
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 26 July 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.905925
This article is part of the Research Topic Social Functions of Bat Vocalizations View all 15 articles
When selecting feeding, hiding, or resting areas, animals face multiple decisions with different fitness consequences. To maximize efficiency, individuals can either collect personal information, or use information gathered and transmitted by other individuals (social information). Within group living species, organisms often specialize in either generating social information or using information gathered by other groups members. That is the case of the Spix’s disk-winged bat, Thyroptera tricolor. This species uses contact calls during roost finding. Social groups are composed by a mix of vocal and non-vocal individuals and those vocal roles appear to be consistent over time. Moreover, their vocal behavior can predict roost finding in natural settings, suggesting that vocal individuals are capable of generating social information that can be used by other group members. To date, however, we do not know if when presented with social information (contact calls) during roost finding, vocal individuals will make more or less use of these cues, compared to non-vocal individuals. To answer this question, we broadcast contact calls from a roost inside a flight cage to test whether vocal individuals could find a potential roost faster than non-vocal individuals when they encounter sounds that signal the presence of a roost site. Our results suggest that non-vocal individuals select roost sites based primarily on social information, whereas vocal individuals do not rely heavily on social information when deciding where to roost. This study provides the first link between vocal behavior and the use of social information during the search for roosting resources in bats. Incorporating ideas of social roles, and how individuals decide when and where to move based on the use of social information, may shed some light on these and other outstanding questions about the social lives of bats.
Animals are constantly faced with the decision of selecting feeding sources and hiding or resting areas; based on those decisions, individuals experience different fitness consequences (Danchin et al., 2004). To maximize efficiency, an organism can either collect personal information by trial-and-error and learning, or it may alternatively use social information; that is, make decisions based on the information gathered and transmitted by other individuals (Danchin et al., 2004; Kurvers et al., 2010). This information can be obtained from cues or signals emitted by successful individuals or by following individuals that are performing specific tasks, such as locating food patches or roost sites. In group living species, individuals can greatly benefit from this information transfer to locate foraging sites and roosts as this allows followers to spend less energy and be less susceptible to predation (Krebs and Davies, 1993).
Within group living species, individuals can specialize in performing specific behavioral tasks (Pruitt and Riechert, 2011). For instance, some species have been reported to exhibit subdivision of labor, in which one or a few group members are in charge of generating information that becomes available to the rest of the group and is vital for efficient or successful acquisition of resources, or they can take leadership roles in resource finding, based on characteristics such as sex, body size, age, group size, personality, or even their vocal behavior (e.g., Pruitt and Riechert, 2011; Sagot et al., 2018). Although it is not well known why a portion of individuals is better at generating social information, some studies suggest that it can be related to various factors, including direct benefits to those individuals that somehow gain by imposing their choices (Jaupart et al., 2003; Conradt and List, 2009), personality traits that are independent of an individual’s knowledge of its surroundings (Johnstone and Manica, 2011), or based on differences in metabolic rates (e.g., Biro and Stamps, 2010).
A common way to share social information about the location of resources, such as food or roosts, is by the use of vocal signals. This is the case of the Pallas’ long-tongued bats (Glossophaga soricina), which are able to socially learn the position of flowers using visual, but also acoustic signals produced by conspecifics, most likely by eavesdropping on acoustic cues (Rose et al., 2016).
Another species that uses social calls to advertise group members about the presence of roost-sites is the Spix’s disk-winged bat, Thyroptera tricolor. This species roosts in furled leaves that are only available for a day (5–31 h; Findley and Wilson, 1974; Vonhof and Fenton, 2004). When an individual finds a roost, it produces a contact call named “response” in reply to “inquiry” calls, produced by flying group members (Chaverri et al., 2010). While most individuals produce inquiry calls (Chaverri et al., 2020), Chaverri and Gillam (2015) found that only a small portion of individuals produce response calls. Moreover, Spix’s disk-winged bats have strong and consistent individual differences in response calling (Chaverri and Gillam, 2015; Chaverri et al., 2020). This means that over time, some individuals do not produce response calls (non-vocal bats), while others consistently produce calls at varying rates (vocal bats). Therefore, groups are composed by a mix of vocal and non-vocal individuals and those vocal roles appear to be consistent over time (Chaverri and Gillam, 2015; Chaverri et al., 2020). Moreover, although this call system facilitates roost location by group members (Sagot et al., 2018), it can be energetically demanding, especially for individuals producing calls at higher rates (Chaverri et al., 2021).
In natural settings, T. tricolor individuals that produce response calls at higher rates are also the ones that are more exploratory (spend more time searching for roosts) and find more leaves (Sagot et al., 2018). This suggests that vocal individuals are capable of generating social information that can be used by other group members. Such tactics, i.e., generating vs. using social information, have been widely explored in the producer-scrounger game, in which individuals either search for food themselves (producer) or make use of information generated by other group members (scrounger; Barnard and Sibly, 1981). In multiple species such as the zebra finch (Taeniopygia guttata), individuals’ tactics tend to be consistent over time and in different conditions (Beauchamp, 2001, 2006), suggesting that the individuals’ personality influences which tactic they use. In the barnacle geese (Branta leucopsis) for instance, individuals with shy personalities tend to associate more often with bolder individuals which are more commonly found at the leading edge of moving groups and are more likely to play the producer tactic (Kurvers et al., 2010). However, shy individuals are capable of approaching feeding areas and moving between patches (Kurvers et al., 2010), suggesting that they can also use personal information, although this is not very common. This tendency of shy individuals to stay closer to other group members instead of generating their own information is often referred to as “sociability” and, at least in the common lizard (Lacerta vivipara), sociable individuals exhibit a positive association with bolder individuals (Cote et al., 2008). To date, however, we do not know if when presented with social information, shy, more sociable individuals will make more use of this information compared to bolder, more exploratory individuals. Because vocal behavior predicts roost finding abilities in T. tricolor (Sagot et al., 2018), in this study we examined the relationship between individual vocal behavior and the use of social information during the location of roost sites. We predicted that vocal individuals, which typically also locate a larger number of roosts and are thus considered to be more exploratory (Sagot et al., 2018), are less likely to follow signals (i.e., response calls) produced by other bats, since they potentially rely more strongly on personal information during the process of finding and selecting roost sites. On the other hand, bats that are non-vocal probably rely on signals produced by other group members to find roosts (social information) and are therefore more receptive to response calls while researching for roosts.
We collected data on T. tricolor’s social behavior at Barú Biological Station in Southwestern Costa Rica. We divided the study in two trial periods (i.e., 2 repetitions): January 6th to January 18th 2018 (trial 1) and July 14th to July 25th 2018 (trial 2). Thyroptera tricolor roosts in social groups (i.e., stable assemblage of individuals that share the same roost at the same time; Vonhof and Fenton, 2004) inside furled leaves of Heliconia spp., Calathea spp., and Musa spp. (Vonhof and Fenton, 2004). We identified potential roosts during the daytime by searching for the characteristic tubular-shaped leaf. The presence of the social group in the leaf was verified through a telescopic mirror. If groups were present, we immediately captured all bats in the roost and we identified them to individual level by reading the unique alphanumeric code of each PIT tag (Biomark, Inc., Boise, ID, United States) previously installed subcutaneously on the back of the animals. We recorded information on sex, age, reproductive condition, weight and forearm length for each individual.
We performed two separate experiments to test whether vocal individuals find a potential roost faster than non-vocal individuals when they encounter sounds that signal the presence of a roost site. The first experiment aimed to collect data on response calling behavior of each individual within different groups. With the second experiment we assessed the effect of acoustic signals (i.e., response calls produced from a speaker inside the leaf) on the individual ability to find the leaf. We performed all the experiments during the day, typically between 9 am and 3 pm, because this is the time when animals have the urgency to search for a leaf if their previous roost becomes unavailable (Chaverri et al., 2010). We captured a total of 182 bats belonging to 45 different groups (Table 1). To gain more statistical power and account for other sources of variation, we recaptured and repeated the experiments on the same individuals whenever possible 6 months later. We called the first repetition “trial 1” and the second “trial 2.” Of the 182 bats captured in trial 1, we were able to recapture 122 individuals during trial 2. Thus, for the analyses we only included bats that were recaptured. On average, we performed experiments on 10 individuals per day. After the experiments, we released all bats in the wild at the end of every daily session after hydrating and feeding them with mealworms (Tenebrio molitor).
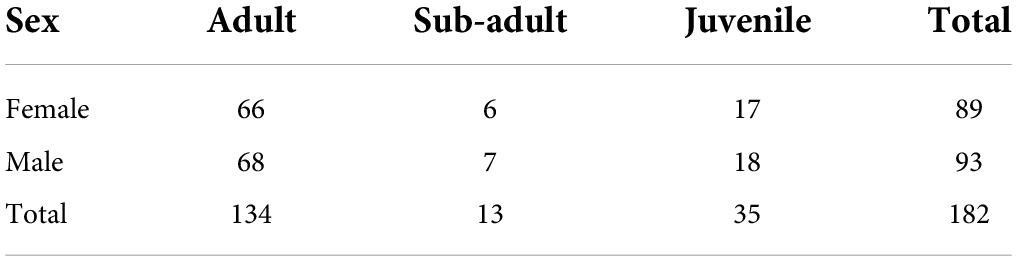
Table 1. Total number of Thyroptera tricolor bats captured for the duration of the study by sex and age.
To record response calls, we placed each bat individually in a suitable leaf (i.e., diameter 4–20 cm; Vonhof and Fenton, 2004) inside a flight cage (9 m × 4 m × 3 m) that was located in the field station. Chaverri et al. (2010) reported that T. tricolor produces response calls only after inquiry calls have been emitted. Therefore, we played back pre-recorded inquiry calls for 5 min to stimulate the emission of response calls. We collected these inquiry calls previously from five individuals belonging to the same group, flying within a flight cage (3 m × 4 m × 9 m) for a total of 1 min; we did not include any of these individuals in our current experiments and thus all test bats were exposed to novel calls. We identified a total of 67 inquiry calls in the 1-min recording (a call rate that lies within the range found in this species; unpublished data) and we ran the playback continuously for 5 min through an UltraSoundGate Player to a broadband loudspeaker (Ultrasonic Omnidirectional Dynamic Speaker Vifa, Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) located outside the leaf. We recorded the response calls with an Avisoft condenser microphone (CM16, Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) through Avisoft’s UltraSoundGate 116Hm connected to a laptop running Avisoft-Recorder software (sampling rate 500 kHz, 16-bit resolution). This procedure was repeated for all individuals. Using Avisoft-SASLab Pro software (Avisoft Bioacoustics, Glienicke/Nordbahn, Germany), we measured the total number of response calls emitted per bat per minute. Previous studies have shown that members of T. tricolor display differential response calling behavior within the social group and each individual behavior is consistent over time (Chaverri and Gillam, 2015). Therefore, we used the number of response calls recorded in this first experiment to assign each member of the group to a vocal category (i.e., vocal vs. non-vocal).
For the same individuals used in experiment 1 we also recorded the time needed to enter a furled leaf from which response calls were being emitted; we used these data as a proxy to gauge receptiveness toward social signals. We used a total of 25 different sound files for this experiment, each coming from a different individual. Files had an average length of 8.77 s, and contained 36.52 response calls (on average) with a call rate of 4.38 calls per second. We presented the same file, on loop mode, to each focal individual.
In a flight cage (9 m × 4 m × 3 m), we positioned a freshly cut furled leaf with an ultrasound loudspeaker (Vifa speaker outside its case, Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) located inside the tubular leaf structure and near the bottom. The loudspeaker was connected to an UltraSoundGate Player as explained above. We released one individual at a time inside the cage and we measured the time needed by the bat to enter the leaf. We ended the experiment if after 5 min the bat did not enter the leaf. We did not add a control repetition, from which no sound or a non-social sound were emitted from the roost, to avoid habituation and/or spatial memory from affecting our results. For this experiment, we only used adult bats because juveniles and sub-adults get tired very quickly and they have a harder time finding roosts as they are still learning. Each adult bat rested for 1 h on average between experiment 1 and 2. Moreover, to determine if there was a difference in the time needed by an individual to enter a tubular leaf with familiar and unfamiliar response calls, we also played response calls from members of the same group.
To determine the effect of familiarity with the response calls (calls produced by individuals of the same group vs. individuals of different groups) on the time spent finding the roost, we performed a paired T-test. We performed a linear mixed model (package lm4, Bates et al., 2015) to determine the effect of sex, trial and the interaction between trial and sex on the time spent finding the roost. We ran this analysis separately for vocal and non-vocal bats. For the model, we used social group as random effect. For vocal bats only, we also used a mixed effect model to determine the effect of number on response calls, trial and the interaction between trial and response calls, on the time spent finding the roost. We used individual as a random effect. Because sex was not significant, we excluded this variable for the analysis. To determine if a change in the vocal behavior (i.e., from vocal to non-vocal and vice versa) of individuals between trial 1 and 2 also affected the time spent finding a roost, we performed a paired- T-test. We performed all the analyses in R 3.0.2.
All sampling protocols followed guidelines approved by the American Society of Mammalogists for capture, handling and care of mammals (Sikes, 2016) and the ASAB/ABS Guidelines for the treatment of animals in behavioral research. This study was conducted in accordance with the ethical standards for animal welfare of the Costa Rican Ministry of Environment and Energy, Sistema Nacional de Áreas de Conservación, permit no. SINAC-ACOPAC-RES-INV-008-2017. Protocols were also reviewed and approved by the University of Costa Rica’s Institutional Animal Care and Use Committee (CICUA-42-2018).
In this study we captured a total of 182 bats and recaptured 122. From all the bats that we recaptured, during trial 1, 59 were vocal and 63 were non-vocal. Six months later (trial 2), 2 of the 59 non-vocal bats (females) became vocal while others remained non-vocal; on the other hand, 12 of the 63 (8 females and 4 males) vocal bats became non-vocal.
Broadcasting response calls from group members vs. non-members did not affect the time spent entering the roost (T = −0.22, df = 20, P = 0.820, Figure 1); that is, time spent flying before entering a leaf did not vary according to which call was broadcast, a familiar or unfamiliar one. For non-vocal bats, both males and females appeared to be equally receptive to response call playback in both trials (Figure 2 and Table 2). The interaction between sex and trial had no effect in the time spent entering the roost (Table 2).
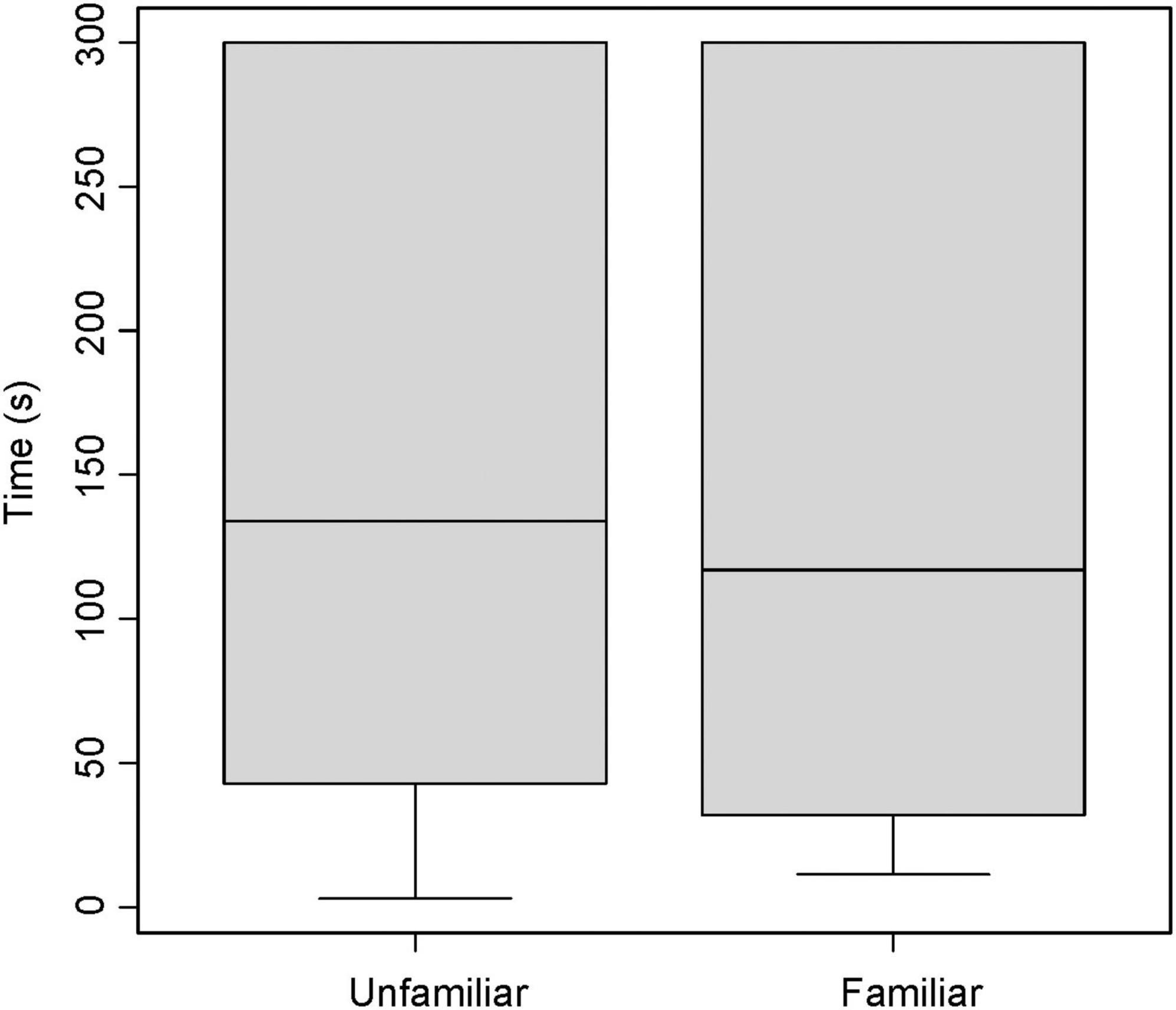
Figure 1. Average time in seconds a Thyroptera tricolor bat spent finding a roost when using an inquiry call playback from a familiar (group member) or unfamiliar (non-group member) bat. Error bars represent standard error.
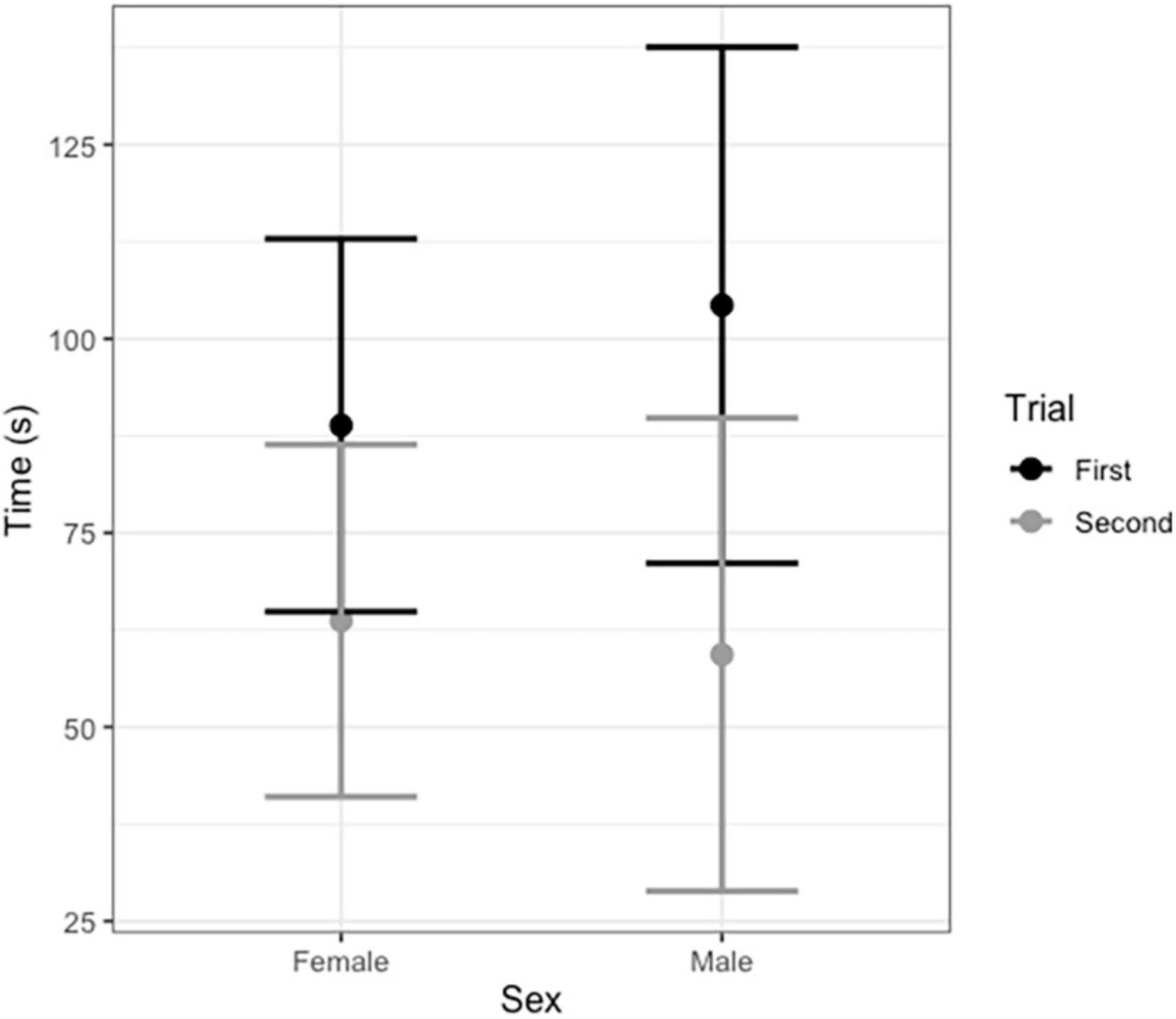
Figure 2. Average time in seconds non-vocal male and female Thyroptera tricolor bats spent finding a roost during the first and second trial (after 6 months). Error bars represent standard error.
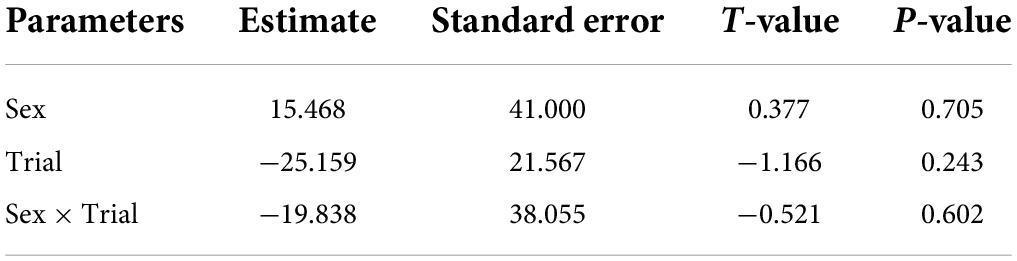
Table 2. Parameter estimated for the linear mixed model estimating the effect of sex, trial and the interaction between trial and vocal sex, on the time spent finding the roost by non-vocal bats.
In vocal bats, females spent significantly more time entering roosts compared to males during trial 1 (Figure 3 and Table 3). However, during trial 2, females entered the roost faster than males (Figure 3 and Table 3). Male behavior did not change significantly between trial 1 and 2 (Figure 3). Furthermore, the number of response calls vocal bats produced, independently of sex, was correlated with the time spent finding the roost (Figure 4 and Table 4). During trial 1, individuals that produced more response calls found the roost faster; however, this effect disappeared during trial 2, as the number of calls produced did not predict the time needed to find the roost. Overall, trial had an effect on the time to find the roost (Table 4). Moreover, the interaction between the number of response calls produced and trial was significant (Figure 4 and Table 4).
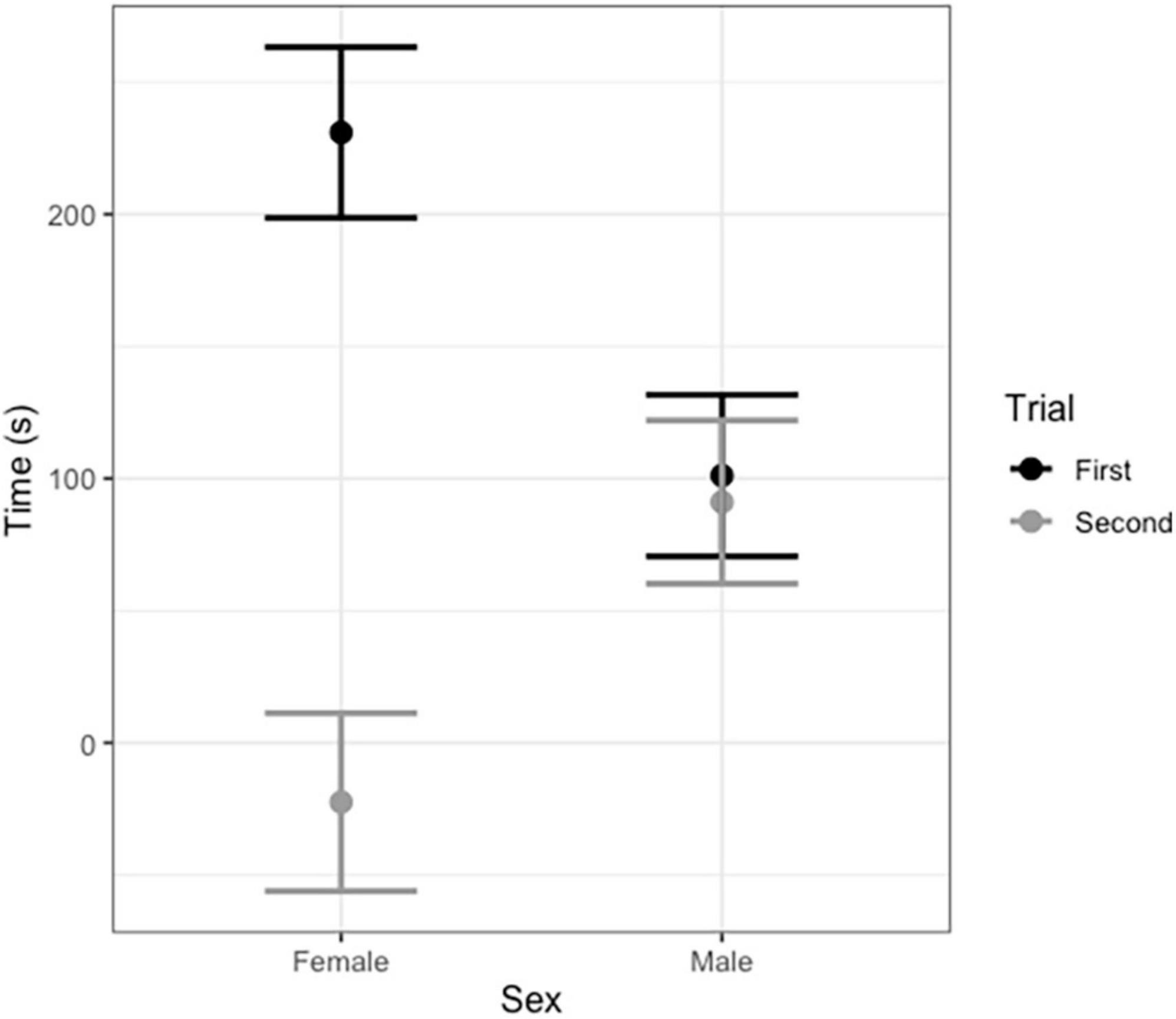
Figure 3. Average time in seconds vocal male and female Thyroptera tricolor bats spent finding a roost during the first and second trial (after 6 months). Error bars represent standard error.
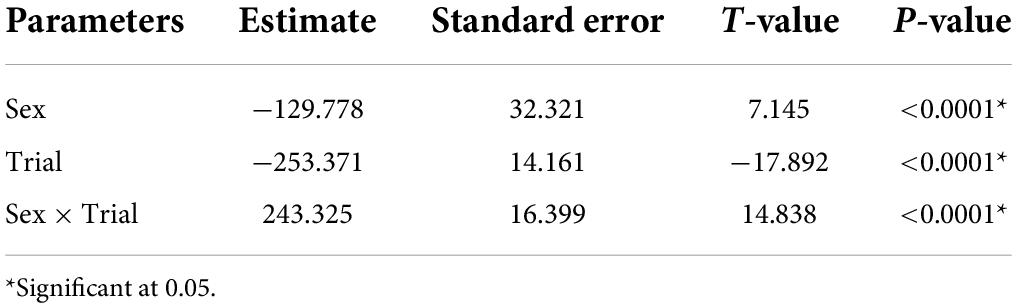
Table 3. Parameter estimated for the linear mixed model estimating the effect of sex, trial and the interaction between trial and vocal sex, on the time spent finding the roost by vocal bats.
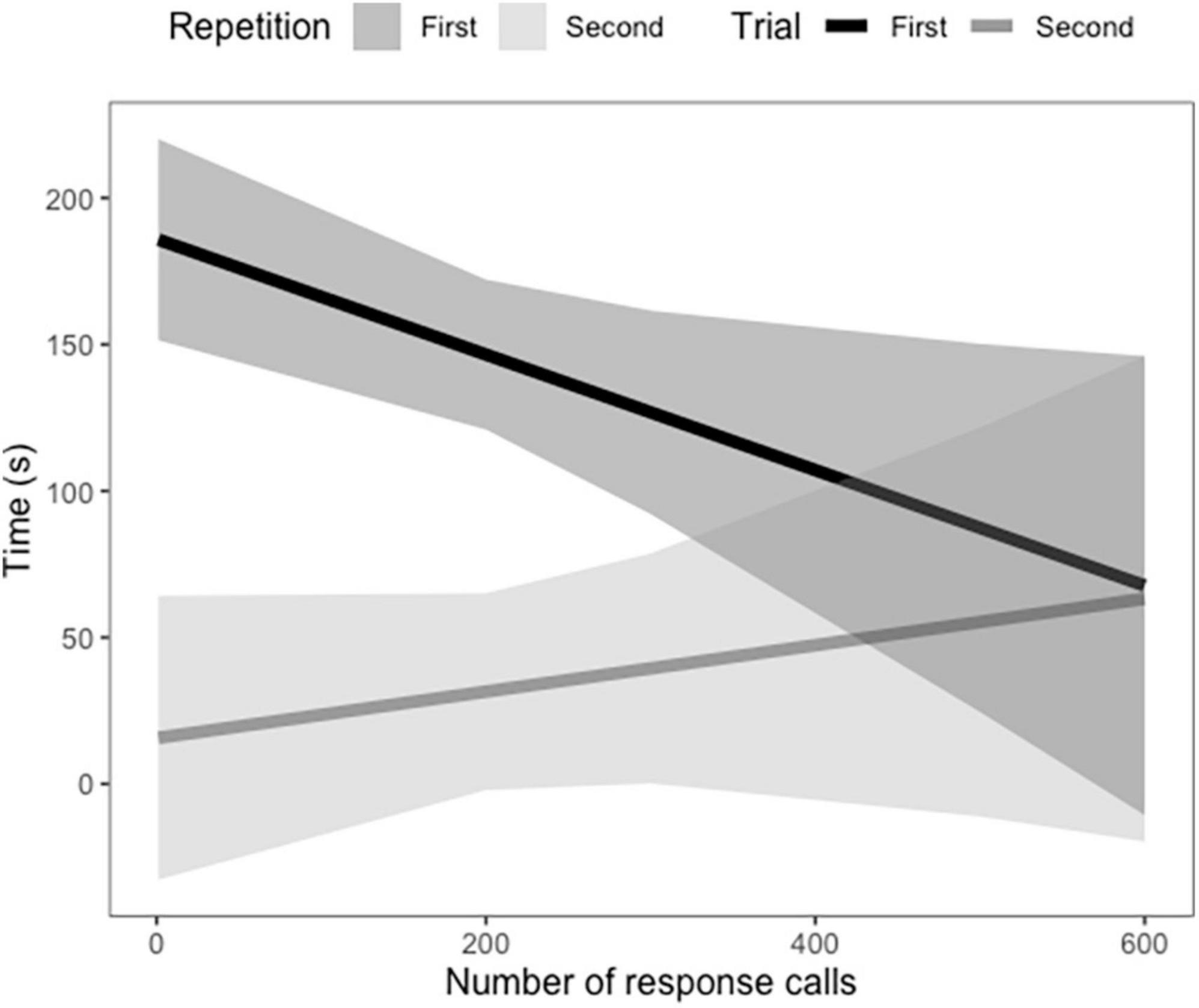
Figure 4. Average time in seconds vocal Thyroptera tricolor bats spent finding a roost based on the number of response calls they produced during trial 1 and 2. Confidence intervals represent the standard error.
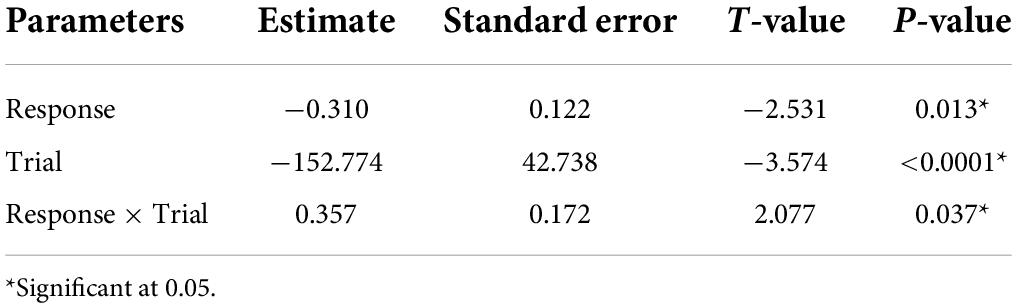
Table 4. Parameter estimated for the linear mixed model estimating the effect of number on response calls, trial and the interaction between trial and response calls, on the time spent finding the roost.
Bats that changed their vocal behavior from trial 1 to 2 also changed how receptive they were to response calls (n = 13) (T = 2.63, df = 12, P = 0.01). Individuals that went from vocal to non-vocal entered the roost faster on trial 2, while bats that went from non-vocal to vocal spent more time entering the roost (Figure 5).
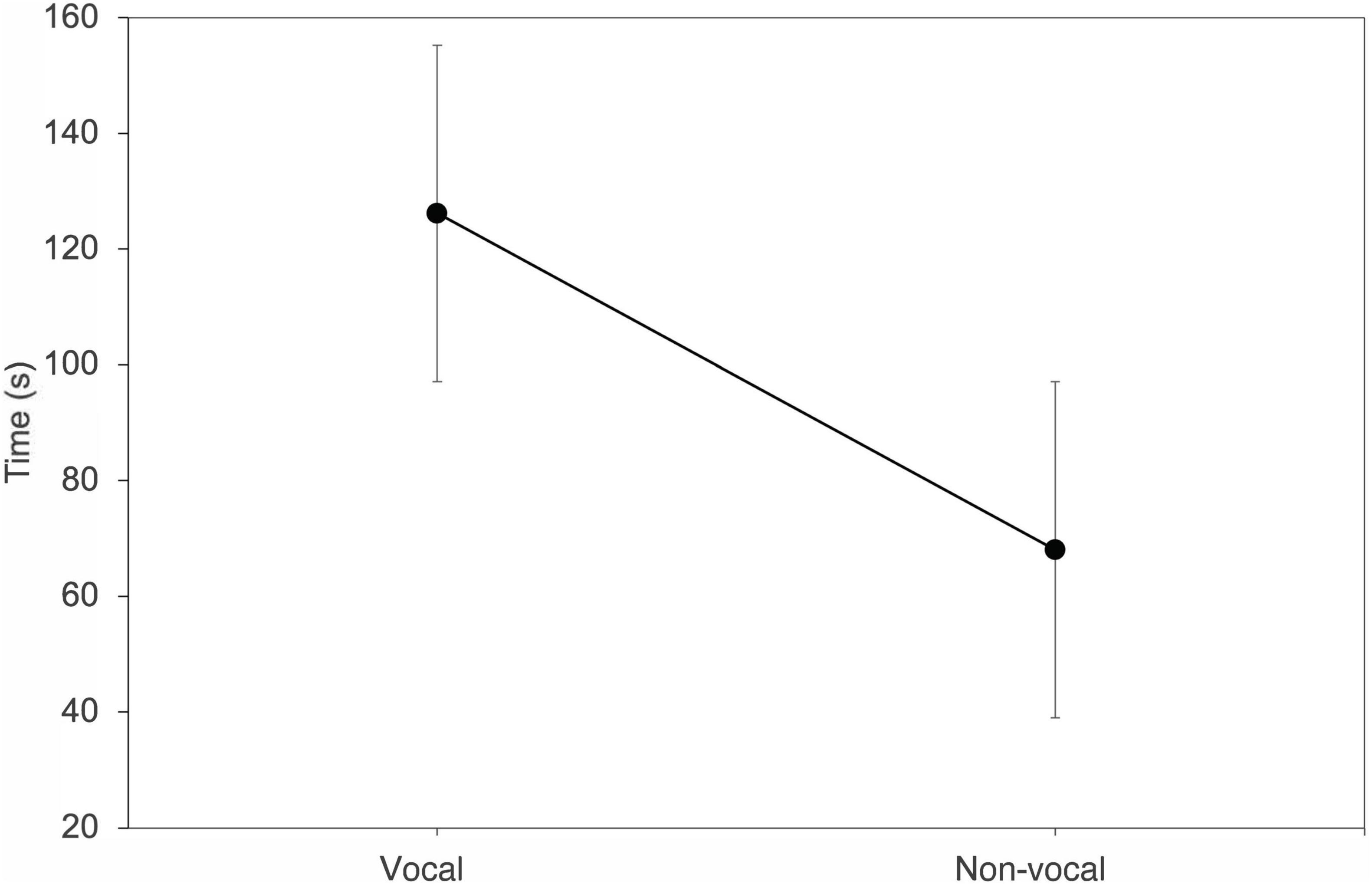
Figure 5. Average change in the time spent finding a roost when Thyroptera tricolor individuals changed from vocal to non-vocal behavior between trial 1 and trial 2. Error bars represent standard error.
Our study provides the first link between vocal behavior and the use of social information during the search for roosting resources. As expected, we found that individuals that differ in their tendency to produce contact calls correspondingly differ in their use of social information when finding roost sites. Specifically, bats that produce more response calls take longer to enter a suitable roost whose position is announced by conspecifics, whereas less vocal individuals very quickly enter the roost. Calling rates and exploratory personalities, in the context of roost finding, are positively associated in T. tricolor (Sagot et al., 2018). Thus, our current results further suggest that non-vocal and less exploratory individuals may more strongly rely on social information for roost-finding compared to vocal and exploratory individuals. Other studies have also established a strong link between exploratory behavior and the use of social information in decision-making. Barnacle geese (Branta leucopsis) and zebra finches (Taeniopygia guttata), for example, exhibit a strong negative link between exploratory behavior and the use of social information when selecting foraging patches or specific food options (Kurvers et al., 2010; Rosa et al., 2012). In contrast, more exploratory three-spined sticklebacks (Gasterosteus aculeatus) readily use social information, estimated as the tendency of naïve animals to join knowledgeable individuals, when searching for food in novel environments (Nomakuchi et al., 2009).
Social groups in T. tricolor are composed by a combination of vocal and non-vocal bats in the context of contact calling (Chaverri et al., 2020). Therefore, while some bats constantly call from within the roost when conspecifics are searching for them, others rarely vocalize; when vocal individuals are inside roosts, this significantly reduces search time for flying group members (Sagot et al., 2018). We still do not have convincing evidence to explain why groups are formed by a combination of vocal and non-vocal bats; however, our present results strongly suggest that this may be partly explained by the use of social information while locating roost sites and its relationship to group cohesion. Vocal bats may be primarily responsible for locating new roost sites on a daily basis, and upon locating one, announce its location to non-vocal group members that are also more responsive to social information. If groups were solely composed of silent bats, roost location would take longer (Sagot et al., 2018) and group members would be unable to locate each other; if groups were composed of only vocal bats, they would similarly dissolve if individuals were non-responsive to the contact calls of other group members. The latter is akin to groups having several knowledgeable individuals who are more heavily influenced by their preferred choices than by those of other individuals while searching for resources, thus inevitably causing groups to split (Couzin et al., 2005).
The results of our study also show that while vocal individuals, especially females, were initially slower at entering roosts based on social information, in subsequent trials they entered roosts significantly faster. Non-vocal bats, however, independently of sex, took a similar amount of time locating the roost in both trials. From previous experiments, it is known that males have a higher chance of being vocal, compared to females (Sagot et al., 2018; Chaverri et al., 2021). However, vocal male and female bats produce similar number of response calls (Sagot et al., 2018). In bats, males typically outperform females in finding objects such as roosts, especially without landmarks that guide them (Schmidtke and Esser, 2011). The same differences have also been found in rodents and primates (Jacobs et al., 1990; Williams and Meck, 1991; Sandstrom et al., 1998; Roof and Stein, 1999; Lacreuse et al., 2005), but to date, there is still a disagreement on the reasons explaining this pattern. One potential reason proposed by Schmidtke and Esser (2011) is that because female bats have to carry their offspring after birth, they might have evolved what is called low-risk navigational strategies (Ecuyer-Dab and Robert, 2004), which is the use of detailed information from multiple spatial landmarks to ensure female and offspring survival. This could also help explain why females did better in the second trial, after they had already been exposed to the flight cage and could use the geometry of the space as a source of information.
Furthermore, multiple studies have shown that exploratory individuals learn to recognize novel objects or situations faster than less exploratory individuals (Blaser and Heyser, 2015); thus, our results suggest that while vocal bats, which are also more exploratory, may largely ignore social information during the location of roost sites, they may locate the roost faster in a second trial regardless of whether the site’s location is announced by a conspecific or not. To provide conclusive evidence for the latter, first it will be necessary to repeat the experiment without broadcasting response calls from the tubular leaf in the flight cage and determine how time to enter the roost decreases for the vocal and non-vocal bats.
Previous studies of call discrimination in T. tricolor have shown that bats searching for a roost site prefer to enter leaves from which response calls of group members are being broadcast, largely avoiding suitable roosts with response calls of non-group members (Chaverri et al., 2013). Therefore, we also tested whether the use of social information in deciding to enter a tubular leaf would differ if we broadcast calls from group and non-group members, the former representing perhaps a more reliable signal than the latter. Surprisingly, we did not find a difference in the time it took a focal bat to enter a leaf when we broadcast a call from a group member vs. a non-group member. This unexpected result could be explained by the fact that we provided no other choice for bats, only a single leaf and acoustic signal. Because bats are extremely vulnerable to predation during the daytime (Speakman, 1991; Speakman et al., 1994), a sense of urgency may have prevailed over the use of a more reliable signal in the decision to occupy a roost site. Accuracy in the process of decision making also varies among personality types (Chittka et al., 2009), so we would expect that “careful” individuals would take longer to enter leaves with unfamiliar calls, whereas “hasty” ones would quickly enter a leaf regardless of the calls broadcasted. We did not see such a trend, at least in relation to vocal and non-vocal personality types, which need not be correlated to behavioral traits related to accuracy during decision-making. Further tests are necessary to determine if there are inter-individual differences in the process of decision-making within groups, with some individuals being more selective, or accurate, than others.
In conclusion, our study shows that personalities related to vocal behavior, specifically calling rates, are linked to the use of social information while searching for roost sites. Unlike other studies that have addressed the use of social information in the process of decision-making, our study is the first to address the question in association to a behavioral trait other than exploration or boldness. Notwithstanding, we have shown in previous studies that vocal and exploratory behaviors are linked in our study system, providing further clues that suggest the need of a more complex multivariate approach to understanding animal personalities and how these affect several processes, including decision-making and group formation. Our study is also novel as it provides clues to understand decision-making using social information in the context of roost-site selection; most studies to date have primarily focused on the selection of food sources or foraging patches. Roost-sites are critically important for the survival of bats, and they are also vital for facilitating social interactions (Kunz, 1982). Many species commonly switch roost sites despite their relative permanency, causing groups to constantly split and reform and thus giving rise to fission-fusion societies (Kerth, 2008); no studies to date have provided conclusive evidence of why this occurs despite its costs of potentially weakening social bonds. Incorporating ideas of animal personalities, and how individuals decide when and where to move based on the use of social information, may shed some light on these and other outstanding questions about the social lives of bats.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/mariasagot/social_information.git.
All sampling protocols followed guidelines approved by the American Society of Mammalogists for capture, handling and care of mammals (Sikes, 2016) and the ASAB/ABS Guidelines for the treatment of animals in behavioral research. This study was conducted in accordance with the ethical standards for animal welfare of the Costa Rican Ministry of Environment and Energy, Sistema Nacional de Áreas de Conservación, permit no. SINAC-ACOPAC-RES-INV-008-2017. Protocols were also reviewed and approved by the University of Costa Rica’s Institutional Animal Care and Use Committee (CICUA-42-2018).
MS and GC: conceptualization. MS, GC, GG, SC-R, and AH-P: methodology and review and editing. MS: formal analysis. MS, GC, and GG: writing draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Ronald Villalobos for logistics support, and Julio Bustamante and Lilliana Rubí Jimenez for their help during research permit application. We would also like to thank Centro Biológico Hacienda Barú for their continuous support of our research.
Barnard, C. J., and Sibly, R. M. (1981). Producers and scroungers: A general model and its application to captive flocks of house sparrows. Anim. Behav. 29, 543–550. doi: 10.1016/S0003-3472(81)80117-0
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beauchamp, G. (2001). Consistency and flexibility in the scrounging behaviour of zebra finches. Can. J. Zool. 79, 540–544.
Beauchamp, G. (2006). Phenotypic Correlates of Scrounging Behavior in Zebra Finches: Role of Foraging Efficiency and Dominance. Ethology 112, 873–878. doi: 10.1111/j.1439-0310.2006.01241.x
Biro, P. A., and Stamps, J. A. (2010). Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653–659. doi: 10.1016/j.tree.2010.08.003
Blaser, R., and Heyser, C. (2015). Spontaneous object recognition: A promising approach to the comparative study of memory. Front. Behav. Neurosci. 9:183. doi: 10.3389/fnbeh.2015.00183
Chaverri, G., Araya-Ajoy, Y. G., and Sagot, M. (2020). Contact calling in context: intra- and intergroup variation in vocalization rates depend on a call’s function. Behav. Ecol. Sociobiol. 74:57. doi: 10.1007/s00265-020-02837-w
Chaverri, G., and Gillam, E. H. (2015). Repeatability in the contact calling system of Spix’s disc-winged bat (Thyroptera tricolor). R. Soc. Open Sci. 2:140197. doi: 10.1098/rsos.140197
Chaverri, G., Gillam, E. H., and Kunz, T. H. (2013). A call-and-response system facilitates group cohesion among disc-winged bats. Behav. Ecol. 24, 481–487. doi: 10.1093/beheco/ars188
Chaverri, G., Gillam, E. H., and Vonhof, M. J. (2010). Social calls used by a leaf-roosting bat to signal location. Biol. Lett. 6, 441–444. doi: 10.1098/rsbl.2009.0964
Chaverri, G., Sandoval-Herrera, N. I., Iturralde-Pólit, P., Romero-Vásquez, A., Chaves-Ramírez, S., and Sagot, M. (2021). The energetics of social signaling during roost location in Spix’s disc-winged bats. J. Exp. Biol 224, jeb238279. doi: 10.1242/jeb.238279
Chittka, L., Skorupski, P., and Raine, N. E. (2009). Speed-accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407. doi: 10.1016/j.tree.2009.02.010
Conradt, L., and List, C. (2009). Group decisions in humans and animals: a survey. Philos. Trans. R. Soc. London B Biol. Sci. 364, 719–742.
Cote, J., Dreiss, A., and Clobert, J. (2008). Social personality trait and fitness. Proc. R. Soc. B Biol. Sci. U.S.A. 275, 2851–2858. doi: 10.1098/rspb.2008.0783
Couzin, I. D., Krause, J., Franks, N. R., and Levin, S. A. (2005). Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516.
Danchin, É, Giraldeau, L. A., Valone, T. J., and Wagner, R. H. (2004). Public information: from nosy neighbors to cultural evolution. Science 305, 487–491. doi: 10.1126/science.1098254
Ecuyer-Dab, I., and Robert, M. (2004). Have sex differences in spatial abilityevolved from male competition for mating and female concern for survival? Cognition 91, 221–257. doi: 10.1016/j.cognition.2003.09.007
Findley, J. S., and Wilson, D. E. (1974). Observations on the neotropical disk-winged bat, Thyroptera tricolor Spix. J. Mammal. 55, 562–571.
Jacobs, L. F., Gaulin, S. J., Sherry, D. F., and HoVman, G. E. (1990). Evolution of spatial cognition: sex-speciWc patterns of spatial behavior predict hippocampal size. Proc. Natl. Acad. Sci. U.S.A. 87, 6349–6352.
Jaupart, C., Langmuir, C., Burton, K., Rands, S. A., Cowlishaw, G., Pettifor, R. A., et al. (2003). Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434. doi: 10.1038/nature01630
Johnstone, R. A., and Manica, A. (2011). Evolution of personality differences in leadership. Proc. Natl. Acad. Sci. U.S.A. 108, 8373–8378. doi: 10.1073/pnas.1102191108
Krebs, J. R., and Davies, N. B. (1993). An Introduction to Behavioural Ecology. Third Edit. Oxford: Blackwell Science.
Kunz, T. H. (1982). “Roosting ecology of bats,” in Ecology of Bats, ed. T. H. Kunz (New York, NY: Plenum Press), 1–50.
Kurvers, R. H. J. M., Prins, H. H. T., van Wieren, S. E., van Oers, K., Nolet, B. A., and Ydenberg, R. C. (2010). The effect of personality on social foraging: shy barnacle geese scrounge more. Proc. R. Soc. B Biol. Sci. U.S.A. 277, 601–608. doi: 10.1098/rspb.2009.1474
Lacreuse, A., Kim, C. B., Rosene, D. L., Killiany, R. J., Moss, M. B., Moore, T. L., et al. (2005). Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta). Behav. Neurosci. 119, 118–126. doi: 10.1037/0735-7044.119.1.118
Nomakuchi, S., Park, P. J., and Bell, M. A. (2009). Correlation between exploration activity and use of social information in three-spined sticklebacks. Behav. Ecol. 20, 340–345. doi: 10.1093/beheco/arp001
Pruitt, J. N., and Riechert, S. E. (2011). How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc. R. Soc. B Biol. Sci. U.S.A. 278, 1209–1215. doi: 10.1098/rspb.2010.1700
Roof, R. L., and Stein, D. G. (1999). Gender differences in Morris water maze performance depend on task parameters. Physiol. Behav. 68, 81–86. doi: 10.1016/s0031-9384(99)00162-6
Rosa, P., Nguyen, V., and Dubois, F. (2012). Individual differences in sampling behaviour predict social information use in zebra finches. Behav. Ecol. Sociobiol. 66, 1259–1265. doi: 10.1007/s00265-012-1379-3
Rose, A., Kolar, M., Tschapka, M., and Knörnschild, M. (2016). Learning where to feed: the use of social information in flower-visiting Pallas’ long-tongued bats (Glossophaga soricina). Anim. Cogn. 19, 251–262. doi: 10.1007/s10071-015-0930-9
Sagot, M., Schöner, C. R., Jago, A. J., Razik, I., and Chaverri, G. (2018). The importance of group vocal behaviour in roost finding. Anim. Behav. 142, 157–164. doi: 10.1038/s41598-018-26122-z
Sandstrom, N. J., Kaufman, J., and Huettel, S. A. (1998). Males and females use different distal cues in a virtual environment navigation task. Brain Res. Cogn. Brain Res. 6, 351–360. doi: 10.1016/s0926-6410(98)00002-0
Schmidtke, D., and Esser, K. H. (2011). Sex matters in echoacoustic orientation: gender differences in the use of acoustic landmarks in Phyllostomus discolor (lesser spear-nosed bat). J. Comp. Physiol. A. 197, 531–539. doi: 10.1007/s00359-010-0573-x
Sikes, R. S. (2016). 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education: J. Mammal. 97, 663–688. doi: 10.1093/jmammal/gyw078
Speakman, J. R. (1991). Why do Insectivorous Bats in Britain Not Fly in Daylight More Frequently? Funct. Ecol. 5:518. doi: 10.2307/2389634
Speakman, J. R., Lumsden, L. F., and Hays, G. C. (1994). Predation rates on bats released to fly during daylight in south-eastern Australia. J. Zool. 233, 318–321. doi: 10.1111/j.1469-7998.1994.tb08593.x
Vonhof, M. J., and Fenton, M. B. (2004). Roost availability and population size of Thyroptera tricolor, a leaf-roosting bat, in northeastern Costa Rica. J. Trop. Ecol. 20, 291–305.
Keywords: Thyroptera tricolor, roosting ecology, contact calls, group living, vocal roles, social roles, information transfer
Citation: Sagot M, Giacomini G, Chaves-Ramírez S, Hernández-Pinsón HA and Chaverri G (2022) Vocal behavior and the use of social information during roost finding. Front. Ecol. Evol. 10:905925. doi: 10.3389/fevo.2022.905925
Received: 28 March 2022; Accepted: 05 July 2022;
Published: 26 July 2022.
Edited by:
Mirjam Knörnschild, Naturwissenschaftliches Museum, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, GermanyReviewed by:
Peter Kanuch, Institute of Forest Ecology, Slovak Academy of Sciences (SAS), SlovakiaCopyright © 2022 Sagot, Giacomini, Chaves-Ramírez, Hernández-Pinsón and Chaverri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Sagot, bWFyaWEuc2Fnb3RAb3N3ZWdvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.