- Department of General Zoology, University of Duisburg-Essen, Essen, Germany
Ansell’s mole-rats (Fukomys anselli) are sexually dimorphic subterranean rodents that live in families consisting of a single breeding pair and their late-dispersing non-breeding offspring. Most individuals exhibit a conspicuous white head patch, which results from integumental depigmentation. Alongside other morphological, physiological, and social characteristics, skin depigmentation in these social rodents mirrors traits that presumably evolved as byproducts from selection against aggression in domestic animals, making them a potential candidate species for a self-domesticated wild mammal. Here we explored whether the expression of the white head patch, sexual dimorphism, and reproductive division of labor are reflected by different personalities in Ansell’s mole-rats. We tested locomotory activity and risk-taking as well as aggression and affiliative behavior in 51 individuals originating from nine captive families in various experimental set-ups. In line with the concept of animal personality, we recovered consistent individual responses over time. While sex had no influence on any tested variable, reproductive status was found to affect risk-taking behavior but not other personality dimensions. Discriminant function analysis revealed that family members clustered more closely together than expected by chance, suggesting that group affiliation rather than sex or social status determines behavioral profiles in this species. Finally, we failed to recover any consistent correlation between head patch expression and behavior, which conflicts with predictions of the self-domestication hypothesis. We argue that many domestication-like traits in Ansell’s mole-rat and its congeners evolved in the framework of subterranean adaptation and call for a cautious application of the self-domestication concept to wild mammals.
Introduction
Ansell’s mole-rat (Fukomys anselli) is a social bathyergid rodent from central Zambia that permanently lives underground in self-excavated tunnel systems (Begall et al., 2021a) extending up to several hundred meters in length (Šklíba et al., 2012). It displays a suit of morphological as well as sensory adaptations to subterranean life, including a cylindrical body shape, reduction of body appendages, and strongly procumbent incisors employed for digging (Begall et al., 2021a). Its eyes are profoundly reduced in size severely constraining visual perception of shapes, size, and movement (Němec et al., 2004). However, Ansell’s mole-rats are able to distinguish between darkness and light (Wegner et al., 2006), and their eyes appear to be involved in sensing the Earth’s magnetic field (Marhold et al., 1997; Caspar et al., 2020). Hearing is confined to low frequencies (Müller and Burda, 1989; Gerhardt et al., 2017), and their somatosensory system is supposedly well developed, as is the case in other subterranean mammals (Park et al., 2007). Odors (such as anogenital scent or urine) help mole-rats to distinguish familiar from unfamiliar conspecifics and to identify family members (Heth et al., 2004; Leedale et al., 2021).
Ansell’s mole-rats, like all its congeners in the genus Fukomys as well as the distantly related naked mole-rat (Heterocephalus glaber), are cooperative breeders that live in groups with high reproductive skew. In Ansell’s mole-rats, families typically comprise about ten individuals, but groups may contain up to 25 animals (Begall et al., 2021a). There is only one monogamous reproductive pair in each family (i.e., breeders, at times also called king and queen), and the offspring (non-breeders or helpers) may remain in their natal family for an extended period of time (Patzenhauerová et al., 2013). The non-reproductive mole-rats contribute to the survival of their younger siblings, for instance, by excavating and maintaining tunnels as well as by retrieving pups that stray away from the communal nest (Begall et al., 2021a). In free living Fukomys mole-rats, breeders are more likely to range in the vicinity (<10 m) of the nest and they also spend more time within the nest compared to non-breeders that may be found at distances of >90 m away from the nest chamber (Lövy et al., 2013; Šklíba et al., 2016). In line with that, breeders of the congeneric Damaraland mole-rat (Fukomys damarensis) have been shown to be less active than non-breeders in the wild (Francioli et al., 2020). However, no significant differences in the locomotor activity between Ansell’s mole-rat breeders and non-breeders could be recovered in the laboratory (Schielke et al., 2012). Due to the reproductive division of labor and strong philopatry of offspring, Ansell’s mole-rats and their congeners have been considered to be eusocial mammals by some authors (Burda et al., 2000; Burland et al., 2002). There is, however, a debate about the applicability of this term, which was originally introduced to describe the lifestyle of social insects such as ants and termites, to mole-rats of the genus Fukomys. It has recently been shown that in wild Damaraland mole-rats, the presence of non-reproductive helpers in a burrow system only weakly increases the fitness of the breeding female (Thorley et al., 2021). Furthermore, Fukomys helpers fundamentally differ from workers in many social insects in not showing evidence for task specialization (F. anselli – Šklíba et al., 2016; F. damarensis – Thorley et al., 2018); a trait that is also lacking in naked mole-rats (Siegmann et al., 2021). Zöttl et al. (2016) showed that the individual cooperative investment of Fukomys helpers increases with age and appears to be a consequence of age-related polyethism as is also the case in other cooperatively breeding mammals, such as meerkats (Suricata suricatta). There were only small differences in the investment of cooperative behavior between the sexes, and if present it was biased towards females. For instance, females provided significantly more alloparental care (a behavior that was very rarely observed overall) and tended to invest more time into nest building and digging than males (within their first year of life – Zöttl et al., 2016). At least in the Damaraland mole-rat, males tend to disperse earlier and more frequently than the more philopatric females (Zöttl et al., 2016; Torrents-Ticó et al., 2018). Males are also more likely to invade family groups to challenge established breeders (Mynhardt et al., 2021). The greater body mass, relative skull dimensions and broader incisors of males might have evolved in the context of such intrasexual conflicts (Caspar et al., 2021b).
Ansell’s mole rats (as well as their congeners) display remarkable longevity, reaching a maximum age of about 22.2 years (Dammann et al., 2011; Begall et al., 2021a). The average (and maximum) lifespan of breeders in captivity is about two times that of non-breeders, resulting in a bimodal aging pattern for the species (Dammann and Burda, 2006). In the congeneric giant mole-rat (Fukomys mechowii) it was recently recovered that cortisol levels in hair are significantly higher in adult non-breeders living together with their parents compared to those of breeders (Begall et al., 2021b). This finding may indicate higher stress levels in non-breeders and could explain the earlier onset of senescence.
Among the many unusual traits of the Ansell’s mole-rat and other Fukomys species are some that mirror characteristics of domesticated mammals. These include markedly low stress hormone levels (Ganem and Bennett, 2004), increased tolerance and lowered aggression toward conspecifics (restricted to group members in captivity, see Begall et al., 2021b; compare Ganem and Bennett, 2004), localized depigmentation of the integument (see below), and brains that are smaller than the average for similar-sized rodents (Kruska and Steffen, 2009). In fact, the combined presence of these traits could be interpreted as evidence for so-called self-domestication in Fukomys.
The concept of self-domestication has originally been invoked to explain various derived phenotypic as well as behavioral characters that differentiate modern humans (Homo sapiens) from their ancestors and other primates (reviewed by Hare (2017); see also Theofanopoulou et al. (2017)). However, it has also been adopted for non-human primates, such as bonobos (Pan paniscus – Hare et al., 2012) and marmosets (Callithrix jacchus – Ghazanfar et al., 2020), with calls to extend it further to other mammalian groups. Self-domestication entails the emergence of specific behavioral and morphological traits in natural populations which are otherwise characteristic for those domesticated by humans (Hare, 2017). The main feature of lineages considered to be self-domesticated is increased social tolerance and reduced aggression (Hare, 2017), importantly mediated by low levels of circulating stress hormones (compare Albert et al., 2009; Wilkins et al., 2014). This reduction in stress hormone levels is hypothesized to chiefly relate to hypofunction of the adrenal glands, which develop from neural crest cells in the mammalian embryo. Due to the action of pleiotropic genes regulating the differentiation and migration of the embryonic neural crest cells, selection against aggression would not only affect the adrenal glands but further give rise to alterations in other neural crest-derived structures, causing localized depigmentation of the integument (although this is not found in humans), as well as a reduction in brain, jaw, and tooth size (Wilkins et al., 2014). The collective of these various altered traits is commonly denoted as the “domestication syndrome” which is supposed to be found in both self-domesticated and human-domesticated lineages (Trut, 1999; Wilkins et al., 2014; but see Lord et al. (2020) for a rebuttal).
Whether the term “self-domestication” is actually fitting for what it aims to describe and how conclusive the available evidence for the hypothesis is, continues to be hotly debated (Sánchez-Villagra and van Schaik, 2019; Losey, 2021). In fact, the only traits constituting the “domestication syndrome” which are universally expressed in domesticated mammal lineages are increased tameness and integumental depigmentation, calling its scope into question (Sánchez-Villagra and van Schaik, 2019).
Among non-synanthrope wild rodents (compare Geiger et al., 2018 for a relevant study on a commensal population of house mice), Fukomys is indeed striking in fitting the self-domestication concept in several crucial aspects. Apart from the aforementioned traits, Fukomys group members provide allomaternal care for the altricial offspring of the family and communicate with each other through an elaborate repertoire of social vocalizations, which surpasses that of most other rodents in complexity (Bednářová et al., 2013). Although not typical of domesticated lineages, these traits have also been hypothesized to characterize alleged self-domesticated species of primates, including humans and marmosets (Ghazanfar et al., 2020).
The most conspicuous domestication-like trait in Ansell’s mole-rats is the white dorsal head spot, which results from local depigmentation of the integument. In most Fukomys species, individuals of both sexes display such a white bles (“occipital patch” – De Graaff, 1964; “blaze” – Burda, 1989). In some species, it might taper caudally along the spine (e.g., F. damarensis, Fukomys micklemi; De Graaff, 1964; pers. obs.) while it is usually constrained to the occipital region of the head in others (e.g., F. anselli, Fukomys foxi – Williams et al., 1983; pers. obs). Yet in other taxa, it is missing altogether or is only found in few individuals of a given population (F. mechowii and Fukomys vandewoestijneae – Caspar et al., 2021a; some species/populations of the F. whytei species group – Faulkes et al., 2017). In all species that express it, the size and shape of the bles varies considerably (e.g., De Graaff, 1964; Williams et al., 1983). Depigmentation in other parts of the integument might occur (particularly on the medial ventrum and the mandibular area) but is never as consistently present as the head patch (pers. obs.). Apart from efforts to assess its potential value for taxonomy, the Fukomys head patch has received little research attention, and both the proximate and ultimate causes for its expression remain enigmatic.
Since partial depigmentation of the integument is often championed as being a hallmark indicator of (self-)domestication (Sánchez-Villagra and van Schaik, 2019), a nuanced examination of its evolutionary ties to social behavior in Fukomys appears warranted. To support the notion of self-domestication, a correlation between head patch expression and other traits considered to derive from selection against aggression should be demonstrable at the individual level (Ghazanfar et al., 2020).
In this study, we aim to explore whether individual personalities are present in Ansell’s mole-rats and how they are linked to sex, social status, and the expression of the white dorsal head patch. Over the past decades, numerous works have demonstrated different temporally stable personalities or behavioral syndromes in populations of vertebrates as well as invertebrates (Gosling, 2001; Sih et al., 2004; Bell et al., 2009; Kralj-Fišer and Schütt, 2014), and it would come as a surprise if Ansell’s mole-rat would not comply to this concept. The current literature on animal personality considers five major temperament trait categories or personality dimensions: aggressiveness, boldness, exploration, activity, and sociability (Sih et al., 2004; Réale et al., 2007; Beckmann and Biro, 2013). Personality studies on rodents so far included species such as domestic guinea-pigs (Cavia porcellus – Zipser et al., 2013), bank voles (Myodes glareolus – Šěchová et al., 2014), ground squirrels (Urocitellus beldingi – Dosmann et al., 2015), and as a representative of the group of subterranean rodents, the Talas tuco-tuco (Ctenomys talarum – Fanjul and Zenuto, 2020). Individuals that show consistent behavior over time and across contexts for two or more behavioral traits are considered to have a personality. However, in some studies only one of these criteria was met (and also just for some behaviors that have been studied) but the concept of animal personality was considered to apply to the species anyway (Zipser et al., 2013; Fanjul and Zenuto, 2020).
Based on the notable sexual dimorphism in body size and weaponry (Caspar et al., 2021b), we expected to find personality differences between the sexes in Ansell’s mole-rats, particularly in feistiness and aggressiveness. Furthermore, we hypothesized that reproductive and non-reproductive individuals represent different behavioral types concerning boldness and/or activity based on previous observations in the wild as well as captivity (Šklíba et al., 2016; Zöttl et al., 2016; Houslay et al., 2020). Since genetic studies have shown that behavioral types are to some extent heritable (van Oers et al., 2005; van Oers and Mueller, 2010), we also expected that individuals of the same family would cluster closer together than chance would suggest. Finally, the self-domestication hypothesis proclaims that more intense integumental depigmentation is indicative of a stronger disruption of neural crest cell migration, predicting that head patch size in mole-rats would correlate with increased docility and social tolerance (Ghazanfar et al., 2020) – at least if this trait is assumed to represent an evolutionary byproduct of selection against aggression. To our best knowledge, this is the first attempt to test the applicability of the self-domestication concept in a non-domesticated rodent species by studying the potential coupling of individual-level personality and morphology.
Materials and Methods
Subjects
A total of 51 Ansell’s mole-rats (F. anselli; karyotype: 2n = 68) from nine families have been studied. This species attains sexual maturity at approximately 18 months (Bappert et al., 2012). We therefore classified animals with an age of at least 548 days at the time of the first experimental trial as adults, and categorized them either as “reproductive” or “non-reproductive” based on social status. Animals that were less than 548 days old at the first trial were classified as “immatures.” The mean age ± SD of reproductive animals (6 males and 6 females) was 3,103±1,279 days (range: 1,915–6,135). Non-reproductive adults (15 males and 5 females) were on average 965±328 (595–1,894) days old. Immature juveniles (11 males and 8 females) had a mean age of 349±173 days (37–531).
All mole-rats were born in captivity at the University of Duisburg-Essen and genealogically derive from founder individuals captured in the vicinity of Lusaka, Zambia, which is the type locality of the species (Begall et al., 2021a). The founder individuals (approximately 150) came to Europe in the course of several expeditions undertaken between the mid 1980s and early 2010s. The animals were kept in glass terraria ranging in size (W × L × H) from 45 cm × 70 cm × 40 cm to 60 cm × 140 cm × 40 cm, depending on family size. The terraria were littered with sawdust and enriched with flower pots serving as nest chambers, and plastic or wooden tubes. Hay and paper tissue strips were regularly provided as nesting material. Room temperature was kept constant at 24 ± 1°C with a relative humidity of 40–50%. Light conditions were regulated with an artificial light-dark cycle (12 L: 12 D, lights on at 8:00 a.m.). Given that Ansell’s mole-rat families behave highly xenophobic in captivity (Begall et al., 2021a), they are kept in isolation from each other.
Experimental Behavioral Assays and Quantification of Head Patch Size
Different dimensions of animal personality were quantified in five experimental set-ups.
Open-Field Test
We used an open-field assay to assess activity and exploration. Each test lasted 5 min and was video-recorded. The animal was placed in the middle of a uniformly illuminated custom-made open-field set-up (80 cm × 80 cm, 8 × 8 = 64 squares with an edge length of 10 cm each), the floor and walls (height: 29 cm) of which were made of non-transparent PVC. We used ANY-maze software (version 5.3, Stoelting group) to measure the covered distance (and speed) of the individuals exploring the open field. Furthermore, we measured the time the animals spent in the center of the field (inner 4 × 4 = 16 squares).
Bubble Wrap Test
To assess risk-taking behavior, we used a custom-made set-up of two cubic boxes (plexiglass, edge length = 20 cm) that were connected by a 40 cm long tunnel (plexiglass, width: 15 cm, height: 10 cm). The tunnel floor was lined with several layers of commercial bubble wrap, covered in plastic foil (width: 15 cm, length: 20 cm, height: 1 cm). Since the bubble wrap covered the tunnel floor from wall to wall, the animals had to walk over it in order to cross the tunnel. One of the boxes (left/right) served as a starting chamber, the identity of which was pseudorandomized. A metal grid closed the entrance to the tunnel, which was opened approximately 10 s after the animal was put into the starting chamber. We measured the latency from opening the starting chamber to the animal first touching the bubble wrap with at least one paw; furthermore, we measured the total time to the completion of the task (from opening the starting chamber until the subject stood with all four paws on the foil). If the animal did not complete the task within 5 min, it was returned to its respective home terrarium. The maximum time of 300 s was noted in this case. We conducted a control test (same set-up without bubble wrap) to investigate whether the bubble wrap was indeed treated as a relevant obstacle by the mole-rats.
Aggressive Encounter
For the aggressive encounter test, we used the same set-up as for the bubble wrap test (but omitting the bubble wrap lining) with an unfamiliar same-sex individual of a different family being put into one of the boxes. A metal grid prevented direct contact between both individuals. The focal animal was put into the starting chamber and after approximately 10 s the metal grid was opened, allowing the focal animal to explore the tunnel leading to the box with the unfamiliar conspecific. We measured the time the focal animal spent near the metal grid separating it from its conspecific, the time the subject lingered at the metal grid when the unfamiliar animal was simultaneously present (close contact), and the number of times the focal animal got in contact with the metal grid. During this assay, we regularly observed behaviors unambiguously indicating aggressiveness or fierce arousal like biting into the grid, intensive sniffing, urinating at the grid, and hopping with the hind feet. Each trial lasted 5 min. The close contact time was measured as an absolute value, but it should be replaced by relative values in future studies.
Affiliative Encounter
We used the same set-up and measured the same variables as for the aggressive encounter assay, but this time a familiar individual (same sex as the focus animal) of the same family was put into box 2. Behaviors observed during the affiliative encounter set-up were friendly (accompanied by contact calls) or neutral but never aggressive.
Handling
Finally, we scored the animals’ evasiveness and docility during handling on a scale of 0–3. While staying in their home terraria, the mole-rats were gently lifted up manually by the experimenter (LB) grasping the skin near the tail-base. The animals were held for approximately 10 s. A maximum of one point was given for each of the following three behaviors, yielding the highest possible score of 3 if all three were observed: Tries to escape handling by elopement; attempts to bite; emits threat vocalizations.
Behavioral Consistency
Behavioral consistency across different contexts was tested for the aggressive and affiliative encounter situations, because for both contexts the same structural set-up has been used and the same variables were measured.
After each trial all materials were cleaned with a mild detergent and dried with paper towels.
We tested each animal twice in the respective assays to check for response consistency over time. At least 4 weeks had to pass between the first and second trial. For each animal, a maximum of one test per day was performed. Not all mole-rats in our sample could be tested in all scheduled assays/trials because some of them were unexpectedly moved to another animal facility and thus unavailable for retests.
The relative size of the white head patch was quantified from photographs of the individual animals. Mole-rats were photographed in containers outside of their home terraria. Each photo was taken with the camera positioned perpendicular to the respective animal, while its head was held outstretched and in parallel to the ground. We used ImageJ (Schneider et al., 2012) to measure the total area of the patch, subsequently divided by the squared inter-aural distance, to arrive at an individual estimate of patch size relative to head dimensions. For each individual, absolute patch size and interaural distance were quantified based on a single photo. The experimenter conducting the behavioral assays (LB) was blind to the aim of the study concerning patch size-related analyses.
Data Analysis
All statistical analyses were performed in R Studio (R Core Team, 2021). Data and model residuals were tested for normal distribution by means of the Shapiro–Wilk test. In case of a detected normal distribution, parametric tests have been used, otherwise the data were transformed by applying log(x + 1) or a square root function. For pairwise comparisons [e.g., results of trial 1 versus results of trial 2, total time to cross the tunnel with bubble wrap versus without (control) pairwise t-tests or paired Wilcoxon-tests have been employed].
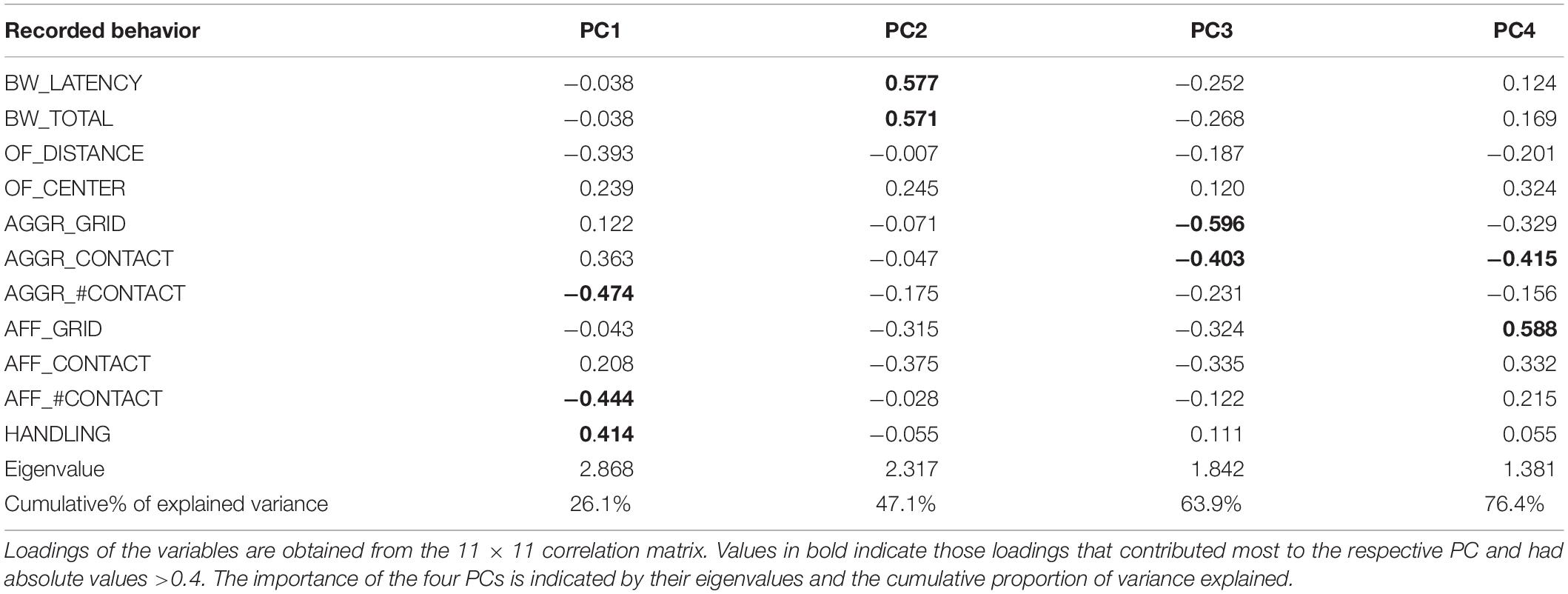
Based on the five experimental set-ups, a total of 11 behaviors (plus one control test) were recorded across two test series (trial 1/trial 2). For each of the eleven behavioral variables, the mean of the two trials obtained for each individual was calculated and analyzed using principal component analysis (PCA), employing the pr.comp()-function of the R package MASS (Venables and Ripley, 2002). Since only complete individual data-sets could be used, the sample size fed into the PCA was confined to 44 individuals. Principal components (PCs) with eigenvalues >1.0 were retained for interpretation and a subsequent discriminant function analysis. Following Martin and Réale (2008), variables with factor loadings >0.4 were considered to contribute greatly to the respective component.
We used the individual PC scores generated by the PCA to perform a leave-one-out cross-validated linear discriminant function analysis. Box’s M test has been employed to test for homogeneity of covariance matrices and the multivariate Shapiro–Wilk test for checking normality. Here, we tested how well sex and status groups could be differentiated based on the behavioral measurements. Furthermore, we tested whether the animals could be reliably assigned to their respective family by using one-tailed exact binomial tests. Since only families with at least four members were considered for these group assignments, the respective dataset was reduced to 34 animals from five different families.
We calculated additive linear models with the behavioral measurements as dependent variables and sex, status (reproductive, non-reproductive, and immature), patch size and age as explanatory variables [function lm() in the R stats package]. The assumptions for linear models have been checked visually, and Gaussian distribution of the residuals was further confirmed by a Shapiro–Wilk test for normality (p > 0.05). We also ran an additive linear model with relative patch size as the dependent variable, taking sex, status, and body mass as explanatory variables into account. This way, we tried to identify factors which might bias head patch expression and thus would need to be addressed in analyses concerning this trait. Since the residuals were not normally distributed even after log transformation, we excluded three outliers. In addition to the linear model, we ran a t-test in order to compare relative patch size between males and females.
Results
Behavioral Consistency Over Time, Habituation, and Interindividual Variation
The values of most behavioral variables measured in trial 1 and trial 2 (except CONTROL and AFF_CONTACT) were moderately but significantly correlated (Table 1) thus showing temporal consistency. Habituation only appeared to have occured for the two variables measured in the open-field test, but not for any other. The coefficients of variations calculated over the means were rather high for each test indicating high interindividual variance. The latencies for crossing the tunnel in the control test (set-up without bubble wrap) were in both trials significantly lower than in those with bubble wrap (paired Wilcoxon test, trial 1: V = 889, p < 0.00001; trial 2: V = 822, p < 0.00001) indicating that the bubble wrap can be considered an obstacle.
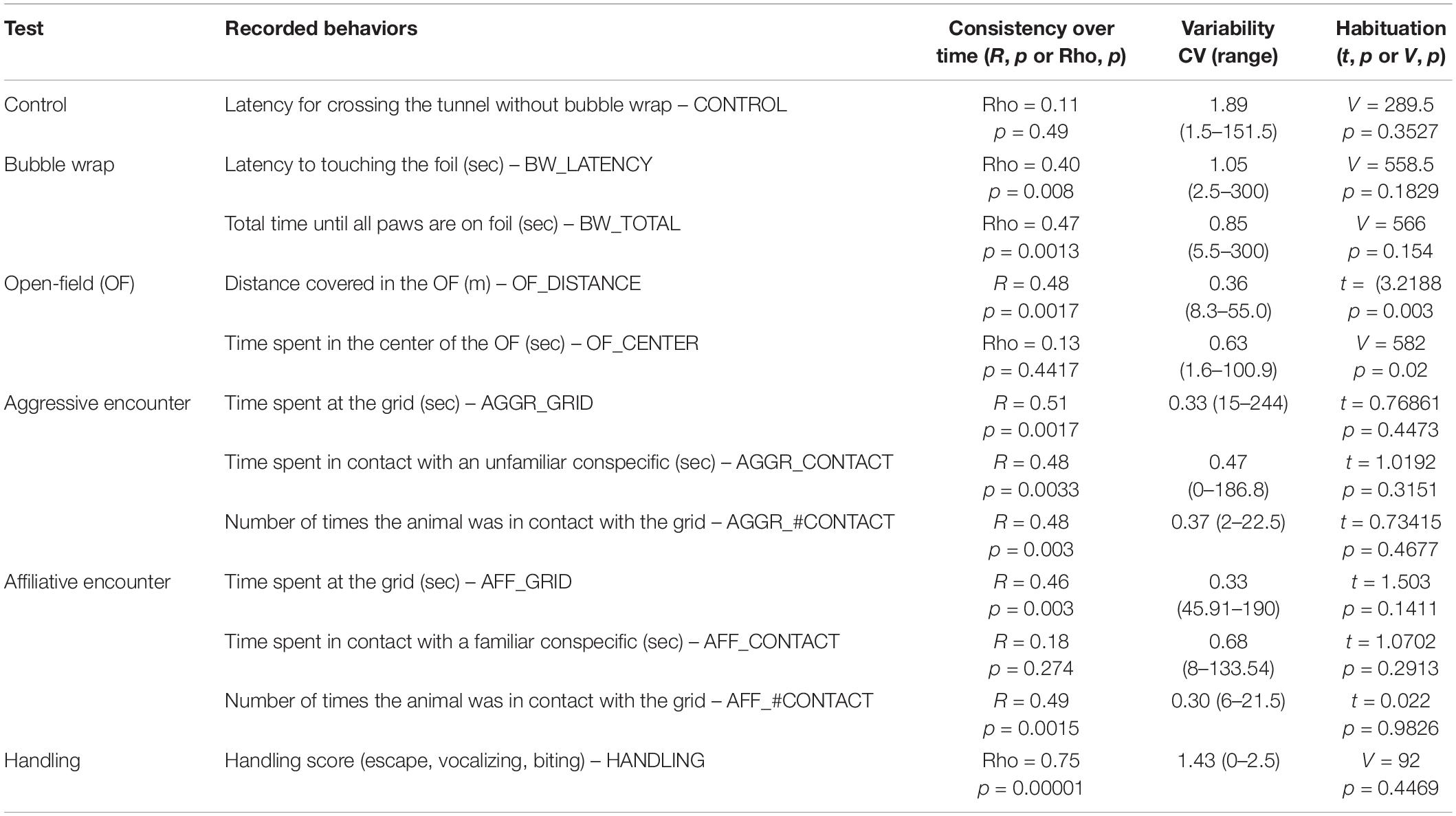
Table 1. Recorded behaviors for n = 51 individuals in five tests (plus control), consistency over time (correlation between trial 1 versus trial 2, Spearman: rho, Pearson: R), variability (coefficient of variation CV measured over mean values), and habituation tests (paired t-test: t or Wilcoxon test: V).
Stability of Behavioral Responses Across Contexts
In the social encounter assays, the time individuals spent near the metal grid when an unfamiliar same-sex conspecific was in the adjacent compartment (AGGR_GRID) did not correlate with the time near the grid when a family member was confined (AFF_GRID) (p = 0.47, r = 0.11). There was, however, a significant moderate correlation (p = 0.035, r = 0.32) for the contact time (i.e., when the other individual was in close contact to the grid as well) for the two set-ups (AGGR_CONTACT and AFF_CONTACT). Also, the number of contacts measured in the two set-ups (AGGR_#CONTACT and AFF_#CONTACT) were significantly correlated (p < 0.0001, r = 0.6).
Principal Component Analysis of Animal Personality Variables
We retained the first four principal components of the PCA that explained 76.4% of the total variance (Table 2). PC1 explained 26.1% of the total variance and loaded highest with handling score opposed to the number of contacts during social encounter assays (measured during both aggressive and affiliative encounters) (Figure 1A). This dimension thus encompasses the shy-bold continuum (docility during handling) and exploration (number of visits at the grid). The second principal component explained 21.1% of variance and encompassed variables related to the bubble wrap assay, therefore aligning with neophobia/exploration. PC3 loaded highest with variables measuring aggressiveness, while the time spent near the grid during affiliative encounters were recovered as the one that loaded highest for PC4, which thus corresponded to sociability. PC5 (loadings for respective variables not shown) had an eigenvalue of 0.86 and loaded significantly with the time spent in the center of the OF (loading factor: −0.66) and the distance covered in the OF (loading factor: −0.44). PC5 can therefore be considered to align with the exploration tendency and general activity level of the subjects.
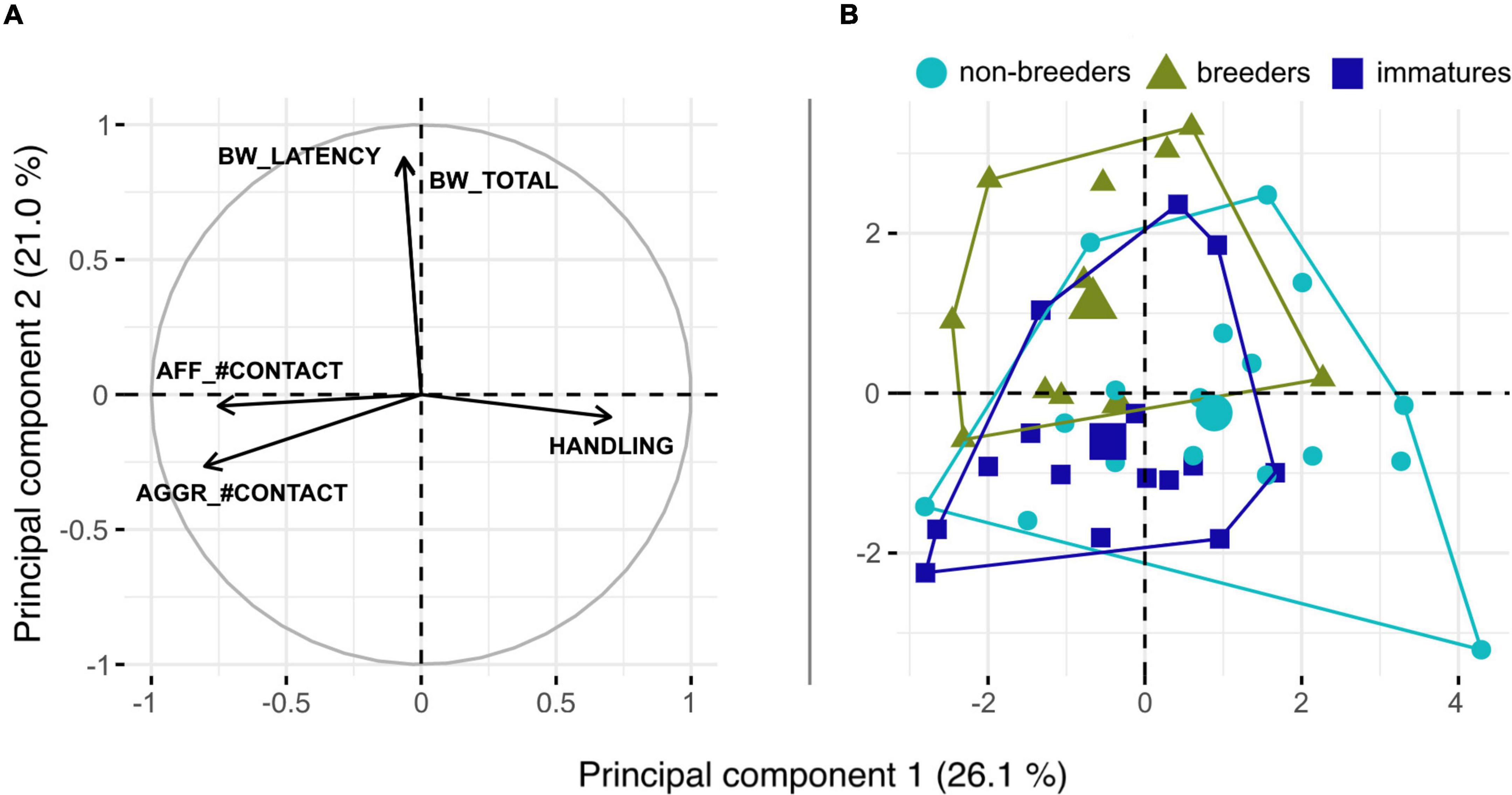
Figure 1. (A) Principal component analysis (PCA) plot showing the five variables that contributed most to the PCA. The arrows for both variables measured during the bubble wrap test, BW_LATENCY and BW_TOTAL, were parallel to each other and almost perpendicular to the variables constituting PC1. (B) PCA plot showing individuals grouped according to their social status. Enlarged symbols mark group centroids.
Linear Discriminant Function Analysis: Effects of Sex, Status, and Family Group
The PCA was not able to separate individuals well regarding sex or status based on our behavioral measurements (Figure 1B). LDA assigned 6% of the females and 89% of the males correctly which in sum did not deviate from chance levels (p > 0.05). For the three reproductive categories, LDA showed a weak trend for correct assignments [non-reproductive adults: 58.8% correct (prior probability 38.6%, p = 0.074), reproductive adults: 50% correct (prior probability 27.3%, p = 0.079), young: 53.3% (prior probability 34.1%, p = 0.099)]. Assignments of individuals to families with at least four family members (n = 5 families with 4–11 family members) yielded mixed results with proportions of correct assignments ranging between 33.3 and 100%. The mean proportion of 61.8% individuals correctly assigned to their respective family differed significantly from randomness (mean prior probability 22.1%, p < 0.000001). Thus, personalities in Ansell’s mole-rats entail a notable family group signal, while effects of sex and reproductive status appear to be negligible overall.
Linear Models: Effects of Sex, Status, and Relative Head Patch Size on Animal Personality
As indicated by the results of the LDA, breeders and non-breeders showed generally similar personalities. However, behavioral responses of reproductive Ansell’s mole-rats differed significantly from that of non-reproductive animals (but not from that of immatures) in the bubble wrap test and the handling assay but not in any of the other experiments (Tables 3, 4). Breeders took about twice as much time to touch the bubble wrap with at least one paw compared to adult non-breeders (t = 2.257, p = 0.0298) and also to stand on the bubble wrap with all four paws (t = 2.273, p = 0.0288). During handling reproductive Ansell’s mole-rats scored significantly lower compared to adult non-reproductive animals (t = −2.251, p = 0.03). Non-reproductive adults and immature animals differed significantly in the time they spent near the metal grid to which an unfamiliar conspecific was close (t = −3.291, p = 0.0021). According to the linear models, sex had no influence on any of the dependent variables except for handling where we found a trend in the additive model (Table 4, F = 3.914, p = 0.055). Wilcoxon signed rank test recovered that males scored significantly higher than females in the handling test (W = 303, p = 0.042). There was a trend for age across all three status groups to have an effect on affiliative behavior (AFF_GRID: t = −1.919, p = 0.062; AFF_CONTACT: t = −1.719, p = 0.093; AFF_#CONTACT: t = −1.848, p = 0.072) but not on any of the other dependent variables. In addition, we used PC1 as a dependent variable including all behavioral measures. The linear model revealed no significant overall effect of sex, status or age (F5,38 = 2.274, p = 0.067), but immature individuals had significantly lower PC1 values compared to non-reproductive adults (t = −2.038, p = 0.049) while there were no significant differences for the other status group comparisons. None of the interactions of sex and status yielded significant results for the behavioral measures or PC1.
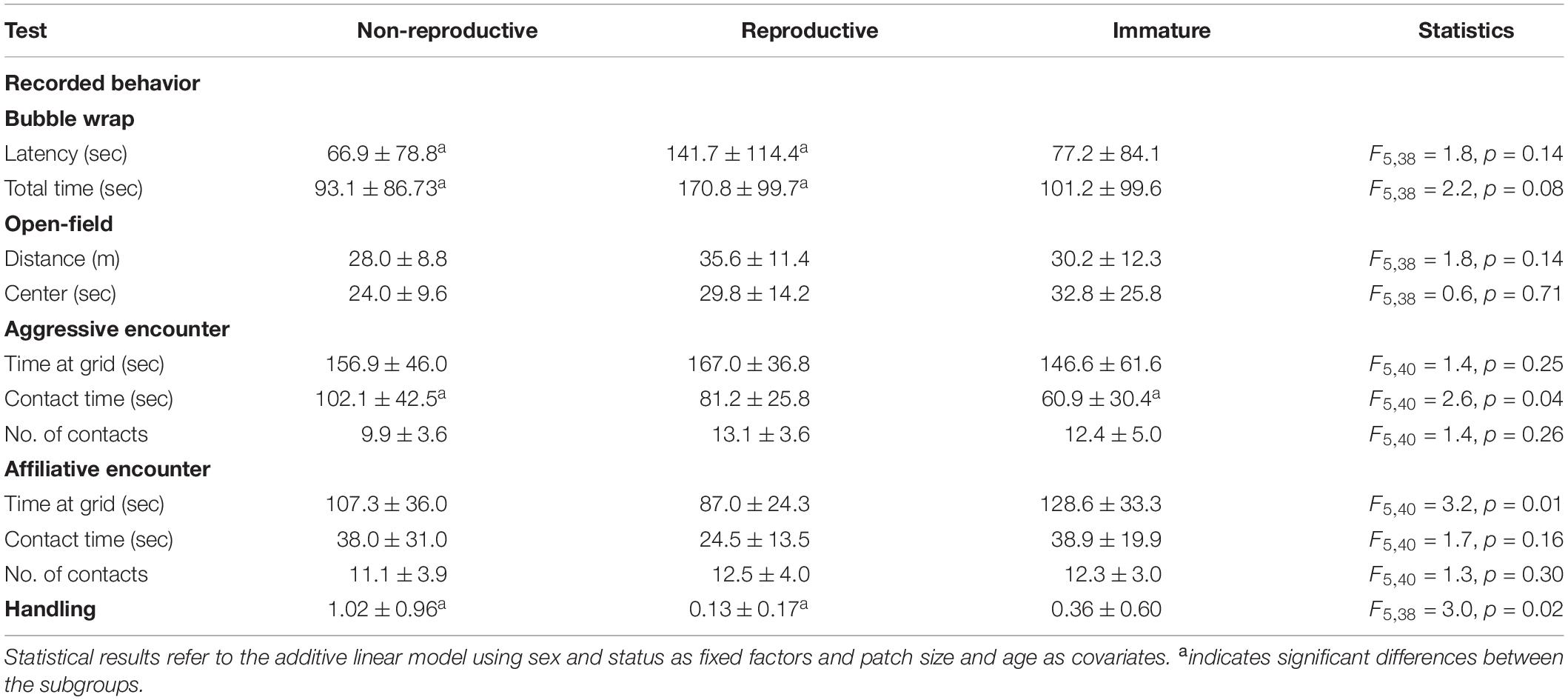
Table 3. Mean values ± SD of 11 behaviors recorded in five tests for the three status groups (non-reproductive adults, reproductive adults, and young).

Table 4. Results of the additive linear models for each behavioral measurement and principal component 1.
Figure 2 gives an overall impression of the relative patch size distribution in our sample itemized for females and males. The respective linear additive model showed no significant effect of sex, status, or body mass on relative head patch size (F4,38 = 1.663, p = 0.179). However, the factor sex was close to the level of significance (t = −2.01; p = 0.0516). Therefore, we additionally conducted a t-test on all subjects for which the relative patch size was available, but again the factor sex was not significant (t = 1.41, p-value = 0.175). The white patch does not change its shape during development, and Ansell’s mole-rats reach their adult body mass at an age of approximately 1 year (Begall and Burda, 1998). In a second analysis, we excluded juveniles younger than 4 months, but included juveniles older than 8 months as most of them were already fully grown. Here, the picture was the same (sex was at the border of significance in the linear additive model, p = 0.064; all other factors had no effect).
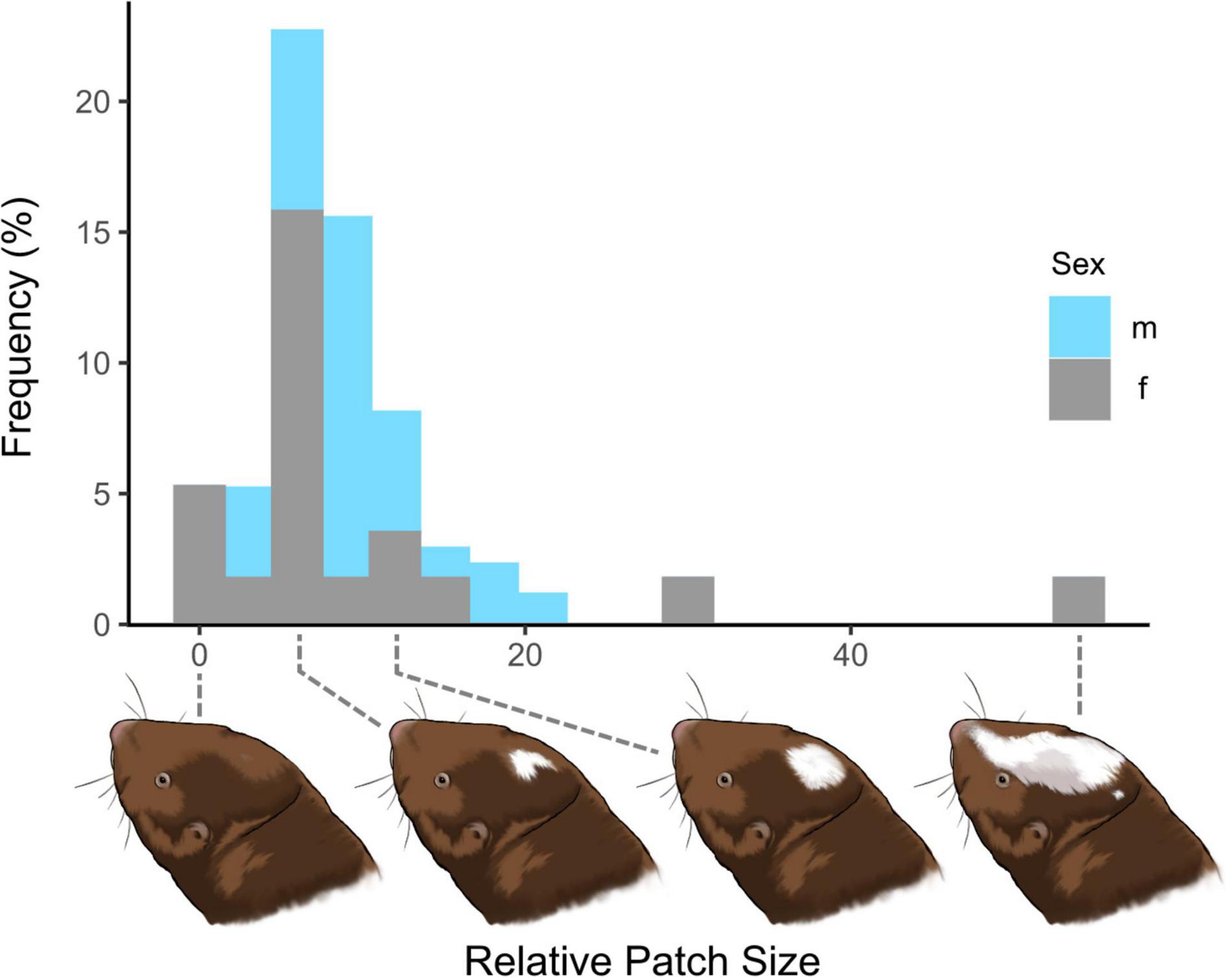
Figure 2. Frequencies of individual relative patch sizes (x-axis) in Ansell’s mole-rats. The patch size distribution in females is more variable than the one in males. Drawings of the heads by Kai R. Caspar.
We were unable to find evidence for consistent links between relative head patch size and any of the quantified behavioral responses or PC1. Animals with larger relative patch size covered more distance in the open field (t = 2.039, p = 0.049) and tended to spend less time at the metal grid during aggressive encounters (t = −2.02, p = 0.05). However, further analysis revealed that for OF_DISTANCE patch size was only decisive in the group of reproductive adults [(patch size: reproductive adults): t = 2.432, p = 0.02] while for AGGR_GRID the group of immature animals skewed the outcome [(patch size: immatures): t = −2.238, p = 0.031]. For other variables, no significant effects of patch size were found.
Discussion
Behavioral Stability
Most of the recorded behaviors (83.3%, 10 out of 12) in this study showed temporal consistency over a period of 4 weeks. Only the control trials (crossing the tunnel without obstacle) and the times an animal spent in close contact to the familiar conspecific in the affiliative behavior assay were not correlated for trial 1 and trial 2. In comparison, in Talas tuco-tucos, only about half of the recorded behaviors (55.5%) where temporally stable (Fanjul and Zenuto, 2020), and emotional behavior (e.g., OF test and dark-light test) in domestic guinea pigs was not consistent over time at all (Zipser et al., 2013). We expect that the time span of 4 weeks between experimental trials was sufficiently long for our subjects to forget about their former test experience. For instance, Burda (1995) demonstrated that non-reproductive Ansell’s mole-rats “forgot” their siblings and started courtship behavior and incestuous mating after a separation of 3 weeks – behaviors otherwise not observed due to a strict incest-avoidance based on individual recognition. We found a significant decrease of measured values, and thus an indication for habituation, only in the two parameters recorded in the open field test (distance traveled in the OF and time spent in the center). It is possible that Ansell’s mole-rats can retain memories related to spatial orientation for a longer period of time. In line with that notion, it was shown that Natal mole-rats [Cryptomys (hottentotus) natalensis] were capable of remembering a relatively simple maze for at least 30 days (but not for 60 days – Du Toit et al., 2012).
We used all three recorded behaviors from the aggressive and affiliative encounter set-ups for testing context stability of responses. Two of these variables showed significant weak to moderate positive correlations. In other personality studies on rodents, context stability also appeared to be rather weak or absent, and it has been argued that it should not be a mandatory aspect to test since it is a rather volatile criterion for behavioral type assessment (Zipser et al., 2013). Overall, however, our data clearly suggest that Ansell’s mole-rats exhibit individually stable behavioral responses and thus personalities.
Personality Dimensions, Sex and Status Dependent Patterns
Unexpectedly, sex did not have an effect on any of the recorded behaviors except for handling, as was shown by the linear models and further supported by the linear discriminant analysis. However, not too much importance should be attached to the higher aggressiveness during handling in males, because handling scores were generally low. Since males are significantly larger than females and also have more formidable weaponry (i.e., hypertrophied jaws and incisors) that presumably evolved in the context of intrasexual competition (Caspar et al., 2021b), we expected that they would spend more time at the grid in the aggressive encounter set-up. On the contrary, males appear not to be generally more aggressive than females, even in situations in which they face unfamiliar conspecifics of the same sex. In line with that, a recent study on closely related giant mole-rats (F. mechowii) found that cortisol levels and thus likely reactive aggressiveness are not elevated in males compared to females in captivity (Begall et al., 2021b). In contrast to that, males of the highland tuco-tuco (Ctenomys opimus), a facultatively social South American subterranean rodent which also exhibits strong sexual dimorphism, show higher concentrations of fecal glucocorticoids than females in the wild (O’Brien et al., 2022). Future research might explore male-male tolerance in Ansell’s mole-rats and their congeners in greater depth and should explore how it aligns with the expression of morphological traits which might aid in intrasexual combat.
The significant difference in the contact time during aggressive encounters found for the three social status groups was skewed by immature animals which spent significantly less time at the grid compared to non-reproductive breeders. This finding was not unexpected because young animals should avoid conflicts due to their greater physical vulnerability. Interestingly, the average time spent at the grid was higher during the aggressive encounter assay compared to the affiliative encounter assay in all three social status groups. Since the animal confined in the box was not in distress, it might not have been appealing for the focal subject to spend more time examining a familiar conspecific – a short olfactory inspection may have sufficed to recognize it as a family member (Heth et al., 2004). It should be noted that additional behaviors recorded during the aggressive and affiliative encounters clearly corresponded to the respective situation (e.g., biting into the grid, intensive sniffing, and vocalizations) but were not quantified here. Nevertheless, such social displays should be considered and analyzed in future analogous studies.
The LDA revealed a trend for correct assignments based on reproductive status. According to the linear models, breeders needed approximately twice as much time in the bubble wrap test as adult non-breeders and therefore appeared to be less explorative and more neophobic. This finding is reflected by the behavior of free-ranging breeders that spend most of the time in the nest area, avoid the inspection of opened tunnels and contribute little to the expansion of the burrow system (Šklíba et al., 2016). It also matches the fact that breeders are usually among the last group members (together with their young) to be captured in the wild (Jacobs et al., 1991; Moolman et al., 1998). Furthermore, breeders act less bold during handling where they scored even lower than immature Ansell’s mole-rats. Otherwise, breeders and non-breeders showed a very similar performance across tasks, which is in line with the assumption that status-dependent behavioral differentiation in these rodents is overall weak (Zöttl et al., 2016) and thus not analogous to patterns found in eusocial invertebrates to which they have been compared to in the past (Burland et al., 2002).
Rather than sex or social status, the LDA recovered family group affiliation as the best available correlate of animal personality in Ansell’s mole-rats. Similar patterns have been found in unpublished previous studies (bachelor theses) on the same species (Karnik, 2014) and also in the congeneric Micklem’s mole-rat and giant mole-rat (F. micklemi – Czajkowski and Jakobi, 2015; F. mechowii – Padberg, 2016). These findings are consistent with the assumption that personality is to some extent inheritable (van Oers et al., 2005; van Oers and Mueller, 2010) and molded by social experience and other environmental conditions. Future work should focus on more detailed comparisons of family-level behavioral types in social mole-rats, particularly in the wild (see Bengston and Dornhaus, 2014, for analogous studies on ant colonies).
Finally, it is noteworthy (compare e.g., Fanjul and Zenuto, 2020) that recorded behaviors relating to social encounters contributed to a higher degree to individual differentiation in Ansell’s mole-rat personality than those concerned with activity (Figure 1A). Our results might be explained by the fact that while these animals are highly sociable, their activity levels are generally low (in captivity, up to 90% of the time is spent resting, Dammann and Burda, 2006; Schielke et al., 2012).
Are Mole-Rats of the Genus Fukomys Self-Domesticated?
We were unable to find consistent correlations between the expression of the white head patch and personality dimensions in Ansell’s mole-rats. For the two variables which showed a marginal significant association, closer inspection revealed individual status groups to skew population-level results (immatures in case of the time an animal spent near the grid in the aggressive encounter assay; breeders regarding the distance covered in the OF). In light of these findings, we currently see no indication of relative head patch size being notably linked to personality, particularly aggressiveness and affiliative tendencies in this species. Given that the self-domestication hypothesis assumes lowered aggression and increased pigmentation defects to be correlated due to their shared dependence on neural crest-derived cell-lines, our results do not conform to it. Available data on potential links between the two traits are currently scarce and inconclusive. Ghazanfar et al. (2020) recently reported a positive correlation between vocal responsiveness, a prosocial trait, and white head patch size in a very small sample of common marmosets. In contrast to that, a domestication study on rats found that although depigmentation evolved in a lab lineage selected for tameness, the quantitative trait locus for white spotting showed no genomic overlap with regions affecting tame behavior (Albert et al., 2009). Furthermore, the presence compared to the absence of spotting was not associated with increased tameness at the individual level (Albert et al., 2009). Apart from that, we are not aware of studies experimentally testing links between personality traits and the expression of depigmentation in species of domesticated or allegedly self-domesticated animals (different from coloration patterns in general, see e.g., Brunberg et al., 2013). More such research will be needed to conclude on how tightly these two factors covariate and whether their potential association differs among mammalian taxa.
Our findings on head patch expression and personality in Ansell’s mole-rats, do not lend support to the idea that Fukomys might be a self-domesticated wild rodent. At the same time, we need to acknowledge that our results represent only a small initial contribution to this issue and cannot rule out a role of self-domestication in Fukomys evolution. An important theoretical restriction of our approach and similar research on other species (compare Ghazanfar et al., 2020) is its insensitivity to ceiling effects. When alleles underlying self-domestication become fixed in the population, it has to be assumed that the residual variation in traits determined by these alleles will become uninformative to identify their dependence on selection against aggression. Yet it is unclear, how to determine whether such a ceiling has been reached in a wild species. Despite these limitations, there appears to be little indication overall that Fukomys is self-domesticated. In fact, we would argue that although Fukomys species do superficially mirror domesticated mammals in several traits summarized under the “domestication syndrome”, they acquired them over the course of adaptation to subterranean life rather than as evolutionary byproducts for selection against aggression. Thus, Fukomys might act as an admonishing example for incautiously assigning self-domestication to lineages of wild mammals. Below, we discuss prevalent ideas and current evidence regarding the evolutionary background of relevant traits occurring in the genus.
The highly variable head patch in Fukomys is one of its most striking domestication-like traits but comparisons with other subterranean rodents suggest that natural selection on pigmentation can explain its presence more parsimoniously than the self-domestication concept would do. Variably expressed head spots and other forms of integumental depigmentation are commonly observed in a number of unrelated lineages of subterranean rodents that comprise diverse social systems. They are found in varying degrees of expression in pocket gophers (Geomys – McCarley, 1951), zokors (Myospalax – Bazhenov and Pavlenko, 2020), blind mole-rats (Nannospalax and Spalax – e.g., Festetics, 1965), root rats and relatives (Cannomys and Tachyoryctes – see e.g., Eisenberg and Maliniak, 1973), and in several lineages of bathyergids other than Fukomys (Bathyergus, Georychus, Heliophobius, rarely in Cryptomys – Bennett and Faulkes, 2000; pers. obs.). Interestingly, frequent light pelage spotting is also well documented in moles (Kamm et al., 2008). Thus, there appears to be a link to subterranean life but evolutionary drivers of depigmentation in Fukomys and other underground-living mammals are not trivial to identify.
Lovegrove et al. (1993) suggested that African mole-rats use the white head patch as a “photon window” in addition to their small eyes to aid the pineal gland in responding to changes in light levels, thus allowing the entrainment of circadian rhythms. To the present day, this idea has remained untested. However, since a bles is not expressed in a number of bathyergids and various other microphthalmic mammal lineages and because depigmentation in subterranean taxa is often not restricted to the forehead, this explanation appears to be unconvincing.
Given the cryptic life of burrowing mammals, a lack of predation pressure could be hypothesized to cause these aberrant pigmentation patterns. Yet, the observation that many subterranean rodents show remarkable pelage color variation that corresponds well to the soils they dwell in Nevo (1979) and evidence that they can fall prey to diverse avian as well as mammalian carnivores, at times at surprisingly high rates (De Graaff, 1964; Németh et al., 2016), argues against negligible predation pressure. In fact, a potential adaptive function of the head spot in some subterranean groups might be aposematism. Contrasting coloration, though particularly in medium-sized mammals, has been prominently hypothesized to aid in signaling feistiness to predators (Caro, 2009), which at least appears well justified for tooth-diggers such as bathyergids and spalacids that are equipped with powerful jaws. However, the extreme individual variability of the head spots in Fukomys and other taxa argues against an aposematic function, since anti-predatory signals are expected to be selected for stability and unambiguity. Hence, although we suggest that the evolution of the Fukomys head patch relates to its ecology based on correlational evidence, we are currently unable to convincingly identify selection pressures (or a lack thereof) underlying its expression. This is possible, however, for other domestication-like traits of the genus.
The extremely low stress hormone levels in Fukomys and other bathergids, one order of magnitude below the ones of epigenetic rodents (Ganem and Bennett, 2004), are at least partially linked to their markedly low basal metabolic rate (Haase et al., 2016). Given that glucocorticoids regulate the mobilization of blood sugar, low levels of adrenal stress hormone release contribute to saving energy during resting between metabolically demanding phases of digging activity (Moshkin et al., 2007). Low metabolic rates and decreased levels of circulating stress hormones have evolved convergently in different lineages of subterranean rodents, irrespective of their social systems (e.g., Ganem and Nevo, 1996; Moshkin et al., 2007). Thus, low stress hormone levels in Fukomys constitute an adaptation to life underground that already evolved in the bathyergid stem-lineage (compare Ganem and Bennett, 2004) rather than from more recent selection against aggression. Nevertheless, although acts of severe conspecific aggression might still occur (though mostly reported from captive settings and so far, only inferred for natural populations – Caspar et al., 2021b) the low stress hormone levels in these animals likely provide a proximate explanation and possibly an exaptation for their remarkably harmonic intragroup lives (Ganem and Nevo, 1996; Ganem and Bennett, 2004; Begall et al., 2021b). Interestingly, social bathyergids (Cryptoms/Fukomys) behave agonistically when encountering a foreign conspecific, but do not show evidence for stress arousal, which contrasts with the responses of Cape mole-rats (Georychus capensis), a solitary species (Ganem and Bennett, 2004). Although the adrenal glands in Ansell’s mole-rats only secrete low hormone concentrations, they are surprisingly large, with an adult mean length of 3.5 mm (Kowalski, 1996). Hence, the average adrenal gland size in Ansell’s mole-rats exceeds that of full-grown laboratory rats (2.9 mm long – Siasios et al., 2021). This is the opposite of what would be expected if domestication-like evolutionary trajectories are assumed for Fukomys (Albert et al., 2009) and also appears to be add odds with the low stress hormone levels described for the genus. Adrenal morphology and physiology in African mole-rats might become an interesting subject for future research.
The subterranean lifestyle of Fukomys could also underlie their relatively small brains (Kruska and Steffen, 2009), because the disproportionate caloric demand of the central nervous system needs to be curbed to sustain energy-intensive digging activity (Kverková et al., 2018). It should be noted, however, that Fukomys brains are not smaller relative to body size or contain less neurons than the brains of solitary African mole-rats and that the bathyergid allometric brain-body ratio does not significantly differ from other rodents (Kverková et al., 2018).
Finally, sophisticated vocal behaviors have been discussed as indicators for self-domestication (Ghazanfar et al., 2020), although they are in fact not apparent in most domestic mammal lineages. Indeed, the vocal communication in Fukomys and other social bathyergids is more elaborate than in other rodents (Bednářová et al., 2013), but this pattern follows a general trend linking increased sociality to greater vocal repertoires in the rodent order (Lima et al., 2018). Vocal communication in African mole-rats is a topic of ongoing research, with particular attention being paid to the naked mole-rat, in which a complex interplay between social variables and vocalizations has been described (Barker et al., 2021). However, since little comparative data on other bathyergids as well as cooperatively-breeding rodents in general is available, it is hard to assess how unusual these behaviors actually are.
Conclusion
Selected behaviors of Ansell’s mole-rats demonstrated a high degree of intraindividual temporal stability, strongly indicating that this subterranean rodent species displays different personalities. In contrast to expectation, sex had no effect on the recorded behaviors, but reproductive status was decisive for some dimensions of personality, with breeders being less explorative than non-reproductive adults. Family affiliation was more strongly correlated with an animal’s personality than sex or reproductive status were.
We found no consistent link between the expression of the white head patch and personality dimensions in Ansell’s mole-rat and thus no indication that this morphological trait evolved in the framework of self-domestication. More data on this issue would nevertheless be crucial to confidently rule out this option. Although a number of traits in Fukomys appear to fit the “(self-)domestication syndrome,” closer inspection suggests them being consequences of adaptation to life underground or extensions of patterns observable among African mole-rats or social rodents in general. Thus, Fukomys might serve as a reminder to be cautious, when trying to explain characteristics of wild mammals by means of self-domestication. This is especially true for cases in which selective comparisons of traits between disparate groups are attempted which might miss the ecological and phylogenetic context in which the respective species evolved.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because animal housing (approved by permit no. 32-2-1180-71/328 Veterinary Office of the City of Essen) as well as all experiments complied with the animal testing regulations of the country where they were performed and were approved by the animal welfare officer. No ethical permissions were necessary. All behavioral tests conformed to the relevant ethical standards and did not harm the animals.
Author Contributions
SB and KRC conceived the study and its methodology. LB performed the behavioral tests. KRC photographed the heads, measured the relevant parameters, and prepared the figures. SB calculated the relative head patch size. SB and LB analyzed the data. All authors interpreted the findings. SB and KRC wrote the first draft, which was reviewed by LB. All authors contributed to the article and approved the submitted version.
Funding
KRC was supported by a Ph.D. fellowship of the German National Academic Foundation (Studienstiftung des deutschen Volkes e.V.). We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank two reviewers and the handling editor AM for their constructive comments on the text.
References
Albert, F. W., Carlborg, O., Plyusnina, I., Besnier, F., Hedwig, D., Lautenschläger, S., et al. (2009). Genetic architecture of tameness in a rat model of animal domestication. Genetics 182, 541–554. doi: 10.1534/genetics.109.102186
Bappert, M.-T., Burda, H., and Begall, S. (2012). To mate or not to mate? mate preference and fidelity in monogamous Ansell’s mole-rats, Fukomys anselli, Bathyergidae. Folia Zool. 61, 71–83. doi: 10.25225/fozo.v61.i1.a11.2012
Barker, A. J., Veviurko, G., Bennett, N. C., Hart, D. W., Mograby, L., and Lewin, G. R. (2021). Cultural transmission of vocal dialect in the naked mole-rat. Science 371, 503–507. doi: 10.1126/science.abc6588
Bazhenov, Y. A., and Pavlenko, M. V. (2020). Distribution of zokors (Myospalax, Rodentia) in Transbaikalia. Biol. Bull. 47, 1235–1244. doi: 10.1134/s1062359020090034
Beckmann, C., and Biro, P. A. (2013). On the validity of a single (boldness) assay in personality research. Ethology 119, 937–947.
Bednářová, R., Hrouzková-Knotková, E., Burda, H., Sedláčk, F., and Šumbera, R. (2013). Vocalizations of the giant mole-rat (Fukomys mechowii), a subterranean rodent with the richest vocal repertoire. Bioacoustics 22, 87–107. doi: 10.1080/09524622.2012.712749
Begall, S., and Burda, H. (1998). Reproductive characteristics and growth rate in the eusocial Zambian common mole-rat (Cryptomys sp., Bathyergidae). Z Säugetierk. 63, 297–306.
Begall, S., Burda, H., and Caspar, K. R. (2021a). Fukomys anselli (Rodentia: Bathyergidae). Mammal. Spec. 53, 160–173. doi: 10.1093/mspecies/seab015
Begall, S., Nappe, R., Hohrenk, L., Schmidt, T. C., Burda, H., Sahm, A., et al. (2021b). Life expectancy, family constellation and stress in giant mole-rats (Fukomys mechowii). Phil. Trans. R. Soc. B Biol. Sci. 376:20200207. doi: 10.1098/rstb.2020.0207
Bell, A. M., Hankison, S. J., and Laskowski, K. L. (2009). The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. doi: 10.1016/j.anbehav.2008.12.022
Bengston, S. E., and Dornhaus, A. (2014). Be meek or be bold? a colony-level behavioural syndrome in ants. Proc. R. Soc. B. 281:20140518. doi: 10.1098/rspb.2014.0518
Bennett, N. C., and Faulkes, C. G. (2000). African Mole-rats: Ecology and Eusociality. Cambridge: Cambridge University Press.
Brunberg, E., Gille, S., Mikko, S., Lindgren, G., and Keeling, L. J. (2013). Icelandic horses with the silver coat colour show altered behaviour in a fear reaction test. Appl. Anim.Behav. Sci. 146, 72–78. doi: 10.1016/j.applanim.2013.04.005
Burda, H. (1989). Reproductive biology (behaviour, breeding, and postnatal development) in subterranean mole-rats, Cryptomys hottentotus (Bathyergidae). Z Säugetierk 54, 360–376.
Burda, H. (1995). Individual recognition and incest avoidance in eusocial common mole-rats rather than reproductive suppression by parents. Experientia 51, 411–413. doi: 10.1007/BF01928906
Burda, H., Honeycutt, R. L., Begall, S., Grütjen, O., and Scharff, A. (2000). Are naked and common mole-rats eusocial and if so, why? Behav. Ecol. Sociobiol. 47, 293–303. doi: 10.1007/s002650050669
Burland, T. M., Bennett, N. C., Jarvis, J. U. M., and Faulkes, C. G. (2002). Eusociality in African mole-rats: new insights from patterns of genetic relatedness in the Damaraland mole-rat (Cryptomys damarensis). Proc. R. Soc. Lond. B 269, 1025–1030. doi: 10.1098/rspb.2002.1978
Caro, T. (2009). Contrasting coloration in terrestrial mammals. Phil. Trans. R. Soc. B Biol. Sci. 364, 537–548. doi: 10.1098/rstb.2008.0221
Caspar, K. R., Moldenhauer, K., Moritz, R. E., Němec, P., Malkemper, E. P., and Begall, S. (2020). Eyes are essential for magnetoreception in a mammal. J. Roy. Soc. Interface 17:20200513. doi: 10.1098/rsif.2020.0513
Caspar, K. R., Burda, H., and Begall, S. (2021a). Fukomys mechowii (Rodentia: Bathyergidae). Mammal. Spec. 53, 145–159. doi: 10.1093/mspecies/seab014
Caspar, K. R., Müller, J., and Begall, S. (2021b). Effects of sex and breeding status on skull morphology in cooperatively-breeding Ansell’s mole-rats and an appraisal of sexual dimorphism in the Bathyergidae. Front. Ecol. Evol. 9:638754. doi: 10.3389/fevo.2021.638754
Czajkowski, J., and Jakobi, R. (2015). Exploration als Persönlichkeitsmerkmal bei Micklem-Graumullen (Fukomys micklemi). Bachelor thesis, Essen: University of Duisburg-Essen.
Dammann, P., and Burda, H. (2006). Sexual activity and reproduction delays aging in a mammal. Curr. Biol. 16, R117–R118. doi: 10.1016/j.cub.2006.02.012
Dammann, P., Šumbera, R., Massmann, C., Scherag, A., and Burda, H. (2011). Extended longevity of reproductives appears to be common in Fukomys mole-rats (Rodentia, Bathyergidae). PLoS One 6:e18757. doi: 10.1371/journal.pone.0018757
De Graaff, O. (1964). A Systematic Revision of the Bathyergidae (Rodentia) of Southern Africa. Ph.D. thesis, Pretoria: University of Pretoria.
Dosmann, A. J., Brooks, K. C., and Mateo, J. M. (2015). Within-individual correlations reveal link between a behavioral syndrome, condition, and cortisol in free-ranging Belding’s ground squirrels. Ethology 121, 125–134. doi: 10.1111/eth.12320
Du Toit, L., Bennett, N. C., Nickless, A., and Whiting, M. J. (2012). Influence of spatial environment on maze learning in an African mole-rat. Anim. Cogn. 15, 797–806. doi: 10.1007/s10071-012-0503-0
Eisenberg, J. F., and Maliniak, E. (1973). Breeding and captive maintenance of the Lesser bamboo rat Cannomys badius. Int. Zoo Yearbook 13, 204–207. doi: 10.1111/j.1748-1090.1973.tb02149.x
Fanjul, M. S., and Zenuto, R. R. (2020). Personality underground: evidence of behavioral types in the solitary subterranean rodent Ctenomys talarum. PeerJ 8:e8490. doi: 10.7717/peerj.8490
Faulkes, C. G., Mgode, G. F., Archer, E. K., and Bennett, N. C. (2017). Relic populations of Fukomys mole-rats in Tanzania: description of two new species F. livingstoni sp. nov. and F. hanangensis sp. nov. PeerJ 5:e3214. doi: 10.7717/peerj.3214
Festetics, A. (1965). Beiträge zur Ethologie, Ökologie und Geographischen Verbreitung der Spalax leucodon Nordmann, 1840. PhD thesis, Vienna: University of Vienna.
Francioli, Y., Thorley, J., Finn, K., Clutton-Brock, T., and Zöttl, M. (2020). Breeders are less active foragers than non-breeders in wild Damaraland mole-rats. Biol. Lett. 16:20200475. doi: 10.1098/rsbl.2020.0475
Ganem, G., and Bennett, N. C. (2004). Tolerance to unfamiliar conspecifics varies with social organization in female African mole-rats. Physiol. Behav. 82, 555–562. doi: 10.1016/j.physbeh.2004.05.002
Ganem, G., and Nevo, E. (1996). Ecophysiological constraints associated with aggression, and evolution toward pacifism in Spalax ehrenbergi. Behav. Ecol. Sociobiol. 38, 245–252. doi: 10.1007/s002650050239
Geiger, M., Sánchez-Villagra, M. R., and Lindholm, A. K. (2018). A longitudinal study of phenotypic changes in early domestication of house mice. R. Soc. Open Sci. 5:172099. doi: 10.1098/rsos.172099
Gerhardt, P., Henning, Y., Begall, S., and Malkemper, E. P. (2017). Audiograms of three subterranean rodent species (genus Fukomys) determined by auditory brainstem potentials reveal extremely poor high-frequency cutoffs. J. Exp. Biol. 220, 4377–4382. doi: 10.1242/jeb.164426
Ghazanfar, A. A., Kelly, L. M., Takahashi, D. Y., Winters, S., Terrett, R., and Higham, J. P. (2020). Domestication phenotype linked to vocal behavior in marmoset monkeys. Curr. Biol. 30, 5026–5032. doi: 10.1016/j.cub.2020.09.049
Gosling, S. D. (2001). From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86. doi: 10.1037/0033-2909.127.1.45
Haase, C. G., Long, A. K., and Gillooly, J. F. (2016). Energetics of stress: linking plasma cortisol levels to metabolic rate in mammals. Biol. Lett. 12:20150867. doi: 10.1098/rsbl.2015.0867
Hare, B. (2017). Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Ann. Rev. Psychol. 68, 155–186. doi: 10.1146/annurev-psych-010416-044201
Hare, B., Wobber, V., and Wrangham, R. (2012). The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. An. Behav. 83, 573–585. doi: 10.1016/j.anbehav.2011.12.007
Heth, G., Todrank, J., Begall, S., Wegner, R. E., and Burda, H. (2004). Genetic relatedness discrimination in a eusocial rodent, Cryptomys anselli molerats. Folia Zool. 53, 269–278.
Houslay, T. M., Vullioud, P., Zöttl, M., and Clutton-Brock, T. H. (2020). Benefits of cooperation in captive Damaraland mole-rats. Behav. Ecol. 31, 711–718. doi: 10.1093/beheco/araa015
Jacobs, D. S., Bennett, N. C., and Jarvis, J. U. M. (1991). The colony structure and dominance hierarchy of the Damaraland mole rat, Cryptomys damarensis (Rodentia: Bathyergidae), from Namibia. J. Zool. 224, 553–576. doi: 10.1111/j.1469-7998.1991.tb03785.x
Kamm, A. A., Feldhamer, G. A., and Reeve, J. D. (2008). Pelage spotting and staining in eastern moles (Scalopus aquaticus). Northeast Nat. 15, 303–308. doi: 10.1656/1092-6194(2008)15[303:psasie]2.0.co;2
Karnik, T. (2014). Studien zur Persönlichkeit bei Graumullen (Fukomys sp.). State examination thesis, Essen: University of Duisburg-Essen.
Kowalski, H. (1996). Morphologie der Nebenniere bei sozial lebenden Graumullen (Cryptomys sp., Bathyergidae, Rodentia). State examination thesis, Essen: University of Essen.
Kralj-Fišer, S., and Schütt, W. (2014). Studying personality variation in invertebrates: why bother? Anim. Behav. 9, 41–52. doi: 10.1016/j.anbehav.2014.02.016
Kruska, D. C., and Steffen, K. (2009). Encephalization of Bathyergidae and comparison of brain structure volumes between the Zambian mole-rat Fukomys anselli and the giant mole-rat Fukomys mechowii. Mammal. Biol. 74, 298–307. doi: 10.1016/j.mambio.2008.04.002
Kverková, K., Bìlíková, T., Olkowicz, S., Pavelková, Z., O’Riain, M. J., Šumbera, R., et al. (2018). Sociality does not drive the evolution of large brains in eusocial African mole-rats. Sci. Rep. 8:9203. doi: 10.1038/s41598-018-26062-26068
Leedale, A. E., Thorley, J., and Clutton-Brock, T. (2021). Odour-based social recognition in Damaraland mole-rats, Fukomys damarensis. Anim. Behav. 79, 83–96. doi: 10.1016/j.anbehav.2021.06.019
Lima, S. G., Sousa-Lima, R. S., Tokumaru, R. S., Nogueira-Filho, S. L., and Nogueira, S. S. (2018). Vocal complexity and sociality in spotted paca (Cuniculus paca). PLoS One 13:e0190961. doi: 10.1371/journal.pone.0190961
Lord, K. A., Larson, G., Coppinger, R. P., and Karlsson, E. K. (2020). The history of farm foxes undermines the animal domestication syndrome. Trends Ecol. Evol. 35, 125–136. doi: 10.1016/j.tree.2019.10.011
Losey, R. J. (2021). Domestication is not an ancient moment of selection for prosociality: insights from dogs and modern humans. J. Soc. Archaeol. 14696053211055475.
Lovegrove, B. G., Heldmaier, G., and Ruf, T. (1993). Circadian activity rhythms in colonies of “blind” mole-rats, Cryptomys damarensis (Bathyergidae). S. Afr. J. Zool. 28, 46–55. doi: 10.1080/02541858.1993.114482
Lövy, M., Šklíba, J., and Šumbera, R. (2013). Spatial and temporal activity patterns of the free-living giant mole-rat (Fukomys mechowii), the largest social bathyergid. PLoS One 8:e55357. doi: 10.1371/journal.pone.0055357
Marhold, S., Wiltschko, W., and Burda, H. (1997). A magnetic polarity compass for direction finding in a subterranean mammal. Naturwiss 84, 421–423. doi: 10.1007/s001140050422
Martin, J. G. A., and Réale, D. (2008). Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim. Behav. 75, 309–318. doi: 10.1016/j.anbehav.2007.05.026
McCarley, W. H. (1951). Color mutations in a small, partially isolated population of pocket gophers (Geomys breviceps). J. Mammal. 32, 338–341. doi: 10.2307/1375666
Moolman, M., Bennett, N. C., and Schoeman, A. S. (1998). The social structure and dominance hierarchy of the highveld mole rat Cryptomys hottentotus pretoriae (Rodentia: Bathyergidae). J. Zool. 246, 193–201. doi: 10.1111/j.1469-7998.1998.tb00148.x
Moshkin, M., Novikov, E., and Petrovski, D. (2007). “Skimping as an adaptive strategy in social fossorial rodents: the mole vole (Ellobius talpinus) as an example,” in Subterranean Rodents: News from Underground, eds S. Begall, H. Burda, and C. E. Schleich (Heidelberg: Springer), 49–60. doi: 10.1007/978-3-540-69276-8_5
Müller, M., and Burda, H. (1989). Restricted hearing range in a subterranean rodent, Cryptomys hottentotus. Naturwiss 76, 134–135. doi: 10.1007/BF00366611
Mynhardt, S., Harris-Barnes, L., Bloomer, P., and Bennett, N. C. (2021). Spatial population genetic structure and colony dynamics in Damaraland mole-rats (Fukomys damarensis) from the southern Kalahari. BMC Ecol. Evol. 21:221. doi: 10.1186/s12862-021-01950-1952
Němec, P., Burda, H., and Peichl, L. (2004). Subcortical visual system of the African mole-rat Cryptomys anselli: to see or not to see? Eur. J. Neurosci. 20, 757–768. doi: 10.1111/j.1460-9568.2004.03510.x
Németh, A., Hegyeli, Z., Sendula, T., Horváth, M., Czabán, D., and Csorba, G. (2016). Danger underground and in the open-predation on blind mole rats (Rodentia: Spalacinae) revisited. Mammal Rev. 46, 204–214. doi: 10.1111/mam.12062
Nevo, E. (1979). Adaptive convergence and divergence of subterranean mammals. Ann. Rev. Ecol Syst. 10, 269–308. doi: 10.1146/annurev.es.10.110179.001413
O’Brien, S. L., Irian, C. G., Bentley, G. E., and Lacey, E. A. (2022). Sex, not social behavior, predicts fecal glucocorticoid metabolite concentrations in a facultatively social rodent, the highland tuco-tuco (Ctenomys opimus). Horm. Behav. 141:105152. doi: 10.1016/j.yhbeh.2022.105152
Padberg, M. (2016). Persönlichkeitsstudien bei Riesengraumullen (Fukomys mechowii). Bachelor thesis, Essen: University of Duisburg-Essen.
Park, T. J., Catania, K. C., Samaan, D., and Comer, C. M. (2007). “Adaptive neural organization of naked mole-rat somatosensation (and those similarly challenged),” in Subterranean Rodents: News from Underground, eds S. Begall, H. Burda, and C. E. Schleich (Heidelberg: Springer), 175–193. doi: 10.1007/978-3-540-69276-8_13
Patzenhauerová, H., Šklíba, J., Bryja, J., and Šumbera, R. (2013). Parentage analysis of Ansell’s mole-rat family groups indicates a high reproductive skew despite relatively relaxed ecological constraints on dispersal. Mol. Ecol. 22, 4988–5000. doi: 10.1111/mec.12434
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Sánchez-Villagra, M. R., and van Schaik, C. P. (2019). Evaluating the self-domestication hypothesis of human evolution. Evol. Anthropol. Issues News Rev. 28, 133–143. doi: 10.1002/evan.21777
Schielke, C. K. M., Begall, S., and Burda, H. (2012). Reproductive state does not influence activity budgets of eusocial Ansell’s mole-rats, Fukomys anselli (Rodentia, Bathyergidae): a study of locomotor activity by means of RFID. Mammal. Biol. 77, 1–5. doi: 10.1016/j.mambio.2011.09.004
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Siasios, A., Delis, G., Tsingotjidou, A., Pourlis, A., and Grivas, I. (2021). Adrenal glands of mice and rats: a comparative morphometric study. Lab. Anim. Online ahead of print. doi: 10.1177/00236772211044352
Šěchová, K., Koskela, E., Mappes, T., Lantova, P., and Boratynski, Z. (2014). On personality, energy metabolism and mtDNA introgression in bank voles. Anim. Behav. 92, 229–237. doi: 10.1016/j.anbehav.2014.04.011
Siegmann, S., Feitsch, R., Hart, D. W., Bennett, N. C., Penn, D. J., and Zöttl, M. (2021). Naked mole-rats (Heterocephalus glaber) do not specialise in cooperative tasks. Ethology 127, 850–864. doi: 10.1111/eth.13160
Sih, A., Bell, A. M., Johnson, J. C., and Ziemba, R. E. (2004). Behavioural syndromes: an integrative overview. Quart. Rev. Biol. 79, 78–277. doi: 10.1086/422893
Šklíba, J., Lövy, M., Burda, H., and Šumbera, R. (2016). Variability of space-use patterns in a free living eusocial rodent, Ansell’s mole-rat indicates age-based rather than caste polyethism. Sci. Rep. 6:37497. doi: 10.1038/srep37497
Šklíba, J., Mazoch, V., Patzenhauerová, H., Hrouzková, E., Lövy, M., Kott, O., et al. (2012). A maze-lover’s dream: burrow architecture, natural history and habitat characteristics of Ansell’s mole-rat (Fukomys anselli). Mammal. Biol. 77, 420–427. doi: 10.1016/j.mambio.2012.06.004
Theofanopoulou, C., Gastaldon, S., O’Rourke, T., Samuels, B. D., Martins, P. T., and Boeckx, C. (2017). Self-domestication in Homo sapiens: insights from comparative genomics. PLoS One 12:e0185306. doi: 10.1371/journal.pone.0185306
Thorley, J., Bensch, H., Finn, K., Clutton-Brock, T., and Zöttl, M. (2021). Fitness of breeders in social Damaraland mole-rats is independent of group size. bioRxiv [preprint] doi: 10.1101/2021.12.08.471794
Thorley, J., Mendonça, R., Vullioud, P., Torrents-Tico, M., Zöttl, M., Gaynor, D., et al. (2018). No task specialization among helpers in Damaraland mole-rats. Anim. Behav. 143, 9–24. doi: 10.1016/j.anbehav.2018.07.004
Torrents-Ticó, M., Bennett, N. C., Jarvis, J. U., and Zöttl, M. (2018). Sex differences in timing and context of dispersal in Damaraland mole-rats (Fukomys damarensis). J. Zool. 306, 252–257. doi: 10.1111/jzo.12602
Trut, L. N. (1999). Early canid domestication: the farm-fox experiment: foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development. Am. Sci. 87, 160–169. doi: 10.1511/1999.2.160
van Oers, K., and Mueller, J. C. (2010). Evolutionary genomics of animal personality. Phil. Trans. R. Soc. Lond. B Biol. Sci. 365, 3991–4000. doi: 10.1098/rstb.2010.0178
van Oers, K., de Jong, G., van Noordwijk, A. J., Kempenaers, B., and Drent, P. J. (2005). Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206. doi: 10.1163/156853905774539364
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S, 4th Edn. New York, NY: Springer.
Wegner, R. E., Begall, S., and Burda, H. (2006). Light perception in ‘blind’ subterranean Zambian mole-rats. Anim. Behav. 72, 1021–1024. doi: 10.1016/j.anbehav.2006.02.018
Wilkins, A. S., Wrangham, R. W., and Fitch, W. T. (2014). The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795–808. doi: 10.1534/genetics.114.165423
Williams, S. L., Schlitter, D. A., and Robbins, L. W. (1983). Morphological variation in a natural population of Cryptomys (Rodentia: Bathyergidae) from Cameroon. Ann. Musée R. l’Afrique Centrale Tervuren 237, 159–172.
Zipser, B., Kaiser, S., and Sachser, N. (2013). Dimensions of animal personalities in guinea pigs. Ethology 119, 970–982.
Keywords: behavioral type, burrowing rodent, neural crest, reproductive skew, sociality
Citation: Begall S, Bottermann L and Caspar KR (2022) Self-Domestication Underground? Testing for Social and Morphological Correlates of Animal Personality in Cooperatively-Breeding Ansell’s Mole-Rats (Fukomys anselli). Front. Ecol. Evol. 10:862082. doi: 10.3389/fevo.2022.862082
Received: 25 January 2022; Accepted: 30 March 2022;
Published: 20 May 2022.
Edited by:
Andrew James Jonathan MacIntosh, Kyoto University, JapanReviewed by:
James Brooks, Kyoto University, JapanMarcelo R. Sanchez, University of Zurich, Switzerland
Copyright © 2022 Begall, Bottermann and Caspar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabine Begall, c2FiaW5lLmJlZ2FsbEB1bmktZHVlLmRl
†These authors have contributed equally to this work
 Sabine Begall
Sabine Begall Lea Bottermann
Lea Bottermann Kai Robert Caspar
Kai Robert Caspar