- Department of Game Management and Wildlife Biology, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Prague, Czechia
This article surveys more than three decades of research on Zambian mole-rats (genus Fukomys, Bathyergidae), pointing out some unanswered questions and untested hypotheses and suggesting approaches to address them. These research proposals range from sensory ecology topics, the main research field, covering different (even not yet identified) senses, orientation in time and space, communication, studies on aging, population dynamics, and the survival strategies of mole-rats during yearly floodings in the Kafue Flats. Discussion includes cryptozoological investigation into the existence of strange mole-rat species in some Zambian localities as reported by local communities, the study of mole-rats in assumed contact (hybrid?) zones of special interest, (cyto)genetic studies of hybrids of selected species, and a non-invasive study of population and family structure and dynamics with help of endoscopes. In each case, there is a rationale, reasoning, hypothesis, and suggested methodical approach.
Introduction
Thirty-seven years ago (1985), in Lusaka, Zambia, I first held a mole-rat, now known as Fukomys anselli, in my hands. In 1986, I was the first to bring these animals out of Africa, to Germany, where I succeeded in breeding them. This began my 33-year-long career, involving (mainly laboratory) multi- and interdisciplinary research on mole-rats. In 1990, my student assistant from the University of Zambia, and later Ph.D. student at the University of Duisburg-Essen, friend and colleague, Mathias Kawalika, moved from Lusaka to Ndola and became acquainted with the local giant mole-rats, now known as Fukomys mechowii. These guinea-pig-sized animals live in larger families, the biology of which was virtually unknown to scholars at that time, though they were familiar to local people. Dr. Kawalika sent me several live animals. The Zambian mole-rats were kept and studied at my home and in institutions where I worked, including J. W. Goethe-University (Zoological Institute, Faculty of Biology and Senckenberg Institute of Anatomy, Faculty of Medicine) in Frankfurt am Main, and at the University Duisburg-Essen (Department of General Zoology, Faculty of Biology) in Essen.
Both species were investigated mainly in the laboratory for diverse aspects of sensory biology, anatomy, physiology, systematics and taxonomy, and behavioral biology. The research was later extended to field studies by former students and colleagues. Existing knowledge on the Zambian mole-rats was recently summarized in Begall et al. (2021) and Caspar et al. (2021a).
Active research on mole-rats had ended with my retirement in 2018, but the baton has been handed down to my students. Because of mainly financial and manpower-related reasons as well as lack of time, several research ideas were never realized, and these hypotheses have never been published. Being convinced that they do not deserve to be forgotten, I would like to share these ideas here in the hope that they might trigger interest and inspire the next generation of young researchers.
Sensory Ecology
Sensory ecology explores “how the animals acquire information and respond to it” (Dusenbery, 1992). Bathyergid mole-rats (as well as other subterranean mammals) are excellent animal models to investigate these questions. They live in a monotonous, uniform, simple sensory environment, deprived of most cues and signals available to animals aboveground, yet they must also orient in space and time, forage, find partners, communicate, and be warned of danger. On one hand, we can expect degeneration of senses because of lack of use, we must also expect specializations and specific adaptations to compensate for handicaps. My interest in the biology of subterranean mammals in general and in their senses, in particular, was drawn in 1979 by a seminal review article by Eviatar (Eibi) Nevo (1979). In the meantime, this field has become a goldmine of research for many students and scholars (reviewed in, e.g., Burda et al., 1990a; Burda, 2006, 2021; Begall et al., 2007a,b; Buffenstein et al., 2021, and numerous papers cited therein and in this article). I would like to point out here some persisting questions.
Hearing
Subterranean mammals rely to a great extent on audition for communication and to be alerted to danger. The only hitherto published reports on burrow acoustics revealed that in the tunnels of blind mole-rats (Spalax), airborne sounds of 440 Hz propagated best whereas lower and higher frequencies were effectively attenuated (Heth et al., 1986) and that in mole-rat (Fukomys) tunnels, low-frequency sounds (200–800 Hz) are not only least attenuated but also their amplitude may be amplified like in a stethoscope (up to twice over 1 m) (Lange et al., 2007). Morpho-functional analyses classified the ear of subterranean mammals as a low-sensitivity and low-frequency device (i.e., Burda et al., 1992, 1989; Burda, 1990; Müller et al., 1989, 1992; Mason, 2001; Schleich and Busch, 2004; Lange and Burda, 2005; Mason et al., 2016; Pleštilová et al., 2016, 2021). Concordantly, hearing (at least in the studied bathyergid, spalacid, and geomyid species) is characterized by low sensitivity and a restricted frequency range of best hearing tuned to low frequencies (0.5–4 kHz) (Müller and Burda, 1989; Heffner and Heffner, 1990, 1992, 1993; Kössl et al., 1996; Brückmann and Burda, 1997; Gerhardt et al., 2018; Barker et al., 2021; Caspar et al., 2021b). Some authors considered the restricted hearing in subterranean mammals vestigial and degenerate due to under-stimulation (Heffner and Heffner, 1990, 1992, 1993). In contrast to this view, moe-rats have a rich (mostly low-frequency) vocal repertoire (see paragraph below) and progressive structural specializations of the middle and inner ear. Thus, other authors have considered these hearing characteristics adaptive, in that hearing sensitivity has decreased during the evolution of subterranean mammals to avoid over-stimulation of the ear in their natural environment (Burda, 2006, 2021; Lange et al., 2007).
We may assume that the local increase in amplitude occurs at the expense of decreasing the amplitude somewhere else and that there may be a specific “stethoscope region” at a certain distance from the sound source, where the amplification effect is particularly pronounced. Given the lowest hearing threshold in Fukomys anselli, 7 dB SPL (Brückmann and Burda, 1997), we may expect that the measured stethoscope effect would amplify the signal, lowering the threshold to 0.5 dB SPL. This would mean that in their natural environment, mole-rats display best hearing sensitivity in a range that is comparable to that of most other mammals, including humans. I do not expect that contact communication calls need to be adapted (either in their intensity or even in their frequency) to the tunnel acoustics. Neither do I expect that hearing is primarily adapted to vocal communication. I expect that hearing is adapted to the tunnel acoustics – i.e., hearing of distant (primarily substrate-borne) acoustic cues provides a warning of danger. Vocalization then must match the given hearing range.
Testing the hearing sensitivity of subterranean rodents in a laboratory in the open field may, thus, not correctly reflect their sensory capacities in natural tunnels where certain signals may be enhanced. In analogy with hearing research on dolphins, which is performed in their natural milieu, in water, the hearing parameters of mole-rats should be studied in their natural acoustic environment, in tunnels.
Vocalization
It is generally expected that vocal-repertoire size correlates positively with group size (e.g., McComb and Semple, 2005). It is thus not surprising that with an average colony size of 78 individuals (Sherman et al., 1992) and 12–18 different adult vocalizations (Pepper et al., 1991; Barker et al., 2021, respectively), the naked mole-rat has been long considered to be the rodent with the largest vocal repertoire. (Note that mechanical sounds and juvenile vocalizations are not considered in this survey.) Later studies showed that many Fukomys species with much smaller mean family sizes (7–12 individuals) have a similarly rich adult repertoire compared to that of the naked mole-rat: 16 adult vocalizations in F. mechowii (Bednářová et al., 2013), 13 in F. anselli, (Credner et al., 1997); 11 in Fukomys darlingi (Dvořáková et al., 2016); 10 in Fukomys micklemi (Vanden Hole et al., 2014), while eight sounds were described in solitary Heliophobius argenteocinereus (Knotková et al., 2009). Thus, the richness of the vocal repertoire in African mole-rats may be influenced not only by group size but also by social complexity and stability (cf. Burda et al., 2000).
Importantly, however, to assess the real richness of the vocalization repertoire we have to study it in all potential social interactions (i.e., also during courtship and during the entire period of care for pups), which is not easy since these animals do not breed in captivity. We may expect that vocalization in solitary animals may be more complex and richer than is known. To give an analogy: let us monitor the vocalization of a lonesome human. Unless she/he talks to herself/himself (soliloquy), we would conclude that the vocal repertoire of this human is much more restricted than the vocal repertoire of humans living (and being monitored) in a group. Moreover, in captivity, we will never be able to simulate all possible interactions and different situations, which may occur in nature. To illustrate the issue: an animal may have a specific call if it encounters a snake but until we have simulated such a situation in the laboratory we will never learn about it. Animals in natural tunnels may elicit sounds that we never hear in the open field.
The other question of interest is whether the pups vocalize in the ultrasonic range like pups of muroid rodents (cf. e.g., Okanoya and Screven, 2018). In many epigenetic rodent species, pups are raised similarly to subterranean rodents in closed underground nests, i.e., in a similar acoustic environment. Also, the morphological substrate for vocalization can be expected to be similar in all rodent pups. The problem is, however, that mothers in mole-rats most probably would not hear and react to ultrasonic calls. Nevertheless, it is possible to produce signals/cues even if we cannot perceive them. Just think of producing smell signals carrying information about our sex, stress, emotions, and diseases, which we cannot perceive but a dog can. Such ultrasonic “signals” do not play a role in communication anymore but they might represent a kind of “atavisms.” However, that ultrasonic vocalization was not found in pups of Heterocephalus glaber (Pepper et al., 1991; Barker et al., 2021). Studies that recorded vocalizations in Fukomys (see above) were limited by the technical equipment used, which did register sounds above 20 kHz.
To summarize, the reason we have not recorded the entire vocalization in solitary mole-rats is because they have no opportunity to reveal it in captivity. Moreover, to make conclusions about the role of vocalization in communication, we need to conduct playback experiments. Studies of vocalization in the ultrasonic range in pups would be of interest. Morpho-functional comparative studies of the vocal apparatus of subterranean rodents enabling them vocalization in the low-frequency range are still missing.
Olfaction
The sense of smell of mole-rats has been studied with respect to olfactory discrimination of familiar and unfamiliar individuals, conspecifics (Heth et al., 2002a,2004), and root kairomones (Heth et al., 2002b; Lange et al., 2005). We expect that the underground environment, which lacks air-currents, is not particularly odorant-rich, and that odorants are predictable. This assumption, however, contradicts the conclusions of a study on the diversity of the study of olfactory receptor subgenome within a single family of olfactory genes (OR7), suggesting that mole-rats can recognize a broad range of odorants (Stathopoulos et al., 2014). Behavioral tests of smell sensitivity and the discrimination capacities of mole-rats are needed to learn what are the specific and convergent olfactory capabilities distinguishing subterranean rodents from their epigeic counterparts.
The idea of “blind” foraging in herbivorous underground dwellers (Lovegrove and Wissel, 1988) has been challenged by showing that subterranean rodents of several species use olfaction to discriminate between soils in which plants had or had not been growing (Heth et al., 2002b; Lange et al., 2005). These laboratory studies should be, however, conducted under (semi)natural conditions in the field to learn which geophytes are present, under which conditions, at which distance, and how precisely they can be targeted in the field.
The randomness versus directionality of food searching in mole-rats could be tested in situ using small beds of diverse plants (carrots and potatoes). These can be established (as controls not vegetated squares will be watered) at given distances from an established burrow system in a random pattern. This potential study could assess the probability that and how long it takes, for the mole-rats to discover and exploit these beds.
Burda et al. (1999a) have shown that subterranean rodents generally start to eat carrots offered to them from the lower end of the root while rodents that are not specialized root eaters display no preference for a particular root end, or they eat from the upper end. Although the adaptive meaning and evolutionary origin of this convergent feeding behavior are understandable, the proximate (sensory) basis enabling distinction between the two root poles remains a challenge for future research. The study demonstrated that subterranean rodents can very finely distinguish between different parts of plant organs and exhibit thus unanticipated sensory abilities. A follow-up study of perceptual mechanisms and cues enabling them to identify the polarity of a root may certainly prove to be of great interest, not only for ecologists and sensory biologists but also for botanists and applied sciences such as pest control.
Vision
The eyes of the African mole-rats are relatively small; however, the optical apparatus, including the retina, contain all the structures of a typical mammalian eye (Peichl et al., 2004). The retina is normally developed and possesses all characteristic layers. The photoreceptor layer is dominated by rods but contains an unusually high proportion of cones (approximately 10%), which solely express short-wavelength-sensitive (SWS) cone photopigment (Peichl et al., 2004). The main problem with the interpretation of the anatomical, physiological, and behavioral findings regarding the visual apparatus of subterranean mammals and the open question is, why the eye in Fukomys is superficial and only quantitatively reduced but qualitatively normally developed, while in some other subterranean mammals, like the blind mole rat, Spalax, it is subcutaneous and structurally degenerated? Why Spalax possess a long wave-sensitive-opsin but no SWS-opsin, whereas the situation in Fukomys is the opposite? Although adaptive explanations as well as non-adaptive ones (the side effects of different adaptations) have been suggested [reviewed in Burda (2021)], they always considered each group (Bathyergidae and Spalacidae) separately and not from the point of view of the otherwise apparent convergence.
Distant Thermoperception
Distant thermoperception in dark underground burrows where the ambient temperature is buffered and uniform would be an advantageous sensory ability to be alerted to conspecifics, intruders, open burrows, and even plant storage organs. A variety of infrared radiation (IR)-detecting receptors are present in animals (e.g., crotalid and boid snakes, vampire bats, and some insects) which aid them in foraging or otherwise enhance their fitness [reviewed in Campbell et al. (2002)]. My former colleague at the University Duisburg-Essen, botanist Prof. Hardy Pfanz, drew my attention to the fact that underground plant organs, roots, bulbs, tubers, etc. act as IR-light conductors (Sun et al., 2005). One can indeed wonder why such a sensory ability has not evolved (or has not yet been discovered) in subterranean mammals?
Decades ago, I have observed that Ansell’s mole-rats were able to detect a warm object (a cup) placed in the cage beside their paths and that they approached it and explored it. The observations were only anecdotal but motivated us (Sabine Begall and me) to design a two-choice test-apparatus and experimental protocol 20 years ago. Students performed pilot experiments to test this ability under controlled laboratory conditions. The results were quite promising and we suggested that the specific nose (rhinarium) of mole-rats might be the seat of putative IR-detectors. Our application for research funding was not successful and the project has been abandoned and subsequently forgotten. The idea of distant thermoperception and IR-detection by mole-rats should be recaptured. It is noteworthy that recently distant thermoperception was recorded also in domestic dogs (Bálint et al., 2020). The respective authors further hypothesized that the dog rhinarium is particularly sensitive to radiating heat thanks to its coldness. It may be of interest and significance in the context of distant thermoperception that the nose area in mole-rats is permanently colder than other body areas (Šumbera et al., 2007).
Spatial Orientation (Dead Reckoning and Magnetoreception)
Simple observations of captive mole-rats moving in their home cages and simple maze experiments reveal very good spatial memory, which seems to be based on proprioception and dead reckoning. Navigation over longer distances in the field is probably assisted by magnetoreception. Maze experiments with mole-rats may be complicated by the fact that the animals seem to learn the maze after one or few trials and the “errors” in the following passes represent explorative trips. Mazes should be also longer and/or more complex than those used for experiments with laboratory rodents. Mole-rats are relatively good swimmers (Hickman, 1978) and a water maze may be an option for an experimental design to study the role of magnetoreception in navigation (cf. Phillips et al., 2013).
Experiments in classical and modified mazes and circular arenas have heuristic potential to study navigation strategies (always considering both: dead reckoning and magnetoreception). Care has to be taken, as in all such experiments generally, that we consider and exclude the possibility of leaving olfactory tracks, providing unconscious cues for piloting (e.g., in that the experimentator waits for the animal at the goal/outlet), magnetic disturbances in buildings (particularly at the floor where iron wires, electric and water installation may be laid).
Experiments like those described by Etienne et al. (1993) should be applied also to mole-rats. Experiments should be done with mirrored mazes to find out whether mole-rats use external (allothetic) cues (e.g., magnetic compass direction) or internal (idiothetic) cues as directional reference. Apart from tube perspex mazes also circular arenas with one or more outlets are suitable for navigation studies in mole-rats. The tube maze may connect the home cage (nesting chamber) with a food chamber or nest-material provisioning chamber (see Section “Conditioning”).
Captive mole-rats prefer to run along the walls of their home cages in a clockwise direction. Having observed this phenomenon, we found it so conspicuous that wanted to use it as a behavioral assay and hypothesized that the moving pattern may be affected by changing the magnetic field. When the animals were put into a circular arena, they started to collect tissue papers from their home cage litter given as substrate into the experimental arena to provide familiar odors and thus alleviate the stress from a new environment and the nest-building assay was born (Burda et al., 1990b). The mole-rats gathered strips of paper and still circled along the arena wall in a clockwise direction. This phenomenon deserves to be studied systematically (and also in the southern hemisphere). On a similar note, the late Graham C. Hickman once suggested monitoring the direction of the spiral coiling of the vertical ventilation burrows (“chimneys”) of subterranean mammals in the southern and northern hemispheres.
The nest-building assay, i.e., recording in which compass direction the rodents build their nests in a circular arena, is a simple experiment and its heuristic potential is not yet exhausted [reviewed in Burda et al. (2020)]. It remains also unclear whether the nest-direction in mole-rats is species- or population-specific (cf. Oliveriusová et al., 2012) or whether it may be entrained (Deutschlander et al., 2003).
Studies of spatial orientation of mole-rats in the field may be inspired by experiments by Kimchi and Terkel (2003a,b), Kimchi et al. (2004) on blind mole rats). Experiments by Eloff (1951) with interrupted or blocked runways should be recapitulated in a systematic way, always noticing the compass direction of the affected tunnel. Homing ability of captured and translocated mole-rats should be studied. Compass direction and straightness of tunnels in newly established burrow systems (e.g., after translocation of a founder pair of animals) should be monitored. The hypothesis of the magnetic field as a heading indicator to keep the course of burrowing (Malewski et al., 2018) should be tested also in localities under high voltage power lines where mole-rats might also occur (cf. Burda et al., 2009).
Chronobiology
Chronobiology (orientation and structuring behavior in time) in African mole-rats has been the subject of several studies in the laboratory and the field. A diversity of cues has been found to function as zeitgebers: especially light (cf. e.g., Oosthuizen et al., 2003), although its biological role underground is not clear, and temperature (Šklíba et al., 2014), although at least in the nest 50 cm and deeper under the surface ambient temperature is buffered and constant (Burda et al., 2007). Besides, that small predictable daily fluctuation of the magnetic field may serve as zeitgeber (cf. Wiltschko and Wiltschko, 1995). Such small variations are masked by electromagnetic disturbances in standard laboratories and animal rooms.
My former colleague at the University Duisburg-Essen, geologist Professor Ulrich Schreiber, drew my attention to another potential and thus far unstudied zeitgeber: periodic circadian oscillation (rise and fall) of the levels of groundwater, the so-called earth tide, which is analogous to sea tides, and also caused by the combined effects of the gravitational forces exerted by the moon and the sun, and the rotation of the earth (Sugisaki, 1981; Sato, 2006). The rising groundwater pushes soil gases up and their pressure in the atmosphere of the underground burrows thus predictably changes during the day. It remains unknown, however, whether mole-rats can detect changes in those gases (carbon dioxide, helium, argon, etc.).
A white head spot (bles) is typical of F. anselli, Fukomys kafuensis, Fukomys damarensis, F. micklemi, and other Fukomys species from southern and western Zambia (southwards of 15th latitude) (cf., Burda et al., 1999b; Van Daele et al., 2004) but is missing in most F. mechowii and in the examined, Fukomys amatus, and Fukomys whytei, F. vandewoestijneae, and Kasama mole-rats (cf., Macholan et al., 1998; Kawalika et al., 2001; Van Daele et al., 2004, 2013; Burda et al., 2005). Even in species where the headspot is present it is variable in size and shape. The adaptive significance of the bles in many Cryptomys and Fukomys species remains obscure but it has been suggested that being an unpigmented part of the skin it may facilitate penetration of light to the pineal organ and thus be involved in photoperception and control of photoperiodicity (Lovegrove et al., 1993). However, chronobiological data for Cryptomys and Fukomys are very ambivalent, and large polymorphisms in the size and shape of the white head spot, even in its very presence, weaken the idea of its functional significance. Nevertheless, having briefly examined the large collection of specimens of mole-rats in the Natural History Museum of Zimbabwe in Bulawayo in 1993 (then under the auspices of Fenton P. D. “Woody” Cotterill), I noticed that there may be a general latitudinal gradient in the size of the bles, decreasing and disappearing from the south toward the equator, reflecting thus also changes in annual photoperiodicity and seasonality of breeding (Figure 1). The bles in museum specimens can be planimetrically measured and the hypothesis of the latitudinal gradient should (and relatively easily could) be statistically tested.

Figure 1. Stuffed specimens of Fukomys mole-rats showing the variability in the size of the headspot (bles) in the collections of the Natural History Museum of Zimbabwe in Bulawayo. (Left) Mole-rats (incl. F. mechowii) from Northern Zambia, (Right) mole-rats (incl. F. damarensis) from Southern Africa.
Nociception
The naked mole-rat became famous for its virtual insensitivity to acid, capsaicin, and histamine. This feature was observed also in some other studied bathyergid species. The phylogenetic distribution of this somatosensory property among bathyergids is inconsistent and the Fukomys species outside Southern Africa have not been examined [reviewed in Lewin et al. (2021)]. I have observed that captive Ansell’s mole-rats learned to accept and eat chili peppers (Habanero) without problems. I suggest that offering mole-rats chili peppers as food complement and observing their behavioral reaction would represent a simple, cheap, non-invasive screening test for the presence or absence of capsaicin insensitivity among species instead of injecting the substances.
“Pumping”
Bednářová et al. (2013) describes the “chest thumping” in Fukomys mechowii. The animals hit rhythmically the floor of the perspex tube in an artificial tunnel system. They produced thus audible vibrations with the highest frequency of the signal at 1.63 kHz and the loudest frequency of the audible part at about 0.45 kHz. The sequences contained from three to four thumps and were separated by breaks of 0.6 s. The mole-rats produced this sound in the closed space of the tube. The sounds were recorded mainly in two situations: during aggressive encounters (males) and feeding (females) – the female entered the tube and started to produce seismic signals after the food was inserted into the terrarium. The function of these signals in giant mole-rats is still unclear. Similar behavior has been, however, reported as so-called “pumping” also in other mole-rat species: in Cryptomys hottentotus (Eloff, 1958; Poduschka, 1978) and in F. damarensis (Bennett and Jarvis, 1988). The authors discussed pumping in the context of warning and antipredator behavior, although no clear conclusion could be made.
I have observed pumping in captive F. anselli and F. kafuensis, in both sexes, but relatively rarely and almost exclusively if the animal was in a tube. It did not matter how long the tube was and whether it was open on one or both ends or closed or whether the animal was in the tube alone or not. Only very seldom have I observed this behavior also in the “open field,” in an animal put into a bucket. Behavior could be described as pumping, not thumping or drumming, and was not audible. It remains to be clarified whether pumping represents signaling or sensing. In both cases, one can imagine that pumping in a tunnel produces slight air currents, which can be perceived by the family members or will be echoed if the tunnel is plugged in front of the animal. Alternatively, the meaning of pumping is not to exhalate (and produce air currents) but to inhale the air (and analyze it). Indeed, this was also the impression of Eloff (1958) who writes: “the abdomen is flattened as if the animal inspires: then the spinal column is curved, bent ventrally and this action is repeated several times.” He further suggested that a break in the tunnel might be detected in this way, through the displacement of a volume of air. The fact that cigarette smoke in the room provoked (though not reproducibly) animals to engage in pumping is interesting and could be of possible relevance.
Conditioning
The testing of sensory capabilities by behavioral methods is based on operant conditioning. Positive reinforcement in mole-rats is not an easy task: they do not drink free water, and reinforcement by food has also several constraints (the animals have very limited body fat reserves and when forced to starve, they very quickly lose water). One possibility would be, rather than feed them at a given place, to provide food pieces that they would repeatedly retrieve into their “storage room.” I have observed, however, that mole-rats are tireless nest builders and use every opportunity to gather and retrieve nest materials (e.g., strips of tissue paper). This material is, contrary to food pieces, odorless, which is its further advantage. It can be used as a reward at the goal of a maze or in association with sensory signals, which would announce that it is available.
Aging
Mole-rats are famous for their longevity and slow aging (cf. Braude et al., 2021; Buffenstein and Craft, 2021). The studies on this aspect of life history would profit if we could study it in the field, without the necessity of long-term capture-recapture studies. For this purpose, we would need a biological marker of age, which could be easily obtained from animals in the field by biopsies without the necessity to sacrifice them. Only recently, an epigenetic age estimator (clock) was developed for the naked mole-rat, enabling us to assess (from skin biopsies) whether epigenome undergoes age-related changes (Horvath et al., 2022).
The Corti organ of the inner ear represents a perfect aging biomarker, accurately quantifiable – unfortunately, the animals have to be sacrificed and preparation of the organ of Corti by the technique of the so-called surface specimens is not a routine method. The number of hair cells is given at birth and in the course of life, the hair cells only die and leave visible scars behind which can be exactly counted (Figure 2). Since mole-rats under natural conditions do not experience any noise overstimulation and are not exposed to ototoxic drugs, all the missing hair cells are expected to be due to physiological aging (cf. also Burda, 1978; Úlehlová et al., 1984).
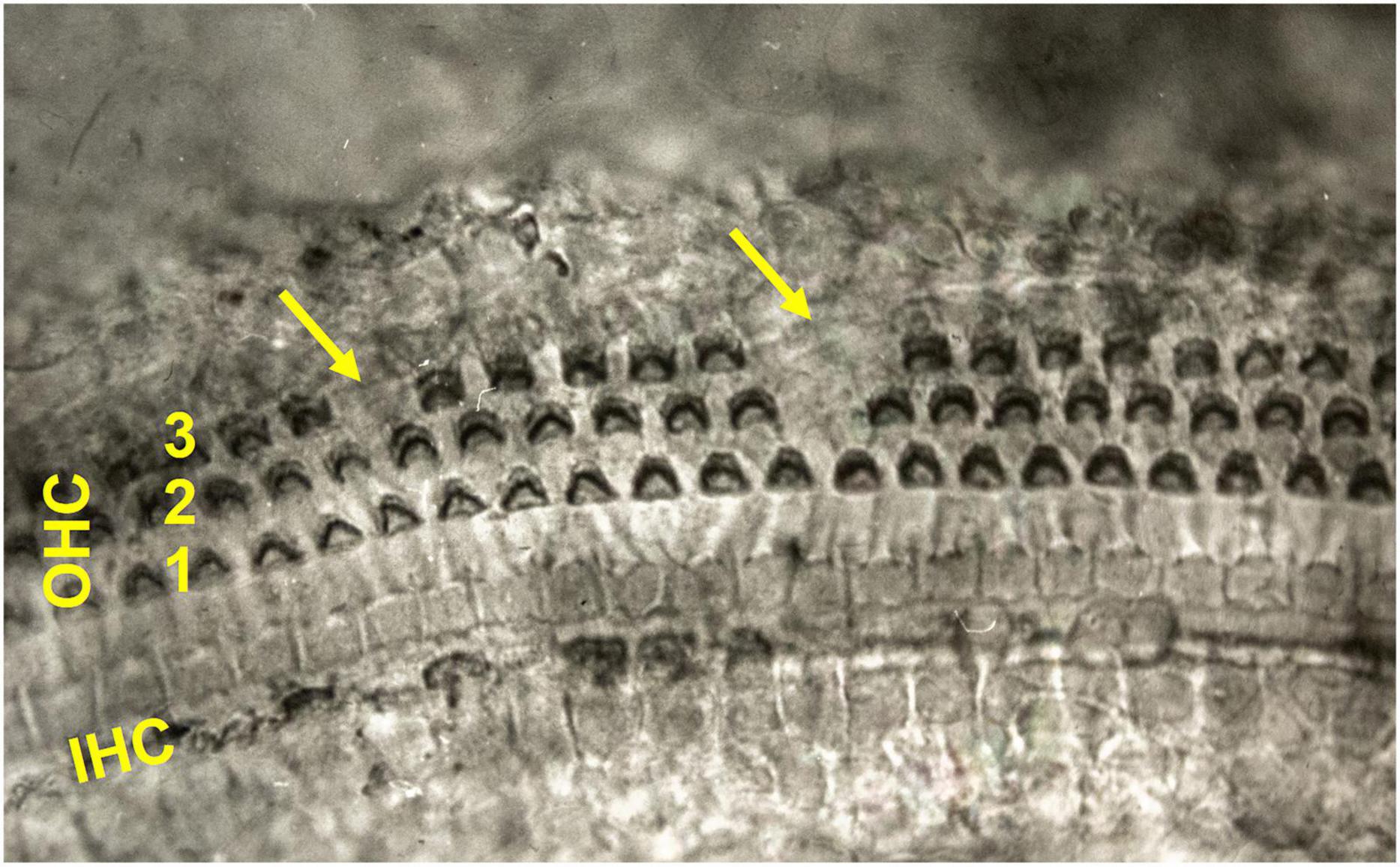
Figure 2. Total surface specimen of the organ of Corti of a shrew (Sorex araneus). The geometrical pattern consists of three rows (1–3) of cuticular plates of outer hair cells (OHC) separated by a row of inner pillar cells from a single row of cuticular plates of inner hair cells (IHC, not in focus). Altogether 4 OHC are missing on account of the physiological involution of the auditory neuroepithelium (arrows). Ehrlich’s hematoxylin × 375. See Burda (1978) and Úlehlová et al. (1984) for further illustrations and description of the phenomenon.
Ecophysiology
Living under hypoxic and hypercapnic conditions is a frequently mentioned stress that subterranean mammals have to cope with (cf. Nevo, 1979, 1999; Braude et al., 2021; Park et al., 2021). There are also many studies providing data on the atmosphere and microclimate (temperature and humidity) in the burrows (cf. Burda et al., 2007; Holtze et al., 2018). I would, however, like to point out that all the measurements were done in unobstructed burrows and empty nest chambers, i.e., without burrow inhabitants being present in close vicinity of the measuring probes.
Microclimate and atmosphere in an occupied nest chamber, i.e., with inhabitants being present, is surely a different one. A digging mole-rat having only a little air volume between its snout and the end of a tunnel in front of it, is also likely exposed to much higher hypoxic and hypercapnic stress than an animal moving in a free superficial runway tunnel.
Speciation, Genetics
Contact Zones
Contact (hybrid?) zones between Fukomys species in Zambia (and elsewhere) require systematic studies. In our laboratory, F. anselli (2n = 68) and F. kafuensis (2n = 58) successfully breed producing reproductive offspring (2n = 63). The mechanism of chromosome hybridization has not been studied.
Distribution of Giant Mole-Rats: A Case for Cryptozoology?
My former student assistant at the University of Zambia, later Ph.D. student, colleague, and friend, Mathias Kawalika (1962-2006), wrote the following paragraphs in his Ph.D. thesis (Kawalika, 2004):
“It remains unclear whether there are hybrid zones between neighboring Fukomys species or not, it remains unclear whether the areas of distribution (of diverse Fukomys species) overlap at some places and whether some of the species occur sympatrically. Indeed, even the map of distribution of the giant mole-rat published by Ansell (1978) (Figure 59), i.e., a species of mole-rats which can be distinguished from other species by its size, needs revision. Ansell himself admits that the record from Malawi is questionable. Also, no giant mole-rats were captured during recent rather intensive mole-rat collecting in the Southern, Western, and North-Western Province (i.e., the strip between the Zambezi and the Kafue rivers) (Van Daele et al., 2004).
However, it should be of interest to note that in Kabwe as well as in Kasama I have been assured by local hunters that there live both, small and giant mole-rats sympatrically. The locals distinguish both species even by name: kakoko and mfuko, respectively. They claimed that even a “super-giant mole-rat” occurs in the area. Similarly, in Chongwe, two types of mole-rats (giant and common) were reported. In Chibale, the sympatric occurrence of C. amatus and C. mechowi could be proved (Scharff, 1998, and own unpublished observations). In Ndola (Chichele) I have captured medium-sized mole-rats which also do not grow in captivity (Burda, personal communication), yet subsequent karyological study revealed a karyotype typical for C. mechowi. It should be noted that in this locality, occurrence of two distinct forms was indicated also by parameters of the burrow system. Thus, I have found at one site (in early June 2000) within an area of 50 m × 25 m, 225 mounds and tunnels having 75–110 mm in diameter, whereas just about 100 m from this sector there were tunnels which ranged from 50 to 75 mm in diameter and there were only about 60 mounds within the same size area. There is no doubt that further field studies are needed to verify or falsify the claims of local hunters and to answer the points raised in this section.
Interviewing of several hunters (in Kasama area) leads to the assumption that there are four types of mole-rats in the area: (a) common (small) mole-rats; (b) giant mole-rats; (c) multi-colored giant mole-rats; (d) white super giant mole-rats. The super giant mole-rats are about the size of small puppies which puts them above 800 g. This was evident from one animal already prepared and cooked in a pot. (Interestingly super-giant mole-rats are recorded also from the Kabwe area.) The hunter (in Bwembya village) revealed that the animal had a white coat which is usually the case for all the super giant animals he has ever caught. The common moles have been described by all hunters as always gray without the white head spot. The giant mole-rats are of two types, the normally colored (as from Ndola) and the multi-colored which usually have a white belly and brown upper colored body. The giant mole-rats (types b, c, and d) can coexist in the same location whereas they cannot and never share their habitat with those of common mole-rats (type a). This was evident also from the size of the burrows inspected. The common mole-rats were more confined to lower altitudes with gray sandy soils whereas the giant were confined to brown soils. The closest distance observed between the habitat of the giant mole-rats and that of the common ones was 1 km and both localities were separated by the escarpment. Local hunters clarified that the super giant mole-rat was less common in comparison to the giant mole-rat. They capture it only occasionally.”
The indigenous knowledge of local mole-rats should not be underestimated, though it has to be viewed critically in some aspects (Burda and Kawalika, 1993). The reports of Mathias Kawalika surely deserve to be taken seriously and to be checked.
Sex Ratio
The subterranean habitat is considered structurally simple and microclimatically stable (Nevo, 1999), thus subterranean mammals may be suitable models for the study of sex-ratio adjustment. There seems to be inconsistency between laboratory and field data as far as the sex ratio is concerned.
The sex ratio among wild captured young (<1 year) giant mole-rats (F. mechowii) was male-biased (1.22) but among adults (>1 year), the proportion of males decreased (0.96) (Kawalika and Burda, 2007). Taking all wild-captured animals together, the overall sex ratio was equal (1.07). Sampling was roughly equally distributed throughout space and time as well as with respect to capture methods so there was probably no bias due to the sampling method. Data on captive families are inconsistent with the field data due to a strong female-biased neonate sex ratio of 0.54 among laboratory-born animals (Scharff et al., 1999).
F. anselli shows a female-biased neonate sex ratio of 0.85 (Begall and Burda, 1998) and even 0.44–0.73 in wild captured animals (Kawalika and Burda, 2007; Sichilima et al., 2011, respectively).
The following hypotheses explain the above findings and should be considered and tested:
(1) The skew toward neonate females is an artifact of captive breeding and does not reflect the natural situation. A reverse (i.e., male-biased) sex ratio should be expected among neonates in the field to explain a higher proportion of males among young wild-captured mole-rats.
(2) With growing older, males disappear from the population. There are indications of a higher dispersal rate of adult males in F. mechowii (Kawalika and Burda, 2007). A male-biased higher dispersal rate has been reported also in F. damarensis (Hazell et al., 2000). It should be, however, noted that a male-biased dispersal rate is deduced from the observed higher aboveground activity of males. A hypothesis should be tested that while males tend to disperse aboveground, females disperse rather underground (cf. Šumbera et al., 2012; Torrents-Ticó et al., 2018; Mynhardt et al., 2021). On the other hand, there is no indication of higher intrinsic mortality in captive male mole-rats (Dammann and Burda, 2007). It is noteworthy that female-female inter-familial encounters (at least in captivity) are more aggressive and lethal (Burda, 1989).
(3) There is a sex-linked age-polyethism, expressed in the higher activity and/or trapability of subadult males. With increasing age, males may become more cautious or less active. However, previous observations on captive animals do not suggest any marked difference in this aspect (own observation, Burda, 1990; Zöttl et al., 2016).
Finding answers to the questions of why sex ratios in newborn captivity-bred giant mole-rats deviate so markedly from equality, may prove to be significant for gaining insights into the ecology of mole-rats in particular, and for understanding phenomena affecting sex-ratios in mammals in general.
The following exciting hypothesis could explain the phenomenon and should be tested: In F. anselli and F. mechowii, two types of females occur: homogametic females: XX and heterogametic females: XY*. Due to the early loss of YY* zygotes, XY* females would produce twice as many phenotypical females as males. XY* females in the S. American field mice (Akodon) have been described already 35 years ago. It was shown that in Akodon, XY* females persist at high frequencies (up to 30%) and are better breeders than XX (Bianchi, 2002). An unusual sex determination system has been found also in an unrelated subterranean rodent: the mole-lemming (Just et al., 2002). Existence of heterogametic (XY*) females at different frequencies in the populations and our captive breeding stock and higher mortality of younger males (observed also in breeding colonies) could explain all the observed phenomena.
Sexual Dimorphism and Age Structure
The question of why there is a marked sexual dimorphism (at least between breeding males and their oldest sons on one side and females on the other side) in Fukomys mole-rats has no clear answer. The mole-rats are monogamous, and because of isolation, family stability, and incest avoidance (Burda, 1995) there is seemingly no significant competition for mating which would act as a sexual selection pressure. Nevertheless, I have observed (Burda, 1995, 1999, own observations) that incest avoidance between mothers and their sons is not as strong as between siblings and between the father and his daughters. This means that there is a competition between the breeding male and his male offspring and thus also between the brothers. This competition may be the motor driving the body size increase in males. An even more significant index signaling the body strength may be, however, the breadth of the (upper) incisors. It should be noted that teeth are testosterone target organs used for signaling dominance status in diverse mammals (baboon or musk deer being familiar examples). I have observed that old breeding males which may be no longer the largest ones in the family still had the broadest incisors. Unfortunately, I have never measured this trait systematically to test the hypothesis. The males themselves may assess this strength index during their typical sparring (playful) fights which have a character of mouth (teeth) wrestling: the animals lock their incisors together and sway from side to side. The males do not bite each other but – in case of serious fights between non-familiar males – they try to break the incisors of the rival. Eventually, the defeated male shows an appeasement posture (Burda, 1989). The fight between two females has a different character: a female does not respect the appeasement postures (if any), does not try to fight mouth-to-mouth but bites the retreating animal (Burda, 1989).
Caspar et al. (2021c) have quantified sexual size dimorphism and have measured also the width of the upper incisors in F. anselli. While the authors have found differences between both sexes, they found no significant differences between breeders and non-breeders. Their study sample consisted of animals of known age and most specimens originated from different families. This approach is not suited to test my hypothesis that the breeding (dominant) male in a family (“king”) can be recognized based on the width of its upper incisors and that the wrestling with locked incisors between males serves to compare the strength and to establish the dominance. The males normally measure their strength in this way with other family members (i.e., within their respective families), not with strangers from different families. Moreover, the study by Caspar et al. (2021c) involved captive animals with non-breeders being on average 58 months old. This situation is most probably not natural. We should expect that in nature, the mean lifespan (coexistence within a family) of a non-breeder is “only” 31 months (Burda et al., 2000). This means that a 58-month-old non-breeder in nature would not be any more competitive with its father but would have been expelled, or “voluntarily” have abandoned its respective family or have died of a different cause before reaching that age.
Based on my observations (Burda, 1989, 1990, 1995, 1999) and the above assumptions we can expect that older male non-breeders (sons) from an established (longer lasting) family will be larger and will have wider upper incisors than the younger males. If they invaded a fledging (smaller) family with a younger “king,” they would be able to defeat him and mate with the “queen,” which is always ready “to have an affair” (Burda, 1995; Bappert et al., 2012). In a family with an older “king,” they will surrender but will be adopted into the family. They might mate also with a non-breeding female as there will be no incest avoidance (Burda, 1989, 1990, 1995). All existing observations about the dispersal and genetic structure of mole-rat families in nature (Patzenhauerová et al., 2013; Torrents-Ticó et al., 2018; Mynhardt et al., 2021) are consistent with conclusions derived from our observations on captive animals (Burda, 1989, 1990, 1995; Bappert et al., 2012).
To avoid any misunderstanding: sporadic copulation in Fukomys mole-rats does by far not guarantee fertilization (Burda, 1990, 1995; Willingstorfer et al., 1998). Moreover, the replacement of the “king” by his son can be expected only when the “king” is already old and weak. At that time, however, the mother (“queen”) is most probably also old and her reproductive potential is reduced (Willingstorfer et al., 1998). Hence, a family bond between mother and son has only a low chance of arising and thriving, and consequently, a much lower chance of being discovered and identified by researchers. On the other hand, taking over a “kingship” by an unfamiliar male invader may frequently escape our attention. This case may affect smaller fledging families with a younger breeding male invaded by an older, bigger male. If this happens, the family has still only a few members (offspring of the founder) and after some time they disappear or are in a minority and the invader could be considered by researchers to be the original founder and “king” of the family who has been probably cuckolded (offspring of the first king would be considered to be the result of extra-pair copulations of the “queen”).
Study of the Structure, Dynamics, and Rhythmicity of Mole-Rat Families in the Field
Most of the information on life histories (reproductive biology, aging, etc.) and the activity of mole-rats have been gained in the laboratory. Thus far, information on wild living mole-rats (and subterranean mammals in general) could only be obtained only using invasive and time consuming methods, requiring long-term projects – capture-marking-recapture methods and telemetry. Because of the apparent time- and manpower-demands as well as technical problems, only a few studies have been undertaken, limited to only a few burrow systems in limited areas.
With Mathias Kawalika, we prepared a new original and innovative method, which would be simple, elegant, and non-invasive and would enable us to survey and monitor many families of mole-rats in their respective nest chambers in situ in different localities over a time span determined only by possibilities of the researchers to revisit the study places. Unfortunately, in 2006 Mathias passed away due to malaria and the project was never realized.
The principle of the projected method is the following (Figure 3):
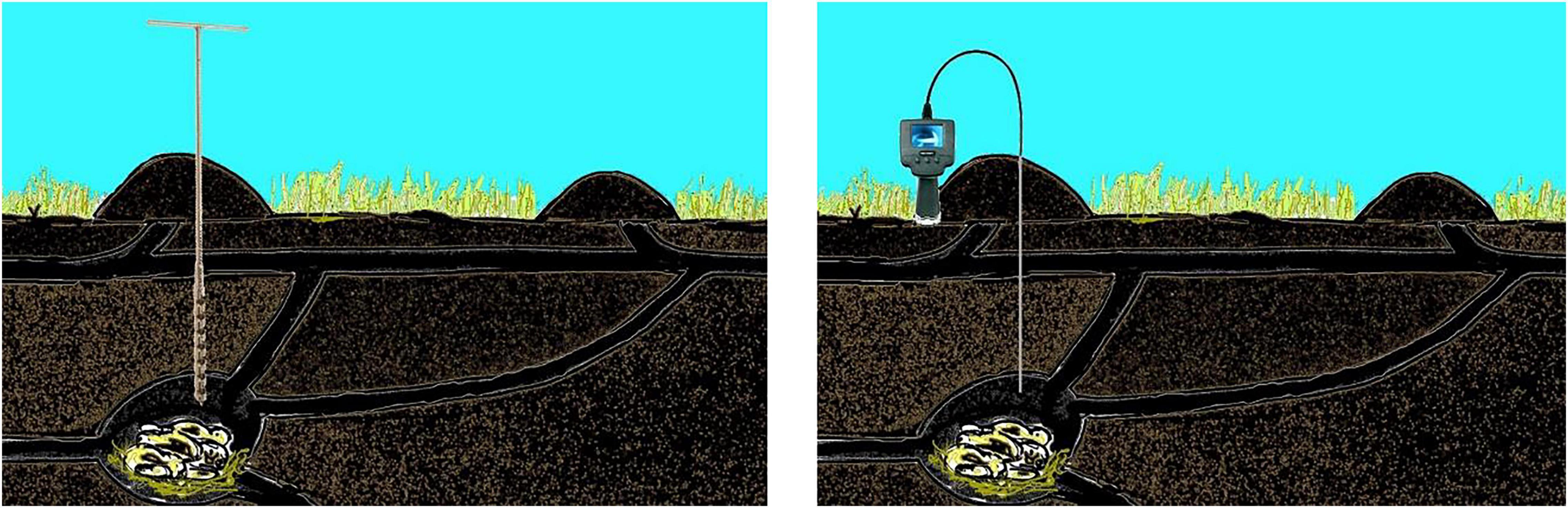
Figure 3. Illustration of the proposed method of nest chamber drilling (Left) and endoscope monitoring (Right).
(a) The nest chamber will be localized (see below).
(b) A chimney will be drilled by a soil driller from above into the ceiling of the nest chamber. The mole-rats are not able to plug a hole in the ceiling of the chamber and are not disturbed by cold and red light. We assumed that the mole-rats would not permanently abandon a nest chamber in the building in which they invested a lot, i.e., they would return after the disturbance due to drilling has ceased.
(c) An endoscope will be introduced, meaning the animals can be counted and video-monitored. Thanks to their white head spots (blesses) they (at least F. anselli) can be recognized individually.
(d) Continuous monitoring of the nest chamber throughout the whole day and in regular time intervals will provide data about the circadian and circannual rhythmicity of individual animals and family dynamics (breeding etc.).
(e) An endoscope will be introduced, and the animals will be counted and video-monitored. In this way, we get information on family size and also the population size in a given area, family (and population) dynamics over a given time period (breeding data, recruitment of new family members, losses), daily and seasonal activity patterns (note that mole-rats in many species can be individually recognized based on the size and form of the head spot).
(f) Temperature, humidity, oxygen, carbon dioxide, and methane concentrations can be measured in situ in occupied nest chambers. These data are thus far generally missing.
Nest Chamber Localization
There are several possibilities to localize the nest, which may be combined.
(a) An experienced local mole-rat hunter will localize the nest chamber. (Mathias Kawalika succeeded to recruit a very experienced mole-rat hunter, Mr. Kawesa from Ndola, who was able to locate nest chambers of mole-rat families.) Should localization of the nest chamber by a hunter fail for whatever reason in some habitats, we might use further complementary approaches to narrow down the nest location.
(b) The telemetry method (an animal is captured at the periphery of the burrow system and collared in order to show us the way to the nest).
(c) Metal search detector can be used in sandy Kalahari soils and involves inserting bait strips of cotton tissues partly into burrows. Metal rings are sewn into these strips. Mole-rats pull down the strips and transport them as nest materials into the nest chamber. The nest is then located by a metal detector.
(d) Sound localization using sensitive spy microphones (note that mole-rats are very vocal animals) (see chapter vocalization above).
(e) Employment of a ground penetrating radar (“georadar”) has become a realistic option in recent years.
Survival Strategies During Floods
Mathias Kawalika noticed that in the Kafue Flats and specifically in the Lochinvar National Park, mole-rats were present in areas that have been regularly seasonally inundated by floods. The question arises of how the mole-rats survive flood events. Local people claimed that mole-rats occupy termite mounds during floods and that animals from different colonies gather peacefully in one place. We did not have the opportunity to test these claims.
Conclusion
The biology of African mole-rats was virtually unknown 50 years ago (cf. Kingdon, 1974). In the last few decades, the number of published papers on mole-rats has grown enormously. Even if they have become the best known wild (and certainly the best known African) rodents in many aspects of biology, there are still many enigmas and mysteries, which will no doubt keep many other scientists busy for their whole academic careers.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because no permit is required for this kind of study which is a theoretical one and is based on published peer-reviewed studies.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
The author received grant “EVA4.0,” No. CZ.02.1.01/0.0/0.0/16_0 19/0000803 financed by OP RDE for funding.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I thank the reviewers and editors for their insightful, constructive comments.
References
Bálint, A., Andics, A., Gácsi, M., Gábor, A., Czeibert, K., Luce, C. M., et al. (2020). Dogs can sense weak thermal radiation. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-60439-y
Bappert, M.-T., Burda, H., and Begall, S. (2012). To mate or not to mate? Mate preference and fidelity in monogamous Ansell’s mole-rats, Fukomys anselli, Bathyergidae. Folia Zool. 61, 71–83. doi: 10.25225/fozo.v61.i1.a11.2012
Barker, A. J., Koch, U., Lewin, G. R., and Pyott, S. J. (2021). Hearing and vocalizations in the naked mole-rat. Adv. Exp. Biol. 1319, 157–196. doi: 10.1007/978-3-030-65943-1_6
Bednářová, R., Hrouzková-Knotková, E., Burda, H., Sedláček, F., and Šumbera, R. (2013). Vocalizations of the giant mole-rat (Fukomys mechowii), a subterranean rodent with the richest vocal repertoire. Bioacoustics 22, 87–107. doi: 10.1080/09524622.2012.712749
Begall, S., and Burda, H. (1998). Reproductive characteristics and growth rate in the eusocial Zambian common mole-rat (Cryptomys sp., Bathyergidae). Z. Säugetierkunde 63, 297–306.
Begall, S., Burda, H., and Caspar, K. (2021). Fukomys anselli (Rodentia: Bathyergidae). Mammal. Spec. 53, 160–173. doi: 10.1093/mspecies/seab015
Begall, S., Burda, H., and Schleich, C. (eds) (2007a). Subterranean Rodents - News from Underground. Heidelberg: Springer.
Begall, S., Lange, S., Schleich, C., and Burda, H. (2007b). “Acoustics, audition and auditory system,” in Subterranean Rodents - News from Underground, eds S. Begall, H. Burda, and C. Schleich (Heidelberg: Springer), 97–111. doi: 10.1007/978-3-540-69276-8_9
Bennett, N. C., and Jarvis, J. U. M. (1988). The social structure and reproductive biology of colonies of the mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae). J. Mammal. 69, 293–302.
Bianchi, N. O. (2002). Akodon sex reversed females: the never ending story. Cytogenet. Gen. Res. 96, 60–65. doi: 10.1159/000063029
Braude, S., Holtze, S., Begall, S., Brenmoehl, J., Burda, H., Dammann, P., et al. (2021). Surprisingly long survival of pre-mature conclusions about naked mole-rat biology. Biol. Rev. 96, 376–393. doi: 10.1111/brv.12660
Brückmann, G., and Burda, H. (1997). Hearing in blind subterranean Zambian common mole-rats (Cryptomys sp., Bathyergidae, Rodentia). J. Comp. Physiol. A 181, 83–88. doi: 10.1007/s003590050095
Buffenstein, R., and Craft, W. (2021). The idiosyncratic physiological traits of the naked mole-rat; a resilient animal model of aging, longevity, and healthspan. Adv. Exp. Biol. 1319, 221–254.
Buffenstein, R., Park, T. J., and Holmes, M. M. (eds) (2021). The Extraordinary Biology of the Naked Mole-Rat. Adv. Exp. Biol. 2021:1319.
Burda, H. (1978). Population der Haarzellen des Cortischen Organs der Spitzmäuse. Zeitschrift für mikroskopisch-anatomische Forschung 92, 514–552.
Burda, H. (1989). Reproductive biology (behaviour, breeding, and postnatal development) in subterranean mole-rats, Cryptomys hottentotus (Bathyergidae). Z. Säugetierkunde 54, 360–376.
Burda, H., Bruns, V., and Nevo, E. (1989). Middle ear and cochlear receptors in the subterranean mole-rat, Spalax ehrenbergi. Hear. Res. 39, 225–230.
Burda, H. (1990). Constraints of pregnancy and evolution of sociality in mole-rats. With special reference to reproductive and social patterns in Cryptomys hottentotus (Bathyergidae, Rodentia). Z. zoologische Systematik und Evolutionsforschung 28, 26–39. doi: 10.1111/j.1439-0469.1990.tb00362.x
Burda, H. (1995). Individual recognition and incest avoidance in eusocial common mole-rats rather than reproductive suppression by parents. Experientia 51, 411–413. doi: 10.1007/BF01928906
Burda, H. (1999). “Syndrome of eusociality in African subterranean mole-rats (Bathyergidae, Rodentia), its diagnosis and aetiology,” in Evolutionary theory and processes: Modern perspectives, ed. S. P. Wasser (NL-Dordrecht: Kluwer Academic Publ), 385–418. doi: 10.1007/978-94-011-4830-6_24
Burda, H. (2006). Ear and eye in subterranean mole-rats, Fukomys anselli (Bathyergidae) and Spalax ehrenbergi (Spalacidae): Progressive specialization or regressive degeneration? Anim. Biol. 56, 475–486. doi: 10.1163/157075606778967847
Burda, H. (2021). in Sensory perception of mole-rats and mole rats: Assessment of a complex natural global evolutionary “experiment”, eds S. Wasser and M. Frenkel-Morgenstern (Amsterdam: New Horizons in Evolution. Elsevier).
Burda, H., Begall, S., Červený, J., Neef, J., and Němec, P. (2009). Extremely low-frequency electromagnetic fields disrupt magnetic alignment of ruminants. Proc. Natl. Acad. Sci. USA 106, 5708–5713. doi: 10.1073/pnas.0811194106
Burda, H., Begall, S., Grütjen, O., Scharff, A., Nevo, E., Beiles, A., et al. (1999a). How to eat a carrot? Convergence in the feeding behaviour of subterranean rodents. Naturwissenschaften 86, 325–327. doi: 10.1007/s001140050625
Burda, H., Zima, J., Scharff, A., Macholan, M., and Kawalika, M. (1999b). The karyotypes of Cryptomys anselli sp. nova and Cryptomys kafuensis sp. nova: new species of the common mole-rat from Zambia (Rodentia, Bathyergidae). Zeitschrift für Saugetierkunde 64, 36–50.
Burda, H., Begall, S., Hart, V., Malkemper, E. P., Painter, M. S., and Phillips, J. B. (2020). “Magnetoreception in Mammals,” in The Senses: A Comprehensive Reference, Vol. Editor, eds B. Fritzsch and H. Bleckmann (Amsterdam: Elsevier, Academic Press), 421–444. doi: 10.1016/b978-0-12-809324-5.24131-x
Burda, H., Bruns, V., and Hickman, G. C. (1992). The ear in subterranean Insectivora and Rodentia in comparison with ground-dwelling representatives. I. Sound conducting system of the middle ear. J. Morphol. 214, 49–61. doi: 10.1002/jmor.1052140104
Burda, H., Bruns, V., and Müller, M. (1990a). Sensory adaptations in subterranean mammals. Progr. Clin. Biol. Res. 335, 269–293.
Burda, H., Marhold, S., Westenberger, T., Wiltschko, W., and Wiltschko, R. (1990b). Magnetic compass orientation in the subterranean rodent Cryptomys hottentotus (Bathyergidae, Rodentia). Experientia 46, 528–530. doi: 10.1007/BF01954256
Burda, H., Honeycutt, R. L., Begall, S., Grütjen, O., and Scharff, A. (2000). Are naked and common mole-rats eusocial and if so, why? Behav. Ecol. Sociobiol. 47, 293–303. doi: 10.1007/s002650050669
Burda, H., and Kawalika, M. (1993). Evolution of eusociality in the Bathyergidae: the case of the giant mole-rat (Cryptomys mechowi). Naturwissenschaften 80, 235–237. doi: 10.1007/BF01175742
Burda, H., Šumbera, R., and Begall, S. (2007). “Microclimate in burrows of subterranean rodents - revisited,” in Subterranean rodents - News from underground, eds S. Begall, H. Burda, and C. Schleich (Heidelberg: Springer), 21–33. doi: 10.1007/978-3-540-69276-8_3
Burda, H., Šumbera, R., Chitaukali, W. N., and Dryden, G. L. (2005). Taxonomic status and remarks on ecology of the Malawian mole-rat, Cryptomys whytei (Thomas, 1897) (Rodentia, Bathyergidae). Acta Theriol. 50, 529–536. doi: 10.1007/bf03192646
Campbell, A. L., Naik, R. R., Sowards, L., and Stone, M. O. (2002). Biological infrared imaging and sensing. Micron 33, 211–225. doi: 10.1016/s0968-4328(01)00010-5
Caspar, K., Burda, H., and Begall, S. (2021a). Fukomys mechowii (Rodentia: Bathyergidae). Mammal. Species 53, 145–159. doi: 10.1093/mspecies/seab014
Caspar, K. R., Heinrich, A., Mellinghaus, L., Gerhardt, P., and Begall, S. (2021b). Evoked auditory potentials from African mole-rats and coruros reveal disparity in subterranean rodent hearing. J. Exp. Biol. 224:jeb243371. doi: 10.1242/jeb.243371
Caspar, K. R., Müller, J., and Begall, S. (2021c). Effects of sex and breeding status on skull morphology in cooperatively breeding Ansell’s mole-rats and an appraisal of sexual dimorphism in the Bathyergidae. Front. Ecol. Evol. 9:355.
Credner, S., Burda, H., and Ludescher, F. (1997). Acoustic communication underground: Vocalization characteristics in subterranean social mole-rats (Cryptomys sp., Bathyergidae). J. Comp. Physiol. A 180, 245–255. doi: 10.1007/s003590050045
Dammann, P., and Burda, H. (2007). “Senescence patterns in African mole-rats (Bathyergidae, Rodentia),” in Subterranean rodents - News from underground, eds S. Begall, H. Burda, and C. Schleich (Heidelberg: Springer), 251–263. doi: 10.1007/978-3-540-69276-8_18
Deutschlander, M. E., Freake, M. J., Borland, S. C., Phillips, J. B., Madden, R. C., Anderson, L. E., et al. (2003). Learned magnetic compass orientation by the Siberian hamster, Phodopus sungorus. Anim. Behav. 65, 779–786. doi: 10.1006/anbe.2003.2111
Dusenbery, D. B. (1992). Sensory Ecology: How Organisms Acquire and Respond to Information. New York, NY: W H Freeman.
Dvořáková, V., Hrouzková, E., and Šumbera, R. (2016). Vocal repertoire of the social Mashona mole-rat (Fukomys darlingi) and how it compares with other mole-rats. Bioacoustics 25, 253–266. doi: 10.1080/09524622.2016.1141117
Eloff, G. (1951). Orientation in the mole-rat Cryptomys. Br. J. Psychol. 2, 134–145. doi: 10.1111/j.2044-8295.1951.tb00285.x
Eloff, G. (1958). Functional and structural degeneration of the eye of the south African rodent mole, Cryptomys bigalkei and Bathyergus maritimus. South Afr. J. Sci. 54, 292–302.
Etienne, A. S., Lambert, S. J., Reverdin, B., and Teroni, E. (1993). Learning to recalibrate the role of dead reckoning and visual cues in spatial navigation. Anim. Learn. Behav. 21, 266–280. doi: 10.3758/bf03197991
Gerhardt, P., Henning, Y., Begall, S., and Malkemper, E. P. (2018). Audiograms of three subterranean rodent species (genus Fukomys) determined by auditory brainstem potentials reveal extremely poor high-frequency cut-offs. J. Exp. Biol. 220, 4377–4382. doi: 10.1242/jeb.175190
Hazell, R. W. A., Bennett, N. C., Jarvis, J. U. M., and Griffin, M. (2000). Adult dispersal in the co-operatively breeding Damaraland mole-rat (Cryptomys damarensis): a case study from the Waterberg region of Namibia. J. Zool. 252, 19–25. doi: 10.1111/j.1469-7998.2000.tb00816.x
Heffner, R. S., and Heffner, H. E. (1990). Vestigial hearing in a fossorial mammal, the pocket gopher (Geomys bursarius). Hear. Res. 46, 239–252. doi: 10.1016/0378-5955(90)90005-a
Heffner, R. S., and Heffner, H. E. (1992). Hearing and sound locatization in blind mole-rats (Spalax ehrenbergi). Hear. Res. 62, 206–216. doi: 10.1016/0378-5955(92)90188-s
Heffner, R. S., and Heffner, H. E. (1993). Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. J. Comp. Neurol. 331, 418–433. doi: 10.1002/cne.903310311
Heth, G., Frankenberg, E., and Nevo, E. (1986). Adaptive optimal sound for vocal communication in tunnels of a subterranean mammal (Spalax ehrenbergi). Experientia 42, 1287–1289. doi: 10.1007/BF01946426
Heth, G., Todrank, J., Begal, S., Braude, S., Koch, R., Zilbiger, Y., et al. (2002b). Odour-guided foraging: “Blind” subterranean rodents do not search “blindly”. Behav. Ecol. Sociob. 52, 53–58. doi: 10.1007/s00265-002-0476-0
Heth, G., Todrank, J., and Burda, H. (2002a). Individual odours and social recognition: similarity in the qualities of individual odours within colonies and across species of African eusocial mole rats (Cryptomys sp.). J. Mammal. 83, 569–575. doi: 10.1644/1545-1542(2002)083<0569:ioswca>2.0.co;2
Heth, G., Todrank, J., Begall, S., Wegner, R., and Burda, H. (2004). Genetic relatedness discrimination in a eusocial rodent, Cryptomys anselli mole-rats. Folia Zool. 53, 269–278.
Hickman, G. C. (1978). Reactions of Cryptomys hottentotus to water (Rodentia: Bathyergidae). Zool. Afr. 13, 319–328. doi: 10.1080/00445096.1978.11447632
Holtze, S., Braude, S., Alemayehu, L., Koch, R., Morhart, M., Szafranski, K., et al. (2018). The microenvironment of naked mole-rat burrows in East Africa. Afr. J. Ecol. 56, 279–289. doi: 10.1111/aje.12448
Horvath, S., Haghani, A., Macoretta, N., Ablaeva, J., Zoller, J. A., Li, C. Z., et al. (2022). DNA methylation clocks tick in naked mole rats but queens age more slowly than nonbreeders. Nat. Aging 2, 46–59. doi: 10.1038/s43587-021-00152-1
Just, W., Baumstark, A., Hameister, H., Schreiner, B., Reisert, I., Hakhverdyan, M., et al. (2002). The sex determination in Ellobius lutescens remains bizarre. Cytogenet. Genome Res. 96, 146–153. doi: 10.1159/000063031
Kawalika, M. (2004). Rodents of Ndola (Copperbelt Province, Zambia). PhD Thesis. Essen: Univ. Duisburg-Essen.
Kawalika, M., and Burda, H. (2007). “Giant mole-rats, Fukomys mechowii, thirteen years on the stage,” in Subterranean rodents - News from underground, eds S. Begall, H. Burda, and C. Schleich (Heidelberg: Springer), 205–219. doi: 10.1007/978-3-540-69276-8_15
Kawalika, M., Burda, H., and Brüggert, D. (2001). “Was Zambia a cradle of the genus Cryptomys (Bathyergidae, Rodentia)? A further new ancestral (?) species of Cryptomys from Zambia,” in African small mammals, eds C. Denys, L. Granjon, and A. Poulet (Paris: Collection Colloques et Seminaires), 253–261.
Kimchi, T., Etienne, A. S., and Terkel, J. (2004). A subterranean mammal uses the magnetic compass for path integration. Proc. Natl. Acad. Sci. U.S.A. 101, 1105–1109. doi: 10.1073/pnas.0307560100
Kimchi, T., and Terkel, J. (2003b). Mole-rats (Spalax ehrenbergi) select bypass burrowing strategies in accordance with obstacle size. Naturwissenschaften 90, 36–39. doi: 10.1007/s00114-002-0383-2
Kimchi, T., and Terkel, J. (2003a). Detours by the blind mole-rat follow assessment of location and physical properties of underground obstacles. Anim. Behav. 66, 885–891. doi: 10.1006/anbe.2003.2267
Kingdon, J. (1974). East African Mammals. An Atlas of Evolution in Africa. Vol II, Pt B, Hares and Rodents. New York, NY: Academic Press.
Knotková, E., Veitl, S., Šumbera, R., Sedláček, F., and Burda, H. (2009). Vocalizations of the silvery mole-rat: comparison of vocal repertoires in subterranean rodents with different social systems. Bioacoustics. 18, 241–257. doi: 10.1080/09524622.2009.9753604
Kössl, M., Frank, G., Burda, H., and Müller, M. (1996). Acoustic distortion products from the cochlea of the blind African mole rat. Cryptomys spec. J. Comp. Physiol. A 178, 427–434. doi: 10.1007/BF00193979
Lange, S., and Burda, H. (2005). Comparative and functional morphology of the middle ear in Zambezian mole-rats (Coetomys–Cryptomys, Bathyergidae). Belg. J. Zool. 135, 5–10.
Lange, S., Burda, H., Wegner, R. E., Dammann, P., Begall, S., and Kawalika, M. (2007). Living in a stethoscope” Acoustics of underground tunnels promotes auditory adaptation in subterranean mole-rats. Naturwissenschaften 94, 134–138. doi: 10.1007/s00114-006-0168-0
Lange, S., Neumann, B., Hagemeyer, P., and Burda, H. (2005). The smell of carrots: kairomone-guided food location in subterranean Zambian mole-rats (Cryptomys spp., Bathyergidae). Folia Zoologica 54, 263–268.
Lewin, G. R., Smith, E. J., Reznick, J., Debus, K., Barker, A. J., and Park, T. J. (2021). “The somatosensory world of the African naked mole-rat,” in The Extraordinary Biology of the Naked Mole-Rat. Advances in Experimental Biology, Vol. 1319, eds R. Buffenstein, T. J. Park, and M. M. Holmes (New York, NY: Springer)197–220. doi: 10.1007/978-3-030-65943-1_7
Lovegrove, B. G., Heldmaier, G., and Ruf, T. (1993). Circadian activity rhythms in colonies of ‘blind’ molerats, Cryptomys damarensis (Bathyergidae). South Afr. J. Zool. 28, 46–55. doi: 10.1080/02541858.1993.11448287
Lovegrove, B. G., and Wissel, C. (1988). Sociality in molerats – metabolic scaling and the role of risk sensitivity. Oecologia 74, 600–606. doi: 10.1007/BF00380059
Macholan, M., Scharff, A., Burda, H., Zima, J., and Grütjen, O. (1998). The karyotype and taxonomic status of Cryptomys amatus (Wroughton, 1907) from Zambia (Rodentia, Bathyergidae). Zeitschrift für Säugetierkunde 63, 186–190.
Malewski, S., Begall, S., Schleich, C. E., Antenucci, C. D., and Burda, H. (2018). Do subterranean mammals use the Earth’s magnetic field as a heading indicator to dig straight tunnels? PeerJ 6:e5819. doi: 10.7717/peerj.5819
Mason, M. J. (2001). Middle ear structures in fossorial mammals: a comparison with non-fossorial species. J. Zool. 255, 467–486. doi: 10.1017/s0952836901001558
Mason, M. J., Cornwall, H. L., and Smith, E. S. J. (2016). Ear structures of the naked mole-rat. Heterocephalus glaber, and its relatives (Rodentia: Bathyergidae). PLoS One 11:e0167079. doi: 10.1371/journal.pone.0167079
McComb, K., and Semple, S. (2005). Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385. doi: 10.1098/rsbl.2005.0366
Müller, M., and Burda, H. (1989). Restricted hearing range in a subterranean rodent, Cryptomys hottentotus (Bathyergidae). Naturwiss 76, 134–135. doi: 10.1007/BF00366611
Müller, M., Burda, H., and Bruns, V. (1989). “Structure and function of the inner ear in subterranean mammals,” in Neural mechanisms of behavior, eds J. Erber, R. Menzel, H. J. Pflüger, and D. Todt (Stuttgart: G Thieme), 144.
Müller, M., Laube, B., Burda, H., and Bruns, V. (1992). Structure and function of the peripheral auditory system in the African mole rat (Cryptomys hottentotus): Evidence for a low frequency acoustic fovea. J. Comp. Physiol. A 17, 469–476. doi: 10.1007/BF00194579
Mynhardt, S., Harris-Barnes, L., Bloomer, P., and Bennett, N. C. (2021). Spatial population genetic structure and colony dynamics in Damaraland mole-rats (Fukomys damarensis) from the southern Kalahari. BMC Ecol. Evol. 21, 1–17. doi: 10.1186/s12862-021-01950-2
Nevo, E. (1979). Adaptive convergence and divergence of subterranean mammals. Annu. Rev. Ecol. Syst. 10, 269–308. doi: 10.1146/annurev.es.10.110179.001413
Nevo, E. (1999). Mosaic evolution of subterranean mammals: Regression, progression and global convergence. Oxford: Oxford Univ. Press, 512.
Okanoya, K., and Screven, L. A. (2018). “Rodent vocalizations: adaptations to physical, social, and sexual factors,” in Rodent Bioacoustics. In: Springer Handbook of Auditory Research, Vol. 67, eds M. Dent, R. Fay, and A. Popper (New York, NY: Springer), 13–41. doi: 10.1007/978-3-319-92495-3_2
Oliveriusová, L., Němec, P., Králová, Z., and Sedláček, F. (2012). Magnetic compass orientation in two strictly subterranean rodents: learned or species-specific innate directional preference? J. Exp. Biol. 215, 3649–3654. doi: 10.1242/jeb.069625
Oosthuizen, M. K., Cooper, H. M., and Bennett, N. C. (2003). Circadian rhythms of locomotor activity in solitary and social species of African mole-rats (family: Bathyergidae). J. Biol. Rhythm. 18, 481–490. doi: 10.1177/0748730403259109
Park, T. J., Smith, E. J., Reznick, J., Bennett, N. C., Applegate, D. T., Larson, J., et al. (2021). African naked mole-rats demonstrate extreme tolerance to hypoxia and hypercapnia. Adv. Exp. Biol. 1319, 255–270. doi: 10.1007/978-3-030-65943-1_9
Patzenhauerová, H., Šklíba, J., Bryja, J., and Šumbera, R. (2013). Parentage analysis of Ansell’s mole-rat family groups indicates a high reproductive skew despite relatively relaxed ecological constraints on dispersal. Mol. Ecol. 22, 4988–5000. doi: 10.1111/mec.12434
Peichl, L., Nìmec, P., and Burda, H. (2004). Unusual cone and rod properties in subterranean African mole-rats (Rodentia, Bathyergidae). Eur. J. Neurosci. 19, 1545–1558. doi: 10.1111/j.1460-9568.2004.03263.x
Pepper, J. W., Braude, S. H., Lacey, E. A., and Sherman, P. W. (1991). “Vocalizations of the naked mole-rat,” in The Biology of the Naked Mole-Rat, eds P. W. Sherman, J. U. M. Jarvis, and R. D. Alexander (New Jersey: Princeton Univ. Press), 243–274.
Phillips, J. B., Youmans, P. W., Muheim, R., Sloan, K. A., Landler, L., Painter, M. S., et al. (2013). Rapid learning of magnetic compass direction by C57BL/6 mice in a 4-armed ‘plus’ water maze. PLoS One 8:e73112. doi: 10.1371/journal.pone.0073112
Pleštilová, L., Hrouzkova, E., Burda, H., Meheretu, Y., and Sumbera, R. (2021). Ear morphology in two root-rat species (genus Tachyoryctes) differing in the degree of fossoriality. J. Comp. Physiol. A 207, 469–478. doi: 10.1007/s00359-021-01489-z
Pleštilová, L., Hrouzková, E., Šumbera, R., and Burda, H. (2016). Does the morphology of the ear of the Chinese bamboo rat (Rhizomys sinensis) show ‘subterranean’ characteristics? J. Morphol. 277, 575–584. doi: 10.1002/jmor.20519
Poduschka, W. (1978). Abwehrreaktion der Mullratte, Cryptomys hottentotus (Lesson, 1826). Säugetierk. Mitt. 26, 260–268.
Sato, K. (2006). Monitoring the underground migration of sequestered carbon dioxide using Earth tides. Energy Conv. Manag. 47, 2414–2423. doi: 10.1016/j.enconman.2005.11.005
Scharff, A. (1998). Systematik und Verhaltensökologie sambischer Sandgräber (Bathyergidae, Rodentia), PhD thesis, Faculty of Biosciences. Essen: University of Essen.
Scharff, A., Begall, S., Grütjen, O., and Burda, H. (1999). Reproductive characteristics and growth of Zambian giant mole-rats, Cryptomys mechowi (Rodentia: Bathyergidae). Mammalia 63, 217–230.
Schleich, C. E., and Busch, C. (2004). Functional morpohology of the middle ear of Ctenomys talarum (Rodentia: Octodontidae). J. Morphology 85, 1–6.
Sichilima, A. M., Bennett, N. C., and Faulkes, C. G. (2011). Field evidence for colony size and aseasonality of breeding and in Ansell’s mole-rat, Fukomys anselli (Rodentia: Bathyergidae). Afr. Zool. 46, 334–339. doi: 10.3377/004.046.0212
Šklíba, J., Lövy, M., Hrouzková, M., Kott, O., and Okrouhlík Šumbera, R. (2014). Social and environmental influences on daily activity pattern in free-living subterranean rodents: The case of a eusocial Bathyergid. J. Biol. Rhyth. 29, 203–214. doi: 10.1177/0748730414526358
Stathopoulos, S., Bishop, J. M., and O’Ryan, C. (2014). Genetic Signatures for Enhanced Olfaction in the African Mole-Rats. PLoS One 9:e93336. doi: 10.1371/journal.pone.0093336
Sugisaki, R. (1981). Deep-seated gas emission induced by the Earth tide: a basic observation for geochemical earthquake prediction. Science 212, 1264–1266. doi: 10.1126/science.212.4500.1264
Šumbera, R., Mazoch, V., Patzenhauerová, H., Lövy, M., Šklíba, J., Bryja, J., et al. (2012). Burrow architecture, family composition and habitat characteristics of the largest social African mole-rat: the giant mole-rat constructs really giant burrow systems. Acta Theriol. 57, 121–130. doi: 10.1007/s13364-011-0059-4
Šumbera, R., Zelová, J., Kunc, P., Knízková, I., and Burda, H. (2007). Patterns of surface temperatures in two mole-rats (Bathyergidae) with different social systems as revealed by IR-thermography. Physiol. Behav. 92, 526–532. doi: 10.1016/j.physbeh.2007.04.029
Sun, Q., Yoda, K., and Suzuki, H. (2005). Internal axial light conduction in the stems and roots of herbaceous plants. J. Exp. Bot. 56, 191–203. doi: 10.1093/jxb/eri019
Torrents-Ticó, M., Bennett, N. C., Jarvis, J. U., and Zott, M. (2018). Sex differences in timing and context of dispersal in Damaraland mole-rats (Fukomys damarensis). J. Zool. 306, 252–257. doi: 10.1111/jzo.12602
Úlehlová, L., Burda, H., and Voldøich, L. (1984). Involution of the auditory neuroepithelium in a tiger (Panthera tigris) and jaguar (Panthera onca). J. Comp. Pathol. 94:153157.
Van Daele, P. A. A. G., Blondé, P., Stjernstedt, R., and Adriaens, D. (2013). A new species of African Mole-rat (Fukomys, Bathyergidae, Rodentia) from the Zaire-Zambezi Watershed. Zootaxa 3636, 171–189. doi: 10.11646/zootaxa.3636.1.7
Van Daele, P. A. A. G., Dammann, P., Kawalika, M., Meier, J.-L., Van De Woestijne, C., and Burda, H. (2004). Chromosomal diversity in Cryptomys mole-rats (Rodentia: Bathyergidae) in Zambia; with the description of new karyotypes. J. Zool. 264, 317–326. doi: 10.1017/s0952836904005825
Vanden Hole, C., Van Daele, P. A. A. G., Desmet, N., Devos, P., and Adriaens, D. (2014). Does sociality imply a complex vocal communication system? A case study for Fukomys micklemi (Bathyergidae, Rodentia). Bioacoustics. 23, 143–160. doi: 10.1080/09524622.2013.841085
Willingstorfer, W., Burda, H., and Winckler, J. (1998). Ovarian growth and folliculogenesis in breeding and non-breeding females of a social rodent, the Zambian common mole-rat, Cryptomys sp. J. Morphol. 237, 33–41. doi: 10.1002/(SICI)1097-4687(199807)237:1<33::AID-JMOR3>3.0.CO;2-P
Keywords: Fukomys, sensory ecology, behavioral ecology, ecophysiology, spatial orientation, Zambia
Citation: Burda H (2022) Zambian Mole-Rats: 33 Years on the Scene and What We Still Do Not Know and How We Could Learn It. Front. Ecol. Evol. 10:866709. doi: 10.3389/fevo.2022.866709
Received: 31 January 2022; Accepted: 08 April 2022;
Published: 19 May 2022.
Edited by:
Susanne Holtze, Leibniz Institute for Zoo and Wildlife Research (LG), GermanyReviewed by:
Gabriel Francescoli, Universidad de la República, UruguayKai Caspar, University of Duisburg-Essen, Germany
Stan Braude, Washington University in St. Louis, United States
Copyright © 2022 Burda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hynek Burda, YnVyZGFAZmxkLmN6dS5jeg==
 Hynek Burda
Hynek Burda