ajp.22074
Koda, H. (2016). “Gibbon songs: Understanding the evolution and development of this unique form of vocal communication,” in Evolution of gibbons and Siamang: Phylogeny, morphology, and cognition, eds U. H. Reichard, H. Hirai, and C. Barelli (New York, NY: Springer), 349–359. doi: 10.1007/978-1-4939-5614-2_15
Koda, H., Lemasson, A., Oyakawa, C., Pamungkas, J., and Masataka, N. (2013). Possible role of mother-daughter vocal interactions on the development of species-specific song in gibbons. PLoS One 8:e71432. doi: 10.1371/journal.pone.0071432
Koda, H., Oyakawa, C., Kato, A., Shimizu, D., Koyama, Y., and Hasegawa, S. (2014). Immature male gibbons produce female-specific songs. Primates 55, 13–17. doi: 10.1007/s10329-013-0390-2
Lemasson, A., Ouattara, K., Petit, E. J., and Zuberbühler, K. (2011). Social learning of vocal structure in a nonhuman primate? BMC Evol. Biol. 11:362. doi: 10.1186/1471-2148-11-362
Marshall, J. T. Jr., and Marshall, E. R. (1976). Gibbons and their territorial songs. Science 193, 235–237. doi: 10.1126/science.193.4249.235
Masataka, N. (1987). The perception of sex-specificity in long calls of the tamarin (Saguinus labiatus labiatus). Ethology 76, 56–64. doi: 10.1111/j.1439-0310.1987.tb00671.x
Merker, B., and Cox, C. (1999). Development of the female great call in Hylobates gabriellae: A case study. Folia Primatol. 70, 97–106. doi: 10.1159/000021680
Mitani, J. C. (1987). Territoriality and monogamy among agile gibbons (Hylobates agilis). Behav. Ecol. Sociobiol. 20, 265–269. doi: 10.1007/BF00292179
Newman, S.-R., Butler, J., Hammond, E. H., and Gray, S. D. (2000). Preliminary report on hormone receptors in the human vocal fold. J. Voice 14, 72–81. doi: 10.1016/s0892-1997(00)80096-x
Oktaviani, R., Kim, S., Cahyana, A., and Choe, J. (2018). “Nutrient composition of the diets of Javan gibbons (Hylobates moloch),” in Proceedings of the IOP Conference Series: Earth and Environmental Science, Vol. 197, (Bristol: IOP Publishing Ltd), 012048. doi: 10.1088/1755-1315/197/1/012048
Pistorio, A. L., Vintch, B., and Wang, X. (2006). Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus). J. Acoust. Soc. Am. 120, 1655–1670. doi: 10.1121/1.2225899
R Core Team (2021). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Raemaekers, J. J., and Raemaekers, P. M. (1985). Field playback of loud calls to gibbons (Hylobates lar): Territorial, sex-specific and species-specific responses. Anim. Behav. 33, 481–493. doi: 10.1016/S0003-3472(85)80071-3
Raemaekers, J. J., Raemaekers, P. M., and Haimoff, E. H. (1984). Loud calls of the gibbon (Hylobates lar): Repertoire, organisation and context. Behaviour 91, 146–189. doi: 10.1163/156853984X00263
Reichard, U. H. (2003). “Social monogamy in gibbons: The male perspective,” in Monogamy: Mating strategies and partnerships in birds, humans and other mammals, eds U. H. Reichard and C. Boesch (Cambridge: Cambridge University Press), 190–213. doi: 10.1017/CBO9781139087247.013
Robinson, J. G. (1979). Vocal regulation of use of space by groups of titi monkeys Callicebus moloch. Behav. Ecol. Sociobiol. 5, 1–15. doi: 10.1007/BF00302691
Roos, C. (2016). “Phylogeny and classification of gibbons (Hylobatidae),” in Evolution of gibbons and siamang: Phylogeny, morphology, and cognition, eds U. H. Reichard, H. Hirai, and C. Barelli (New York, NY: Springer), 151–165. doi: 10.1007/978-1-4939-5614-2_7
Schilling, D. (1984). “Song bouts and duetting in the concolor gibbon,” in The lesser apes : Evolutionary and behavioural biology, eds H. Preuschoft, D. J. Chivers, W. Y. Brockelman, and N. Creel (Edinburgh: Edinburgh University Press), 390–403.
Takahashi, D. Y., Liao, D. A., and Ghazanfar, A. A. (2017). Vocal learning via social reinforcement by infant marmoset monkeys. Curr. Biol. 27, 1844–1852.e6. doi: 10.1016/j.cub.2017.05.004
Tenaza, R. R. (1976). Songs, choruses and countersinging of Kloss’ gibbons (Hylobates klossii) in Siberut Island, Indonesia. Z. Tierpsychol. 40, 37–52. doi: 10.1111/j.1439-0310.1976.tb00924.x
Tilson, R. L. (1981). Family formation strategies of Kloss’s gibbons. Folia Primatol. 35, 259–287. doi: 10.1159/000155979
Tomaszycki, M. L., Davis, J. E., Gouzoules, H., and Wallen, K. (2001). Sex differences in infant rhesus macaque separation–rejection vocalizations and effects of prenatal androgens. Horm. Behav. 39, 267–276. doi: 10.1006/hbeh.2001.1659
Treesucon, U. (1984). Social development of young gibbons (Hylobates lar) in Khao Yai National Park, Thailand. MSc. thesis. Bangkok: Mahidol University.
van Schaik, C. P., and Isler, K. (2012). “Life-history evolution in primates,” in The evolution of primate societies, eds J. C. Mitani, J. Call, P. M. Kappeler, R. A. Palombit, and J. B. Silk (Chicago, IL: The University of Chicago Press), 220–244.
Whitten, A. J. (1982). The ecology of singing in Kloss gibbons (Hylobates klossii) on Siberut Island, Indonesia. Int. J. Primatol. 3, 33–51. doi: 10.1007/BF02693489
Winter, P., Schott, D., Ploog, D., and Handley, P. (1973). Ontogeny of squirrel monkey calls under normal conditions and under acoustic isolation. Behaviour 47, 230–239. doi: 10.1163/156853973x00085
Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168. doi: 10.2307/1938423
Yang, K. L. (2022). Raven lite: Interactive sound analysis software (Version 2.0.3) [Computer software]. Ithaca, NY: The Cornell Lab of Ornithology.
Yi, Y., Fichtel, C., Ham, S., Jang, H., and Choe, J. C. (2020). Fighting for what it’s worth: Participation and outcome of inter-group encounters in a pair-living primate, the Javan gibbon (Hylobates moloch). Behav. Ecol. Sociobiol. 74, 1–15. doi: 10.1007/s00265-020-02879-0
Keywords: co-singing, immature, duet acoustics, Javan gibbon, vocal development
Citation: Yi Y, Choi A, Lee S, Ham S, Jang H, Oktaviani R, Mardiastuti A and Choe JC (2022) Transient co-singing of offspring and mothers in non-duetting Javan gibbons (Hylobates moloch). Front. Ecol. Evol. 10:910260. doi: 10.3389/fevo.2022.910260
Received: 01 April 2022; Accepted: 18 July 2022;
Published: 17 August 2022.
Edited by:
Dena Jane Clink, Cornell University, United StatesReviewed by:
Pengfei Fan, Sun Yat-sen University, ChinaMichal Hradec, Czech University of Life Sciences Prague, Czechia
Valeria Torti, University of Turin, Italy
Copyright © 2022 Yi, Choi, Lee, Ham, Jang, Oktaviani, Mardiastuti and Choe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoonjung Yi, eWkueW9vbmp1bmdAZ21haWwuY29t; Jae C. Choe, amFlY2hvZTlAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Yoonjung Yi
Yoonjung Yi Ahyun Choi
Ahyun Choi Saein Lee
Saein Lee Soojung Ham2
Soojung Ham2 Haneul Jang
Haneul Jang