- 1College of Wildlife and Protected Area, Northeast Forestry University, Harbin, China
- 2Academy of Inventory and Planning, National Forestry and Grassland Administration, Beijing, China
- 3State Key Laboratory of Biological Control, School of Life Sciences and School of Ecology, Sun Yat-sen University, Guangzhou, China
Large carnivores maintain the balance of ecosystems. Understanding distribution and population changes are necessary prerequisites for scientific conservation strategy. The east of Jilin Province is the habitat of endangered Amur tiger (Panthera tigris altaica). The Chinese government has focused the monitoring on protecting the Amur tiger. However, little is known about Asiatic black bear (ABB, Ursus thibetanus) distribution, population dynamics in the wild, and protection awareness of local residents in Jilin Province, China. We conducted a integrative survey in mountain areas of eastern Jilin to determine ABB distribution. We explored the drivers of the distribution of ABB in Jilin using logstic regression, we further predicted the habitat suitability and potential suitable habitat of the ABB. Totally, we surveyed 112 grids (15 km × 15 km) from November 2015 to January 2019. Logistic regression analysis revealed that the main factors driving ABB distribution in Jilin are forest coverage, distance from protected areas, distance from main roads (railways and highways), and distance from water bodies. The results of questionnaire survey showed that the local residents’ understanding of ABB distribution is congruent with our field research. They believed that the number of ABBs has gradually increased in the past ten years. Nevertheless, the local residents have a negative attitude toward the ABBs, which may adversely affect efforts to protect them, possibly leading to more conflicts between humans and bears. Therefore, there is a need to consider ways to change the attitude of the locals through the strengthening of the protection propaganda and advocating management as being critical for the protection of ABBs. Our research provides a scientific basis for future conservation planning. We recommend taking local people’s attitude into consideration during conservation management strategy making to reduce human-bear conflicts and promote the coexistence of humans and bears.
Introduction
The spatial distribution of species is the basis of almost all aspects of biodiversity management, including protecting rare species, monitoring invasive species, identifying biodiversity hotspots, and delineating valuable protected areas (Franklin, 2010). Species distribution might vary with resources. While the consequent change of species distribution might in turn cause the change of resources (Cardinale et al., 2012; Keil et al., 2015; Jarzyna and Jetz, 2017; Kopatz et al., 2021). Apex predators play an irreplaceable effect on ecosystem stability (Schmitz, 2008; Rodríguez-Lozano et al., 2015; Antiqueira et al., 2018). Bears are top predators and omnivores, feeding on various types of food, including berries, plant roots, nuts, insects, ungulates, and dead animals. As a scavenger, bears feed on decaying food (especially animal carcasses), which can control the spread of diseases and pests (Aguilera-Alcalá et al., 2020). It not only scavenging but also promotes material circulation (Moleon et al., 2014). Bears are an effective seed disperser for some plants, of which swallowing and expelling can improve the seed germination rate (Enders and Vander Wall, 2012; Koike et al., 2012; García-Rodríguez et al., 2021). However, bears play an essential role in the whole land ecosystem. The conflict between this large carnivore and humans is a common problem, especially When both occur in close proximity or share a common space. For example, although the Sanjiangyuan is vast and sparsely populated, more and more bears have visited human settlements, causing losses of life and property to local herders (Dai et al., 2020). In turn, railway, road, garbage, and recreational activities threaten bears’ survival (Morales-González et al., 2020). Therefore, studying spatial distribution is beneficial to improving the conservation and management of bears and preventing human-bear conflicts.
According to IUCN (Garshelis and Steinmetz, 2020) the habitat of Asiatic black bears (ABB,Ursus thibetanus) is reportedly fragmented, and a declining population trend has been observed. The increasingly intense resource (e.g., timber and mining) utilization and the expansion of man-made facilities (e.g., railways and roads) has led to fragmentation and deterioration of forested habitat, which may become population barriers, the formation of isolated populations, or even disappearance (Gregório et al., 2020; Hooker et al., 2020; Lázaro et al., 2020; Kopatz et al., 2021; Saura, 2021). Fragmentation of the habitat can lead to a decrease in the niche width of top predators, or even the collapse of the entire system (Layman et al., 2007). As typical forest-dwelling animals, the decline of the habitat quality brings threats to the survival of ABBs. We can understand the status of ABB’s habitat and protect ABB more effectively by understanding the distribution of ABB and its driving factors.
The northeast region of China is the main distribution area of ABBs. And the forest ecosystem is their primary habitat (Reid et al., 1991; Ma and Hu, 1994). In the 1980s, timber, highways, railways, and a rapidly increasing human population led to the fragmentation of forest habitats. In addition, the development of the live bear bile harvesting industry severely threatened the wild population. In the past, the population was estimated mainly based on interviews from residents, management administrations, and transect surveys (Ma et al., 1998; Piao et al., 2012). Because individuals are challenging to track, we estimate population change indirectly through the monitoring of the change of population distribution range (i.e., presence-absence; Pollock, 2006). Effective ABB monitoring is critical and especially useful for relevant wildlife management action plans.
The east of Jilin Province is the habitat of endangered Amur tiger (Panthera tigris altaica). The Chinese government has focused the monitoring on protecting the Amur tiger, neglecting ABB. We choose Jilin Province of China as the research area since past wildlife surveys suggest that bears are distributed across the province. Our research objectives are as follows: (1) We map the distribution of ABBs across the province to provide a reference for monitoring future population trends. (2) What are the driving factors for the distribution pattern? (3) We estimate the trend of ABBs population change and attitudes toward ABBs conservation. We hope our result will be incorporated into future conservation planning.
Materials and Methods
Study Area
Jilin Province is located in the hinterland of Northeast China and is the geographic center of Northeast Asia (E: 121° 38′∼ 131° 19′, N:40° 50′∼ 46° 19′), with a total area of 187,400 km2 (Figure 1). The difference between the eastern mountainous region and the central and western plain in landform is noticeable. The prominent mountains are Changbai mountain, Dahei Mountain, Zhangguangcailing, Hadaling, Laoling, Mudanling. The eastern region of the landcover is composed of mixed coniferous and broad-leaved forest, broad-leaved forest, and coniferous forest. The central and western regions are dominated by farmland, followed by grassland. Therefore, deemed as a suitable habitat for ABBs, the eastern forest region is selected as the main survey area.
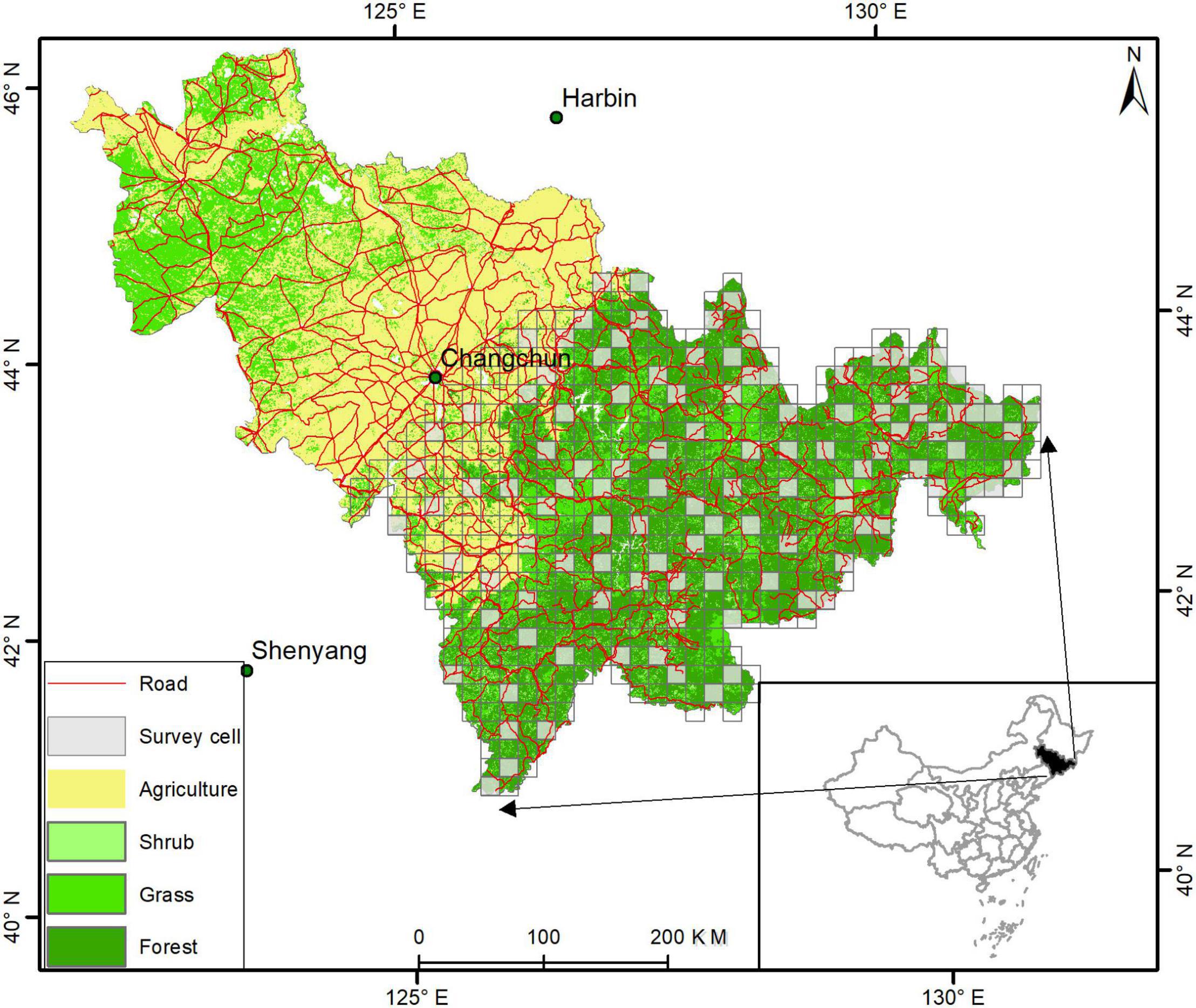
Figure 1. Grids (15 km × 15 km) where the survey was taken (gray cells; n = 112) during 2015–2019 in Jilin Province, China.
Sample Method
We used ArcGIS software [version 10.6, produced by Environmental Systems Research Institute (ESRI)] to divide eastern Jilin Province into 15 km × 15 km grids (n = 540, Figure 1). The size of each grid was based on the largest known home range of ABBs in Asia. Copernicus global land service was used to obtain different land use types in Jilin Province. The following land use types were identified: forest, shrub, grass, agriculture, water, and other non-forest types. We used ArcGIS 10.6 to calculate various land-use types in every 15 km × 15 km grid. The National Basic Geographic Information Center obtained a digital elevation model (DEM1) and calculated the average elevation in each grid. The National Catalogue Service for Geographic Information obtained roadway data2 in Jilin Province and used ArcGIS to calculate the total road length in each grid. Similarly, The National Catalogue Service for Geographic Information was used to obtain data on villages (see footnote 2) in Jilin Province and calculated the number of villages in each grid using ArcGIS. We only considered the three following parameters when sampling each grid: proportion of forest cover, average altitude, and roadway length. However, all parameters were used in analyzing the data for each grid.
To make the sampling more evenly distributed, stratified random sampling was performed on the grids in the eastern part of Jilin Province. All the grids are arranged according to the values of forest cover, average altitude, and road density from low to high (based on the length of roadways). Values for each factor were categorized into three levels: low, medium, and high. There are the same or a similar number of grids on each level. Therefore, all the grids can be divided into 27 types according to the three factors and three grades of each attribute (see Table 1 for the values of each type and factor). Approximately 20% of the grids were randomly selected from each type of grid (a total of 27 types) as survey grids. In total, we selected 112 grids for investigation (Figure 1), which accounted for approximately 20.74% of all grids in Jilin Province (n = 540).
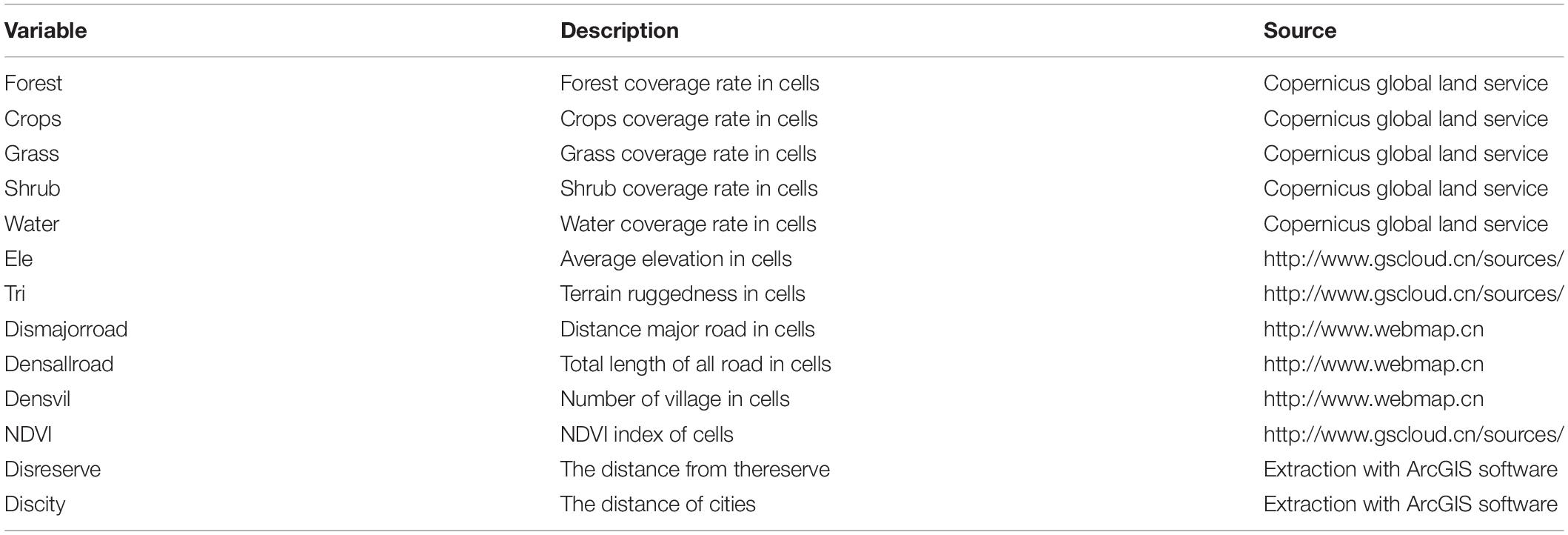
Table 1. The candidate variables in logistic regression models predicting distributions of Asiatic black bear in Jilin Province.
Field Survey
Our survey of each grid comprised of two methods: interview and field surveys. When investigators arrived at each grid, they first visit the village or the related administration of forestry nearby, focusing mainly on their awareness of whether ABBs inhabitated the nearby forest, as well as other information about bear, such as frequency and type of witness (animal, sign, conflict encounters, etc.). When the species was claimed to be observed, we asked the interviewee to lead us to the location to look for signs for verification. We considered claw marks on trees, feeding platforms, feces, den, infrared camera images, and footprints to be positive evidence of bear activity (Hwang, 2003; Kelly and Holub, 2008; Sun et al., 2015; Du, 2018). When we directly found a sign of ABBs, we marked the grid as containing bears.
Supposethe locals cannot provide valid information regarding ABBs. In that case, we use forest stand map, satellite map and select area with the best forest stand with the help of the local administration of forestry (Mongolian oak forest, fir forest, berry, nut, etc. Graber and White, 1983, Nozaki et al., 1983, Raine and Kansas, 1990) to design a 5-km “U” transect that 2 km from the foot of the mountain to the mountain top by the vertical contour line, 1km by the parallel contour line, and 2 km from the top of the mountain to the vertical contour line of the mountain bottom. If there is no evidence of ABBs’ activity found in the transect. To satisfy relatively accurate detection probability, we searched for sign along five transects (100 m × 20 m) in the best bear habitat that we could find. Transects were conducted along elevational contours. Three people, spread across the width of the transect, walked up to and closely inspected every tree within 10 m of the transect line, looking for bear claw marks and also examined the ground for scats, tracks or digging signs. If we found positive evidence of bear activity along any transect, we considered the cell occupied; otherwise, we considered it unoccupied (Liu, 2009; Liu et al., 2009).
In recent years, forestry administrations have set up camera traps to photograph wild animals in some protected areas. We collected all the photos and videos from the survey grid. Two professionals were responsible for selecting the images with ABBs or signs of their activity. The grid is marked as ‘present’ if they both find valid evidence.
Visiting Survey
We classified the local residents according to their age, gender, occupation, and educational background and ensure that the interviewees are randomly selected and distributed evenly in each category. We prepared pictures of ABBs, brown bears, wild boars, red deer, roe deer, Amur tigers, leopards, and ABBs’ signs so that interviewees could attempt to identify ABBs and their signs from the pictures. If the ABBs could be accurately determined, the questionnaire is deemed as effective. Hunters, forest rangers, non-timber collectors, and other residents who are familiar with the local situations were also interviewed.
We used a semi-structured interview procedure on local villagers. The questions were mainly based on the framework of the social questionnaire we designed. We casually asked these questions and took notes instead of the structured questionnaire to avoid unnecessary tension. The majority of the questions were open-ended, instead of giving multiple choice questions, to prevent missing possible answers. When the interviewees gathered, we asked them separately to avoid mutual interference. The primary purpose was to confirm whether ABBs were present near the village and the trend of the ABBs’ population in the past ten years from information provided by the locals. We investigated whether there had been human-bear conflict incidences in the area and the reason for it. The general attitude of residents toward ABBs and the corresponding reasons were recorded. Apart from that, each interviewee was asked a series of questions by the survey team about poaching and the bile trade of ABBs.
Distribution Model
We used binary logistic regression to identify the key environmental factors associated with occupied and unoccupied cells. We used the following 13 factors as independent variables: forest, agriculture, meadow, thickets, and water area (as a proportion of the grid area; Water), average elevation (Ele), the total length of main roadways (railway, national highway, provincial highway, and motorway) (Densallroad), the total length of all roads, number of towns and villages (Densvil), NDVI index (NDVI), terrain ruggedness (Tri), distance of the grid to the nearest reserve (Disreserve), distance to the major road (Dismajorroad), and distance to the nearest city (Discity) (Rogers, 1993; Baruch-Mordo et al., 2008; Liu et al., 2009; Carter et al., 2010). The roads and the number of towns in grids were the best factors that we could use to reflect the disturbance of human activities on ABBs.
Factors with a correlation greater than 0.7 (Figure 2) and the VIF larger than four (Crops, Grass, Densvil, NDVI) were removed, leaving nine (Forest, Shrub, Water, Ele, Tri, Dismajorroad, Densallroad, Disreserve, and Discity) as influencing factors for predicting the distribution of ABBs.
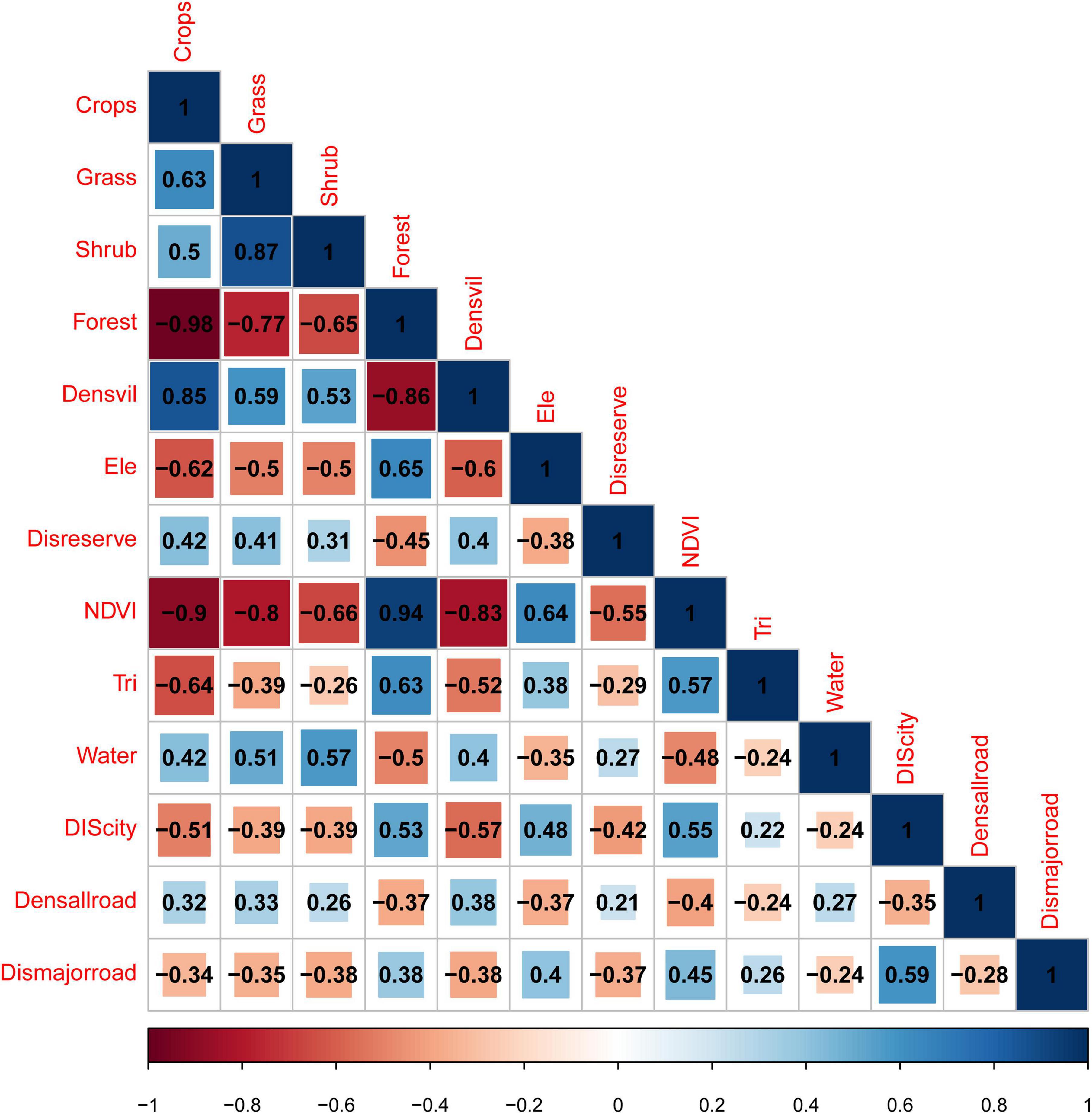
Figure 2. Factors with a correlation greater than 0.7 were removed, leaving Shrub, Forest Ele, Disreserve, Tri, Water, Discity, Densallroad, Dismajorroad.
We built a binary logistic regression model. We ranked models by the values of Akaike’s Information Criterion (AIC) (Akaike, 1981) and considered models with ΔAIC less than 2 as optimal (Burnham and Anderson, 2002). We evaluated the number of occurrences for each variable in the optimal models (i.e., with ΔAIC < 2). Whereas, no optimal model was found. Therefore, we used weighted average method. We chose the models average with cumulative weight higher than 95%.
To predict occupancy of unsurveyed grids using the models, we set the prediction probability threshold to a number from 0.1 to 1.0, with intervals of 0.1. We ran a logistic regression model at each threshold and calculated the rate that the model correctly predicted the grid with the presence or absence. Adding the sensitivity and specificity, the maximum sum value of sensitivity and specificity was determined as the optimal threshold. The probability obtained by the model based on the optimal threshold was divided into grids with or without present. The threshold value predicted by the model is 0.508.
The relative operating characteristic (ROC) (Pearce and Ferrier, 2000) area under the curve (AUC) (Jiménez-Valverde, 2012) was used to determine the discriminative capacity of the model for grids with or without ABBs’ distribution. AUC can be used as a good indicator to measure the model’s discriminative capacity because the value was not affected by the value of the predicted probability threshold.
Results
Distribution and Landscape Variables
We surveyed 112 grids and found evidence of bears in 43 grids and no evidence in 69 grids. The logistic regression model was constructed with the cumulative weight >95% model average results. ROC test results showed that the AUC was 0.845 (>0.5), indicating that the model is effective.
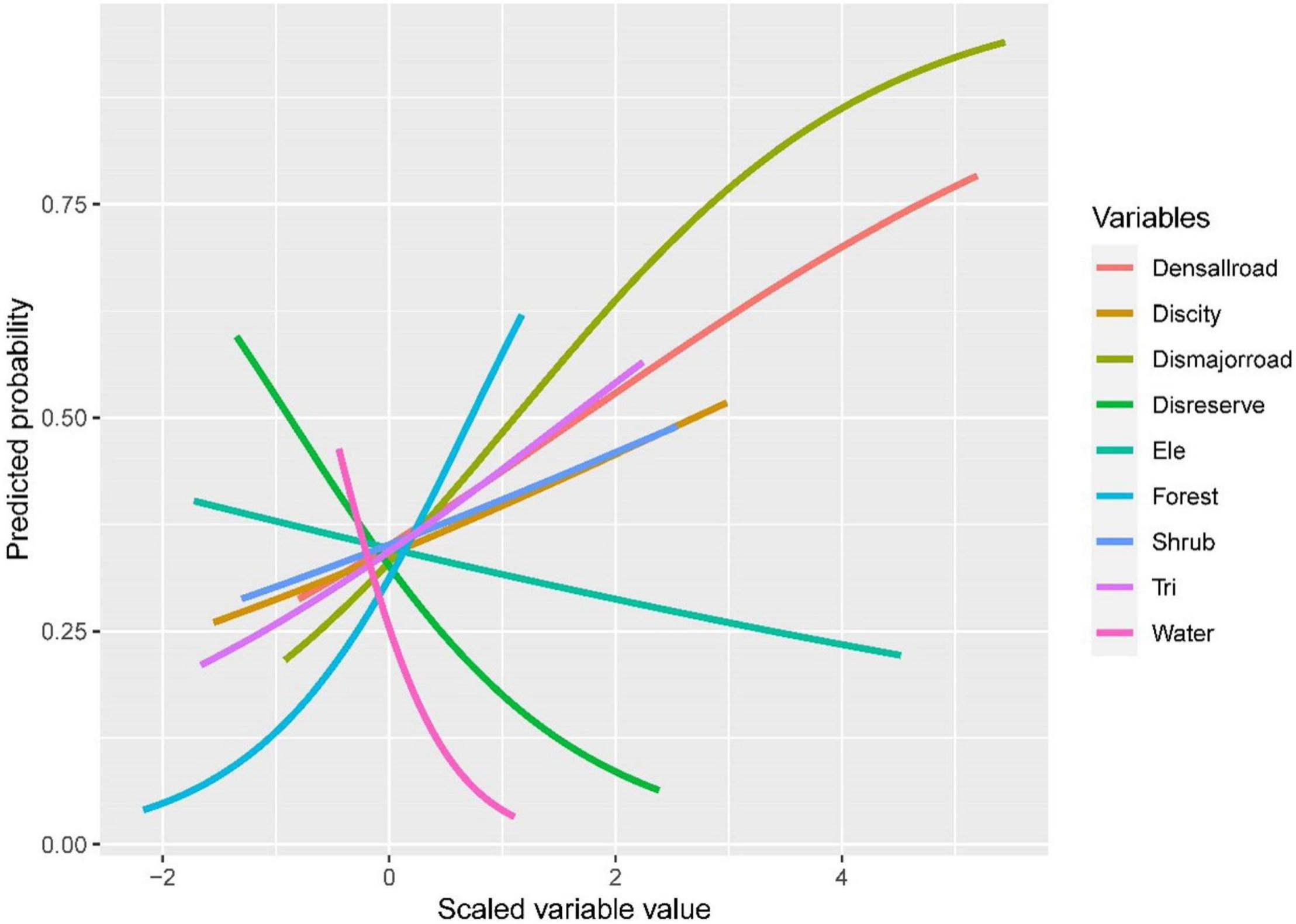
Shrub, Forest, Tri, Discity, Densallroad, Dismajorroad were positively correlated with the presence of ABBs. However, Disreserve, Ele, and Water were negatively associated with the distribution of ABBs (Figure 3). The cumulative weight of each factor showed that the importance of Forest, Dismajorroad, Disreserve, Water was more significant. These four factors mainly affected the distribution of ABBs. While other factors made low contributions to the distribution of ABBs. Thus, we believed that they exert a low impact on distribution (Table 2). Forests are closely related to ABB’s distribution, and the forest coverage rate directly affects distribution. Water is one of the indispensable conditions for animal survival. Our results show that when selecting habitat, ABBs tend to avoid the main road, which is consistent with other studies and supports the anthropogenic risk avoidance hypothesis (Gantchoff et al., 2019).
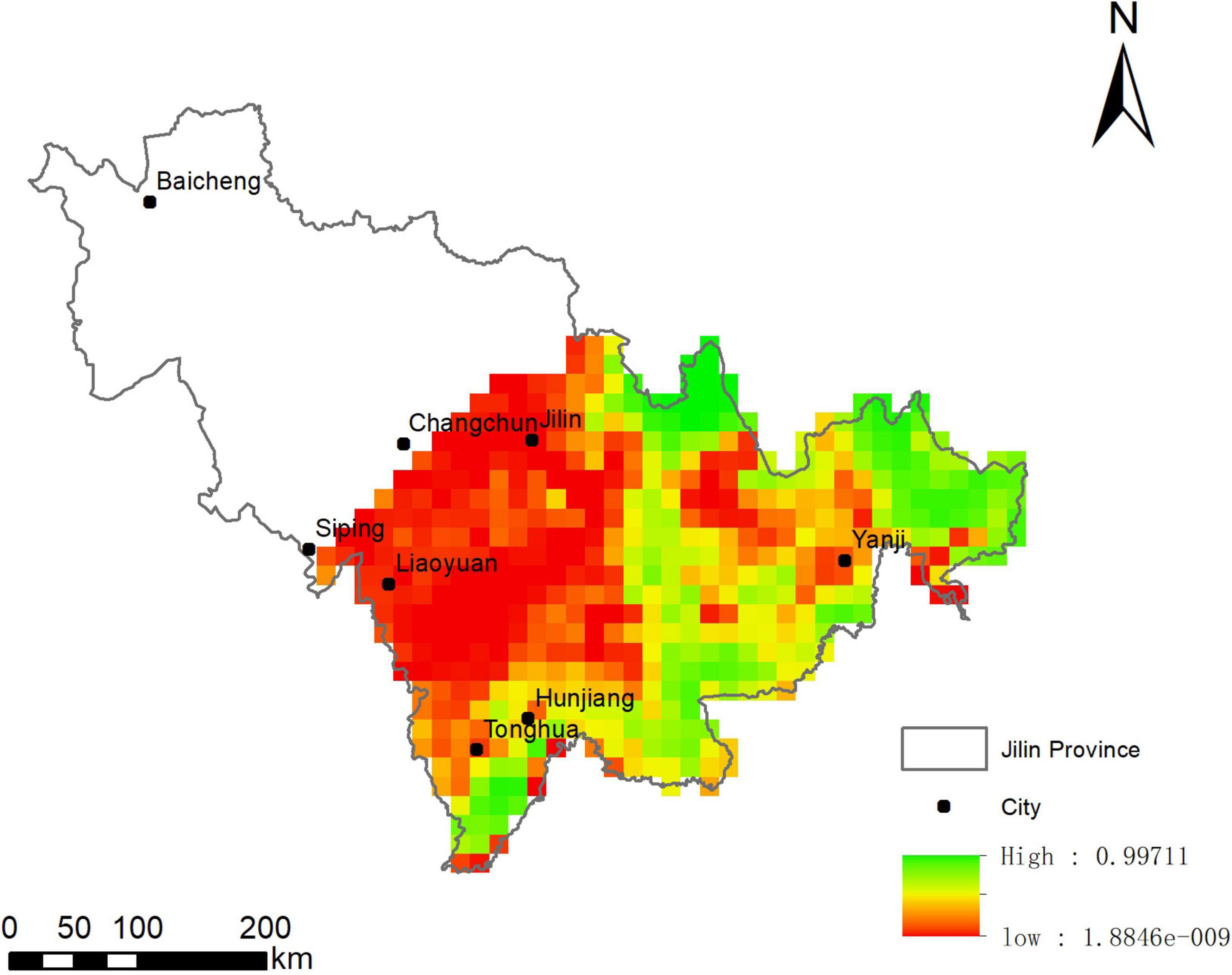
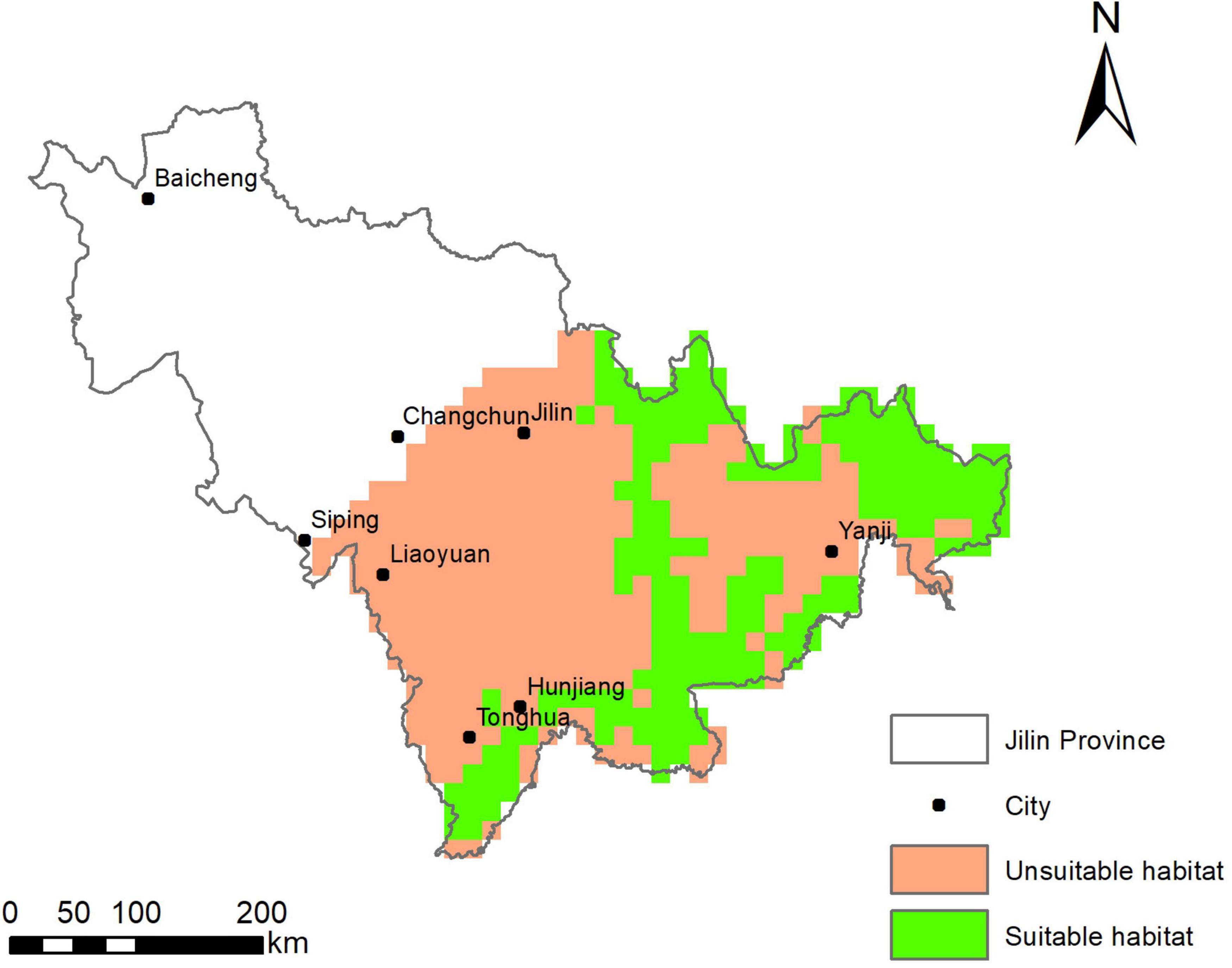
The habitat suitability for ABBs predicted by logistic regression in Jilin province was shown in Figure 4. The region is divided into 540 grids, in which 112 grids are randomly selected as the investigation target. The results of prediction modeling showed that 275 grids contained habitat suitable for ABBs, accounting for 50.9% of the entire sampling area (Figure 5).
Comparison of Field and Questionnarire Survey Results
The questionnaire survey is a key component of the field survey because the relevant information about ABBs provided by interviewees is crucial to the latter. We identified three possible levels of awareness regarding ABBs. When the responses provided by an interviewee are consistent with the field results, his awareness level is considered as high; when the answers provided by an interviewee are inconsistent with the field, his awareness level is considered as medium; when an interviewee cannot provide ABB-related information, his awareness level is considered as low.
The statistical results show that among 319 questionnaires, 41 show unawareness of black bear-relevant information, eight manifest information inconsistent with field results, and 270 display information consistent with field results. Questionnaires identified as “unaware” are not considered as valid questionnaires. Questionnaires with results consistent with the field survey results account for 97.1% of total questionnaires, which manifests that local resident understand the existence of ABBs.
Status and Trend of Population
About 176 of 319 local resident judged the population dynamics in the past decade. Among those 176 residents, 115 claimed ABB population had increased, five said it was stable, and ten asserted it was in decline. The questionnaires show that 65.3% of the interviewees believe the population of ABBs has increased in the past ten years; 3.4% think the population has stabilized, and 31.3% think the population has decreased. The number of people who believe the increase of the ABB population is twice as much as the number of people who hold the opposite opinion (Table 3).
In our field investigation in “presence” grids, 115 respondents accurately provided the distribution information (High), 25 respondents did not know the distribution information (Low), and three respondents claimed there were no ABBs (Middle). These respondents also had different understandings of the population dynamics (Table 4).
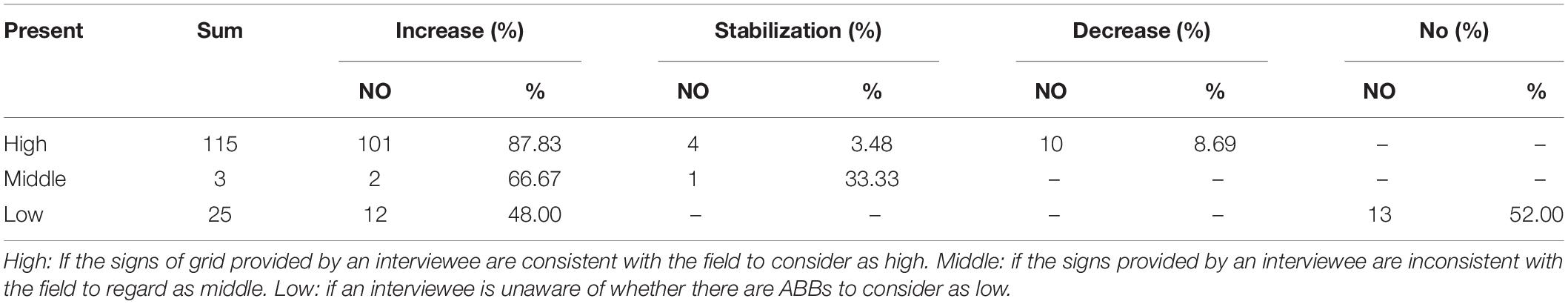
Table 4. Results of reported trends of the Asiatic Black Bear population size in the past ten years’ questionnaires in “presence” grids.
In the field investigation in “absence” grids, 155 respondents accurately provided the distribution information (High), 16 respondents did not know the distribution information (Low), and five claimed there were no ABBs (Middle). And the interviewees’ understanding of the population dynamics of ABBs also differed (Table 5).
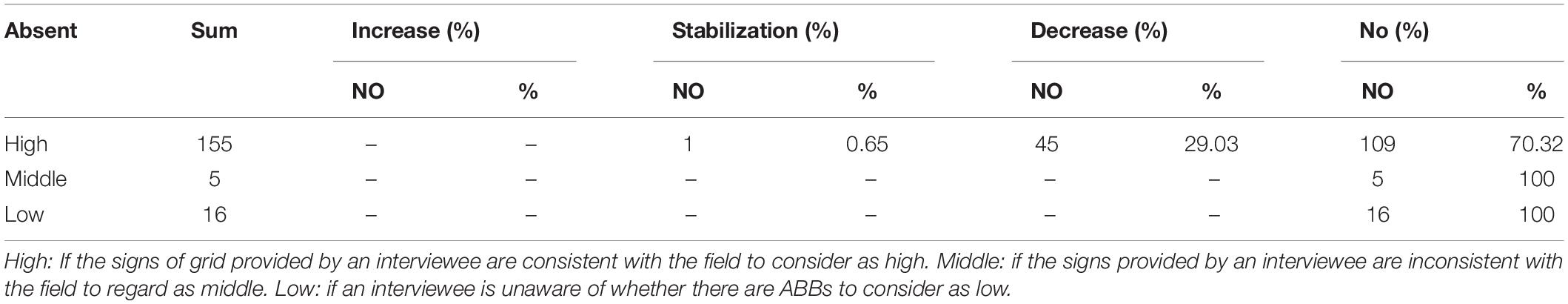
Table 5. Results of reported trends of the Asiatic Black Bear population size in the past ten years’ questionnaires in “absence” grids.
Attitude Towards Black Bears
The attitudes of interviewees toward ABBs were divided into three categories: positive, neutral, and negative. About 236 of the 355 respondents expressed their attitude. Interviewees with a positive attitude accounted for 19% of the total; 43.2% expressed neutral attitude; and 37.8% expressed negative attitude (Table 6). People with a neutral attitude mainly live in grids without ABB distribution. They hold a neutral attitude because they have not been in contact with ABBs and are not familiar with them. Interviewees with a positive attitude believed that ABBs are a nationally protected species and an integral part of the ecosystem; while people with a negative attitude consider ABBs as highly aggressive animals with vicious appearance and cause damage to crops and livestock.
To understand what affects the attitudes of local residents, factors are divided into different types, including age (younger than 35; between 35 and 55; older than 55), gender (male; female), education (educated; uneducated), understanding of ABBs (low; medium; high), and living in the presence or absence grid (presence; absence).
Interviewees without explicit attitudes due to the ABB’s absence are excluded from the analysis. And results show that, among the five factors, only the presence or absence in the grid is significantly correlated with the locals’ positive or negative attitude (χ2 = 6.784, df = 1, P = 0.009), which means negative attitude is strongly related to the presence of ABBs in the area.
Discussion
We made a detailed distribution map (Figure 5) for the ABBs in the eastern mountainous area from the research, which is the most accurate until now. In the past, more than 20 years ago, only the county level was taken as the minimum research unit for ABBs. It also cannot accurately describe the factors affecting the distribution of ABBs, of which the data was collected by mainly interviewing without on-the-spot verification, leading to possible errors (Zou and Ma, 1997). The area of the county-level administrative units varies greatly from 1,142.65 to 9,016 km2, hence it is actually of little significance and relevance for the conservation planning of ABBs. In comparison, our study is more practical for the planning of suitable habitat protection. Our method is based on a random sampling of 27 combinations of three factors that affect the distribution of ABBs. The survey grids are set according to research purposes instead of administrative boundaries. The results provide a reference for the current distribution and a basis for the protection of suitable habitats in Jilin Province. We suggest that similar surveys should be conducted on a five-year basis and attention should be continuously paid to the changes of the distribution of ABBs, especially in areas presently not covered by our survey (McShea et al., 2022).
Compared with DNA capture-recapture method (Miller et al., 1987; Whittington and Sawaya, 2015; Morehouse and Boyce, 2016; Murphy et al., 2017), the distribution range survey is more straightforward and cost-effective (Liu et al., 2009). It is also a more practical method for the grassroot conservation practitioners. According to our visits and field surveys, local residents are familiar with ABBs and their distribution changes. Therefore, we believe that future protection and management could rely on the local administration of forestry, and, more importantly, local residents.
The landfrom of western region of Jilin Province is mainly plains, except for some wetlands and grasslands, most of which are farmland. And the forest coverage rate there is less than 20%. We believe that such habitat is not suitable for the long-term survial of ABBs. Thus, we chose the eastern mountainous area for this research. The forest coverage rates in the 540 selected grids are all greater than 20%. our results suggests that forest coverage is the main factor affecting the existence of ABBs, which is consistent with the results of many studies that forest is indispensable for ABBs (Colton et al., 2021; Tomita and Hiura, 2021). Of course, the prediction results show that ABBs appear in 50.9% of the grids, not in the remaining nearly half of the grids, which we think might be related to the quality of the forest, especially available food (Clark et al., 1994; Costello and Sage, 1994). Hibernators like the black bears are faced with a particular need to consume large amounts of food and store fat before hibernation to provide energy overwinter (Farley and Robbins, 1995; Manchi and Swenson, 2005; Robbins et al., 2012). In order to hibernate and obtain high-quality food resources, black bears will choose migration to satisfy their food needs (Noyce and Garshelis, 2011). So the availability of food is very important. Previous studies have shown that logging destroys stand composition and reduces berry abundance, leading to a decrease in edible food for bears (Bergstedt and Milberg, 2001). Forest logging has a significant impact on the availability of food for bears. Commercial forestry shapes the distribution and abundance of food for bears and consequently affects bear foraging patterns (Hertel et al., 2016). Resources limit the choice of animal habitat. Female black bears have higher requirements for the spatial distribution of resources with moderate resource thresholds and low levels of resource depression (Mitchell and Powell, 2007). Food resources also affect black bear reproduction and offspring rearing (Noyce and Garshelis, 1994; Costello et al., 2009). Our results found that the distribution of ABBs is negatively correlated with the distance from the protected area, which shows that the reserve is the most suitable habitat for ABBs. The quality of habitat in the protected area is higher than that outside due to forbidding logging, strict management, regular patrol, and legislative protection (Watson et al., 2014). Therefore, habitat quality is closely related to the distribution of black bears, and restoring forest coverage rate and habitat quality is the prerequisite for protecting black bears.
Roads are one of the main factors that threaten bears’ survival (Jacobson et al., 2016), especially highways, which fragment the habitat and risk lives (death by animal collisions) (Find’o et al., 2019; Pollock, 2019). Our research results show that the ABBs avoid the main road, which is consistent with many studies. Black bears avoid roads with heavy traffic (Find’o et al., 2019). Major roads (highways and train tracks) connect big cities with heavy traffic, and railings on train tracks also hinder bear migration. However, the density of roads in the grid is positively correlated with the distribution. This result does not align with the current research trend (Bischof et al., 2017), but some studies show that ABBs may choose small roads (Duquette et al., 2017). Bears did not shy away from the unpaved roads, frequently crossing and moving around the unpaved roads (Graham et al., 2010; Stillfried et al., 2015). The roads in our study were mainly rural trails with less traffic and were used only in spring (planting) and autumn (harvesting),and the cumulative weighting of road density indicates the impact is low (0.227 m/km2). Since our survey grid area is large, the actual road density is very low in the grids. Furthermore, the roads in the grid are not evenly distributed, and most are small unpaved roads. Perhaps, the ABBs have more habitat choices in areas where the roads are scattered. Nevertheless, more fine-scale road and habitat data should be examined.
Water is one of the indispensible conditions for the survival of wild animals. Our result found that the distribution of ABBs is negatively correlated with the area of water in the grid, which does not indicate that water is not essential to ABBs. The mountainous areas in eastern Jilin Province are closer to the ocean, affected by the maritime climate, where there is plenty of precipitation in summer and snow in winter forming more forest creeks and puddles to provide enough water for bears. Therefore, the eastern mountainous area is not short of water. The water factor selected is the area of water resources in grid. Larger areas are generally rivers (e.g., Suifen River, Songhua River Basin, Dongliao River Basin, Yalu River, Tumen River) and lakes (e.g., Changbai Mountain Tianchi). The riparian are almost occupied by farmland, water conservancy project (reservoir), aquaculture (fish or frog ponds), roads, cities, and residential areas, which may have deterred the ABBs from visiting. Urbanization is likely to increase animal collisions and conflicts between humans and animals, which not only threatens the survival of animals but also is adverse to their habitats (Baruch-Mordo et al., 2014; Hung et al., 2017). Although the cities may provide food for them, their threat is increasing (Penteriani et al., 2018). Our results also show that ABBs avoid cities, but the cumulative weight of the factor is low, which may be caused by the small scale and low proportion of cities.
The coverage of shrubs is a positive factor for ABBs’ distribution. The berries in the shrubs are abundant and are the favorite food of bears in autumn (McLellan and McLellan, 2015). Therefore, the shrubs may be a positive driver of their distribution. The vegetation changes as the altitude increases. High-altitude areas are generally occupied by coniferous forests, alpine meadows, and bare lands, where there is less food resources for ABBs (Milakovic et al., 2012), resulting in a drop in the abundance of ABBs.
The local residents’ judgment on the presence of ABBs matches the results of our field investigations to demonstrate that people in this area are, to a large extent, aware of the distribution of ABBs. Among the interviewees who believe the population has declined, 82% live in the area without ABBs distribution, And only 18% live in ABB-distributed area, who are all over 35 years old. Interviewees who do not have a clear memory about the past ten years but often recall it 30 years ago when they were children and claim there were back then. They believe the population has decreased, but there is no accurate information about the population trend in the past ten years.
In “presence” areas, interviewees who believe the population has increased are 11.5 times more than those who hold the opposite opinion, demonstrating that the population has risen in “presence” areas in the past ten years. In addition, among the ten interviewees who considered it to have decreased, eight did not provide any judgment but were advised by others, which we did not regard as valid.
The main signs, as given by the interviewees, for the perceived increase in ABBs are, first, the number of traces in the distribution area has increased; second, news about ABBs harming local resident has increased; third, more images of ABBS were captured by camera traps.
Possible reasons for the increase in ABB maybe, first, the nationwide ban of guns in 1996; second, the implementation of wildlife protection laws, especially in Jilin Province, where hunting was banned in the 1990s; third, the total forest area has increased due to the implementation of The Natural Forest Protection Project, fourth, protected areas were established; fifth, after the ban on logging, the number of forestry workers has dramatically reduced, and even some areas are uninhabited, so overall human interference has dwindled.
As such, we believe that the local residents’ understanding of ABBs is relatively accurate. In areas with ABBs’ distribution, most people believe that the number of ABBs has increased, and they have given reasonable explanations. However, we found that most local resident dislike ABBs. The main reason is that they damage crops and livestock, and hurt and threaten people. We have also found that the presense of ABBs is an essential factor in determining attitudes. In recent years, there have been media reports of black bears hurting people in some areas, which has frightened many people. Therefore, we believe that the increase of ABB population or the expansion of their distribution may lead to intensified human-bear conflicts. Although humans may not pose a threat to ABBs since the current laws protects wild animals, the emergence of human-wildlife conflicts will inevitably lead to crop and livelihood losses and the resulting retaliation may thus pose a threat to ABBs. We propose that publicity campaigns and conservation education should be strengthened over time. The management of ABBs should be enhanced to reduce the risk of escalating human-bear conflicts (Kaczensky et al., 2004; Eklund et al., 2020; Oražem et al., 2021). Our result can provide a reference for future wildlife management planning and formulate an appropriate plan to monitor and deal with human-bear conflicts as soon as possible to better deal with the relationship between conservation and interests. Jilin Province started the Natural Forest Protection Project and restored grain plots to forested lands in the 1990s. At the same time, logging was banned entirely in 2015. We believe that, with time, the quality of the forest will gradually recover, which will lead to an expanding distribution of ABBs.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
DH was mainly responsible for the field survey, data analysis, and manuscript writing. ZX was responsible for data analysis and manuscript revision. ZM supervised manuscript writing and helped with data analysis. RX was accountable for manuscript revision and field investigation coordination of various management departments. TL helped revise the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank The National Forestry and Grassland Administration of China for its funding support. Thanks to the IUCN expert for guidance to manuscript design, and discussion of the results. Thanks to Dave Garshelis, Hank Jenkins, Brendan Moyle, Wang Haibin, and Amy Hinsley. Thanks to the Jilin Provincial staff for cooperating with the investigation. Thanks to our project team members who conducted the field survey.
Footnotes
References
Aguilera-Alcalá, N., Morales-Reyes, Z., Martín-López, B., Moleón, M., and Sánchez-Zapata, J. A. (2020). Role of scavengers in providing non-material contributions to people. Ecol. Indic. 117:106643. doi: 10.1016/j.ecolind.2020.106643
Akaike, H. (1981). Likelihood of a model and information criteria. J. Econom. 16, 3–14. doi: 10.1016/0304-4076(81)90071-3
Antiqueira, P. A. P., Petchey, O. L., and Romero, G. Q. (2018). Warming and top predator loss drive ecosystem multifunctionality. Ecol. Lett. 21, 72–82.
Baruch-Mordo, S., Breck, S. W., Wilson, K. R., and Theobald, D. M. (2008). Spatiotemporal distribution of black bear−human conflicts in Colorado, USA. J. Wildl. Manag. 72, 1853–1862. doi: 10.2193/2007-442
Baruch-Mordo, S., Wilson, K. R., Lewis, D. L., Broderick, J., Mao, J. S., and Breck, S. W. (2014). Stochasticity in natural forage production affects use of urban areas by black bears: implications to management of human-bear conflicts. PLoS One 9:e85122. doi: 10.1371/journal.pone.0085122
Bergstedt, J., and Milberg, P. (2001). The impact of logging intensity on field-layer vegetation in Swedish boreal forests. For. Ecol. Manag. 154, 105–115. doi: 10.1016/S0378-1127(00)00642-3
Bischof, R., Steyaert, S. M., and Kindberg, J. (2017). Caught in the mesh: roads and their network−scale impediment to animal movement. Ecography 40, 1369–1380. doi: 10.1111/ecog.02801
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference A Practical Information-Theoretic Approach, Edn 2 Edn. Berlin: Springer Science & Business Media.
Cardinale, B. J., Duffy, J. E., Gonzalez, A., Hooper, D. U., Perrings, C., Venail, P., et al. (2012). Biodiversity loss and its impact on humanity. Nature 486, 59–67.
Carter, N. H., Brown, D. G., Etter, D. R., and Visser, L. G. (2010). American black bear habitat selection in northern Lower Peninsula, Michigan, USA, using discrete-choice modeling. Ursus 21, 57–71. doi: 10.2192/09GR011.1
Clark, J. D., Clapp, D. L., Smith, K. G., and Ederington, B. (1994). Black bear habitat use in relation to food availability in the interior highlands of Arkansas. Bears Their Biol. Manag. 9, 309–318. doi: 10.2307/3872716
Colton, C. P., Coops, N. C., and Burton, A. C. (2021). Grizzly bear (Ursus arctos) responses to forest harvesting: a review of underlying mechanisms and management recommendations. For. Ecol. Manag. 497:119471. doi: 10.1016/j.foreco.2021.119471
Costello, C. M., Creel, S. R., Kalinowski, S. T., Vu, N. V., and Quigley, H. B. (2009). Determinants of male reproductive success in American black bears. Behav. Ecol. Sociobiol. 64, 125–134. doi: 10.1007/s00265-009-0828-0
Costello, C. M., and Sage, R. W. Jr. (1994). Predicting black bear habitat selection from food abundance under 3 forest management systems. Bears Their Biol. Manag. 9, 375–387. doi: 10.2307/3872724
Dai, Y., Hacker, C. E., Zhang, Y., Li, Y., Li, J., Xue, Y., et al. (2020). Conflicts of human with the Tibetan brown bear (Ursus arctos pruinosus) in the Sanjiangyuan region, China. Glob. Ecol. Conserv. 22:e01039. doi: 10.1016/j.gecco.2020.e01039
Du, H. (2018). The Denning Habitat of Asiatic Black Bear (Ursus thibetanus) During Winter in the Wangqing Region, Jilin Prov. China. Master thesis. Harbin: Northeast Forestry University.
Duquette, J. F., Belant, J. L., Wilton, C. M., Fowler, N., Waller, B. W., and Beyer, D. E. Jr., et al. (2017). Black bear (Ursus americanus) functional resource selection relative to intraspecific competition and human risk. Can. J. Zool. 95, 203–212. doi: 10.1139/cjz-2016-0031
Eklund, A., Johansson, M., Flykt, A., Andrén, H., and Frank, J. (2020). Drivers of intervention use to protect domestic animals from large carnivore attacks. Hum. Dimens. Wildl. 25, 339–354. doi: 10.1080/10871209.2020.1731633
Enders, M. S., and Vander Wall, S. B. (2012). Black bears Ursus americanus are effective seed dispersers, with a little help from their friends. Oikos 121, 589–596. doi: 10.1111/j.1600-0706.2011.19710.x
Farley, S. D., and Robbins, C. T. (1995). Lactation, hibernation, and mass dynamics of American black bears and grizzly bears. Can. J. Zool. 73, 2216–2222. doi: 10.1139/z95-262
Find’o, S., Skuban, M., Kajba, M., Chalmers, J., and Kalaš, M. (2019). Identifying attributes associated with brown bear (Ursus arctos) road-crossing and Roadkill sites. Can. J. Zool. 97, 156–164. doi: 10.1139/cjz-2018-0088
Franklin, J. (2010). Mapping Species Distributions: Spatial Inference and Prediction. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511810602
Gantchoff, M. G., Beyer, D., and Belant, J. L. (2019). Reproductive class influences risk tolerance during denning and spring for American black bears (Ursus americanus). Ecosphere 10:e02705. doi: 10.1002/ecs2.2705
García-Rodríguez, A., Albrecht, J., Szczutkowska, S., Valido, A., Farwig, N., and Selva, N. (2021). The role of the brown bear Ursus arctos as a legitimate megafaunal seed disperser. Sci. Rep. 11:1282. doi: 10.1038/s41598-020-80440-9
Garshelis, D., and Steinmetz, R. (2020). Ursus thibetanus (amended version of 2016 assessment). IUCN Red List Threat. Species 2020:e.T22824A166528664.
Graber, D. M., and White, M. (1983). Black bear food habits in Yosemite National Park. Bears Biol. Manage. 5, 1–10.
Graham, K., Boulanger, J., Duval, J., and Stenhouse, G. (2010). Spatial and temporal use of roads by grizzly bears in west-central Alberta. Ursus 21, 43–56. doi: 10.2192/09GR010.1
Gregório, I., Barros, T., Pando, D., Morante, J., Fonseca, C., and Ferreira, E. (2020). Paths for colonization or exodus? New insights from the brown bear (Ursus arctos) population of the Cantabrian Mountains. PLoS One 15:e0227302. doi: 10.1371/journal.pone.0227302
Hertel, A. G., Steyaert, S. M., Zedrosser, A., Mysterud, A., Lodberg-Holm, H. K., Gelink, H. W., et al. (2016). Bears and berries: species-specific selective foraging on a patchily distributed food resource in a human-altered landscape. Behav. Ecol. Sociobiol. 70, 831–842. doi: 10.1007/s00265-016-2106-2
Hooker, M. J., Jared, D. A., Warren, R. J., Miller, K. V., and Chamberlain, M. J. (2020). Characterizing American black bear (Ursus americanus) highway crossing locations in central Georgia. J. Southeast Assoc. Fish Wildl. Agencies 7, 227–237.
Hung, K.-L. J., Ascher, J. S., and Holway, D. A. (2017). Urbanization-induced habitat fragmentation erodes multiple components of temporal diversity in a Southern California native bee assemblage. PLoS One 12:e0184136. doi: 10.1371/journal.pone.0184136
Hwang, M.-H. (2003). Ecology of Asiatic Black Bears and People-Bear Interactions in Yushan National Park, Taiwan. Minneapolis, MN: University of Minnesota.
Jacobson, S. L., Bliss-Ketchum, L. L., de Rivera, C. E., and Smith, W. P. (2016). A behavior−based framework for assessing barrier effects to wildlife from vehicle traffic volume. Ecosphere 7:e01345. doi: 10.1002/ecs2.1345
Jarzyna, M. A., and Jetz, W. (2017). A near half−century of temporal change in different facets of avian diversity. Glob. Change Biol. 23, 2999–3011. doi: 10.1111/gcb.13571
Jiménez-Valverde, A. (2012). Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 21, 498–507. doi: 10.1111/j.1466-8238.2011.00683.x
Kaczensky, P., Blazic, M., and Gossow, H. (2004). Public attitudes towards brown bears (Ursus arctos) in Slovenia. Biol. Conserv. 118, 661–674. doi: 10.1016/j.biocon.2003.10.015
Keil, P., Storch, D., and Jetz, W. (2015). On the decline of biodiversity due to area loss. Nat. Commun. 6:8837. doi: 10.1038/ncomms9837
Kelly, M. J., and Holub, E. L. (2008). Camera trapping of carnivores: trap success among camera types and across species, and habitat selection by species, on Salt Pond Mountain, Giles County, Virginia. Northeast. Nat. 15, 249–262. doi: 10.1656/1092-6194(2008)15[249:CTOCTS]2.0.CO;2
Koike, S., Morimoto, H., Kozakai, C., Arimoto, I., Yamazaki, K., Iwaoka, M., et al. (2012). Seed removal and survival in Asiatic black bear Ursus thibetanus faeces: effect of rodents as secondary seed dispersers. Wildl. Biol. 18, 24–34.
Kopatz, A., Kleven, O., Kojola, I., Aspi, J., Norman, A. J., Spong, G., et al. (2021). Restoration of transborder connectivity for Fennoscandian brown bears (Ursus arctos). Biol. Conserv. 253:108936. doi: 10.1016/j.biocon.2020.108936
Layman, C. A., Quattrochi, J. P., Peyer, C. M., and Allgeier, J. E. (2007). Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944. doi: 10.1111/j.1461-0248.2007.01087.x
Lázaro, A., Fuster, F., Alomar, D., and Totland, Ø. (2020). Disentangling direct and indirect effects of habitat fragmentation on wild plants’ pollinator visits and seed production. Ecol. Appl. 30:e02099. doi: 10.1002/eap.2099
Liu, F. (2009). Research on Spatial Distribution Patterns of Asiatic Black Bear (Ursus thibetanus) and Human-Bear Interactions in Sichuan Province. Ph.D. thesis. Beijing: Peking University.
Liu, F., McShea, W., Garshelis, D., Zhu, X., Wang, D., Gong, J., et al. (2009). Spatial distribution as a measure of conservation needs: an example with Asiatic black bears in south−western China. Divers. Distrib. 15, 649–659. doi: 10.1111/j.1472-4642.2009.00571.x
Ma, Y., Xu, L., and Hu, J. (1998). On the Resources and Conservation of Bears in China. Life Sci. Res. 3, 205–211.
Manchi, S., and Swenson, J. E. (2005). Denning behaviour of Scandinavian brown bears Ursus arctos. Wildl. Biol. 11, 123–132. doi: 10.2981/0909-6396(2005)11[123:DBOSBB]2.0.CO;2
McLellan, M. L., and McLellan, B. N. (2015). Effect of season and high ambient temperature on activity levels and patterns of grizzly bears (Ursus arctos). PLoS One 10:e0117734. doi: 10.1371/journal.pone.0117734
McShea, W. J., Hwang, M.-H., Liu, F., Li, S., Lamb, C., McLellan, B., et al. (2022). Is the delineation of range maps useful for monitoring Asian bears? Glob. Ecol. Conserv. 35:e02068. doi: 10.1016/j.gecco.2022.e02068
Milakovic, B., Parker, K. L., Gustine, D. D., Lay, R. J., Walker, A. B., and Gillingham, M. P. (2012). Seasonal habitat use and selection by grizzly bears in northern British Columbia. J. Wildl. Manag. 76, 170–180. doi: 10.1002/jwmg.235
Miller, S. D., Becker, E. F., and Ballard, W. B. (1987). Black and brown bear density estimates using modified capture-recapture techniques in Alaska. Bears Their Biol. Manag. 7, 23–35. doi: 10.2307/3872604
Mitchell, M. S., and Powell, R. A. (2007). Optimal use of resources structures home ranges and spatial distribution of black bears. Anim. Behav. 74, 219–230.
Moleon, M., Sanchez-Zapata, J. A., Margalida, A., Carrete, M., Owen-Smith, N., and Donazar, J. A. (2014). Humans and scavengers: the evolution of interactions and ecosystem services. Bioscience 64, 394–403. doi: 10.1093/biosci/biu034
Morales-González, A., Ruiz-Villar, H., Ordiz, A., and Penteriani, V. (2020). Large carnivores living alongside humans: brown bears in human-modified landscapes. Glob. Ecol. Conserv. 22:e00937. doi: 10.1016/j.gecco.2020.e00937
Morehouse, A. T., and Boyce, M. S. (2016). Grizzly bears without borders: spatially explicit capture–recapture in Southwestern Alberta. J. Wildl. Manag. 80, 1152–1166. doi: 10.1002/jwmg.21104
Murphy, S. M., Augustine, B. C., Ulrey, W. A., Guthrie, J. M., Scheick, B. K., McCown, J. W., et al. (2017). Consequences of severe habitat fragmentation on density, genetics, and spatial capture-recapture analysis of a small bear population. PLoS One 12:e0181849. doi: 10.1371/journal.pone.0181849
Noyce, K. V., and Garshelis, D. L. (1994). Body size and blood characteristics as indicators of condition and reproductive performance in black bears. Bears Their Biol. Manag. 9, 481–496. doi: 10.2307/3872736
Noyce, K. V., and Garshelis, D. L. (2011). Seasonal migrations of black bears (Ursus americanus): causes and consequences. Behav. Ecol. Sociobiol. 65, 823–835. doi: 10.1007/s00265-010-1086-x
Nozaki, E., Azuma, S., Aoi, T., Torii, H., Ito, T., and Maeda, K. (1983). Food habits of Japanese black bear. Bears Biol. Manage. 5, 106–109.
Oražem, V., Smolej, T., and Tomažič, I. (2021). Students’ attitudes to and knowledge of brown bears (Ursus arctos L.): can more knowledge reduce fear and assist in conservation efforts? Animals 11:1958. doi: 10.3390/ani11071958
Pearce, J., and Ferrier, S. (2000). Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 133, 225–245. doi: 10.1016/S0304-3800(00)00322-7
Penteriani, V., Delgado, M. D. M., Krofel, M., Jerina, K., Ordiz, A., Dalerum, F., et al. (2018). Evolutionary and ecological traps for brown bears Ursus arctos in human−modified landscapes. Mamm. Rev. 48, 180–193. doi: 10.1111/mam.12123
Piao, Z., Piao, L., Wang, Z., Luo, Y., Wang, C., and Sui, Y. (2012). Population size variation of black bear (Ursus thibetanus) and brown bear (U. arctos) between 1986 to 2010 in the Changbai Mountain Nature Reserve, China. Chin. J. Zool. 47, 66–72.
Pollock, J. F. (2006). Detecting population declines over large areas with presence−absence, time−to−encounter, and count survey methods. Conserv. Biol. 20, 882–892. doi: 10.1111/j.1523-1739.2006.00342.x
Pollock, S. Z. (2019). The Influence of a Railway on Grizzly Bears (Ursus arctos) in Canada’s Rocky Mountain Parks. Doctoral Distraction. Berlin: Springer.
Raine, R. M., and Kansas, J. L. (1990). Black bear seasonal food habits and distribution by elevation in Banff National Park, Alberta. Bears Biol. Manage. 8, 297–304.
Reid, D., Jiang, M., Teng, Q., Qin, Z., and Hu, J. (1991). Ecology of the Asiatic Black Bear (Ursus thibetanus) in Sichuan, China. New York, NY: Walter de Gruyter. doi: 10.1515/mamm.1991.55.2.221
Robbins, C. T., Lopez-Alfaro, C., Rode, K. D., Tøien, Ø., and Nelson, O. L. (2012). Hibernation and seasonal fasting in bears: the energetic costs and consequences for polar bears. J. Mammal. 93, 1493–1503. doi: 10.1644/11-MAMM-A-406.1
Rodríguez-Lozano, P., Verkaik, I., Rieradevall, M., and Prat, N. (2015). Small but powerful: top predator local extinction affects ecosystem structure and function in an intermittent stream. PLoS One 10:e0117630. doi: 10.1371/journal.pone.0117630
Rogers, L. L. (1993). “The role of habitat quality in the natural regulation of black bear populations,” in Proceedings of the Western Black Bear Workshop, Yosemite National Park, CA, 95–102.
Saura, S. (2021). The habitat amount hypothesis implies negative effects of habitat fragmentation on species richness. J. Biogeogr. 48, 11–22. doi: 10.1111/jbi.13958
Schmitz, O. J. (2008). Effects of predator hunting mode on grassland ecosystem function. Science 319, 952–954. doi: 10.1126/science.1152355
Stillfried, M., Belant, J. L., Svoboda, N. J., Beyer, D. E., and Kramer-Schadt, S. (2015). When top predators become prey: black bears alter movement behaviour in response to hunting pressure. Behav. Processes 120, 30–39. doi: 10.1016/j.beproc.2015.08.003
Sun, H., Li, L., Yin, Y., Lu, X., Zhou, S., Tian, J., et al. (2015). Monitoring of mammals in the distribution area of Amur tiger with automatic camera. For. Sci. Technol. 40, 51–55.
Tomita, K., and Hiura, T. (2021). Reforestation provides a foraging habitat for brown bears (Ursus arctos) by increasing cicada Lyristes bihamatus density in the Shiretoko world heritage site. Can. J. Zool. 99, 205–212. doi: 10.1139/cjz-2020-0222
Watson, J. E., Dudley, N., Segan, D. B., and Hockings, M. (2014). The performance and potential of protected areas. Nature 515, 67–73.
Whittington, J., and Sawaya, M. A. (2015). A comparison of grizzly bear demographic parameters estimated from non-spatial and spatial open population capture-recapture models. PLoS One 10, e0134446. doi: 10.1371/journal.pone.0134446
Keywords: Asiatic black bear, spatial distribution, population dynamics, conservation awareness, Ursus thibetanus
Citation: Hairong D, Xiaoliang Z, Minghai Z, Xiangdong R and Lee TM (2022) Spatial Distribution and Conservation Strategies of Large Carnivores in Human-Dominated Landscape: A Case Study of Asiatic Black Bear in Jilin, China. Front. Ecol. Evol. 10:882282. doi: 10.3389/fevo.2022.882282
Received: 23 February 2022; Accepted: 11 May 2022;
Published: 01 June 2022.
Edited by:
Guangyu Wang, University of British Columbia, CanadaReviewed by:
Sathyakumar Sambandam, Wildlife Institute of India, IndiaJennifer Vonk, Oakland University, United States
Copyright © 2022 Hairong, Xiaoliang, Minghai, Xiangdong and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Minghai, emhhbmdtaW5naGFpMjAwNEAxMjYuY29t; Ruan Xiangdong, eGRydWFuQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Du Hairong
Du Hairong Zhi Xiaoliang1†
Zhi Xiaoliang1† Ruan Xiangdong
Ruan Xiangdong Tien Ming Lee
Tien Ming Lee