- 1Department of Biology, Illinois College, Jacksonville, IL, United States
- 2Museum of Natural Sciences, Pinar del Rio, Cuba
Most bat species are highly social and utilize a variety of calls to communicate with each other including distress calls that may warn other bats of potential threats. The function of these calls in different species varies and could include eliciting help or acting as a warning signal to stay away. In this study, Cuban fruit-eating bats, Brachyphylla nana, were captured from La Barca Cave in Guanahacabibes National Park, Cuba and distress calls were recorded to examine call structure and variability among different bats. We used Avisoft SASlab pro to analyze 14 different spectral and temporal characteristics of the calls and utilized factor analysis to reduce the dimensionality in the data set and assess variability in call structure. The recorded calls and a pink noise control were used in a playback experiment inside the cave to analyze how bats respond to distress calls. An infrared video camera and ultrasonic microphone were used during the playback to determine if there were any changes in bat behavior, such as an increase in calls observed, bats flying by the speaker, or bats leaving the area. Our results suggest that call structure is variable with limited evidence that call characteristics are unique to specific individuals. Our playbacks suggest that these calls serve a social function in that the number of bats approaching the speaker increased during distress call playbacks relative to the control. Future work will include building on these results to further explore Brachyphylla nana social behavior including anti-predatory behavior and social communication.
Introduction
Anti-predator benefits of living in a social group for animals include a passive dilution effect, reducing any individual’s risk of being attacked as group size increases, and active mechanisms of predator deterrence including mobbing and warning signals or distress calls in response to predator exposure. Depending on the species and the level of threat, distress calls may serve multiple functions, which are not mutually exclusive. These include soliciting help from conspecific group members, warning other group members to stay away, startling the predator and increasing the chance of release, or attracting other predators causing a distraction and giving the calling individual a chance to escape (e.g., Hill, 1986; Branch and Freeberg, 2012).
From a signal design perspective, the acoustic structure of a distress call is predicted to match its function. For instance, a distress call that plays a more prominent role in attracting conspecifics for assistance may be localizable and have complex frequency and temporal characteristics to provide information (e.g., facilitate individual recognition of the caller, provide information about the potential predator etc.). For example, birds have been shown to respond to distress calls from conspecifics by mobbing the potential predator, with call structure conveying the level of urgency (Griesser, 2009). On the other hand, calls that function to induce a startle response, serve as a warning to conspecifics or heterospecifics, or attract additional predators that could threaten the capturing individual may be louder and less complex. For example, evidence suggests that distress call elements, primarily the prominence of the fundamental frequency, may be conserved from an evolutionary perspective, indicated by the positive response of deer mothers to playback stimuli consisting of distress calls from distantly related taxa (Lingle and Riede, 2014).
Bats are a highly diverse group of mammals with most species exhibiting some level of sociality ranging from species that live in small family units to species that form colonies of individuals numbering in the millions (Kerth, 2008). Bats are a particularly interesting group to study the influence of acoustic communication on social behavior, given that echolocating bats rely on sound for navigation and prey capture and thus have evolved sophisticated mechanisms for sound production and sound detection. Recent studies have examined the functional aspect of distress calls in bats by investigating both the frequency and temporal characteristics of the call as well as behavioral responses of conspecifics to playbacks of recorded calls (Chaverri et al., 2018). For example, distress calls show shared acoustic properties and elicit reactions from conspecifics and heterospecifics in both closely related species of pipistrelle bats (Pipistrellus nathusii, P. pipistrellus, and P. pygmaeus) (Russ et al., 2004) and bats that are more distantly related (Huang et al., 2018). A playback study conducted by Carter et al. (2015) found that distress calls in Molossus molossus elicit investigative behaviors from conspecifics but not to the extent that they were mobbing the perceived threat. Finally, in a study by González-Palomares et al. (2021) they found a difference in both the acoustic structure of distress calls and calling behavior between males and females with males producing more distress calls in response to a potential threat that are louder, lower in frequency, and contain a greater proportion of syllables with fast amplitude modulations.
Bats that inhabit cave environments face various potential predatory threats including owls, rodents, and snakes which hang from high spaces and catch bats in the air as they fly by (Dinets, 2017). The Cuban fruit-eating bat (Brachyphylla nana) is a social species which is commonly found in caves in tropical regions of the Caribbean roosting in large mix sexed groups in deeper areas of the cave where temperatures are more stable (Swanepoel and Genoways, 1983). While the echolocation calls of B. nana have been studied in the laboratory (Macias et al., 2006), less work has been done on social communication in their natural environment. Brachyphylla nana exhibits a diverse repertoire of social vocalizations while in the roost as well as a highly repetitive and loud distress call when held in the hand by a researcher (Manuel de la Cruz Mora et al., 2014). However, the acoustic structure of the call including the level of information provided by the call as well as the potential response of conspecifics to calling has not been studied thoroughly in the field. The purpose of this study was to examine distress calls in B. nana by investigating the acoustic structure of distress calls in terms of both inter and intra-individual variability and to utilize playbacks in a natural setting to provide insight into call function.
Methods
Distress Call Recording
Field work was conducted at La Barca Cave in Guanahacabibes Peninsula, Cuba over the course of 4 days in January 2017. Brachyphylla nana were captured inside the cave using hand nets while they were roosting on the cave ceiling or flying in the air. Each captured bat’s sex, reproductive condition, forearm length (measured to the nearest 0.1 mm using a vernier caliper), and age (adult or juvenile), were recorded. Captured bats were taken individually to a remote open cavern in the cave to collect distress calls and avoid interference from other bats. The recording location is an isolated cavern near an entrance to the cave which is not typically occupied by roosting bats and is approximately 150 m from the capture location. For the recordings, one researcher held the bat by hand approximately 8 m away from an ultrasound microphone (Avisoft UltraSoundGate 116 Hme) which was held by a second experimenter. To elicit distress calls, the bats were held by the scruff of the neck with the wings positioned toward the back while their ventral side was lightly massaged which caused the bat to emit a series of vocalizations while the mouth was exposed and oriented toward the microphone. We collected a minimum of one recording from each bat which consisted of at least 30 s of continuous vocalizations. After recordings were obtained, the bats were released. We marked individual bats on the wing using a dark marker to avoid re-capturing and recording the bats on successive sampling days.
Distress Call Analysis
The recordings were analyzed using Avisoft SASLab Pro software (Figure 1). We used the software’s automatic parameter measurement tool to extract temporal measurements (e.g., duration of each element, interval between elements) and frequency measurements (e.g., peak frequency, peak amplitude, minimum frequency, maximum frequency, bandwidth, and entropy) for a total of 14 variables measured (Table 1). For the analysis, we measured distress calls from 10 females and 10 males, and selected recordings based on signal to noise ratio, eliminating recordings which were overloaded. Each bat produced distress calls that contained multiple call elements (range 62–257). Since the number of elements per recording varied widely, we randomly selected 20 call elements from each recording for statistical analysis using a random number generator. All call elements shared the same basic structure (Figure 1). To reduce the dimensionality of the dataset we used a factor analysis with varimax rotation conducted using SPSS V.28 (IBM) to identify five factors that account for 72% of the variance in the data. The five factors were then used in a variance component analysis to estimate the variance explained by differences between calls recorded from males and females, differences among calls given by different bats, and differences among call elements within a single bat recording using maximum likelihood estimation. In addition to the distress call analysis, we recorded and analyzed echolocation calls from 3 females and 3 males during release after distress call recording to compare variability in a different type of vocalization in this species. We measured 12 temporal and frequency variables from each recording using Avisoft SASLab Pro and performed a similar factor analysis procedure in SPSS to extract five factors that explained over 87% of the variance in the data.
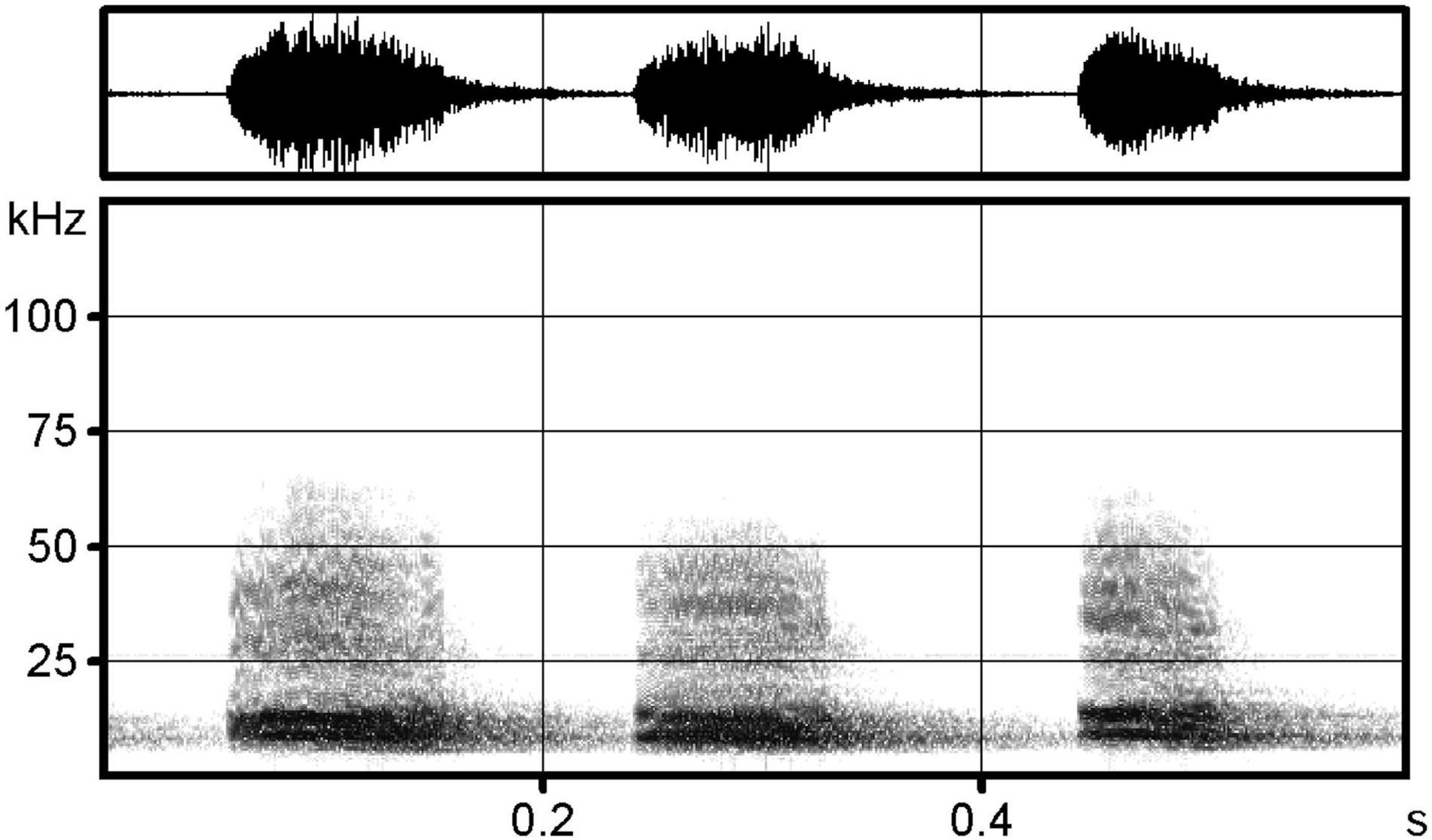
Figure 1. Spectrogram showing elements from a typical Brachyphylla nana distress call (FFT = 512, Hamming Window, 50% overlap).
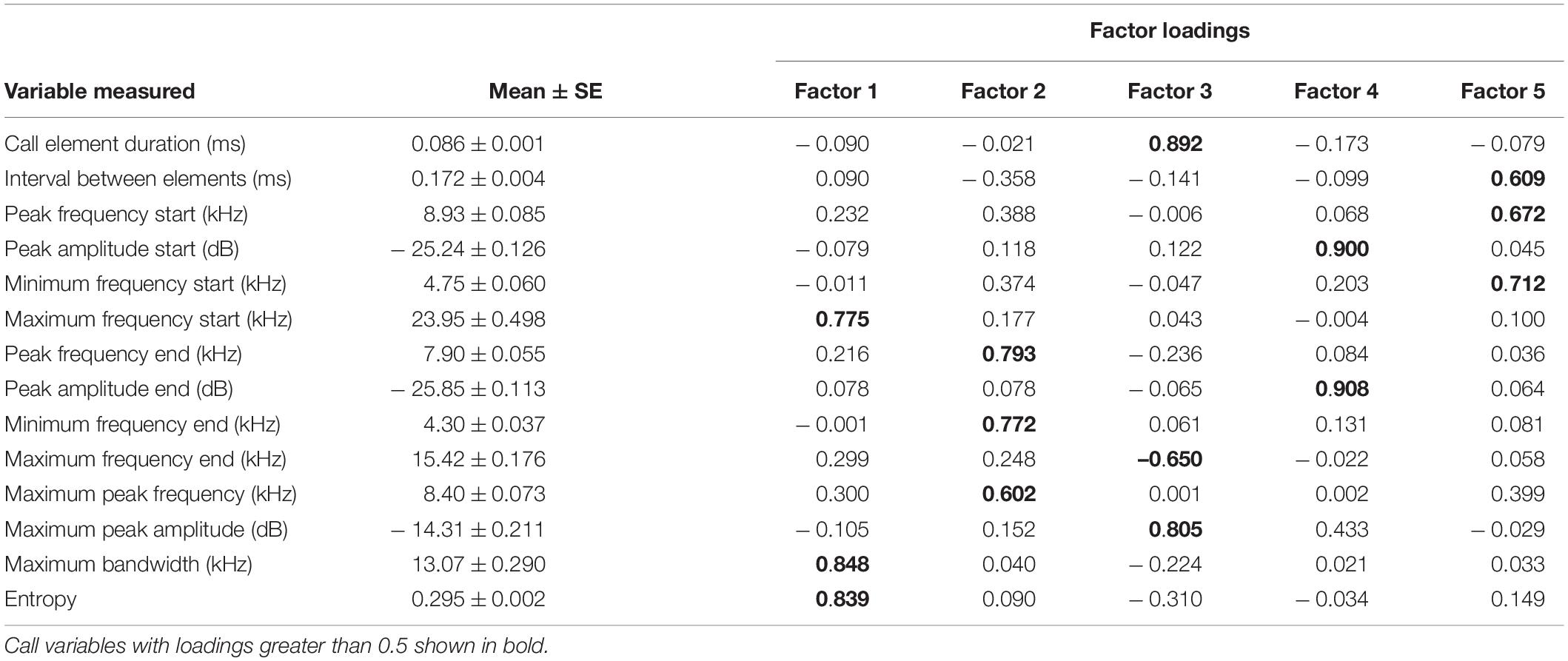
Table 1. Descriptive statistics and varimax-rotated factor loadings for the acoustic variables measured from Brachyphylla nana distress call spectrograms.
Distress Call Playback
We constructed playback files from recorded distress calls (N = 7 generated from recordings of 3 females and 4 males) using the program RavenPro 1.5 (Cornell Lab of Ornithology). Distress call stimuli consisted of 10 s of distress call elements at a rate and amplitude consistent with what bats produced during recording sessions (avg. approx. 6 elements per second) followed by 10 s of silence, repeating for a 60 s playback file. For a control, we assembled a file with elements of pink noise (similar to white noise but with greater intensity of lower frequencies) substituted for the distress call elements, but otherwise following the same rate, duration, and amplitude. Each playback trial consisted of 1 min of silence followed by the 1 min of playback of distress or control treatments. Each trial was videotaped using a Sony NightShot camera focused on the speaker (Jam Audio Bluetooth speaker) which was suspended from a rope on a pole approximately 2.5 m in the air and was illuminated using an infrared spotlight. The playback files were standardized to have amplitudes similar to distress calls made by recorded bats using a sound meter. In total, we conducted six trials on two consecutive days (2 trials on day 1 and 4 trials on day 2) each consisting of one distress playback sequence and one control sequence with a 5-min interval between trials). For the analysis, we measured bat activity by observing the number of bats that passed in close proximity to the speaker (within approximately 2 m) as well as examining recordings from an ultrasound microphone (Avisoft UltraSoundGate 116 Hme) which was pointed toward the speaker and could thus allow us to identify bats that echolocated as they oriented toward the speaker. Our response variable was recorded as the activity level (either bat passes or echolocation) identified during the playback period (distress or control) minus the pre-trial silence period. Thus, a positive number indicates the treatment increased bat activity, while a negative number or near zero number indicates that the treatment lowered activity or had no effect. Both bat passes by the speaker and echolocation calls were analyzed using a non-parametric Mann-Whitney U-Test conducted using SPSS (α = 0.05).
Results
Our results indicate that the acoustic structure of B. nana distress calls exhibits both significant intra-individual variation (range 16.9–93% of the variance explained by differences among call elements of a recorded bat’s call) and to a lesser extent inter-individual variation (range 6.6–82.9% of the variance explained by differences among calls from different bats). Variables such as peak amplitude, peak frequency, and maximum frequency appear to be informative as they loaded prominently on factors that explain a significant amount of the variance among calls from different bats (e.g., Factor 4 associated with amplitude characteristics of the call explained greater than 80% of the variance among calls from different bats). Very little of the variance is explained by differences among calls recorded from males and females (range 0–0.1%).
In comparison, the analysis of the echolocation calls showed somewhat less but comparable inter-individual variation (range 8.3–64%) and significant intra-individual variation (range 24–87.5%).
The results of our playback experiment indicate that bats both approached and inspected the speaker during the distress call playback trials more so than the control playback trials based on the video data (Figure 2) and the acoustic data (Figure 3), although based on the non-parametric Mann-Whitney U-test, these differences are not statistically significant (p = 0.240 for approaches, p = 0.065 for calling data). While the results were apparent from the data set as a whole, these differences are at least partially driven by the findings from initial trials with strong responses to the distress playback relative to the control followed by reduced activity and limited responses to both treatments in later trials indicating that bats may have avoided the area after exposure to the distress call treatment.
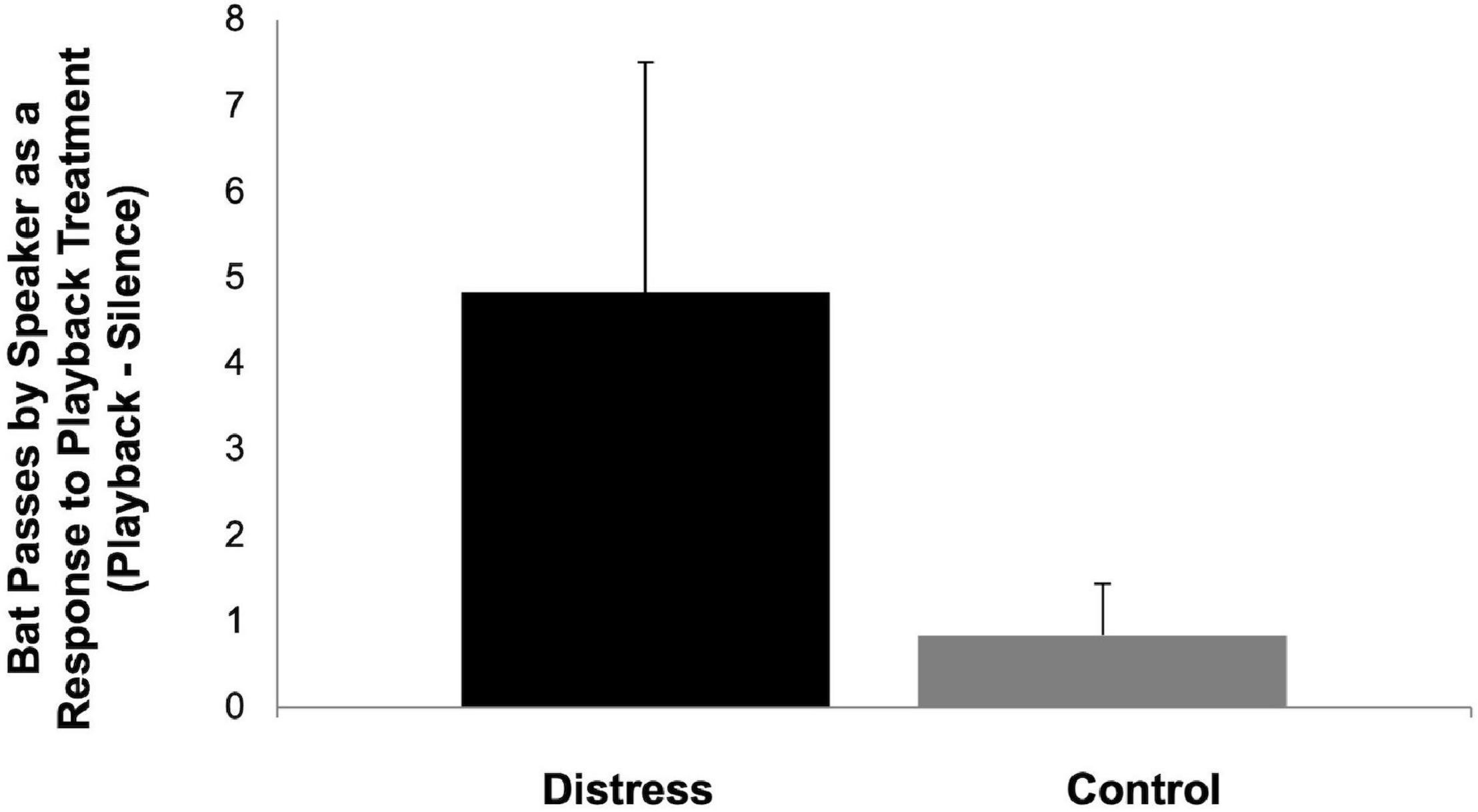
Figure 2. Effect of playback treatment on bat passes near speaker shown as the mean change in responsiveness (passes during playback period—silent period). Error bars represent the standard error of the mean.
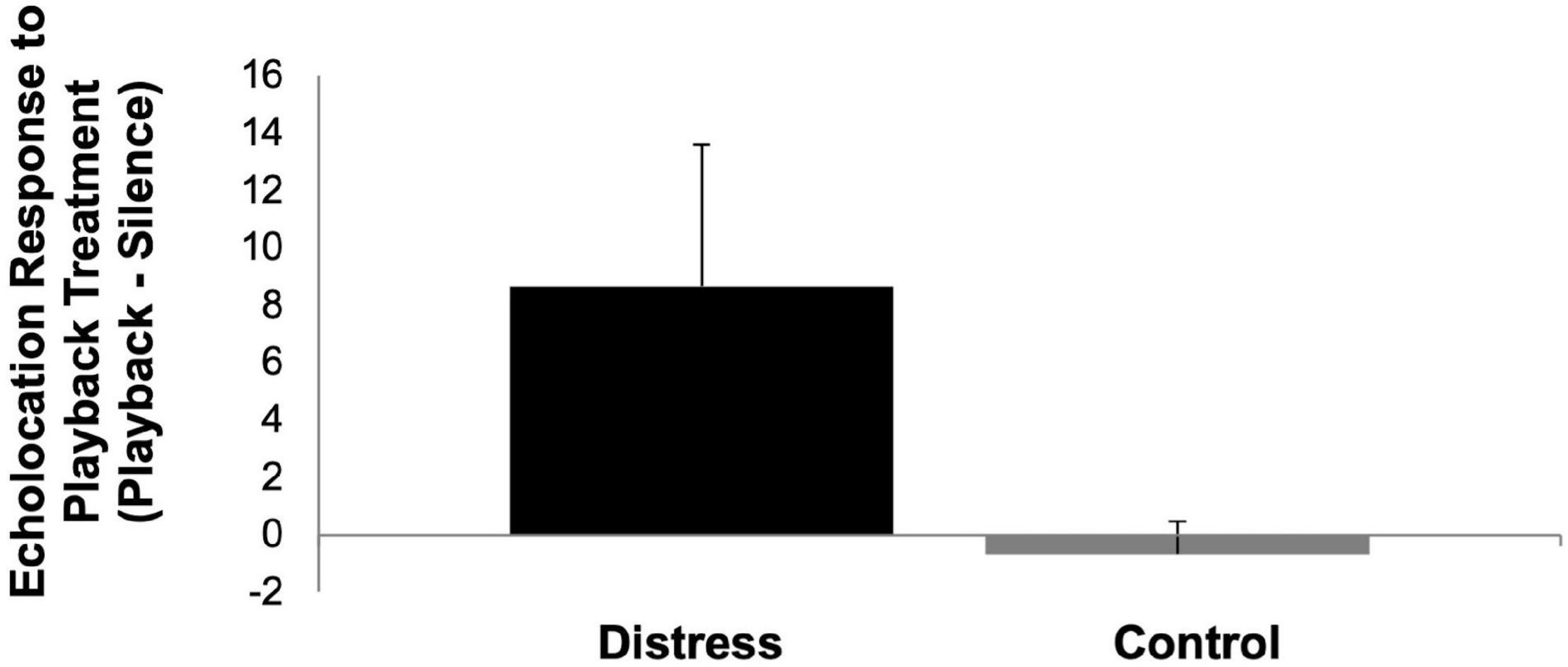
Figure 3. Effect of playback treatment on echolocation calling behavior in Brachyphylla nana shown as the mean change in responsiveness (echolocation calls during playback period—silent period). Error bars represent the standard error of the mean.
Discussion
We found that distress calls in B. nana, while highly variable both within and among different bats, exhibit a biological function in that they seem to communicate information to either conspecifics or heterospecifics given the responses observed during the playback trials; which while not statistically significant, are in the predicted direction with increased responses during distress call treatments vs. the control. In addition, the limited individual specificity in terms of the acoustic structure of distress calls as well as a lack of differentiation in acoustic structure of the call between males and females in B. nana fits the function of a general alarm or distress call to attract attention from conspecifics but not necessarily provide information about individual identity. Further, given that both bat response to playbacks and activity in general waned in the later playback trials, this supports that calls may serve as a warning to conspecifics to avoid a particular area, although given the limited number of trials in this study, further playback trials will be necessary to more thoroughly test this hypothesis. As Carter et al. (2015) reported for Molossus molossus, we saw little evidence that bats exhibited any mobbing behavior as a response to the distress call treatment. Based on the video, bats that approached the speaker flew in close proximity to it, but did not appear to make contact or fly toward the speaker in an aggressive fashion as animals that exhibit mobbing behavior typically do. A recent study by Eckenweber and Knörnschild (2016) examined the response of Saccopteryx bilineata to distress calls based on proximity to the day roost with more intense responses found when calls were played back near the roost vs. when played back near their foraging ground. In this study, the playback trials were conducted in a cavern near the day roost of B. nana which could explain the initial strong responses we found although B. nana are also known to respond to the distress calls of bats captured in mist nets placed in a foraging corridor (personal observation).
It should be stated that our video evidence is limited to observing bats approaching and flying near the speaker and does not allow us to identify if the responding species is B. nana or another similarly sized bat found in the cave (e.g., Artibeus jamaicensis). Thus, we could not test for the possibility that heterospecifics respond to the call as has been shown in other studies (e.g., Russ et al., 2004; Huang et al., 2018). In addition, the study was conducted in one cave only. While the social structure of B. nana is not well studied, it would be fruitful to examine the responses of bats to distress calls from a different social group. In a study on distress calls in least horseshoe bats (Rhinolophus pusillus), Wu et al. (2019) found that playback response was dependent on colony affiliation with non-colony calls eliciting a stronger response. In addition, at the time of the recording and playback, all bats were non-reproductive. One area we hope to explore in the future is to conduct recordings and playbacks after the pups have been born. The levels to which juveniles give distress calls is not known, but given the prominence of calls in adults it seems likely they do. If so, examining response to playbacks of pup distress calls in comparison to adult distress calls will be an interesting avenue for future research. Finally, we presented bats with just one stimulus to respond to; the playback of a distress call. The responses of bats could change if an additional stimulus is added to the experiment, for example a predator model which could enhance the perceived threat in the presence of a distress call. To our knowledge, these types of studies have not been thoroughly conducted in bats and could thus significantly add to what we know about anti-predatory behavior in different bat species.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Institutional Review Board and Institutional Animal Care and Use Committee of Illinois College and the Scientific Experts Committee of ECOVIDA (Centro de Investigaciones y Servicios Ambientales) and the National Center of Biodiversity of Cuba.
Author Contributions
BA designed the study, conducted field work, analyzed the data, and wrote the manuscript. JD designed the study, conducted field work, and assisted with editing the manuscript. JR conducted field work, analyzed the data, and assisted with editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding for transportation for the Illinois College faculty and students was provided by the Tillery Student-Faculty Research Collaboration Fund, the Richard Fry Faculty Collaboration Award, and the Alice Engelbach Endowment for Peace Studies.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This collaboration would not have been possible without the persistent effort and hard work of Steven Gardner, Department of World Languages and Cultures—Illinois College, Lawrence Zettler, Department of Biology—Illinois College, and Ernesto Mújica, Soroa Botanical Garden, Pinar del Rio Cuba. We also wish to acknowledge the following Illinois College students who assisted with the research: Eve Bahler, Allison McNamara, Adam Herdman, and Connor Melton. We also thank Andy Stice for his assistance with field equipment. Finally, we wish to thank José L. Camejo (Parque National Guanahacabibes) who helped make this work possible. Finally, we wish to thank the reviewers for their helpful feedback to improve this manuscript.
References
Branch, C. L., and Freeberg, T. M. (2012). Distress calls in tufted titmice (Baeolophus bicolor): Are conspecifics or predators the target? Behav. Ecol. 23, 854–862. doi: 10.1093/beheco/ars041
Carter, G., Schoeppler, D., Manthey, M., Knörnschild, M., and Denzinger, A. (2015). Distress calls of a fast-flying bat (Molossus molossus) provoke inspection flights but not cooperative mobbing. PLoS One 10:e0136146. doi: 10.1371/journal.pone.0136146
Chaverri, G., Ancillotto, L., and Russo, D. (2018). Social communication in bats. Biol. Rev. Camb. Philos. Soc. 93, 1938–1954. doi: 10.1111/brv.12427
Dinets, V. (2017). Coordinated hunting by Cuban boas. Anim. Behav. Cogn. 4, 24–29. doi: 10.12966/abc.02.02.2017
Eckenweber, M., and Knörnschild, M. (2016). Responsiveness to conspecific distress calls is influenced by day-roost proximity in bats (Saccopteryx bilineata). R. Soc. Open Sci. 3:160151. doi: 10.1098/rsos.160151
González-Palomares, E., López-Jury, L., Wetekam, J., Kiai, A., García-Rosales, F., and Hechavarria, J. C. (2021). Male Carollia perspicillata bats call more than females in a distressful context. R. Soc. Open Sci. 8:202336. doi: 10.1098/rsos.202336
Griesser, M. (2009). Mobbing calls signal predator category in a kin group-living bird species. Proc. R. Soc. B 276, 2887–2892. doi: 10.1098/rspb.2009.0551
Hill, G. E. (1986). The function of distress calls given by tufted titmice (Parus bicolor): an experimental approach. Anim. Behav. 34, 590–598. doi: 10.1016/s0003-3472(86)80128-2
Huang, X., Metzner, W., Zhang, K., Wang, Y., Luo, B., Sun, C., et al. (2018). Acoustic similarity elicits responses to heterospecific distress calls in bats (Mammalia: Chiroptera). Anim. Behav. 146, 143–154. doi: 10.1016/j.anbehav.2018.10.018
Kerth, G. (2008). Causes and consequences of sociality in bats. Bioscience 58, 737–746. doi: 10.1641/b580810
Lingle, S., and Riede, T. (2014). Deer mothers are sensitive to infant distress vocalizations of diverse mammalian species. Am. Nat. 184, 510–522. doi: 10.1086/677677
Macias, S., Mora, E. C., Garcia, A., and Yarelys, M. (2006). Echolocation behavior of Brachyphylla nana (Chiroptera: Phyllostomidae) under laboratory conditions. Caribb. J. Sci. 42, 114–120. doi: 10.18474/0749-8004-48.2.114
Manuel de la Cruz Mora, J., Mora, E. C., and Macias, S. (2014). Clasificación de los diseños espectro-temporales de las llamadas de estrés de seis especies de murciélagos cubanos. Cubazoo 26, 14–20.
Russ, J. M., Jones, G., Mackie, I. J., and Racey, P. A. (2004). Interspecific responses to distress calls in bats (Chiroptera: Vespertilionidae): a function for convergence in call design? Anim. Behav. 67, 1005–1014. doi: 10.1016/j.anbehav.2003.09.003
Swanepoel, P., and Genoways, H. H. (1983). Brachyphylla nana. Mamm. Species 206, 1–3. doi: 10.2307/3503872
Keywords: distress call, cuban fruit bat, Brachyphylla, playback, communication
Citation: Arnold B, De La Cruz Mora JM and Roesch J (2022) Assessing the Structure and Function of Distress Calls in Cuban Fruit-Eating Bats (Brachyphylla nana). Front. Ecol. Evol. 10:907751. doi: 10.3389/fevo.2022.907751
Received: 30 March 2022; Accepted: 06 May 2022;
Published: 24 May 2022.
Edited by:
Kirsten Bohn, Johns Hopkins University, United StatesReviewed by:
Gabriel Francescoli, Universidad de la República, UruguayPatrícia Ferreira Monticelli, University of São Paulo, Brazil
Copyright © 2022 Arnold, De La Cruz Mora and Roesch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bryan Arnold, QnJ5YW4uYXJub2xkQGljLmVkdQ==
 Bryan Arnold
Bryan Arnold Jose Manuel De La Cruz Mora
Jose Manuel De La Cruz Mora Joseph Roesch1
Joseph Roesch1