
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 04 May 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.909983
This article is part of the Research Topic Effects of Non-Random Sources of Alteration on Biodiversity and Ecosystem Functioning View all 19 articles
 Weitang Zhu1,2
Weitang Zhu1,2 Jing Liu1
Jing Liu1 Qihui Li1
Qihui Li1 Peng Gu3*
Peng Gu3* Xiaohui Gu4
Xiaohui Gu4 Lingling Wu4
Lingling Wu4 Yang Gao4
Yang Gao4 Jun Shan5
Jun Shan5 Zheng Zheng6
Zheng Zheng6 Weizhen Zhang1*
Weizhen Zhang1*
Microorganisms can both indicate the water quality characteristics and the health of the aquatic environment, which have an important influence on the cycling of organic carbon, nitrogen (N), and phosphorus (P) in nature. In this study, we took Taihu Lake, a typical eutrophic lake in China, as the research object, and monitored the northern (Changzhou City) and southern (Changxing County) regions of Taihu Lake for three consecutive years (2019–2021), respectively. We also analyzed the microbial diversity in sediments, and then summarized the effects of different nutrient environments on microorganisms in the aquatic environment. The results showed that the pollution level in the northern part of Taihu Lake was higher than that in the southern region of Taihu Lake, where the pollution was mainly in summer (June–July). The pollution in the southern region of Taihu Lake is relatively stable between 2019 and 2021; the changes in the northern part of Taihu Lake are relatively significant. Microbial diversity in the study area was negatively correlated with the degree of eutrophication of water bodies; microbial abundance was positively correlated with nutrient levels. The functional difference analysis indicated that the microorganisms in the sediments of Taihu Lake in the study area were involved in the nutrient transport and transformation, and played an important role in the purification of the lake water body. This study reveals the relationship between water eutrophication and microbial diversity, and then provides a theoretical basis for the management of eutrophic lakes.
The concept of eutrophication was first introduced by Lindeman in 1942, and the study concluded that the eutrophic stage of a water body is the last stage of the natural evolution of a normal lake (Lindeman, 1942). The water body reaches the eutrophication level when the nutrient total phosphorus (TP) > 0.02 mg/L and nitrate nitrogen (NO3–-N) > 0.3 mg/L (Namsaraev et al., 2018). Eutrophication of water bodies was once compared to “ecological cancer,” which destroys the ecosystem of water bodies and causes the ecological imbalance of water bodies by making the community structure homogeneous (Ramachandra and Solanki, 2007). Freshwater eutrophication is a widespread global environmental problem and a consequence of intense human activities (Wang et al., 2019). Currently, 54% of Asian lakes, 53% of European lakes, 48% of North American lakes, 41% of South American lakes and 28% of African lakes are eutrophic (Zhang et al., 2020).
According to the process of eutrophication formation, there are two types of eutrophication, natural and man-made (Conty and Bécares, 2013). Natural eutrophication mainly refers to the continuous deposition of soil erosion, evaporation, precipitation, river entrained alluvium, and aquatic debris at the bottom of water caused by natural environment without human interference, and the enrichment of nutrients in water bodies, which in turn leads to the development of water bodies from poor nutrients to eutrophication, gradually evolving into swamps and eventually into land. The process of eutrophication under natural conditions is very slow and takes hundreds or even tens of thousands of years to complete (Cui et al., 2021; Padedda et al., 2021). Nutrients contained in industrial, agricultural, and domestic wastewater have caused the eutrophication of lake water, degrading the quality of the water and the aquatic ecosystem (Liu and Wang, 2016). From the perspective of primary production of water bodies, eutrophication can be further classified into the following four categories: hypertrophic, eutrophic, mesotrophic, and oligotrophic (Kim et al., 2021; Wang et al., 2021). N and P were regarded as the limiting factors to the density of aquatic organisms and the main reduction objects especially for the cultural eutrophic lakes (Yuan et al., 2011). The sources of nutrients include two categories: external sources include the discharge of urban living and production wastewater (point source pollution) and lake runoff, farmland surface runoff, lake precipitation, dustfall, farming bait, etc. (non-point source pollution); internal sources are mainly the dead decay of aquatic plants and animals, and nutrients released from sediments. In addition, the destruction of the water body ecosystem is also one of the reasons for the deterioration of the water body. A good biological community structure of the aquatic environment is not only a good indicator of the health of the water, but also a guarantee of the self-purification of the water (Schindler et al., 1987; Madsen and Cedergreen, 2002; Rahaman and Sinha, 2013; Coluccio et al., 2021; Younes et al., 2021).
As an important part of the water ecosystem, microorganisms play a very important role in the migration and transformation of nutrients, organic matter and other materials at the water-sediment interface. Microorganisms can influence the nutrient status in the water environment, and when the external environment in the aquatic environment changes, the species and abundance of the microbial community will also change (Moshiri and Crumpton, 1977; Phelps et al., 1994; Jonah et al., 2015; Lakshmipathi et al., 2019; Vincent et al., 2021). At present, studies on microbial communities have mainly focused on the vertical distribution characteristics of the microorganisms in sediments within a specific region, and relatively few studies have been conducted on the dominant populations and microbial community structure under different trophic states of the environment (Sangakkara, 1997; Powlson et al., 2001; Haller et al., 2011; Liu et al., 2015).
In this study, the water quality and nutrient content in sediments of Taihu Lake, a typical eutrophic lake in China, were monitored for three consecutive years in the northern (Changzhou City) and southern (Changxing County) regions of Taihu Lake, respectively, and the microbial diversity in sediments was analyzed. And then the effects of different nutrient environments on microorganisms in the aquatic environment were summarized. This study can provide valuable experience for the study of biodiversity in eutrophic lakes.
Taihu Lake is the third largest freshwater lake in China, which is located in the core area of Yangtze River Delta. In recent years, with the rapid economic development and population growth as well as frequent human activities, industrial wastewater, and domestic sewage continuously flow into the lake, making the eutrophication problem of Taihu Lake more and more serious. In this study, lake water and surface sediment samples were collected from six points (N1–N6; S1–S6) each in North and South Taihu Lake for subsequent water quality analysis and microbiological analysis from 2019 to 2021, respectively, and the sample points were collected as shown in Figure 1.
At the sampling site, a portable dissolved oxygen meter was used to determine the dissolved oxygen (DO) and water temperature (WT). And then a pH meter was used to determine the pH of the water body. The rest of the physical and chemical properties were determined in accordance with the national standard method within 3 days after sampling, mainly including: permanganate index (CODMn), total nitrogen (TN), total phosphorus (TP), nitrate nitrogen (NO3–-N), ammonia nitrogen (NH3-N). According to the national standard, the determination of CODMn was done by the acidic method (GB/T11892-1989), the determination of TN was done by the alkaline potassium persulfate extinction UV spectrophotometric method (GB/T11894-1989), the determination of TP was done by the ammonium molybdate spectrophotometric method (GB/T11893-1989), the determination of NO3–-N was done by the phenol disulfate method (GB/T7480-1987), and the determination of NH3-N was done by the nano reagent colorimetric method (GB/T7479-1987).
Total DNA extraction was performed according to the EZNA soil kit (Omega Bio-tek, Norcross, GA, United States) instructions. DNA concentration and purity were determined using NanoDrop2000, and DNA extraction quality was measured by 1% agarose gel electrophoresis; 5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) primers for polymerase chain reaction (PCR) amplification of the V3–V4 variable region. The amplification procedure was: pre-denaturation at 95°C for 3 min, 27 cycles (denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 45 s), and extension at 72°C for 10 min (PCR: ABI GeneAmp 9700). The amplification system was 20 μl, 4 μl 5*FastPfu buffer, 2 μl 2.5 mM dNTPs, 0.8 μl primer (5 μM), 0.4 μl FastPfu polymerase; 10 ng DNA template (Zhang et al., 2020).
The PCR product was recovered using a 2% agarose gel, purified using AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States), eluted with Tris–HCl, and detected by 2% agarose electrophoresis. Detection quantification was performed using QuantiFluorTM-ST (Promega, Madison City, Wisconsin, United States). The purified amplified fragment was constructed into a library of PE 2*300 according to the standard operating protocol of the Illumina MiSeq platform (Illumina, San Diego, CA, United States). The steps of constructing the library: (a) connecting the “Y” shaped liknker; (b) using magnetic beads to remove the self-ligating fragment of the linker; (c) enriching the library template by PCR amplification; (d) denaturing the sodium hydroxide, producing single-stranded DNA fragments. Sequencing using Illumina’s MiSeq PE300 platform (Zhang et al., 2020).
Statistical analysis was performed using Origin Pro 9.0 software for Windows (OriginLab, Northampton, MA, United States). An analysis of the structure and abundance of the bacterial community distribution in surface sediments was performed using the free online platform Majorbio I-Sanger Cloud Platform (Zhang et al., 2020).
The changes in water quality monitored from January 2019 to November 2021 are shown in Figure 2. The values of WT in South and North Taihu Lake are 6.2°C–31.4°C (mean value 19.7°C) and 3.5–32.1°C (mean value 20.1°C), respectively. There is no significant difference between the WT in South and North Taihu Lake, but the temperature difference is large in all quarters, with certain characteristics of time and space distribution. The highest temperature of each year is concentrated in July–September, and the lowest temperature in January. The values of pH are 6.49–8.45 (mean value 7.29) and 6.89–8.88 (mean value 7.66), with a small trend of pH variation, and no obvious pH exceedance was found. The DO levels ranged from 4.00 to 10.71 mg/L (mean value 6.85 mg/L) and 4.55 to 11.33 mg/L (mean value 7.48 mg/L), respectively. The maximum concentration of DO in South Taihu Lake in winter and the lowest in summer and autumn, and the minimum concentration of DO in North Taihu Lake in winter and the maximum in summer. The conductivity of South Taihu Lake ranged from 15.1 to 48.9 ms/m (average value 34.9 ms/m), and the conductivity fluctuated irregularly from year to year, with the lowest conductivity in autumn. The turbidity of North Taihu Lake ranged from 10.22 to 47.01 NTU (mean value 28.98 NTU), and the overall turbidity was high, especially in summer and autumn, indicating that the water body was turbid.
The changes of water quality nutrient salts in the southern and northern regions of Taihu Lake, observed with the data monitored from January 2019 to November 2021, are shown in Figure 3. The annual CODMn in South and North Taihu Lake ranged from 2.833 to 5.767 mg/L (mean value 4.214 mg/L) and 17.817 mg/L to 46.208 mg/L (mean value 23.838 mg/L), respectively; the amount of NH3-N ranged from 0.045 to 1.337 mg/L (mean value 0.259 mg/L), 0.315 to 2.937 mg/L (average value 1.417 mg/L); the amounts of TN were 0.720–5.083 mg/L (average value 2.830 mg/L), 3.075–10.815 mg/L (average value 7.625 mg/L); the amounts of TP were 0.044–0.177 mg/L (average value 0.082 mg/L), 0.204–0.964 mg/L (average value 0.574 mg/L); NO3-N in North Taihu Lake is 1.514–6.255 mg/L (average value 3.298 mg/L).
According to the Environmental Quality Standard for Surface Water (GB3818-2002), the amounts of CODMn and TN in North Taihu Lake exceeded the limits of Class V water standard. The amount of NH3-N in spring and summer exceeds the standard limit for Class V water, and the amount of TP also exceeds the standard limit for Class V water most of the time. The amount of TP in South Taihu Lake exceeds the standard limit of Class V water, and the amount of TN also exceeds the standard limit of Class V water most of the time. It can be seen that both South and North Taihu Lake are polluted to different degrees. While comparing the nutrient levels of water in North and South Taihu Lake, it can be found that North Taihu Lake is more polluted and has higher N and P content. This is consistent with the conclusion of higher pollution in the northern part of Taihu Lake mentioned in previous studies (Cheng et al., 2005; Chen et al., 2018; Luo et al., 2020).
The data monitored from January 2019 to November 2021 were used to observe the changes of sediment nutrient salts in the southern and northern regions of Taihu Lake. In Figure 4, it can be obtained that the annual CODMn in South and North Taihu Lake ranged from 19.143 to 45.764 mg/L (mean value 28.472 mg/L) and 33.370 to 79.924 mg/L (mean value 51.796 mg/L), respectively; the amount of NH3-N ranged from 0.324 to 2.819 mg/L (mean value 1.299 mg/L) and 0.483–1.515 mg/L (mean value 0.869 mg/L); the amounts of NO3-N were 1.529–5.994 mg/L (mean value 3.207 mg/L), 0.524–9.297 mg/L (mean value 3.597 mg/L); the amounts of TN were 3.058–8.259 mg/L (mean value 5.500 mg/L), 4.608–10.744 mg/L (mean value 8.114 mg/L); the amounts of TP were 0.242–0.953 mg/L (mean value 0.577 mg/L), 0.548–1.212 mg/L (mean value 0.947 mg/L). The TP in South Taihu Lake exceeded the standard limit of Class V water except for July every year, and the TP in North Taihu Lake exceeded the standard limit of Class V water. It can be seen that South and North Taihu Lake have been polluted to some extent, and the contents of CODMn, TN and TP in North Taihu Lake are basically higher than those in South Taihu Lake, indicating that the pollution in North Taihu Lake is more serious.
The community structure of various samples at different taxonomic levels in the sampling sites of North and South Taihu Lake was analyzed statistically. The top 20 phyla of relative abundance of bacteria in the samples at the phylum level are shown individually, and the relative abundance distribution of the dominant bacterial groups in the sediments of South (S) and North (N) Taihu Lake at the phylum level is shown in Figure 5. Among them, the phyla with greater abundance are Proteobacteria, Cyanobacteria, Bacteroidetes, Actinobacteria, etc. The sum of the relative abundance of these phyla accounted for more than 80% in each sampling site, and the first phylum in relative abundance in each sampling site was Proteobacteria, indicating that the species composition of microorganisms in the sediment samples of South and North Taihu Lake at the phylum level had some similarity.
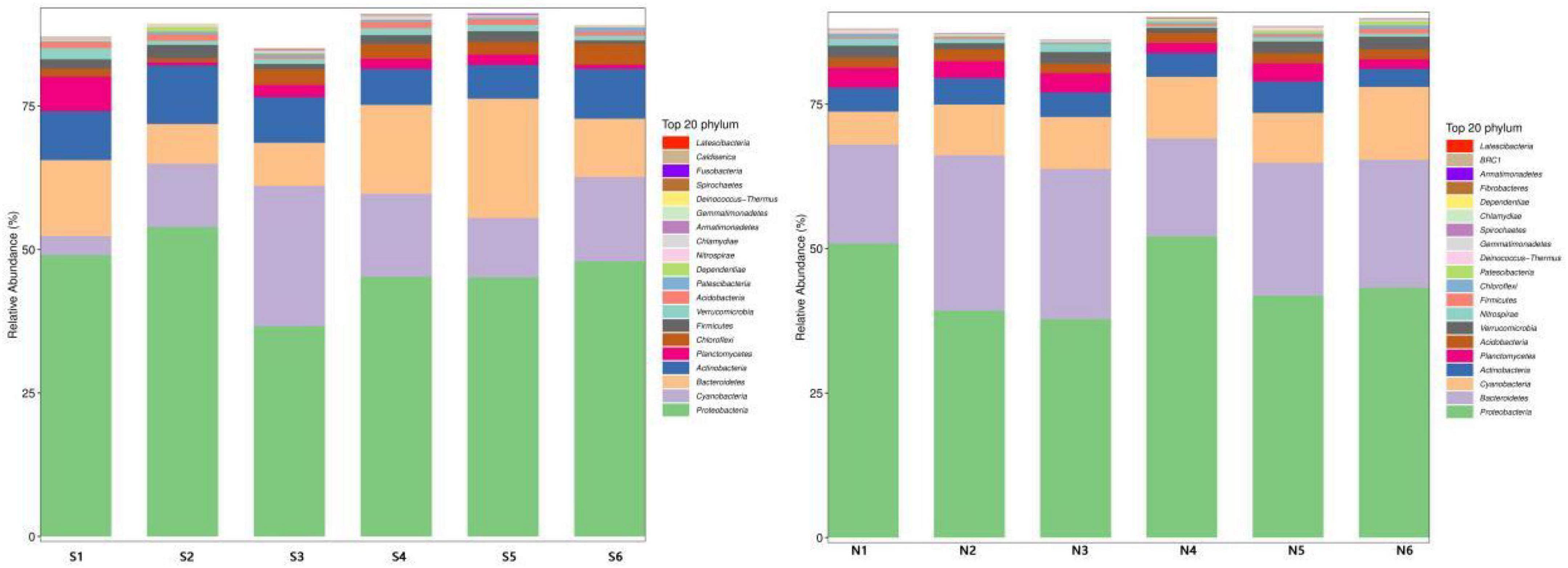
Figure 5. Changes in microbial communities at the phylum level in the sediments of South (S) and North Taihu Lake (N).
The main flora of S1 in the southern part of Taihu Lake were Proteobacteria (49.03%), Bacteroidetes(13.22%), Actinobacteria(8.52%); S2 were Proteobacteria (53.93%), Cyanobacteria(11.01%), Actinobacteria(10.21%); S3 were Proteobacteria (36.66%), Cyanobacteria(24.44%), Actinobacteria (7.96%); S4 were Proteobacteria (45.26%), Bacteroidetes(15.49%), Cyanobacteria(14.47%); S5 were Proteobacteria (45.15%), Bacteroidetes(20.78%), Cyanobacteria(10.36%); S6 were Proteobacteria (47.96%), Cyanobacteria(14.70%), Bacteroidetes (10.14%).
The sampling sites N1–N6 located in the northern part of Taihu Lake were all in the phylum Proteobacteria > Bacteroidetes > Cyanobacteria, with relatively low diversity, and the proportions of the three main phyla were N1 (50.90, 17.13, 5.70%); N2 (39.29, 26.87, 8.79%); N3 (37.80, 25.99, 8.99%); N4 (52.13, 16.97, 10.65%); N5 (41.86, 22.98, 8.67%); N6 (43.26, 22.13, 12.61%). Although the distribution of dominant phyla was the same at each sampling site, the relative abundance content of each phylum varied significantly. And the species composition of microorganisms in sediment samples from North and South Taihu Lake at the phylum level was somewhat different; the microbial diversity in South Taihu Lake was higher than that in North Taihu Lake, indicating that microbial diversity was negatively correlated with the concentration of nutrients. However, there are evidence that the abundance and biomass of various microbial components increases with the eutrophication of aquatic ecosystems (Conty and Bécares, 2013).
Microbial diversity is a key element to respond to the state of the ecosystem, and there are significant differences in microbial community diversity in different ecological environments (Wu et al., 2015; Pastorelli et al., 2021). In order to obtain more information about the bacterial community, the standardized processing method of row processing was used based on the Euclidean distance between the relative abundance of dominant species at each sample genus level, and the species clustering algorithm was average, based on the pheatmap package to draw the dominant. The clustering heat map of the dominant species based on the pheatmap package is shown in Figure 6.
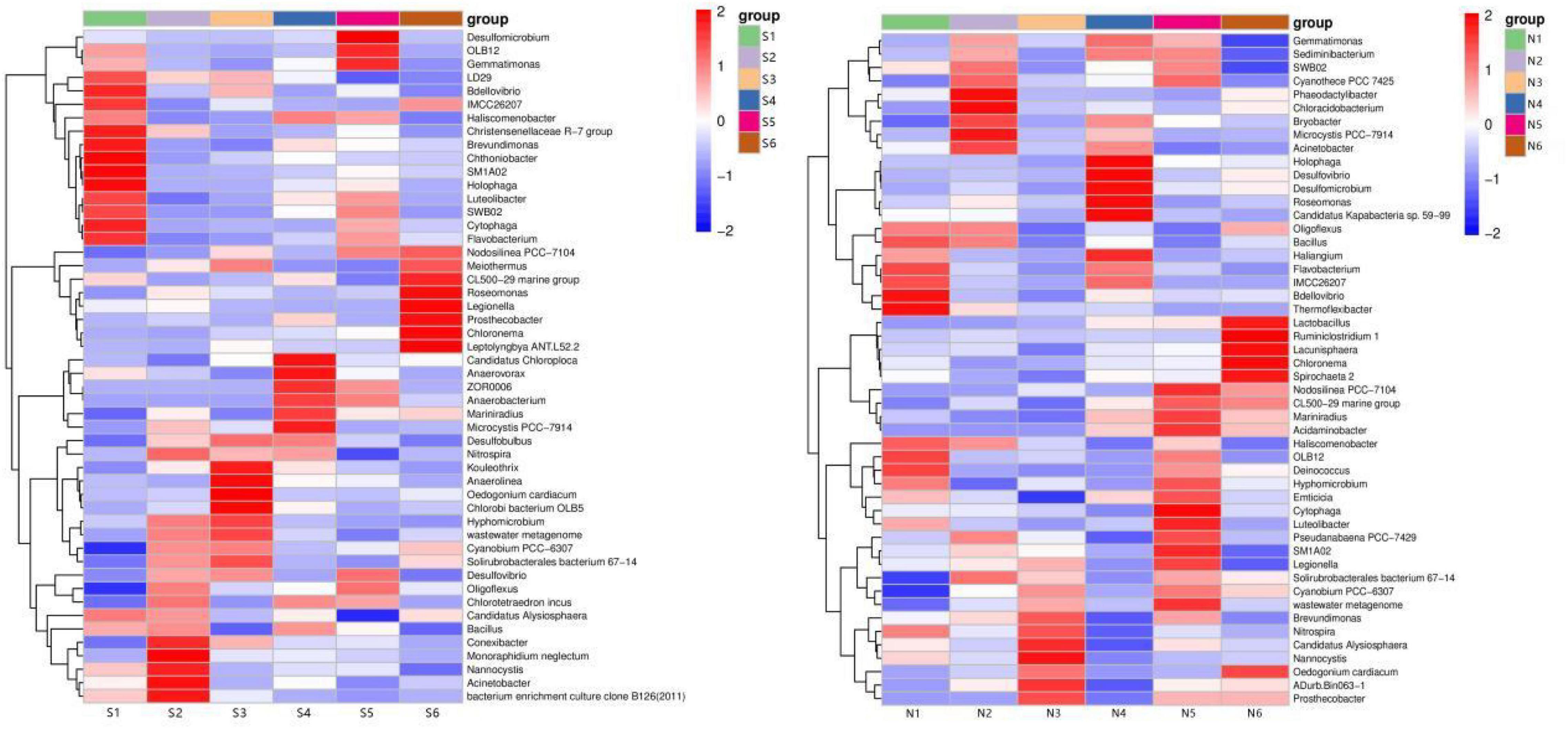
Figure 6. Genus-level heat map-cluster analysis of microbial communities in sediments from South (S) and North Taihu Lake (N).
At the genus level in South Taihu Lake, SM1A02, Holophaga and Acinetobacter, Bacterium enrichment culture clone B126 were preferentially clustered, and the abundance composition of the six samples between these two groups was similar, with SM1A02 and Holophaga having the highest abundance at S1. And the highest abundance was found at S2 for Bacteroides and Bacterium enrichment culture clone B126. At the genus level in North Taihu Lake, Candidatus Alyslosphaera and Nannocystis clustered preferentially before clustering with Nitrospira spp. The content of these three genera was mainly concentrated in N3, reflecting a high similarity.
Combined with the results of PICRUST2 analysis, the metabolic pathway abundance statistics were plotted using the normalized pathway abundance table, as shown in Figure 7. The right side of the graph shows the first level pathway to which the pathway belongs, the left side shows the second level pathway, and the bar graphs in the figure express the abundance of each pathway. The first level pathways include Biosynthesis, Degradation/Utilization/Assimilation, Detoxification, Generation of Precursor Metabolite and Energy, Glycan Pathways, Maclromolecule Modification and Metabolic Clusters. The metabolic pathways involved in microorganisms in the sediments of the Taihu Lake study area were mainly biosynthetic, including Amino Acid Biosynthesis, Carbohydrate Biosynthesis, Cofactor/Prosthetic Group/Electron Carrier/Vitamin Biosynthesis, Fatty Acid and Lipid Biosynthesis, Nucleoside and Nucleotide Biosynthesis are the main metabolic pathways of biosynthesis.
Among the Degradation/Utilization/Assimilation pathways, most of the secondary pathways were in balanced abundance, with the highest abundance of Nucleoside and Nucleotide Degradation. In the pathway of precursor metabolite and energy production, fermentation and TCA cycle (tricarboxylic acid cycle) were in higher abundance. The above functional differences suggest that sediment microorganisms in Taihu Lake in the study area are involved in nutrient transport and transformation, and play an important role in lake water purification.
The physical and chemical indexes such as pH, TN, TP, and CODMn of the Taihu Lake show that the pollution degree in the north of Taihu Lake is higher than that in the south. And the pollution mainly occurred in summer. The pollution in southern Taihu Lake was relatively stable from 2019 to 2021. The microbial communities in sediments of the northern and southern Taihu Lake were studied. The results showed that the microbial community structure in sediment samples showed obvious spatial diversity and similarity. The difference is that the relative abundance content of each phylum is different at each sampling point. The diversity of microorganisms was inversely proportional to the degree of eutrophication, while the abundance of microorganisms was positively correlated with the level of nutrients in the study area. The functional differences indicate that the microorganisms in the sediments of Taihu Lake in the study area participate in the migration and transformation of nutrients, which plays an important role in the purification of lake.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
WZu: conceptualization, data curation, and writing – original draft. JL: methodology and software. QL: conceptualization, methodology, and resources. XG: investigation and data curation. LW: investigation and supervision. YG: conceptualization and resources. JS: methodology and data curation. ZZ: conceptualization and supervision. PG: conceptualization, methodology and supervision. WZa: conceptualization, methodology and supervision. All authors contributed to the article and approved the submitted version.
This study was supported by the Chengdu University of Technology Research Startup Fund (10912-KYQD2020-08431), Taihu Lake Cyanobacteria Treatment Technology Project of Wuxi City (2021SQ005903201), and National Environmental Protection Key Laboratory of Cooperative Control and Joint Remediation of Water and Soil Pollution Open Fund Project (GHB-2020-010).
XG, LW, and YG were employed by Jiangsu Dongfang Ecological Dredging Engineering Co., Ltd. JS was employed by Changzhou Clear Water and Turquoise Waves Environmental Protection Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chen, X., Wang, Y. H., Ye, C., Zhou, W., Cai, Z. C., Yang, H., et al. (2018). Atmospheric nitrogen deposition associated with the eutrophication of Taihu Lake. J. Chem-ny. 2018, 1–10. doi: 10.1155/2018/4017107
Cheng, B., Zhang, Z., Chen, L., Yuan, Z. H., and Sun, X. R. (2005). Eutrophication of Taihu Lake and pollution from agricultural non-point sources in Lake Taihu Basin. J. Agro-environ. Sci. 24, 118–124. doi: 10.1109/ETFA.2005.1612741
Coluccio, K. M., Morgan, L. K., and Santos, I. R. (2021). Resolving groundwater sources to a coastal lagoon using major ions, nutrients and stable isotopes. Environ. Earth. Sci. 80, 1–15. doi: 10.1007/s12665-021-09880-4
Conty, A., and Bécares, E. (2013). Unimodal patterns of microbial communities with eutrophication in mediterranean shallow lakes. Hydrobiologia 700, 257–265. doi: 10.1007/s10750-012-1235-5
Cui, L. J., Jiang, Z. J., Huang, X. P., Chen, Q. M., Wu, Y. C., Liu, S. L., et al. (2021). Eutrophication reduces seagrass contribution to coastal food webs. Ecosphere 12:e03626. doi: 10.1002/ecs2.3626
Haller, L., Tonolla, M., Zopfi, J., Peduzzi, R., Wildi, W., and Poté, J. (2011). Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water. Res. 45, 1213–1228. doi: 10.1016/j.watres.2010.11.018
Jonah, A. E., Solomon, M. M., Ikpe, D. I., and Etim, I. G. (2015). Macro nutrients determination and bateriological status assessment of water and sediment samples from Ohii Miri River in Abia State. Nigeria. Int. J. Eng. Innov. Res. 4:383.
Kim, D. K., Yang, C., Parsons, C. T., Bowman, J., Theysmeyer, T., and Arhonditsis, G. B. (2021). Eutrophication management in a Great Lakes wetland: examination of the existence of alternative ecological states. Ecosphere 12:e03339. doi: 10.1002/ecs2.3339
Lakshmipathi, R. N., Subramanyam, B., and Prasad, B. D. (2019). Microorganisms, organic matter recycling and plant health. Plant. Health. Under. Biotic. Stress. 187–212. doi: 10.1007/978-981-13-6043-5_10
Lindeman, R. L. (1942). Experimental simulation of winter anaerobiosis in a Senescent Lake. Ecology 23, 1–13. doi: 10.2307/1930867
Liu, X. J., and Wang, H. L. (2016). Dianchi Lake, China: geological formation, causes of eutrophication and recent restoration efforts. Aquat. Ecosyst. Health 19, 40–48. doi: 10.1080/14634988.2016.1145022
Liu, Y., Shi, X. Y., Chen, M. H., Li, B. G., Qi, G. H., and Zhang, X. M. (2015). Effects of different water conversation measures on soil nutrients and microorganisms in newly planted walnut orchard in degraded drought mountain land. Bull. Soil. Water. Conserv. 35, 218–222.
Luo, J. H., Pu, R., Duan, H. T., Ma, R. H., Mao, Z. G., Zeng, Y., et al. (2020). Evaluating the influences of harvesting activity and eutrophication on loss of aquatic vegetations in Taihu Lake. China. Int. J. Appl. Earth. Obs. 87:102038. doi: 10.1016/j.jag.2019.102038
Madsen, T. V., and Cedergreen, N. (2002). Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich stream. Freshwater. Biol. 47, 283–291. doi: 10.1046/j.1365-2427.2002.00802.x
Moshiri, G. A., and Crumpton, W. G. (1977). Interrelationships Between Certain Microorganisms and Some Aspects of Sediment-Water Nutrient Exchange in Two Bayou Estuaries. Phases I and II. U.S. Government Science and Technology Report.
Namsaraev, Z., Melnikova, A., Ivanov, V., Komova, A., and Teslyuk, A. (2018). Cyanobacterial bloom in the world largest freshwater lake Baikal. IOP. Conf. Ser: Earth. Env. Sci. 121:032039.
Padedda, B. M., Lugliè, A., Lai, G. G., Giadrossich, F., Satta, C. T., and Pulina, S. (2021). Land-based impact of nutrient loads and eutrophication on an ancient Mediterranean natural lake. Hydrology 9:7. doi: 10.3390/hydrology9010007
Pastorelli, R., Paletto, A., Agnelli, A. E., Lagomarsino, A., and Meo, I. D. (2021). Microbial diversity and ecosystem functioning in deadwood of black pine of a temperate forest. Forests 12:1418. doi: 10.3390/f12101418
Phelps, T. J., Pfiffner, S. M., Sargent, K. A., and White, D. C. (1994). Factors influencing the abundance and metabolic capacities of microorganisms in Eastern Coastal Plain sediments. Microb. Ecol. 28, 351–364. doi: 10.1007/BF00662028
Powlson, D. S., Hirsch, P. R., and Brookes, P. C. (2001). The role of soil microorganisms in soil organic matter conservation in the tropics. Nutr. Cycl. Agroecosys. 61, 41–51.
Rahaman, S., and Sinha, A. C. (2013). Effect of water regimes and organic sources of nutrients for higher productivity and nitrogen use efficiency of summer rice (Oryza sativa). Afr. J. Agr. Res. 8, 6189–6195. doi: 10.5897/AJAR12.885
Ramachandra, T. V., and Solanki, M. (2007). Ecological assessment of lentic water bodies of Bangalore. Ministry. Sci. Technol. 25:96.
Sangakkara, U. R. (1997). “Root dynamics and nutrient uptake efficiencies of mung bean as affected by organic matter and effective microorganisms,” in Proceedings of the 5th International Conference on Kyusei Nature Farming, Bangkok, 23–26.
Schindler, D. W., Hesslein, R. H., and Turner, M. A. (1987). Exchange of nutrients between sediments and water after 15 years of experimental eutrophication. Can. J. Fish. Aquat. Sci. 44, s26–s33. doi: 10.1139/f87-277
Vincent, S. C. T., Jennerjahn, T., and Ramasamy, K. (2021). Sources, types, and effects of nutrients (N and P) in coastal sediments. Microb. Communities. Coast. Sediment. Struct. Funct. 47–78.
Wang, H., Molinos, J. G., Heino, J., Zhang, H., Zhang, P. Y., and Xu, J. (2021). Eutrophication causes invertebrate biodiversity loss and decreases cross-taxon congruence across anthropogenically-disturbed lakes. Environ. Int. 153:106494. doi: 10.1016/j.envint.2021.106494
Wang, W. D., Liu, W. Y., Wu, D., Wang, X. X., and Zhu, G. B. (2019). Differentiation of nitrogen and microbial community in the littoral and limnetic sediments of a large shallow eutrophic lake (Chaohu Lake. China). J. Soil. Sediment. 19, 1005–1016.
Wu, W. X., Wang, L., Liao, Y., and Huang, B. Q. (2015). Microbial eukaryotic diversity and distribution in a river plume and cyclonic eddy-influenced ecosystem in the South China Sea. Microbiologyopen 4, 826–840. doi: 10.1002/mbo3.282
Younes, M., Aggett, P. J., Aguilar, F., Crebelli, R., Dusemund, B., Filipic, M., et al. (2021). Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources (Revision 1)1. EFSA. J. 19:e06552. doi: 10.2903/j.efsa.2021.6552
Yuan, H. Z., Shen, J., Liu, E. F., Wang, J. J., and Meng, X. H. (2011). Assessment of nutrients and heavy metals enrichment in surface sediments from Taihu Lake, a eutrophic shallow lake in China. Environ. Geochem. Health 33, 67–81. doi: 10.1007/s10653-010-9323-9
Keywords: nutrient levels, microbial diversity, sediments, eutrophic shallow, lake
Citation: Zhu W, Liu J, Li Q, Gu P, Gu X, Wu L, Gao Y, Shan J, Zheng Z and Zhang W (2022) Effects of Nutrient Levels on Microbial Diversity in Sediments of a Eutrophic Shallow Lake. Front. Ecol. Evol. 10:909983. doi: 10.3389/fevo.2022.909983
Received: 31 March 2022; Accepted: 12 April 2022;
Published: 04 May 2022.
Edited by:
Chao Wang, Pearl River Fisheries Research Institute (CAS), ChinaReviewed by:
Jirong Bai, Changzhou Institute of Technology, ChinaCopyright © 2022 Zhu, Liu, Li, Gu, Gu, Wu, Gao, Shan, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Gu, cGVuZ2d1QGppYW5nbmFuLmVkdS5jbg==; Weizhen Zhang, emhhbmd3ejE1QGZ1ZGFuLmVkdS5jbg==; orcid.org/0000-0001-8601-7230
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.