- 1Department of Biology and Marine Science, Jacksonville University, Jacksonville, FL, United States
- 2Graduate Program, Department of Biological Sciences, Texas Tech University, Lubbock, TX, United States
- 3Wildlife Conservation Society, Bolivia Program, La Paz, Bolivia
Temporally coordinated interactive vocalizations are important means of communication between individuals in various animal taxa. In mammals, interactive calling and singing can be highly synchronized to create either overlapping or antiphonal duets while in others, competitors antagonistically vocalize, engaging in counter-singing. Among non-primate mammals these vocalizations are considered rare and poorly understood. We provide an overview of antiphonal calling, duetting and counter-singing in non-primate mammals. Many of these coordinated vocalizations play a role in social interactions and allow mammals to convey information to other members of the social unit in visually inaccessible environments. South American Bamboo rats Dactylomys spp. are arboreal bamboo specialists found in dense bamboo thickets in Bolivia, Peru, Ecuador, Brazil and Colombia. These nocturnal rodents are rarely seen but can be easily heard because of their loud and distinctive staccato vocalizations. We provide some evidence that Bamboo rats engage in duetting, and as such they provide another case of a mammalian species, in which to investigate temporally coordinated interactive singing. We urge researchers to work toward common definitions of temporally coordinated vocalizations and to search for more mammals that utilize such vocalizations.
Introduction
Mammals produce a diverse array of vocalizations ranging from single note contact calls to highly synchronized, multiple note songs (Fitch, 2006). Whether simple or complex, mammal vocalizations convey vital information to conspecifics. While calls, such as contact and alarm calls, are short with few notes and function in a variety of daily activities songs are typically more complex consisting of several notes, and used in mating and territorial displays (Boughman and Moss, 2003; Marler, 2004). Interactive vocal communication has evolved in several mammalian groups, including primates, rodents, bats, and cetaceans (Logue and Krupp, 2016; Vernes, 2017; Terleph et al., 2018; de Reus et al., 2021; De Gregorio et al., 2022). Antiphonal vocalizations, counter-singing, and duetting have been thoroughly documented and studied in birds (for a review of duetting in birds see Hall, 2009), but less so in mammals, with most of the attention given to primates (Adret et al., 2018; Clink and Lau, 2020). Broadening research to include mammals other than primates will allow us to test hypotheses on the function and evolution of these vocalizations.
Across all taxa, interactive vocalizations and turn-taking (Levinson, 2016), have received more attention in recent years, with researchers trying to assess appropriate frameworks to study and analyze interactive vocalizations. The disparity in definitions of these types of vocalizations makes comparative studies difficult, with some researchers suggesting a focus on the stepwise process between individuals involved in back-and-forth communication (who, when and how does turn-taking occur), whereas others suggest a focus on the temporal and rhythmic elements of vocalizations (Pika et al., 2018; Ravignani et al., 2019; de Reus et al., 2021). We suggest and utilize the term temporally coordinated interactive vocalizations (TCIVs hereafter) to encompass both the dynamics between signaling mammals as well as the rhythmic and temporal component of the vocalizations themselves. We provide an overview of the literature on non-primate mammal vocalizations that fall under this umbrella. Across the literature different terms are utilized and we adhere to the terms utilized by individual researchers. We classify TCIVs into three categories (antiphonal vocalizations, duets, and counter-vocalizations). These categories are based on the literature we examined but we realize that some overlap may occur and may not reflect current definitions. Antiphonal vocalizations are the broadest category and involve call and response occurring at regular intervals between two or more individuals (Yoshida and Okanoya, 2005; Filippi et al., 2019). Counter-vocalizations occur between specific individuals and territorial mammals may engage in counter-calling (or counter-singing), in which rival individuals call (or sing) back and forth in a non-overlapping fashion (Banerjee et al., 2019). These types of vocalizations may or may not involve turn-taking in which individuals adjust vocalizations based on the behavior of the other participant, including overlap avoidance (Demartsev et al., 2018; Pika et al., 2018; Okobi et al., 2019). Duetting has been examined extensively in birds and early definitions drawn from the avian literature frequently described duetting as coordinated vocalizations in mated pairs (Todt and Naguib, 2000; Hall, 2009). Duets may or may not overlap, and current definitions of duetting vary (see Pika et al., 2018). As noted by Langmore (2002), a more accurate definition should focus on acoustic features of duets and not just the participants. Our definition of duetting follows the literature that characterizes duets as coordinated, predictable, repetitive, stereotypical vocalizations between two or more individuals, often bonded individuals (Langmore, 2002; Clink et al., 2020; Nieder and Mooney, 2020).
Antiphonal Vocalizations, Duets and Counter-Vocalizations in Non-Primate Mammals
Mammals from diverse orders, with varying habitats, activity periods, and social and mating systems engage in TCIVs (Table 1). The functions of these calls vary greatly, and categories were selected post-hoc after examining the literature. Researchers used behavioral data, including temporal and spatial aspects of vocalizations and individuals, and playback experiments to elucidate function. Below is a brief discussion of the different vocalizations and their functions in non-primate mammals.
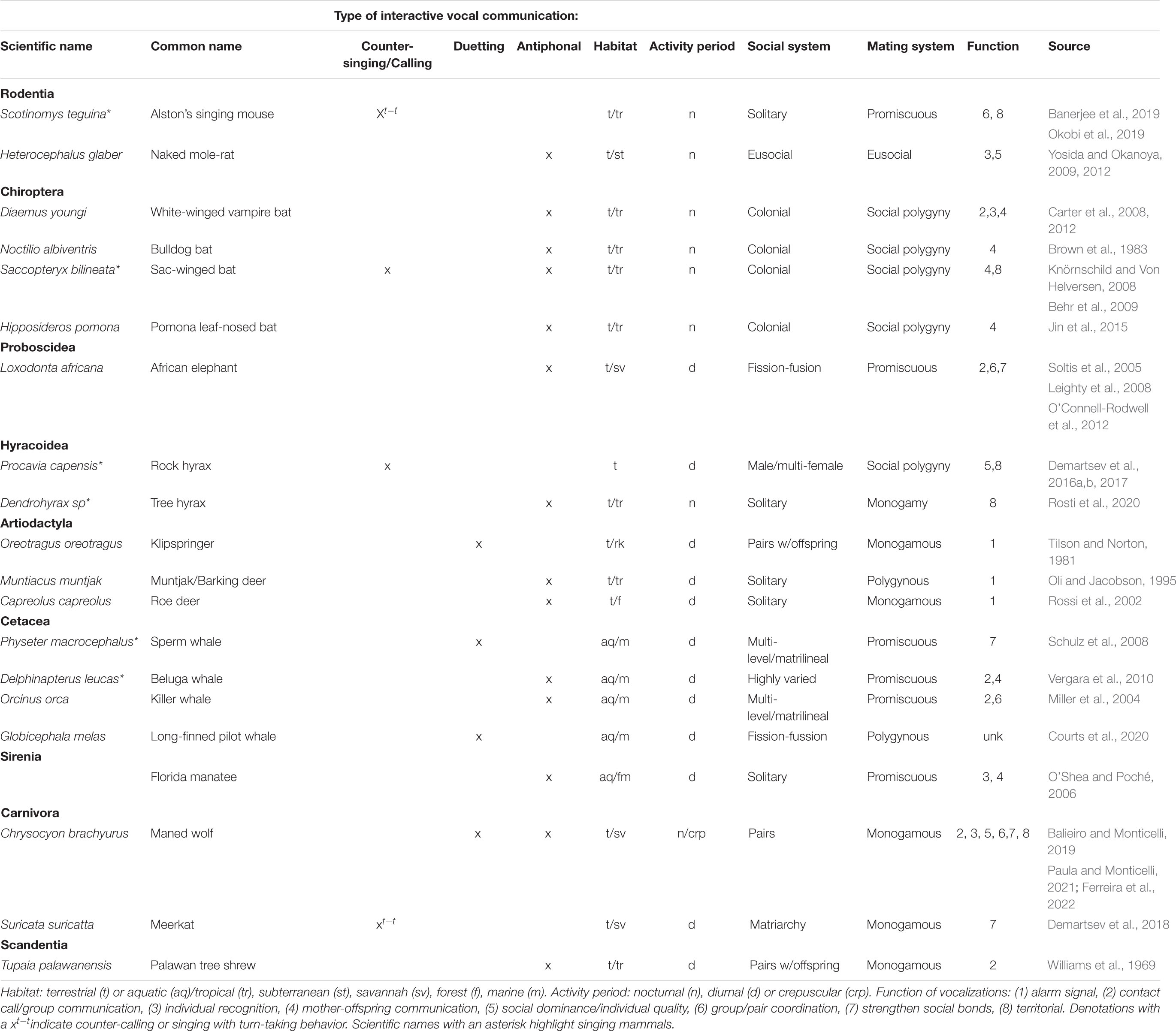
Table 1. Examples of non-primate mammals engaging in temporally coordinated interactive vocal communication.
Antiphonal Vocalizations
In mammals, antiphonal vocalizations (or the use of the term antiphonal) is more prevalent in the literature than duetting and counter-singing, and antiphonal calls serve as contact calls that can encode information about individual identity and condition. For example, naked-mole rats Heterocephalus glaber can identify individuals as well as social rank within their colony (Yosida and Okanoya, 2009). In group living mammals, antiphonal vocalizations can also help individuals coordinate with conspecifics. White-winged vampire bats Diaemus youngi can discern individuals and thus their spatial relationship in the colony via antiphonal calling (Carter et al., 2008). African elephants Loxodonta africana use antiphonal rumbles to monitor individuals and coordinate group movement, with antiphonal calling increasing as elephants disperse away from one another (Leighty et al., 2008; O’Connell-Rodwell et al., 2012). Moreover, female elephants exchange rumbles more with close social partners (Soltis et al., 2005). Similarly, pulsed “type A” calls of beluga whales Delphinapterus leucas enable group cohesion and closely related individuals use similar variants of these calls (Vergara et al., 2010). Other examples of antiphonal calls between related individual include calls between mother and offspring in belugas, as well as in several bat species (bulldog bat Noctilio albiventris, sac-winged bat Saccopteryx bilineata, Pomona leaf-nosed bat Hipposideros pomona) and the Florida manatee Trichechus manatus latirostris (Brown et al., 1983; O’Shea and Poché, 2006; Knörnschild and Von Helversen, 2008; Jin et al., 2015).
While antiphonal calls function largely to facilitate social interactions among conspecifics, in Artiodactyls antiphonal calls function as important pursuit deterrent signals to predators. The antiphonal calls of Indian muntjac Muntiacus muntjak and Roe deer Capreolus capreolus intensify alarm signals, confusing predators as multiple individuals call and respond from different locations (Oli and Jacobson, 1995; Rossi et al., 2002). Klipspringer Oreotragus oreotragus elicit short vocal exchanges, females following males swiftly (30 ms intercal interval) in loud and repetitive alarm calls (Tilson and Norton, 1981). While these calls have been traditionally seen as alarm calls, they could also aid in pair and group coordination.
Duets
Unlike the call and response of antiphonal vocalizations, duetting requires two individuals to coordinate vocalizations in a precisely timed manner (Langmore, 2002; Nieder and Mooney, 2020; de Reus et al., 2021). Duets begin with one individual vocalizing, followed by another individual vocalizing, sometimes simultaneously with the first. Duetting by non-primate mammals appears to be rarer in the literature. Within social units of sperm whale. Physeter macrocephalus, individual whales will produce codas after another whale begins to sing and in some instances the codas will overlap (Schulz et al., 2008). As with many antiphonal calls, duets in sperm whales help to maintain social bonds. Long finned pilot whales (Globicephala melas) also produce vocal duets, yet their function is unknown (Courts et al., 2020). The duets of maned wolves Chrysocyon brachyurus also have a social function. Maned wolves live in dispersed pairs that actively defend a territory and their individually identifiable roar-barks produced as loose duets (2 s inter-call intervals on average) may help them facilitate and strengthen pair bonds necessary for this defense (Emmons, 2013; Balieiro and Monticelli, 2019; Paula and Monticelli, 2021). Recent work with maned wolves describe their interactive roar-barks as counter-calling, not duetting. This behavior is most frequent during the mating season and when young are present, suggesting a function in not only pair bonding, but also in care of the young (Ferreira et al., 2022). The function of duetting is still poorly understood and debated for many taxa and as seen in birds it may serve more than one function (Hall, 2009).
Counter-Vocalizations and Turn-Taking
Unlike duetting that involves coordination between members of the same social group, counter-vocalizations involve back and forth vocalizations, often between territorial rivals. Many mammals sing in a territorial fashion and counter-singing has been documented in sac-winged bats Saccopteryx bilineata, singing mice Scotinomys teguina and rock hyraxes Procavia capensis (Behr et al., 2009; Demartsev et al., 2016a,b, Demartsev et al., 2017; Banerjee et al., 2019; Okobi et al., 2019). As with other coordinated vocalizations, they can contain information about caller identity; however, these vocal duels have only been documented in males and function in sexual advertisement and territorial interactions. The singing behavior of rock hyraxes has been well documented, and 25% of all singing is male-male counter singing (Demartsev et al., 2016b). Tree hyraxes Dendrohyrax sp., unlike rock hyraxes, are solitary and monogamous, and although they sing, their coordinated communication occurs in antiphonal territorial calls. In a recent study of tree hyrax vocalizations, 75% of thwack call sequences involved counter-calling between two or more individuals (Rosti et al., 2020). It is important to note that these two closely related, yet socially and ecologically distinct species, both have coordinated vocalizations, one in song and the other in calls. Future comparative studies of hyrax species, as well as across and within mammalian groups in general, could lead to insights on the evolution of coordinated vocalizations.
Turn-taking behavior does not occur in all instances of counter-singing, but as documented in singing mice, individuals mediate their response to other individuals, regulating their vocalizations and altering their behavior in response (Banerjee et al., 2019; Okobi et al., 2019). Similarly, meerkats engage in turn-taking “sunning vocalizations” when several group members sun individual meerkat calls avoid overlap with other vocalizing meerkats (Demartsev et al., 2018).
In addition to clearer definitions of TCIVs, researchers must identify which mammals engage in this behavior and how it affects each vocalizing individual. While duetting and other coordinated vocalizations have been traditionally viewed and examined as a collective behavior, they occur at both the level of the individual and the collective pair or group (Hall, 2004; Logue and Krupp, 2016; Ravignani et al., 2019; Clink et al., 2020; Clink and Lau, 2020). It is likely that many more mammals engage in TCIVs than is currently recognized. Next, we present observational data of bamboo rats Dactylomys spp., a neotropical bamboo specialist, from Ecuador and Bolivia as an example of an understudied mammal that engages in TCIVs.
Perspectives From Bamboo Rat Vocalizations
South American bamboo rats Dactylomys spp. are nocturnal, arboreal bamboo specialists found in dense bamboo thickets in Bolivia, Peru, Ecuador, Brazil, and Colombia (La Val, 1976; Emmons, 1981; Dunnum and Salazar-Bravo, 2004; Bezerra et al., 2007). Dactylomys spp. are rarely seen but can be easily heard due to their loud and distinctive staccato vocalizations that start at dusk (La Val, 1976; Emmons, 1981). Vocalizations are thought to be territorial calls; however, the spatial and social relationship between individuals within and between bamboo patches has not been thoroughly investigated. Little is known about this secretive species due to its nocturnal cryptic behavior and the dense bamboo thickets in which they inhabit, yet Dactylomys spp. might provide a good comparative model to study TCIVs. Emmons (1981) first described duetting behavior in the Amazonian bamboo rat Dactylomys dactylinus and noted two distinct call types: loud staccato “L calls” given by males, often followed by softer grunting “A calls” given by females. She also noted call and responses between males producing the loud “L calls.”
Our preliminary investigations of D. dactylinus in Ecuador and D. boliviensis in Bolivia provide more evidence for TCIVs. In July 2010 and July 2011, ENV surveyed D. dactylinus populations and recorded their vocalizations at Wildsumaco Wildlife Sanctuary in Ecuador, (00° 41.250’ S, 77° 36.049’ W; ∼1400 m elevation). Between 2015 and 2017, as part of the “Identidad Madidi Project” led by Wildlife Conservation Society in Bolivia, NBH and her team observed and recorded D. boliviensis at five sites inside Madidi National Park (14.1892° S, 68.3339° W; 200–1700 m elevation). At both Wildsumaco and Madidi, bamboo rats were heard and seen in bamboo patches (Guadua spp). Ten bamboo patches, ranging in size from 25 to 500 m2, often consisting of several clusters of bamboo within a matrix of other vegetation, were identified at Wildsumaco. Some patches were relatively close to one another (∼15 m), but could be as far apart as 100–200 m. At Madidi, bamboo patches could also have different extensions and were generally localized in wet soils along running water, but their distribution and size was not quantified as it was part of a larger survey. Identification of individuals on most nights was difficult given the dense vegetation and the rats cryptic behavior, as Emmons (1981) notes they move silently one foot at a time making vocalizations the only means to identify if individuals were present. At both Wildsumaco and Madidi loud staccato calls and soft grunting calls, referred to as “L” and “A” calls by Emmons (1981), occurred during the night, between 19:00 and 04:00. Males were visually identified and observed producing the staccato “L calls” twice at Madidi and once at Wildsumaco.
Audio recordings at both sites were made with Marantz PMD 661/671 Digital recorders (sampling rate: 44.1 kHz; resolution: 16-bit) and Sennheiser ME 66 directional microphones. At Madidi several locations were visited and a total of five recordings (one per site) were made. Most recordings were incomplete as they often began once individuals had already started vocalizing. For example, at one location. patches were visited for seven nights in a row and on some nights no bamboo rats could be heard responding to vocalizations of the focal individual and in other instances calling could be heard, but a distance away from it and only on one night was a complete vocalization recorded. Three focal bamboo patches at Wildsumaco were observed for three nights in July 2010 and a total of 19 recordings were made. In line with Emmons’ work (1981), our observational data suggest that a single pair resides in each bamboo patch and individuals within patches vocalized approximately every 10 min. At Madidi, vocalizations occurred after longer time intervals, approximately every 45 min. It is unclear what prompts bamboo rats to vocalize; whether it is in response to vocalizations given by rats in other patches is uncertain. Playback of a previous recording was attempted at two sites in Madidi to see if individuals were present in patches, and while at one location a bamboo rat responded, no response was elicited at the other site. Listening to their calls in the forest suggests that the rats are calling and responding to one another in different patches, as seen in other counter-singing mammals, but tests must be conducted to ascertain the true nature of vocalizations to ensure what we perceive as coordinated behavior is not due to random chance. The loud vocalizations of bamboo rats may help them identify and be aware of other rats’ location; given their highly specialized low nutrient diet of bamboo, proper spacing and low energy communication networks may be selected for Emmons, 1981.
Overlapping duets were recorded at both site; however, this was a rare occurrence, and because of limited observations and few recordings, it is uncertain if this is a seasonal behavior, occurs year-round or whether it is tied to reproduction. Recordings of duets made at Wildsumaco and Madidi were visualized with Raven bioacoustic software (Figure 1). Despite the lack of individual identification, elements of these three vocalizations reveal structural features that are found in other mammals that engage in TCIVs. At both Wildsumaco and Madidi, soft grunting calls in response to the loud staccato L calls–the latter featuring a typical increase in inter-note intervals–could be viewed as interactive duets between members of the same patch (Figures 1A,B). These intra-patch male-female duets may aid in coordination and social bonding. In one instance two individuals engaged in loud staccato vocalizations (Figure 1C). Individuals in different bamboo patches may counter vocalize in a more competitive territorial manner and inter-patch male-male vocalizing may be a form of counter-singing announcing and delimiting borders, especially when territorial rivals come in close proximity to one another. Experimentation is needed to discern the true nature of TCIVs in bamboo rats.
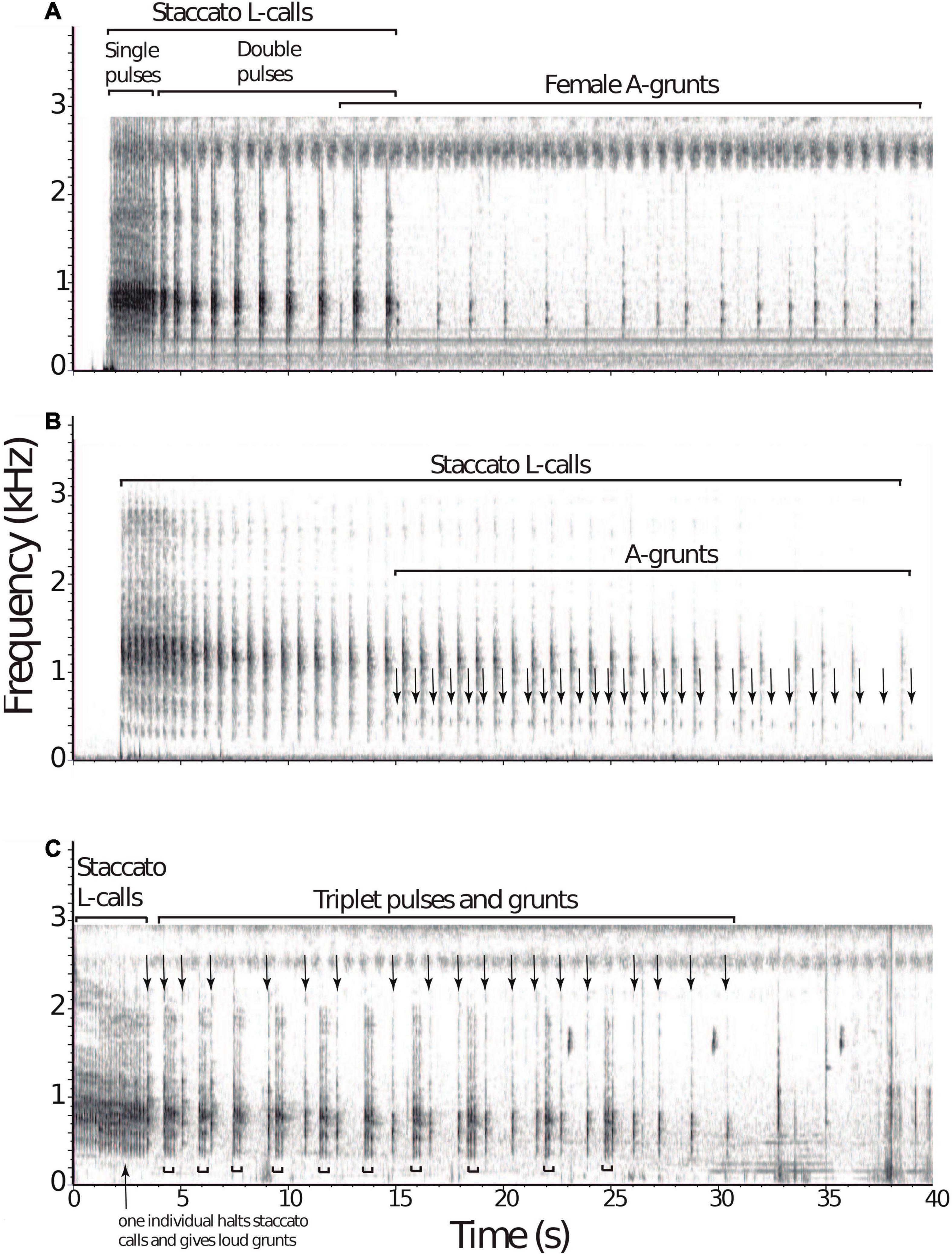
Figure 1. Examples of vocal exchanges in Neotropical bamboo rats. At Wildsumaco, two instances of duetting in the Amazonian bamboo rat, Dactylomys dactylinus were recorded. (A) The first instance involved loud staccato “L calls” by a male, starting with single pulses followed shortly after by doubles pulses emitted at a decreasing rate. A power spectrum analysis of L-calls (both single and double pulses; n = 23) revealed one peak of energy at 801 Hz. After the eighth double pulse, soft grunting “A calls” by another individual followed, assumed to be a female based on Emmons’ previous work. Spectral analysis of A-calls (n = 16) revealed two peaks of energy at 559 and 743 Hz. (B) At Madidi, a similar duetting pattern was recorded from the Bolivian bamboo rat, D. boliviensis. A male gave 21 loud staccato “L calls,” followed by a soft grunting “A calls” from another individual. A power spectrum analysis of L-calls (n = 21) revealed one peak of energy at 1193 Hz. Note the decrease in call rate of the staccato vocalization. (C) In the second instance at Wildsumaco, both individuals engaged in the loud staccato calls “L calls.” After 10 pulses by the first individual, another individual joined in with loud L calls that ceased shortly after, followed by single loud pulses while the other individual continued staccato L calls in triplets, answered by the grunts. A power spectrum analysis of the first 10 pulses revealed one peak of energy at 754 Hz. Spectrograms were prepared with Raven-Lite software (v. 2.0.3, Cornell Laboratory of Ornithology, Ithaca, NY, United States), using a window size of 2048 points. Spectral analysis of calls was performed using the Audacity software (v. 2.4.2).
The potential duetting behavior detected in Dactylomys spp. might be the result of ecological features unique to these rodents. Duetting and antiphonal vocalizations evolved in several mammals that need to communicate over long distances in dense forest environments, like those inhabited by the bamboo rats. The social structure of bamboo rats is not completely understood; they are thought to live in family groups with a single male (and female and offspring) occupying a single bamboo patch (Eisenberg, 1989). Investigations of the closely related Brazilian bamboo rat Kannabateomys amblyonyx have found variation in mating systems (either polygynous or monogamous) depending on resources, including number of females and bamboo patch availability (Silva et al., 2008). It is likely that the genus Dactylomys is monogamous and as seen in monogamous primates, (e.g., tarsiers, titi monkeys and hylobatids), duetting may help to strengthen pair bonds, coordinate movement, as well as send territorial information to other conspecifics in the area.
Discussion and Future Directions
Much of the literature on TCIVs, including antiphonal vocalizations and duets, contains confounding terminology. With multiple definitions of antiphonal vocalizations, duets, antiphonal duet, duet calls, songs and duet singing, forming a cohesive framework for discerning patterns and testing hypotheses can be challenging (Filippi et al., 2019; De Gregorio et al., 2022). Regardless of terminology, these TCIVs are rarely investigated in non-primate mammals. The disparity in ecology and sociality of the different mammals herein discussed, makes it difficult to discern if commonalities exist in these important interactive vocalizations. However, two main themes emerge for antiphonal communication and duetting. First, most of the species discussed live in habitats in which visual proximity is restricted, whether it is a dense tropical forest or a vast ocean. The second theme is the highly social nature of these calls. Highly social mammals may require TCIVs to maintain and reinforce social relationships amongst group members, similar to vocal grooming in primates, the more complex mammalian societies become the more complex their vocal repertoire and vocalizations may become (Dunbar, 2012). There is some evidence for this in bats, in which antiphonal vocalizations and counter singing have been identified (Knörnschild, 2014). Knörnschild et al. (2020) showed a positive relationship between the information contained in the contact and isolation calls of bat species and the size of the specie’s social group suggesting a link between social and vocal complexity across bat species.
Vocal communication that encodes specific information about individuals and functions in maintaining social relationships may be selected for, regardless of social system, in visually isolated, yet social species. For example, sperm whales are highly social, yet visually restricted from group members in the ocean environment, and duets help to reinforce their social bonds (Schulz et al., 2008). The question then is why do we see so little of this type of interactive communication in mammals? Duetting is common in bonded pairs and the scarcity of monogamy and shared parental responsibilities may also account for less mammalian representation (Lukas and Clutton-Brock, 2013). Many group living mammals may be in close visual proximity to other group members, making these sorts of communicative channels unnecessary. Turn-taking vocalizations may however evolve in social units in which individuals can see and alter their and other’s behavior with their vocalizations, as is the case with meerkats (Demartsev et al., 2018). Counter-singing is even rarer than duetting and its rarity might stem from how rare singing generally is in mammals, which has only been investigated in a few taxa. It may be that mammals, relying heavily on scents, are simply less vocal than other groups like birds that rely heavily on vision and sound; or more interactive communication is occurring in mammals, but we have yet to detect it with studies. The human auditory range is limited, and mammals frequently produce and perceive sound at frequencies beyond human auditory abilities (Heffner and Heffner, 2018). Both infrasound and ultrasound are used by mammals in terrestrial and aquatic habitats (Martin et al., 2017) and detection of these vocalizations require specialized bioacoustics monitoring equipment and this fact may help to explain the paucity of data (Ladich and Winkler, 2017; Romero-Mujalli et al., 2021).
Bamboo rats produce loud, audible vocalizations, and they might engage in TCIVs. Evidence for duetting exists, but the frequency, causes and adaptive value of this behavior have yet to be deciphered. Most of what is known about bamboo rat vocalizations comes from anecdotal field recordings by ornithologists and through Emmons (1981) work in Ecuador. The data we collected in both Wildsumaco and Madidi were observational in nature and not intended or designed to test specific hypotheses and in the case of Madidi the records were part of an integrated inventory of wildlife at the park. One difficulty in studying bamboo rats is their nocturnal secretive nature and the dense vegetation in which they reside. At both study sites bamboo rats were seldom seen and often when they were spotted, they froze and stopped vocalizing, making it difficult to follow individuals and gather behavioral data. We expect bamboo rat calls will vary depending on the different ecological and social factors, including vegetation structure, seasonality, population density, reproductive stage, etc. Dialects between and within populations may also exist and be another confounding variable when comparing different species and populations. New passive recording technologies may provide a solution, with arrays of recorders in bamboo patches researchers may be able to answer questions about the timing and frequency of these behaviors (see Szymański et al., 2021). Duetting may be more common during the breeding season, which is unknown, and further analysis of vocalizations could provide information on the temporal elements of the vocalizations themselves as well as inter- and intraspecific and population differences. Duetting may have several functions in bamboo rats and non-primate mammals in general. Playback experiments may shed light on the territorial nature of these vocalizations, whether counter calling exists, and how and if bamboo rats respond differently to individuals within their patch (social unit), in nearby patches (neighbors) and those further away (strangers). Mammals with complex vocal communication should be sought out and investigated to determine their prevalence, as well as to test hypotheses on the ecological and evolutionary pressures leading to TCIVs. Bamboo rats are just one example of a mammal whose conspicuous vocalizations have yet to be fully examined.
Mammalian species that live in visually restrictive habitats and require complex vocalizations to maintain long-term social relationships are likely to utilize TCIVs. Several mammal groups may be ideal targets for future research, including close relatives of mammals known to produce TCIVs. For example, forest dwelling elephant species, including the African forest elephant Loxodonta cyclotis and Asian elephants Elephas maximus produce rumble vocalizations like African elephants, but reside in slightly different habitats with differing visibilities (Pardo et al., 2019). Canids, including gray wolves Canis lupus and jackal species (C. aureus, Lupulella adusta, and L. mesomelas) are also highly vocal and social (Moehlman, 1987; Jenner et al., 2011; Zaccaroni et al., 2012). Like maned wolves, jackals are monogamous, but display variation in social complexity within and across species (Moehlman, 1987). Comparative studies of closely related species may shed light on the evolution of TCIVs. Rock and tree hyraxes mentioned earlier live in very different habitats with different social structures, yet both utilize TCIVs. Spotted hyenas Crocuta crocuta living in fission-fusion societies utilize long distance “whoop” calls and comparisons could be made with the solitary and monogamous striped hyena Hyaena hyaena (Mills, 1989; Holekamp et al., 2007; Califf et al., 2020). In addition to seeking out new species and conducting comparative studies, examination of the physiological mechanisms underpinning call emission and sound reception should be undertaken. The future of the field of mammal vocalization is promising and insights from diverse taxa will strengthen our understanding of antiphonal calls, duets and counter-singing.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because observational study, review not required by institution.
Author Contributions
EV and NH collaborated on the outline and direction of the manuscript. EV gathered information on coordinated vocalizations in mammals, as well as collected and analyzed vocalizations from Wildsumaco, and wrote the manuscript. NH provided vocalizations and observational data from Madidi, as well as reference material and, provided feedback. Both authors read and approved the final version of the manuscript before submission.
Funding
Identidad Madidi was an interinstitutional project led by Wildlife Conservation Society, Bolivia Program, with funds from the Gordon and Betty Moore Foundation. EV received funding for work at Wildsumaco from Jacksonville University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Travis Knowles and Daniela Arenas-Viveros for their feedback on the initial manuscript. Patrice Adret and reviewers made significant suggestion and provided editorial assistance to improve iterations of the manuscript; we thank them for their insight and patience. Additionally, Patrice Adret assisted with the spectrogram analysis and figure creation. NH was grateful for the support of the environmental authorities in Bolivia (DGBAP and SERNAP). The project in Madidi was conducted under the authorization # MMAYA/VMABCCGDF/DGABAP 353/2014 and 130/2019. NH also thanks Rob Wallace, the Director of the Greater Madidi Tambota Program, Wildlife Conservation Society, Bolivia Program for all the support during the Identidad Madidi Project. Ornithologist Victor Hugo García helped NH with recording bamboo rats in the field and the laboratory cleaning of the calls, and Marisol Hidalgo Cossio and the Identidad Madidi team for on the ground support during field work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.906546/full#supplementary-material
References
Adret, P., Dingess, K. A., Caselli, C. B., Vermeer, J., Martínez, J., Luna Amancio, J. C., et al. (2018). Duetting Patterns of Titi Monkeys (Primates, Pitheciidae: Callicebinae) and Relationships with Phylogeny. Animals 8:178. doi: 10.3390/ani8100178
Balieiro, F., and Monticelli, P. F. (2019). Evidence of individual discrimination in the maned wolf long-distance extended-bark. Behav. Processes 158, 219–227. doi: 10.1016/j.beproc.2018.12.004
Behr, O., Knörnschild, M., and von Helversen, O. (2009). Territorial counter-singing in male sac-winged bats (Saccopteryx bilineata): low-frequency songs trigger a stronger response. Behav. Ecol. Sociobiol. 63, 433–442.
Bezerra, A. M. R., da Silva, N. J. Jr., and Marinho-Filho, J. (2007). The amazon bamboo rat Dactylomys dactylinus (Rodentia: Echimydae: Dactylomyinae) in the cerrado of central Brazil. Biota Neotrop. 7, 235–237. doi: 10.1590/S1676-06032007000100030
Boughman, J. W., and Moss, C. F. (2003). “Social sounds: vocal learning and development of mammal and bird calls,” in Acoustic Communication, eds A. M. Simmons, R. R. Fay, and A. N. Popper (New York, NY: Springer), 138–224.
Brown, P. E., Brown, T. W., and Grinnell, A. D. (1983). Echolocation, Development, and Vocal Communication in the Lesser Bulldog Bat, Noctilio albiventris. Behav. Ecol. Sociobiol. 13, 287–298.
Califf, K. J., Green, D. S., Wagner, A. P., Scribner, K. T., Beatty, K., Wagner, M. E., et al. (2020). Genetic relatedness and space use in two populations of striped hyenas (Hyaena hyaena). J. Mammal. 101, 361–372.
Carter, G. G., Logsdon, R., Arnold, B. D., Menchaca, A., and Medellin, R. A. (2012). Adult Vampire Bats Produce Contact Calls When Isolated: Acoustic Variation by Species, Population, Colony, and Individual. PLoS One 7:e38791. doi: 10.1371/journal.pone.0038791
Carter, G. G., Skowronski, M. D., Faure, P. A., and Fenton, B. (2008). Antiphonal calling allows individual discrimination in white-winged vampire bats. Anim. Behav. 76, 1343–1355.
Clink, D. J., and Lau, A. R. (2020). Adherence to Menzerath’s Law is the exception (not the rule) in three duetting primate species. R. Soc. Open Sci. 7:201557. doi: 10.1098/rsos.201557
Clink, D. J., Tasirin, J. S., and Klinck, H. (2020). Vocal individuality and rhythm in male and female duet contributions of a nonhuman primate. Curr. Zool. 66, 173–186. doi: 10.1093/cz/zoz035
Courts, R., Erbe, C., Wellard, R., Boisseau, O., Jenner, K. C., and Jenner, M. N. (2020). Australian long-finned pilot whales (Globicephala melas) emit stereotypical, variable, biphonic, multi-component, and sequenced vocalisations, similar to those recorded in the northern hemisphere. Sci. Rep. 10:20609. doi: 10.1038/s41598-020-74111-y
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. 34, 205–219. doi: 10.1080/03949370.2021.2015451
de Reus, K., Soma, M., Anichini, M., Gamba, M., de Heer Kloots, M., Lense, M., et al. (2021). Rhythm in dyadic interactions. Philos. Trans. R. Soc. B 376:20200337.
Demartsev, V., Bar Ziv, E., Shani, U., Goll, Y., Koren, L., and Geffen, E. (2016a). Harsh vocal elements affect counter-singing dynamics in male rock hyrax. Behav. Ecol. 27, 1397–1404.
Demartsev, V., Ilany, A., Barocas, A., Bar Ziv, E., Schnitzer, I., Koren, L., et al. (2016b). A mixed strategy of counter-singing behavior in male rock hyrax vocal competitions. Behav. Ecol. Sociobiol. 70, 2185–2193.
Demartsev, V., Ilany, A., Kershenbaum, A., Geva, Y., Margalit, O., Schnitzer, I., et al. (2017). The progression pattern of male hyrax songs and the role of climactic ending. Sci. Rep. 7:2794. doi: 10.1038/s41598-017-03035-x
Demartsev, V., Strandburg-Peshkin, A., Ruffner, M., and Manser, M. (2018). Vocal Turn-Taking in Meerkat Group Calling Sessions. Curr. Biol. 28, 3661–3666.e3. doi: 10.1016/j.cub.2018.09.065
Dunbar, R. I. (2012). Bridging the bonding gap: the transition from primates to humans. Philos. Trans. R. Soc. B 367, 1837–1846.
Eisenberg, J. F. (1989). Mammals of the Neotropics, The Northern Neotropics Volume 1. Chicago, IL: University of Chicago Press.
Emmons, L. (1981). Morphological, ecological, and behavioral adaptations for arboreal browsing in Dactylomys dactylinus (Rodentia. Echimyidae). J. Mammal. 62, 183–189.
Emmons, L. (2013). The Maned Wolves of Noel Kempff Mercado National Park. Washington, DC: Smithsonian Institution Scholarly Press.
Ferreira, L. S., Sábato, V., Pinheiro, T. A., Neto, E., Rocha, L. H., Baumgarten, J., et al. (2022). Long-Distance Counter Calling in Maned Wolves: Friends or Foes? Animals 12:1081.
Filippi, P., Hoeschele, M., Spierings, M., and Bowling, D. L. (2019). Temporal modulation in speech, music, and animal vocal communication: evidence of conserved function. Ann. N.Y. Acad. Sci. 1453, 99–113.
Hall, M. L. (2004). A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430.
Heffner, H. E., and Heffner, R. S. (2018). “The evolution of mammalian hearing,” in To the Ear and Back – Advances in Auditory Biophysics, eds C. Bergevin and S. Puria (Melville, NY: American Institute of Physics Publishing), 130001–130008.
Holekamp, K., Benson-Amram, S., Theis, K., and Greene, K. (2007). Sources of variation in the long-distance vocalizations of spotted hyenas. Behaviour 144, 557–584.
Jenner, N., Groombridge, J., and Funk, S. M. (2011). Commuting, territoriality and variation in group and territory size in a black-backed jackal population reliant on a clumped, abundant food resource in Namibia. J. Zool. 284, 231–238.
Jin, L., Yang, S., Kimball, R. T., Xie, L., Yue, X., Luo, B., et al. (2015). Do pups recognize maternal calls in pomona leaf-nosed bats, Hipposideros pomona? Anim. Behav. 100, 200–207.
Knörnschild, M., Fernandez, A. A., and Nagy, M. (2020). Vocal information and the navigation of social decisions in bats: is social complexity linked to vocal complexity? Funct. Ecol. 34, 322–331.
Knörnschild, M., and Von Helversen, O. (2008). Nonmutual vocal mother–pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009.
Ladich, F., and Winkler, H. (2017). Acoustic communication in terrestrial and aquatic vertebrates. J. Exp. Biol. 220, 2306–2317.
Langmore, N. E. (2002). Vocal duetting: definitions, discoveries and directions. Trends Ecol. Evol. 17, 451–452.
La Val, R. K. (1976). Voice and Habitat of Dactylomys dactylinus (Rodentia: Echimyidae) in Ecuador. J. Mammal. 57, 402–404.
Leighty, K. A., Soltis, J., Wesolek, C. M., and Savage, A. (2008). Rumble vocalizations mediate interpartner distance in African elephants, Loxodonta africana. Anim. Behav. 76, 1601–1608.
Levinson, S. C. (2016). Turn-taking in Human Communication – Origins and Implications for Language Processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Logue, D. M., and Krupp, D. B. (2016). Duetting as a collective behavior. Front. Ecol. Evol. 4:7. doi: 10.3389/fevo.2016.00007
Lukas, D., and Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677
Marler, P. (2004). Bird calls: their potential for behavioral neurobiology. Ann. N.Y. Acad. Sci. 1016, 31–44.
Martin, K., Tucker, M. A., and Rogers, T. L. (2017). Does size matter? Examining the drivers of mammalian vocalizations. Evolution 7, 249–260. doi: 10.1111/evo.13128
Miller, P. J., Shapiro, A. D., Tyack, P. L., and Solow, A. R. (2004). Call-type matching in vocal exchanges of free-ranging resident killer whales, Orcinus orca. Anim. Behav. 67, 1099–1107.
Mills, M. G. (1989). “The comparative behavioral ecology of hyenas: the importance of diet and food dispersion,” in Carnivore Behavior, Ecology, and Evolution, ed. J. L. Gittleman (Boston, MA: Springer), 125–142.
Moehlman, P. D. (1987). Social Organization in Jackals: The complex social system of jackals allows the successful rearing of very dependent young. Am. Sci. 75, 366–375.
Nieder, A., and Mooney, R. (2020). The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Philos. Trans. R. Soc. B 375:20190054. doi: 10.1098/rstb.2019.0054
O’Connell-Rodwell, C. E., Wood, J. D., Wyman, M., Redfield, S., Puria, S., and Hart, L. A. (2012). Antiphonal vocal bouts associated with departures in free-ranging African elephant family groups (Loxodonta africana). Bioacoustics 21, 215–224.
Okobi, D. E., Banerjee, A., Matheson, A. M. M., Phelps, S. M., and Long, M. A. (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983–988. doi: 10.1126/science.aau9480
Oli, M. K., and Jacobson, H. A. (1995). Vocalizations of barking deer (Muntiacus muntjak) in Nepal. Mammalia 59, 179–186.
O’Shea, T. J., and Poché, L. B. Jr. (2006). Aspects of underwater sound communication in Florida manatees (Trichechus manatus latirostris). J. Mammal. 87, 1061–1071.
Pardo, M. A., Poole, J. H., Stoeger, A. S., Wrege, P. H., O’Connell-Rodwell, C. E., Padmalal, U. K., et al. (2019). Differences in combinatorial calls among the 3 elephant species cannot be explained by phylogeny. Behav. Ecol. 30, 809–820.
Paula, B., and Monticelli, P. (2021). Maned wolf duet? The first record of two maned wolves vocalizing simultaneous. Acad. Lett. [Epub ahead of print]. doi: 10.20935/AL1720
Pika, S., Wilkinson, R., Kendrick, K. H., and Vernes, S. C. (2018). Taking turns: bridging the gap between human and animal communication. Proc. R. Soc. B 285:20180598. doi: 10.1098/rspb.2018.0598
Ravignani, A., Verga, L., and Greenfield, M. D. (2019). Interactive rhythms across species: the evolutionary biology of animal chorusing and turn-taking. Ann. N. Y. Acad. Sci. 1453, 12–21.
Romero-Mujalli, D., Bergmann, T., Zimmermann, A., and Scheumann, M. (2021). Utilizing DeepSqueak for automatic detection and classification of mammalian vocalizations: a case study on primate vocalizations. Sci. Rep. 11:24463. doi: 10.1038/s41598-021-03941-1
Rossi, I., Mauri, L., Laficara, S., and Apollonio, M. (2002). Barking in roe deer (Capreolus capreolus): seasonal trends and possibile functions. Hystrix 13, 13–18.
Rosti, H., Pihlström, H., Bearder, S., Pellikka, P., and Rikkinen, J. (2020). Vocalization analyses of nocturnal arboreal mammals of the Taita Hills Kenya. Diversity 12:473. doi: 10.3390/d12120473
Schulz, T. M., Whitehead, H., Gero, S., and Rendell, L. (2008). Overlapping and matching of codas in vocal interactions between sperm whales: insights into communication function. Anim. Behav. 76, 1977–1988.
Silva, R. B., Vieira, E. M., and Izar, P. (2008). Social monogamy and biparental care of the neotropical southern bamboo rat (Kannabateomys amblyonyx). J. Mammal. 89, 1464–1472.
Soltis, J., Leong, K., and Savage, A. (2005). African elephant vocal communication I: antiphonal calling behavior among affiliated females. Anim. Behav. 70, 579–587.
Szymański, P., Olszowiak, K., Wheeldon, A., Budka, M., and Osiejuk, T. S. (2021). Passive acoustic monitoring gives new insight into year-round duetting behaviour of a tropical songbird. Ecol. Indic. 122:107271.
Terleph, T. A., Malaivijitnond, S., and Reichard, U. H. (2018). Male white-handed gibbons flexibly time duet contributions. Behav. Ecol. Sociobiol. 72:16.
Tilson, R. L., and Norton, P. M. (1981). Alarm duetting and pursuit deterrence in an African antelope. Am. Nat. 118, 455–462.
Todt, D., and Naguib, M. (2000). Vocal interactions in birds: the use of song as a model in communication. Adv. Study Behav. 29, 247–296.
Vergara, V., Michaud, R., and Barrett-Lennard, L. (2010). What can captive whales tell us about their wild counterparts? Identification, usage, and ontogeny of contact calls in belugas (Delphinapterus leucas). Int. J. Comp. Psychol. 23, 278–309.
Vernes, S. C. (2017). What bats have to say about speech and language. Psychon. Bull. Rev. 24, 111–117. doi: 10.3758/s13423-016-1060-3
Williams, H. W., Sorenson, M. W., and Thompson, P. (1969). Antiphonal calling of the tree shrew Tupaia palawanensis. Folia Primatol. 11, 200–205. doi: 10.1159/000155269
Yoshida, S., and Okanoya, K. (2005). Evolution of turn-taking: a bio-cognitive perspective. Cogn. Stud. 12, 153–165.
Yosida, S., and Okanoya, K. (2009). Naked mole-rat is sensitive to social hierarchy encoded in antiphonal vocalizations. Ethology 115, 823–831.
Yosida, S., and Okanoya, K. (2012). Bilateral lesions of the medial frontal cortex disrupt recognition of social hierarchy during antiphonal communication in naked mole-rats (Heterocephalus glaber). J. Comp. Physiol. A. 198, 109–117. doi: 10.1007/s00359-011-0692-z
Keywords: antiphonal vocalizations, mammal duets, counter-singing, Dactylomys spp., temporally coordinated interactive vocalizations
Citation: Vanderhoff EN and Bernal Hoverud N (2022) Perspectives on Antiphonal Calling, Duetting and Counter-Singing in Non-primate Mammals: An Overview With Notes on the Coordinated Vocalizations of Bamboo Rats (Dactylomys spp., Rodentia: Echimyidae). Front. Ecol. Evol. 10:906546. doi: 10.3389/fevo.2022.906546
Received: 28 March 2022; Accepted: 15 June 2022;
Published: 07 July 2022.
Edited by:
Patrice Adret, Universidad Autónoma Gabriel René Moreno, BoliviaReviewed by:
Vlad Demartsev, University of Konstanz, GermanyCharles T. Snowdon, University of Wisconsin-Madison, United States
Copyright © 2022 Vanderhoff and Bernal Hoverud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. Natasha Vanderhoff, bnZhbmRlcjRAanUuZWR1
 E. Natasha Vanderhoff
E. Natasha Vanderhoff N. Bernal Hoverud
N. Bernal Hoverud