- 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bengaluru, India
- 2Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, India
- 3Department of Botany, University of Kashmir, Kargil, India
- 4Division of Evolutionary Biology, Ludwig-Maximilians-Universität München, Planegg, Germany
- 5National Institute of Pharmaceutical Education and Research, Guwahati, India
- 6Department of Chemistry, Govt. Degree College Eidgah (Affiliated to Cluster University Srinagar), Srinagar, India
- 7Department of Botany, University of Ladakh, Ladakh, India
Adaptation to changing environmental conditions is a driver of plant diversification. Elevational gradients offer a unique opportunity for investigating adaptation to a range of climatic conditions. The use of specialized metabolites as volatile and phenolic compounds is a major adaptation in plants, affecting their reproductive success and survival by attracting pollinators and protecting themselves from herbivores and other stressors. The wormseed Artemisia brevifolia can be found across multiple elevations in the Western Himalayas, a region that is considered a biodiversity hotspot and is highly impacted by climate change. This study aims at understanding the volatile and phenolic compounds produced by A. brevifolia in the high elevation cold deserts of the Western Himalayas with the view to understanding the survival strategies employed by plants under harsh conditions. Across four sampling sites with different elevations, polydimethylsiloxane (PDMS) sampling and subsequent GCMS analyses showed that the total number of volatile compounds in the plant headspace increased with elevation and that this trend was largely driven by an increase in compounds with low volatility, which might improve the plant’s resilience to abiotic stress. HPLC analyses showed no effect of elevation on the total number of phenolic compounds detected in both young and mature leaves. However, the concentration of the majority of phenolic compounds decreased with elevation. As the production of phenolic defense compounds is a costly trait, plants at higher elevations might face a trade-off between energy expenditure and protecting themselves from herbivores. This study can therefore help us understand how plants adjust secondary metabolite production to cope with harsh environments and reveal the climate adaptability of such species in highly threatened regions of our planet such as the Himalayas.
Introduction
Altitude plays an essential role in determining the type of organisms living in a particular ecosystem, as both biotic, as well as abiotic factors change with an increase in elevation. With an increase in altitude, organisms face extreme abiotic changes, such as temperature decrease, irradiation increase (especially UV-B irradiation that increases 18% per 1,000 m altitude (Blumthaler et al., 1997), wind speed increase, snow cover increase, oxygen content decrease, drier soils, frozen soils in winter, atmospheric pressure decrease, and others (Körner, 2003). Plants cope with such situations by producing chemical compounds like phenolics, alkaloids, flavonoids, tannins, and terpenoids as specialized metabolites (Wink, 2018). These specialized metabolites are synthesized by plants as volatile or non-volatile compounds and have evolved certain physiological and ecological functions which help the plant adapt to different environmental conditions (Holopainen et al., 2018).
Volatile organic compounds (VOCs) synthesized by plants mediate interactions with pollinators, herbivores, pathogens and pests, microbes, and other surrounding plants (Bouwmeester et al., 2019). VOCs, help to attract beneficial organisms such as pollinators (Schiestl, 2015; Proffit et al., 2020) and natural enemies of herbivores (Amo et al., 2013; Aartsma et al., 2017; Turlings and Erb, 2018), as well as repel the herbivores themselves (De Moraes et al., 2001). The VOCs can inhibit the growth and development of plant pathogens (Neri et al., 2007; Marques et al., 2014; Quintana-Rodriguez et al., 2018), and produce allelopathic effects on competitive plant species (Arimura et al., 2010; Santonja et al., 2019). Volatile blends can also result in chemotypes of the same plant species under different conditions (Karban et al., 2014, 2016). VOCs can further act as a priming stimulus and activate defense responses against both biotic and abiotic stress (Brilli et al., 2019). Abiotic stress such as light and temperature (Monson et al., 1992; Loreto et al., 2006), salt (Loreto and Delfine, 2000), drought (Fortunati et al., 2008), UV-B radiation (Harley et al., 1996; Tiiva et al., 2007), and atmospheric CO2 (Scholefield et al., 2004) can also lead to emission of VOCs from plants. Plants also synthesize non-volatile phenolic compounds like phenolic acids and flavonoids for defense against abiotic stress and acclimatization to harsh environmental conditions (Sharma et al., 2019a; Samec et al., 2021). In addition, phenolic compounds play a crucial role in protection against biotic stressors such as attack by pathogens (bacteria, fungi, and viruses) and insects (Wallis and Galarneau, 2020).
The higher altitudes in the Ladakh region in the Western Himalayas are often referred to as “cold desert” (Kumar et al., 2011b) because diurnal and seasonal fluctuations in temperature have been observed with temperatures varying from −40°C in winters to about + 35°C in summer (Shafiq et al., 2016), and the area is semi-arid with very sparse vegetation. The precipitation is usually low with a mean annual precipitation of 68 mm in winter and 102 mm in summer, during the period of 1901–2000 (Shafiq et al., 2016). The altitude ranges from 2,800 to 6,700 meters above sea level (masl), which differentiates the region from the rest of the earth’s cold deserts (Schmidt and Nüsser, 2017). This region covers a geographical area of about 98,000 sq km and lies between 32°15″ and 36°15″N latitude and 75°15″–80°15″E longitude (Kala, 2011). The combination of extreme altitudinal and climatic conditions makes this region particularly suitable for examining how plants use specialized metabolites to adapt to harsh environments. Such alpine ecosystems are further considered to be especially sensitive to warming and, hence, it is relevant to understand trait plasticity and how species can cope with extreme climatic changes.
Artemisia is one of the largest genera of the Asteraceae family and consists of shrubby herbs, with about 500 species of Artemisia reported across the globe (Shah, 2014). Many of the Artemisia species are medicinal and aromatic plant species. Some Artemisia species are reported from the Western Himalayas, one such as A. brevifolia Wall ex DC. (syn. Seriphidium brevifolium, commonly referred to as wormseed) (Kumar et al., 2011a). This species is commonly found in the Ladakh region across elevational gradients. At higher altitudes, it is usually found as a stand-alone species. Pollination in A. brevifolia is reported to occur both via anemophily as well as entomophily (Bharti et al., 2019), and this dual mode of pollination might also help in the acclimatization of A. brevifolia to the harsh climatic conditions of the region.
Here we use a variety of field and laboratory chemo-analytical techniques to examine the diversity and concentration of phytochemical constituents produced by A. brevifolia at different elevations of the Ladakh region. Specialized metabolites play a crucial role in survival, helping to ameliorate various stresses and maintaining ecological interactions in the given niche. The presence and concentration of these compounds are also known to be affected by the abiotic environment (Radwan et al., 2017; Kopaczyk et al., 2020; Mahdavi et al., 2020; Pagadala Damodaram et al., 2021; Peron et al., 2021). This study can therefore help us understand how species adapt their specialized metabolite production to cope with harsh environments such as high-altitude cold deserts, and can also shed light on the climate adaptability of such species in highly threatened regions of our planet such as the Himalayas.
Materials and Methods
Study Sites
Four study sites were selected in different parts and at different altitudes (2,897–4,446 masl) of the Ladakh region. However, two of our study sites were at approximately the same altitude (2,897–2,927 masl), but at different geographical locations (Table 1). At each site, 20 fully grown mature plants with no obvious sign of stress, herbivory, pathogen attack, or other damage were randomly selected for sampling, which was performed July 1–9 and August 20–23 2019. The plants did not exhibit any obvious signs of stress and we did not observe microstructural changes such as trichome or stomata differences. However, we did observe that plants at higher altitudes were small in overall size. Polydimethylsiloxane (PDMS) tube samples were collected for analysis of VOCs from all individual plants. Furthermore, 6 out of the 20 sampled plants from each site were selected at random and 5 young and 5 mature leaves were collected from each of those plants for analysis of phenolic defense compounds. The samples for VOCs, as well as phenolic defense compounds at each site, were collected in the morning from about 7:00 to 11:00 a.m.
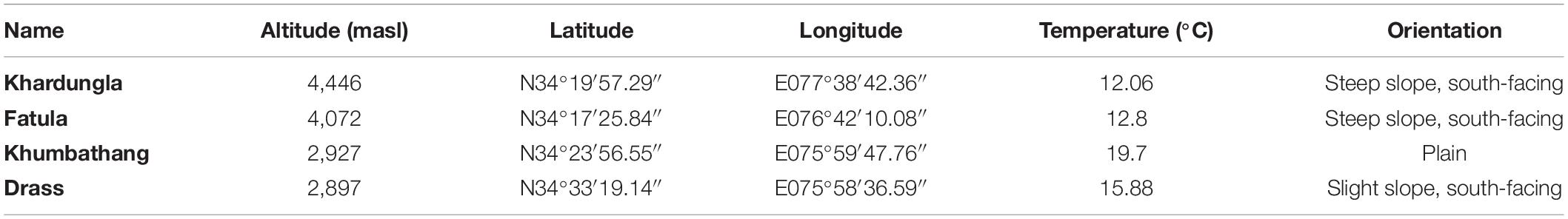
Table 1. Study sites in the Ladakh region (average temperature as measured during sample collection).
Solid-Phase Extraction for Volatile Compounds
The collection of volatile organic compounds was performed using Solid-phase extraction (SPE) as described in Nordström et al. (2017) and Nair et al. (2018). Briefly, SPE was performed using polydimethylsiloxane (PDMS) tubes (Carl Roth, Karlsruhe, Germany) with a length of approximately 5–8 mm. The tubes were soaked in a 100 ml beaker containing a mixture of HPLC grade Acetonitrile and Methanol in the ratio of 1:1 for 4 h. The tubes were dried and then conditioned using a Gerstel Tube Conditioner (C-200) (Gerstel, Mülheim an der Ruhr, Germany) by heating the tubes with a constant supply of nitrogen gas. The above procedure was repeated to obtain complete conditioning of the PDMS tubes (Nair et al., 2018). After conditioning, the tubes were flushed with Nitrogen gas to remove impurities. The tubes were then transferred to inert Amber glass vials and stored in the freezer until further use. PDMS tubes were placed on sterilized wire inside a plastic cup, at a distance of 3 inches from the plant for about 4 h (as in Nordström et al., 2017). After sampling, the tubes were transferred and stored in labeled and sterilized glass vials. Two tubes taken as control were exposed to the environment and then transferred to an empty glass vial as an environmental control.
Thermal Desorption System
Thermal desorption was performed using a Gerstel Thermal Desorption Unit system (TDU). Two tubes were placed into the TDU by a Gerstel Multipurpose Sampler (MPS) controlled by Gerstel MAESTRO software. Inside the TDU, the temperature was increased from 30 to 200°C with a rate of 100°C per minute. Desorbed volatiles were transferred to a Cooled Injection System (CIS) maintained at −50°C using liquid Nitrogen. The total time for the whole process of TDU-CIS was roughly 13 min.
GC-MS Analysis
Volatile analysis was carried out using an Agilent 7890B GC system (Agilent Technologies, Santa Clara, US) coupled with 5977A MSD quadruple mass spectrometer using an HP-5 MS column at an initial temperature of 40°C for 1 min increased to 180°C at 5°C/min and then increased to 270°C at 25°C/min and held for 5 min, for a total run time of about 37.6 min. Helium was used as carrier gas at a flow rate of 1 ml/min. Ionization energy of 70 eV was used for electron impact ionization. Analysis was performed using Agilent MassHunter Qualitative Analysis version B.07.00. Chromatograms of samples were compared with those of controls to check for environmental contaminants as described by Nordström et al. (2017) and Nair et al. (2018). The different VOCs were identified by comparing their spectra, molecular weight, and Kovats-Retention index (RI) values with NIST and authentic standards. Relative ratios were calculated by dividing peak areas of the detected compounds by the area of the internal standard (Octamethylcyclotetrasiloxane) to compare compounds across elevations (Nordström et al., 2017).
Non-volatile Phenolic Compounds Analysis
The collected leaves were packed with silica gel in zip-lock bags and dried at room temperature, then transferred to the lab and stored in the refrigerator at 4°C until extraction (Nybakken et al., 2008). For extraction of phenolic compounds, the leaves were crushed in liquid nitrogen and transferred to pre-weighed Eppendorf vials for weighing. 50 mg of the weighed sample was added to 500 μl of 90% MeOH and vortexed. After sonication for 2 min, the samples were centrifuged at 14,800 rpm for 5 min. Ten microliter of supernatant was then injected into the HPLC-PDA system for further analysis.
For the analysis of phenolic compounds, a Shimadzu Nexera UHPLC system (Shimadzu Corporation, Kyoto, Japan) was used with an Agilent Eclipse Plus C18, 5 μm, 250 mm × 4.6 mm column. The samples were eluted using 10 mM ammonium acetate in water (0.1%FA) and acetonitrile (0.1%FA) as the mobile phase. The temperature of the auto-sampler was kept at 10°C whereas the column oven temperature was 45°C. The samples were eluted using a gradient of acetonitrile as shown in Supplementary Table 1. A photodiode array detector was used for detecting the phenolic compounds with a range of 190–600 nm. The identification of compounds was performed according to their retention times and UV-spectra, quantified at 270 nm, and their respective concentrations were calculated using standards. A total of 15 standards were used (Supplementary Table 2 and Supplementary Figure 1): caffeic acid, catechin, chlorogenic acid, coumaric acid, coumarin, epicatechin, ferulic acid, gallic acid, kaempferol, myricetin, naringenin, picein, quercetin, taxifolin, and vanillic acid. Most of these compounds (except for epicatechin, ferulic acid, picein, and taxifolin) were reported from various Artemisia species, namely A. armeniaca, A. incana, A. tournefortiana, A. haussknechtii, A. scoparia, A. annua, and A. absinthium (Ferreira et al., 2010; Lee et al., 2013; Kursat et al., 2015). Caffeic acid, catechin, chlorogenic acid, coumaric acid, ferulic acid, myricetin, picein, quercetin, and vanillic acid were also reported from some alpine plants such as Bistorta vivipara, Dryas octopetala, Salix reticulate, and Lobelia rhynchopetalum (Nybakken et al., 2008; Lemma et al., 2019). The calibration curve for our 15 standards was prepared using MeOH as a solvent to get a concentration of 1 mg/ml. 10 μl each of 4 standards (catechin, chlorogenic acid, epicatechin, and myricetin) and 5 μl each of remaining 11 standards were pooled together to reach a volume of 1 ml 90% MeOH using LC-MS grade water and 100% MeOH. Then the mixtures were serially diluted (twofold) in 90% MeOH to get a set of concentrations that allows for an 8-point calibration curve (for catechin, chlorogenic acid, epicatechin and myricetin: 100, 50, 25, 12.5, 6.25, 3.125, 1.5625, and 0.78125 ng on column; and for the other 11 standards: 50, 25, 12.5, 6.25, 3.125, 1.5625, 0.78125, and 0.390625 ng on column).
Statistical Analyses
All analyses were performed in R, version 3.6.1 (R Core Team, 2019). VOC data from one sample replicate from Khumbathang was excluded from the analyses, as 6 compounds were uniquely detected in this sample, a sign of contamination (Supplementary Table 3). We fitted several statistical models to our VOC data. To determine how elevation affected the presence/absence of single VOCs, we used generalized linear models (GLMs) with binomial error structure, including elevation, compound names, and their interaction as fixed effects. VOCs detected in samples from only one elevation (considering Drass (2,897 m) and Khumbathang (2,927 m) as the same elevation) were excluded from this analysis (Supplementary Table 3). We fitted an additional GLM of equal structure to the presence/absence data, where instead of the compound names, we included the natural logarithm of their vapor pressure as a covariate (see Supplementary Table 12). We also modeled the effect of elevation on the total number of VOCs detected per sample, using a GLM with Poisson error structure, with only elevation as a fixed effect. To determine how elevation affects the relative ratio of individual compounds, we fitted a linear model (LM) to the natural logarithm of the relative ratio of a compound (resulting in a normally distributed response variable), including elevation, compound names, and their interaction as fixed effects. For this model, ratios of 0 (i.e., compound not detected) were not included. For our data on phenolic defense compounds, we fitted the same types of model structures, except that we didn’t fit a model for the effect of vapor pressure. Also, instead of approximating the relative ratio of a compound (as for VOCs), we could use actual concentration values. Estimated marginal means (EMMs) and their 95% CIs were extracted from all models using the emmeans package (Lenth, 2019), which was also used to perform statistical tests. Test statistics were based on the link-scale of the respective GLM. All p-values for slopes of interaction terms involving individual compounds (from logistic regression models and models for concentration or relative ratio) were corrected using the “false-discovery rate” method. PCAs were performed on presence/absence data (for VOCs) and concentration data (for phenolic compounds; note that values were not log-transformed as for the LM, however, they were scaled and centered). For the former, we used the logisticPCA package (Landgraf and Lee, 2020).
Results
Chemical Variability of Artemisia brevifolia Volatile Organic Compounds at Different Elevations
Across the four sampling sites with different elevations, a total of 24 volatile compounds were identified in the plant headspace using SPE and GC-MS analysis (Supplementary Table 3). The total number of volatile compounds in the plant headspace increased significantly with elevation (Figure 1A, Poisson GLM, n = 79, z-value = 3.228, p = 0.001). The low elevation sites in Drass (2,897 masl, mean number of compounds = 2.00) and Khumbathang (2,927 masl, mean number of compounds = 3.11) had a lower number of VOCs per sample than the higher elevation sites Fatula (4,072 masl, mean number of compounds = 3.50) and Khardungla (4,446 masl, mean number of compounds per sample = 4.05). This increase can be partly explained by a significant effect of the two-way interaction between elevation and vapor pressure on the probability that a compound is detected in a sample (binomial GLM, n = 79, z-value = −2.272, p = 0.023). Compounds with lower vapor pressure (i.e., lower volatility) were more likely to be detected in samples taken from higher elevation (Figure 1B and Supplementary Figure 2).
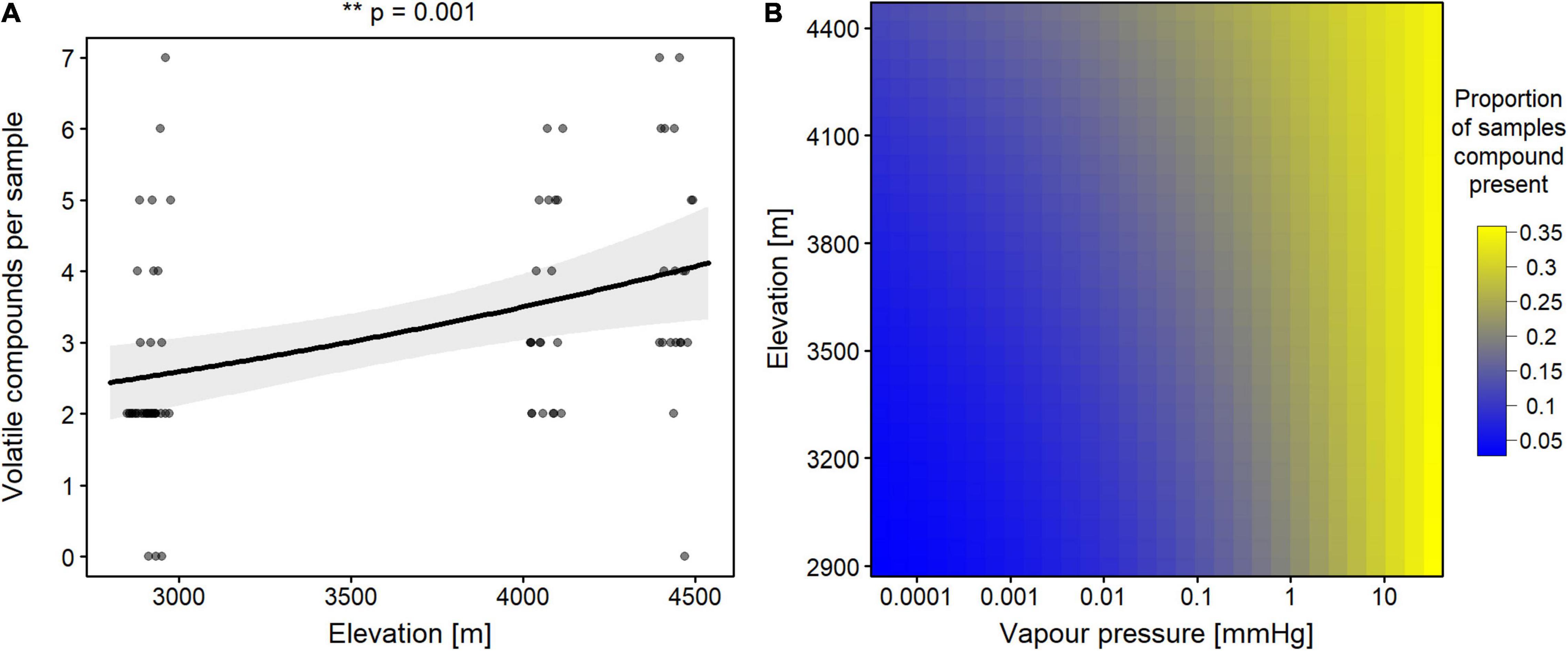
Figure 1. Effect of elevation and vapor pressure on overall presence of volatile organic compounds (VOCs). (A) Effect of elevation on number of VOCs detected per sample. Solid lines show the estimated marginal means from the GLM and the gray-shaded area shows the 95% confidence interval. Raw data is displayed as dots (n = 79), slightly jittered to avoid overlap. The significance of the effect is highlighted with common asterisk notation. (B) GLM estimates for the two-way interaction between elevation and vapor pressure on the probability that a compound was detected in a sample (n = 79). One of the squares represents a combination of values of elevation and vapor pressure. The color of a square shows the magnitude of the estimated proportion, with yellow squares denoting higher proportions. The vapor pressure axis was logarithmized.
The major compounds detected were eucalyptol/cineole and (L)-camphor. Eucalyptol/cineole was present across all elevations and in almost all samples. (L)-camphor, however, was only common in samples from the lower three sites, but entirely absent in Khardungla samples (4,446 masl). These compounds (together with alpha-and beta-thujone) also showed the highest relative ratios when present in a sample (Supplementary Figure 3 and Supplementary Table 10). The presence or absence of most VOCs in the headspace of a sample was significantly impacted by the elevation of the sampling site (Figure 2 and Supplementary Table 4). The presence of beta-thujone, alpha-thujone, 2,3-dichlorobenzaldehyde, geranyl isovalerate, 3-thujanone, camphor, and 2-hexadecanol increased significantly in samples with altitude of the sampling site, whereas the presence of (L)-camphor decreased significantly in samples with altitude (Figure 2; see statistical tests after correction for multiple testing in Supplementary Table 4). In a logistic PCA based on the presence/absence of compounds, samples clustered by elevation, and clusters of similar elevation were closer to each other than to other clusters (Supplementary Figure 4; loadings in Supplementary Table 5). The relative ratio of 57% of the analyzed VOCs increased with elevation (Supplementary Figure 5). However, for the only compound where a significant effect of elevation was visible (eucalyptol/cineole), the trend was negative (Supplementary Figure 5 and Supplementary Table 6, n = 79, t-ratio = −4.06, p < 0.001).
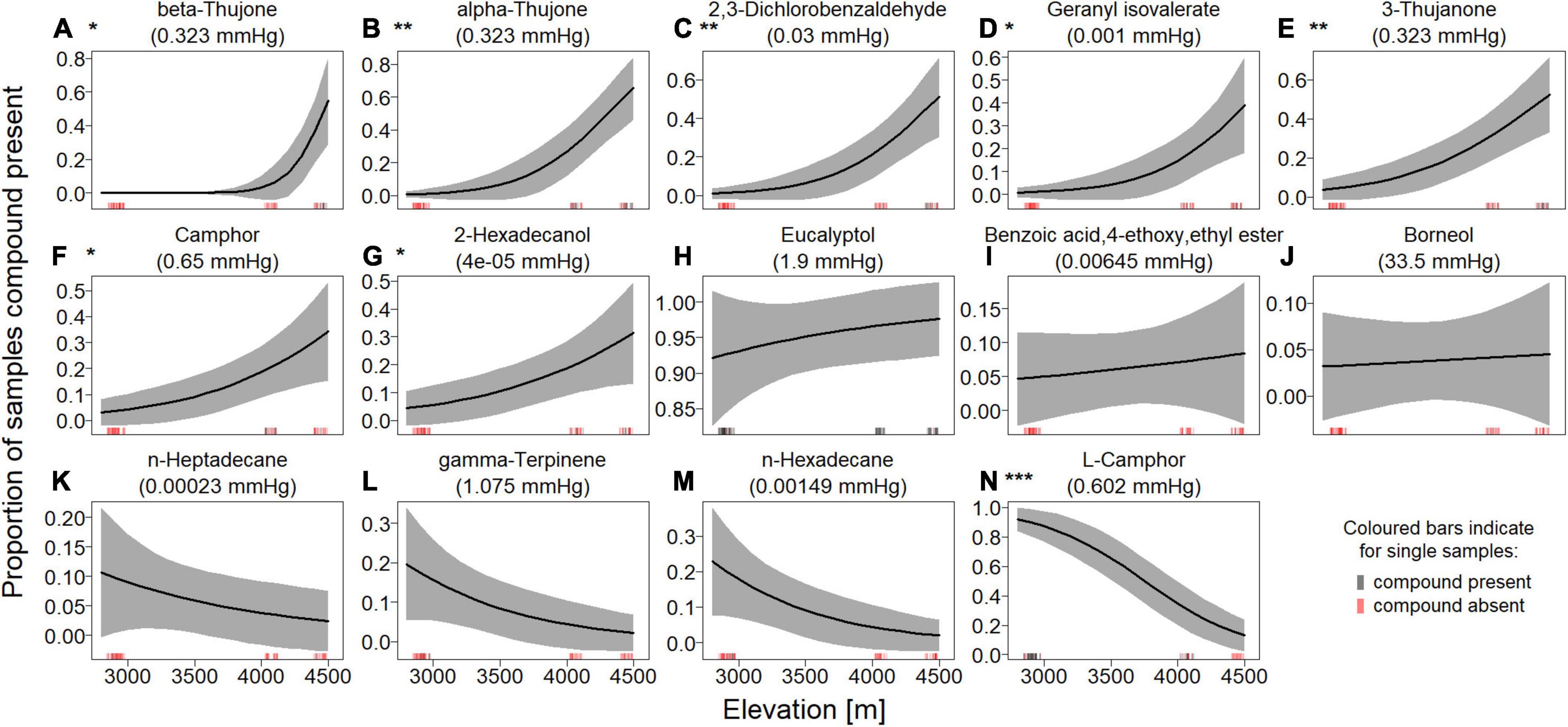
Figure 2. Effect of elevation on presence/absence of different volatile organic compounds (A–N). Solid lines show the estimated marginal means from the GLM and the gray-shaded area shows the 95% confidence interval. Compounds were sorted from top left to bottom right, according to their slope (on the logit-scale). Raw data is displayed at the lower end of each graph, with red lines denoting that a compound was not detected at a certain elevation and black lines denoting that it was detected (each n = 79). The lines are slightly jittered to avoid overlap. Significant effects are highlighted with common asterisk notation next to the panel label.
Chemical Variability of Artemisia brevifolia Phenolic (Defense) Compounds at Different Elevations
Out of 15 possible phenolic compounds used as standards, we detected 8 compounds in our A. brevifolia leaf samples (Supplementary Figure 6). Most of the detected compounds were phenolic acids, with the most highly concentrated compound being chlorogenic acid, followed by caffeic acid and coumaric acid, while the concentration of flavonoids was either low, such as for catechin and picein (Supplementary Figure 6 and Supplementary Table 11), or the compounds were not detected (coumarin, epicatechin, kaempferol, myricetin, naringenin, quercetin, and taxifolin). The concentration of individual compounds was highly affected by elevation (Figure 3). The concentration of most phenolic defense compounds decreased as elevation increased, in both young and mature leaves and this effect was significant in caffeic acid (young and mature leaves) as well as picein (only mature leaves; all statistical tests and p-values after correction for multiple testing can be found in Supplementary Table 7). We found no effect of elevation on total number of phenolic defense compounds detected in both young (Supplementary Figure 7A, n = 24, z-value = −0.202, p = 0.840) and mature leaves (Supplementary Figure 7B, n = 24, z-value = −0.095, p = 0.924). Similarly, the presence/absence of no single compound was affected by elevation (Supplementary Table 8). In PCAs based on compound concentration performed separately for samples from young and mature leaves, samples clustered by location and, to some extent, by elevation (Supplementary Figure 8, loadings in Supplementary Table 9).
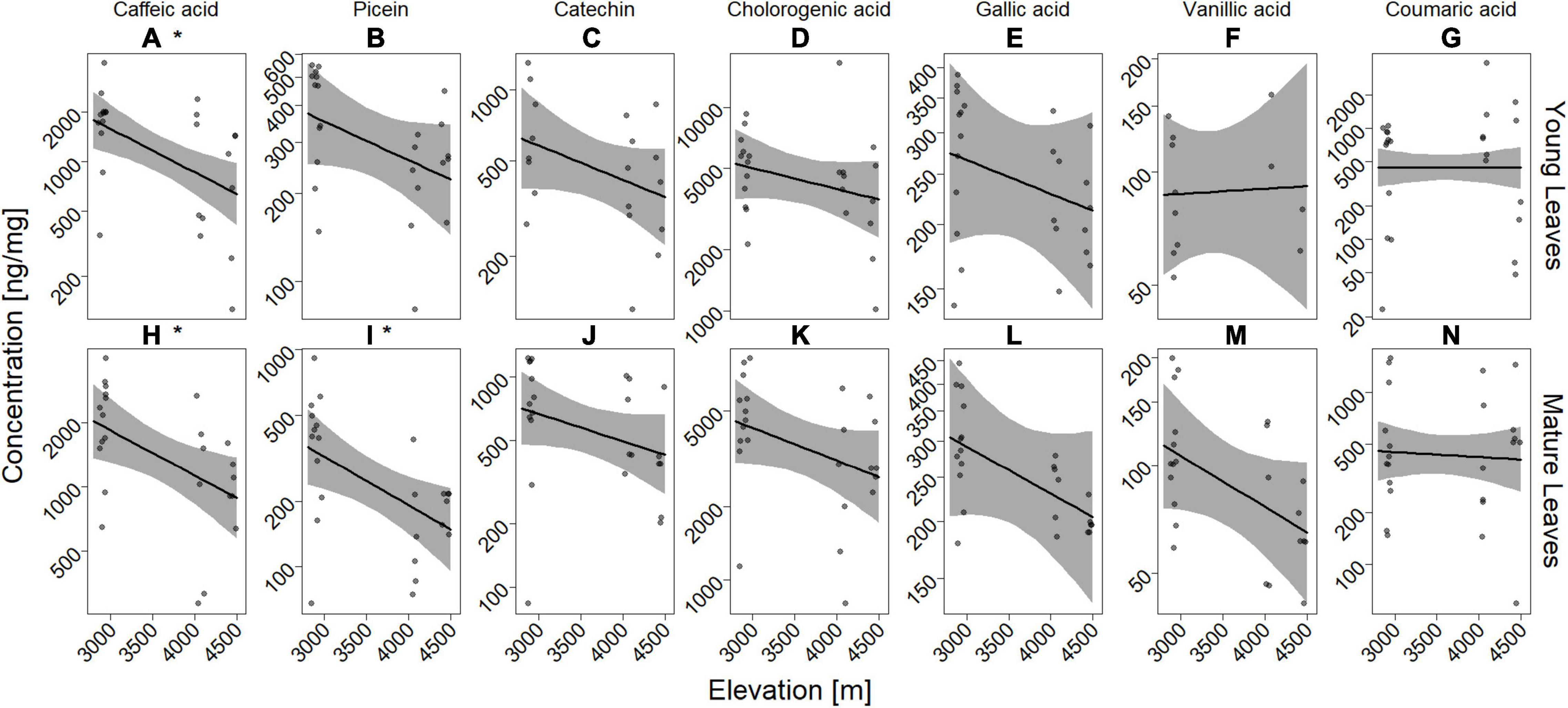
Figure 3. Concentration of phenolic defense compounds decreases with elevation in young (A–G) and mature (H–N) leaves. Solid lines show the estimated marginal means from the GLM and the gray-shaded area shows the 95% confidence interval. Raw data is displayed as dots (each n = 24), slightly jittered to avoid overlap. Significant effects are highlighted with common asterisk notation next to the panel label. The concentration axis was logarithmized.
Discussion
Elevational gradients of the Ladakh region of Western Himalaya offer a unique opportunity for investigating the adaptation of plants to a range of climatic conditions, as biotic and abiotic factors including resource availability sharply change with an increase in elevation in this cold desert region. The present study aimed to understand the impact of altitude on volatile and phenolic compounds produced by A. brevifolia with the view to understanding the survival strategies employed by this plant under these harsh climatic conditions. Our results show that the number of VOCs per plant increased with elevation, and VOCs of low volatility were more abundant at higher altitudes. However, the plants showed lower concentrations of phenolic compounds at higher elevations but no differences in their number. Two sites with similar elevations (i.e., Drass and Khumbathang) were included to examine the effect of sampling locations independent of elevation. The similarity in terms of concentration and composition of VOCs and phenolic compounds between these sites suggests that altitude was the major driving force causing variation.
Our results show that at higher altitudes of Khardungla (4,446 masl) the VOCs of low volatility were more abundant compared to lower altitudes of Drass (2,897 masl). Among the VOCs, we found that several compounds known to protect from oxidative stress increased in presence with elevation such as alpha-thujone (Németh and Huong Thi Nguyen, 2020), camphor (Suprasanna and Variyar, 2013), 2-hexadecanol (Shahzad et al., 2021) and geranyl isovalerate (Severino et al., 2007). Terpenoids, which comprised the major constituents of VOCs in our plants, are known to provide an adaptive strategy against abiotic stress such as drought, heat, and light stress (Vickers et al., 2009; Haberstroh et al., 2018). Small doses of monoterpenes such as camphor, eucalyptol, and thujone under UV-induced mutagenesis were reported to stimulate error-free DNA repair processes and acted as bio-antimutagens (Nikolić et al., 2011a,b), suggesting the role of these monoterpenes in the acclimatization of plants at high elevational ranges with high UV-B irradiation. In the sage plant (Salvia officinalis), monoterpenes (namely eucalyptol, camphor, and alpha/beta-thujone) accumulated under drought stress (Nowak et al., 2010) and their concentration doubled after 2 days of drought (Radwan et al., 2017). However, at the end of 2 weeks of continuous drought stress, the concentration of eucalyptol and camphor remained stationary, while the concentration of alpha/beta-thujone showed a steady increase (Radwan et al., 2017). These reports suggest the possible defensive roles of monoterpenes such as camphor, eucalyptol, and thujones against drought and UV radiation, and this might be one of the reasons that the presence of these monoterpenes increased with elevation in our results.
Our study further indicates that the concentration of phenolic compounds decreased in both young and mature leaves of A. brevifolia with an increase in elevation. In many plant species, the content of phenolic compounds such as phenolic acids and flavonoids has been reported to increase under abiotic stress conditions (drought, heavy metal pollution, salinity, high/low temperature, and ultraviolet radiations) by enhancing the activity of the phenylpropanoid biosynthetic pathway (Mahdavi et al., 2015; Sharma et al., 2016, 2019b; Wang et al., 2019). Phenolic acids in plants are powerful antioxidants as they mediate the scavenging of harmful reactive oxygen species when a plant is experiencing a combination of abiotic stresses (Bistgani et al., 2019; Chen et al., 2019). Furthermore, there was an increase in the content of total phenol and flavonoid in Thalictrum foliolosum with increasing elevation, and the content of phenolic acids such as caffeic acid derivatives in Arnica montana also increased (Spitaler et al., 2006; Pandey et al., 2018). Therefore, the decrease in the concentration of these compounds in our study with increasing elevation is initially puzzling.
The unpredicted decrease in concentration in phenolics might be caused by the extreme conditions these plants are experiencing. Albert et al. (2009) reported in Arnica montana that enhanced UV-B radiation at higher altitudes might not contribute significantly to shifting the compositions of phenolic compounds, but that temperature might. A study in grapevine leaves found that the total content of phenolics, phenolic acids (caffeic acids, p-coumaric acid, and ferulic acid), and their antioxidant activity decreased under prolonged exposure to cold stress, which might be a survival strategy deployed to reduce the energy expenditure under such unfavorable environmental conditions (Król et al., 2015).
Secondly, the reduction in phenolic defense compounds might also reflect the reduced biodiversity at higher elevations. Phenolic acids such as chlorogenic acid, caffeic acid, gallic acid, etc. are efficient defense molecules against insect herbivores (Tosovic, 2017; Kundu and Vadassery, 2019; Punia et al., 2021). The abundance as well as the species richness of herbivorous Orthoptera decreased with elevation in the Central Alps (Pitteloud et al., 2020), and the highest species richness of insect herbivores was reported at elevations between 2,000 and 3,000 masl, in Hengduan Mountains in southwestern China (Wu et al., 2021). The decrease in herbivorous insects will also decrease herbivory pressure along elevation gradients; this should lead to an overall reduction in plant defenses or a lower level of plant resistance at high elevations (Pellissier et al., 2012). Finally, phenolic acids such as gallic acid, chlorogenic acid, caffeic acid, coumaric acid, hydroxybenzoic acid, ferulic acid, etc. (Li et al., 2010) also play an essential role in the communication of plants, by acting as a signaling molecule in plant-microbe interactions (Mandal et al., 2010). Phenolics were also reported from root exude of wild oat roots into their rhizospheric soil and these might adversely affect the growth of other plants grown in this soil (Iannucci et al., 2013). Fewer plant and microbial interactions could also impact phenolic concentrations as elevation increases.
Our results suggest that the changes in composition and concentration of phytochemical constituents might help A. brevifolia to survive the harsh conditions of the high altitudes and extreme drought of the overall Ladakh region to become one of the dominant plant species in the higher elevation sites studied. Further, examination of the expression of various pathway genes and antioxidant profiles vis-a-vis rhizomicrobiome at different altitudes are currently under investigation, and controlled transplantation studies could additionally assess whether changes in chemical profile are directly impacted by elevation, and subsequently, impact plant survival. Plant volatile compounds and specialized metabolites play an important role in a plant’s ecology and their diversity and concentration can be affected by changes in the abiotic environment. This can either be a passive process, directed by environmental constraints, or an active process, where plants tune their chemical profiles to the needs imposed by the environment (Shamala et al., 2020). Climate change progresses rapidly and alpine ecosystems are considered to be particularly affected. Understanding phytochemical plasticity in a dominant shrub species of the Western Himalayas can give us an idea of how organisms can survive under changing climatic conditions.
Data Availability Statement
The original contributions presented in the study as well as code for the analyses are included in the article/Supplementary Material. Raw data files are available from the Dryad Digital Repository: 10.5061/dryad.m63xsj447.
Author Contributions
NN, SO, and BM designed the study. MH, MI, and JK collected the data. NN, MH, and SR did the sample analysis. AH and NN analyzed the data. NN, AH, MH, SO, BM, and SK wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by NCBS-TIFR and a SERB Ramanujan Fellowship to SO, along with the Department of Atomic Energy, Government of India, under project nos. 12-R&D-TFR-5.04-0800 and 12-R&D-TFR-5.04-0900.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to University Grants Commission, New Delhi for fellowships and MS facility of Center for Cellular and Molecular Platforms C-CAMP, Bengaluru for carrying out HPLC analysis. BM acknowledges the support and encouragement provided by S.K. Mehta, Hon’ble Vice-Chancellor of the University of Ladakh.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.864728/full#supplementary-material
References
Aartsma, Y., Bianchi, F. J. J. A., van der Werf, W., Poelman, E. H., and Dicke, M. (2017). Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 216, 1054–1063. doi: 10.1111/nph.14475
Albert, A., Sareedenchai, V., Heller, W., Seidlitz, H. K., and Zidorn, C. (2009). Temperature is the key to altitudinal variation of phenolics in Arnica montana L. cv. ARBO. Oecologia 160, 1–8. doi: 10.1007/s00442-009-1277-1
Amo, L., Jansen, J. J., van Dam, N. M., Dicke, M., and Visser, M. E. (2013). Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol. Lett. 16, 1348–1355. doi: 10.1111/ele.12177
Arimura, G. I., Shiojiri, K., and Karban, R. (2010). Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 71, 1642–1649. doi: 10.1016/j.phytochem.2010.06.021
Bharti, U., Sharma, E., Parihar, J., and Sharma, N. (2019). Genetic System of Artemisia maritima L.: An Overexploited Medicinal Species Under Stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 89, 1373–1378. doi: 10.1007/s40011-018-1057-y
Bistgani, Z. E., Hashemi, M., DaCosta, M., Craker, L., Maggi, F., and Morshedloo, M. R. (2019). Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 135, 311–320. doi: 10.1016/J.INDCROP.2019.04.055
Blumthaler, M., Ambach, W., and Ellinger, R. (1997). Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B Biol. 39, 130–134. doi: 10.1016/S1011-1344(96)00018-8
Bouwmeester, H., Schuurink, R. C., Bleeker, P. M., and Schiestl, F. (2019). The role of volatiles in plant communication. Plant J. 100, 892–907. doi: 10.1111/tpj.14496
Brilli, F., Baccelli, I., and Loreto, F. (2019). Exploiting Plant Volatile Organic Compounds (VOCs) in Agriculture to Improve Sustainable Defense Strategies and Productivity of Crops. Front. Plant Sci. 1:264. doi: 10.3389/fpls.2019.00264
Chen, Z., Ma, Y., Yang, R., Gu, Z., and Wang, P. (2019). Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 288, 368–376. doi: 10.1016/J.FOODCHEM.2019.02.131
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. (2001). Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580. doi: 10.1038/35069058
Ferreira, J. F., Luthria, D. L., Sasaki, T., and Heyerick, A. (2010). Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15, 3135–3170. doi: 10.3390/molecules15053135
Fortunati, A., Barta, C., Brilli, F., Centritto, M., Zimmer, I., Schnitzler, J.-P., et al. (2008). Isoprene emission is not temperature-dependent during and after severe drought-stress: a physiological and biochemical analysis. Plant J. 55, 687–697. doi: 10.1111/j.1365-313X.2008.03538.x
Haberstroh, S., Kreuzwieser, J., Lobo-Do-Vale, R., Caldeira, M. C., Dubbert, M., and Werner, C. (2018). Terpenoid emissions of two Mediterranean woody species in response to drought stress. Front. Plant Sci. 9:1071. doi: 10.3389/fpls.2018.01071
Harley, P., Deem, G., Flint, S., and Caldwell, M. (1996). Effects of growth under elevated UV-B on photosynthesis and isoprene emission in Quercus gambelii and Mucuna pruriens. Glob. Chang. Biol. 2, 149–154. doi: 10.1111/j.1365-2486.1996.tb00060.x
Holopainen, J. K., Virjamo, V., Ghimire, R. P., Blande, J. D., Julkunen-Tiitto, R., and Kivimäenpää, M. (2018). Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 9:1445. doi: 10.3389/fpls.2018.01445
Iannucci, A., Fragasso, M., Platani, C., Papa, R., Woo, S. H., and Paul, N. C. (2013). Plant growth and phenolic compounds in the rhizosphere soil of wild oat (Avena fatua L.). Front. Plant Sci. 4:509. doi: 10.3389/fpls.2013.00509
Kala, C. P. (2011). Floral Diversity and Distribution in the High Altitude Cold Desert of Ladakh. J. Sustain. For. 30, 360–369. doi: 10.1080/10549811.2011.534036
Karban, R., Wetzel, W. C., Shiojiri, K., Ishizaki, S., Ramirez, S. R., and Blande, J. D. (2014). Deciphering the language of plant communication: Volatile chemotypes of sagebrush. J. Physiol. 204, 380–385. doi: 10.1111/nph.12887
Karban, R., Wetzel, W. C., Shiojiri, K., Pezzola, E., and Blande, J. D. (2016). Geographic dialects in volatile communication between sagebrush individuals. Ecology 97, 2917–2924. doi: 10.1002/ecy.1573
Kopaczyk, J. M., Warguła, J., and Jelonek, T. (2020). The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 180:104197. doi: 10.1016/j.envexpbot.2020.104197
Körner, C. (2003). Alpine Plant Life. Berlin: Springer Berlin Heidelberg, doi: 10.1007/978-3-642-18970-8
Król, A., Amarowicz, R., and Weidner, S. (2015). The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J. Plant Physiol. 189, 97–104. doi: 10.1016/J.JPLPH.2015.10.002
Kumar, J., Mishra, G. P., Naik, P. K., Murkute, A. A., and Srivastava, R. B. (2011b). Genomic DNA isolation from Artemisia species grown in cold desert high altitude of India. African J. Biotechnol. 10, 7303–7307. doi: 10.4314/ajb.v10i37
Kumar, G. P., Kumar, R., Chaurasia, O. P., and Singh, S. B. (2011a). Current status and potential prospects of medicinal plant sector in trans-Himalayan Ladakh. J. Med. Plants Res. 5, 2929–2940. doi: 10.5897/JMPR.9000420
Kundu, A., and Vadassery, J. (2019). Chlorogenic acid-mediated chemical defence of plants against insect herbivores. Plant Biol. 21, 185–189. doi: 10.1111/plb.12947
Kursat, M., Irfan, E. M., Yilmaz, O., Civelek, S., Demir, E., and Turkoglu, I. (2015). Phytochemical contents of five Artemisia species. Notulae Scient. Biolog. 7, 495–499. doi: 10.15835/nsb.7.4.9683
Landgraf, A. J., and Lee, Y. (2020). Dimensionality reduction for binary data through the projection of natural parameters. J. Multivar. Anal. 180:104668. doi: 10.1016/j.jmva.2020.104668
Lee, Y. J., Thiruvengadam, M., Chung, I. M., and Nagella, P. (2013). Polyphenol composition and antioxidant activity from the vegetable plant’Artemisia absinthium’L. Australian J. Crop Sci. 7, 1921–1926.
Lemma, B., Grehl, C., Zech, M., Mekonnen, B., Zech, W., Nemomissa, S., et al. (2019). Phenolic compounds as unambiguous chemical markers for the identification of keystone plant species in the bale mountains. Ethiopia. Plants 8:228. doi: 10.3390/plants8070228
Lenth, R. (2019). emmeans: Estimated marginal means, aka least-squares means. R package version 1.4.6.
Li, Z.-H., Wang, Q., Ruan, X., Pan, C.-D., and Jiang, D.-A. (2010). Phenolics and plant allelopathy. Molecules 15, 8933–8952. doi: 10.3390/molecules15128933
Loreto, F., Basrta, C., Brilli, F., and Nogues, I. (2006). On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell Environ. 29, 1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x
Loreto, F., and Delfine, S. (2000). Emission of Isoprene from Salt-Stressed Eucalyptus globulus Leaves. Plant Physiol. 123, 1605–1610. doi: 10.1104/pp.123.4.1605
Mahdavi, A., Moradi, P., and Mastinu, A. (2020). Variation in Terpene profiles of thymus vulgaris in water deficit stress response. Molecules 25:1091. doi: 10.3390/molecules25051091
Mahdavi, V., Farimani, M. M., Fathi, F., and Ghassempour, A. (2015). A targeted metabolomics approach toward understanding metabolic variations in rice under pesticide stress. Anal. Biochem. 478, 65–72. doi: 10.1016/J.AB.2015.02.021
Mandal, S. M., Chakraborty, D., and Dey, S. (2010). Plant Signaling & Behavior Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 5, 359–368. doi: 10.4161/psb.5.4.10871
Marques, J. P. R., Amorim, L., José Silva-Junior, G., Bellato Spó Sito, M., and Appezzato-da Gloria, B. (2014). Structural and biochemical characteristics of citrus flowers associated with defence against a fungal pathogen. AoB Plants 7, 1–10. doi: 10.1093/aobpla/plu090
Monson, R. K., Jaeger, C. H., Adams, W. W., Driggers, E. M., Silver, G. M., and Fall, R. (1992). Relationships among Isoprene Emission Rate, Photosynthesis, and Isoprene Synthase Activity as Influenced by Temperature. Plant Physiol. 98, 1175–1180. doi: 10.1104/pp.98.3.1175
Nair, J. V., Shanmugam, P. V., Karpe, S. D., Ramakrishnan, U., and Olsson, S. (2018). An optimized protocol for large-scale in situ sampling and analysis of volatile organic compounds. Ecol. Evol. 8, 5924–5936. doi: 10.1002/ece3.4138
Németh, E. Z., and Huong Thi Nguyen. (2020). Thujone, a widely debated volatile compound: what do we know about it? Phytochem. Rev. 19, 405–423. doi: 10.1007/s11101-020-09671-y
Neri, F., Mari, M., Brigati, S., and Bertolini, P. (2007). Fungicidal Activity of Plant Volatile Compounds for Controlling Monilinia laxa in Stone Fruit. Plant Dis. 91, 30–35. doi: 10.1094/PD-91-0030
Nikolić, B., Mitić-Ćulafić, D., Vuković-Gačić, B., and Knežević-Vukčević, J. (2011a). Modulation of genotoxicity and DNA repair by plant monoterpenes camphor, eucalyptol and thujone in Escherichia coli and mammalian cells. Food Chem. Toxicol. 49, 2035–2045. doi: 10.1016/J.FCT.2011.05.015
Nikolić, B., Mitić-Ćulafić, D., Vuković-Gačić, B., and Knežević-Vukčević, J. (2011b). The antimutagenic effect of monoterpenes against UV- irradiation-, 4NQO- and t-BOOH-induced mutagenesis in coli. Arch. Biol. Sci. 63, 117–128. doi: 10.2298/ABS1101117N
Nordström, K., Dahlbom, J., Pragadheesh, V. S., Ghosh, S., Olsson, A., Dyakova, O., et al. (2017). In situ modeling of multimodal floral cues attracting wild pollinators across environments. PNAS 114, 13218–13223. doi: 10.5061/dryad.s7jb3
Nowak, M., Kleinwächter, M., Manderscheid, R., Weigel, H. J., and Selmar, D. (2010). Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J. Appl. Bot. Food Qual. 83, 133–136.
Nybakken, L., Klanderud, K., and Totland, O. (2008). Simulated environmental change has contrasting effects on defensive compound concentration in three alpine plant species. Arctic, Antarct. Alp. Res. 40, 709–715. doi: 10.1657/1523-0430(07-103)[nybakken]2.0.CO;2
Pagadala Damodaram, K. J., Gadad, H. S., Parepally, S. K., Vaddi, S., Ramanna Hunashikatti, L., and Bhat, R. M. (2021). Low moisture stress influences plant volatile emissions affecting herbivore interactions in tomato. Solanum lycopersicum. Ecol. Entomol. 46, 637–650. doi: 10.1111/een.13012
Pandey, G., Khatoon, S., Pandey, M. M., and Rawat, A. K. S. (2018). Altitudinal variation of berberine, total phenolics and flavonoid content in Thalictrum foliolosum and their correlation with antimicrobial and antioxidant activities. J. Ayurveda Integr. Med. 9, 169–176. doi: 10.1016/j.jaim.2017.02.010
Pellissier, L., Loı..c Fiedler, K., Ndribe, C., Dubuis, A., Pradervand, J.-N., Guisan, A., et al. (2012). Shifts in species richness, herbivore specialization, and plant resistance along elevation gradients. Ecol. Evol. 2, 1818–1825. doi: 10.1002/ece3.296
Peron, A., Kaser, L., Fitzky, A. C., Graus, M., Halbwirth, H., Greiner, J., et al. (2021). Combined effects of ozone and drought stress on the emission of biogenic volatile organic compounds from Quercus robur L. Biogeosciences 18, 535–556. doi: 10.5194/bg-18-535-2021
Pitteloud, C., Descombes, P., Sànchez-Moreno, S., Kergunteuil, A., Ibanez, S., Rasmann, S., et al. (2020). Contrasting responses of above- and below-ground herbivore communities along elevation. Oecologia 194, 515–528. doi: 10.1007/s00442-020-04778-7
Proffit, M., Lapeyre, B., Buatois, B., Deng, X., Arnal, P., Gouzerh, F., et al. (2020). chemical signal is in the blend: bases of plant-pollinator encounter in a highly specialized interaction. Sci. Rep. 10, 1–11. doi: 10.1038/s41598-020-66655-w
Punia, A., Chauhan, N. S., Singh, D., Kesavan, A. K., Kaur, S., and Sohal, S. K. (2021). Effect of gallic acid on the larvae of Spodoptera litura and its parasitoid Bracon hebetor. Sci. Rep. 11, 1–11. doi: 10.1038/s41598-020-80232-1
Quintana-Rodriguez, E., Rivera-Macias, L. E., Adame-Alvarez, R. M., Torres, J. M., and Heil, M. (2018). Shared weapons in fungus-fungus and fungus-plant interactions? Volatile organic compounds of plant or fungal origin exert direct antifungal activity in vitro. Fungal Ecol. 33, 115–121. doi: 10.1016/j.funeco.2018.02.005
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Radwan, A., Kleinwächter, M., and Selmar, D. (2017). Impact of drought stress on specialised metabolism: Biosynthesis and the expression of monoterpene synthases in sage (Salvia officinalis). Phytochemistry 141, 20–26. doi: 10.1016/j.phytochem.2017.05.005
Samec, D., Karalija, E., Sola, I., Vujci, V., Bok, V., and Salopek-Sondi, B. (2021). The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 10, 1–24. doi: 10.3390/plants10010118
Santonja, M., Bousquet-Mélou, A., Greff, S., Ormeño, E., and Fernandez, C. (2019). Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 9, 8201–8213. doi: 10.1002/ece3.5390
Schiestl, F. P. (2015). Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 206, 571–577. doi: 10.1111/nph.13243
Schmidt, S., and Nüsser, M. (2017). Changes of High Altitude Glaciers in the Trans-Himalaya of Ladakh over the Past Five Decades (1969–2016). Geosciences 7:27. doi: 10.3390/geosciences7020027
Scholefield, P. A., Doick, K. J., Herbert, B. M. J., Hewitt, C. N. S., Schnitzler, J.-P., Pinelli, P., et al. (2004). Impact of rising CO2 on emissions of volatile organic compounds: isoprene emission from Phragmites australis growing at elevated CO2 in a natural carbon dioxide spring. Plant, Cell Environ. 27, 393–401. doi: 10.1111/j.1365-3040.2003.01155.x
Severino, J. F., Stich, K., and Soja, G. (2007). Ozone stress and antioxidant substances in Trifolium repens and Centaurea jacea leaves. Environ. Pollut. 146, 707–714. doi: 10.1016/j.envpol.2006.04.006
Shafiq, M., Bhat, M., and Rasool, R. (2016). Variability of Precipitation regime in Ladakh region of India from 1901-2000. J. Climatol. Weather Forecast. 4:2. doi: 10.4172/2332-2594.1000165
Shahzad, R., Ewas, M., Harlina, P. W., Ullah Khan, S., Nie, X., and Nishawy, E. (2021). β-Sitosterol differentially regulates key metabolites for growth improvement and stress tolerance in rice plants during prolonged UV-B stress. J. Genet. Eng. Biotechnol. 19, 1–16. doi: 10.1186/s43141-021-00183-6
Shamala, L. F., Zhou, H.-C., Han, Z.-X., Wei, S., Han, W., Fu, X., et al. (2020). UV-B Induces Distinct Transcriptional Re-programing in UVR8-Signal Transduction, Flavonoid, and Terpenoids Pathways in Camellia sinensis. Front. Plant Sci. 11, 1–15. doi: 10.3389/fpls.2020.00234
Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M., and Zheng, B. (2019a). Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 24, 1–22. doi: 10.3390/molecules24132452
Sharma, A., Yuan, H., Kumar, V., Ramakrishnan, M., Kohli, S. K., Kaur, R., et al. (2019b). Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 179, 50–61. doi: 10.1016/J.ECOENV.2019.03.120
Sharma, A., Thakur, S., Kumar, V., Kanwar, M. K., Kesavan, A. K., Thukral, A. K., et al. (2016). Pre-sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 1:1569. doi: 10.3389/fpls.2016.01569
Spitaler, R., Schlorhaufer, P. D., Ellmerer, E. P., Merfort, I., Bortenschlager, S., Stuppner, H., et al. (2006). Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO. Phytochemistry 67, 409–417. doi: 10.1016/j.phytochem.2005.11.018
Suprasanna, P., and Variyar, P. S. (2013). Coping Abiotic Stress with Plant Volatile Organic Chemicals (PVOCs): A Promising Approach in Crop Improvement. Boston, MA: Springer, 295–306. doi: 10.1007/978-1-4614-7028-1_9
Tiiva, P., Rinnan, R., Faubert, P., Räsänen, J., Holopainen, T., Kyrö, E., et al. (2007). Isoprene emission from a subarctic peatland under enhanced UV-B radiation. New Phytol. 176, 346–355. doi: 10.1111/j.1469-8137.2007.02164.x
Tosovic, J. (2017). Spectroscopic features of caffeic acid: Theoretical study. Kragujev. J. Sci. 39, 99–108. doi: 10.5937/kgjsci1739099t
Turlings, T. C. J., and Erb, M. (2018). Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 63, 433–452. doi: 10.1146/annurev-ento-020117-043507
Vickers, C. E., Gershenzon, J., Lerdau, M. T., and Loreto, F. (2009). A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 5, 283–291. doi: 10.1038/nchembio.158
Wallis, C. M., and Galarneau, E. R.-A. (2020). Phenolic Compound Induction in Plant-Microbe and Plant-Insect Interactions: a Meta-Analysis. Front. Plant Sci. 11:2034. doi: 10.3389/fpls.2020.580753
Wang, L., Shan, T., Xie, B., Ling, C., Shao, S., Jin, P., et al. (2019). Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 272, 530–538. doi: 10.1016/j.foodchem.2018.08.085
Wink, M. (2018). Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction? Front. Physiol. 9:364. doi: 10.3389/fphys.2018.00364
Keywords: elevation, volatile organic compounds, phenolic defense compounds, Western Himalayas, Artemisia brevifolia
Citation: Nataraj N, Hussain M, Ibrahim M, Hausmann AE, Rao S, Kaur S, Khazir J, Mir BA and Olsson SB (2022) Effect of Altitude on Volatile Organic and Phenolic Compounds of Artemisia brevifolia Wall ex Dc. From the Western Himalayas. Front. Ecol. Evol. 10:864728. doi: 10.3389/fevo.2022.864728
Received: 28 January 2022; Accepted: 13 April 2022;
Published: 12 May 2022.
Edited by:
Michael Rostás, Georg August University, GermanyReviewed by:
Christoph Crocoll, University of Copenhagen, DenmarkShahinoor Rahman, Georg August University, Germany
Copyright © 2022 Nataraj, Hussain, Ibrahim, Hausmann, Rao, Kaur, Khazir, Mir and Olsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bilal Ahmad Mir, bWVlcmJpbGFsODJAZ21haWwuY29t; Shannon B. Olsson, c2hhbm5vbkBuaWNlLm5jYnMucmVzLmlu
†Present address: Nandita Nataraj, Department of Evolutionary Neuroethology, Max Planck Institute for Chemical Ecology, Jena, Germany
‡These authors have contributed equally to this work
 Nandita Nataraj
Nandita Nataraj Manzoor Hussain
Manzoor Hussain Mohd Ibrahim
Mohd Ibrahim Alexander E. Hausmann
Alexander E. Hausmann Srinivas Rao
Srinivas Rao Satwinderjeet Kaur
Satwinderjeet Kaur Jabeena Khazir
Jabeena Khazir Bilal Ahmad Mir
Bilal Ahmad Mir Shannon B. Olsson
Shannon B. Olsson