
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 17 June 2022
Sec. Paleontology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.900687
This article is part of the Research TopicSeed Behavior in Response to Extreme EnvironmentsView all 7 articles
 Natalia V. Bazhenova1,2†
Natalia V. Bazhenova1,2† Xin-Kai Wu3†
Xin-Kai Wu3† Tatiana M. Kodrul4
Tatiana M. Kodrul4 Natalia P. Maslova2
Natalia P. Maslova2 Maria V. Tekleva2
Maria V. Tekleva2 Sheng-Lan Xu1
Sheng-Lan Xu1 Jian-Hua Jin1,5*
Jian-Hua Jin1,5*Anatomical characters of Cenozoic pine seed cones are known mainly from North American fossils, while data on cone anatomy of Cenozoic species from Asia remain scarce. To date, only one seed cone of Pinus from the Miocene of eastern China has been studied using micro-computed tomography (micro-CT). A new fossil-species, Pinus prehwangshanensis sp. nov., of mummified seed cones from the upper Pleistocene of South China is described using a combination of scanning electron microscopy (SEM) and micro-CT. The new fossil-species combines a mosaic of seed cone morphological and anatomical characters observed in the group of closely related East Asian extant species of subgenus Pinus, section Pinus, subsection Pinus, comprising Pinus taiwanensis, Pinus hwangshanensis, Pinus luchuensis, Pinus thunbergii, and Pinus densiflora. The data obtained indicate that the characteristic anatomical features of this group were formed no later than the end of the Pleistocene. Based on the external seed cone morphology, the East Asian pine fossils confirm the existence of floristic exchange between continental Asia and the Japan archipelago prior to the formation of the Sea of Japan and later, in the middle Miocene to the late Pliocene, when the connection between the Japanese islands and Eurasian continent became re-established. Pollen grains associated with the new fossil-species are similar to those of some extant pine species related to P. thunbergii. A taxonomic and ecological analysis of the Pleistocene plant taxa from the Maoming Basin suggests that the regional climate was a humid subtropical monsoon with hot wet summers and cool dry winters, similar to the present-day climate of northeastern Vietnam.
As the largest genus of extant conifers, Pinus L. is widely distributed in the Northern Hemisphere with only one species present in the Southern Hemisphere (Eckenwalder, 2009; Farjon, 2017), but the data on the origin of modern sections and species of this genus are quite fragmentary. The most ancient reliable remains of Pinus are known from the Lower Cretaceous Chaswood Formation (Valanginian, about 133–140 Ma) of Nova Scotia, Canada (Falcon-Lang et al., 2016), and the Speeton Clay Formation (Hauterivian–Barremian transition, 131–129 Ma) of Yorkshire, UK (Ryberg et al., 2012). Cretaceous records of seed cones assigned to the genus are very scanty (Alvin, 1960; Miller and Malinky, 1986; Saiki, 1996). Cenozoic occurrences become more abundant through time but are still rather rare around the world, and fossil seed cones are not always suitable for a detailed study in terms of their preservation. The anatomical features of the Cenozoic seed cones are known for a small number of Pinus representatives from the USA and Canada (Miller, 1969, 1973, 1974, 1978; Underwood and Miller, 1980; Banks et al., 1981; Stockey, 1983, 1984; McKown et al., 2002; Klymiuk et al., 2011; Ryberg et al., 2012).
Nine Pinus species were described previously from the Cenozoic of China based on three-dimensionally preserved seed cones. The late Eocene Pinus maomingensis Xu, Jin, Zhou, Kodrul et Naugolnykh (subgenus Strobus) was reported from the Maoming Basin in Guangdong Province (Xu Q.-Q. et al., 2015), and the late Eocene-early Oligocene Pinus prototabulaeformis Tao et Wang (subgenus Pinus) was described from Hebei Province (Tao and Wang, 1983; Ding et al., 2013). All other fossil-species reported from China are dated to the Neogene and belong to the subgenus Pinus. Recently, exquisitely preserved seed cones and associated leaves from the lower Miocene of Weichang County in Hebei Province were described as Pinus weichangensis T.-M. Yi et C.-S. Li (Li et al., 2022). Three fossil-species, namely, Pinus preyunnanensis X.H. Xu et B.N. Sun (Xu X.-H. et al., 2015), Pinus premassoniana Ding et Sun (Ding et al., 2013), and Pinus speciosa Li (Nanjing Institute of Geology Mineral Resources (NIGMR)., 1982), are known from the upper Miocene Shengxian Formation of Zhejiang Province. The fossil-species, Pinus prekesiya Xing, Liu et Zhou, also found in the same deposits (Xu X.-H. et al., 2015), was originally described from the upper Miocene Xiaolongtan Formation in Yunnan Province (Xing et al., 2010a,b). More recently, another seed cone and associated leaves from this formation were assigned to the extant species Pinus massoniana Lamb. (Zhang et al., 2015). A further extant species, Pinus yunnanensis Franch., was described from the upper Pliocene in Eryuan County of Yunnan Province (Tao and Kong, 1973). Of all these Chinese fossil-species, only the anatomy of the seed cone of P. prekesiya from the upper Miocene of Zhejiang Province has been studied using X-ray computed tomography (Xing et al., 2010b).
Poor preservation often deprives fossil material of the diagnostic features that are used to distinguish extant taxa. This also significantly impedes the classification of fossil Pinus species. Seed cone anatomy, along with morphology, is most frequently used in the systematics of fossil Pinaceae (Miller, 1976; Yamada and Yamada, 2017), and in this study, a new fossil-species of Pinus from the upper Pleistocene of South China is described based on well-preserved mummified seed cones. The combined use of scanning electron microscopy (SEM) and micro-computed tomography (micro-CT) facilitates the detailed study of seed cone anatomical and morphological features. This study confirms the diagnostic significance of cone seed scale anatomy of Pinus for classification above the species level. A unique combination of characters indicates that the new fossil-species is closely related to East Asian extant species of subgenus Pinus, section Pinus, subsection Pinus. We have also discussed the implication of the new fossil-species for phytogeography and ecology.
The mummified seed cones were collected from the Zhenjiang opencast mine (21°52′47.5″N; 110°40′06.3″E) within the Maoming Basin, located northwest of Maoming City in southwestern Guangdong Province, South China (Figure 1). The fossiliferous strata overlie, with an angular unconformity, the upper Eocene Huangniuling Formation. The plant-bearing deposits were dated to the late Pleistocene using the accelerator mass spectrometry 14C dating of fossil wood and fruit samples (28,660 ± 140 and 26,860 ± 130 BP, respectively) at the Beta Analytic Testing Laboratory, Miami, Florida, USA (Huang et al., 2021a). From bottom to top, the measured sedimentary succession with a total thickness of about 2.5 m is composed of yellow poorly sorted sands, black massive clays, gray sandy mudstones, and grayish-yellow mudstones and siltstones that presumably represent floodplain environments. A total of 24 seed cones were obtained mainly from a layer of yellow sands exposed at the bottom of study section. The three-dimensionally preserved mummified cones are predominantly closed, but cones in the initial stage of dehiscence are also present.
Figure 1. Map showing plant fossil locality (asterisk) in the Maoming Basin, Guangdong Province, South China.
The specimens were mechanically cleaned from the rock using needles and brushes. Fossil cones were photographed using a Panasonic GX7 with a Leica DG MacroElmarit 1:2.8/45 mm macro lens and an Olympus SZX10 stereo microscope with a Tucsen H-694CICE digital camera at the Museum of Biology, Sun Yat-sen University, Guangzhou, China. All photographs were optimized for size, color, and contrast using Adobe Photoshop CC 2018. A micro-CT system was used to study the internal cone structure, specifically a Zeiss 520 Versa 3-D X-ray microscope at the Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences, Nanjing, China. Transverse breaks of the cone axis and cone scales were mounted on SEM stubs with carbon tape, sputtered with palladium and gold, and examined using a Tescan SEM at the Borissiak Paleontological Institute, Moscow, Russia. Cone scale fragments were prepared using hydrochloric and hydrofluoric acids and treated with KOH. The obtained cone scale cuticles were mounted on aluminum stubs, coated with palladium, and examined using the Tescan SEM at a high vacuum.
This study follows the taxonomy of Farjon (2017) for extant species of Pinus. The terminology for the morphological description of pine seed cones follows Klaus (1980, 1989).
All fossil cones are curated in the Museum of Biology of Sun Yat-sen University, Guangzhou, China, with specimen numbers beginning MMRH.
Order PINALES Gorozh., 1904
Family PINACEAE Spreng. ex F. Rudolphi, 1830
Genus Pinus L., 1753
Subgenus Pinus L., 1753
Section Pinus Gernandt, Geada López, Ortiz García et Liston, 2005
Subsection Pinus Gernandt, Geada López, Ortiz García et Liston, 2005
Fossil-species Pinus prehwangshanensis Bazhenova, Wu et Jin
sp. nov. (Figures 2, 3, 4B–F, 5–10).

Figure 2. Seed cones of Pinus prehwangshanensis sp. nov. (A) Specimen MMRH186. (B) An enlargement in (A) showing the apophyses with perexcentromucronate umbo. (C) Specimen MMRH169. (D) Holotype, specimen MMRH170. (E) Specimen MMRH181. (F) Specimen MMRH174. (G) Specimen MMRH182. (H) An upper part of dehiscent seed cone, specimen MMRH171. (I) A seed cone base, specimen MMRH169. (J) A seed cone base, specimen MMRH181. (K) Specimen MMRH168.
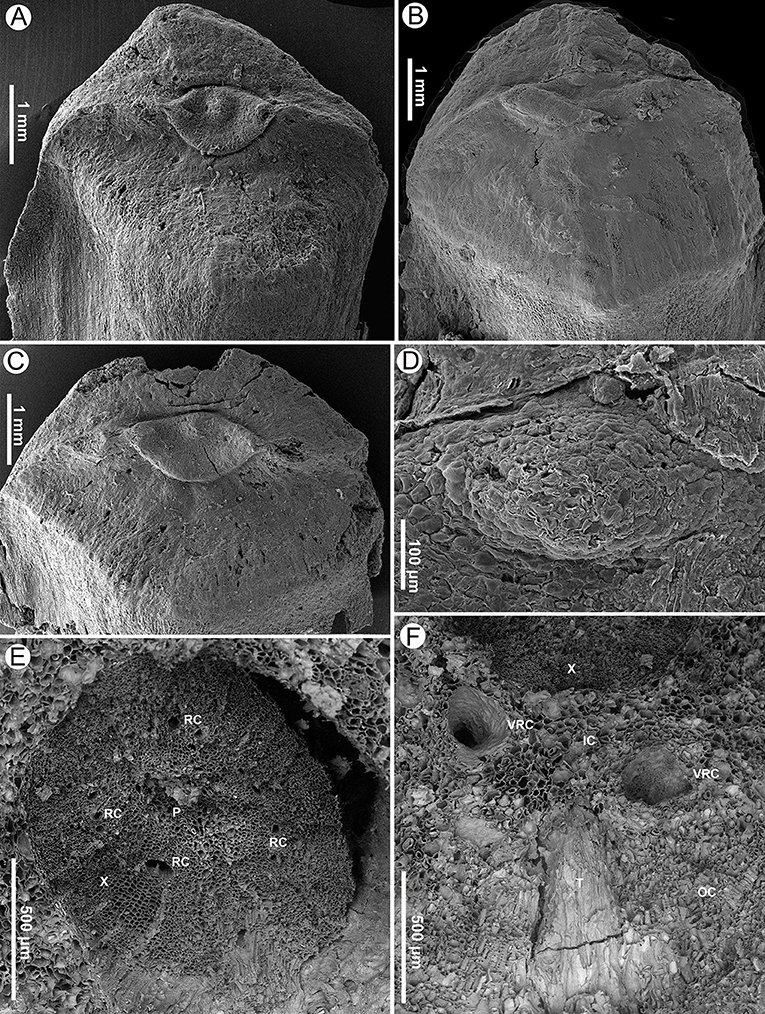
Figure 3. Seed scales and transverse sections of a cone axis of Pinus prehwangshanensis sp. nov., specimen MMRH171, SEM. (A–C) Apophyses with perexcentromucronate umbo. (D) Mucro, an enlargement in (B). (E) Transverse section of a cone axis showing a vascular cylinder (P – pith; X – xylem; RC – resin canals). (F) Transverse section of a cone axis cortex (X – xylem; IC – inner cortex; OC – outer cortex; T – trace of bract-scale vascular complex, VRC – vertical resin canals).
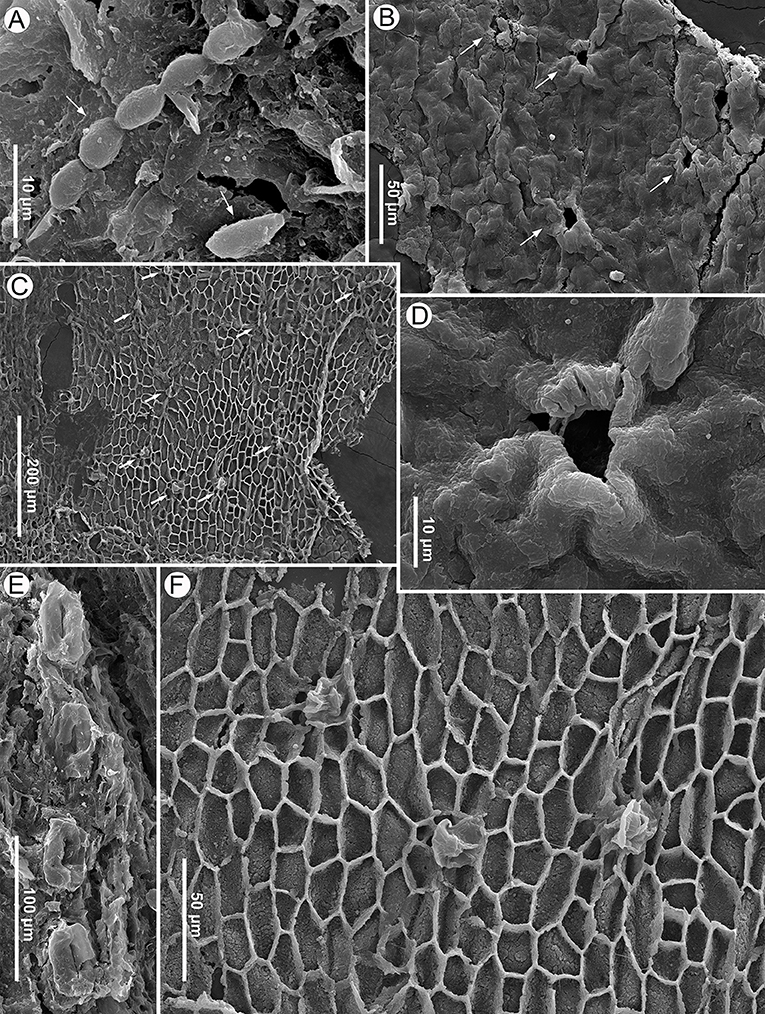
Figure 4. Seed scale cuticle of Pinus prehwangshanensis sp. nov. and fungal remains, specimen MMRH171, SEM. (A) Conidia (arrows) of fungus similar to Cladosporium on seed scale surface. (B) Cuticle of apophysis surface with rare stomata (arrows), outer view. (C) Cuticle of apophysis surface with monocyclic stomata (arrows), inner view. (D) Stoma with papillose subsidiary cells, an enlargement in (B). (E) A distinct row of stomata on an abaxial surface of a seed scale. (F) Stomata, an enlargement in (C).
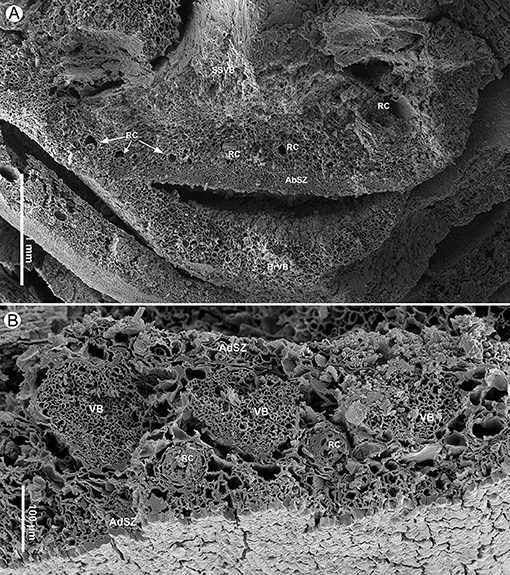
Figure 5. Bract and seed scale anatomy of Pinus prehwangshanensis sp. nov., specimen MMRH171, SEM. (A) Transverse section of a bract-scale complex near its base showing the anatomy of a seed scale (SSVB – seed scale vascular bundles; RC – resin canals; AbSZ – abaxial sclerified zone) and a bract (BrVB – bract vascular bundles) separated from a seed scale by a medial pouch. (B) Transverse section of a seed scale in its lower third showing a sclerified cortex (AbSZ – abaxial sclerified zone; AdSZ – adaxial sclerified zone), slightly ellipsoidal to rounded vascular bundles (VB) without pronounced xylem rays and abaxial resin canals (RC).
A closed seed cone, MMRH 170, designated here (Figures 2D, 8–10).
The Museum of Biology of the Sun Yat-sen University, Guangzhou, China.
Zhenjiang opencast mine, Maoming Basin, Guangdong Province, China.
Late Pleistocene.
The specific epithet is the combination of the prefix pre (from lat. prae – before) and the specific epithet of extant species Pinus hwangshanensis.
Seed cones, narrowly ovoid to ovoid-oblong, symmetrical, 43–47 mm long, 17–19 mm in diameter, 2.0–3.1 cone length/diameter ratio, with short peduncle. Bract lingulate, lacking keel. Seed scale apophyses in middle part of cone rhombic to (rarely) pentagonal, often with rounded upper margin, flat to slightly raised, with pronounced transverse keel. Umbos dorsal, widely-ellipsoidal, slightly depressed, perexcentromucronate; mucro persistent. Pith parenchymatous, with rare slightly sclerified cells. Secondary xylem with well-developed resin canals, marking boundary between two growth rings. Inner cortex parenchymatous, with up to 18 vertical resin canals; outer cortex slightly sclerified. Vascular strands of bract-scale complex united at origin, forming cylindrical trace. Bract trace separating from seed scale trace in the inner part of outer cortex. Vascular bundle reaching less than one half of bract length, accompanied by two small indistinct lateral resin canals. Outer part of seed scale cortex slightly sclerified. Vascular bundles in seed scale slightly ellipsoidal to rounded, with indistinct xylem rays; cellular rows in xylem unclear. Vascular bundles of seed scale usually with well-developed resin canals. Resin canals at seed scale base initially abaxial, then alternating with vascular bundles, distally forming adaxial and abaxial rows relative to vascular bundles. Resin canals with sclerified epithelial cells and ring of slightly sclerified encircling cells. Seeds ellipsoidal, slightly flattened; wing subtriangular, persistent.
Closed seed cones are narrowly ovoid to ovoid-oblong, 43–47 mm long, 17–19 mm in diameter, with a 2.0–3.1 cone length/diameter ratio. The short peduncle is up to 2 mm long and 2–3 mm in diameter (Figures 2I,J).
A bract is small, lingulate, and lacking a keel, with slightly sclerified tissues. It separates from a seed scale by a medial pouch (Figures 5A, 8B,D–F, 10A,D,E).
Seed scales are woody, with a maximum of 15 mm length and 4–5 mm wide at a scale base (Figures 5A, 10A,B–D), bearing two inverted seeds; the interseminal ridge is low and poorly developed. Apophyses in the middle part of a cone are rhombic to (rarely) pentagonal, up to 6 mm high, up to 10 mm wide (Figures 2A–H,K, 3A–C), often with a rounded upper margin. Apophyses are flat or slightly raised, with a well-developed transverse keel. Umbos in the middle part of a cone are dorsal (somewhat shifted to the upper part of apophysis), but they can be central at the cone base. Umbos are widely ellipsoidal, 1.4–3.0 mm wide, 0.8–1.5 mm high, and often slightly depressed, bearing a conical perexcentral persistent mucro of up to 450 μm high (Figures 2B, 3A–D).
The apophysis surface is well cutinized. The epidermal cells are small, slightly longitudinally elongated, 12–50 μm long, 5–15 μm wide, with straight anticlinal walls and convex outer periclinal walls (Figures 4C,F). Stomata are sufficiently rare, unevenly spaced, and predominantly longitudinally orientated (Figures 4B,C,F). They are monocyclic, with 7–8 subsidiary cells, bearing small proximal papillae (Figures 4B,D,F). The length of the cutinized parts of guard cells is about 25 μm, and their width is approximately the same.
The abaxial surface of a seed scale below the apophysis is thinly cutinized, and its epidermis is composed of longitudinally elongated cells (Figure 6C). The abaxial surface has distally rare chains of longitudinally orientated stomata with well-developed bean-shaped guard cells 50–60 μm long; the width of the guard cell pair is about 30 μm (Figure 4E).
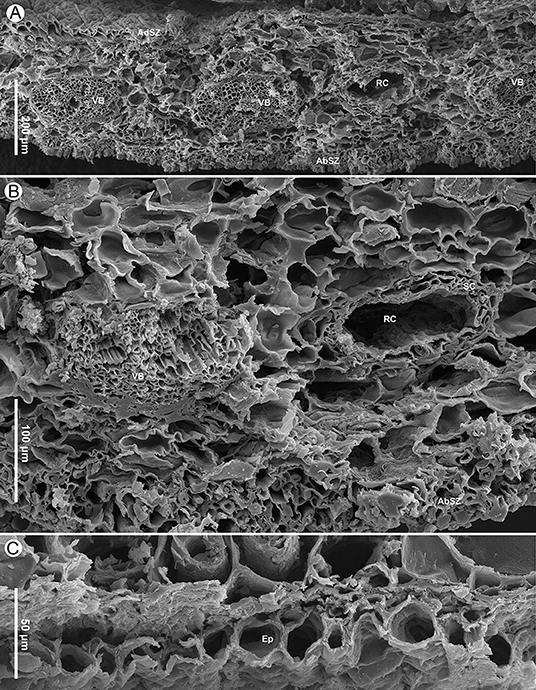
Figure 6. Seed scale anatomy of Pinus prehwangshanensis sp. nov., specimen MMRH171, SEM. (A) Transverse section of a seed scale in the middle part showing a sclerified cortex (AbSZ – abaxial sclerified zone; AdSZ – adaxial sclerified zone) and vascular bundles (VB) alternated with resin canals (RC). (B) Transverse section of a seed scale showing a vascular bundle (VB) and a resin canal (RC) with two concentric rows of sclerified cells (SC); note a sclerified zone (AbSZ) in the abaxial cortex. (C) A seed scale cross-section showing abaxial epidermal cells (Ep).
The cone axis is up to 7 mm in diameter near the cone base, tapering toward the peduncle and the apex (Figure 10A). The cone axis consists of a well-developed pith up to 1,500 μm in diameter near the cone base, diminishing gradually toward the apex, a ring of endarch xylem up to 550 μm wide, and a parenchymatous cortex up to 2,400 μm wide (Figures 3E, 8A–D, 10A). The pith is mainly parenchymatous with rare slightly sclerified cells. Pith cells are up to 25 μm in diameter. The secondary xylem of the cone axis has well-developed vertical resin canals of 30–50 μm in diameter and uniseriate xylem rays (Figure 3E). Resin canals are located at about one-third of the distance from the inner to the outer margin of the secondary xylem and mark the boundary between two growth rings. The diameter of tracheids varies from 20 μm in the proximal part of a growth ring to 3 μm in its distal part. Phloem is not preserved.
The inner cortex is composed of large parenchyma cells up to 90 μm in diameter and comprises a ring of vertical resin canals (Figures 3F, 8). The outer cortex is slightly sclerified, up to 180 μm thick, and composed of cells 5–50 μm in diameter (Figure 3F). The boundary between the inner and outer cortex is indistinct.
Cylindrical vascular bundles within a cone bract-scale complex originate from the xylem cylinder at a right angle in a tight spiral. Each vascular bundle, accompanied by two resin canals diverging from a pair of vertical resin canals (Figure 3F), enters into the bases of the bract-scale complexes and then gives off pairs of collateral bundles, vascularizing a bract and seed scale, respectively (Figures 8E,F, 9, 10). Two resin canals bifurcate and give rise to resin canals of a seed scale and bract (Figures 10B–D). The bract is supplied by an ellipsoidal vascular bundle up to 320 μm wide (Figures 5A, 9B–D, 10B–E). This vascular bundle reaches less than half the length of the bract free part and is accompanied by two small indistinct lateral resin canals (Figures 10C,E). The free part of the bract is about 3.5 mm long and 2.5 mm wide (Figures 5A, 10A,E). The vascular bundles of a seed scale are arranged in one row (Figures 5B, 6A, 8). There are up to 16 vascular bundles in the middle part of a scale. They are endarch, slightly ellipsoidal to rounded, with 200–300 μm in diameter, and without pronounced xylem rays; cellular rows in xylem are unclear (Figures 6A,B, 7A). The protoxylem in the vascular bundles of a seed scale is underlain by a layer of sclerified cells up to 10–20 μm thick (Figures 5B, 6B, 7A). The tracheids vary in size, are 2–12 μm in diameter, and are rounded to polygonal in the transverse section, with walls of 1–2 μm thick. The vascular bundles usually contain a well-developed resin canal of 25–30 μm in diameter (Figures 7A,B). Resin canals in the ground tissue of a seed scale are 50–200 μm in diameter, with sclerified epithelial cells and a ring of slightly sclerified encircling cells (Figure 6B). The resin canals in the seed scale basal part are abaxial (Figures 5A,B), and then move upward, where they alternate with vascular bundles (Figure 6A). Near the apophysis base, the resin canals occupy adaxial and abaxial positions relative to the vascular bundles (Figure 7C); the vascular bundles and resin canals here become smaller. The seed scales are about 450–550 μm thick in their middle parts. The outer part of a seed scale cortex is sclerified and is composed of cells 10–25 μm in diameter. The abaxial outer sclerified zone of the cortex is about 220 μm thick at the seed scale base, tapering distally to about 70 μm. This layer is replaced upward by parenchymatous tissues that are composed of cells with 25–70 μm diameter (Figures 6A,B). The adaxial cortex sclerified zone is up to 45 μm thick (Figures 5B, 6A).
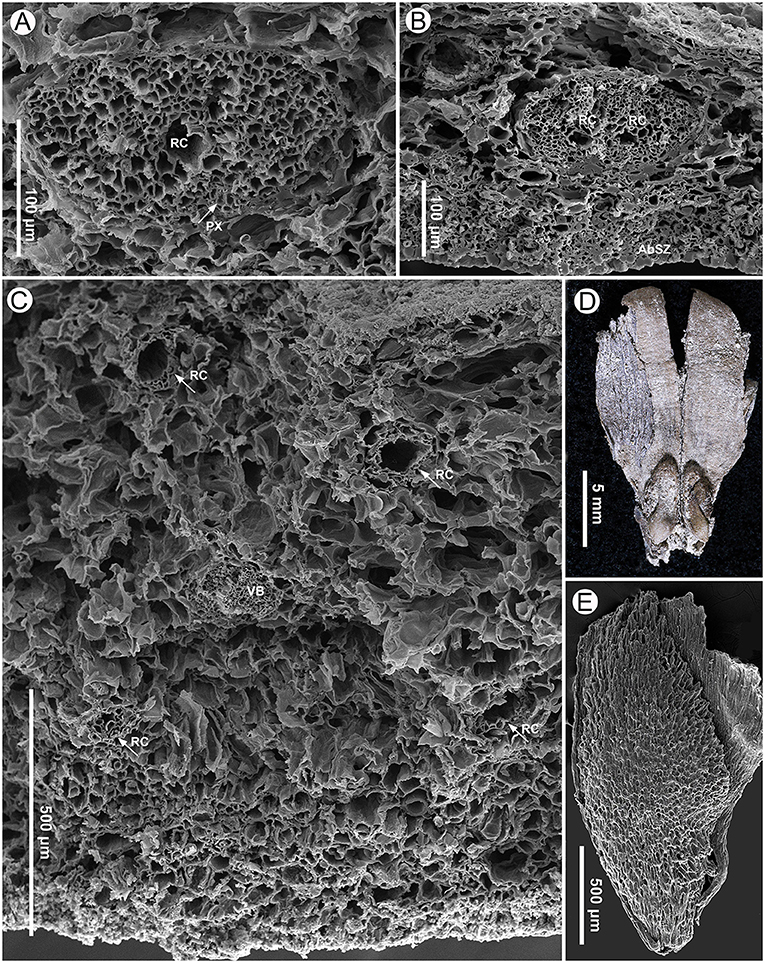
Figure 7. Seed scale anatomy (specimen MMRH171) and seeds (specimen MMRH188) of Pinus prehwangshanensis sp. nov. (A) A vascular bundle of a seed scale with a resin canal (RC) and well-preserved protoxylem (PX, indicated by arrow), SEM. (B) A bifurcating vascular bundle with two resin canals (RC); note a sclerified zone (AbSZ) in the abaxial cortex, SEM. (C) Abaxial and adaxial resin canals (RC, indicated by arrows) in the distal dilated part of a seed scale near the apophysis base and their arrangement relative to a vascular bundle (VB), SEM. (D) A fragment of seed scale with two winged seeds. (E) A seed showing cells of the sclerotesta, SEM.
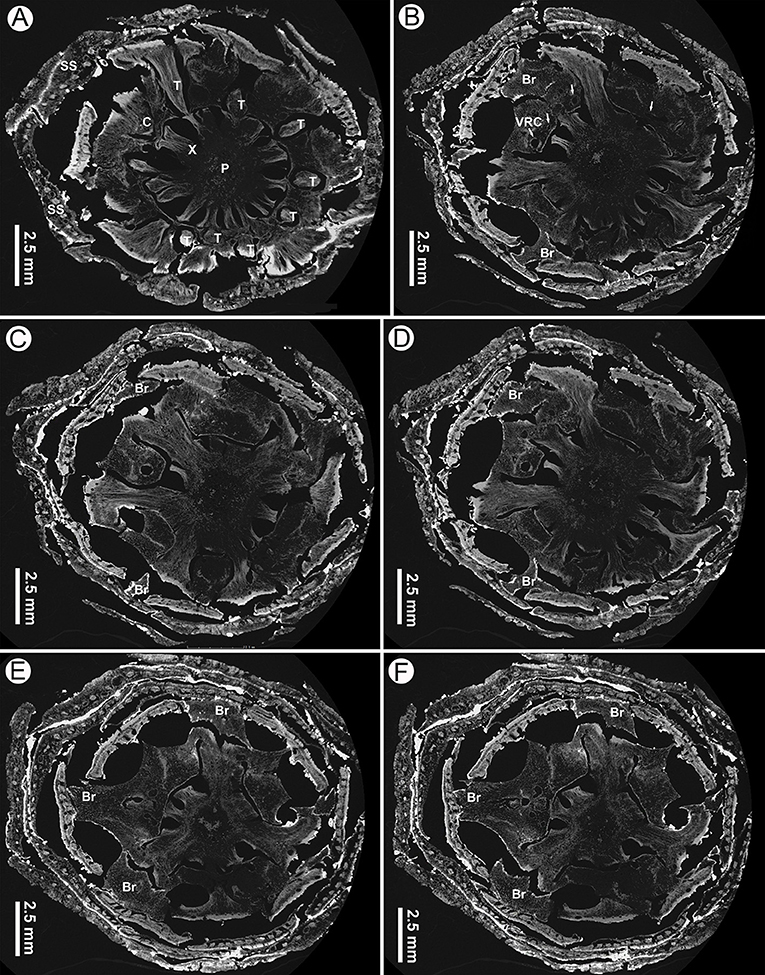
Figure 8. Virtual transverse sections of a seed cone of Pinus prehwangshanensis sp. nov., holotype, specimen MMRH170, micro-CT. (A) Traces of bract-scale vascular complexes (T) diverging from a vascular cylinder of cone axis; note a pith (P), xylem (X), cortex (C), and seed scales (SS). (B) Bracts (Br) separating from a seed scale by a medial pouch and vertical resin canals (VRC, indicated by arrows). (C–F) Bracts (Br) separating from a seed scale by a medial pouch.
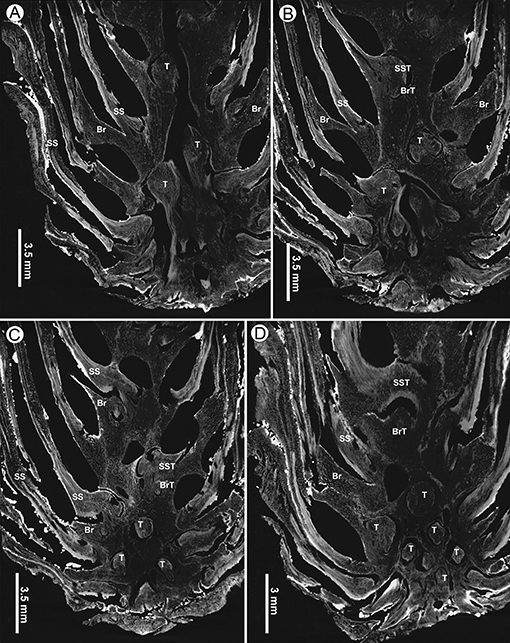
Figure 9. Virtual tangential sections near base of a seed cone of Pinus prehwangshanensis sp. nov., holotype, specimen MMRH170, micro-CT. (A–D) Circular traces (T) of bract-scale vascular complexes diverging from the axial vascular cylinder and supplying seed scales (SS) and bracts (Br); note the traces of vascular bundles to seed scales (SST) and bracts (BrT).
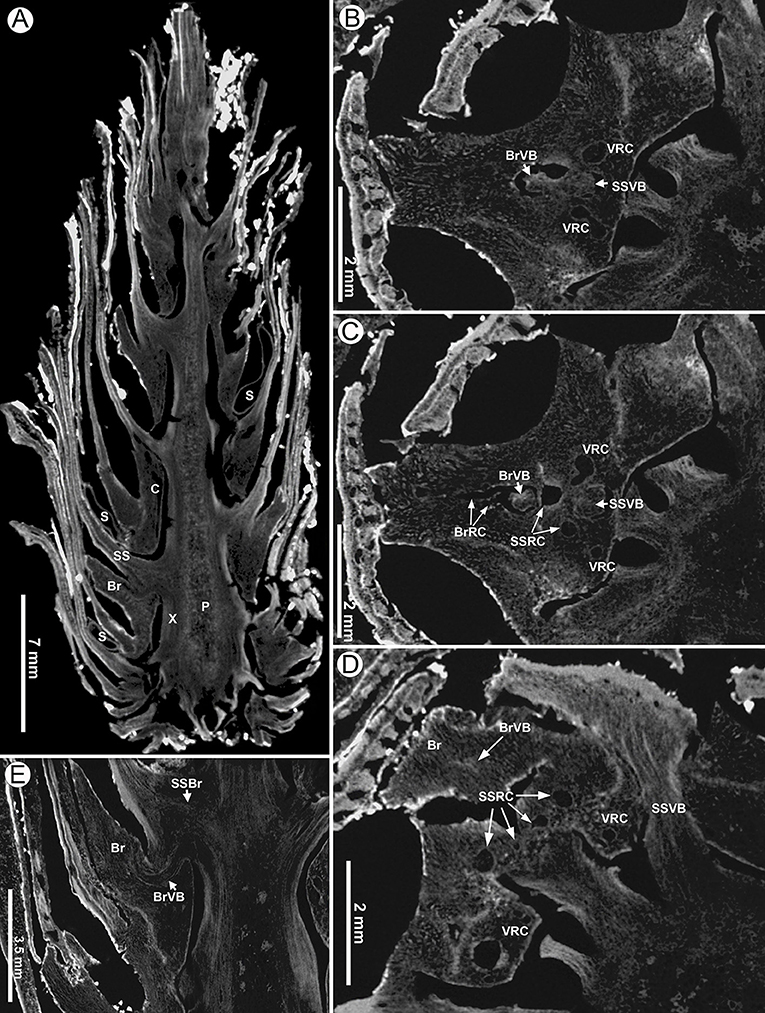
Figure 10. Virtual sections of a seed cone of Pinus prehwangshanensis sp. nov., holotype, specimen MMRH170, micro-CT. (A) Longitudinal section of a seed cone showing a cone axis (P – pith, X –xylem, C – cortex) and numerous bract-scale complexes (SS –seed scale, Br – bract) bearing seeds (S). (B,C) Two lateral resin canals (VRC) giving rise to resin canals of a seed scale (SSRC) and bract (BrRC); note the vascular bundles of a seed scale (SSVB) and bract (BrVB) (enlargements in Figures 8E,F, respectively). (D) A bract (Br) separated from a seed scale by a medial pouch, a vascular bundle of a bract (BrVB) is visible; note a vascular bundle of a seed scale near its base (SSVB), resin canals of a seed scale (SSRC), and two vertical resin canals (VCR) (an enlargement in Figure 8D). (E) Longitudinal section of a seed cone showing a bract (Br) and vascular bundles of a bract (BrVB) and a seed scale (SSVB).
Seeds are ellipsoidal, slightly flattened, 2–5 mm long, 1.5–2.5 mm wide, with a persistent subtriangular wing up to 12 mm long (Figures 7D,E). The testa is thin (Figure 10A). The seed sarcotesta is composed of longitudinally orientated polygonal cells of 45–140 μm long and 10–45 μm wide; the sclerotesta is thin and composed of more or less isodiametric cells of 10–50 μm in diameter (Figure 7E).
Pollen grains found on the seed scales of P. prehwangshanensis are similar to those of some extant pine species related to Pinus thunbergii Parl., and may belong to the same plant as the new fossil-species. All pollen grains were united in one group, except for one pollen grain that looked different (referred to here as ?Pinus pollen grain, Figure 11A). The ?Pinus pollen grain (Figure 11A) is bisaccate, bilaterally symmetrical, and heteropolar. The corpus is elliptical in polar view, plano-convex in lateral view, and measures 36.3 × 23.1 μm (width × length). The grain length (equatorial diameter) is 41.3 μm with sacci. Sacci are elliptical in polar view, biconvex in lateral view, and both sacci are of similar size and shape. The left saccus length is 36.3 μm and the right saccus length is 34.4 μm. The cappa is granulate-foveolate with large, weakly expressed rugulae, while the sacci are densely foveolate. The cappa-saccus transition is rather clear, abruptly changing from one pattern to another with clearly expressed rugulae that may also be an artifact of dehydration.
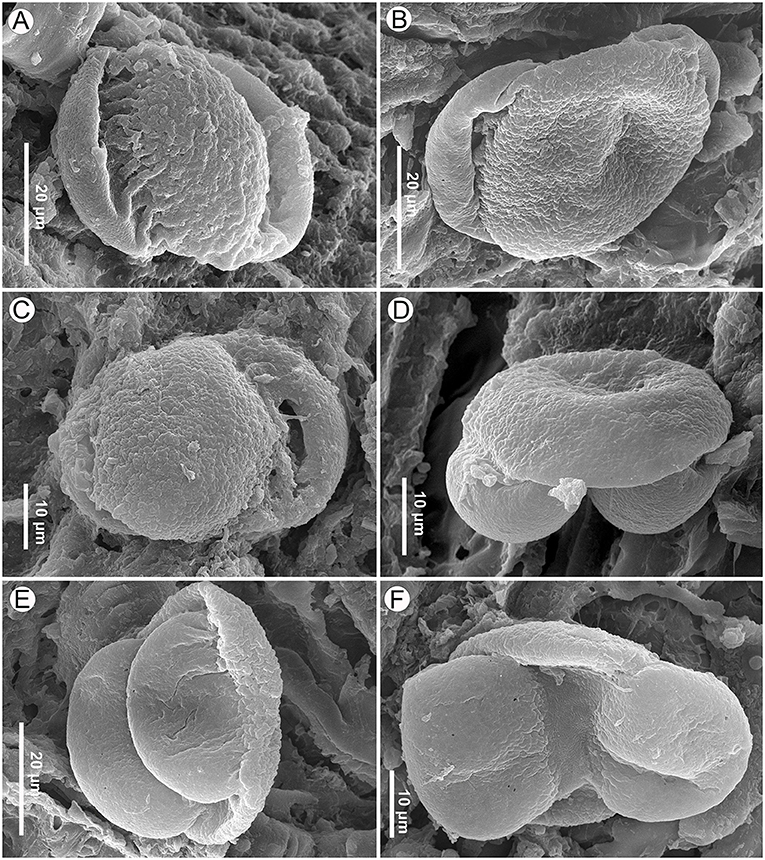
Figure 11. Pinus pollen on the seed scale surface of Pinus prehwangshanensis sp. nov., specimen MMRH171, SEM. (A) ?Pinus pollen, proximal view, granulate-foveolate, coarsely rugulate cappa sculpture, and deeply folded rugulae in the cappa-saccus transition are seen. (B,C) Pinus pollen, proximal view, verrucate-rugulate cappa sculpture is seen. (D,E) Pinus pollen, lateral view, a sharp change in the sculpture pattern is seen in the cappa-saccus transition. (F) Pinus pollen, distal view, the leptoma membrane is finely rugulate.
Other pollen grains are bisaccate, bilaterally symmetrical, and heteropolar (Figures 11B–F). The corpus is elliptical in polar view, plano-convex in lateral view, and measures 32 (21.1–43.4) × 29 (17.9–40) × 8.5 (6.9–10) μm (width × length × depth). The pollen width (polar axis) is 23.4 (15.0–27.6) μm with sacci, and the pollen length (equatorial diameter) is 46.2 (36.5–54.7) μm with sacci. The sacci are elliptical in polar view, biconvex in lateral view, both sacci are of the similar size and shape, and they are considered here left and right sacci for convenience. The left saccus is 30.6 (25.0–35.9) × 20.4 (14.2–25.9) × 7.5 μm, while the right saccus is 30.6 (26.7–35.0) × 18.1 (11.9–25.0) × 6.5 μm. A thinned area on the distal side of the corpus represents a leptoma of about 3.62 (1.8–5.6) μm wide (Figure 11F). The leptoma membrane is rugulate (based on two photos).
The cappa is verrucate-rugulate (Figures 11B–D), and the sacci are foveolate, with abundant or less sparsely distributed foveolae (Figures 11B–F). The cappa-saccus transition is rather clear, abruptly changing from one pattern to another (Figures 11D,E).
Apart from pollen grains, conidium chains of hyphomycetes were found on the inner surface of the cone scales. Conidia are slightly ellipsoid in shape and 5–6 μm in length (Figure 4A).
There are a total of 24 specimens of mummified three-dimensionally preserved seed cones and seeds (i.e., MMRH 168, MMRH 169, MMRH 170, MMRH 171, MMRH 172, MMRH 173, MMRH 174, MMRH 175, MMRH 176, MMRH 177, MMRH 178, MMRH 179, MMRH 180, MMRH 181, MMRH 182, MMRH 183, MMRH 184, MMRH 185, MMRH 186, MMRH 187, MMRH 188, MMRH 189, MMRH 190, MMRH 191).
According to Xing et al. (2010a), the perexcentromucronate type of umbo exists in seven extant species belonging to section Pinus, subsections Pinus and Pinaster (sensu Gernandt et al., 2005). Three species of subsection Pinus with perexcentromucronate umbo, Pinus kesiya Royle ex Gordon, P. massoniana, and P. yunnanensis, are distributed in Southeast and East Asia. Indistinct xylem rays in vascular bundles of seed scales are characteristic only for a group of pines closely related to the new fossil-species and those are extant species from subsection Pinus (Figure 12): Pinus hwangshanensis W.Y. Hsia, which is endemic to the mountains of eastern China, Pinus luchuensis Mayr from Ryukyu islands of Japan, Pinus taiwanensis Hayata from Taiwan Island of China, P. thunbergii from the southern Korean Peninsula and Honshu, Kyushu, and Shikoky islands of Japan, and Pinus densiflora Siebold et Zucc. from Japan, northeastern China, the Korean Peninsula, and southeastern Russia (Farjon, 2017; Yamada and Yamada, 2017). Moreover, in these species, as well as in the new fossil-species, resin canals are fringed with two concentric rows of sclerified cells. Apophyses of their seed scales are rhombic, often possessing a rounded upper margin, and transversally keeled. Umbos in these species are excentromucronate, and, according to Yamada et al. (2014), two species, P. thunbergii and P. densiflora, also possess perexcentromucronate umbos. However, Li et al. (2022) defined the umbo types of P. thunbergii and P. densiflora as denticulatomucronate and excentroerectomucronate, respectively. Several species of this group of related extant pines, P. hwangshanensis, P. luchuensis, P. taiwanensis, and P. thunbergii, form a clade in molecular phylogenetic trees (Wang and Szmidt, 1993; Wang et al., 1999; Gernandt et al., 2005; Yamada and Yamada, 2017). P. densiflora and P. thunbergii are closely related and form a natural hybrid named P. x densithunbergii (Farjon, 2017).
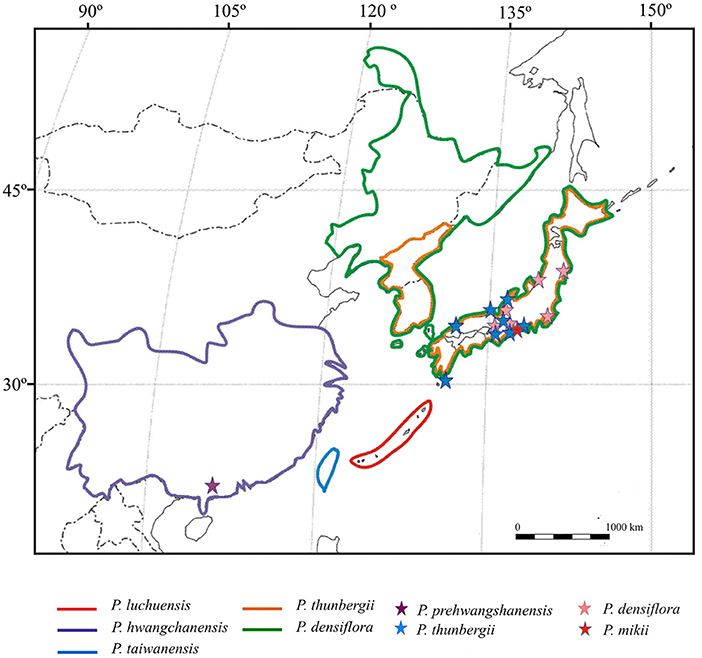
Figure 12. Distribution of the extant species closely related to the new fossil-species Pinus prehwangshanensis (after Forest et al., 2018; species areas are bounded by color lines), and occurrences of related fossil-species (after Miki, 1957; Yamada et al., 2015; occurrences are marked by asterisks).
The new fossil-species is most similar to P. hwangshanensis in seed cone morphology; however, extant species possesses narrowly ovoid cones, 3–6 cm long, with nearly flat widely ellipsoidal depressed umbos bearing minute persistent exentroerectomucronate mucros (Li et al., 2022). Seeds in P. hwangshanensis are ellipsoidal-obovate and slightly flattened (Farjon, 2017). The size of the resin canals within seed scales in the new fossil-species and the configuration of cells encircling these canals are most similar to those of P. hwangshanensis. However, the vascular bundles in P. hwangshanensis consist of more ordered thick-walled tracheids and lack resin canals (Yamada and Yamada, 2017).
P. prehwangshanensis shares several common morphological characters with P. luchuensis. Seed cones of this extant species are narrowly ovoid, 4–5 cm long, apophyses are slightly raised, and the mucro forms a small acute prickle. The arrangement of tracheids in the vascular bundle is very similar to that seen in the new fossil-species, but P. luchuensis lacks resin canals in seed scale vascular bundles (Yamada and Yamada, 2017). Moreover, the diameter of the resin canals in the seed scale cortex is half that of the new fossil-species. In addition, P. luchuensis differs in having slightly larger diameter cones with pyramidal umbo (Farjon, 2017).
Extant species P. thunbergii, known since the Pleistocene (Yamada et al., 2014), is similar to the new fossil-species in having resin canals in the vascular bundles of the seed scales (Yamada and Yamada, 2017). Resin canals in the seed scale cortex of these two species have similar sizes. However, the seed cones of P. thunbergii are ovoid-conical, 4–6 (7) cm long, with an umbo often lacking a mucro (Farjon, 2017), and the tracheids in the seed scale vascular bundles are arranged in distinct rows (Yamada and Yamada, 2017). In addition, seeds of P. thunbergii are obovate, unlike those of the new fossil-species.
In P. taiwanensis, cones are narrowly ovoid, (3) 4–9 (10) cm long. Overall, they are larger than the cones of the new fossil-species; apophyses are flat to slightly raised, and the mucro is often deciduous (Farjon, 2017) in contrast to the persistent mucro in the new fossil-species. Resin canals in the cortex of the seed scales are small, and they are lacking in vascular bundles. The shape of vascular bundles is similar to that in the new fossil-species, but tracheid walls are significantly thicker, and tracheids exhibit more regular rows.
Two extant species with perexcenromucronate umbos, P. kesiya and P. yunnanensis, show a close phylogenetic affinity (Wang et al., 1999; Gernandt et al., 2005) and similarity in foliage and seed cone morphology (Fu et al., 1999). In contrast to P. prehwangshanensis, both these species possess a rather protruding umbo with a well-defined vallum (Fu et al., 1999; Erwin and Schorn, 2006). In addition, P. yunnanensis is distinguished in having cross-keeled cone scale apophyses (Xing et al., 2010a).
Extant species P. densiflora is also known in the paleobotanical record since the Pleistocene (Yamada et al., 2014). Cones of this species are ovoid-conical, 3–6 cm long. As with the new fossil-species, P. densiflora exhibits adaxial resin canals in its seed scales (Yamada and Yamada, 2017). However, the extant species possesses a strongly sclerified cone axis outer cortex (McKown et al., 2002), a character that is absent in P. prehwangshanensis. It is apparent that the new fossil-species displays a mosaic of characters seen in several extant Pinus species, but it does not show complete similarity to any one of these species (Table 1).
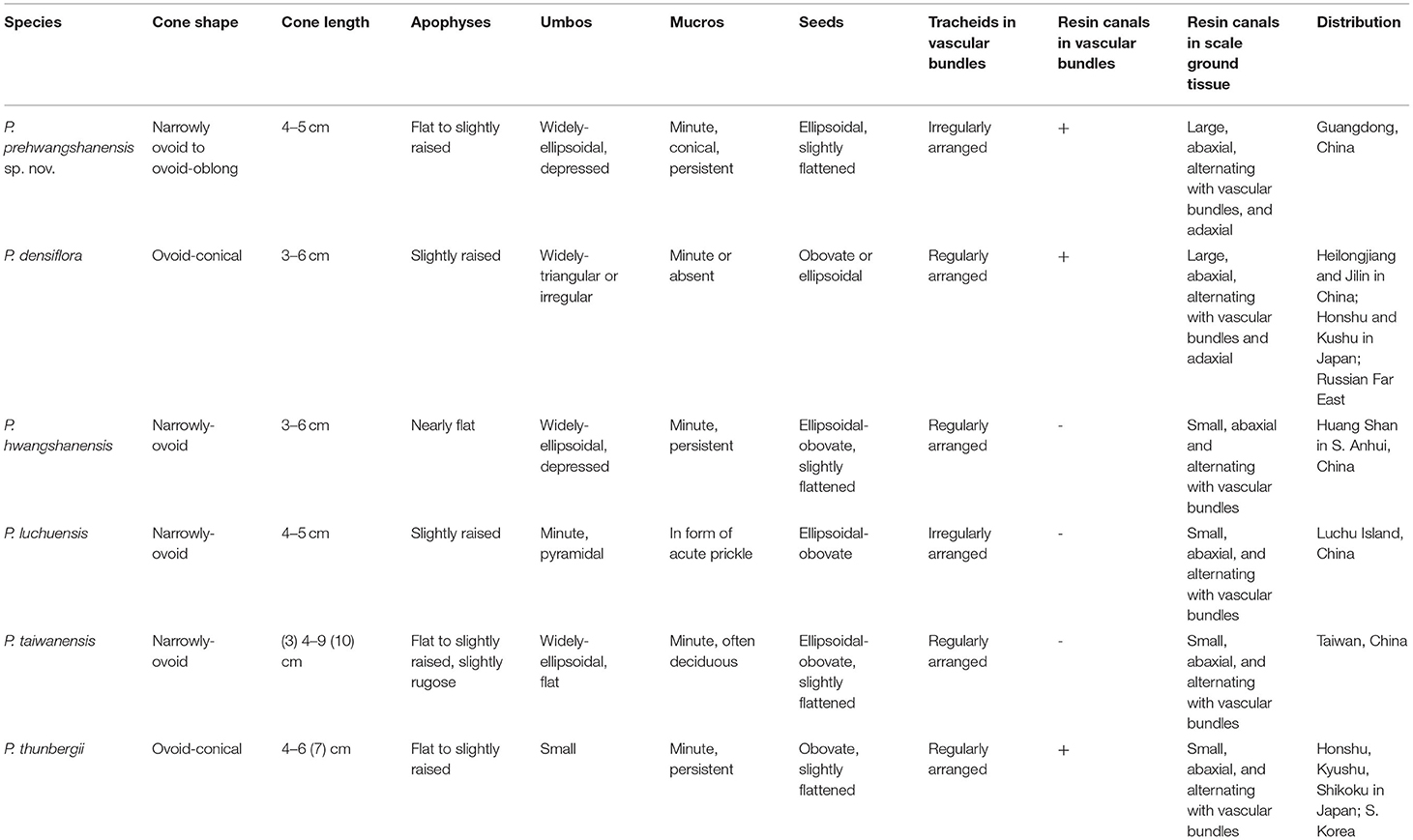
Table 1. Comparison of the seed cones of Pinus prehwangchanensis sp. nov. and related extant species of Pinus.
Seed cones of the fossil-species, P. massoniana from the Miocene of Yunnan Province, China (Zhang et al., 2015), are similar to those of the new fossil-species in their overall shape, apophysis morphology, and perexcentral mucro. However, the upper part of the umbo in fossil P. massoniana is raised, while the umbo in the new fossil-species is rather flat. The anatomy of the seed scales of fossil P. massoniana is unknown, but similar to the new fossil-species, the vascular bundles in the modern P. massoniana possess rounded outlines in transverse section, usually contain resin canals, and are composed of poorly ordered tracheids. The vascular bundles in the extant P. massoniana differ from those of P. prehwangshanensis in having distinct xylem rays and resin canals without a second ring of sclerified cells (Yamada and Yamada, 2017).
P. premassoniana (Ding et al., 2013) is morphologically less similar to the new fossil-species than P. massoniana, since its cones are ovate, with a small umbo strongly shifted to the upper margin of the apophysis and having a markedly raised upper half.
P. weichangensis from the lower Miocene of Weichang, Hebei Province (Li et al., 2022), differs distinctly from the new fossil-species in the cone length/diameter ratio (1.6–2.0 in P. weichangensis vs. 2.0–3.1 in P. prehwangshanensis) and having predominantly denticulatomucronate or tectoid umbos.
Similar to the new fossil-species, the seed cones of two fossil-species, Pinus mikii T. Yamada, M. Yamada et Tsukagoshi from the upper Miocene of Japan (Yamada et al., 2015) and P. prekesiya from the upper Miocene of Yunnan, China (Xing et al., 2010a), possess minute perexcentral mucros. However, the cones of these species differ from those of P. prehwangshanensis in having distinctly vallate apophyses. Moreover, their seed cones are larger and wider than the cones of the new fossil-species.
Cones of Pinus oligolepis Miki from the lower Pliocene-middle Pleistocene of Japan (Yamada et al., 2014) are noticeably smaller than those of the new fossil-species, and are ovate in shape, with slightly raised perexcentromucronate umbos, bearing obtusely hooked mucros.
Pinus fujiii (Yasui) Miki from the upper Miocene of Japan is the only Cenozoic Asiatic species with known seed scale anatomy (Yasui, 1928; Yamada et al., 2015; Yamada and Yamada, 2017). This fossil-species differs significantly from the new fossil-species in terms of cone morphology and anatomy. The seed cones of P. fujiii possess uncinate centromucronate apophyses, vascular bundles that are reniform, distinct xylem rays, and resin canals composed of non-sclerified epithelial cells.
P. prototabulaeformis Tao et Wang from the upper Eocene-lower Oligocene of China is distinguished from the new fossil-species by having significantly larger seed cones, as well as by displaying raised apophyses with protruding pyramidal umbos and bearing well-developed denticulate mucros (Tao and Wang, 1983).
The seed cone of Pinus sp. 1 from the upper Oligocene or lower Miocene of Nong Ya Plong Basin, Thailand (Grote and Srisuk, 2021), is very similar to the cones of the new fossil-species in shape, but further comparison is difficult due to poor preservation of the fossil from Thailand.
The European species Pinus salinarum (Partsch) Zablocki from the middle Miocene of Poland displaying perexcentromucronate umbos is characterized by relatively large ovoid cones with an asymmetrical cone base and apophyses with distinctly protruding umbos (Mai, 1986; Klaus, 1989). Another European middle-upper Miocene species, Pinus brevis Ludwig (Mai, 2004; Kowalsky, 2017), has broadly elliptic cones, often with protruding apophyses at the cone base. Apophyses in this species are distinctly vallate.
Most of the anatomically preserved seed cones of North American pines (Miller, 1969, 1973, 1974, 1978; Underwood and Miller, 1980; Banks et al., 1981; Stockey, 1983, 1984; McKown et al., 2002; Klymiuk et al., 2011; Ryberg et al., 2012) distinctly differ from those of the new fossil-species in that they lack perexcentromucronate umbos and have vascular bundles with regularly arranged tracheids. The seed cones of Pinus baylei Erwin et Schorn from the Paleogene of Idaho with unknown anatomical characters are the only American fossil cones with perexcentromucronate umbos (Erwin and Schorn, 2006). This species differs from P. prehwangshanensis in having larger and wider cones with prominent umbos bearing sharp-pointed mucros.
There is no absolute certainty that all the observed pollen grains were produced by the same Pinus species, but they appear to be similar (Figures 11B–F). Another problem is that each pollen grain is available for description only in a predefined view and most of the pollen grains are compressed; therefore, few characters are seen. The attribution of ?Pinus pollen grain to Pinus is rather conventional as it is based on one pollen grain from a predefined perspective.
In general, the studied pollen grains are smaller than those of most known fossil or extant species, although the size range for any given species might vary between different studies (e.g., Chen, 1988; Chen and Wang, 1999). Pollen grains of P. taiwanensis and P. luchuensis are similar in size. The sculpture pattern of the cappa is similar to that of pollen grains of P. densiflora and, to a lesser extent, P. thunbergii, P. luchuensis, and P. massoniana (Chen, 1988; Chen and Wang, 1999; Fujiki et al., 2005; Miyoshi et al., 2011; Song et al., 2012).
Conidia of hyphomycetes found on the inner surface of the cone scales are very similar to the widespread genus Cladosporium Link (Ascomycota), which is a common infecting agent on recent, as well as fossil, Pinus leaves and cones (e.g., Kowalczyk et al., 2004; Zalar et al., 2007; Paul and Yu, 2008; Bensch et al., 2010; Chungu et al., 2010).
Based on a study by Yamada and Yamada (2017), several anatomical characters of Pinus cone ovuliferous scales are useful for classification above the species level. The new fossil-species from the Maoming Basin is related to the group of extant pine species within the subsection Pinus that has its diversity center in East Asia. As mentioned above, round vascular bundles of seed scales with indistinct xylem rays and two rows of sclerified epithelial cells in resin canals are shared by P. prehwangshanensis and extant P. taiwanensis, P. hwangshanensis, P. luchuensis, P. thunbergii, and P. densiflora (Yamada and Yamada, 2017). Furthermore, the occurrence of resin canals in the vascular bundles of a seed scale is a particular characteristic of two extant species, P. densiflora and P. thunbergii (Yamada and Yamada, 2017), as well as P. prehwangshanensis. The new fossil-species exhibits a mosaic of seed cone morphological and anatomical characters found in this group of related extant species (Table 1). A relatively weakly sclerified seed cone outer cortex, as well as cells encircling resin canals, may be regarded as archaic features in the fossil-species from the Maoming Basin.
Fossil data show that Pinus species related to P. prehwangshanensis sp. nov. appeared in continental Asia no later than the late Pleistocene (Figure 12). Among these extant pines, only P. densiflora and P. thunbergii are recognized in the fossil record (Yamada et al., 2014). However, the anatomical characters of these fossils from the Pliocene-Pleistocene of Japan are unknown; therefore, their assignment to extant species has not been fully confirmed. Based on external seed cone morphology, Li et al. (2022) demonstrated a close resemblance of P. weichangensis from the lower Miocene of Hebei Province with P. thunbergii and supposed that P. thunbergii, native to Japan and South Korea, may have been derived from a continental Asian species, such as P. weichangensis. Yamada et al. (2015) inferred an affinity of another fossil-species, P. mikii, from the upper Miocene of Japan with extant P. thunbergii, as well as P. hwangshanensis and Pinus tabuliformis distributed in China. According to Yamada et al. (2015), this inference suggests phytogeographic relationships between Japan and China during the Miocene. However, it remains unclear where the ancestor of these extant pines originated. The occurrence of P. prototabulaeformis in the upper Eocene-lower Oligocene of China, which has a close similarity with extant P. tabuliformis (Tao and Wang, 1983; Ding et al., 2013), contradicts the hypothesis that the Neogene species, P. mikii, is the ancestor of certain continental Asian species. Overall, the fossil data confirm the existence of floristic exchange between continental Asia and Japan prior to the formation of the Sea of Japan and later, in the middle Miocene to the late Pliocene, when the connection between the Japanese islands and the Eurasian continent became re-established (Yabe et al., 2019).
Modern relatives of the new fossil-species grow under broadly varying conditions, predominantly in mixed forests on the slopes of mountains, but sometimes on marine coasts. The extant species P. hwangshanensis occurs at 600–3,400 m elevation as pure stands in open areas on mountain slopes and ridges, and as co-dominant of Fagaceae in mixed warm-temperate and montane forests (Fu et al., 1999). This pine can also colonize valleys and spread southward to where tropical rainforest communities have been destroyed by human activity (Farjon, 2017).
The week sclerification of seed cone tissues may indicate that P. prehwangshanensis grew under more humid and warm conditions compared to its modern relatives. Upper Pleistocene fossiliferous deposits from the Maoming Basin contain abundant plant detritus, fruits and seeds of angiosperms, and mummified woods. To date, the identified fossil reproductive structures of angiosperms include endocarps of Elaeocarpus (Elaeocarpaceae) and Canarium (Burseraceae) as well as acorns of Fagaceae. Fossil woods from this locality are assigned to Keteleeria sp. (Pinaceae) and the extant species Liquidambar formosana Hance (Altingiaceae) (Huang et al., 2021a,b). Most of the taxa accompanying P. prehwangshanensis are widespread in the modern evergreen broad-leaved and mixed forests of warm-temperate to subtropical Eastern Asia (e.g., Chen et al., 2010; Ashton and Zhu, 2020). Based on the growth ring analysis of fossil wood of Keteleeria sp. and L. formosana, and that of extant species Keteleeria davidiana (C.E. Bertrand) Beissn., Huang et al. (2021a,b) inferred that in late Pleistocene, the Maoming Basin region experienced a monsoon-influenced humid subtropical climate with hot wet summers and cool dry winters. This climate is similar to present-day conditions seen in northeastern Vietnam, but with less strongly expressed precipitation seasonality. According to the Köppen-Geiger classification of global climates (Köppen, 1936; Peel et al., 2007), the prevailing type of climate in northeastern Vietnam is type Cwa (Beck et al., 2018), which is clearly defined by dry winters and hot wet summers.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
J-HJ organized the fieldwork, led the data acquisition, and conceived the study. X-KW and S-LX performed the micro-CT study. NB and NM performed the SEM study. NB analyzed and interpreted the data and drafted the manuscript. MT analyzed the pollen. NM interpreted the data on fossil fungi. TK, MT, NM, J-HJ, and X-KW contributed to the initial manuscript preparation. NB, TK, and X-KW prepared the figures. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 41872015 and 42111530024 for J-HJ), the State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Paleontology, CAS) (Grant No. 193118 for J-HJ), the Russian Foundation for Basic Research (RFBR, Grant No. 19-04-00046 for NB and NM and partly for TK and No. 21-54-53001 for NB, NM, and MT), the State program (Grant No. 0135-2019-0045, Geological Institute, Russian Academy of Sci. for TK), and the China Postdoctoral Science Foundation (Grant No. 2021M693589 for X-KW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors sincerely thank Robert A. Spicer (The Open University, UK) for giving helpful suggestions, Anatoly V. Bazhenov (Borissiak Paleontological Institute of the Russian Academy of Sciences) for valuable discussion, Alexei B. Herman (Geological Institute of the Russian Academy of Sciences) for his help and guidance in photographing seed cones, and Roman A. Rakitov (Borissiak Paleontological Institute of the Russian Academy of Sciences) for helpful assistance with SEM. The authors also thank Lutz Kunzmann and Harald Schneider for their helpful comments that improved the manuscript.
Alvin, K. L. (1960). Further conifers of the Pinaceae from the Wealden Formation of Belgium. Mem. Inst. Roy. Sci. Nat. Belg. 146, 1–39.
Ashton, P., and Zhu, H. (2020). The tropical-subtropical evergreen forest transition in East Asia: An exploration. Plant Divers. 42, 255–280. doi: 10.1016/j.pld.2020.04.001
Banks, H. P., Ortiz-Sotomayor, A., and Hartman, C. M. (1981). Pinus escalantensis, sp. nov., a new permineralized cone from the Oligocene of British Columbia. Bot. Gaz. 142, 286–293. doi: 10.1086/337225
Beck, H. E., Zimmermann, N. E., McVicar, T. R., Vergopolan, N., Berg, A., and Wood, E. F. (2018). Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data. 5:180214. doi: 10.1038/sdata.2018.214
Bensch, K., Groenewald, J. Z., Dijksterhuis, J., Starink-Willemse, M., Andersen, B., Summerell, B. A., et al. (2010). Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol. 67, 1–94. doi: 10.3114/sim.2010.67.01
Chen, S.-H. (1988). A scanning electron microscope survey of common airborne pollen grains in Taipei, Taiwan. Taiwania. 33, 75–108.
Chen, S.-H., and Wang, Y.-F. (1999). Pollen flora of Yuenyang Lake Nature Preserve, Taiwan (I). Taiwania. 44, 82–136.
Chen, Y., Ni, J., and Herzschuh, U. (2010). Quantifying modern biomes based on surface pollen data in China. Glob. Planet. Change. 74, 114–131. doi: 10.1016/j.gloplacha.2010.09.002
Chungu, D., Muimba-Kankolongo, A., Wingfield, M. J., and Roux, J. (2010). Identification of fungal pathogens occurring in eucalypt and pine plantations in Zambia by comparing DNA sequences. Forestry. 83, 507–515. doi: 10.1093/forestry/cpq033
Ding, S. T., Wu, J. Y., Chen, J. L., Yang, Y., Yan, D. F., and Sun, B. N. (2013). Needles and seed cones of Pinus premassoniana sp. nov., and associated pollen cone from the upper Miocene in East China. Rev. Palaeobot. Palynol. 197, 78–89. doi: 10.1016/j.revpalbo.2013.05.004
Erwin, D. M., and Schorn, H. E. (2006). Pinus baileyi (section Pinus, Pinaceae) from the Paleogene of Idaho, USA. Am. J. Bot. 93, 197–205. doi: 10.3732/ajb.93.2.197
Falcon-Lang, H., Mages, V., and Collinson, M. (2016). The oldest Pinus and its preservation by fire. Geology. 44, 303–306. doi: 10.1130/G37526.1
Farjon, A. (2017). A Handbook of the World's Conifers. Leiden, Boston: Brill. doi: 10.1163/9789004324510
Forest, F., Moat, J., Baloch, E., Brummitt, N. A., Bachman, S. P., Ickert-Bond, S., et al. (2018). Gymnosperms on the EDGE. Sci. Rep. 8:6053. doi: 10.1038/s41598-018-24365-4
Fu, L. G., Li, N., and Mill, R. R. (1999). “Pinaceae”, in Flora of China, eds Z. Y. Wu, and P. H. Raven (Beijing: Science Press) 11–52.
Fujiki, T., Zhou, Z., and Yasuda, Y. (2005). The Pollen Flora of Yunnan, China. New Delhi: Roli Books.
Gernandt, D. S., Gaeda López, G., Ortiz García, S., and Liston, A. (2005). Phylogeny and classification of Pinus. Taxon. 54, 29–42. doi: 10.2307/25065300
Grote, P. J., and Srisuk, P. (2021). Fossil Pinus from the cenozoic of Thailand. Rev. Palaeobot. Palynol. 295:104501. doi: 10.1016/j.revpalbo.2021.104501
Huang, L.-L., Jin, J.-H., and Oskolsky, A. A. (2021a). Mummified fossil of Keteleeria from the late Pleistocene of maoming basin, South China, and its phytogeographical and paleoecological implications. J. Syst. Evol. 59, 198–215. doi: 10.1111/jse.12540
Huang, L.-L., Jin, J.-H., Quan, C., and Oskolsky, A. A. (2021b). New occurrences of Altingiaceae fossil woods from the Miocene and upper Pleistocene of South China with phytogeographic implications. J. Palaeogeogr. 10, 482–493. doi: 10.1016/j.jop.2021.11.001
Klaus, W. (1980). Neue Beobachtungen zur Morphologie des Zapfens von Pinus und ihre Bedeutung für die Systematik, Fossilbestimmung, Arealgestaltung und Evolution der Gattung. Plant Syst. Evol. 134, 137–171. doi: 10.1007/BF00986796
Klaus, W. (1989). Mediterranean pines and their history. Plant Syst. Evol. 162, 133–163. doi: 10.1007/BF00936915
Klymiuk, A. A., Stockey, R. A., and Rothwell, G. W. (2011). The first organismal concept for an extinct species of Pinaceae: Pinus arnoldii Miller. Int. J. Plant Sci. 172, 294–313. doi: 10.1086/657649
Köppen, W. (1936). “Das geographische System der Klimate”, in Handbuch der Klimatologie, Köppen, W., and Geiger, R. (eds). Berlin: Gebrüder Bornträger. p. 1–44.
Kowalczyk, A., Kacprzak, M., and Mańka, M. (2004). Fungi inhabiting Scots pine (Pinus sylvestris) seeds in various stages of extraction process. Phytopathol. Pol. 33, 53–70.
Kowalsky, R. (2017). Miocene carpological floras of the Konin region (Central Poland). Acta Palaeobot. 57, 39–100. doi: 10.1515/acpa-2017-0007
Li, Y., Yi, T.-M., Grote, P. J., An, P.-C., Zhu, Y.-B., Zhang, Z.-Y., et al. (2022). A new species of Pinus (Pinaceae) from the Miocene of Weichang, Hebei Province, China and its evolutionary significance. Hist. Biol. 34, 885–896. doi: 10.1080/08912963.2021.1952197
Mai, D. H. (1986). Über Typen und Originale tertiärer Arten von Pinus. L. (Pinaceae) in mitteleuropäischen Sammlungen – Ein Beitrag zur Geschichte der Gattung in Europa. Feddes Repert. 97, 571–605. doi: 10.1002/fedr.4910970904
Mai, D. H. (2004). Die miozänen und pliozänen Floren aus Nordostbrandenburg und Südwestmecklenburg. Palaeontogr. Abt. B 269, 1–130. doi: 10.1127/palb/269/2004/1
McKown, A. D., Stockey, R. A., and Schweger, C. E. (2002). New species of Pinus subgenus Pinus subsection Contortae from Pliocene. Int. J. Plant Sci. 163, 687–697. doi: 10.1086/340425
Miki, S. (1957). Pinaceae of Japan, with special reference to its remains. J. Inst. Polytech. Osaka City Univ. Ser. D. 8, 221–272.
Miller, C. N. (1969). Pinus avonensis, a new species of petrified cones from the Oligocene of Western Montana. Am. J Bot. 56, 972–978. doi: 10.1002/j.1537-2197.1969.tb09748.x
Miller, C. N. (1973). Silicified cones and vegetative remains of Pinus from the Eocene of British Columbia. Contrib. Mus. Paleontol. Univ. Michigan. 24, 101–118.
Miller, C. N. (1974). Pinus wolfei, a new petrified cone from the Eocene of Washington. Am. J. Bot. 61, 772–777. doi: 10.1002/j.1537-2197.1974.tb12300.x
Miller, C. N. (1976). Early evolution in the Pinaceae. Rev. Palaeobot. Palynol. 21, 101–117. doi: 10.1016/0034-6667(76)90024-5
Miller, C. N. (1978). Pinus burtii, a new species of petrified cones from the Miocene of Martha's Vineyard. Bull. Torrey Bot. Club. 105, 93–97. doi: 10.2307/2484425
Miller, C. N., and Malinky, J. M. (1986). Seed cones of Pinus from the Late Cretaceous of New Jersey, U.S.A. Rev. Palaeobot. Palynol. 46, 257–272. doi: 10.1016/0034-6667(86)90018-7
Miyoshi, N., Fujiki, T., and Kimura, H. (2011). Pollen Flora of Japan. Sapporo: Hokkaido Univ. Press.
Nanjing Institute of Geology and Mineral Resources (NIGMR). (1982). Paleontological Atlas of East China, Part 3, Volume of Mesozoic and Cenozoic. Beijing: Geological Publishing House.
Paul, N. C., and Yu, S. H. (2008). Two species of endophytic Cladosporium in pine trees in Korea. Mycobiol. 36, 211–216. doi: 10.4489/MYCO.2008.36.4.211
Peel, M. C., Finlayson, B. L., and McMahon, T. A. (2007). Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644. doi: 10.5194/hess-11-1633-2007
Rudolphi, F. K. L. (1830). Systema orbis vegetabilium: quod gratiosi medicorum ordinis consensu et auctoritate. Typis F. Guil. Kunike.
Ryberg, P. E., Rothwell, G. W., Stockey, R. A., Hilton, J., Mapes, G., and Riding, J. B. (2012). Reconsidering relationships among stem and crown group Pinaceae: oldest record of the genus Pinus from the Early Cretaceous of Yorkshire, United Kingdom. Int. J. Plant Sci. 173, 917–932. doi: 10.1086/667228
Saiki, K. (1996). Pinus mutoi (Pinaceae), a new species of permineralized seed cone from the Upper Cretaceous of Hokkaido, Japan. Am. J. Bot. 83, 1630–1636. doi: 10.1002/j.1537-2197.1996.tb12821.x
Song, U., Park, J., and Song, M. (2012). Pollen morphology of Pinus (Pinaceae) in northeast China. For. Sci. Techol. 8, 179–186. doi: 10.1080/21580103.2012.704973
Stockey, R. A. (1983). Pinus driftwoodensis sp. n. from the Early Tertiary of British Columbia. Bot. Gaz. 144, 148–156. doi: 10.1086/337355
Stockey, R. A. (1984). Middle Eocene Pinus remains from British Columbia. Bot. Gaz. 145, 262–274. doi: 10.1086/337455
Tao, J. R., and Kong, Z. C. (1973). The fossil flora and spore–pollen assemblage of Sanying coal series of Eryuan, Yunnan. Acta Bot. Sin. 15, 120–126.
Tao, J. R., and Wang, Q. Z. (1983). Fossil Pinus in Laiyuan Xian, Hebei Province. Acta Phytotax. Sin. 21, 108–109.
Underwood, C., and Miller, C. N. (1980). Pinus buchananii, a new species based on a petrified cone from the Oligocene of Washington. Am. J. Bot. 67, 1132–1135. doi: 10.1002/j.1537-2197.1980.tb07745.x
Wang, X. R., and Szmidt, A. E. (1993). Chloroplast DNA-based phylogeny of Asian Pinus species (Pinaceae). Plant Syst. Evol. 188, 197–211. doi: 10.1007/BF00937728
Wang, X. R., Tsumura, Y., Yoshimaru, H., Nagasaka, K., and Szmidt, A. E. (1999). Phylogenetic relationships of Eurasian pines (Pinus, Pinaceae) based on chloroplast rbcL, matK, rpl20-rpl18 spacer, and trnV intron sequences. Am. J. Bot. 86, 1742–1753. doi: 10.2307/2656672
Xing, Y. W., Liu, Y. S., Su, T., Jacques, F. M., and Zhou, Z. K. (2010a). Pinus prekesiya sp. nov. from the upper Miocene of Yunnan, southwestern China and its biogeographical implications. Rev. Palaeobot. Palynol. 160, 1–9. doi: 10.1016/j.revpalbo.2009.12.008
Xing, Y. W., Liu, Y. S., Su, T., and Zhou, Z. K. (2010b). Application of the X-ray CT scanning technique on a late Miocene pine cone from Yunnan, China. Acta Palaeontol. Sin. 49, 133–137.
Xu, Q.-Q., Zhou, W.-J., Kodrul, T. M., Naugolnykh, S. V., and Jin, J.-H. (2015). Late Eocene white pines (Pinus subgenus Strobus) from southern China. Sci. Rep. 5, 16390. doi: 10.1038/srep16390
Xu, X.-H., Wang, Z.-X., Yang, G.-L., Wang, J., Yang, Y., Ma, F.-J., et al. (2015). Two Pinus species from the upper Miocene in Zhejiang, China and their palaeobiogeographic significance. Rev. Palaeobot. Palynol. 215, 68–75. doi: 10.1016/j.revpalbo.2015.01.003
Yabe, A., Jeong, E., Kim, K., and Uemura, K. (2019). Oligocene–Neogene fossil history of Asian endemic conifer genera in Japan and Korea. J. Syst. Evol. 57, 114–128. doi: 10.1111/jse.12445
Yamada, M., and Yamada, T. (2017). Ovuliferous scale anatomy of Pinus species and its value for classification above the species level. Bot. J. Linn. Soc. 183, 633–643. doi: 10.1093/botlinnean/box006
Yamada, T., Yamada, M., and Tsukagoshi, M. (2014). Fossil records of subsection Pinus (genus Pinus, Pinaceae) from the Cenozoic in Japan. J. Plant Res. doi: 10.1007/s10265-013-0621-z
Yamada, T., Yamada, M., and Tsukagoshi, M. (2015). Taxonomic revision of Pinus fujiii (Yasui) Miki (Pinaceae) and its implications for the phytogeography of the section Trifoliae in East Asia. PLoS ONE. 10:e0143512. doi: 10.1371/journal.pone.0143512
Yasui, K. (1928). Studies on the structure of lignite, brown coal, and bituminous coal in Japan. J. Fac. Sci. Imp. Univ. Tokyo. Sect 3 Bot. 3, 381–468.
Zalar, P. D., De Hoog, G., Schroers, H. J., Crous, P. W., Groenewald, J. Z., and Gunde-Cimerman, N. (2007). Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Stud. Mycol. 58, 157–183. doi: 10.3114/sim.2007.58.06
Zhang, J.-W., D'Rozario, A., Adams, J. M., Liang, X.-Q., Jacques, F. M. B., Su, T., et al. (2015). The occurrence of Pinus massoniana Lambert (Pinaceae) from the upper Miocene of Yunnan, SW China and its implications for paleogeography and paleoclimate. Rev. Palaeobot. Palynol. 215, 57–67. doi: 10.1016/j.revpalbo.2014.11.006
Keywords: associated pollen, anatomy, Maoming Basin, micro-computed tomography, morphology, Pinaceae, Pleistocene, seed cone
Citation: Bazhenova NV, Wu X-K, Kodrul TM, Maslova NP, Tekleva MV, Xu S-L and Jin J-H (2022) Mummified Seed Cones of Pinus prehwangshanensis sp. nov. (Subgenus Pinus, Pinaceae) From the Upper Pleistocene of Guangdong, South China: Taxonomical Significance and Implication for Phytogeography and Ecology. Front. Ecol. Evol. 10:900687. doi: 10.3389/fevo.2022.900687
Received: 21 March 2022; Accepted: 26 April 2022;
Published: 17 June 2022.
Edited by:
Hang Sun, Kunming Institute of Botany (CAS), ChinaReviewed by:
Lutz Kunzmann, Museum of Mineralogy and Geology, GermanyCopyright © 2022 Bazhenova, Wu, Kodrul, Maslova, Tekleva, Xu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Hua Jin, bHNzampoQG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.