
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 08 July 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.897132
This article is part of the Research TopicProximate and Ultimate Approaches to Behavior in a Changing WorldView all 8 articles
Large-carnivore populations have experienced significant declines in the past centuries in extended parts of the world. Habitat loss, fragmentation, and depletion of natural resources are some of the main causes of this decline. Consequently, behavioral flexibility, enabling the exploitation of anthropogenic food resources in highly disturbed human-dominated landscapes, is becoming critical for the survival of large carnivores. These behavioral changes increase the potential for human-large carnivore conflict and can further intensify carnivore persecution. Here, we examine how land cover types (representing a gradient of anthropogenic disturbance) alter the behavior of striped hyenas (Hyaena hyaena) in a human-dominated landscape in Israel, and whether differences in life history between males and females affect their reaction to such disturbances and consequently their level of exposure to humans. We used a Hidden Markov Model on GPS-tracking data for seven striped hyenas to segment individual-night trajectories into behavioral states (resting, searching, and traveling). We then used multinomial logistic regression to model hyenas’ behavioral state as a function of the interaction between land cover and sex. Females traveled less than males both in terms of average distance traveled per hour, per night, and nightly net displacement. Most steps were classified as “searching” for females and as “traveling” for males. Female hyenas spent a higher proportion of time in human-dominated areas and a lower proportion in natural areas compared to males, and they were also more likely to be found close to settlements than males. Females changed their time allocation between natural and human-dominated areas, spending more time resting than traveling in natural areas but not in human-dominated ones; males spent more time searching than resting in human-dominated areas but were equally likely to rest or search in natural ones. The differences in life history between male and female hyenas may reflect different motivations for space use as a means to optimize fitness, which affects their exposure to humans and therefore their potential involvement in human-hyenas conflict. Understanding the mechanisms that lead to behavioral change in response to human disturbance is important for adaptive management and promoting human large-carnivores co-existence in general.
Large-carnivore populations have experienced significant declines in the past two centuries in extended parts of the world (Ripple et al., 2014). Habitat loss, fragmentation, and depletion of natural resources are some of the main causes of these declines (Di Minin et al., 2016). The fact that carnivores usually occupy large ranges is forcing many of them to inhabit multi-use landscapes outside protected areas. Therefore, the ability to use human-dominated landscapes is becoming critical for the survival of large carnivores in the Anthropocene (Carter and Linnell, 2016; Gantchoff et al., 2020).
According to the movement ecology framework, animal movement is shaped both by factors relating to the individual – i.e., internal state (why to move), motion capacity (how to move), and navigation capacity (where to move), and by external biotic and abiotic factors that interact with the individual (Nathan et al., 2008). The physiological and psychological drivers of movement (internal state) comprise a balance between the motivation to find resources like food, shelter, or mates on the one hand, and the need to avoid predation or competition on the other (Doherty and Driscoll, 2018). Habitat modifications by humans cause changes to resource availability, which may alter the trade-off between acquiring resources and avoiding conflict, which in turn, can be reflected in an animal’s movement behavior (Allen and Singh, 2016; Doherty and Driscoll, 2018). Examples include reduced search and commute time, allowing animals to travel less to find food (Fleming and Bateman, 2018; Tucker et al., 2018) and acquire energy faster (Beckmann and Berger, 2003), or increased foraging within residential development areas when natural food is scant (Johnson et al., 2020).
In addition to habitat loss and resource depletion, human persecution is often a primary cause of large-carnivore mortality (Bunnefeld et al., 2006; Ripple et al., 2014). Large-carnivore persecution may be the result of damage to humans, livestock, or property, or just due to the belief that carnivores can cause such damage (Støen et al., 2015; Bleyhl et al., 2021) even if it doesn’t match any actual degree of risk (Dickman, 2010; Ordiz et al., 2019). A carnivore’s attraction to predictable anthropogenic food resources, such as anthropogenic waste, domestic animals, roadkill, and deliberate feeding in human-dominated landscapes (Bateman and Fleming, 2012; Dubois and Fraser, 2013; Fleming and Bateman, 2018) may result in a loss of fear, increased tolerance to human presence, and even aggressive behaviors (Orams, 2002; Beckmann and Berger, 2003; Dubois and Fraser, 2013; Elfström et al., 2014). All of these behavioral changes may increase the perceived conflict between carnivores and humans and can further intensify the persecution of carnivores (Støen et al., 2015; Bleyhl et al., 2021).
In many species, motivation for space use may differ between the sexes, since a male’s reproductive success is generally limited by access to female mates, whereas females optimize their fitness by improving their access to food (Clutton-Brock and Harvey, 1978; Klug, 2011). The resulting differences in male and female behavior are likely to affect the trade-off between foraging and the risks associated with human-dominated landscapes (Bunnefeld et al., 2006). Consequently, while in some large carnivores, like felids, males are more likely to be involved in human-wildlife conflict (Loveridge et al., 2010), the high energetic demands of females during gestation and while lactating may attract them to human-dominated areas where food availability is higher (Bunnefeld et al., 2006; Wilmers et al., 2013). Such differences in male and female behavior can also lead to seasonal variation in conflict according to sex (Teichman et al., 2013).
Some species, populations, or specific individuals may develop behaviorally flexible strategies to avoid spatial or temporal overlap with humans, and still efficiently use anthropogenic resources at the same time, by altering their movement behavior or habitat use (Bunnefeld et al., 2006; Schuette et al., 2013; Wilmers et al., 2013; Ordiz et al., 2017; Doherty et al., 2021). These may include increased speed (Habib et al., 2021), temporal partitioning such as reduced daytime activity (Tigas et al., 2002), shift to nocturnality (Wang et al., 2015; Wheat and Wilmers, 2016; Gaynor et al., 2018), avoidance of periods when humans are most active (Valeix et al., 2012; Oriol-Cotterill et al., 2015a,b), increased vigilance when close to humans (Pangle and Holekamp, 2010), and use of protective cover when near humans (Boydston et al., 2003; Suraci et al., 2019). Behavioral flexibility of large carnivores in close association with humans may moderate the impact of anthropogenic changes to the environment (Wong and Candolin, 2015) and serve as the key to human-carnivore coexistence. This knowledge may also be used by the public and by conservation practitioners to mitigate conflict, as well as for better management practices (Ordiz et al., 2019). Thus, understanding how carnivores move in and use anthropogenic landscapes can provide crucial information for the management of human-carnivore interactions.
The striped hyena (Hyaena hyaena) is a large carnivore whose global conservation status is Near Threatened with a decreasing global population trend (Abi-Said and Dloniak, 2015). In the Mediterranean region, its IUCN conservation status is Vulnerable due to human-caused mortality (Jdeidi et al., 2010). While the striped hyena has the widest distribution of the four species in the family Hyaenidae, it is the least studied among them (Watts and Holekamp, 2007). As opportunistic omnivores, striped hyenas often exploit anthropogenic food sources and are regarded as a commensal species to humans (Yom-Tov, 2003). Their diet consists of mostly carrion, from both natural and anthropogenic sources (e.g., cattle, hens, turkeys, cats, and dogs), but also live vertebrate and invertebrates, garbage, and plant material (grass, fruit, pods, leaves, seeds, and grains) (Hofer, 1998; Yom-Tov, 2003; Alam and Khan, 2015; Bhandari et al., 2020; Pérez-Claros and Coca-Ortega, 2020). A flexible diet, along with the ability to live in diverse habitats and adjust to habitat modifications, makes the species highly suitable for the anthropogenic environment. Indeed, as with other large carnivores (e.g., spotted hyenas Crocuta crocuta, black bears Ursus americanus, pumas Puma concolor, and gray wolves Canis lupus; Bateman and Fleming, 2012; bobcat Lynx rufus and coyote Canis latrans; Šálek et al., 2015), striped hyenas are often observed near or within human settlements, including urban areas, in search of food (Yom-Tov, 2003; Abi-Said and Abi-Said, 2007; Monchot and Mashkour, 2010; Singh et al., 2010; Akay et al., 2011; Alam et al., 2014; Bhandari et al., 2021). Striped hyenas are also heavily persecuted throughout their geographic range, due to superstitious fears, prejudice, the attribute of magical properties, lack of knowledge, and misinterpretation of their behavior (Boneh, 1987; Frembgen, 1998; Hofer, 1998; Qarqaz et al., 2004; Tourani et al., 2012). In some countries, deliberate or unintentional persecution is one of the dominant reasons for striped hyena population decline (Hofer, 1998; Tourani et al., 2012). This evokes a constant trade-off between striped hyenas’ need to avoid humans, and their motivation to use easily accessible anthropogenic food resources (Schoener, 1971).
In most large carnivores, including hyaenid species, males disperse more often and to longer distances compared to females (Greenwood, 1980; Trochet et al., 2016; Bartoń et al., 2019; Holekamp and Sawdy, 2019; but see Wagner et al., 2008). This sex-biased dispersal often results in female kin occurring in closer proximity to one another than to their male counterparts (Greenwood, 1980; Smale et al., 1997). Females striped hyenas appear to be the main ones responsible for the care of the young (Kruuk, 1976; Bouskila, 1984; Nissim, 1986; Mendelssohn and Yom-Tov, 1999; Watts and Holekamp, 2007). Adult males have been spotted nearby dens (Nissim, 1986; Reichmann, 2005; Califf et al., 2020; Personal observation), sometimes interacting with the cubs, but their involvement is believed to be mainly derived from territorial defense (Davidar, 1990; Wagner, 2006; Watts and Holekamp, 2007; Wagner et al., 2008), though food provisioning by the father has also been recorded (Califf et al., 2020).
In Israel, striped hyenas can be found across the country (Israel Nature and Parks Authority, 2020), and their interactions with humans are becoming more frequent. The country’s small area, closed borders, and high human population growth rate lead to an increase in natural habitat destruction and modification in favor of development (Lotan et al., 2019). The area that holds the greatest potential for conflict between striped hyenas and humans in Israel today is located within the central district of the country. This is because the area is characterized by a low number of nature reserves, national parks, and agriculture on one hand, and high population density, built-up areas, and fragmentation of natural landscapes on the other.
Here, we used tracking data of four female and three male striped hyenas from the central district of Israel to examine how (1) land cover types (representing a gradient of anthropogenic disturbance) alter the behavior of striped hyenas and (2) whether differences in life-history between male and female hyenas affect the way they react to human disturbances and consequently their level of exposure to humans.
The study was carried out in the center of Israel (31°53′55.5″N 35°00′39.3″E), between the Shfela lowlands (which border to the west with the dense and developed coastal strip of Israel), the Judaean mountains (northwest to Jerusalem) and Judea and Samaria to the east (Figure 1). The natural vegetation consists primarily of Mediterranean forests, woodlands, and scrub. Other carnivores that can be found in the area include the golden jackal (Canis aureus), the red fox (Vulpes vulpes), as well as feral dogs (Canis lupus familiaris). Both jackals and feral dogs were spotted chasing hyenas away, but only dogs were seen involved in fatal attacks on hyenas. The landscape is highly fragmented and composed of a complex mosaic of cities, industrial areas, roads and railways, rural settlements, natural areas, managed planted forests, and agricultural areas (including open areas such as fields and orchards, and closed areas such as greenhouses and livestock sheds). In addition, the security fence between Israel and the Palestinian Authority, which was built over what was once open and continuous landscapes, bisects the eastern part of our study area and generates an impassable barrier for terrestrial wildlife east-west movements. The fence also has an impact on wildlife north-south movements inside Israel as it limits the open landscape available, which in turn, creates bottlenecks for wildlife crossings (Rotem, 2014). The human population density in the area ranges from 904 to 3189 habitants/km2 (data refer to 2019, the current density is expected to be higher; Central Bureau of Statistics, 2020). Even though the study area resides mainly within the boundaries of Israel’s national ecological corridor (including core areas), the corridor status is non-binding, nor are there any binding guidelines for the management of these areas. As an outcome, many of the natural areas within our study area are subjected to intense anthropogenic development and consequently to severe habitats degradation (Gabai and Zanzuri, 2019).
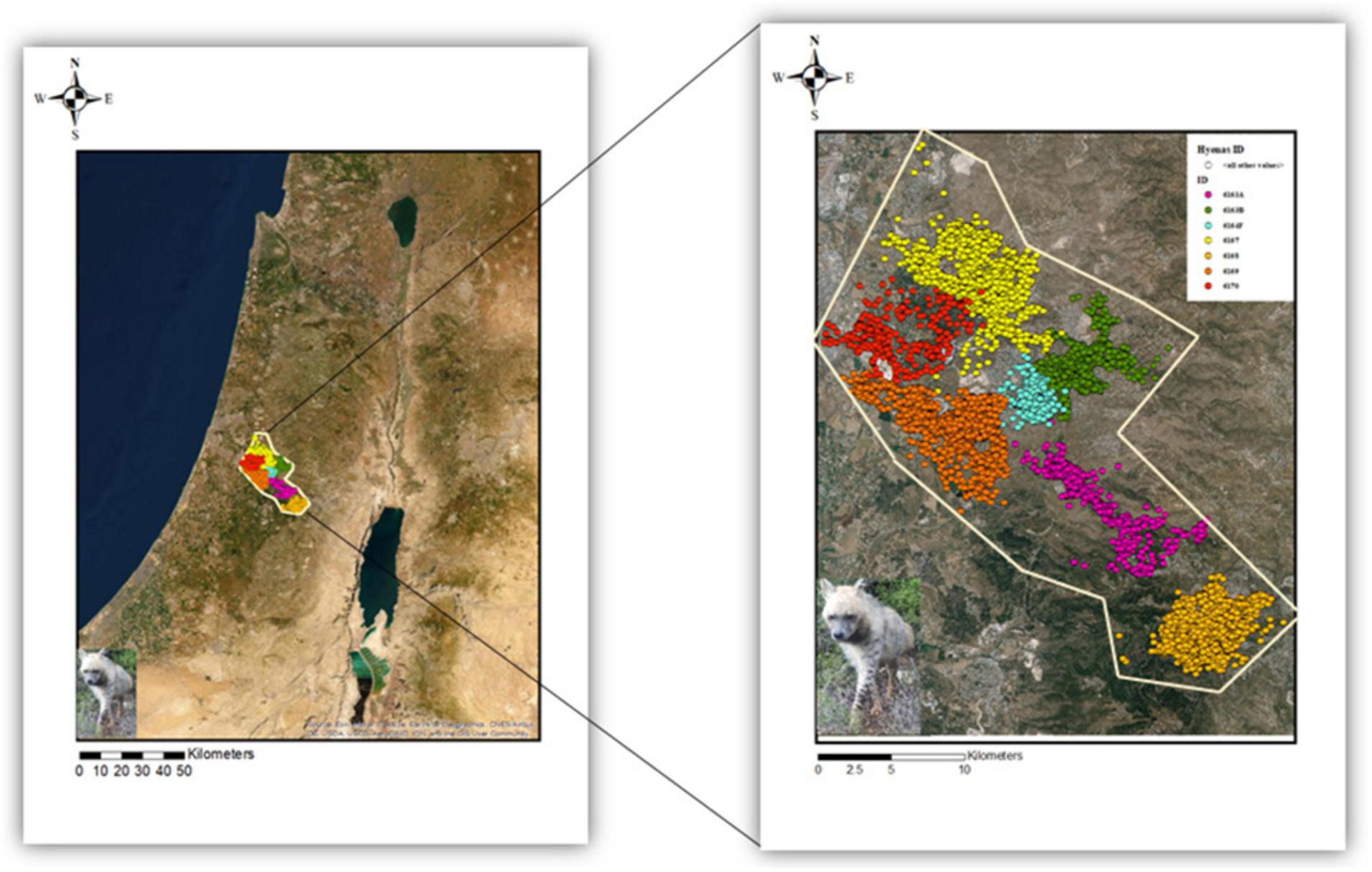
Figure 1. Study area in the central district of Israel. GPS locations for each of the seven striped hyenas are marked in distinct colors. 6163A, 6163B, 6164F, and 6170 are females, while 6167, 6168, and 6169 represent males’ ID.
From January 2018 to April 2019, we captured seven free-ranging striped hyenas (Figure 2), four females (two subadults and two adults), and three males (one subadult and two adults). We placed all traps within a 15 km radius of the city of Modiin. We used Victor #3 softcatch leg-hold traps (Woodstream Co., Lititz, PA, United States), which were monitored by a 128X wireless trail camera (ATC Technologies, Haifa, Israel). The capturing and handling of the hyenas were done by experienced personnel from the Israel nature and parks authorities (INPA). Captured hyenas were anesthetized using an intramuscular injection of Domitor-Ketamine Hydrochloride-Midazolam mix at a dose of 0.046, 2, and 5 mg/kg, respectively, body weight. Hyenas were then fitted with a Tellus 2 small GPS Iridium collar equipped with a drop-off (built-in release) mechanism (650 g; Followit, Lindesberg, Sweden), and an ear tag (Ritchey Livestock, Brighton, CO, United States).

Figure 2. An adult striped hyena female 6163A caught in a camera trap after being fitted with a GPS collar and an ear tag.
Striped hyenas are mainly nocturnal, therefore, we used nightly GPS data (between 6 PM and 8 AM) at 1-h resolution to study the movement behavior of tracked hyenas. We standardized tracks by rounding timestamps to the nearest minute by adding or subtracting up to 3 s. We split data into individual-nights and discarded any individual-night with <7 locations out of the expected 15. For each timestamp, we calculated time from solar midnight as the decimal number of hours from the midpoint between sunset and sunrise at the corresponding geographical location on that date. Thus, timestamps between 6 PM and the solar midnight were assigned a negative value, those between the solar midnight and 8 AM were assigned a positive value. We intersected each location with spatial data including land cover (GIS layer provided by the Survey of Israel, 2020©), settlements (Survey of Israel, 2020©), farming facilities (Ministry of agriculture, 2021), JNF (Jewish National Fund) land and campsites (JNF, 2021), and roads (Survey of Israel, 2020©). We synthesized data into a simplified land cover variable that included four categories: anthropogenic, roads, agricultural, and natural. The “anthropogenic” land cover included locations within settlement boundaries, locations in “Artificial,” “Built-up,” and “Lawn or garden” classes, or locations within 50 m of a farming facility or a JNF campsite. The “roads” land cover included locations within 50 m of any paved road. The “agriculture” land cover included locations in “Vineyards,” “Fruit trees,” “Olive trees,” “Palm trees,” “Cultivated,” or “Uncultivated” classes. The “natural” land cover included locations within the “Natural vegetation” or “Natural non-vegetated” land cover types. For each location, we calculated the distance to the edge of the nearest settlement using the function “distance” in the R (R Core Team, 2021) package “raster” (Hijmans et al., 2015) and assigned a value of 0 for locations within a settlement boundary.
We used a Hidden Markov Model (HMM; Patterson et al., 2008; Langrock et al., 2012) to segment individual-night trajectories into behavioral states based on the time series of step lengths (the linear distance between two consecutive GPS points) and turning angles (the angle between two consecutive steps). We used a Gamma distribution to model step lengths and a von Mises distribution to model turning angles (Langrock et al., 2012). We defined initial parameter values for three behavioral states:
• State 1 (resting): gamma with mean = 50 m (corresponding to very little displacement), standard deviation = 50 m, and zero mass parameter = 0.5 (corresponding to high probability of no movement); von Mises with mean = π rad (corresponding to turning back) and concentration = 0.1 (corresponding to nearly uniform turn-angle distribution).
• State 2 (searching): gamma with mean = 1,000 m (corresponding to medium displacement), standard deviation = 2,500 m, and zero mass parameter = 0.001 (corresponding to very low probability of no movement); von Mises with mean = π rad (corresponding to turning back) and concentration = 0.5 (corresponding to a moderately mean-centered distribution of turn angles).
• State 3 (traveling): gamma with mean = 3,000 m (corresponding to high displacement) and standard deviation = 5,000 m and zero mass parameter = 0.001 (corresponding to very low probability of no movement); von Mises with mean = 0 rad (corresponding to not turning – positive directional persistence) and concentration = 0.99 (corresponding to a highly mean-centered distribution of turn angles).
We modeled the transition probabilities between states as a function of time from midnight. We used the Viterbi algorithm to assign each step to the most likely behavioral state based on the HMM output (Zucchini et al., 2017). We conducted this analysis in R (R Core Team, 2021) using functions from the package “momentuHMM” (McClintock and Michelot, 2018).
We used multinomial logistic regression to model the hyenas’ behavioral state (resting, searching, or traveling) as a function of the interaction between land cover and sex, using the individual ID as a random effect. We used 1,000 iterations of empirical bootstrapping (sampling with replacement from the full dataset, sample size = 16,016 data points) to calculate 95% confidence intervals around mean estimate values. We conducted this analysis in R using package “mclogit” (Elff, 2021).
The final GPS dataset included tracks for seven hyenas (four females and three males), for a total of 16,019 locations and 1,187 individual-nights, covering an area measuring approximately 815 km2. The HMM estimated parameters for the three behavioral states as follow:
• State 1 (resting): gamma with mean = 14.32 m, standard deviation = 11.52 m, and zero mass parameter = 0.00; von Mises with mean = −3.06 rad and concentration = 0.35 (Figure 3).
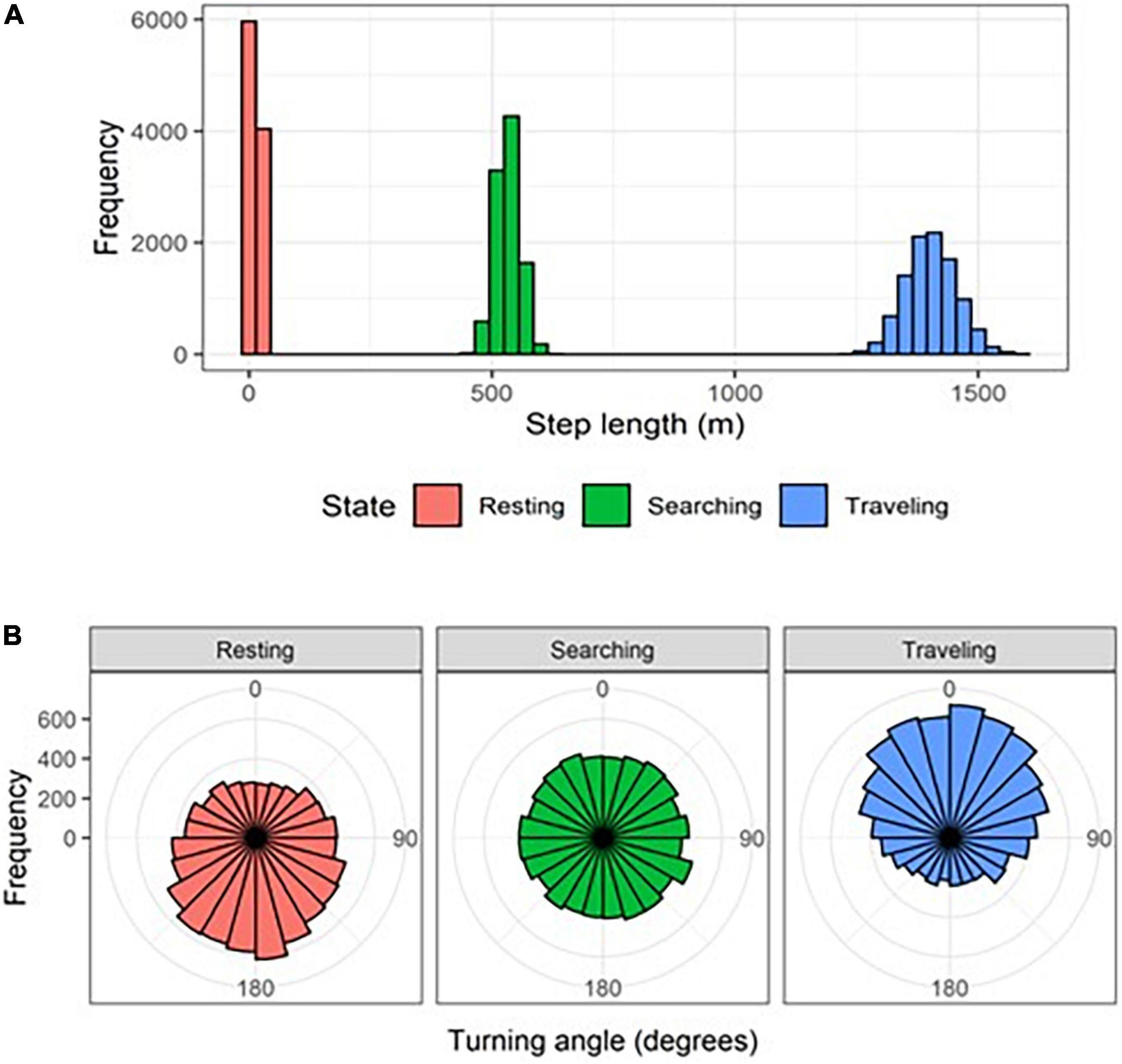
Figure 3. Distributions of (A) step lengths and (B) turning angles for striped hyenas in the Central District of Israel in three behavioral states identified using a Hidden-Markov Model.
• State 2 (searching): gamma with mean = 532.52 m, standard deviation = 458.75 m, and zero mass parameter = 0.00; von Mises with mean = 1.39 rad and concentration = 0.03 (Figure 3).
• State 3 (traveling): gamma with mean = 1403.30 m, standard deviation = 704.60 m, and zero mass parameter = 0.00; von Mises with mean = 0.06 rad and concentration = 0.53 (Figure 3).
Hyenas were more likely to be resting than searching at the beginning of the night, and least likely to be traveling (see Supplementary Figure 1). Transition probabilities from resting to searching or traveling and from searching to traveling were negatively correlated with time from solar midnight, whereas those from searching to resting and from traveling to resting or searching were positively correlated with time from solar midnight (see Supplementary Table 1 and Supplementary Figure 2).
Across all-land cover types, female hyenas were more likely to be searching than resting or traveling, whereas male hyenas were more likely to be traveling than resting or searching (Figure 4). In addition to this general pattern, females were more likely to travel than rest in anthropogenic areas, agricultural areas, and in proximity to roads, and more likely to rest than travel in natural areas (Figure 4). Males were more likely to search than rest in anthropogenic areas, agricultural areas, and in proximity to roads, and equally likely to search or rest in natural areas (Figure 4).
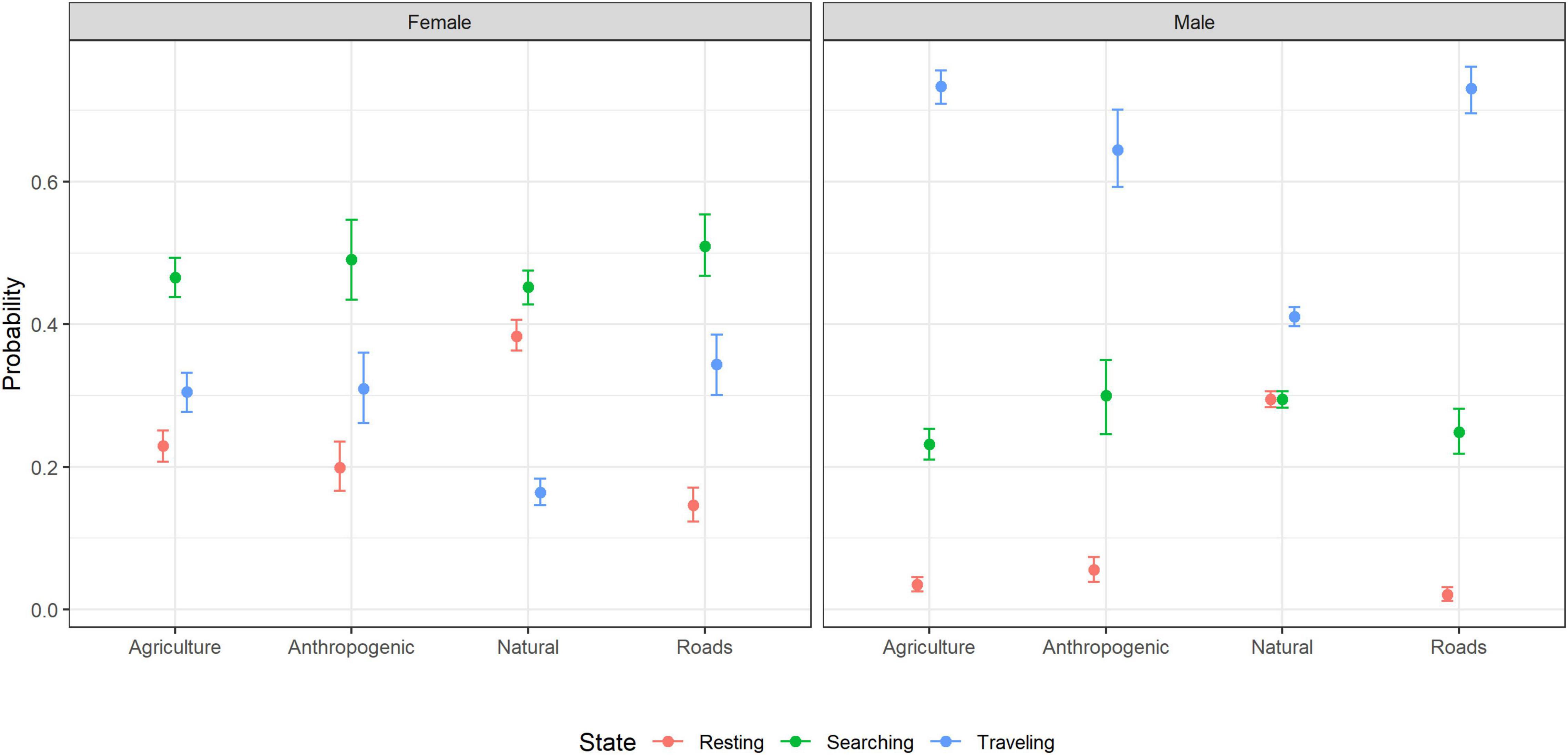
Figure 4. The probability of male and female striped hyenas being in each behavioral state as a function of land cover in the Central District of Israel estimated using multinomial logistic regression. Points indicate the MLE and error bars indicate 95% confidence intervals.
Female hyenas had a higher proportion of steps than males in agricultural (26 and 14%, respectively) and anthropogenic areas (24 and 10%, respectively), and a lower proportion of steps in natural areas compared to males (41 and 67%, respectively; Pearson’s Chi-squared test 545.94, d.f. = 3, p < 0.001). The proportion of steps in the proximity of roads was 9% for both sexes (Figure 5). In addition, females’ hyenas were more likely to be found close to settlements compared to males (average distance of 350 and 641 m, respectively).
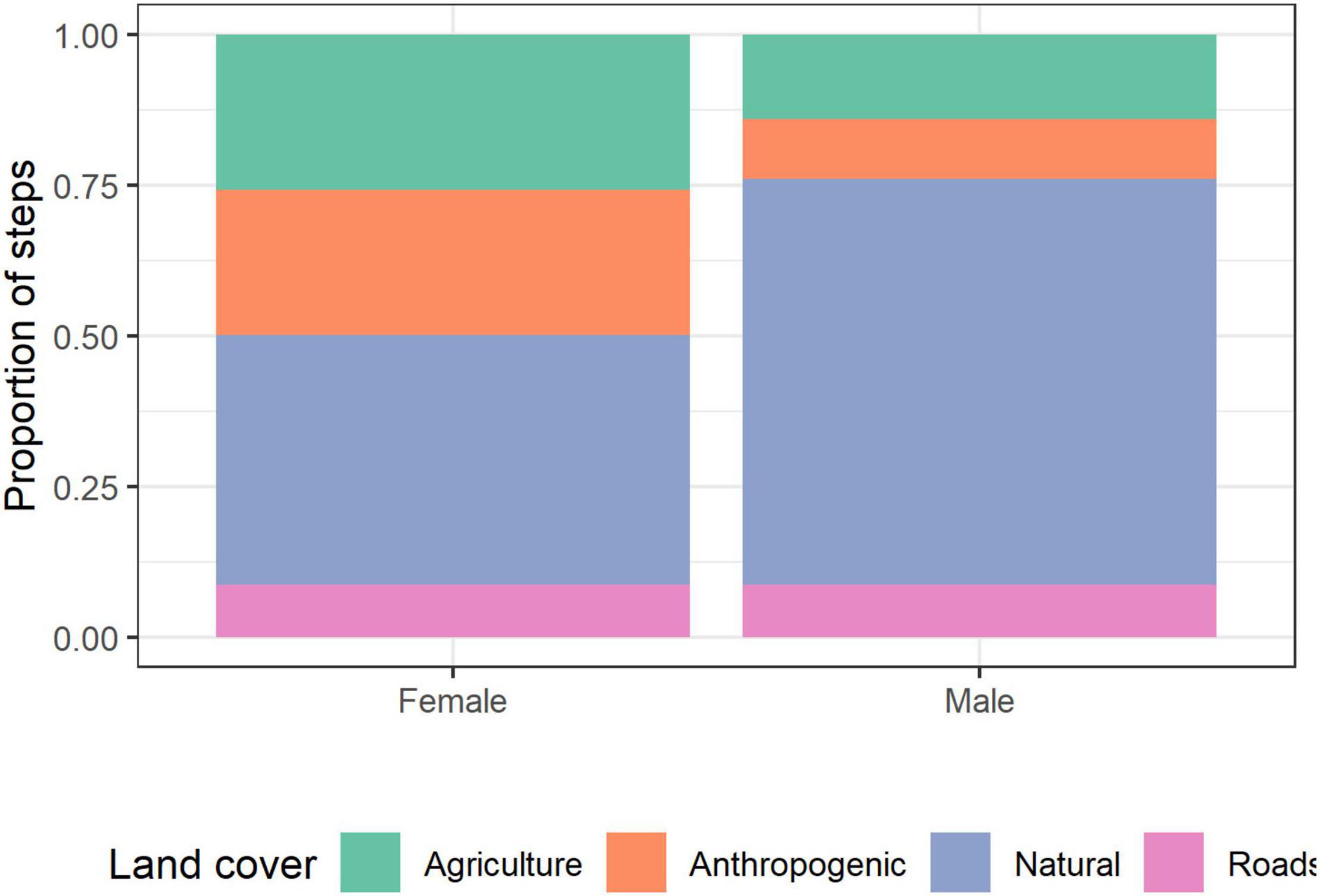
Figure 5. The proportion of GPS locations in each land cover type for male and female striped hyenas in the Central District of Israel.
Females traveled less than males in terms of average distance traveled per hour (592 and 951 m, respectively; Welch two-sample T-test = −30.746, p < 0.001), average distance traveled per night (6,954 and 11,837 m, respectively; Welch two-sample T-test = −21.961, p < 0.001), and nightly net displacement (2,779 and 4,071 m, respectively; Welch two-sample T-test = −27.003, p < 0.001; Figure 6).
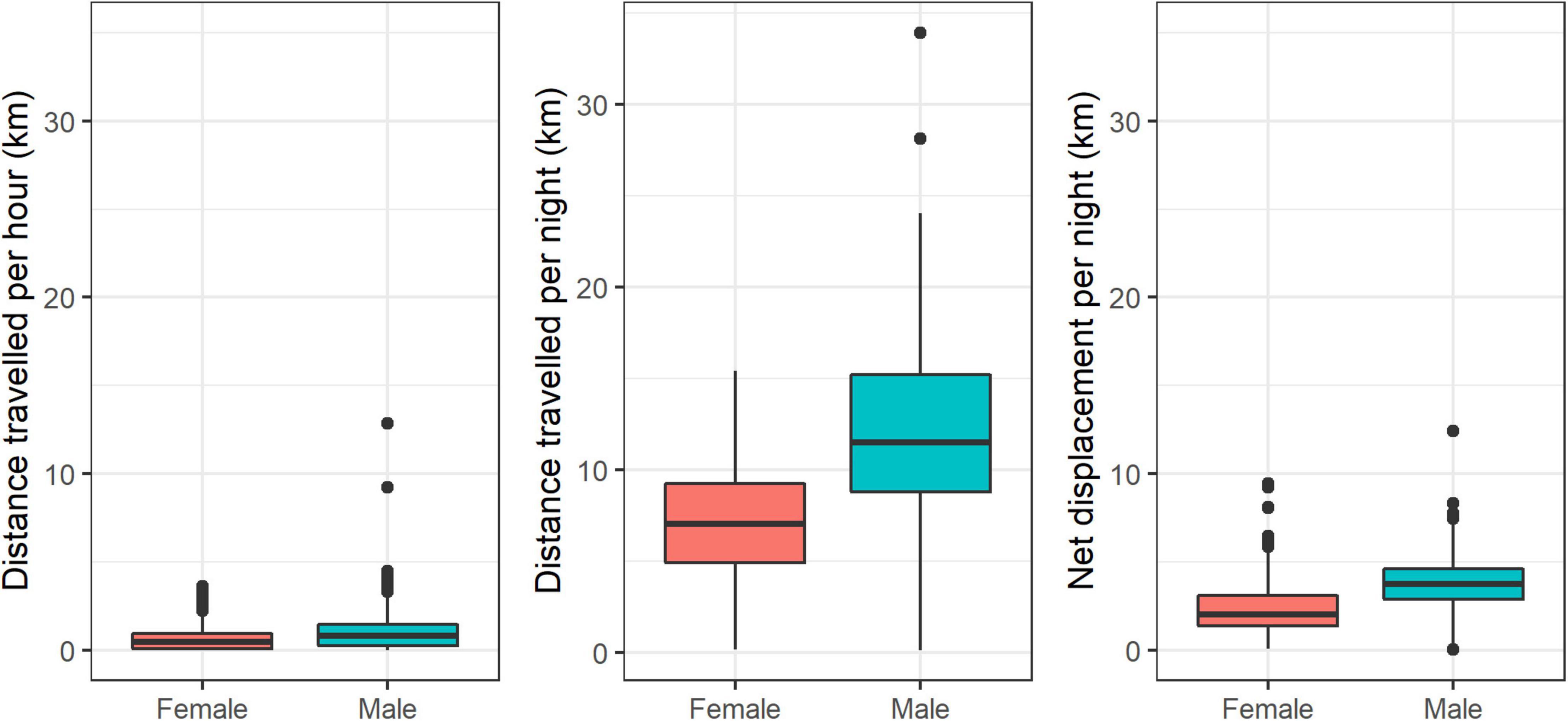
Figure 6. Distributions of movement metrics for male and female striped hyenas in the Central District of Israel. Left panel: distance traveled per hour; center panel: distance traveled per night; right panel: nightly net displacement.
Humans are dominating substantial parts of our planet, and protected areas are often too small to sustain viable populations of large carnivores. This makes human-large carnivore interactions inevitable. Here, we show that male and female hyenas show different behaviors in anthropogenic and agricultural areas compared to natural ones, indicating that females are more prone to conflict at the human-wildlife interface than males. The percentage of steps in human-dominated areas out of the total number of steps was almost twice as large in females (59%) than in males (33%). Moreover, even at times when males were found in anthropogenic areas, they were much more likely to just rapidly pass through the area, rather than spend enough time to be able to utilize available food resources within such areas. Direct and rapid movements through a particular area may reflect a low motivation to stay in a habitat perceived as suboptimal (Doherty and Driscoll, 2018).
Hyenas did not use anthropogenic and natural landscapes in the same way. Females changed their time allocation between natural and human-dominated areas, spending more time resting than traveling in natural areas, but not in human-dominated ones, while males spent more time searching than resting in human-dominated areas, and were equally likely to rest or search in natural ones. This implies that striped hyenas use natural areas for a variety of behaviors – resting, foraging, and traveling – while human-dominated areas are predominantly used for searching (by females) or traveling (by males). Altogether, it seems that striped hyenas of both sexes differentiate between human-dominated and natural areas and use the latter as a refuge (Singh et al., 2010). By using natural areas, even when they are not protected, limited in size, and adjacent to settlements, as stepping-stones, striped hyenas can survive even in a highly fragmented landscape, which stresses the importance of remnant natural fragments (Singh et al., 2010, 2014; Gantchoff et al., 2020; Bhandari et al., 2021). The fact that males can be found further away from settlements than females, and are less likely to stay and feed in human-dominated areas, emphasizes the need to identify important areas to preserve for each sex specifically (Zeller et al., 2018) while considering their different motivational states (Maiorano et al., 2017; Picardi et al., 2021). In this case, it seems that preserving the open areas adjacent to settlements will be more beneficial for females, while preserving extended areas further away from settlements will be beneficial for males. Agricultural lands were also extensively used by hyenas of both sexes. Other than in Israel (Kruuk, 1976; Nissim D, personal communication, January 21, 2022), the use of agricultural areas by striped hyenas was documented in India (Karanth and Chellam, 2009; Athreya et al., 2013), Turkey (Akay et al., 2011), and Iran (Djamali et al., 2020). This is another indication of the importance of keeping connectivity outside of protected areas, and the significance of integrating agricultural areas within ecological corridors (Gastón et al., 2016; Gantchoff et al., 2020).
Female reproductive state can also affect foraging behavior, leading females to take more risks while feeding in human-dominated areas (Bunnefeld et al., 2006; Wilmers et al., 2013). For example, female pumas have a higher chance of getting into conflict while they are accompanied by dependent kittens and their energetic needs are at their peak, leading them to forage in risky areas during winter (Teichman et al., 2013). Nevertheless, in brown and black bears (Ursus arctos, Ursus thibetanus), the presence of females with cubs and subadults near settlement can also be explained by reduced intraspecies predation risk, using the human-dominated environment as a shield against adult males, rather than due to food availability and quality (Elfström et al., 2014).
Our findings indicate that male and female striped hyenas show two different movement tactics. Males tend to travel longer distances and move faster, minimizing the risk of encounters with humans by traveling quickly through human-dominated areas and spending most of their time in natural areas. Females, on the other hand, are at greater risk of encountering people because they spend a greater proportion of time in human-dominated areas and move slower (presumably, to forage) while they are there. Although the movement strategy of males seems to be more energetically costly, males of this species are less involved in providing for their young relative to females, and therefore they need to invest less energy in foraging. Females, on the other hand, are likely to invest more energy in foraging as the main providers for their young, as well as in protecting the young while they accompany them on their foraging trips (Kruuk, 1976; Nissim, 1986). This strategy may lead the females to save energy by moving less – both in terms of their geographic range (net displacement) and total travel distance, as well as in terms of reduced speed. Females may also spend more time searching for food, because even anthropogenic food is more spatially clumped than food found in the wild, each local patch (e.g., garbage bin), may still contain only a small amount of food, hence the necessity to continually search for foraging opportunities.
We note that differences in behavioral classification between males and females could be also due to different baseline movement rates rather than to actual differences in behavior (life history). Nevertheless, the biology of the species supports the life-history hypothesis. Male and female hyenas are similar in body size (Kruuk, 1976) and therefore, there is no reason to expect longer steps for males a priori, supporting the hypothesis that the difference we found in average step length between males and females is likely due to different time allocation between behaviors characterized by longer (e.g., traveling) versus shorter (e.g., resting) step lengths. In addition, since females are the main providers for their young, it is most likely that their energetic demands will be higher than that of males.
In Israel, eight recorded human-striped hyena interactions (all between 2014 and 2021 except one that occurred in 1983), escalated into a meaningful conflict that demanded the interference of the INPA. Four of these incidents involved striped hyaenas that resided within cities, two involved high association with rural settlements, and two were related to a cattle/sheep ranch. Mitigation methods included translocation (which has not proven to be effective), the use of deterrents and aversion, diversionary feeding, and providing information to the public regarding hyena’s behavior (via national and local media, social media, and lectures). In seven out of the eight incidents, it was possible to determine the sex of the hyena involved, and in all these cases it was a female. Two separate conflict incidents included two females arriving on site – an adult and a subadult, which were likely related (a mother and daughter). Our findings provide evidence in support of these anecdotal observations that females tend to be at higher risk of conflict with humans than males.
The increased overlap between hyenas and humans in the highly disturbed area in the center of Israel, along with the females’ involvement in conflicts, may be amplified with time (Figure 7). A female’s high demand for food (during pregnancy, lactation, and as the main food provider for the young), is supplied by predictable and easily accessible anthropogenic food. Then, since in most mammals, including hyaenid species, males disperse more often and to longer distances compared to females (Greenwood, 1980; Trochet et al., 2016; Bartoń et al., 2019; Holekamp and Sawdy, 2019; but see Wagner et al., 2008), females kin are often found in closer proximity to one another than to their male counterparts (Greenwood, 1980). Relaxed competition for food may promote tolerance and even cooperation between these females (Califf et al., 2020). This may include assistance in raising cubs, and even den-sharing (Bouskila, 1984; Nissim, 1986; Mandal et al., 2018; Califf et al., 2020; Personal observation). Accordingly, two and even three females with their young have indeed been documented foraging together (Bouskila, 1984; Nissim, 1986). Younger females benefit from staying with their mother for extended periods as this may allow for more knowledge transfer from adult females. Thus, as mothers transfer knowledge to their daughters, the young females may continue utilizing anthropogenic resources, regardless of their reproductive state.
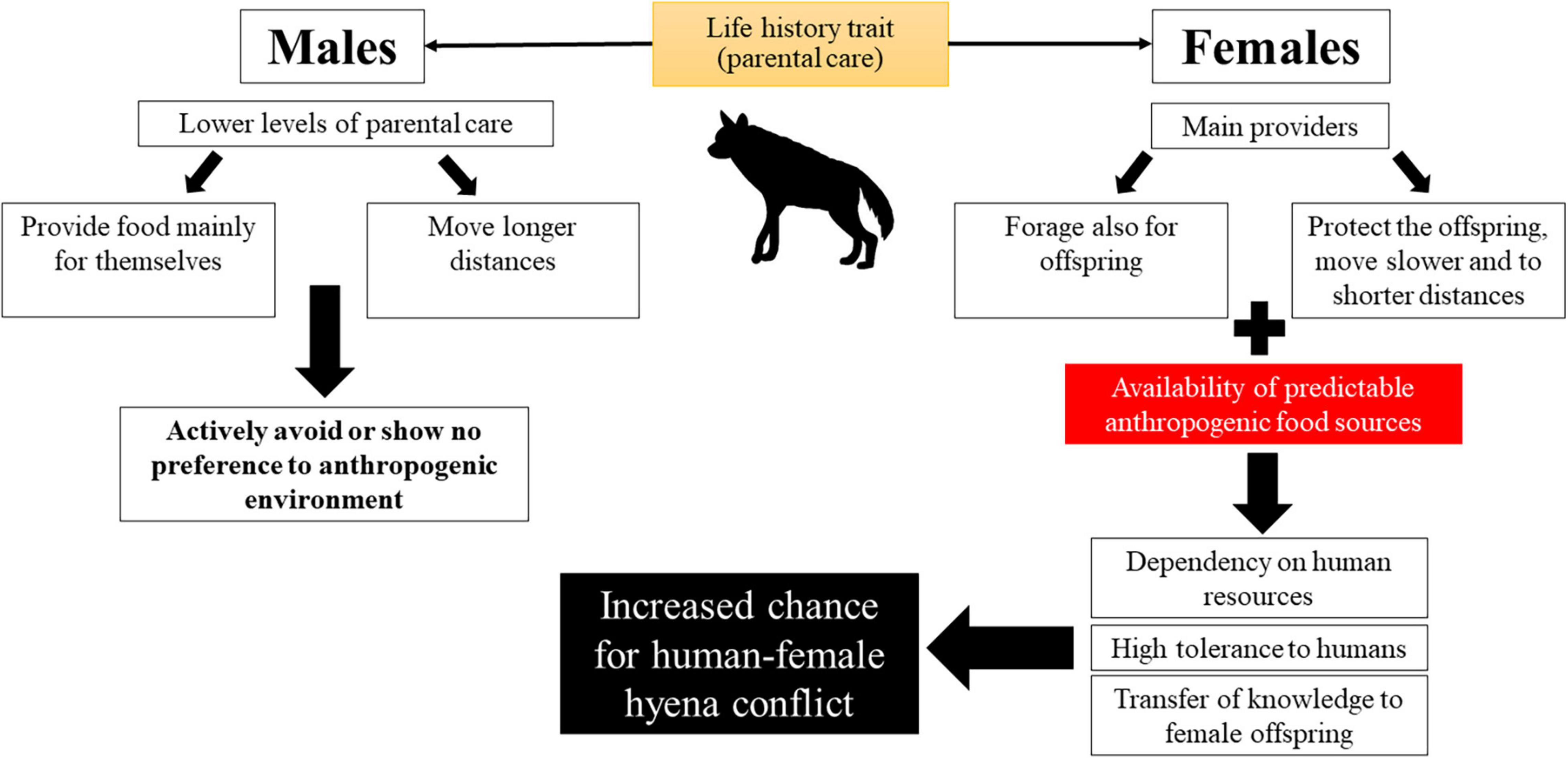
Figure 7. A conceptual model for the combined effects of adding anthropogenic food subsidies on males and females striped hyenas’ behavior and risk of human-wildlife conflict.
Since human-striped hyenas spatial overlap is only going to increase, it is of great importance to share scientific findings regarding hyenas’ behavior not only with conservation practitioners but also with the general public and with policymakers as part of carnivore’ conservation programs (Treves and Karanth, 2003; Bhandari and Chalise, 2016). Providing information to the public regarding the behavior of a species that is usually misunderstood and suffers from a bad reputation can assist us in reducing fear and decreasing the chances that human-wildlife interactions will turn into conflict. Our findings suggest that female striped hyenas are more likely to get into conflict, and hence, the information provided to the public should focus on female hyenas and the reasons they are approaching human settlements. The factors that drive females to spend more time in human-dominated areas require further investigation. However, we recommend that, in a case of a conflict with a female hyena within an anthropogenic landscape, conservation practitioners may include the use of adaptive management in accordance with the female reproductive state: for example, for young females with no indication of cubs, deterrents and/or aversive conditioning should be used, while for mothers with higher energetic needs, a combination of the former with temporal diversionary feeding at dens area could be implemented. In this case, local knowledge regarding hyena sightings, cub presence, and den locations could all assist conservation practitioners in reducing conflict. In extreme cases, and only as a last resort (Massei and Cowan, 2014), sterilization may prove to be effective in conflict mitigation (Bromley and Gese, 2001; Fredrickson and Hedrick, 2006; Massei and Cowan, 2014). In this case, the female’s energetic needs will be reduced, which may affect her nuisance behavior, and knowledge transfer to the next generation will be prevented.
While moving less and relying on predictable anthropogenic food sources can save energy, living in high proximity to humans and feeding on anthropogenic food waste may come with other costs. This may be reflected in high cortisol levels (Støen et al., 2015), poor health (Murray et al., 2015), low fertility, or reduced survival of adults and offspring (Bunnefeld et al., 2006; Wong and Candolin, 2015; Johnson et al., 2020) that inhabit anthropogenic environments. Future work on striped hyenas subjected to high anthropogenic pressure should therefore include physiological measurements as well as long-term monitoring of individual survival. This, in turn, can assist us in determining whether the tolerance behavior exhibited by this large carnivore is adaptive, or whether it represents an evolutionary trap for striped hyenas.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Israel Nature and Parks Authority, as well as by the University Committee for the Ethical Care and Use of Animals in Experiments at Ben-Gurion University of the Negev (Authorization Number IL-80-12-2016).
EB-Z and OB-T conceived and design the study. EB-Z and AK conducted the fieldwork. EB-Z organized the database and wrote the first draft of the manuscript with input from OB-T. SP and TA performed the statistical analysis. SP wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was funded by an internal grant from Ben-Gurion University of the Negev and by the Israel Nature and Parks Authority. EB-Z was funded by a fellowship from the Swiss Institute for Dryland Environmental and Energy Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Yariv Malihi, the Central District Ecologist for the INPA for his collaboration and for promoting this project. We also thank Roni King and Ariel Kedem, Yair Friedberg, and Ohad Mass, for assisting with fieldwork whenever needed.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.897132/full#supplementary-material
Abi-Said, M., and Dloniak, S. M. D. (2015). Hyaena hyaena. The IUCN Red List of Threatened Species 2015:e.T10274A45195080.
Abi-Said, M. R., and Abi-Said, D. M. (2007). Distribution of the Striped Hyaena (Hyaena hyaena syriaca Matius, 1882) (Carnivora: Hyaenidae) in urban and rural areas of Lebanon. Zool. Middle East 42, 3–14. doi: 10.1080/09397140.2007.10638241
Akay, A. E., Inac, S., and Yildirim, I. C. (2011). Monitoring the local distribution of striped hyenas (Hyaena hyaena L.) in the Eastern Mediterranean Region of Turkey (Hatay) by using GIS and remote sensing technologies. Environ. Monit. Assess. 181, 445–455. doi: 10.1007/s10661-010-1840-6
Alam, M., Khan, J., Kushawa, S., Agrawal, R., Pathak, B., and Kumar, S. (2014). Assessment of suitable habitat of near threatened striped hyena (Hyaena hyaena Linnaeus, 1758) using Remote Sensing and Geographic Information System. Asian J. Geoinform. 14, 1–10.
Alam, M. S., and Khan, J. A. (2015). Food habits of striped hyena (Hyaena hyaena) in a semi-arid conservation area of India. J. Arid. Land 7, 860–866. doi: 10.1007/s40333-015-0007-2
Allen, A. M., and Singh, N. J. (2016). Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 3:155. doi: 10.3389/fevo.2015.00155
Athreya, V., Odden, M., Linnell, J. D. C., Krishnaswamy, J., and Karanth, U. (2013). Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS One 8:57872. doi: 10.1371/journal.pone.0057872
Bartoń, K. A., Zwijacz-Kozica, T., Zięba, F., Sergiel, A., and Selva, N. (2019). Bears without borders: long-distance movement in human-dominated landscapes. Glob. Ecol. Conserv. 17:e00541. doi: 10.1016/J.GECCO.2019.E00541
Bateman, P. W., and Fleming, P. A. (2012). Big city life: carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Beckmann, J. P., and Berger, J. (2003). Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. J. Zool. 261, 207–212. doi: 10.1017/S0952836903004126
Bhandari, S., Bhusal, D. R., Psaralexi, M., and Sgardelis, S. (2021). Habitat preference indicators for striped hyena (Hyaena hyaena) in Nepal. Glob. Ecol. Conserv. 27:e01619. doi: 10.1016/J.GECCO.2021.E01619
Bhandari, S., and Chalise, M. K. (2016). People’s attitudes toward Striped Hyaena (Hyaena hyaena Linnaeus, 1758) (Mammalia: Carnivora: Hyaenidae) conservation in lowland Nepal. J. Threat. Taxa 8, 9125–9130. doi: 10.11609/jott.2518.8.9.9125-9130
Bhandari, S., Morley, C., Aryal, A., and Shrestha, U. B. (2020). The diet of the striped hyena in Nepal’s lowland regions. Ecol. Evol. 10, 7953–7962. doi: 10.1002/ece3.6223
Bleyhl, B., Ghoddousi, A., Askerov, E., Bocedi, G., Breitenmoser, U., Manvelyan, K., et al. (2021). Reducing persecution is more effective for restoring large carnivores than restoring their prey. Ecol. Appl. 31:5. doi: 10.1002/eap.2338
Bouskila, Y. (1984). The foraging groups of the striped hyena (Hyaena hyaena syriaca). Carnivore 7, 2–12.
Boydston, E. E., Kapheim, K. M., Watts, H. E., Szykman, M., and Holekamp, K. E. (2003). Altered behaviour in spotted hyenas associated with increased human activity. Anim. Conserv. 6, 207–219. doi: 10.1017/S1367943003003263
Bromley, C., and Gese, E. M. (2001). Surgical sterilization as a method of reducing coyote predation on domestic sheep. J. Wildlife Manag. 2001, 510–519.
Bunnefeld, N., Linnell, J. D. C., Odden, J., van Duijn, M. A. J., and Andersen, R. (2006). Risk taking by Eurasian lynx (Lynx lynx) in a human-dominated landscape: Effects of sex and reproductive status. J. Zool. 270, 31–39. doi: 10.1111/j.1469-7998.2006.00107.x
Califf, K. J., Green, D. S., Wagner, A. P., Scribner, K. T., Beatty, K., Wagner, M. E., et al. (2020). Genetic relatedness and space use in two populations of striped hyenas (Hyaena hyaena). J. Mammal. 101, 361–372. doi: 10.1093/jmammal/gyz165
Carter, N. H., and Linnell, J. D. C. (2016). Co-Adaptation Is Key to Coexisting with Large Carnivores. Trends Ecol. Evol. 31, 575–578. doi: 10.1016/j.tree.2016.05.006
Central Bureau of Statistics (2020). Central Bureau of Statistics. Statistical Abstract of Israel 2020. No. 71, Table 2.1. Retrieved from https://www.cbs.gov.il/he/publications/doclib/2020/2.shnatonpopulation/02_01.xls (accessed August 18, 2021).
Clutton-Brock, and Harvey, P. H. (1978). Mammals, resources and reproductive strategies. Nature 273, 191–195.
Davidar, E. R. C. (1990). Observation at a hyena Hyaena hyaena den. J. Bomb. Nat. Hist. Soc. 87, 445–447.
Di Minin, E., Slotow, R., Hunter, L. T. B., Montesino Pouzols, F., Toivonen, T., Verburg, P. H., et al. (2016). Global priorities for national carnivore conservation under land use change. Sci. Rep. 6:23814. doi: 10.1038/srep23814
Dickman, A. J. (2010). Complexities of conflict: The importance of considering social factors for effectively resolving human-wildlife conflict. Anim. Conserv. 13, 458–466. doi: 10.1111/j.1469-1795.2010.00368.x
Djamali, M., Mashkour, M., Akhani, H., Belkacem, D., Gambin, B., Leydet, M., et al. (2020). Pollen analysis of present-day striped hyena (Hyaena hyaena) scats from central Iran: Implications for dryland paleoecology and animal paleoethology. Rev. Palaeobot. Palynol. 281:104277. doi: 10.1016/J.REVPALBO.2020.104277
Doherty, T. S., and Driscoll, D. A. (2018). Coupling movement and landscape ecology for animal conservation in production landscapes. Proc. R. Soc. B 285:20172272. doi: 10.1098/rspb.2017.2272
Doherty, T. S., Hays, G. C., and Driscoll, D. A. (2021). Human disturbance causes widespread disruption of animal movement. Nat. Ecol. Evol. 5, 513–519. doi: 10.1038/s41559-020-01380-1
Dubois, S., and Fraser, D. (2013). A framework to evaluate wildlife feeding in research, wildlife management, tourism and recreation. Animals 3, 978–994. doi: 10.3390/ani3040978
Elff, M. (2021). mclogit: Multinomial Logit Models, with or without Random Effects or Overdispersion (0.8.7.3) [Computer software]. Available online at: https://CRAN.R-project.org/package=mclogit (accessed date 2022-04-19).
Elfström, M., Zedrosser, A., Støen, O. G., and Swenson, J. E. (2014). Ultimate and proximate mechanisms underlying the occurrence of bears close to human settlements: review and management implications. Mamm. Rev. 44, 5–18. doi: 10.1111/j.1365-2907.2012.00223.x
Fleming, P. A., and Bateman, P. W. (2018). Novel predation opportunities in anthropogenic landscapes. Anim. Behav. 138, 145–155. doi: 10.1016/J.ANBEHAV.2018.02.011
Fredrickson, R. J., and Hedrick, P. W. (2006). Dynamics of hybridization and introgression in red wolves and coyotes. Conserv. Biol. 20, 1272–1283. doi: 10.1111/j.1523-1739.2006.00401.x
Frembgen, J. W. (1998). The Magicality of the Hyena: beliefs and Practices in West and South Asia. Asian Folklore Stud. 57:2.
Gabai, O., and Zanzuri, A. (2019). The Threats to Israel’s Ecological Corridors. The Society for the Protection of Nature in Israel (SPNI) report (in Hebrew). Tel-Aviv: Society for the Protection of Nature in Israel.
Gantchoff, M., Laura, C., and Belant, J. (2020). Planning for carnivore recolonization by mapping sex-specific landscape connectivity. Glob. Ecol. Conserv. 21:869. doi: 10.1016/j.gecco.2019.e00869
Gastón, A., Blázquez-Cabrera, S., Garrote, G., Mateo-Sánchez, M. C., Beier, P., Simón, M. A., et al. (2016). Response to agriculture by a woodland species depends on cover type and behavioural state: insights from resident and dispersing Iberian lynx. J. Appl. Ecol. 53, 814–824. doi: 10.1111/1365-2664.12629
Gaynor, K. M., Hojnowski, C. E., Carter, N. H., and Brashares, J. S. (2018). The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235. doi: 10.1126/science.aar7121
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Habib, B., Ghaskadbi, P., Khan, S., Hussain, Z., and Nigam, P. (2021). Not a cakewalk: insights into movement of large carnivores in human-dominated landscapes in India. Ecol. Evol. 11, 1653–1666. doi: 10.1002/ece3.7156
Hijmans, R. J., Van Etten, J., Cheng, J., Mattiuzzi, M., Sumner, M., Greenberg, J. A., et al. (2015). Package ‘raster’. R package, 734.
Hofer, H. (1998). “Stripped hyaena Hyaena (hyaena) hyaena (Linnaeus, 1758),” in Hyaenas: Status Survey and Conservation Action Plan, eds H. Hofer and G. Mills (Gland: IUCN), 21–26.
Holekamp, K. E., and Sawdy, M. A. (2019). The evolution of matrilineal social systems in fissiped carnivores. Philosoph. Transact. R. Soc. B: Biol. Sci. 374:20180065. doi: 10.1098/rstb.2018.0065
Israel Nature and Parks Authority (2020). INPA Database. Unpublished Internal Organization Data. Jerusalem: Israel Nature and Parks Authority.
Jdeidi, T., Masseti, M., Nader, I., de Smet, K., and Cuzin, F. (2010). Hyaena hyaena. IUCN Red List Threatened Species 2010:E.T10274A3188449.
JNF. (2021). Farming facilities (in Hebrew). Available online at: https://kkl-open-data-hub-kkl.opendata.arcgis.com (Accessed May 25, 2021).
Johnson, H. E., Lewis, D. L., and Breck, S. W. (2020). Individual and population fitness consequences associated with large carnivore use of residential development. Ecosphere 11:3098. doi: 10.1002/ecs2.3098
Karanth, K. U., and Chellam, R. (2009). Carnivore conservation at the crossroads. Oryx 43, 1–2. doi: 10.1017/S003060530843106X
Klug, H. (2011). Animal Mating Systems. In eLS. Hoboken, NJ: John Wiley & Sons, Ltd, doi: 10.1002/9780470015902.a0022553
Kruuk, H. (1976). Feeding and social behaviour of the striped hyaena (Hyaena vulgaris Desmarest). East Afr. Wildlife J. 14, 91111. doi: 10.1111/j.1365-2028.1976.tb00155.x
Langrock, R., King, R., Matthiopoulos, J., Thomas, L., Fortin, D., and Morales, J. M. (2012). Flexible and practical modeling of animal telemetry data: Hidden Markov models and extensions. Ecology 93, 2336–2342. doi: 10.1890/11-2241.1
Lotan, A., Grossbard, S., Safriel, U., and Feitelson, E. (2019). Ecosystems and Human Wellbeing – A National Assessment. Hamaarag-Israel’s National Nature Assessment Program. The Steinhardt Museum of Natural History. Israel: Tel-Aviv University.
Loveridge, A. J., Wang, S. W., Frank, L. G., and Seidensticker, J. (2010). “People and wild felids: conservation of cats and management of conflicts,” in Biology and conservation of wild felids, Vol. 2010, eds D. W. Macdonald and A. J. Loveridge (Oxford: Oxford Univ. Press), 161–195.
Maiorano, L., Boitani, L., Chiaverini, L., and Ciucci, P. (2017). Uncertainties in the identification of potential dispersal corridors: the importance of behaviour, sex, and algorithm. Basic Appl. Ecol. 21, 66–75. doi: 10.1016/J.BAAE.2017.02.005
Mandal, D., Qureshi, Q., and Sanka, K. (2018). “Solitary yet social: does resource heterogeneity govern striped hyena sociality?,” in Meeting Presented at the17th International Behavioral Ecology Congress, (ISBE).
Massei, G., and Cowan, D. (2014). Fertility control to mitigate human-wildlife conflicts: a review. Wildlife Res. 41, 1–21. doi: 10.1071/WR13141
McClintock, B. T., and Michelot, T. (2018). momentuHMM: R package for generalized hidden Markov models of animal movement. Methods Ecol. Evol. 9, 1518–1530. doi: 10.1111/2041-210X.12995
Mendelssohn, H., and Yom-Tov, Y. (1999). Fauna Palaestina: Mammalia of Israel, 205–211. Jerusalem: The Israel Academy of Sciences and Humanities.
Ministry of agriculture (2021). Agricultural vegetation (in Hebrew). Available online at: https://data1-moag.opendata.arcgis.com (Accessed May 25, 2021).
Murray, M., Edwards, M. A., Abercrombie, B., Cassady, C., and Clair, S. (2015). Poor health is associated with use of anthropogenic resources in an urban carnivore. Proc. R. Soc. B 282:20150009. doi: 10.1098/rspb.2015.0009
Nathan, R., Getz, W. M., Revilla, E., Holyoak, M., Kadmon, R., Saltz, D., et al. (2008). A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. 105:80037505. doi: 10.1073/pnas.0800375105
Nissim, D. (1986). Feeding station “hyenas cliff”, Kalia, Israel 1983-86. Internal Israel Nature and Park Authorities (INPA) Report (in Hebrew). Unpublished. Jerusalem: Israel Nature and Park Authorities.
Orams, M. B. (2002). Feeding wildlife as a tourism attraction: a review of issues and impacts. Tour. Manag. 23, 281–293. doi: 10.1016/S0261-5177(01)00080-2
Ordiz, A., Moen, G. K., Sæbø, S., Stenset, N., Swenson, J. E., and Støen, O. G. (2019). Habituation, sensitization, or consistent behavioral responses? Brown bear responses after repeated approaches by humans on foot. Biolog. Conserv. 232, 228–237. doi: 10.1016/j.biocon.2019.01.016
Ordiz, A., Støen, O. G., Delibes, M., and Swenson, J. E. (2017). Staying cool or staying safe in a human-dominated landscape: which is more relevant for brown bears? Oecologia 185, 191–194. doi: 10.1007/s00442-017-3948-7
Oriol-Cotterill, A., Macdonald, D. W., Valeix, M., Ekwanga, S., and Frank, L. G. (2015a). Spatiotemporal patterns of lion space use in a human-dominated landscape. Anim. Behav. 101, 27–39. doi: 10.1016/J.ANBEHAV.2014.11.020
Oriol-Cotterill, A., Valeix, M., Frank, L. G., Riginos, C., and Macdonald, D. W. (2015b). Landscapes of Coexistence for terrestrial carnivores: The ecological consequences of being downgraded from ultimate to penultimate predator by humans. Oikos 124, 1263–1273 doi: 10.1111/oik.02224
Pangle, W. M., and Holekamp, K. E. (2010). Lethal and nonlethal anthropogenic effects on spotted hyenas in the Masai Mara National Reserve. J. Mammal. 91, 154–164. doi: 10.1644/08-mamm-a-359r.1
Patterson, T. A., Thomas, L., Wilcox, C., Ovaskainen, O., and Matthiopoulos, J. (2008). State–space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. doi: 10.1016/J.TREE.2007.10.009
Pérez-Claros, J. A., and Coca-Ortega, C. (2020). Canines and carnassials as indicators of sociality in durophagous hyaenids: Analyzing the past to understand the present. PeerJ. 8:10541. doi: 10.7717/peerj.10541
Picardi, S., Coates, Peter, Kolar, J., O’Neil, S., Mathews, S., et al. (2021). Behavioural state-dependent habitat selection and implications for animal translocations. J. Appl. Ecol. 59, 624–635. doi: 10.1111/1365-2664.14080
Qarqaz, M. A., Baker, M. A. A., and Amr, Z. S. (2004). Status and ecology of the Striped Hyaena, Hyaena hyaena, in Jordan. Zool. Midd. East 33, 87–92. doi: 10.1080/09397140.2004.10638067
Reichmann, A. (2005). Striped Hyena in Northern Israel. Internal Israel Nature and Park Authorities (INPA) report (in Hebrew). Unpublished. The Northern district.
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world’s largest carnivores. Science 343:1241484. doi: 10.1126/science.1241484
Rotem, D. (2014). The Effect of Fencing on Open Space – Policy and Recommendations for Action. Israel Nature and Park Authorities report (in Hebrew). Jerusalem: Israel Nature and Park Authorities.
Šálek, M., Drahníková, L., and Tkadlec, E. (2015). Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mamm. Rev. 45, 1–14. doi: 10.1111/mam.12027
Schuette, P., Wagner, A. P., Wagner, M. E., and Creel, S. (2013). Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biolog. Conserv. 158, 301–312. doi: 10.1016/j.biocon.2012.08.008
Singh, P., Gopalaswamy, A. M., and Karanth, K. U. (2010). Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. J. Mammal. 91, 1152–1159. doi: 10.1644/09-MAMM-A-159.1
Singh, R., Qureshi, Q., Sankar, K., Krausman, P. R., Goyal, S. P., and Nicholson, K. L. (2014). Population density of striped hyenas in relation to habitat in a semi-arid landscape, western India. Acta Theriol. 59, 521–527. doi: 10.1007/s13364-014-0187-8
Smale, L., Nunes, S., and Holekamp, K. E. (1997). Sexually Dimorphic Dispersal in Mammals: patterns, Causes, and Consequences. Adv. Study Behav. 26, 181–250. doi: 10.1016/S0065-3454(08)60380-0
Støen, O. G., Ordiz, A., Evans, A. L., Laske, T. G., Kindberg, J., Fröbert, O., et al. (2015). Physiological evidence for a human-induced landscape of fear in brown bears (Ursus arctos). Physiol. Behav. 152, 244–248. doi: 10.1016/j.physbeh.2015.09.030
Suraci, J. P., Frank, L. G., Oriol-Cotterill, A., Ekwanga, S., Williams, T. M., and Wilmers, C. C. (2019). Behavior-specific habitat selection by African lions may promote their persistence in a human-dominated landscape. Ecology 100:2644. doi: 10.1002/ecy.2644
R Core Team (2021). R: A language and environment for statistical computing (Version 4.0. 5)[Programming language]. Vienna: R Foundation for Statistical Computing.
Teichman, K. J., Cristescu, B., and Nielsen, S. E. (2013). Does sex matter? Temporal and spatial patterns of cougar-human conflict in British Columbia. PLVS One 8:e74663. doi: 10.1371/journal.pone.0074663
Tigas, L. A., van Vuren, D. H., and Sauvajot, R. M. (2002). Behavioral responses of bobcats and coyotes to habitat fragmentation and corridors in an urban environment. Biological Conservation 108, 299–306. doi: 10.1016/S0006-3207(02)00120-9
Tourani, M., Moqanaki, E. M., and Kiabi, B. H. (2012). Vulnerability of striped hyaenas, Hyaena hyaena, in a human-dominated landscape of central iran. Zool. Middle East 56, 133–136. doi: 10.1080/09397140.2012.10648948
Treves, A., and Karanth, K. U. (2003). Human-Carnivore Conflict and Perspectives on Carnivore Management Worldwide. Conserv. Biol. 17, 1491–1499.
Trochet, A., Courtois, E. A., Stevens, V. M., Baguette, M., Chaine, A., Schmeller, D. S., et al. (2016). Evolution of sex-biased dispersal. Q. Rev. Biol. 91, 297–320.
Tucker, M. A., Böhning-gaese, K., Fagan, W. F., Fryxell, J. M., Moorter, B., van, et al. (2018). Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359, 466–469. doi: 10.1126/science.aam9712
Valeix, M., Hemson, G., Loveridge, A. J., Mills, G., and Macdonald, D. W. (2012). Behavioural adjustments of a large carnivore to access secondary prey in a human-dominated landscape. J. Appl. Ecol. 49, 73–81. doi: 10.1111/j.1365-2664.2011.02099.x
Wagner, A. P. (2006). Behavioral ecology of the striped hyena (Hyaena hyaena). Ph.D. thesis. Bozeman: Montana State University.
Wagner, A. P., Frank, L. G., and Creel, S. (2008). Spatial grouping in behaviourally solitary striped hyaenas, Hyaena hyaena. Anim. Behav. 75, 1131–1142. doi: 10.1016/J.ANBEHAV.2007.08.025
Wang, Y., Allen, M. L., and Wilmers, C. C. (2015). Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biolog. Conserv. 190, 23–33. doi: 10.1016/j.biocon.2015.05.007
Watts, H. E., and Holekamp, K. E. (2007). Hyena societies. Curr. Biol. 17, R657–R660. doi: 10.1016/j.cub.2007.06.002
Wheat, R. E., and Wilmers, C. C. (2016). Habituation reverses fear-based ecological effects in brown bears (Ursus arctos). Ecosphere 7:7. doi: 10.1002/ecs2.1408
Wilmers, C. C., Wang, Y., Nickel, B., Houghtaling, P., Shakeri, Y., Allen, M. L., et al. (2013). Scale Dependent Behavioral Responses to Human Development by a Large Predator, the Puma. PLoS One 8:4. doi: 10.1371/journal.pone.0060590
Wong, B., and Candolin, U. (2015). Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. doi: 10.1093/beheco/aru183
Yom-Tov, Y. (2003). Body sizes of carnivores commensal with humans have increased over the past 50 years. Funct. Ecol. 17, 323–327. doi: 10.1046/j.1365-2435.2003.00735.x
Zeller, K. A., Jennings, M. K., Winston Vickers, T., Ernest, H. B., Cushman, S. A., Boyce, W. M., et al. (2018). Are all data types and connectivity models created equal? Validating common connectivity approaches with dispersal data. Sci. J. 2018:12742. doi: 10.1111/ddi.12742
Keywords: large carnivores, Hidden Markov Model, behavioral states, Anthropocene, human-wildlife conflict, parental care, life history, behavioral flexibility
Citation: Bar-Ziv E, Picardi S, Kaplan A, Avgar T and Berger-Tal O (2022) Sex Differences Dictate the Movement Patterns of Striped Hyenas, Hyaena hyaena, in a Human-Dominated Landscape. Front. Ecol. Evol. 10:897132. doi: 10.3389/fevo.2022.897132
Received: 15 March 2022; Accepted: 22 June 2022;
Published: 08 July 2022.
Edited by:
Tasmin Rymer, James Cook University, AustraliaReviewed by:
Gaurav Dhiman, Government Bikram College of Commerce, IndiaCopyright © 2022 Bar-Ziv, Picardi, Kaplan, Avgar and Berger-Tal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Einat Bar-Ziv, ZWluYXQuYmFyLnppdkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.